Abstract
Background
Since the approval of tyrosine kinase inhibitors, angiogenesis inhibitors and immune checkpoint inhibitors, the treatment landscape for advanced renal cell carcinoma (RCC) has changed fundamentally. Today, combined therapies from different drug categories have a firm place in a complex first‐line therapy. Due to the large number of drugs available, it is necessary to identify the most effective therapies, whilst considering their side effects and impact on quality of life (QoL).
Objectives
To evaluate and compare the benefits and harms of first‐line therapies for adults with advanced RCC, and to produce a clinically relevant ranking of therapies. Secondary objectives were to maintain the currency of the evidence by conducting continuous update searches, using a living systematic review approach, and to incorporate data from clinical study reports (CSRs).
Search methods
We searched CENTRAL, MEDLINE, Embase, conference proceedings and relevant trial registries up until 9 February 2022. We searched several data platforms to identify CSRs.
Selection criteria
We included randomised controlled trials (RCTs) evaluating at least one targeted therapy or immunotherapy for first‐line treatment of adults with advanced RCC. We excluded trials evaluating only interleukin‐2 versus interferon‐alpha as well as trials with an adjuvant treatment setting. We also excluded trials with adults who received prior systemic anticancer therapy if more than 10% of participants were previously treated, or if data for untreated participants were not separately extractable.
Data collection and analysis
All necessary review steps (i.e. screening and study selection, data extraction, risk of bias and certainty assessments) were conducted independently by at least two review authors. Our outcomes were overall survival (OS), QoL, serious adverse events (SAEs), progression‐free survival (PFS), adverse events (AEs), the number of participants who discontinued study treatment due to an AE, and the time to initiation of first subsequent therapy. Where possible, analyses were conducted for the different risk groups (favourable, intermediate, poor) according to the International Metastatic Renal‐Cell Carcinoma Database Consortium Score (IMDC) or the Memorial Sloan Kettering Cancer Center (MSKCC) criteria. Our main comparator was sunitinib (SUN). A hazard ratio (HR) or risk ratio (RR) lower than 1.0 is in favour of the experimental arm.
Main results
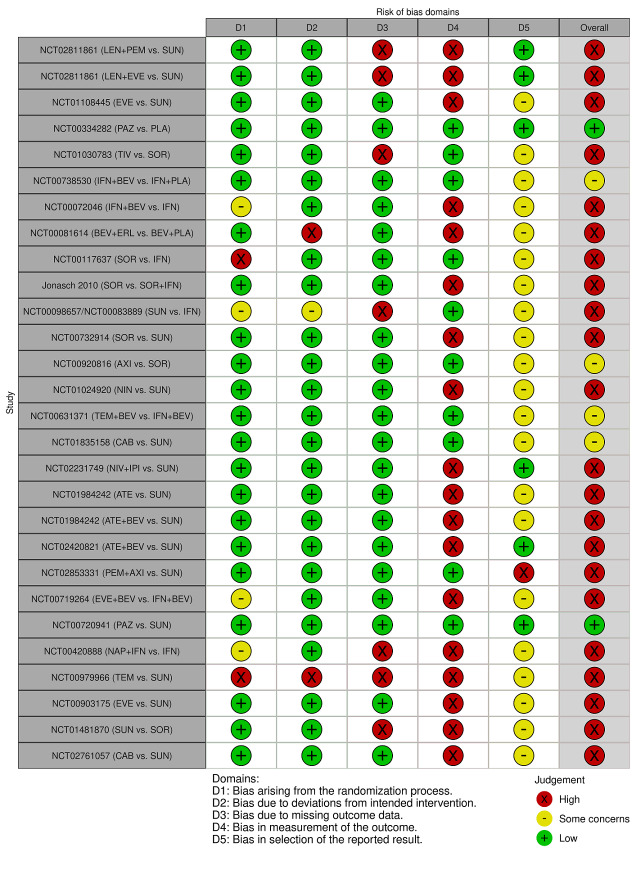
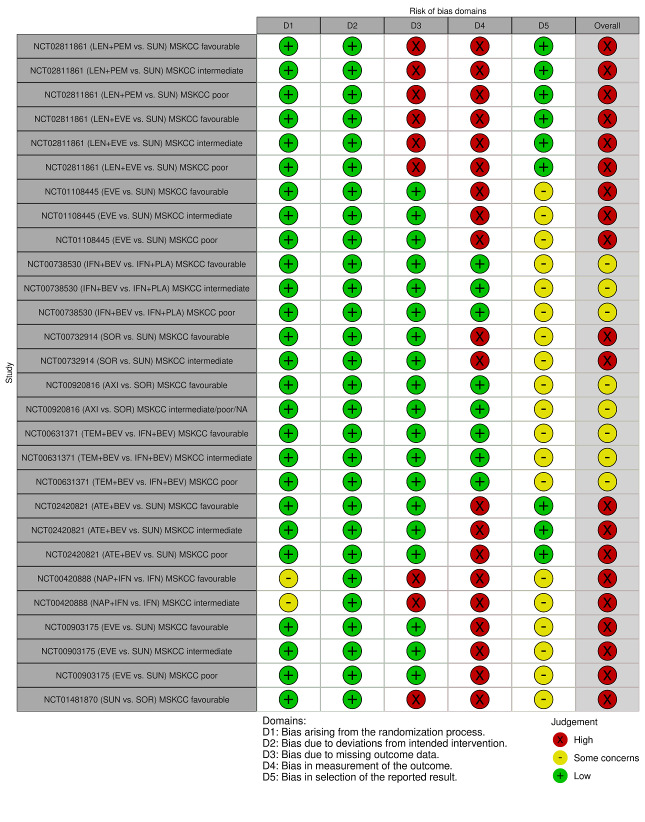
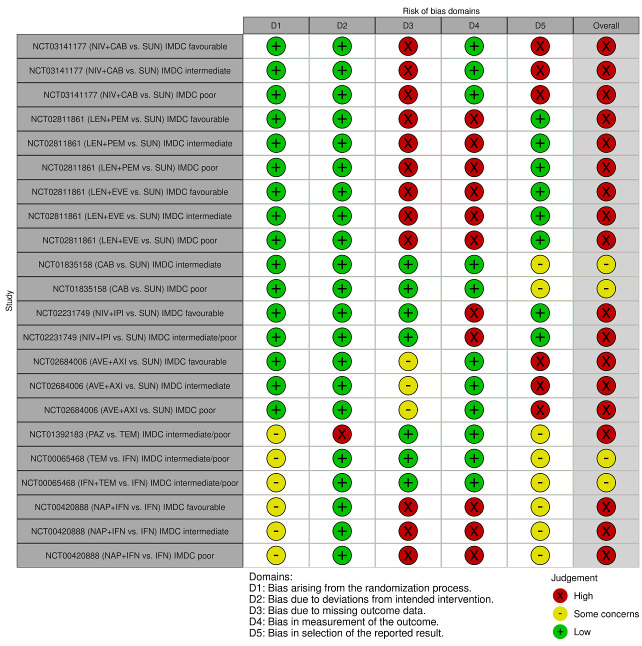
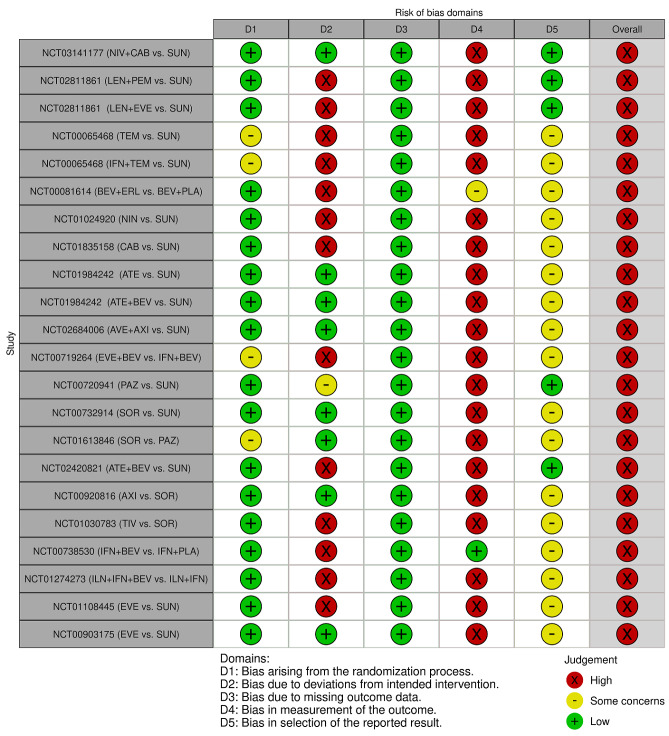
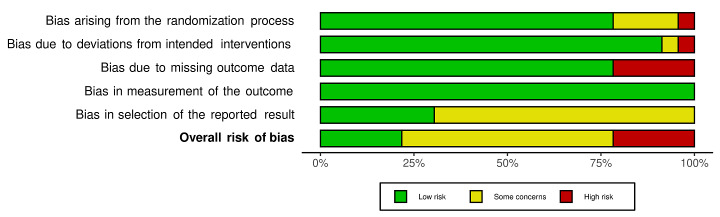
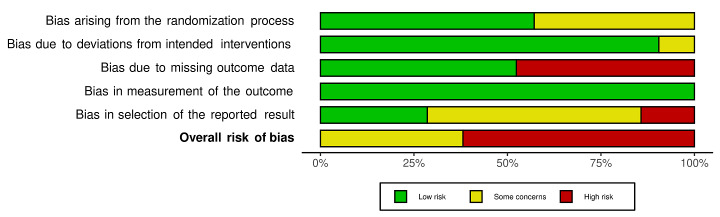
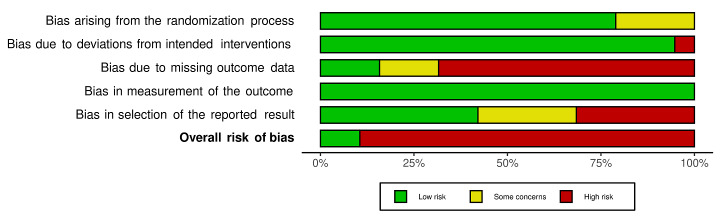
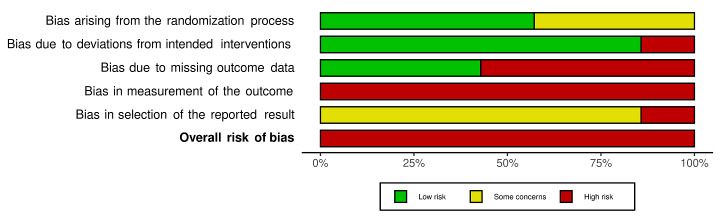
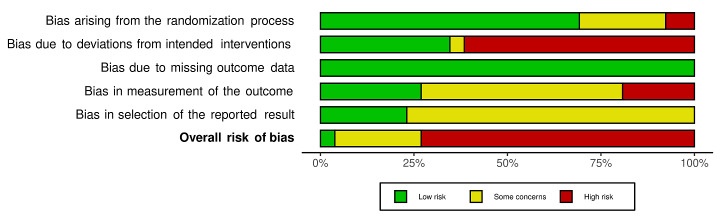
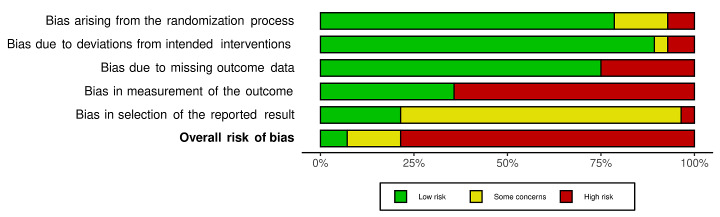
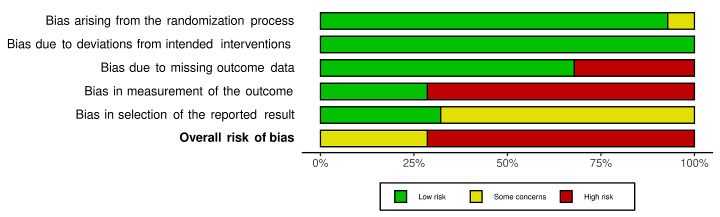
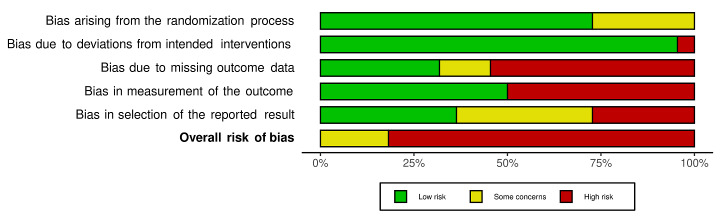
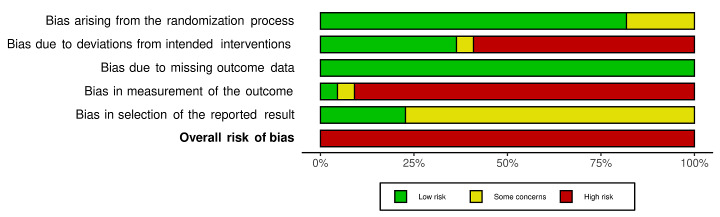
We included 36 RCTs and 15,177 participants (11,061 males and 4116 females). Risk of bias was predominantly judged as being 'high' or 'some concerns' across most trials and outcomes. This was mainly due to a lack of information about the randomisation process, the blinding of outcome assessors, and methods for outcome measurements and analyses. Additionally, study protocols and statistical analysis plans were rarely available.
Here we present the results for our primary outcomes OS, QoL, and SAEs, and for all risk groups combined for contemporary treatments: pembrolizumab + axitinib (PEM+AXI), avelumab + axitinib (AVE+AXI), nivolumab + cabozantinib (NIV+CAB), lenvatinib + pembrolizumab (LEN+PEM), nivolumab + ipilimumab (NIV+IPI), CAB, and pazopanib (PAZ). Results per risk group and results for our secondary outcomes are reported in the summary of findings tables and in the full text of this review. The evidence on other treatments and comparisons can also be found in the full text.
Overall survival (OS)
Across risk groups, PEM+AXI (HR 0.73, 95% confidence interval (CI) 0.50 to 1.07, moderate certainty) and NIV+IPI (HR 0.69, 95% CI 0.69 to 1.00, moderate certainty) probably improve OS, compared to SUN, respectively. LEN+PEM may improve OS (HR 0.66, 95% CI 0.42 to 1.03, low certainty), compared to SUN. There is probably little or no difference in OS between PAZ and SUN (HR 0.91, 95% CI 0.64 to 1.32, moderate certainty), and we are uncertain whether CAB improves OS when compared to SUN (HR 0.84, 95% CI 0.43 to 1.64, very low certainty). The median survival is 28 months when treated with SUN. Survival may improve to 43 months with LEN+PEM, and probably improves to: 41 months with NIV+IPI, 39 months with PEM+AXI, and 31 months with PAZ. We are uncertain whether survival improves to 34 months with CAB. Comparison data were not available for AVE+AXI and NIV+CAB.
Quality of life (QoL)
One RCT measured QoL using FACIT‐F (score range 0 to 52; higher scores mean better QoL) and reported that the mean post‐score was 9.00 points higher (9.86 lower to 27.86 higher, very low certainty) with PAZ than with SUN. Comparison data were not available for PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI, and CAB.
Serious adverse events (SAEs)
Across risk groups, PEM+AXI probably increases slightly the risk for SAEs (RR 1.29, 95% CI 0.90 to 1.85, moderate certainty) compared to SUN. LEN+PEM (RR 1.52, 95% CI 1.06 to 2.19, moderate certainty) and NIV+IPI (RR 1.40, 95% CI 1.00 to 1.97, moderate certainty) probably increase the risk for SAEs, compared to SUN, respectively. There is probably little or no difference in the risk for SAEs between PAZ and SUN (RR 0.99, 95% CI 0.75 to 1.31, moderate certainty). We are uncertain whether CAB reduces or increases the risk for SAEs (RR 0.92, 95% CI 0.60 to 1.43, very low certainty) when compared to SUN. People have a mean risk of 40% for experiencing SAEs when treated with SUN. The risk increases probably to: 61% with LEN+PEM, 57% with NIV+IPI, and 52% with PEM+AXI. It probably remains at 40% with PAZ. We are uncertain whether the risk reduces to 37% with CAB. Comparison data were not available for AVE+AXI and NIV+CAB.
Authors' conclusions
Findings concerning the main treatments of interest comes from direct evidence of one trial only, thus results should be interpreted with caution. More trials are needed where these interventions and combinations are compared head‐to‐head, rather than just to SUN. Moreover, assessing the effect of immunotherapies and targeted therapies on different subgroups is essential and studies should focus on assessing and reporting relevant subgroup data. The evidence in this review mostly applies to advanced clear cell RCC.
Keywords: Adult; Female; Humans; Male; Axitinib; Carcinoma, Renal Cell; Carcinoma, Renal Cell/drug therapy; Nivolumab; Sunitinib
Plain language summary
Initial treatment for adults with advanced kidney cancer (renal cell carcinoma)
Abbreviations
• renal cell carcinoma (RCC)
• avelumab (AVE)
• axitinib (AXI)
• cabozantinib (CAB)
• ipilimumab (IPI)
• lenvatinib (LEN))
• nivolumab (NIV)
• pazopanib (PAZ)
• pembrolizumab (PEM)
• sunitinib (SUN)
Key messages
• When making treatment decisions, it is important to think about whether drugs lengthen life, and whether they decrease or increase harmful side effects.
• The findings in this review apply mostly to advanced renal cell carcinoma (RCC) with a clear cell component.
What is advanced RCC, and how is it treated?
RCC is a type of kidney cancer. It is more common in older people and in men than in women. This is because age (≥60 years) and male sex put people at higher risk of getting it. Other risk factors include body weight, smoking, a history of kidney stones and high blood pressure. More than half of people with RCC discover they have it from routine health check‐ups, because many do not have symptoms in the early stages. When symptoms appear, they can impact people's quality of life and day‐to‐day activities. Before 2005, drugs for treatment of advanced RCC were few and treatments caused many side effects. Now, there are new types of drugs: immunotherapy (use people’s own immune system to find and destroy cancer cells), or targeted therapy (interferes with molecules that are responsible for helping cancer cells to grow, divide, and spread). Combinations of these drugs are used for therapy. With these drugs, people may live longer, with a good quality of life and fewer or milder side effects. These drugs are evaluated in clinical studies with people with RCC.
What did we want to find out?
We wanted to use the most up‐to‐date information from clinical studies to measure the benefits and harms of different treatments for people with advanced RCC. We also wanted to learn if the drugs worked better for some people than others.
What did we do?
We searched for studies that explored different drugs that are immunotherapies or targeted therapies. We examined these in adults (≥18 years) with advanced RCC who receive their first therapy. We compared these drugs to the drug SUN, which is a widely used targeted drug and a commonly used comparator drug in studies. We used a standardised process to assess the quality of the findings and our certainty in them. We rated our certainty in the findings based on factors such as study methods, the number of participants in them, and the precision of study results.
What did we find?
We found 36 studies with 4116 women and 11,061 men, around 60 years of age, with advanced RCC. Most people had ≥2 metastatic sites. We found 22 drugs and 17 combinations of drugs that were measured in the studies. We also performed analyses for different risk groups of advanced RCC. We present and discuss our results for the different risk groups, drugs and combinations in the main text of this review, plus further outcomes. Below we present our main results for our primary outcomes, when all risk groups are combined. We focus on selected drugs (and combinations) (PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI, CAB alone, PAZ alone) that are currently recommended in international guidelines for the treatment of advanced RCC. We report their impact on survival, quality of life and serious side effects.
How long do people live?
People live an average of 28 months when treated with SUN. In comparison, people may live an average of 43 months with LEN+PEM, probably 41 months with NIV+IPI, probably 39 months with PEM+AXI, and probably 31 months with PAZ alone. We are uncertain whether people live an average of 34 months with CAB alone. We do not have information for AVE+AXI and NIV+CAB.
How do people rate their quality of life?
People who receive PAZ alone reported a higher level of quality of life than people who receive SUN, but we are uncertain about the findings. We do not have information for PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI or CAB alone.
What is people's risk for serious side effects?
People who receive SUN have an average risk of 40% for experiencing serious side effects. In comparison, the average risk is probably: 61% with LEN+PEM, 57% with NIV+IPI, 52% with PEM+AXI, and 40% with PAZ. We are uncertain whether the risk is on average 37% with CAB alone. We do not have information for AVE+AXI and NIV+CAB.
What are the limitations of the evidence?
More studies are needed where these new drugs (and combinations) are not only compared to SUN alone, but also to each other. We lack information on the comparative benefits and harms of these drugs in different people, e.g. when comparing men with women, or different histology types of RCC (e.g. clear cell type, papillary type, sarcomatoid type).
How up to date is this evidence?
We conducted our last search for studies in February 2022 and incorporated the most recent study results into this review.
Summary of findings
Summary of findings 1. Summary of findings table for all risk groups combined.
| First‐line therapy for adults with advanced renal cell carcinoma | |||||||
|
Population: people with a confirmed diagnosis of advanced renal cell carcinoma (combined risk groups) without previous systemic anticancer therapy Setting: outpatient Interventions: pembrolizumab + axitinib (PEM+AXI), avelumab + axitinib (AVE+AXI), nivolumab + cabozantinib (NIV+CAB), lenvatinib + pembrolizumab (LEN+PEM), nivolumab + ipilimumab (NIV+IPI), pazopanib (PAZ), cabozantinib (CAB) Comparator: sunitinib (SUN) | |||||||
| Effect estimates (hazard ratio (HR) or risk ratio (RR) < 1 favours intervention) and 95% confidence intervals (CI). Main comparator is SUN1 | |||||||
| Outcomes |
№ of participants (trials) in the network |
Intervention |
Relative effect (95% CI) of the network meta‐analyses |
Anticipated absolute effects (95% CI) |
Certainty of the evidence (GRADE) |
Interpretation of findings | |
| Risk with SUN1,2,3 |
Risk with intervention4 |
||||||
|
Overall survival (OS) ‐ Network (subnet 1) included 19 pairwise comparisons ‐ Median follow‐up across trials5: 32.2 months ‐ Median OS with SUN across trials2 in this network: 28.7 months |
9705 (17 RCTs) |
PEM + AXI | HR 0.73 (0.50 to 1.07)6 |
28.7 months | 39.3 months (26.8 to 57.4) |
⊕⊕⊕⊝ moderatea |
PEM+AXI probably improve OS, when compared to SUN. |
| AVE + AXI | n.a.7 | ‐ | ‐ | ‐ | |||
| NIV + CAB | n.a.7 | ‐ | ‐ | ‐ | |||
| LEN + PEM | HR 0.66 (0.42 to 1.03)6 |
43.5 months (27.9 to 68.3) |
⊕⊕⊝⊝ lowa, b |
LEN+PEM may improve OS, when compared to SUN. | |||
| NIV+IPI | HR 0.69 (0.69 to 1.00)6 | 41.6 months (28.7 to 41.6) | ⊕⊕⊕⊝ moderatec |
NIV + IPI probably improve OS, when compared to SUN. | |||
| CAB | HR 0.84 (0.43 to 1.64)6 |
34.2 months (17.5 to 66.7) |
⊕⊝⊝⊝ very lowd, e |
We are uncertain whether CAB improves OS, when compared to SUN. | |||
| PAZ | HR 0.91 (0.64 to 1.32)6 |
31.5 months (21.7 to 44.8) |
⊕⊕⊕⊝ moderatef |
There is probably little or no difference in OS between PAZ and SUN. | |||
|
Quality of life (QoL) We reported this outcome narratively in this review. Here, long‐term results (i.e., at the end of treatment) are presented. In the comparison PAZ versus SUN, QoL was measured using FACIT‐F (score range 0‐52; higher scores represent better QoL). |
‐ | PEM + AXI | n.a.7 | ‐ | ‐ | ‐ | ‐ |
| AVE + AXI | n.a.7 | ‐ | ‐ | ‐ | ‐ | ||
| NIV + CAB | n.a.7 | ‐ | ‐ | ‐ | ‐ | ||
| LEN + PEM | n.a.7 | ‐ | ‐ | ‐ | ‐ | ||
| NIV+IPI | n.a.7 | ‐ | ‐ | ‐ | ‐ | ||
| CAB | n.a.7 | ‐ | ‐ | ‐ | ‐ | ||
| PAZ | ‐ | The mean post‐score of the control group was 29.5. | One RCT (N = 2) reported that the mean post‐score of the intervention group was 9.00 points higher (9.86 lower to 27.86 higher) than that of the control group. | ⊕⊝⊝⊝ very low g, h |
We are uncertain whether PAZ compared to SUN improves quality of life. | ||
|
Serious adverse events (SAEs) ‐ Network included 31 pairwise comparisons ‐ Mean risk with SUN across trials3 included in this network: 40.7% |
10,709 (22 RCTs) |
PEM + AXI | RR 1.29 (0.90 to 1.85)6 |
40.7% | 52.5% (36.6 to 75.3) |
⊕⊕⊕⊝ moderatef |
PEM+AXI probably increase slightly the risk for SAEs, when compared to SUN. |
| AVE + AXI | n.a.7 | ‐ | ‐ | ‐ | |||
| NIV + CAB | n.a.7 | ‐ | ‐ | ‐ | |||
| LEN + PEM | RR 1.52 (1.06 to 2.19) |
61.9% (43.1 to 89.1) |
⊕⊕⊕⊝ moderateb |
LEN+PEM probably increase the risk for SAEs, when compared to SUN. | |||
| NIV+IPI | RR 1.40 (1.00 to 1.97)6 |
57% (40.7 to 80.2) |
⊕⊕⊕⊝ moderateb |
NIV+IPI probably increase the risk for SAEs, when compared to SUN. | |||
| CAB | RR 0.92 (0.60 to 1.43)6 |
37.4% (24.4 to 58.2) |
⊕⊝⊝⊝ very lowb, i |
We are uncertain whether CAB reduces or increases the risk for SAE, when compared to SUN. | |||
| PAZ | RR 0.99 (0.75 to 1.31 6 |
40.3% (30.5 to 53.3) |
⊕⊕⊕⊝ moderatef |
There is probably little or no difference in the risk for SAEs between PAZ and SUN. | |||
|
Progression‐free survival (PFS) ‐ Network (subnet 1) included 27 pairwise comparisons ‐ Median follow‐up across trials5: 9.1 months ‐ Median PFS with SUN across trials2 in this network: 7.9 months |
11,737 (25 RCTs) |
PEM + AXI | HR 0.68 (0.52 to 0.89)6 |
9.2 months | 13.5 months (10.3 to 17.7) |
⊕⊕⊕⊝ moderateb |
PEM+AXI probably improve slightly PFS, when compared to SUN. |
| AVE + AXI | n.a.7 | ‐ | ‐ | ‐ | |||
| NIV + CAB | n.a.7 | ‐ | ‐ | ‐ | |||
| LEN + PEM | HR 0.39 (0.29 to 0.53)6 |
23.6 months (17.3 to 31.7) |
⊕⊕⊕⊝ moderateb |
LEN+PEM probably improve PFS, when compared to SUN. | |||
| NIV+IPI | HR 0.89 (0.68 to 1.16)6 |
10.3 months (7.9 to 13.5) |
⊕⊕⊝⊝ lowb, f |
There may be little or no difference between NIV+IPI and SUN in improving PFS. | |||
| CAB | HR 0.54 (0.37 to 0.76)8 |
17.0 months (12.1 to 24.9) |
⊕⊕⊝⊝ lowb, d |
CAB may improve PFS, when compared to SUN. | |||
| PAZ | HR 1.05 (0.81 to 1.36)6 |
8.8 months (6.8 to 11.3) |
⊕⊕⊕⊝ moderatef |
There probably is little or no difference in PFS between PAZ and SUN. | |||
|
Adverse events (AEs) (grade 3 or 4) ‐ Network included 19 pairwise comparisons ‐ Mean risk with SUN across trials3 in this network: 70.6% |
6909 participants (13 RCTs) |
PEM + AXI | n.a.7 | 70.6% | ‐ | ‐ | ‐ |
| AVE + AXI | RR 1.00 (0.92 to 1.08)6 | 70.6% (64.9 to 76.2) |
⊕⊕⊕⊝ moderateb |
There probably is little or no difference in the risk for AEs between AVE+AXI and SUN. | |||
| NIV + CAB | RR 1.07 (0.97 to 1.17)6 |
75.5% (68.5 to 82.6) |
⊕⊕⊕⊝ moderateb |
There probably is little or no difference in the risk for AEs between NIV+CAB and SUN. | |||
| LEN + PEM | RR 1.15 (1.06 to 1.25)6 |
81.2% (74.8 to 88.2) |
⊕⊕⊕⊝ moderateb |
LEN+PEM probably increase slightly the risk for AEs (grade 3 or 4), when compared to SUN. | |||
| NIV+IPI | n.a.7 | ‐ | ‐ | ‐ | |||
| CAB | RR 1.04 (0.83 to 1.31)6 |
73.4% (58.6 to 92.5) |
⊕⊝⊝⊝ very lowb, j |
We are uncertain whether CAB reduces or increases the risk for AEs, when compared to SUN. | |||
| PAZ | RR 1.02 (0.96 to 1.09)6 |
72% (67.7 to 76.9) |
⊕⊕⊕⊝ moderateb |
There probably is little or no difference in the risk for AEs between PAZ and SUN. | |||
|
Time to initiation of first subsequent therapy This outcome was not reported as a time‐to‐event outcome. Instead, authors of the trials reported the number of participants who received subsequent anticancer therapy after discontinuation of trial treatment. |
861 (1 RCT) |
PEM + AXI | RR 0.72 (0.64 to 0.81)6 |
65%3 | 46.8% (41.6 to 52.6) |
⊕⊕⊝⊝ lowk |
PEM+AXI may reduce the risk for subsequent therapy, when compared to SUN. |
| 886 (1 RCT) |
AVE + AXI | RR 0.61 (0.52 to 0.72)6 |
51%3 | 31.1% (26.5 to 36.7) |
⊕⊕⊝⊝ lowk |
AVE+AXI may reduce the risk for subsequent therapy, when compared to SUN. | |
| 651 (1 RCT) |
NIV + CAB | RR 0.57 (0.44 to 0.75)6 |
33%3 | 18.8% (14.5 to 24.7) |
⊕⊝⊝⊝ very lowb,k |
We are uncertain whether NIV+CAB reduce the risk for subsequent therapy, when compared to SUN. | |
| 712 (1 RCT) |
LEN + PEM | RR 0.57 (0.48 to 0.68)6 |
60%3 | 34.2% (28.8 to 40.8) |
⊕⊝⊝⊝ very lowb, k |
We are uncertain whether LEN+PEM reduce the risk for subsequent therapy, when compared to SUN. | |
| 1096 (1 RCT) |
NIV+IPI | RR 0.86 (0.79 to 0.94)6 |
70% | 60.2% (55.3 to 65.8) |
⊕⊕⊝⊝ lowk |
NIV+IPI may reduce the risk for subsequent therapy, when compared to SUN. | |
| 151 (1 RCT) |
CAB | RR 0.93 (0.74 to 1.16)6 |
64% | 59.5% (47.4 to 74.2) |
⊕⊝⊝⊝ very lowb, k, j |
We are uncertain whether CAB reduces or increases the risk for subsequent therapy, when compared to SUN. | |
| ‐ | PAZ | n.a.7 | ‐ | ‐ | ‐ | ||
| CI: confidence interval; HR: hazard ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
1 Basis for the assumed risks
2 The risk of SUN for OS and PFS was obtained from the included trials in the networks, respectively, and estimated by calculating the mean of all available medians for SUN
3 Mean risk for AEs and SAEs, respectively, was estimated by dividing the total events under SUN‐therapy by the total of participants treated with SUN across all trials in the network. For TFST, the risk for SUN was calculated using the number of events / number of participants for SUN in the respective trial.
4 Methods of calculating the assumed risks in the intervention group:
‐ For OS and PFS: The median survival in the intervention group was calculated using the methods by Tierney 2007: Corresponding median survival in the intervention group (in months) = comparator group median survival time (in months) divided by the HR. Upper and lower confidence limits for the corresponding intervention risk were obtained by replacing HRs by their upper and lower confidence limits, respectively.
‐ For AEs and SAEs: The assumed risk in the intervention group was calculated with the formula available in the Cochrane Handbook. For the meta‐analytic RR and assumed comparator risk (ACR) the corresponding intervention risk is obtained per 1000: 1000 x ACR x RR. Upper and lower confidence limits for the corresponding intervention risk were obtained by replacing RRs by their upper and lower confidence limits, respectively.
5 Median follow‐up across trials in the networks for OS and PFS, respectively, was estimated by calculating the mean of all available medians
6 Only direct evidence from one trial.
7 Not applicable, comparison not available.
8 Only direct evidence from two trials.
a Downgraded by 1 level for imprecision because of a wide CI and upper CI limit suggests no difference between interventions.
b Downgraded by 1 level for study limitations because the one trial contributing all direct evidence is at high risk of bias.
c Downgraded by 1 level for imprecision because upper CI limit suggests no difference between interventions.
d downgraded by 1 level for indirectness because in one trial, 7% of the total study population received previous systemic therapy.
e Downgraded by 2 levels for imprecision because of a very wide CI that includes values that favour either of the compared treatments, and evidence stems from only one trial with 90 participants.
f Downgraded by 1 level for imprecision because of a wide CI that favours either of the compared treatments.
g Downgraded by 2 levels for study limitations due to a high risk of bias.
h Downgraded by 2 levels for imprecision because of a very wide CI that includes values that favour either of the compared treatments, and evidence stems from only one trial with four participants analysed.
i Downgraded by 2 levels for imprecision because of a very wide CI that includes values that favour either of the compared treatments, and evidence stems from only one trial with 157 participants.
j Downgraded by 2 levels for imprecision because of a wide CI that includes values that favour either of the interventions, and the evidence stems from only one trial with 157 participants.
k Downgraded by 2 levels for indirectness due to indirect measurement of outcome of interest.
Summary of findings 2. Summary of findings table for the favourable risk groups (according to IMDC and MSKCC).
| First‐line therapy for adults with advanced renal cell carcinoma | |||||||
|
Population: people with a confirmed diagnosis of advanced renal cell carcinoma (RCC) and a favourable risk according to the International Metastatic RCC Database Consortium (IMDC) and Memorial Sloan‐Kettering Cancer Center (MSKCC) risk models Setting: outpatient Interventions: pembrolizumab + axitinib (PEM+AXI), avelumab + axitinib (AVE+AXI), nivolumab + cabozantinib (NIV+CAB), lenvatinib + pembrolizumab (LEN+PEM), nivolumab + ipilimumab (NIV+IPI), cabozantinib (CAB), pazopanib (PAZ) Comparator: sunitinib (SUN) | |||||||
| Effect estimate (hazard ratio (HR) < 1 favours intervention) and 95% confidence intervals (CI). Main comparator is SUN1 | |||||||
| Outcomes |
№ of participants (trials) in the network |
Intervention | Relative effect (95% CI) of the network meta‐analyses | Anticipated absolute effects (95% CI) |
Certainty of the evidence (GRADE) |
Interpretation of findings | |
|
Risk with SUN1,2 |
Risk with intervention3 | ||||||
| IMDC risk group | |||||||
|
Overall survival (OS) ‐ Network (subnet 1) included 5 pairwise comparisons ‐ Median follow‐up across trials4: 35 months ‐ Median OS with SUN could not be estimated from data of the included trials in this network. We used the reported median survival from mdalc5 for IMDC favourable risk groups |
933 (4 RCTs) |
PEM + AXI | n.a.7 | 43.25 months | ‐ | ‐ | ‐ |
| AVE + AXI | HR 0.66 (0.36 to 1.22)6 |
65.4 months (35.4 to 120.0) |
⊕⊕⊝⊝ lowa, b |
AVE+AXI may improve OS, when compared to SUN. | |||
|
NIV + CAB |
HR 0.94 (0.46 to 1.92)6 |
45.9 months (22.5 to 93.9) |
⊕⊝⊝⊝ very lowa, c |
We are uncertain whether NIV+CAB improve or decrease OS, when compared to SUN. | |||
| LEN + PEM | HR 1.15 (0.55 to 2.40)6 |
37.7 months (18.0 to 78.5) |
⊕⊕⊝⊝ lowd |
There may be little or no difference in OS between LEN+PEM and SUN. | |||
| NIV+IPI | HR 0.93 (0.62 to 1.40)6 |
46.4 months (30.8 to 69.7) |
⊕⊕⊕⊝ moderateb |
There probably is little or no difference in OS between NIV+IPI and SUN. | |||
| CAB | n.a.7 | ‐ | ‐ | ‐ | |||
| PAZ | n.a.7 | ‐ | ‐ | ‐ | |||
| Serious adverse events | Subgroup data not available. | ||||||
| Quality of life | Subgroup data not available. | ||||||
|
Progression‐free survival (PFS) ‐ Network (subnet 1) included 5 pairwise comparisons ‐ Median follow‐up across trials4: 35 months ‐ Median PFS with SUN across trials2 in this network: 20.9 months |
933 (4 RCTs) |
PEM + AXI | n.a.7 | 20.9 months |
‐ | ‐ | ‐ |
| AVE + AXI | HR 0.71 (0.49 to 1.02)6 |
29.4 months (20.5 to 42.6) | ⊕⊕⊝⊝ lowa, e |
AVE+AXI may improve PFS, when compared to SUN. | |||
|
NIV + CAB |
HR 0.58 (0.36 to 0.93)6 |
36.0 months (22.5 to 58.0) | ⊕⊕⊝⊝ lowa,f |
NIV+CAB may improve PFS, when compared to SUN. | |||
| LEN + PEM | HR 0.41 (0.28 to 0.61)6 |
51.0 months (34.3 to 74.6) | ⊕⊕⊝⊝ low a,f |
LEN+PEM may improve PFS, compared to SUN. | |||
| NIV+IPI | HR 1.84 (1.29 to 2.62)6 |
11.3 months (7.8 to 16.2) |
⊕⊕⊕⊝ moderatea |
NIV+IPI probably reduce PFS, when compared to SUN. | |||
| CAB | n.a.7 | ‐ | ‐ | ‐ | |||
| PAZ | n.a.7 | ‐ | ‐ | ‐ | |||
| Adverse events (grade 3 to 4) | Subgroup data not available. | ||||||
| Time to initiation of first subsequent therapy | Subgroup data not available. | ||||||
| MSKCC risk group | |||||||
|
Overall survival (OS) ‐ Network (subnet 1) included 3 pairwise comparisons ‐ Median follow‐up across trials4: 26.6 months ‐ Median OS with SUN across trials2 in this network: 43.6 months |
594 (2 RCTs) |
PEM + AXI |
n.a.7 | 43.6 months |
‐ | ‐ |
‐ |
|
AVE + AXI |
n.a.7 | ‐ | ‐ |
‐ | |||
|
NIV + CAB |
n.a.7 | ‐ | ‐ |
‐ | |||
|
LEN + PEM |
HR 0.86 (0.38 to 1.93)6 |
50.7 months (22.6 to 114.7) | ⊕⊝⊝⊝ very lowa, d |
We are uncertain whether LEN+PEM improve OS, when compared to SUN. | |||
| NIV+IPI | n.a.7 | ‐ | ‐ | ‐ | |||
| CAB | n.a.7 | ‐ | ‐ | ‐ | |||
| PAZ | HR 0.88 (0.63 to 1.21)6 |
49.5 months (36.0 to 69.2) | ⊕⊕⊝⊝ lowa, b |
There may be little or no difference between PAZ and SUN. | |||
| Serious adverse events (SAEs) | Subgroup data not available. | ||||||
| Quality of life (QoL) | Subgroup data not available. | ||||||
|
Progression‐free survival (PFS) ‐ Network (subnet 1) included 7 pairwise comparisons ‐ Median follow‐up across trials4: 25 months ‐ Median PFS with SUN across trials2 in this network: 13.7 months |
784 (6 RCTs) |
PEM + AXI | n.a.7 | 13.7 months | ‐ | ‐ | ‐ |
| AVE + AXI | n.a.7 | ‐ | ‐ | ‐ | |||
| NIV + CAB | n.a.7 | ‐ | ‐ | ‐ | |||
| LEN + PEM | HR 0.36 (0.11 to 1.23)6 |
38.0 months (11.1 to 124.5) | ⊕⊝⊝⊝ very lowa, d |
We are uncertain whether LEN+PEM improve PFS, when compared to SUN. | |||
| NIV+IPi | n.a.7 | ‐ | ‐ | ‐ | |||
| CAB | n.a.7 | ‐ | ‐ | ‐ | |||
| PAZ | n.a.7 | ‐ | ‐ | ‐ | |||
| Adverse events (grade 3 to 4) | Subgroup data not available. | ||||||
| Time to initiation of first subsequent therapy | Subgroup data not available. | ||||||
| CI: confidence interval; HR: hazard ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
1 Basis for the assumed risks
2 The risk of SUN for OS and PFS was obtained from the included trials in the networks, respectively, and estimated by calculating the mean of all available medians for SUN
3 Method of calculating the assumed risks in the intervention group for survival outcomes: The median survival in the intervention group was calculated using the methods by Tierney 2007: Corresponding median survival in the intervention group (in months) = comparator group median survival time (in months) divided by the HR. Upper and lower confidence limits for the corresponding intervention risk were obtained by replacing HRs by their upper and lower confidence limits, respectively.
