Objectives:
Current literature is lacking a comprehensive review of data on dietary interventions in blood pressure (BP) management in sub-Saharan African countries. We assessed the association of dietary and other lifestyle interventions with BP-lowering effects in populations within sub-Saharan Africa.
Methods:
We performed a systematic review and random-effects meta-analysis to determine the impact of dietary and lifestyle interventions on SBP and DBP in sub-Saharan Africa. We searched the MEDLINE, EMBASE, and Web of Science databases. We included intervention studies that were randomized and nonrandomized conducted in Africans residing in sub-Saharan Africa investigating diet and other lifestyle, physical activity, weight loss, tobacco, and alcohol cessation modifications. We determined the effect of diet and other lifestyle interventions on SBP and DBP. We expressed effect size as weighted mean difference and 95% confidence interval (CI).
Main results
: We identified six studies with a total of 1412 individuals, 38% males, mean age of 52.8 years (SD = 11.5). The weighted mean difference of dietary and other lifestyle interventions on SBP and DBP was −7.33 mmHg, (95% CI: −9.90 to −4.76, P < 0.001) and −2.98 mmHg, (95% CI: −4.28 to −1.69, P < 0.001), respectively. In the metaregression analyses, the duration of the interventions did not have any effect on changes in SBP and DBP.
Principal conclusion
: Dietary modifications showed a beneficial overall improvement in SBP and DBP in Africans. However, aside from low-salt interventions, studies on dietary potassium, healthy dietary patterns, and lifestyle modifications have not been investigated extensively in Africans and are in critical need. In addition, researchers will need to consider the settings (rural, urban, or semiurban) and the predominant existing dietary habits while designing studies on dietary interventions in sub-Saharan Africa.
PROSPERO registration:
CRD42020207923.
Keywords: diet, hypertension, interventions, lifestyle, sub-Saharan Africa
INTRODUCTION
Hypertension is an important public health problem worldwide because it is a cardinal risk factor for cardiovascular morbidity and mortality. SBP is the leading contributor to disability-adjusted life years [1,2]. A worldwide study of 19.1 million people showed that in the 40 years between 1975 and 2015, the burden of hypertension and other noncommunicable diseases has shifted from high-income to low-income regions such as sub-Saharan Africa [3–7].
The number of adults with hypertension is projected to increase to 1.56 billion by the year 2025 [8]. Both genetic and environmental factors may affect blood pressure (BP) [9]. Of the environmental factors affecting BP, dietary factors have a predominant role in BP homeostasis [9]. The African diet is largely made up of leafy greens, root vegetables, tubers like cassava, potatoes, sweet potatoes, yams, and grains like beans, lentils, and black-eyed peas. Starches and whole grains include maize, corn, millet, rice, sorghum, injera, barley, couscous, and fonio, and protein from meat, poultry, milk, and eggs. Comparatively, the typical US diet consists of ultra-processed food, which is high in saturated fat, sodium, added sugar, lower than recommended fruits, vegetables, whole grains, and lower than normal dietary fiber. The US diet is also high in saturated fatty acids and red meat [10]. Since some sub-Saharan countries are geographically and economically disparate from high-income countries, it is important to investigate which dietary and other lifestyle interventions have been successfully implemented to lower BP in various sub-Saharan countries.
In the US, increased potassium intake, reduced sodium intake, and consumption of dietary patterns such as the dietary approach to stop hypertension (DASH) lower BP [11–13]. However, the current literature is lacking a comprehensive review of data on dietary and lifestyle interventions reducing BP in sub-Saharan Africa. Therefore, the objective of this study is to determine the effect of dietary and lifestyle interventions on SBP and DBP in Africa.
METHODS
The current review is based on a protocol registered on PROSPERO: CRD42020207923. We performed a systematic review and metanalysis of dietary and lifestyle interventions on SBP and DBP in sub-Saharan Africa, guided by PRISMA Statement [14].
Systematic review
Search strategy
We utilized a predefined, comprehensive, and sensitive search strategy combining Medline, EMBASE, and Web of Science. We also used the African filter developed and tested on MEDLINE and EMBASE with the names of countries in Africa to obtain the maximum possible number of studies [15]. This filter included the names of each sub-Saharan African country and shortened terms to capture studies from regions. Table S1 shows our search strategy for the metanalysis.
