Abstract
Background
Renal glycogen synthase kinase-3 beta (GSK3β) overactivity has been associated with a diverse range of kidney diseases. GSK3β activity in urinary exfoliated cells was reported to predict the progression of diabetic kidney disease (DKD). We compared the prognostic value of urinary and intrarenal GSK3β levels in DKD and nondiabetic chronic kidney disease (CKD).
Methods
We recruited 118 consecutive biopsy-proved DKD patients and 115 nondiabetic CKD patients. Their urinary and intrarenal GSK3β levels were measured. They were then followed for dialysis-free survival and rate of renal function decline.
Results
DKD group had higher intrarenal and urinary GSK3β levels than nondiabetic CKD (p < 0.0001 for both), but their urinary GSK3β mRNA levels were similar. Urinary p-GSK3β level is statistically significantly correlated with the baseline estimated glomerular filtration rate (eGFR), but urinary GSK3β level by ELISA, its mRNA level, the p-GSK3β level, or the p-GSK3β/GSK3β ratio had no association with dialysis-free survival or the slope of eGFR decline. In contrast, the intrarenal pY216-GSK3β/total GSK3β ratio significantly correlated with the slope of eGFR decline (r = −0.335, p = 0.006) and remained an independent predictor after adjusting for other clinical factors.
Conclusion
Intrarenal and urinary GSK3β levels were increased in DKD. The intrarenal pY216-GSK3β/total GSK3β ratio was associated with the rate of progression of DKD. The pathophysiological roles of GSK3β in kidney diseases deserve further studies.
Keywords: Podocyte, Biomarker, Diabetes, Proteinuria
Introduction
Chronic kidney disease (CKD) is an important and costly noncommunicable disease [1]. Although the kidney consists of multiple cell types that are arranged in a delicate 3-dimensional architecture, podocyte has long been implicated as the primary focus of many kidney diseases [2, 3], and markers of podocyte injury have been extensively studied as biomarkers of kidney diseases [4–6]. However, renal tubular cell is the most abundant cell type in the kidney; proximal tubular cells are particularly active metabolically and play a pivotal role in the progression of many kidney diseases [7, 8]. Recently, glycogen synthase kinase-3 beta (GSK3β) has emerged as a valuable marker of both glomerular and tubular injury.
Glycogen synthase kinase-3 is a highly conserved, ubiquitously expressed serine/threonine protein kinase, which was originally identified as a key transducer of the insulin signaling cascade that regulates glycogenesis [9, 10]. The enzyme has two highly homologous isoforms, alpha and beta, of which GSK3β is predominantly expressed both in the glomeruli and proximal tubular cells of the renal cortex [11, 12]. Renal GSK3β overactivity has been associated with a diverse range of kidney diseases. For example, analysis of publicly available kidney transcriptome database demonstrated that patients with progressive CKD exhibited GSK3β overexpression in the renal tubulointerstitial space [13]. Treatment of renal tubular epithelial cells in vitro by transforming growth factor beta-1 (TGF-β1) augmented GSK3β expression and led to cellular dedifferentiation, accumulation of extracellular matrix, and overproduction of other profibrotic cytokines [13]. In a mouse model of progressive CKD, GSK3β ablation in renal tubules mitigated the profibrogenic plasticity of renal tubular epithelial cells, with attenuation of interstitial fibrosis and tubular atrophy [13]. Patients with diabetic kidney disease (DKD) had an increased expression of GSK3β in glomeruli and renal tubules, and the magnitude of increase correlated with the severity of DKD [14]. More importantly, the activity of GSK3β in urinary exfoliated cells was an independent risk factor for the progression of renal impairment in diabetic patients [14], suggesting that GSK3β level in urinary sediment is a promising biomarker for the progression of DKD. In the present study, we compared the prognostic value of urinary and intrarenal GSK3β levels in DKD and nondiabetic CKD.
Patients and Methods
The study was approved by the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (approval number CRE-2019.283). All study procedures were in compliance with the Declaration of Helsinki, and all subjects provided written informed consent. We recruited 118 consecutive patients who had kidney biopsy-proved DKD (the DKD group) and 115 nondiabetic patients with CKDs (73 with typical features of hypertensive nephrosclerosis, 42 with non-specific glomerulosclerosis) as the nondiabetic CKD group. All kidney biopsy specimens were assessed by a single experienced pathologist (F.M.L.). A whole-stream early morning urine was collected on the day of kidney biopsy for gene expression and biochemical studies. We also reviewed the demographic and clinical data including serum creatinine and proteinuria. The estimated glomerular filtration rate (eGFR) was calculated by a standard equation [15].