4 Median follow‐up across trials in the networks for OS and PFS, respectively, was estimated by calculating the mean of all available medians
5 Median OS with SUN could not be estimated from data of the included in this network. We used the reported median survival from mdalc for IMDC favourable risk groups, which is comparable to MSKCC favourable risk groups under SUN therapy
6 Only direct evidence from one trial.
7 Not applicable, comparison not available.
a Downgraded by 1 level for study limitations because the one trial contributing all direct evidence is at high risk of bias.
b Downgraded by 1 level for imprecision because of a wide CI that includes values that favour either of the compared treatments.
c Downgraded by 2 levels for imprecision because of a very wide CI that includes values that favour either of the compared treatments, and evidence stems from only one trial with 146 participants.
d Downgraded by 2 levels for imprecision because of a very wide CI that includes values that favour either of the compared treatments.
e Downgraded by 1 level for imprecision because of a wide CI and upper CI limit suggests no difference.
f Downgraded by 1 level for imprecision because evidence stems from only one trial with < 150 participants.
Summary of findings 3. Summary of findings for the intermediate and poor risk groups (according to IMDC and MSKCC).
| First‐line therapy for adults with advanced renal cell carcinoma | |||||||
|
Population: people with a confirmed diagnosis of advanced renal cell carcinoma (RCC) and an intermediate or poor risk according to the International Metastatic RCC Database Consortium (IMDC) and Memorial Sloan ‐Kettering Cancer Center (MSKCC) risk models Setting: outpatient Interventions: pembrolizumab + axitinib (PEM+AXI), avelumab + axitinib (AVE+AXI), nivolumab + ipilimumab (NIV+IPI), nivolumab + cabozantinib (NIV+CAB), lenvatinib + pembrolizumab (LEN+PEM), cabozantinib (CAB), pazopanib (PAZ) Comparator: sunitinib (SUN) | |||||||
| Effect estimate (hazard ratio (HR) < 1 favours intervention) and 95% confidence intervals (CI). Main comparator is SUN1 | |||||||
| Outcomes |
№ of participants (trials) in the network |
Intervention | Relative effect (95% CI) of the network meta‐analyses | Anticipated absolute effects (95% CI) |
Certainty of the evidence (GRADE) |
Interpretation of findings | |
|
Risk with SUN1,2 |
Risk with intervention3 | ||||||
| IMDC risk groups | |||||||
|
Overall survival (OS) ‐ Network (subnet 1) included 10 pairwise comparisons ‐ Median follow‐up across trials4: 35.1 months ‐ Median OS with SUN across trials2 in this network: 23.9 months |
2908 (5 RCTs) |
PEM + AXI | n.a.5 | 23.9 months | ‐ | ‐ | ‐ |
| AVE + AXI | HR 0.73 (0.48 to 1.11)6 |
32.7 months (21.5 to 49.8) | ⊕⊕⊝⊝ lowa, b |
AVE+AXI may improve OS, when compared to SUN. | |||
|
NIV + CAB |
HR 0.60 (0.37 to 0.96)6 |
39.8 months (24.9 to 64.6) | ⊕⊕⊕⊝ moderatea |
NIV+CAB probably improve OS, when compared to SUN. | |||
| LEN + PEM | HR 0.55 (0.33 to 0.91)6 |
43.4 months (26.3 to 72.4) | ⊕⊕⊕⊝ moderatea |
LEN+PEM probably improve OS, when compared to SUN. | |||
| NIV + IPI | HR 0.65 (0.38 to 1.10)6 |
36.8 months (21.7 to 62.9) | ⊕⊕⊕⊝ moderateb |
NIV+IPI probably improve OS, when compared to SUN. | |||
| CAB | HR 0.80 (0.42 to 1.52)6 |
29.8 months (15.7 to 56.9) |
⊕⊝⊝⊝ very lowa, c |
CAB may improve slightly OS, when compared to SUN. | |||
| PAZ | n.a.5 | ‐ | ‐ | ‐ | |||
| Quality of life | Subgroup data not available. | ||||||
| Serious adverse events | Subgroup data not available. | ||||||
|
Progression‐free survival (PFS) ‐ Network (subnet 1) included 11 pairwise comparisons ‐ Median follow‐up across trials4: 34.5 months ‐ Median PFS with SUN across trials2 in this network: 6.0 months |
2908 (5 RCTs) |
PEM + AXI | n.a.5 | 6.0 months | ‐ | ‐ | ‐ |
| AVE + AXI | HR 0.60 (0.43 to 0.84)6 |
10.0 months (7.1 to 13.9) |
⊕⊕⊕⊝ moderatea |
AVE+AXI probably improve PFS, when compared to SUN. | |||
|
NIV + CAB |
HR 0.48 (0.34 to 0.69)6 |
12.5 months (8.7 to 17.6) | ⊕⊕⊕⊝ moderatea |
NIV+CAB probably improve PFS, when compared to SUN. | |||
| LEN + PEM | HR 0.36 (0.24 to 0.54)6 |
16.6 months (11.1 to 25.0) | ⊕⊕⊕⊝ moderatea |
LEN+PEM probably improve PFS, when compared to SUN. | |||
| NIV + IPI | HR 0.74 (0.49 to 1.11)6 |
8.1 months (5.4 to 12.2) |
⊕⊕⊝⊝ lowa, b |
There may be little or no difference in PFS between NIV+IPI and SUN. | |||
| CAB | HR 0.46 (0.27 to 0.79)6 |
13.0 months (7.6 to 22.2) |
⊕⊕⊕⊝ moderated |
CAB probably improves PFS, when compared to SUN. | |||
| PAZ | n.a.5 | ‐ | ‐ |
‐ | |||
| Adverse events (grade 3 or 4) | Subgroup data not available. | ||||||
| Time to initiation of first subsequent therapy | Subgroup data not available. | ||||||
| MSKCC risk groups | |||||||
|
Overall survival (OS) ‐ Network included 15 pairwise comparisons ‐ Median follow‐up across trials4: 36.4 months ‐ Median OS with SUN across trials2 in this network: 18.2 months |
3937 (7 RCTs) |
PEM + AXI | n.a.5 | 18.2 months | ‐ | ‐ | ‐ |
| AVE + AXI | n.a.5 | ‐ | ‐ | ‐ | |||
| NIV + CAb | n.a.5 | ‐ | ‐ | ‐ | |||
|
LEN + PEM |
HR 0.63 (0.46 to 0.86)6 |
28.9 months (21.2 to 39.6) | ⊕⊕⊕⊝ moderatea |
LEN+PEM probably improve OS, when compared to SUN. | |||
| NIV + IPI | n.a.5 | ‐ | ‐ | ‐ | |||
| CAB | n.a.5 | ‐ | ‐ | ‐ | |||
| PAZ | HR 0.89 (0.75 to 1.06)6 |
20.4 months (17.2 to 24.3) |
⊕⊕⊝⊝ lowa, b |
There may be little or no difference in OS between PAZ and SUN. | |||
| Quality of life (QoL) | Subgroup data not available. | ||||||
| Serious adverse events | Subgroup data not available. | ||||||
|
Progression‐free survival (PFS) ‐ Network (subnet 1) included 10 pairwise comparisons ‐ Median follow‐up across trials4: 25 months ‐ Median PFS with SUN across trials2 in this network: 5.4 months |
1522 (5 RCTs) |
PEM + AXI | n.a.5 | 5.4 months | ‐ | ‐ | ‐ |
| AVE + AXI | n.a.5 | ‐ | ‐ | ‐ | |||
| NIV + CAB | n.a.5 | ‐ | ‐ | ‐ | |||
| LEN + PEM | HR 0.33 (0.17 to 0.62)6 |
16.4 months (8.7 to 31.8) | ⊕⊕⊕⊝ moderatea |
LEN+PEM probably improve PFS, when compared to SUN. | |||
| NIV + IPI | n.a.5 | ‐ | ‐ | ‐ | |||
| CAB | n.a.5 | ‐ |
‐ | ‐ | |||
| PAZ | n.a.5 | ‐ | ‐ | ‐ | |||
| Adverse events (grade 3 or 4) | Subgroup data not available. | ||||||
| Time to initiation of first subsequent therapy | Subgroup data not available. | ||||||
| CI: confidence interval; HR: hazard ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
1 Basis for the assumed risks.
2 The risk of SUN for OS and PFS was obtained from the included trials in the networks, respectively, and estimated by calculating the mean of all available medians for SUN.
3 Method of calculating the assumed risks in the intervention group for survival outcomes: The median survival in the intervention group was calculated using the methods by Tierney 2007: Corresponding median survival in the intervention group (in months) = comparator group median survival time (in months) divided by the HR. Upper and lower confidence limits for the corresponding intervention risk were obtained by replacing HRs by their upper and lower confidence limits, respectively.
4 Median follow‐up across trials in the networks for OS and PFS, respectively, was estimated by calculating the mean of all available medians.
5 Not applicable, comparison not available.
6 Only direct evidence from only one trial.
a Downgraded by 1 level for study limitations because the one trial contributing all direct evidence is at high risk of bias.
b Downgraded by 1 level for imprecision because of a wide CI that favours either of the compared treatments.
c Downgraded by 2 levels for imprecision because of a very wide CI that includes values that favour either of the compared treatments, and evidence stems from only one trial with 157 participants.
d Downgraded by 1 level for imprecision because the evidence stems from only one trial with 157 participants.
Background
Description of the condition
In 2020, it was estimated that 431,288 people were diagnosed with kidney cancer worldwide (ASCO 2022). The most common type of kidney cancer is renal cell carcinoma (RCC) (ASCO 2021). In the USA for example, kidney cancers account for 5% of all cancers in men and 3% of cancers in women (American Cancer Society 2022). It is estimated that in 2022, 79,000 new cases of kidney cancer (including the renal pelvis) will be diagnosed in the USA (50,290 estimated new cases in men and 28,710 estimated new cases in women) and that 13,920 people will die from this disease (American Cancer Society 2022; Siegel 2022). Males are twice as likely to be diagnosed with kidney cancer (with a lifetime risk for developing kidney cancer being 2.02%), as compared to females (with a lifetime risk for developing kidney cancer being 1.03%) (American Cancer Society 2022). The number of deaths in the USA in 2022 is estimated to be 13,920: 8,960 for men and 4,960 for women (American Cancer Society 2022; Siegel 2022). The five‐year relative survival rates of all stages (i.e. local, regional, distant) are estimated at 76% (American Cancer Society 2022). For Germany, the Robert Koch Institute reported a kidney cancer incidence of 14,830 new cases in the year 2018, with an incidence rate of 15.4% in men and 7.6% in women. The mortality rate due to kidney cancer was 4.5% for men and 1.9% for women (Robert Koch Institute 2021). Moreover, kidney cancer was the most frequent tumour site for 3.5% of men and 2.4% of women in Germany (Robert Koch Institute 2021). For 2022, the Robert Koch Institute predicts 14,500 new cases of kidney cancer (36% in women and 64% in men).
With a 2:1 ratio, RCC presents predominantly in men and commonly develops after the 60th year of life (Rini 2009). Besides gender and age, further risk factors include an increased body mass index (BMI) (i.e. increased body weight) and active as well as passive smoking (Capitanio 2019; Rini 2009; Scelo 2018; Robert Koch Institute 2021). Important co‐morbidity associated with an increased risk for developing this type of kidney cancer include hypertension, a history of kidney stones, type 2 diabetes, increased use of certain analgesics such as non‐aspirin non‐steriodal anti‐inflammatory drugs, and several chronic liver and kidney diseases (Capitanio 2019; Rini 2009; Robert Koch Institute 2021; Scelo 2018). Physical activity is associated with a decreased risk of RCC (Robert Koch Institute 2021). Other factors, which may be protectively related to a risk for developing RCC, are fruit and vegetable and moderate alcohol consumption (Capitanio 2019; Rini 2009).
Staging of RCC is performed in accordance with the Union International Cancer Control (UICC) tumour, node, and metastasis (TNM) classification system (UICC 2017). First, the TNM system is used for classifying the tumour, where T stands for tumour (i.e. size and extent of the tumour); N for nodes (i.e. whether the cancer has spread to nearby lymph nodes); M for metastasis (i.e. whether the cancer spread to other organs (e.g. bones, brain, lungs). Thus, each category provides detailed information about the cancer, and a number (i.e. 1, 2 or 3) is assigned to each category, with a higher number indicating a more advanced cancer. Second, by combining these three categories and assigning a number to each, the overall cancer stage is determined (so‐called group staging). Stages I to III are considered to be local or locoregional disease (depending on the group staging according to the TNM system: stage I includes T1; stage II includes T2; stage III includes T3 or T1‐T3, and N1), and stage IV, which involves tumour spread beyond the renal/Gerota's fascia and/or distant metastases, to be advanced disease (stage IV includes T4 or N2 or M1) (Brierley 2016; Escudier 2019). While the overall five‐year survival rates are approximately 76% (American Cancer Society 2022), the rates decrease drastically to 71% amongst individuals with locoregional disease (stage II and III, i.e. when the cancer has spread outside the kidney to nearby tissue and/or nearby lymph nodes), and to 14% for those with metastatic disease (stage IV, i.e. has spread to distant parts of the body) (ASCO 2022). Around a third of those affected will present with advanced disease. Furthermore, every fourth patient receiving treatment for localised RCC (stage I) will relapse and eventually develop distant metastases (???Choueiri 2017b; Dabestani 2016; Sun 2011).
Renal cell carcinoma is characterised by a variety of subtypes, the most common of which amongst adults are the clear cell type (75%), the papillary type (10%), and the chromophobe type (5%) (Lopez‐Beltran 2009; Warren 2018). Of these three subtypes, the clear cell type is associated with the worst prognosis (Lopez‐Beltran 2009; Warren 2018). For clear cell and papillary RCC, grading with prognostic value is commonly done by the International Society of Urological Pathology (ISUP) tumour grading system, which is adopted by the World Health Organization (WHO) and, therefore, also considered the ISUP/WHO grading classification system (Delahunt 2019). The validity of the grading systems with regard to the correlation of grade and outcome has not been shown for other subtypes, but these systems can be applied for descriptive purposes (Delahunt 2019). The ISUP/WHO grading system includes four stages, with classification based on the nucleus of the tumour cell: tumour cell nucleoli is absent or not clearly visible and basophilic at 400× magnification (grade 1); tumour cell nucleoli is clearly visible and eosinophilic at 400× magnification and visible but not prominent at 100× magnification (grade 2); tumour cell nucleoli is clearly visible and eosinophilic at 100× magnification (grade 3); tumour showing extreme nuclear pleomorphism, tumour giant cells and/or the presence of any proportion of tumour showing sarcomatoid and/or rhabdoid dedifferentiation (grade 4) (Delahunt 2019).
Renal cell carcinomas present in both local symptoms, including haematuria or flank pain, and systemic symptoms evoked, inter alia, through metastases. The latter may include, for example, hypercalcaemia, hypertension, erythrocytosis (increased numbers of red blood cells), and fever (Rini 2009). Nevertheless, renal cell carcinomas primarily present asymptomatically, meaning that today over half of renal cell carcinomas are discovered incidentally (Escudier 2019). Once advanced, they are associated with many symptoms, reduced health‐related quality of life, and fatigue in those affected, especially when the disease progresses (de Groot 2018). For example, in a qualitative survey 46% of 287 participants reported psychiatric symptoms such as depressive symptoms and post‐traumatic stress disorder. Due to poor survival rates, advanced renal cell carcinoma puts an immense burden on healthcare systems (Thekdi 2015).
Individuals with advanced RCC are categorised into favourable, intermediate, or poor risk groups. These are the common risk groups as defined by the International Metastatic RCC Database Consortium (IMDC) and the Memorial Sloan Kettering Cancer Center (MSKCC). The IMDC model (also known as Heng's model) determines the risk group based on the presence of six clinical factors: <1 year from time of diagnosis to systemic treatment; Karnofsky performance status < 80%; haemoglobin < lower limit of normal; corrected calcium > upper limit of normal; neutrophils > upper limit of normal; platelets > upper limit of normal). For every factor that applies, one point (+1) is added. The risk group is then based on the total sum of points appointed (i.e., favourable risk = 0 points, intermediate risk = 1 to 2 points, poor risk = 3 to 6 points) (www.mdcalc.com/). The MSKCC model (also known as the Motzer model) includes five clinical factors: time from diagnosis to systemic treatment <1 year; haemoglobin < lower limit of normal; calcium >10 mg/dL (>2.5 mmol/L); lactate dehydrogenase (LDH) > 1.5x upper limit of normal; Karnofsky performance status <80%). The risk group is also based on the total sum of points appointed (i.e., favourable risk = 0 points, intermediate risk = 1 to 2 points, poor risk = 3 to 5 points) (www.mdcalc.com/).
Description of the intervention
Before 2005, treatment options for advanced RCC were limited to immunotherapies such as the cytokine therapies interferon (IFN)‐alpha and interleukin (IL)‐L. These are associated with many adverse events and with partial or complete remission rates of approximately 12%, they benefit only a small percentage of participants (Coppin 2004). Nowadays, targeted therapies such as tyrosine kinase inhibitors, and immunotherapies, such as immune checkpoint inhibitors, have emerged as an effective alternative, and the benefit of standard approaches, such as sunitinib or temsirolimus, over cytokine therapies with regard to mortality, quality of life, and adverse events in advanced renal cell carcinoma has been indicated (Unverzagt 2017). Multiple drugs such as sunitinib, sorafenib, bevacizumab, nivolumab, pazopanib, axitinib, cabozantinib, and everolimus have therefore been approved by the US Food and Drug Administration (FDA), mostly for second‐line therapy, but several of them have been approved for first‐line treatment as well. However, further novel therapeutic options could be associated with increased toxicities, which require consideration within an organised framework (Qin 2018).
For the first‐line treatment setting, the National Comprehensive Cancer Network (NCCN) (Motzer 2022), the European Association of Urology (EAU) (Ljungberg 2022), the European Society for Medical Oncology (ESMO) guideline (Powles 2021), and the German guideline (Leitlinienprogramm Onkologie) all recommend the combination of pembrolizumab + axitinib (PEM + AXI) as the treatment option across all risk groups (i.e. favourable‐, intermediate‐ or poor risk) for first‐line therapy of advanced clear cell RCC. In addition, for the favourable risk group, the guidelines by NCCN, ESMO and EAU also list the combinations lenvatinib + pembrolizumab (LEN + PEM) or nivolumab + cabozantinib (NIV + CAB) as additional options (Ljungberg 2022; Motzer 2022; Powles 2021). For the intermediate‐ or poor risk groups, additional options can also be NIV + CAB, LEN + PEM or nivolumab + ipilimumab (NIV + IPI) (Ljungberg 2022; Motzer 2022; Powles 2021). The German guideline also lists NIV+IPI as an additional option for the intermediate or poor risk groups (Leitlinienprogramm Onkologie). In addition, the German guideline and the NCCN also suggest avelumab + axitinib (AVE + AXI) across all risk groups (Leitlinienprogramm Onkologie; Motzer 2022). Recommendations are also provided for situations when immune checkpoint inhibitors cannot be administered or tolerated. In such cases, targeted therapy is another option: pazopanib (PAZ) for IMDC favourable or intermediate/poor risk groups (Ljungberg 2022), and additionally cabozantinib (CAB) or sunitinib (SUN) for intermediate‐, and poor‐risk groups (Ljungberg 2022). The NCCN guideline recommends CAB, PAZ or SUN across all risk groups as possible options (Motzer 2022). The German guideline recommends bevacizumab + interferon (BEV+IFN), PAZ, SUN or tivozanib (TIV) for the favourable risk group; TIV, SUN, PAZ, CAB, or alternatively BEV+IFN for the intermediate risk group; CAB, SUN, or alternatively PAZ or temsirolimus (TEM) for the poor risk group, in cases where checkpoint inhibitors cannot be administered or tolerated (Leitlinienprogramm Onkologie). It should be noted that the recommendations of the German guidelines and the EAU guidelines are specifically for the IMDC risk groups.
Due to the high cost of targeted drugs and novel immunotherapeutic agents in cancer care, the economic burden of treatment of advanced RCC is enormous. Swallow 2018 reported additional cost per month of overall survival of USD 49,000 for cabozantinib and USD 24,000 for nivolumab compared to everolimus. On the other hand, Edwards 2018 analysed data from more than 4000 relapsed participants and showed that everolimus is cost‐effective compared to best supportive care, with an incremental cost‐effectiveness ratio (ICER) of GBP 45,000 per quality‐adjusted life‐year (QALY), as it is likely to be considered an end‐of‐life treatment. They reported that cabozantinib compared to everolimus might not be cost‐effective, with an ICER of GBP 126,000 per QALY. In their economic analysis, nivolumab performed even worse than cabozantinib, as it was more costly but less effective.
How the intervention might work
In immunotherapy, which has as its primary aim to enhance the response of the immune system to the tumour cells, the classic, non‐specific immunotherapeutic agents interleukin‐2 (IL‐2)—and especially interferon‐alpha (INF‐a)—have largely been replaced by novel agents. More advanced immunotherapeutics such as nivolumab, atezolizumab, and ipilimumab target specific immune checkpoints. Together with its ligand 1 (PD‐L1), the programmed cell death protein 1 (PD‐1) inhibits the immune response, the release of cytokines, and the cytotoxic function of T‐cell lymphocytes (Harshman 2014). This PD‐1—PD‐L1 pathway is used by most renal cell carcinoma tumour cells to avoid the immune system (Aguiar 2018; Choueiri 2017b;?? Harshman 2014). Nivolumab, a monoclonal antibody, directly targets and binds the PD‐1 receptor, thus stimulating the immune response against cancer cells. Another monoclonal antibody, atezolizumab, targets the PD‐1—PD‐L1 pathway by binding PD‐L1, which then further prevents interaction of the receptor and its ligand (Keir 2007). Besides the PD‐1—PD‐L1 pathway, the cytotoxic T ‐ymphocyte‐associated antigen 4 (CTLA‐4) pathway has gained relevance in the treatment of renal cell carcinoma. The monoclonal antibody ipilimumab targets the CTLA‐4 receptor, which is responsible for the regulation of tumour‐specific T cell lymphocytes, and stimulates the immune response by inhibiting the regulatory function of CTLA‐4 (Aguiar 2018; Sanchez‐Gastaldo 2017).
Besides immunotherapeutic approaches, targeted therapies, which are aimed directly at preventing the growth and/or spread of cancer cells by targeting specific proteins or genes, are today an integral component of the treatment of advanced renal cell carcinoma. An effective target for such approaches is the vascular endothelial growth factor (VEGF) pathway that affects tumour angiogenesis, growth, and survival (Aguiar 2018). The monoclonal antibody and angiogenesis inhibitor bevacizumab directly targets and neutralizes VEGF. Another common target specifically used by tyrosine kinase inhibitors is the VEGF receptor (VEGFR). Its neutralization inhibits angiogenesis as well. Because most tyrosine kinase inhibitors do not focus on the VEGF pathway only, for example to overcome resistance of the tumour to VEGFR inhibition alone, many of them are considered multikinase inhibitors (Aguiar 2018; Sanchez‐Gastaldo 2017). This group includes the agents sunitinib, sorafenib, pazopanib, axitinib, and cabozantinib (Sanchez‐Gastaldo 2017). Another important target for targeted approaches in the treatment of renal cell carcinoma is the mechanistic target of rapamycin (mTOR) pathway, which triggers cell growths and division. More precisely, mTOR is itself part of a protein complex which performs important tasks in cell growth and proliferation and subsequently in tumour angiogenesis and survival (Sabatini 2006). Both temsirolimus and everolimus inhibit the function of mTOR, and by these means deactivate the associated protein complexes (Sanchez‐Gastaldo 2017). Among the afore‐outlined agents, combinations within and across groups and mechanisms involved are common. INF‐a, for example, is used in combination with bevacizumab, and has shown lower mortality rates as well as reduced side effects compared to INF‐a alone, whereas it has not shown a difference in combination with temsirolimus compared to temsirolimus alone (Unverzagt 2017).
Why it is important to do this review
Our preliminary searches of the literature identified a great number of trials, including many ongoing trials that will be completed within the next years. In fact, we are aware of at least 36 published randomised controlled trials (RCTs) involving more than 10,000 participants, as well as 19 ongoing trials that have been registered in trial registries. This highlights the importance of a living systematic review approach, which applies all the detailed methods recommended by Cochrane, and is updated and republished whenever new evidence relevant to the review is identified (Elliott 2014). Such systematic and continuous updates of the available evidence ensure that recent findings are rapidly integrated into the body of evidence to support recommendations given in guidelines and to contribute to an up‐to‐date and high‐grade decision support for effective therapeutic strategies for the individual patient.
However, recommendations can be complicated when economic arguments are introduced into discussions on the best strategy, because the related costs differ enormously per treatment option. This dissent provides the rationale for a network meta‐analytic approach to the existing evidence for all available first‐line therapy regimens. Although we are aware of several recently conducted network meta‐analyses, none of these have analyzed indirect comparisons of all evaluable treatment options.
Lastly, as a critically necessary innovation within Cochrane, we planned to integrate evidence identified from clinical study reports (CSRs) into our systematic review and favoured this new source of evidence, where available, over the journal publication of eligible trials. Furthermore, as publication bias might influence all subsequent analyses and conclusions, all potential relevant trial registries were searched in detail to detect each conducted trial evaluating eligible drugs.
Objectives
The primary objective of this systematic review with network meta‐analysis (NMA) was to evaluate and compare the benefits and harms of first‐line therapies for adults with advanced renal cell carcinoma (RCC), and to produce a clinically relevant ranking of therapies.
The secondary objectives were to maintain the currency of the evidence by conducting continuous update searches, using a living systematic review approach, and to incorporate data from clinical study reports (CSRs).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), both parallel‐group RCTs and cross‐over RCTs, in this review. For cross‐over trials, we only extracted data from the first treatment period. We excluded cluster‐RCTs as these do not fit with the aim of this review as we are interested in treatment benefit and harm in individuals, rather than in group effects. We also excluded quasi‐randomised trials.
Where a clinical study report (CSR) for an individual eligible trial was available, we extracted available data on trial design and trial results from the CSR instead of the respective journal publications.
There was no limitation on trial eligibility with respect to the length of follow‐up in individual trials.
Types of participants
We included trials involving adult participants (18 years of age or older) with a confirmed diagnosis of advanced renal cell carcinoma and (RCC) without previous systemic anticancer therapy, irrespective of gender and ethnicity of participants. Because first‐line therapy only relate to participants with metastatic renal cell carcinoma, only trials including participants with metastatic disease were eligible.
We also included trials with previously treated participants in the total trial population if results for the previously untreated participants were separately extractable. However, when sufficient subgroup data were unavailable for untreated participants, we still extracted results from the entire trial population if less than 10% of participants have received previous systemic anti‐cancer treatment.
Types of interventions
We included trials evaluating at least one of the following therapeutics without restrictions on the dose, dosage form, frequency, or duration of treatment, for example as shown below.
-
Targeted therapy
Tyrosine kinase inhibitor (e.g. sunitinib, sorafenib, pazopanib, axitinib, cabozantinib, savolitinib, anlotinib)
mTOR inhibitor (e.g. temsirolimus, everolimus)
Angiogenesis inhibitor (e.g. bevacizumab, levantinib)
-
Immunotherapy
Checkpoint inhibitors (e.g. atezolizumab, avelumab, nivolumab, ipilimumab, pembrolizumab)
Interferon
Interleukin
Placebo
any combination of the above (e.g. nivolumab + ipilimumab, avelumab + axitinib, pembrolizumab + axitinib)
We included trials evaluating at least one targeted therapy or immunotherapy in at least one intervention arm to provide up‐to‐date results. We excluded trials evaluating these agents in an adjuvant setting. We also excluded trials that assessed the comparison of interleukin versus interferon only. Instead, we only included trials with interleukin and interferon when given in combination with another substance (e.g. interferon‐alpha (IFN‐a) + bevacizumab) or when compared to another substance (e.g. IFN‐a versus sunitinib).
We analysed interventions for favourable‐risk groups separately from interventions for intermediate‐ and poor‐risk groups (intermediate‐ and poor‐risk groups were combined). Moreover, we analysed risk groups according to IMDC and MSKCC criteria separately (see Differences between protocol and review). All interventions were analysed using direct and indirect comparisons. When no direct evidence from randomised trials was available, but the trials were considered sufficiently similar with respect to the participant population, indirect estimates of intervention effects were obtained by means of network calculations. In the protocol of this review, we pre‐specified that different doses of the same drug will be combined to single drug categories if these would differ. However, most interventions were administered at the same dose across trials (see Table 1 in Results).
We included sunitinib as our main comparator as it is a widely used tyrosine kinase inhibitor and is often used as the comparator drug in trials. For the transitivity assumption to hold true, we assessed the administration routes, the dosage and the discontinuation rates of this comparator in each trial (Salanti 2012). In the protocol of this review we had pre‐specified that we would create networks of trials with the same administration route and average dose if these would differ. However, in all included trials that assessed sunitinib, the drug was provided via the same administration route (oral) and the administration dose was 50mg/ day in all trials (see Table 1 in Results).
Types of outcome measures
We included all trials fulfilling the inclusion criteria defined above, irrespective of the reported outcomes. To inform this review and to ensure that we assess outcomes that are most relevant to adults with advanced renal cell carcinoma, during the protocol development of this review, patients and patient representatives were invited in a two‐hour session to discuss relevant outcomes from their perspectives. The following outcomes and order of outcomes (i.e., primary and secondary outcomes) were prioritised together with the patients and patient representatives during the workshop.
Primary outcomes
Overall survival (OS), defined as the time from random treatment assignment to death from any cause
Quality of life (QoL), assessed with validated and reliable instruments
Serious adverse events (SAEs)*, assessed as the number of participants with at least one event
We prioritised OS, QoL, and SAEs as our primary outcomes together with the participants and patient representatives, who regarded these outcomes as most relevant, and also because they are a direct measure of treatment benefit. Furthermore, OS can be considered the most robust endpoint as it does not require blinding.
*An adverse event that results in death or is life‐threatening.
Secondary outcomes
Progression‐free survival (PFS), defined as the time interval from randomisation to the first confirmed disease progression, disease relapse, or death from any cause, or to the last time point of follow‐up
Adverse events (AEs), assessed as the number of participants with at least one event
Number of participants who discontinued study treatment due to an AE
We included PFS as a secondary outcome as it is commonly used to assess stable disease.
With regard to AEs, we assessed severity grades 3 and 4** in the number of participants with at least one AE. We only extracted data on AEs that were labelled as 'all‐cause' AEs; hence, we did not extract data when AEs were labelled as 'treatment‐related'. In addition, we put a special focus on specific AEs that were regarded as most relevant by the participants and patient representatives. These included: hand‐foot syndrome, fatigue, diarrhoea, vomiting, loss off appetite, weight loss, mucous membrane damage (generic term; we looked at mucosal inflammation and stomatitis separately), insomnia, and depression. We extracted data for these specific AEs separately.
In the protocol for this review, we had stated that we would, additionally, extract all individual AEs reported in the included studies, as well as their frequency of occurrence. However, this was not feasible (see Differences between protocol and review).
**Severity grading according to Common Terminology Criteria for Adverse Events (CTCAE). Trials usually report grades 3 and 4 together, and a severe AE (grade 3 or 4) does not necessarily need to be considered serious.
Time to initiation of the first subsequent anticancer therapy (TFST), defined as the time from initiation of first‐line chemotherapy until the start of subsequent therapy or death
Method and timing of outcome measurement
We analysed OS and PFS as time‐to‐event outcomes, and included results representing the longest follow‐up time available. The outcome TFST was not reported as a time‐to‐event outcome in the included trials. Pooling of this outcome was not feasible, so we report results narratively.
For QoL, we initially accepted all validated instruments, and we would have calculated standardised mean differences (SMD) instead of mean difference (MD) when scales used between trials differed (see Measures of treatment effect). However, during the conduct of this review, we decided to prioritise scales for the assessment of this outcome because we initially identified a total of 25 scales and sub‐scales across trials that were used to assess QoL. Due to this high heterogeneity, we decided to prioritise scales that are most clinically relevant and used in clinical daily practice. To prioritise QoL‐scales, two review authors (AA, ET) first created a list of all scales that were reported in the included trials, which was then provided to two co‐authors with a clinical background (AH, PD), who ranked them by assigning them to either low priority, medium priority or high priority based on clinical relevance. Prioritisation was further guided by a third clinician (PM) on the author team and there was discussion amongst author team members (AA, AH, ET, PM, PD) via teleconference. Ultimately, the following scales were prioritised to extract data for QoL:
the Functional Assessment of Cancer Therapy Kidney Cancer Symptom Index – Disease Related Symptoms (FKSI‐DRS);
the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐C30 (QLQ‐C30);
the EuroQol Visual Analogue Scale (EQ‐VAS);
the Functional Assessment of Cancer Therapy General (FACT‐G);
the Functional Assessment of Chronic Illness Therapy Fatigue (FACIT‐F).
We grouped the measurement time points of QoL into those measured directly after initiation of treatment up to four weeks after initiation treatment, medium‐term outcomes (1 month up to 12 months after initiation of treatment), and longer‐term outcomes (over one year after initiation of treatment). Where available, we also extracted data at the end of treatment.
We included all other outcome categories for the observational periods reported in the CSRs or trial publications. We planned to include AEs and SAEs occurring during active treatment as well as long‐term AEs and SAEs. However, we were not able to extract long‐term AEs or SAEs, and we could also not group the timing of outcome measurements as we had pre‐specified in the protocol, because in the publications of the trials it was not stated which time points were being reported. Hence, for AEs and SAEs, we extracted data for events that occurred during the time of treatment.
Outcomes to be included in GRADE summary of findings table
During the development of this protocol, participants and patient representatives were invited to share their opinions and perspectives regarding the most patient‐relevant outcome measures to be included in this review. The most relevant outcome categories, to be included in summary of findings tables, were OS, QoL, SAEs, PFS, AEs, and TFST.
Search methods for identification of studies
We adapted all search strategies for electronic database searches and searching other sources from those suggested in the Cochrane Handbook for Systematic Reviews of Interventions and in accordance with the specified recommendations therein (Lefebvre 2019). We applied no language restriction in order to reduce language bias. All abstracts were available in English.
Electronic searches
Searching for clinical study reports
For this systematic review, the inclusion of trial design and results data from clinical study reports (CSRs) was preferred above the respective journal publications. The search method was initiated by the identification of the sponsors of the included clinical trials. This was done by referring to the clinicaltrials.gov platform (www.clinicaltrials.gov/). After identification of the respective sponsor, the possibility of a direct request for CSRs was checked. Furthermore, the availability of the CSRs on the manufacturer’s platform was verified. To complement the search method, the following data platforms were enclosed for the search of the CSRs: the European Medicines Agency (EMA) ‘clinical data platform’ (clinicaldata.ema.europa.eu/web/cdp/home), the Yale University Open Data Access (YODA) platform (yoda.yale.edu/), the clinical data study request (CSDR) platform (clinicalstudydatarequest.com), and the Vivli platform (https://vivli.org). Initially, the FDA platform was intended to be included, however it was indicated to have insufficient data, as the platform remained in its pilot stage during the search process. The EMA ‘clinical data platform’ was searched for active substances of the included clinical trials. This search offered an overview of all available trials encompassing the respective active substances, and subsequently we screened the search for the trials included in this review and for available CSRs to these trials. The YODA platform allows utilising the NCT (i.e. the clinicaltrials.gov registry number) within the search process. This approach was exclusively performed for this particular platform. The CSDR platform was used to search and request for CSRs. The search process was done by searching for active substances of the included clinical trials. The CSDR platform only offers CSRs from its members; hence, requests are also only possible to be made if the sponsor of all included clinical trials is an official member of the platform. The pharmaceutical company Bayer is excluded from this particular case, as it is a member of the CSDR, however does not offer the opportunity to take in requests. Two types of requests were offered by the CSDR: 1. datasets that are not yet shared on the CSDR platform and 2. trial documents only. Almost all requests that were made throughout this search process included both types. In total, 21 requests were made, and 19 requests included both types. The final platform utilised for this search method was Vivli. The search process included searching for key terms such as “renal”, “kidney”, and the active substances, and complementary the NCT was used to find available CSR. One request on the Vivli platform was made.
