Abstract
Background:
Patients living in rural communities experience difficulty accessing specialized medical care. Rural patients with cancer present with more advanced disease, have reduced access to treatment and have poorer overall survival than urban patients. This study’s aim was to evaluate outcomes of patients with gastric cancer living in rural and remote areas versus urban and suburban communities in the context of an established care corridor to a tertiary care centre.
Methods:
All patients treated for gastric cancer at the McGill University Health Centre during 2010–2018 were included. Travel, lodging and cancer care coordination were provided for patients from remote and rural areas and coordinated centrally by dedicated nurse navigators servicing these regions. Statistics Canada’s remoteness index was used to categorize patients into a rural and remote group and an urban and suburban group.
Results:
A total of 274 patients were included. Compared with patients from urban and suburban areas, patients from rural and remote areas were younger and their clinical tumour stage was higher at presentation. The number of curative resections and palliative surgeries and rate of nonresection were comparable (p = 0.96). Overall, disease-free and progression-free survival were comparable between the groups, and having locally advanced cancer correlated with poorer survival (p < 0.001).
Conclusion:
Although patients with gastric cancer from rural and remote areas had more advanced disease at presentation, their treatment patterns and survival were comparable to those of patients from urbanized areas in the context of a publicly funded care corridor to a multidisciplinary specialist cancer centre. Equitable access to health care is necessary to diminish any preexisting disparities among patients with gastric cancer.
Abstract
Contexte:
Les malades des communautés rurales ont de la difficulté à accéder à des soins médicaux spécialisés. Les personnes cancéreuses des milieux ruraux ont une maladie plus avancée, un accès moindre au traitement et une moins bonne survie globale comparativement aux malades des milieux urbains. Cette étude avait pour but de comparer les résultats chez les malades aux prises avec un cancer de l’estomac qui vivent en région rurale ou éloignée et chez ceux qui vivent en ville ou en banlieue dans le contexte d’un corridor de services dument affilié à un centre de soins tertiaires.
Méthodes:
Nous avons inclus tous les malades traités pour un cancer de l’estomac au Centre universitaire de santé McGill de 2010 à 2018. Les déplacements, l’hébergement et la coordination des traitements oncologiques étaient fournis aux patients de régions rurales et éloignées, sous la coordination centrale d’un personnel infirmier pivot attitré desservant ces régions. L’indice d’éloignement de Statistique Canada a servi à catégoriser les malades selon qu’ils appartenaient à un groupe de région rurale et éloignée ou à un groupe de région urbanisée.
Résultats:
En tout, 274 patients ont été inclus. Comparativement aux malades de la ville ou de la banlieue, les malades des régions rurales et éloignées étaient plus jeunes, et le stade clinique de leur tumeur était plus élevé au moment de consulter. Le nombre de résections à visée curative, de chirurgies palliatives et le taux de non-résection étaient comparables (p = 0,96). Globalement les groupes ont enregistré des taux comparables de survie sans maladie et de survie sans progression, et les cancers localement avancés étaient en corrélation avec une moins bonne survie (p < 0,001).
Conclusion:
Même si les malades aux prises avec un cancer de l’estomac provenant de régions rurales et éloignées avaient une maladie plus avancée au moment de consulter, leurs modalités thérapeutiques et leur survie ont été comparables à celles des malades de régions urbanisés dans le contexte d’un corridor de services financés à même les fonds publics donnant accès à un centre d’oncologie multidisciplinaire. Un accès équitable aux soins de santé est nécessaire pour réduire les disparités existantes entre les malades atteints d’un cancer de l’estomac.
Approximately 20% of North Americans (70 million people) live in rural communities.1–3 Among patients with cancer, residence in a rural area has been correlated with more advanced disease stage at diagnosis and poorer overall survival than residence in an urban area.4–11 This relationship has been demonstrated for many types of cancers worldwide and for gastric cancer in China.12
People residing in rural areas have limited access to diagnostic and treatment services and increased travel costs, which together represent substantial barriers to early diagnosis, access to optimal cancer treatment and compliance with prescribed treatment regimens.13–15 In addition, many patients from rural areas choose not to avail themselves of necessary health care services when out-of-pocket costs are high.16 For patients with cancer in particular, a lack of service coordination can lead to fragmented care, loss of patients to follow-up and failure to access appropriate services.14
To address the challenges experienced by rural patients in accessing necessary health care services, the provincial health care authority in the province of Quebec, Canada, has established care corridors between rural and urban areas to facilitate access to specialized health care services.14 Patients residing in remote areas who require health care services not available close to home are provided transportation to and from urban areas, room and board during treatment and multidisciplinary support services as needed. For patients with cancer, these services also include access to a dedicated oncology nursing case manager to coordinate their care across specialties and treatment sites.14
As gastric cancer is relatively rare in Canada, multidisciplinary care is essentially available only in highly urbanized centres. Although provincially funded transit and lodging services have been offered to rural patients with gastric cancer for nearly a decade, the impact of this care model on the outcomes of these patients has not been evaluated, to our knowledge. The goal of this study, therefore, was to examine the impact of a publicly funded corridor of care on gastric cancer outcomes by comparing rural and urban patients treated at a centralized referral centre.