RNA Extraction
The method of mRNA extraction and quantification in urinary sediment has been described previously [16]. Briefly, urine samples were centrifuged immediately after collection at 3,200 g, for 15 min at 4°C. The supernatant was removed, the pellet suspended in 1.5 mL diethyl pyrocarbonate treated phosphate buffered saline, and then centrifuged at 120,00 g for 5 min at 4°C. The washed pellet was resuspended in lysis buffer (RNeasy®; Qiagen, Germantown, MD, USA) and then purified using an RNeasy mini kit (Qiagen). The cDNA was prepared by the SuperScript™ IV First-Strand Synthesis System (ThermoFisher, Germany).
GSK3β mRNA Expression Level
Quantitation of GSK3β mRNA was performed by the StepOnePlus real-time quantitative polymerase chain reaction system (Applied Biosystems, Foster City, CA, USA), using TaqMan™ Fast Advanced Master Mix (ThermoFisher), with a final volume of 10 μL per reaction. Commercially available Taqman primers and probes, including two unlabeled PCR primers and one FAM dye-labeled TaqMan MGB probe, were used for GSK3β and β-actin genes (all from ThermoFisher). Each sample was run in triplicate. Results were analyzed with the use of Sequence Detection software, version 1.9 (Applied Biosystems). Gene expression for each signal was calculated by using the difference-in-threshold-cycle procedure. For the quantification of the target mRNA abundance, differences of threshold cycles between target genes were calculated by 2−ΔΔCT method.
Urine GSK3β and p-GSK3β Level
Urine GSK3β levels were determined by a commercially available ELISA kit (Bioassay Technology Laboratory, China) following the manufacturer’s protocol. The urinary levels were adjusted to urinary creatinine level, which was measured by a creatinine colorimetric assay kit (Millipore Sigma, USA). Each sample was measured in duplicates. An ELISA kit for phosphorylated GSK3β (p-GSK3β) (Cell Signaling Technology, Danvers, MA, USA) was used to quantify the phosphoprotein present. In this part of the experiment, lysates were processed and the assays performed according to manufacturer’s instructions. Bicinchoninic acid assay (BCA) was performed on each lysate, and the lysates were diluted such that 20 μg of protein lysate was used in each ELISA assay. The amount of phosphoprotein in ng per 20 μg of total protein lysate was then determined by comparing the measured 450 nm absorbance of the sample to the standard curve.
Intrarenal GSK3β and pY216-GSK3β Level
Intrarenal GSK3β levels were determined by Western blot study with β-actin level as the reference. The protein electrophoresis, transfer apparatus, and acrylamide gel were obtained from Bio-Rad (Hercules, CA, USA). Total protein from the kidney biopsy specimen was washed with radioimmunoprecipitation assay buffer containing protease inhibitor cocktail. The protein concentrations were quantified by a BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China). Individual proteins were then separated from 20 μg of total protein extract by acrylamide gel electrophoresis in Mini-PROTEAN® Cell (Hercules Bio-Rad, CA, USA) transferred to Hybond-P PVDF membrane and then probed with primary antibodies against GSK3β (1:1,000, Abcam, Cambridge, UK), pY216-GSK3β (1:1,000, Abcam, Cambridge, UK), and β-actin (1:1,000, Abcam, Cambridge, UK). The corresponding secondary antibody was obtained from Abcam. The membrane was exposed to Amersham Hyperfilm Blue. The areas of the bands were estimated by the software Image J. The protein expression level of a sample was calculated by dividing the area of the protein interested by the area of β-actin of the same sample.
Morphometric Study of Kidney Biopsy
The method of morphometry study of renal scarring has been described in previous studies [17, 18]. Briefly, Jones’ silver staining was performed on 5 μm thick sections of renal biopsy specimen. Semi-quantitative computerized image analysis was performed with the Leica Twin Pro image analysis system (Leica Microsystems, Wetzlar, Germany), which was connected to a Leica DC500 digital camera on a Leica DMRXA2 microscope with a ×40 objective (final calibration: 0.258 mm/pixel). Image analysis was performed by MetaMorph 4.0 image-analyzing software (Universal Imaging Corporation™, Downingtown, PA, USA). Ten glomeruli and 10 randomly selected areas were assessed in each patient, and the average percentage of scarred glomerular and tubulointerstitial areas, as represented by the area with positive silver staining, were computed.
Outcome Measures
All patients were followed for at least 12 months. All patients were followed by nephrologists, and their treatment was not affected by the study. Kidney function was monitored every 3 months. The primary outcome measures are dialysis-free survival and renal event-free survival. Renal event was defined as death from any cause, need for dialysis, or ≥40% decline in eGFR as compared to baseline. Secondary outcome measure includes the rate of eGFR decline, which was calculated by the least-squares regression method.
