Abstract
The mainstay of treatment for adult patients with gliomas, glioneuronal and neuronal tumors consists of combinations of surgery, radiotherapy, and chemotherapy. For many systemic cancers, targeted treatments are a part of the standard of care, however, the predictive significance of most of these targets in central nervous system (CNS) tumors remains less well-studied. Despite that, there is increasing use of advanced molecular diagnostics that identify potential targets, and tumor-agnostic regulatory approvals on targets also present in CNS tumors have been granted. This raises the question of when and for which targets it is meaningful to test in adult patients with CNS tumors. This evidence-based guideline reviews the evidence available for targeted treatment for alterations in the RAS/MAPK pathway (BRAF, NF1), in growth factor receptors (EGFR, ALK, fibroblast growth factor receptor (FGFR), neurotrophic tyrosine receptor kinase (NTRK), platelet-derived growth factor receptor alpha, and ROS1), in cell cycle signaling (CDK4/6, MDM2/4, and TSC1/2) and altered genomic stability (mismatch repair, POLE, high tumor mutational burden (TMB), homologous recombination deficiency) in adult patients with gliomas, glioneuronal and neuronal tumors. At present, targeted treatment for BRAF p.V600E alterations is to be considered part of the standard of care for patients with recurrent gliomas, pending regulatory approval. For approved tumor agnostic treatments for NTRK fusions and high TMB, the evidence for efficacy in adult patients with CNS tumors is very limited, and treatment should preferably be given within prospective clinical registries and trials. For targeted treatment of CNS tumors with FGFR fusions or mutations, clinical trials are ongoing to confirm modest activity so far observed in basket trials. For all other reviewed targets, evidence of benefit in CNS tumors is currently lacking, and testing/treatment should be in the context of available clinical trials.
Keywords: Adults, EANO, Guideline, Glioma, Targeted treatments
Key Points.
Panel diagnostics are a more efficient way to identify rare genetic variants than target-specific assays.
At present, only the clinical benefit in patients with BRAF p.V600E mutant recurrent CNS tumors is sufficiently well established to consider this part of standard of care.
Despite tumor agnostic regulatory approval for NTRK fusion inhibiting agents in patients with NTRK fusions and of pembrolizumab in ‘tumor mutational burden high’ tumors, the evidence of benefit of these targeted treatments in CNS tumors is limited and treatment should be given in prospective clinical registries/trials.
In adult gliomas, glioneuronal and neuronal tumors, for the other targets covered in this guideline, clinical benefit of treatment for these targets in CNS tumors has not been established and testing for the target is only relevant if a clinical trial is available.
Summary.
This EANO (European Association of Neuro-Oncology) guideline provides a comprehensive overview of targets for targeted treatment in gliomas, glioneuronal and neuronal tumors. It allows a rational and cost-effective testing strategy by building the recommendations on the evidence of the clinical benefit of targeted treatment of molecularly selected central nervous system tumors in adults.
The therapeutic options for patients with primary brain tumors remain limited, especially at the time of tumor progression after radiotherapy and/or chemotherapy. Precision oncology holds great promises for cancer patients, but targeted treatments have not yet altered the standard of care for patients diagnosed with central nervous system (CNS) tumors. Increasingly, next-generation sequencing (NGS) panels are ordered to identify targeted treatment options in patients that have exhausted first-line or standard-of-care options. With the increasing availability and coverage of multigene sequencing panels in routine diagnostics of these panels, the rate of detection of molecular alterations in CNS tumors with possible therapeutic implications is also increasing. However, the clinical evidence of the actionability of those alterations ranges from established therapeutic efficacy in CNS tumors or in other cancers to hypothetical targets with presumed clinical significance based on preclinical evidence from in vivo or in vitro research only. In addition, not all alterations even within the same gene have the same predictive significance across cancer types: targeting a molecular alteration found actionable in some cancers may result in a different magnitude of benefit in other cancer types. Precision oncology provides a formal framework to address these challenges through standardized variant interpretation, annotation, and joint clinical decision-making in molecular boards. To identify brain tumor patients that possibly benefit from precision oncology approaches, the present guideline aims to provide evidence-based recommendations for predictive molecular testing for patients with CNS tumors. It covers how to test for predictive genetic alterations, how to report findings, how to attribute pathogenetic significance to findings, how to attribute clinical significance to findings, and when to test.
Thereafter the guideline reviews the spectrum of genetic alterations that may have potential therapeutic implications in CNS tumors, beyond routine diagnostics according to the WHO 2021 classification.1 The guideline does not cover all tumor types of the WHO 2021 CNS tumor classification, but focuses on gliomas, glioneuronal and neuronal tumors diagnosed in adults. The specific targets included in this guideline are NF1 (outside neurofibromatosis type 1), BRAF, Anaplastic lymphoma kinase (ALK), epidermal growth factor receptor (EGFR), FGFR1-3, MET, NTRK1-3, platelet-derived growth factor receptor alpha (PDGFRA), ROS1, MDM2/4, cyclin-dependent kinases 4 (CDK4)/6, TSC1/2, and MDM2/4. In addition, the significance of high tumor mutational burden (TMB), DNA mismatch repair (MMR), DNA polymerase, and homologous recombination deficiency (HRD) are reviewed. In the core text, the integrated recommendations on testing and treatment for each alteration are presented, and the in-depth literature review that provides the basis for the recommendation on each target is presented in the accompanying supplement. The relevant literature until May 31, 2022, has been selected by the expert authors, according to EANO standard operating procedures.
Molecular Testing: How to Test
The molecular testing strategy depends on the tumor entity, the patient's clinical status and stage of the disease, the available clinical studies, their entry requirements, and local resources. The individual situation defines the most suitable testing approach: confirmation of a very frequent alteration (eg, diagnosing a BRAF fusion in a pilocytic astrocytoma or proof of the presence of a BRAF p.V600E mutation in a tumor for study inclusion) may be conducted by a target-specific assay. In contrast, the identification of diverse targetable alterations (eg, in recurrent glioblastoma with no further treatment options or in a rare tumor type with no established standard protocol) may be best achieved through a broad sequencing approach. In most CNS tumor types in adult patients, the prevalence of individual targets of interest is low (<5% of cases) and many occur in a mutually exclusive manner. In tumors with low target prevalence, next-generation sequencing (NGS), which allows simultaneously assessing multiple targets, is preferred over time-consuming, tissue-consuming, and expensive sequential testing by target-specific assays.
Immunohistochemistry
The usefulness of immunohistochemistry as a screening tool for therapeutically targetable molecular alterations in CNS tumors is restricted to a few exceptions. Companion diagnostic grade immunohistochemical assays for targets in primary CNS tumors do not exist. Certain immunohistochemical screening assays used in general pathology are of limited or no use in CNS tumors, eg, endogenous neurotrophic tyrosine receptor kinase (NTRK) expression hinders immunohistochemical screening for NTRK fusions in the nervous system and is therefore not recommended.2BRAF p.V600E mutations can be identified by a mutation-specific antibody.3 Immunohistochemical staining can be used as a screening method for ALK and ROS1 fusions, but positive results should be confirmed by additional molecular tests. FGFR3:TACC3 fusions are associated with increased FGFR3 protein expression and IHC proved useful in prescreening diffuse gliomas.4 Different MET antibodies have been tested to detect MET overexpression as an indicator of MET amplifications in gliomas, but standardized cutoff criteria and protocols do not exist and overexpression in IHC cannot replace gene amplification testing.5 Loss or reduced expression of hamartin (TSC1) or tuberin (TSC2) has been described in SEGAs, but the sensitivity and specificity of such immunohistochemical analysis for the detection of TSC1 and TSC2 alterations need further elucidation, similar to the loss of neurofibromin caused by deleterious NF1 alterations.6,7 Immunohistochemistry can be used to diagnose MMR deficiency, which is detected by the loss of expression of MMR proteins in tumor cells (or in both non-neoplastic and neoplastic cells in the case of constitutional mismatch repair deficiency (CMMRD).8
Detection of Amplification
High and low-level amplification (eg, EGFR, CDK4/6, MDM2/4, MET, PDGFRA) can be identified using a variety of techniques including single gene methods such as FISH, digital droplet polymerase chain reaction (ddPCR), qPCR, or high-throughput methods such as array comparative genome hybridization, (low pass) whole genome, exome sequencing and targeted NGS gene panels. Genome-wide DNA methylation arrays can also identify copy number gains, though the detection sensitivity in samples with low-level copy number gains and samples with a low tumor purity is unclear. An advantage of the array and whole genome sequencing or whole exome sequencing methods is their ability to distinguish focal high-level amplification against low-level gains due to trisomy or polysomy. Moreover, additional genetic alterations (eg, extracellular or tyrosine kinase domain mutations, fusions, exon skipping, or deletions) can be detected simultaneously.