Eligibility
We included interventional studies that were either randomized control trials (RCTs) or nonrandomized pre and post-intervention studies, investigating lifestyle interventions for BP management in sub-Saharan Africa. Studies published up till November 2021 were considered. We restricted our studies to those that included participants aged 18 years and above. We defined hypertension as SBP of more than 140 mmHg and DBP of more than 90 mmHg, and/or self-reported antihypertensive medication use. We included interventions that lasted at least 3 weeks.
We excluded studies of the population of Africans living outside Africa. We also excluded studies involving pregnant women.
The interventions were dietary and lifestyle modifications in studies conducted in Africans living in sub-Saharan Africa. We included interventions lasting at least 3 weeks. The search strategy for interventions included but was not limited to diet and dietary patterns, such as the DASH diet, Mediterranean diet, carbohydrate, high-protein, low-fat, vegetarian, low-sodium, low-glycemic index, and paleolithic diets. Interventions were selected whether or not they were applied alone or in combination with drugs or other lifestyle interventions such as physical activity, tobacco cessation, or alcohol intake modification.
Controls/comparator
We considered usual care or control or other treatment modalities for high BP if available as comparators. Otherwise, we included studies that showed results at baseline and after an intervention was applied.
Data extraction process
Two independent investigators (A.Z. and R.V.) reviewed the articles, titles, and abstracts of the full texts where relevant. The final inclusion was based on full article review. In addition, the reviewers screened references of the selected articles to identify additional articles of interest. When there were disagreements, we consulted a third reviewer (T.I.), and a consensus was reached.
We extracted data from eligible studies using organized data extraction sheets in a single excel file. Information was collected on authors, country, year of publication, language of publication, type of publication, and study design (randomized trial or cross-over trial). We also included age, sex, sample size, diagnostic criteria for hypertension, mean baseline SBP and DBP, mean BMI, and how BP was measured. Other factors considered were body weight, medication use, especially antihypertensive medication, dietary interventions, dietary protocols, dietary assessment methods, and physical activity status. We examined the presence of associated comorbidities cardiovascular disease, type 2 diabetes, the presence of control groups, the dietary intervention of choice, specification of control groups (if present), loss to follow-up, and funding source.
Metanalysis
Data synthesis
The mean change from baseline in the levels of the outcome variables of interest (SBP and DBP) and standard error (SE) for both intervention and control groups were used to calculate the effect size. The effect size was defined as the weighted mean difference calculated by net changes in measurements (change scores). For RCTs, change scores were calculated as measurement at the end of follow-up in the treatment group minus measure at the end of follow-up in the control group. For nonrandomized pre and postintervention studies, individuals’ BP was measured before and after interventions. Change scores were calculated as differences in the post-intervention measurement minus baseline measurement. Where only the standard deviation was reported, we calculated the SE. We derived SEs from these effect estimates using the formulas from the Cochrane training handbook. We used discrete likelihood methods for the metanalysis of proportions and rates. For studies with sufficient data, we performed meta-analyses by random-effects models (DerSimonian–Laird method) to determine the pooled relative effect of each intervention relative to every other intervention in terms of postintervention values or changes from baseline scores of different lifestyle interventions.
To assess for publication bias, we used the funnel plot as a visual measure of precision against the average intervention effect and Begg's rank correlation, and Egger's weighted regression [16]. A Cochrane risk of bias tool was also used to evaluate any methodological bias in the studies. We assessed for selection bias, performance bias, detection bias, attrition bias, and reporting bias. Each trial was then classified as low, high, or unclear risk of bias.