Statistical Analysis
Statistical analysis was performed by SPSS for Windows software version 17.0 (SPSS, Chicago, IL, USA). All the results were presented in mean ± SD for data normally distributed and median (lower and upper quartiles) for the others. Since data on gene expression levels were highly skewed, we used the Mann-Whitney U test to compare gene expression levels between groups and Spearman’s rank-order correlations to test associations between gene expression levels and other parameters. Data were further analyzed with log rank test with dialysis-free survival and renal event-free survival. In addition to GSK3β levels, the multivariate Cox regression model was constructed by age, sex, diagnosis group (DKD vs. CTL), baseline eGFR, and proteinuria. In view of the univariate analysis result, a multivariate linear regression model was constructed to adjust for sex, age, baseline eGFR, proteinuria, the severity of glomerulosclerosis and tubulointerstitial fibrosis, and the intrarenal pY216-GSK3β/GSK3β ratio to determine the independent predictor of the rate of renal function decline. A p value of below 0.05 was considered statistically significant. All probabilities were two tailed.
Results
A total of 233 patients were enrolled, including 118 patients with biopsy-proved DKD and 115 nondiabetic CKD. Their baseline clinical, biochemical, and pathological information is summarized in Table 1. In essence, the DKD group was older and had more proteinuria than the control group, but there was no significant difference in any histological parameter between the groups.
Table 1.
Baseline demographic and clinical data
| DKD | Nondiabetic CKD | p value | |
|---|---|---|---|
| Patients, n | 118 | 115 | |
| Sex (M:F) | 80:38 | 56:59 | 0.041a |
| Age, years | 61.3±12.3 | 55.4±15.1 | 0.031b |
| Serum creatinine, μmol/L | 217.9±162.2 | 273.3±228.5 | 0.036b |
| eGFR, mL/min/1.73 m2 | 41.4±31.3 | 38.0±32.0 | 0.528b |
| Proteinuria, g/day | 3.2±2.4 | 2.6±2.1 | 0.007b |
| Histological damage (%) | |||
| Glomerulosclerosis | 32.7±21.7 | 25.8±22.5 | 0.144b |
| Tubulointerstitial fibrosis | 30.2±17.4 | 23.8±22.8 | 0.584b |
DKD, diabetic kidney disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Data were compared by aχ2 test and bStudent’s t test.
Urinary and Intrarenal GSK3β Levels
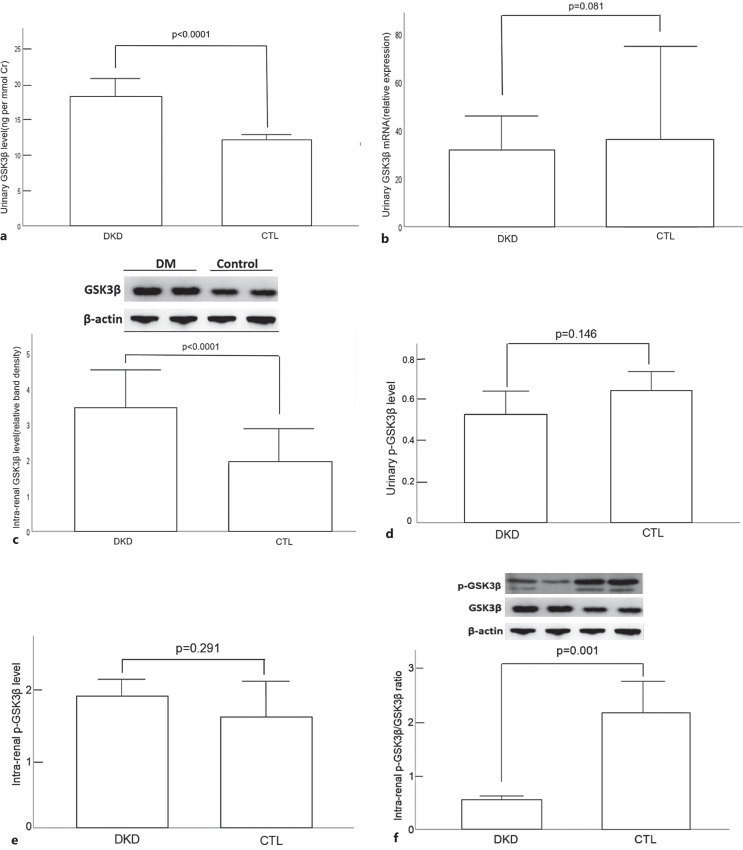
The urinary GSK3β level by ELISA, its corresponding urinary mRNA level, and intrarenal level by Western blotting are summarized and compared between DKD and nondiabetic CKD groups in Figure 1a–c, respectively. The urinary p-GSK3β level by ELISA, intrarenal p-GSK3β level by Western blotting, and the intrarenal pY216-GSK3β/total GSK3β ratio by Western blotting are summarized and compared between DKD and nondiabetic CKD groups in Figure 1d–f, respectively. The urinary GSK3β level and intrarenal GSK3β level in the DKD group were significantly higher than those of the nondiabetic CKD group. In contrast, the intrarenal pY216-GSK3β/GSK3β ratio in the nondiabetic CKD group was significantly higher than those of the DKD group. However, there was no significant difference in urinary GSK3β mRNA expression between the two groups. The internal correlation of urinary and intrarenal GSK3β levels is summarized in Table 2. In essence, only urinary GSK3β level by ELISA was significantly negative correlated with intrarenal level by Western blotting.
