Abstract
Background
Terminology to describe extent of resection in glioblastoma is inconsistent across clinical trials. A surgical classification system was previously proposed based upon residual contrast-enhancing (CE) tumor. We aimed to (1) explore the prognostic utility of the classification system and (2) define how much removed non-CE tumor translates into a survival benefit.
Methods
The international RANO resect group retrospectively searched previously compiled databases from 7 neuro-oncological centers in the USA and Europe for patients with newly diagnosed glioblastoma per WHO 2021 classification. Clinical and volumetric information from pre- and postoperative MRI were collected.
Results
We collected 1,008 patients with newly diagnosed IDHwt glioblastoma. 744 IDHwt glioblastomas were treated with radiochemotherapy per EORTC-26981/22981 (TMZ/RT→TMZ) following surgery. Among these homogenously treated patients, lower absolute residual tumor volumes (in cm3) were favorably associated with outcome: patients with “maximal CE resection” (class 2) had superior outcome compared to patients with “submaximal CE resection” (class 3) or “biopsy” (class 4). Extensive resection of non-CE tumor (≤5 cm3 residual non-CE tumor) was associated with better survival among patients with complete CE resection, thus defining class 1 (“supramaximal CE resection”). The prognostic value of the resection classes was retained on multivariate analysis when adjusting for molecular and clinical markers.
Conclusions
The proposed “RANO categories for extent of resection in glioblastoma” are highly prognostic and may serve for stratification within clinical trials. Removal of non-CE tumor beyond the CE tumor borders may translate into additional survival benefit, providing a rationale to explicitly denominate such “supramaximal CE resection.”
Keywords: classification, EOR, glioblastoma, outcome, surgical resection
Key Points.
The proposed RANO categories provide a tool to stratify extent of resection in glioblastoma.
Residual contrast-enhancing (CE) and non-CE tumor is associated with outcomes.
The prognostic value of residual tumor volume is independent from molecular or clinical markers.
Importance of the Study.
Extent of resection is associated with overall survival in glioblastoma patients. However, terminology to describe extent of resection varies substantially across clinical studies. Based on a molecularly and clinically well-defined cohort of 1008 IDHwt glioblastoma patients from 7 centers in the US and Europe, we designed an easy-to-use yet highly prognostic classification system entitled “RANO categories for extent of resection in glioblastoma” resting upon residual tumor volume on postoperative MRI. Here, categories describing smaller volumes of postoperative contrast-enhancing (CE) but also non-CE tumor were associated with improved outcomes. The prognostic value of the RANO classes was retained on a uni- and multivariate analysis when stratifying for clinical and molecular markers including O6-methylguanine-DNA methyltransferase promotor methylation status. The RANO categories may therefore serve as a stratification tool for clinical trials on glioblastoma.
In glioblastoma, microsurgical resection—whenever possible—forms the basis for further medical therapies and represents the standard of care which is followed by radiochemotherapy.1,2 Prior studies provided evidence that larger extent of resection (EOR) is associated with more favorable outcome.3–7 However, the terminology used to describe EOR has been inconsistently applied across clinical studies; hampering comparative analyses between different reports or institutions. Here, EOR has often been defined based upon the proportion of removed tumor although more recent studies suggest that the absolute residual tumor volume might be prognostically more relevant.5,8,9 Based on these considerations, we previously proposed an evidence-based classification system to standardize terminology for EOR in glioblastoma.10 This classification system incorporates the relative tumor volume reduction (in percentage), but more importantly also the absolute residual tumor volume (in cm3).
Whereas former studies mainly focused on resection of contrast-enhancing (CE) tumor, the concept of “supramaximal” resection beyond CE tumor borders emerges.11,12 Despite recent evidence supporting an additional survival benefit when removal of surrounding non-CE tumor is provided,5,9,13 the cut-off values for non-CE tumor volumes which need to be removed to convey a prognostic benefit have not been systematically determined. As such, our previously proposed classification system remained descriptive and lacked a clear volumetric definition for such a “supramaximal” resection.10 To fill this gap, we, the multi-center and -professional RANO resect group, retrospectively assembled a large cohort of clinically, volumetrically, and molecularly well-annotated patients with newly diagnosed glioblastoma per WHO 2021 classification. We explored the prognostic utility of our previously proposed surgical classification system in a clinical setting of high-volume centers. Moreover, we analyzed how much non-CE tumor may need to be removed to translate into a survival benefit, thus refining the potential definition of “supramaximal” resection. The resulting classification system was then refined for simplicity and tested and potential confounders using a multivariate model for its prognostic relevance and potential confounders.
Methods
Clinical metadata were collected after approval from the institutional review boards or ethics committees at each participating center and sent for centralized analysis to the main study center at the Ludwig-Maximilians-University (Munich, Germany). Centralized data storage and analysis was approved by the ethics committee of the Ludwig-Maximilians-University (AZ:21-0996). PRISMA guidelines were followed whenever applicable (Supplementary Table 1). Details on the study protocol and population, volumetrics, endpoints, and statistics are given in Supplementary Methods 1.
Study Population
RANO resect investigators queried institutional databases at 7 neuro-oncological centers in Europe and the US for patients with newly diagnosed glioblastoma (Figure 1A). Patients were selected based on the following criteria: (1) tissue-based diagnosis of previously untreated IDH-wildtype glioblastoma meeting the WHO 2021 classification14; (2) pre- and postoperative MRI available for review (including contrast-enhanced T1- and T2/FLAIR-sequences); and (3) follow-up of ≥ 3 months after histopathological diagnosis of glioblastoma. Patients were consecutively treated at the individual institutions and no further search criteria were applied to avoid the introduction of unnecessary confounders. A standardized set of demographic, clinical, and volumetric information were extracted from the databases (Supplementary Table 2).
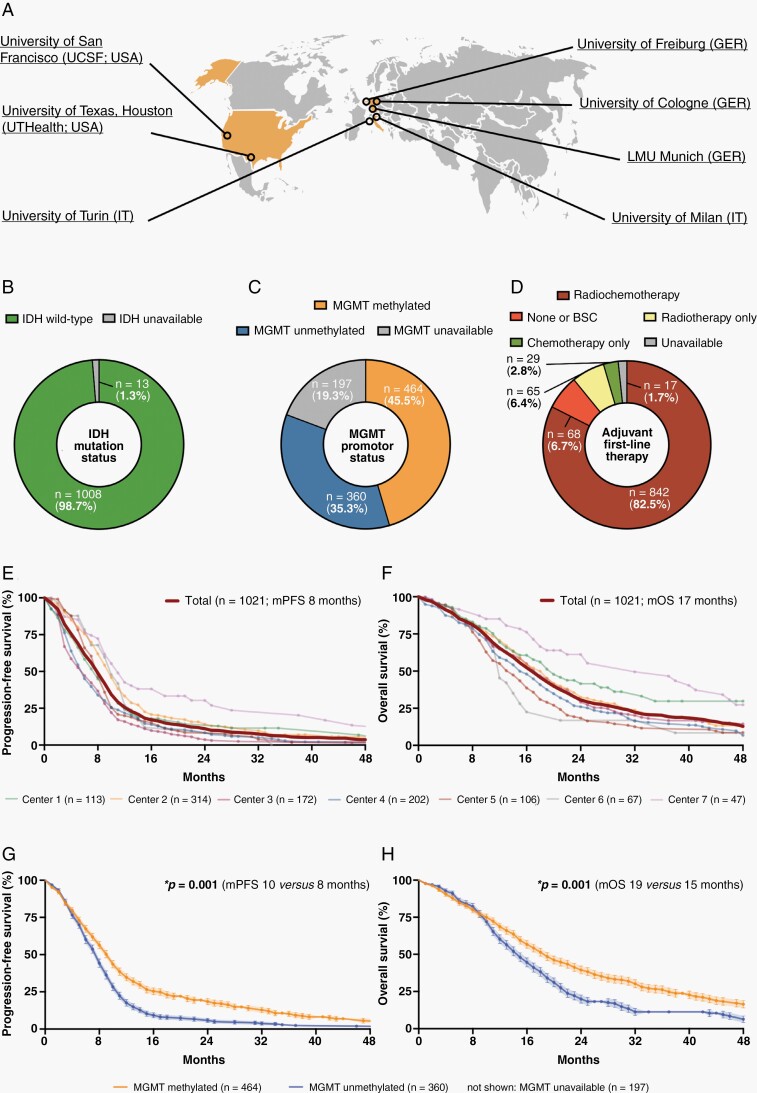
Fig. 1.
