Supplemental Digital Content is available in the text.
Keywords: hepatic hemangiomas, cutaneous hemangiomas, consumptive hypothiroidsm
Introduction:
Consumptive hypothyroidism (CH) is a rare and potentially overlooked complication of hepatic hemangiomas (HH) overexpressing the enzyme deiodinase, which converts thyroxine (T4) to reverse triiodothyronine (rT3).
Materials and methods:
Here, we report a case series of 3 patients and a systematic review of the literature.
Results:
Hypothyroidism (mean serum TSH 52.03 mIU/L) was detected at a mean age of 4.6 months (range 3–6) in 3 infants with infantile hepatic hemangiomas, treated with thyroxine (mean dose 12 µg/kg/day). All received treatment with propranolol (1–3 mg/kg/day) from the mean age of 4 months. Hormonal treatment was stopped at a mean age of 20 months (range 12–30). Hypothyroidism reoccurred in a patient concurrently with the increase of liver lesions, requiring liver transplantation (LT) at age 39 months.
Literature review retrieved 42 studies (48 patients): HH (n = 43) were isolated in 24 infants and associated with cutaneous hemangiomas in 19. Hemangiomas were only cutaneous in 5.
In the first 43 patients, hypothyroidism was detected at a mean age of 1 month; 21 of 43 patients were prescribed propranolol alone (n = 8) or associated with other medicaments (n = 13); 2 of 43 patients underwent LT. Hormonal treatment consisted of T4 in 35 of 43 patients and T3 in 10.
CH associated with only cutaneous and extrahepatic visceral hemangiomas (n = 5), detected at a mean age of 7 months (TSH mean levels at diagnosis of 150.3 mIU/L). Three of 5 patients received treatment with propranolol ± other medicaments. All 5 patients were treated with T4.
Conclusions:
Periodical thyroid function assessment is necessary in patients with hepatic hemangiomas, particularly when lesions’ size and number increase rapidly.
What Is Known
Consumptive hypothyroidism (CH) is a rare form of hypothyroidism due to thyroid hormone inactivating enzyme type 3 (Deiodinase) overexpressed by hepatic/hepatic and cutaneous hemangiomas, and occasionally by some otherextrahepatic visceral hemangiomas.
Early recognition and substitutive hormonal treatment are important in terms of neurocognitive outcome.
Medical treatment for the hemangiomas and reduction in tumor burden leads to CH resolution in most cases.
What Is New
Pediatric hepatologists should recognize the importance of periodicalassessments of thyroid function in patients with hepatic hemangiomas
INTRODUCTION
Hepatic hemangiomas (infantile and congenital) are benign vascular tumors affecting 4% to 5% of Caucasian infants (1). These are classified into unifocal, multifocal, and diffuse (2). Infantile hepatic hemangiomas (IHH), characterized by glucose transporter 1 (glut-1) cytoplasmic immunostaining positivity, proliferate after birth from newborn period until 6–12 months of age with gradual involution until 3–9 years of age (3). Possible complications are high-output cardiac failure, liver failure, abdominal compartment syndrome, failure to thrive, and acquired consumptive hypothyroidism (CH). CH is a rare form of hypothyroidism resulting from overexpression of Thyroid Hormone Inactivating Enzyme type 3 (deiodinase) by vascular endothelium, resulting in conversion of thyroxine (T4) to reverse triiodothyronine (rT3) and T3 to diiodothyronine. CH is mainly associated with hepatic, rarely cutaneous hemangiomas, and some other tumors. It was first described in 2000 by Huang et al, in a 6-week-old infant with multiple hepatic hemangiomas (4). Afterwards, other authors described this form of hypothyroidism associated with hepatic hemangiomas, and a direct relationship between CH, tumor size, and D3 activity has been hypothesized. The expression of D3 (deiodinase type 3) in hemangioma is induced by basic fibroblast growth factor and vascular endothelial growth factor. Its activity increases paralleling the increase of tumor size regardless of its location as demonstrated by the normalization of rT3 levels after (medical or surgical) treatment of the lesion (5).
From the earlier, it follows that the gold standard for the diagnosis of CH is the demonstration of D3 activity on tumor tissue, but biopsy is rarely performed in patients with hepatic hemangiomas due to the risks associated with the procedure. CH has also been associated with extrahepatic hemangiomas, such as cutaneous and parotid hemangiomas, and benign neonatal hemangiomatosis.
Here, we report a case series of 3 patients: 2 affected by multifocal IHH and 1 with diffuse IHH, all complicated with CH. We also systematically reviewed previous pediatric cases reported in the literature.