Additional analyses of subgroups or subsets
We used the I2 value to determine the heterogeneity of the studies. Where heterogeneity or inconsistency existed, we used subgroup analyses and metaregression analyses to identify possible sources. Low, moderate, and high I2 values were 25, 50, and 75%, respectively. When heterogeneity was substantial, we used a prediction interval rather than a confidence interval (CI) to capture uncertainty around the effect estimate. We explored the potential impact of intervention duration on calculated weighted mean difference using meta-regression. A mixed-effects model was applied with study length centered to 2 months for the meta-regression. To evaluate the influence of each study on the overall effect size, a sensitivity analysis was conducted using the leave-one-out method. We analyzed the data by leaving out one study at a time to see if the results changed. This would indicate if there was one specific study driving the results (i.e. removing one study each time and repeating the analysis).
RESULTS
Review process
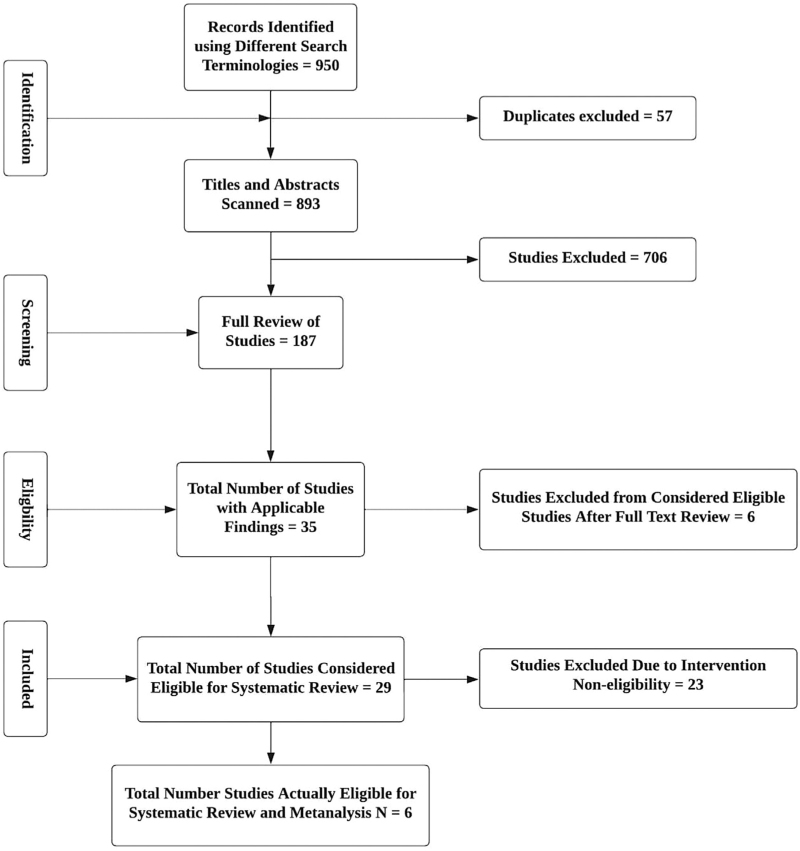
We identified 950 records using the different search terminologies. Figure 1 shows the selection process for articles included in the final systematic review and meta-analysis. After removing duplicates (n = 57), we scanned titles and abstracts of the remaining 893 studies. After excluding 706 irrelevant studies, we selected 187 studies for the full review. After further examination of the studies, we included six studies in the final systematic review and meta-analysis (Fig. 1).
FIGURE 1.
Process of study selection for the meta-analysis on the impact of dietary and lifestyle interventions on blood pressure management in sub-Saharan Africa. This figure shows the process of study selection for the meta-analysis of the impact of dietary and lifestyle interventions on blood pressure in sub-Saharan Africa: study selection for meta-analysis shows the identification, screening, eligibility, and inclusion of participants.
The six intervention-type studies were RCTs (n = 5) and one nonrandomized pre and post-intervention study (n = 1). Details on the type of studies, countries of location, and mean age and SD of the studies included in the metanalysis are seen in Table 1. We included a total of 1412 individuals, 38% of the entire population was male (n = 548), and the mean age of the population was 52.8 years (SD = 11.5).
TABLE 1.