Ultimately, we identified two CSRs to two trials (NCT00334282; NCT00720941) and one scientific summary result to one trial (NCT01064310) through the CSDR platform. The CSRs and the scientific result summary were used for data extraction and to inform risk of bias assessment.
Electronic database searches
We searched the following databases/sources to identify eligible trials.
-
Databases of medical literature:
Cochrane Library, including the Central Register of Controlled Trials (CENTRAL), 2022 issue 02, see Appendix 1 and Appendix 2);
MEDLINE (Ovid, from 1946 up to 9 February 2022, see Appendix 3 and Appendix 4);
Embase (from 1974 up to 9 February 2022, see Appendix 5 and Appendix 6).
-
Conference proceedings of annual meetings of the following societies (included in CENTRAL):
American Society of Clinical Oncology (ASCO);
European Society of Medical Oncology (ESMO).
As publication bias might influence all subsequent analyses and conclusions, we searched all potential relevant trial registries in detail to detect ongoing as well as completed studies that have not yet been published. It is mandatory today for the type of studies eligible for inclusion in this review to provide results at least in the study registry (United States Congress 2007; World Medical Association). When results were not published elsewhere, data from the trial registries were extracted and analysed.
-
Trial registries to identify ongoing trials and results of completed trials (up to 9 February 2022), see Appendix 7 and Appendix 8:
ISRCTN registry (www.isrctn.com);
EU Clinical Trials Register (www.clinicaltrialsregister.eu/ctr-search/search);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/);
WHO ICTRP (https://www.who.int/clinical-trials-registry-platform).
Living systematic review considerations
We first conducted baseline review searches in February and October 2020. Starting from December 2020, after publication of the protocol for this review, we ran monthly update searches until April 2021 (Figure 1). Together with the clinical experts on this review, we decided to stop the update‐searches in April 2021 in order to be able to finalise data extraction and risk of bias assessments. However, one final update search was conducted on 9 February 2022, as searches for intervention reviews should not be older than 12 months at publication.
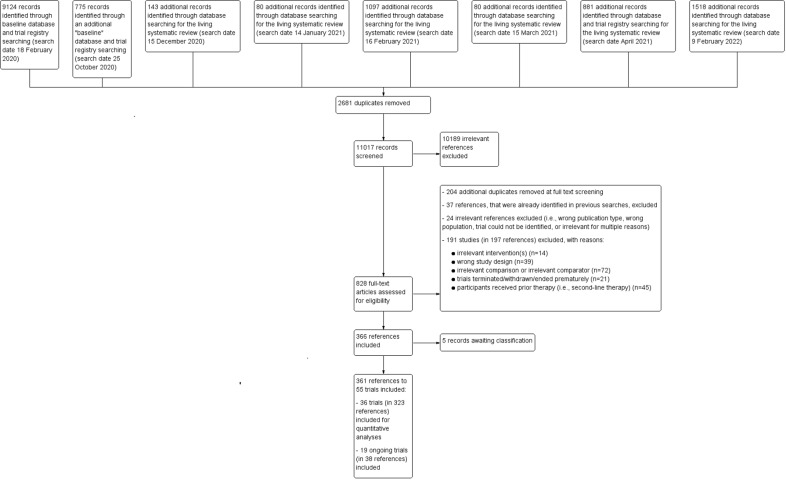
1.
Flow diagram
Search strategies for electronic databases were reviewed yearly to ensure that they reflected any terminology changes in the topic area, the databases, or the eligibility criteria of the review. In addition, our primary search strategy (see Appendix 4), developed by our Information Specialist, was peer‐reviewed by another Information Specialist (see Acknowledgements). We searched trial registries every six months.
Searching other resources
If needed, we would have extended the electronic searches by handsearching the references of all identified trials and relevant review articles. However, all relevant trials and articles were identified by our electronic searches.
Living systematic review considerations
We planned to search additional sources only yearly, as novel RCTs in this field are included in study registers or databases and thus were identified by our electronic searches.
Data collection and analysis
Selection of studies
Three review authors (AAa, MG, VP) screened citations retrieved by the baseline searches. The following monthly/update searches were screened by two review authors (AAa, BB). All records were assessed immediately for eligibility by reading the abstracts using Covidence software (Covidence). In case of disagreement on the relevance of a citation, we obtained the full‐text of the respective article for further review. We then eliminated all articles that did not meet the eligibility criteria and obtained the full‐text articles of the remaining articles. We proceeded similarly with the electronically and manually gathered registry entries as well as any reports identified from CSR databases. Subsequently, the full‐text articles were screened. Both at title and abstract screening and at full‐text screening, the four review authors (AA, BB, MG, VP) screened the references independently and any disagreements were resolved by discussion.
We documented the overall numbers of trials identified, excluded, and included at every stage of the search and screening of the literature in a PRISMA flow diagram (Figure 1).
We listed all eligible trials in the Characteristics of included studies section of the full review irrespective of whether measured outcome data were reported in a way that allows inclusion into a quantitative analysis. We recorded excluded trials in the Excluded studies section; trials that are ongoing with no results available in the Characteristics of ongoing studies section; and trials that are completed with no result data available, and where eligibility for inclusion was unclear, in the Studies awaiting classification section. We considered completed trials for which no results are available narratively in our publication bias judgements (see Assessment of reporting biases).
Clinical study report considerations
Besides our primary search for eligible and available CSR in the databases of pharmaceutical manufacturers, the EMA database, the FDA database, the YODA database and the CSDR, we searched specifically for additional reports on the trials identified by our searches. When a CSR that was linked to a primary trial publication could be retrieved, we preferred any data given in the report over the respective data from the clinical trial publication. For informational purposes, we would have reported, if found, discrepancies between the CSR and the clinical study publication in a separate table.
Living systematic review considerations
Two review authors (AA, BB) screened any new citations retrieved by the monthly searches immediately for eligibility by reading the abstracts and following all afore‐outlined steps. With every update search, we documented overall numbers of additionally identified trials and references in an updated PRISMA flow diagram (Moher 2009) (Figure 1).
Data extraction and management
We performed data extraction in accordance with the guidelines proposed by Cochrane (Li 2019). In total, five review authors were involved in data extraction of outcome data and trial characteristics (AAa, BB, CH, ET, ND), and independently extracted data from CSRs and study publications using a standardised data extraction form. Each outcome was extracted by two review authors independently; extractions were then compared to detect and resolve any discrepancies.
We extracted the following items.
General information: author, title, source, publication date, country, language, duplicate publications.
Quality assessment: (see Assessment of risk of bias in included studies).
Trial characteristics: trial design, aims, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, subgroup analysis, statistical methods, treatment cross‐overs, compliance with assigned treatment, length of follow‐up, time point of randomisation.
Participant characteristics: age, gender, number of participants recruited/allocated/evaluated, participants lost to follow‐up, stage of disease, histologic type, site of metastases, concomitant therapy.
Interventions: type, dosage, duration, and administration route of therapy; type, dosage, duration, and administration route of therapy in control arm; concomitant therapy; duration of follow‐up.
Outcomes: all outcomes mentioned above (including assessment of causality, relationship between intervention and adverse drug reaction, how severity or seriousness was measured).
Additional information: sponsorship/funding for the trial, potential conflicts of interest, trial registry record information (e.g. NCT numbers).
For cross‐over RCTs, we only extracted results from the first treatment period (i.e. before treatment cross‐over).
Some of the above‐mentioned characteristics (age, sex, histologic type, site of metastases (i.e. lung, bone, liver), administration route and dosage of substances) are potential effect modifiers for which we extracted data to check for validity of the transitivity assumption (see Assessment of heterogeneity).
We collated all reports of the same trial so that each trial, rather than each report, was the unit of interest. This applied to trial publications, conference abstracts, trial registry information and CSRs/scientific result summaries. For all studies, except three, we used published data only (e.g. published in full‐text articles, abstracts or on trial registries). For the other three studies (NCT00720941; NCT00334282; NCT01064310), we used unpublished data from CSRs/scientific result summaries).
Assessment of risk of bias in included studies
We used the Risk of Bias 2.0 (RoB 2) tool to assess the risk of bias in the underlying trial results of the included RCTs (Sterne 2019). For cross‐over RCTs, from which we extracted data from the first treatment period, we also used the standard RoB 2 tool for parallel‐group RCTs, as suggested in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a).
Of interest in this review was the effect of the assignment to the intervention (the intention‐to‐treat effect); hence, we performed all assessments with RoB 2 on this effect. We assessed the risk of bias of all trials that contributed results to the analyses of the outcomes overall survival (OS), : progression‐free survival (PFS), adverse events (AEs) and serious adverse events (SAEs). As we initially assumed that analyses for quality of life (QoL) would also be feasible, we assessed the risk of bias for this outcome as well (time point: QoL at the end of treatment), although we ended up reporting this outcome narratively in this review. Furthermore, risk of bias was assessed for the total population (i.e. all risk groups combined) for the following outcomes: OS, PFS, AEs, SAEs and QoL. For OS and PFS, we additionally assessed the risk of bias for each risk group (i.e. favourable, intermediate or poor risk group per International Metastatic RCC Database Consortium (IMDC) or Memorial Sloan Kettering Cancer Center (MSKCC)) separately.
In total, four review authors (AAa, BB, ET, ND) were involved in the risk of bias assessments. Two review authors independently assessed the risk of bias for a specific outcome result, and assessments were then compared to detect disagreements. When disagreements arose and the review authors were unable to reach a consensus by discussion, a third review author was consulted to reach a final decision. We assessed the following types of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), using the RoB 2 Excel tool (available at riskofbiasinfo.org):
Bias arising from the randomisation process.
Bias due to deviations from the intended interventions.
Bias due to missing outcome data.
Bias in measurement of the outcome.
Bias in selection of the reported result.
To address these types of bias, we employed the signalling questions recommended in RoB 2 and made a judgement using the following options;
'yes': if there is firm evidence that the question is fulfilled in the trial (i.e. the trial is at low or high risk of bias for the given direction of the question);
'probably yes': a judgement has been made that the question is fulfilled in the trial (i.e. the trial is at low or high risk of bias for the given direction of the question);
'no': if there is firm evidence that the question is unfulfilled in the trial (i.e. the trial is at low or high risk of bias for the given direction of the question);
'probably no': a judgement has been made that the question is unfulfilled in the trial (i.e. the trial is at low or high risk of bias for the given direction of the question);
'no information' if the trial report provides insufficient information to permit a judgement.
We used the algorithms proposed by RoB 2 to assign each domain one of the following levels of bias.
Low risk of bias
Some concerns
High risk of bias
Subsequently, we derived a 'Risk of bias' rating for each prespecified outcome in each trial in accordance with the following suggestion.
'Low risk of bias': the trial is judged to be at low risk of bias for all domains for this result.
'Some concerns': the trial is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain.
'High risk of bias': the trial is judged to be at high risk of bias in at least one domain for the result OR the trial is judged to have some concerns for multiple domains in such a way that substantially lowers our confidence in the results.
We stored and presented our consensus decisions for the signalling questions of RoB 2 in the appendices (Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13). We used the online available visualisation software robvis to summarise and visually present our assessments. We created traffic light plots (domain‐level judgements) and summary plots (distribution of judgements within each domain) for each outcome.
Measures of treatment effect
Relative treatment effect
We conducted all analyses on the effect of the randomised intervention (intention‐to‐treat effect).
To estimate effects in binary outcomes, we extracted the number of participants and events per arm and calculated risk ratios (RRs) with corresponding 95% confidence intervals (CIs) for each trial individually. However, this was not possible for one outcome reported in this review ('TFST') because, firstly, the definition of this outcome varied between trials. Furthermore, it was unclear which time points were being reported. Therapies for advanced renal cell carcinoma are usually long‐term therapies, and people may stop therapy at very varying time points. Hence, this would have led to high heterogeneity and thus, pooling data were not feasible. Therefore, we decided to report results for this outcome narratively in a tabular form.
For time‐to‐event outcomes, we extracted hazard ratios (HRs) and their corresponding measures of statistical uncertainty to directly retrieve an HR and a corresponding 95% CI for each individual trial. If this information had not been included in individual trial reports, we would have used the methodology proposed by Parmar 1998 and Tierney 2007 to reconstruct HRs indirectly from the information given in the trial report. We considered the following hierarchy of direct and indirect reconstruction methods, according to which HR from individual trials is preferred (Tudur 2001).
Unadjusted direct estimates (e.g. log HR and variance).
Indirect calculation 1: log HR and CI.
Indirect calculation 2: log‐rank P value and number of events.
Indirect calculation 3: estimating the log HR and variance from survival curves.
It is important to note here that the directly extracted HR as well as its different reconstruction methods produce either adjusted or unadjusted HR. For our calculations, we preferred unadjusted HR. If we would have needed to reconstruct HRs, we would have conducted sensitivity analyses to explore the effect of different reconstruction methods on our findings whenever necessary (see Sensitivity analysis). For two trials, however, we had to recalculate the CI to obtain a 95% CI, as one trial (NCT01108445) provided a 80% CI and the second trial (NCT00732914) provided a 90% CI.
For both binary and time‐to‐event outcomes, we clearly indicated the direction of the effect in the SoF table along with the individually reported outcomes (i.e. 'RR or HR smaller than 1.0 favours the intervention'), as this has led to confusion and flawed reporting of review results in the past (Skoetz 2019).
For continuous outcomes we had planned to use the mean difference (MD) when the same instruments were used for assessments; otherwise we would have calculated the standardised mean difference (SMD) with corresponding 95% CIs. We would have interpreted an SMD of zero as equivalent effects between the experimental and the control intervention. Depending on whether an improvement in the outcome of interest was associated with a higher or lower score, an SMD greater or lower than zero would be associated with a positive effect of the experimental intervention over the control intervention. This would have applied to the outcome QoL.
Absolute treatment effect
In addition to the relative measures of treatment effect outlined above, we presented absolute effect measures (Skoetz 2020) for every network estimate. To keep the SoF simple, understandable and reader‐friendly, we refrained from adding number needed to treat for an additional beneficial (NNTB) outcome/ number needed to treat for an additional harmful (NNTH) outcome to the SoF (see Differences between protocol and review).
Unit of analysis issues
Trials with multiple treatment groups
As recommended in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a), for trials with multiple treatment groups, we planned to combine arms as long as they could be regarded as subtypes of the same intervention. When arms could not be pooled this way, we included multi‐arm trials using a network meta‐analysis approach that accounted for the within‐trial correlation between the effect sizes by re‐weighting all comparisons of each multi‐arm trial (Rücker 2012; Rücker 2014). For pairwise meta‐analyses, if conducted, we would have treated multi‐arm trials as multiple independent comparisons and not combine these data in any analyses.
Cross‐over trials
For cross‐over RCTs, we only extracted results from the first treatment period, thereby treating these trials as parallel‐group RCTs for network meta‐analysis (Higgins 2019c).
Dealing with missing data
As suggested in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022), we planned to take the following steps to deal with missing data.
When only percentages but no absolute number of events were reported for binary outcomes, we calculated numerators using percentages. When data were not reported numerically but were reported graphically, we tried to estimate missing data from figures.
If needed, we would have contacted the original investigators to request relevant missing data. If the number of participants evaluated for a given outcome would not have been reported, we would have used the number of participants randomised per treatment arm as the denominator. If estimates for mean and standard deviations had been missing, we would have calculated these statistics from reported data, using the approaches described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b). If standard deviations had been missing and if we would have not been able to calculate them from the reported data, we would have calculated values according to a validated imputation method (Furukawa 2006).
For binary and continuous outcomes, if needed, we would have performed sensitivity analyses based on assumptions to assess how robust the analysis results are to missing data (Guyatt 2017). We addressed the potential impact of missing data in the risk of bias assessment, the grading of the evidence, and the discussion. As there is currently no procedure available permitting the quantitative assessment of the sensitivity of time‐to‐event outcomes to issues of missing data, we used the visual inspection of survival curves to evaluate any potential bias introduced by missing outcome data on such outcomes. We regarded this in the risk of bias section of the review, our grading of the outcomes, and the discussion.
Assessment of heterogeneity
Assessment of clinical and methodological heterogeneity within treatment comparisons
We planned to evaluate the presence of clinical and methodological heterogeneity through subgroup and sensitivity analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of transitivity across treatment comparison
To assess the adequacy of the assumption of transitivity, we evaluated whether the included interventions were similar when they were evaluated in RCTs with different designs: for example, whether double‐drug combinations are administered the same way in trials comparing them to other double‐drug combinations and in those comparing double‐drug combinations to triple‐drug combinations. Further potential effect modifiers of interest were age, sex, histology type, site of metastases (i.e. lung, bone, liver), administration routes, and dosage of substances. We evaluated the transitivity assumption by visually assessing the distribution of these across the different pairwise comparisons and explored their potential influence in subgroup analyses, whenever possible (see Subgroup analysis and investigation of heterogeneity).
Assessment of statistical heterogeneity and inconsistency
A critical prerequisite for a valid network analytic approach to the available evidence is consistency of effects within the network. This refers to sufficient agreement amongst the direct and indirect effect on the same comparisons (White 2012; Puhan 2014). To evaluate the presence of heterogeneity and inconsistency in the entire network, we provided the generalised heterogeneity statistic Qtotal and the generalised I2 statistic, as described in (Schwarzer 2015). We used the decomp.design command in the R package netmeta for decomposition of the heterogeneity statistic into a Q statistic for assessing the heterogeneity between trials with the same design, and a Q statistic for assessing design inconsistency to identify the amount of heterogeneity/inconsistency within as well as between designs (R Core Team 2019; Rücker 2019).
To evaluate the presence of inconsistency locally, we compared direct and indirect treatment estimates of each treatment comparison. This served as a check for consistency of a network meta‐analysis (Dias 2010). For this purpose, we used the netsplit command in the R package netmeta, which enables the splitting of the network evidence into direct and indirect contributions (R Core Team 2019; Rücker 2019). For each treatment comparison, we presented direct and indirect treatment estimates plus the network estimate using forest plots. In addition, for each comparison, we gave the Z value and P value of test for disagreement (direct versus indirect). It should be noted that in a network of evidence there may be many loops, and with multiple testing there is an increased likelihood to find an inconsistent loop by chance. We were, therefore, cautious in deriving conclusions from this approach.
Furthermore, we created a net heat plot (Krahn 2013), a graphical tool for locating inconsistency in network meta‐analysis.
We planned to explore possible sources of heterogeneity by performing prespecified sensitivity and subgroup analyses, whenever possible (Subgroup analysis and investigation of heterogeneity; Sensitivity analysis). In addition, we reviewed the evidence base and discussed the potential role of unmeasured effect modifiers in order to identify additional sources of heterogeneity.
We interpreted I2 values according to Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022), as follows:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% represents considerable heterogeneity.
We used the P value of the Chi2 test only for describing the extent of heterogeneity and not for determining statistical significance. In addition, we reported Tau2, the between‐study variance in random‐effects meta‐analysis.
Assessment of reporting biases
We searched trial registries to identify completed trials that have not been published elsewhere in order to minimise or determine publication bias. In the protocol for this review, we pre‐specified that for meta‐analyses involving at least 10 trials, we would explore potential and small‐study effects by generating a funnel plot and assessing it using a linear regression test (Egger 1997). We would have considered a P value of < 0.1 as significant for this test. However, in this review, we did not have direct comparisons that involved more than 10 trials.
As the identified evidence was sufficient, and a natural common comparator exists for the interventions in a single outcome, we planned to use a 'comparison‐adjusted' funnel plot to support our judgements on potential for publication bias within the network meta‐analysis (Chaimani 2012; Chaimani 2013). This type of funnel plot allows for the inclusion of all trials in a given network regardless of the respective interventions under trial. However, creating such a funnel‐plot was not feasible for this review (see Differences between protocol and review). Hence, in accordance with the advice given in the Cochrane Handbook, our judgements on potential publication bias were primarily non‐statistical (Chaimani 2019).
Data synthesis
We included all eligible trials in our analyses, but conducted sensitivity analyses according to risk of bias ratings (low bias/some concerns versus high risk of bias; see Sensitivity analysis) for our primary outcomes. We analysed interventions for favourable‐risk groups separately from interventions for intermediate‐ and poor‐risk groups. Furthermore, we analysed risk groups according to the MSKCC criteria separately from risk groups according to the IMDC criteria.
Direct comparison of interventions
If data had been insufficient to be combined in network meta‐analyses (e.g. in the case of inconsistency), and the clinical and methodological characteristics of individual studies sufficiently homogeneous, we would have performed pairwise meta‐analyses with an overall estimate, according to the recommendations provided in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). Had we conducted pairwise meta‐analyses, we would have used R package meta for statistical analyses (R Core Team 2019; Schwarzer 2007). We would have used a random‐effects model, and calculated corresponding 95% CIs for all analyses, as some heterogeneity in trial design, interventions, and outcome measurements can be expected.
To outline the available direct evidence (even when network meta‐analyses were conducted), we provided forest plots for pairwise comparisons, but without giving an overall estimate.
Indirect and mixed comparison of interventions
We considered the data to be sufficiently similar to be combined and, therefore, performed network meta‐analyses using the frequentist weighted least squared approach described by Rücker 2015. We used the R package netmeta for statistical analyses (R Core Team 2019; Rücker 2019), and we used a random‐effects model, taking into account the correlated treatment effects in multi‐arm trials. We assumed a common estimate for the heterogeneity variance across the different comparisons. We created and provided visual network plots (i.e., network graphs) for all analyses of our primary and secondary outcomes to evaluate the extent to which treatments are connected within a network, and also to visually present whenever networks were not fully connected. When a network was not fully connected and consisted of two or more sub‐networks, we analysed each sub‐network separately. Analyses were conducted whenever a sub‐network included more than one trial. We provided visual network plots and forest plots with results for each sub‐network analysis. In the network plots, any two treatments were connected by a line when there was at least one trial comparing the two treatments. The line width represents the number of trials within a comparison, while the plot width represents the number of participants within that comparison. For each comparison, we gave the estimated treatment effect along with its 95% CI. We graphically presented the results using forest plots, with sunitinib (SUN) as the reference treatment.
To evaluate the transitivity assumption, we visually assessed the distribution of important effect modifiers across the different pairwise comparisons and explored their potential influence in subgroup analyses, whenever possible (see Assessment of heterogeneity). To check for consistency, we compared direct and indirect treatment estimates of each treatment comparison (see Assessment of heterogeneity). Our assessment and judgement of potential publication bias was primarily non‐statistical (see Assessment of reporting biases).
Relative treatment ranking
We obtained a ranking of treatment options using P‐scores (Rücker 2015). P‐scores allow ranking treatments on a continuous 0‐to‐1 scale in a frequentist network meta‐analysis. We provided a ranking of treatments for the outcomes OS, SAEs, PFS, all‐cause grade 3 or 4 AEs (including the individual AEs explored in this review), and for the outcome number of participants who discontinued treatment due to an AE. Data for the outcomes QoL and TFST were not analysed, hence, we could not calculate P‐scores for these outcomes as we had initially planned.
Subgroup analysis and investigation of heterogeneity
We intended to perform subgroup analyses for our primary outcomes OS, SAE and QoL and for the following characteristics:
age of participants (≤ 65 versus > 65);
sex of participants (male versus female);
histology type (clear cell type, papillary type, sarcomatoid type);
nephrectomy (yes versus no);
radiotherapy (yes versus no);
follow‐up times (< 5 years versus ≥ 5 years);
site of metastases (lung, bone, liver);
administration routes (oral versus intravenous);
dosages (clinically relevant dose categories).
However, most subgroup analyses were not possible due to the distribution of these characteristics in the included trials, and a lack of reporting on subgroup data (for more details see Differences between protocol and review).
Sensitivity analysis
We planned to conduct sensitivity analyses for our primary outcomes OS, QoL, and SAEs.
To test the robustness of the results, we conducted fixed‐effect network meta‐analyses. As a post‐hoc decision, this was also completed for the outcome PFS.
Furthermore, we conducted sensitivity analyses on quality components (overall low risk of bias or some concerns versus overall high risk of bias) and sensitivity analyses on whether the assumption of proportional hazards underlying the HR had been tested and was justified in primary trials.
We had also planned to explore the influence of trial design (blinded trials versus unblinded trials) and the influence of completed but not published trials. For time‐to‐event outcomes, we had planned to use sensitivity analyses to explore the robustness of our findings should variable techniques to reconstruct HR from primary trial reports be necessary. However, some of these pre‐specified sensitivity analyses were not possible (for more details see Differences between protocol and review).
Methods for future updates
We planned to review the scope and methods approximately yearly, or more frequently if appropriate in light of potential changes in the topic area or the evidence included in the review (e.g. when additional comparisons, interventions, subgroups, or outcomes, or new review methods become available) (Garner 2016).
Summary of findings and assessment of the certainty of the evidence
Summary of findings tables
We include 'Summary of findings (SoF) tables to present the main findings of the review in a transparent and simple tabular format. In particular, we included key information concerning the certainty of the evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes. We reported the following outcomes and time points in the SoF table: OS and PFS (as time‐to‐event) at the longest follow‐up available and AEs and SAEs that occurred during treatment. The outcomes QoL and TFST were not meta‐analysed, so we reported them narratively in the SoF.
We initially planned to create two networks (one for the favourable‐risk group and one combined for the intermediate‐ and poor‐risk groups) and also to present one SoF table, respectively, for participants with a favourable risk and one combined for participants with an intermediate or poor risk. During the conduct of this review, however, we decided to additionally analyse and report results for the IMDC and MSKCC risk groups separately, as well as to provide a combined analysis (and SoF) of all risk groups combined (i.e. an overall analysis with the total trial populations); for more details, see Differences between protocol and review). Thus, in this review, we provided three SoF tables: one for the total trial population (all risk groups combined); one for the favourable risk group (separated by IMDC and MSKCC); and one for the intermediate and poor risk groups (separated by IMDC and MSKCC).
As SUN was our main comparator in this review, it was also chosen as the main comparator in all SoF tables. Moreover, for all SoF tables, we chose the clinically most relevant interventions that are currently recommended across all risk groups in four clinical practice guidelines (ESMO, EAU, NCCN and the German guideline; see Description of the intervention). This resulted in seven (combinations of) substances that we chose for the SoF tables: PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI, CAB alone, PAZ alone.
Certainty of the evidence
One review author (AA) independently rated the certainty of the evidence for each outcome. Another review author (VP) independently checked the assessments and then the two authors met to discuss and finalise the assessments. We used the GRADE approach to rank the certainty of the evidence and the guidelines provided in Chapter 15 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019). More precisely, we used the GRADE network meta‐analysis approach by Salanti 2014 to assess the certainty of the evidence and included our final judgements in the SoF tables.
The GRADE approach to assess the certainty of the body of evidence for each outcome of a network meta‐analysis uses five domains: trial limitations (risk of bias of included trials, using the overall 'risk of bias' judgement as derived from the RoB 2 Excel tool), indirectness (relevance to the review question), inconsistency (looking at heterogeneity and incoherence), imprecision (e.g. confidence intervals), and publication bias (Chaimani 2019). GRADE ratings of the evidence are interpreted as follows.
High: we are very confident that the true effect lies close to that of the effect estimate.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the effect estimate, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the effect estimate.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the effect estimate.
The GRADE system uses the following criteria for assigning a rating to a body of evidence (Schünemann 2019).
High: randomised trials or double‐upgraded observational trials.
Moderate: downgraded randomised trials or upgraded observational trials.
Low: double‐downgraded randomised trials or observational trials.
Very low: triple‐downgraded randomised trials, downgraded observational trials, or case series/case reports.
We downgraded the certainty of the evidence as follows.
Serious (−1) or very serious (−2) limitation to trial quality.
Important inconsistency (−1).
Some (−1) or major (−2) uncertainty about directness.
Imprecise or sparse data (−1) or very imprecise (i.e. very wide confidence interval) (‐2).
High probability of reporting bias (−1).
In the protocol for this review we stated that we will use GRADEpro GDT for the GRADE assessment; however, this was not feasible (see Differences between protocol and review).
Living systematic review considerations
At protocol stage, we proposed an approach for updating this review (see Differences between protocol and review). However, due to restricted funding an update of the review is currently not planned.
Results
Description of studies
Results of the search
The overall numbers of trials screened, included and excluded, are documented in a PRISMA flow diagram (Figure 1).
We identified a total of 13,689 records through our living systematic review approach. We conducted baseline searches in February and in October 2020. Between December 2020 and April 2021, we conducted monthly database searches according to the living systematic review approach. Baseline searches and update searches in the trial registries were also conducted in February 2020, in October 2020 and in April 2021. We conducted one final update search in databases and trial registries in February 2022.
After removal of 2681 duplicates, we screened a total of 11,017 records. At title and abstract screening, we regarded 10,189 records as irrelevant and excluded these. As a result, we screened 828 full‐text articles. At full‐text screening, we identified another 204 duplicates, and 37 references that were already found in previous update searches, so we excluded these as well. Another 221 references were excluded at full text screening with reasons (see Characteristics of excluded studies). Hence, we included 366 relevant references. Thereof, five references (trials) are still awaiting classification (see Characteristics of studies awaiting classification). Ultimately, we included 36 trials (in 323 references) (see Characteristics of included studies) and 19 ongoing trials (in 38 references) (see Ongoing studies).
Clinical study reports
We identified clinical study reports for two trials (NCT00720941; NCT00334282) and one scientific result summary for one trial (NCT01064310) through the CSDR platform. We used these documents as our primary sources to extract relevant data and to inform our risk of bias assessments.
Included studies
We included a total of 55 trials that met our pre‐specified inclusion criteria. Of these, 36 trials were included in quantitative analyses and narrative reporting (see Characteristics of included studies); 19 trials were classified as ongoing (see Characteristics of ongoing studies).
In these 36 included trials, a total of 15,177 participants (11,061 males; 4116 females) from 53 countries were included. Thirty‐three trials were multi‐centre trials. The median age of participants ranged from 55 to 68 years.
Design
All 36 trials were RCTs, out of which 32 were two‐arm trials; the remaining four trials were three‐arm trials (NCT00065468; NCT01984242; NCT02811861; NCT00619268). Six trials were cross‐over RCTs (NCT00732914; NCT00903175; NCT01392183; NCT01481870; NCT01613846; NCT00117637) and we extracted data from the first period (before cross over) whenever possible. Most trials were open‐label (non‐blinded), except for one trial (NCT00081614), which was double‐blinded (participants and investigators) and two trials (NCT00334282; NCT01064310), which were blinded quadruple (participants, care providers, investigators and outcome assessors). In one trial, blinding was not reported (Jonasch 2010). Three studies were placebo‐controlled (NCT00334282; NCT00081614; NCT00738530).
Sample size
The smallest trial had a sample size of N = 22 and the largest trial had a sample size of N = 1110.
Locations
Most trials were multi‐centre trials (33 multi‐centre trials, three single‐centre trials) and included participants from Europe, North‐ and South America, Asia, Australia, Africa and the Pacific region. 28 trials included participants from European Countries (NCT00065468; NCT00098657/NCT00083889; NCT00117637; NCT00334282; NCT00420888; NCT00609401; NCT00619268; NCT00631371; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT00979966; NCT01024920; NCT01030783; NCT01064310; NCT01108445; NCT01274273; NCT01613846; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177), 24 trials from northern America (Jonasch 2010; NCT00065468; NCT00072046; NCT00081614; NCT00098657/NCT00083889; NCT00117637; NCT00126594; NCT00631371; NCT00719264; NCT00720941; NCT00903175; NCT00920816; NCT01030783; NCT01108445; NCT01392183; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02761057; NCT02811861; NCT02853331; NCT03141177), 15 trials from Asia (NCT00334282; NCT00631371; NCT00719264; NCT00720941; NCT00738530; NCT00903175; NCT00920816; NCT01030783; NCT01481870; NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177), 13 trials from southern America (NCT00065468; NCT00098657/NCT00083889; NCT00334282; NCT00631371; NCT00719264; NCT00903175; NCT00920816; NCT01030783; NCT02231749; NCT02420821; NCT02684006; NCT02853331; NCT03141177), 12 trials from Australia (NCT00065468; NCT00098657/NCT00083889; NCT00334282; NCT00631371; NCT00720941; NCT00738530; NCT00903175; NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT03141177), five trials from Africa (NCT00065468; NCT00334282; NCT00631371; NCT00719264; NCT00920816), and three trials from the Pacific region (NCT00065468; NCT00334282; NCT02684006).
Participants
All trials included participants with advanced renal cell carcinoma (RCC), and all participants were above the age of 18 years. All trials explored first‐line treatment and most included treatment‐naive participants (i.e. participants who have not received prior systemic anticancer treatment). Both treatment‐naive and previously treated participants were included in seven trials. However, for two trials, where more than 10% of participants were previously treated, we were able to extract data for the treatment‐naive participants only for some outcomes (NCT00334282; NCT01030783). For four trials, separate data for the treatment‐naive participants was not extractable. However, in one trial, 7% of the trial population received previous systemic therapy (NCT02761057); in another trial, 4% of the trial population previously received therapy (NCT01392183); and in two trials, 3% of the trial population received prior therapy (NCT00732914; NCT00420888). This is less than our pre‐defined threshold of 10%, meaning we included these trials in our analyses. The results of one trial were not included in any analyses because, although it was stated in the methods that participants who had not received prior systemic therapy were eligible, 90% of the trial population that was ultimately included have had some prior anticancer therapy, but without further details about what this therapy consisted of (NCT01064310).
Risk groups
In most trials, the total trial population included all risk groups (i.e. favourable, intermediate or poor risk groups), according to either IMDC or MSKCC criteria. In three trials, the total trial population included only intermediate and poor risk groups (NCT01392183; NCT00065468; NCT01835158) and in four trials, the total trial population included only favourable and intermediate risk groups (NCT00081614; NCT00420888; NCT01481870; NCT01064310).
Histology type
In 18 trials, only participants with clear cell renal cell carcinoma were included (NCT01030783; Jonasch 2010; NCT00072046; NCT00098657/NCT00083889; NCT00117637; NCT00126594; NCT00720941; NCT00903175; NCT00920816; NCT01024920; NCT01030783; NCT01274273; NCT01392183; NCT01481870; NCT02231749; NCT02684006; NCT02853331; NCT03141177). In 10 trials, most participants (80% to 90%) had clear cell carcinoma (NCT00065468; NCT00334282; NCT00609401; NCT00619268; NCT00631371; NCT01064310; NCT01984242; NCT00609401; NCT00719264; NCT01613846). In two trials, at least 50% of participants had clear cell carcinoma (NCT00081614; NCT00738530). Another two trials mostly included participants with clear cell carcinoma, of which some had sarcomatoid features (NCT02811861; NCT02420821). One trial included only participants with non‐clear cell carcinoma (NCT01108445); another trial included both participants with clear cell or non‐clear (papillary) carcinoma (NCT00420888), and in another trial, 79% of participants had non‐clear cell (papillary) carcinoma (NCT00979966). One trial included several subtypes (NCT02761057).
Sites of metastases
In 26 trials, more than one metastatic site was reported in each trial, including the lung, lymph nodes, bones, liver and/or kidney (Jonasch 2010; NCT00072046; NCT00098657/NCT00083889; NCT00117637; NCT00334282; NCT00609401; NCT00619268; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT01024920; NCT01030783; NCT01108445; NCT01274273; NCT01392183; NCT01481870; NCT01613846; NCT02231749; NCT02420821; NCT02761057; NCT02811861; NCT02853331; NCT03141177). In two trials, only participants with bone or brain metastases (NCT01835158) or metastases in the central nervous system (NCT00631371) were included. In the remaining eight trials, the sites of the metastases were not reported. However, these trials reported the following: 80% of participants had ≥ 2 metastatic sites (NCT00065468); participants with metastatic RCC were eligible and had one or two tumour sites (NCT02684006); only participants with metastatic disease were eligible (NCT00081614; NCT00126594); 86.5% had metastatic disease and 13.5% a locally advanced stage (NCT00979966); 73% had two metastatic sites, 26% had 1 or none (NCT01064310); participants with metastatic or unresectable locally advanced RCC were eligible (NCT00420888; NCT01984242).
Nephrectomy
In all trials but one (NCT00979966, information not provided) it was either reported that participants had previously received a nephrectomy (either full or partial), or prior nephrectomy was generally expected by the inclusion criteria of the trials, but without further information about how many participants actually have had a prior nephrectomy.
Radiotherapy
In eight trials, participants in both arms had received prior radiotherapy (NCT00117637; NCT00631371; NCT00720941; NCT00732914; NCT01064310; NCT02231749; NCT02853331; NCT03141177). In 11 trials, prior radiotherapy was generally allowed (based on the inclusion criteria of the trials), but had to be completed at least two, three or four weeks (depending on the trial) prior to initiation of the first cycle of systemic treatment in the trial (NCT00065468; NCT00081614; NCT00126594; NCT00903175; NCT00920816; NCT00979966; NCT01024920; NCT01613846; NCT01984242; NCT02420821; NCT02811861). In the remaining 17 trials, no information about prior radiotherapy was provided.
Interventions
We identified 22 drugs and 17 different combinations in the included trials. In 16 trials, the substance sunitinib (main comparator in this review) was assessed in the comparator arm; in three trials, it was assessed in the experimental arm. In all 19 trials, sunitinib was administered via the same administration route (oral) and the same dose (50 mg/day). Discontinuation rates of sunitinib were high in most trials: in two trials, less than 20% of participants discontinued treatment; in four trials, 50% to 80% of participants discontinued treatment; and in the remaining 13 trials, between 80% to 100% of participants discontinued sunitinib treatment.
As for the other interventions, all were administered via the same administration route and most were also administered at the same dose (see Table 1). For more details per trial, see Characteristics of included studies.
Duration of therapy
In most trials, therapy was provided as continuous therapy, meaning therapy was continued as long as there was no disease progression, unacceptable toxicity (intolerable adverse events), clinical deterioration, loss of clinical benefit or withdrawal of consent. In three trials, participants were allowed to continue therapy despite disease progression if evidence of clinical benefit was observed according to the trial investigators (NCT02420821; NCT02684006; NCT00920816). In seven trials, therapy (either all or certain drugs) was provided for a fixed period: for 24 months (NCT00081614); for 18 months (NCT00420888); in one cross‐over study, period 1 lasted for 10 weeks (NCT01064310); bevacizumab was administered for a maximum of one year (NCT01274273); pembrolizumab was administered for a maximum of 35 cycles in two trials (NCT02853331; NCT02811861); nivolumab was administered for a maximum of two years (NCT03141177). In five trials, specific information about the treatment duration was not provided (NCT00126594; NCT00719264; NCT00979966; NCT01613846; NCT01984242).
