Effective host defense against microbial invasion requires an innate immune system whose response is both rapid and independent of prior exposure (25). The neutrophil is a central cellular effector of the innate immune system whose importance to host defense is manifest in the increased frequency and severity of infections in patients who have defects in neutrophil quantity or quality.
The mechanisms by which neutrophils exert their antimicrobial activity have been under investigation since the seminal work of Eli Metchnikoff, who demonstrated the phagocytic activity of these cells at the beginning of the 20th century. Activated neutrophils increase oxygen consumption during inflammatory responses in what has been termed the “respiratory burst,” reflecting assembly of a multicomponent neutrophil oxidase which transfers electrons to molecular oxygen, forming toxic radicals (6). Defects in the phagocyte oxidase proteins underlie chronic granulomatous disease (CGD), which is characterized by increased frequency of infections with certain bacterial and fungal pathogens (38). Nitric oxide is another small, readily diffusible antimicrobial mediator generated by activated neutrophils (54).
Over the past two decades, there has been increasing recognition of oxygen-independent killing mechanisms relating to the following observations: (i) neutrophils from CGD patients are capable of killing a variety of microorganisms (52), (ii) normal neutrophils deprived of oxygen in vitro are also able to efficiently kill certain bacterial pathogens (52), (iii) crude acid extracts of neutrophils possess direct microbicidal activity which is oxygen independent (24), and (iv) cationic proteins and peptides isolated from such extracts are able to directly kill microorganisms in vitro (29). Encouraged by these observations, investigators have made use of protein chromatography and molecular cloning in order to isolate, sequence, and define a growing number of neutrophil granule-derived antimicrobial proteins and peptides (15, 22, 23).
Neutrophil protein and peptide antibiotics are deployed by degranulation either extracellularly into inflammatory fluids or intracellularly into the phagolysosome, thereby exposing microorganisms to high concentrations of these agents. The antimicrobial proteins and peptides share in common a net positive charge which contributes to electrostatic interactions with negatively charged microbial surface components. However, despite similar charges, these agents vary markedly in size and structure as well as the mechanisms and selectivities of their cytotoxic actions. Here the focus is on a remarkably selective anti-infective component of human neutrophils known as the bactericidal/permeability-increasing protein (BPI).
MOLECULAR ASPECTS
Originally isolated by Elsbach, Weiss, and colleagues two decades ago (50), BPI is a 55-kDa protein found in the primary (azurophilic) granules of human neutrophils and has also been detected on the neutrophil cell surface. More recently, BPI has also been detected at lower levels in the specific granules of eosinophils (4). BPI selectively exerts multiple anti-infective activities against gram-negative bacteria: (i) cytotoxicity via sequential damage to bacterial outer and inner lipid membranes (36), (ii) neutralization of gram-negative bacterial lipopolysaccharide (LPS) (endotoxin) (37), and (iii) opsonization of bacteria to enhance phagocytosis by neutrophils (27).
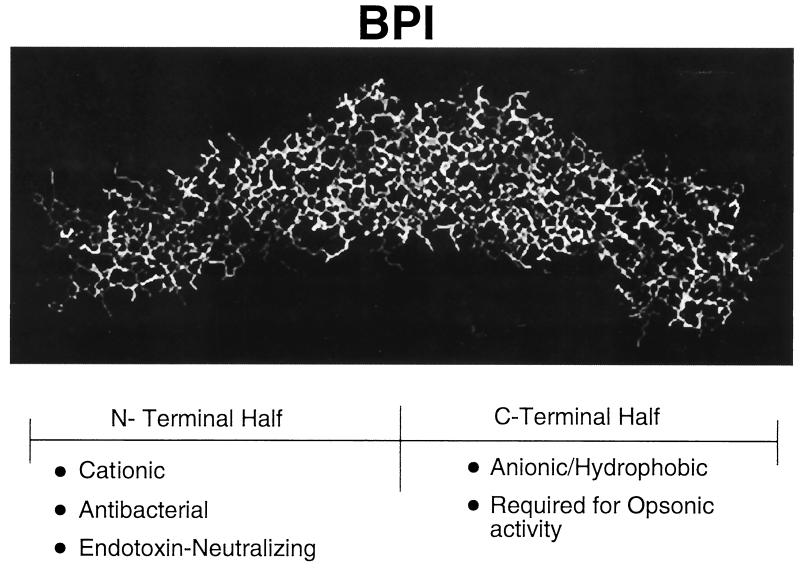
Structural characterization of the BPI molecule has provided insight into its function. Cloning and sequencing of the BPI cDNA has revealed a primary structure whose N-terminal half contains a high proportion of basic and hydrophilic residues and whose C-terminal half contains acidic and hydrophilic residues (18). Consistent with this primary structure, the recently defined crystal structure of BPI reveals a symmetric bipartite structure characterized by N- and C-terminal regions, each of which contains lipid-binding apolar pockets (3) (Fig. 1). Whereas the cationic N-terminal region of BPI possesses both antibacterial and endotoxin-neutralizing properties, the ability of BPI to opsonize gram-negative bacteria appears to require its C-terminal end (27).
