Abstract
In many peripheral and central neurons, A-type K+ currents, IA, have been identified and shown to be key determinants in shaping action potential waveforms and repetitive firing properties, as well as in the regulation of synaptic transmission and synaptic plasticity. The functional properties and physiological roles of native neuronal IA, however, have been shown to be quite diverse in different types of neurons. Accumulating evidence suggests that this functional diversity is generated by multiple mechanisms, including the expression and subcellular distributions of IA channels encoded by different voltage-gated K+ (Kv) channel pore-forming (α) subunits, interactions of Kv α subunits with cytosolic and/or transmembrane accessory subunits and regulatory proteins and post-translational modifications of channel subunits. Several recent reports further suggest that local protein translation in the dendrites of neurons and interactions between IA channels with other types of voltage-gated ion channels further expands the functional diversity of native neuronal IA channels. Here, we review the diverse molecular mechanisms that have been shown or proposed to underlie the functional diversity of native neuronal IA channels.
Keywords: Kv4 channels, Kv1 channels, Kv channel accessory subunits, posttranslational regulation of Kv channels, Kv12 channels
Introduction
Rapidly activating and inactivating, A-type K+ currents (IA), initially characterized by Connor and Stevens (1971a, 1971b) in invertebrate neurons, are now well recognized as key determinants in the regulation of excitability in a wide variety of vertebrate peripheral and central neurons. In addition, electrophysiological studies have revealed that IA subserves a variety of physiological roles in neurons, from the regulation of action potential durations and repetitive firing rates to controlling the backpropagation (into dendrites) of action potentials and the modulation of synaptic transmission and synaptic plasticity (Johnston and others 2000; Kim and Hoffman 2008). Considerable evidence now suggests, however, that the detailed biophysical properties, the molecular determinants and the functional roles of IA are actually quite variable in different types of neurons. Here, we review the physiological roles and the molecular and functional diversity of native neuronal IA channels and discuss the various regulatory mechanisms that have been shown or postulated to contribute to this diversity.
Molecular and Functional Diversity of Native IA Channels
Studies in heterologous expression systems have demonstrated that at least six of the genes encoding voltage-gated K+ (Kv) channel pore-forming (α) subunits can generate rapidly activating and inactivating K+ currents with properties similar to neuronal IA, suggesting that the functional diversity of native IA is generated, at least in part, by the expression of different and/or multiple Kv α subunits and Kv α subunit–encoded channels. Indeed, accumulating evidence suggests that the molecular determinants and the functional properties of native neuronal IA channels are distinct in different types of neurons. The Kv4.2 α subunit, for example, is robustly expressed in hippocampal and cortical pyramidal neurons as well as in cerebellar granule cells and is one of the main contributors to the generation of IA in these cells (Kim and others 2005; Nadin and Pfaffinger 2010; Nerbonne and others 2008; Norris and Nerbonne 2010; Shibata and others 2000; Yuan and others 2005). In dorsal root ganglion neurons, in contrast, Kv4.2 expression is negligible, suggesting that Kv4.2-encoded channels do not contribute to IA in these cells (Phuket and Covarrubias 2009). Multiple Kv α subunits, including Kv4.1, Kv4.3, Kv3.4, and Kv1.4, that can generate IA-type channels, however, are highly expressed in dorsal root ganglion neurons and have been suggested to contribute to the generation of IA in these cells (Cao and others 2010; Chien and others 2007; Phuket and Covarrubias 2009; Rasband and others 2001).
In cortical pyramidal neurons, the Kv4.2, Kv4.3, and Kv1.4 α subunits have all been suggested to contribute to IA (Norris and Nerbonne 2010), and IA channels encoded by these different Kv α subunits have been shown to play unique roles in the regulation of the intrinsic excitability of these cells (Carrasquillo and others 2012). Kv4.2-encoded IA channels, for example, contribute to the input resistances, the current thresholds for action potential generation and the repolarization of action potentials in cortical pyramidal cells. Kv4.3-encoded channels, in contrast, contribute to action potential repolarization without affecting input resistances or current thresholds for action potential generation. IA channels encoded by Kv1.4 also contribute to the regulation of resting membrane potentials and the current thresholds for action potential generation but do not measurably affect action potential durations. Taken together, these results demonstrate that native neuronal Kv4.2-, Kv4.3-, and Kv1.4-encoded IA channels in cortical pyramidal cells function over different voltage ranges and differentially regulate resting and active membrane properties (Carrasquillo and others 2012).
