Abstract
Daptomycin is a novel lipopeptide antibiotic with potent bactericidal activity against most clinically important gram-positive bacteria, including resistant strains. Daptomycin has been shown to have an effect on skeletal muscle. To guide the clinical dosing regimen with the potential for the least effect on skeletal muscle, two studies were conducted with dogs to compare the effects of repeated intravenous administration every 24 h versus every 8 h for 20 days. The data suggest that skeletal-muscle effects were more closely related to the dosing interval than to either the maximum concentration of the drug in plasma or the area under the concentration-time curve. Both increases in serum creatine phosphokinase activity and the incidence of myopathy observed at 25 mg/kg of body weight every 8 h were greater than those observed at 75 mg/kg every 24 h despite the lower maximum concentration of drug in plasma. Similarly, the effects observed at 25 mg/kg every 8 h were greater than those observed at 75 mg/kg every 24 h at approximately the same area under the concentration-time curve from 0 to 24 h. Once-daily administration appeared to minimize the potential for daptomycin-related skeletal-muscle effects, possibly by allowing for more time between doses for repair of subclinical effects. Thus, these studies with dogs suggest that once-daily dosing of daptomycin in humans should have the potential to minimize skeletal-muscle effects. In fact, interim results of ongoing clinical trials, which have focused on once-daily dosing, appear to be consistent with this conclusion.
Daptomycin is a novel lipopeptide antibiotic with proven in vitro bactericidal activity against most clinically relevant gram-positive bacteria, including resistant pathogens for which there are very few therapeutic alternatives (Clinical Microbiology Institute, Wilsonville, Oreg., data on file; M. J. Rybak, E. Hershberger, and T. Moldovan, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-146, 1998). This activity, along with rapid, concentration-dependent bactericidal activity, a long postantibiotic effect, a low rate of drug resistance, and linear pharmacokinetics, makes daptomycin an attractive choice for empiric therapy for serious gram-positive infections (4, 11; N. Oliver, T. Andrew, J. A. Silverman, and T. Li, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-117, 1998). Nonclinical and clinical results to date have been promising, with phase 2 clinical trials demonstrating potential efficacy against complicated skin and soft-tissue infections as well as bloodstream infections (bacteremia) (9). Additional phase 3 clinical trials for these indications are currently under way.
Nonclinical and early clinical studies conducted with daptomycin prior to 1999 had not yet established the optimal dosing regimen. In phase 2 clinical trials, doses as high as 6 mg/kg of body weight per day (3 mg/kg every 12 h [q12h]) have been shown to be potentially effective against some types of infections, and the incidence of adverse effects was low and comparable to that of conventional therapy (9). Although higher doses may be needed to effectively treat more deep-seated infections, such as endocarditis, a total daily dose of 8 mg/kg administered as a fractionated dose of 4 mg/kg q12h resulted in adverse skeletal-muscle effects in two of five subjects after 7 and 11 days of dosing. While this adverse effect was mild and fully reversible, it is important to identify the dose regimen that minimizes the potential for this effect while maximizing the potential for clinical efficacy.
Animal studies have shown that daptomycin-induced myopathy is specific to skeletal muscle and is distinct from other myopathies. No pathological changes have been observed in the cardiac (heart) or smooth muscle of animals treated for as long as 6 months (Toxicology Reports no. 14 and 28, Lilly Research Laboratories, Greenfield, Ind.). The skeletal myopathy is characterized by minimal degeneration, with regeneration in the absence of fibrosis. Although high-dose treatment with daptomycin is associated with minimal inflammation secondary to the degenerative changes, the inflammation did not contribute to further muscle damage. In addition, the adverse skeletal-muscle effects associated with daptomycin were not progressive and were readily reversible. Thus, daptomycin-induced myopathy is distinct from inflammatory myopathies (characterized by prominent inflammation and fibrosis), dystrophies (characterized by clinical muscle weakness, fibrosis, and/or atrophy), inherited metabolic or congenital myopathies (characterized by various skeletal-muscle structural alterations), and rhabdomyolysis (characterized by widespread muscle necrosis and renal failure). The absence of rhabdomyolysis is consistent with the lack of nephrotoxicity in dogs, even at the highest dose level tested (75 mg/kg/day) (Toxicol. Rep. no. 14, Lilly).
