Abstract
Although inherited bleeding disorders (IBDs) affect both females and males, this review of the preoperative diagnosis and management of IBDs focuses on genetic and gynecologic screening, diagnosis and management of affected and carrier females. A PubMed literature search was conducted, and the peer-reviewed literature on IBDs was evaluated and summarized. Best-practice considerations for screening, diagnosis and management of IBDs in female adolescents and adults, with GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) evidence level and ranking of recommendation strength, are presented. Health care providers need to increase their recognition of and support for female adolescents and adults with IBDs. Improved access to counselling, screening, testing and hemostatic management is also required. Patients should be educated and encouraged to report abnormal bleeding symptoms to their health care provider when they have a concern. It is hoped that this review of preoperative IBD diagnosis and management will enhance access to women-centred care to increase patients’ understanding of IBDs and decrease their risk of IBD-related morbidity and mortality.
Abstract
Même si les troubles héréditaires de la coagulation affectent les femmes et les hommes, la présente revue sur leur diagnostic et leur prise en charge en contexte préopératoire s’intéresse au dépistage génétique et gynécologique, au diagnostic et à la prise en charge des femmes atteintes et porteuses. Nous avons interrogé la base de données PubMed et nous avons évalué et résumé les articles révisés par des pairs au sujet des troubles héréditaires de la coagulation. Nous présentons un aperçu des pratiques optimales en matière de dépistage et de diagnostic des troubles héréditaires de la coagulation chez les adolescentes et les femmes au moyen du système GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) qui évalue la qualité des données probantes et la force des recommandations. Les professionnels de la santé doivent mieux reconnaître les troubles héréditaires de la coagulation chez les adolescentes et les femmes afin de mieux soutenir cette population. Un meilleur accès au counseling, au dépistage, aux tests et à la prise en charge de l’hémostase s’impose également. Il faut renseigner les patientes et les encourager à informer leur médecin de tout saignement anormal qui les inquiète. Nous espérons que cette revue du diagnostic et de la prise en charge préopératoires des troubles héréditaires de la coagulation facilitera l’accès à des soins centrés sur les femmes et aidera ces dernières à être mieux renseignées afin de réduire le risque de morbidité et de mortalité associé aux troubles héréditaires de la coagulation.
The pathway to high-quality and safe care for people with inherited bleeding disorders (IBDs) continues to be challenging. Patients at risk may encounter obstacles in accessing appropriately resourced, high-quality comprehensive care centres for innovative evidence-based treatment.1 The IBDs that affect female surgical care include inherited von Willebrand disease (vWD) and X-linked hemophilia A and B carrier status, as well as rare platelet disorders.
Focused genetic and hematologic education related to IBDs is required for the surgical health care provider. Enhanced medical and nursing training curricula and public awareness are necessary as well.2,3
Although IBDs affect both female and male patients, this review focuses on preoperative surgical screening, diagnosis and management of affected or carrier females. The review provides an evidence-based diagnostic and genetic classification and management pathway to help provide a framework for reducing IBD morbidity in affected adolescent and adult females undergoing scheduled surgical procedures.
Methods
A scoping review methodology was used. The review was focused primarily on IBDs in adolescent and adult females and pregnant people with vWD, hemophilia A and B carrier status, and inherited platelet disorders. Keywords, report titles, peer-reviewed articles with reference review and author names were used in the search strategy. PubMed was searched for relevant articles in English with the keywords “von Willebrand disease,” “hemophilia A,” “hemophilia B,” “inherited platelet disorders,” “screening criteria,” “diagnostic criteria,” “genetic testing,” “laboratory testing,” “major surgery,” “minor surgery,” “pregnancy,” “menorrhagia” and “prophylaxis.” The scoping review identified adequate data sources for this evidence-based focused review of the topic.
Present health care status of females with inherited bleeding disorders
The impact of IBDs is substantial. The clinical issues of bleeding severity, determination of the patient’s individual risk related to surgery and pregnancy, disease transmission, testing and reproductive decisions contribute to health-related quality of life.4–17 Compared to females without IBDs, those with IBDs have lower values for bleeding control, vitality, and physical and social functioning, and increased pain (joint bleeding/heavy menstrual bleeding) and psychosocial problems (guilt, anxiety, depression, work and school absenteeism, isolation and difficult social relationships).4–17 Women’s occupational opportunities are substantially affected, related to the person’s disease severity and, in the case of familial hemophilia, the potential need to undertake a role as caregiver.4–17 Patient access to specialist care is commonly reported as an obstacle.4–17
Knowledge translation regarding male hemophilia A and hemophilia B outcomes is important for informed-consent counselling of female hemophilia carriers. Both hemophilia A and hemophilia B have transitioned from a disease of childhood to one of adulthood. Life expectancy has increased from 11 years in the mid-1900s to the present 55–63 years owing to management with coagulation factor concentrates. There are long-term clinical concerns associated with hemophilia outcomes, including chronic viral infections with liver disease, joint impairment with bone disease, cardiovascular disease, cancers, chronic kidney disease, covert cerebral microbleeds, impaired sexuality and development of immune factor inhibitors.18
Primary risk screening in female adolescents and adults with an abnormal bleeding history
Health care providers need to increase their recognition of and support for female adolescents and adults with IBDs. Improved access to counselling, screening, testing and hemostatic management is also required. Patients should be educated and encouraged to report abnormal bleeding symptoms to their health care provider when they have a concern.
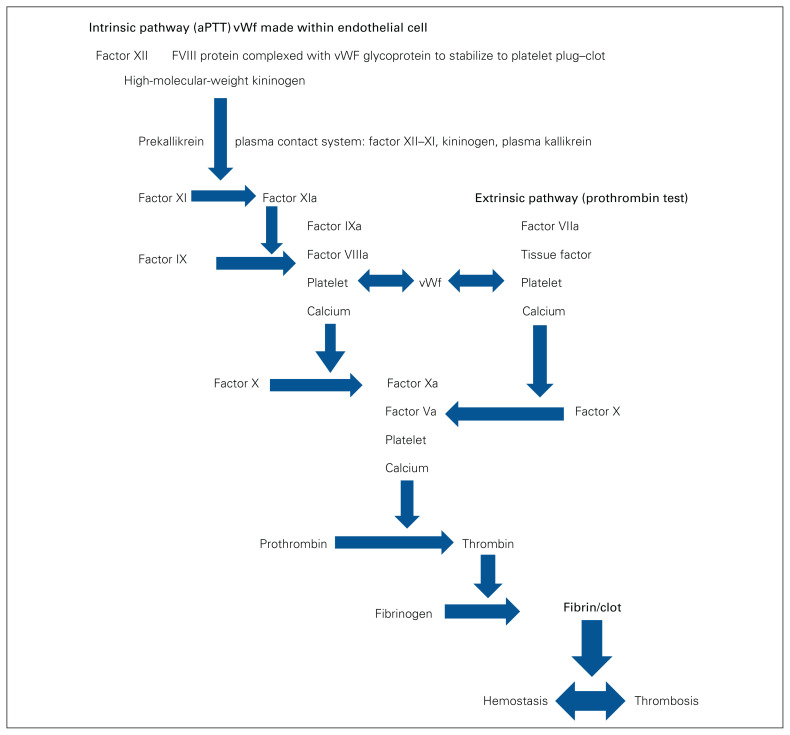
The genetic impact, definition and coagulation testing factors of IBDs affecting female adolescents and adults are summarized in Table 1.2,19–32 The coagulation cascade summarizes the factors and their interactions to achieve hemostasis–thrombosis balance33 (Figure 1).
Table 1.
Genetic impact, definition and coagulation testing of inherited bleeding disorders
| Disorder | Genetic impact | Definition | Coagulation testing |
|---|---|---|---|
| von Willebrand disease2,19,20 |
|
|
|
| Hemophilia A21–23 |
|
|
|
| Hemophilia B24–26 |
|
|
|
| Factor XII deficiency27 | — | — |
|
| Factor XIII deficiency28 | Autosomal recessive |
|
|
| Inherited platelet disorders29–31 |
|
|
|
| Rare coagulation factor deficiencies |
|
|
|
| α2-antiplasmin deficiency32 | Rare, but prevalence increased with consanguinity |
|
ADP = adenosine diphosphate; aPTT = activated partial thromboplastin time; INR = International Normalized Ratio; vWF = von Willebrand factor.
Fig. 1.
Traditional coagulation cascade model.33 aPTT = activated partial thromboplastin time; vWF = von Willebrand factor.
Menorrhagia is the most common symptom reported by female adolescents and adults with IBDs. The clinical descriptions that indicate serious menorrhagia are soaking through menstrual pads or tampons within 1 hour, soaking through bedclothes, decreased ferritin level and anemia (microcytic; hemoglobin level < 10 g/dL) and, as an objective measurement, a score less than 100 on a pictorial bleeding assessment chart (PBAC).34
Health care providers must consider a bleeding disorder in adolescent females with bleeding problems, as 50% of patients have some form of coagulopathy.35,36 Many females diagnosed with an IBD as an adult will have been missed as an adolescent.
Evaluation
Evaluation of females with an IBD consists of the following elements:22,23
-
History and physical examination
♦ Current clinical status (characterize bleeding phenotype, complete a bleeding assessment tool)
♦ History of excessive bleeding (determine whether history is lifelong)
♦ History of joint pain/swelling (identify undiagnosed hemarthrosis)
♦ History of chronic diseases (identify nongenetic causes)
♦ Reproductive history (assess fertility)
-
Family history and pedigree
♦ Family history from reliable relatives (determine inheritance patterns)
♦ Laboratory results of affected relatives (determine severity in males)
Verbal review of the use of nonwarfarin and over-the-counter medications, as certain agents have an associated bleeding risk (Table 2).33
Table 2.