Mutation Detection
For the detection of the hotspot, mutations targeted assays (eg, Sanger sequencing, pyrosequencing, ddPCR) may be used. For genes in which mutations are not confined to specific exons (eg, EGFR, MET, PDGFRA, NF1, and mTOR/TSC1/2), the size of the coding sequence along with a large number of exons makes classical sequencing efforts to detect mutations difficultly; these are preferably identified by DNA NGS. Depending on the size of the panel and the availability of matched normal (germline) control samples, the number of variants can be substantial. Alterations include known pathogenic driver mutations, splice or truncating mutations as well as missense variants, many of which are of unknown significance. Variant interpretation and target prioritization involve consideration of gene-specific (eg, proto-oncogene vs. tumor suppressor gene, +/− biallelic inactivation) and tumor type-specific aspects as well as clonality (ie, clonal vs. subclonal variant). Currently available public databases mainly comprise information on non-CNS tumor types and may be of limited use for variant interpretation in CNS tumors. For deleterious alterations, especially relevant for tumor suppressor genes (eg, NF1, TSC1/2, and MMR genes), potentially relevant aberrations may be missed by short-read NGS sequencing (eg, large deletions, complex gene rearrangements). Furthermore, some genomic regions are hard to sequence and align (eg, because of high guanine-cytosine content, and highly repetitive regions).
Fusion Detection
Therapeutically relevant gene fusions in BRAF, FGFR1-3, MET, NTRK1-3, and PDGFRA usually involve various fusion partners and genomic breakpoints. Except for BRAF fusions in pilocytic astrocytoma, most fusions in adult CNS tumor patients are rare and mutually exclusive. Fusion and break-apart FISH probes are available for NTRK1-3, but FISH cannot determine if the fusion results in a productive in-frame chimeric transcript. While FISH assays allow for a fusion-partner agnostic detection of a specific fusion per probe, RT-PCR approaches are further limited as they require knowledge of the fusion partner. In most CNS tumor types, time- and resource-consuming sequential testing of several targets would be necessary, which renders both methods less attractive than NGS approaches, especially in entities with a low prevalence of fusions. Fusions may also be detected by amplicon-based or hybrid capture-based sequencing of DNA or RNA. Fusion breakpoints are often located in intronic regions, which makes it challenging to detect them from DNA sequencing and requires whole genome sequencing or custom NGS panels including intronic regions of the genes of interest. Amplicon-based sequencing will only allow the detection of a known and limited number of fusion partners. Commercial non-hybrid capture-based NGS panels—including those designed for non-CNS tumors and regularly used in general pathology departments—often do not contain all fusion partners relevant for CNS tumors. Anchored multiplex PCR or hybrid capture-based approaches instead allow for the detection of fusions in an agnostic manner and are most suitable for fusion detection in CNS tumors involving a plethora of fusion partners.
Hints for certain types of gene fusions can be identified in DNA copy number variation plots calculated from DNA methylation arrays (ie, Illumina EPIC array) and should generally prompt further genetic testing, except for the frequently detected KIAA1549::BRAF fusion on chromosome 7q34 in pilocytic astrocytomas and high-grade astrocytoma with piloid features. Fusions may appear either as small focal gains (eg, RAF1 duplication in SRGAP3::RAF1 fusion on chromosome 3p) or focal losses (eg, BRAF partial deletion in FAM131B::BRAF fusion) usually partially encompassing the gene of interest.9 Several gene amplifications are associated with fusions of the same gene (eg, EGFR, PDFGRA, and MET), thus fusion testing may be considered for amplified cases.
TMB, POLE, and MMR Deficiency
TMB can be accurately inferred using panels covering at least 0.6 Mb.10–12 Harmonization efforts for TMB calculation, reporting and interpretation are ongoing. TMB reporting should include a brief description of the inclusion and exclusion criteria of variants used for the TMB calculation (eg, minimum read depth and allele frequency, +/− synonymous variants).13 Most whole exome-based studies have used the cutoff of 10 mutations per Mb to define TMB-high (TMB-H); however, this cutoff cannot be extrapolated to all NGS panels as the discrimination of samples with TMB-H varies across different panels, with panel size, gene content, and bioinformatics pipelines contributing to variability.11 The advantage of DNA sequencing using large panels is that it can also provide the mutational status of the genes associated with DNA polymerase and MMR deficiency (ie, POLE, POLD1, MSH2, MSH6, MLH1, and PMS2) as well as “mutational signatures” (ie, specific mutational patterns) occurring in the sample of interest (eg, polymerase or MMR deficiency, alkylating agent exposure). Diagnosis of MMR deficiency using classical microsatellite instability (MSI) assays is unreliable in gliomas as MMR-deficient gliomas are often classified as microsatellite stable using tests developed for colorectal and other cancer types.8,14,15
Germline Testing
Genetic counseling should be done prior to germline testing, in view of the implications for patients when being diagnosed with a germline mutation. Diagnostic criteria have been developed and can guide clinicians to select patients that are more likely to carry germline defects.16 If deleterious mutations in tumor suppressor genes relevant in the context of cancer predisposition syndromes (eg, NF1, TSC1/2) are detected in tumor-only sequencing at variant allele frequencies suggestive of a potential germline alteration, genetic counseling may be considered as well, especially in young adults. Genetic counseling should further be considered for all patients diagnosed with pretreatment TMB-H glioma (especially in the presence of polymerase or MMR gene aberrations consistent with a constitutional event).
How to Report Findings
Nucleotide sequence variants are reported as changes relative to reference sequences. The use of the term “variant” or “alteration” is preferred over “mutation,” as the latter term tends to imply that the detected DNA sequence change has a disease-related significance which may be true for some variants but not for others, and of many variants the significance is unknown. In addition, defining a variant as a (somatic) mutation in cancer with certainty would require sequencing-matched constitutional DNA, while the use of “variant” is also used to describe sequences deviating from the reference genome. With modern sequencing panels, many alterations of unclear functional significance outside known (hotspot) driver variants are detected (“variants of unknown significance”), even in genes with a well-established role in tumorigenesis/oncogenesis and with pathogenic variants described. For the description of sequence variants, standardized wording developed by the Human Genome Variation Society should be used as well as information on the reference genome build (currently mostly CRCh19/hg19 or CRCh38/hg38), with changes in the coding DNA reference sequence (c.), the protein reference sequence (p.), and the transcript ID being provided.17 The molecular pathology report should contain the list of genes/ regions of genes covered by the NGS panel and further information necessary to assess sequence quality and assay limitations. In addition, information relevant to the interpretation of tumor heterogeneity and actionability in molecular tumor boards should be added (ie, mean coverage, coverage at variant site, technical detection limits, tumor cell content, variant allele frequency, predicted copy number variation). The assessment of the relevance of a variant involves 2 levels: (1) A biological interpretation (ie, effect on the translated protein, pathogenic/oncogenic potential, diagnostic implication, prognostic significance), and (2) A clinical interpretation (ie, actionable and predictive potential of a variant) usually assessed by an interdisciplinary molecular tumor board.