Studies of dietary and lifestyle interventions on lifestyle interventions on blood pressure management in sub-Saharan Africa
| First author (year of publication) | Country | Mean age (SD) (years) | Total sample size | Male (%) | Intervention | Control | Study type | Duration | |
| 1. | Siervo (2020) | Tanzania (rural) | 60.7 ± 6.3 | 33 | 17 | Combined intervention (N = 11): high-nitrate beetroot juice (∼400 mg) and folic acid (∼5 mg); Single intervention (N = 12): high-nitrate beetroot juice (∼400 mg) and placebo | Nitrate-depleted beetroot juice and placebo (n = 10) | RCT double-blind placebo | 60 days |
| 2. | Schouw (2020) | South Africa (urban) | 42.7 ± 9.7 | 137 | 64 | Healthy choices at work program focused on food services, physical activity, health and wellness services, and managerial support | Individuals were their own controls – paired pre and post-intervention | Pre and postintervention | 24 months |
| 3. | Babiker (2018) | Sudan (urban) | 50.09 ± 9.3 | 91 | 19.7 | Consumption of 30-g gum Arabic (n = 46) (fiber) | Consumption of 5-g Pectin (N = 45) | RCT double-blinded, placebo-controlled | 3 months |
| 4. | Charlton (2008) | South Africa (periurban) | 61.7 ± 7.9 | 80 | 16.3 | 8-week provision of six food items with a modified cation content (salt replacement (SOLO), bread, margarine, stock cubes, soup mix, and a flavor enhancer) and 500 ml of mass (fermented milk)/d.(low-salt, high-potassium, and low-fat and dairy) | The control diet provided the same quantities of the targeted foods but of standard commercial composition and 500 ml/day of artificially sweetened drink | RCT | 8 weeks |
| 5. | Cappuccio (2006) | Ghana (rural) | 54.7 ± 11.3 | 1013 | 38 | Health promotion activity which included dietary prevention of hypertension, advice not to add salt to food, limiting the amount of salted fish and beef consumed | Health promotion message on prevention of malaria and diarrhea and enhancing awareness of diabetes and hypertension. No mention of dietary interventions and hypertension | Cluster randomized trial | 6 months |
| 6. | Forrester (2005) | Nigeria (rural) | 46.6 ± 8.3 | 58 | 58.6 | Low (50 meq) reduction from baseline or high-salt diet (64 meq of salt) for 3 weeks followed by a 2-week wash-out and then crossover for 3 weeks | Baseline line state (recorded difference in high-salt vs. low-salt diet) | RCT with crossover design | 3 weeks then crossover total = 6 weeks |
RCT, randomized control trial.
Systematic review
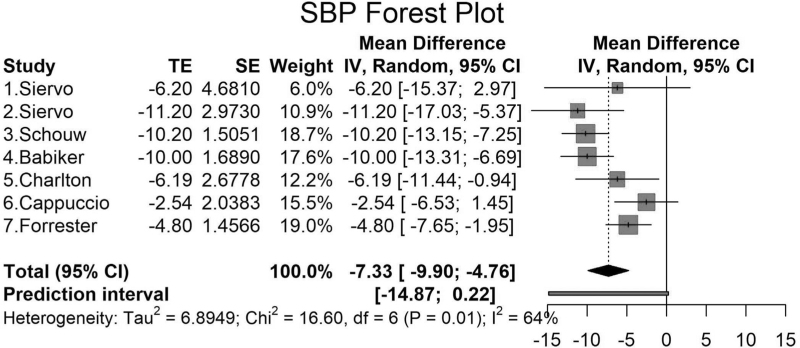
Siervo et al.[17] showed in a pilot RCT that inorganic nitrate supplementation (in beetroot juice) with and without folic acid significantly reduced SBP, but DBP was only significantly reduced in the nitrate and placebo groups. Using a work-based wellness program with dietary and lifestyle changes, Schouw et al.[18] found significant reductions in SBP and DBP. A high-fiber intervention by Babiker et al.[19] using gum Arabic (30-g gum Arabic vs. 5-g Pectin) on BP also showed a significant reduction in SBP but not in DBP (Figs. 2 and 3).
FIGURE 2.