Table 1. Interventions in the included trials
| Drug substance | Administration route | Dose | Combinations with other drugs in the included trials |
| Atezolizumab (ATE) | intravenous infusion | 1200 mg | ATE+BEV |
| Avelumab (AVE) | intravenous infusion | 10 mg | AVE+AXI PEM+AXI |
| Axitinib (AXI) | oral administration | 5 mg | AVE+AXI |
| Bevacizumab (BEV) | intravenous infusion | 10 mg | BEV+ERL IFN+BEV TEM+BEV ATE+BEV EVE+BEV |
| Cabozantinib (CAB) | oral administration | 60 mg | NIV+CAB |
| Crizotinib (CRI) | oral administration | 60 mg | ‐ |
| Erlotinib (ERL) | oral administration | 150 mg | BEV+ERL |
| Everolimus (EVE) | oral administration | 5 mg or 10 mg | EVE+BEV LEN+EVE |
| Interferon‐alpha (IFN) | subcutaneous injection | 0.5 MIU; or 3 MIU; or 6 MIU; or 9 MIU | IFN+BEV SOR+IFN NAP+IFN IFN+TEM ILN+IFN ILN+IFN+BEV |
| Interleukin (ILN) | subcutaneous injection | 2.4 MIU | SOR+ILN ILN+IFN |
| Ipilimumab (IPI) | intravenous infusion | 1mg | NIV+IPI |
| Lenvatinib (LEN) | oral administration | 18 mg; or 20 mg | LEN+PEM LEN+EVE |
| Naptumomab (NAP) | intravenous infusion | 15 mg | NAP+IFN |
| Nintedanib (NIN) | oral administration | 200 mg | ‐ |
| Nivolumab (NIV) | intravenous infusion | 3 mg or 240 mg | NIV+IPI NIV+CAB |
| Pazopanib (PAZ) | oral administration | 800 mg | ‐ |
| Pembrolizumab (PEM) | intravenous infusion | 200 mg | PEM+AXI LEN+PEM |
| Savolitinib (SAV) | oral administration | 600 mg | ‐ |
| Sorafenib (SOR) | oral administration | 400 mg | SOR+IFN SOR+ILN |
| Sunitinib (SUN) (main comparator in this review) |
oral administration | 50 mg | ‐ |
| Temsirolimus (TEM) | intravenous infusion | 15 mg or 25 mg | IFN+TEM TEM+BEV |
| Tivozanib (TIV) | oral administration | 1.5 mg | ‐ |
Outcome Measures
Primary outcomes
Overall survival
Overall survival (OS) was reported in 32 out of 36 trials included in this review (Jonasch 2010; NCT00065468; NCT00072046; NCT00081614; NCT00098657/NCT00083889; NCT00334282; NCT00420888; NCT00609401; NCT00619268; NCT00631371; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT00979966; NCT01024920; NCT01030783; NCT01108445; NCT01392183; NCT01481870; NCT01613846; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02761057; NCT02811861; NCT02853331; NCT03141177). Trials reported outcome data on OS for the total trial population and/or for individual risk groups according to IMDC and/or MSKCC. Whenever possible, we extracted data for the individual risk groups.
Quality of life
As stated in the Methods, we prioritised scales for the assessment of this quality of life (QoL). The final prioritisation included the following scales: FKSI‐DRS; EORTC‐QLQ‐C30; EQ‐VAS; FACT‐G; FACIT‐F.
Quality of life was assessed in a total of 22 trials, out of which 15 trials assessed this outcome using at least one of our prioritised scales. However, we could only extract or estimate data from seven trials (NCT00098657/NCT00083889; NCT00720941; NCT00920816; NCT01108445; NCT02231749; NCT03141177; NCT00903175). For the remaining eight trials, extracting the results was not possible for the following reasons.
In one trial, QoL should have been assessed with FACT‐G, but we could not find results anywhere (NCT01392183).
In another trial, QoL should have been assessed with FACIT‐F, but we could not find results anywhere (NCT01613846).
Separate data for treatment‐naive participants were not reported in two trials (NCT00334282; NCT01030783).
In one trial, 'time to definitive deterioration' analyses were reported, which were not a focus of this review (NCT00719264).
In another trial, 'time to first deterioration' analyses were reported, which were not a focus of this review (NCT02811861).
For one trial, we did not extract data because of a discrepancy between methods and reported results (NCT01064310).
For one trial, it was not possible to estimate data from the provided graphs (NCT00631371).
Where data extraction was possible, we extracted from a variety of different sources, including the full‐text publications (NCT01108445; NCT02231749; NCT03141177; NCT00903175); the trial registry (clinicaltrials.gov) (NCT00920816); both the full‐text publication and trial registry (NCT00098657/NCT00083889); the clinical study report (NCT00720941) and the scientific result summary (NCT01064310). For two of these trials, we tried to estimate data for the DRS‐scale from the graphs (NCT03141177; NCT00903175). In NCT00903175, the EORTC‐scale was also reported, but data could not be estimated. In NCT03141177, only TTD results for EQ‐5D‐VAS were reported.
We extracted (or estimated) data for the following scales.
FKSI‐DRS in five trials (NCT00098657/NCT00083889; NCT00920816; NCT01108445; NCT03141177; NCT00903175).
EQ‐5D‐VAS in three trials (NCT00098657/NCT00083889; NCT00920816; NCT02231749).
FACT‐G in two trials NCT00098657/NCT00083889; NCT02231749).
FACIT‐F in one trial (NCT00720941).
It was not possible to extract or estimate data for EORTC‐QLQ‐C30 from any trial. Furthermore, QoL was assessed only in the total trial populations (all risk groups combined), meaning the outcome was not assessed in the individual risk groups separately.
Serious adverse events
Serious adverse events (SAEs) were reported in 27 out of 36 included trials (NCT01108445; NCT00738530; NCT00631371; NCT01835158; NCT02231749; NCT00720941;NCT00719264; NCT00903175;NCT01984242; NCT02420821; NCT00117637;NCT00619268; NCT00732914; NCT01613846; NCT00979966; NCT02853331; NCT02811861; NCT00065468; NCT00098657/NCT00083889; NCT00920816; NCT01024920; NCT00126594; NCT00334282; NCT01064310; NCT01030783; NCT02761057; NCT01392183). Serious adverse events were reported for the total population only; meaning they were not reported for the individual risk groups separately.
Evaluable data for SAEs was available for only 22 trials (NCT01108445; NCT00738530; NCT00631371; NCT01835158; NCT02231749; NCT00720941; NCT00719264; NCT00903175; NCT01984242; NCT02420821; NCT00117637; NCT00619268; NCT00732914; NCT01613846; NCT00979966; NCT02853331; NCT02811861; NCT00065468; NCT00098657/NCT00083889; NCT00920816; NCT01024920; NCT00126594). The remaining five trials were not evaluable due to the following reasons: only treatment‐related SAEs were reported in one trial (NCT02761057); SAEs were not extractable for treatment‐naive participants in two trials that included more than 10% of previously treated participants (NCT00334282; NCT01030783); data for SAEs that occurred during the first treatment period was not extractable for one cross‐over trial (NCT01392183). Results of one trial were not presented because we were unsure whether participants were treatment‐naive (discrepancy between methods and results in the trial) (NCT01064310). Nine trials did not report SAEs (NCT03141177; NCT00420888; NCT01481870; NCT00081614; Jonasch 2010; NCT00072046; NCT00609401; NCT01274273; NCT02684006).
If available, data for SAEs were preferably extracted from the trial registries (clinicaltrials.gov; clinicaltrialsregister.eu), where we extracted the number of participants with at least one SAE. Furthermore, we assumed that this was the most current data. It was not explicitly stated whether all‐cause or treatment‐related SAEs were reported, but we strongly assumed that all‐cause SAEs were reported on the trial registries. This applied to 18 trials (NCT01108445; NCT00631371; NCT00738530; NCT01835158; NCT02231749; NCT00720941; NCT00065468; NCT00098657/NCT00083889; NCT00903175; NCT00719264; NCT00117637;NCT01984242; NCT00920816; NCT01024920; NCT02853331; NCT02811861; NCT00126594; NCT00979966). Only for four trials, data for all‐cause SAEs in the number of participants with at least one SAE were extracted from the respective publications (NCT00619268; NCT01613846; NCT00732914; NCT02420821).
Secondary outcomes
Progression‐free survival
Progression‐free survival (PFS) was reported in 34 out of 36 trials included in this review (Jonasch 2010; NCT00065468; NCT00072046; NCT00081614; NCT00098657/NCT00083889; NCT00117637; NCT00334282; NCT00420888; NCT00609401; NCT00619268; NCT00631371; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT00979966; NCT01024920; NCT01030783; NCT01108445; NCT01274273; NCT01392183; NCT01481870; NCT01613846; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02761057; NCT02811861; NCT02853331; NCT03141177). Trials reported outcome data on PFS for the total trial population and/or for individual risk groups according to IMDC and/or MSKCC. Whenever possible, we extracted data for the individual risk groups.
Adverse events
Adverse events (AEs) were reported in all included trials (N = 36). However, evaluable data for all‐cause grade 3 or 4 AEs was available for only 18 trials (NCT00065468; NCT00081614; NCT00719264; NCT00720941; NCT00732914; NCT01024920; NCT01613846; NCT01835158; NCT01984242; NCT02420821; NCT02684006; NCT02811861; NCT03141177; NCT00738530; NCT00920816; NCT01030783; NCT01108445; NCT01274273). One of these trials did not report individual AEs, meaning we could only extract data for the total number of participants with at least one grade 3 or 4 AE for this trial (NCT01984242). Another five of these trials did not report the total number of participants with at least one grade 3 or 4 AE, meaning we could only extract data for individual grade 3 or 4 AEs. (NCT00738530; NCT00920816; NCT01030783; NCT01108445; NCT01274273). Moreover, AEs were reported for the total population only; meaning they were not reported for the individual risk groups separately.
Eleven of the 18 trials reported AEs of "grade 3 or 4" (NCT01835158; NCT00732914; NCT01613846; NCT01984242; NCT02420821; NCT00719264; NCT00081614; NCT00720941; NCT00065468; NCT01274273; NCT01108445). The remaining seven trials reported AEs of "grade 3 or higher" and we assumed that grade 5 was not included as grade 5 AEs should be regarded as serious adverse events (NCT03141177; NCT02684006; NCT02811861; NCT01024920; NCT01030783; NCT00920816; NCT00738530). Lastly, in two of these trials, it was not explicitly stated whether all‐cause or treatment‐related AEs were reported, but we assumed all‐cause (NCT00065468; NCT00081614).
For the remaining 18 trials, this outcome was not evaluated in this review for the following reasons: only treatment‐related AEs were reported in 10 trials (NCT00072046; NCT00098657/NCT00083889; NCT00117637; NCT00126594; NCT00420888; NCT00979966; NCT02231749; NCT02853331; NCT02761057; NCT00609401); the event rate of AEs was reported in three trials (NCT01392183; NCT00903175; NCT00631371); in one trial, it was unclear whether the event rate or the number of participants with one event was reported (Jonasch 2010); AEs were not extractable for treatment‐naive participants in one trial that included more than 10% of previously treated participants (NCT00334282); only all‐grade AEs were reported in one trial (NCT00619268); for one cross‐over trial, it was unclear which treatment period where the AEs occurred was reported (NCT01481870). Lastly, results of one trial were not presented because we were unsure whether participants were treatment‐naive (discrepancy between methods and results in the trial) (NCT01064310).
Reporting of individual grade 3 or 4 AEs was common. All individual AEs that were of special interest in this review were reported: hand‐food syndrome was reported in 10 trials (NCT00720941; NCT00920816; NCT01024920; NCT01030783; NCT01108445; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177); fatigue in 14 trials (NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00920816; NCT01024920; NCT01030783; NCT01108445; NCT01274273; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177); diarrhoea in 16 trials (NCT00081614; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00920816; NCT01024920; NCT01030783; NCT01108445; NCT01274273; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177; NCT00065468); vomiting in10 trials (NCT00720941; NCT00920816; NCT01024920; NCT01108445; NCT01274273; NCT01835158; NCT02684006; NCT02811861; NCT03141177; NCT00065468); loss of appetite in 11 trials (NCT00719264; NCT00720941; NCT00732914; NCT00920816; NCT01024920; NCT01108445; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177); weight loss in 12 trials (NCT00719264; NCT00720941; NCT00920816; NCT01024920; NCT01108445; NCT01274273; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177; NCT00065468); insomnia in two trials (NCT00720941; NCT01108445); depression in two trials (NCT00738530; NCT01274273). For mucous membrane damage, the following were reported: mucosal inflammation in four trials (NCT00720941; NCT01108445; NCT02684006; NCT03141177) and stomatitis in t12 trials (NCT00719264; NCT00720941; NCT00732914; NCT01024920; NCT01108445; NCT01274273; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177; NCT00065468).
Number of participants who discontinued study treatment due to an Adverse event
This outcome was reported in 34 out of 36 included trials. Results were reported for the total population only; meaning they were not reported for the individual risk groups separately.
However, data for this outcome were evaluable for only 30 trials (NCT01108445; NCT00732914; NCT01613846; NCT00920816; NCT01024920; NCT00979966; NCT00719264; NCT00903175; NCT01481870; NCT00631371; NCT01835158; NCT02231749; NCT00720941; NCT01984242; NCT00081614; NCT01030783; NCT00117637; Jonasch 2010; NCT00065468; NCT00072046; NCT00098657/NCT00083889; NCT00609401; NCT00619268; NCT01274273; NCT01392183; NCT02420821; NCT02684006; NCT02853331; NCT03141177; NCT02811861). Data from two trials were not evaluable due to the following reasons: discontinuations due to treatment‐related AEs were reported in one trial (NCT02761057) and discontinuations due to AEs were not extractable for treatment‐naive participants in another trial that included more than 10% of previously treated participants (NCT00334282). Results of one trial were not included in the analysis because we were unsure whether participants were treatment‐naive (discrepancy between methods and results in the trial) (NCT01064310). Lastly, one trial did not report the data in a way in which it would evaluable (NCT00738530). Two trials did not report this outcome (NCT00420888; NCT00126594).
Time to initiation of first subsequent therapy
None of the included trials reported this outcome as a time‐to‐event outcome. However, 19 trials (NCT00081614; NCT00098657/NCT00083889; NCT00609401; NCT00619268; NCT00719264; NCT00732914; NCT00738530; NCT00920816; NCT01024920; NCT01108445; NCT01274273; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177) reported the number of participants who received any subsequent anticancer therapy after discontinuing study treatment. As reporting between trials was heterogenous, for example in terms of definition of this outcome and the timing of reporting being unclear, we refrained from pooling data in quantitative analyses and reported the results narratively in tabular form instead.
Description of studies awaiting classification
We included five trials that still await classification. Of these, two trials are still active (but not recruiting) (NCT01217931; NCT03541902). Another two trials are completed but not yet published (NCT01688973; NCT01829841). For the latter two trials we are awaiting results to see how many participants have received prior therapy, and whether results for treatment‐naive may be reported separately, in order to be able to make a decision about inclusion into this review. Lastly, thus far one trial is only published as an abstract and does not yet provide enough information for us to be able to decide whether it is eligible for inclusion into this review (Liu 2017). For more details per trial, see Characteristics of studies awaiting classification.
Description of ongoing studies
We identified 19 ongoing trials that would be eligible for inclusion into this review once results are published. One of these trials is still ongoing (EUCTR2008‐000928‐71‐IT); seven are active (but not recruiting) (NCT02210117; NCT03260894; NCT03729245; NCT03873402; NCT03937219; NCT02996110; NCT04540705); 10 are still recruiting (NCT03075423; NCT03592472; NCT03793166; NCT04090710; NCT04203901; NCT04394975; NCT04523272; NCT04736706; NCT05043090; UMIN 000012522); and one trial is not yet recruiting (NCT05096390). For more details per trial, see Characteristics of ongoing studies.
Excluded studies
After title and abstract screening, we excluded a total of 10,189 records that did not match our inclusion criteria (see Figure 1).
At full‐text stage, we excluded 191 trials (in a total of 197 references) after detailed evaluation. The trials were excluded for the following reasons:
Irrelevant intervention(s), for example interventions included a cancer vaccine, hormone therapy, adjuvant therapy or chemotherapy agents that are not relevant to this review (Aass 2005; Adler 1987; Bex 2017; Demirci 1999; Eisen 2019; EUCTR2008‐002667‐13‐DE 2008; Euctr2015‐002133‐22‐FR; Gruenwald 2020; Haas 2016; NCT02960906; NCT03829111; NCT00467025; Ravaud 2016; Richards 1977).
Wrong study design, for example single‐arm trials, dose‐finding trials, cohort trials or non‐randomised trials (Abdel 2018; Amin 2018; Amin 2018a; Barrios 2009; Bracarda 2007; Buckley 2019; Cirkel 2016; Cirkel 2017; Climent 2020; Collinson 2018; Colomba 2021; Conter 2013; Epaillard 2020; Euctr 2006‐003429‐95‐ES; Feldman 2020; Feldman 2020a; Gedye 2021; Hutson 2006; Hutson 2021; ISRCTN95351638; Jeon 1999; Larkin 2019; Lee 2020; Lee 2021; McDermott 2020; Minasian 1993; NCT01408004; NCT01444807; NCT02127710; NCT00835978; NCT00100906; NCT03173560; Nosov 2010; Nosov 2012; Plimack 2015; Sternberg 2013; Taylor 2020; Taylor 2020a; Voss 2015).
Irrelevant comparisons or irrelevant comparator (Atkins 1991; Atkins 1993; Atzpodien 1997; Atzpodien 1997a; Atzpodien 1999; Atzpodien 2001; Atzpodien 2004; Atzpodien 2006; Berg 1998; Boccardo 1998; Cole 2003; Collinson 2012; de Mulder 1991; Dexeus 1988; Dexeus 1989; Dubois 1997; Elhilali 2000; Escudier 2005; Euctr2007‐002556‐41‐AT; Figlin 1998; Figlin 1999; Foon 1988; Fossa 1989; Fossa 1992; Gleave 1997; Gleave 1997a; Gleave 1998; Gore 2008; Gore 2010; Hainsworth 2015; Hainsworth 2016; Han 2002; Harima 1990; Henriksson 1998; Jayson 1998; JPRN‐jRCTs031180024; Kinouchi 2004; Kinouchi 2006; Law 1995; Lindskog 2020; Lissoni 1993; Liu 2012; Lummen 1996; Madhusudan 2004; McDermott 2001; McDermott 2005; Mickisch 2001; Motzer 2001; Naglieri 1998; NCT00002737; NCT00005966; NCT00019539; NCT00027664; NCT00053820; NCT00416871; NCT01164228; Negrier 1996; Negrier 1997; Negrier 1998; Negrier 2000; Negrier 2006; Negrier 2007; Negrier 2008; Passalacqua 2010; Pyrhonen 1995; Pyrhonen 1996; Pyrhonen 1999; Rini 2011; Rini 2012; Rpcec 2017; Trump 2004; Verzoni 2018; Witte 1995).
Trials were terminated (DRKS00010309 2016; Figlin 2014; Figlin 2014a; Figlin 2017; Figlin 2018; Figlin 2020; NCT00491738; NCT01673386; NCT03035630; Rexer 2017; Rodriguez‐Vida 2020; Tannir 2016; Wood 2013), ended prematurely (Euctr2006‐002851‐33‐AT; Euctr2006‐005751‐16‐NL; Euctr2012‐001730‐33‐ES; Euctr2018‐001495‐38‐FR; NCT00873236; NCT02014636; NCT00709995) or were withdrawn (NCT01616186).
Participants have previously received therapy, i.e. trials assessed second‐line therapy (Beaumont 2009; Beaumont 2011; Cella 2016; Choueiri 2017; Choueiri 2020; Choueiri 2020a; Flaherty 2015; Gao 2017; Gao 2019; Ghiorghiu 2018; Jager 2005; JPRN‐JapicCTI‐122014; JPRN‐UMIN000001995; McDermott 2013; Molina 2009; Mulders 2012; NCT00378703; NCT00073307; NCT01223027; NCT01664182; NCT01727089; NCT01727336; NCT01793636; NCT02667886; NCT02724020; NCT03092856; NCT03095040; NCT03501381; NCT03595124; NCT03095040; NCT04195750; NCT04300140; Pal 2015; Pal 2021a; Ravaud 2006; Szarek 2021; Thiam 2010; Twardowski 2015; Twardowski 2017; Voss 2019; Wright 2020; Yang 2002; Yang 2003; Zhou 2016; Zhou 2019).
Risk of bias in included studies
Detailed risk of bias assessments (domain judgements and support for judgements) can be found in the appendices (Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13). Further details (including answers to the signalling questions of the RoB 2 tool) are available in a supplementary file (Aldin 2023).
Overall survival (OS)
Bias assessment of OS (domain judgements and support for judgements) is reported in Appendix 9 and visually presented in Figure 2 (traffic light plot) and Figure 53 in Appendix 14 (summary plot) for the total population; in Figure 3 (traffic light plot) and Figure 54 in Appendix 14 (summary plot) for the Memorial Sloan Kettering Cancer Center (MSKCC) risk groups; and in Figure 4 (traffic light plot) and Figure 55 in Appendix 14 (summary plot) for the International Metastatic RCC Database Consortium (IMDC) risk groups. This outcome was predominantly judged as 'some concerns' mainly due to missing study protocols and statistical analyses plans (SAPs). For the majority of the remaining trials, OS was judged as 'high risk of bias' due to the lack of information about missing outcome data, the randomisation process and allocation concealment. Risk of bias judgement differed between the total population and the risk groups for only one trial: whereas OS for the total population was judged as 'low risk of bias', OS per risk group was judged as 'high risk of bias' because this subgroup analysis was conducted as post‐hoc analysis (NCT00720941).
2.
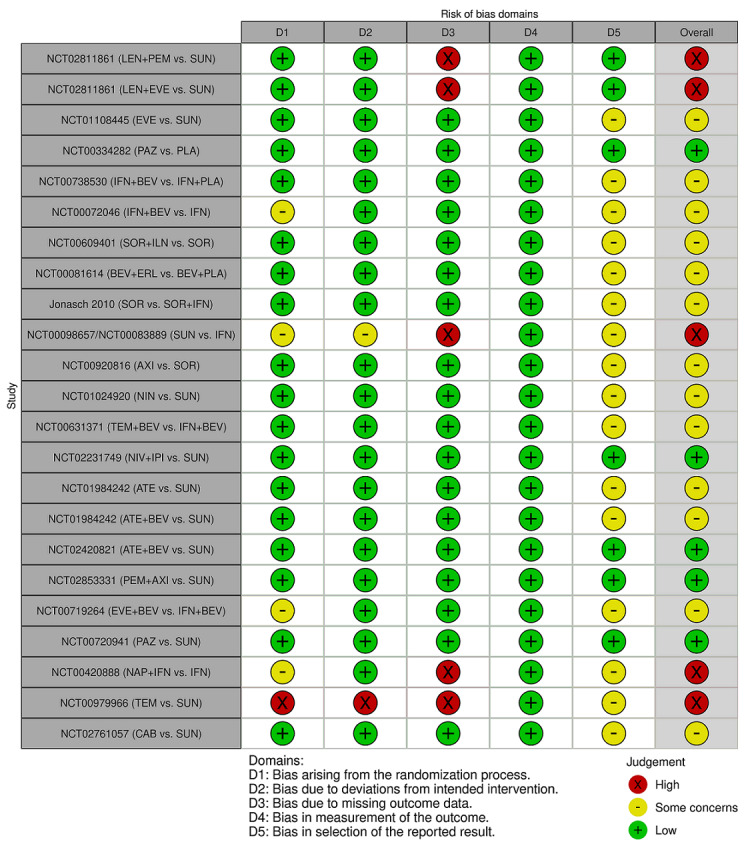
Traffic light plot for OS for all risk groups combined
3.
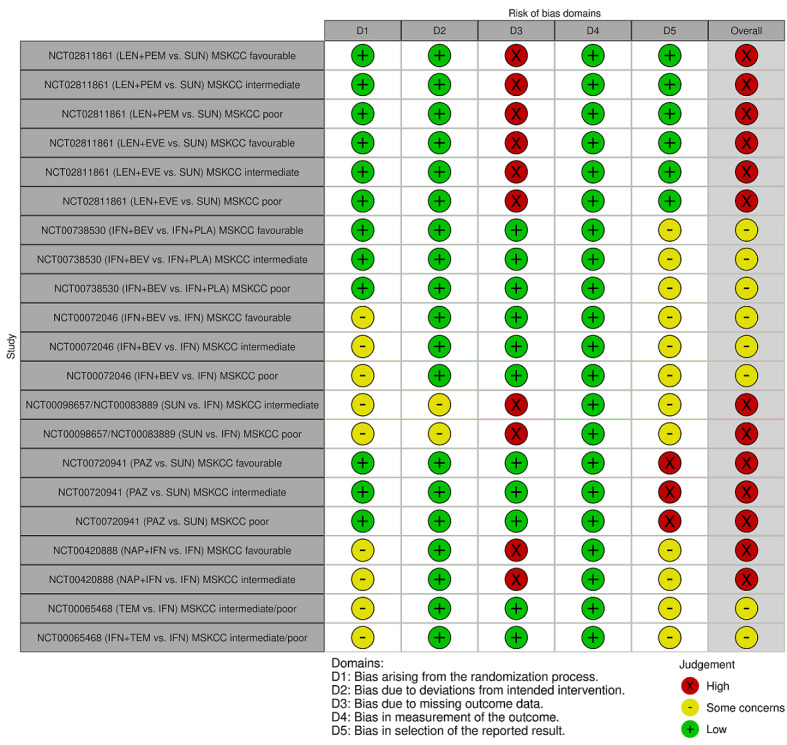
Traffic light plot for OS per MSKCC favourable, intermediate, poor risk
4.
Traffic light plot for OS per IMDC favourable, intermediate, poor risk
Quality of life (QoL)
The outcome QoL is presented in Appendix 10 and visually presented in Figure 5 (traffic light plot) and Figure 56 in Appendix 14 (summary plot). It was also predominantly judged as 'high risk of bias' mainly due to the outcome assessors’ awareness of the assigned interventions, which is owed to the nature of self‐reported questionnaires and participants (the outcome assessors) not being blinded to the intervention received in open‐label (non‐blinded) trials, as well as due to the high number of participants without outcome data at the end of treatment (time point for which risk of bias was assessed).
5.
Traffic light plot for QoL for all risk groups combined at the end of treatment
Serious adverse events (SAEs)
The outcome SAEs is reported in Appendix 11 and visually presented in Figure 6 (traffic light plot) and Figure 57 in Appendix 14 (summary plot). This outcome was predominantly judged as 'high risk of bias' mainly due to the lack of information about method of analysis and method of outcome measurement. In few cases, risk of bias was judged as 'high risk' due to the lack of information about the randomisation process and allocation concealment.
6.
Traffic light plot for SAEs for all risk groups combined
Progression‐free survival (PFS)
Bias assessment of PFS is reported in Appendix 12 and visually presented in Figure 7 (traffic light plot) and Figure 58 in Appendix 14 (summary plot) for the total population; in Figure 8 (traffic light plot) and Figure 59 in Appendix 14 (summary plot) for the MSKCC risk groups; and in Figure 9 (traffic light plot) and Figure 60 in Appendix 14 (summary plot) for the IMDC risk groups. This outcome was predominantly judged as 'high risk of bias' mainly due to the lack of information about missing outcome data and allocation concealment as well as the outcome assessors’ probable or evident awareness of the assigned interventions. There were no differences in the risk of bias judgement between the total population and the risk groups.
7.
Traffic light plot for PFS for all risk groups combined
8.
Traffic light plot for PFS per MSKCC favourable, intermediate, poor risk
9.
Traffic light plot for PFS per IMDC favourable, intermediate, poor risk
Adverse events (AEs)
Bias assessment of the outcome AEs is reported in Appendix 13 and visually presented in Figure 10 (traffic light plot) and Figure 61 in Appendix 14 (summary plot). This outcome was continuously judged as 'high risk of bias' mainly due to the outcome assessors’ awareness of the assigned interventions as well as the lack of information about method of analysis and method of outcome measurement.
10.
Traffic light plot for all‐cause grade 3 or 4 AEs for all risk groups combined
Publication bias
We searched trial registries to identify completed trials that have not been published elsewhere in order to determine publication bias. Thereby, we identified 19 ongoing trials (see Characteristics of ongoing studies). Furthermore, we identified three trials that were completed but have not been published yet: one trial (NCT01688973) was completed in 2019, and we are awaiting publication of results in order to be able to make a decision about inclusion or exclusion of the trial in this review, as participants may have received up to one prior systemic therapy; the second trial (NCT01829841) was completed in 2018 and results are yet to be published; for the third trial (Liu 2017), we only found an abstract. We listed these three trials in the Studies awaiting classification.
Out of the 36 trials included in analyses for this review, for one trial (NCT00126594), we were able to extract data on the outcome adverse events, which were published in the 'Results' section on the trial registry (https://clinicaltrials.gov/). We only identified one publication related to the trial, in which retrospective analyses of a subgroup of participants who were initially included in the RCT was conducted. However, these analyses were not of interest for our review, and we could not find a full‐text publication of the RCT.
Allocation
All risk of bias assessments (domain judgements and support for judgements) can be found in the appendices (Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13). Further details are available in a supplementary file (Aldin 2023).
Blinding
All risk of bias assessments (domain judgements and support for judgements) can be found in the appendices (Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13). Further details are available in a supplementary file (Aldin 2023).
Incomplete outcome data
All risk of bias assessments (domain judgements and support for judgements) can be found in the appendices (Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13). Further details are available in a supplementary file (Aldin 2023).
Selective reporting
All risk of bias assessments (domain judgements and support for judgements) can be found in the appendices (Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13). Further details are available in a supplementary file (Aldin 2023).
Other potential sources of bias
All risk of bias assessments (domain judgements and support for judgements) can be found in the appendices (Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13). Further details are available in a supplementary file (Aldin 2023).
Effects of interventions
See: Table 1; Table 2; Table 3
Main findings
The main findings of this review are reported in the Table 1 for the combined risk groups, in the Table 2 for the favourable risk groups (and separately according to the International Metastatic RCC Database Consortium (IMDC) and the Memorial Sloan Kettering Cancer Center (MSKCC) criteria) and in the Table 3 for the intermediate and poor risk groups (and separately according to IMDC and MSKCC criteria). The main comparator in our review was SUN. For the SoF tables, we chose the clinically most relevant treatments that are currently recommended across all risk groups in four clinical practice guidelines (European Society for Medical Oncology (ESMO), European Association of Urology (EAU), National Comprehensive Cancer Network (NCCN) and the German guideline; see Description of the intervention): PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI, CAB alone, PAZ alone. For the description of results below, we also focused on these prioritised treatments. The results for the analyses of all available treatments and comparisons per outcome can be found in the figures 11 to 107 as well as in the additional tables 1 to 19. In the results section below, for each outcome, the corresponding figures and tables are linked.
Transitivity
The included trials were similar with regard to clinical and methodological characteristics, therefore we assumed that the transitivity assumption holds and conducted analyses for the outcomes OS, SAEs, PFS, AEs, and the number of participants who discontinued treatment due to an AE. All trials were RCTs and most were open‐label (non‐masked). For cross‐over trials, we extracted data from the first period of treatment for results to be comparable. The same definitions for OS and PFS were used across trials. For analysing potential harms, we made sure that data were as comparable as possible (for more information see 'Outcome measures' in the section Included studies). All interventions were administered via the same administration route across all trials, and most interventions were administered at the same dose (see Table 1 in Included studies). Particularly our main comparator SUN was administered via the same route and at the same dosis in all trials that included SUN. Discontinuation rates of SUN were high: in 13 out of 19 trials in which SUN was administered, between 80% to 100% of participants who received SUN discontinued treatment.
All trials included participants with advanced and metastatic renal cell carcinoma (RCC), and participants in most trials had several metastatic sites. All participants were above the age of 18 years and both sexes (males and females) were included in all trials. The median age was approximately 60 years across trials. Furthermore, all trials explored first‐line treatment and 80% of trials included only treatment‐naive participants. For the remaining trials, we extracted data for the treatment‐naive population whenever possible. Eighteen trials included only people with clear cell carcinoma and 14 trials mostly clear cell carcinoma, whereas the remaining four trials included non‐clear cell carcinoma. In all trials but one, participants had previously received a nephrectomy and in most trials, prior radiotherapy was previously administered. Lastly, with regard to the risk groups, we conducted separate analyses for the different risk groups according to the different criteria (IMDC or MSKCC) whenever possible in order for results to be even more comparable.
Primary outcomes
Overall survival
Overall survival (OS) was reported in 32 trials (29 two‐arm trials and three three‐arm trials) (Jonasch 2010; NCT00065468; NCT00072046; NCT00081614; NCT00098657/NCT00083889; NCT00334282; NCT00420888; NCT00609401; NCT00619268; NCT00631371; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT00979966; NCT01024920; NCT01030783; NCT01108445; NCT01392183; NCT01481870; NCT01613846; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02761057; NCT02811861;NCT02853331; NCT03141177). However, evaluable data for OS was available for only 26 trials; the remaining six trials were not evaluable for this outcome for different reasons: one trial included more than 10% of previously treated participants, and separate data for treatment‐naive participants was not reported for this outcome (NCT01030783); one trial did not report this outcome in a way that it would have been evaluable and estimating data were not possible (NCT00619268); four trials were cross‐over trials that did not report outcome data after the first period (NCT00732914; NCT01613846; NCT00903175; NCT01481870).
As for the 26 trials that were evaluable for this outcome, some provided data for the total population (i.e. all risk groups combined) and the different risk groups (according to MSKCC or IMDC criteria) separately, at the longest follow‐up available. Other trials provided data either only for the total population or only for the different risk groups, at the longest follow‐up available. With regard to the three three‐arm trials, we did not combine the different arms but rather treated these as multiple independent comparisons.
Results for all risk groups combined
We analysed data on the combined risk groups (i.e. the total trial population) from 21 trials (Jonasch 2010; NCT00072046; NCT00081614; NCT00098657/NCT00083889; NCT00334282; NCT00420888; NCT00609401; NCT00631371; NCT00719264; NCT00720941; NCT00738530; NCT00920816; NCT00979966; NCT01024920; NCT01108445; NCT01984242; NCT02231749; NCT02420821; NCT02761057; NCT02811861; NCT02853331). Thereof, two three‐arm trials were included, each presenting two pairwise comparisons (we did not have data for the third comparison). A total of 10,304 participants were included in the analyses. Figure 62 in Appendix 15 outlines the available direct evidence (23 pairwise comparisons). The network was not fully connected and consisted of three sub‐networks (Figure 11). We conducted network meta‐analysis for the sub‐networks 1 and 2. Sub‐network 3 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, are shown in Table 4 and Figure 12, per subnetwork.
11.
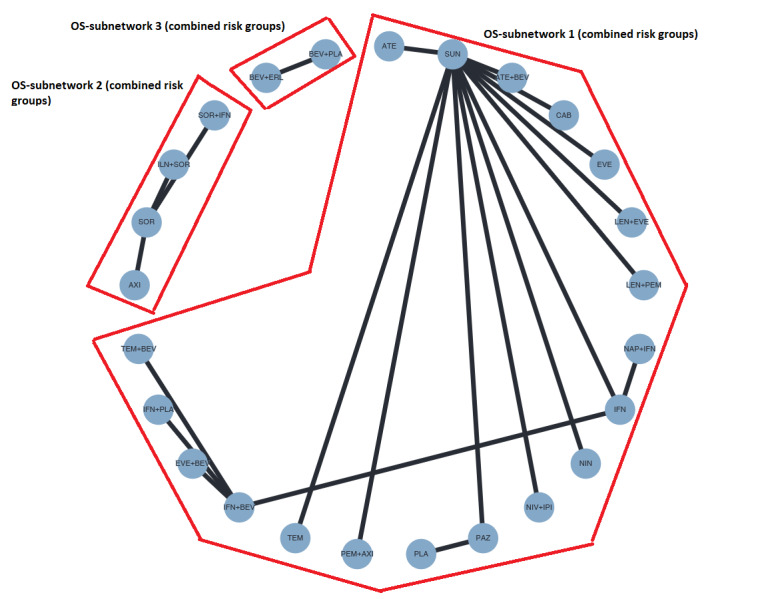
Network graph for OS (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments.