CASE SERIES
Patient 1
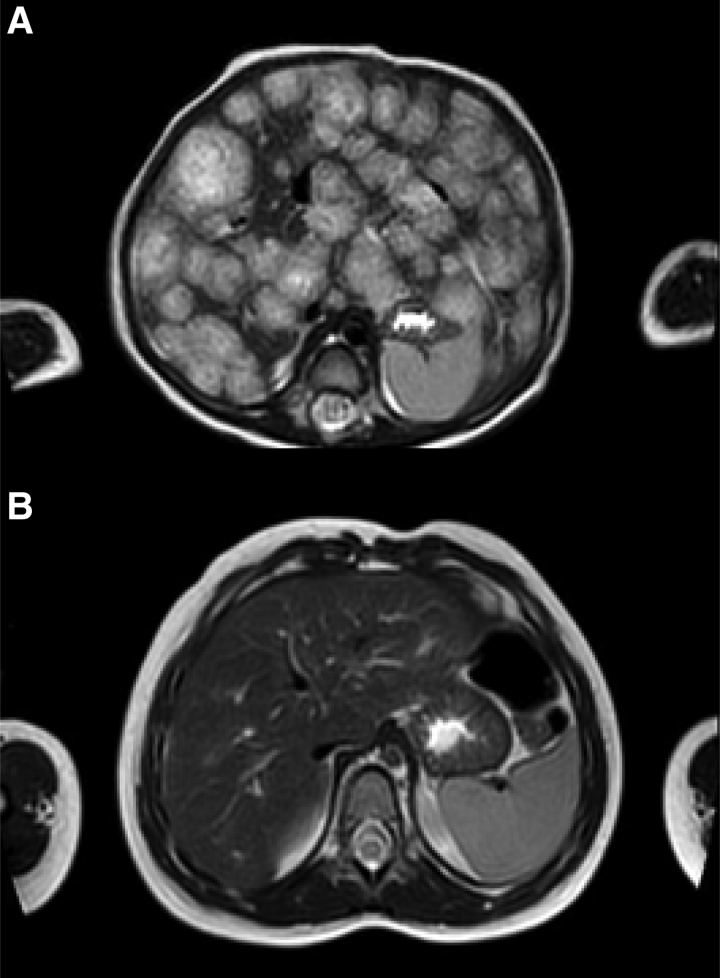
A full-term female infant presented at 3 months of age with abdominal distension and poor feeding. Extended neonatal screening (ENS) was reported normal. A little cutaneous hemangioma was present on the forehead. Abdominal distension was noted on examination, with the liver edge palpable 2 to 3 cm below the costal margin. Ultrasound revealed an enlarged liver with multiple contextual pseudonodular macro areas (maximum diameter = 3 cm). Magnetic resonance imaging (MRI) was indicative of hepatic multifocal hemangiomas. Liver function tests (LFTs) were normal. Thyroid US was normal. Thyroid-stimulating hormone (TSH) values were markedly elevated at 58.7 mIU/L. She was therefore treated with good clinical response for severe hypothyroidism with levothyroxine (10 µg/kg/day) suspended at the 13th month of life, and propranolol (1–3 mg/kg/day), stopped at 22 months. At 4 years of age, she had normal development; control MRI, after 19 months of treatment with propranolol and after 10 months of treatment with levothyroxine, showed absent liver hemangiomas (Fig. 1A,B).
FIGURE 1.
MRI of the abdomen in one of our patients (patient 1), before (A) and after (B) 19 months of treatment with propranolol/10 months of treatment with levothyroxine. The T2-weighted axial MRI images shows the regression of a diffuse infantile hepatichemangioma with innumerable T2 hyperintense masses throughout the liver with central hypointense central regions. MRI = magnetic resonance imaging.
Patient 2
M. is a preterm male infant, born at 26 weeks after twin pregnancy. During the hospitalization in the Neonatal Intensive Care Unit (NICU), multiple cutaneous hemangiomas appeared (n = 6, of which 3 on the trunk and 3 in the lower limbs; the largest in the right thigh measuring 1 × 2 cm). Extended neonatal screening (ENS) and LFTs was reported normal. He was diagnosed with hypothyroidism (TSH: 36,700 mIU/ml) and started treatment with levothyroxine (12 µg/kg/day) at 1 month of life. He was evaluated at 5 months in a dermatology clinic for his cutaneous hemangiomas, and abdominal ultrasound revealed an enlarged liver with hepatic multifocal hemangiomas. Thyroid US was normal. He started propranolol therapy (1–2 mg/kg/day) until the hepatic picture was normalized after 7 months (control US). He was weaned off levothyroxine progressively with reduction to 5 µg/kg/day until discontinuation at the age of 1 year and 9 months and is currently on no medications since 3 years, with normal neurological development.