Attributing Pathogenetic Significance to Findings
The pathogenic significance attributed to detected sequence variants is based on various sources such as databases where variants, their frequency, and significance are reported, including germline frequency in the general population, the similarity of the variant to known pathogenetic variants, localization of the abnormality (eg, known hotspot), predicted impact on the function of the protein and functional assays.18,19 For the reporting of germline sequencing results, the American College of Medical Genetics updated a classification scheme and nomenclature for constitutional variants but so far no international consensus on the reporting and interpretation of somatic variants in cancer has been reached.18–20 A commonly used 5-tiered denomination scheme for somatic variants distinguishes between “pathogenic/relevant,” “likely pathogenic/likely relevant,” “uncertain significance,” “likely benign,” and “benign.” Recently, a new recommendation for the classification of pathogenicity (oncogenicity) of somatic variants in cancer, adopted from the 2015 American College of Medical Genetics classification for germline variants, has been proposed to harmonize standards in reporting (Table 1).19 For variant frequencies within specific tumor entities the databases require information on precise tumor diagnoses. The majority of NGS data available in public databases provide information mainly on non-CNS tumor entities. The quality of data, granularity of annotation, and curation of datasets are highly variable. For most neuro-oncological entities covered in this review, we were not able to extract meaningful data from such databases, mostly because the tumor diagnoses were outdated and did not cover the diverse spectrum of WHO 2021 CNS tumor classes.
Table 1.
Objective and Wording in the 2015 guideline of the American Society of Medical Genetics, the 2022 Joint Recommendations of Clinical Genome Resource (ClinGen), Cancer Genomics Consortium (CGC), and Variant Interpretation for Cancer Consortium (VICC); the 2017 Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists; and the ESMO Scale for Clinical Actionability of molecular Targets
| Guideline | American College of Medical Genetics 201520 | ClinGen/CGC/VICC19 | AMP/ASCO/CAP 201718 | ESCAT21 |
|---|---|---|---|---|
| Objective | Classification of germline variants | Classification of the pathogenicity of somatic variants in cancer (oncogenicity) | Classification of somatic variants in cancer based on the evidence available supporting their value as clinical targets | Classification of somatic variants in cancer based on the evidence available supporting their value as clinical targets |
| Used wording |
Pathogenic
Likely Pathogenic Uncertain significance Likely benign Benign |
Oncogenic
Likely oncogenic Uncertain significance Likely benign Benign |
Variants of strong clinical significance
-Level A: FDA-approved therapy -Level B: well-powered studies with consensus in the field Variants of potential clinical significance -Level C: FDA-approved therapies for different tumor types or investigational therapies -Level D preclinical trials or a few case reports Variants of unknown clinical significance Benign or Likely benign variants |
Tier I: ready for routine use: Alteration-drug match is associated with improved outcomes in clinical trials Tier II: Investigational: alteration-drug the match is associated with antitumor activity, but the magnitude of the benefit is unknown Tier III: Hypothetical: alteration-drug match suspected to improve outcome based on clinical trial data in other tumor type(s) or with similar molecular alteration |
| Role | Diagnostic | Diagnostic/prognostic | Therapeutic/predictive | Therapeutic/predictive |
Attributing Clinical Significance to Findings
Certain genetic alterations provide meaningful targets for medical treatment. To address the difference in the level of evidence of the potential clinical benefit of genetic alterations, various guidelines have provided scoring systems for targets identified as part of the diagnostic process.18,21,22 Although they follow similar approaches, there is variability in these scales. Since the attribution is partly based on the regulatory approval of drugs, decisions of regulatory agencies may also affect the scoring and thus different attributions may exist in different countries (eg, tier I: Strong clinical significance, FDA-approved therapy; Table 1).18 This guideline uses the widely accepted ESMO Scale of Clinical Actionability for molecular Targets (ESCAT), which assigns levels of evidence for the actionability of identified potential targets (Table 1, 2).21 The tiers of this scale (levels I–V) follow the levels of evidence commonly used to develop guidelines from results of clinical trials, with tiers related to the quality of the available clinical evidence. This tier system goes from evidence obtained in randomized clinical trials within the disease of interest, via randomized trials in other cancers, uncontrolled studies, and experimental agents in clinical trials to evidence obtained in the laboratory setting. These levels indicate the clinical benefit that may be expected from targeted treatments, This paper will only consider targets within levels I–III of the ESCAT evidence tier (“ready for routine use”, “investigational”, “hypothetical target”). Also, drugs directed for some targets have been (conditionally) approved in a tumor-agnostic setting (eg, larotrectinib and entrectinib in NTRK fusion-positive tumors; in the US dabrafenib and trametinib for BRAF p.V600E mutant tumors and pembrolizumab for tumors with high tumor mutational burden). However, for some of these approvals, such as the approval for pembrolizumab, the evidence of benefit in patients with glioma and other CNS tumors is weak, either in terms of the number of treated patients or of the activity observed. In the presence of such limited knowledge, the recommendation of this guideline is to treat such patients only within clinical trials or prospective registries.
Table 2.
The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT)21
| ESCAT Evidence Tier | Required Level of Evidence | Clinical Value Class | Clinical Implication | |
|---|---|---|---|---|
| Ready for routine use | I: Alteration-drug match is associated with improved outcome in clinical trials |
IA: prospective, randomized clinical trials show the alteration-drug match in a specific tumortype results in a clinically meaningful improvement of a survival endpoint | Drug administered to patients with the specific molecular alteration has led to improved clinical outcome in prospective clinical trial(s) |
Access to the treatment should be considered standard of care |
| IB: prospective, non-randomized clinical trials show that the alteration-drug match in a specific tumor type, results in clinically meaningful benefit as defined by ESMO MCBS 1.1 | ||||
| IC: clinical trials across tumor types or basket clinical trials show clinical benefits associated with the alteration-drug match, with similar benefits observed across tumor types | ||||
| Investigational | II: alteration-drug match is associated with antitumor activity, but the magnitude of benefit is unknown | IIA: retrospective studies show patients with the specific alteration in a specific tumor type experience clinically meaningful benefit with the matched drug compared with alteration-negative patients | Drug administered to a molecularly defined patient population is likely to result in clinical benefit in a given tumor type, but additional data are needed | Treatment to be considered “preferable” in the context of evidence collection either as a prospective registry or as a prospective clinical trial |
| IIB: prospective clinical trial(s) show the alteration-drug match in a specific tumor type results in increased responsiveness when treated with a matched drug, however, no data currently available on survival endpoints | ||||
| Hypothetical target | III: alteration-drug match suspected to improve outcome based on clinical trial data in other tumor type(s) or with similar molecular alteration | IIIA: clinical benefit demonstrated in patients with the specific alteration (as tiers I and II above) but in a different tumor type. Limited/ absence of clinical evidence available for the patient-specific cancer type or broadly across cancer types | Drug previously shown to benefit the molecularly defined subset in another tumor type (or with a different mutation in the same gene), efficacy, therefore, is anticipated for but not proved | Clinical trials to be discussed with patients |
| IIIB: an alteration that has a similar predicted functional impact as an already studied tier I abnormality in the same gene or pathway, but does not have associated supportive clinical data | ||||
| IV: preclinical evidence of actionability | IVA: evidence that the alteration or a functionally similar alteration influences drug sensitivity in preclinical in vitro or in vivo models | Actionability is predicted based on preclinical studies, no conclusive clinical data are available | Treatment should “only be considered” in the context of early clinical trials. Lack of clinical data should be stressed to patients | |
| IVB: actionability predicted in silico | ||||
| Combination development | V: alteration-drug match is associated with objective response, but without clinically meaningful benefit | Prospective studies show that targeted therapy is associated with objective responses, but this does not lead to improved outcome | Drug is active but does not prolong PFS or OS, probably in part due to mechanisms of adaptation | Clinical trials assessing drug combination strategies could be considered |
| X: lack of evidence for actionability | No evidence that the genomic alteration is therapeutically actionable | There is no evidence, clinical or preclinical, that a genomic alteration is a potential therapeutic target | The finding should not be taken into account for clinical decision |
Reprinted with permission from Ref 21. ESMO, European Society for Medical Oncology.