FIG. 1.
Crystal structure of BPI. The BPI molecule has a boomerang shape composed of two structurally similar domains. The N-terminal half of the protein is rich in basic residues and possesses the antibacterial and antiendotoxic activities of the molecule, whereas the hydrophobic (anionic) C-terminal half is required for opsonic activity. Apolar lipid-binding pockets are present in each half of the molecule and are believed to be important for interactions with LPS acyl chains.
Antibacterial action.
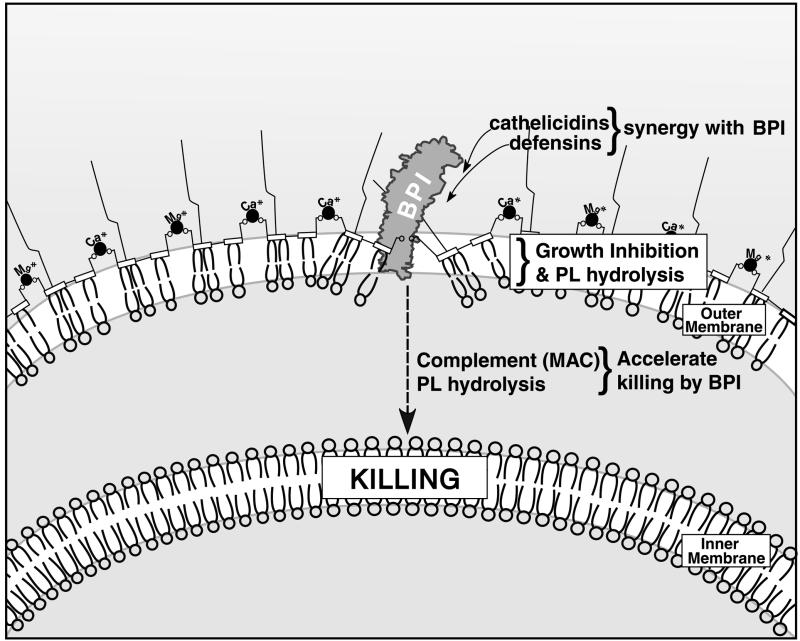
With few exceptions (28), BPI's cytotoxic activity is selectively manifest toward gram-negative bacteria, including some encapsulated, serum-resistant clinical isolates such as Escherichia coli K1/r. BPI does not manifest cytotoxicity against gram-positive bacteria, fungi, or mammalian cells. The selectivity of BPI's action toward gram-negative bacteria has been attributed to its high affinity (in the nanomolar range) for the lipid A moiety of LPS or endotoxin (16). LPSs, which are the major components of the outer leaflet of the gram-negative bacterial outer membrane, are stabilized by a regular array of divalent ions that serve to cross-link the negatively charged LPS molecules. Binding of BPI to gram-negative bacteria competitively displaces these outer membrane calcium and magnesium ions (34). Studies with LPS model membranes also suggest that BPI displaces divalent cations, thereby perturbing the regular arrangement of LPS molecules, causing membrane rupture, an increase in the membrane current, and a change in transmembrane potential (Fig. 2) (55).
FIG. 2.
Action of BPI on the gram-negative bacterial membrane. The gram-negative bacterial outer and inner membranes are depicted. Positively charged residues in the N terminus of BPI are thought to bind to negatively charged LPS phosphate groups, displacing the divalent cations that normally stabilize the outer membrane. Hydrophobic interactions of BPI's apolar lipid-binding pockets with LPS acyl chains are also believed to contribute to this disruption. The early effects of BPI on bacteria, including growth inhibition and activation of bacterial phospholipid (PL) hydrolysis, are enhanced in the presence of antimicrobial peptides of the cathelicidin and defensin families. Killing of bacteria is believed to require penetration of BPI to the inner membrane, a time-dependent process which is accelerated by the complement membrane attack complex (MAC) as well as by bacterial phospholipid hydrolysis.
Binding of BPI to gram-negative bacteria is followed by time-dependent progression of BPI-mediated membrane damage (Fig. 2). Early effects of BPI on gram-negative bacteria include permeabilization of the bacterial membrane to small hydrophobic molecules (e.g., β-lactam antibiotics), rendering bacterial phospholipids susceptible to hydrolysis by both exogenous (host) and endogenous (bacterial) phospholipases, and bacterial growth inhibition. Late effects of BPI are manifest as irreversible growth inhibition (killing) and coincide with the effects on the bacterial inner membrane, where critical components of the bacterium's biochemical machinery reside (36).
Gram-negative bacteria have variable sensitivities to BPI's bactericidal activity, with species such as Klebsiella pneumoniae demonstrating significant resistance (32a, 51). Gram-negative bacteria respond to BPI's action by up-regulating LPS repair (53). Studies of isogenic strains of Proteus mirabilis differing only in LPS chain length reveal that expression of long-chain LPS confers relative BPI resistance, presumably reflecting stearic hindrance of BPI access to the lipid A moiety of the LPS molecules (5). It has been argued that acquired resistance to BPI action would be unlikely, given the essential role played by the lipid A target to the viability of gram-negative bacteria. However, several considerations prompt concern about the possibility of resistance to BPI: (i) resistance of gram-negative bacteria to polymyxin B, an antibiotic that also binds to lipid A, is mediated by changes in LPS core structure (20); (ii) several investigators have demonstrated that a variety of gram-negative pathogens alter their LPS composition and structure as a means of resistance to other neutrophil-derived cationic peptides (19, 21); and (iii) there have been no published reports of the possible effect of serial passage of bacteria in media containing relatively low concentrations of BPI.
