Objectives:
Autoimmune hepatitis (AIH) can lead to progressive fibrosis in patients refractory to conventional therapy with prednisolone and azathioprine. The use of mammalian target of rapamycin (mTOR) inhibitors has recently emerged in refractory AIH, but no data have been published about everolimus in pediatric AIH to date. Our aim was to share our experience about everolimus as a second-/third-line therapy in pediatric AIH.
Methods:
Pretransplant AIH patients aged 0–18 years who received everolimus therapy from 2014 to 2021 were retrospectively identified. All patients underwent regular plasma monitoring of everolimus trough levels to avoid toxicity and assess adherence. Special attention was paid to the clinical and biochemical occurrence of everolimus-related adverse events.
Results:
We report six difficult-to-treat AIH patients who received everolimus therapy for 8–46 months (median 28 months). No side effects were reported when everolimus plasma trough levels were in the therapeutic range. Liver transaminases improved in 5 of 6 patients at everolimus introduction and significantly decreased at the last follow-up (FU) in our cohort (P < 0.05). None of our patients achieved complete biochemical remission at the last FU and 3 of 6 admitted to have suboptimal adherence to therapy.
Conclusions:
Our data bring preliminary safety for the use of everolimus as a second-/third-line therapy in pediatric AIH. Although liver transaminases improved in our cohort, prospective studies are needed to determine if everolimus can induce long-term remission.
Keywords: autoimmune hepatitis, corticosteroids, everolimus, mTOR inhibitors, pediatric
What Is Known
New second-line therapies are needed in pediatric autoimmune hepatitis (AIH) refractory to conventional immunosuppressive therapy with prednisolone and azathioprine.
The use of mammalian target of rapamycin (mTOR) inhibitors has recently emerged in refractory AIH.
What Is New
Our work suggests that everolimus can be safely used in difficult-to-treat pediatric AIH.
INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disorder, which leads to progressive fibrosis and cirrhosis. Conventional immunosuppressive therapy consists in prednisolone and azathioprine, the latter being used alone as well for long-term maintenance after remission (1,2). Treatment alternatives such as mycophenolate mofetil (MMF, in replacement of azathioprine), budesonide, cyclosporine, or tacrolimus can be administered in the event of intolerable adverse effects or persistent disease activity despite standard therapy. The use of second-line therapies is based on case series reports and experience rather than on randomized controlled trials, which explains why the level of current guidelines recommendations about rescue therapies is low (1,3). The efficacy of those medications is variable in difficult-to-treat patients, and therefore, there is a need for developing new treatment alternatives.
The use of mammalian target of rapamycin (mTOR) inhibitors has recently emerged in refractory AIH (4). A central element in AIH pathogenesis is the loss of immunoregulation related to defective regulatory T cells (5,6). mTOR inhibitors sirolimus and everolimus were originally administered to prevent rejection in solid organ transplantation based on their antiproliferative properties on T cells (7). However, those drugs induce a selective proliferation of regulatory T cells, making them very promising tools in AIH treatment strategies (8,9). mTOR inhibitors also decrease dendritic cells and lymphocytes B maturation, result in a reduced antibodies production, and induce autophagy (10). Sirolimus and everolimus have related chemical structures but display different properties, including differences in pharmacokinetics and drug–target protein interactions (11). Very scarce and rather disappointing retrospective case reports have been published about sirolimus in refractory AIH. Two reports of five and two adult patients respectively showed sustained liver transaminases improvement in 2 of 5 and 0 of 2 patients, with side effects justifying sirolimus withdrawal in one patient (12,13). A pediatric report about sirolimus in AIH showed biochemical improvement in 2 of 4 patients (14). The only case series published about everolimus in difficult-to-treat AIH showed liver transaminases improvement in 6 of 7 adult patients (15). Those 6 patients had their corticosteroids doses reduced and 4 of them displayed complete biochemical response to everolimus. A follow-up (FU) liver biopsy was performed after 3–5 years of everolimus therapy in the 4 patients who responded to the treatment, showing histological stability in 2 patients and fibrosis reduction in the other 2. No major side effects were reported. To our knowledge, no data were ever published about everolimus in pediatric AIH.