Mean difference and standard errors for the net effect of dietary and other lifestyle interventions on SBP (meta-analysis). The mean differences in SBP and 95% confidence intervals and standard errors are shown for the intervention and control groups for the populations analyzed in the meta-analysis. First, results are reported in mmHg. Horizontal lines are reported as 95% confidence interval. Second, studies (1) and (2) by Siervo are the same study with two intervention groups [17], include two groups (high-nitrate beetroot juice with folic acid (1) and high-nitrate beetroot juice without folic acid (2). Third, Chi2, chi-square statistic; CI, confidence interval; df, degree of freedom; I2, I-square heterogeneity statistic; IV, weighted mean difference; SE, standard error of treatment Effect; Tau2, between-study variance; TE, treatment effect.
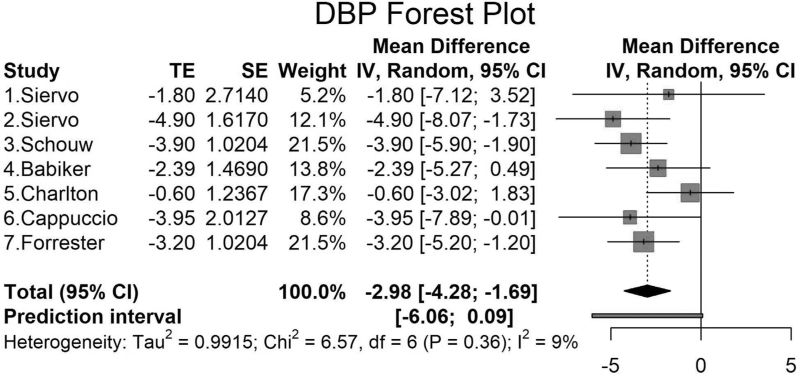
FIGURE 3.
Mean difference and standard errors for the net effect of dietary and other lifestyle interventions on DBP (meta-analysis). The mean differences in DBP and 95% confidence intervals and standard errors are shown for the intervention and control groups for the populations analyzed in the meta-analysis. First, results are reported in mmHg. Horizontal lines are reported as 95% confidence interval. Second, studies 1 and 2 by Siervo are the same study with two intervention groups [17], include two groups (high-nitrate beetroot juice with folic acid (1) and high-nitrate beetroot juice without folic acid (2). Third, Chi2, chi-square statistic; CI, confidence interval; df, degree of freedom; I2, I-square heterogeneity statistic; IV, weighted mean difference; SE, standard error of treatment effect; Tau2, between-study variance; TE, treatment effect.
Charlton et al.[20] tested salt replacement substitutes in six specified foods in an RCT and found a significant reduction in SBP but not DBP (Figs. 2 and 3). Cappuccio et al.[21] reported a large cluster randomized trial involving (n = 1013) over 6 months, using a health promotion activity counseling on low-salt diet and BP. The study by Cappuccio using a health promotion activity counseling on low-salt diet and BP showed a significant change in DBP but not SBP (Fig. 2) [11]. The study by Forrester et al.[22] measured changes in BP on a high-sodium vs. low-sodium diet and found a significantly lower SBP and DBP −4.8 mmHg (95% CI: −7.65 to −1.95) and −3.20 mmHg (95% CI: −5.20 to −1.20), respectively (Figs. 2 and 3).
Not all studies characterized the number of individuals with hypertension at baseline [18]. Siervo et al.[17] reported that 39 of 47 participants had grade 1 and 2 hypertension at baseline, and five individuals who started BP medication during the trial. Babiker et al.[19] reported a baseline prevalence of hypertension of 30.4 and 13.3% in the intervention and control groups, respectively. The study by Charlton et al. in South Africa included individuals with mild-to-moderate hypertension (SBP ≤ 160 mmHg and DBP ≤ 95 mmHg) at baseline [19,20]. In the study by Cappuccio et al.[21], there were 30% of hypertensive individuals in the intervention group and 28% of hypertensives in the control group. The study by Babiker et al.[19] focused on individuals with type 2 diabetes. Only one study, Babiker et al.[19] reported a change in BMI with the high-fiber gum Arabica intervention in addition to SBP reduction.