Baseline characteristics of the entire study cohort including 1,021 glioblastoma patients. (A) Schematic localization of the 7 participating neuro-oncological centers. (B–D) Distribution of IDH mutation status (B), MGMT promotor status (C), and first-line therapies (D) across the entire study cohort (n = 1,021). (E–H) Kaplan–Meier estimates of progression-free survival (E and G) and OS (F and H) for the entire study cohort (n = 1,021). Curves are given for patients stratified according to the respective study center (E and F) and MGMT promotor status (G and H). Points: deceased/censored patients; shading: SEM.
Volumetric Image Analysis and Inter-Rater Variability
Tumor volumes were quantified on pre- and postoperative MRI (obtained ≤ 72 h after surgery whenever possible).15 Tumor volumes were delineated using the preferred institutional software (BrainLab Smartbrush, Philips IntelliSpace Discovery, 3D Slicer). Total CE tumor was measured on contrast-enhanced T1-sequences and non-CE tumor (defined as signal alterations beyond the enhancing tumor borders) on FLAIR (if not available: T2)-sequences. Raters ensured that postoperative FLAIR/T2-abnormalities were not surgically induced edema or ischemia. We recorded absolute volumes (in cm3), and calculated relative volume reduction (in percentage) as follows: “[(volumepostoperative)/volumepreoperative)] × 100.” To allow quantification of the inter-rater variability across the different study centers, an identical set of 12 pre- and postoperative MRIs was analyzed at each study center and data were re-transferred to the main study center for further analysis.
Patient Classification According to EOR
The previously proposed classification system for EOR in glioblastoma formed the basis for initial patient stratification.10 Patients were classified based upon the relative tumor volume reduction (in percentage) and absolute residual tumor volume (in cm3) as follows:
“supramaximal resection of CE tumor”: beyond CE tumor borders (cut-off values remain to be defined);
“complete resection of CE tumor”: removal of all CE tumor;
“near total resection of CE tumor”: 95%–99.9% CE tumor reduction + ≤1 cm3 residual CE tumor;
“subtotal resection of CE tumor”: 80%–94.9% CE tumor reduction + ≤5 cm3 residual CE tumor;
“partial resection of CE tumor”: <80% CE tumor reduction ± >5 cm3 residual CE tumor (administered for mass effect-related symptoms); or
“biopsy”: no tumor reduction (intervention done for tissue-based diagnosis only).
Statistics
Continuous variables were tested for normal distribution and equal variance using the D’Agostino–Pearson-test. Differences between 2 groups were analyzed by the unpaired Student’s t-test, and differences between multiple groups were analyzed by a 1-way ANOVA. For nonparametric data, we used the Mann–Whitney U-test for 2 groups and the Kruskal–Wallis test for multiple groups. The relation between pre- and postoperative tumor volumes was assessed using the Pearson correlation coefficient (r), and prediction models were built using simple linear regression. Data are expressed as mean ± SEM, and range is given. Categorical variables are described in absolute numbers and percentages. Relationships between categorical variables were analyzed using the χ2-test. For univariate survival analysis, Kaplan–Meier survival estimates and log-rank tests were calculated. Progression was assessed per RANO criteria.16 For multivariate survival analysis, Cox proportional hazard regression models were constructed to compute hazard ratios (HR) and 95% confidence intervals (CI). Markers were first assessed on univariate analysis and forwarded into the multivariate model if significant on univariate analysis. Assumptions of proportional hazards and linearity were confirmed using scaled Schoenfeld residuals (vs time) and deviance residuals. Inter-rater agreement on volumetrics was evaluated by determining the intraclass correlation coefficient (ICC; reliability: <0.5: poor, 0.5–0.75: moderate, 0.75–0–9: good, >0.9: excellent). Statistical analyses were performed using Prism (v9.3.1; GraphPad Software Inc., San Diego, CA) and Stata statistical software (v17.0; StataCorp LLC., College Station, TX). The significance level was set at P ≤ .05. Coded data can be accessed upon qualified request from the authors.
Results
Baseline Patient Characteristics
Data from 1021 glioblastoma patients diagnosed between 2003 and 2022 were collected (Table 1). In the entire cohort, male-to-female ratio was 1:0.7, mean age at diagnosis was 61.5 ± 0.4 years (18–97 years), and median preoperative Karnofsky Performance Status (KPS) was 80% (20%–100%). All tumors were assigned to WHO grade 4 meeting WHO 2021 classification,14 IDH-wildtype was documented in 1,008 patients (97.8%; unavailable in 13 patients, 1.3%). O6-methylguanine-DNA methyltransferase (MGMT) promotor status, when reported, was methylated in 464 patients (45.5% of entire cohort; 56.3% of MGMT reported) and unmethylated in 360 patients (35.3% of entire cohort; 43.7% of MGMT reported) (Figure 1B and C). Surgical resection was well tolerated with 161 patients (15.8%) experiencing new neurologic deficits which were generally mild. The vast majority of patients underwent postoperative radiochemotherapy (82.5%; including regimens utilizing temozolomide, lomustine, or both) following surgical intervention (Figure 1D). Median progression-free survival was 8 (CI: 8–9) months (860 patients with progressive disease; 84.2%) and median overall survival (OS) was 17 (CI: 16–18) months (681 deceased patients; 66.7%) at a median follow-up of 38 (CI: 33–45) months consistent with recent outcome data for IDH-wildtype glioblastoma (Figure 1E and F).17,18 At time of final data collection, 161 (15.8%) patients were not progressive with a median follow-up of 7 (CI:5–9) months and 340 patients (33.3%) were alive with a median follow-up of 12 (CI:10–13) months including 141 patients lost to follow-up (not seen for ≥ 12 months). Among patients with reported MGMT promotor status, methylation was strongly associated with favorable outcomes (Figure 1G and H).19,20
Table 1.