1. NMA results for OS (combined risk groups).
| Results of network meta‐analysis for outcome overall survival (combined risk groups). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 19. No. of pairwise comparisons: 19. No. of treatments: 19. No. of designs: 18 Heterogeneity/Inconsistency: Q=1.81, df=1, P = 0.18; I² = 44.6%, Tau² = 0.0284 Treatment Effects + 95%‐CIs (Hazard Ratios, random effects model): | ||||||||||||||||||
| LEN+PEM | . | . | . | . | . | . | . | 0.66 [0.42, 1.03] | . | . | . | . | . | . | . | . | . | . |
| 0.96 [0.54, 1.70] | NIV+IPI | . | . | . | . | . | . | 0.69 [0.48, 1.00] | . | . | . | . | . | . | . | . | . | . |
| 0.90 [0.50, 1.62] | 0.95 [0.56, 1.60] | PEM+AXI | . | . | . | . | . | 0.73 [0.50, 1.07] | . | . | . | . | . | . | . | . | . | . |
| 0.79 [0.35, 1.75] | 0.82 [0.38, 1.76] | 0.87 [0.40, 1.88] | CAB | . | . | . | . | 0.84 [0.43, 1.64] | . | . | . | . | . | . | . | . | . | . |
| 0.72 [0.41, 1.28] | 0.75 [0.45, 1.26] | 0.80 [0.47, 1.35] | 0.92 [0.43, 1.97] | PAZ | 1.01 [0.63, 1.62] | . | . | 0.92 [0.64, 1.32] | . | . | . | . | . | . | . | . | . | . |
| 0.73 [0.35, 1.53] | 0.76 [0.38, 1.53] | 0.81 [0.40, 1.64] | 0.93 [0.38, 2.28] | 1.01 [0.63, 1.62] | PLA | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.72 [0.33, 1.54] | 0.75 [0.36, 1.55] | 0.79 [0.38, 1.65] | 0.91 [0.37, 2.28] | 0.99 [0.48, 2.05] | 0.98 [0.41, 2.34] | NIN | . | 0.92 [0.49, 1.72] | . | . | . | . | . | . | . | . | . | . |
| 0.67 [0.19, 2.41] | 0.70 [0.20, 2.46] | 0.74 [0.21, 2.61] | 0.86 [0.22, 3.38] | 0.93 [0.27, 3.26] | 0.92 [0.24, 3.52] | 0.94 [0.24, 3.62] | TEM | 0.98 [0.30, 3.24] | . | . | . | . | . | . | . | . | . | . |
| 0.66 [0.42, 1.03] | 0.69 [0.48, 1.00] | 0.73 [0.50, 1.07] | 0.84 [0.43, 1.64] | 0.91 [0.64, 1.32] | 0.91 [0.50, 1.65] | 0.92 [0.49, 1.72] | 0.98 [0.30, 3.24] | SUN | 0.99 [0.72, 1.36] | . | . | . | 0.94 [0.52, 1.70] | 0.89 [0.36, 2.21] | . | 0.87 [0.57, 1.33] | 0.82 [0.56, 1.21] | . |
| 0.65 [0.38, 1.13] | 0.68 [0.42, 1.11] | 0.72 [0.44, 1.19] | 0.83 [0.40, 1.75] | 0.91 [0.56, 1.47] | 0.90 [0.46, 1.77] | 0.91 [0.45, 1.84] | 0.97 [0.28, 3.35] | 0.99 [0.72, 1.36] | ATE+BEV | . | . | . | . | . | . | . | . | . |
| 0.64 [0.28, 1.44] | 0.67 [0.30, 1.45] | 0.70 [0.32, 1.55] | 0.81 [0.31, 2.12] | 0.88 [0.40, 1.92] | 0.87 [0.35, 2.17] | 0.89 [0.35, 2.25] | 0.95 [0.24, 3.76] | 0.96 [0.48, 1.92] | 0.97 [0.46, 2.07] | EVE+BEV | 0.99 [0.64, 1.53] | . | . | . | . | . | . | . |
| 0.63 [0.32, 1.26] | 0.66 [0.35, 1.26] | 0.70 [0.36, 1.34] | 0.80 [0.34, 1.89] | 0.87 [0.46, 1.66] | 0.86 [0.39, 1.93] | 0.88 [0.39, 2.00] | 0.94 [0.25, 3.47] | 0.95 [0.56, 1.63] | 0.96 [0.52, 1.79] | 0.99 [0.64, 1.53] | IFN+BEV | 1.00 [0.69, 1.46] | . | . | 0.91 [0.62, 1.33] | . | 0.86 [0.60, 1.24] | . |
| 0.63 [0.29, 1.39] | 0.66 [0.31, 1.39] | 0.70 [0.33, 1.49] | 0.80 [0.31, 2.04] | 0.87 [0.41, 1.84] | 0.86 [0.36, 2.10] | 0.88 [0.36, 2.17] | 0.94 [0.24, 3.66] | 0.95 [0.50, 1.83] | 0.96 [0.47, 1.99] | 0.99 [0.56, 1.76] | 1.00 [0.69, 1.46] | TEM+BEV | . | . | . | . | . | . |
| 0.62 [0.30, 1.30] | 0.65 [0.32, 1.30] | 0.69 [0.34, 1.39] | 0.79 [0.32, 1.94] | 0.86 [0.43, 1.73] | 0.85 [0.37, 1.98] | 0.87 [0.37, 2.05] | 0.92 [0.24, 3.51] | 0.94 [0.52, 1.70] | 0.95 [0.49, 1.86] | 0.98 [0.39, 2.42] | 0.99 [0.45, 2.19] | 0.99 [0.41, 2.38] | ATE | . | . | . | . | . |
| 0.59 [0.22, 1.61] | 0.62 [0.23, 1.64] | 0.65 [0.24, 1.74] | 0.75 [0.24, 2.31] | 0.82 [0.31, 2.17] | 0.81 [0.27, 2.39] | 0.82 [0.27, 2.47] | 0.87 [0.20, 3.92] | 0.89 [0.36, 2.21] | 0.90 [0.34, 2.35] | 0.93 [0.30, 2.89] | 0.94 [0.33, 2.67] | 0.94 [0.31, 2.86] | 0.95 [0.32, 2.79] | EVE | . | . | . | . |
| 0.57 [0.26, 1.26] | 0.60 [0.28, 1.27] | 0.63 [0.30, 1.35] | 0.73 [0.29, 1.86] | 0.79 [0.38, 1.68] | 0.79 [0.32, 1.91] | 0.80 [0.32, 1.97] | 0.85 [0.22, 3.33] | 0.87 [0.45, 1.67] | 0.88 [0.42, 1.81] | 0.90 [0.51, 1.61] | 0.91 [0.62, 1.33] | 0.91 [0.53, 1.55] | 0.92 [0.38, 2.22] | 0.97 [0.32, 2.97] | IFN+PLA | . | . | . |
| 0.57 [0.31, 1.06] | 0.60 [0.34, 1.05] | 0.63 [0.36, 1.12] | 0.73 [0.33, 1.62] | 0.80 [0.46, 1.39] | 0.79 [0.38, 1.64] | 0.80 [0.38, 1.70] | 0.85 [0.24, 3.03] | 0.87 [0.57, 1.33] | 0.88 [0.52, 1.49] | 0.90 [0.40, 2.03] | 0.91 [0.46, 1.80] | 0.91 [0.42, 1.99] | 0.92 [0.45, 1.91] | 0.97 [0.36, 2.65] | 1.00 [0.46, 2.18] | LEN+EVE | . | . |
| 0.54 [0.30, 0.97] | 0.57 [0.33, 0.96] | 0.60 [0.35, 1.03] | 0.69 [0.32, 1.49] | 0.75 [0.44, 1.28] | 0.74 [0.37, 1.51] | 0.76 [0.36, 1.57] | 0.80 [0.23, 2.83] | 0.82 [0.56, 1.21] | 0.83 [0.50, 1.36] | 0.85 [0.48, 1.51] | 0.86 [0.60, 1.24] | 0.86 [0.51, 1.46] | 0.87 [0.43, 1.76] | 0.92 [0.34, 2.46] | 0.95 [0.56, 1.60] | 0.94 [0.53, 1.67] | IFN | 0.93 [0.63, 1.37] |
| 0.50 [0.25, 1.01] | 0.52 [0.27, 1.01] | 0.55 [0.28, 1.08] | 0.64 [0.27, 1.52] | 0.70 [0.36, 1.34] | 0.69 [0.31, 1.55] | 0.70 [0.30, 1.61] | 0.74 [0.20, 2.78] | 0.76 [0.44, 1.31] | 0.77 [0.41, 1.44] | 0.79 [0.39, 1.57] | 0.80 [0.47, 1.36] | 0.80 [0.41, 1.53] | 0.81 [0.36, 1.80] | 0.85 [0.30, 2.45] | 0.88 [0.45, 1.69] | 0.87 [0.44, 1.75] | 0.93 [0.63, 1.37] | NAP+IFN |
|
Subnet 2 No. of studies: 3. No. of pairwise comparisons: 3. No. of treatments: 4. No. of designs: 3 Heterogeneity/Inconsistency: Q=0, df=0, p=n.a.; I²=n.a., Tau²=n.a. Treatment Effects + 95%‐CIs (Hazard Ratios, random effects model): | ||||||||||||||||||
| ILN+SOR | . | 0.91 [0.59, 1.41] | . | |||||||||||||||
| 0.91 [0.54, 1.56] | AXI | 1.00 [0.73, 1.36] | . | |||||||||||||||
| 0.91 [0.59, 1.41] | 0.99 [0.73, 1.36] | SOR | 0.51 [0.22, 1.19] | |||||||||||||||
| 0.47 [0.18, 1.21] | 0.51 [0.21, 1.26] | 0.51 [0.22, 1.19] | SOR+IFN | |||||||||||||||
12.
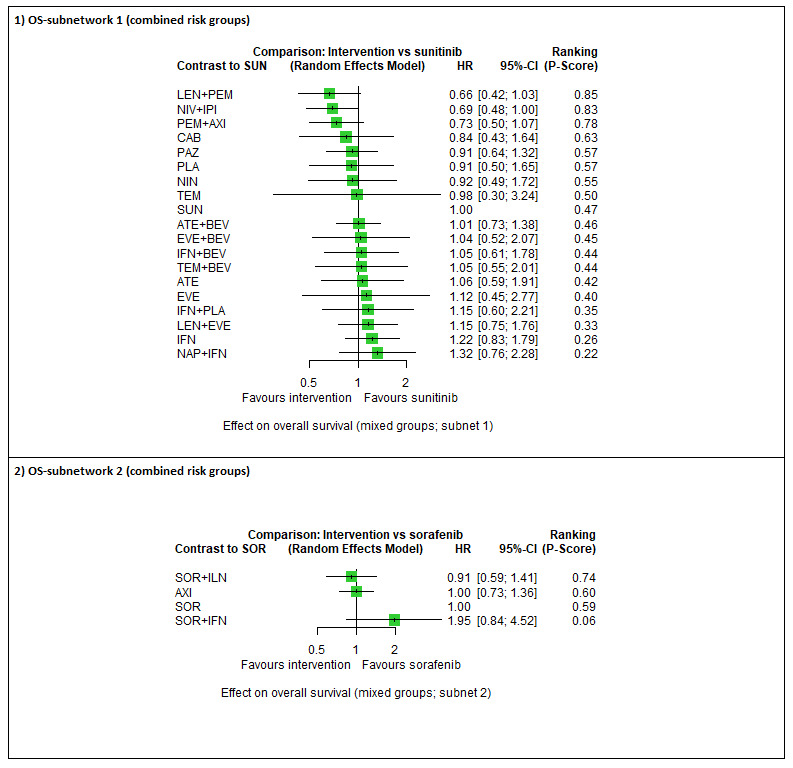
Forest plot for OS (all risk groups combined). 1) OS‐subnetwork 1. Reference treatment: sunitinib (SUN); 2) OS‐subnetwork 2. Reference treatment: sorafenib (SOR). Treatments are ordered by P‐score (descending).
In sub‐network 1, we observed moderate between‐study heterogeneity (Q = 1.81, df = 1, P = 0.18; I² = 44.6%, Tau² = 0.0284). We found that LEN+PEM may improve OS (hazard ratio (HR) 0.66, 95% confidence interval (CI) 0.42 to 1.03), low certainty). The combinations NIV+IPI (HR 0.69, 95% CI 0.69 to 1.00, moderate certainty, P‐score 0.83) and PEM+AXI (HR 0.73, 95% CI 0.50 to 1.07, moderate certainty, P‐score 0.78) probably improve OS when compared to SUN alone (P 0.47), respectively. We are uncertain whether CAB alone improves OS (HR 0.84, 95% CI 0.43 to 1.64, very low certainty, P‐score: 0.63) when compared to SUN alone, and there is probably little or no difference in OS between PAZ alone (HR 0.91, 95% CI 0.64 to 1.32, moderate certainty, P‐score: 0.57) and SUN alone. We have no comparison data for AVE+AXI and NIV+CAB. In the ranking of treatments, LEN+PEM (P‐score: 0.85) was the best treatment option, and NAP+IFN was the worst option (P‐score: 0.22) (Figure 12). For this sub‐network, the fixed‐effect model yielded somewhat different results (see Sensitivity analysis).
In sub‐network 2, each pairwise comparison was reported by a single trial only, so no heterogeneity statistics could be calculated. Here, SOR alone was the comparator treatment, and the ranking of treatments suggested that SOR+ILN (P‐score: 0.74) was the best treatment option (Figure 12).
Results for MSKCC favourable risk group
We analysed data on the favourable risk group according to the MSKCC criteria from five trials (1175 participants) (NCT00072046; NCT00420888; NCT00720941; NCT00738530; NCT02811861). Figure 63 in Appendix 15 outlines the available direct evidence (six pairwise comparisons). The network was not fully connected and consisted of two sub‐networks (Figure 13). We conducted network meta‐analysis for both networks. Results for all network comparisons, including the ranking of treatments, are shown in Table 5 and Figure 14. In both networks, each pairwise comparison was reported by a single trial only, so no heterogeneity statistics could be calculated.
13.
Network graph for OS (MSKCC favourable risk group). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
2. NMA results for OS (MSKCC favourable risk groups).
| Results of network meta‐analysis for outcome overall survival (MSKCC favourable risk group). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 3. No. of pairwise comparisons: 3. No. of treatments: 4. No. of designs: 3 Heterogeneity/Inconsistency: Q = 0, df = 0, p=n.a.; I²=n.a., Tau²=n.a. Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | |||
| LEN+EVE | . | . | 0.54 [0.21, 1.37] |
| 0.62 [0.23, 1.65] | PAZ | . | 0.88 [0.63, 1.21] |
| 0.63 [0.18, 2.16] | 1.02 [0.43, 2.44] | LEN+PEM | 0.86 [0.38, 1.93] |
| 0.54 [0.21, 1.37] | 0.88 [0.63, 1.21] | 0.86 [0.38, 1.93] | SUN |
|
Subnet 2 No. of studies: 3. No. of pairwise comparisons: 3. No. of treatments: 4. No. of designs: 3 Heterogeneity/Inconsistency: Q = 0, df = 0, P = n.a.; I²=n.a., Tau² = n.a. Treatment Effects + 95%‐CIs (Hazard Ratios, random effects model): | |||
| IFN+BEV | . | 0.92 [0.62, 1.37] | 0.90 [0.64, 1.25] |
| 0.93 [0.58, 1.49] | NAP+IFN | . | 0.96 [0.69, 1.34] |
| 0.92 [0.62, 1.37] | 0.99 [0.53, 1.83] | IFN+PLA | . |
| 0.89 [0.64, 1.25] | 0.96 [0.69, 1.34] | 0.97 [0.58, 1.64] | IFN |
14.
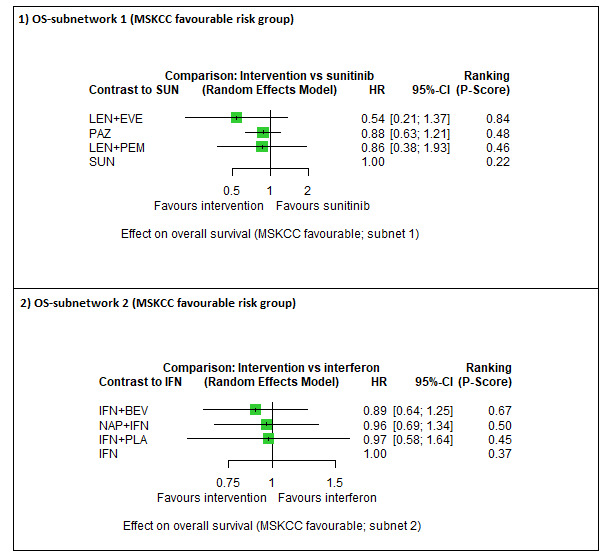
Forest plot for OS (MSKCC favourable risk group). 1) OS‐subnetwork 1. Reference treatment: sunitinib (SUN); 2) OS‐subnetwork 2. Reference treatment: interferon‐alpha (IFN). Treatments are ordered by P‐score (descending).
We are uncertain whether LEN+PEM improves OS (HR 0.86, 95% CI 0.38 to 1.93, very low certainty, P‐score: 0.46) when compared to SUN alone (P‐score: 0.22). There may be little or no difference in OS between PAZ alone (HR 0.88, 95% CI 0.63 to 1.21, low certainty, P‐score: 0.48) and SUN alone. We have no comparison data for AVE+AXI, NIV+CAB, PEM+AXI, NIV+IPI and CAB alone. In the ranking of treatments, LEN+EVE was the best treatment option (P‐score: 0.84) and SUN alone (P‐score: 0.22) the worst option (Figure 14).
In sub‐network 2, where IFN alone was the comparator treatment, the ranking of treatments suggested that IFN+BEV was the best treatment option (P‐score: 0.67) and IFN alone the worst option (P‐score: 0.37).
Results for IMDC favourable risk group
We analysed data on the favourable risk group according to the IMDC criteria from five trials (1007 participants) (NCT00420888; NCT02231749; NCT02684006; NCT02811861; NCT03141177). Figure 64 in Appendix 15 outlines the available direct evidence (six pairwise comparisons). The network was not fully connected and consisted of two sub‐networks (Figure 15). We conducted network meta‐analysis for subnetwork 1; subnetwork 2 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, are shown in Table 6 and Figure 16.
15.
Network graph for OS (IMDC favourable risk group). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
3. NMA results for OS (IMDC favourable risk group).
| Results of network meta‐analysis for outcome overall survival (IMDC favourable risk group). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 5. No. of pairwise comparisons: 5. No. of treatments: 6. No. of designs: 5 Heterogeneity/Inconsistency: Q = 0, df = 0, P = n.a.; I²=n.a., Tau² = n.a. Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | |||||
| AVE+AXI | . | . | . | 0.66 [0.36, 1.22] | . |
| 0.71 [0.34, 1.48] | NIV+IPI | . | . | 0.93 [0.62, 1.40] | . |
| 0.70 [0.27, 1.80] | 0.99 [0.43, 2.25] | NIV+CAB | . | 0.94 [0.46, 1.92] | . |
| 0.65 [0.24, 1.77] | 0.92 [0.38, 2.22] | 0.93 [0.32, 2.68] | LEN+EVE | 1.01 [0.46, 2.20] | . |
| 0.66 [0.36, 1.22] | 0.93 [0.62, 1.40] | 0.94 [0.46, 1.92] | 1.01 [0.46, 2.20] | SUN | 0.87 [0.42, 1.82] |
| 0.57 [0.22, 1.50] | 0.81 [0.35, 1.88] | 0.82 [0.29, 2.28] | 0.88 [0.30, 2.57] | 0.87 [0.42, 1.82] | LEN+PEM |
16.
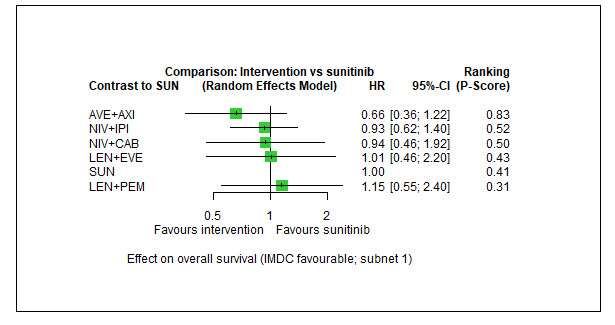
Forest plot for OS (IMDC favourable risk group). 1) OS‐subnetwork 1. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
In sub‐network 1, each pairwise comparison was reported by a single trial only, so no heterogeneity statistics could be calculated. We found that AVE+AXI may improve OS (HR 0.66, 95% CI 0.36 to 1.22, low certainty, P‐score: 0.83) when compared to SUN alone (P‐score: 0.41). There probably is little or no difference in OS between NIV+IPI (HR 0.93, 95% CI 0.62 to 1.40, moderate certainty, P‐score: 0.52) and SUN alone, and there may be little or no difference in OS between LEN+PEM (HR 1.15, 95% CI 0.55 to 2.40, low certainty, P‐score: 0.31) and SUN alone. We are uncertain whether NIV+CAB improves or decreases OS (HR 0.94, 95% CI 0.46 to 1.92, very low certainty in the evidence, P‐score: 0.50) when compared to SUN alone. We have no comparison data for PEM+AXI, CAB alone and PAZ alone. In the ranking of treatments, AVE+AXI was the best treatment option (P‐score: 0.83) and LEN+PEM was the worst option (P‐score: 0.31) (Figure 16).
Results for MSKCC Intermediate and poor risk groups
We analysed data on the intermediate and poor risk groups according to the MSKCC criteria from seven trials (3937 participants) (NCT00065468; NCT00072046; NCT00098657/NCT00083889; NCT00420888; NCT00720941; NCT00738530; NCT02811861). Figure 65 in Appendix 15 outlines the available direct evidence (15 pairwise comparisons). The network was fully connected (Figure 17). Results for all network comparisons, including the ranking of treatments, are shown in Table 7 and Figure 18. No heterogeneity (Q=1.45, df = 6, P = 0.96; I² = 0%, Tau² = 0.0) was detected in this network.
17.
Network graph for OS (MSKCC intermediate and poor risk groups). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
4. NMA results for OS (MSKCC intermediate and poor risk groups).
| Results of network meta‐analysis for outcome overall survival (MSKCC intermediate and poor risk groups). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. No. of studies: 15. No. of pairwise comparisons: 15. No. of treatments: 10. No. of designs: 9 Heterogeneity/Inconsistency: Q = 1.45, df = 6, P = 0.96; I² = 0%, Tau² = 0.0 Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | |||||||||
| LEN+PEM | . | 0.63 [0.46, 0.86] | . | . | . | . | . | . | . |
| 0.71 [0.49, 1.02] | PAZ | 0.89 [0.75, 1.06] | . | . | . | . | . | . | . |
| 0.63 [0.46, 0.86] | 0.89 [0.75, 1.06] | SUN | . | . | . | 0.81 [0.61, 1.07] | 0.77 [0.61, 0.96] | . | . |
| 0.62 [0.40, 0.97] | 0.88 [0.61, 1.25] | 0.98 [0.72, 1.35] | TEM | . | . | . | 0.78 [0.63, 0.97] | . | . |
| 0.57 [0.37, 0.88] | 0.80 [0.57, 1.13] | 0.91 [0.67, 1.21] | 0.92 [0.69, 1.22] | IFN+BEV | . | . | 0.85 [0.70, 1.02] | 0.83 [0.67, 1.04] | . |
| 0.52 [0.33, 0.81] | 0.73 [0.51, 1.05] | 0.83 [0.61, 1.13] | 0.84 [0.62, 1.14] | 0.91 [0.69, 1.21] | IFN+TEM | . | 0.93 [0.75, 1.15] | . | . |
| 0.51 [0.33, 0.77] | 0.72 [0.51, 1.00] | 0.81 [0.61, 1.07] | 0.82 [0.54, 1.24] | 0.89 [0.59, 1.34] | 0.98 [0.64, 1.48] | LEN+EVE | . | . | . |
| 0.48 [0.33, 0.71] | 0.68 [0.51, 0.91] | 0.77 [0.61, 0.96] | 0.78 [0.63, 0.97] | 0.85 [0.70, 1.02] | 0.93 [0.75, 1.15] | 0.95 [0.67, 1.37] | IFN | . | 0.88 [0.68, 1.15] |
| 0.48 [0.29, 0.77] | 0.67 [0.45, 1.01] | 0.75 [0.52, 1.09] | 0.77 [0.53, 1.10] | 0.83 [0.67, 1.04] | 0.91 [0.64, 1.31] | 0.94 [0.59, 1.49] | 0.98 [0.74, 1.31] | IFN+PLA | . |
| 0.43 [0.27, 0.68] | 0.60 [0.41, 0.89] | 0.68 [0.48, 0.96] | 0.69 [0.49, 0.97] | 0.75 [0.55, 1.03] | 0.82 [0.59, 1.15] | 0.84 [0.54, 1.32] | 0.88 [0.68, 1.15] | 0.90 [0.61, 1.33] | NAP+IFN |
18.
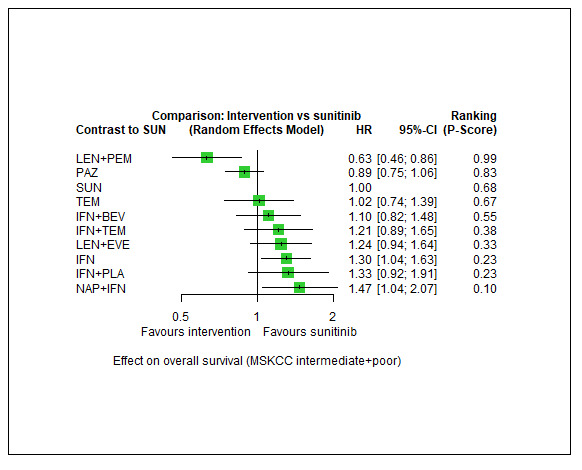
Forest plot for OS (MSKCC intermediate and poor risk groups). 1) OS‐network. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
The combination LEN+PEM probably improves OS (HR 0.63, 95% CI 0.46 to 0.86, moderate certainty, P‐score: 0.99), when compared to SUN alone (P‐score: 0.68). There may be little or no difference in OS between PAZ alone (HR 0.89, 95% CI 0.75 to 1.06, low certainty, P‐score: 0.83) and SUN alone. We have no comparison data for PEM+AXI, AVE+AXI, NIV+IPI, NIV+CAB, and CAB alone. In the ranking of treatments, LEN+PEM was the best treatment option (P‐score 0.99) and NAP+IFN was the worst option (P‐score: 0.10) (Figure 18).
Results for IMDC intermediate and poor risk groups
We analysed data on the intermediate and poor risk groups according to the IMDC criteria from seven trials (3416 participants) (NCT00420888; NCT01392183; NCT01835158; NCT02231749; NCT02684006; NCT02811861; NCT03141177). Figure 66 in Appendix 15 outlines the available direct evidence (13 pairwise comparisons). The network was not fully connected and consisted of three sub‐networks (Figure 19). We conducted network meta‐analysis for sub‐networks 1 and 2; sub‐network 3 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, are shown in Table 8 and Figure 20.
19.
Network graph for OS (IMDC intermediate and poor risk groups). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
5. NMA results for OS (IMDC intermediate and poor risk groups).
| Results of network meta‐analysis for outcome overall survival (IMDC intermediate and poor risk groups). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 10. No. of pairwise comparisons: 10. No. of treatments: 7. No. of designs: 6 Heterogeneity/Inconsistency: Q = 9.1, df = 4, P = 0.059; I² = 56.1%, Tau² = 0.0635 Treatment Effects + 95%‐CIs (Hazard rratios, random‐effects model): | |||||||
| LEN+PEM | . | . | . | . | 0.55 [0.33, 0.91] | . | |
| 0.91 [0.46, 1.83] | NIV+CAB | . | . | . | 0.60 [0.37, 0.96] | . | |
| 0.84 [0.40, 1.75] | 0.92 [0.45, 1.86] | NIV+IPI | . | . | 0.65 [0.38, 1.10] | . | |
| 0.75 [0.39, 1.45] | 0.82 [0.44, 1.54] | 0.89 [0.46, 1.75] | AVE+AXI | . | 0.73 [0.48, 1.11] | . | |
| 0.68 [0.30, 1.55] | 0.75 [0.34, 1.66] | 0.81 [0.35, 1.87] | 0.91 [0.42, 1.96] | CAB | 0.80 [0.42, 1.52] | . | |
| 0.55 [0.33, 0.91] | 0.60 [0.37, 0.96] | 0.65 [0.38, 1.10] | 0.73 [0.48, 1.11] | 0.80 [0.42, 1.52] | SUN | 0.93 [0.58, 1.48] | |
| 0.51 [0.25, 1.01] | 0.55 [0.28, 1.07] | 0.60 [0.30, 1.22] | 0.67 [0.36, 1.26] | 0.74 [0.33, 1.64] | 0.93 [0.58, 1.48] | LEN+EVE | |
|
Subnet 2 No. of studies: 2. No. of pairwise comparisons: 2. No. of treatments: 2. No. of designs: 1 Heterogeneity/Inconsistency: Q=0.12, df=1, p=0.73; I²=0%, Tau²=0 Treatment Effects + 95%‐CIs (Hazard Ratios, random effects model): | |||||||
| IFN | 0.85 [0.68, 1.05] | ||||||
| 0.85 [0.68, 1.05] | NAP+IFN | ||||||
20.
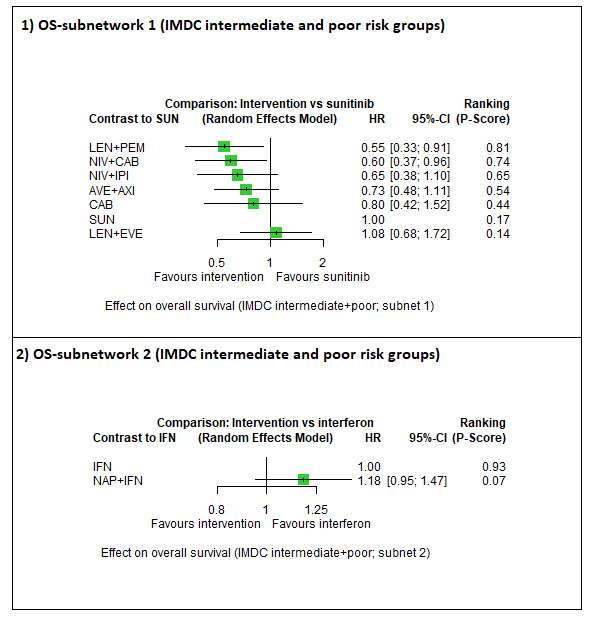
Forest plot for OS (IMDC intermediate and poor risk groups). 1) OS‐subnetwork 1. Reference treatment: sunitinib (SUN); 2) OS‐subnetwork 2. Reference treatment: interferon‐alpha (IFN). Treatments are ordered by P‐score (descending).
In sub‐network 1, we observed moderate between‐study heterogeneity (Q = 9.1, df=4, P = 0.059; I² = 56.1%, Tau² = 0.0635). The combinations LEN+PEM (HR 0.55, 95% CI 0.33 to 0.91, moderate certainty, P‐score: 0.81), NIV+CAB (HR 0.60, 95% CI 0.37 to 0.96, moderate certainty, P‐score: 0.74) and NIV+IPI (HR 0.65, 95% CI 0.38 to 1.10, moderate certainty, P‐score: 0.65) probably improve OS when compared to SUN alone (P‐score: 0.17), respectively. The combination AVE+AXI may improves OS (HR 0.73, 95% CI 0.48 to 1.11, low certainty, P‐score: 0.54), and CAB alone may improves slightly OS (HR 0.80, 95% CI 0.42 to 1.50, low certainty, P‐score: 0.44), when compared to SUN alone, respectively. We have no comparison data for PEM+AXI and PAZ alone. In the ranking of treatments, LEN+PEM (P‐score: 0.81) was the best treatment option, and LEN+EVE was the worst option (P‐score: 0.14) (Figure 20).
In sub‐network 2, only one pairwise comparison by a singe trial only was reported, so no heterogeneity statistics could be calculated. Here, IFN alone was the comparator treatment, and the ranking of treatments suggested that IFN alone was the best treatment option (P‐score: 0.93) and NAP+IFN the worst option (P‐score: 0.07).
Quality of life
Pooling data were not feasible for the outcome quality of life (QoL), so we reported results in a tabular form. Results for the different time points are reported for every scale where data were extractable. The time point of main interest for this review was QoL at the end of treatment, which is reported below in Table 2. Results for other time points are reported in the additional tables: short‐term results (one month after initiation of treatment) are reported in Table 9; mid‐term results (six months after initiation of treatment) are reported in Table 10; mid‐term results (12 months after initiation of treatment) are reported in Table 11; long‐term results (approximately 24 months after initiation of treatment) are reported in Table 12; long‐term results (at the end of treatment) are reported in Table 13.
6. Short‐term results (1 month after initiation of treatment) for QoL (all risk groups combined).
| Trial |
time point of measurement (in months) |
Intervention (N analysed) | Intervention post mean score (SD) | Comparator (N analysed) | Comparator post mean score (SD) |
| Scale: FKSI‐DRS (score range 0‐36; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 1 | SUN (N = 348) | 27.73 (5.080) | IFN (N = 317) | 26.68 (5.195) |
| Scale: EQ‐5D‐VAS (score range 0‐100; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 1 | SUN (N=347) | 69.35 (18.992) | IFN (N=315) | 67.66 (20.058) |
| Scale: FACT‐G (score range 0‐108; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 1 | SUN (N=348) | 42.71 (8.959) | IFN (N=317) | 40.93 (9.292) |
| Scale: FACIT‐F (score range 0‐52; higher scores represent better QoL) | |||||
| NCT00720941 | 1 | PAZ (N=388) | 35.2 (11.67) | SUN (N=393) | 33.8 (12.56) |
Comparisons including SUN are bold. The scales are listed in no particular order.
7. Mid‐term results (6 months after initiation of treatment) for QoL (all risk groups combined).
| Trial |
time point of measurement (in months) |
Intervention (N analysed) | Intervention post mean score (SD) | Comparator (N analysed) | Comparator post mean score (SD) |
| Scale: FKSI‐DRS (score range 0‐36; higher scores represent better QoL) | |||||
| NCT01108445 | 6.9 | EVE (N=13) | 29.8 (3.76) | SUN (N = 19) | 27.6 (4.37) |
| NCT00098657/NCT00083889 | 6.4 | SUN (N=242) | 29.43 (4.280) | IFN (N = 109) | 28.37 (4.726) |
| NCT00920816 | 6.4 | AXI (N=131) | 28.557 (4.308) | SOR (N = 60) | 30.296 (3.890) |
| Scale: EQ‐5D‐VAS (score range 0‐100; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 6.4 | SUN (N=240) | 75.13 (16.771) | IFN (N=104) | 72.57 (16.007) |
| NCT00920816 | 6.4 | AXI (N=131) | 71.031 (19.081) | SOR (N = 60) | 73.183 (16.674) |
| Scale: FACT‐G (score range 0‐108; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 6.4 | SUN (N=241) | 82.23 (15.124) | IFN (N = 106) | 80.60 (15.527) |
| Scale: FACIT‐F (score range 0‐52; higher scores represent better QoL) | |||||
| NCT00720941 | 6.5 | PAZ (N=230) | 38.8 (9.55) | SUN (N = 226) | 36.2 (10.26) |
Comparisons including SUN are bold. The scales are listed in no particular order.
8. Mid‐term results (12 months after initiation of treatment) for QoL (all risk groups combined).
| Trial |
time point of measurement (in months) |
Intervention (N analysed) | Intervention post mean score (SD) | Comparator (N analysed) | Comparator post mean score (SD) |
| Scale: FKSI‐DRS (score range 0‐36;higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 12 | SUN (N = 166) | 29.72 (4.245) | IFN (N=46) | 29.36 (4.418) |
| NCT00920816 | 12 | AXI (N = 95) | 29.579 (4.186) | SOR (N=37) | 31.027 (3.790) |
| Scale: EQ‐5D‐VAS (score range 0‐100; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 12 | SUN (N=168) | 76.24 (15.740) | IFN (N = 46) | 76.57 (17.924) |
| NCT00920816 | 12 | AXI (N = 95) | 73.147 (17.546) | SOR (N = 37) | 75.108 (18.371) |
| Scale: FACT‐G (score range 0‐108; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 12 | SUN (N = 166) | 82.71 (15.276) | IFN (N = 46) | 83.14 (17.067) |
| Scale: FACIT‐F (score range 0‐52; higher scores represent better QoL) | |||||
| NCT00720941 | 12 | PAZ (N = 138) | 39.5 (9.36) | SUN (N = 144) | 37.7 (8.32) |
Comparisons including SUN are bold. The scales are listed in no particular order.
9. Long‐term results (approximately 24 months after initiation of treatment) for QoL (all risk groups combined).
| Trial |
time point of measurement (in months) |
Intervention (N analysed) | Intervention post mean score (SD) | Comparator (N analysed) | Comparator post mean score (SD) |
| Scale: EQ‐5D‐VAS (score range 0‐100; higher scores represent better QoL) | |||||
| NCT02231749 | 23.7 | NIV+IPI (N = 342) |
(mean (SD) not reported) mean change 10.07 (4.35 to 15.80) |
SUN (N = 351) |
(mean (SD) not reported) mean change 6.40 (‐1.36 to 14.16) |
| Scale: FACT‐G (score range 0‐108; higher scores represent better QoL) | |||||
| NCT02231749 | 23.7 | NIV+IPI (N = 352) |
(mean (SD) not reported) mean change 4.77 (1.73 to 7.82) |
SUN (N = 356) |
(mean (SD) not reported) mean change ‐4.32 (‐8.54 to ‐0.11) |
| Scale: FACIT‐F (score range 0‐52; higher scores represent better QoL) | |||||
| NCT00720941 | 23.5 | PAZ (N=39) | 39.9 (8.33) | SUN (N = 36) | 37.7 (8.46) |
Comparisons including SUN are bold. The scales are listed in no particular order.
10. Long‐term results (at the end of treatment) for QoL.
| Trial |
time point of measurement (in months) |
Intervention (N analysed) | Intervention post mean score (SD) | Comparator (N analysed) | Comparator post mean score (SD) |
| Scale: FKSI‐DRS (score range 0‐36; higher scores represent better QoL) | |||||
| NCT01108445 | 40 | EVE (N = 33) | 26.6 (6.85) | SUN (N = 47) | 26.6 (6.13) |
| NCT00098657/NCT00083889 | 28.5 | SUN (N = 53) | 29.44 (4.210) | IFN (N = 351) | 29.22 (7.694) |
| NCT00920816 | 24.6 | AXI ( N =72) | 26.556 (5.487) | SOR (N=95) | 26.786 (5.982) |
| Scale: EQ‐5D‐VAS (score range 0‐100; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 28.5 | SUN (N=54) | 76.85 (16.863) | IFN (N = 352) | 75.44 (25.060) |
| NCT00920816 | 24.6 | AXI (N = 71) | 67.254 (19.495) | SOR (N=94) | 67.048 (22.570) |
| Scale: FACT‐G (score range 0‐108; higher scores represent better QoL) | |||||
| NCT00098657/NCT00083889 | 28.5 | SUN (N = 52) | 84.62 (16.257) | IFN (N = 351) | 79.54 (26.109) |
| Scale: FACIT‐F (score range 0‐52; higher scores represent better QoL) | |||||
| NCT00720941 | 38 | PAZ (N = 2) | 38.5 (13.59) | SUN (N = 2) | 29.5 (0.71) |
Long‐term results (at the end of treatment)
As pre‐specified in the protocol of this review, we assessed the risk of bias for QoL at the end of treatment (see Table 13 for results, Risk of bias in included studies and Appendix 10). All trials were overall judged to have a 'high risk of bias' mainly due to the outcome assessors’ awareness of the assigned interventions, which is owed to the nature of self‐reported questionnaires and due to the trials' design (open‐label, non‐masked trials) as well as due to the high number of participants without outcome data at the end of treatment. In most comparisons including SUN, across all scales, participants in the experimental groups seemed to achieve a higher score in the post‐intervention assessments compared to participants in the comparator arm.
One RCT measured QoL using FACIT‐F (score range 0 to 52; higher scores mean better QoL) and reported that the mean post‐score was 9.00 points higher (9.86 lower to 27.86 higher, very low certainty) with PAZ than with SUN. Comparison data were not available for PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI, and CAB alone.
Serious adverse events
Serious adverse events (SAEs) were not consistently reported across trials. To be able to meta‐analyse results, we only considered SAEs when the number of participants with at least one SAE was reported. We did not consider cumulated events. Serious adverse events were assessed in 22 trials (NCT00065468; NCT00098657/NCT00083889; NCT00117637; NCT00126594; NCT00619268; NCT00631371; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT00979966; NCT01024920; NCT01108445; NCT01613846; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02811861; NCT02853331) (18 two‐arm trials, four three‐arm trials), for a total of 10,709 participants. All trials provided data on SAE for all risk groups combined only. Figure 67 in Appendix 15 outlines the available direct evidence (31 comparisons). The network was fully connected (Figure 21). Results for all network comparisons, including the ranking of treatments, are shown in Table 14 and Figure 22. We observed substantial heterogeneity (Qtotal= 15.40, df = 6, P = 0.017; Qwithin=3.44, df=1, P = 0.064; Qbetween=11.96, df=5, P = 0.035; I² = 61.0%, Tau² = 0.0256) in the network.