Metanalysis
Figure 2 shows the forest plots for the mean differences and SEs for the net effect of dietary and lifestyle interventions on SBP. Overall, the net SBP change ranged from −2.54 to −11.2 mmHg. The overall pooled net effect of dietary and other lifestyle interventions on SBP was −7.33 mmHg (95% CI: −9.90 to −4.76). We found that the largest net SBP reduction was in a study in a placebo-controlled, double-blind trial, testing high-nitrate beetroot juice with folic acid/placebo vs. nitrate-depleted beetroot juice and placebo to lower BP [17].
Overall, the net DBP change ranged from −0.60 to −4.90 mmHg (Fig. 3). The overall pooled net effect of diet and lifestyle interventions on DBP was −2.98 mmHg (95% CI: −4.28 to −1.69), as seen in Fig. 3.
The estimated effect size for the impact of dietary and lifestyle on SBP and DBP was robust when we performed a sensitivity analysis leaving out each study (Fig. S3A, Fig. S3B). If the P value of the new model leaving out a study is more than 0.05 (or other alpha level as indicated), then the overall results are sensitive to the study taken out.
We found moderate heterogeneity between our studies for SBP (I2 = 64%, P = 0.01) but not for DBP (I2 = 9%, P = 0.36) (Figs. 2 and 3). The funnel plots show that there was no evidence of bias in the studies for SBP or DBP (Fig. S1). The metaregression analyses showed that there was no significant effect of the duration of the intervention on the changes in SBP or DBP in the populations (Fig. S2). Four of the studies provided sample size calculations, and three out of four of the studies provided power analyses that were based on estimates, and one of the studies was a feasibility trial. Two studies did not provide sample size analyses (Table S2). Overall, there was one high-quality study and others with some concerns for bias.
DISCUSSION
The current meta-analysis of six intervention studies and a total of 1412 participants in sub-Saharan Africa showed that there was a significant reduction in SBP and DBP with dietary interventions. The interventions tested in the metanalysis included low-salt diets, high-nitrate beetroot juice, and programs involving dietary counseling and behavioral modifications.
Gay et al.[23] reported a meta-analysis of 24 trials with a total of 23 858 participants, the overall net effect on SBP and DBP was −3.07 mmHg (95% CI −3.85 to −2.30) and −1.81 mmHg (95% CI −2.24 to −1.38), respectively. However, the study from Gay et al. did not focus on studies based in sub-Saharan Africa. Our metanalysis showed an overall higher net difference in SBP and DBP; however, compared with other studies done in other regions, we are limited by the small sample sizes resulting in larger CIs around our pooled effects. Our study also differs from other meta-analysis where DASH-type dietary patterns rich in fruits and vegetables or a ‘combination’ diet rich in fruits, vegetables, and low-fat dairy products with reduced saturated and total fat showed the lowest reduction in BP [23]. We were not able to make inferences on interventions in sub-Saharan because of the paucity of studies.
Our metaregression analyses showed that the duration of the intervention did not significantly change the SBP or DBP. Other trials examining dietary and lifestyle interventions and BP have shown similar findings [23,24]. However, it is possible that because this is a subgroup analysis, we may be missing an existing effect of the duration of the intervention on SBP or DBP.
Although the majority of our population were women (62%), we did not find any sex-based differences in BP. Data from the National Health and Nutrition Examination Survey in the US shows us that hypertensive women are significantly more likely to be treated than men but less likely to achieve BP control [25]. However, post-hoc analysis of the Comparison of three Combination Therapies in lowering Blood Pressure in Black Africans (CREOLE) trial showed that African women might have significantly lower BP compared with men after treatment for 6 months [26]. Therefore, it is plausible that the treatment effect may have been larger than expected because we have a higher percentage of women in our population. We did not find any sex difference in dietary and lifestyle control for BP in our aggregated data or in the individual-level data.