Fig. 1.
Comparison of GSK3β levels: urinary level by ELISA (a); urinary mRNA level (b); intrarenal level by Western blotting (c); urinary p-GSK3β level by ELISA (d); intrarenal p-GSK3β level by Western blotting (e); and intrarenal pY216-GSK3β/total GSK3β ratio by Western blotting (f), between diabetic kidney disease (DKD) and nondiabetic chronic kidney disease (CKD) groups. Error bars denote standard deviations. Data were compared by Mann-Whitney U test.
Table 2.
Internal correlation of urinary and intrarenal GSK3β levels
| Urinary level by ELISA | Intrarenal level | |
|---|---|---|
| Urinary level by ELISA | ||
| Entire cohort | / | r = −0.075, p = 0.475 |
| DKD group | / | r = −0.247, p = 0.027 |
| Nondiabetic CKD group | / | r = 0.115, p = 0.721 |
| Urinary mRNA level | ||
| Entire cohort | r = 0.110, p = 0.106 | r = 0.034, p = 0.746 |
| DKD group | r = 0.060, p = 0.521 | r = −0.061, p = 0.588 |
| Nondiabetic CKD group | r = −0.105, p = 879 | r = −0.025, p = 0.938 |
DKD, diabetic kidney disease; CKD, chronic kidney disease.
Person’s correlation coefficients are depicted.
Relation with Clinical and Histological Parameters
The correlation between urinary GSK3β level by ELISA, its corresponding urinary mRNA level, its p-GSK3β level by ELISA, or its ratio and clinical and histological parameters is summarized in Table 3. The urinary p-GSK3β level is statistically significantly correlated with the baseline eGFR. There was a significant but only modest correlation between baseline eGFR and GSK3β mRNA expression in DKD patients. The urinary p-GSK3β/GSK3β ratio was not correlated with any clinical or histological parameters in any group.
Table 3.
Relation between GSK3β levels and clinicopathological parameters
| eGFR | Proteinuria | Glomerulosclerosis | Tubulointerstitial fibrosis | |
|---|---|---|---|---|
| (A) Urinary levels | ||||
| Urinary level by ELISA | ||||
| Entire cohort | r = −0.057, p = 0.406 | r = 0.020, p = 0.775 | r = 0.069, p = 0.434 | r = 0.075, p = 0.390 |
| DKD group | r = 0.013, p = 0.893 | r = 0.058, p = 0.546 | r = −0.160, p = 0.085 | r = −0.169, p = 0.069 |
| Nondiabetic CKD group | r = −0.010, p = 0.922 | r = 0.015, p = 0.885 | r = −0.293, p = 0.122 | r = −0.234, p = 0.213 |
| Urinary mRNA level | ||||
| Entire cohort | r = 0.096, p = 0.149 | r = 0.063, p = 0.357 | r = 0.067, p = 0.435 | r = 0.018, p = 0.836 |
| DKD group | r = 0.230, p = 0.012 | r = 0.057, p = 0.552 | r = −0.080, p = 0.393 | r = −0.024, p = 0.798 |
| Nondiabetic CKD group | r = 0.140, p = 0.155 | r = 0.129, p = 0.201 | r = −0.238, p = 0.213 | r = −0.223, p = 0.236 |
| Urinary p-GSK3β level | ||||
| Entire cohort | r = −0.054, p = 0.447 | r = −0.053, p = 0.464 | r = −0.029, p = 0.745 | r = −0.034, p = 0.699 |
| DKD group | r = 0.325, p = 0.001 | r = −0.100, p = 0.323 | r = −0.016, p = 0.869 | r = −0.021, p = 0.259 |
| Nondiabetic CKD group | r = −0.109, p = 0.292 | r = −0.071, p = 0.500 | r = 0.159, p = 0.437 | r = −0.047, p = 0.818 |