21.
Network graph for SAEs (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
11. NMA results for SAEs (all risk groups combined).
| Results of network meta‐analysis for outcome serious adverse events (all risk groups combined). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. No. of studies: 22. No. of pairwise comparisons: 30. No. of treatments: 21. No. of designs: 21. Qtotal=15.40, df=6, p=0.017; Qwithin= 3.44, df=1, P = 0.064; Qbetween=11.96, df=5, P = 0.035; I² = 61.0%, Tau² = 0.0256 Treatment Effects + 95%‐CIs (Risk ratios, random‐effects model): | ||||||||||||||||||||
| TEM | 0.80 [0.54, 1.17] | . | 0.67 [0.46, 0.98] | . | . | . | . | . | 0.97 [0.45, 2.09] | . | . | . | . | . | . | . | . | . | . | . |
| 0.89 [0.63, 1.26] | IFN | . | 0.84 [0.59, 1.21] | . | . | . | 0.83 [0.53, 1.30] | . | 0.57 [0.39, 0.83] | . | . | . | . | . | . | . | . | . | . | . |
| 0.84 [0.36, 1.94] | 0.94 [0.43, 2.07] | IFN+PLA | . | . | . | . | . | . | . | . | . | . | . | 0.55 [0.36, 0.85] | . | . | . | . | . | . |
| 0.71 [0.50, 1.03] | 0.80 [0.56, 1.14] | 0.85 [0.36, 2.00] | IFN+TEM | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.68 [0.42, 1.11] | 0.77 [0.52, 1.13] | 0.81 [0.37, 1.77] | 0.96 [0.57, 1.60] | EVE | . | . | . | . | 0.91 [0.70, 1.19] | . | . | . | . | . | . | . | . | . | . | . |
| 0.68 [0.37, 1.23] | 0.76 [0.45, 1.28] | 0.81 [0.34, 1.90] | 0.95 [0.51, 1.76] | 0.99 [0.59, 1.65] | CAB | . | . | . | 0.92 [0.60, 1.43] | . | . | . | . | . | . | . | . | . | . | . |
| 0.69 [0.31, 1.52] | 0.77 [0.37, 1.61] | 0.82 [0.30, 2.22] | 0.96 [0.43, 2.16] | 1.01 [0.49, 2.08] | 1.02 [0.45, 2.28] | NIN | . | . | 0.91 [0.46, 1.79] | . | . | . | . | . | . | . | . | . | . | . |
| 0.64 [0.41, 1.00] | 0.72 [0.52, 0.99] | 0.76 [0.35, 1.66] | 0.89 [0.56, 1.43] | 0.93 [0.64, 1.36] | 0.94 [0.56, 1.58] | 0.93 [0.45, 1.93] | SOR | 0.99 [0.68, 1.43] | 1.08 [0.74, 1.58] | 0.97 [0.64, 1.45] | . | . | 0.74 [0.44, 1.23] | . | . | . | . | . | . | . |
| 0.63 [0.39, 1.02] | 0.71 [0.49, 1.03] | 0.75 [0.34, 1.65] | 0.88 [0.54, 1.45] | 0.92 [0.63, 1.35] | 0.93 [0.55, 1.57] | 0.92 [0.44, 1.91] | 0.99 [0.74, 1.32] | PAZ | 0.99 [0.71, 1.40] | . | . | . | . | . | . | . | . | . | . | . |
| 0.62 [0.41, 0.94] | 0.70 [0.52, 0.94] | 0.75 [0.36, 1.55] | 0.87 [0.56, 1.36] | 0.91 [0.70, 1.19] | 0.92 [0.60, 1.43] | 0.91 [0.46, 1.79] | 0.98 [0.75, 1.28] | 0.99 [0.75, 1.31] | SUN | . | 0.78 [0.47, 1.30] | 0.78 [0.54, 1.12] | . | 0.69 [0.36, 1.31] | 0.77 [0.58, 1.03] | . | 0.72 [0.50, 1.04] | 0.71 [0.51, 1.00] | 0.70 [0.38, 1.27] | 0.66 [0.46, 0.94] |
| 0.62 [0.34, 1.13] | 0.69 [0.41, 1.16] | 0.74 [0.31, 1.78] | 0.86 [0.46, 1.61] | 0.90 [0.52, 1.57] | 0.91 [0.47, 1.76] | 0.90 [0.39, 2.07] | 0.97 [0.64, 1.45] | 0.98 [0.59, 1.62] | 0.99 [0.61, 1.61] | SOR+IFN | . | . | . | . | . | . | . | . | . | . |
| 0.58 [0.32, 1.07] | 0.65 [0.38, 1.12] | 0.70 [0.29, 1.64] | 0.82 [0.43, 1.53] | 0.85 [0.51, 1.44] | 0.86 [0.46, 1.62] | 0.85 [0.38, 1.92] | 0.91 [0.54, 1.54] | 0.93 [0.54, 1.57] | 0.93 [0.60, 1.47] | 0.95 [0.49, 1.84] | ATE | . | . | . | 0.74 [0.47, 1.16] | . | . | . | . | . |
| 0.48 [0.28, 0.84] | 0.54 [0.34, 0.87] | 0.58 [0.26, 1.31] | 0.68 [0.38, 1.20] | 0.71 [0.45, 1.11] | 0.72 [0.41, 1.27] | 0.71 [0.33, 1.52] | 0.76 [0.48, 1.19] | 0.77 [0.49, 1.22] | 0.78 [0.54, 1.12] | 0.79 [0.43, 1.44] | 0.83 [0.47, 1.48] | PEM+AXI | . | . | . | . | . | . | . | . |
| 0.47 [0.24, 0.93] | 0.53 [0.29, 0.96] | 0.56 [0.22, 1.43] | 0.66 [0.33, 1.32] | 0.69 [0.37, 1.30] | 0.70 [0.34, 1.44] | 0.69 [0.28, 1.67] | 0.74 [0.44, 1.23] | 0.75 [0.42, 1.34] | 0.75 [0.42, 1.34] | 0.76 [0.40, 1.47] | 0.81 [0.39, 1.68] | 0.97 [0.49, 1.92] | AXI | . | . | . | . | . | . | . |
| 0.46 [0.22, 0.95] | 0.52 [0.27, 1.00] | 0.55 [0.36, 0.85] | 0.64 [0.31, 1.35] | 0.67 [0.35, 1.29] | 0.68 [0.33, 1.42] | 0.67 [0.27, 1.65] | 0.72 [0.38, 1.38] | 0.73 [0.38, 1.40] | 0.74 [0.41, 1.33] | 0.75 [0.35, 1.61] | 0.79 [0.38, 1.66] | 0.95 [0.47, 1.90] | 0.98 [0.43, 2.23] | IFN+BEV | . | 0.96 [0.65, 1.42] | . | . | 0.91 [0.68, 1.22] | . |
| 0.48 [0.29, 0.80] | 0.54 [0.36, 0.81] | 0.57 [0.26, 1.26] | 0.67 [0.40, 1.14] | 0.71 [0.48, 1.04] | 0.71 [0.42, 1.20] | 0.70 [0.34, 1.46] | 0.75 [0.51, 1.12] | 0.76 [0.51, 1.14] | 0.77 [0.58, 1.03] | 0.78 [0.44, 1.38] | 0.83 [0.54, 1.27] | 0.99 [0.63, 1.58] | 1.02 [0.54, 1.94] | 1.05 [0.54, 2.02] | ATE+BEV | . | . | . | . | . |
| 0.44 [0.19, 1.00] | 0.49 [0.23, 1.06] | 0.53 [0.29, 0.94] | 0.62 [0.27, 1.42] | 0.64 [0.30, 1.37] | 0.65 [0.28, 1.50] | 0.64 [0.24, 1.71] | 0.69 [0.32, 1.47] | 0.70 [0.33, 1.50] | 0.70 [0.35, 1.43] | 0.71 [0.30, 1.69] | 0.75 [0.33, 1.75] | 0.91 [0.41, 2.02] | 0.93 [0.37, 2.33] | 0.96 [0.65, 1.42] | 0.91 [0.42, 1.96] | EVE+BEV | . | . | . | . |
| 0.45 [0.26, 0.78] | 0.50 [0.32, 0.80] | 0.54 [0.24, 1.22] | 0.63 [0.35, 1.11] | 0.66 [0.42, 1.03] | 0.66 [0.38, 1.18] | 0.65 [0.30, 1.41] | 0.70 [0.45, 1.11] | 0.71 [0.45, 1.13] | 0.72 [0.50, 1.04] | 0.73 [0.40, 1.34] | 0.77 [0.43, 1.38] | 0.93 [0.55, 1.55] | 0.95 [0.48, 1.88] | 0.98 [0.49, 1.96] | 0.93 [0.59, 1.48] | 1.02 [0.46, 2.27] | LEN+EVE | . | . | 0.91 [0.64, 1.29] |
| 0.45 [0.26, 0.76] | 0.50 [0.32, 0.78] | 0.53 [0.24, 1.19] | 0.62 [0.36, 1.09] | 0.65 [0.43, 1.00] | 0.66 [0.38, 1.15] | 0.65 [0.30, 1.38] | 0.70 [0.45, 1.08] | 0.71 [0.46, 1.10] | 0.71 [0.51, 1.00] | 0.72 [0.40, 1.31] | 0.76 [0.44, 1.34] | 0.92 [0.56, 1.51] | 0.95 [0.49, 1.84] | 0.97 [0.49, 1.92] | 0.93 [0.59, 1.44] | 1.01 [0.46, 2.23] | 0.99 [0.60, 1.63] | NIV+IPI | . | . |
| 0.42 [0.21, 0.85] | 0.47 [0.25, 0.89] | 0.50 [0.30, 0.84] | 0.59 [0.28, 1.21] | 0.61 [0.33, 1.15] | 0.62 [0.30, 1.27] | 0.61 [0.25, 1.48] | 0.65 [0.35, 1.23] | 0.66 [0.35, 1.26] | 0.67 [0.38, 1.19] | 0.68 [0.32, 1.44] | 0.72 [0.35, 1.49] | 0.86 [0.44, 1.70] | 0.89 [0.39, 2.00] | 0.91 [0.68, 1.22] | 0.87 [0.46, 1.65] | 0.95 [0.58, 1.55] | 0.93 [0.47, 1.84] | 0.94 [0.48, 1.83] | TEM+BEV | . |
| 0.41 [0.24, 0.71] | 0.46 [0.29, 0.73] | 0.49 [0.22, 1.11] | 0.57 [0.32, 1.02] | 0.60 [0.38, 0.94] | 0.61 [0.34, 1.07] | 0.60 [0.28, 1.29] | 0.64 [0.41, 1.01] | 0.65 [0.41, 1.03] | 0.66 [0.46, 0.94] | 0.67 [0.36, 1.22] | 0.70 [0.39, 1.26] | 0.85 [0.51, 1.41] | 0.87 [0.44, 1.72] | 0.89 [0.45, 1.79] | 0.85 [0.54, 1.35] | 0.93 [0.42, 2.07] | 0.91 [0.64, 1.29] | 0.92 [0.56, 1.51] | 0.98 [0.50, 1.94] | LEN+PEM |
22.

Forest plot for SAEs (all risk groups combined). Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
PEM+AXI probably increase slightly the risk for SAEs (risk ratio (RR) 1.29, 95% CI 0.90 to 1.85, moderate certainty, P‐score: 0.31), when compared to SUN alone (P‐score: 0.59). The combinations LEN+PEM (RR 1.52, 95% CI 1.06 to 2.19, moderate certainty, P‐score: 0.17) and NIV+IPI (RR 1.40, 95% CI 1.00 to 1.97, moderate certainty, P‐score: 0.23) probably increase the risk for SAEs when compared to SUN alone, respectively. We are uncertain whether CAB alone reduces or increases the risk for SAE (RR 0.92, 95% CI 0.60 to 1.43, very low certainty, P‐score: 0.65) when compared to SUN alone, and there is probably little or no difference in the risk for SAEs between PAZ alone (RR 0.99, 95% CI 0.75 to 1.31, moderate certainty, P‐score: 0.59) and SUN alone. Comparison data were not available for AVE+AXI and NIV+CAB. In the ranking of treatments, TEM alone (P‐score: 0.94) was the best treatment option, and LEN+PEM the worst option (P‐score: 0.17) (Figure 22). The fixed‐effect model yielded somewhat different results (Sensitivity analysis).
There are closed loops in the network (Figure 21). Figure 68 in Appendix 15 depicts the forest plot of splitting direct and indirect evidence. There was no significant difference between direct and indirect estimates (data not shown). The net heat plot showed negligible signs for inconsistency (Figure 69 in Appendix 15).
Secondary outcomes
Progression‐free survival
Progression‐free survival (PFS) was reported in 34 trials (31 two‐arm trials and three three‐arm trials) (Jonasch 2010; NCT00065468; NCT00072046; NCT00081614; NCT00098657/NCT00083889; NCT00117637; NCT00334282; NCT00420888; NCT00609401; NCT00619268; NCT00631371; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT00979966; NCT01024920; NCT01030783; NCT01108445; NCT01274273; NCT01392183; NCT01481870; NCT01613846; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02761057; NCT02811861; NCT02853331; NCT03141177). However, evaluable data for PFS was available for only 30 trials; the remaining four trials were not evaluable for this outcome for different reasons: one trial was a cross‐over trial that did not report outcome data after the first period (NCT01613846); three trials did not report this outcome in a way that it would have been evaluable and estimating data were not possible (NCT00609401; NCT00619268; NCT01274273).
As for the 30 trials that were evaluable for this outcome, some provided data for the total population (i.e. all risk groups combined) and the different risk groups (according to MSKCC and/or IMDC criteria) separately, at the longest follow‐up available, while some trials provided data for either the total population or for the different risk groups only. With regard to the three three‐arm trials, we did not combine the different arms but rather treated these as multiple independent comparisons.
Results for all risk groups combined
We analysed data on the combined risk groups from 26 trials (11,840 participants) (Jonasch 2010; NCT00072046; NCT00081614; NCT00098657/NCT00083889; NCT00117637; NCT00334282; NCT00420888; NCT00631371; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT00979966; NCT01024920; NCT01030783; NCT01108445; NCT01481870; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02761057; NCT02811861; NCT02853331). Thereof, two three‐arm trials were included, each presenting two pairwise comparisons (we did not have data for the third comparison). Figure 70 in Appendix 15 outlines the available direct evidence (28 pairwise comparisons). The network was not fully connected and consisted of two sub‐networks (Figure 23). We conducted network meta‐analysis for subnetwork 1; subnetwork 2 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, are shown in Table 15 and Figure 24.
23.
Network graph for PFS (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. The green lines highlight the one available closed loop.
12. NMA results for PFS (all risk groups combined).
| Results of network meta‐analysis for outcome progression‐free survival (combined risk groups). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 27. No. of pairwise comparisons: 27. No. of treatments: 23. No. of designs: 23. Qtotal= 6.93, df=5, P = 0.23; Qwithin= 2.02, df = 4, p=0.73; Qbetween=4.91, df = 1, P = 0.027; I²=27.9%, Tau² = 0.0155 Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | ||||||||||||||||||||||
| LEN+PEM | . | . | . | . | . | 0.39 [0.29, 0.53] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.73 [0.46, 1.16] | CAB | . | . | . | . | 0.54 [0.37, 0.76] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.60 [0.39, 0.91] | 0.82 [0.52, 1.31] | LEN+EVE | . | . | . | 0.65 [0.48, 0.87] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.57 [0.38, 0.86] | 0.79 [0.50, 1.23] | 0.96 [0.64, 1.42] | PEM+AXI | . | . | 0.68 [0.52, 0.89] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.45 [0.31, 0.65] | 0.61 [0.40, 0.93] | 0.74 [0.51, 1.08] | 0.78 [0.55, 1.10] | ATE+BEV | . | 0.87 [0.70, 1.10] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.44 [0.29, 0.65] | 0.60 [0.39, 0.94] | 0.73 [0.49, 1.09] | 0.76 [0.53, 1.11] | 0.98 [0.69, 1.39] | NIV+IPI | 0.89 [0.68, 1.16] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.39 [0.29, 0.53] | 0.54 [0.37, 0.76] | 0.65 [0.48, 0.87] | 0.68 [0.52, 0.89] | 0.87 [0.70, 1.10] | 0.89 [0.68, 1.16] | SUN | 0.95 [0.73, 1.23] | . | . | 0.89 [0.53, 1.50] | . | . | 0.84 [0.55, 1.28] | . | . | 0.71 [0.55, 0.92] | 0.79 [0.60, 1.04] | . | 0.57 [0.22, 1.47] | 0.54 [0.41, 0.71] | . | . |
| 0.37 [0.25, 0.55] | 0.51 [0.33, 0.79] | 0.62 [0.42, 0.92] | 0.65 [0.45, 0.94] | 0.83 [0.59, 1.17] | 0.85 [0.58, 1.23] | 0.95 [0.73, 1.23] | PAZ | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.40 [0.25, 0.63] |
| 0.36 [0.22, 0.60] | 0.50 [0.29, 0.86] | 0.60 [0.36, 1.00] | 0.63 [0.38, 1.03] | 0.81 [0.50, 1.30] | 0.82 [0.50, 1.35] | 0.93 [0.61, 1.40] | 0.97 [0.60, 1.59] | TIV | . | . | . | . | . | . | . | . | 0.76 [0.54, 1.06] | . | . | . | . | . |
| 0.35 [0.21, 0.61] | 0.49 [0.27, 0.86] | 0.59 [0.35, 1.01] | 0.62 [0.37, 1.04] | 0.79 [0.48, 1.31] | 0.81 [0.48, 1.36] | 0.91 [0.58, 1.42] | 0.95 [0.57, 1.60] | 0.98 [0.59, 1.63] | AXI | . | . | . | . | . | . | . | 0.77 [0.53, 1.12] | . | . | . | . | . |
| 0.35 [0.19, 0.63] | 0.48 [0.25, 0.90] | 0.58 [0.32, 1.05] | 0.61 [0.34, 1.09] | 0.78 [0.44, 1.37] | 0.79 [0.44, 1.42] | 0.89 [0.53, 1.50] | 0.94 [0.53, 1.67] | 0.96 [0.50, 1.87] | 0.98 [0.50, 1.95] | NIN | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.33 [0.21, 0.53] | 0.46 [0.28, 0.76] | 0.56 [0.35, 0.88] | 0.58 [0.37, 0.91] | 0.75 [0.49, 1.14] | 0.76 [0.49, 1.18] | 0.85 [0.60, 1.22] | 0.90 [0.58, 1.39] | 0.92 [0.55, 1.54] | 0.94 [0.55, 1.62] | 0.96 [0.51, 1.79] | IFN+BEV | . | . | 0.92 [0.65, 1.29] | 0.91 [0.69, 1.20] | . | . | . | . | 0.71 [0.55, 0.92] | 0.63 [0.48, 0.83] | . |
| 0.32 [0.01, 17.44] | 0.44 [0.01, 24.04] | 0.54 [0.01, 29.06] | 0.56 [0.01, 30.33] | 0.72 [0.01, 38.92] | 0.73 [0.01, 39.70] | 0.83 [0.02, 44.22] | 0.87 [0.02, 46.82] | 0.89 [0.02, 48.10] | 0.91 [0.02, 49.16] | 0.92 [0.02, 51.21] | 0.97 [0.02, 52.33] | SOR+IFN | . | . | . | . | 0.85 [0.02, 45.10] | . | . | . | . | . |
| 0.33 [0.20, 0.55] | 0.45 [0.26, 0.78] | 0.55 [0.33, 0.91] | 0.57 [0.35, 0.94] | 0.73 [0.45, 1.19] | 0.75 [0.45, 1.23] | 0.84 [0.55, 1.28] | 0.88 [0.54, 1.45] | 0.91 [0.50, 1.64] | 0.92 [0.50, 1.71] | 0.94 [0.48, 1.84] | 0.98 [0.57, 1.71] | 1.02 [0.02, 55.79] | ATE | . | . | . | . | . | . | . | . | . |
| 0.31 [0.17, 0.55] | 0.42 [0.23, 0.77] | 0.51 [0.29, 0.91] | 0.53 [0.30, 0.93] | 0.69 [0.40, 1.18] | 0.70 [0.40, 1.22] | 0.78 [0.48, 1.29] | 0.82 [0.47, 1.44] | 0.85 [0.46, 1.57] | 0.86 [0.45, 1.64] | 0.88 [0.43, 1.80] | 0.92 [0.65, 1.29] | 0.95 [0.02, 52.25] | 0.93 [0.49, 1.79] | EVE+BEV | . | . | . | . | . | . | . | . |
| 0.30 [0.18, 0.52] | 0.42 [0.23, 0.74] | 0.51 [0.29, 0.87] | 0.53 [0.31, 0.89] | 0.68 [0.41, 1.13] | 0.69 [0.41, 1.17] | 0.78 [0.49, 1.22] | 0.82 [0.48, 1.37] | 0.84 [0.47, 1.51] | 0.85 [0.46, 1.57] | 0.87 [0.44, 1.73] | 0.91 [0.69, 1.20] | 0.94 [0.02, 51.51] | 0.92 [0.50, 1.72] | 0.99 [0.64, 1.54] | TEM+BEV | . | . | . | . | . | . | . |
| 0.28 [0.19, 0.41] | 0.38 [0.25, 0.59] | 0.46 [0.31, 0.68] | 0.48 [0.34, 0.70] | 0.62 [0.44, 0.88] | 0.63 [0.44, 0.92] | 0.71 [0.55, 0.92] | 0.75 [0.52, 1.08] | 0.77 [0.47, 1.25] | 0.78 [0.47, 1.32] | 0.80 [0.45, 1.42] | 0.83 [0.54, 1.29] | 0.86 [0.02, 46.67] | 0.85 [0.52, 1.39] | 0.91 [0.52, 1.59] | 0.92 [0.55, 1.54] | EVE | . | . | . | . | . | . |
| 0.27 [0.19, 0.40] | 0.37 [0.24, 0.58] | 0.45 [0.31, 0.67] | 0.48 [0.33, 0.68] | 0.61 [0.44, 0.85] | 0.62 [0.43, 0.89] | 0.70 [0.55, 0.89] | 0.73 [0.52, 1.05] | 0.76 [0.54, 1.06] | 0.77 [0.53, 1.12] | 0.78 [0.44, 1.39] | 0.82 [0.56, 1.21] | 0.85 [0.02, 45.10] | 0.83 [0.51, 1.36] | 0.89 [0.53, 1.50] | 0.90 [0.56, 1.45] | 0.98 [0.69, 1.40] | SOR | . | . | 1.14 [0.75, 1.74] | . | . |
| 0.26 [0.16, 0.41] | 0.35 [0.21, 0.59] | 0.43 [0.27, 0.69] | 0.45 [0.28, 0.71] | 0.58 [0.37, 0.89] | 0.59 [0.37, 0.92] | 0.66 [0.46, 0.95] | 0.69 [0.44, 1.09] | 0.71 [0.42, 1.20] | 0.73 [0.42, 1.26] | 0.74 [0.39, 1.39] | 0.77 [0.53, 1.13] | 0.80 [0.01, 43.36] | 0.78 [0.45, 1.38] | 0.84 [0.50, 1.41] | 0.85 [0.53, 1.36] | 0.92 [0.59, 1.45] | 0.94 [0.63, 1.41] | NAP+IFN | . | 0.92 [0.70, 1.22] | . | . |
| 0.22 [0.08, 0.60] | 0.30 [0.11, 0.84] | 0.37 [0.14, 1.00] | 0.39 [0.14, 1.04] | 0.50 [0.19, 1.32] | 0.51 [0.19, 1.36] | 0.57 [0.22, 1.47] | 0.60 [0.22, 1.60] | 0.61 [0.22, 1.73] | 0.63 [0.22, 1.79] | 0.64 [0.22, 1.88] | 0.66 [0.24, 1.83] | 0.69 [0.01, 41.24] | 0.68 [0.24, 1.91] | 0.72 [0.25, 2.11] | 0.73 [0.26, 2.09] | 0.80 [0.30, 2.13] | 0.81 [0.30, 2.16] | 0.86 [0.31, 2.39] | TEM | . | . | . |
| 0.24 [0.16, 0.35] | 0.32 [0.21, 0.50] | 0.39 [0.27, 0.58] | 0.41 [0.29, 0.59] | 0.53 [0.38, 0.74] | 0.54 [0.38, 0.77] | 0.61 [0.48, 0.77] | 0.64 [0.45, 0.91] | 0.66 [0.42, 1.02] | 0.67 [0.42, 1.07] | 0.68 [0.38, 1.20] | 0.71 [0.55, 0.92] | 0.74 [0.01, 39.50] | 0.72 [0.44, 1.18] | 0.77 [0.50, 1.19] | 0.78 [0.53, 1.14] | 0.85 [0.60, 1.21] | 0.87 [0.65, 1.15] | 0.92 [0.70, 1.22] | 1.07 [0.40, 2.84] | IFN | . | . |
| 0.21 [0.12, 0.36] | 0.29 [0.16, 0.51] | 0.35 [0.20, 0.60] | 0.37 [0.22, 0.62] | 0.47 [0.28, 0.78] | 0.48 [0.28, 0.81] | 0.54 [0.34, 0.85] | 0.57 [0.34, 0.95] | 0.58 [0.32, 1.04] | 0.59 [0.32, 1.09] | 0.60 [0.30, 1.20] | 0.63 [0.48, 0.83] | 0.65 [0.01, 35.70] | 0.64 [0.35, 1.19] | 0.69 [0.44, 1.07] | 0.69 [0.47, 1.03] | 0.76 [0.45, 1.27] | 0.77 [0.48, 1.24] | 0.82 [0.51, 1.31] | 0.95 [0.33, 2.71] | 0.89 [0.61, 1.30] | IFN+PLA | . |
| 0.15 [0.08, 0.27] | 0.20 [0.11, 0.38] | 0.25 [0.14, 0.45] | 0.26 [0.14, 0.46] | 0.33 [0.19, 0.59] | 0.34 [0.19, 0.61] | 0.38 [0.23, 0.64] | 0.40 [0.25, 0.63] | 0.41 [0.21, 0.80] | 0.42 [0.21, 0.83] | 0.43 [0.20, 0.89] | 0.45 [0.24, 0.84] | 0.46 [0.01, 25.58] | 0.45 [0.23, 0.89] | 0.49 [0.24, 1.00] | 0.49 [0.25, 0.98] | 0.53 [0.30, 0.95] | 0.54 [0.31, 0.97] | 0.58 [0.31, 1.09] | 0.67 [0.23, 1.98] | 0.63 [0.35, 1.11] | 0.71 [0.36, 1.41] | PLA |
24.
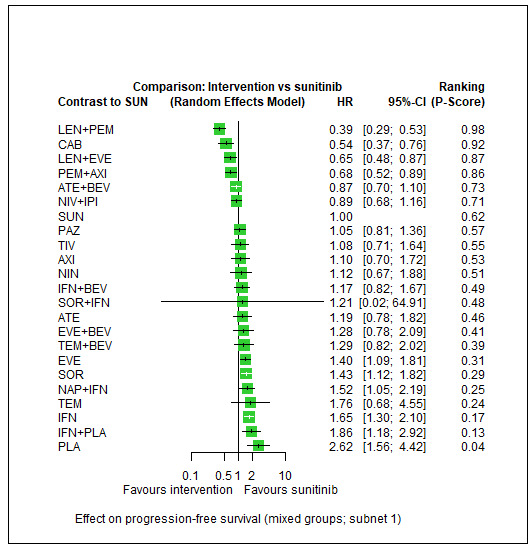
Forest plot for PFS (all risk groups combined). 1) PFS‐subnetwork 1. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
In subnetwork 1, we observed little between‐study heterogeneity (Qtotal = 6.93, df = 5, P = 0.23; Qwithin = 2.02, df = 4, P = 0.73; Qbetween = 4.91, df = 1, P = 0.027; I² = 27.9%, Tau² = 0.0155). The combination LEN+PEM (HR 0.39, 95% CI 0.29 to 0.53, moderate certainty, P‐score: 0.98) probably improves PFS, and CAB alone may improve PFS (HR 0.54, 95% CI 0.37 to 0.76, low certainty, P‐score: 0.92), when compared to SUN alone (P‐score: 0.62), respectively. PEM+AXI probably improve slightly PFS (HR 0.68, 95% CI 0.52 to 0.89, moderate certainty, P‐score: 0.86), when compared to SUN alone. There probably is little or no difference in PFS between PAZ alone (HR 1.05, 95% CI 0.81 to 1.36, moderate certainty, P‐score: 0.57) and SUN alone, and there may be little or no difference in PFS between NIV+IPI (HR 0.89, 95% CI 0.68 to 1.16, low certainty, P‐score: 0.71) and SUN alone. Comparison data were not available for AVE+AXI and NIV+CAB. In the ranking of treatments, LEN+PEM (P‐score: 0.98) was the best treatment option, and IFN+PLA the worst option (0.13) (Figure 24).
As shown in Figure 23, there was one closed loop in the network. Figure 71 in Appendix 15 depicts the forest plot of splitting direct and indirect evidence. Results suggested that there is no difference between direct and indirect estimates (P = 0.083 (data not shown)). The net heat plot showed small signs for inconsistency (Figure 72 in Appendix 15).
Results for MSKCC favourable risk group
We analysed data on the favourable risk group according to the MSKCC criteria from nine trials (1410 participants) (NCT00420888; NCT00631371; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT01108445; NCT01481870; NCT02811861). Of these, one three‐arm trial was included, presenting two pairwise comparisons (we did not have data for the third comparison). One additional trial (NCT00920816) did not report a confidence interval, and it was not possible to reconstruct it, so we excluded this trial from this analysis. Figure 73 in Appendix 15 outlines the available direct evidence (10 pairwise comparisons). The network was not fully connected and consisted of three sub‐networks (Figure 25). We conducted network meta‐analysis for the sub‐networks 1 and 2; subnetwork 3 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, are shown in Table 16 and Figure 26, per subnetwork.
25.
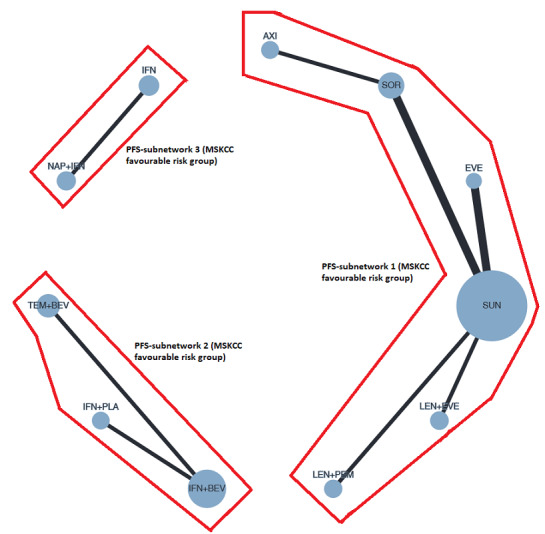
Network graph for PFS (MSKCC favourable risk group). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
13. NMA results for PFS (MSKCC favourable risk group).
| Results of network meta‐analysis for outcome progression‐free survival (MSKCC favourable risk group). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 7. No. of pairwise comparisons: 7. No. of treatments: 6. No. of designs: 5 Heterogeneity/Inconsistency: Q = 6.16, df = 2, P = 0.046; I² = 67.6%, Tau² = 0.3473 Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | |||||||
| LEN+PEM | . | 0.36 [0.11, 1.23] | . | . | . | ||
| 0.80 [0.14, 4.54] | LEN+EVE | 0.45 [0.13, 1.53] | . | . | . | ||
| 0.36 [0.11, 1.23] | 0.45 [0.13, 1.53] | SUN | . | 0.59 [0.23, 1.55] | 0.50 [0.19, 1.33] | ||
| 0.28 [0.04, 2.10] | 0.35 [0.05, 2.61] | 0.79 [0.16, 3.81] | AXI | . | 0.64 [0.18, 2.23] | ||
| 0.21 [0.04, 1.02] | 0.27 [0.06, 1.26] | 0.59 [0.23, 1.55] | 0.75 [0.12, 4.78] | EVE | . | ||
| 0.18 [0.04, 0.87] | 0.23 [0.05, 1.08] | 0.50 [0.19, 1.33] | 0.64 [0.18, 2.23] | 0.85 [0.22, 3.32] | SOR | ||
|
Subnet 2 No. of studies: 2. No. of pairwise comparisons: 2. No. of treatments: 3. No. of designs: 2 Heterogeneity/Inconsistency: Q = 0.0, df=0, P = n.a.; I² = n.a., Tau² = n.a. Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | |||||||
| IFN+BEV | 0.83 [0.59, 1.18] | 0.60 [0.42, 0.85] | |||||
| 0.83 [0.59, 1.18] | TEM+BEV | . | |||||
| 0.60 [0.42, 0.85] | 0.72 [0.44, 1.18] | IFN+PLA | |||||
26.
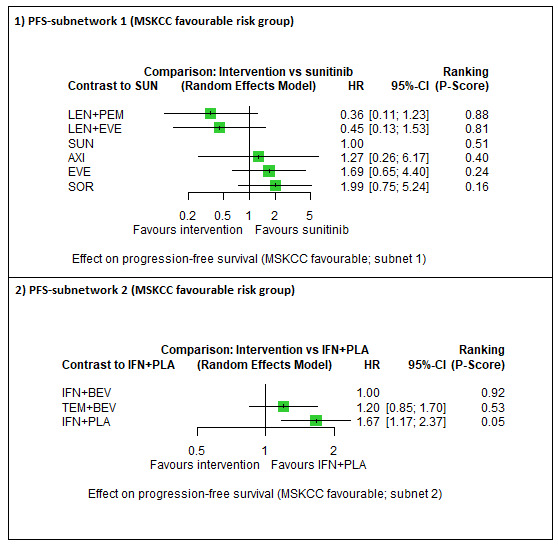
Forest plot for PFS (MSKCC favourable risk group). 1) PFS‐subnetwork 1. Reference treatment: sunitinib (SUN); 2) PFS‐subnetwork 2. Reference treatment: interferon‐alpha + bevacizumab (IFN+BEV). Treatments are ordered by P‐score (descending).
In sub‐network 1, we observed substantial heterogeneity (Q = 6.16, df = 2, P = 0.046; I² = 67.6%, Tau² = 0.3473). We are uncertain whether LEN+PEM (HR 0.36, 95% CI 0.11 to 1.23, very low certainty, P‐score: 0.88) improves PFS when compared to SUN alone (P‐score: 0.51). Comparison data were not available for PEM+AXI, AVE+AXI, NIV+CAB, NIV+IPI, PAZ alone and CAB alone. In the ranking of treatments, LEN‐PEM (P‐score: 0.88) was the best treatment option, whereas SOR alone was the worst option (P‐score: 0.16) (Figure 26). The fixed‐effect model yielded different results (see Sensitivity analysis).
In subnetwork 2, each pairwise comparison was reported by a single trial only, so no heterogeneity statistics could be calculated. Here, IFN+BEV was the comparator, and the ranking of treatments suggested that IFN+BEV was the best treatment option (P‐score: 0.92) whereas IFN+PLA was the worst option (P‐score: 0.05).
Results for IMDC favourable risk groups
We analysed data on the favourable risk group according to the IMDC criteria from five trials (1007 participants) (NCT00420888; NCT02231749; NCT02684006; NCT02811861; NCT03141177). Thereof, one three‐arm trial was included, presenting two pairwise comparisons (we did not have data for the third comparison). Figure 74 in Appendix 15 outlines the available direct evidence (six pairwise comparisons). The network was not fully connected and consisted of two sub‐networks (Figure 27). We conducted network meta‐analysis for subnetwork 1. Subnetwork 2 contained only one trial, so no further analyses were conducted. Results for all network comparisons in subnet 1, including the ranking of treatments, are shown in Table 17 and in Figure 28.
27.
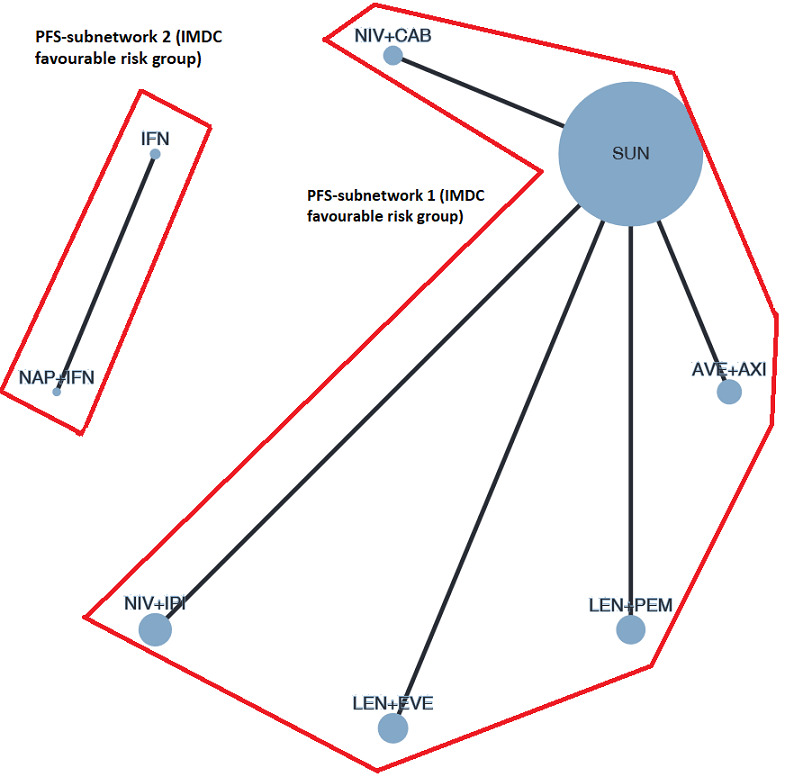
Network graph for PFS (IMDC favourable risk group). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
14. NMA results for PFS (IMDC favourable risk group).
| Results of network meta‐analysis for outcome progression‐free survival (IMDC favourable risk group). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 5. No. of pairwise comparisons: 5. No. of treatments: 6. No. of designs: 5 Heterogeneity/Inconsistency: Q = 0, df=0, P = n.a.; I²=n.a., Tau² = n.a. Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | |||||
| LEN+PEM | . | . | . | 0.41 [0.28, 0.61] | . |
| 0.75 [0.43, 1.29] | LEN+EVE | . | . | 0.55 [0.38, 0.80] | . |
| 0.71 [0.38, 1.31] | 0.95 [0.52, 1.74] | NIV+CAB | . | 0.58 [0.36, 0.93] | . |
| 0.58 [0.34, 0.99] | 0.77 [0.46, 1.31] | 0.82 [0.45, 1.49] | AVE+AXI | 0.71 [0.49, 1.02] | . |
| 0.41 [0.28, 0.61] | 0.55 [0.38, 0.80] | 0.58 [0.36, 0.93] | 0.71 [0.49, 1.02] | SUN | 0.54 [0.38, 0.77] |
| 0.22 [0.13, 0.38] | 0.30 [0.18, 0.50] | 0.32 [0.17, 0.57] | 0.39 [0.23, 0.64] | 0.54 [0.38, 0.77] | NIV+IPI |
28.