Characteristics of the Unadjusted Study Cohort for Glioblastoma WHO Grade 4 From Seven Neuro-oncological Institutions
| Study centers | Center 1 | Center 2 | Center 3 | Center 4 | Center 5 | Center 6 | Center 7 | Total | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 113 | 314 | 172 | 202 | 106 | 67 | 47 | 1,021 | ||
| Demographics | Age (years) | 60.1 ± 1 | 62.4 ± 1 | 59.5 ± 1 | 62.8 ± 1 | 64.0 ± 1 | 59.4 ± 1 | 55.7 ± 2 | 61.5 ± 0.4 | *.001 |
| M:F ratio | 1:0.7 | 1:0.7 | 1:0.8 | 1:0.6 | 1:0.9 | 1:0.5 | 1:0.8 | 1:0.7 | .642 | |
| Clinical markers | Pre-OP KPS (median, range) | 80 (50–100) | 80 (20–100) | 70 (40–100) | 90 (30–100) | 90 (40–100) | 90 (60–100) | 90 (70–100) | 80 (20–100) | *.001 |
| Post-OP KPS (median, range) | 80 (20–100) | n.a. | 80 (50–100) | 80 (10–100) | 90 (50–100) | 90 (70–100) | 80 (70–100) | 80 (10–100) | *.001 | |
| New postoperative deficit (n, %) | 17 (15.0%) | 54 (17.2%) | 13 (7.6%) | 43 (21.3%) | 10 (9.4%) | 13 (19.4%) | 11 (23.4%) | 161 (15.8%) | *0.001 | |
| IDH status (n, %) | wildtype | 113 (100%) | 314 (100%) | 172 (100%) | 189 (93.6%) | 106 (100%) | 67 (100%) | 47 (100%) | 1,008 (98.7%) | n. appl. |
| mutated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| n.a. | 0 | 0 | 0 | 13 (6.4%) | 0 | 0 | 0 | 13 (1.3%) | ||
| MGMT promotor (n, %) | methylated | 56 (49.6%) | 211 (67.2%) | 5 (2.9%) | 95 (47.0%) | 37 (34.9%) | 27 (40.3%) | 33 (70.2%) | 464 (45.5%) | *.001 |
| nonmethylated | 57 (50.4%) | 101 (32.2%) | 4 (2.3%) | 106 (52.5%) | 45 (42.5%) | 35 (52.2%) | 12 (25.5%) | 360 (35.3%) | ||
| n.a. | 0 | 2 (0.6%) | 163 (94.8%) | 1 (0.5%) | 24 (22.6%) | 5 (7.5%) | 2 (4.3%) | 197 (19.3%) | ||
| TERT promotor (n, %) | wildtype | 14 (12.4%) | 17 (5.4%) | 30 (17.4%) | 4 (2.0%) | 0 | 0 | 5 (10.6%) | 70 (6.9%) | *.001 |
| mutated | 98 (86.7%) | 151 (48.1%) | 124 (72.1%) | 3 (1.5%) | 0 | 8 (11.9%) | 12 (25.5%) | 396 (38.8%) | ||
| n.a. | 1 (0.9%) | 146 (46.5%) | 18 (10.5%) | 195 (96.5%) | 106 (100%) | 59 (88.1%) | 30 (63.8%) | 555 (54.4%) | ||
| Localization (n, %) | (sub-)cortical | 88 (77.9%) | 242 (77.1%) | 130 (75.6%) | 133 (65.8%) | 95 (89.6%) | 51 (76.1%) | 33 (70.2%) | 772 (75.6%) | *.001 |
| deep-seated | 13 (11.5%) | 16 (5.1%) | 24 (14.0%) | 49 (24.3%) | 4 (3.8%) | 11 (16.4%) | 9 (19.2%) | 126 (12.3%) | ||
| multifocal | 12 (10.6%) | 54 (17.2%) | 18 (10.5%) | 20 (9.9%) | 7 (6.6%) | 5 (7.5%) | 5 (10.6%) | 121 (11.9%) | ||
| n.a. | 0 | 2 (0.6%) | 0 | 0 | 0 | 0 | 0 | 2 (0.2%) | ||
| dominant hemisphere | 56 (49.6%) | 165 (52.6%) | 80 (46.5%) | 112 (55.5%) | 46 (43.4%) | 40 (59.7%) | 15 (31.9%) | 514 (50.3%) | *.001 | |
| Tumor volumes (mL; mean ± SEM) | Pre-OP CE | 29.4 ± 2 | 39.9 ± 2 | 29.9 ± 2 | 27.7 ± 2 | 35.5 ± 3 | 28.2 ± 3 | 25.5 ± 4 | 32.7 ± 1 | *.001 |
| Pre-OP non-CE | 20.3 ± 1 | 42.6 ± 2 | 107.7 ± 5 | 69.3 ± 4 | 60.0 ± 4 | 49.7 ± 4 | 54.0 ± 6 | 59.2 ± 2 | *.001 | |
| Post-OP CE | 9.1 ± 2 | 1.7 ± 0.2 | 2.8 ± 0.4 | 9.7 ± 1 | 1.1 ± 0.2 | 1.6 ± 1 | 0.2 ± 0.1 | 1.5 ± 0.1 | *.001 | |
| Post-OP non-CE | 8.9 ± 1 | 26.6 ± 2 | 58.9 ± 4 | 58.2 ± 3 | 34.1 ± 3 | 28.0 ± 3 | 26.8 ± 4 | 34.5 ± 1 | *.001 | |
| Adjuvant therapy (n, %) | RT alone | 14 (12.4%) | 8 (2.6%) | 3 (1.7%) | 21 (10.4%) | 11 (10.4%) | 7 (10.5%) | 1 (2.1%) | 65 (6.4%) | *.001 |
| EORTC-26981/22981 | 67 (59.3%) | 247 (78.7%) | 129 (75.0%) | 155 (76.7%) | 74 (69.8%) | 56 (83.6%) | 30 (63.8%) | 758 (74.2%) | ||
| CeTeG | 8 (7.1%) | 0 | 0 | 15 (7.4%) | 0 | 0 | 0 | 23 (2.3%) | ||
| TMZ alone | 15 (13.3%) | 3 (1.0%) | 3 (1.7%) | 0 | 6 (5.7%) | 0 | 2 (4.3%) | 29 (2.8%) | ||
| EORTC-26981/22981 + study drug | 0 | 29 (9.2%) | 25 (14.5%) | 0 | 0 | 0 | 7 (14.9%) | 61 (6.0%) | ||
| none or BSC | 9 (8.0%) | 23 (7.3%) | 12 (7.0%) | 11 (5.5%) | 3 (2.8%) | 3 (4.5%) | 7 (14.9%) | 68 (6.7%) | ||
| n.a. | 0 | 4 (1.3%) | 0 | 0 | 12 (11.3%) | 1 (1.5%) | 0 | 17 (1.7%) | ||
| Outcome | PFS (months; CI) | 8 (6–10) | 10 (9–11) | 6 (5–7) | 6 (5–7) | 8 (8–9) | 10 (9–12) | 11 (9–18) | 8 (8–9) | *.001 |
| OS (months; CI) | 20 (15–29) | 18 (16–20) | 18 (16–20) | 15 (13–18) | 14 (11–16) | 12 (12–15) | 32 (19–45) | 17 (16–18) | *.001 |
Characteristics are given for patients with glioblastoma WHO grade 4 as stratified according to the study center the data were transferred from and are summarized for all patients (total; n = 1,021). Differences between the study centers were analyzed using a 1-way ANOVA (for parametric data) or the Kruskal–Wallis test (for nonparametric data) for continuous variables; and categorical variables were assessed by the χ2 test. Kaplan–Meier estimates and log-rank testing were performed for survival analyses. P-values are given, and asterisks indicate P ≤ .05. BSC, best supportive care; CeTeG, TMZ + CCNU/RT→TMZ + CCNU; CCNU, lomustine; CI, 95% CI.; EORTC-26981/22981, TMZ/RT→TMZ; F, female; KPS, Karnofsky Performance Score; M, male; n.a., not available for review; n. appl., not applicable; PFS, progression-free survival; RT, radiotherapy; TERT, telomerase reverse transcriptase promotor; TMZ, temozolomide.
