Abstract
Background
We evaluated O-(2-[18F]fluoroethyl)-l-tyrosine (FET) PET and MRI for early response assessment in recurrent glioma patients treated with lomustine-based chemotherapy.
Methods
Thirty-six adult patients with WHO CNS grade 3 or 4 gliomas (glioblastoma, 69%) at recurrence (median number of recurrences, 1; range, 1–3) were retrospectively identified. Besides MRI, serial FET PET scans were performed at baseline and early after chemotherapy initiation (not later than two cycles). Tumor-to-brain ratios (TBR), metabolic tumor volumes (MTV), the occurrence of new distant hotspots with a mean TBR >1.6 at follow-up, and the dynamic parameter time-to-peak were derived from all FET PET scans. PET parameter thresholds were defined using ROC analyses to predict PFS of ≥6 months and OS of ≥12 months. MRI response assessment was based on RANO criteria. The predictive values of FET PET parameters and RANO criteria were subsequently evaluated using univariate and multivariate survival estimates.
Results
After treatment initiation, the median follow-up time was 11 months (range, 3–71 months). Relative changes of TBR, MTV, and RANO criteria predicted a significantly longer PFS (all P ≤ .002) and OS (all P ≤ .045). At follow-up, the occurrence of new distant hotspots (n ≥ 1) predicted a worse outcome, with significantly shorter PFS (P = .005) and OS (P < .001). Time-to-peak changes did not predict a significantly longer survival. Multivariate survival analyses revealed that new distant hotspots at follow-up FET PET were most potent in predicting non-response (P < .001; HR, 8.578).
Conclusions
Data suggest that FET PET provides complementary information to RANO criteria for response evaluation of lomustine-based chemotherapy early after treatment initiation.
Keywords: amino acid PET, CCNU, glioblastoma, nitrosourea
Key Points.
Both RANO criteria and FET PET metrics allow the prediction of a significantly longer survival time.
In direct comparison with FET PET metrics, response based on RANO criteria appeared to especially predict a longer OS.
The occurrence of distant and metabolically active hotspots on FET PET during lomustine-based chemotherapy proved to be the strongest predictor for non-response.
Information of both imaging modalities may improve clinical decision-making (eg, dis- or continuation of nitrosourea-based chemotherapy).
Importance of the Study.
In glioma patients at recurrence, lomustine-based chemotherapy represents the standard care especially in the most countries of Europe, where bevacizumab is not approved. Currently, changes in conventional contrast-enhanced MRI during follow-up according to the Response Assessment in Neuro-Oncology (RANO) criteria is frequently used for response assessment. However, in randomized clinical trials with recurrent glioblastoma patients using a lomustine control arm, objective response rates, including complete and partial responses, are only around 10%. Here, we showed that RANO criteria and FET PET metrics provide complementary information for the evaluation of response to lomustine-based chemotherapy. On one hand, response based on RANO criteria appeared to especially predict a longer overall survival than FET PET imaging changes. On the other hand, the occurrence of new distant FET PET hotspots at follow-up were most potent in predicting non-response. Therefore, this may help to improve clinical decision-making (eg, dis- or continuation of nitrosourea-based chemotherapy) since the nitrosourea lomustine is frequently associated with significant adverse events such as hematological toxicity.
In glioma patients at recurrence, lomustine chemotherapy represents the standard care especially in Europe, where bevacizumab is not approved (except Switzerland). Lomustine is applied either as monotherapy or in combination with procarbazine and vincristine (PCV regimen) or with procarbazine only (PC regimen).1 In the current guideline of the European Association of Neuro-Oncology (EANO), lomustine is recommended for use in patients with glioblastoma and WHO CNS grade 3 astrocytoma at recurrence.2 Furthermore, lomustine is probably the critical component of the PCV regimen, which has become the standard of care for newly diagnosed patients with IDH-mutated glioma of the WHO CNS grade 2.3
Of note, in clinical trials using lomustine as a control arm, the progression-free survival (PFS) rate at 6 months was only in the range of 20%, and the overall survival (OS) ranged from 6 to 9 months.4 Besides, the nitrosourea lomustine is frequently associated with significant adverse events, most frequently with hematological toxicity, for example, thrombocytopenia. For example, in the REGOMA trial, the grade 3 or 4 hematological toxicity rate was 38% in the lomustine control arm.5
For these reasons, assessing the response early after lomustine chemotherapy initiation is of considerable clinical importance for further treatment decisions (eg, the continuation of lomustine). Currently, contrast-enhanced conventional MRI is the method of choice for response assessment. Precisely, changes in contrast enhancement are frequently used to assess treatment response according to the criteria defined by the Response Assessment in Neuro-Oncology (RANO) Working Group.6 However, in randomized clinical trials with recurrent glioblastoma patients using a lomustine control arm, objective response rates, including complete and partial responses, are only in the range of 10%.4 Thus, further information on treatment effects derived from other imaging modalities is desirable.
In addition to conventional MRI, PET imaging using the radiolabeled amino acid O-(2-[18F]fluoroethyl)-l-tyrosine (FET) provides valuable clinical information for treatment response assessment in glioma patients in both the newly diagnosed and recurrence setting.7 The biological mechanism responsible for FET uptake within gliomas is obtained by specific amino acid transporters especially belonging to the system of L-type amino acid transporters (LAT), particularly the subtypes LAT1 and LAT2.8,9 An experimental study suggested that the over-expression of LAT1 considerably facilitates the influx of FET in glioma cells.10 On the other side, FET seems to be a poor efflux substrate of LAT1. Consequently, the entrapment of FET in glioma cells is most probably related to this asymmetry.10
Its added clinical value has been demonstrated for various treatment regimens, including radiotherapy with concomitant temozolomide,11 adjuvant temozolomide,12 bevacizumab-based therapies,13–15 or experimental therapies, for example, regorafenib.16 A recent study suggested that FET PET is also valuable in assessing response to immunotherapy using checkpoint inhibitors in patients with brain metastases.17 Importantly, irrespective of the treatment regimen, metabolic responders identified using FET PET had a significantly longer PFS.11–13,17
Except for an initial study,18 data on the FET PET value for evaluating response to lomustine-based chemotherapy remain scarce. Therefore, we evaluated the response to lomustine-based chemotherapy using FET PET compared to contrast-enhanced MRI in patients with WHO CNS grade 3 or 4 gliomas at recurrence to identify the optimal parameter to predict an early response.