Medications and over-the-counter medicines that entail a risk of increased bleeding33
| Class; agent | Mechanism |
|---|---|
| Antiplatelet drugs | |
| Acetylsalicylic acid | Inhibition of COX-1 and COX-2/thrombin generation |
| Clopidogrel/ticlopidine | Inhibition of P2Y12 receptor on platelet surface |
| Dipyridamole | Blocks cellular uptake of adenosine with antiplatelet due to extracellular adenosine |
| NSAIDs | Block COX-1 and COX-2 |
| Unfractionated heparin | Thrombin inactivation via antithrombin III binding sites |
| Low-molecular-weight heparin (multiple products) | Thrombin inactivation via antithrombin III binding sites |
| Fondaparinux | Inhibition of thrombin generation |
| Direct thrombin inhibitors | |
| Argatroban | Reversible and direct inhibition of circulating and fibrin-bound thrombin |
| Bivalirudin | |
| Hirudin | |
| Lepirudin | |
| Ancrod (purified snake venom protein) | Defibrinogenation |
| Fibrinolytics | |
| Anistreplase | Convert plasminogen to plasmin (fibrinolytic)Alteplase |
| Reteplase | |
| Tenecteplase | |
| Steptokinase | |
| Urokinase | |
| Drotrecogin alfa | Vitamin K–dependent antithrombotic serine protease that directly inhibits factors Va and VIIIa |
| Glycoprotein IIb–IIIa inhibitors | |
| Abciximab | Inhibition of platelet aggregation by binding of soluble fibrinogen to platelet receptor glycoprotein IIb–IIIa |
| Eptifibatide | |
| Tirofiban | |
| Herbal medicines | |
| Anise | Potential for thrombocytopenia |
| Garlic | |
| Ginger | |
| Ginseng | |
| Ginkgo biloba | |
| Feverfew | |
| Herbal Plus | |
NSAID = nonsteroidal anti-inflammatory drug.
The screening criteria used to identify a possible diagnosis of IBD are as follows:34,35
-
Adolescents
♦ Menses lasting longer than 7 days, bleeding through a pad or tampon in 1 hour, clots greater than 3 cm in diameter, or bleeding that results in anemia or low iron status
♦ Bleeding requiring a transfusion
♦ Unmanageable heavy menstrual bleeding
♦ Prolonged bleeding from small wounds lasting more than 15 minutes or recurrent bleeding after 7 days
-
Adults: the above criteria as well as the following:
♦ History of heavy or prolonged bleeding after a procedure or surgical intervention (e.g., tooth extraction, surgery, delivery)
♦ Two or more of the following conditions: epistaxis once or twice per month, epistaxis lasting more than 10 minutes, frequently bleeding gums or family history of bleeding symptoms
♦ Unexpected postsurgical bleeding, history of hemorrhagic ovarian or corpus luteum cyst
♦ Failure of response to conventional hormonal management of menorrhagia (combined estrogen–progesterone or progesterone alone).
Validated tools are available for clinical bleeding history scoring, including a bleeding assessment tool (BAT) and a pictorial scoring system for pads and tampons (PBAC) used during bleeding episodes.36–40 The ISTHBAT (International Society on Thrombosis and Haemostasis – Bleeding Assessment Tool) comprises 14 categories for assessing bleeding symptoms retrospectively; a high bleeding score is associated with the presence of an IBD.37,38 The PBAC is a prospective scoring system developed as a semiquantitative evaluation of menstrual blood loss that considers the number of sanitary products used, the degree to which these products are soiled with blood, the number and size of blood clots passed, and the number of flooding episodes.39,40 These tools provide useful methods for establishing the amount of bleeding, which can then can be compared after therapy initiation.
Hematology and laboratory studies
Hematology screening of plasma should include the following elements:33–35
Complete blood count
Prothrombin time
Activated partial thromboplastin time
-
Factor VIII (FVIII) level decrease:
♦ vWD profile (rule out vWD)
♦ Factor V level (rule out factor V and FVIII deficiency)
♦ FVIII binding to von Willebrand factor (vWF) (rule out vWD type 2N)
♦ FVIII inhibitor (evaluate for possible acquired hemophilia)
Factor IX (FIX) level decrease: factor II, factor VII, factor X (rule out genetic or acquired combined vitamin K–dependent clotting factor deficiency)
FIX inhibitor (evaluate for possible acquired hemophilia)
von Willebrand panel (vWF activity–ristocetin cofactor test, vWF antigen level, FVIII level)
Fibrinogen level
Possible platelet function and/or aggregation studies, and thyroid function testing if the above screening gives normal results (normal platelet count 150–450 000 × 109/L; normal values in Alberta: thyroid-stimulating hormone level 0.20–6.50 mIU/L, free T4 level 10.0–25.0 pmol/L, free T3 level [age ≥ 18 yr] 3.0–6.5 pmol/L)
A hematology consultation may be required based on the laboratory screening results.
Additional genetic studies after identification of primary etiology20,23,26
Paternity testing (rule out nonpaternity)
Karyotype with high resolution of X (identify X chromosome abnormality)
Sequencing of FVIII and FIX (identify point mutations)
Multiplex ligation-dependent probe amplification (identify gene deletion or duplication)
X chromosome inactivation studies (identify nonrandom inactivation).
Common etiologies and diagnoses identified in females with inherited bleeding disorders
von Willebrand disease2,19,20,41–44
von Willebrand disease is the single most common IBD, with a laboratory risk-defined prevalence of 1% and a symptomatic prevalence of 0.1%. Autosomal dominant and recessive inheritance patterns are dependent on the vWD classification (Table 1).
The classical vWD triad association consists of mucocutaneous bleeding, family history of bleeding and a laboratory finding of a vWF deficiency. Directed questioning for pregnancy-related bleeding, epistaxis and heavy menstrual periods should be considered. The most commonly reported symptoms are heavy menstrual bleeding (75%–100% of patients), excessive bruising (60%–80%), oropharyngeal bleeding (64%), epistaxis (56%) and bleeding after dental surgery (24%–26%).44
In vWD, the bleeding tendency is due primarily to the inherited deficiency or dysfunction of the multimeric glycoprotein vWF, causing abnormal platelet–vessel wall interactions and defective formation of the platelet plug (primary hemostasis).
Diagnosis
There are pre-analytical variables that can affect the result of screening for an increased vWF level, such as delays in transportation or processing, estrogen level, pregnancy, physical or mental stress, and other medical conditions. Testing is required to evaluate quantitative and qualitative “diagnostic” results based on specific laboratory controls. The screening and diagnostic tests are prothrombin time, activated partial thromboplastin time, FVIII coagulant activity, ristocetin cofactor activity and vWF antigen concentration.2,19,20,41,42
Pregnancy is a prothrombotic physiologic event, and the hyperestrogen environment can increase vWF and FVIII levels by up to 300%–400%. These physiologic changes may minimize potential bleeding events, but pregnant people with vWD still have an increased risk for peripartum hemorrhage and the need for red cell transfusion. In addition, there is an increased risk of recurrent miscarriage.43
X-linked hemophilia A and B carrier status
Hemophilia is an X-linked IBD, and females can be mutation carriers with variable levels of FVIII (hemophilia A) or FIX (hemophilia B). Female hemophilia carriers can be classified as obligate (daughters of affected male, women with > 1 affected son, women with 1 affected son and an affected brother or uncle) or possible (women with 1 affected son and no family history, daughters of an obligate carrier and woman with a maternal family history of hemophilia). A de novo germ cell X chromosome mutation is found in 30%–50% of newborn boys with hemophilia21–26,45–47 (Table 1).
There are important characteristic differences between hemophilia A and B, such as hemostatic functional impact (cofactor in hemophilia A, enzyme in hemophilia B), genetic variant impact (null variant in 80% of cases of hemophilia A, missense variant in > 60% of hemophilia B cases) and risk of development of severe treatment-related inhibition of coagulation (in 35%–40% of cases of hemophilia A and 4%–5% of cases of hemophilia B).46
Genetic mechanisms explain most of the reported clinical variability. Possible causes of FVIII and FIX deficiency in women and girls are23,26,48–51
Homozygosity (2 identical hemophilia alleles) by consanguinity, chance or de novo mutation(s)
Compound heterozygosity (2 different hemophilia alleles) due to unrelated hemophilia families, de novo second mutation or 2 de novo mutations
Hemizygosity (1 hemophilia allele and 1 normal allele) by single X chromosome 45,X/46,XY mosaicism with complete androgen insensitivity or complete gonadal dysgenesis, or X chromosome deletion of the FVIII or FIX gene
Heterozygosity (1 hemophilia allele, 1 normal allele) by inheritance in hemophilia-affected family, or 1 new mutation followed by skewed X inactivation (unknown or random), preferential X inactivation due to X chromosome abnormality, cell viability disorder or specific allele of the X-inactive-specific transcript gene, or other inherited skewed X inactivation
Other genetic causes: vWD, particularly type 2N *autosomal recessive or dominant; factor V and FVIII deficiency (autosomal recessive); combined vitamin K–dependent clotting factor (II, VII, IX, XI) deficiency (autosomal recessive)
Nongenetic cause: acquired hemophilia due to FVIII or FIX inhibitors, or vitamin K deficiency.
Diagnosis
Female hemophilia A carriers have an FVIII deficiency, mild in 94% of cases, moderate in 3% and severe in 3%. The circulating plasma FVIII concentration observed in hemophilia A carriers ranges from normal to levels close to those of males with hemophilia, with a median level of 55% of normal (range 4%–136%).52
Female hemophilia B carriers have an FIX deficiency. The median FIX level is 45% of normal (range 19%–114%); 30% of patients have levels less than 40% of normal. The data for hemophilia B carrier morbidity are limited compared to those for hemophilia A carrier status.25
A new nomenclature for female hemophilia carriers aimed at improving diagnosis and management has been defined.53 It distinguishes 5 clinically relevant categories: women/girls with mild, moderate or severe hemophilia (FVIII/FIX level > 0.05 IU/mL and < 0.40 IU/mL, 0.01–0.05 IU/mL and < 0.01 IU/mL, respectively) and symptomatic and asymptomatic hemophilia carrier status (FVIII/FIX level ≥ 0.40 IU/mL with and without a bleeding phenotype, respectively). With the new nomenclature, women attending US hemophilia treatment centres were identified as having mild (FVIII 16.0%, FIX 23.7%), moderate (FVIII 1.8%, FIX 0.86%) or severe (FVIII 0.49%, FIX 0.42%) hemophilia.23 The proportion of women receiving hemophilia treatment in each category was 17.8%, 1.4% and 0.5%, respectively.