Serum creatine phosphokinase (CPK) activity appears to be a sensitive marker of daptomycin-induced myopathy. Upon isozyme analysis during clinical investigations, the CPK elevations were shown to be related to release from skeletal muscle, and not from heart or brain (9); this result is in agreement with the microscopic findings in animals demonstrating that myopathy is specific to skeletal muscle. The leakage of CPK from the myocytes is presumed to be mediated via membrane perturbations, consistent with daptomycin's lipophilic nature, antimicrobial mechanism of action, and inability to penetrate the cell membrane (2).
Two nonclinical studies (referred to below as study A and study B) were undertaken to determine the effects of pharmacokinetic parameters on daptomycin-related skeletal-muscle effects. These studies were conducted with dogs because this species, like humans, exhibits skeletal-muscle effects evident through CPK increases and muscle weakness (9). The objective of the studies was to identify the potentially safest clinical dosing regimen by assessing the relationship between skeletal-muscle effects and either the maximum concentration of the drug in plasma (Cmax) or the total daily exposure (area under the concentration-time curve from 0 to 24 h [AUC0–24]). Study A investigated the hypothesis that the muscle effects of daptomycin are related to Cmax and thus can be mitigated by reducing Cmax via fractionation of the total daily dose. Study B assessed whether the Cmax at the no-observable-effect level for skeletal muscle effects was constant regardless of dosing frequency.
MATERIALS AND METHODS
Daptomycin, derived from the fermentation of a strain of Streptomyces roseosporus, was provided by Cubist Pharmaceuticals, Inc. (Cambridge, Mass.) as lyophilized bulk drug (lot 444BYO 13.05; purity, 95.2%). Dosing solutions were prepared daily in bicarbonate-buffered saline (pH 6.0 to 7.0) and were stored refrigerated and protected from light until dose administration.
Seven groups of four male beagle dogs received either daptomycin or bicarbonate-buffered saline by bolus intravenous injection for 20 consecutive days. Animals were approximately 6 to 9 months of age at study initiation. Animals in study A were randomized to receive saline q8h or daptomycin at dose regimens of 25 mg/kg q24h, 75 mg/kg q24h, or 25 mg/kg q8h. Animals in study B were randomized to receive saline q8h or daptomycin at 5 mg/kg either q24h or q8h. In previous studies, regimens of 25 and 75 mg/kg q24h were associated with adverse effects on skeletal muscle; repeated administration at 5 mg/kg q24h produced no adverse effects (Toxicol. Rep. no. 14 and 15, Lilly). A dosing interval of 8 h is equivalent to approximately 3 half-lives in dogs (t1/2, 2.5 h). This dosing interval was selected to maintain comparable Cmax values for the same dose administered q8h versus q24h and comparable AUC0–24 values for the same total daily dose administered in fractionated doses as opposed to a single daily dose.
All animals in the studies were observed twice daily for mortality and clinical signs of adverse effects. Body weights were measured, and detailed physical examinations were conducted weekly beginning 1 week prior to study initiation and again just prior to sacrifice. Food consumption was measured daily beginning 2 weeks prior to study initiation and throughout the 20-day treatment period.
Plasma daptomycin concentrations were determined for estimation of Cmax and AUC0–24 values at steady state. Blood samples (∼2 ml) were collected prior to and approximately 0.08 (study B only), 0.25, 0.5, 1, 2, 4, 8, 12, 16, 20, and 24 h after dose administration on the 19th or 20th day of dosing. Plasma samples were prepared and stored at approximately −20°C. Plasma concentrations were determined by high-performance liquid chromatography (7). Cmax values were empirically determined from concentration data, and AUC0–24 was calculated by linear trapezoidal summation.