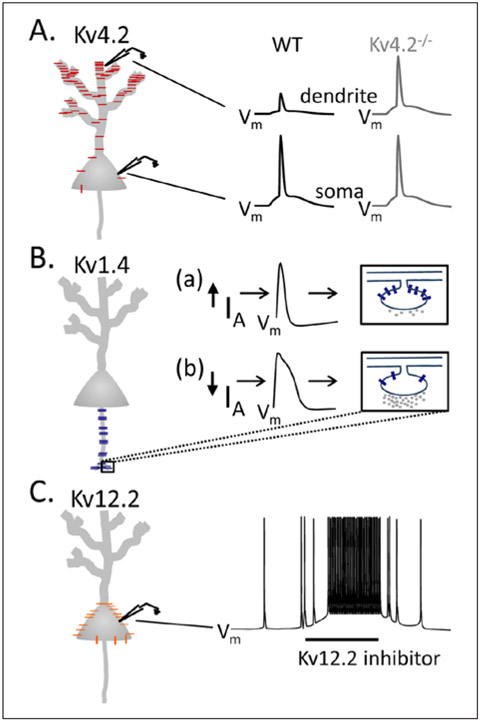
Similar to the observations in cortical pyramidal neurons, IA is also encoded by multiple Kv α subunits in hippocampal pyramidal neurons (Angelova and Muller 2009; Cai and others 2004; Chen and others 2006; Cooper and others 1998; Jenkins and others 2011; Kim and others 2005; Sheng and others 1992; Zhang and others 2010). In addition, the subcellular distribution of IA channels encoded by each of the different Kv α subunits is unique (Fig. 1) and has distinct functional consequences. Kv4.2-encoded IA channels, for example, are expressed in the soma and dendrites of hippocampal pyramidal neurons, in a gradient that increases from the proximal to distal segments (Chen and others 2006; Sheng and others 1992) (Fig. 1A). As a result of this unique subcellular distribution pattern, Kv4.2-encoded IA channels function to regulate the amplitudes of backpropagating action potentials into dendrites and contribute to the regulation of synaptic integration in these cells (Cai and others 2004; Kim and others 2005). In contrast to the somatodendritic expression of Kv4.2 (Sheng and others 1992), Kv1.4 has been reported to be localized preferentially in axons and presynaptic terminals (Angelova and Muller 2009; Cooper and others 1998; Jenkins and others 2011; Sheng and others 1992), where Kv1.4-encoded IA channels have been proposed to play important roles in the modulation of neurotransmitter release and presynaptic facilitation (Fig. 1B). Recent studies have also revealed that Kv12.2-encoded channels also contribute to a component of IA in hippocampal pyramidal cells (Zhang and others 2010). While the subcellular distribution of Kv12.2-encoded channels has not been characterized, electrophysiological experiments have clearly demonstrated that Kv12.2-encoded IA channels (unlike Kv4.2-encoded IA channels) contribute to the regulation of somatic resting membrane potentials and repetitive firing rates (Zhang and others 2010), suggesting that Kv12.2-encoded channels are preferentially expressed in the soma of hippocampal pyramidal neurons (Fig. 1C).
Figure 1.