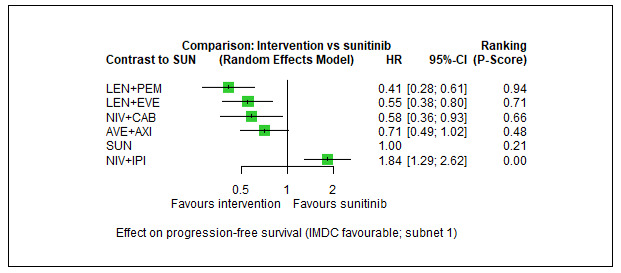
Forest plot for PFS (IMDC favourable risk group). 1) PFS‐subnetwork 1. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
In sub‐network 1, each pairwise comparison was reported by a single trial only, so no heterogeneity statistics could be calculated. The combinations LEN+PEM (HR 0.41, 95% CI 0.28 to 0.61, low certainty, P‐score: 0.94), NIV+CAB (HR 0.58, 95% CI 0.36 to 0.93, low certainty, P‐score: 0.66) and AVE+AXI (HR 0.71, 95% CI 0.49 to 1.02, low certainty, P‐score: 0.48) may improve PFS when compared to SUN alone (P‐score: 0.21, respectively. The combination NIV+IPI probably reduces PFS (HR 1.84, 95% CI 1.29 to 2.62, moderate certainty, P‐score: 0.00), when compared to SUN. Comparison data were not available for PAZ alone, CAB alone and PEM+AXI. In the ranking of treatments, LEN+PEM was the best treatment option (P‐score: 0.94) and NIV+IPI was the worst (P‐score: 0.00) (Figure 28).
Results for MSKCC intermediate and poor risk groups
We analysed data on the intermediate and poor risk groups according to the MSKCC criteria from eight trials (2797 participants) (NCT00420888; NCT00631371; NCT00732914; NCT00738530; NCT00903175; NCT00920816; NCT01108445; NCT02811861). Of these, one three‐arm trial was included, presenting two pairwise comparisons (we did not have data for the third comparison). Figure 75 in Appendix 15 outlines the available direct evidence (15 pairwise comparisons). The network was not fully connected and consisted of three sub‐networks (Figure 29). We conducted network meta‐analysis for sub‐networks 1 and 2. Subnetwork 3 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, are shown in Table 18 and Figure 30.
29.
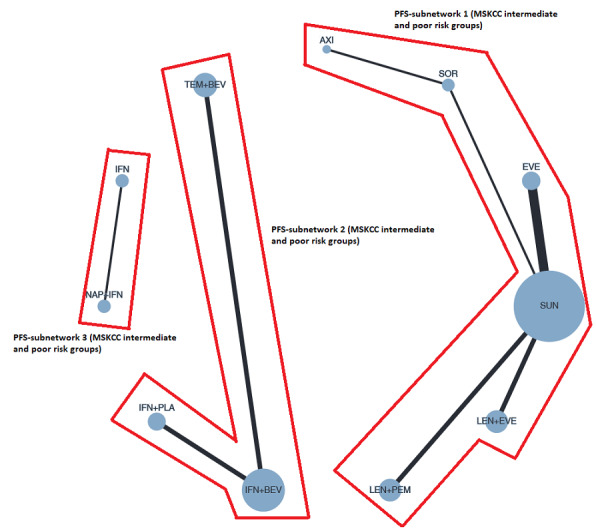
Network graph for PFS (MSKCC intermediate and poor risk groups). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
15. NMA results for PFS (MSKCC intermediate and poor risk groups).
| Results of network meta‐analysis for outcome progression‐free survival (MSKCC intermediate and poor risk groups). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 10. No. of pairwise comparisons: 10. No. of treatments: 6. No. of designs: 5 Heterogeneity/Inconsistency: Q = 14.40, df = 5, P = 0.013; I² = 65.3%, Tau² = 0.1433 Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | |||||||
| LEN+PEM | . | . | 0.33 [0.17, 0.62] | . | . | ||
| 0.45 [0.19, 1.10] | LEN+EVE | . | 0.72 [0.39, 1.33] | . | . | ||
| 0.34 [0.09, 1.32] | 0.76 [0.20, 2.88] | AXI | . | 0.83 [0.35, 1.96] | . | ||
| 0.33 [0.17, 0.62] | 0.72 [0.39, 1.33] | 0.95 [0.29, 3.08] | SUN | 0.88 [0.39, 1.97] | 0.84 [0.52, 1.34] | ||
| 0.29 [0.10, 0.81] | 0.63 [0.23, 1.75] | 0.83 [0.35, 1.96] | 0.88 [0.39, 1.97] | SOR | . | ||
| 0.27 [0.12, 0.61] | 0.60 [0.28, 1.31] | 0.79 [0.22, 2.82] | 0.84 [0.52, 1.34] | 0.95 [0.37, 2.43] | EVE | ||
|
Subnet 2 No. of studies: 2. No. of pairwise comparisons: 2. No. of treatments: 3. No. of designs: 2 Heterogeneity/Inconsistency: Q = 2.79, df = 2, P = 0.247; I²=28.3%, Tau² = 0.0175 Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | |||||||
| IFN+BEV | 0.99 [0.74, 1.32] | 0.60 [0.45, 0.82] | |||||
| 0.99 [0.74, 1.32] | TEM+BEV | . | |||||
| 0.60 [0.45, 0.82] | 0.61 [0.40, 0.93] | IFN+PLA | |||||
30.
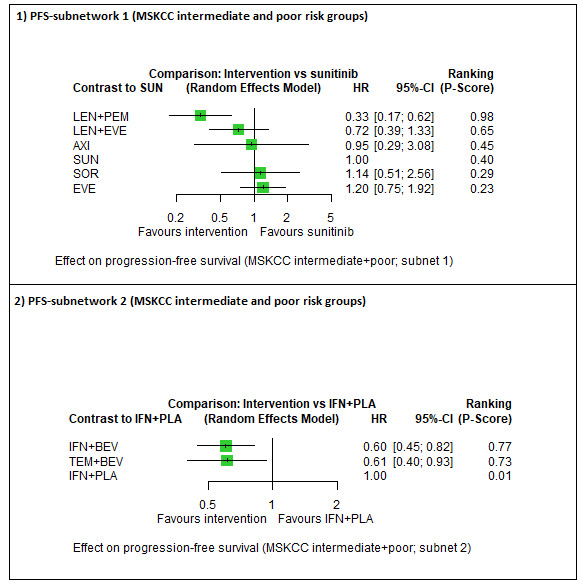
Forest plot for PFS (MSKCC intermediate and poor risk groups). 1) PFS‐subnetwork 1. Reference treatment: sunitinib (SUN); 2) PFS‐subnetwork 2. Reference treatment: interferon‐alpha + placebo (IFN+PLA). Treatments are ordered by P‐score (descending).
In sub‐network 1, we observed substantial between‐study heterogeneity (Q = 14.40, df=5, P = 0.013; I² = 65.3%, Tau² = 0.1433). The combination LEN+PEM probably improves PFS (HR 0.33, 95% CI 0.17 to 0.62, moderate certainty, P‐score: 0.98) when compared to SUN alone (P‐score: 0.40). Comparison data were not available for PEM+AXI, AVE+AXI, NIV+IPI, NIV+CAB, PAZ alone and CAB alone. In the ranking of treatments, LEN+PEM was the best treatment option (P‐score: 0.98), and EVE alone was the worst option (P‐score: 0.23) (Figure 30). The fixed‐effect model yielded somewhat different results (Sensitivity analysis).
In sub‐network 2, where IFN+PLA was the comparator treatment, we observed moderate between‐study heterogeneity (Q=2.79, df=2, P = 0.247; I² = 28.3%, Tau² = 0.0175). In the ranking of treatments, IFN+BEV was the better treatment option (P‐score: 0.77) and IFN+PLA the worst (P‐score: 0.01). The fixed‐effect model yielded slightly different results (Sensitivity analysis).
Results for IMDC intermediate and poor risk groups
We analysed data on the intermediate and poor risk groups according to the IMDC criteria from eight trials (4042 participants) (NCT00065468; NCT00420888; NCT01392183; NCT01835158; NCT02231749; NCT02684006; NCT02811861; NCT03141177). Of these, two three‐arm trials were included, each presenting two pairwise comparisons (we did not have data for the third comparison). Figure 76 in Appendix 15 outlines the available direct evidence (16 pairwise comparisons). The network was not fully connected and consisted of two sub‐networks (Figure 31). We conducted network meta‐analysis for both networks. Results for all network comparisons, including the ranking of treatments, are shown in Table 19 and Figure 32, per subnetwork.
31.
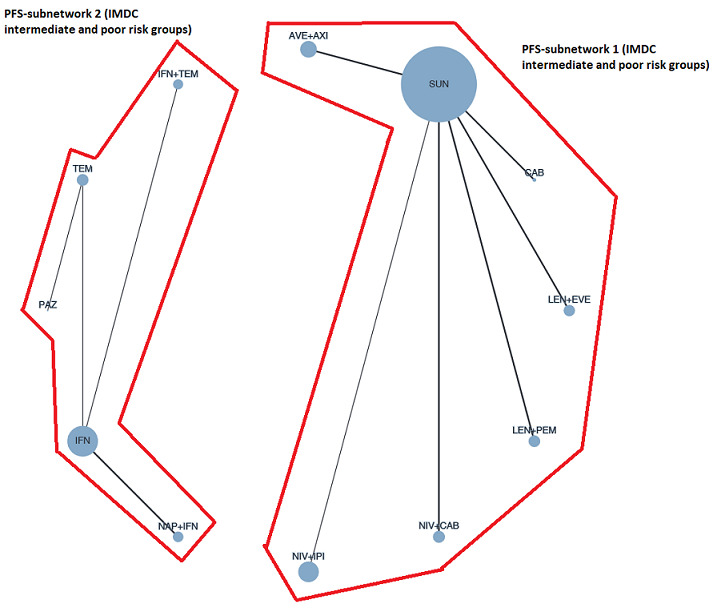
Network graph for PFS (IMDC intermediate and poor risk groups). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
16. NMA results for PFS (IMDC intermediate and poor risk groups).
| Results of network meta‐analysis for outcome progression‐free survival (IMDC intermediate and poor risk groups). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 11. No. of pairwise comparisons: 11. No. of treatments: 7. No. of designs: 6 Heterogeneity/Inconsistency: Q = 8.7, df = 5, P = 0.12; I² = 42.5%, Tau² = 0.0357 Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | ||||||||||
| LEN+PEM | . | . | . | . | . | 0.36 [0.24, 0.54] | ||||
| 0.78 [0.40, 1.52] | CAB | . | . | . | . | 0.46 [0.27, 0.79] | ||||
| 0.75 [0.43, 1.29] | 0.96 [0.51, 1.81] | NIV+CAB | . | . | . | 0.48 [0.34, 0.69] | ||||
| 0.60 [0.35, 1.02] | 0.77 [0.41, 1.45] | 0.81 [0.50, 1.32] | AVE+AXI | . | . | 0.60 [0.43, 0.84] | ||||
| 0.52 [0.30, 0.92] | 0.67 [0.35, 1.29] | 0.70 [0.42, 1.18] | 0.87 [0.52, 1.44] | LEN+EVE | . | 0.69 [0.47, 1.01] | ||||
| 0.49 [0.27, 0.87] | 0.63 [0.32, 1.22] | 0.65 [0.38, 1.13] | 0.81 [0.48, 1.37] | 0.93 [0.53, 1.63] | NIV+IPI | 0.74 [0.49, 1.11] | ||||
| 0.36 [0.24, 0.54] | 0.46 [0.27, 0.79] | 0.48 [0.34, 0.69] | 0.60 [0.43, 0.84] | 0.69 [0.47, 1.01] | 0.74 [0.49, 1.11] | SUN | ||||
|
Subnet 2 No. of studies: 5. No. of pairwise comparisons: 5. No. of treatments: 5. No. of designs: 4 Heterogeneity/Inconsistency: Q 0.47, df = 1, P = 0.50; I² = 0%, Tau² = 0.0 Treatment Effects + 95%‐CIs (Hazard ratios, random‐effects model): | ||||||||||
| PAZ | 0.73 [0.45, 1.19] | . | . | . | ||||||
| 0.73 [0.45, 1.19] | TEM | . | . | 0.74 [0.60, 0.91] | ||||||
| 0.71 [0.40, 1.25] | 0.97 [0.73, 1.31] | IFN+TEM | . | 0.76 [0.62, 0.94] | ||||||
| 0.56 [0.32, 0.98] | 0.76 [0.57, 1.02] | 0.78 [0.58, 1.05] | NAP+IFN | 0.97 [0.79, 1.19] | ||||||
| 0.54 [0.32, 0.92] | 0.74 [0.60, 0.91] | 0.76 [0.62, 0.94] | 0.97 [0.79, 1.19] | IFN | ||||||
32.
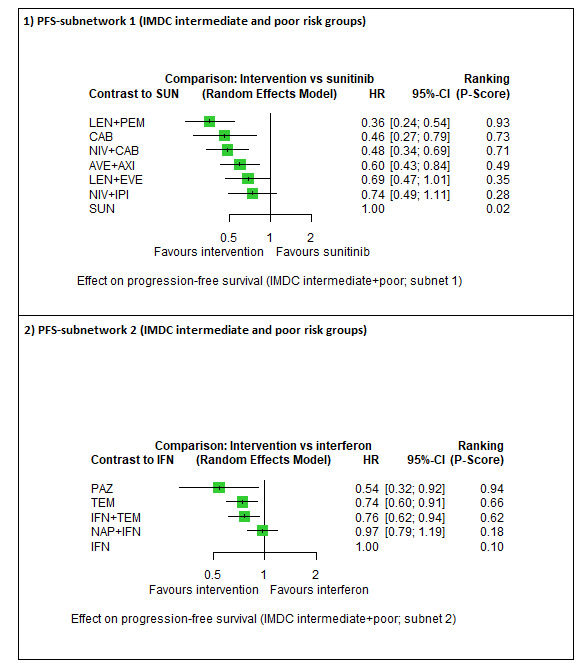
Forest plot for PFS (IMDC intermediate and poor risk groups). 1) PFS‐subnetwork 1. Reference treatment: sunitinib (SUN); 2) PFS‐subnetwork 2. Reference treatment: interferon‐alpha (IFN). Treatments are ordered by P‐score (descending).
In sub‐network 1, we observed moderate between‐study heterogeneity (Q=8.7, df = 5, P = 0.12; I² = 42.5%, Tau² = 0.0357). Cabozantinib alone (HR 0.46, 95% CI 0.27 to 0.79, moderate certainty, P‐score: 0.73), and the combinations LEN+PEM (HR 0.36, 95% CI 0.24 to 0.54, moderate certainty, P‐score: 0.93), NIV+CAB (HR 0.48, 95% CI 0.34 to 0.69, moderate certainty, P‐score: 0.71) and AVE+AXI (HR 0.60, 95% CI 0.43 to 0.84, moderate certainty, P‐score: 0.49) probably improve PFS when compared to SUN alone (P‐score: 0.02), respectively. There may be little or no difference in PFS between NIV+IPI (HR 0.74, 95% CI 0.49 to 1.11, low certainty, P‐score: 0.28) and SUN alone. Comparison data were not available for PEM+AXI and PAZ alone. In the ranking of treatments, LEN+PEM was the best treatment option (P‐score: 0.93), and SUN alone was the worst option (P‐score: 0.02) (Figure 32). The fixed‐effect model yielded little differences (Sensitivity analysis).
In subnetwork 2, where IFN alone was the comparator treatment, we did not observe between‐study heterogeneity (Q = 0.47, df = 1, P = 0.50; I² = 0%, Tau² = 0.0). The ranking of treatments suggested that PAZ alone was the best treatment option (P‐score: 0.94), whereas IFN alone was the worst option (P‐score: 0.10).
Adverse events
Adverse events (AEs) were not consistently reported across trials. To be able to meta‐analyse results, we could only consider AEs when the number of participants with at least one all‐cause event of grade 3 or 4 was reported. We did not consider cumulated events or treatment‐related AEs. All‐cause AEs were assessed in a total of 18 trials (NCT00065468; NCT00081614; NCT00719264; NCT00720941; NCT00732914; NCT01024920; NCT01613846; NCT01835158; NCT01984242; NCT02420821; NCT02684006;NCT02811861; NCT03141177; NCT00738530; NCT00920816; NCT01030783; NCT01108445; NCT01274273) (15 two‐arm trials, three three‐arm trials), for a total of 8423 participants. One of these trials did not report individual AEs, meaning we could only extract data for the total number of participants with at least one grade 3 or 4 AE (NCT01984242). Another five trials did not report the total number of participants with at least one grade 3 or 4 AE, meaning we could only extract data for individual grade 3 or 4 AEs (NCT00738530; NCT00920816; NCT01030783; NCT01108445; NCT01274273). In the three‐arm trials, only two comparisons (arm A versus arm C and arm B versus arm C) were reported, so we manually added a third comparison (arm A versus arm B).
Analysis of all‐cause AEs (grade 3 or 4)
We conducted a combined analysis of all‐cause grade 3 or 4 AEs in all risk groups combined from 13 trials (NCT00065468; NCT00081614; NCT00719264; NCT00720941; NCT00732914; NCT01024920; NCT01613846; NCT01835158; NCT01984242; NCT02420821; NCT02684006; NCT02811861; NCT03141177), for a total of 6909 participants. Thereof, three three‐arms trials were included, each presenting three pairwise comparisons (NCT00065468; NCT01984242; NCT02811861). In one trial, AEs occurring in >2% of participants were reported; in another trial the frequency was >3%; in three trials >10% of participants; in four trials >20% of participants; in one trial AEs in >25% of the participants; for three trials, the frequency was not reported. Figure 77 in Appendix 15 outlines the available direct evidence (19 pairwise comparisons). The network was not fully connected and consisted of four sub‐networks (Figure 33). We conducted network meta‐analysis for subnetwork 1. Sub‐networks 2, 3 and 4 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, are shown in Table 20 and Figure 34.
33.
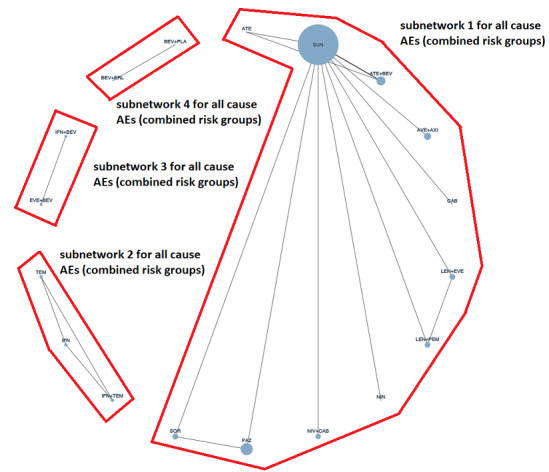
Network graph for all‐cause AEs (grades 3‐4; all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
17. NMA results for AEs (all risk groups combined).
| Results of network meta‐analysis for outcome all‐cause AEs (grades 3 and 4). Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 10. No. of pairwise comparisons: 14. No. of treatments: 11. No. of designs: 10. Qtotal= 0.31, df = 2, P =0.85; Qwithin= 0.0, df = 0, P = n.a.; Qbetween=0.31, df = 2, P = 0.85; I² = 0.0%, Tau² = 0.0 Treatment Effects + 95%‐CIs (Risk ratios, random‐effects model): | ||||||||||
| ATE | 0.63 [0.47, 0.83] | . | . | . | 0.58 [0.44, 0.76] | . | . | . | . | . |
| 0.64 [0.49, 0.83] | ATE+BEV | . | . | . | 0.89 [0.81, 0.97] | . | . | . | . | . |
| 0.70 [0.44, 1.11] | 1.09 [0.74, 1.61] | NIN | . | . | 0.82 [0.56, 1.20] | . | . | . | . | . |
| 0.59 [0.44, 0.78] | 0.92 [0.80, 1.06] | 0.84 [0.57, 1.26] | SOR | . | 0.99 [0.85, 1.14] | 0.92 [0.78, 1.09] | . | . | . | . |
| 0.57 [0.43, 0.75] | 0.89 [0.79, 1.01] | 0.82 [0.55, 1.21] | 0.97 [0.84, 1.12] | AVE+AXI | 1.00 [0.92, 1.08] | . | . | . | . | . |
| 0.57 [0.44, 0.74] | 0.89 [0.81, 0.97] | 0.82 [0.56, 1.20] | 0.97 [0.86, 1.08] | 1.00 [0.92, 1.08] | SUN | 0.98 [0.92, 1.05] | 0.96 [0.77, 1.21] | 0.94 [0.85, 1.03] | 0.87 [0.80, 0.95] | 0.86 [0.80, 0.94] |
| 0.56 [0.43, 0.73] | 0.87 [0.78, 0.97] | 0.80 [0.54, 1.18] | 0.95 [0.84, 1.06] | 0.98 [0.88, 1.09] | 0.98 [0.92, 1.05] | PAZ | . | . | . | . |
| 0.55 [0.39, 0.77] | 0.85 [0.67, 1.09] | 0.78 [0.50, 1.22] | 0.93 [0.72, 1.20] | 0.96 [0.75, 1.22] | 0.96 [0.77, 1.21] | 0.98 [0.77, 1.24] | CAB | . | . | . |
| 0.53 [0.40, 0.70] | 0.83 [0.73, 0.95] | 0.77 [0.52, 1.13] | 0.91 [0.78, 1.05] | 0.93 [0.82, 1.06] | 0.94 [0.85, 1.03] | 0.96 [0.85, 1.07] | 0.98 [0.76, 1.25] | NIV+CAB | . | . |
| 0.50 [0.38, 0.65] | 0.77 [0.69, 0.87] | 0.71 [0.48, 1.05] | 0.84 [0.73, 0.97] | 0.87 [0.77, 0.98] | 0.87 [0.80, 0.95] | 0.89 [0.80, 0.99] | 0.91 [0.71, 1.15] | 0.93 [0.82, 1.05] | LEN+PEM | 0.99 [0.93, 1.06] |
| 0.49 [0.37, 0.64] | 0.77 [0.68, 0.87] | 0.70 [0.48, 1.04] | 0.83 [0.73, 0.96] | 0.86 [0.76, 0.97] | 0.86 [0.80, 0.94] | 0.88 [0.79, 0.98] | 0.90 [0.71, 1.14] | 0.92 [0.81, 1.04] | 0.99 [0.93, 1.06] | LEN+EVE |
34.
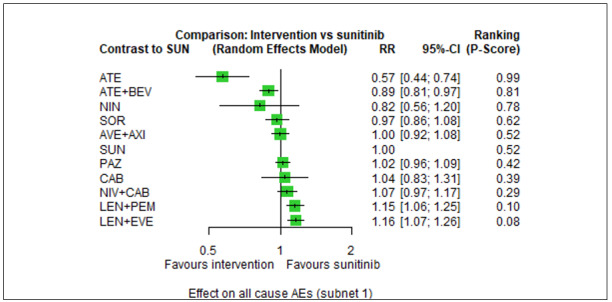
Forest plot for all‐cause AEs (grades 3‐4; all risk groups combined). Subnet 1. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
In sub‐network 1, we did not observe between‐study heterogeneity (Qtotal = 0.31, df = 2, P = 0.85; Qwithin = 0.0, df = 0, P = n.a.; Qbetween = 0.31, df=2, P = 0.85; I² = 0.0%, Tau² = 0.0). The combination LEN+PEM probably increases slightly the risk for AEs (RR 1.15, 95% CI 1.06 to 1.25, moderate certainty, P‐score: 0.10) when compared to SUN alone (P‐score: 0.52). We found that there probably is little or no difference in the risk for AEs between AVE+AXI (RR 1.00, 95% CI 0.92 to 1.08, moderate certainty, P‐score: 0.52) and NIV+CAB (RR 1.07, 95% CI 0.97 to 1.17, moderate certainty, P‐score: 0.29), when compared to SUN alone, respectively. We are uncertain whether CAB alone reduces or increases the risk for AEs (HR 1.04, 95% CI 0.83 to 1.31, very low certainty, P‐score: 0.39), when compared to SUN alone. There is probably little or no difference in the risk for AEs between PAZ alone (RR 1.02, 95% CI 0.96 to 1.09, moderate certainty, P‐score: 0.42) and SUN alone. Comparison data were not available for PEM+AXI and NIV+IPI. In the ranking of treatments, ATE alone was the best treatment option (P‐score: 0.99), and LEN+EVE was the worst option(P‐score: 0.08) (Figure 34).
As shown in Figure 33, there are closed loops in this network. The forest plot of splitting direct and indirect evidence is depicted in Figure 78 in Appendix 15. There was no significant difference between direct and indirect estimates (P = 0.7148) for ATE alone versus SUN alone and ATE alone versus ATE+BEV; P = 0.6702 for PAZ alone versus SOR alone, PAZ alone versus SUN alone and SOR alone versus SUN alone (data not shown)). The net heat plot showed no signs for inconsistency (Figure 79 in Appendix 15).
Analyses of individual AEs
Hand‐food syndrome
Hand‐food syndrome was assessed in 10 trials (NCT00720941; NCT00920816; NCT01024920; NCT01030783; NCT01108445; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177) (nine two‐arm trials, one three‐arm trial), for a total of 5029 participants. One two‐arm trial reported zero events and was therefore excluded from the analyses (NCT01024920). Hence, analyses were conducted with 4933 participants. Figure 80 in Appendix 15 outlines the available direct evidence (11 pairwise comparisons). The network was fully connected (Figure 35). Results for all network comparisons, including the ranking of treatments, is shown in Figure 36. Heterogeneity statistics could not be calculated because each pairwise comparison was reported by a single trial only.
35.
Network graph for the AE hand‐foot‐syndrome (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
36.
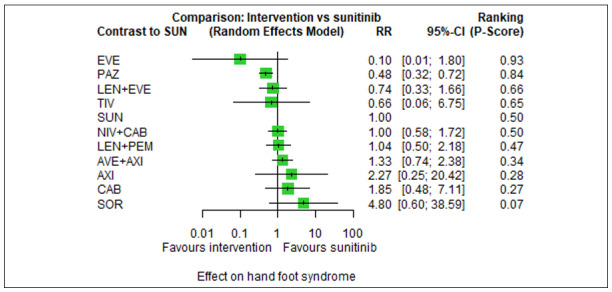
Forest plot for the AE hand‐foot‐syndrome (all risk groups combined). Reference treatment in the network: sunitinib (SUN). Treatments are ordered by P‐score (descending).
The evidence suggests a substantially smaller risk with PAZ alone (RR 0.48, 95% CI 0.32 to 0.72, P‐score: 0.84) when compared to SUN alone (P‐score: 0.50). The combinations NIV+CAB (RR 1.00, 95% CI 0.58 to 1.72, P‐score: 0.50), LEN+PEM (RR 1.04, 95% CI 0.50 to 2.18, P‐score: 0.47), AVE+AXI (RR 1.33, 95% CI 0.74 to 2.38, P‐score: 0.34) and CAB alone (RR 1.85, 95% CI 0.48 to 7.11, P‐score: 0.27) reduce or increase the risk for hand‐foot syndrome, when compared to SUN alone, respectively. Comparison data were not available for PEM+AXI and NIV+IPI. In the ranking of treatments, EVE alone was the best treatment option (P‐score: 0.93) and SOR alone was the worst treatment option (P‐score: 0.07) (Figure 36).
Fatigue
Fatigue was assessed in 14 trials (NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00920816; NCT01024920; NCT01030783; NCT01108445; NCT01274273; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177) (13 two‐arm trials, one three‐arm trial), for a total of 6502 participants. Figure 81 in Appendix 15 outlines the available direct evidence (16 pairwise comparisons). The network was not fully connected and consisted of three sub‐networks (Figure 37). We conducted analyses for subnets 1 and 2; subnetwork 3 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, is shown in Figure 38.
37.
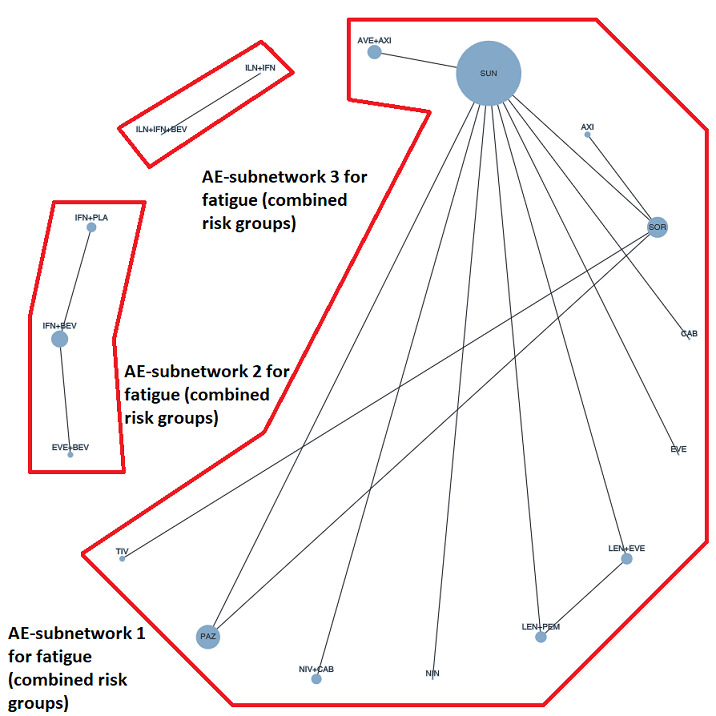
Network graph for the AE fatigue (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
38.
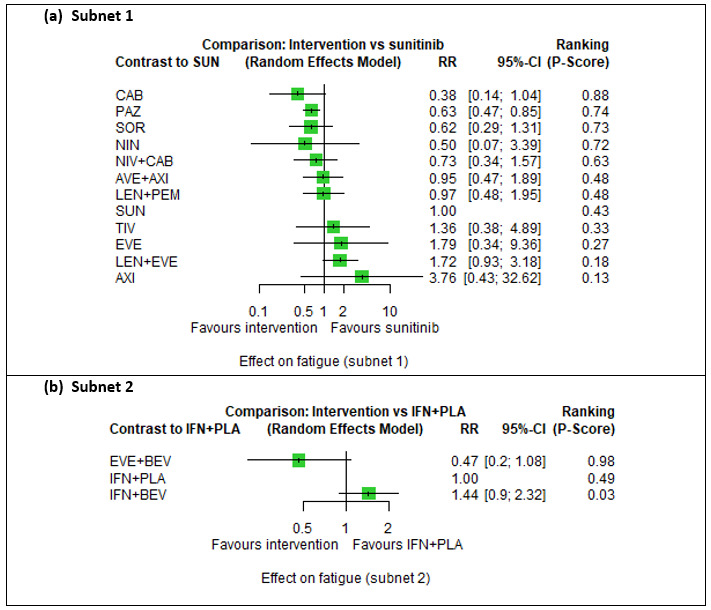
Forest plot for the AE fatigue (all risk groups combined). a) Subnetwork 1. Reference treatment: sunitinib (SUN). b) Subnetwork 2. Reference treatment: interferon‐alpha + placebo (IFN+PLA). Treatments are ordered by P‐score (descending).
In sub‐network 1, we did not observe between‐study heterogeneity (Qtotal=0.0, df = 1, P = 0.97; Qwithin=0.0, df = 0, P = n.a.; Qbetween=0.0, df=1, P = 0.97; I² = 0.0%, Tau² = 0.0). Cabozantinib alone reduces or increases the risk for fatigue (RR 0.38, 95% CI 0.14 to 1.04, P‐score: 0.88) when compared to SUN alone (P‐score: 0.43). The evidence suggests a substantially smaller risk with PAZ alone (RR 0.63, 95% CI 0.47 to 0.85, P‐score: 0.74) compared to SUN alone. The combinations NIV+CAB (RR 0.73, 95% CI 0.34 to 1.57, P‐score: 0.63), AVE+AXI (RR 0.95, 95% CI 0.47 to 1.89, P‐score: 0.48), LEN+PEM (RR 0.97, 95% CI 0.48 to 1.95, P‐score: 0.48) decrease or increase the risk for fatigue, when compared to SUN alone, respectively. Comparison data were not available for PEM+AXI and NIV+IPI. In the ranking of treatments, CAB alone was the best treatment option (P‐score: 0.88) and AXI alone was the worst treatment option (P‐score: 0.13) (Figure 38).
In sub‐network 2, heterogeneity statistics could not be calculated because each pairwise comparison was reported by a single trial only. Here, IFN+PLA was the comparator treatment, and the results suggested a lower risk for fatigue with EVE+BEV compared to IFN+PLA (RR 0.47, 95% CI 0.2 to 1.08). In the ranking of treatments, EVE+BEV was the best treatment option (P‐score: 0.98) and IFN+BEV was the worst option (P‐score: 0.03).
Diarrhoea
Diarrhoea was assessed in 16 trials (NCT00081614; NCT00719264; NCT00720941; NCT00732914; NCT00738530; NCT00920816; NCT01024920; NCT01030783; NCT01108445; NCT01274273; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177; NCT00065468) (15 two‐arm trials, two three‐arm trials), for a total of 7222 participants. Figure 82 in Appendix 15 outlines the available direct evidence (20 pairwise comparisons). The network was not fully connected and consisted of five sub‐networks (Figure 39). We conducted analyses for sub‐networks 1 and 2; sub‐networks 3, 4 and 5 contained only one trial each, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, is shown in Figure 40.
39.
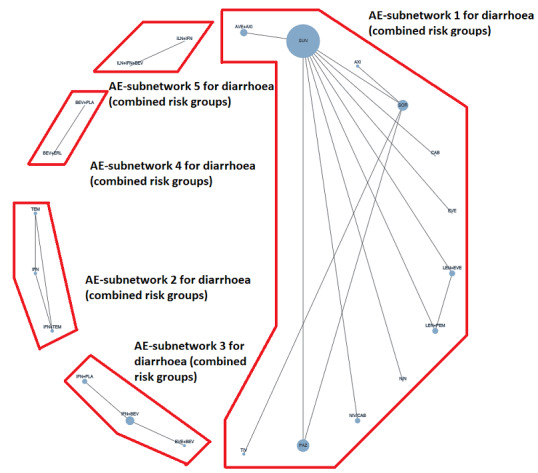
Network graph for the AE diarrhoea (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
40.
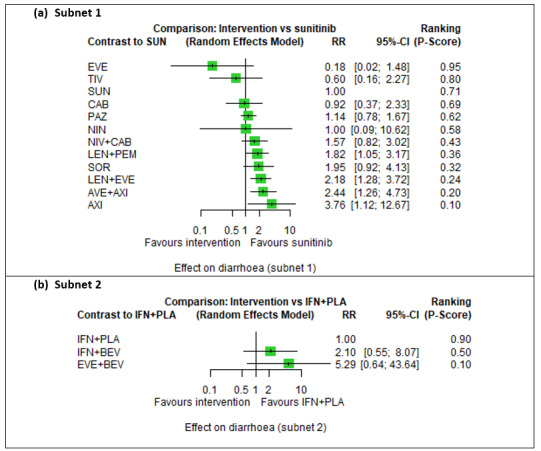
Forest plot for the AE diarrhoea (all risk groups combined). a) Subnetwork 1. Reference treatment: sunitinib (SUN); b) Subnetwork 2. Reference treatment: interferon‐alpha + placebo (IFN+PLA). Treatments are ordered by P‐score (descending).
In sub‐network 1, we did not observe between‐study heterogeneity (Qtotal = 0.05, df=1, P = 0.83; Qwithin = 0.0, df = 0, P = n.a. ; Qbetween = 0.05, df = 1, P = 0.83; I² = 0.0%, Tau² = 0.0). Cabozantinib alone (RR 0.92, 95% CI 0.37 to 2.33, P‐score: 0.69), PAZ alone (RR 1.14, 95% CI 0.78 to 1.67, P‐score: 0.62) and NIV+CAB (RR 1.57, 95% CI 0.82 to 3.02, P‐score: 0.43) reduce or increase the risk for diarrhoea, when compared to SUN alone (P‐score: 0.71), respectively. The evidence suggests that LEN+PEM (RR 1.82, 95% CI 1.05 to 3.17, P‐score: 0.36) and AVE+AXI (RR 2.44, 95% CI 1.26 to 4.73, P‐score: 0.20) substantially increase the risk for diarrhoea, when compared to SUN, respectively. Comparison data were not available for PEM+AXI and NIV+IPI. In the ranking of treatments, EVE alone was the best treatment option (P‐score: 0.95) and AXI alone was the worst treatment option (P‐score: 0.10) (Figure 40).
As shown in Figure 39, there are closed loops in the network. The forest plot of splitting direct and indirect evidence is depicted in Figure 83 in Appendix 15. There was no significant difference between direct and indirect estimates (P = 0.8272 (data not shown)). The net heat plot showed no signs for inconsistency (Figure 84 in Appendix 15).
In sub‐network 2, heterogeneity statistics could not be calculated because each pairwise comparison was reported by a single trial only. Here, IFN+PLA was the comparator treatment, and the ranking of treatments suggested that IFN+PLA was the best treatment option (P‐score: 0.90) and EVE+BEV the worst option (P‐score: 0.10).
Vomiting
Vomiting was assessed in 10 trials (NCT00720941; NCT00920816; NCT01024920; NCT01108445; NCT01274273; NCT01835158; NCT02684006; NCT02811861; NCT03141177; NCT00065468) (eight two‐arm trials, two three‐arm trials), for a total of 5035 participants. Figure 85 in Appendix 15 outlines the available direct evidence (14 pairwise comparisons). The network was not fully connected and consisted of four subnets (Figure 41). We conducted analyses for subnetwork 1; sub‐networks 2, 3 and 4 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, is shown in Figure 42.
41.