Methods
Study population
All patients who presented with gastric adenocarcinoma to the Montreal General Hospital from January 2010 to December 2018 were identified from a prospectively collected database. Patients with Siewert III tumours were included while patients with esophageal and Siewert I and II tumours were excluded. All patients were managed according to the FLOT4 trial protocol with preoperative taxane-based triplet chemotherapy or were enrolled in clinical systemic therapy trials as available. Demographic and outcomes data were collected prospectively and verified by a review of the patient chart and electronic medical record. Ethics approval was obtained from the McGill University Health Centre Research Ethics Board (file nos. 2019–5085 and 2020–5981).
Geographic distribution
The remoteness index is a composite measure of community size and distance from major cities that was developed by Statistics Canada.17 A remoteness index below 0.1 was defined as urban and suburban (U) while a remoteness index of 0.1 or higher was defined as rural and remote (R). Raw data were obtained from Statistics Canada, and the 2016 remoteness index was used for each census subdivision.
Care corridor
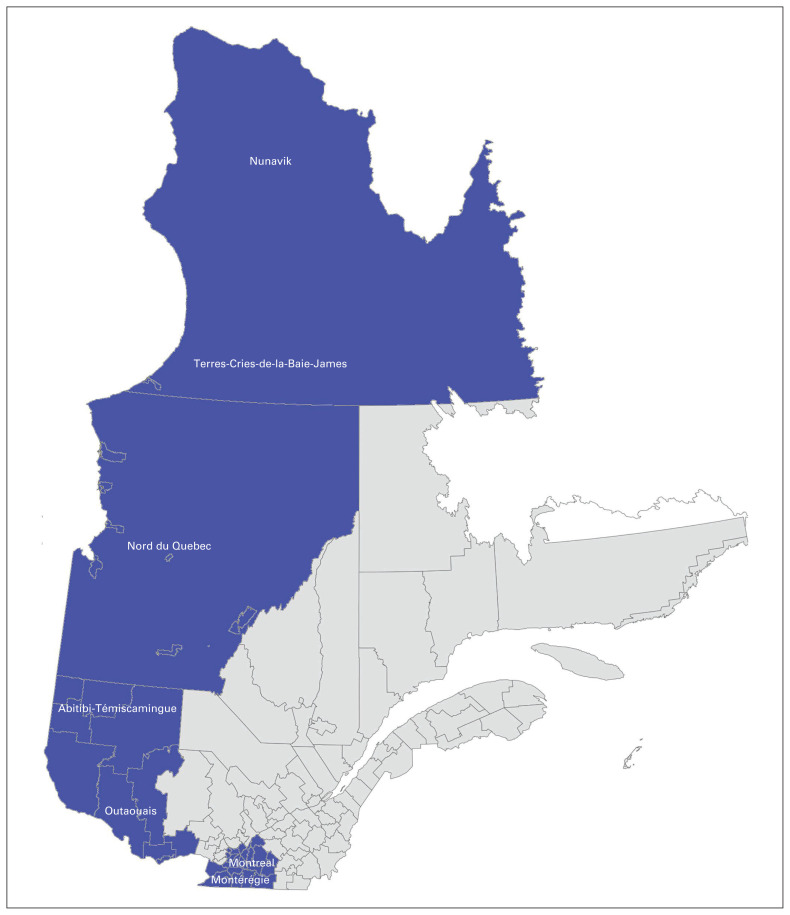
The McGill University Health Centre (MUHC) provides specialized care to more than 1.8 million Quebecers living across 63% of the province’s land mass.18,19 The catchment area serviced by the MUHC extends from Nunavik in the far north to the United States border (Figure 1).19 Travel, lodging and cancer care coordination are provided for all patients who live in remote areas while they are receiving care at the MUHC.
Fig. 1.
Catchment area of McGill University Health Centre in Montréal, Quebec.
The MUHC has 18 oncology pivot nurses (nurse navigators) who are assigned to all patients (R and U) by their cancer type and place of residence; they coordinate the integrated network of multidisciplinary teams to provide complete biologic, psychological, sociological and spiritual care for patients with cancer and their families to provide a highly effective interface between the patient and their family, the care team and the various health services.20–22 These nurses coordinate blood work, consultations, systematic therapy and follow-up appointments and address patient concerns throughout their care trajectory. Their services are covered by Canada’s universal, publicly funded health care system.23,24 Through the services they provide, the pivot nurses offer individualized care that reflects the patient’s preferences, expectations and needs by communicating with all of the care providers to facilitate a consistent vision throughout the patient’s care trajectory.22,25 The care corridor model has been in place for many years for numerous diseases and has been in a mature form for gastric cancer since at least 2010.