The greatest reduction in BP was seen in the study by Siervo et al.[17], testing the effect of high-nitrate beetroot juice. Dietary inorganic nitrate is a substrate for nitric oxide, which is found in green leafy vegetables and beetroot and has been shown to be a nutritional antihypertensive agent [17,27]. The Siervo study was a randomized controlled feasibility trail that had three arms, with the high-nitrate and placebo groups showing the greatest difference [17]. The next greatest reduction was seen in the ‘Healthy choices at work’ study in South Africa that paired 137 employees and involved pre and post-intervention and focused on food services, physical activity, health and wellness services, and managerial support [18]. However, this study has its limitations of being a pre and postintervention study without a control group, and the randomization was not completed. Existing studies in the metanalysis focused primarily on sodium intake, and there was less focus on interventions that included other dietary nutrients like increased potassium to reduce BP. Two of the studies tested community-based educational and behavioral interventions to reduce BP [11,20].
Previous studies have assumed that because of the low number of processed foods consumed in sub-Saharan rural and semiurban areas, interventions should focus on community-based health promotion of dietary and lifestyle intervention [28,29]. However, with the increase in the fast-food industry and adoption of Western diets in sub-Saharan Africa, future intervention community-based dietary counseling/behavior modifications may not be adequate for more urban-type settings. Future studies may need to pilot what interventions would be appropriate for different settings in settings (rural, semi-urban, or urban areas). Involvement of the food industry in the urban settings may become increasingly important.
Our study shows significant clinical and public health implications. First, aside from medications, dietary interventions are effective for lowering SBP and DBP in African populations. With the increase in westernized diets and processed foods, interventions employed in sub-Saharan Africa may need to be tailored to suit populations in rural, semiurban, or urban settings. Finally, some of the challenges which may need to be considered in the design of future studies will be adherence to interventions, prevention of contamination across groups, the small size of many of the African studies. We need larger RCTs, including cluster designs, on the African continent that are rigorously conducted, like the Salt Substitute and Stroke Study and the China Rural Health Initiative Salt Reduction Study [30].
We conducted a rigorous search of publications showing the impact of dietary interventions on BP in Africa. We used three comprehensive databases to complete our search. We had rigorous inclusion and exclusion criteria limiting studies to those that involved RCTs or nonrandomized pre and postintervention studies. Despite the strengths in the approach and methodology of our systematic review and metanalysis, we found that very few studies met inclusion and exclusion criteria, thus limiting our metanalysis to only six studies. The metanalysis needs to be interpreted with caution because we did not have many studies included in the final analyses, and there was significant heterogeneity for SBP within the studies.
In conclusion, overall, there was a pooled effect on the reduction in both SBP and DBP from all the studies in the metanalysis, which considered an array of dietary modifications. Aside from dietary sodium interventions, there is a need for studies focused on other dietary nutrients like potassium, healthy dietary patterns, and lifestyle interventions like physical activity, smoking, and alcohol cessation. In addition, it may be beneficial to scale up studies to larger RCTs tailored for populations in the rural, urban, semiurban areas. Large high-quality trials in sub-Saharan Africa addressing the impact of dietary interventions on BP will be important in informing clinical practice and perhaps policy decision-making.
ACKNOWLEDGEMENTS
The authors would like to thank Elizabeth Jenkins, Boston University Alumni Medical Library, for putting together the search terms for the metanalysis. The authors would also like to thank the Boston University Master of Science in Statistical Practice Consulting Group for the assistance with the analysis.
T.O.I. is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K23DK119542 and the Department of Medicine, Boston Medical Center. Funding for this work was received from the National Institute of Health (NIH) to T.O.I. DK-119542.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Abbreviations: BP, blood pressure; CKD, chronic kidney disease
Supplemental digital content is available for this article.