| p-GSK3β/total GSK3β ratio | ||||
| Entire cohort | r = −0.006, p = 0.932 | r = −0.060, p = 0.415 | r = −0.035, p = 0.692 | r = −0.051, p = 0.562 |
| DKD group | r = 0.049, p = 0.620 | r = −0.040, p = 0.693 | r = −0.010, p = 0.917 | r = 0.015, p = 0.881 |
| Nondiabetic CKD group | r = −0.066, p = 0.532 | r = −0.002, p = 0.983 | r = 0.035, p = 0.865 | r = −0.096, p = 0.633 |
| (B) intrarenal levels | ||||
| Total intrarenal GSK3β | ||||
| Entire cohort | r = 0.146, p = 0.164 | r = 0.052, p = 0.631 | r = 0.187, p = 0.086 | r = 0.152, p = 0.165 |
| DKD group | r = 0.100, p = 0.375 | r = 0.016, p = 0.893 | r = 0.012, p = 0.913 | r = −0.060, p = 0.596 |
| Nondiabetic CKD group | r = −0.231, p = 0.470 | r = 0.323, p = 0.332 | r = −0.352, p = 0.562 | r = −0.448, p = 0.450 |
| Intrarenal pY216-GSK3β level | ||||
| Entire cohort | r = 0.266, p = 0.013 | r = −0.219, p = 0.053 | r = −0.046, p = 0.681 | r = −0.137, p = 0.220 |
| DKD group | r = 0.259, p = 0.020 | r = −0.201, p = 0.085 | r = −0.064, p = 0.575 | r = −0.166, p = 0.144 |
| Nondiabetic CKD group | r = 0.384, p = 0.453 | r = −0.789, p = 0.113 | r = 0.918, p = 0.260 | r = 0.858, p = 0.344 |
| pY216-GSK3β/total GSK3β ratio | ||||
| Entire cohort | r = −0.124, p = 0.255 | r = −0.188, p = 0.097 | r = −0.140, p = 0.210 | r = −0.144, p = 0.197 |
| DKD group | r = 0.303, p = 0.006 | r = −0.218, p = 0.062 | r = −0.053, p = 0.642 | r = −0.149, p = 0.190 |
| Nondiabetic CKD group | r = 0.575, p = 0.232 | r = −0.693 p = 0.195 | r = −0.352, p = 0.562 | r = 0.866, p = 0.333 |
DKD, diabetic kidney disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Person’s correlation coefficients are depicted.
The correlation between intrarenal total GSK3β, pY216-GSK3β level, and their ratio by Western blotting with clinical parameters is summarized in Table 3. In fact, there was a significant but only modest correlation between baseline eGFR and intrarenal pY216-GSK3β level in DKD patients. The pY216-GSK3β/total GSK3β ratio significantly correlated with the baseline eGFR in the DKD group. In contrast, there was no correlation between the intrarenal total GSK3β or pY216-GSK3β and clinical parameters in the nondiabetic CKD group.
Relation with Clinical Outcome
The entire cohort was followed for an average 30.2 ± 31.2 months. During this period, none of the patients died; 106 patients progressed to dialysis-dependent kidney failure, and another 44 patients had 40% decline in GFR. The average rate of eGFR decline was −9.7 ± 24.2 mL/min/1.73 m2 per year. The relation between urinary and intrarenal GSK3β levels and clinical outcome by univariate analysis is summarized in Table 4. In essence, urinary GSK3β level by ELISA, its mRNA level, the p-GSK3β level, or the p-GSK3β/GSK3β ratio had no association with dialysis-free survival or the slope of eGFR decline in DKD or nondiabetic CKD groups.
Table 4.