Inherited platelet disorders
Inherited platelet disorders consist of a broad spectrum of medical conditions that include quantitative platelet disorders, which can present as increased or decreased platelet numbers, and qualitative platelet disorders, which affect platelet function. Inherited functional platelet disorders are rare, are commonly autosomal recessive and present usually in consanguineous populations29–31,54–57 (Table 1). A history of mucocutaneous bleeding or periprocedural hemorrhage with no other secondary etiology or family history should raise clinical suspicion of an inherited platelet disorder.
An important distinction is that acquired disorders of platelet function are more common than inherited disorders. Antiplatelet drugs are the most common etiology of acquired disorders. Clinical conditions associated with acquired platelet dysfunction are uremia, hepatic cirrhosis, myeloma, polycythemia vera and essential thrombocythemia.58
Diagnosis
The diagnosis is challenging and commonly delayed. The defects in platelet aggregation and activation can be from platelet receptors, platelet signalling, platelet granule release, platelet cytoskeletal remodelling or platelet hematopoiesis. Molecular studies have identified up to 40 genes as being causative of inherited thrombocytopenia, but in 50% of cases, no genetic basis has been identified.31 Whole blood electronic counters are used to evaluate platelet count and size, and a blood smear (with or without staining technology) is also required. For platelet function assessment, the classic testing, such as aggregometry, secretion assays, flow cytometry, Western blotting and electron microscopy, is time consuming and expensive, and interpretation of the results is complex.31
Quantitative platelet disorders associated with an inherited thrombocytopenia etiology are a large heterogeneous group of rare platelet disorders characterized by low platelet count, leading to bleeding and syndromic and systemic manifestations. The prevalence is estimated at 1 per 100 000, but many patients are undiagnosed.30
Inherited qualitative (functional) platelet disorders affect normal platelet function at different stages of initial hemostasis: adhesion (Bernard–Soulier syndrome), activation, granule secretion (Hermansky–Pudlak syndrome, Quebec platelet disorder), signal transduction, aggregation for platelet thrombus formation (Glanzmann thrombasthenia) and coagulation factor activation (Scott syndrome).30
Coagulation cascade and the drugs, medications and blood products used in management
Understanding the coagulation cascade is important for IBD care in order to implement individualized management choices. Figure 1 summarizes the intrinsic and extrinsic systems that result in the development of fibrin-based clotting, with normal balance between the thrombosis and hemostasis states.
Female adolescents and adults with IBDs have a spectrum of bleeding risk based on the genetics and physiology of the coagulation factor imbalance. The deficiency factors include vWF, coagulation factors VIII, IX, XI, VII, XIII and V, and platelet abnormalities. These deficient coagulation factors can be harvested from donated human plasma sources, through technology for recombinant products and with specific HLA platelet selection techniques.2,33,59
The products used for deficiency replacement are as follows: FVIII, recombinant FVIII concentrate; FIX, recombinant FIX concentrate; plasma-derived factors V, VII, XI, XIII concentrate; fresh frozen plasma (can be used if factor XI concentrate is unavailable, as it contains all the coagulation factors); vWF concentrate; tranexamic acid; and desmopressin (Table 3).2,59
Table 3.
| Concentrate drug product | Important effects | Why used | How used |
|---|---|---|---|
| Factor VIII deficiency: recombinant factor VIII concentrate | — | Genetic deficiency | Intravenous transfusion |
| Factor IX deficiency: recombinant factor IX concentrate | |||
| Factor V deficiency: factor concentrate | |||
| Factor VII deficiency | |||
| Factor XIII deficiency | |||
| Factor XI deficiency: plasma-derived factor XI concentrate* | |||
| Tranexamic acid |
|
Inhibits fibrinolysis |
|
| Desmopressin |
|
|
|
| vWF concentrate† | — |
|
Intravenous transfusion |
WD = von Willebrand disease; vWF = von Willebrand factor.
If factor XI concentrate is not available, fresh frozen plasma can be used, as it has all the coagulation factors.
The size of vWF multimers and therefore their activity are regulated by the plasma enzyme ADAMTS13, which cleaves vWF in the A2 domain.
Traditional antithrombotic therapies (anticoagulation, antiplatelet) may be required in females with IBDs needing other surgical and medical treatments.2,55–57,59–63 The available anticoagulation drugs are used to reduce the risk of thrombosis but result in a concomitantly increased risk of bleeding (Table 4).61,62 Although perioperative management of patients who are receiving antiplatelet therapy is a common clinical occurrence, there are minimal high-quality studies to direct, continue or interrupt antiplatelet therapy, as well as concerning the role of bridging therapy.63
Table 4.
Summary of traditional antithrombotic therapies for use in elective surgical management of inherited bleeding disorders*
| Therapy | Mechanism | Delivery route | Indication |
|---|---|---|---|
| Anticoagulant | |||
| Warfarin, phenprocoumon | Inhibition of vitamin K–dependent carboxylation of factors II, VII, IX, X |
Oral | Prevention and treatment of venous and arterial thrombosis/thromboembolism |
| Unfractionated heparin, low-molecular-weight heparin | Activation of antithrombin | Intravenous or subcutaneous | Prevention and treatment of venous and arterial thrombosis/thromboembolism |
| Direct oral anticoagulants (direct thrombin or factor Xa inhibitors) | Inhibition of thrombin or factor Xa | Oral | Prevention and treatment of venous and arterial thrombosis/thromboembolism |
| Antiplatelet | |||
| Acetylsalicylic acid | Inhibition of cyclooxygenase | Oral | Secondary prophylaxis of infarction or ischemic stroke |
| Continuing acetylsalicylic acid before elective surgery | Inhibition of cyclooxygenase | Oral |
|
| Thienopyridine | P2Y receptor antagonist | Oral | Secondary prophylaxis of infarction or ischemic stroke |
Management
Management strategies for IBDs must consider both episodic and preventive approaches for patient care. Compared to the general population, patients with IBDs are at 1.5 and 5 times greater risk for hemorrhage and transfusion, respectively.64–67 Short-term prophylaxis is warranted mainly to provide effective hemostatic coverage to patients undergoing surgery or invasive procedures, at the time of labour and delivery, or with recurrent excessive menstrual bleeding. Long-term prophylaxis is used to prevent bleeding in patients at increased risk for frequent and spontaneous bleeding in the joints, nose or gastrointestinal tract (Table 3).2,59
Menorrhagia
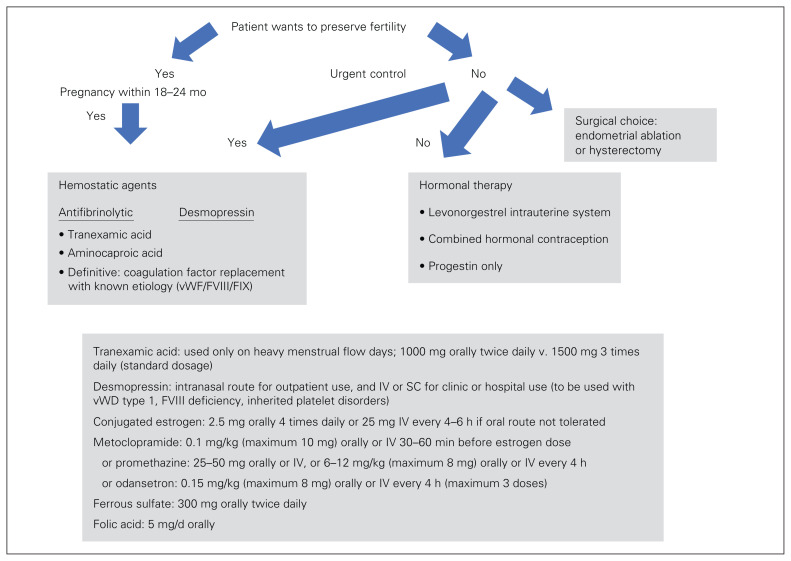
The initial clinical presentation of an IBD in female adolescents and adults is commonly associated with menorrhagia. The primary management pathway and drug or replacement products that may be required before diagnosis of an IBD etiology are summarized in Figure 234 and Table 3.2,59 Educational counselling is required for the informed-consent process. With an IBD diagnosis, discussion of treatment and choice needs to include the patient’s long-term fertility choice, contraception needs, IBD etiology, bleeding severity, and social or religious factors.
Fig. 2.
Decision guide for acute heavy menstrual bleeding (defined as any menstrual bleeding that interferes with daily activity [work, housework, leisure or sport, social activity] during most menstrual periods; bleeding is significant for any 1 of these scenarios: changing pads within 2 h, bleeding lasting ≥ 7 d, clot size > 1 cm with “flooding,” pictorial blood assessment chart score > 100).34 F = factor; IV = intravenously; SC = subcutaneously; vWD = von Willebrand disease; vWF = von Willebrand factor.
Hormonal therapy is indicated for women who want to preserve their fertility options but are not planning an immediate pregnancy (within 12–18 mo). Evidence-based assessment of hormonal therapy in patients with IBDs is limited (Table 5).68 Additional risk counselling may be required, as some women with IBDs have an increased risk of thromboembolic events (afibrinogenemia), and factor replacement may be required for women with a severe bleeding phenotype if they do not respond to medical therapy.
Table 5.