Patients and Methods
Patients
From 2015 to 2021, we retrospectively identified patients with histomolecularly characterized glioma who (1) had completed at least one line of pretreatment including resection, radiotherapy, alkylating chemotherapy, or combinations thereof, (2) had progressive MRI findings according to the RANO criteria,6 (3) were treated with a lomustine-based chemotherapy, and (4) underwent serial MR and FET PET imaging for response assessment (ie, at baseline and after the second cycle, or in the case of clinical disease progression and/or early progression on MRI before the second cycle).
Lomustine-based chemotherapy consisted of lomustine as monotherapy (90–110 mg/m2 body surface area on day 1 of a 42-day cycle) or the combination of lomustine with procarbazine (PC regimen; lomustine 90–110 mg/m2 body surface area on day 1, and procarbazine 60 mg/m2 body surface area on days 8–21 of a 56-day cycle). FET PET was performed not later than 7–10 days after MR imaging and not later than 7 days before treatment initiation.
The local ethics committee approved the retrospective analysis of neuroimaging data. There was no conflict with the Declaration of Helsinki. Before PET imaging, all patients had given written informed consent for the PET investigation and data usage for scientific purposes.
Follow-Up
Patients were assessed clinically by neurological examination and the Karnofsky Performance Score at baseline, every 8–12 weeks during the treatment, and every 8–12 weeks after treatment completion. After the last FET PET scan, a contrast-enhanced conventional MRI was performed every 8–12 weeks. The PFS was defined as the time interval between initiation of lomustine-based chemotherapy and tumor progression with clinical deterioration and MRI findings consistent with Progressive Disease according to RANO criteria.6 The latter situation prompted discontinuation of lomustine-based chemotherapy. The OS was defined as the time interval between the initiation of lomustine-based chemotherapy and death.
Conventional MR Imaging
Following the International Standardized Brain Tumor Imaging Protocol (BTIP),19 MR imaging was performed using a 1.5 T or 3.0 T MRI scanner with a standard head coil before and after administration of a gadolinium-based contrast agent (0.1 mmol/kg body weight). The imaging protocol comprised 3D isovoxels acquired in T1-weighted, 2D T2-weighted, and 2D fluid-attenuated inversion recovery-weighted sequences. MRI changes at first follow-up compared to the baseline scan were assigned by an experienced neuroradiologist (C.K.) according to the RANO criteria.6 The criteria for Stable Disease, Partial Response, and Complete Response were considered as response to lomustine-based chemotherapy.
FET PET Imaging
As described previously, the amino acid FET was produced via nucleophilic 18F-fluorination with a radiochemical purity of greater than 98%, molar radioactivity greater than 200 GBq/µmol, and a radiochemical yield of about 60%.20 According to international guidelines for brain tumor imaging using radiolabeled amino acid analogues,21 patients fasted for at least four hours before the PET measurements. All patients underwent a dynamic PET scan from 0 to 50 min after injection of 3 MBq of FET per kg of body weight at baseline (within 7 days before starting lomustine-based chemotherapy) and after the second cycle of lomustine-based chemotherapy. PET imaging was performed either on an ECAT Exact HR+ PET scanner in 3-dimensional mode (n = 64 scans; Siemens, Erlangen, Germany; axial field-of-view, 15.5 cm) or simultaneously with 3T MR imaging using a BrainPET insert (n = 8 scans; Siemens, Erlangen, Germany; axial field-of-view, 19.2 cm). The BrainPET is a compact cylinder that fits into the bore of the Magnetom Trio MR scanner.22 Iterative reconstruction parameters were 16 subsets, 6 iterations using the OSEM algorithm for the ECAT HR+ PET scanner and two subsets, and 32 iterations using the OPOSEM algorithm for the BrainPET. Data were corrected for random, scattered coincidences, dead time, and motion for both systems. Attenuation correction for the ECAT HR+ PET scan was based on a transmission scan, and for the BrainPET scan on a template-based approach.22 The reconstructed dynamic data sets consisted of 16 time frames (5 × 1 min; 5 × 3 min; 6 × 5 min) for both scanners. To optimize the comparability of the results related to the influence of the two different PET scanners, reconstruction parameters, and post-processing steps, a 2.5 mm 3D Gaussian filter was applied to the BrainPET data before further processing. In phantom experiments using spheres of different sizes to simulate lesions, this filter kernel demonstrated the best comparability between PET data obtained from the ECAT HR+ PET and the BrainPET scanner.23
FET PET Data Analysis
For evaluating FET data, summed PET images from 20 to 40 min after injection were used. FET metabolic tumor volumes (MTV) and mean tumoral FET uptake were determined by a three-dimensional auto-contouring process using a threshold of 1.6 using the software PMOD (Version 4.3, PMOD Technologies Ltd.). This cut-off was based on a biopsy-controlled study in glioma patients and differentiated best between tumoral and peritumoral tissue.24 Using a regions-of-interest (ROI) analysis, maximum and mean tumor-to-brain ratios (TBRmax, TBRmean) were calculated by dividing the mean and maximum standardized uptake value (SUV) of the tumor ROI by the mean SUV of a larger ROI placed in the semioval center of the contralateral unaffected hemisphere including white and grey matter.21
To evaluate new hotspot regions at follow-up, every lesion with a TBRmean > 1.6 without spatial connection to (distant to) the tumor ROI at baseline was defined as a hotspot.