Functional consequences of the differential (subcellular) distributions of Kv α subunit-encoded native IA channels in hippocampal pyramidal neurons. Schematic representation of Kv4.2-encoded (red), Kv1.4-encoded (blue), and Kv12.2-encoded (orange) IA channel distributions in hippocampal pyramidal neurons. (A) Kv4.2-encoded IA channels (red) are expressed in the soma and dendrites of hippocampal pyramidal neurons, in a gradient that increases from the proximal to distal segments (Chen and others 2006; Sheng and others 1992), and that regulates the amplitudes of backpropagating action potentials (bAPs) in the dendrites. In wild type (WT) neurons, the amplitudes of bAPs in distal dendrites are attenuated, compared with the soma. In (Kv4.2−/−) hippocampal neurons lacking Kv4.2, the amplitudes of bAPs are increased (Chen and others 2006). (B) Kv1.4-encoded IA channels (blue) are localized in axons and presynaptic terminals (Angelova and Muller 2009; Cooper and others 1998; Jenkins and others 2011; Sheng and others 1992). (Ba, Bb) Schematics illustrating model for Kv1.4-mediated modulation of neurotransmitter release. Increased Kv1.4-encoded IA in presynaptic terminals leads to briefer action potentials and less neurotransmitter release. Decreased Kv1.4-encoded IA, in contrast, leads to prolonged action potentials and higher neurotransmitter release. (C) Proposed distribution of Kv12.2-encoded IA channels (orange), with high expression levels in the soma. Bath application of a Kv12.2 inhibitor depolarizes somatic resting membrane potentials and increases repetitive firing rates in hippocampal pyramidal neurons (Zhang and others 2010).
Native Channels in Macromolecular Complexes
Accumulating evidence suggests that native neuronal IA channels function in macromolecular protein complexes comprising one or more Kv α subunit (in the same sub-family) together with cytosolic and/or transmembrane accessory subunits and regulatory proteins that influence channel stability, localization, and properties. Similar to Kv α subunits, there are multiple types of Kv channel accessory subunits that have been suggested, based largely on the results obtained from heterologous co-expression studies, to function in the generation of native neuronal IA channels. Importantly, however, accumulating evidence suggests that, similar to the Kv α subunits, the physiological roles of IA channel accessory subunits also vary with cell type. Targeted deletion of the cytosolic accessory subunit K+ channel interacting protein 2 (KChIP2), for example, reportedly alters the densities, voltage-dependent properties and the recovery from inactivation of IA in hippocampal neurons, increasing spontaneous firing rates and lowering the current thresholds for repetitive firing (Wang and others 2013). In cortical pyramidal and basolateral amygdala neurons, in contrast, targeted deletion of KChIP2 neither measurably affects IA nor does it affect the intrinsic resting membrane properties or repetitive firing (Norris and others 2010; Wang and others 2013). Additional studies, however, suggest that the lack of effect of loss of KChIP2 on IA in cortical pyramidal neurons likely reflects compensatory effects of other members of this family, KChIP3 and KChIP4 (Norris and others 2010). Similar cell-type specificity has been reported for the recently identified Kv4 channel accessory subunit, Navβ1 (Marionneau and others 2012). Loss of Navβ1 increases the excitability of cortical pyramidal neurons but does not measurably affect the excitability of hippocampal pyramidal neurons (Marionneau and others 2012; Patino and others 2009).
It has been suggested recently that Kv channel accessory subunits play distinct roles in the functional regulation of IA channels in different cellular compartments (Fig. 2). The single transmembrane Kv4 channel accessory protein, dipeptidyl peptidase-like protein 6 (DPP6), for example, has been suggested to be critical for generating the characteristic IA gradient in hippocampal pyramidal neurons (Fig. 2A). Interestingly, targeted deletion of DPP6 reduces IA densities and alters the amplitudes of backpropagating action potentials in the distal dendrites of hippocampal pyramidal neurons without altering IA densities or intrinsic excitability in the soma (Sun and others 2011). In cerebellar granule cells, in contrast, acute “knockdown” of DPP6 was shown to drastically reduce IA densities and to alter intrinsic excitability in the soma (Nadin and Pfaffinger 2010). Whether IA is functionally expressed in the dendrites of granule cells, however, has not been determined. Taken together, these results suggest distinct functional roles for DPP6 and, in addition, suggest that compartmentalization of function of DPP6 is cell type specific (Fig. 2B). Whether cell type-specific compartmentalization of function is unique to DPP6 or whether this mechanism is important in the functional regulation of IA channels by other Kv accessory subunits remains to be determined.
Figure 2.