REFERENCES
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997; 349:1269–1276. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lauer JA, Hutubessy RC, Niessen L, Tomijima N, Rodgers A, et al. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. Lancet 2003; 361:717–725. [DOI] [PubMed] [Google Scholar]
- 3.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017; 389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addo J, Smeeth L, Leon DA. Hypertension in sub-Saharan Africa: a systematic review. Hypertension 2007; 50:1012–1018. [DOI] [PubMed] [Google Scholar]
- 5.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet 2011; 377:568–577. [DOI] [PubMed] [Google Scholar]
- 6.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 7.Devi P, Rao M, Sigamani A, Faruqui A, Jose M, Gupta R, et al. Prevalence, risk factors and awareness of hypertension in India: a systematic review. J Hum Hypertens 2013; 27:281–287. [DOI] [PubMed] [Google Scholar]
- 8.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223. [DOI] [PubMed] [Google Scholar]
- 9.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006; 47:296–308. [DOI] [PubMed] [Google Scholar]
- 10.Urquiaga I, Guasch V, Marshall G, San Martín A, Castillo O, Rozowski J, et al. Effect of Mediterranean and Occidental diets, and red wine, on plasma fatty acids in humans. An intervention study. Biol res 2004; 37:253–261. [DOI] [PubMed] [Google Scholar]
- 11.Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens 1991; 9:465–473. [DOI] [PubMed] [Google Scholar]
- 12.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 1997; 277:1624–1632. [DOI] [PubMed] [Google Scholar]
- 13.Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens 2003; 17:471–480. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pienaar E, Grobler L, Busgeeth K, Eisinga A, Siegfried N. Developing a geographic search filter to identify randomised controlled trials in Africa: finding the optimal balance between sensitivity and precision. Health Inform Libr J 2011; 28:210–215. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 17.Siervo M, Shannon O, Kandhari N, Prabhakar M, Fostier W, Köchl C, et al. Nitrate-rich beetroot juice reduces blood pressure in Tanzanian adults with elevated blood pressure: a double-blind randomized controlled feasibility trial. J Nutr 2020; 150:2460–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schouw D, Mash R, Kolbe-Alexander T. Changes in risk factors for noncommunicable diseases associated with the ‘Healthy choices at work’ programme, South Africa. Glob Health Action 2020; 13:1827363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babiker R, Elmusharaf K, Keogh MB, Saeed AM. Effect of Gum Arabic (Acacia Senegal) supplementation on visceral adiposity index (VAI) and blood pressure in patients with type 2 diabetes mellitus as indicators of cardiovascular disease (CVD): a randomized and placebo-controlled clinical trial. Lipids Health Dis 2018; 17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlton KE, Steyn K, Levitt NS, Peer N, Jonathan D, Gogela T, et al. A food-based dietary strategy lowers blood pressure in a low socio-economic setting: a randomised study in South Africa. Public Health Nutr 2008; 11:1397–1406. [DOI] [PubMed] [Google Scholar]
- 21.Cappuccio FP, Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB. A community programme to reduce salt intake and blood pressure in Ghana. BMC Public Health 2006; 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrester T, Adeyemo A, Soarres-Wynter S, Sargent L, Bennett F, Wilks R, et al. A randomized trial on sodium reduction in two developing countries. J Hum Hypertens 2005; 19:55–60. [DOI] [PubMed] [Google Scholar]
- 23.Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension 2016; 67:733–739. [DOI] [PubMed] [Google Scholar]
- 24.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002; 136:493–503. [DOI] [PubMed] [Google Scholar]
- 25.Gu Q, Burt VL, Paulose-Ram R, Dillon CF. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among US adults with hypertension: data from the National Health and Nutrition Examination Survey 1999–2004. Am J Hypertens 2008; 21:789–798. [DOI] [PubMed] [Google Scholar]
- 26.Ojji DB, Shedul GL, Sani M, Ogah OS, Dzudie A, Barasa F, et al. A differential response to antihypertensive therapy in African men and women: insights from the CREOLE trial. Am J Hypertens 2022; 35:551–560. [DOI] [PubMed] [Google Scholar]
- 27.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. New Engl J Med 2006; 355:2792–2793. [DOI] [PubMed] [Google Scholar]
- 28.Cappuccio FP, Plange-Rhule J, Phillips RO, Eastwood JB. Prevention of hypertension and stroke in Africa. Lancet 2000; 356:677–678. [DOI] [PubMed] [Google Scholar]
- 29.Cappuccio FP, Miller MA. Cardiovascular disease and hypertension in sub-Saharan Africa: burden, risk and interventions. Intern Emerg Med 2016; 11:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neal B, Tian M, Li N, Elliott P, Yan LL, Labarthe DR, et al. Rationale, design, and baseline characteristics of the Salt Substitute and Stroke Study (SSaSS) – a large-scale cluster randomized controlled trial. Am Heart J 2017; 188:109–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.