Considering the lack of data about the use of mTOR inhibitors in pediatric AIH and the promising results obtained with everolimus in adults, our aim was to share our experience about everolimus as a second-/third-line therapy in pediatric AIH. We report 6 AIH/autoimmune sclerosing cholangitis (ASC) children who received everolimus due to poor response to other medications or to related adverse events.
METHODS
Inclusion Criteria
AIH patients who received everolimus (Certican; Novartis, Switzerland) therapy between 2014 and 2021 were retrospectively identified from the Pediatric Hepatology database of the Cliniques Universitaires Saint-Luc (Brussels, Belgium). The inclusion criteria were: diagnosis of definite AIH or ASC according to the ESPGHAN juvenile AIH/ASC scoring system (score ≥ 8, retrospectively applied), patients aged 0–18 at the time of everolimus introduction and pretransplant state (1). Everolimus was introduced in patients refractory to first-/second-line therapies or in patients who suffered from adverse events related to those medications. This research project was assessed and approved by the Biomedical Ethics Committee of the Université catholique de Louvain (Brussels, Belgium; no. 2020/04SEP/445).
Everolimus Regimen and Monitoring
Everolimus was introduced in replacement of azathioprine/MMF or in addition to other immunosuppressant, depending on the patients’ clinical and biochemical situations. Based on previous reports in pediatric liver transplantation, everolimus initial dose was 0.8–1.6 mg/m2/d (16,17). The initial dose was subsequently adapted according to clinical and biochemical response to achieve plasma trough levels of 3–6 ng/ml, as published in adult AIH (15). Everolimus plasma trough levels >2 ng/ml were tolerated in patients who reached biochemical stability and in patients with multiple immunosuppressive therapy to limit infectious side effects. Patients were submitted to regular monitoring of everolimus plasma trough levels to avoid drug toxicity and to assess the adherence. Side effects and disease evolution were investigated clinically and biochemically every 2 weeks at everolimus introduction. Once patients reached stability, clinical and biochemical controls were progressively reduced. Laboratory testing included liver and hematological parameters, kidney function, and blood glucose. Special attention was payed to the clinical and biological occurrence of everolimus most frequent side effects observed in the pediatric population: infections (upper respiratory tract, pneumonia, gastroenteritis, Epstein-Barr), diarrhea, vomiting, mouth ulceration, chronic cough, dermatitis, edema, dyslipidemia, proteinuria, and increase of hepatic enzymes (16,18).
Statistical Analysis
Statistical analysis was conducted using GraphPad Prism 5.0 (GraphPad Software; La Jolla, CA). Continuous variables were presented as mean ± standard deviation or as median (range). The Wilcoxon signed-rank nonparametric test was used to compare repeated measures between subgroups. A two-tailed P ≤0.05 was considered to indicate statistical significance for all analyses.
RESULTS
Description of the Study Population
Six AIH/ASC patients received everolimus therapy between 2014 and 2021. Clinical, biochemical, and histological characteristics of the study population at the time of diagnosis are summarized in Table 1. Three of the 4 AIH type 1 patients had positive pANCA, but showed no signs of sclerosing cholangitis in magnetic resonance cholangiograpy nor in liver biopsy. None of our patients had associated IBD. All patients displayed important fibrosis with incomplete/complete cirrhosis at diagnosis (Metavir score F3-F4). No biopsy was performed in our cohort during the course of everolimus therapy.
TABLE 1.