It has been argued that intracellular killing of certain gram-negative bacteria by whole neutrophils is an oxygen-independent process which is largely BPI dependent because neutrophil-mediated killing of E. coli (i) is manifest in neutrophils from patients with CGD, (ii) occurs in normal neutrophils deprived of oxygen, (iii) follows similar kinetics as killing by pure BPI, (iv) is, similarly to pure BPI, enhanced by phospholipid hydrolysis as well as by the complement system, and (v) is largely inhibited in neutrophil extracts by the addition of neutralizing anti-BPI serum (10). However, the complexity of the neutrophil's bactericidal armamentarium (i.e., the presence of multiple other antimicrobial proteins and peptides [14, 22, 29]) makes it difficult to ascribe activity to a single agent. In fact, there are multiple examples of synergistic interactions when isolated agents are tested in combination in vitro (2, 32).
Although the activity of neutrophil extracts against E. coli is BPI dependent, the bactericidal capacities of neutrophil extracts and inflammatory fluids exceed those predicted by their BPI contents, suggesting a contribution by additional antibacterial agents. Whereas early effects of BPI on gram-negative bacteria are synergistically enhanced by members of the cathelicidin and defensin antimicrobial peptide families (11), killing of E. coli by both purified BPI and whole neutrophils is enhanced by the complement membrane attack complex (35) (Fig. 2). BPI also enhances the action of bacterial and host phospholipases on E. coli, resulting in enhanced hydrolysis of bacterial phospholipids and acceleration of progression to killing.
Endotoxin neutralizing activity.
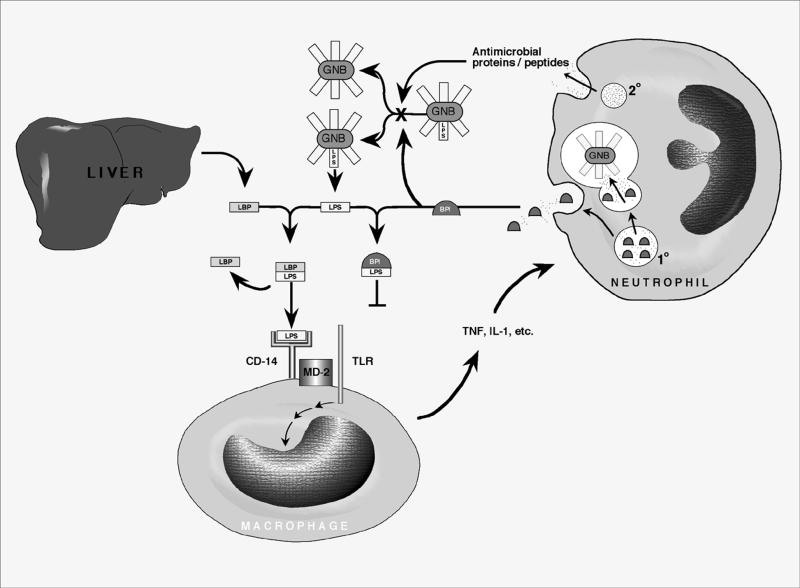
High-affinity (approximately in the nanomolar range) binding of BPI to the lipid A moiety common to all gram-negative bacterial LPSs suggests that it is a classic “pattern recognition” molecule of the innate immune system (25). BPI's ability to potently inhibit endotoxin is opposite that of its structural homologue, the LPS-binding protein (LBP), which is a liver-produced acute-phase reactant that greatly amplifies LPS-mediated inflammatory signaling (13, 44) (Fig. 3). BPI and LBP genes colocalize with phospholipid transfer protein to human chromosome 20 and have similar exon-intron structures, suggesting that they are, together with cholesterol ester transfer protein, divergent members of a family of lipid-binding proteins.
FIG. 3.
Mechanisms of antibacterial and antiendotoxic activities of neutrophils against gram-negative bacteria (GNB). The presence of gram-negative bacteria in normally sterile body compartments is signaled by bacterial surface LPS (endotoxin). LPS is specifically bound by the plasma LBP, a plasma constituent which is secreted by the liver. The LBP-LPS complex is recognized by the macrophage CD-14 receptor, which, via an adaptor protein such as MD-2, activates a Toll-like receptor protein (TLR) that transduces a signal leading to secretion of proinflammatory mediators including tumor necrosis factor (TNF) and interleukin 1 (IL-1). Neutrophils migrate to sites of infection, where they deploy their granule-associated antimicrobial arsenal both intracellularly within the phagolysosome and extracellularly by degranulation. Antimicrobial proteins and peptides from both compartments participate in bacterial growth inhibition. The role of BPI is highlighted in that it binds to LPS with a high affinity, thereby preventing formation of the LBP-LPS complex and markedly decreasing the proinflammatory effects of endotoxin.