Historically, drugs that have shown clinical benefit in randomized clinical trials are considered for registration and reimbursement in that disease. Today, several new and often rare neuro-oncological tumor types have been delineated for which randomized controlled trials are difficult to conduct or simply not feasible and the activity of new therapeutic strategies is often evaluated in terms of objective response rate (ORR) in single-arm studies or basket trials on multiple tumor types characterized by the same actionable mutation. This strategy may nowadays lead to regulatory approval.23,24 An alternative single-arm study design for very rare cancers, which is also considered by EMA, is a within-patient time-to-progression or progression-free survival (PFS) analysis (or the combination), requiring superiority of time-to-progression and/or PFS of the experimental treatment over the time-to progression /PFS obtained with prior standard treatment.25–29
Regardless of the study design used, especially single-arm studies require tools that systematically evaluate the magnitude of benefit to the experimental treatment demonstrated in them. The ESMO ESMO–Magnitude of Clinical Benefit Scale is a tool that weighs the clinical evidence also when obtained in single-arm studies based on ORR, Duration of objective Response (DoR; complete and partial response) and durable PFS.30 In this scale, for single-arm studies the highest grade of 3 is awarded to studies demonstrating either median PFS >6 months or an ORR >60%; or an ORR between 20% and 60% and DoR >9 months (Table 3). If the quality of life outcomes are reported, the score can be adapted, eg, the score is decreased in the presence of >30% grade 3-4 toxicities impacting daily well-being. This paper will use the ESMO-MCBS to score the activity of (combination of) drugs on targets; this score will only be attributed to results from prospective trials on targets with an ESCAT score of I or II (Table 1).
Table 3.
ESMO Magnitude of Clinical Benefit Scale (MCBS) Form 3: Evaluation Scale for Benefit as Observed in Single Arm Studies in “Orphan Diseases” and for Diseases With “High Unmet Need” With Primary Outcome Progression-Free Survival or Objective Response Rate. Non-Curative Setting Grading 5 and 4 Indicates a Substantial Magnitude of Clinical Benefit31
| Grade | Criteria |
|---|---|
| 3 | PFS ≥ 6 months ORR (PR + CR) ≥ 60% ORR (PR + CR) ≥ 20% - < 60% AND Duration of response ≥ 9 months |
| 2 | PFS ≥ 3 - < 6 months ORR (PR + CR) ≥ 40% - < 60% ORR (PR + CR) ≥ 20% - < 40% AND Duration of response ≥ 6 - < 9 months |
| 1 | PFS 2 - < 3 months ORR (PR + CR) ≥ 20%–< 40% AND Duration of response < 6 months ORR (PR + CR) ≥ 10% - < 20% AND Duration of response ≥ 6 months |
Adjustments: Final adjusted magnitude of clinical benefit grade.
Downgrade 1 level if there are ≥30% grade 3–4 toxicities impacting daily well-being*.
Upgrade 1 level if improved Quality of Life.
Upgrade 1 level for confirmatory, adequately sized, phase 4 experience.
Abbreviations: PFS: Progression-Free Survival; ORR: Objective response rate. ESMO: European Society for Medical Oncology.
Molecular Testing: When to Test?
The large number of potential targets raises the issue when testing is warranted. This concerns both the timing (at the time of initial diagnosis or at recurrence), and the evidence required to make testing for a specific target clinically meaningful. Many potential targets may indeed be present but are either rare or of no or unclear clinical significance, or both. Considerations that give guidance here are the relevance of the target (eg, as expressed in the ESCAT scale, level I as opposed to level II and III), the magnitude of the clinical benefit obtained in a responding patient population (ESMO-MCBS), the frequency with which the target occurs, the complexity and costs of the diagnostic assay, the routine institutional diagnostic approach (eg, single assays versus standard panel assays) and the availability of clinical trials.21,30 Here, health economics come into the equation, with the caveat that for most targets and accompanying drugs, no data exist for CNS tumor patients. If a target is “hypothetical” according to the ESCAT scale (ESCAT III) and treatment should only be considered in the context of early clinical trials, it makes no sense to test separately for that target unless a clinical trial is readily available. However, if such a target is “routinely” assessed by the used panel assay, and the target is found to be present, more intensive searches for trials aiming at that specific alteration are possible. This argues against “named patient programs” for ESCAT scale III/IV targets without systematic information on the outcome being collected within a prospective registry, but also warns against overinterpretation of case reports given the risk of bias from these reports and the potential exposure of subsequent patients as a result of “promising” but unconfirmed case reports. For rare targets, with drugs of only limited effectiveness and more complex and costly diagnostic assays, testing may present an inappropriate use of resources. Testing at the time of first diagnosis is especially meaningful if no well-established standard of care exists for that entity and/or a potential meaningful treatment for a target in that entity is likely, in which case upfront treatment could be considered. Examples of this are frequently BRAF p.V600E mutated tumors like ganglioglioma and PXA. Overall, molecular testing of targets for non-approved and experimental treatments should principally be considered when standard treatments have been exhausted for the respective tumor type. Whenever possible molecular testing should be done from the most recent tumor tissue sample, as molecular alterations may change over time; but also over time the development of newer methodologies justifies waiting with analysis until clinically indicated.31 The risk and benefits of performing dedicated neurosurgical interventions for the sole purpose of tissue acquisition for molecular testing for ESCAT scale III or IV targets need to be carefully weighed given the absence of established benefit.
Targets of Potential Interest
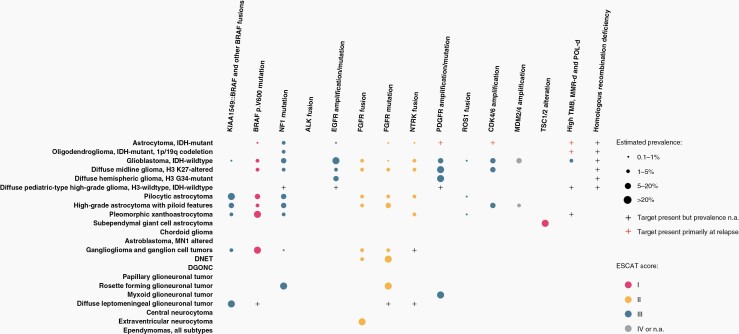
Targets covered in this review were selected because of their potential actionability in gliomas, glioneuronal and neuronal tumors. Based on these considerations, the clinical relevance of the following targets are described in detail: BRAF; NTRK; fibroblast growth factor receptor (FGFR), mTOR/TSC1/TSC2; EGFR; MET, CDK4/6, ALK, NF1, PDGFRA, ROS1, and MDM2/4. In addition, high TMB, HDR, and POLE or MMR deficiency are discussed. IDH mutations were not part of the current effort, these are part of WHO diagnostics and although trials on IDH as a target are ongoing IDH is not considered here. The full review of all targets summarizing (1) its role in cancer, (2) type and frequency in gliomas, glioneuronal and neuronal tumors in adults, (3) testing methods, (4) the alteration as a therapeutic target gliomas, glioneuronal and neuronal tumors in adults, and (5) Integrated recommendations on testing and treatment) is presented in the supplemental files, for space restrictions the main text is limited to the integrated recommendations on testing and treatment for each alteration. However, the supplement is an integral and essential part of this guideline and should be consulted when considering a target for testing or treatment. Table 4 summarizes the ESCAT score per target and the ESMO-MCBS of treatments with an ESCAT score of I or II. Figure 1 presents an overview of frequencies of the molecular alterations per WHO 2021 tumor type and the ESCAT score of the alteration.