Effects on skeletal muscle were evaluated through two parameters: CPK activity and microscopic examination of the muscles. Blood samples for CPK determination were collected twice prior to treatment initiation and every 4 days throughout the treatment period. Blood was collected at the time of presumed maximal effect on CPK (i.e., 2 h postdosing) as opposed to the traditional approach of collection just prior to the next dose, which would have resulted in unequal times postdosing for the q8h and q24h regimens. Serum CPK activity was determined by a creatine phosphate/ADP assay as modified by Szasz et al. (8), using a Hitachi 911 serum chemistry analyzer and Boehringer Mannheim (Indianapolis, Ind.) reagents (catalog no. 45006).
Skeletal and cardiac muscles were examined for histopathological changes. Animals were euthanized by an intravenous injection of sodium pentobarbital followed by exsanguination. Regardless of dosing regimen, necropsy was performed at a constant interval of 12 h after the time the next dose would have been administered. The minor difference in time interval between the last dose and necropsy for the different regimens had no effect on the results of the microscopic evaluation because complete regeneration of myofibers is a 3-week process. The diaphragm, left and right quadriceps femoris, left and right gastrocnemius, and left and right triceps muscles (seven skeletal-muscle sites per animal) and the heart were collected and preserved in 10% neutral buffered formalin. After fixation, tissues were trimmed and processed into paraffin blocks. Cross sections (5 to 8 μm) were stained with hematoxylin and eosin, coded, and examined blindly for microscopic lesions. Lesions were graded for severity on a scale of 1 (minimal), 2 (mild), 3 (moderate), and 4 (marked). The scarcity of lesions in study B necessitated the use of a severity scale more sensitive than that used in study A; a grade of extremely minimal severity was also included.
Body weight, body weight change, food consumption, and CPK values were subjected to a one-way analysis of variance, followed by Dunnett's test. In addition, day 8 CPK values were analyzed using Duncan's test. The incidence of microscopic lesions was analyzed using Fisher's exact test to assess for intergroup differences.
RESULTS
All animals in the studies appeared normal and healthy throughout the treatment period. There were no clinical signs or any changes in body weight or food consumption that would suggest a test article-related effect.
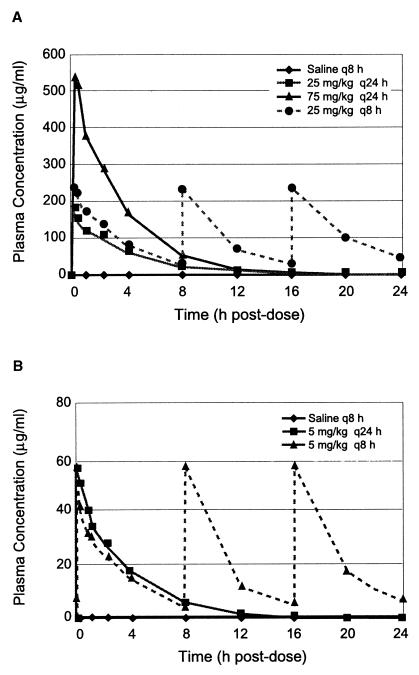
Pharmacokinetic evaluations demonstrate dose proportionality with low accumulation upon dose fractionation. In study A, the Cmax for 75 mg/kg q24h was 3.3 times that for 25 mg/kg q24h and 2.3 times that for 25 mg/kg q8h (Fig. 1A; Table 1). Daily exposure (AUC0–24) was dose proportional at a constant dosing interval (q24h) but slightly higher (1.37 times) upon administration of the same daily dose of 75 mg/kg on a fractionated regimen (i.e., 25 mg/kg q8h). Thus, in study A, the association of Cmax with skeletal-muscle effects can be evaluated by comparing the effects at dosing regimens (75 mg/kg q24h versus 25 mg/kg q8h) with similar AUC values and different Cmax values. Further, the effects of the AUC can be assessed at 25 mg/kg q24h versus 25 mg/kg q8h because Cmax values were comparable for the two regimens. In study B, administration of 5 mg/kg resulted in a Cmax of 58 μg/ml regardless of the dosing interval (q8h versus q24h (Fig. 1B; Table 1). Tripling of the daily dose by administering 5 mg/kg q8h instead of q24h resulted in a 2.3-times-higher AUC. Thus, in study B, a threshold effect can be assessed despite different total daily doses because Cmax was unaffected by dosing frequency.