In an effort to study the mechanisms of LBP- and BPI-mediated endotoxin modulation, investigators have used chemically extracted LPSs which form aggregates (micelles). Whereas LBP acts to disperse such LPS aggregates, delivering LPS to the cellular CD-14/Toll-like receptor complex (Fig. 3), BPI apparently increases the sizes of LPS aggregates and prevents LBP from recruiting LPS monomers for delivery to CD-14 (45). Of note, BPI has a greater affinity for LPS than does LBP (57). Since LPS bound by BPI is incapable of being recognized by LBP and of being bound by the macrophage LPS receptor complex (Fig. 3), all the myriad proinflammatory effects of endotoxin are inhibited by BPI, including LPS-mediated cytokine release, endothelial damage, coagulation, and nitric oxide release. Although multiple cationic proteins and peptides have demonstrated antiendotoxic activity when tested in artificial media in vitro (43), BPI is notable for its ability to neutralize endotoxin in biologic fluids even in the presence of LBP (31). Thus, BPI may contribute to down-regulation of the proinflammatory effects of gram-negative bacteria and endotoxin. Although gram-negative bacteria vary in their susceptibilities to BPI's bactericidal action, BPI readily neutralizes endotoxin from all gram-negative bacterial strains tested whether presented as isolated LPS or whole bacteria (51). In endotoxin neutralization assay systems, BPI is also markedly more effective than anti-LPS antibodies such as HA-1A and E5 (1).
Opsonic activity.
BPI has recently been shown to possess opsonic activity toward gram-negative bacteria which requires both the N- and C-terminal domains of the protein (27). Bacteria exposed to physiologically relevant concentrations of BPI (10 to 100 nM) are readily ingested by human neutrophils. Such opsonic activity is accompanied by mobilization of myeloperoxidase-mediated oxidative metabolism, suggesting possible collaboration between BPI and oxygen-dependent mechanisms of neutrophils. Whether BPI-coated bacteria are more readily ingested secondary to BPI-mediated alterations in the bacterial membrane or via a specific BPI receptor mechanism is as yet unclear.
ACTIVITY IN BIOLOGIC FLUIDS
BPI is bactericidal and neutralizes endotoxin at nanomolar concentrations not only in artificial laboratory media but also in blood, plasma, and serum. In vivo, neutrophils activated by a variety of inflammatory stimuli (including LPS, tumor necrosis factor alpha, and clotting) release BPI by degranulation into inflammatory fluids. Addition of a neutralizing anti-BPI serum blocks the bactericidal activity of rabbit inflammatory (ascitic) fluid against encapsulated gram-negative bacteria, suggesting that such activity is BPI dependent (49). Although LBP concentrations normally exceed those of BPI in normal plasma, the BPI/LBP ratio is greatly elevated at inflammatory sites with a robust influx of neutrophils (e.g., abscesses) (42). Consistent with these findings, plasma BPI levels are elevated in critically ill children and adults, especially those with sepsis (58). BPI is also released at the colonic mucosa of patients with inflammatory bowel disease (39) which is associated with anti-BPI antibodies in perinuclear antineutrophil cytoplasmic antibody-positive ulcerative colitis (48). Anti-BPI antibodies have also been detected in patients with cystic fibrosis (60). The relevance of such antibodies to the pathophysiology of these diseases is unclear.
CLINICAL APPLICATION
The action of BPI against gram-negative bacteria and their endotoxins which has been documented in vitro has recently been tested in animal models and with humans. A recombinant 21-kDa N-terminal BPI fragment (rBPI21) (26) expresses both the antibacterial and antiendotoxic activities of the holoprotein and has been demonstrated to have beneficial effects, either alone or in synergistic combination with conventional antibiotics, in animal models of sepsis, pneumonia, endotoxemia, and burns. Intravenous administration of rBPI21 reduces the mortality rate in multiple animal models (mice, rats, and baboons) of gram-negative bacterial infection. Although rBPI21 consistently neutralizes endotoxin, its bactericidal activity has been variable. In animal models, gram-negative bacterial strains vary in their susceptibilities to rBPI21. For example, BPI reduced the rate of mortality among mice injected with the rough strain E. coli J5 but was unable to reduce the rate of mortality when strains with long-chain LPSs such as E. coli O111:B4 and O7:K1 were injected (12).
There is also evidence that BPI may be a useful adjunct to conventional antibiotics. In a rabbit model of E. coli O7:K1 bacteremia, treatment with the conventional antibiotic cefamandole is associated with a rapid release of endotoxin, marked cytokine release, and significant mortality (33). Addition of rBPI21 together with cefamandole in this rabbit model markedly decreases the level of cytokine release and reduces the rate of mortality. rBPI21 also ameliorates hypercoagulability after hemorrhagic shock in a rat model, presumably by neutralizing translocated gut endotoxin, thereby inhibiting LPS-mediated induction of plasminogen-activator inhibitor-1 and tissue factor (59).