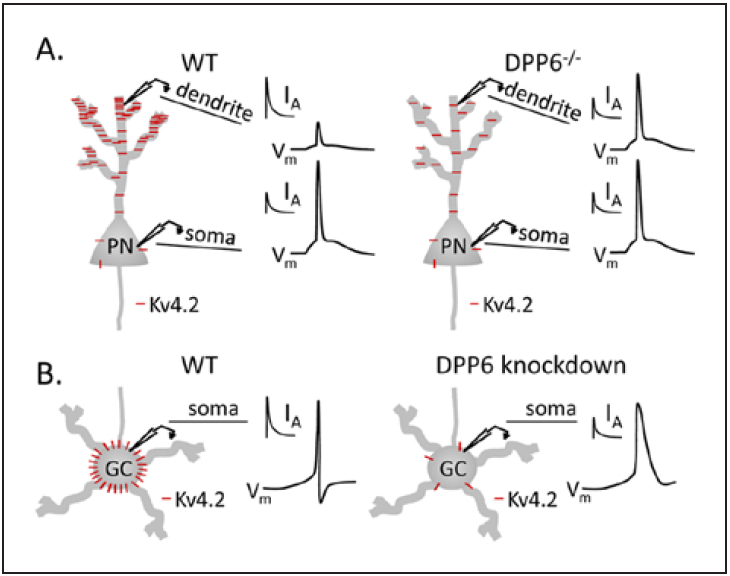
Cell type–specific modulation of Kv4.2-encoded IA channels by accessory (dipeptidyl peptidase-like protein 6 [DPP6]) subunits. Schematic representation of Kv4.2 (red) distribution in hippocampal pyramidal neurons (PN) and cerebellar granule cells (GC) in wild type (WT) mice and in mice lacking DPP6. (A) In WT neurons, Kv4.2 (red) expression and IA densities display a somatodendritic gradient, increasing from the proximal to distal segments (Chen and others 2006; Sheng and others 1992). Targeted deletion of DPP6 (DPP6−/−) eliminates the somatodendritic gradient of Kv4.2 expression and IA densities and increases the amplitudes of bAPs without altering IA or intrinsic excitability in the soma (Sun and others 2011). (B) In WT cerebellar granule cells, in contrast, IA is robustly expressed in the soma and acute “knockdown” of DPP6 reduces somatic IA densities and alters action potential waveforms recorded from the soma (Nadin and Pfaffinger 2010).
Differential modulation of the biophysical properties of native neuronal IA channels by individual accessory subunits also contributes to the functional diversity of IA channels. Targeted deletion of KChIP2, for example, shifts the V1/2 of steady-state inactivation of IA to hyperpolarized potentials but does not affect the voltage dependence of channel activation (Wang and others 2013). Acute “knockdown” or targeted deletion of DPP6, in contrast, shifts the voltage-dependences of IA activation and steady-state inactivation to more depolarized potentials (Kim and others 2008; Nadin and Pfaffinger 2010; Sun and others 2011). Similarly, while acute “knockdown” or targeted deletion of DPP6 slows the kinetics of IA activation and inactivation (Kim and others 2008; Nadin and Pfaffinger 2010; Sun and others, 2011), targeted deletion of KChIP2 or Navβ1 does not measurably affect the kinetics of IA activation and inactivation (Marionneau and others 2012; Wang and others 2013). The biochemical mechanisms underlying IA channel modulation by different Kv channel accessory subunits are also distinct. Considerable evidence suggests, for example, that Kv4 protein stability is dependent on the expression of KChIPs and that the expression KChIPs is also dependent on the expression of Kv4 α subunits (Foeger and others 2012; Menegola and Trimmer 2006; Norris and others 2010). Similarly, it has also been reported that Kv4 (and KChIP) protein stability is dependent on the association of Kv4 α subunits with DPP6 (Nadin and Pfaffinger 2010). DPP6 protein expression in the hippocampus, cortex and cerebellum, however, is only mildly reduced after the loss Kv4.2, suggesting that stabilization of the DPP6 protein is independent of Kv4.2 (Nadin and Pfaffinger 2010).