Characteristics of the study population at the time of AIH diagnosis
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| AIH type | Type 1 | Type 1 | Type 1 | Type 1 | ASC | ASC |
| Gender | F | F | F | F | M | F |
| Age (years) | 11 | 12 | 9 | 14 | 11 | 12 |
| Juvenile AIH/ASC score* | 9 | 11 | 11 | 10 | 12 | 11 |
| Autoantibodies | ANA | ANA | ANA | ANA | ANA | ANA |
| SMA | SMA | SMA | pANCA | SMA | SMA | |
| pANCA | pANCA | pANCA | pANCA | |||
| γ-globulins (g/L) (<15) | NA | 41.4 | 31.7 | 71.5 | 44.4 | 23.1 |
| ALT (UI/L) (<35) | 1520 | 95 | 1062 | 255 | 311 | 206 |
| AST (UI/L) (<35) | 1380 | 106 | 990 | 592 | 399 | 142 |
| GGT (UI/L) (<40) | 568 | 33 | 69 | 167 | 201 | 324 |
| Fibrosis (Metavir score) | F3 | F4 | F3 | F3-F4 | F3 | F3 |
| Other immune diseases | None | Coombs-positive hemolytic anemia | Celiac disease | Coombs-positive hemolytic anemia, AI pancreatitis | None | None |
*Definite AIH/ASC: score ≥ 8 (4).
AI = autoimmune; AIH = autoimmune hepatitis; ALT = alanine aminotransferase; ANA = antinuclear antibodies; ASC = autoimmune sclerosing cholangitis; AST = aspartate aminotransferase; GGT = gamma-glutamyl transferase; LKM-1 = anti-liver kidney microsome type 1 antibodies; NA = not available; pANCA = perinuclear antineutrophil cytoplasmic antibodies; SMA = antismooth muscle antibodies.
Everolimus Introduction
Conventional therapy with methylprednisolone and azathioprine had been tried in all patients before everolimus introduction, as well as budesonide (n = 1) and MMF (n = 4), the latter in replacement of azathioprine. Both ASC patients received ursodeoxycholic acid therapy from the moment of the diagnosis. The reason for everolimus introduction was poor response to first- and/or second-line medications in 4 patients requiring corticosteroids maintenance therapy (Table 2). Important side effects of corticosteroids/azathioprine or MMF justified the change of medication in the 2 other patients. Patient no. 2 suffered from diabetes secondary to high-dose methylprednisolone. She subsequently presented a cutaneous reaction and upper limb paresthesia that resolved at azathioprine withdrawal. Those side effects did not recur when azathioprine was added to everolimus therapy 2 years later. Patient no. 6 had intense abdominal pain that only resolved at MMF therapy interruption. Everolimus replaced azathioprine or MMF except for patients no. 3 and 4, who had everolimus added to standard therapy with methylprednisolone and azathioprine due to very poor control of the disease. Median FU duration under everolimus therapy was 28 months (range 8–46 months).
TABLE 2.
Everolimus introduction and follow-up
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Reason for EV introduction | Poor control | Side effects | Poor control | Poor control | Poor control | Side effects |
| Age at EV introduction (years) | 14 | 12 | 9 | 15 | 16 | 16 |
| FU under EV (months) | 30 | 46 | 15 | 8 | 27 | 28 |
| EV mean plasma trough levels (ng/ml) | 3.3 ± 0.8 | 3.1 ± 2 | 2.6 ± 0.5 | 1.5 ± 0.6 | 3.8 ± 3.4 | 5.8 ± 3.2 |
| EV side effects | No | No | No | No | No | Yes |
| Admitted adherence issues | No | Yes | No | Yes | Yes | No |
| Number of EV plasma trough levels < 2 ng/ml | 0/8 (0%) | 5/21 (24%) | 1/12 (8%) | 6/8 (75%) | 4/12 (33%) | 0/12 (0%) |
| Treatment before EV introduction | MMF, mPred | Aza | Aza, mPred | Aza, mPred | Aza, mPred | MMF, mPred |
| Treatment at EV introduction | EV, | EV | EV, | EV, | EV, | EV, |
| mPred | Aza, | Aza, | mPred | mPred | ||
| mPred | mPred | |||||
| Treatment at last FU | EV, | EV, | EV, | EV, | EV | EV, |
| Budes | Aza, | Aza | Aza, | mPred | ||
| Budes | mPred | |||||
| Steroid dosage during EV therapy (mg/kg/d) | 0.5 → 0.1 | 0 → 0.1 | 0.1 → 0 | 0.1 | 0.3 → 0 | 0.03 |
Aza = azathioprine; Budes = budesonide; EV = everolimus; FU = follow-up; MMF = mycophenolate mofetil; mPred = methylprednisolone; NA = not available.