As described previously,25 time-activity curves (TACs) of FET uptake (mean SUV) in the tumor were generated by the application of a spherical volume-of-interest (VOI) with a volume of 2 mL centered on the voxel with the maximum tumor uptake and the reference ROI as described above to the entire dynamic dataset. A reference TAC was generated by placing a reference ROI in the unaffected brain tissue as reported.25 For TAC evaluation, the time-to-peak (TTP; time in minutes from the beginning of the dynamic acquisition up to the maximum SUV of the lesion) was determined.25 In cases with steadily increasing FET uptake without identifiable peak uptake, we defined the end of the dynamic PET acquisition as TTP.
Neuropathological Tumor Classification and Analysis of Molecular Markers
All tumors were histomolecularly classified according to the World Health Organization (WHO) Classification of Tumors of the Central Nervous System of 2021.26 For molecular biomarker analysis, tumor DNA was extracted from formalin-fixed and paraffin-embedded tissue samples with a histologically estimated tumor cell content of 80% or more. To assess the isocitrate dehydrogenase (IDH) mutation status, the presence of an IDH1-R132H mutation was evaluated by immunohistochemistry using a mutation-specific antibody in a standard immunohistochemical staining procedure as reported.27,28 If immunostaining for IDH1-R132H remained negative, the mutational hotspots at codon 132 of IDH1 and codon 172 of IDH2 were directly sequenced as reported.29,30 The MGMT promoter methylation status was assessed by methylation-specific PCR, as described elsewhere.30
Statistical Analyses
Descriptive statistics are provided as mean and standard deviation or median and range. The Student’s t-test was used to compare two groups. The Mann–Whitney rank-sum test was used when variables were not normally distributed. The diagnostic performance of MRI for predicting a favorable PFS and OS was calculated using 2 × 2 contingency tables; The Pearson’s chi-squared test determined statistical significance.
The values of relative changes of the PET parameters TBRmax, TBRmean, MTV, and TTP to predict a significantly longer PFS and OS as an indicator for response to lomustine-based chemotherapy were assessed by receiver operating characteristic (ROC) curve analyses using a favorable PFS and OS as reference. A favorable outcome was defined as a PFS ≥ 6 months and an OS ≥ 12 months. These thresholds were adopted from studies investigating lomustine and other agents in patients with recurrent glioblastoma.4,5,31 Decision cut-off was considered optimal when the product of paired values for sensitivity and specificity reached its maximum. As a measure of the test’s diagnostic quality, the area under the ROC curve (AUC), its standard error, and level of significance were determined.
Univariate survival analyses were performed using Kaplan–Meier estimates. The log-rank test was used to compare the median PFS and OS between the subgroups. Patients were censored if the event (tumor progression or death) had not occurred at the time of data evaluation (March 2022). Parameters that were significant in univariate analyses were included in multivariate models. Multivariate Cox proportional hazards models were constructed to test the relationship between relative changes of FET PET parameters and other decisive prognostic and predictive factors (ie, RANO criteria, the extent of resection, MGMT promoter methylation, and IDH mutation status) for a favorable survival as an indicator for response to lomustine-based chemotherapy. This analysis was performed for each FET PET imaging parameter separately. Hazard ratios and their 95%-confidence intervals were calculated. P-values of .05 or less were considered significant. Statistical analyses were performed using SPSS statistics (Release 28.0.1.0, SPSS Inc., Chicago, IL, USA).
Results
Patients
According to the search criteria, we identified 36 adult patients (mean age, 54 ± 13 years; age range, 21–82 years; 16 females) with WHO CNS grade 3 or 4 gliomas at recurrence (glioblastoma, 69%) and a Karnofsky performance status ≥ 70%. Initial glioma diagnoses were distributed as follows: WHO CNS grade 4 glioblastoma, IDH-wildtype, n = 25; WHO CNS grade 4 H3 K27-mutant diffuse midline glioma, n = 1; WHO CNS grade 4 astrocytoma, IDH-mutant, n = 2; WHO CNS grade 3 astrocytoma, IDH-mutant, n = 2; WHO CNS grade 3 oligodendroglioma, IDH-mutant, 1p/19q co-deletion, n = 6. Eighteen patients (50%) had a methylated MGMT promoter. The median number of recurrences before initiating lomustine-based chemotherapy was 1 (range, 1–3). Most patients (n = 25; 69%) were treated with lomustine-based chemotherapy at first recurrence. 33% of the patients (n = 12) were treated with lomustine monotherapy, and 68% (n = 24) with the PC regimen. The rate of patients with two and three recurrences was 25% (n = 9) and 6% (n = 2), respectively. Further details on demographics, neuropathological diagnoses, pretreatment, clinical follow-up parameters, and the patient’s survival are listed in Supplementary Tables 1 and 2.
All 36 patients completed FET PET and MR imaging at baseline and follow-up. Static and dynamic FET PET parameters at baseline and after two cycles of lomustine-based chemotherapy and their relative changes are listed in Supplementary Table 3. At the time of data evaluation, tumor progression had occurred in 32 patients (89%) and death in 31 patients (86%). The median PFS after initiation of lomustine-based chemotherapy was 3 months (range, 1–71 months), and the median OS was 11 months (range, 3–71 months).
Imaging Changes Following Lomustine-Based Chemotherapy
According to RANO criteria, two patients (6%) had a Partial Response, in 13 patients (36%) MRI findings were consistent with Stable Disease, and 21 patients (58%) had a Progressive Disease (Supplementary Table 3). None of the patients had a Complete Response on MRI. In patients with Partial Response or Progressive Disease on MRI, FET PET findings (ie, TBRmax or MTV changes) were highly congruent (100% and 91%, respectively).