Posttranslational Modification of Channel Subunits
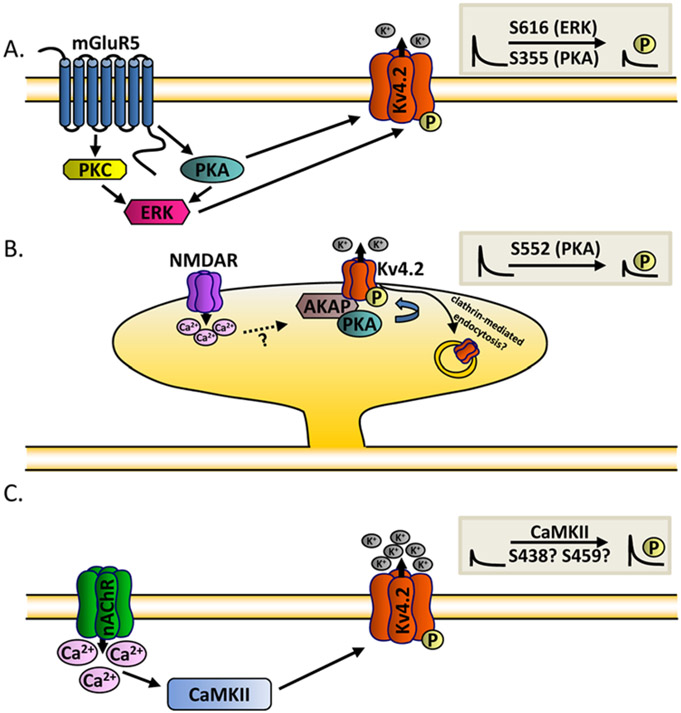
The functional diversity of native neuronal IA channels is further expanded by posttranslational modifications of pore-forming and accessory channel subunits, which influence channel properties, densities and subcellular localization. Indeed, considerable evidence suggests that multiple upstream signaling pathways function in concert to fine-tune the properties of IA, thereby modulating intrinsic neuronal excitability and synaptic transmission/plasticity (Fig. 3). In hippocampal and dorsal horn neurons, for example, extracellular-regulated kinase (ERK)–mediated phosphorylation of Kv4.2 decreases IA densities (Hu and others 2003; Hu and others 2007; Yuan and others 2002) (Fig. 3A). Attenuation of IA increases the excitability of dorsal horn neurons (Hu and others 2007; Hu and Gereau 2003) and increases the amplitudes of backpropagating action potentials in the distal dendrites of hippocampal neurons (Yuan and others 2002). It has also been reported that ERK-mediated phosphorylation of Kv4.2 (at serine 616) is downstream of the activation of the metabotropic glutamate receptor 5 (mGluR5) (Hu and others 2007) and is localized to excitatory neurons in the dorsal horn of the spinal cord (Hu and Gereau 2011). In both hippocampal and dorsal horn neurons, IA is also regulated by the activation of protein kinase A (PKA) and protein kinase C (PKC) in an ERK-dependent manner (Hu and others 2007; Hu and others 2003; Yuan and others 2002). Direct phosphorylation of Kv4.2 (at Ser 355) by PKA, however, has also been shown to decrease IA densities in hippocampal neurons (Hammond and others 2008). Interaction of Kv4.2 with the A-kinase anchoring protein 79/150 (AKAP79/150) mediates the anchoring of PKA and regulates Kv4.2 surface expression and excitability in the hippocampus (Lin and others 2011). Interestingly, PKA-mediated phosphorylation of Kv4.2 (at S552) can be driven by neuronal activity and, in addition, promotes the internalization of Kv4.2, resulting in the redistribution of the (Kv4.2) protein from the spines to the dendritic shafts and soma (Hammond and others 2008; Kim and others 2007). The activity-dependent redistribution of Kv4.2 is controlled through clathrin-mediated endocytosis and requires NMDA receptor activation and Ca2+ influx (Kim and others 2007). Whether activity-dependent redistribution of Kv4.2 requires the anchoring of PKA by AKAP79/150 remains to be determined.
Figure 3.