Our care coordinator ensures all new referrals are either booked to be seen within 1 week of referral or complete all workup before their clinic appointment if possible, including organizing imaging or endoscopy if not yet done. If a patient comes without full workup, we have urgent endoscopy and imaging spots dedicated to patients with gastric cancer to ensure staging is complete within 1 week of contact with our team. Patients from remote regions where resources are scarce are housed locally for as long as they need specialized care.26
The Division of Thoracic and Upper Gastrointestinal (UGI) Surgery at the Montreal General Hospital is one of the highest volume centres in North America; it sees patients from all over the province of Quebec and some out-of-province patients who come to MUHC for specialized care not available elsewhere in Canada.27 MUHC is the central tertiary referral centre for remote parts of Northern Quebec, so we see most patients with gastric cancer from this region. For in-between regions that have community hospitals with a general surgeon, it is possible that some patients with gastric cancer are being treated locally and do not come to MUHC. If they are referred to MUHC, we always do the surgery at Montreal General Hospital; their preoperative chemotherapy may be delivered at their local hospital.
All new gastric cancer diagnoses are discussed at the multidisciplinary UGI tumour board at MUHC, even if they are referred for oncology care closer to home. A care plan is developed, and new patients with cancer are always seen urgently. Treatment often begins within 1 week of the patient being seen in the clinic to minimize delays.27 After patients are assessed by the surgical team, the care for those requiring chemotherapy or radiation therapy or both is coordinated with oncology services at MUHC or, in many cases, with an oncology centre close to the patient’s home.27 This coordination is done with the help of the pivot nurses, who help patients navigate their cancer care at every step.27
The Enhanced Recovery After Surgery (ERAS) Program at MUHC has standardized patient care while ensuring adherence to international standards.27 The goal of ERAS is to facilitate patients’ return to independent functioning and effective adjustment to postoperative changes.27 The ERAS pathways include guidelines for all members of the team: surgeons, anesthesiologists, nurses, dietitians and patients.27 Patient information booklets are given to patients and family members to help them understand what to expect both pre- and postoperatively.27
Data collection
The primary outcome was overall survival. Secondary outcomes included patient and tumour characteristics, operative outcomes, 30-day complications, mortality and disease-free and progression-free survival. All data were collected prospectively and verified by a thorough review of paper and electronic medical records. Age-adjusted Charlson Comorbidity Index (CCI) score was used to categorize age and comorbidities before treatment.28–31 Tumour stage was classified according to the eighth edition of the American Joint Committee on Cancer Staging Manual for clinical, post-treatment and pathologic stage.32 Node-negative and T1–2 stage tumours were classified as early-stage cancer while any node-positive disease and T3–4 stage cancer was classified as locally advanced cancer.33 Provincial cancer registry data were used to verify survival status and date of death for all patients.
Statistical analysis
Data were analyzed using 2-tailed Mann–Whitney U tests for nonparametric variables, t tests for parametric variables and Fisher exact or χ2 tests for categorical variables. Kaplan–Meier curves and log-rank tests were used for survival analysis. Prism 8.0.2 (GraphPad) was used for data analysis. In addition, R Core Team (2013) was used for Cox proportional hazards regression analysis. Multivariate analysis was performed using clinical variables for which there was a statistically significant difference between groups on univariate analysis, with the following exceptions: patients’ birthplace was not included as it was not adequately captured retrospectively, and patients’ sex was added, despite being similar between groups, to account for potential sex bias. A p value of less than 0.05 was used to determine statistical significance.