FIG. 1.
Plasma daptomycin concentrations as determined by high-performance liquid chromatography on the 20th and 19th days of dosing in study A (A) and study B (B), respectively.
TABLE 1.
Pharmacokinetics and myopathy findings
| Dose regimen | Cmax ± SD (μg/ml) | AUC0–24 ± SD (μg · h/ml) | Day 8 CPK activity ± SD (U/liter)a | Incidence and severity of microscopic findings
|
||
|---|---|---|---|---|---|---|
| Skeletal muscle (including diaphragm)
|
Heart: no. of sites with myofiber degeneration total number of sites | |||||
| No. of sites with myofiber degeneration-regeneration/ total no. of sites | Severityb | |||||
| Study A | ||||||
| Control (saline q8h) | 265 ± 182 | 1/28 | Minimal | 0/4 | ||
| 25 mg/kg q24h | 165 ± 44 | 682 ± 44 | 994 ± 1,401 | 3/28 | Minimal | 0/4 |
| 75 mg/kg q24h | 540 ± 112 | 1,840 ± 374 | 991 ± 421 | 8/28c | Minimal | 0/4 |
| 25 mg/kg q8h | 238 ± 22 | 2,526 ± 197 | 3,996 ± 3,151d | 15/28ce | Minimal | 0/4 |
| Study B | ||||||
| Control (saline q8h) | 152 ± 23 | 0/28 | 0/4 | |||
| 5 mg/kg q24h | 58 ± 6 | 180 ± 42 | 157 ± 30 | 4/28 | Extremely minimal; NOELf | 0/4 |
| 5 mg/kg q8h | 58 ± 7 | 412 ± 78 | 483 ± 553 | 21/28cg | Extremely minimal | 0/4 |
CPK values for most dogs peaked on day 8.
The scarcity of lesions in study B necessitated the use of a more-sensitive severity scale than that used in study A. By standard evaluation criteria, lesions may not have been detectable in study B because the number of fibers affected was only 1 to 5 in 10,000.
Significantly different from the control group at a P value of 0.05, using Fisher's exact test.
Significantly different from the control group at a P value of 0.05, using Duncan's test.
Significantly different from the 25-mg/kg q24h group at a P value of 0.05, using Fisher's exact test.
NOEL, no-observable-effect level. The severity and incidence of lesions at 5 mg/kg q24h are considered comparable to those for the historical controls.
Significantly different from the 5-mg/kg q24h group at a P value of 0.05, using Fisher's exact test.