Evidence-based assessment of hormonal therapy in patients with inherited bleeding disorders68
| Hormonal product | Efficacy | Other clinical factors |
|---|---|---|
| Levonorgestrel-releasing intrauterine system | Most effective therapy for heavy menstrual bleeding |
|
| Combined hormonal contraception (birth control pill, transdermal patch and vaginal ring) | Effective for contraception and menses suppression |
|
| Progesterone only (pill, intramuscular medroxyprogesterone or intradermal implant) | Reduces menstrual blood loss with irregular bleeding | — |
von Willebrand disease
Surgical prophylaxis is required for major and minor surgical procedures in patients with vWD.44 The American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation and the World Federation of Hemophilia formed a multidisciplinary guideline panel to establish evidence-based guidelines intended to support patients, clinicians and health care professionals in their decisions about management of vWD. Following are their clinical consensus-based recommendations for vWD management.69
Prophylaxis therapy
-
Recommendation 1: in patients with vWD with a history of severe and frequent bleeds, the panel suggests the use of long-term prophylaxis rather than no prophylaxis.
Desmopressin challenge (trial and administration):
Recommendation 2A: desmopressin is a valid treatment option for patients with vWD type 1 with a baseline vWF level less than 0.30 IU/mL. The panel suggests a trial of desmopressin and treating with desmopressin based on the results over not performing a trial and treating with tranexamic acid or coagulation factor concentrate.
Recommendation 2B: for these patients, the panel suggests against treating with desmopressin in the absence of desmopressin trial results.
Antithrombotic therapy (as required)
Recommendation 3: in patients with vWD and cardiovascular disease who require treatment with antiplatelet agents or anticoagulant therapy, the panel suggests giving the necessary therapy over no treatment.
Recommendation 6A: the panel suggests using either hormonal therapy (combined hormonal contraception) or levonorgestrel-releasing intrauterine system), or tranexamic acid over desmopressin to treat women with heavy menstrual bleeding who do not wish to conceive.
Recommendation 6B: the panel suggests using tranexamic acid over desmopressin to treat women with heavy menstrual bleeding who wish to conceive.
Major surgery
Recommendation 4A: the panel suggests targeting both FVIII and vWF activity levels of 0.50 IU/mL or greater for at least 3 days after surgery.
Recommendation 4B: the panel suggests against using only FVIII level of 0.50 IU/mL or greater as a target level for at least 3 days after surgery.
General surgical options are appropriate for women with IBD who do not want to preserve their fertility options. Multidisciplinary counselling should be used for informed consent and choice. Endometrial ablation is a minimally invasive procedure that is appropriate for women with no uterine or pelvic disorders, as it reduces blood loss substantially in women with IBDs. Hysterectomy provides a definitive surgical treatment for women with IBDs and heavy menstrual bleeding, or uterine or pelvic disorders. There is an operative and anesthesia risk, estimated at 1%–3% for hysterectomy. Women with IBDs are at increased risk for perioperative bleeding, subsequent red cell transfusion and potential interventional radiology. This hemorrhagic complication risk is important to consider as part of the informed-choice process (Figure 2).34
Minor surgery (invasive procedures)
Recommendation 5A: in patients undergoing minor surgery or minor invasive procedures, the panel suggests increasing vWF activity levels to 0.50 IU/mL or greater with desmopressin or factor concentrate with the addition of tranexamic acid over raising vWF levels to 0.50 IU/mL or greater with desmopressin or factor concentrate alone.
Recommendation 5B: in patients with vWD type 1 with baseline vWF activity levels greater than 0.30 IU/mL and a mild bleeding phenotype who are undergoing minor dental mucosal procedures, the panel suggests giving tranexamic acid alone over increasing vWF activity levels to 0.50 IU/mL or greater with any intervention.
Other von Willebrand disease bleeding events
In severe trauma or moderate to severe bleeding, if the coagulation factor level is less than 1%–30% or less than 30%–60%, the use of factor replacement dependent on the percent deficiency level can be considered.41,42
X-linked hemophilia A and B carrier status
Many female hemophilia A carriers are at risk for abnormal bleeding, but these carriers are underrecognized by health care providers, and their bleeding symptoms are underreported.70 Low FVIII levels are consistently associated with clinically significant bleeding and correlate with female skewed X chromosome inactivation. Bleeding can occur in some hemophilia A carriers with normal FVIII levels.70 Chaudhury and colleagues45 reported that females with mild hemophilia (FVIII/FIX) and a bleeding phenotype (traumatic/joint/clinical bleeding) were managed with factor concentrates or antifibrinolytics, but heavy menstrual bleeding was more difficult to control.
Nongynecologic bleeding occurs in female hemophilia carriers owing to the genetic mechanisms described, and their bleeding management may be similar to that for males with hemophilia. Prophylactic management for affected female carriers minimizes bleeding risk and morbidity in joints and the central nervous system (increased with age > 60 yr and hypertension). Prophylactic management should consist of replacement of the appropriate coagulation factor after the diagnosis has been identified and an individualized prophylaxis regimen has been determined based on the patient’s peak–trough FVIII levels. An accessible app (WAPPS-hemo) is available to assist in managing the patient’s regimen.54 The prophylactic use of noncoagulation factor therapy (emicizumab) can be considered as more evidence accrues.52
Inherited platelet disorders
Potential curative treatments for inherited platelet disorders include allogenic hematopoietic stem cell transplantation and gene therapy.
Technologic diagnostic improvements have not been translated into clinical management. Therapies to increase platelet count, mitigate platelet dysfunction and definitively correct inherited thrombocytopenia continue to be used.30,31,55
Outpatient management29,71
As a first-line option, initial control will be obtained with desmopressin in 40% of cases and with an oral contraceptive pill in 30%.71 At 2 years, control will be achieved with an oral contraceptive pill or desmopressin in 50% of cases.71 Additional options for the 50% of cases not controlled by either of these medications include
Nonhormonal therapy: specific factor replacement, antifibrinolytic agent (tranexamic acid or aminocaproic acid) or desmopressin
Hormonal therapy: combined estrogen–progestin product or progestin only
Combination depends on the patient’s contraception needs, whether an estrogen product is contraindicated, the patient’s ability to tolerate an estrogen product, and major adverse effects or sensitivities (there is no real risk in using an antifibrinolytic with estrogen).
Directed procedural/surgical management via bleeding risk assessment31
-
Prevention/prophylaxis of mild to moderate inherited platelet disorder bleeding
♦ Topical measure: compression with gelatin sponges for wounds, epistaxis and bleeding after dental surgery
♦ Antifibrinolytic drugs: treatment before procedure and for 3–5 days afterward: 1) tranexamic acid, 15–25 mg/kg orally every 8 hours; 2) desmopressin, 150 μg/dose intranasally, 0.3 μg/kg subcutaneously or 0.2–0.3 μg/kg in saline (4 μg/mL) intravenously over 30 minutes starting 1 hour before procedure.
-
Prevention of moderate to severe inherited platelet disorder bleeding
♦ Antifibrinolytic drugs: severe bleeding, tranexamic acid, 10 mg/kg intravenously every 8 hours
♦ Platelet transfusion: essential; leukocyte-depleted platelet concentrate is preferable; recommended preprocedure platelet count is greater than 30 × 109/L for dental or minor surgery, greater than 50–80 × 109/L for major surgery or vaginal or cesarean delivery, and greater than 100 × 109/L for eye or central nervous system surgery
♦ Recombinant active factor VII: approved for Glanzmann thrombasthenia; off label for Bernard–Soulier syndrome; potentially useful in combination with antifibrinolytics for control of bleeding in childbirth in women with severe platelet dysfunction; 90–120 μg/kg intravenously before the procedure and then every 90–120 minutes until hemostatic control is attained.
Pregnancy
Obstetric management for women with vWD, hemophilia carriers and those with inherited platelet disorders requires counselling (preferably before conception), choice, informed consent and multidisciplinary care providers (Table 3,2,59 Table 6,2,40,59,72–81 Table 7,2,40,59,72–81 Table 842,82–90). There are substantial hemostatic changes in a normal pregnancy whose role is to preserve placental function and prevent excessive bleeding at delivery.63
Table 6.
Pregnancy and percutaneous procedure (pregnancy or gynecology) management protocols for patients with inherited bleeding disorders2,40,59,72–81
| Condition | Primary coagulation issue | Female bleeding phenotype | Pregnancy: factor change ante partum and/or at delivery* | Percutaneous procedure† | ||
|---|---|---|---|---|---|---|
| Product | Time before/after | Further dosing; regional anesthesia | ||||
| von Willebrand disease | ||||||
| Type 1 | vWF level decreased | Mild to moderate | Increase vWF-containing concentrates or desmopressin | vWF-containing factor VIII concentrate | ≤ 4 h/1–3 d |
|
| Type 2 | Dysfunctional vWF | Variable/moderate | Small increase in vWF-containing concentrates | vWF-containing factor VIII concentrate | ≤ 4 h/1–3 d |
|
| Type 3 | vWF absent | Severe | No increase in vWF-containing concentrates | vWF-containing factor VIII concentrate | ≤ 2 h/> 3–5 d |
|
| Hemophilia A | Factor VIII level decreased | Mild to moderate, with factor VIII level < 40 IU/dL |
|
Recombinant factor VIII | ≤ 2 h/> 3–5 d |
|
| Hemophilia B | Factor IX level decreased | Mild to moderate, with factor IX level < 40 IU/dL |
|
Recombinant factor IX | ≤ 2 h/> 3–5 d |
|
| Glanzmann thrombasthenia | Platelet function disorder | Associated with severe bleeding phenotype |
|
— | — | |
| Bernard–Soulier syndrome | Platelet receptor abnormality | Associated with severe bleeding phenotype |
|
— | — | |
| Factor XI deficiency | Factor XI level decreased | Highly variable but increased with factor XI level < 15 IU/dL |
|
|
≤ 8 h/1–3 d |
|
HLA = human leukocyte antigen; vWF = von Willebrand factor.
If factor level is less than 40 IU/dL.
Pregnancy-related (prenatal diagnosis, regional anesthesia), gynecologic or pelvic endoscopy.