Results
A total of 274 patients underwent treatment for gastric cancer at our centre between 2010 and 2018. The majority (n = 219, 80%) were from urban and suburban communities (U), while 55 (20%) had a home address in a rural or remote area (R). Those living in rural regions were younger (63 [standard deviation (SD) 12] yr v. 69 [SD 13] yr; p = 0.023), more likely to have been born in Canada (45 [82%] v. 70 [32%]; p < 0.001) and more likely to have higher disease stage at presentation (118 U patients [53%] v. 38 R patients [69%] had stage III or IV disease; p = 0.003). The proportion of patients with incurable disease at presentation was similar between the 2 groups (60 U patients [27%] v. 10 R patients [18%], p = 0.22). Body mass index and comorbidities were comparable between the groups. Male sex predominated in both groups. Similar rates of regional and distant metastases and neoadjuvant chemotherapy administration were observed in the 2 groups. Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics by study group
| Characteristic | No. (%) of patients;* residence | p value | |
|---|---|---|---|
| Urban and suburban n = 219 |
Rural and remote n = 55 |
||
| Male sex | 145 (66) | 37 (67) | 0.88 |
| Age, yr, mean ± SD | 69 ± 13 | 63 ± 12 | 0.023 |
| Place of birth | < 0.001 | ||
| Canada | 70 (32) | 45 (82) | |
| Region not specified | 54 (25) | 5 (9) | |
| Western Europe | 46 (21) | 1 (2) | |
| Eastern Europe | 25 (11) | 3 (5) | |
| Asia | 15 (7) | 1 (2) | |
| Africa | 6 (3) | 0 (0) | |
| Central or South America | 3 (1) | 0 (0) | |
| Body mass index, kg/m2, mean ± SD | 25 ± 5 | 26 ± 4 | 0.70 |
| Charlson Comorbidity Index | 0.33 | ||
| Mild (1–2) | 8 (4) | 2 (4) | |
| Moderate (3–4) | 43 (19) | 16 (29) | |
| Severe (≥ 5) | 166 (74) | 37 (66) | |
| Signet ring cell carcinoma | 35 (16) | 12 (22) | > 0.99 |
| Tumour stage at diagnosis† | 0.003 | ||
| 0 | 2 (1) | 1 (2) | |
| I | 38 (17) | 7 (13) | |
| II | 48 (22) | 4 (7) | |
| III | 54 (25) | 27 (49) | |
| IV | 64 (29) | 11 (20) | |
| Clinical T stage | 0.30 | ||
| Tis | 2 (1) | 1 (2) | |
| T1 | 17 (8) | 4 (7) | |
| T2 | 29 (13) | 3 (5) | |
| T3 | 110 (50) | 33 (60) | |
| T4 | 47 (21) | 9 (16) | |
| Clinical N stage | 0.33 | ||
| N0 | 92 (42) | 18 (33) | |
| N+ | 111 (51) | 30 (55) | |
| Clinical M stage | 0.22 | ||
| M0 | 144 (66) | 40 (73) | |
| M1 | 60 (27) | 10 (18) | |
| Site of metastasis | 0.23 | ||
| Peritoneum | 23 (11) | 7 (13) | |
| Liver | 2 (1) | 0 (0) | |
| Nonregional lymph nodes | 6 (3) | 1 (2) | |
| Bone | 0 (0) | 1 (2) | |
| Neoadjuvant chemotherapy | 115 (53) | 34 (62) | 0.23 |
| Neoadjuvant radiotherapy | 8 (4) | 2 (4) | 1.00 |
M = distant metastasis; N = lymph node metastasis; SD = standard deviation; T = tumour extension.
Unless indicated otherwise.
According to the eighth edition of the American Joint Committee on Cancer Staging Manual.32
Table 2 depicts tumour characteristics and surgical approach for the patients in each study group. Rates of curative-intent surgeries (139 U patients [63%] v. 36 R patients [65%]; p = 0.96), palliative surgeries (33 U patients [15%] v. 8 R patients [15%]; p = 0.96) and nonresections (47 U patients [21%] v. 11 R patients [20%]; p = 0.96) were similar between the groups. Among patients undergoing surgery, surgical approach, type of procedure, duration of surgery, estimated blood loss, postoperative complications and length of stay (6 [interquartile range (IQR) 4–10] d for U patients v. 6 [IQR 5–7] d for R patients; p = 0.50) were comparable in the 2 groups.
Table 2.
Surgical details by study group
| Characteristic | No. (%) of patients;* residence | p value | |
|---|---|---|---|
| Urban and suburban n = 219 |
Rural and remote n = 55 |
||
| Surgery | 0.96 | ||
| Curative intent | 139 (63) | 36 (65) | |
| Palliative | 33 (15) | 8 (15) | |
| None | 47 (21) | 11 (20) | |
| Time from diagnosis to surgery, d, median (IQR) | 71 (35–125) | 86 (34–121) | 0.91 |
| Approach | 0.36 | ||
| Minimally invasive | 56 (26) | 14 (25) | |
| Open | 110 (49) | 29 (52) | |
| Converted | 5 (2) | 3 (5) | |
| Procedure | 0.21 | ||
| Subtotal gastrectomy | 93 (42) | 23 (42) | |
| Total gastrectomy | 50 (23) | 14 (25) | |
| Proximal gastrectomy | 10 (5) | 4 (7) | |
| Extended total gastrectomy | 12 (3) | 5 (9) | |
| Gastrojejunal bypass | 3 (1) | 0 (0) | |
| Feeding jejunostomy | 3 (1) | 0 (0) | |
| Surgery duration, min, median (IQR) | 161 (137–187) | 164 (150–191) | 0.33 |
| Estimated blood loss, mL, median (IQR) | 200 (100–400) | 350 (138–625) | 0.11 |
| Length of stay, d, median (IQR) | 6 (4–10) | 6 (5–7) | 0.50 |
| 30-day complications | 0.55 | ||
| CD 0 | 65 (30) | 20 (36) | |
| CD 1–2 | 66 (30) | 19 (35) | |
| CD 3–4 | 35 (16) | 8 (15) | |
| CD 5 | 6 (3) | 0 (0) | |
CD = Clavien–Dindo score; IQR = interquartile range.