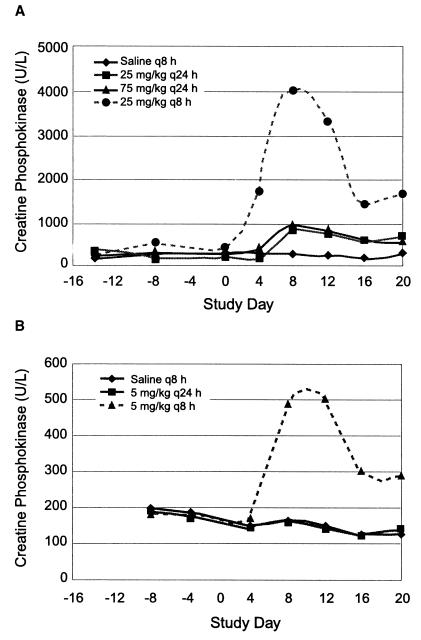
Elevations in serum CPK activity were highest upon dose fractionation as opposed to once-daily dosing. Daptomycin administration resulted in serum CPK elevations in the absence of clinical signs of adverse skeletal-muscle effects. Throughout the treatment period in study A, CPK activities were similar at 25 mg/kg q24h and 75 mg/kg q24h, despite the increase in total daily dose (Fig. 2A). However, an approximately fourfold increase in mean CPK activities was observed in animals dosed at 25 mg/kg q8h compared with those dosed at 75 mg/kg q24h, even though the total daily dose for these two regimens was the same (Fig. 2A; Table 1). CPK values generally reached peak elevations after approximately 8 days of dosing and then declined, despite continued treatment for an additional 12 days. In study B, CPK increases (three- to fourfold above baseline) were evident upon administration of the 5-mg/kg dose three times per day (q8h) whereas, as expected on the basis of previous data, no CPK elevations were observed upon administration of this dose once per day (Fig. 2B; Table 1).
FIG. 2.
Daily serum CPK activities 2 h postdosing in study A (A) and study B (B).
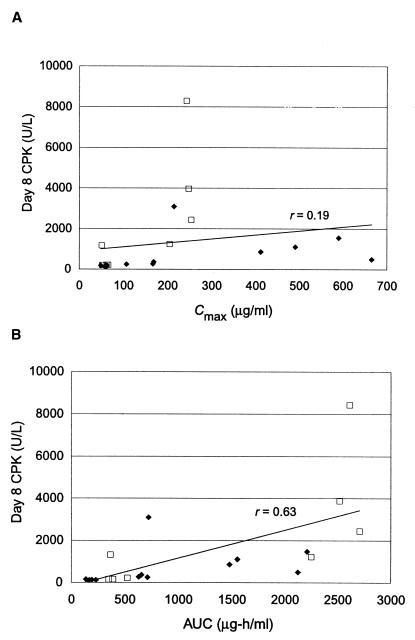
The impact of dosing frequency on skeletal-muscle effects is evident upon graphical analyses of the day 8 CPK levels versus pharmacokinetic values for individual dogs. Correlation between Cmax and CPK activity is poor (r = 0.19), and a twofold increase in CPK activity was observed only upon a sixfold increase in the Cmax (Fig. 3A). In addition, the q8h regimen resulted in several unexpectedly high CPK values, suggesting that dose fractionation leads to greater toxicity than does once-daily administration. Correlation between CPK activity and AUC, although better than that for Cmax, is still marginal (r = 0.63) (Fig. 3B). Again, the q8h regimens appear to have the greatest influence on the dose response.
FIG. 3.
Graphical analysis of day 8 pharmacokinetic values for individual dogs. (A) Correlation between the CPK activity and the Cmax. (B) Correlation between the CPK activity and the AUC for all dose regimens (q24h and q8h) and for q24h regimens alone. Solid line, regression line for all regimens; ♦, q24h regimens; □, q8h regimens.
Microscopic changes in skeletal muscle occurred with all dosing regimens, with increased incidences apparent in groups that received daptomycin three times daily (Table 1). The changes consisted primarily of minimal or extremely minimal degeneration and regeneration of myofibers. The incidence of myofiber lesions increased twofold upon dose fractionation of the 75-mg/kg daily dose to 25 mg/kg q8h; although not statistically significant, this increase was considered to be biologically relevant. A fivefold increase in the incidence of degeneration-regeneration was observed when either 5 or 25 mg/kg was administered q8h compared with q24h. Minimal, nonsuppurative inflammation was evident in the affected skeletal muscle; however, this response was secondary to degeneration. Changes were observed less frequently in the diaphragm than in other muscles. No microscopic changes were noted in the heart of any animal in either study.