Everolimus Monitoring and Side Effects
The mean everolimus plasma trough levels in our cohort was 3.4 ± 1.4 ng/ml. Three patients admitted to have suboptimal adherence to therapy and repeatedly displayed low/undetectable everolimus plasma trough levels (Table 2). During the first month following everolimus introduction, all patients were adherent according to their everolimus plasma trough levels. No clinical side effects were reported during the course of everolimus therapy when plasma trough levels were in the therapeutic range. Specifically, none of our patients suffered from severe/frequent infections, mouth ulcerations, chronic cough, or dermatitis. Patient no. 6 had an elevation of aspartate aminotransferase (AST) levels associated to high everolimus plasma trough levels (10.6 ng/ml) one month after everolimus introduction. Her AST levels improved shortly after adapting everolimus regimen. Everolimus was not associated to dyslipidemia, hyperglycemia, or renal function alteration/proteinuria in our cohort.
Corticosteroids During Everolimus Therapy
Methylprednisolone was stopped in 2 patients during the course of everolimus therapy: patient no. 3 due to biochemical stability and patient no. 5 in an attempt to improve the adherence to the rest of the treatment (Table 2). Two patients (no. 4 and 6) were receiving a well-tolerated low dose of methylprednisolone at everolimus introduction, which was unchanged to avoid subsequent relapse. Budesonide and azathioprine were added to the treatment of patient no. 2 due to insufficient disease control under everolimus alone and to previous diabetes secondary to methylprednisolone therapy. Finally, budesonide replaced methylprednisolone in patient no. 1 due to dependence to steroids.
Clinical and Biochemical Evolution During Everolimus Therapy
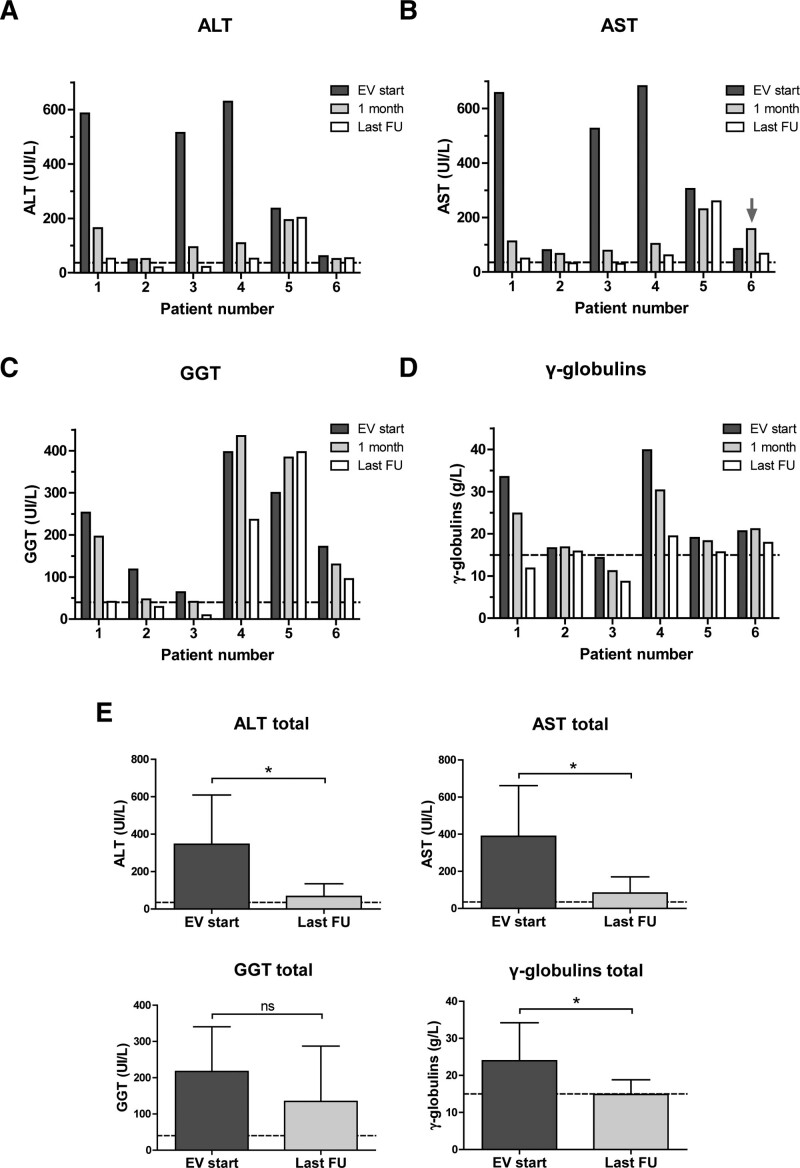