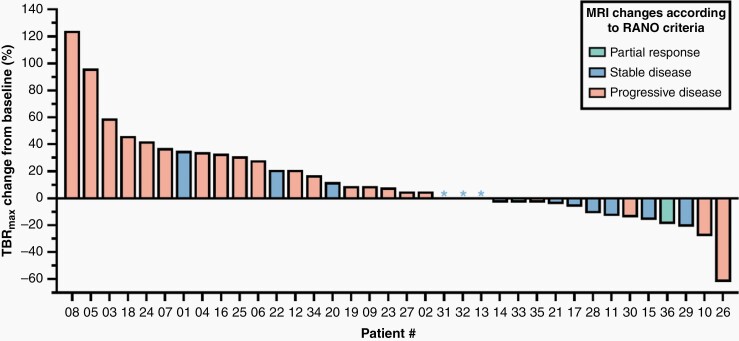
Notably, in three patients with Stable Disease on MRI after two cycles of lomustine-based chemotherapy and a favorable OS of ≥ 12 months (patients #17, #28, #29), the metabolic activity on FET PET, as assessed by TBRmax, decreased in the range of 6–21% (Figure 1). In 2 of 21 patients with Progressive Disease on MRI (patients #26 and #35), FET PET findings were discrepant. In these patients, reductions either of TBRmax or MTV after two cycles of lomustine-based chemotherapy were associated with an OS of ≥ 12 months (Figure 1). In two further patients (patients #1, #22) with a relative increase of TBRmax and an OS < 12 months, MRI changes were consistent with Stable Disease (Figure 1).
Figure 1.
Waterfall plot of responses based on changes of the static FET uptake parameter maximum tumor-to-brain ratio (TBRmax) in relation to MRI responses according to RANO criteria. Relative changes of TBRmax after two cycles of lomustine-based chemotherapy are plotted on the y-axis. In relation to the individual metabolic response on FET PET, patient columns on the x-axis are color-coded assigned to the respective MRI changes according to RANO criteria (ie, green = Partial Response; blue = Stable Disease; orange = Progressive Disease). Basically, the most patients with Progressive disease on MRI showed an increase in TBRmax, and those with Partial response had a decrease in TBRmax. Notably, considerable discrepancies, that is, increasing metabolic activity on FET PET and unchanged MRI (eg, patients #1, #22), and decreasing metabolic activity and stable (eg, patients #17, #28, #29) or progressive MRI changes (patients #26 and #35) could be observed.
Univariate Survival Analysis Regarding Changes in Imaging Parameters During Lomustine-Based Chemotherapy
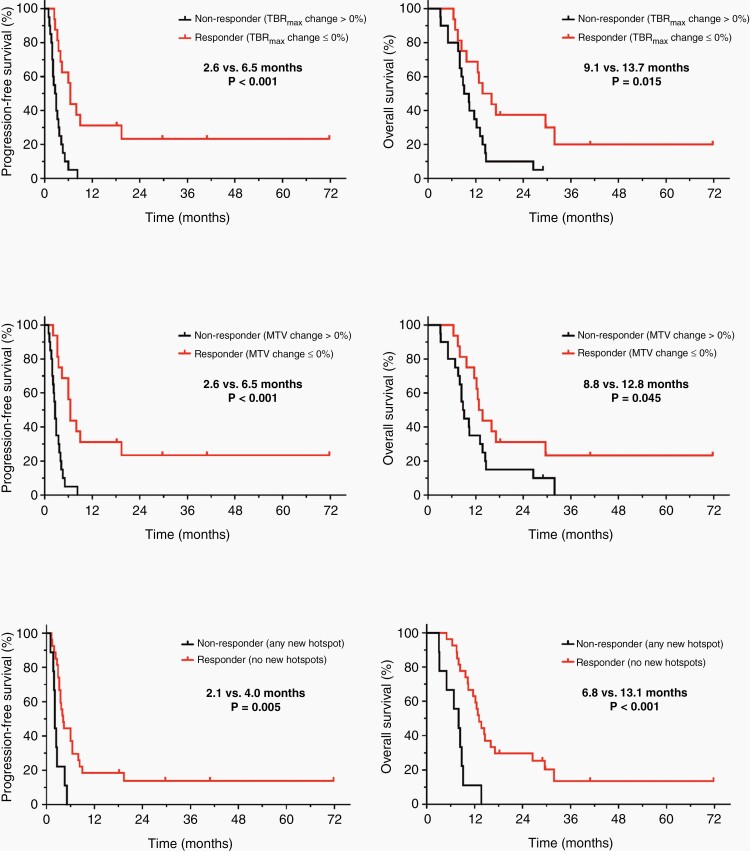
The results of the ROC analyses regarding changes in FET PET parameters during lomustine-based chemotherapy for predicting a favorable PFS of ≥ 6 months or an OS of ≥ 12 months are presented in Supplementary Tables 4 and 5. Relative changes in static FET PET parameters TBRmean (6.0 vs. 2.9 months; P = .002), TBRmax (6.5 vs. 2.6 months; P < .001), and MTV (6.5 vs. 2.6 months; P < .001) predicted a significantly longer PFS (Supplementary Table 6; Figures 2 and 3). Relative changes in TBRmean, TBRmax, and MTV after two cycles of lomustine-based chemotherapy also predicted a significantly longer OS (TBRmean, 17.1 vs. 9.7 months; P = .035; TBRmax, 13.7 vs. 9.1 months; P = .015; and MTV, 12.8 vs. 8.8 months; P = .045) (Supplementary Table 6; Figures 2 and 3). Changes in the dynamic FET PET parameter TTP were not significant regarding the prediction of both PFS and OS. Furthermore, the occurrence of any new FET hotspot at follow-up predicted both a shorter PFS (2.1 vs. 4.0 months; P = .005) and OS (6.8 vs. 13.1 months; P < .001) (Supplementary Table 6; Figures 2 and 3).
Figure 2.