Relation between GSK3β Levels and clinic outcome
| Dialysis-free survivala | Renal event-free survivala | Slope of eGFR declineb | |
|---|---|---|---|
| (A) Urinary levels | |||
| Urinary level by ELISA | |||
| Entire cohort | 1.00 (0.99–1.02), p = 0.693 | 1.00 (0.98–1.02), p = 0.849 | r = −0.029, p = 0.698 |
| DKD group | 0.99 (0.98–1.02), p = 0.858 | 1.00 (0.98–1.02), p = 0.957 | r = −0.004, p = 0.970 |
| Nondiabetic CKD group | 0.94 (0.87–1.01), p = 0.102 | 0.89 (0.81–0.99), p = 0.031 | r = 0.081, p = 0.474 |
| Urinary mRNA level | |||
| Entire cohort | 1.02 (0.86–1.22), p = 0.808 | 0.96 (0.80–1.14), p = 0.623 | r = −0.011, p = 0.876 |
| DKD group | 1.06 (0.84–1.33), p = 0.645 | 0.95 (0.76–1.18), p = 0.625 | r = 0.020, p = 0.851 |
| Nondiabetic CKD group | 0.96 (0.74–1.24), p = 0.754 | 0.97 (0.73–1.30), p = 0.849 | r = −0.074, p = 0.507 |
| Urinary p-GSK3β level | |||
| Entire cohort | 0.80 (0.52–1.23), p = 0.307 | 0.92 (0.65–1.32), p = 0.655 | r = 0.018, p = 0.816 |
| DKD group | 0.72 (0.42–1.21), p = 0.212 | 0.83 (0.53–1.30), p = 0.412 | r = 0.020, p = 0.856 |
| Nondiabetic CKD group | 1.07 (0.58–1.99), p = 0.829 | 1.02 (0.60–1.73), p = 0.941 | r = −0.096, p = 0.372 |
| p-GSK3β/total GSK3β ratio | |||
| Entire cohort | 0.00 (0.00–33.92), p = 0.278 | 0.00 (0.00–30.51), p = 0.553 | r = 0.062, p = 0.382 |
| DKD group | 0.00 (0.00–24.18), p = 0.242 | 0.00 (0.00–26.23), p = 0.399 | r = 0.041, p = 0.712 |
| Nondiabetic CKD group | 369.50 (0.00–62.00), p = 0.735 | 1,058.93 (0.00–57.61), p = 0.829 | r = −0.093, p = 0.401 |
| (B) Intrarenal levels | |||
| Total GSK3β | |||
| Entire cohort | 1.14 (0.76–1.70), p = 0.534 | 1.02 (0.695–1.51), p = 0.905 | r = −0.220, p = 0.054 |
| DKD group | 0.86 (0.48–1.54), p = 0.619 | 0.94 (0.51–1.72), p = 0.850 | r = −0.122, p = 0.328 |
| Nondiabetic CKD group | 0.14 (0.00–6.83), p = 0.139 | 1.59 (0.21–12.10), p = 0.655 | r = 0.135, p = 0.730 |
| pY216-GSK3β | |||
| Entire cohort | 0.93 (0.67–1.29), p = 0.652 | 1.24 (0.91–1.68), p = 0.168 | r = −0.228, p = 0.058 |
| DKD group | 0.93 (0.67–1.30), p = 0.670 | 1.26 (0.92–1.72), p = 0.152 | r = −0.221, p = 0.075 |
| Nondiabetic CKD group | 0.62 (0.04–9.73), p = 0.731 | 1.14 (0.11–11.70), p = 0.915 | r = −0.632, p = 0.368 |
| pY216-GSK3β/total GSK3β ratio | |||
| Entire cohort | 0.95 (0.51–1.76), p = 0.870 | 1.21 (0.72–2.02), p = 0.477 | r = 0.069, p = 0.568 |
| DKD group | 0.82 (0.26–1.2.53), p = 0.724 | 2.47 (0.89–6.82), p = 0.081 | r = −0.335, p = 0.006 |
| Nondiabetic CKD group | 0.64 (0.01–24.17), p = 0.811 | 1.61 (0.08–33.8), p = 0.759 | r = −0.600, p = 0.400 |
DKD, diabetic kidney disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Results are depicted as aunadjusted hazard ratio (95% confidence interval) and bPearson’s correlation coefficient.
For intrarenal levels, the total GSK3β, pY216-GSK3β, or their ratio do not predict dialysis-free survival or renal event-free dialysis survival in the DKD group. Intrarenal Y216-GSK3β had a modest but insignificant correlation with the rate of eGFR decline. The pY216-GSK3β/total GSK3β ratio significantly correlated with the slope of eGFR decline (r = −0.335, p = 0.006). After adjusting for other clinical parameters, the intrarenal pY216-GSK3β/total GSK3β ratio remained an independent predictor of the slope of eGFR decline (unstandardized B −24.1, 95% confidence interval −9.4 to −38.8, p = 0.002). In contrast, intrarenal total GSK3β, pY216-GSK3β level, or their ratio were not associated with dialysis-free survival or the slope of eGFR decline in nondiabetic CKD patients.
Discussion
GSK3β, a highly conserved, redox-sensitive serine/threonine protein kinase, is centrally involved in insulin signaling as well as a number of other cellular signal pathways that are crucial for pathophysiological processes related to kidney injury, repair, and regeneration [19]. Notably, GSK3β is a critical regulator of cellular function in podocytes [20]. The role of GSK3β in DKD is an active area of research. Existing evidence suggests that GSK3β dysregulation is related to type 2 diabetes mellitus. GSK3β overexpression and hyperactivity were reported in the muscles of diabetic patients and in the adipose tissues of obese diabetic mice [21–23]. In the kidney, GSK3β mediates podocyte autonomous injury by integrating multiple podocytopathic signaling pathways [24, 25]. Activation of GSK3β by sodium nitroprusside ameliorates diabetes-induced kidney injury in diabetic mice [26]. More recently, GSK3β activation in the kidney and in urinary exfoliated cells was reported to be associated with the development or progression of DKD [14].