Table 7.
| Item | Hemophilia A | Hemophilia B |
|---|---|---|
| Factor levels |
|
|
| Obstetric clinical area | ||
| Second- and third-trimester activity | Multidisciplinary consultations and labour and delivery planning are required | As for hemophilia A |
| Second stage before delivery | Tranexamic acid, 1000 mg orally | |
| Anesthesia |
|
|
| Low level of coagulation factor (VIII/IX) | FVIII level < 0.8 IU/dL: FVIII coagulation factor replacement to achieve > 100–150 IU/dL at delivery, or desmopressin, 0.3 μg/kg (prepregnancy weight) nasally or subcutaneously after cord clamping* |
|
| Delivery |
|
|
| Postpartum hemorrhage | Prophylaxis therapy: coagulation factor replacement is most common therapy; desmopressin, plasma or tranexamic acid rarely used |
|
| Postpartum period |
|
|
FIX = factor IX; FVIII = factor VIII.
Desmopressin is safe in the first and second trimesters, but safety at delivery has not been shown (risk of fluid overload and hyponatremia/fluid restriction). Saline use and sodium evaluation are required.
Table 8.
Preconception or intrapartum counselling and informed consent for female hemophilia A and B carriers
| Study | Summary |
|---|---|
| Hermans et al.,42 2018 | Description of the evolution of practice, and unmet needs and options for girls and women in families with hemophilia, as well as the clinical and laboratory characteristics during pregnancy. |
| Hermans et al.,82 2022 | Choosing the optimal treatment using an integrated, patient-oriented approach to shared decision-making between patients and clinicians. |
| Mannucci,83 2020 | Despite the major advances in prophylaxis obtained with extended half-life factor products and nonfactor therapies, break-through bleeding has not been fully eliminated, and treatment is still physically and psychologically invasive, even when the subcutaneous route of administration is used. |
| Delgado-Flores et al.,84 2022 | Results suggest that prophylactic treatment (at low, intermediate or high dosages) is superior to episodic treatment for bleeding prevention. For patients with hemophilia A, bleeding rates seem to have a dose–response effect. |
| Ellsworth et al.,85 2021 | Review of background, rationale and potential of nonfactor therapies, as well as pitfalls and expected limitations. Discussion of factor mimetic therapy (emicizumab), siRNA therapeutics, tissue factor pathway inhibitors and serine protease targets. |
| Kenet et al.,86 2021 | Despite high adherence to prophylaxis, the continued occurrence of spontaneous and joint bleeding events requiring treatment, and impaired physical functioning was evident in this study. These results illustrate real-world shortcomings associated with regular FVIII prophylaxis for a cohort of patients with severe hemophilia A, for whom additional hemostatic options were needed. |
| Witarto et al.,87 2022 | This analysis indicates that rurioctocog alfa pegol could serve as a safe and effective alternative for bleeding prophylaxis in previously treated patients with hemophilia A; however, there are limited direct comparison studies. Moreover, this therapy appears to have low immunogenicity, which further increases the safety profile of the drug in such clinical conditions. |
| George,88 2021 | Current clinical hemophilia gene therapy efforts are focused largely on the use of systemically administered recombinant adeno-associated viral (rAAA) vectors for FVIII or FIX gene addition. |
| Arruda et al.,89 2021 | Gene therapy for hemophilia A and B has benefited from advancements in the field of general gene therapy, such as the development of adeno-associated viral vectors and disease-specific breakthroughs, such as the identification of B-domain deleted FVIII and hyperactive FIX Padua. It has also benefited from hemophilia B clinical studies that revealed critical safety concerns related to immune responses to the vector capsid not anticipated in preclinical models. |
| Gualtierotti et al.,90 2022 | The management of pain in patients with hemophilia requires more standardization. Pain management is based on analgesics such as paracetamol, the first-line treatment of acute and chronic pain in adults and children, in association with opioids in adults. Since NSAIDs inhibit platelet function, short courses of these drugs are required. Local treatment with intra-articular injections of corticosteroids is an option for refractory cases. Physiotherapy has a role after hemarthrosis and for long-term management of chronic pain for both pediatric and adult patients. |
FIX = factor IX; FVIII = factor VIII; NSAID = nonsteroidal anti-inflammatory drug; siRNA = small interfering RNA.
An individualized protocol for the management of labour and delivery care in women with IBDs is required based on the identified individualized maternal and fetal risk (Table 3, Tables 6–8). Factor VIII levels are not a good predictor of bleeding risk in hemophilia A carriers, as there are no significant differences in FVIII levels for heterozygous carriers of mutations classified as mild, moderate and severe for hemophilia A.77,78
Prenatal diagnosis techniques may be required for fetal assessment in women with IBDs after routine serum-based aneuploidy screening. No evidence-based series to determine invasive procedure risks for pregnant people with IBDs were identified. Prenatal diagnosis procedures have been used for maternal management decisions in 50% of female hemophilia carriers.72,91 In patients with vWD, early genetic testing of the fetus is not routinely recommended owing to gene size and molecular testing, as well as the lack of clinical implication (other than for delivery and neonatal risk), except for couples with a child with autosomal recessive vWD type 3.72 These couples should have preconception or early pregnancy counselling regarding prenatal testing. Pregnant people with severe autosomal dominant vWD have a 50% chance of having an affected child. For patients with vWD type 1–3, amniocentesis testing at 30–32 weeks can be considered to identify significant fetal risk for labour and delivery management.72
Preconception counselling of female hemophilia carriers (obligate or highly possible) is recommended, if possible, to identify the causative mutation, measure FVIII and FIX levels, provide information for the management of pregnancy and delivery, and discuss the pregnant person’s options and choice in the case of an affected male fetus. Prenatal diagnosis techniques (chorionic villus sampling, amniocentesis, plasma cell-free placental DNA screening [noninvasive prenatal screening]) and the availability of assisted reproductive technology are options to inform hemophilia carriers’ reproductive choice.92 Plasma cell-free placental DNA screening for a Y chromosome via Y-specific polymerase chain reaction is an early prenatal screening option. If a male fetus is identified, chorionic villus sampling to test for the hemophilia mutation can be done. This procedure is normally done at 10–14 weeks’ gestation, via transcervical or transabdominal techniques under ultrasonography guidance. Vaginal bleeding or fetal loss may occur (rate 0.5%–3%). Placental tissue has a 1%–2% biologic risk of confined placental mosaicism.
Second-trimester screening with ultrasonography will identify the gender of the fetus; if male, amniocentesis under ultrasonography guidance to test for the hemophilia mutation can be done.12,60,72 This procedure is normally performed at 15–18 weeks but can be done into the third trimester. Amniotic fluid, with amniocyte cells, is used for testing. Procedural risks include minimal bleeding risk, fetal loss (< 1%) and premature rupture of membranes (2%–3%). Direct studies are carried out with the focus on identifying the responsible mutation (intron 22 inversion) in the carrier’s FVIII gene through full sequencing of the gene. The intron 22 inversion mutation of FVIII accounts for 45% of mutations associated with severe hemophilia, and large deletions and duplications are present in 6% of cases; complex rearrangements involving both inversion and deletion/duplication and mosaicism for Inv22 are rare. If the hemophilia mutation testing is not predicted to be severe, the FVIII gene can be sequenced directly. Indirect studies are important if the carrier’s gene testing does not detect a mutation. The FVIII gene is examined for normal variants or polymorphisms within the family to identify which X chromosome is associated with the family hemophilia. Indirect studies always complement direct studies.
In noninvasive prenatal screening, placental cell-free DNA present in the maternal plasma is used for fetal testing (Y chromosome sex; X chromosome analysis). If assisted reproductive technology is used, preimplantation genetic diagnosis can be performed (blastomere or trophectoderm biopsy, with fluorescence in situ hybridization, polymerase chain reaction or next-generation sequencing).92 Pregnancy outcomes are facility specific, but generic results indicate delivery in 18% of oocyte retrieval procedures and 25% per embryo transfer procedure.93
Directed prelabour anesthesia consultations are recommended for management of regional anesthesia block in labour, with individualized risk assessment and planning. Treatment options of desmopressin acetate or recombinant vWF/FVIII Haemate P (intermediate-purity vWF/FVIII concentrate) (CSL Behring) should normalize coagulation factors and decrease maternal bleeding risk in patients with vWD.94–99
Regarding neuraxial anesthesia, the panel recommends the following:69
Recommendation 7: in women with vWD for whom neuraxial anesthesia during labour is deemed suitable, the panel suggests targeting a vWF activity level of 0.50–1.50 IU/mL over targeting an activity level of more than 1.50 IU/mL to allow neuraxial anesthesia.
Directed obstetrical delivery management for fetal vWD type 1–3 and hemophilia A and B risk (using a stratification process for estimated obstetric bleeding risk levels of mild, moderate and high [Table 3, Tables 6–8]) suggests that, in pregnant people with associated mild or unlikely risk, a recommendation for avoidance of ventouse use and external cephalic version, and selected use of rotational forceps, fetal scalp blood sampling and fetal scalp electrodes should be considered.2,40,59,72–81 In those with associated moderate risk, care providers should consider a recommendation for avoidance of use of midcavity or rotational forceps, ventouse use procedure and external cephalic version, and selected use of fetal scalp blood sampling and fetal scalp electrodes. In those with associated high risk, a recommendation for avoidance of use of all forceps, external cephalic version, ventouse procedure, fetal scalp blood sampling and fetal scalp electrodes should be considered.
Regarding the postpartum period, the panel recommends the following:69
Recommendation 8: the panel suggests the use of tranexamic acid over not using tranexamic acid in women with vWD type 1 or low vWF levels (which may apply to vWD type 2 and 3) during the postpartum period.