Unless indicated otherwise.
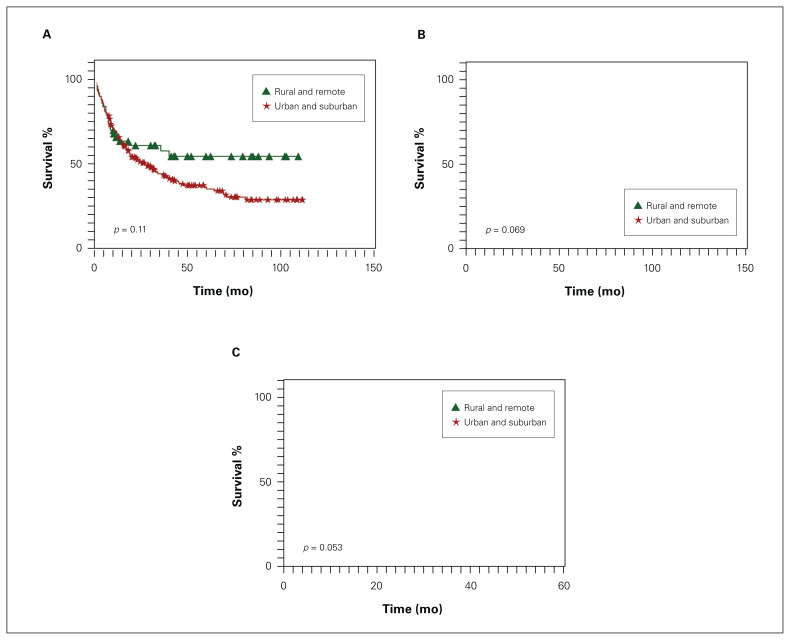
Oncologic outcomes are presented in Table 3. Final pathologic stage, lymph node involvement, presence of distant metastases and follow-up time did not vary by group. Overall survival, disease-free survival and progression-free survival were comparable between the 2 groups (Figure 2). Stage-based overall and disease-free survival were also similar. These findings were mirrored on multivariate analysis as well; residence in rural and remote regions was not associated with worse overall, disease-free or progression-free survival, while having locally advanced cancer at presentation negatively affected overall and disease-free survival regardless of proximity to an urban centre (Table 4).
Table 3.
Oncologic outcomes by study group
| Outcome | No. (%) of patients;* residence | p value | |
|---|---|---|---|
| Urban and suburban n = 219 |
Rural and remote n = 55 |
||
| Tumour size,† cm, median (IQR) | 4.0 (2.5–6.5) | 5.0 (2.7–8.2) | 0.07 |
| Location of tumour | 0.74 | ||
| Antrum | 47 (21) | 8 (15) | |
| Body | 32 (15) | 8 (15) | |
| Cardia | 8 (4) | 2 (4) | |
| Pylorus | 1 (0.5) | 0 (0) | |
| Lauren classification | 0.14 | ||
| Intestinal | 44 (20) | 7 (13) | |
| Diffuse | 26 (12) | 9 (16) | |
| Mixed | 9 (4) | 0 (0) | |
| Pathologic stage | 0.31 | ||
| 0 | 2 (1) | 0 (0) | |
| I | 51 (23) | 11 (20) | |
| II | 25 (11) | 9 (16) | |
| III | 61 (28) | 13 (24) | |
| IV | 34 (16) | 14 (25) | |
| Grade | 0.97 | ||
| I | 14 (6) | 3 (5) | |
| II | 59 (27) | 14 (25) | |
| III | 128 (58) | 32 (58) | |
| pT stage | 0.014 | ||
| T0 | 4 (2) | 0 (0) | |
| T1 | 34 (16) | 9 (16) | |
| T2 | 27 (12) | 3 (5) | |
| T3 | 42 (19) | 22 (40) | |
| T4 | 66 (30) | 12 (22) | |
| pN stage | 0.36 | ||
| N0 | 57 (26) | 15 (27) | |
| N1 | 26 (12) | 5 (9) | |
| N2 | 34 (16) | 6 (11) | |
| N3 | 55 (25) | 21 (38) | |
| Total no. of lymph nodes resected, median (IQR) | 28 (21–39) | 28 (21–43) | 0.64 |
| No. of positive lymph nodes, median (IQR) | 2 (0–9) | 5 (0–16) | 0.23 |
| LV invasion | 79 (36) | 21 (38) | 0.27 |
| PN invasion | 65 (30) | 22 (40) | 0.34 |
| pM stage | 0.67 | ||
| M0 | 142 (65) | 37 (67) | |
| M1 | 31 (14) | 10 (18) | |
| Site of metastasis | 0.12 | ||
| Peritoneum | 23 (11) | 7 (13) | |
| Liver | 2 (1) | 0 (0) | |
| Nonregional lymph nodes | 6 (3) | 1 (2) | |
| Bone | 0 (0) | 1 (2) | |
| Invasion into surrounding structure | 0 (0) | 1 (2) | |
| pCR | 4 (3) | 0 (0) | 0.58 |
| Follow-up time, mo, median (IQR) | 18 (6–37) | 11 (6–39) | 0.40 |
IQR = interquartile range; LV = lymphovascular; M = distant metastasis; N = lymph node metastasis; p = pathologic or post-treatment; pCR = pathologic complete response; PN = perineural; T = tumour extension.