Table 4.
Target Genes, Most Frequent Gene Alterations and Their ESMO Scale of Clinical Actionability of Molecular Targets Score, Drugs With an Established or Investigational role in That Target, ESMO Magnitude of Clinical Benefit Score (MCBS), and the Clinical Setting in Which Treatment can be Considered. ESMO-MCBS are not Provided for Targets That are Considered ESCAT III (= hypothetical target), for Some ESCAT II Targets Data are Insufficient for ESMO-MCBS
| Gene | Type of Gene Abnormality | ESCAT | Drugs | ESMO-MCBS | Setting |
|---|---|---|---|---|---|
| RAS/MAPK pathway | |||||
| BRAF | |||||
| p.V600E mutation | IB | dabrafinib/trametinib | 3 | standard of care at PD | |
| vemurafinib | 3 | ||||
| dabrafinib | |||||
| KIAA1549::BRAF fusion | IIIA | selumetinib | No data | trial | |
| novel BRAF inhibitors | trial | ||||
| NF1 | somatic alterations (non-germline) | IIIA | MEK inhibitors | trial | |
| Growth factor receptors | |||||
| ALK | fusions, mutations | IIIA | crizotinib, alectinib, ceritinib | registry/trial | |
| EGFR | amplifications, fusion, mutations | IIIA | novel agents | trial | |
| FGFR | FGFR1,3 fusions, FGFR1 mutations N546K, K656E | IIB | erdafitinib | 2 | registry/trial |
| pemigatinib | No data | trial | |||
| MET | exon 14 skipping, fusions, amplifications, | IIIA | MET inhibitors | trial | |
| NTRK 1-3 | fusion, mutations | IIB | larotrectinib, entrectinib | No data | registry/trial |
| PDGFRA | amplifications, mutations, fusions | IIIA | PDGFRA inhibitors | trial | |
| ROS1 | fusions | IIIA | crizotinb, entrectinib, etc | trial | |
| Cell cycle | |||||
| CDK4/6 | amplification | IIIA | CDK4/6 inhibitors | trial | |
| MDM2/4 | amplification | IV | Various investigational agents | trial | |
| TSC1/2 | |||||
| SEGA as part of TSC syndrome | IA | everolimus sirolimus |
2 No data |
standard of care trial |
|
| TSC1/TSC2 outside of TSC syndrome | IIIB | mTOR inhibitors | trial | ||
| Altered genomic stability/DNA repair | |||||
| MMR deficiency | |||||
| MMR deficiency, POLE alterations, TMB-high germline | IIIA | nivolumab, pembrolizumab, ipilimumab | registry/trial | ||
| MMR deficiency, POLE alterations, TMB-high treatment-induced | IIIB | nivolumab, pembrolizumab, ipilimumab | trial | ||
| HR deficiency | IDH, (BRCA1, BRCA2, and RAD51) | NA | PARP inhibitors | trial | |
ESCAT short descriptions: ESCAT I: Access to the treatment should be considered standard of care; ESCAT II: Treatment to be considered preferable in the context of evidence collection either as a prospective registry or as a prospective clinical trial; ESCAT III: Clinical trials to be discussed with patients. Abbreviations: NA: not available; PD: a progressive disease. ESMO: European Society for Medical Oncology.
Figure 1.
Overview of molecular targets found in gliomas, glioneuronal tumors, and neuronal tumors of adults and associated ESCAT score.
Notes: - Numbers are rough estimates based on literature and public databases, data on rare or new subtypes for which only a few samples have been characterized may evolve. Whenever feasible, results have been translated into tumor types as they are defined according to the WHO 2021 central nervous system tumor classification. This may be responsible for variations in biomarker prevalence compared to past studies. Definitions of variants may vary between different studies (eg, rare mutations outside known hotspots of which the somatic status is unknown and oncogenic potential has not been determined). Single cases or discordant reports are not included in the table.
Relevant Alterations for Gliomas, Glioneuronal and Neuronal Tumors in Adults: Integrated Recommendations for Testing and Treatment
RAS/Mitogen-Activated Protein Kinase Pathway
BRAF mutations.—
Ganglioglioma, pleomorphic xantroastrocytoma WHO grade 2 and 3, adolescent patients with diffuse midline astrocytomas (diencephalon, brainstem)32 should be tested for BRAF p.V600E mutations once treatment is required after surgery. Pilocytic astrocytoma should be tested for KIAA1549::BRAF fusions and if negative, once treatment is required after surgery for other BRAF alterations. The epithelioid subtype of glioblastoma, IDH-wildtype carries BRAF alterations in around 50% of cases and molecularly resembles PXA.33 Some tumors with morphological features of glioblastoma and BRAF p.V600E mutations were upon further studies reclassified as PXA, these tumors were often located in the medial temporal lobe.
Access to dabrafinib/trametinib treatment should be considered in patients with progressive BRAF p.V600E mutant glial tumors after standard of care. In the United States, dabrafenib and trametinib are approved in adult and pediatric patients 6 years of age and older with unresectable or metastatic solid tumors with BRAF p.V600E mutation who have progressed following prior treatment and have no satisfactory alternative treatment option; registration in Europe and other countries is pending (ESCAT 1B; ESMO-MCBS grade 3). BRAF p.V600E mutant tumors of lower-grade histologies are likely to benefit more in terms of response rate and duration. In some of these very rare disease entities, the standard of care is actually ill-defined with prospective studies lacking and treatment recommendations inferred from other glial tumors. Upfront targeted treatment with BRAF or BRAF/MEK inhibition could be considered in these entities in specific situations (large tumors, no well-established standard of care) but preferably inside clinical trials or clinical registries. Patients with KIAA1549::BRAF fusion-positive pilocytic astrocytoma recurrent after standard of care should be considered for trials on MEK inhibitors or type II RAF inhibitors (ESCAT IIIA).