Patient 3
Second child, born at term from normal pregnancy, ENS reported normal, was hospitalized at the age of 6 months for evidence of pallor and abdominal distension, referable to massive hepatomegaly, associated with thrombocytopenia and coagulopathy (Kasabath-Merritt syndrome). The ultrasound examination revealed multiple expansive nodules located in the II–III, VII–VIII, and VI segments, subsequently diagnosed as IHH at liver biopsy. Thyroid US was normal. Liver mass was first treated with steroids, vincristine, with tendency to regression. At that time, TSH was found markedly high at 60.7 mIU/L, requiring treatment for severe hypothyroidism with levothyroxine (15 µg/kg/day). After clinical improvement, he was switched to propranolol at the age of 9 months (3 mg/kg/day). The good clinical response allowed to stop hormone therapy at age 24 months. However, hypothyroidism reoccurred at the age of 32 months together with the enlargement of 2 liver nodules and no longer responsive to medical treatment. A new laparoscopic biopsy of the liver confirmed the diagnosis of Glut-1 positive IHH. However, because of the risk of malignancy reported in previous cases of IHH relapsing (6, 7), we referred the child to a Liver Transplant Center. At the age 3 years, the patient underwent liver transplantation (LT), having considered the risk of developing hepatic angiosarcoma (8). Liver histology confirmed the diagnosis of IHH but revealed some foci of Kaposiform hemangioendotelioma. After LT, he stopped levothyroxine therapy due to the complete normalization of the thyroid profile. He presented mild developmental delay, probably related to spectrum autism disorder.
LITERATURE REVIEW
We searched within the PubMed academic medical database. Search strategy was formulated around terms for “Consumptive hypothyroidism” and “Hemangioma AND Hypothyroidism.” Systematic search of the databases of literature was performed with no language or data restrictions. To be eligible for inclusion, studies had to describe a case of consumptive hypothyroidism associated with hemangioma in children. Study details and quality characteristics were independently extracted by two of the authors for all the articles and in a stepwise approach, first by reading the title, then by reviewing the abstract, and finally by revising the full text, where appropriate (Figure S1 http://links.lww.com/PG9/A96). At the end of revision, 42 studies were selected including 48 patients.
CH was found in infants with “multifocal/diffuse hepatic hemangiomas” (24 cases), and “hepatic and cutaneous hemangiomas” (19 cases). Hypothyroidism was detected at a mean age of 1 month. Mean levels of TSH at diagnosis were in the range 15–475 mIU/L. Neonatal screening, showed CH in 7 cases, normal results in 13, in the remaining cases, it was not reported. Among these 43 patients, 8 patients received treatment with propranolol, 13 propranolol in combination with other medicaments, and 21 did not received propranolol. Two patients underwent liver transplantation; 35 of 43 patients were treated with T4 and 10 with T3 (Table 1).
Literature review also found CH is associated with nonhepatic hemangiomas: parotid hemangioma (2 cases), benign neonatal hemangiomatosis (1 patient), internal acoustic meatus hemangioma and cutaneous hemangioma (1 patient), large (12 × 10 cm) cutaneous hemangioma (1 patient). CH was associated with only cutaneous and extrahepatic visceral hemangiomas, detected at a mean age of 7 months (TSH mean levels at diagnosis of 150.3 mIU/L); 3 of 5 patients received treatment with propranolol ± other medicaments, all 5 patients were treated with T4 (Table 2).
DISCUSSION
CH is a rare form of hypothyroidism (to date, more than 40 cases of CH secondary to HH have been reported). It caused by the high expression of the D3 isoenzyme by the vascular tumor, which transforms thyroid hormones into inactive form during the proliferative phase of IHH (4).
The etiology of elevated D3 activity in IHH is not fully known. It was assumed that endothelial cells in infantile hemangiomas and placenta share some immunohistochemical markers (50), including GLUT1, specific marker of IHH (51), and it has been proposed that IH could be derived from placental angioblasts sharing similar characteristics such as high D3 activity and self-limited growth (31). Generally, CH has poor response to the usual doses of levothyroxine(10-15/kg/day in infants with congenital hypothyroidism) (52). T3 is useful in case of severe consumptive hypothyroidism. When hypothyroidism is very severe, large doses of thyroid hormone are necessary to normalize the T4 level. Since T4 is rapidly converted to the inactive rT3 form, combined therapy with T3 may be necessary (53).
Our cases of hepatic hemangiomas complicated by CH were multifocal in two and diffuse in one of three patients. In the first two infants early medical management led to good hepatic outcomes, while in the third a liver transplant was required. Noteworthy, differently from the first 2 cases, patient 3 had not cutaneous hemangiomas.