Four patients had very high liver transaminases plasma levels at everolimus introduction (between 7-fold and 20-fold elevation) and showed biochemical improvement within one month of therapy without normalization (2- to 5-fold elevation of alanine aminotransferase [ALT] after 1 month) (Fig. 1A, B). The other two patients (no. 2 and 6) had up to 3-fold elevation of transaminases when everolimus was introduced due to side effects related to other medications. The transaminases levels of patient no. 2 very slightly decreased after one month, while patient no. 6 had an initial elevation of AST levels associated to high everolimus plasma trough levels. Gammaglobulins levels evolution during everolimus therapy were variable and GGT levels tended to improve after one month except for patients no. 4 (AIH type 1) and 5 (ASC) (Fig. 1C, D).
FIGURE 1.
Evolution of biochemical parameters during the course of everolimus therapy. A) ALT plasma levels. Normal levels < 35 UI/L (discontinued line); (B) AST plasma levels. Normal levels < 35 UI/L (discontinued line). The grey arrow shows the AST elevation that was associated to high everolimus plasma levels; (C) GGT plasma levels. Normal levels < 40 UI/L (discontinued line); (D) γ-globulins plasma levels. Normal levels < 15 g/L (discontinued line); (E) Evolution of the whole cohort biochemical parameters between everolimus introduction and the last FU. Discontinued lines represent the upper limit of normal levels for each parameter. Data are presented as mean ± standard deviation; *P < 0.05. ALT = alanine aminotransferase; AST = aspartate aminotransferase; EV = everolimus; FU = follow-up; GGT = γ-glutamyl transferase; ns = not significant.
At last FU, all but one patient had a marked decrease (n = 3) or normalization (n = 2) of ALT levels (Fig. 1A). The remaining patient with ASC (patient no. 5) had no biochemical improvement in the context of poor adherence to therapy. When we considered the whole cohort, ALT, AST, and gammaglobulins levels significantly improved at the last FU compared to the levels observed at everolimus introduction (P < 0.05) (Fig. 1E). Four patients still displayed moderately elevated gammaglobulins at last FU (maximum 1.3-fold increase), and none of our patients achieved a persistent normalization of autoantibodies titers during FU. Clinically, all patients were in remission except for patient no. 5 who still presented intense fatigue.