Somatic Neurofibromin 1 gene (NF1) mutations.—
Routine testing for somatic NF1 variants is currently not recommended for patients with sporadic adult-type diffuse gliomas (ESCAT IIIA). Testing for somatic NF1 variants should be considered in patients with recurrent H3-K27-altered diffuse midline glioma, in glioblastoma IDH-wildtype, and in tumor types with a high prevalence of NF1 mutations (eg, high-grade astrocytoma with piloid features, high-grade glioma with pleomorphic and pseudopapillary features, and rosette-forming glioneuronal tumors), in whom standard treatment options are exhausted and with clinical trial options available (ESCAT IIIA). In patients with progressive pilocytic astrocyma in whom standard treatment options are exhausted and with clinical trial options available, somatic NF1 testing is recommended unless a canonical BRAF alteration has already been identified or a diagnosis of neurofibromatosis type 1 is established. Genetic counseling should be considered for patients in whom NF1 alterations were identified in tumors of the NF1 spectrum even if the clinical criteria of neurofibromatosis type 1 are not fulfilled. This should be done prior to germline testing given the consequences of the NF1 diagnosis.34
Growth Factor Receptors
ALK alterations.—
The published data indicate that activating ALK fusions or mutations do not play a role in the pathogenesis of the vast majority of primary CNS tumors, except for the very rare cases of infant-type hemispheric gliomas in newborns. Because of the rare occurrence, general testing for ALK alterations is not recommended and targeted treatment with ALK inhibitors is of limited value in gliomas, glioneuronal and neuronal tumors in adults and the vast majority of pediatric-type CNS tumor patients (ESCAT IIIA): treatment should only be considered in the context of clinical trials or prospective registries once standard treatment options are exhausted.
EGFR gene alterations.—
Detection of EGFR gene amplification represents an important diagnostic biomarker for IDH-wildtype glioblastoma that suffices for the diagnosis even in the absence of typical histological features.1 In addition, the detection of EGFR mutation or, rarely, EGFR amplification serves as a diagnostic biomarker for the EGFR-mutant subtype of diffuse midline glioma, H3 K27-altered. However, despite the large number of clinical trials examining the efficacy of EGFR inhibitors, antibodies, antibody-drug conjugates, and vaccines in gliomas, none has demonstrated clinical activity (ESCAT X). We, therefore, recommend investigating EGFR inhibitors on entities that have thus far not/poorly been studied (including the rare cases with mutations affecting the tyrosine kinase domain, eg, in a subset of EGFR-mutant thalamic diffuse midline glioma, H3 K27-altered) (ESCAT IIIA) or with agents with novel mechanisms of action (or novel combinations) that may be active against glioblastoma-associated EGFR variants or EGFR amplification. Outside the routine diagnostics for EGFR alterations required for appropriate WHO diagnosis, testing for EGFR alterations should be limited to patients who have exhausted standard therapy but who are still in a good enough clinical condition and with clinical trial options available.
FGFR gene alterations.—
FGFR represents an ESCAT IIB target in glioma and glioneuronal tumors, RGNT, pilocytic astrocytoma, DNET, and midline diffuse gliomas in adults should be tested for FGFR1 mutation If options for targeted therapies are sought. FGFR1::TACC1 fusions are present in the majority of extraventricular neurocytomas where they can serve to support the diagnosis. In patients with IDH-wildtype glioblastoma, attention should be paid to histological features associated with FGFR3::TACC3 fusion. Immunohistochemical staining for FGFR3 expression can be used as prescreening for glioblastoma, IDH wildtype. Positive cases (less than 10%) should then be molecularly tested for FGFR3::TACC3 fusion. Glioma and glioneuronal tumor patients with progressive disease who have exhausted standard therapy but who are still in a good enough clinical condition and with clinical trial options available are candidates for testing for FGFR alterations. If diagnosed with an FGFR variant, treatment is to be considered, preferably in a clinical study or as part of a prospective registry if registered drugs are available.
Mesenchymal-Epithelial Transition factor (MET)/Hepatocyte Growth Factor Receptor alterations.—
In adult patients, MET testing is to be considered in patients with recurrent glioblastomas, IDH-wildtype (CNS WHO grade 4) and with recurrent astrocytoma, IDH-mutant (CNS WHO grade 3 or 4), in patients with tumors with a methylation profile of a diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype, in patients with diffuse midline glioma, H3 K27-altered, and in patients with diffuse hemispheric glioma, H3 G34-mutant; in whom standard treatment options are exhausted, that is still in a good enough clinical condition and with clinical trial options available (ESCAT IIIA). For patients with tumors with MET amplification, fusion, or exon skipping alteration meeting trial inclusion criteria, treatment in a clinical trial should be considered. No clinically relevant MET alterations have so far been reported in oligodendroglioma.
NTRK-fusion
Adult patients with primary CNS tumors who would qualify for further treatment and who have exhausted standard therapy but are still in a good clinical condition are candidates for NTRK fusion testing. A limitation of testing to certain histologies is at present not possible; NTRK fusions are diagnosed across many types of glioma but are more prevalent in hemispheric gliomas of infants. For glioma, despite the tumor agnostic approval by EMA and the FDA for the treatment of NTRK fusion-positive cancers, NTRK fusions are ESCAT IIB. An ESMO-MCBS is not possible until more clinical data become available. In patients with a recurrent NTRK-fusion-positive CNS tumor and with the standard of care therapies exhausted treatment with an approved NTRK inhibitor is recommended, with a strong preference for treatment in a clinical study or as part of a registry. Whether glioma patients with NTRK fusions detected at diagnosis should receive such inhibitors early on and without or with standard therapy remains controversial.
PDGFRA gene alterations.—
Testing for PDGFRA p.K385L mutation is useful as a diagnostic marker for the rare cases of myxoid glioneuronal tumors. Various PDGFRA alterations (high-level gene amplifications and sequence variants) are found at a significant frequency in IDH-wildtype glioblastomas, diffuse midline gliomas with H3 K27 alteration, pediatric-type diffuse high-grade glioma, H3-wildtype and IDH-wildtype, and at lower frequency in IDH-mutant astrocytomas. Despite a significant number of clinical trials examining the efficacy of PDGFRA inhibitors in unselected glioma patients, none has demonstrated clinical activity, and reports on efficacy based on molecularly selected tumors are scarce (ESCAT IIIA). We, therefore, recommend testing for this target only in patients in whom standard treatment options are exhausted, that are still in a good enough clinical condition and with clinical trial options available. Trials on PDGFR inhibitors should focus on entities that have thus far not been or only poorly been investigated, or that are using novel agents in the context of early clinical trials and in trials with molecular entry criteria aiming at specific PDGFR alterations.
Proto-oncogene tyrosine-protein kinase-1 (ROS1) fusions.—
In adult patients with glioma, glioneuronal or neuronal tumors ROS1 fusion is an ESCAT IIIA target, testing for the target should only be considered in patients in whom standard treatment options are exhausted, who are still in a good enough clinical condition and with clinical trial options available. If a ROS1 fusion is identified as part of a broader more general NGS screening, treatment should be considered within a clinical trial or a prospective registry.
Cell Cycle
CDK4 and cyclin-dependent kinases 6 amplification.—
Testing for CDK4 or CDK6 amplification is of limited significance for adult patients with gliomas, glioneuronal, and neuronal tumors. Early clinical trials on patients with recurrent IDH-wildtype glioblastoma selecting for RB positivity alone, or in combination with CDKN2A loss or CDK4 amplification have not shown efficacy of monotherapy with CDK4/6 inhibitors (ESCAT IIIA).35–38 Testing for CDK4/6 amplification might be considered in patients with gliomas or neuronal/glioneuronal tumors in whom standard treatment options are exhausted, who are still in a good enough clinical condition and with clinical trial options available.
Mouse Double Minute 2, 4 (MDM2/MDM4) gene amplification.—
MDM2 or MDM4 alterations (amplifications) are found at a significant frequency in IDH-wild type glioblastomas. Clinical trials examining the efficacy of MDM2 or MDM4 inhibitors in other solid tumors have shown limited signs of clinical activity, and a small cohort of MDM2 amplified glioblastoma patients failed to demonstrate clinical activity (ESCAT IV). Testing for MDM2/MDM4 amplification is therefore only to be considered in the context of an available early clinical trial with molecular entry criteria aiming at MDM2/MDM4 amplification, in glioblastoma patients who have exhausted standard therapy but who are still in a good enough clinical condition.