Current guidelines recommend screening with abdominal ultrasonography in the presence of 5 or more cutaneous hemangiomas because risk of IHH increases along with increasing numbers of cutaneous lesions (54). However, IHH can also be found in the presence of one or no cutaneous hemangioma as seen in our patients 1 and 3, suggesting the importance of abdominal US for all children with any cutaneous lesions and systemic compromise. In this regard, difficulties in feeding and abdominal distension can be red flags and require further investigation.
Although neonatal screening was normal in all three patients and congenital hypothyroidism was excluded; however, a severe form of hypothyroidism was detected on thyroid function test assessment. The level of rT3 could be useful in cases nonresponder to T4 treatment; however, it is not always feasible in clinical practice. We did not measure the level of rT3 nor the activity of tissue D3 (due to the risk of bleeding from biopsy), but the diagnosis of consumption hypothyroidism associated with the presence of IHH was inferred from the high TSH presenting values, the poor response to the usual doses of levothyroxine and the gradual normalization of the thyroid profile concomitant with the reduction of the hemangiomatous mass. This is consistent with the first cases described by Huang et al. (4) and also with data of our systematic review (Table 1). Literature search also found that CH may be occasionally observed in some patients with cutaneous hemangiomas only and extrahepatic visceral hemangiomas (Table 2).
TABLE 1.
Summary of the systematic review of the literature with studies reporting CH in patients with hepatic hemangiomas
| References | Sex, age | Hemangioma | TSH onset mU/L | Neonatal screening congenital hypot. | Treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Size (cm) | Description | Lev. | Lio. | Propr. | Other | ||||
| Joshi et al (9) | F, 3 m | HH | N.R. | Diffuse | 75 | N.R. | Yes | Yes | Yes | Prednisolone + IFNα |
| Kim et al (10) | M, 1 m | IHH | N.R. | Diffuse | 100 | Normal | Yes | No | Yes | Prednisolone |
| Macchiaiolo et al (11) | F, 2 m | HH+ CuH | N.R. | Diffuse | 15 | N.R. | No | No | Yes | No |
| Verma et al (12) | F, 4 m | HH+1CuH | 2.6 × 4.8 | Multiple | 17.5 | CH | Yes | No | Yes | No |
| Osada et al (13) | M, 4 m | IHH+ 1subCuH | N.R., 2 | Multiple | 561 | Normal | Yes | Yes | Yes | No |
| Acharya et al (14) | F, 20 d | HHE+ CuH | 1- 3,N.R. | Multiple | 100 | N.R. | Yes | No | Yes | Prednisolone |
| Simsek et al (15) | M, 4 m | IHH+ 1 CuH | N.R., <0.5 | Multiple | 177 | Normal | Yes | No | Yes | Methyl-pr. |
| Al-Ghamdi et al (16) | M, 2 m | HH | N.R. | Multiple | 281 | N.R. | Yes | No | Yes | No |
| Campbell et al (17) | F, 11 d | HH+3 CuH | N.R. | Multiple | 115.4 | N.R. | Yes | No | Yes | No |
| Takai et al (18) | M, 6 d | HHE | N.R. | Diffuse | 54.7 | N.R. | N.R | No | Yes | Prednisolone |
| Weber P. et al (19) | F, 7 m | HHE | N.R. | Diffuse | 24 | N.R. | Yes | Yes | No | VCR + Steroids |
| Nguyen et al (20) | M, 2 m | HH + 5 CuH | N.R. | Multiple | 54 | Normal | No | No | Yes | No |
| Higuchi et al (21) | M, 11 m | HH | 4 × 2.5 | Multifocal | 17.7 | Normal | No | Yes | Yes | No |
| Al Tasseh et al (22) | M, 3.5 m | HH+1CuH | 0.5-0.42, 1.5 × 1.5 |
Diffuse | 220 | N.R. | Yes | No | Yes | No |