Of note, endotoxemia has also been documented in critically ill patients without detectable gram-negative bacterial infection, raising the possibility that translocation of endotoxin across an intestinal wall that has been damaged in the setting of shock contributes to the pathophysiology of multiple shock states (41).
Phase I studies.
Studies performed to assess the safety of administering recombinant N-terminal congeners of BPI to humans indicate that the protein is well tolerated and nonimmunogenic (56). rBPI21 given intravenously to subjects who have received endotoxin is able to markedly inhibit LPS-induced cytokine release (46), coagulant responses (47), and pathophysiologic changes such as alteration of cardiac index (9).
Phase II studies.
Phase II studies of rBPI21 as treatment for infections and endotoxemia include studies of rBPI21 as treatment for intra-abdominal infections (endotoxin formation by invading bacteria), hemorrhagic trauma (endotoxin translocation secondary to decreased intestinal barrier integrity), and liver resection (decreased endotoxin clearance). Open-label administration of rBPI21 to 26 children admitted to pediatric intensive care units with fulminant meningococcemia was associated with a reduced rate of mortality relative to that predicted by clinical prognostic scores, interleukin 6 levels, and the rate for historical controls (17). In addition, trauma patients with infectious complications associated with blood loss experienced a reduced incidence of pneumonia and adult respiratory distress syndrome following treatment with rBPI21 (8).
Phase III studies.
Two phase III double-blind placebo-controlled trials of rBPI21 for the treatment of hemorrhagic trauma and fulminant meningococcemia have now been concluded.
The hemorrhagic trauma trial was discontinued due to insufficient activity (without any safety concerns). Analysis and speculation as to the reason(s) for the insufficient effect in that study must await publication of the study data, including the levels of endotoxin and cytokines in the plasma of this patient population.
Results of a prospective, double-blinded, placebo-controlled phase III trial of rBPI21 involving 393 children ages 2 weeks to 18 years of age presenting with severe meningococcal sepsis have recently been published (28a). The study was underpowered to detect significant differences in mortality (7.4% in the BPI group and 9.9% in the placebo group; P = 0.48). However, treatment with rBPI21 was associated with a lesser number of multiple severe amputations (3.2 versus 7.4%; P = 0.067) and better functional outcome at day 60 (77.3 versus 66.3%; P = 0.019). Since the patient populations were well balanced in this study, the data suggest that rBPI21 is beneficial in reducing the complications of meningococcal sepsis.
Future directions.
In addition to possible studies in a variety of infectious disease settings, other clinical indications for which BPI may be considered are those in which gram-negative bacteremia and/or endotoxemia occur in the setting of relative BPI deficiency. Such deficiency can be due to neutropenia, whether due to overwhelming sepsis or chemotherapy, including myeloablative therapy prior to bone marrow transplantation, wherein endotoxemia has recently been implicated as a key precipitant of graft-versus-host disease (7). For example, an LBP-BPI hybrid has shown efficacy in reducing the rate of mortality in an animal model of neutropenic Pseudomonas sepsis (40). Of note, a recent study has indicated that neutrophils derived from newborn cord blood have significantly less BPI than those derived from adults, correlating with the diminished activity of newborn neutrophils against the encapsulated clinical pathogen E. coli K1/r (30). These observations raise the possibility that supplementing BPI levels with rBPI21 may be beneficial for newborns with gram-negative bacteremia and/or endotoxemia (32a) or other patient populations exhibiting BPI deficiencies.
CONCLUSIONS
Increasing appreciation of the role played by endotoxemia in the pathophysiology of bacterial infections suggests that optimal treatment will require targeting not only bacterial viability but endotoxic activity as well. The neutrophil-derived protein antibiotic BPI is a unique innate defense molecule which binds to LPSs (endotoxins) of the gram-negative bacterial membrane, leading to neutralization of bacterial endotoxic activity, opsonization, and bacterial growth arrest. A recombinant N-terminal BPI fragment, rBPI21, has antiendotoxic and antibacterial activities that can be demonstrated in biologic fluids and animal models. Human clinical trials have indicated that rBPI21 is safe, without significant immunogenicity or toxicity. Although a biologics license application has not yet been submitted for rBPI21, evidence of clinical benefit has been noted for multiple indications, and other studies are being planned. Judicious enhancement of this arm of innate immunity may eventually prove to be of clinical benefit to selected patients.
ACKNOWLEDGMENTS
My graduate research was supported by the Medical Scientist Training Program under National Institutes of Health (NIH) training grant 5T32GM07308 from the National Institute of General Medical Sciences. Currently, my research is supported by NIH/National Center for Research Resources/General Clinical Research Center Grant M01RR02172 at the Children's Hospital of Boston, as well as grants from the Children's Hospital of Boston, the Dana Farber Cancer Research Institute, the American Academy of Pediatrics, and XOMA (U.S.) LLC.
I thank Philip Pizzo, physician-in-chief of the Children's Hospital of Boston, and Donald Goldmann for support and encouragement.