Stratification According to the Classification System and Prognostic Implications of CE Tumor Resection
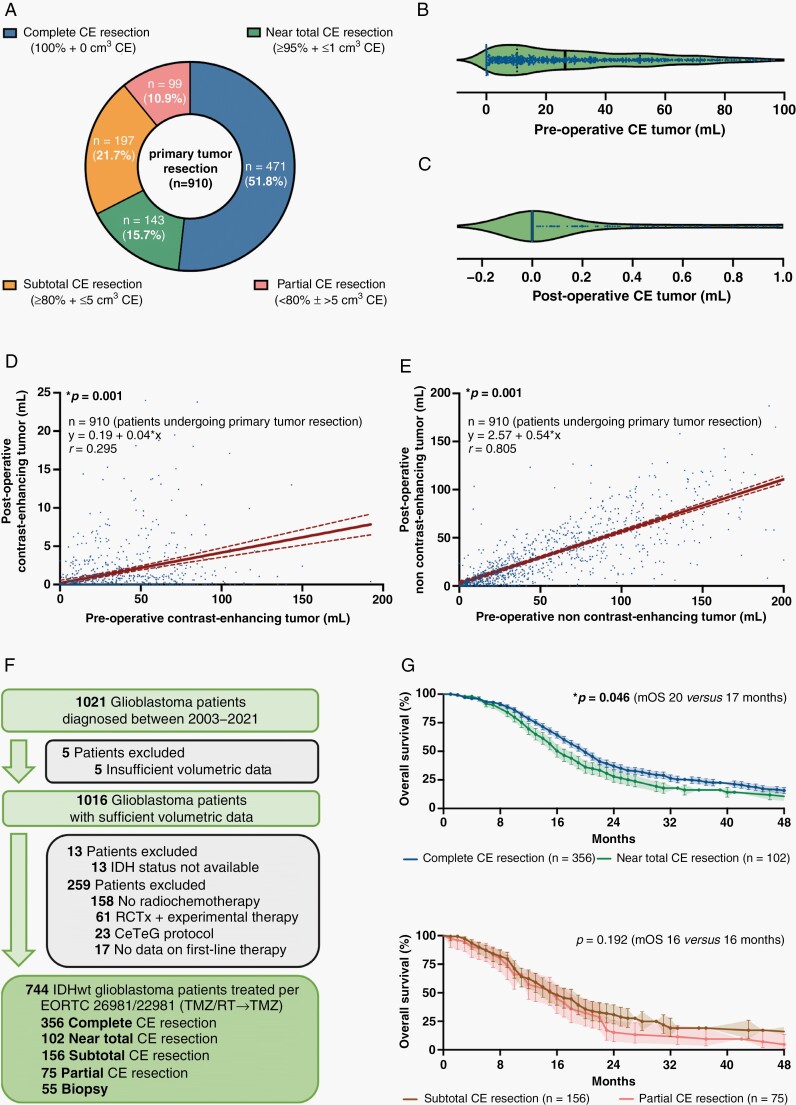
Sufficient volumetric information to analyze surgical results as measured by extent of CE tumor resection was available in 1,016 patients, including 910 individuals (910/1,016 patients, 89.6%) in whom microsurgical tumor resection was provided and 106 individuals (106/1,016 patients, 10.4%) who underwent a stereotactic biopsy. Based on the previously proposed classification system,10 patients undergoing primary tumor resection were stratified into 1 of 4 categories: “complete CE resection” (471/910 patients, 51.8%), “near total CE resection” (143/910 patients, 15.7%), “subtotal CE resection” (197/910 patients, 21.7%), and “partial CE resection” (99/910 patients, 10.9%) (Figure 2A). Median preoperative CE tumor volume was 26.4 ± 1.0 cm3 (0–192 cm3) in patients undergoing microsurgical resection. No residual CE tumor was detected in most cases with a median postoperative CE volume of 0 ± 0.1 cm3 (0–45 cm3) and a median percentage of 100 ± 0.4% (0%–100%) CE tumor resected, with 493 patients having some residual CE tumor (Figure 2B and C). Notably, pre- and postoperative CE tumor volumes correlated (r = 0.295; P = .001) and a regression analysis predicted a postoperative increase of 0.04 cm3 residual CE tumor volume per each cm3 of preoperative CE tumor volume (β 1: 0.04) (Figure 2D). The positive correlation was even more pronounced for non-CE tumor volumes (r = 0.805; P = .001), and with each cm3 preoperative non-CE volume an increase of 0.54 cm3 residual non-CE tumor volume was estimated (β 1: 0.54) (Figure 2E).
Fig. 2.
Stratification according to extent of CE tumor resection and prognostic implications. (A) Stratification of all patients undergoing microsurgical tumor resection (n = 910) according to the previously proposed classification system based on extent of CE tumor resection. (B and C) Pre-(B) and postoperative CE tumor volume (C; in cm3) after microsurgical tumor resection (n = 910). Median ± 95% CI. (D and E) Simple linear regression analyses comparing the pre- to the postoperative tumor volumes for CE (D) and non-CE tumor (E) in patients undergoing microsurgical tumor resection (n = 910). Pearson correlation coefficients (r), calculated equations including slope β 1, and P-values are given. Dotted lines: 95% CI. (F) Schematic representation of the formation of a patient cohort exclusively including IDH-wildtype glioblastomas homogenously treated per EORTC-26981/22981-protocol (n = 744). (G and H) Kaplan–Meier estimates of OS in IDH-wildtype glioblastomas treated per EORTC-26981/22981-protocol (n = 744). Patients stratified to the category “complete CE resection” were compared to “near total CE resection” (G), and patients stratified to the category “subtotal CE resection” were compared to “partial CE resection” (H). Points: deceased/censored patients; shading: SEM.
To study the prognostic implications of patient stratification according to extent of CE tumor resection, we isolated a cohort of the 744 IDH-wildtype glioblastoma patients who were homogenously treated as per EORTC-26981/22981-protocol (TMZ/RT→TMZ) (Figure 2F).17 Here, we found that patients with either “complete CE resection” had superior survival compared to patients with “subtotal CE resection” [20 (CI:18–21) vs 16 (CI:14–19) months: HR:0.75, CI:0.6–1.0; P = .024] or “partial CE resection” [20 (CI:18–21) vs 16 (CI:12–18) months: HR:0.54, CI:0.4–0.8; P = .001]. Notably, we found no evidence for differences in outcome between the groups “subtotal CE resection” and “partial CE resection” [16 (CI:14–19) vs 16 (CI:12–18) months: HR:0.8, CI:0.6–1.1; P = .192]; however, we detected survival differences between the groups “complete CE resection” and “near total CE resection” [20 (CI:18–21) vs 17 (CI:15–20) months: HR:0.74, CI:0.6–1.0; P = .046] (Figure 2G and H).
Exploring the Prognostic Relevance of Non-CE Tumor Resection to Define “Supramaximal CE Resection”
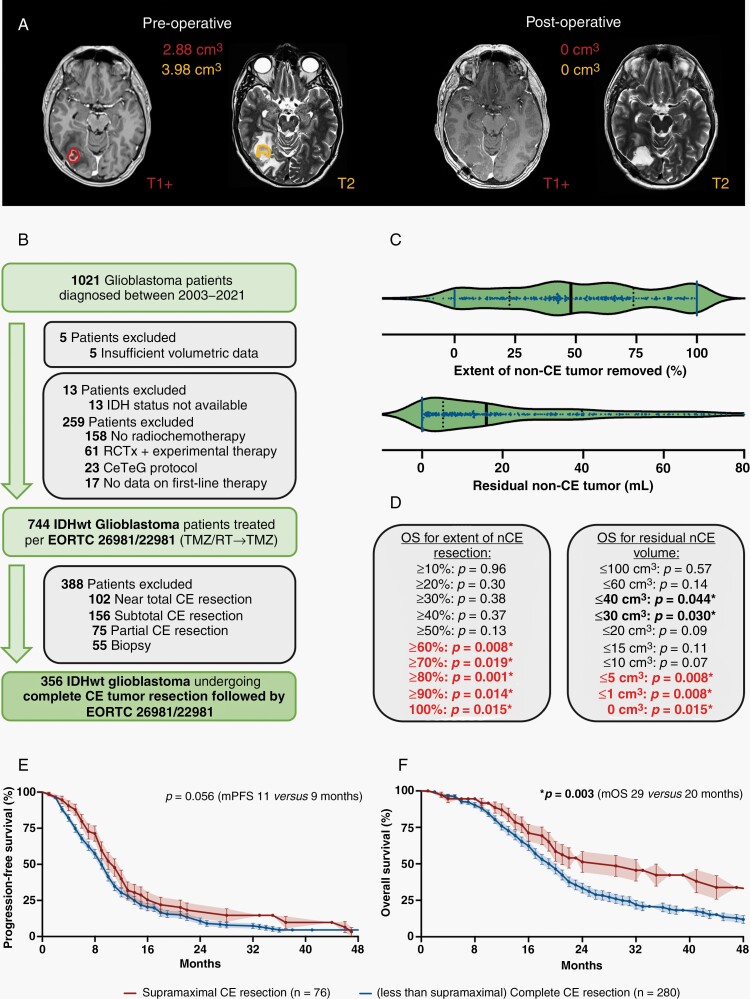
Favorable outcomes in patients with “complete CE resection” compared to patients with “near total CE resection” might be due to the inclusion of patients undergoing additional resection of non-CE tumor (Figure 3A). To explore whether patients undergoing such a “supramaximal CE resection” beyond CE tumor borders experience a distinct survival benefit, we selected all individuals with complete resection of CE IDH-wildtype glioblastoma treated per EORTC-26981/22981-protocol (n = 356; Figure 3B). Among the patients initially assigned as “complete CE resection,” median non-CE tumor volume beyond the CE tumor core was 32.9 ± 2.5 cm3 (0–322 cm3) prior to surgery. After resection, median remaining non-CE tumor volume was 16.0 ± 1.6 cm3 (0–190 cm3) and median relative non-CE tumor reduction was 48.0 ± 3.7% (0%–100%) (Figure 3C). On univariate analysis, larger extent of non-CE tumor resection quantified either as relative reduction of tumor volume or as absolute residual tumor volume was associated with prolonged OS. Cut-off values for the non-CE tumor volume needed to be removed to achieve a prognostic benefit were > 60% non-CE tumor or ≤ 5 cm3 residual non-CE tumor (Figure 3D), validating a previously reported cut-off.21 Patients meeting both cut-off values had substantially better outcomes compared to patients in which complete resection of CE tumor but less extent of non-CE tumor resection was achieved [median OS: 29 (CI:20–44) vs 20 (CI:18–21) months: HR:0.62, CI:0.5–0.8; P = .003; Figure 3E and F]. This finding provides a rationale to denote such patients as “supramaximal CE resection.” Notably, the rate of new postoperative neurologic deficits was not increased in such patients. We did not detect outcome differences anymore between the groups “complete CE resection” and “near total CE resection” when individuals with “supramaximal CE resection” were subtracted from the group of patients with “complete CE resection.”