| Varrasso et al (23) | F, 4 m | HH | N.R. | Diffuse | 69.96 | N.R. | Yes | No | Yes | Predn. |
| Wasserman et al (24) | F, 49 d | HH+1CuH | N.R., 0.15 | Diffuse | 123 | Normal | Yes | Yes | Yes | Predn. +VCR |
| Wijeratne et al, 2014(25) | F, 36 d | HHE+ CuH | >0.25 | Multiple | 37.6 | Normal | Yes | No | Yes | Prednisolone |
| Sun et al (26) | F, 21 d | HH+ CuH | N.R. | Multiple | nr | N.R. | Yes | No | Yes | Steroids |
| Vergine et al (27) | F, 2 m | HH + subCuH | N.R. | Multiple | 21 | Normal | Yes | No | Yes | Predn. + VCR+ Cycloph. |
| Imteyaz et al (28) | F, 4 m | HH | N.R | Multiple | 14.2 | N.R | N.R. | No | No | Prednisone |
| Emir et al (29) | F, 15 d | HH+ 1 CuH | 3 × 3.5 2 × 1 |
Multiple | 74.2 | CH | Yes | No | Yes | Methyl-pr. |
| Jassam et al (30) | M, 56 d | HHE | N.R. | Multiple | 138 | Normal | Yes | No | No | Prednisolone + IFNα + HA ligation |
| Yeh et al (31) | F, 42 d | HH+ CuH | N.R. | Diffuse | 68 | N.R. | Yes | No | No | Methyl-pr.+ pred. +VCR |
| M, 21 d | N.R. | 19.8 | N.R. | No | No | No | Prednisolone +VCR + Cycloph. | |||
| M, 35 d | N.R. | 31.4 | N.R. | Yes | No | Yes | Methyl-pr. | |||
| F, 14 d | N.R. | 55.8 | N.R. | Yes | No | Yes | Methyl-pr.+Prednisolone+VCR | |||
| Bessho et al (32) | F, 4 m | HH | N.R. | Diffuse | 42.5 | CH | Yes | No | No | Prednisolone + IFNα+SG |
| Çetinkaya et al (33) | M, 28 d | HH | 3 | Multiple | 150 | CH | Yes | Yes | No | Methyl-pr. + IFN α |
| Peters et al (34) | M, 28 d | HH+3CuH | N.R. | Multiple | 66.2 | CH | Yes | Yes | No | VCR+Dexa. |
| Mouat et al (35) | F, 21 d | HHE | N.R. | Massive | 17 | Normal | Yes | No | No | Predn. |
| Cho et al (36) | M, 10m | HH | N.R. | Multiple | 18.98 | Normal | Yes | No | No | Prednisolone |
| Kalpatthi et al, 2007(37) | M, 4 m | HH+ CuH | N.R. | Multiple | 53.3 | Normal | Yes | No | No | Prednisolone |
|
Lee et al (38) Balazas et al (39) |
F, 42 d | HHE | N.R. | Multiple | 182 | Normal | Yes | Yes | No | Predn.+ IFNα +Hydroc. +VCR +Cytoxan+ LT |
| Güven et al (40) | F, 3 m | HHE | N.R. | Multiple | 100 | N.R. | Yes | Yes | No | Methyl-pr. |
| Ho et al (41) | F, 3 m | HHE | N.R. | Multiple | 90 | N.R. | Yes | Yes | No | Predn. |
| Konrad et al (42) | M, 63 d | HH | N.R. | Multiple | 100 | Normal | Yes | No | No | No |
| Ayling et al (43) | F, 56 d | HHE | N.R. | Multiple | 220 | N.R. | No | No | No | No |
| M, 10 d | HHE | N.R. | Multicentric | 35 | CH | Yes | No | No | LT | |
| M, 42 d | HHE | N.R. | N.R. | 200 | CH | Yes | No | No | HA ligation | |
| F, 4 m | HHE | N.R. | N.R | 475 | N.R. | Yes | No | No | HA ligation | |
| F, 4 m | HHE | N.R. | N.R | 16 | N.R. | N.R. | No | N.R. | HA ligation | |
| Mason et al (44) | F | HH | N.R. | Multiple | 155.9 | N.R. | Yes | No | No | Methyl-pr.+embolization |
| Huang et al (4) | M, 42 d | Hepatic | N.R. | Multiple | 156 | N.R. | Yes | Yes | No | Prednisolone + IFNαHA ligation |
CuH = cutaneous hemangioma; Cycloph. = cyclophosphamide; d. = days; Dexa. = dexamethasone; F. = female; HA = hepatic artery; HH = hepatic hemangioma; HHE = hepatic hemangio-endothelioma; IFNa = interferon alpha; Hydroc. = hydrocortisone; Hypot. = hypothyroidism; IHH = infantile hepatic hemangioma; Levo = levothyroxine; Lio. = liothyronine; LT = liver transplantation; M. = male; m. = months; Methyl-pr. = methyl-prednisolone; N.R. = not reported; Pred. = prednisone; Prop. = propranolol; SG = surgical resection; VCR = vincristine.
TABLE 2.