DISCUSSION
We report 6 AIH/ASC patients aged 9–16 years, who received everolimus as a rescue therapy due to disease poor control despite first-/second-line medications or to intolerable side effects. No adverse events were reported in our cohort when everolimus plasma trough levels were in the therapeutic range. Everolimus improved liver transaminases in our patients without inducing biochemical remission.
Plasma monitoring of everolimus was regularly performed to avoid drug toxicity and to verify the adherence. None of our patients suffered from clinical/biochemical adverse events associated to everolimus when plasma trough levels were in the therapeutic range. In particular, no dyslipidemia, proteinuria, frequent/severe infections, mouth ulcerations, chronic cough, or dermatitis were reported in our cohort. Everolimus plasma trough levels were kept in the inferior limit of the therapeutic range in patients receiving multiple immunosuppressive therapy, to limit infectious side effects. This might have contributed to the very low occurrence of clinical/biochemical adverse events associated to everolimus in our cohort. Three of our 6 patients admitted to have suboptimal adherence and displayed a high variability in everolimus plasma trough levels during FU. However, those patients did not suffer from any side effects when adherence was good and everolimus plasma trough levels were in the therapeutic range. We observed that adherence seemed to be better when patients were seen more regularly due to therapeutic changes or poor disease control. Based on this observation, a systematic closer FU in nonadherent patients could maybe help to improve adherence issues. Nonadherence remains a major problem in the management of juvenile AIH and made the assessment of everolimus efficacy as a long-term remission inducer even more challenging in our cohort (19).
Liver transaminases improved in 5 of 6 patients within 1 month of everolimus introduction, when adherence was good in all patients. The last patient had initial elevation of AST associated to high trough levels of plasma everolimus, which resolved once everolimus dosage returned in the target range. At the last FU, 5 of 6 patients showed liver transaminases improvement, of which 2 AIH type 1 patients displayed ALT complete normalization. The last ASC patient had insignificant biochemical improvement in the context of adherence issues, and still suffered from important fatigue at the last FU. When we considered our whole cohort, ALT, AST, and gammaglobulins levels improved between everolimus introduction and the last FU. Still, existing guidelines define biochemical remission as the normalization of liver transaminases and γ-globulins levels, in the presence of low or negative autoantibodies titles (1, 20). According to this definition, none of our patients achieved biochemical remission at the last FU. No FU biopsies were performed during the course of everolimus therapy in our cohort.
Important limitations of our study are the retrospective design and the small number of included patients. The retrospective study design and the complexity of the clinical cases explain that the patients’ therapeutic regimens varied during the course of everolimus therapy. Those differences must be noted as they limit the ability to evaluate the efficacy of everolimus alone. In addition, our cohort includes AIH type 1 and ASC patients, offering interesting information but further limiting the number of patients for each subtype. ASC patients have poorer prognosis as compared to AIH type 1 due to the progression of biliary lesions despite immunosuppressive therapy (21,22). Our AIH type 1 patients seemed to have a better biochemical response to everolimus as compared to ASC, but larger studies are needed to corroborate those preliminary results. The mean FU duration of patients receiving everolimus therapy was 28 months in our cohort, which allowed us to obtain appreciable information about the clinical and biochemical evolution of our patients. Due to the rarity of the condition and the lack of published data about everolimus in pediatric AIH, we believe that our small case series report is a substantial contribution to the existing literature.