Kaplan–Meier curves for PFS and OS separated by relative changes of the maximum tumor-to-brain ratio (TBRmax) (top row), metabolic tumor volume (MTV) (middle row), and the of occurrence of new hotspots (bottom row) on FET PET after two cycles of lomustine-based chemotherapy. Responders on FET PET defined by any decrease or an unchanged TBRmax and/or MTV at follow-up compared to baseline had a significantly longer PFS (both 6.5 vs. 2.6 months; P < .001) and OS (TBRmax, 13.7 vs. 9.1 months; P = .015; MTV, 12.8 vs. 8.8 months; P = .045) than non-responders (ie, patients with an increase of TBRmax and/or MTV at follow-up compared to baseline). Non-responders on FET PET defined by the occurrence of any new distant hotspot at follow-up compared to baseline had a significantly shorter PFS (2.1 vs. 4.0 months, P = .005) and OS (6.8 vs. 13.1 months, P < .001) than patients without any new distant hotspots at follow-up.
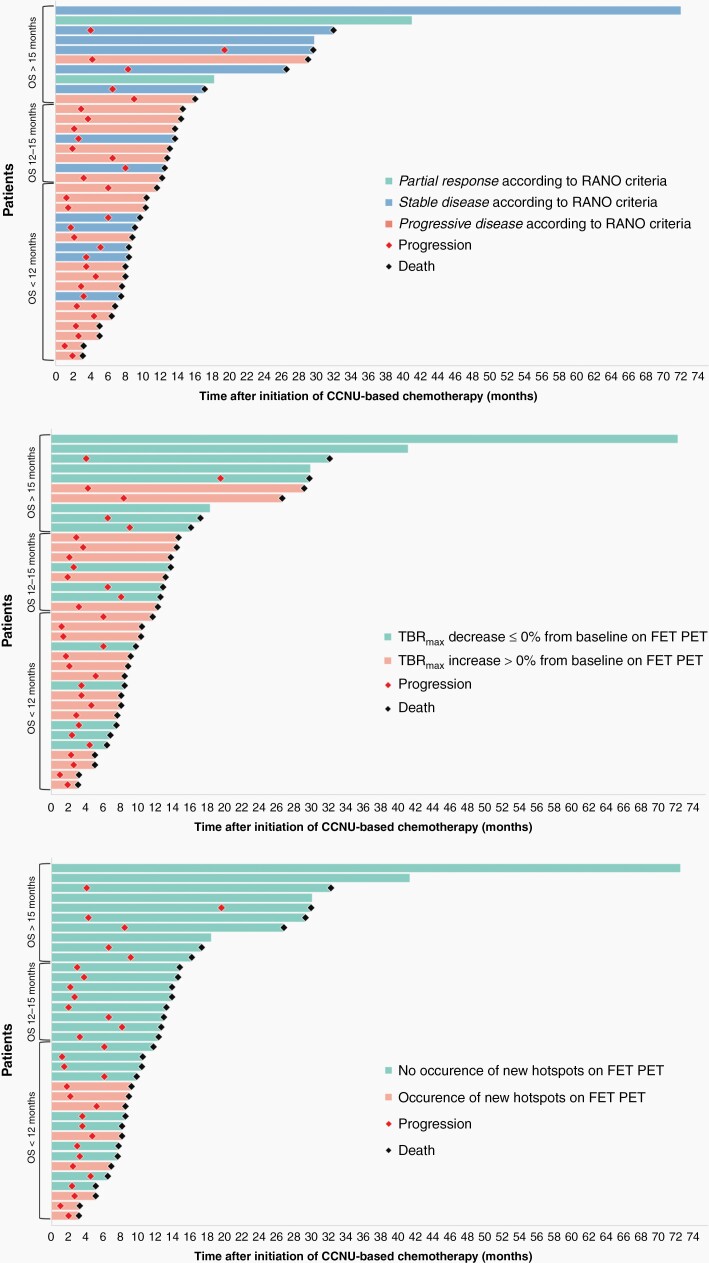
Figure 3.
Swimmer plots of each individual patient after initiation of lomustine-based chemotherapy. Patient bars are sorted by the OS and are color-coded based on changes in RANO criteria (top row), maximum tumor-to-brain ratio (TBRmax) (middle row), and the occurrence of new hot spots on FET PET (bottom row). Of note, the occurrence of new hotspots are present only in patients with an OS < 12 months, indicating its high positive predictive value for OS.
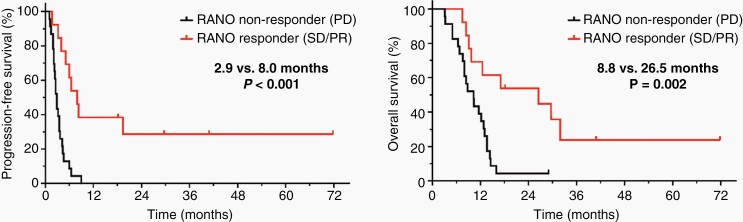
MRI changes according to RANO criteria (ie, MRI findings consistent with Stable Disease, Partial Response, or Complete Response compared to Progressive Disease) predicted a significantly longer PFS and OS (PFS, 8.0 vs. 2.9 months; P < .001; and OS, 26.5 vs. 8.8 months; P = .002) (Supplementary Table 6; Figures 3 and 4).
Figure 4.
Kaplan–Meier curves for PFS and OS separated by MRI changes according to RANO criteria after two cycles of lomustine-based chemotherapy. Responders (ie, MRI changes consistent with Complete/Partial Response or Stable Disease according to RANO criteria) had a significantly longer PFS (8.0 vs. 2.9 months; P < .001) and OS (26.5 vs. 8.8 months; P = .002) than non-responders (ie, Progressive Disease according to RANO criteria).