Summary of the systematic review of the literature with studies reporting CH in patients with extra-hepatic hemangiomas
| References | Sex, age | Hemangioma | TSH onset mU/L | Neonatal screening | Treatment | ||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Size (cm) | Lev. | Lio. | Propr. | Other | ||||
| Igarashi et al (45) | M, 26 d | I.A.M. + CuH | 1 cm 44 × 22 × 7 mm |
15.32 | Normal | Yes | No | Yes | Laser-therapy |
| Chakraborty et al (46) | M, 2.5 y | CuH | 12 × 10 cm | 76 | N.R. | Yes | N.R. | N.R. | N.R. |
| De corti et al (47) | M, 48 d | Parotid IH | 4cm | 8.28 | Normal | Yes | No | Yes | No |
| Metwalley et al (48) | M, 8 m | BNH | 5- 10 mm | 176 | Normal | Yes | No | No | No |
| Vigone et al (49) | F, 7 d | Parotid IH | N.R. | 476 | + for CH | Yes | No | Yes | Corticosteroid |
+ = positive; BNH = Benign neonatal hemangiomatosis; CH = congenital hypothyroidism; CuH = cutaneous hemangioma; d. = days; F. = female; I.A.M. = internal acoustic meatus; Levo = levothyroxine; Lio. = liothyronine; M. = male; m. = months; N.R. = not reported; Prop. = propranolol.
CONCLUSIONS
CH should be investigated in all patients with IHH and possibly also in those with large-sized cutaneous hemangiomas and extrahepatic visceral hemangiomas. Thyroid function should be periodically assessed particularly when the size and number of hemangiomas increase rapidly. Early substitutive hormone treatment may avoid lower neurocognitive outcome, which is likely in those infants with more severe hypothyroidism. In addition, future studies should investigate the incidence and effects of propranolol treatment on thyroid dysfunction. On the other hand, an acquired infantile hypothyroidism, not recalled at neonatal screening, can hide an unrecognized hepatic hemangioma. So, unexplained hypothyroidism could represent a red flag for an underlying IHH. This hypothesis requires other studies to be supported.
ETHICS STATEMENT
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
ACKNOWLEDGMENTS
We are grateful to prof. Pietro Vajro for advice during the study and writing.
Supplementary Material
Footnotes
The authors report funding and no conflicts of interest.
Supplemental digital content is available for this article.
REFERENCES
- 1.Wassef M, Blei F, Adams D, et al. ; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:e203–14. [DOI] [PubMed] [Google Scholar]
- 2.Christison-Lagay ER, Burrows PE, Alomari A, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62–7; discussion 67. [DOI] [PubMed] [Google Scholar]
- 3.Iacobas I, Phung TL, Adams DM, et al. Guidance document for hepatic hemangioma (infantile and congenital) evaluation and monitoring. J Pediatr. 2018;203:294–300.e2. [DOI] [PubMed] [Google Scholar]
- 4.Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–9. [DOI] [PubMed] [Google Scholar]
- 5.Luongo C, Trivisano L, Alfano F, et al. Type 3 deiodinase and consumptive hypothyroidism: a common mechanism for a rare disease. Front Endocrinol (Lausanne). 2013;4:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann O, Fabre M, Franchi S, et al. Widening spectrum of liver angiosarcoma in children. J Pediatr Gastroenterol Nutr. 2011;53:615–9. [DOI] [PubMed] [Google Scholar]
- 7.Rutten C, Ladarre D, Ackermann O, et al. Spontaneous evolution patterns of focal congenital hepatic hemangiomas: a case series of 25 patients. Pediatr Radiol. 2022;52:1048–60. [DOI] [PubMed] [Google Scholar]
- 8.Grassia KL, Peterman CM, Iacobas I, et al. Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer. 2017;64:e26627. [DOI] [PubMed] [Google Scholar]
- 9.Joshi K, Bolia R, Poddar U, et al. Consumptive hypothyroidism due to diffuse hepatic hemangiomas treated with propranolol therapy. Indian Pediatr. 2020;57:366–8. [PubMed] [Google Scholar]
- 10.Kim YH, Lee YA, Shin CH, et al. A Case of Consumptive Hypothyroidism in a 1-Month-Old Boy with Diffuse Infantile Hepatic Hemangiomas. J Korean Med Sci. 2020;35:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macchiaiolo M, Markowich AH, Diociaiuti A, et al. Diffuse infantile hepatic hemangiomas in a patient with Beckwith-Wiedemann syndrome: a new association? Am J Med Genet A. 2020;182:1972–6. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Jain R, Babbar N, et al. Multiple infantile hepatic hemangiomas leading to consumptive hypothyroidism successfully treated with propranolol: a case report. J Family Med Prim Care. 2020;9:5759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osada A, Araki E, Yamashita Y, et al. Combination therapy of propranolol, levothyroxine, and liothyronine was effective in a case of severe consumptive hypothyroidism associated with infantile hepatic hemangioma. Clin Pediatr Endocrinol. 2019;28:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acharya S, Giri PP, Das D, et al. Hepatic hemangioendothelioma: a rare cause of congenital hypothyroidism. Indian J Pediatr. 2019;86:306–7. [DOI] [PubMed] [Google Scholar]