Neonatal management has identified that the vWF level is raised at birth, but neonates with severe vWD type 1 and vWD types 2 and 3 have continued low vWF and are at risk for bleeding complications.72 Prenatal risk assessment with amniocentesis at 30–32 weeks can be considered for optimal neonatal planning.72 Cord blood testing for FVIII and vWF antigen and activity is recommended. Cranial imaging and short-term prophylaxis with factor concentrate should be considered neonates with vWD type 3, especially with a history of traumatic delivery. Oral vitamin K administration is recommended for neonates with vWD type 2 or 3. The smallest-gauge needles should be used for later newborn vaccination dosing. The risks and issues for the neonate and during the first year of life should be reviewed with the parents before discharge.2,59,72
Female hemophilia B carriers do not exhibit the hemostatic acute phase reactant elevations with FVIII, and this carrier state may be associated with increased risk of postpartum hemorrhage.25 Postpartum hemorrhage rates have been found to be higher for carriers with a severe hemophilia mutation (54%) compared to those with a mild mutation (29%).78–81
Best-practice considerations
Best-practice considerations for screening, diagnosis and management of IBDs in female adolescents and adults are presented in Table 9.
Table 9.
Best-practice considerations for screening, diagnosis and management of inherited bleeding disorders in female adolescents and adults
| Consideration no. | Consideration | Quality of evidence/strength of recommendation* |
|---|---|---|
| General | ||
| 1 | IBDs should be considered in the differential diagnosis of all female adolescents and adults presenting with menorrhagia. | High/strong |
| 2 | The understanding and recognition of the increased morbidity and low health-related quality of life among female adolescents and adults with menorrhagia, regardless of the etiology, is important for improving patient-oriented health outcomes. | High/strong |
| 3 | Adolescents and adults with IBDs should have timely access to regional–provincial hematologic, genetic, obstetric, gynecologic and pediatric multidisciplinary services for screening, diagnosis, education, care and continuing health care support. | High/weak |
| Screening | ||
| 4 | Primary care or gynecologic care providers: a chart defining the minimum criteria for significant bleeding, a history-related bleeding severity scoring tool (ISTH-BAT [International Society on Thrombosis and Haemostasis – Bleeding Assessment Too]) and a validated graphical scoring system tool (pictorial bleeding assessment chart) are simple and practical methods that can be used by patients to quantify their blood loss. | High/strong |
| 5 | Primary care provider should review in detail a list of the patient’s prescription and nonprescription (including over-the-counter and naturopathic products) medications to identify possible medications and products that increase the risk of bleeding. | Low/weak |
| 6 | Primary care providers with adolescent or adult patients presenting with menorrhagia or with positive results from the validated screening assessment tools, or both, should consider obtaining a laboratory bleeding assessment panel (complete blood count, platelet count, ferritin level, fibrinogen level, prothrombin time test and International Normalized Ratio), activated partial thromboplastin time and vWD investigations (factor VIII level, vWF antigen level and functional assay). If a low platelet count is present, further platelet testing (functional and aggregation) should be considered. Thyroid screening can be included if screening testing gives normal results. | Moderate/strong |
| 7 | Primary care or pediatric care providers: female adolescents in families with a history of vWD or other IBDs should be tested before the onset of menses to determine whether they have inherited the disease, to allow both the patient and her family to prepare for her first and subsequent menstrual periods. | Moderate/strong |
| 8 | Primary care or pediatric care providers: when possible, investigations should be done before oral contraceptive therapy is instituted, as the hormonally induced increase in factor VIII and vWF levels may mask the diagnosis. | Moderate/strong |
| Diagnosis | ||
| 9 | Adolescents and adults with a confirmed diagnosis of vWD should be described based on the quantitative (type 1 autosomal dominant, type 3 autosomal recessive) or qualitative (type 2, 2A, 2B, 2M, 2N) evidenced-based subtype. | Moderate/strong |
| 10 | Adolescents and adults with a confirmed diagnosis of a hemophilia A or B phenotype should be described (and re-evaluated as required) based on the clinical risk category criteria (mild, moderate, severe, symptomatic carrier, asymptomatic carrier). | Moderate/strong |
| 11 | Adolescents and adults with a confirmed diagnosis of a hemophilia A or B phenotype should be described based on their genetic inheritance mechanism (homozygosity, compound heterozygosity, hemizygosity, heterozygosity, other). | Moderate/strong |
| 12 | Adolescent and adults with an inherited platelet disorder should be described based on the specific platelet etiology, given the very low prevalence, the association with people from a consanguineous union, and the variable quantitative and qualitative pathology. | Moderate/strong |
| Management | ||
| 13 | Treatment of menorrhagia in adolescents and adults with IBDs should be individualized according to the defined inherited etiology. | Moderate/weak |
| 14 | Primary care providers or gynecologic care providers: an IBD is not a contraindication to hormonal therapy (oral contraceptives [II-IB], depot medroxyprogesterone acetate [II-3B], danazol [II-2B], gonadotrophin-releasing hormone analogues [II-3B]), or local treatments (levonorgestrel-releasing intrauterine system [II-IB]) and nonhormonal therapy (tranexamic acid [II IB]), as well as desmopressin (II-IB). These therapies represent first-line treatment. Blood products should not be used for patients with mild bleeding disorders (III-A). |
High/strong High/strong |
| 15 | In women with IBDs who no longer want to preserve their fertility, conservative surgical therapy (ablation) and hysterectomy may be options. | High/strong |
| 16 | To minimize the risk of intraoperative and postoperative hemorrhage, coagulation factors should be corrected preoperatively and monitored postoperatively. | Moderate/strong |
| 17 | Pregnancy care providers for women with IBDs should adopt a multidisciplinary consultation and care approach, including primary care, obstetrics, maternal–fetal medicine, internal medicine, anesthesia and neonatology. A copy of the multidisciplinary planning recommendations should be given to the patient, and she should be instructed to present it to the health care provider admitting her to the birthing location, as well as ensuring a directed copy is provided to the birthing location. Women with severe bleeding disorders or with a fetus at risk for a severe bleeding disorder should give birth in a hospital (level 3) or where there is access to consultants in obstetrics, anesthesiology, hematology and pediatrics. |
High/strong |
| 18 | Pregnancy care providers: owing to the varied etiologies of IBDs, the third-trimester and intrapartum prophylactic or treatment use of coagulation factor replacement, use of tranexamic acid or desmopressin, and platelet transfusion must be clearly agreed on and documented by the team in the second trimester; it may be altered later owing to clinical changes in the pregnancy. | Moderate/weak |
| 19 | Pregnancy care providers: vacuum extraction, use of forceps and fetal scalp electrodes, and fetal scalp blood sampling should be avoided if the fetus is known or thought to be at risk for a congenital bleeding disorder. Cesarean delivery should be performed for obstetric indications only. | High/strong |
| 20 | Pregnancy anesthesia providers: epidural anesthesia and spinal anesthesia are contraindicated if there is a coagulation defect. There is no contraindication to regional anesthesia if coagulation is normalized. The decision to use regional anesthesia should be made on an individual basis. | High/strong |
| 21 | Pregnancy: the risk of early and late postpartum hemorrhage is increased in women with bleeding disorders. Women with IBDs should be advised about the possibility of excessive postpartum bleeding and instructed to report this to their care provider immediately. | Moderate/strong |
| 22 | Neonatology: intramuscular injections, surgery and circumcision should be avoided in neonates at risk for a severe IBD until adequate investigation and preparation are possible. | High/strong |
IBD = inherited bleeding disorder; vWD = von Willebrand disease; vWF = von Willebrand factor.
GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) system.
New management considerations
With the management goal of treating the primary deficiency in vWD — vWF — the novel approach of replacing only vWF was introduced about a decade ago. After the manufacture of a concentrate fractionated from human plasma and another obtained through recombinant DNA technology, clinical trials showed that vWF products correct not only the primary vWF deficiency, but also the secondary FVIII coagulant activity deficiency safely and effectively in surgical cohorts.100 Other clinical areas, such as pediatrics, long-term prophylaxis and recurrent gastrointestinal bleeding due to angiodysplasia, have not been as well researched.
There have been major advancements in management and treatment options for X-linked hemophilia, with prolonged half-life FVIII and FIX concentrates, nonfactor therapies (emicizumab, anti-tissue factor pathway inhibitor antibodies, siRNA targeting of antithrombin) and gene therapy. Fassel and McGuinn101 reported that these new molecules significantly reduced the burden of hemophilia and improved the quality of life for patients with severe disease. Prophylaxis with FVIII replacement remains the standard of care for male hemophilia A, as nonreplacement products such as prophylactic emicizumab, given subcutaneously every 4 weeks, are not suitable for acute bleeding episodes or management in major surgery.80,81,102
Additional treatments to increase platelet count stability, transiently before surgery or via invasive interventions, are57
Splenectomy: for use in Wiskott–Aldrich syndrome or X-linked thrombocytopenia only; may reduce bleeding but increase immunodeficiency and rate of severe infections
Eltrombopag/romiplostim: recommended for use in Wiskott–Aldrich syndrome, MYH9-related disease and thrombocytopenia related to the ankyrin repeat domain 26 gene only.
Limitations
Limitations of this scoping review are the quality and quantity of the subject-based publications sorted for review, as the selection and elimination of the publications were done by a single author.
Conclusion
Health care providers need to increase their recognition of and support for female adolescents and adults with IBDs. Improved access to counselling, screening, testing and hemostatic management is also required. Patients should be educated and encouraged to report abnormal bleeding symptoms to their health care provider when they have a concern. It is hoped that this review of preoperative IBD diagnosis and management will enhance access to women-centred care to increase patients’ understanding of IBDs and decrease their risk of IBD-related morbidity and mortality.
Footnotes
Competing interests: The author reports travel support to attend Society of Obstetricians and Gynaecologists of Canada (SOGC) meetings. He is the 2022–2023 SOGC president. No other competing interests were declared.