Multiple upstream signaling pathways function in concert to fine-tune the properties of IA. (A) Extracellular-regulated kinase (ERK)– and protein kinase A (PKA)–mediated phosphorylation of Kv4.2 is downstream of the activation of the metabotropic glutamate receptor 5 (mGluR5) and decreases IA densities (Hammond and others 2008; Hu and others 2003; Hu and others 2007; Yuan and others 2002). (B) Interaction of Kv4.2 with the A-kinase anchoring protein (AKAP) mediates the anchoring of PKA and decreases Kv4.2 surface expression (Lin and others 2011). Neuronal activity redistributes Kv4.2 from spines to dendritic shafts and soma through clathrin-mediated endocytosis and requires N-methyl-d-aspartate (NMDA) receptor activation and Ca2+ influx (Kim and others 2007). (C) Ca2+/calmodulin-dependent kinase II (CaMKII)–dependent phosphorylation of Kv4.2 (Shal in Drosophila) and increased IA density (Ping and Tsunoda 2012; Varga and others 2004). Activation of α7 nicotinic acetylcholine receptors (nAChR) and increased Ca2+ influx results in downstream CaMKII-dependent phosphorylation of Kv4.2 and in increases in IA densities.
An upstream signaling pathway that includes the Ca2+/calmodulin-dependent kinase II (CaMKII) has also been identified as a modulator of native Kv4.2-encoded IA channels in hippocampal neurons (Varga and others 2004) (Fig. 3C). Recent studies in cultured Drosophila neurons further suggest that CaMKII-dependent phosphorylation of native IA channels plays a critical role in synaptic homeostasis (Ping and Tsunoda 2012). In response to a prolonged (24 hours) period of synaptic inactivity, for example, IA densities are increased in the soma and distal dendrites of excitatory motor neurons and the expression of the Shal protein, the Drosophila homolog of mammalian Kv4, is upregulated (Ping and Tsunoda 2012). The homeostatic upregulation of IA in response to synaptic inactivity stabilizes postsynaptic potentials. Inactivity-dependent increases in IA are dependent on upregulation of the Drosophila α7 (Dα7) nicotinic acetylcholine receptors (nAChR), increases in Ca2+ influx and the activation of CaMKII, suggesting that CaMKII-dependent phosphorylation of IA channels drives the (inactivity dependent) observed increases in IA (Ping and Tsunoda 2012) (Fig. 3C). Interestingly, these results are consistent with previous studies demonstrating that the expression of constitutively active CaMKII increases IA densities and decreases neuronal excitability in mammalian neurons (Varga and others 2004).
Novel Mechanisms Regulating Native IA Channels
Local Translation of Kv4.2 in Dendrites
It was recently reported that Kv4.2 is translated locally in the dendrites of hippocampal neurons and that the fragile X mental retardation protein (FMRP), which is an RNA binding protein, regulates the translation and, therefore, the dendritic protein expression, of Kv4.2 by associating with the 3′ and 5′ untranslated regions (UTRs) of Kv4.2 mRNA (Gross and others 2011). Conflicting results have been reported, however, about the net effect of the association of FMRP with the 3′ UTR of Kv4.2 mRNA on dendritic Kv4.2 protein expression (Gross and others 2011; Lee and others 2011). Immunohistochemical and biochemical studies conducted by Gross and others (2011), for example, revealed that targeted deletion of FMRP decreases total and cell surface Kv4.2 protein expression in hippocampal and cortical neurons, suggesting that the association of FMRP with Kv4.2 mRNA promotes Kv4.2 protein expression. Biochemical and immunohistochemical experiments performed by Lee and others. (2011), however, yielded completely opposite results. These investigators reported that the in vivo loss of FMRP increases dendritic Kv4.2 protein levels in hippocampal neurons, suggesting that the association of FMRP with Kv4.2 mRNA suppresses Kv4.2 protein expression. Technical differences in the experiments, such as the primary antibodies used for the immunostaining, might underlie the substantive difference in the results reported by these two groups. In spite of these contradicting results, a novel and important finding from both studies is that FMRP associates with Kv4.2 mRNA and that that Kv4.2 is locally translated in the dendrites of hippocampal and cortical pyramidal neurons.
It was also reported by Lee and others (2011) that the 3′ UTR of Kv4.2 mRNA is sufficient for dendritic targeting and that the in vivo loss of FMRP does not affect dendritic targeting of Kv4.2 mRNA. Additional biochemical and pharmacological studies demonstrated that upstream activation of N-methyl-d-aspartate (NMDA) receptors results in dephosphorylation of both mammalian target of rapamycin (mTOR) and FMRP in a process that is dependent on phosphoprotein phosphatase 1 (Lee and others 2011). Dephosphorylation of FMRP, in turn, results in increased dendritic Kv4.2 protein expression and in alterations in synaptic plasticity that can be reversed by pharmacologically reducing Kv4-encoded IA (Lee and others 2011), observations that are clearly consistent with the suggestion that dendritic Kv4.2-encoded IA channels contributes to synaptic plasticity.