- 15.Simsek E, Demiral M, Gundoğdu E. Severe consumptive hypothyroidism caused by multiple infantile hepatic haemangiomas. J Pediatr Endocrinol Metab. 2018;31:823–7. [DOI] [PubMed] [Google Scholar]
- 16.Al-Ghamdi AH. Consumptive hypothyroidism in a two month old infant secondary to hepatic haemangiomas. Sudan J Paediatr. 2018;18:71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell V, Beckett R, Abid N, et al. Resolution of consumptive hypothyroidism secondary to infantile hepatic hemangiomatosis with a combination of propranolol and levothyroxine. J Clin Res PediatrEndocrinol. 2018;10:294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai A, Iehara T, Miyachi M, et al. Successful treatment of a hepatic-hemangioendothelioma infant presenting with hypothyroidism and tetralogy of Fallot. PediatrNeonatol. 2018;59:216–8. [DOI] [PubMed] [Google Scholar]
- 19.Weber Pasa M, SelbachScheffel R, Borsatto Zanella A, et al. Consumptive hypothyroidism: case report of hepatic hemangioendotheliomas successfully treated with vincristine and systematic review of the syndrome. Eur Thyroid J. 2017;6:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen T, Orchard D, Zacharin M. Liver haemangiomas and consumptive hypothyroidism in association with three cutaneous haemangiomas. J Paediatr Child Health. 2017;53:1226–8. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi S, Takagi M, Hasegawa Y. Use of liothyronine without levothyroxine in the treatment of mild consumptive hypothyroidism caused by hepatic hemangiomas. Endocr J. 2017;64:639–43. [DOI] [PubMed] [Google Scholar]
- 22.Al Tasseh F, El-Khansa M, Abd O, et al. Diffuse hepatic hemangioma with single cutaneous hemangioma: an alerting occurrence. Clin Case Rep. 2017;5:887–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varrasso G, Schiavetti A, Lanciotti S, et al. Propranolol as first-line treatment for life-threatening diffuse infantile hepatic hemangioma: a case report. Hepatology. 2017;66:283–5. [DOI] [PubMed] [Google Scholar]
- 24.Wasserman JD, Mahant S, Carcao M, et al. Vincristine for successful treatment of steroid-dependent infantile hemangiomas. Pediatrics. 2015;135:e1501–5. [DOI] [PubMed] [Google Scholar]
- 25.Wijeratne NG, Kao KT, Simm PJ, et al. A baby boy with hypothyroidism and hemangioendothelioma. Clin Chem. 2014;60:818–21. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Karmazyn B, Lin J, et al. Newborn with an underrecognized triad: skin lesion, abdominal distention, and hypothyroidism. J Pediatr. 2014;164:419–20. [DOI] [PubMed] [Google Scholar]
- 27.Vergine G, Marsciani A, Pedini A, et al. Efficacy of propranolol treatment in thyroid dysfunction associated with severe infantile hepatic hemangioma. Horm Res Paediatr. 2012;78:256–60. [DOI] [PubMed] [Google Scholar]
- 28.Imteyaz H, Karnsakul W, Levine MA, et al. Unusual case of hypothyroidism in an infant with hepatic hemangioma. J Pediatr Gastroenterol Nutr. 2012;54:692–5. [DOI] [PubMed] [Google Scholar]
- 29.Emir S, Ekici F, İkiz MA, et al. The association of consumptive hypothyroidism secondary to hepatic hemangioma and severe heart failure in infancy. Turk PediatriArs. 2016;51:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jassam N, Visser TJ, Brisco T, et al. Consumptive hypothyroidism: a case report and review of the literature. Ann Clin Biochem. 2011;48(Pt 2):186–9. [DOI] [PubMed] [Google Scholar]
- 31.Yeh I, Bruckner AL, Sanchez R, et al. Diffuse infantile hepatic hemangiomas: a report of four cases successfully managed with medical therapy. Pediatr Dermatol. 2011;28:267–75. [DOI] [PubMed] [Google Scholar]
- 32.Bessho K, Etani Y, Ichimori H, et al. Increased type 3 iodothyronine deiodinase activity in a regrown hepatic hemangioma with consumptive hypothyroidism. Eur J Pediatr. 2010;169:215–21. [DOI] [PubMed] [Google Scholar]