Fig. 3.
Prognostic value of non-CE tumor resection beyond CE tumor borders. (A) Axial brain MRI with contrast-enhanced T1- (left on each panel) and T2-weighted (right on each panel) sequences demonstrating a right temporo-occipital glioblastoma. On preoperative imaging (left panel), CE (red) and non-CE tumors (orange) are delineated. Note that non-CE tumor was defined as T2/FLAIR-alterations beyond the enhancing tumor borders not corresponding to surgically induced edema or ischemia. On postoperative imaging (right panel), complete resection of CE and non-CE tumors was achieved. (B) Schematic representation of the formation of a patient cohort exclusively including IDH-wildtype glioblastomas homogenously treated per EORTC-26981/22981-protocol and in which complete CE resection was provided (n = 365). (C) Extent of non-CE tumor removed (upper panel; in percentage) and postoperative non-CE tumor volume (lower panel; in cm3) in IDH-wildtype glioblastomas treated per EORTC-26981/22981-protocol and in which complete CE resection was provided (n = 365). Median ± 95%CI. (D) Univariate analyses for OS in IDH-wildtype glioblastomas treated per EORTC-26981/22981-protocol and in which complete CE resection was provided (n = 365) when stratified according to relative reduction of non-CE tumor (left panel; in percentage) or postoperative non-CE tumor volume (right panel; in cm3). P-values are given; asterisks and bold letters indicate P ≤ .05. (E and F) Kaplan–Meier estimates of progression-free survival (E) and OS (F) in IDH-wildtype glioblastomas treated per EORTC-26981/22981-protocol and in which complete CE resection was provided (n = 365). Curves are given for patients with “supramaximal" CE resection (red; defined as ≥ 60% reduction of non-CE tumor volume + ≤5 cm3 residual non-CE tumor) and less than “supramaximal" CE resection (blue). Points: deceased/censored patients; shading: SEM.
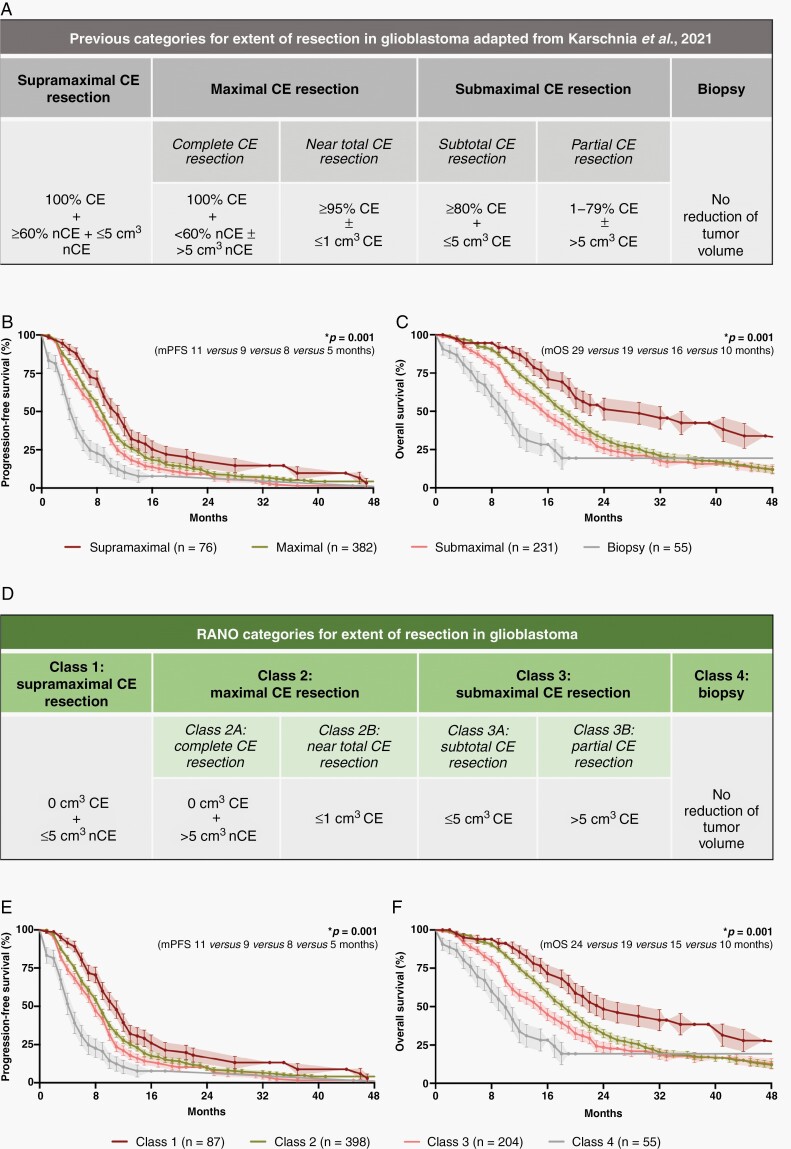
Refining the Classification System Into the “RANO Categories for EOR in Glioblastoma”
Incorporating the newly introduced category “supramaximal CE resection,” the prognostic utility of the previously proposed classification system was tested in the 744 IDH-wildtype glioblastoma patients homogenously treated per EORTC-26981/22981-protocol. Acknowledging comparable outcomes of individuals with “complete CE resection”/“near total CE resection” or “subtotal CE resection”/“partial CE resection,” these patients were summarized as “maximal CE resection” (“complete CE resection”/“near total CE resection”) or “submaximal CE resection” (“subtotal CE resection”/“partial CE resection”) (Figure 4A). When applying the resulting classification system for EOR, the respective categories reflected distinct survival outcomes: patients stratified to “supramaximal CE resection” had superior outcomes compared to patients with “maximal CE resection,” whereas the latter group was superior to patients with “submaximal CE resection,” and patients designated as “biopsy” had least favorable progression-free survival [11 (CI:9–13) vs 9 (CI:8–10) vs 8 (CI:7–9) vs 5 (CI:4–6) months; P = .001; Figure 4B] and OS [median OS: 29 (CI:20–44) vs 19 (CI:17–20) vs 16 (CI:14–18) vs 10 (CI:8–12] months; P = .001; Figure 4C].
Fig. 4.
Stratification systems to describe EOR in glioblastoma. (A) Previously proposed classification system by Karschnia et al. (in Eur J Cancer, 2021) based upon the relative reduction of tumor volume (in percentage) and the absolute residual tumor volume (in cm3) on postoperative MRI. (B and C): Kaplan–Meier estimates of progression-free survival (B) and OS (C) in IDH-wildtype glioblastomas treated per EORTC-26981/22981-protocol (n = 744). Patients were stratified according to the categories proposed in the classification system. Points: deceased/censored patients; shading: SEM. (D) The developed RANO categories for EOR in glioblastoma which solely base upon the absolute residual tumor volume (in cm3) on postoperative MRI. (E and F): Kaplan–Meier estimates of progression-free survival (E) and OS (F) in IDH-wildtype glioblastomas treated per EORTC-26981/22981-protocol (n = 744). Patients were stratified according to the RANO categories. Note that the prognostic value of the previously proposed classification system (A) is retained in the RANO categories (D) although the relative reduction of tumor volume (in percentage) has been removed as a classification criterion. Points: deceased/censored patients; shading: SEM.