In conclusion, we present the first pediatric report about everolimus in difficult-to-treat AIH type 1/ASC patients. Liver transaminases improved in 5 of 6 patients following everolimus introduction and significantly decreased at the last FU. Everolimus was safe when plasma trough levels were in the therapeutic range, but did not allow to achieve biochemical remission in our cohort. Our results bring preliminary safety for the use of everolimus as a second-/third-line therapy in pediatric AIH, and open the way to a proper controlled trial.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Mieli-Vergani G, Vergani D, Baumann U, et al. Diagnosis and management of pediatric autoimmune liver disease: ESPGHAN hepatology committee position statement. J Pediatr Gastroenterol Nutr. 2018;66:345–360. [DOI] [PubMed] [Google Scholar]
- 2.Johnson PJ, McFarlane IG, Williams R. Azathioprine for long-term maintenance of remission in autoimmune hepatitis. N Engl J Med. 1995;333:958–963. [DOI] [PubMed] [Google Scholar]
- 3.Mack CL, Adams D, Assis DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the american association for the study of liver diseases. Hepatology. 2020;72:671–722. [DOI] [PubMed] [Google Scholar]
- 4.Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: standard treatment and systematic review of alternative treatments. World J Gastroenterol. 2017;23:6030–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mieli-Vergani G, Vergani D, Czaja AJ, et al. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. [DOI] [PubMed] [Google Scholar]
- 6.Ferri S, Longhi MS, De Molo C, et al. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52:999–1007. [DOI] [PubMed] [Google Scholar]
- 7.Neuhaus P, Klupp J, Langrehr JM. mTOR inhibitors: an overview. Liver Transpl. 2001;7:473–484. [DOI] [PubMed] [Google Scholar]
- 8.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. [DOI] [PubMed] [Google Scholar]
- 9.Gedaly R, De Stefano F, Turcios L, et al. mTOR inhibitor everolimus in regulatory T Cell expansion for clinical application in transplantation. Transplantation. 2019;103:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klawitter J, Nashan B, Christians U. Everolimus and sirolimus in transplantation-related but different. Expert Opin Drug Saf. 2015;14:1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatrath H, Allen L, Boyer TD. Use of sirolimus in the treatment of refractory autoimmune hepatitis. Am J Med. 2014;127:1128–1131. [DOI] [PubMed] [Google Scholar]
- 13.Rubin JN, Te HS. Refractory autoimmune hepatitis: beyond standard therapy. Dig Dis Sci. 2016;61:1757–1762. [DOI] [PubMed] [Google Scholar]
- 14.Kurowski J, Melin-Aldana H, Bass L, et al. Sirolimus as rescue therapy in pediatric autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 2014;58:e4–e6. [DOI] [PubMed] [Google Scholar]
- 15.Ytting H, Larsen FS. Everolimus treatment for patients with autoimmune hepatitis and poor response to standard therapy and drug alternatives in use. Scand J Gastroenterol. 2015;50:1025–1031. [DOI] [PubMed] [Google Scholar]
- 16.Ganschow R, Ericzon BG, Dhawan A, et al. Everolimus and reduced calcineurin inhibitor therapy in pediatric liver transplant recipients: results from a multicenter, prospective study. Pediatr Transplant. 2017;21. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen D, Briem-Richter A, Sornsakrin M, et al. The use of everolimus in pediatric liver transplant recipients: first experience in a single center. Pediatr Transplant. 2011;15:510–514. [DOI] [PubMed] [Google Scholar]
- 18.Dumortier J, Couchonnal E, Lacaille F, et al. mTOR inhibitors in pediatric liver transplant recipients. Clin Res Hepatol Gastroenterol. 2019;43:403–409. [DOI] [PubMed] [Google Scholar]
- 19.Kerkar N, Annunziato RA, Foley L, et al. Prospective analysis of nonadherence in autoimmune hepatitis: a common problem. J Pediatr Gastroenterol Nutr. 2006;43:629–634. [DOI] [PubMed] [Google Scholar]
- 20.Manns MP, Czaja AJ, Gorham JD, et al. ; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. [DOI] [PubMed] [Google Scholar]
- 21.Gregorio GV, Portmann B, Karani J, et al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544–553. [DOI] [PubMed] [Google Scholar]
- 22.Jannone G, Stephenne X, Castanares-Zapatero D, et al. Fibrosis and inflammation histology scores predict disease remission in pediatric autoimmune hepatitis. JSM Hepatitis. 2017;2:1006. [Google Scholar]