TSC1/TSC2/mTOR alterations.—
In tumors that unequivocally qualify as SEGA, mTOR pathway inhibition is part of the standard of care if surgical removal is not possible (ESCAT IA, ESMO-MCBS 3); however, in patients that are not (yet) known for TS and with a histologically ambiguous tumor, demonstration of a TSC1 or TSC2 mutation is advised before considering this treatment. In TS patients, everolimus may improve seizure control. For other glial and glioneuronal tumors, assessing mutations in mTOR, TSC1/TSC2 and mTOR pathway activation should only be considered in patients in whom standard treatment options are exhausted, that still in a good enough clinical condition and with clinical trial options available (ESCAT IIIB).
Altered Genomic Stability
High tumor mutational burden, DNA mismatch repair, and polymerase proofreading deficiency alterations.—
In patients with newly diagnosed adult-type diffuse glioma, testing for TMB-H, DNA polymerase, and MMR deficiency should be considered in young adults, tumors with unusual histological or molecular features (eg, tumors with severe pleomorphism and/or giant cell features, tumors not falling into classic molecular subtypes, or associated with a DNA methylation pattern suggestive of MMR deficiency in particular “diffuse pediatric-type high-grade glioma RTK1”), and patients with a personal or familial history suggestive of germline DNA polymerase or MMR deficiency.39 Prior to testing for this, genetic counseling is advised.
In patients with recurrence of the tumor after treatment with an alkylating agent, testing of the recurrent tumor for TMB/MMR deficiency is relevant only for patients with IDH-mutant gliomas, MGMT promoter methylated IDH-wildtype glioblastoma, or patients who initially responded to alkylating agents and in the context of available clinical trials or access to anti-PD1 therapies (ESCAT III). Despite the registration or reimbursement of anti-PD1 therapies in some areas, in the absence of positive prospective trial data anti-PD1 treatment of post-treatment TMB-H glioma is best limited to clinical trials and prospective registries after standard of care are exhausted in patients in a good enough clinical condition.40
HRD alterations.—
In the almost complete absence of mutations affecting genes directly involved in HR in gliomas there is no rationale to test for HRD alterations. If a HRD alteration is nonetheless identified, treatment should only be considered in patients in whom standard treatment options are exhausted, who are still in a good enough clinical condition and within a prospective clinical trial. For other brain tumors including IDH mutated gliomas, clinical trials on PARP inhibitors should only be considered in patients in whom standard treatment options are exhausted, and who are still in a good enough clinical condition (ESCAT IIIA).
Conclusion
The wealth of diagnostic opportunities in modern oncology has led to the identification of alterations in genes that are targets for treatment. The number of clinically relevant targets predictive for benefit of targeted treatment in primary CNS tumors in adults remains limited, however, with only BRAF p.600V having solid evidence of clinical benefit. Other targets that show efficacy in other tumor types leading to tumor agnostic regulatory approvals (in particular tumors with NTRK fusions and TMB-high tumors) lack sufficient evidence of benefit to targeted treatment in adult patients with primary CNS tumors. It is therefore of vital importance that patients eligible for these therapies are treated within prospective registries and clinical trials. Some initial data suggest limited activity of targeted treatments in CNS tumors with FGFR alterations, confirmatory studies are needed for solid conclusions. For other targets, treatment remains investigational, and thus testing should be limited to the context of available clinical trials.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Acknowledgment
The contribution of Diego Prost in the preparation of the figures of this manuscript is acknowledged.
Contributor Information
David Capper, Department of Neuropathology, Charité — Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany; German Cancer Consortium (DKTK), Partner Site Berlin, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Guido Reifenberger, Institute of Neuropathology and German Cancer Consortium (DKTK), Partner Site Essen/Düsseldorf, Heinrich Heine University, Medical Faculty University Hospital Düsseldorf, Moorenstrasse 5, D-40225 Düsseldorf, Germany.
Pim J French, Department of Neurology, Brain Tumor Center at Erasmus MC Cancer Institute, University Medical Center Rotterdam, The Netherlands.
Leonille Schweizer, Institute of Neurology (Edinger Institute), University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany; German Cancer Consortium (DKTK), Partner Site Frankfurt/Mainz, German Cancer Research Center (DKFZ), Heidelberg, Germany; Frankfurt Cancer Institute (FCI), Frankfurt am Main, Germany.
Michael Weller, Department of Neurology, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland.
Mehdi Touat, Sorbonne Université, Inserm, CNRS, UMR S 1127, Institut du Cerveau, ICM, AP-HP, Hôpitaux Universitaires La Pitié Salpêtrière - Charles Foix, Service de Neurologie 2-Mazarin, Paris, France.
Simone P Niclou, Department of Cancer Research, NORLUX Neuro-Oncology Laboratory, Luxembourg Institute of Health, 6A, rue Nicolas Ernest-Barblé, L-1210 Luxembourg.
Philipp Euskirchen, Department of Neuropathology, Charité — Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany; Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Klinik für Neurologie, Charitéplatz 1, 10117 Berlin, Germany; Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Charité Comprehensive Cancer Center, Charitéplatz 1, 10117 Berlin, Germany.
Christine Haberler, Division of Neuropathology and Neurochemistry, Department of Neurology, Medical University of Vienna, Vienna, Austria.
Monika E Hegi, Neuroscience Research Center, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Sebastian Brandner, Department of Neurodegenerative Disease, Queen Square Institute of Neurology, University College London London, UK; Division of Neuropathology, The National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, London, UK.
Emilie Le Rhun, Department of Neurosurgery and Neurology, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland.
Roberta Rudà, Division of Neurology, Castelfranco Veneto/Treviso Hospital; Division of Neuro-Oncology, Department of Neuroscience, University of Turin, Turin, Italy.
Marc Sanson, Sorbonne Université, Inserm, CNRS, UMR S 1127, Institut du Cerveau, ICM, AP-HP, Hôpitaux Universitaires La Pitié Salpêtrière - Charles Foix, Service de Neurologie 2-Mazarin, Paris, France.
Ghazaleh Tabatabai, Department of Neurology & Interdisciplinary Neuro-Oncology, University Hospital Tübingen and Hertie Institute for Clinical Brain Research, Eberhard Karls University Tübingen; Center for Neuro-Oncology, Comprehensive Cancer Center Tübingen-Stuttgart, University Hospital Tübingen; German Cancer Consortium (DKTK), partner site Tübingen, Eberhard Karls University Tübingen.
Felix Sahm, Department of Neuropathology, University Hospital Heidelberg, CCU Neuropathology, Heidelberg, Germany; German Consortium for Translational Cancer Research (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Patrick Y Wen, Center For Neuro-Oncology, Dana-Farber Cancer institute and Harvard Medical School, Boston, USA.
Pieter Wesseling, Department of Pathology, Amsterdam University Medical Centers/VUmc (De Boelelaan 1117, 1081 HV) Amsterdam, The Netherlands; Laboratory for Childhood Cancer Pathology, Princess Máxima Center for Pediatric Oncology (Heidelberglaan 25, 3584 CS Utrecht, The Netherlands.
Matthias Preusser, Division of Oncology, Department of Medicine I, Medical University of Vienna, Vienna, Austria.
Martin J van den Bent, Department of Neurology, Brain Tumor Center at Erasmus MC Cancer Institute, University Medical Center Rotterdam, The Netherlands.