We next aimed to simplify the classification system to improve practicability without losing prognostic relevance. Hereby, the calculation of relative reduction of tumor volume (in percentage) is particularly challenging given the need for thorough volumetric image analysis of both pre- and postoperative imaging. Thus, we analyzed whether the prognostic value is retained when relying solely on postoperative tumor volumes as a stratification criterium. We defined four resection classes (class 1: “supramaximal CE resection,” class 2: “maximal CE resection,” class 3: “submaximal CE resection,” class 4: “biopsy”) based on the residual CE and non-CE tumor volume (in cm3) (Figure 4D). Each resection class identified with distinct outcomes for progression-free survival [11 (CI:9–13) vs 9 (CI:8–10) vs 8 (CI:7–9) vs 5 (CI:4–6) months; P = .001; Figure 4E] and OS [24 (CI:20–41) vs 19 (CI:17–20) vs 15 (CI:12–17) vs 10 (CI:8–12) months; P = .001; Figure 4F]. The prognostic relevance held true when the resection classes were individually tested against each other. This easy-to-use yet highly prognostic stratification system was termed “RANO categories for EOR in glioblastoma.”
Controlling for Confounding Effects: Multivariate Analysis, Second-Line Therapies, and Inter-Rater Variability
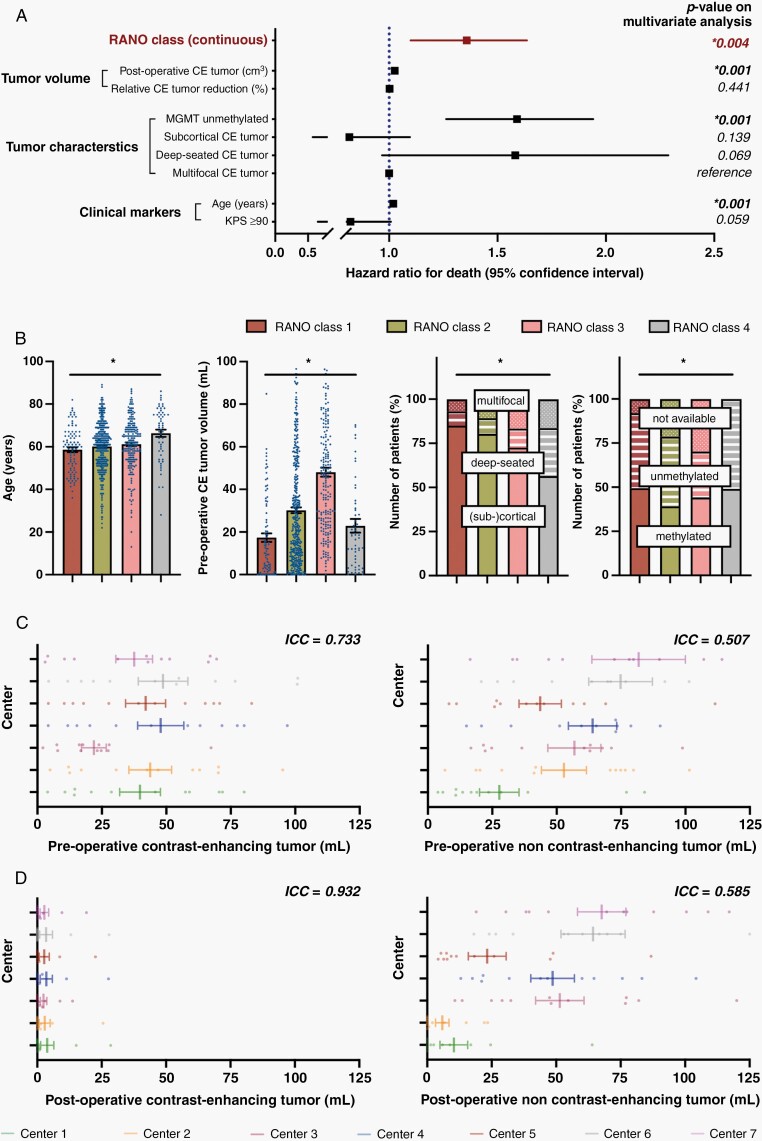
In univariate models, stratification according to these newly defined RANO categories (per higher class: HR:1.46, CI:1.3–1.7; P = .001), postoperative CE volume (per cm3: HR:1.04, CI:1.0–1.1; P = .001), relative CE tumor reduction (per percentage: HR:0.99, CI:0.9–1.0; P = .001), age (per year: HR:1.02, CI:1.0–1.1; P = .001), preoperative KPS (≥90%: HR:0.76, CI:0.6–0.9; P = .003), tumor localization [subcortical (reference: multifocal): HR:0.67, CI:0.5–0.9; P = .004], and MGMT promotor methylation status (unmethylated: HR:1.53, CI:1.3–1.9; P = .001) were associated with OS (Supplementary Table 3). Importantly, the prognostic value of the RANO categories was retained (HR:1.34, CI:1.1–1.6; P = .004) when included in a multivariate model (as continuous variable) with the other univariately significant variables (Figure 5A). When stratification according to the newly defined RANO categories was included as categorical variable in the multivariate analysis and RANO class 1 was set as reference level, decreased outcomes were confirmed across the RANO classes (RANO class 2: HR:1.58, CI:1.1–2.3; P = .013/RANO class 3: HR:1.89, CI:1.2–2.9; P = .003/RANO class 4: HR:2.4, CI:0.7–10.9; P = .205). Interestingly, postoperative CE tumor volume (in cm3) was also significant in the multivariate model (HR:1.03, CI:1.0–1.0) whereas relative CE tumor reduction (in percentage) lost prognostic relevance. It remains to be noted that several factors including age, preoperative CE volume, tumor localization, and MGMT promotor methylation status were associated with EOR measured per RANO categories (Figure 5B). Although such factors might have not confounded analyses on the prognostic role for the RANO categories as they were incorporated into multivariate testing, they may predispose to larger extents of resection in a real-world clinical setting (although the role for MGMT promotor methylation status in this context remains puzzling).
Fig. 5.
Multivariate analysis and determination of inter-rater variability to control for potential confounding effects. (A) Multivariate analysis using a Cox proportional hazard regression model estimating the hazard ratio for death of numerous factors which were of significance on univariate analysis. Note that the prognostic value of the RANO classes and absolute residual CE tumor volume (in cm3) was conserved, whereas the prognostic relevance of the relative reduction of CE tumor volume (in percentage) was lost. “Multifocal CE tumor” served as reference for “deep-seated CE tumor” and “subcortical CE tumor.” Hazard ratio ± 95% CI. (B) Distribution of age (left panel), preoperative CE tumor volume (second to left panel), tumor localization (second to right panel), and MGMT promotor status (right panel) across the 4 different RANO categories. Asterisks indicate P ≤ .05 when all four groups were tested together using a Kruskal–Wallis test (for continuous data) or a χ2-test (for categorical variables). Mean ± SEM for continuous data. (C and D): Volumetric analyses of twelve identical pre-(C) and postoperative (D) MRIs by the seven participating centers (color-coded). ICCs (<0.5: poor reliability, 0.5–0.75: moderate reliability, 0.75–0–9: good reliability, >0.9: excellent reliability) are reported for pre-and postoperative CE (left panel) as well as non-CE tumor (right panel). Note that ICCs are higher for post- than for preoperative imaging and for CE than for non-CE tumor. Mean ± SEM.
Therapeutic approaches for first progression varied between patients when stratified per RANO categories: whereas individuals with initially higher EOR (ie, RANO class 1–2) underwent more often (re-)radiation or re-resection, patients which initially had biopsy (ie, RANO class 4) were frequently transferred for palliative care (Supplementary Table 4).
Quantifying the inter-rater variability across the study centers, we found moderate inter-rater agreement on CE tumor (ICC:0.733) and only very modest agreement for non-CE tumor (ICC:0.507) (Figure 5C). Importantly, the consistency between the raters was considerable higher when assessed for postoperative imaging with excellent agreement on CE tumor (ICC:0.932) and moderate agreement on non-CE tumor (ICC:0.585) (Figure 5D).