In the univariate survival analysis for the subgroup of patients with IDH-wildtype glioblastoma, changes of static FET PET parameters (ie, TBRmax, TBRmean, MTV), the dynamic FET PET parameter TTP, and MRI changes according to RANO criteria predicted a significant longer PFS. In addition, RANO criteria and the absence of new hotspots at follow-up on FET PET predicted a significantly longer OS (P = .013 and P = .001, respectively) (Supplementary Table 7).
Multivariate Survival Analysis Regarding Changes in Imaging Parameters During Lomustine-Based Chemotherapy
Relative changes in the static FET PET parameters TBRmax and MTV predicted a significantly longer PFS (P = .029 and P = .001, respectively) independent of the extent of resection, MGMT promoter methylation, IDH mutation status, and MRI changes according to RANO criteria. In contrast, none of the static FET PET parameters were independent predictors of OS (P > .05). On the other hand, the absence of any new FET hotspot after two cycles of lomustine-based chemotherapy predicted a significantly longer PFS (P = .019) and OS (P < .001) and was the most significant parameter independent of the extent of resection, MGMT promoter methylation, IDH mutation status, and MRI changes according to RANO criteria. MRI changes according to RANO criteria were significant predictors for both PFS (P = .002) and OS (P = .013) independent of the extent of resection, MGMT promoter methylation, IDH mutation status. Supplementary Table 8 provides a summary of all results of the multivariate analysis.
In the multivariate survival analysis for the subgroup of patients with IDH-wildtype glioblastoma only, the absence of any new FET hotspot after two cycles of lomustine-based chemotherapy also predicted a significantly longer OS (P = .005) and was the most significant parameter (RANO criteria, P = .034) (Supplementary Table 9).
Discussion
One main finding of the present study is that metabolic changes of imaging parameters derived from FET PET early after initiation of lomustine-based chemotherapy for treating patients with recurrent gliomas of the WHO CNS grades 3 or 4 seem to be of clinical value in predicting the patient’s response to therapy and outcome. In particular, relative reductions of static FET PET parameters such as the MTV or TBRmax could identify metabolic responders with a significantly longer PFS and OS than non-responders (Figures 2 and 5). Additionally, the occurrence of new distant hotspots on FET PET after initiation of lomustine-based chemotherapy had the highest significance level in predicting non-response and an unfavorable outcome. Moreover, these FET PET parameter’s predictive values appear to be independent of other strong prognostic and predictive factors such as the extent of resection at initial glioma diagnosis, MGMT promoter methylation status, IDH mutation status, and RANO criteria.
Figure 5.
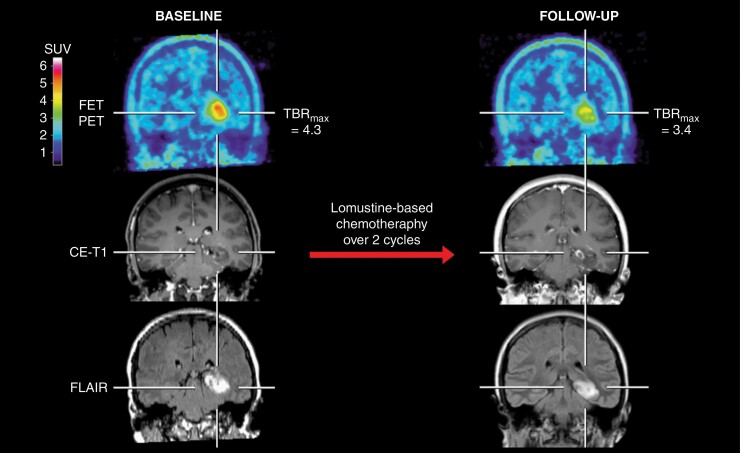
Contrast-enhanced MRI and FET PET of a 31-years-old female patient (patient #29) with an astrocytoma (IDH-mutant, WHO CNS grade 3, MGMT promoter methylated) treated with lomustine-based chemotherapy. After two cycles, the contrast-enhancing lesion progressed slightly. In contrast, the follow-up FET PET showed, relative to the baseline scan, a considerably decreased metabolic activity of 21% as assessed by a relative reduction of maximum tumor-to-brain ratios (baseline, 4.3; follow-up, 3.4) and indicated metabolic response. Without clinical deterioration or treatment change, the patient had a favorable PFS and OS (both not reached; 71.8 months at the time of data evaluation).
In direct comparison with FET PET metrics, response based on RANO criteria appeared to especially predict a longer OS. On the other hand, the occurrence of new distant FET PET hotspots at follow-up were most potent in predicting non-response. Thus, RANO criteria and FET PET metrics provide complementary information for the evaluation of response to lomustine-based chemotherapy (Figures 3 and 6) and may help to improve clinical decision-making (eg, dis- or continuation of nitrosourea-based chemotherapy) since the nitrosourea lomustine is frequently associated with significant adverse events such as hematological toxicity.
Figure 6.
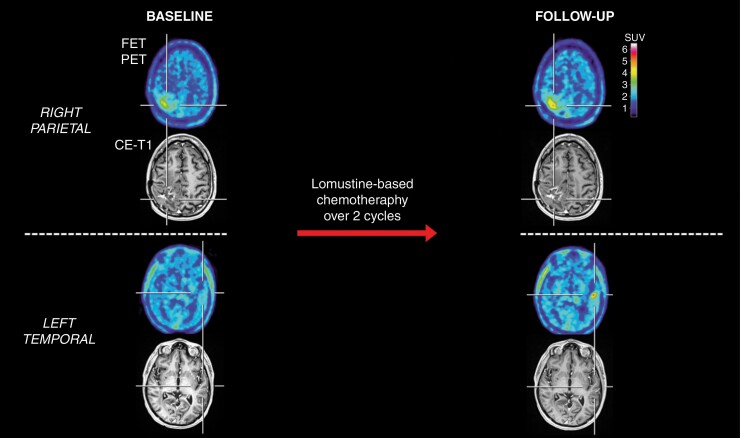
Patient with a right parietal glioblastoma with an unfavorable survival (patient #22). After two cycles of lomustine chemotherapy, the contrast-enhancing lesion on MRI appeared unchanged (Stable Disease according to RANO criteria) compared to the baseline MRI scan. In contrast, the corresponding FET PET at follow-up showed, relative to the baseline scan, an increased maximum tumor-to-brain ratio (TBRmax) and metabolic tumor volume (MTV) (relative increase, 21% and 14%, respectively). Additionally, a new distant hotspot lesion at follow-up was observed in the left temporal lobe on FET PET but not MRI. The patient had an unfavorable outcome with a PFS of 1.7 months and an OS of 9.1 months.