REFERENCES
- 1.Arditi M, Zhou J, Huang S H, Luckett P M, Marra M N, Kim K S. Bactericidal/permeability-increasing protein protects vascular endothelial cells from lipopolysaccharide-induced activation and injury. Infect Immun. 1994;62:3930–3936. doi: 10.1128/iai.62.9.3930-3936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson J M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beamer L J, Carroll S F, Eisenberg D. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science. 1997;276:1861–1864. doi: 10.1126/science.276.5320.1861. [DOI] [PubMed] [Google Scholar]
- 4.Calafat J, Janssen H, Tool A, Dentener M A, Knol E F, Rosenberg H F, Egesten A. The bactericidal/permeability-increasing protein (BPI) is present in specific granules of human eosinophils. Blood. 1998;91:4770–4775. [PubMed] [Google Scholar]
- 5.Capodici C, Chen S, Sidorczyk Z, Elsbach P, Weiss J. Effect of lipopolysaccharide (LPS) chain length on interactions of bactericidal/permeability-increasing protein and its bioactive 23-kilodalton NH2-terminal fragment with isolated LPS and intact Proteus mirabilis and Escherichia coli. Infect Immun. 1994;62:259–265. doi: 10.1128/iai.62.1.259-265.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanock S, Banna J, Smith R, Babior B. The respiratory burst oxidase. J Biol Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- 7.Cooke K, Hill G, Crawford J, Bungard D, Brinson Y, Delmonte J, Ferrara J. Tumor necrosis factor-α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Investig. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Winter R, Von der Mohlen M, Van Lieshout H, Wedel N, Nelson B, Friedmann N, Delemarre B, van Deventer S. Recombinant endotoxin binding protein (rBPI23) attenuates endotoxin-induced circulatory changes in humans. J Inflamm. 1995;45:193–206. [PubMed] [Google Scholar]
- 9.Demetriades D, Smith J, Jacobsen L, Moncure M, Minei J, Nelson B, Scannon P. Bactericidal/permeability-increasing protein (rBPI21) in patients with hemorrhage due to trauma: results of a multicenter phase II clinical trial. rBPI21 Acute Hemorrhagic Trauma Study Group. J Trauma-Injury Infect Crit Care. 1999;46:667–676. doi: 10.1097/00005373-199904000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Elsbach P, Weiss J. Role of the bactericidal/permeability-increasing protein in host defence. Curr Opin Immunol. 1998;10:45–49. doi: 10.1016/s0952-7915(98)80030-7. [DOI] [PubMed] [Google Scholar]
- 11.Elsbach P, Weiss J, Levy O. Integration of antimicrobial host defenses: role of the bactericidal/permeability-increasing protein. Trends Microbiol. 1994;2:324–328. doi: 10.1016/0966-842x(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 12.Evans T J, Carpenter A, Moyes D, Martin R, Cohen J. Protective effects of a recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in an animal model of gram-negative sepsis. J Infect Dis. 1995;171:153–160. doi: 10.1093/infdis/171.1.153. [DOI] [PubMed] [Google Scholar]
- 13.Fenton M J, Golenbock D T. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 14.Ganz T, Lehrer R I. Antibiotic peptides from higher eukaryotes: biology and applications. Mol Med Today. 1999;5:292–297. doi: 10.1016/s1357-4310(99)01490-2. [DOI] [PubMed] [Google Scholar]
- 15.Ganz T, Lehrer R I. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Gazzano-Santoro H, Parent J B, Grinna L, Horwitz A, Parsons T, Theofan G, Elsbach P, Weiss J, Conlon P J. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–4761. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giroir B P, Quint P A, Barton P, Kirsch E A, Kitchen L, Goldstein B, Nelson B J, Wedel N J, Carroll S F, Scannon P J. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet. 1997;350:1439–1443. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 18.Gray P W, Flaggs G, Leong S R, Gumina R J, Weiss J, Ooi C E, Elsbach P. Cloning of the cDNA of a human neutrophil bactericidal protein. Structural and functional correlations. J Biol Chem. 1989;264:9505–9509. [PubMed] [Google Scholar]
- 19.Groisman E, Aspedon A. The genetic basis of microbial resistance to antimicrobial peptides. Methods Mol Biol. 1997;78:205–215. doi: 10.1385/0-89603-408-9:205. [DOI] [PubMed] [Google Scholar]
- 20.Gunn J, Lim K, Krueger J, Kim K, Guo L, Hackett M, Miller S. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol. 1998;27:1171–1182. doi: 10.1046/j.1365-2958.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 21.Hancock R. Antibacterial peptides and the outer membranes of gram-negative bacilli. J Med Microbiol. 1997;46:1–3. doi: 10.1099/00222615-46-1-1. [DOI] [PubMed] [Google Scholar]