References
- 1.Page D. Setting the scene: historical overview of challenges and what led to advances in comprehensive care in developed countries, the Canadian experience. Haemophilia 2020;26:4–5. [DOI] [PubMed] [Google Scholar]
- 2.Winikoff R, Scully MF, Robinson KS. Women and inherited bleeding disorders — a review with a focus on key challenges foe 2019. Transfus Apher Sci 2019;58:613–22. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Best C, Dunn S, et al. No. 292 – abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can 2018;40:e391–415. [DOI] [PubMed] [Google Scholar]
- 4.Sanigorska A, Chaplin S, Holland M, et al. The lived experience of women with a bleeding disorder: a systematic review. Res Pract Thromb Haemost 2022;6:e12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hoorn ES, Houwing ME, Arashi WA, et al. Patient-reported outcomes in autosomal inherited bleeding disorders: a systematic literature review. Haemophilia 2022;28:197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual A. Patient Voice: Women with haemophilia also exist. Lancet Haematol 2021;8:e18. [DOI] [PubMed] [Google Scholar]
- 7.von der Lippe C, Frich JC, Harris A, et al. Treatment of hemophilia: a qualitative study of mothers’ perspectives. Pediatr Blood Cancer 2017; 64:121–7. [DOI] [PubMed] [Google Scholar]
- 8.Lindvall K, von Mackensen S, Elmstahl S, et al. Increased burden on caregivers of having a child with haemophilia complicated by inhibitors. Pediatr Blood Cancer 2014;61:706–11. [DOI] [PubMed] [Google Scholar]
- 9.Wiedebusch S, Pollmann H, Siegmund B, et al. Quality of life, psychological strains and coping in parents of children with haemophilia. Haemophilia 2008;14:1014–22. [DOI] [PubMed] [Google Scholar]
- 10.Limperg PF, Haverman L, Peters M, et al. Psychological functioning of mothers of boys with haemophilia. Haemophilia 2016;22:e57–60. [DOI] [PubMed] [Google Scholar]
- 11.Torres-Ortuno A. Hemophilia carriers: quality of life and management at different life stages. Blood Coagul Fibrinolysis 2020;31:S12–4. [DOI] [PubMed] [Google Scholar]
- 12.Mingot Castellano ME. General concepts on hemophilia A and on women carrying the disease. Blood Coagul Fibrinolysis 2020;31:S1–3. [DOI] [PubMed] [Google Scholar]
- 13.Cousino MK, Hazen R. Parenting stress among caregivers of children with chronic illness: a systematic review. J Pediatr Psychol 2013; 38:809–28. [DOI] [PubMed] [Google Scholar]
- 14.Dunn NF, Millar R, Griffioen A, et al. Carrier testing in haemophilia A and B: adult carriers’ and their partners’ experiences and their views on the testing of young females. Haemophilia 2008;14:584–92. [DOI] [PubMed] [Google Scholar]
- 15.Perez L. Hemophilia carriers and women with coagulopathies: challenges in the occupational arena. Blood Coagul Fibrinolysis 2020;31: S15–6. [DOI] [PubMed] [Google Scholar]
- 16.Punt MC, Aalders TH, Bloemenkemp KWM, et al. The experiences and attitudes of hemophilia carriers around pregnancy: a qualitative systematic review. J Thromb Haemost 2020;18:1626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitaker S, Aiston H, Hung WT, et al. Haemophilia Carriers Experience Study (CARES): a mixed method exploration into the experience of women who are carriers of haemophilia. Haemophilia 2021; 27:848–53. [DOI] [PubMed] [Google Scholar]
- 18.Hodroj MH, Hasbani GE, Al-Shamsi HO, et al. Clinical burden of hemophilia in older adults: beyond bleeding risk. Blood Rev 2022;53: 100912. [DOI] [PubMed] [Google Scholar]
- 19.Harris NS, Pelletier JP, Marin MJ, et al. Von Willebrand factor and disease: a review for laboratory professionals. Crit Rev Clin Lab Sci 2022;59:241–56. [DOI] [PubMed] [Google Scholar]
- 20.Laffan M, Sathar J, Johnsen JM. Von Willebrand disease: diagnosis and treatment, treatment of women, and genomic approach to diagnosis. Haemophilia 2021;27:66–74. [DOI] [PubMed] [Google Scholar]
- 21.Seaman CD, Xavier F, Ragni MV, et al. Hemophilia A (factor VIII deficiency). Hematol Oncol Clin North Am 2021;35:1117–29. [DOI] [PubMed] [Google Scholar]
- 22.Pasca S, Zanon E. Haemophilia A/B carriers: haemorrhagic burden of disease and open issues. Blood Transfus 2020;18:496–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller CH, Bean CJ. Genetic causes of haemophilia in women and girls. Haemophilia 2021;27:e164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konkle BA, Fletcher SN, Hemophilia A. 2000. Sept. 21 [updated 2022 Oct. 27]. In Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews [Internet]. Seattle: University of Washington; 1993–2023. Available: https://www.ncbi.nlm.nih.gov/books/NBK1116/ (accessed 2023 Apr. 3). [Google Scholar]
- 25.Staber J, Croteau SE, Davis J, et al. The spectrum of bleeding in women and girls with haemophilia B. Haemophilia 2018;24:180–5. [DOI] [PubMed] [Google Scholar]
- 26.Miller CH. The clinical genetics of hemophilia B (factor IX deficiency). Appl Clin Genet 2021;14:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei B, Liang C, Feng H. Congenital hemophilia A with low activity of factor XII: a case report and literature review. Ital J Pediatr 2021; 47:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asahina T, Kobayashi T, Takeuchi K, et al. Congenital blood coagulation factor XIII deficiency and successful deliveries: a review of the literature. Obstet Gynecol Surv 2007;62:255–60. [DOI] [PubMed] [Google Scholar]
- 29.Pennesi CM, Quint EH, Rosen MW, et al. Outpatient management of heavy menstrual bleeding in adolescent and young women with inherited platelet function disorders. J Pediatr Adolesc Gynecol 2020; 33:489–93. [DOI] [PubMed] [Google Scholar]
- 30.Karasneh J, Christoforou J, Walker JS, et al. World Workshop on Oral Medicine VII: bleeding control interventions for invasive dental procedures in patients with inherited functional platelet disorders: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol 2022; 133:412–31. [DOI] [PubMed] [Google Scholar]
- 31.Bastida JM, Gonzalez-Porras JR, Rivera J, et al. Role of thrombopoietin receptor agonists in inherited thrombocytopenia. Int J Mol Sci 2021;22:4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batsuli G, Kouides P. Rare coagulation factor deficiencies (factors VII, X, V, and II). Hematol Oncol Clin North Am 2021;35:1181–96. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman LH. Causes and consequences of critical bleeding and mechanisms of blood coagulation. Pharmacotherapy 2007;27(9 Pt 2): 45S–56S. [DOI] [PubMed] [Google Scholar]
- 34.James AH. Diagnosis and management of women with bleeding disorders — international guidelines and consensus from an international expert panel. Haemophilia 2011;17(Suppl 1):3–5. [DOI] [PubMed] [Google Scholar]
- 35.Bradley LD, Gueye NA. The medical management of abnormal uterine bleeding in reproductive-aged women. Am J Obstet Gynecol 2016;214:31–44. [DOI] [PubMed] [Google Scholar]
- 36.Kai J, Dutton B, Vinogradova Y, et al. Medical treatment for heavy menstrual bleeding in primary care: ten-year data from the ECLIPSE trial. Br J Gen Pract 2022;72:e857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elbatarny M, Mollah S, Grabell J, et al. Normal range of bleeding scores from the ISTH-BAT: adult and pediatric data from the Merging Project. Haemophilia 2014;20:831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ISTH-SSC bleeding assessment tool. Available: https://bleedingscore.certe.nl (accessed 2023 Apr. 3).