Similar findings were reported in studies evaluating FET PET for assessing response to alkylating chemotherapy (predominantly temozolomide) in patients with non-enhancing gliomas, usually with a WHO CNS grade 2.18,32,33 The main finding of these studies is that a more substantial reduction of the MTV (range of decrease, 10–25%) predicted a significantly longer survival or a favorable clinical course, that is, an improvement of seizure control.33 In contrast, our study shows that an unchanged MTV at follow-up after start of lomustine-based chemotherapy was already associated with a prolonged PFS and OS. Reasons for that remain unclear and are most probably related to various factors such as a different metabolic behavior on amino acid PET of the treated WHO CNS grade 2 gliomas,34,35 the applied chemotherapy regimen, and the disease stage (ie, newly diagnosed tumor or at recurrence). Furthermore, in two of these studies,18,33 response evaluation using MRI according to the RANO criteria for low-grade gliomas36 was not significant in predicting a significantly longer PFS or OS.
The value and robustness of relative changes of the FET PET-derived parameters MTV and TBRmax for response assessment have already been shown for radiotherapy with concomitant temozolomide chemotherapy,11 adjuvant temozolomide maintenance chemotherapy,12 and bevacizumab plus lomustine chemotherapy.13 However, in these studies, mainly performed in newly diagnosed glioblastoma patients, MRI response to treatment according to RANO criteria for high-grade gliomas was not significant in predicting either PFS or OS.6 Even a lesser reduction of the contrast enhancement in one study (ie, ≤ −25%) could not predict a significantly longer survival.11
In contrast to these studies, response assessment according to RANO criteria for high-grade gliomas6 proved to be a strong predictor in the present study although only 6% of the patients had objective responses (two patients had a Partial Response, none of the patients had a Complete Response). In contrast, in more than the half of the patients (4 of 7 patients; 57%) with Stable Disease on MRI after two cycles of lomustine-based chemotherapy and a favorable OS of ≥ 12 months, the metabolic activity on FET PET decreased considerably, thereby allowing a more objective response assessment. In addition, two patients with an OS of ≥ 12 months had Progressive Disease on MRI, whereas the metabolic activity on FET PET decreased. Importantly, that more intuitive reduction of metabolic activity as an additional sign of response—already on a purely visual level—may improve clinical decision-making.
A possible explanation for the predictive value of RANO criteria is that radiotherapy was not part of the treatment for the patients at recurrence included in the present study. In these patients, pretreatment with radiotherapy was already performed at least 6 months before the recurrence. Thus, radiotherapy-induced changes such as pseudoprogression, which typically occurs weeks within the first 12 weeks after radiotherapy completion37 and considerably hampers MRI-based response assessment, were less likely to occur.
Another notable finding of this study is the outstanding predictive value of new hotspots on FET PET following lomustine-based chemotherapy for an unfavorable PFS and OS, highlighting its value for treatment response evaluation, particularly by identifying non-responders (Figure 6). Notably, new hotspot occurrence remained the most significant parameter in the multivariate analysis. To our knowledge, the predictive value of new distant hotspots on FET PET has not been reported yet. Of note, most patients with glioblastoma at initial diagnosis have MRI-defined local disease and maintain this pattern notwithstanding multiple recurrences irrespective of the applied treatment.38,39 Thus, the occurrence of distant new hotspots on FET PET during treatment may help diagnose disease progression earlier.
Potential limitations of our study might be the retrospective character and treatment heterogeneity, that is, treatment with lomustine monotherapy or the PC regimen. Further multicenter trials, especially in a prospective setting to evaluate chemotherapy effects in glioma patients, are warranted to validate our data.
In summary, FET PET-derived imaging parameters provide complementary information to RANO criteria to assess the response to lomustine-based chemotherapy in glioma patients at recurrence and may predict the patient’s outcome early after treatment initiation. RANO criteria and relative changes in static FET PET parameters such as MTV and TBRmax can be used to identify responders, whereas the occurrence of new hotspots on FET PET following lomustine-based chemotherapy strongly correlates with non-response. An important next step to confirm our initial results on FET PET for response assessment of lomustine-based chemotherapy in a higher number of patients, ideally in a prospective setting.
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Contributor Information
Michael M Wollring, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany.
Jan-Michael Werner, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Elena K Bauer, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Caroline Tscherpel, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany.
Garry S Ceccon, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Philipp Lohmann, Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany; Department of Stereotaxy and Functional Neurosurgery, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Gabriele Stoffels, Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany.
Christoph Kabbasch, Institute of Radiology, Division of Neuroradiology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Roland Goldbrunner, Department of General Neurosurgery, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Center of Integrated Oncology (CIO), Universities of Aachen, Bonn, Cologne, and Duesseldorf, Germany.
Gereon R Fink, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany.
Karl-Josef Langen, Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany; Department of Nuclear Medicine, RWTH Aachen University Hospital, Aachen, Germany; Center of Integrated Oncology (CIO), Universities of Aachen, Bonn, Cologne, and Duesseldorf, Germany.
Norbert Galldiks, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany; Center of Integrated Oncology (CIO), Universities of Aachen, Bonn, Cologne, and Duesseldorf, Germany.