Unless indicated otherwise.
Greatest dimension of tumour as measured by pathologist.
Fig. 2.
Kaplan–Meier survival curves by patients’ primary residence for (A) overall, (B) disease-free and (C) progression-free survival.
Table 4.
Multivariate analyses for overall, disease-free and progression-free survival*
| Variable | Overall survival | Disease-free survival | Progression-free survival | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, yr | 0.993 (0.979–1.007) | 0.31 | 1.022 (0.994–1.051) | 0.12 | 1.002 (0.979–1.025) | 0.90 |
|
| ||||||
| Female v. male sex | 1.066 (0.748–1.520) | 0.72 | 0.751 (0.379–1.489) | 0.41 | 1.306 (0.779–2.190) | 0.31 |
|
| ||||||
| Locally advanced v. early stage† | 3.291 (1.887–5.738) | < 0.001 | 4.313 (1.813–10.259) | < 0.001 | — | — |
|
| ||||||
| Rural or remote v. urban or suburban residence | 0.622 (0.383–1.010) | 0.06 | 0.472 (0.186–1.200) | 0.12 | 0.536 (0.250–1.149) | 0.11 |
CI = confidence interval; HR = hazard ratio.
Clinical variables with a statistically significant difference between groups in univariate analysis were included in this model.
Not applicable for progression-free survival since all patients had locally advanced cancer.
Discussion
The health disadvantage of rural and remote populations is multifactorial. The worse cancer survival observed with rurality may be partially due to higher rates of poverty, lower levels of education and worse health literacy among rural than urban dwellers, which in turn affect health behaviours and the use of screening and other health care services.34,35 The distance that patients in rural and remote areas need to travel for care is associated with several barriers: patients may incur travel and accommodation costs, they may need to take time off work and secure childcare coverage, they may be separated from their support networks and they may lack knowledge on how to access the health system. These barriers may all contribute to delayed presentation and ultimately worse outcomes for patients with cancer from rural and remote areas.3,13,36 Ready access to specialized medical services and subsidies for treatment and transportation expenses may help mitigate these effects.37,38
This study shows that within the context of a publicly funded corridor of care, rural patients with gastric cancer can achieve outcomes similar to those of patients who live close to a specialist centre. Even though the rural and remote patients in this series had more advanced disease at presentation, they had similar treatment patterns in terms of neoadjuvant therapy and surgical intervention. This, in turn, was reflected in the fact that their overall, disease-free and progression-free survival was similar to that of their urban counterparts. Our findings are noteworthy as rurality has often been associated with delayed diagnosis and decreased survival among patients with cancer.
Similar to our findings, many studies have demonstrated that increased travel distance to a specialist hospital and treatment facilities is associated with increased cancer stage at diagnosis.10,13,39,40 An evaluation of women in the US who were living in remote communities near the US–Mexico border found that they had a higher stage of cervical cancer at diagnosis, although this type of cancer is largely preventable with vaccination and screening.5 A Danish population-based study showed increased risk of being diagnosed with high-risk breast cancer among patients who resided in rural areas because of reduced access to screening mammography and lower attendance rates compared with urban patients.6 In the United Kingdom, delay of diagnosis has been partially associated with poorer access to services among patients with cancer from rural areas.41 Our findings are consistent with these studies, as tumours were more often locally advanced among rural patients in this series, potentially because of poorer access to diagnostic tests in remote communities.
Once rural patients received a diagnosis, however, their care trajectories and oncologic outomes were similar in this series to those of urban dwellers. This contrasts with numerous studies from various regions globally in which increased proximity to urban centres has been associated with improved survival for patients with cancer.13,34,42 In contrast, rural patients enrolled in an American clinical trial, and therefore subjected to uniform treatment strategies, had overall, progression-free and cancer-specific survival similar to that of urban patients.3 Our work reaffirms this concept by demonstrating that equivalent outcomes can be achieved for rural and urban patients with gastric cancer in the context of a centralized program that reduces financial and geographic barriers to accessing specialized care.