The results of our present study are in line with but slightly different from the report of Liang et al. [14]. Our study showed that urinary and intrarenal GSK3β levels are specifically elevated in DKD as compared to nondiabetic CKD, and urinary sediment GSK3β mRNA level had a modest but significant correlation with eGFR in DKD but not nondiabetic CKD, both of which were consistent with the findings of the previous study [14]. In our present study, neither urinary GSK3β level or its mRNA expression was associated with the rate of progression of DKD, while Liang et al. [14] showed that urinary phosphorylated GSK3β (p-GSK3β) predicted DKD progression [14]. In this study, we also found that urinary sediment p-GSK3β level correlated with the baseline eGFR. More importantly, we found that the intrarenal pY216-GSK3β/total GSK3β ratio level was associated with the slope of eGFR decline in DKD patients. The pathophysiological role of pY216-GSK3β in the progression of DKD deserves further study.
An important edge of our study was the inclusion of a large group of nondiabetic CKD patients for comparison because a major objective of our study was to determine whether total or activated GSK3β level is a specific marker of DKD or a generic marker of kidney damage irrespective of the underlying cause. We found that the urinary GSK3β level and intrarenal GSK3β level in the DKD group were significantly higher than those of the nondiabetic CKD group, suggesting that the abnormal GSK3β level was at least partly specific for DKD. However, there was no significant difference in urinary GSK3β mRNA expression between the two groups.
Our study carries several limitations. First, we did not have a control group with normal kidney biopsy. Nonetheless, we found no correlation between GSK3β level (either intrarenal or urinary) and any clinical parameter in the nondiabetic group, suggesting that GSK3β level is not affected by kidney damage per se. Second, unlike the previous study of Liang et al. [14], we only measured the total GSK3β level in urinary supernatant due to the limitation of experimental design and the availability of urine sample. Finally, the kidney biopsy of nondiabetic CKD, and probably DKD, may represent a heterogeneous group, although we did exclude cases with obvious immune deposit or underlying causes. Further studies are necessary to explore the expression of GSK3β in specific kidney diseases and its relationship with their disease progression.
In summary, our results showed that intrarenal and urinary GSK3β levels were increased in DKD as compared to nondiabetic CKD. Neither urinary GSK3β nor its p-GSK3β levels predicted the progression of DKD, and the prognostic value of the urinary GSK3β level in nondiabetic CKD was also limited. However, the intrarenal pY216-GSK3β/total GSK3β ratio was associated with the rate of progression of DKD. Our findings indicated that urinary GSK3β may not be a suitable biomarker for DKD, but the pathophysiological roles of GSK3β in DKD progression deserve further studies.
Statement of Ethics
The study was approved by the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (approval number CRE-2019.283). All study procedures were in compliance with the Declaration of Helsinki, and all subjects provided written informed consent.
Conflict of Interest Statement
The authors declare no other conflict of interest.
Funding Sources
This study was supported by the Research Grant Council Research Impact Fund (project reference R4012-18) and the Chinese University of Hong Kong research accounts 6905134, 2410026, and 3133200. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Research idea and study design, data analysis/interpretation, and statistical analysis: L.Z. and C.C.S.; data acquisition: L.Z., W.W.S.F., G.C.S.C., and J.K.C.N.; supervision or mentorship: K.M.C. and C.C.S. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding Statement
This study was supported by the Research Grant Council Research Impact Fund (project reference R4012-18) and the Chinese University of Hong Kong research accounts 6905134, 2410026, and 3133200. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015 Feb 24;313(8):837–46. 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerjaschki D. 2015 homer W. Smith award: the podocyte from periphery to center stage. J Am Soc Nephrol. 2016 Nov;27(11):3266–70. 10.1681/asn.2016040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torban E, Braun F, Wanner N, Takano T, Goodyer PR, Lennon R, et al. From podocyte biology to novel cures for glomerular disease. Kidney Int. 2019 Oct;96(4):850–61. 10.1016/j.kint.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 4. Zeng L, Szeto CC. Urinary podocyte markers in kidney diseases. Clin Chim Acta. 2021 Dec;523:315–24. Epub 2021 Oct 16. 10.1016/j.cca.2021.10.017. [DOI] [PubMed] [Google Scholar]