Channel–Channel Macromolecular Protein Complexes
Several recent studies have also suggested that the biophysical properties of native neuronal IA channels are also modulated through interactions with other types of ion channels. The interaction of voltage-gated Ca2+ (Cav) channels encoded by α subunits of the Cav3 subfamily with Kv4.2-encoded IA channels, for example, has been shown to regulate the biophysical properties of Kv4.2-encoded IA channels and intrinsic excitability in cerebellar stellate cells (Anderson and others 2010). Although the mechanisms underlying the functional interaction between Cav3- and Kv4-encoded channels are not known, a role for the KChIP3 accessory subunit has been revealed.
It was also demonstrated recently that the voltage-gated Na+ (Nav) channel accessory subunit, Navβ1, binds to Kv4.2 α subunits and modulates Kv4.2-encoded IA and intrinsic excitability in cortical pyramidal neurons (Marionneau and others 2012). Similarly, recent reports have demonstrated that “knockdown” of the accessory subunit DPP6 alters the densities and biophysical properties of IA and also the densities of IK(SO) and the Nav currents (Nadin and Pfaffinger 2010; Nadin and Pfaffinger 2013). Although these studies clearly suggest that Kv4-encoded IA channels interact biochemically and functionally with other channels in the brain, the structural bases and the physiological roles of these channel–channel interactions remain to be elucidated.
Summary, Conclusions, and Future Directions
Substantial progress has been made in recent years in the identification of the molecular mechanisms underlying the generation of IA in different neuronal cell types and the molecular mechanisms that regulate the biophysical properties, the subcellular distributions, and the functioning of native neuronal IA channels. It is now clear, for example, that multiple Kv α subunits contribute to the generation of native neuronal IA channels and that IA channels encoded by these different Kv α subunits are differentially distributed and play distinct physiological roles in the regulation of intrinsic excitability and synaptic transmission. It is also now known that the association of Kv α subunits with cytosolic and/or transmembrane accessory subunits strongly influences the biophysical properties and the subcellular distributions of native neuronal IA channels and that posttranslational modifications of Kv channel subunits through multiple upstream signaling pathways further expands and fine-tunes the physiological roles of IA. Further efforts are clearly needed to fully define the cell type–specific mechanisms that underlie the diversity in the physiological roles and functional compartmentalization of native neuronal IA channels in different populations of neurons.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge the support provided by the National Institute for Neurological Disorders and Stroke (R01 NS-03676 to JMN and F32 NS-065581 to YC).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, and others. 2010. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci 13(3):333–7. [DOI] [PubMed] [Google Scholar]
- Angelova PR, Muller WS. 2009. Arachidonic acid potently inhibits both postsynaptic-type Kv4.2 and presynaptic-type Kv1.4 IA potassium channels. Eur J Neurosci 29(10):1943–50. [DOI] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. 2004. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44(2):351–64. [DOI] [PubMed] [Google Scholar]
- Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL. 2010. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem 114(5):1460–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo Y, Burkhalter A, Nerbonne JM. 2012. A-type K+ channels encoded by Kv4.2, Kv4.3 and Kv1.4 differentially regulate intrinsic excitability of cortical pyramidal neurons. J Physiol 590(Pt 16):3877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, and others. 2006. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci 26(47):12143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML. 2007. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 27(37):9855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. 1971a. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J Physiol 213(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. 1971b. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol 213(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. 1998. Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J Neurosci 18(3):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foeger NC, Norris AJ, Wren LM, Nerbonne JM. 2012. Augmentation of Kv4.2-encoded currents by accessory dipeptidyl peptidase 6 and 10 subunits reflects selective cell surface Kv4.2 protein stabilization. J Biol Chem 287(12):9640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong D, Jeromin A, Bassell G. 2011. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci 31(15):5693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Lin L, Sidorov MS, Wikenheiser AM, Hoffman DA. 2008. Protein kinase A mediates activity-dependent Kv4.2 channel trafficking. J Neurosci 28(30):7513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW 4th. 2007. Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci 27(48):13181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Gereau RW 4th. 2003. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol 90(3):1680–8. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Gereau RW 4th. 2011. Metabotropic glutamate receptor 5 regulates excitability and Kv4.2-containing K+ channels primarily in excitatory neurons of the spinal dorsal horn. J Neurophysiol 105(6):3010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Glauner KS, Gereau RW 4th. 2003. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol 90(3):1671–9. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, McIntyre JC, Zhang L, Anantharam A, Vesely ED, Arendt KL, and others. 2011. Subunit-dependent axonal trafficking of distinct alpha heteromeric potassium channel complexes. J Neurosci 31(37):13224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM, and others. 2000. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol 525(Pt 1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hoffman DA. 2008. Potassium channels: newly found players in synaptic plasticity. Neuroscientist 14(3):276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. 2007. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 54(6):933–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, and others. 2008. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol 100(4):1835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wei DS, Hoffman DA. 2005. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol 569(Pt 1):41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson-Baldwin A, and others. 2011. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron 72(4):630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sun W, Kung F, Dell’Acqua ML, Hoffman DA. 2011. AKAP79/150 impacts intrinsic excitability of hippocampal neurons through phospho-regulation of A-type K+ channel trafficking. J Neurosci 31(4):1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marionneau C, Carrasquillo Y, Norris AJ, Townsend RR, Isom LL, Link AJ, and others. 2012. The sodium channel accessory subunit Navbeta1 regulates neuronal excitability through modulation of repolarizing voltage-gated K+ channels. J Neurosci 32(17):5716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegola M, Trimmer JS. 2006. Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain. J Neurosci 26(47):12137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin BM, Pfaffinger PJ. 2010. Dipeptidyl peptidase-like protein 6 is required for normal electrophysiological properties of cerebellar granule cells. J Neurosci 30(25):8551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin BM, Pfaffinger PJ. 2013. A new TASK for dipeptidyl peptidase-like protein 6. PLoS One 8(4):e60831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne JM, Gerber BR, Norris A, Burkhalter A. 2008. Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J Physiol 586(6):1565–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Foeger NC, Nerbonne JM. 2010. Interdependent roles for accessory KChIP2, KChIP3, and KChIP4 subunits in the generation of Kv4-encoded IA channels in cortical pyramidal neurons. J Neurosci 30(41):13644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Nerbonne JM. 2010. Molecular dissection of I(A) in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv4.3, and Kv1.4 alpha-subunits. J Neurosci 30(14):5092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, and others. 2009. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci 29(34):10764–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuket TR, Covarrubias M. 2009. Kv4 channels underlie the subthreshold-operating A-type K-current in nociceptive dorsal root ganglion neurons. Front Mol Neurosci 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Y, Tsunoda S. 2012. Inactivity-induced increase in nAChRs upregulates Shal K= channels to stabilize synaptic potentials. Nat Neurosci 15(1):90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. 2001. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 98(23):13373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Tsaur ML, Jan YN, Jan LY. 1992. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron 9(2):271–84. [DOI] [PubMed] [Google Scholar]
- Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K. 2000. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J Neurosci 20(11):4145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Maffie JK, Lin L, Petralia RS, Rudy B, Hoffman DA. 2011. DPP6 establishes the A-type K+ current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 71(6):1102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, and others. 2004. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci 24(14):3643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, He XP, Li Q, Madison RD, Moore SD, McNamara JO, and others. 2013. The auxiliary subunit KChIP2 is an essential regulator of homeostatic excitability. J Biol Chem 288(19):13258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. 2002. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci 22(12):4860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Burkhalter A, Nerbonne JM. 2005. Functional role of the fast transient outward K+ current IA in pyramidal neurons in (rat) primary visual cortex. J Neurosci 25(40):9185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bertaso F, Yoo JW, Baumgartel K, Clancy SM, Lee V, and others. 2010. Deletion of the potassium channel Kv12.2 causes hippocampal hyperexcitability and epilepsy. Nat Neurosci 13(9):1056–8. [DOI] [PMC free article] [PubMed] [Google Scholar]