Limitations
Limitations of this work include the relatively small sample size of the rural and remote group, selection bias and the retrospective nature of this analysis. Patients in both groups who failed to present for treatment or were not referred because of very advanced disease at presentation may not have been captured. However, the proportion of people living in rural Quebec according to national Census data corresponds to the sample size observed in this group, suggesting that both groups are representative of the general population. Surgical and oncologic outcomes were mostly similar among urban and rural patients, but the small sample size in the rural group may limit the interpretation of this result. Collection of patient-reported outcomes was minimal in the past, but these data are actively being collected at our institution now. Finally, as we conducted a single-centre study, our results may not be generalizable to other populations. Nonetheless, the findings can be extrapolated to other regions with universal health care as we captured data from the largest province in Canada in terms of land mass. As Canada is also the second largest country in the world and comprises a highly multiethnic population, it is reasonable to infer that similar results could be found in other countries where distances between rural and urban centres may be smaller and the population more culturally and linguistically homogeneous.
Conclusion
This study shows that in the context of a publicly funded care corridor to a multidisciplinary specialist cancer centre, patients with gastric cancer in remote and rural areas have similar outcomes to those of patients residing in urban and suburban areas.
Acknowledgements
The authors thank Aya Siblini for assisting with securing research ethics review board approval and Kyle Zullo, Saleh Almatar and Lina Abdrabo for assisting with data extraction. They are grateful to Nick Newstead for sharing these data.
Footnotes
Presentation at the 61st Annual Meeting of the Society for Surgery of the Alimentary Tract, Chicago, Ill., May 2–5, 2020, cancelled because of the COVID-19 pandemic
Competing interests: None declared.
Contributors: A. Kammili designed the study. D. Morency acquired the data, which all authors analyzed. A. Kammili wrote the article, which all authors critically revised. All authors gave final approval of the version to be published.
References
- 1.Statistics Canada. Canada goes urban. Ottawa: Statistics Canada; 2018. Jun. 17. Available: https://www150.statcan.gc.ca/n1/pub/11-630-x/11-630-x2015004-eng.htm (accessed 2019 May 31). [Google Scholar]
- 2.United States Census Bureau. New census data show differences between urban and rural populations. Washington (DC): United States Census Bureau; 2016. Dec. 8. Available: https://www.census.gov/newsroom/press-releases/2016/cb16-210.html (accessed 2020 May 4). [Google Scholar]
- 3.Unger JM, Moseley A, Symington B, et al. Geographic distribution and survival outcomes for rural patients with cancer treated in clinical trials. JAMA Netw Open 2018;1:e181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monroe AC, Ricketts TC, Savitz LA. Cancer in rural versus urban populations: a review. J Rural Health 1992;8:212–20. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SS, Richards TB, Nasseri K, et al. Cervical cancer incidence in the United States in the US–Mexico border region, 1998–2003. Cancer 2008;113(Suppl):2964–73. [DOI] [PubMed] [Google Scholar]
- 6.Dalton SO, During M, Ross L, et al. The relation mentions between socioeconomic and demographic factors and tumour stage in women diagnosed with breast cancer in Denmark, 1983–1999. Br J Cancer 2006;95:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashibe M, Kirchhoff AC, Kepka D, et al. Disparities in cancer survival and incidence by metropolitan versus rural residence in Utah. Cancer Med 2018;7:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jemal A, Ward E, Wu X, et al. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiol Biomarkers Prev 2005;14:590–5. [DOI] [PubMed] [Google Scholar]
- 9.Klein J, Ji M, Rea NK, et al. Differences in male breast cancer stage, tumor size at diagnosis, and survival rate between metropolitan and nonmetropolitan regions. Am J Mens Health 2011;5:430–7. [DOI] [PubMed] [Google Scholar]
- 10.Zahnd WE, Fogleman AJ, Jenkins WD. Rural–urban disparities in stage of diagnosis among cancers with preventive opportunities. Am J Prev Med 2018;54:688–98. [DOI] [PubMed] [Google Scholar]
- 11.Maslach D, Krzyzak M, Szpak A, et al. Differences in results of breast cancer curative treatment between urban/rural female population in Podlaskie Voivodship of Poland before introduction of the National Cancer Control Programme. Ann Agric Environ Med 2013;20:68–71. [PubMed] [Google Scholar]
- 12.Booth S, Bakhishli G, Iyoriobhe P, et al. Pre-operative anaemia is associated with inferior outcomes and increased use of red cell transfusion in patients undergoing hysterectomy. Br J Haematol 2018;181:145.28107562 [Google Scholar]
- 13.Afshar N, English DR, Milne RL. Rural–urban residence and cancer survival in high-income countries: a systematic review. Cancer 2019;125:2172–84. [DOI] [PubMed] [Google Scholar]
- 14.Walsh J, Harrison JD, Young JM, et al. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res 2010;10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendren S, Chin N, Fisher S, et al. Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J Natl Med Assoc 2011;103:701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalo MB, House L, Santiago K, et al. Access to care in cancer: barriers and challenges. J Clin Oncol 2017;35:33. [Google Scholar]
- 17.Alasia A, Bédard F, Bélanger J, et al. Measuring remoteness and accessibility: a set of indices for Canadian communities. Ottawa: Statistics Canada; 2017. [Google Scholar]
- 18.McGill University Health Centre. Excellence in cancer care 2020. Available: https://muhc.ca/cancer/profile/cancer-care-mission-0 (accessed 2020 May 4).