Disclosure Statement
DC has received research support from Novocure. DC receives royalties for sales of mutation-specific antibodies H09 (IDH1 R132H) and VE1 (BRAF V600E). A patent for DNA methylation based tumor profiling has been filed; GR, PJF, have nothing to disclose; LS received honoraria for lectures from Molecular Health; MW has received research grants from Quercis and Versameb, and honoraria for lectures or advisory board participation or consulting from Bayer, Medac, Merck (EMD), Novartis, Orbus, and Philogen; MT has a consulting or advisory role with Servier, Novocure, Integragen, and Taiho Oncology, and research funding from Sanofi; SPN, PE, CH, MH, SB have nothing to disclose; ELR has received a research grant from Bristol Meyer Squibb and honoraria for lectures or advisory board from Adastra, Bayer, Janssen, Leo Pharma, Pierre Fabre, and Seattle Genetics; RR has received honoraria for lectures, consultation or advisory board from UCB, Bayer, Novocure, Genenta; MS reports research support from AstraZeneca, and received honoraria for consultation or advisory from Genenta and Abbvie; GT has served on advisory boards of AbbVie, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb; received consulting fees from AbbVie, Bayer; received speaker fees from Medac and Novocure; FS has received honoraria from Bayer, Illumina; Patent and royalties thereof for a method for classification of cancer; PYW has received research support from Astra Zeneca/Medimmune, Beigene, Celgene, Chimerix, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Puma, Servier, Vascular Biogenics, VBI Vaccines and honoraria for advisory board participation on consultation from Astra Zeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Karyopharm, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, VBI Vaccines; PW has nothing to disclose; MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals; MvdB has received honoraria for consultancy or advisory board participation from the following companies: Servier, Boehringer, Carthera, Genenta, Nerviano, Chimerix, Astra Zeneca, Fore Biotherapeutics, and Roche.
References
- 1. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marchiò C, Scaltriti M, Ladanyi M, et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol. 2019;30(9):1417–1427. [DOI] [PubMed] [Google Scholar]
- 3. Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122(1):11–19. [DOI] [PubMed] [Google Scholar]
- 4. Schittenhelm J, Ziegler L, Sperveslage J, et al. FGFR3 overexpression is a useful detection tool for FGFR3 fusions and sequence variations in glioma. Neurooncol Pract. 2021;8:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kwak Y, Kim SI, Park CK, et al. C-MET overexpression and amplification in gliomas. Int J Clin Exp Pathol. 2015;8:14932–14938. [PMC free article] [PubMed] [Google Scholar]
- 6. Jóźwiak S, Kwiatkowski D, Kotulska K, et al. Tuberin and hamartin expression is reduced in the majority of subependymal giant cell astrocytomas in tuberous sclerosis complex consistent with a two-hit model of pathogenesis. J Child Neurol. 2004;19(2):102–106. [DOI] [PubMed] [Google Scholar]
- 7. Reuss DE, Habel A, Hagenlocher C, et al. Neurofibromin specific antibody differentiates malignant peripheral nerve sheath tumors (MPNST) from other spindle cell neoplasms. Acta Neuropathol. 2014;127(4):565–572. [DOI] [PubMed] [Google Scholar]
- 8. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capper D, Stichel D, Sahm F, et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol. 2018;136(2):181–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vega DM, Yee LM, McShane LM, et al. Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: phase II of the Friends of Cancer Research TMB Harmonization Project. Ann Oncol. 2021;32(12):1626–1636. [DOI] [PubMed] [Google Scholar]
- 12. Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merino DM, McShane LM, Fabrizio D, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J ImmunoTher Cancer. 2020;8:e000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wimmer K, Kratz CP, Vasen HF, et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium “care for CMMRD” (C4CMMRD). J Med Genet. 2014;51:355–365. [DOI] [PubMed] [Google Scholar]
- 15. Bakry D, Aronson M, Durno C, et al. Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: report from the constitutional mismatch repair deficiency consortium. Eur J Cancer. 2014;50(5):987–996. [DOI] [PubMed] [Google Scholar]
- 16. Aronson M, Colas C, Shuen A, et al. Diagnostic criteria for constitutional mismatch repair deficiency (CMMRD): recommendations from the international consensus working group. J Med Genet. 2022;59:318–327. [DOI] [PubMed] [Google Scholar]
- 17. den Dunnen JT, Dalgleish R, Maglott DR, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016;37(6):564–569. [DOI] [PubMed] [Google Scholar]
- 18. Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horak P, Griffith M, Danos AM, et al. Standards for the classification of pathogenicity of somatic variants in cancer (oncogenicity): joint recommendations of Clinical Genome Resource (ClinGen), Cancer Genomics Consortium (CGC), and Variant Interpretation for Cancer Consortium (VICC). Genet Med. 2022;24:986–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29(9):1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leichsenring J, Horak P, Kreutzfeldt S, et al. Variant classification in precision oncology. Int J Cancer. 2019;145(11):2996–3010. [DOI] [PubMed] [Google Scholar]
- 23. Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mullard A. BRAF plus MEK inhibitor combo secures tumour-agnostic FDA approval. Nat Rev Drug Discov. 2022. 10.1038/d41573-022-00117-y [DOI] [PubMed] [Google Scholar]
- 25. Italiano A, Nanda S, Briggs A, et al. Larotrectinib versus prior therapies in tropomyosin receptor kinase fusion cancer: an intra-patient comparative analysis. Cancers. 2020;12(11):3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. (CHMP) EMACfMPfHU. Guideline on the Evaluation of Anticancer Medicinal Products in Man; 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-anticancer-medicinal-products-man-revision-5_en.pdf.
- 27. Bailey CH, Jameson G, Sima C, et al. . Progression-free survival decreases with each subsequent therapy in patients presenting for phase I clinical trials. J Cancer. 2012;3:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Von Hoff DD, StephensonJJ, Jr, Rosen P, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28(33):4877–4883. [DOI] [PubMed] [Google Scholar]
- 29. Mellinghoff IK, Ellingson BM, Touat M, et al. Ivosidenib in isocitrate dehydrogenase 1–mutated advanced glioma. J Clin Oncol. doi: 10.1200/JCO.19.03327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cherny NI, Dafni U, Bogaerts J, et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28(10):2340–2366. [DOI] [PubMed] [Google Scholar]
- 31. Barthel FP, Johnson KC, Varn FS, et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576(7785):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lassaletta A, Zapotocky M, Mistry M, et al. Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol. 2017;35:2934–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furuta T, Miyoshi H, Komaki S, et al. Clinicopathological and genetic association between epithelioid glioblastoma and pleomorphic xanthoastrocytoma. Neuropathology. 2018;38(3):218–227. [DOI] [PubMed] [Google Scholar]
- 34. Nix JS, Blakeley J, Rodriguez FJ.. An update on the central nervous system manifestations of neurofibromatosis type 1. Acta Neuropathol. 2020;139:625–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller TW, Traphagen NA, Li J, et al. Tumor pharmacokinetics and pharmacodynamics of the CDK4/6 inhibitor ribociclib in patients with recurrent glioblastoma. J Neurooncol. 2019;144(3):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tien AC, Li J, Bao X, et al. A phase 0 trial of ribociclib in recurrent glioblastoma patients incorporating a tumor pharmacodynamic- and pharmacokinetic-guided expansion cohort. Clin Cancer Res. 2019;25(19):5777–5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor JW, Parikh M, Phillips JJ, et al. Phase-2 trial of palbociclib in adult patients with recurrent RB1-positive glioblastoma. J Neurooncol. 2018;140(2):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Golebiewska A, Hau AC, Oudin A, et al. Patient-derived organoids and orthotopic xenografts of primary and recurrent gliomas represent relevant patient avatars for precision oncology. Acta Neuropathol. 2020;140(6):919–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suwala AK, Stichel D, Schrimpf D, et al. Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol. 2021;141:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.