- 5. Szeto CC, Lai KB, Chow KM, Szeto CYK, Yip TWC, Woo KS, et al. Messenger RNA expression of glomerular podocyte markers in the urinary sediment of acquired proteinuric diseases. Clin Chim Acta. 2005 Nov;361(1–2):182–90. 10.1016/j.cccn.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 6. Wang G, Lai FM, Kwan BC, Lai KB, Chow KM, Li PK, et al. Podocyte loss in human hypertensive nephrosclerosis. Am J Hypertens. 2009 Mar;22(3):300–6. 10.1038/ajh.2008.360. [DOI] [PubMed] [Google Scholar]
- 7. Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol. 2016 Jul 1;311(1):F145–61. 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018 Mar;93(3):568–79. 10.1016/j.kint.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 9. Xu W, Ge Y, Liu Z, Gong R. Glycogen synthase kinase 3β dictates podocyte motility and focal adhesion turnover by modulating paxillin activity: implications for the protective effect of low-dose lithium in podocytopathy. Am J Pathol. 2014;184(10):2742–56. 10.1016/j.ajpath.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev. 2001;101(8):2527–40. 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 11. Li C, Ge Y, Dworkin L, Peng A, Gong R. The β isoform of GSK3 mediates podocyte autonomous injury in proteinuric glomerulopathy. J Pathol. 2016;239(1):23–35. 10.1002/path.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou S, Wang P, Qiao Y, Ge Y, Wang Y, Quan S, et al. Genetic and pharmacologic targeting of glycogen synthase kinase 3β reinforces the Nrf2 antioxidant defense against podocytopathy. J Am Soc Nephrol. 2016;27(8):2289–308. 10.1681/ASN.2015050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen B, Wang P, Liang X, Jiang C, Ge Y, Dworkin LD, et al. Permissive effect of GSK3 on profibrogenic plasticity of renal tubular cells in progressive chronic kidney disease. Cell Death Dis. 2021 Apr 30;12(5):432. 10.1038/s41419-021-03709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang X, Wang P, Chen B, Ge Y, Gong AY, Flickinger B, et al. Glycogen synthase kinase 3β hyperactivity in urinary exfoliated cells predicts progression of diabetic kidney disease. Kidney Int. 2020 Jan;97(1):175–92. 10.1016/j.kint.2019.08.036. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G, Szeto CC. Methods of microRNA quantification in urinary sediment. Methods Mol Biol. 2013;1024:211–20. 10.1007/978-1-62703-453-1_17. [DOI] [PubMed] [Google Scholar]
- 17. Wang G, Lai FM, Chow KM, Kwan BC, Pang WF, Luk CC, et al. Urinary mRNA levels of ELR-negative CXC chemokine ligand and extracellular matrix in diabetic nephropathy. Diabetes Metab Res Rev. 2015 Oct;31(7):699–706. 10.1002/dmrr.2654. [DOI] [PubMed] [Google Scholar]
- 18. Wei PZ, Kwan BC, Chow KM, Cheng PM, Luk CC, Lai KB, et al. Urinary mitochondrial DNA level in non-diabetic chronic kidney diseases. Clin Chim Acta. 2018 Sep;484:36–9. 10.1016/j.cca.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 19. Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116(Pt 7):1175–86. 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hurcombe JA, Hartley P, Lay AC, Ni L, Bedford JJ, Leader JP, et al. Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function. Nat Commun. 2019 Jan 24;10(1):403. 10.1038/s41467-018-08235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes. 2000;49:263–71. 10.2337/diabetes.49.2.263. [DOI] [PubMed] [Google Scholar]
- 22. Nikoulina SE, Ciaraldi TP, Mudaliar S, Carter L, Johnson K, Henry RR. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes. 2002;51(7):2190–8. 10.2337/diabetes.51.7.2190. [DOI] [PubMed] [Google Scholar]
- 23. Henriksen EJ, Kinnick TR, Teachey MK, O’Keefe MP, Ring D, Johnson KW, et al. Modulation of muscle insulin resistance by selective inhibition of GSK-3 in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab. 2003;284(5):E892–900. 10.1152/ajpendo.00346.2002. [DOI] [PubMed] [Google Scholar]
- 24. Gong R, Rifai A, Ge Y, Chen S, Dworkin LD. Hepatocyte growth factor suppresses proinflammatory NFkappaB activation through GSK3beta inactivation in renal tubular epithelial cells. J Biol Chem. 2008;283(12):7401–10. 10.1074/jbc.M710396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li C, Ge Y, Peng A, Gong R. The redox sensitive glycogen synthase kinase 3β suppresses the self-protective antioxidant response in podocytes upon oxidative glomerular injury. Oncotarget. 2015;6(37):39493–506. 10.18632/oncotarget.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mariappan MM, Prasad S, D’Silva K, Cedillo E, Sataranatarajan K, Barnes JL, et al. Activation of glycogen synthase kinase 3β ameliorates diabetes-induced kidney injury. J Biol Chem. 2014;289(51):35363–75. 10.1074/jbc.M114.587840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.