- 33.Çetinkaya S, Kendirci HN, Ağladioğlu SY, et al. Hypothyroidism due to hepatic hemangioendothelioma: a case report. J Clin Res PediatrEndocrinol. 2010;2:126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters C, Langham S, Mullis PE, et al. Use of combined liothyronine and thyroxine therapy for consumptive hypothyroidism associated with hepatic haemangiomas in infancy. Horm Res Paediatr. 2010;74:149–52. [DOI] [PubMed] [Google Scholar]
- 35.Mouat F, Evans HM, Cutfield WS, et al. Massive hepatic hemangioendothelioma and consumptive hypothyroidism. J Pediatr Endocrinol Metab. 2008;21:701–3. [DOI] [PubMed] [Google Scholar]
- 36.Cho YH, Taplin C, Mansour A, et al. Case report: consumptive hypothyroidism consequent to multiple infantile hepatic haemangiomas. Curr Opin Pediatr. 2008;20:213–5. [DOI] [PubMed] [Google Scholar]
- 37.Kalpatthi R, Germak J, Mizelle K, et al. Thyroid abnormalities in infantile hepatic hemangioendothelioma. Pediatr Blood Cancer. 2007;49:1021–4. [DOI] [PubMed] [Google Scholar]
- 38.Lee TC, Barshes NR, Agee EE, et al. Resolution of medically resistant hypothyroidism after liver transplantation for hepatic hemangioendothelioma. J Pediatr Surg. 2006;41:1783–5. [DOI] [PubMed] [Google Scholar]
- 39.Balazs AE, Athanassaki I, Gunn SK, et al. Rapid resolution of consumptive hypothyroidism in a child with hepatic hemangioendothelioma following liver transplantation. Ann Clin Lab Sci. 2007;37:280–4. [PubMed] [Google Scholar]
- 40.Güven A, Aygun C, Ince H, et al. Severe hypothyroidism caused by hepatic hemangioendothelioma in an infant of a diabetic mother. Horm Res. 2005;63:86–9. [DOI] [PubMed] [Google Scholar]
- 41.Ho J, Kendrick V, Dewey D, et al. New insight into the pathophysiology of severe hypothyroidism in an infant with multiple hepatic hemangiomas. J Pediatr Endocrinol Metab. 2005;18:511–4. [DOI] [PubMed] [Google Scholar]
- 42.Konrad D, Ellis G, Perlman K. Spontaneous regression of severe acquired infantile hypothyroidism associated with multiple liver hemangiomas. Pediatrics. 2003;112:1424–6. [DOI] [PubMed] [Google Scholar]
- 43.Ayling RM, Davenport M, Hadzic N, et al. Hepatic hemangioendothelioma associated with production of humoral thyrotropin-like factor. J Pediatr. 2001;138:932–5. [DOI] [PubMed] [Google Scholar]
- 44.Mason KP, Koka BV, Eldredge EA, et al. Perioperative considerations in a hypothyroid infant with hepatic haemangioma. Paediatr Anaesth. 2001;11:228–32. [DOI] [PubMed] [Google Scholar]
- 45.Igarashi A, Hata I, Yuasa M, et al. A case of an infant with extremely low birth weight and hypothyroidism associated with massive cutaneous infantile hemangioma. J Pediatr Endocrinol Metab. 2018;31:1377–80. [DOI] [PubMed] [Google Scholar]
- 46.Chakraborty PP, Bera M, Patra S, et al. Consumptive hypothyroidism in solitary cutaneous haemangioma. BMJ Case Rep. 2017;2017:bcr2017221366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Corti F, Crivellaro C, Zanon GF, et al. Consumptive hypothyroidism associated with parotid infantile hemangioma. J Pediatr Endocrinol Metab. 2015;28:467–9. [DOI] [PubMed] [Google Scholar]
- 48.Metwalley KA, Farghaly HS. Consumptive hypothyroidism in an Egyptian baby with benign neonatal hemangiomatosis: a case report. J Med Case Rep. 2013;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vigone MC, Cortinovis F, Rabbiosi S, et al. Difficult treatment of consumptive hypothyroidism in a child with massive parotid hemangioma. J Pediatr Endocrinol Metab. 2012;25:153–5. [DOI] [PubMed] [Google Scholar]
- 50.North PE, Waner M, Mizeracki A, et al. A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol. 2001;137:559–70. [PubMed] [Google Scholar]
- 51.Mo JQ, Dimashkieh HH, Bove KE. GLUT1 endothelial reactivity distinguishes hepatic infantile hemangioma from congenital hepatic vascular malformation with associated capillary proliferation. Hum Pathol. 2004;35:200–9. [DOI] [PubMed] [Google Scholar]
- 52.Wassner AJ. Pediatric hypothyroidism: diagnosis and treatment. Paediatr Drugs. 2017;19:291–301. [DOI] [PubMed] [Google Scholar]
- 53.Luongo C, Trivisano L, Alfano F, et al. Type 3 deiodinase and consumptive hypothyroidism: a common mechanism for a rare disease. Front Endocrinol (Lausanne). 2013;4:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horii KA, Drolet BA, Frieden IJ, et al. ; Hemangioma Investigator Group. Prospective study of the frequency of hepatic hemangiomas in infants with multiple cutaneous infantile hemangiomas. Pediatr Dermatol. 2011;28:245–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.