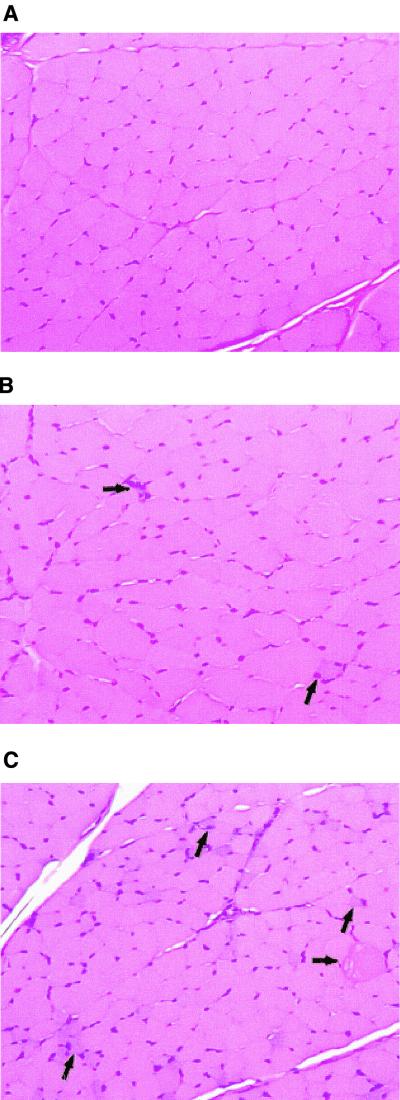
The severity of the muscle lesions observed was minimal at all dose regimens, irrespective of the increase in CPK activity. Only degenerative and regenerative changes were evident, with no necrosis, cell lysis, or fibrosis, regardless of the dose regimen. After 20 days of dosing at 25 mg/kg q8h, myofiber degeneration was minimal and was not accompanied by fibrosis (Fig. 4C). This dose regimen was associated with a peak (day 8) CPK increase of 4,000 IU/liter compared with the saline control value of 265 IU/liter. The microscopic lesions evident after administration of 75 mg/kg q24h were similar in severity to those observed at 25 mg/kg q8h; however, the number of myofibers affected was lower at 75 mg/kg q24h (Fig. 4B). At 5 mg/kg q24h and 5 mg/kg q8h, lesions were extremely minimal based on the number of fibers affected (1 to 5 of 10,000 fibers, or less than 0.05%). Because the number of fibers affected was so low, extensive examination was required to detect the lesions at 5 mg/kg q24h and 5 mg/kg q8h. Therefore, the incidence of affected muscles in study B was artificially inflated in comparison with that in study A. According to the evaluating pathologist (K.S.R.), the lesions observed at 5 mg/kg q24h were considered to be comparable to those for historical controls (i.e., the no-observable-effect level) and those at 5 mg/kg q8h were not considered to be biologically significant.
FIG. 4.
Histopathological lesions in the skeletal muscle. Shown are photomicrographs of cross sections of the left triceps muscles of dogs at a total magnification of ×183. (A) Treatment, saline q8h. (B) Treatment, daptomycin at 75 mg/kg q24h. A regenerative fiber (upward arrow) and degenerative fibers with foci of inflammatory cells (right arrow) are present. (C) Treatment, daptomycin at 25 mg/kg q8h. Multiple regenerative fibers characterized by enlarged nuclei, slightly basophilic cytoplasm, and small cross-sectional diameters (upward arrows), as well as a swollen, hyalinized, degenerative fiber (right arrow), are present.