Discussion
More extensive tumor resection has been shown to be associated with outcome.22,23 However, no classification system to accurately stratify glioblastoma patients according to EOR is available. Here, the RANO resect group proposes an easy-to-use and prognostic stratification system entitled “RANO categories for EOR in glioblastoma.”
Based on a large international cohort of IDH-wildtype glioblastoma patients diagnosed per WHO 2021 classification and homogenously treated per current standard of care,14 we verified the prognostic value of the RANO categories. Patients assigned to RANO classes reflecting higher EOR had more favorable outcome, and the difference between each of the 4 main classes was estimated with a HR of 1.34 using a multivariate model. The introduction of additional subclasses (ie, class 2A/B and class 3A/B) allows more detailed substratification which is descriptive in nature but not prognostically relevant per se.24,25 Importantly, additional removal of non-CE tumor beyond CE borders translated into additional survival benefit which provides a rationale to denominate such a “supramaximal CE resection” in the setting of clinical trials. Our observation is corroborated by a large retrospective, multi-center cohort study reporting that IDH-wildtype glioma patients with <5.4 cm3 non-CE tumor volume (and almost no residual CE tumor) experience particularly beneficial outcome.9 It is striking that we encountered a similar cut-off of residual non-CE tumor tissue which conveys a prognostic benefit, and we have proposed to define RANO class 1 (“supramaximal CE resection”) accordingly. Together with other studies,5 our results contradict prior reports suggesting that IDH-wildtype glioblastoma patients do not benefit from resection beyond CE tumor borders.26,27 Further validation in prospective cohorts is warranted (eg, by incorporating the RANO classes into clinical trials).
We ruled out that our findings on the prognostic role of the RANO categories were confounded by molecular markers (ie, MGMT promotor methylation status) or clinical factors (eg, preoperative tumor volume, new postoperative neurological deficits, and eloquent tumor localization) using a meticulous statistical approach. The beneficial association between greater EOR and more favorable outcome therefore cannot be solely explained by the assumption that lower EOR is a surrogate marker for glioblastomas with closer proximity to critical brain regions (and inherently worse prognosis), although less eloquent localization was indeed associated with more extensive resection. Moreover, pre- and postoperative volumes for CE and non-CE tumors were directly correlated suggesting that larger tumors are likely to invade eloquent areas, limiting extensive resection.28,29 Although surgically induced neurologic deficits were not linked to less favorable outcome, deficits were generally mild. This might reflect the mindset that profound deficits jeopardize survival,6,30,31 and preventing these should be prioritized over EOR.1 Thus, the favorable effects of “supramaximal CE resection” on survival are probably limited to patients with less eloquent localized tumors. Notably, patients with initially larger EOR underwent more often tumor-directed therapies for recurrence whereas biopsy patients were frequently transferred to palliative care. It is unclear whether this inequality in second-line therapies contributes to the distinct outcomes between the RANO classes or it is the natural history of more extensive disease per se which is being observed. The latter assumption is supported by the fact that no second-line therapy was shown to improve survival in randomized clinical trials.1 As MGMT promotor methylation was more frequently encountered in RANO class 1 than in RANO class 2, it remains also puzzling whether MGMT methylated tumors are indeed more amenable to “supramaximal CE resection” which needs to be elucidated in future studies.
Previous studies frequently quantified EOR by determining the relative reduction of tumor volume (in percentage) as a measurement of surgical efficacy3,32; however, absolute residual tumor volume (in cm3) might be of higher importance from an oncological standpoint.10,33 We identified both factors as univariately significant, but only residual CE tumor volume retained prognostic value in our multivariate model.8,9,34 Therefore, the RANO categories stratify patients solely on the volume of residual tumor. This approach may also correspond to a more reliable stratification when assessed by different raters, as our inter-rater agreement was particularly good for postoperative imaging as characterized by substantially higher ICC for both CE and non-CE tumors. Moreover, the level of technical effort would be reduced as volumetric analysis of preoperative imaging would become unnecessary. We encountered considerable larger inter-rater variability for non-CE tumor compared to CE tumor,35 which might be interpreted as supportive of centralized imaging reviews for studies focusing on non-CE tumor volumes. Further studies providing uniform definitions for non-CE tumor on imaging are warranted to standardize imaging protocols and measurements. This might also pave the way to the emerging field of AI-supported, automated tumor segmentation.
Since the WHO 2021 classification restricts the diagnosis of glioblastoma WHO grade 4 to IDH-wildtype tumors,14 we only included such patients in our analysis. Therefore, we cannot comment on whether these new RANO categories have prognostic relevance for IDH-mutant tumors which may morphologically resemble all features of glioblastoma on MRI yet have more favorable outcomes. In turn, tumors previously characterized as low-grade astrocytomas on histology may now be denominated as glioblastomas WHO grade 4 defined by the presence of qualifying molecular features.14 Given that these “molecular” glioblastomas may present with little-to-no contrast enhancement on initial imaging, it remains unclear whether the RANO categories are of descriptive and prognostic value in such entities.27 These “molecular” glioblastomas might have been included in our present cohort as they would have matched our inclusion criteria; however, the recorded data do not allow us to clearly extract how many of those patients would have been classified as tumors other than glioblastoma when applying the WHO 2016 classification.14
It remains to be noted that our cohort predominantly compromises glioblastoma patients undergoing microsurgical resection rather than biopsy (or no surgery at all) given the surgical nature of our study, thus not necessarily depicturing the surgical landscape which is being offered to all affected patients. The benefits of surgery also include tissue acquisition to guide therapy based on molecular markers, and we cannot comment on outcome differences between patients undergoing biopsy relative to those managed with palliative care. Also, we have accumulated a selected group of well-characterized patients rather than presenting the entity of individuals being treated at our institutions during the observation period, and we believe that we have randomly captured less than every second patient which would be suitable for our analysis given the retrospective nature of our study. This reflects in the seemingly distinct outcomes between individual study centers and an uncommon MGMT promotor status distribution (as one would have expected higher number of MGMT unmethylated patients), and we can therefore not exclude some selection bias; also, our statistical approach did not control for center-specific effects besides the predefined clinical, molecular, and volumetric data on our uni- and multivariate analysis.
Collectively, the RANO resect group herein introduces the easy-to-use yet highly prognostic “RANO categories for EOR in glioblastoma” to stratify glioblastoma patients according to EOR. The proposed classes are based on measurements of residual CE as well as non-CE tumor volume, and may serve for stratification and overall design of clinical trials.
Supplementary Material
Acknowledgments
The authors thank all patients who contributed to the results of this study. Parts of the study were presented as an oral abstract at the 2022 Annual Meeting of the American Society for Clinical Oncology (ASCO).
Contributor Information
Philipp Karschnia, Department of Neurosurgery, Ludwig-Maximilians-University, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany.
Jacob S Young, Department of Neurosurgery & Division of Neuro-Oncology, University of San Francisco, San Francisco, California, USA.
Antonio Dono, Department of Neurosurgery, McGovern Medical School at UT Health Houston , Houston, Texas, USA.
Levin Häni, Department of Neurosurgery, University of Freiburg , Freiburg, Germany.
Tommaso Sciortino, Division for Neuro-Oncology, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
Francesco Bruno, Division of Neuro-Oncology, Department of Neuroscience, University of Turin, Turin, Italy.
Stephanie T Juenger, Department of Neurosurgery, University of Cologne , Cologne, Germany.
Nico Teske, Department of Neurosurgery, Ludwig-Maximilians-University, Munich, Germany.
Ramin A Morshed, Department of Neurosurgery & Division of Neuro-Oncology, University of San Francisco, San Francisco, California, USA.
Alexander F Haddad, Department of Neurosurgery & Division of Neuro-Oncology, University of San Francisco, San Francisco, California, USA.
Yalan Zhang, Department of Neurosurgery & Division of Neuro-Oncology, University of San Francisco, San Francisco, California, USA.
Sophia Stoecklein, Department of Radiology, University Hospital, LMU Munich , Munich, Germany.
Michael Weller, Department of Neurology, University Hospital and University of Zurich, Zurich, Switzerland.
Michael A Vogelbaum, Department of NeuroOncology, Moffitt Cancer Center, Tampa, Florida, USA.