- 39.Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol 1990;97:734–9. [DOI] [PubMed] [Google Scholar]
- 40.Rodeghiero F, Pabinger I, Ragni M, et al. Fundamentals for a systematic approach to mild and moderate inherited bleeding disorders: an EHA consensus report. Hemasphere 2019;3:e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colonne CK, Reardon B, Curnow J, et al. Why is misdiagnosis of von Willebrand disease still prevalent and how can we overcome it? A focus on clinical considerations and recommendations. J Blood Med 2021;12:755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermans C, Kulkarni R. Women with bleeding disorders. Haemophilia 2018;24:29–36. [DOI] [PubMed] [Google Scholar]
- 43.Eladly F, Miesbach W. Von Willebrand disease — specific aspects in women. Hamostaseologie 2022;42:330–6. [DOI] [PubMed] [Google Scholar]
- 44.Weyand AC, Flood VH. Von Willebrand disease. Hematol Oncol Clin North Am 2021;35:1085–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaudhury A, Sidonio R, Jain N, et al. Women and girls with haemophilia and bleeding tendencies: outcomes related to menstruation, pregnancy, surgery, and other bleeding episodes from a retrospective chart review. Haemophilia 2021;27:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidonio RF, Jr, Malec L. Hemophilia B (factor IX deficiency). Hematol Oncol Clin North Am 2021;35:1143–55. [DOI] [PubMed] [Google Scholar]
- 47.Hart DP, Matino D, Astermark J, et al. International consensus recommendations on the management of people with haemophilia B. Ther Adv Hematol 2022;13:20406207221085202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garagiola I, Mortarino M, Siboni SM, et al. X chromosome inactivation: a modifier of factor VIII and IX plasma levels and bleeding phenotype in haemophilia carriers. Eur J Hum Genet 2021;29: 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dardik R, Avishai E, Lalezari S, et al. Molecular mechanisms of skewed X-chromosome inactivation in female hemophilia patients — lessons from wide genome analyses. Int J Mol Sci 2021;22:9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vidal F. State of the art of genetic studies in hemophilia carriers. Blood Coagul Fibrinolysis 2020;31:S4–5. [DOI] [PubMed] [Google Scholar]
- 51.Swystun LL, James P. Using genetic diagnostics in hemophilia and von Willebrand disease. Hematology Am Soc Hematol Educ Program 2015;2015:152–9. [DOI] [PubMed] [Google Scholar]
- 52.Plug I, Mauser-Bunschote EP, Brocker-Vriends AHUT, et al. Bleeding in carriers of haemophilia. Blood 2006;108:52–6. [DOI] [PubMed] [Google Scholar]
- 53.van Galen KP, d’Oiron R, James P, et al. A new hemophilia carrier nomenclature to define hemophilia in women and girls: communication from the SSC of the ISTH. J Thromb Haemost 2021;19:1883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McEneny-King A, Yeung CH, Edginton AN, et al. Clinical application of Web Accessible Population Pharmacokinetic Service–Hemophilia (WAPPS-Hemo): patterns of blood sampling and patient characteristics among clinician users. Haemophilia 2020;26: 56–63. [DOI] [PubMed] [Google Scholar]
- 55.Rao I, Crisafulli L, Paulis M, et al. Hematopoietic cells from pluripotent stem cells: hope and promise for the treatment of inherited blood disorders. Cells 2022;11:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Punt MC, Schuitema PCE, Bloemenkamp KWM, et al. Menstrual and obstetrical bleeding in women with inherited platelet receptor defects — a systematic review. Haemophilia 2020;26:216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai FD, Battinelli EM. Inherited platelet disorders. Hematol Oncol Clin North Am 2021;35:1069–84. [DOI] [PubMed] [Google Scholar]
- 58.Hassan AA, Kroll MH. Acquired disorders of platelet function. Hematology Am Soc Hematol Educ Program 2005;403–8. [DOI] [PubMed] [Google Scholar]
- 59.Dunkley S, Curtin JA, Marren AJ, et al. Updated Australian consensus statement on management of inherited bleeding disorders in pregnancy. Med J Aust 2019;210:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manderstedt E, Lind-Halldén C, Ljung R, et al. Identification of F8 rearrangements in carrier and non-carrier mothers of haemophilia A patients [letter]. Haemophilia 2021;27:e654–8. [DOI] [PubMed] [Google Scholar]
- 61.Mailer RK, Kuta P, Renne T. An update on safe anticoagulation. Hamostaseologie 2022;42:65–72. [DOI] [PubMed] [Google Scholar]
- 62.Alkhalil M, Kuzemczak M, Bell A, et al. A practical approach to prescribing antiplatelet therapy in patients with acute coronary syndromes. CMAJ 2022;194:E205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah S, Urtecho M, Firwana M, et al. Perioperative management of antiplatelet therapy: a systematic review and meta-analysis. Mayo Clin Prac Inn Qual Out 2022;6:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franchini M, Seidizadeh O, Mannucci PM. Prophylactic management of patients with von Willebrand disease. Ther Adv Hematol 2021;12:20406207211064064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simionescu AA, Buinoiu NF, Berbec N. Von Willebrand disease type 2 in pregnancy — a critical clinical association. Transfus Apher Sci 2017;56:269–71. [DOI] [PubMed] [Google Scholar]
- 66.James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost 2007;5:1165–9. [DOI] [PubMed] [Google Scholar]
- 67.James PD, Lillicrap D. Von Willebrand disease: clinical and laboratory lessons learned from the large von Willebrand disease studies. Am J Hematol 2012;87:S4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Djambas Khayat C, Gouider E, von Mackensen S, et al. Heavy menstrual bleeding in women with inherited bleeding disorders. Haemophilia 2020;26:16–9. [DOI] [PubMed] [Google Scholar]
- 69.Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv 2021;5:301–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cygan PH, Kouides PA. Regulation and importance of factor VIII levels in hemophilia A carriers. Curr Opin Hematol 2021;28:315–22. [DOI] [PubMed] [Google Scholar]
- 71.Thorne JG, James PD, Reid RL. Heavy menstrual bleeding: Is tranexamic acid a safe adjunct to combined hormonal contraception? Contraception 2018;98:1–3. [DOI] [PubMed] [Google Scholar]
- 72.Leebeek FWG, Duvekot J, Kruip MJHA. How I manage pregnancy in carriers of hemophilia and patients with von Willebrand disease. Blood 2020;136:2143–50. [DOI] [PubMed] [Google Scholar]
- 73.Falcon Rodriguez MF. Carriers of haemophilia: pregnancy, childbirth, and postpartum. Blood Coagul Fibrinolysis 2020;31:S9–11. [DOI] [PubMed] [Google Scholar]
- 74.Wolf S, Infirri SS, Batty P, et al. Postpartum bleeding in women with inherited bleeding disorders: a matched cohort study. Blood Coagul Fibrinolysis 2020;31:452–8. [DOI] [PubMed] [Google Scholar]
- 75.Punt MC, Waning ML, Mauser-Bunschoten EP, et al. Maternal and neonatal bleeding complications in relation to peripartum management in hemophilia carriers: a systematic review. Blood Rev 2021;49: 100826. [DOI] [PubMed] [Google Scholar]
- 76.Togioka BM, Burwick RM, Kujovich JL. Delivery and neuraxial technique outcomes in patients with hemophilia and in hemophilia carriers: a systematic review. J Anesth 2021;35:288–302. [DOI] [PubMed] [Google Scholar]
- 77.Olsson A, Hellgren M, Bertorp E, et al. Clotting factor level is not a good predictor of bleeding in carriers of haemophiia A and B. Blood Coagul Fibrinolysis 2014;25:471–5. [DOI] [PubMed] [Google Scholar]
- 78.Collins AEI, Curry N, Raza-Burton S, et al. Factor VIII levels and bleeding according to factor 8(F8) mutation in pregnant carriers of haemophiia A: a multicentre retrospective cohort study. Br J Haematol 2021;193:397–400. [DOI] [PubMed] [Google Scholar]
- 79.Wong J, George RB, Hanley CM, et al. Tranexamic acid: current use in obstetrics, major orthopedic, and trauma surgery. Can J Anaesth 2021;68:894–917. [DOI] [PubMed] [Google Scholar]
- 80.Aledort L, Mannucci PM, Schramm W, et al. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus 2019;17:479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dargaud Y, Janbain M. Clinical utility of subcutaneous factor VIII replacement therapies in hemophilia A: a review of the evidence. J Blood Med 2021;12:1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hermans C, Noone D, Benson G, et al. Hemophilia treatment in 2021: choosing the “optimal” treatment using an integrative, patient-oriented approach to shared decision-making between patients and clinicians. Blood Rev 2022;52:100890. [DOI] [PubMed] [Google Scholar]
- 83.Mannucci PM. Hemophilia therapy: the future has begun. Haematologica 2020;105:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delgado-Flores CJ, Garcia-Gomero D, Salvador-Salvador S, et al. Effects of replacement therapies with clotting factors in patients with hemophilia: a systematic review and meta-analysis. PLoS One 2022; 17: e0262273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ellsworth P, Ma A. Factor-mimetic and rebalancing therapies in hemophilia A and B: The end of factor concentrates? Hematology Am Soc Hematol Educ Program 2021;2021:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kenet G, Chen YC, Lowe G, et al. Real-world rates of bleeding, factor VIII use, and quality of life in individuals with severe haemophilia A receiving prophylaxis in a prospective, noninterventional study. J Clin Med 2021;10:5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witarto BS, Visuddho V, Witarto AP, et al. Efficacy, safety, and immunogenicity of rurioctocog alfa pegol for prophylactic treatment in previously treated patients with severe hemophilia A: a systematic review and meta-analysis of clinical trials. F1000Res 2022; 10:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.George LA. Hemophilia gene therapy: Ushering in a new treatment paradigm? Hematology Am Soc Hematol Educ Program 2021;2021: 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arruda VR, Weber J, Samelson-Jones BJ. Gene therapy for inherited bleeding disorders. Semin Thromb Hemost 2021;47:161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gualtierotti R, Tafuri F, Arcudi S, et al. Current and emerging approaches for pain management in hemophilic arthropathy. Pain Ther 2022;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martensson A, Tedgard U, Ljung R. Prenatal diagnosis of haemophilia in Sweden now more commonly used for psychological preparation than termination of pregnancy. Haemophilia 2014;20:854–8. [DOI] [PubMed] [Google Scholar]
- 92.García-Lozano JC, Lozano-Arana MD. Prenatal diagnostic techniques and IVF in patients with coagulopathies. Blood Coagul Fibrinolysis 2020;31:S6–8. [DOI] [PubMed] [Google Scholar]
- 93.De Rycke M, Goossens V, Kokkali G, et al. ESHIRE PGD Consortium data collection XIV–XV: cycles from January 2011 to December 2012 with pregnancy follow-up to October 2013. Hum Reprod 2017;32:1974–94. [DOI] [PubMed] [Google Scholar]
- 94.Miesbach W. Perioperative management for patients with von Willebrand disease: defining the optimal approach. Eur J Haematol 2020;105:365–77. [DOI] [PubMed] [Google Scholar]
- 95.Castaman G. How I treat von Willebrand disease. Thromb Res 2020; 196:618–25. [DOI] [PubMed] [Google Scholar]
- 96.Castaman G, Linari S. Obstacles to early diagnosis and treatment of inherited von Willebrand disease: current perspectives. J Blood Med 2021;12:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Turan O, Abdul Kadir R. Pregnancy in special populations: challenges and solutions practical aspects of managing von Willebrand disease in pregnancy. Hematology Am Soc Hematol Educ Program 2021; 2021:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seidizadeh O, Peyvandi F, Mannucci PM. Von Willebrand disease type 2N: an update. J Thromb Haemost 2021;19:909–16. [DOI] [PubMed] [Google Scholar]
- 99.Rashid A, Moiz B, Karim F, et al. Use of ISTH bleeding assessment tool to predict inherited platelet dysfunction in resource constrained settings. Scand J Clin Labora Invest 2016;76:373–8. [DOI] [PubMed] [Google Scholar]
- 100.Mannucci PM. New therapies for von Willebrand disease. Hematology 2019;2019:590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fassel H, McGuinn C. Haemophilia: factoring in new therapies. Br J Haematol 2021;194:835–50. [DOI] [PubMed] [Google Scholar]
- 102.Kloosterman F, Zwagemaker AF, Abdi A, et al. Hemophilia management: huge impact of a tiny difference. Res Pract Thromb Haemost 2020;4:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]