DISCUSSION
The results of these investigative studies with dogs suggest that adverse skeletal-muscle effects associated with daptomycin are primarily related to dosing frequency and are not related to peak plasma concentrations. Both parameters of muscle effects assessed (CPK activity and microscopic changes) increased two- to fourfold upon fractionation of the daily dose from 75 mg/kg q24h to 25 mg/kg q8h. This difference was apparent despite the lower Cmax at 25 mg/kg q8h. Administration of dose regimens resulting in comparable Cmax values (i.e., 5 mg/kg q8h versus 5 mg/kg q24h, and 25 mg/kg q8h versus 25 mg/kg q24h) also led to disparate degrees of myopathy, suggesting that the effects are not driven by Cmax alone. Similarly, a disproportionate increase in the incidence of myopathy relative to the AUC0–24 was observed upon dose fractionation versus a dose increase. Although a threefold dose increase (i.e., 75 versus 25 mg/kg q24h) led to a threefold increase in both the AUC and the incidence of myopathy, dose fractionation (75 mg/kg q24h to 25 mg/kg q8h) resulted in a two- to fourfold increase in myopathy, with only a 37% increase in the AUC. Thus, neither the Cmax nor the AUC appears to be the key pharmacokinetic parameter determining daptomycin-associated skeletal-muscle effects in dogs.
Skeletal-muscle effects appear to be related to the duration of time between doses. In comparison with dose fractionation, once-daily dosing resulted in more time at low plasma drug concentrations, which may have led to more time for repair and, therefore, less potential for untoward effects. For example, at a dose regimen of 25 mg/kg q8h, the plasma drug concentrations never fell below 27 μg/ml, the trough value for this regimen (Fig. 1A). In contrast, plasma drug concentrations for the 75-mg/kg q24h regimen were below this level for approximately 12 h prior to administration of the next dose. This daily period of minimal exposure may allow for repair of subclinical damage to myofibers, which may explain why the once-daily dosing regimen (75 mg/kg q24h) was associated with less toxicity than was fractionated dosing (25 mg/kg q8h).
The hypothesis of dosing schedule-dependent repair may explain data from previous studies with dogs. The full reversibility of CPK elevations and microscopic lesions after cessation of dosing provides evidence that repair occurs (Toxicol. Rep. no. 14 and 28, Lilly). The presence of regenerative changes in skeletal muscle at the end of the dosing period indicates that repair is an ongoing process (Toxicol. Rep. no. 14 and 28, Lilly). The lack of progression of toxicity upon once-daily dosing at a constant dose for 1 to 6 months suggests that ongoing repair is sufficient to minimize accumulation of damage at this dosing interval (Toxicol. Rep. no. 14 and 28, Lilly). The increase in CPK activities with a q8h regimen versus a q24h regimen suggests that repair is less complete at 8 h postdosing than at 24 h postdosing, leading to accumulation of damage at the shorter dosing interval.
The pattern of CPK elevations over time may reflect the induction of myofiber tolerance to daptomycin. CPK elevations peaked at day 8 and decreased thereafter despite continued treatment. Because daptomycin does not enter the cytoplasm of mammalian cells (2) and microscopic evaluation on day 20 revealed no fibrosis indicative of prior cell lysis, daptomycin is presumed to cause leakage of intracellular CPK from the affected myofibers via membrane perturbations. The decrease in CPK levels after day 8 may reflect a reduction in the number of affected myofiber cells. This hypothesis is based on the finding of regeneration at all daptomycin dose regimens tested in these studies, as well as evidence that regenerated myofibers may be less sensitive to chemically induced damage than is mature muscle tissue (1, 10). Decreased sensitivity of regenerating fibers is also suggested by the low percentage of degenerative myofibers, as shown in Fig. 4. The decrease in serum CPK activities after 8 days of treatment is consistent with skeletal-muscle adaptation associated with repeated exercise (3).
Extrapolation of the results in dogs to humans suggests that the current clinical dose regimen of daptomycin should not be associated with adverse muscle effects. The degree of the daptomycin-related muscle effect is postulated to be related to the time allowed for repair between doses. The interval between doses in relation to the half-life of daptomycin in a given species defines the available repair time. Therefore, comparison of daptomycin's effects across species requires that the ratio of the dosing interval to the half-life be constant. For instance, administration q8h in dogs would be equivalent to administration q24h in humans, based on a dosing interval of 3 half-lives for both species. A dose regimen of 25 mg/kg q8h in dogs results in four- to sixfold-greater exposure than does the phase 3 clinical dose regimen of 4 mg/kg q24h, based on the comparison of Cmax values of 238 versus 55 μg/ml and AUC values of 2,526 versus 385 μg · h/ml. Although daptomycin exhibits significant plasma protein binding (∼90%), no adjustments are necessary for cross-species comparison because binding is independent of both concentration and animal species (5, 6; A. Louie, P. Kaw, W. Liu, N. Jumbe, M. H. Miller, and G. L. Drusano, submitted for publication; N. Safdar, D. R. Andes, and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1769, 1999). Inasmuch as the 25-mg/kg q8h regimen in dogs was associated with significant CPK elevations but only minimal microscopic myopathy, the clinical dose regimens under investigation (4 and 6 mg/kg q24h) are not anticipated to be associated with any clinically relevant skeletal-muscle effects.