Juergen Beck, Department of Neurosurgery, University of Freiburg , Freiburg, Germany.
Nitin Tandon, Department of Neurosurgery, McGovern Medical School at UT Health Houston , Houston, Texas, USA.
Shawn Hervey-Jumper, Department of Neurosurgery & Division of Neuro-Oncology, University of San Francisco, San Francisco, California, USA.
Annette M Molinaro, Department of Neurosurgery & Division of Neuro-Oncology, University of San Francisco, San Francisco, California, USA.
Roberta Rudà, Division of Neuro-Oncology, Department of Neuroscience, University of Turin, Turin, Italy; Division of Neurology, Castelfranco Veneto and Treviso Hospital, Treviso, Italy.
Lorenzo Bello, Division for Neuro-Oncology, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
Oliver Schnell, Department of Neurosurgery, University of Freiburg , Freiburg, Germany.
Yoshua Esquenazi, Department of Neurosurgery, McGovern Medical School at UT Health Houston , Houston, Texas, USA.
Maximilian I Ruge, Department of Stereotactic and Functional Neurosurgery, Centre for Neurosurgery, University Hospital Cologne , Cologne, Germany.
Stefan J Grau, Department of Neurosurgery, University of Cologne , Cologne, Germany.
Mitchel S Berger, Department of Neurosurgery & Division of Neuro-Oncology, University of San Francisco, San Francisco, California, USA.
Susan M Chang, Department of Neurosurgery & Division of Neuro-Oncology, University of San Francisco, San Francisco, California, USA.
Martin van den Bent, Department of Neurology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Joerg-Christian Tonn, Department of Neurosurgery, Ludwig-Maximilians-University, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, Munich, Germany.
Funding
Nothing to report. No unpublished papers were cited.
Conflict of interest statement. M.W.-Research grants: Apogenix, Quercis. Honoraria/advisory board participation/consulting: Bayer, Medac, Merck (EMD), Nerviano-Medical-Sciences, Novartis, Orbus, Philogeny-Mabs. M.A.V.-Indirect equity/patient royalty interests: Infuseon Therapeutics. Honoraria: Celgene, Tocagen, Blue Earth Diagnostics. N.Ta.-Research grant: Medtronic; Founder: BrainDynamics; Advisory Board: Nervonik, BrainGrade. R.R.-Honoraria/advisory board/consulting: UCB, Bayer, Novocure, Genenta. M.v.d.B.-Consultant: Celgene, BMS, Agios, Boehringer, Abbvie, Bayer, Carthera, Nerviano, Genenta. J.C.T.-Research grants: Novocure, Munich-Surgical-Imaging; Royalties: Springer Publisher. P.K.; J.C.Y.; A.D.; L.H.; T.S.; F.B.; S.T.J.; N.T.; R.A.M.; A.F.H.; Y.Z.; S.S.; J.B.; S.H.J.; A.M.M.; L.B.; O.S.; Y.E.; M.I.R.; S.J.G.; M.S.B.; S.M.C.-None.
Authorship statement. Study concept/design: P.K., S.M.C., M.v.d.B., J.C.T.; Data collection: P.K., J.S.Y., A.D., L.H., T.S., F.B., S.T.J., N.T., R.A.M., A.F.H., Y.Z., S.H.J., M.I.R., R.R., L.B., O.S., Y.E., S.J.G., A.M.M., M.S.B., S.M.C., J.C.T.; Analysis/interpretation: P.K., S.M.C., M.v.d.B., J.C.T.; Statistics: P.K., A.M., J.C.T.; Manuscript drafting: P.K., S.M.C., M.v.d.B., J.C.T.; Manuscript revising: P.K., J.S.Y., A.D., L.H., T.S., F.B., S.T.J., N.T., R.A.N., A.F.H., Y.Z., S.S., M.W., M.A.V., J.B., N.Ta., S.H.J., A.M.M., R.R., L.B., O.S., Y.E., M.I.R., S.J.G., M.S.B., S.M.C., M.v.d.B., J.C.T.
References
- 1. Weller M, van den Bent M, Preusser M, et al. . EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2020; 18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wen PY, Weller M, Lee EQ, et al. . Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020; 22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown TJ, Brennan MC, Li M, et al. . Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016; 2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gessler F, Bernstock JD, Braczynski A, et al. . Surgery for glioblastoma in light of molecular markers: impact of resection and MGMT promoter methylation in newly diagnosed IDH-1 wild-type glioblastomas. Neurosurgery. 2019; 84(1):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Incekara F, Smits M, van der Voort SR, et al. . The association between the extent of glioblastoma resection and survival in light of MGMT promoter methylation in 326 patients with newly diagnosed IDH-wildtype glioblastoma. Front Oncol. 2020; 10:1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aabedi AA, Young JS, Zhang Y, et al. . Association of neurological impairment on the relative benefit of maximal extent of resection in chemoradiation-treated newly diagnosed isocitrate dehydrogenase wild-type glioblastoma. Neurosurgery. 2022; 90(1):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellingson BM, Wen PY, Cloughesy TF. Evidence and context of use for contrast enhancement as a surrogate of disease burden and treatment response in malignant glioma. Neuro Oncol. 2018; 20(4):457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grabowski MM, Recinos PF, Nowacki AS, et al. . Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014; 121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 9. Molinaro AM, Hervey-Jumper S, Morshed RA, et al. . Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020; 6(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karschnia P, Vogelbaum MA, van den Bent M, et al. . Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur J Cancer. 2021; 149:23–33. [DOI] [PubMed] [Google Scholar]
- 11. de Leeuw CN, Vogelbaum MA. Supratotal resection in glioma: a systematic review. Neuro Oncol. 2019; 21(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esquenazi Y, Friedman E, Liu Z, Zhu JJ, Hsu S, Tandon N. The survival advantage of “Supratotal” resection of glioblastoma using selective cortical mapping and the subpial technique. Neurosurgery. 2017; 81(2):275–288. [DOI] [PubMed] [Google Scholar]
- 13. Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016; 124(4):977–988. [DOI] [PubMed] [Google Scholar]
- 14. Louis DN, Perry A, Wesseling P, et al. . The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vogelbaum MA, Jost S, Aghi MK, et al. . Application of novel response/progression measures for surgically delivered therapies for gliomas: response assessment in neuro-oncology (RANO) Working Group. Neurosurgery. 2012; 70(1):234–243. [DOI] [PubMed] [Google Scholar]
- 16. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 17. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 18. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017; 318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 20. Hegi ME, Genbrugge E, Gorlia T, et al. . MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of four clinical trials. Clin Cancer Res. 2019; 25(6):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molinaro AM, Hervey-Jumper S, Morshed RA, et al. . Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020; 6(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stummer W, Reulen HJ, Meinel T, et al. . Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008; 62(3):564–576. [DOI] [PubMed] [Google Scholar]
- 23. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 24. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006; 7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 25. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008; 358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 26. Beiko J, Suki D, Hess KR, et al. . IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014; 16(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Lucas CG, Young JS, et al. . Prospective genomically-guided identification of “early/evolving” and “undersampled” IDH-wildtype glioblastoma leads to improved clinical outcomes. Neuro Oncol. 2022;noac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016; 130(2):269–282. [DOI] [PubMed] [Google Scholar]
- 29. Kocher M, Jockwitz C, Lohmann P, et al. . Lesion-function analysis from multimodal imaging and normative brain atlases for prediction of cognitive deficits in glioma patients. Cancers (Basel). 2021; 13(10) :2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rahman M, Abbatematteo J, De Leo EK, et al. . The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg. 2017; 127(1):123–131. [DOI] [PubMed] [Google Scholar]
- 31. Johnson DR, Sawyer AM, Meyers CA, O’Neill BP, Wefel JS. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2012; 14(6):808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011; 115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 33. Ellingson BM, Abrey LE, Nelson SJ, et al. . Validation of postoperative residual contrast-enhancing tumor volume as an independent prognostic factor for overall survival in newly diagnosed glioblastoma. Neuro Oncol. 2018; 20(9):1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. . Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014; 16(1):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Visser M, Müller DMJ, van Duijn RJM, et al. . Inter-rater agreement in glioma segmentations on longitudinal MRI. Neuroimage Clin. 2019; 22:101727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.