- 19.McGill University Faculty of Medicine and Health Sciences. Serving diverse regions. Available: https://www.mcgill.ca/medicine/health-care/ruisss-mcgill (accessed 2020 May 4).
- 20.McGill University Health Centre Libraries. Accommodations near the Glen Site [updated 22 Mar. 2018]. Available: https://www.muhclibraries.ca/patients/health-topics/accommodations-glen/ (accessed 2020 May 4).
- 21.McGill Travel Services. Travel services. Available: https://www.mcgill.ca/travelservices/accommodations (accessed 2020 May 4).
- 22.McGill University Health Centre. The pivot nurse. Available: https://muhc.ca/cancer/page/pivot-nurse-indispensable-resource (accessed 2020 Apr. 30).
- 23.Rakick JS. Canada’s universal-comprehensive healthcare system. Hosp Top 1991;69:14–9. [DOI] [PubMed] [Google Scholar]
- 24.Martin D, Miller AP, Quesnel-Vallee A, et al. Canada’s universal health-care system: achieving its potential. Lancet 2018;391:1718–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saucier A, Biron A. Reaching professional consensus on pivot nurse in oncology interventions in the goal of staff planning in Quebec. Can Oncol Nurs J 2018;28:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inuulitisivik Health Center. Organisation. 20 Jan 2021. Available: https://www.inuulitsivik.ca/organisation-en-ca/?lang=en (accessed 2020 Apr. 30).
- 27.Division of Thoracic and Upper Gastrointestinal Surgery. Esophageal and gastric cancer. Available: https://www.mcgill.ca/thoracic/esophageal-and-gastric-cancer (accessed 2020 May 4).
- 28.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 29.Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 2014;100:288–94. [DOI] [PubMed] [Google Scholar]
- 30.Huang YQ, Gou R, Diao YS, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B 2014;15:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moro-Sibilot D, Aubert A, Diab S, et al. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J 2005;26:480–6. [DOI] [PubMed] [Google Scholar]
- 32.Rice TW, Patil DT, Blackstone EH. 8th editionAJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Sur 2017;6:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roses RE, Datta J, You YN. Defining the optimal treatment of locally advanced gastric cancer. Chicago: Bulletin of the American College of Surgeons; 2019. May 1. Available: https://bulletin.facs.org/2019/05/defining-the-optimal-treatment-of-locally-advanced-gastric-cancer/ (accessed 2020 May 4). [Google Scholar]
- 34.Ireland MJ, March S, Crawford-Williams F, et al. A systematic review of geographical differences in management and outcomes for colorectal cancer in Australia. BMC Cancer 2017;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KB, Humphreys JS, Wilson MG. Addressing the health disadvantage of rural populations: How does epidemiological evidence inform rural health policies and research? Aust J Rural Health 2008;16:56–66. [DOI] [PubMed] [Google Scholar]
- 36.Moen EL, Kapadia NS, O’Malley AJ, et al. Evaluating breast cancer care coordination at a rural National Cancer Institute Comprehensive Cancer Center using network analysis and geospatial methods. Cancer Epidemiol Biomarkers Prev 2019;28:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorin SS, Haggstrom D, Han PKJ, et al. Cancer care coordination: a systematic review and meta-analysis of over 30 years of empirical studies. Ann Behav Med 2017;51:532–46. [DOI] [PubMed] [Google Scholar]
- 38.Passwater C, Itano J. Care coordination: overcoming barriers to improve outcomes for patients with hematologic malignancies in rural settings. Clin J Oncol Nurs 2018;22:549–54. [DOI] [PubMed] [Google Scholar]
- 39.Ambroggi M, Biasini C, Del Giovane C, et al. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist 2015;20:1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell NC, Elliott AM, Sharp L, et al. Rural and urban differences in stage at diagnosis of colorectal and lung cancers. Br J Cancer 2001;84:910–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neal RD, Allgar VL. Sociodemographic factors and delays in the diagnosis of six cancers: analysis of data from the “National Survey of NHS Patients: Cancer”. Br J Cancer 2005;92:1971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Dziegielewski PT, Jean Nguyen TT, et al. The effects of geography on survival in patients with oral cavity squamous cell carcinoma. Oral Oncol 2015;51:578–85 [DOI] [PubMed] [Google Scholar]