- 22.Hancock R, Chapple D. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock R E. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch J. Phagocytin: a bactericidal substance from polymorphonuclear leukocytes. J Exp Med. 1956;103:589–621. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman J, Kafatos F, Janeway C, Ezekowitz R. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz A H, Leigh S D, Abrahamson S, Gazzano-Santoro H, Liu P S, Williams R E, Carroll S F, Theofan G. Expression and characterization of cysteine-modified variants of an amino-terminal fragment of bactericidal/permeability-increasing protein. Protein Expr Purif. 1996;8:28–40. doi: 10.1006/prep.1996.0071. [DOI] [PubMed] [Google Scholar]
- 27.Iovine N M, Elsbach P, Weiss J. An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains. Proc Natl Acad Sci USA. 1997;94:10973–10978. doi: 10.1073/pnas.94.20.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan A, Lambert L J, Remington J, Araujo F. Recombinant bactericidal/permeability-increasing protein (rBPI21) in combination with sulfadiazine is active against Toxoplasma gondii. Antimicrob Agents Chemother. 1999;43:758–762. doi: 10.1128/aac.43.4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Levin M, Quint P A, Goldstein B, Barton P, Bradley J S, Shemie S D, Yeh T, Kim S S, Cafaro D P, Scannon P J, Giroir B P the rBPI21 Meningococcal Sepsis Study Group. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. Lancet. 2000;356:961–967. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 29.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol. 1996;56:263–277. doi: 10.1111/j.1600-0609.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 30.Levy O, Martin S, Eichenwald E, Ganz T, Valore E, Carroll S, Lee K, Goldmann D, Thorne G. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein (BPI) Pediatrics. 1999;104:1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 31.Levy O, Ooi C E, Elsbach P, Doerfler M E, Lehrer R I, Weiss J. Antibacterial proteins of granulocytes differ in interaction with endotoxin. Comparison of bactericidal/permeability-increasing protein, p15s, and defensins. J Immunol. 1995;154:5403–5410. [PubMed] [Google Scholar]
- 32.Levy O, Ooi C E, Weiss J, Lehrer R I, Elsbach P. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J Clin Investig. 1994;94:672–682. doi: 10.1172/JCI117384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Levy O, Sisson R, Kenyon J, Eichenwald E, Macone A, Goldmann D. Enhancement of neonatal innate defense: effects of adding an N-terminal recombinant fragment of bactericidal/permeability-increasing protein (rBPI21) on growth and tumor necrosis factor-inducing activity of gram-negative bacteria tested in neonatal cord blood ex vivo. Infect Immun. 2000;68:5120–5125. doi: 10.1128/iai.68.9.5120-5125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Y, Leach W J, Ammons W S. Synergistic effect of a recombinant N-terminal fragment of bactericidal/permeability-increasing protein and cefamandole in treatment of rabbit gram-negative sepsis. Antimicrob Agents Chemother. 1996;40:65–69. doi: 10.1128/aac.40.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannion B A, Kalatzis E S, Weiss J, Elsbach P. Preferential binding of the neutrophil cytoplasmic granule-derived bactericidal/permeability increasing protein to target bacteria. Implications and use as a means of purification. J Immunol. 1989;142:2807–2812. [PubMed] [Google Scholar]
- 35.Mannion B A, Weiss J, Elsbach P. Separation of sublethal and lethal effects of polymorphonuclear leukocytes on Escherichia coli. J Clin Investig. 1990;86:631–641. doi: 10.1172/JCI114755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannion B A, Weiss J, Elsbach P. Separation of sublethal and lethal effects of the bactericidal/permeability increasing protein on Escherichia coli. J Clin Investig. 1990;85:853–860. doi: 10.1172/JCI114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marra M N, Wilde C G, Griffith J E, Snable J L, Scott R W. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J Immunol. 1990;144:662–666. [PubMed] [Google Scholar]
- 38.Meischl C, Roos D. The molecular basis of chronic granulomatous disease. Spring Semin Immunopathol. 1998;19:417–434. doi: 10.1007/BF00792600. [DOI] [PubMed] [Google Scholar]
- 39.Monajemi H, Meenan J, Lamping R, Obradov D O, Radema S A, Trown P W, Tytgat G N, Van Deventer S J. Inflammatory bowel disease is associated with increased mucosal levels of bactericidal/permeability-increasing protein. Gastroenterology. 1996;110:733–739. doi: 10.1053/gast.1996.v110.pm8608882. [DOI] [PubMed] [Google Scholar]
- 40.Opal S, Palardy J, Jhung J, Donsky C, Romulo R, Parejo N, Marra M. Activity of lipopolysaccharide-binding protein–bactericidal/permeability-increasing protein fusion peptide in an experimental model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1995;39:2813–2815. doi: 10.1128/aac.39.12.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Opal S, Scannon P, Vincent J, White M, Carroll S, Palardy J, Parejo N, Pribble J, Lemke J. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 42.Opal S M, Palardy J E, Marra M N, Fisher C J, Jr, McKelligon B M, Scott R W. Relative concentrations of endotoxin-binding proteins in body fluids during infection. Lancet. 1994;344:429–431. doi: 10.1016/s0140-6736(94)91767-1. [DOI] [PubMed] [Google Scholar]