Funding
The Cologne Clinician Scientist-Program (CCSP) of the Deutsche Forschungsgemeinschaft (DFG, FI773/15-1), Germany, supported this work.
Disclosure
Related to the present work, the authors disclosed no potential conflicts of interest.
References
- 1. Ahn S, Kim YI, Shin JY, et al. Clinical feasibility of modified procarbazine and lomustine chemotherapy without vincristine as a salvage treatment for recurrent adult glioma. Oncol Lett 2022;23(4):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 4. Weller M, Le Rhun E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat Rev. 2020;87:102029. [DOI] [PubMed] [Google Scholar]
- 5. Lombardi G, De Salvo GL, Brandes AA, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20(1):110–119. [DOI] [PubMed] [Google Scholar]
- 6. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 7. Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langen KJ, Stoffels G, Filss C, et al. Imaging of amino acid transport in brain tumours: positron emission tomography with O-(2-[(18)F]fluoroethyl)-l-tyrosine (FET). Methods. 2017;130:124–134. [DOI] [PubMed] [Google Scholar]
- 9. Vettermann FJ, Diekmann C, Weidner L, et al. L-type amino acid transporter (LAT) 1 expression in (18)F-FET-negative gliomas. EJNMMI Res. 2021;11(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habermeier A, Graf J, Sandhofer BF, et al. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-l-tyrosine (FET). Amino Acids. 2015;47(2):335–344. [DOI] [PubMed] [Google Scholar]
- 11. Galldiks N, Langen K, Holy R, et al. Assessment of treatment response in patients with glioblastoma using [18F]Fluoroethyl-l-Tyrosine PET in comparison to MRI. J Nucl Med. 2012;53(7):1048–1057. [DOI] [PubMed] [Google Scholar]
- 12. Ceccon G, Lohmann P, Werner JM, et al. Early treatment response assessment using (18)F-FET PET compared with contrast-enhanced MRI in glioma patients after adjuvant temozolomide chemotherapy. J Nucl Med. 2021;62(7):918–925. [DOI] [PubMed] [Google Scholar]
- 13. Galldiks N, Dunkl V, Ceccon G, et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur J Nucl Med Mol Imaging. 2018;45(13):2377–2386. [DOI] [PubMed] [Google Scholar]
- 14. Hutterer M, Nowosielski M, Putzer D, et al. O-(2-18F-fluoroethyl)-l-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52(6):856–864. [DOI] [PubMed] [Google Scholar]
- 15. Galldiks N, Rapp M, Stoffels G, et al. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-l-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40(1):22–33. [DOI] [PubMed] [Google Scholar]
- 16. Galldiks N, Werner JM, Tscherpel C, Fink GR, Langen KJ. Imaging findings following regorafenib in malignant gliomas: FET PET adds valuable information to anatomical MRI. Neurooncol Adv. 2019;1(1):vdz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galldiks N, Abdulla DSY, Scheffler M, et al. Treatment monitoring of immunotherapy and targeted therapy using (18)F-FET PET in patients with melanoma and lung cancer brain metastases: initial experiences. J Nucl Med. 2021;62(4):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suchorska B, Unterrainer M, Biczok A, et al. (18)F-FET-PET as a biomarker for therapy response in non-contrast enhancing glioma following chemotherapy. J Neurooncol. 2018;139(3):721–730. [DOI] [PubMed] [Google Scholar]
- 19. Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamacher K, Coenen HH. Efficient routine production of the 18F-labelled amino acid O-2-18F fluoroethyl-l-tyrosine. Appl Radiat Isot. 2002;57(6):853–856. [DOI] [PubMed] [Google Scholar]
- 21. Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herzog H, Langen KJ, Weirich C, et al. High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin. 2011;50(2):74–82. [DOI] [PubMed] [Google Scholar]
- 23. Lohmann P, Herzog H, Rota Kops E, et al. Dual-time-point O-(2-[(18)F]fluoroethyl)-l-tyrosine PET for grading of cerebral gliomas. Eur Radiol. 2015;25(10):3017–3024. [DOI] [PubMed] [Google Scholar]
- 24. Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(Pt 3):678–687. [DOI] [PubMed] [Google Scholar]
- 25. Galldiks N, Stoffels G, Filss C, et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17(9):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20(1):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118(5):599–601. [DOI] [PubMed] [Google Scholar]
- 29. Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. [DOI] [PubMed] [Google Scholar]
- 30. Felsberg J, Rapp M, Loeser S, et al. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res. 2009;15(21):6683–6693. [DOI] [PubMed] [Google Scholar]
- 31. Wick W, Gorlia T, Bendszus M, et al. Lomustine and Bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 32. Wyss M, Hofer S, Bruehlmeier M, et al. Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol. 2009;95(1):87–93. [DOI] [PubMed] [Google Scholar]
- 33. Roelcke U, Wyss MT, Nowosielski M, et al. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro Oncol. 2016;18(5):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galldiks N, Unterrainer M, Judov N, et al. Photopenic defects on O-(2-[18F]-fluoroethyl)-l-tyrosine PET: clinical relevance in glioma patients. Neuro Oncol. 2019;21(10):1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rapp M, Heinzel A, Galldiks N, et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. 2013;54(2):229–235. [DOI] [PubMed] [Google Scholar]
- 36. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 37. Galldiks N, Kocher M, Langen KJ. Pseudoprogression after glioma therapy: an update. Expert Rev Neurother. 2017;17(11):1109–1115. [DOI] [PubMed] [Google Scholar]
- 38. Chamberlain MC. Radiographic patterns of relapse in glioblastoma. J Neurooncol. 2011;101(2):319–323. [DOI] [PubMed] [Google Scholar]
- 39. Oh J, Sahgal A, Sanghera P, et al. Glioblastoma: patterns of recurrence and efficacy of salvage treatments. Can J Neurol Sci. 2011;38(4):621–625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.