Daptomycin-related skeletal-muscle effects in humans do not appear to be Cmax driven, based on interim results of an ongoing phase 2 trial. In this trial, no significant adverse muscle effects were observed in 26 patients receiving daptomycin at a regimen of 6 mg/kg q24h (M. F. DeBruin and F. P. Tally, 4th Decennial Int. Conf. Nosocom. Healthcare-Assoc. Infect., poster P-S2-37, 2000). The projected steady-state Cmax for this regimen (∼85 μg/ml) is slightly higher than that achieved at 4 mg/kg q12h (i.e., a Cmax of 70 to 80 μg/ml), a regimen at which skeletal-muscle weakness was observed in two of five subjects (9). Thus, extension of dosing schedule-dependent myopathy of daptomycin in dogs to humans suggests that once-daily dosing should result in a lower incidence of skeletal-muscle effects in patients than the same total daily dose administered on a fractionated regimen.
REFERENCES
- 1.Benoit P W, Belt W D. Destruction and regeneration of skeletal muscle after treatment with a local anaesthetic, bupivacaine (Marcaine®) J Anat. 1970;107:547–556. [PMC free article] [PubMed] [Google Scholar]
- 2.Canepari P, Boaretti M, del Mar Lleó M, Satta G. Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin ( LY146032) Antimicrob Agents Chemother. 1990;34:1220–1226. doi: 10.1128/aac.34.6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarkson P, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24:512–520. [PubMed] [Google Scholar]
- 4.Hanberger H, Nilsson L E, Maller R, Isaksson B. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob Agents Chemother. 1991;35:1710–1716. doi: 10.1128/aac.35.9.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaatz G W, Seo S M, Reddy V N, Bailey E M, Rybak M J. Daptomycin compared with teicoplanin and vancomycin for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1990;34:2081–2085. doi: 10.1128/aac.34.11.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee B L, Sachdeva M, Chambers H F. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob Agents Chemother. 1991;35:2505–2508. doi: 10.1128/aac.35.12.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybak M J, Bailey E M, Lamp K C, Kaatz G W. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob Agents Chemother. 1992;36:1109–1114. doi: 10.1128/aac.36.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szasz G, Gruber W, Bernt E. Creatine kinase in serum. 1. Determination of optimum reaction conditions. Clin Chem. 1976;22:650–656. [PubMed] [Google Scholar]
- 9.Tally F P, Zeckel M, Wasilewski M M, Carini C, Berman C L, Drusano G L, Oleson F B., Jr Daptomycin: a novel agent for Gram-positive infections. Exp Opin Investig Drugs. 1999;8:1223–1238. doi: 10.1517/13543784.8.8.1223. [DOI] [PubMed] [Google Scholar]
- 10.Van Vleet J F, Ferrans V J, Herman E. Cardiovascular and skeletal muscle systems. In: Haschek W M, Rousseaux C G, editors. Handbook of toxicologic pathology. San Diego, Calif: Academic Press, Inc.; 1991. pp. 539–624. [Google Scholar]
- 11.Woodworth J R, Nyhart E H, Jr, Brier G L, Wolny J D, Black H R. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob Agents Chemother. 1992;36:318–325. doi: 10.1128/aac.36.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]