- 43.Scott M, Vreugdenhil A, Buurman W, Hancock R, Gold M. Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol. 2000;164:549–553. doi: 10.4049/jimmunol.164.2.549. [DOI] [PubMed] [Google Scholar]
- 44.Tobias P, Tapping R, Gegner J. Endotoxin interactions with lipopolysaccharide-responsive cells. Clin Infect Dis. 1999;28:476–481. doi: 10.1086/515163. [DOI] [PubMed] [Google Scholar]
- 45.Tobias P S, Soldau K, Iovine N M, Elsbach P, Weiss J. Lipopolysaccharide (LPS)-binding proteins BPI and LBP form different types of complexes with LPS. J Biol Chem. 1997;272:18682–18685. doi: 10.1074/jbc.272.30.18682. [DOI] [PubMed] [Google Scholar]
- 46.von der Mohlen M A, Kimmings A N, Wedel N I, Mevissen M L, Jansen J, Friedmann N, Lorenz T J, Nelson B J, White M L, Bauer R, et al. Inhibition of endotoxin-induced cytokine release and neutrophil activation in humans by use of recombinant bactericidal/permeability-increasing protein. J Infect Dis. 1995;172:144–151. doi: 10.1093/infdis/172.1.144. [DOI] [PubMed] [Google Scholar]
- 47.von der Mohlen M, van Deventer S, Levi M, van den Ende B, Wedel N, Nelson B, Friedmann N, ten Cate J. Inhibition of endotoxin-induced activation of the coagulation and fibrinolytic pathways using a recombinant endotoxin-binding protein (rBPI23) Blood. 1995;85:3437–3443. [PubMed] [Google Scholar]
- 48.Walmsley R S, Zhao M H, Hamilton M I, Brownlee A, Chapman P, Pounder R E, Wakefield A J, Lockwood C M. Antineutrophil cytoplasm autoantibodies against bactericidal/permeability-increasing protein in inflammatory bowel disease. Gut. 1997;40:105–109. doi: 10.1136/gut.40.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinrauch Y, Foreman A, Shu C, Zarember K, Levy O, Elsbach P, Weiss J. Extracellular accumulation of potently microbicidal bactericidal/permeability-increasing protein and p15s in an evolving sterile rabbit peritoneal inflammatory exudate. J Clin Investig. 1995;95:1916–1924. doi: 10.1172/JCI117873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss J, Elsbach P, Olsson I, Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978;253:2664–2672. [PubMed] [Google Scholar]
- 51.Weiss J, Elsbach P, Shu C, Castillo J, Grinna L, Horwitz A, Theofan G. Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J Clin Investig. 1992;90:1122–1130. doi: 10.1172/JCI115930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss J, Kao L, Victor M, Elsbach P. Oxygen-independent intracellular and oxygen-dependent extracellular killing of Escherichia coli S15 by human polymorphonuclear leukocytes. J Clin Investig. 1985;76:206–212. doi: 10.1172/JCI111947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss J, Muello K, Victor M, Elsbach P. The role of lipopolysaccharides in the action of the bactericidal/permeability-increasing neutrophil protein on the bacterial envelope. J Immunol. 1984;132:3109–3115. [PubMed] [Google Scholar]
- 54.Wheeler M A, Smith S D, Garcia-Cardena G, Nathan C F, Weiss R M, Sessa W C. Bacterial infection induces nitric oxide synthase in human neutrophils. J Clin Investig. 1997;99:110–116. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiese A, Brandenburg K, Carroll S F, Rietschel E T, Seydel U. Mechanisms of action of bactericidal/permeability-increasing protein BPI on reconstituted outer membranes of gram-negative bacteria. Biochemistry. 1997;36:10311–10319. doi: 10.1021/bi970177e. [DOI] [PubMed] [Google Scholar]
- 56.Wiezer M, Langendoen S, Meijer C, Bauer R, White M, Carroll S, Meyer S, Thijs L, van Leeuwen P. Pharmacokinetics of a recombinant amino terminal fragment of bactericidal/permeability-increasing protein (rBPI21) after liver surgery in rats and humans. Shock. 1998;10:161–166. doi: 10.1097/00024382-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Wilde C G, Seilhamer J J, McGrogan M, Ashton N, Snable J L, Lane J C, Leong S R, Thornton M B, Miller K L, Scott R W, et al. Bactericidal/permeability-increasing protein and lipopolysaccharide (LPS)-binding protein. LPS binding properties and effects on LPS-mediated cell activation. J Biol Chem. 1994;269:17411–17416. [PubMed] [Google Scholar]
- 58.Wong H R, Doughty L A, Wedel N, White M, Nelson B J, Havrilla N, Carcillo J A. Plasma bactericidal/permeability-increasing protein concentrations in critically ill children with the sepsis syndrome. Pediatr Infect Dis. 1995;14:1087–1091. doi: 10.1097/00006454-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita M. Bactericidal/permeability-increasing protein ameliorates hypercoagulability after hemorrhagic shock. Thromb Res. 1997;87:323–329. doi: 10.1016/s0049-3848(97)00134-5. [DOI] [PubMed] [Google Scholar]
- 60.Zhao M H, Jayne D R, Ardiles L G, Culley F, Hodson M E, Lockwood C M. Autoantibodies against bactericidal/permeability-increasing protein in patients with cystic fibrosis. Queen's J Med. 1996;89:259–265. doi: 10.1093/qjmed/89.4.259. [DOI] [PubMed] [Google Scholar]