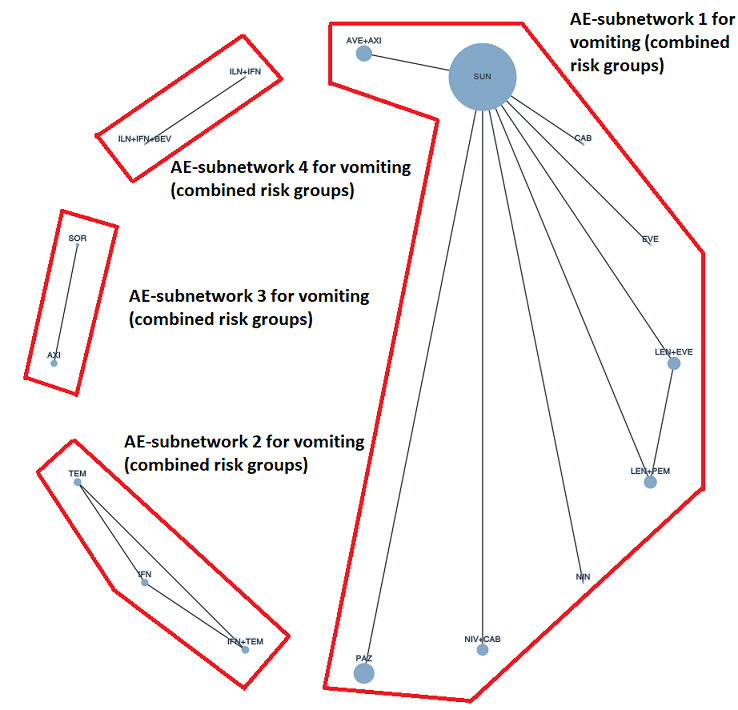
Network graph for the AE vomiting (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
42.
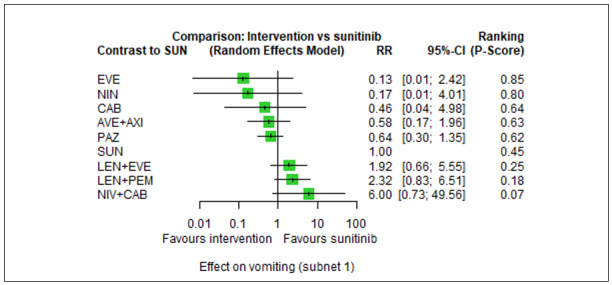
Forest plot for the AE vomiting (all risk groups combined). Subnetwork 1. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
In sub‐network 1, each comparison was reported by a single trial only, so no heterogeneity statistics could be calculated. We found that CAB alone (RR 0.46, 95% CI 0.04 to 4.98, P‐score: 0.64), AVE+AXI (RR 0.58, 95% CI 0.17 to 1.96, P‐score: 0.63), PAZ alone (RR 0.64, 95% CI 0.30 to 1.35, P‐score. 0.62), LEN+PEM (RR 2.32, 95% CI 0.83 to 6.51, P‐score: 0.18) and NIV+CAB (RR 6.00, 95% CI 0.73 to 49.56, P‐score: 0.07) decrease or increase the risk for vomiting, when compared to SUN alone (P‐score: 0.45), respectively. Comparison data were not available for PEM+AXI and NIV+IPI. In the ranking of treatments, EVE alone was the best treatment option (P‐score: 0.85) and NIV+CAB was the worst treatment option (P‐score: 0.07) (Figure 42).
Loss of appetite
Loss of appetite was assessed in 11 trials (NCT00719264; NCT00720941; NCT00732914; NCT00920816; NCT01024920; NCT01108445; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177) (10 two‐arm trials, one three‐arm trial), for a total of 5381 participants. Figure 86 in Appendix 15 outlines the available direct evidence (13 pairwise comparisons). However, one trial (NCT01024920) was excluded from analyses because zero events were reported. Hence, data were analysed for 5285 participants. The network was not fully connected and consisted of two subnets (Figure 43). We conducted analyses for subnetwork 1; subnetwork 2 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, is shown in Figure 44.
43.
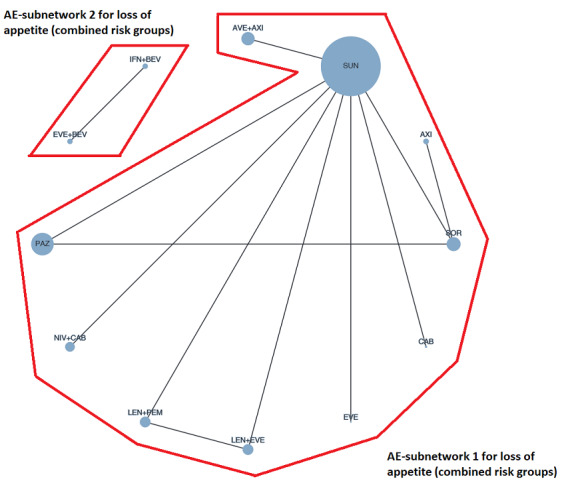
Network graph for the AE loss of appetite (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments. Line width: number of trials. Plot width: number of participants.
44.
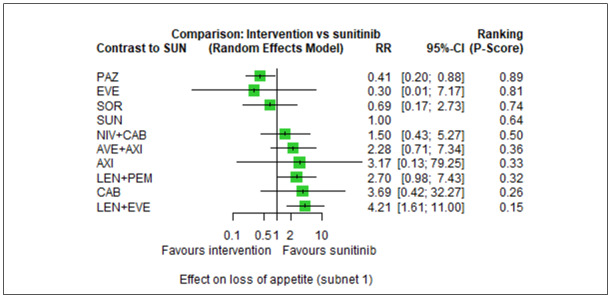
Forest plot for the AE loss of appetite (all risk groups combined). Subnetwork 1. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
In sub‐network 1, we did not observe between‐study heterogeneity (Qtotal = 0.44, df = 1, P = 0.51; Qwithin = 0.0, df = 0, P = n.a.; Qbetween = 0.44, df = 1, P = 0.51; I² = 0.0%, Tau² = 0.0). The evidence suggests a lower risk for loss of appetite with PAZ alone (RR 0.41, 95% CI 0.20 to 0.88) compared to SUN alone (P‐score: 0.64). We found that NIV+CAB (RR 1.50, 95% CI 0.43 to 5.27, P‐score: 0.50), AVE+AXI (RR 2.28, 95% CI 0.71 to 7.34, P‐score: 0.36), LEN+PEM (RR 2.70, 95% CI 0.98 to 7.43, P‐score: 0.32), and CAB alone (RR 3.69, 95% CI 0.42 to 32.27, P‐score: 0.26) reduce or increase the risk for loss of appetite, when compared to SUN alone, respectively. WComparison data were not available for PEM+AXI and NIV+IPI. In the ranking of treatments, PAZ alone was the best treatment option (P‐score: 0.89), whereas LEN+EVE was the worst treatment option (P‐score: 0.15) (Figure 44).
As shown in Figure 43, there are closed loops in the network. Figure 87 in Appendix 15 depicts the forest plot of splitting direct and indirect evidence. There was no significant difference between direct and indirect estimates (P = 0.5071 (data not shown)). The net heat plot showed negligible signs for inconsistency (Figure 88 in Appendix 15).
Weight loss
Weight loss was assessed in 12 trials (NCT00719264; NCT00720941; NCT00920816; NCT01024920; NCT01108445; NCT01274273; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177; NCT00065468) (10 two‐arm trials, two three‐arm trials), for a total of 5762 participants. Figure 89 in Appendix 15 outlines the available direct evidence (16 pairwise comparisons). However, one trial (NCT01108445) was excluded from analyses because zero events were reported. Hence, data were analysed for 5654 participants. The network was not fully connected and consisted of four sub‐networks (Figure 45). We conducted analyses for subnetwork 1; sub‐networks 2, 3 and 4 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, is shown in Figure 46.
45.
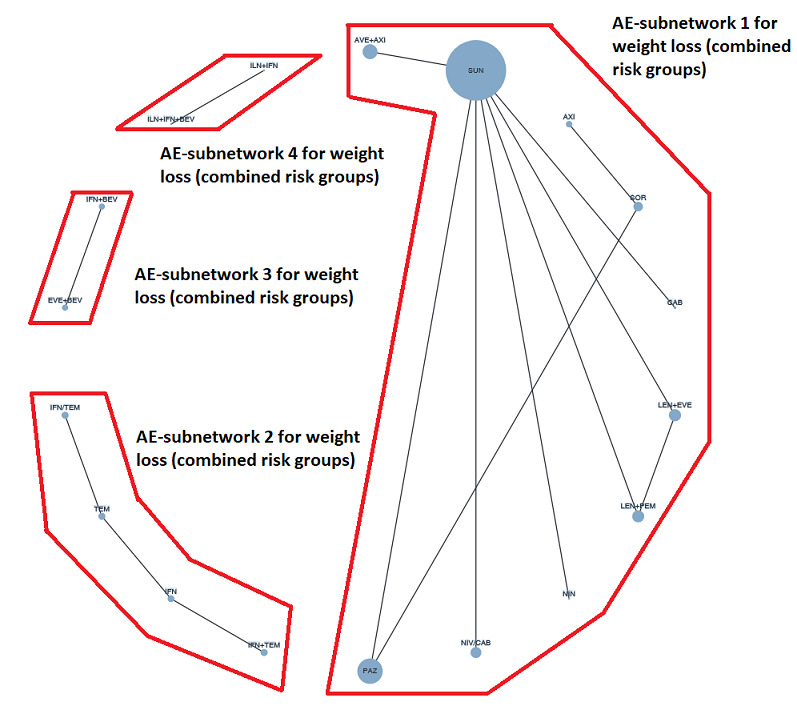
Network graph for the AE weight loss (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments.
46.
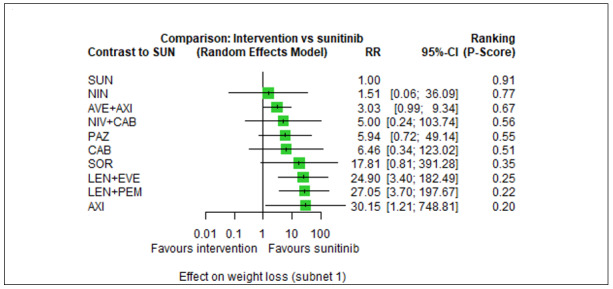
Forest plot for the AE weight loss (all risk groups combined). Subnetwork 1. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
In sub‐network 1, heterogeneity statistics could not be calculated because each comparison was reported by single trial only. The evidence showed that no treatment had a lower risk for weight loss compared to SUN alone. In the ranking of treatments, SUN alone was the best treatment option (P‐score: 0.91) and AXI alone was the worst treatment option (P‐score: 0.20) (Figure 46). The risk with SUN alone was substantially lower when compared to LEN+PEM (RR 0.04, 95% CI 0.01 to 0.27, P‐score: 0.22). We found that NIV+CAB (RR 5.00, 95% CI 0.24 to 103.74, P‐score: 0.56), AVE+AXI (RR 3.03, 95% CI 0.99 to 9.34, P‐score: 0.67), and CAB alone (RR 6.46, 95% CI 0.34 to 123.02, P‐score: 0.51) reduce or increase the risk for weight loss, when compared to SUN alone, respectively. Comparison data were not available for PEM+AXI and NIV+IPI.
Stomatitis
Stomatitis was assessed in 12 trials (NCT00719264; NCT00720941; NCT00732914; NCT01024920; NCT01108445; NCT01274273; NCT01613846; NCT01835158; NCT02684006; NCT02811861; NCT03141177; NCT00065468) (10 two‐arm trials, two three‐arm trials), for a total of 5830 participants. Figure 90 in Appendix 15 outlines the available direct evidence (16 pairwise comparisons). However, one trial (NCT01274273) was excluded from analyses because zero events were reported. Hence, data were analysed for 5712 participants. The network was not fully connected and consisted of three sub‐networks (Figure 47). We conducted analyses for subnetwork 1; sub‐networks 2 and 3 contained only one trial, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, is shown in Figure 48.
47.
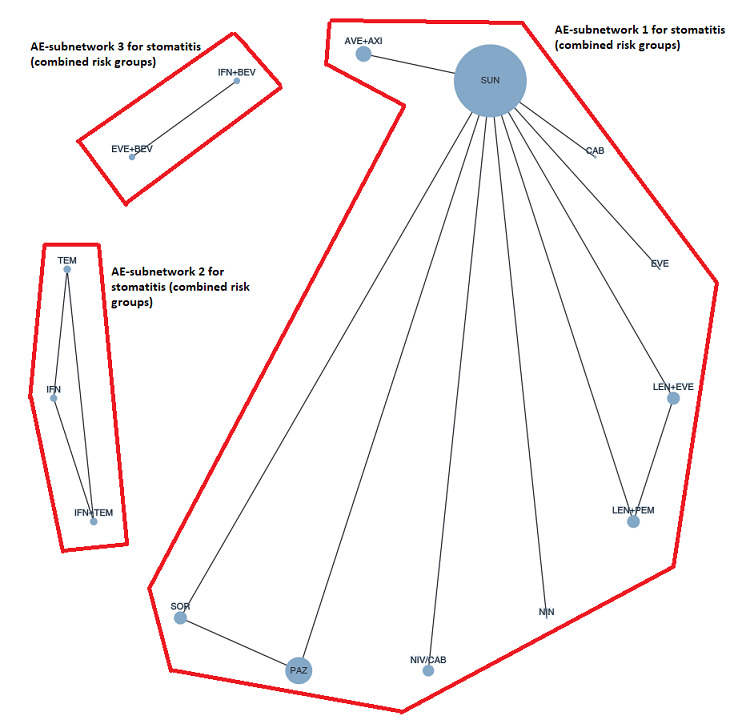
Network graph for the AE stomatitis (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments.
48.
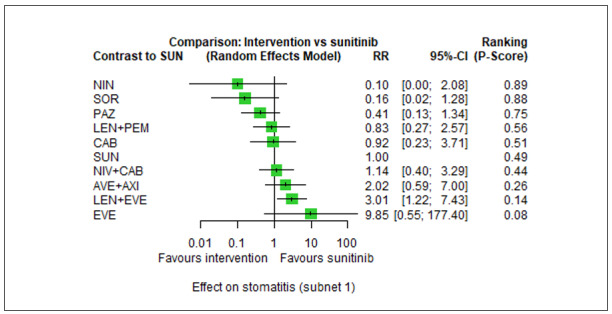
Forest plot for the AE stomatitis (all risk groups combined). Subnetwork 1. Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
In sub‐network 1, we did not observe important between‐study heterogeneity (Qtotal = 1.02, df = 1, P = 0.31; Qwithin = 0.0, df = 0, P = n.a.; Qbetween = 1.02, df = 1, P = 0.31; I² = 2%, Tau² = 0.0302). We found that LEN+PEM (RR 0.83, 95% CI 0.27 to 2.57, P‐score: 0.56), NIV+CAB (RR 1.14, 95% CI 0.40 to 3.29, P‐score: 0.44), AVE+AXI (RR 2.02, 95% CI 0.59 to 7.00, P‐score: 0.26), PAZ alone (RR 0.41, 95% CI 0.13 to 1.34, P‐score: 0.75) and CAB alone (RR 0.92, 95% CI 0.23 to 3.71, P‐score: 0.51) reduce or increase the risk for stomatitis, when compared to SUN alone (P‐score: 0.49), respectively. Comparison data were not available for PEM+AXI and NIV+IPI. In the ranking of treatments, NIN alone (P‐score: 0.89) was the best treatment option, and EVE alone was the worst treatment option (P‐score: 0.08) (Figure 48).
As shown in Figure 47, there are closed loops in the network. Figure 91 in Appendix 15 depicts the forest plot of splitting direct and indirect evidence. There was no significant difference between direct and indirect estimates (P = 0.3173 (data not shown)). The net heat plot showed negligible signs for inconsistency (Figure 92 in Appendix 15).
Mucosal inflammation
Mucosal inflammation was assessed in four two‐arm trials (NCT00720941; NCT01108445; NCT02684006; NCT03141177), for a total of 2723 participants. Figure 93 in Appendix 15 outlines the available direct evidence (four pairwise comparisons). The network was fully connected (Figure 49); results for all network comparisons, including the ranking of treatments, is shown in Figure 50. Because each comparison in this network was reported by a single trial only, heterogeneity statistics could not be calculated.
49.
Network graph for the AE mucosal inflammation (all risk groups combined). Any two treatments are connected by a line when there is at least one trial comparing the two treatments.
50.
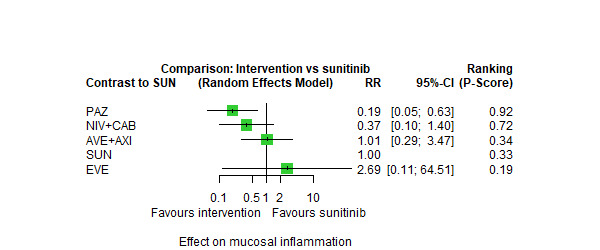
Forest plot for the AE mucosal inflammation (all risk groups combined). Reference treatment: sunitinib (SUN). Treatments are ordered by P‐score (descending).
The evidence suggests a substantially lower risk for mucosal inflammation with PAZ alone (RR 0.19, 95% CI 0.05 to 0.63, P‐score: 0.92) compared to SUN alone (P‐score: 0.33). The combinations NIV+CAB (RR 0.37, 95% CI 0.10 to 1.40, P‐score: 0.72) and AVE+AXI (RR 1.01, 95% CI 0.29 to 3.47, P‐score: 0.34) reduce or increase the risk for mucosal inflammation, when compared to SUN alone, respectively. Comparison data were not available for PEM+AXI, NIV+IPI, LEN+PEM, and CAB alone. In the ranking of treatments, PAZ alone was the best treatment option (P‐score: 0.92) and EVE alone was the worst option (P‐score: 0.19) (Figure 50).
Insomnia
Insomnia was assessed in two two‐arm trials (NCT00720941; NCT01108445), for a total of 1210 participants. In NCT00720941, participants in the experimental arm received PAZ alone and in NCT01108445, participants in the experimental arm received EVE alone. In both trials, SUN alone was the comparator treatment. We were not able to analyse results as the trials reported null events (i.e. no participant in any arm had an event).
Depression
Depression was assessed in two two‐arm trials (NCT00738530; NCT01274273), for a total of 759 participants. We were not able to analyse results as the two trials were not connected in the network. In NCT00738530, IFN+BEV (experimental arm, N=337) versus IFN+PLA (control arm, N = 304) were compared. Ten participants in the experimental arm and four participants in the control arm had at least one grade 3 or 4 event. In NCT01274273, ILN+IFN+BEV (experimental arm, N = 59) versus ILN+IFN (control arm, N = 59) were compared. It was reported that no participant in the experimental arm had an event, whereas one participant in the control arm had at least one event of grade 3 or 4.
Number of participants who discontinued study treatment due to an adverse effect (AE)
The number of participants who discontinued study treatment due to an AE was assessed for 30 trials (NCT00920816; NCT00979966; NCT01024920; NCT01108445; NCT01613846; Jonasch 2010; NCT00065468; NCT00072046; NCT00081614; NCT00098657/NCT00083889; NCT00117637; NCT00609401; NCT00619268; NCT00631371; NCT00719264; NCT00720941; NCT00732914; NCT00903175; NCT01030783; NCT01274273; NCT01392183; NCT01481870; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177) (27 two‐arm trials, three three‐arm trials), for a total of 13,110 participants. Figure 94 in Appendix 15 outlines the available direct evidence (36 comparisons). The network was not fully connected and consisted of three sub‐networks (Figure 51). We conducted network meta‐analysis for subnetwork 1. Sub‐networks 2 and 3 contained only one trial each, so no further analyses were conducted. Results for all network comparisons, including the ranking of treatments, are shown in Table 21 and Figure 52.
51.
Network graph for the outcome Number of participants who discontinued treatment due to an AE (combined risk groups). Any two treatments are connected by a line when there is at least one trial comparing the two treatments.
18. NMA results for Number of participants who discontinued study treatment due to an AE (all risk groups combined).
| Results of network meta‐analysis for the outcome Number of participants who discontinued treatment due to an AE. Treatments are ordered by P‐Score (descending). Only subnetworks with >1 designs. Upper triangle: direct estimate. Lower triangle: network estimate. Subnet 1 No. of studies: 28. No. of pairwise comparisons: 34. No. of treatments: 24. No. of designs: 25. Qtotal= 18.14, df = 8, P = 0.020; Qwithin= 3.27, df = 3, P = 0.35; Qbetween= 14.87, df = 5, P = 0.011; I²=55.9%, Tau² = 0.1029 Treatment Effects + 95%‐CIs (Risk ratios, random‐effects model): | |||||||||||||||||||||||
| TIV | . | . | . | . | . | 0.51 [0.24, 1.05] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.89 [0.28, 2.80] | AVE+AXI | . | . | . | 0.57 [0.27, 1.20] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.67 [0.16, 2.79] | 0.75 [0.19, 2.92] | ATE | . | . | 0.76 [0.24, 2.36] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.53 [0.22, 1.32] | 0.60 [0.24, 1.49] | 0.80 [0.23, 2.80] | PAZ | . | 1.24 [0.64, 2.43] | 0.72 [0.35, 1.47] | . | . | . | 0.69 [0.11, 4.31] | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.55 [0.16, 1.88] | 0.61 [0.19, 1.94] | 0.82 [0.19, 3.45] | 1.02 [0.37, 2.85] | CAB | 0.92 [0.38, 2.22] | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.50 [0.21, 1.20] | 0.57 [0.27, 1.20] | 0.76 [0.24, 2.36] | 0.95 [0.56, 1.60] | 0.92 [0.38, 2.22] | SUN | 1.42 [0.80, 2.54] | 1.02 [0.55, 1.87] | 1.00 [0.28, 3.62] | 0.89 [0.45, 1.79] | 0.40 [0.02, 9.32] | 0.86 [0.42, 1.74] | 0.78 [0.39, 1.56] | . | . | 0.71 [0.34, 1.47] | 0.68 [0.33, 1.41] | 0.51 [0.26, 1.01] | 0.29 [0.09, 0.98] | . | 0.41 [0.21, 0.82] | . | . | 0.23 [0.07, 0.72] |
| 0.51 [0.24, 1.05] | 0.57 [0.24, 1.38] | 0.76 [0.22, 2.61] | 0.95 [0.56, 1.63] | 0.93 [0.34, 2.53] | 1.01 [0.63, 1.62] | SOR | . | . | . | . | . | . | 0.72 [0.20, 2.58] | 0.71 [0.28, 1.78] | . | . | . | 0.75 [0.28, 1.96] | . | . | . | . | . |
| 0.51 [0.18, 1.48] | 0.58 [0.22, 1.51] | 0.77 [0.21, 2.80] | 0.96 [0.43, 2.15] | 0.94 [0.32, 2.74] | 1.02 [0.55, 1.87] | 1.01 [0.47, 2.18] | EVE | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.50 [0.11, 2.38] | 0.57 [0.13, 2.51] | 0.76 [0.14, 4.21] | 0.95 [0.24, 3.80] | 0.92 [0.19, 4.39] | 1.00 [0.28, 3.62] | 0.99 [0.25, 3.90] | 0.98 [0.24, 4.08] | NIN | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.45 [0.15, 1.37] | 0.51 [0.18, 1.40] | 0.67 [0.18, 2.56] | 0.84 [0.35, 2.02] | 0.82 [0.27, 2.53] | 0.89 [0.45, 1.79] | 0.89 [0.38, 2.05] | 0.88 [0.35, 2.21] | 0.89 [0.21, 3.85] | PEM+AXI | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.43 [0.13, 1.45] | 0.49 [0.14, 1.65] | 0.65 [0.15, 2.88] | 0.81 [0.30, 2.19] | 0.79 [0.21, 2.92] | 0.86 [0.33, 2.25] | 0.85 [0.32, 2.23] | 0.84 [0.27, 2.64] | 0.86 [0.17, 4.29] | 0.96 [0.29, 3.16] | TEM | . | . | . | . | . | . | . | 0.51 [0.22, 1.22] | . | . | . | 0.36 [0.15, 0.83] | . |
| 0.43 [0.14, 1.33] | 0.48 [0.17, 1.36] | 0.65 [0.17, 2.47] | 0.81 [0.34, 1.96] | 0.79 [0.26, 2.45] | 0.86 [0.42, 1.74] | 0.85 [0.36, 1.99] | 0.84 [0.33, 2.15] | 0.86 [0.20, 3.73] | 0.96 [0.36, 2.59] | 1.00 [0.30, 3.31] | NIV+CAB | . | . | . | . | . | . | . | . | . | . | . | . |
| 0.39 [0.13, 1.19] | 0.44 [0.16, 1.22] | 0.59 [0.16, 2.23] | 0.74 [0.31, 1.76] | 0.72 [0.24, 2.20] | 0.78 [0.39, 1.56] | 0.77 [0.34, 1.78] | 0.77 [0.31, 1.93] | 0.78 [0.18, 3.36] | 0.88 [0.33, 2.33] | 0.91 [0.28, 2.98] | 0.91 [0.34, 2.45] | IFN | . | . | . | . | . | . | . | . | . | . | . |
| 0.36 [0.08, 1.59] | 0.41 [0.09, 1.94] | 0.55 [0.09, 3.23] | 0.68 [0.17, 2.74] | 0.67 [0.13, 3.39] | 0.72 [0.18, 2.83] | 0.72 [0.20, 2.58] | 0.71 [0.16, 3.17] | 0.72 [0.11, 4.72] | 0.81 [0.17, 3.75] | 0.84 [0.17, 4.18] | 0.84 [0.18, 3.93] | 0.92 [0.20, 4.27] | AXI | . | . | . | . | . | . | . | . | . | . |
| 0.36 [0.11, 1.17] | 0.41 [0.11, 1.45] | 0.54 [0.12, 2.52] | 0.68 [0.24, 1.96] | 0.66 [0.17, 2.57] | 0.72 [0.26, 2.01] | 0.71 [0.28, 1.78] | 0.71 [0.21, 2.34] | 0.72 [0.14, 3.74] | 0.80 [0.23, 2.79] | 0.84 [0.22, 3.16] | 0.84 [0.24, 2.93] | 0.92 [0.27, 3.18] | 0.99 [0.21, 4.81] | SOR+IFN | . | . | . | . | . | . | . | . | . |
| 0.36 [0.11, 1.11] | 0.40 [0.14, 1.14] | 0.53 [0.14, 2.06] | 0.67 [0.27, 1.64] | 0.65 [0.21, 2.05] | 0.71 [0.34, 1.47] | 0.70 [0.29, 1.67] | 0.69 [0.27, 1.80] | 0.71 [0.16, 3.10] | 0.79 [0.29, 2.17] | 0.82 [0.25, 2.76] | 0.83 [0.30, 2.28] | 0.91 [0.33, 2.47] | 0.98 [0.21, 4.61] | 0.98 [0.28, 3.48] | LEN+PEM | 0.96 [0.47, 1.95] | . | . | . | . | . | . | . |
| 0.34 [0.11, 1.06] | 0.38 [0.14, 1.09] | 0.51 [0.13, 1.98] | 0.64 [0.26, 1.57] | 0.63 [0.20, 1.96] | 0.68 [0.33, 1.41] | 0.67 [0.28, 1.60] | 0.67 [0.26, 1.72] | 0.68 [0.16, 2.98] | 0.76 [0.28, 2.08] | 0.79 [0.24, 2.65] | 0.79 [0.29, 2.19] | 0.87 [0.32, 2.37] | 0.94 [0.20, 4.42] | 0.95 [0.27, 3.34] | 0.96 [0.47, 1.95] | LEN+EVE | . | . | . | . | . | . | . |
| 0.26 [0.09, 0.78] | 0.29 [0.11, 0.79] | 0.39 [0.10, 1.45] | 0.48 [0.20, 1.14] | 0.47 [0.16, 1.43] | 0.51 [0.26, 1.01] | 0.51 [0.22, 1.16] | 0.50 [0.20, 1.25] | 0.51 [0.12, 2.19] | 0.57 [0.22, 1.51] | 0.60 [0.18, 1.94] | 0.60 [0.22, 1.59] | 0.65 [0.25, 1.72] | 0.71 [0.15, 3.26] | 0.71 [0.21, 2.45] | 0.72 [0.27, 1.96] | 0.75 [0.28, 2.03] | NIV+IPI | . | . | . | . | . | . |
| 0.24 [0.09, 0.68] | 0.27 [0.10, 0.78] | 0.36 [0.09, 1.42] | 0.46 [0.20, 1.03] | 0.45 [0.14, 1.41] | 0.48 [0.23, 1.01] | 0.48 [0.23, 0.99] | 0.47 [0.18, 1.24] | 0.48 [0.11, 2.13] | 0.54 [0.20, 1.49] | 0.56 [0.26, 1.23] | 0.56 [0.20, 1.57] | 0.62 [0.23, 1.70] | 0.67 [0.15, 2.92] | 0.67 [0.21, 2.16] | 0.68 [0.24, 1.93] | 0.71 [0.25, 2.01] | 0.95 [0.35, 2.58] | IFN | . | . | 0.81 [0.41, 1.62] | 0.70 [0.33, 1.50] | 0.77 [0.34, 1.74] |
| 0.23 [0.06, 0.88] | 0.25 [0.06, 1.00] | 0.34 [0.07, 1.70] | 0.42 [0.13, 1.40] | 0.41 [0.10, 1.75] | 0.45 [0.14, 1.41] | 0.44 [0.14, 1.39] | 0.44 [0.12, 1.61] | 0.45 [0.08, 2.51] | 0.50 [0.13, 1.91] | 0.52 [0.16, 1.74] | 0.52 [0.14, 2.01] | 0.57 [0.15, 2.18] | 0.62 [0.11, 3.46] | 0.62 [0.14, 2.70] | 0.63 [0.16, 2.46] | 0.66 [0.17, 2.56] | 0.88 [0.23, 3.32] | 0.93 [0.37, 2.34] | EVE+BEV | . | 0.87 [0.42, 1.80] | . | . |
| 0.21 [0.07, 0.63] | 0.23 [0.08, 0.64] | 0.31 [0.08, 1.18] | 0.39 [0.16, 0.93] | 0.38 [0.12, 1.16] | 0.41 [0.21, 0.82] | 0.41 [0.18, 0.94] | 0.40 [0.16, 1.02] | 0.41 [0.10, 1.77] | 0.46 [0.17, 1.23] | 0.48 [0.15, 1.57] | 0.48 [0.18, 1.29] | 0.53 [0.20, 1.40] | 0.57 [0.12, 2.63] | 0.57 [0.16, 1.98] | 0.58 [0.21, 1.59] | 0.60 [0.22, 1.65] | 0.80 [0.30, 2.12] | 0.85 [0.31, 2.34] | 0.92 [0.24, 3.50] | ATE+BEV | . | . | . |
| 0.20 [0.06, 0.62] | 0.22 [0.07, 0.70] | 0.30 [0.07, 1.25] | 0.37 [0.14, 0.96] | 0.36 [0.10, 1.26] | 0.39 [0.16, 0.95] | 0.39 [0.16, 0.94] | 0.38 [0.13, 1.12] | 0.39 [0.08, 1.86] | 0.44 [0.14, 1.35] | 0.46 [0.17, 1.19] | 0.46 [0.15, 1.42] | 0.50 [0.16, 1.53] | 0.54 [0.11, 2.57] | 0.54 [0.15, 1.94] | 0.55 [0.18, 1.74] | 0.58 [0.18, 1.81] | 0.76 [0.25, 2.33] | 0.81 [0.46, 1.44] | 0.87 [0.42, 1.80] | 0.95 [0.31, 2.93] | IFN+BEV | . | 0.84 [0.42, 1.68] |
| 0.16 [0.05, 0.56] | 0.18 [0.05, 0.65] | 0.25 [0.05, 1.12] | 0.31 [0.11, 0.88] | 0.30 [0.08, 1.14] | 0.33 [0.12, 0.89] | 0.32 [0.12, 0.87] | 0.32 [0.10, 1.04] | 0.33 [0.06, 1.67] | 0.36 [0.11, 1.24] | 0.38 [0.17, 0.85] | 0.38 [0.11, 1.30] | 0.42 [0.12, 1.41] | 0.45 [0.09, 2.29] | 0.45 [0.12, 1.76] | 0.46 [0.13, 1.59] | 0.48 [0.14, 1.66] | 0.64 [0.19, 2.14] | 0.67 [0.32, 1.42] | 0.73 [0.22, 2.38] | 0.79 [0.23, 2.69] | 0.83 [0.33, 2.13] | IFN+TEM | . |
| 0.16 [0.05, 0.51] | 0.19 [0.06, 0.58] | 0.25 [0.06, 1.02] | 0.31 [0.12, 0.78] | 0.30 [0.09, 1.03] | 0.33 [0.14, 0.77] | 0.32 [0.14, 0.77] | 0.32 [0.11, 0.92] | 0.33 [0.07, 1.53] | 0.37 [0.12, 1.10] | 0.38 [0.15, 1.00] | 0.38 [0.13, 1.16] | 0.42 [0.14, 1.25] | 0.45 [0.10, 2.12] | 0.46 [0.13, 1.60] | 0.46 [0.15, 1.42] | 0.48 [0.16, 1.48] | 0.64 [0.22, 1.90] | 0.68 [0.37, 1.24] | 0.73 [0.29, 1.85] | 0.80 [0.27, 2.39] | 0.84 [0.47, 1.49] | 1.01 [0.39, 2.61] | TEM+BEV |
52.
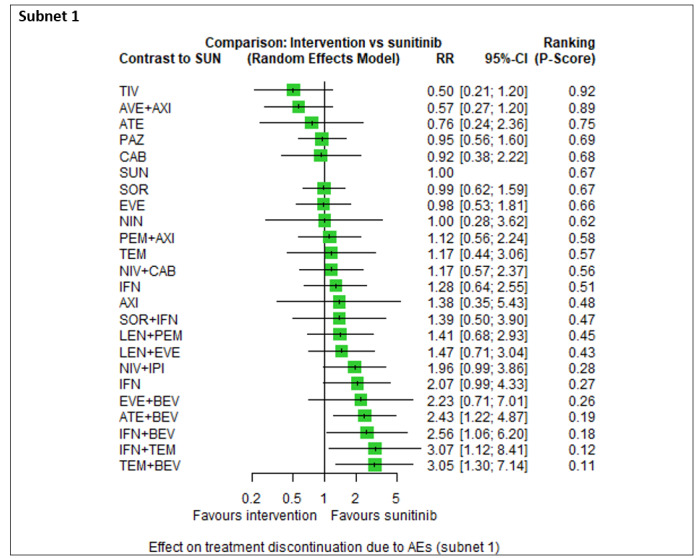
Forest plot for the outcome Number of participants who discontinued treatment due to an AE (combined risk groups). Any two treatments are connected by a line when there is at least one trial comparing the two treatments.
For sub‐network 1, we observed moderate heterogeneity (Qtotal = 18.14, df = 8, P = 0.020; Qwithin = 3.27, df = 3, P = 0.35; Qbetween = 14.87, df = 5, P = 0.011; I² = 55.9%, Tau² = 0.1029). The only closed loops in this subnet contained of multi‐arm trials, so inconsistency could not be checked. The evidence suggests that AVE+AXI (P‐score: 0.89), PAZ alone (P‐score: 0.69), CAB alone (P‐score: 0.68), PEM+AXI (P‐score: 0.58), NIV+CAB (P‐score: 0.56), LEN+PEM (P‐score: 0.45), and NIV+IPI (P‐score: 0.28) decrease or increase the risk for study discontinuation due to an AE, when compared to SUN alone, respectively. For this outcome, the best treatment option was TIV alone (P‐score: 0.92) and the worst option was TEM+BEV (P‐score: 0.11).
Time to initiation of the first subsequent anticancer therapy
None of the included trials reported the outcome TFST as a time‐to‐event outcome. However, 19 trials (NCT00081614; NCT00098657/NCT00083889; NCT00609401; NCT00619268; NCT00719264; NCT00732914; NCT00738530; NCT00920816; NCT01024920; NCT01108445; NCT01274273; NCT01835158; NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177) reported the number of participants who received some subsequent anticancer therapy after discontinuing study treatment. In one trial (NCT00072046) there were discrepancies in the numbers reported in texts and tables, so we refrained from reporting the results. However, reporting of the outcome was very heterogenous: different definitions were provided; different types of therapy reported (subsequent therapy could include only systemic therapy or systemic therapy, radiotherapy or other); participants may have received more than one subsequent therapy. Furthermore, the timing of reporting was not reported in the trials. Hence, we refrained from pooling data and reported results narratively for all available interventions and comparisons in Table 22.
19. Results for TFST (all risk groups combined, narratively reported).
| Trial | Definition of subsequent therapy | Participants | Intervention (N randomised) | n with subsequent anticancer treatment | Comparator (N randomised) | n with subsequent anticancer treatment |
| Comparisons including SUN (in no particular order) | ||||||
| NCT00098657/NCT00083889 | "any poststudy cancer treatment for patients who discontinued from the trial" | all risk groups (according to MSKCC) | SUN (N=375) | n=182 | IFN (N=375) | n=213 |
| NCT00619268 comparison 1 | "second‐line therapy after study treatment failure" | all risk groups (according to MSKCC) | TEM+BEV (N=88) | n=61 | SUN (N=42) | n=20 |
| NCT00619268 comparison 2 | "second‐line therapy after study treatment failure" | all risk groups (according to MSKCC) | IFN+BEV (N=41) | n=27 | SUN (N=42) | n=20 |
| NCT01108445 | "subsequent therapy after progression" | all risk groups (according to MSKCC) | EVE (N=57) | n=33 | SUN (N=51) | n=36 |
| NCT01835158 | "subsequent anticancer therapy" | intermediate and poor risk groups (according to IMDC) | CAB (N=79) | n=51 | SUN (N=72) | n=50 |
| NCT02231749 | "any subsequent therapy" | all risk groups (according to IMDC) | NIV+IPI (N=550) | n=330 | SUN (N=546) | n=382 |
| NCT01984242 comparison 1** | "subsequent therapy for patients who progressed" | all risk groups (according to MSKCC) | ATE (N=103) | n=62 | SUN (N=101) | n=79 |
| NCT02420821 | "subsequent systemic treatment" | all risk groups (according to MSKCC) | ATE+BEV (N=454) | n=193 | SUN (N=461) | n=238 |
| NCT02684006 | "subsequent anticancer therapy" | all risk groups (according to IMDC) | AVE+AXI (N=442) | n=138 | SUN (N=444) | n= 227 |
| NCT02853331 | "subsequent anticancer therapy" | all risk groups (according to IMDC) | PEM+AXI (N=432) | n=204 | SUN (N=429) | n=281 |
| NCT00732914 | "subseuqent therapy for patients who discontinued the study after first‐line therapy" (cross over trial) | all risk groups (according to MSKCC) | SOR (N=74)*** | n=13 | SUN (N=100)*** | n=24 |
| NCT01024920 | "post‐study anticancer therapy" | all risk groups (according to MSKCC) | NIN (N=64) | n=25 | SUN (N=32) | n=8 |
| NCT03141177 | "subsequent anticancer therapy" | all risk groups (according to IMDC) | NIV+CAB (N=323) | n=61 | SUN (N=328) | n=108 |
| NCT02811861 comparison 1 | "any subsequent therapy" | all risk groups (according to MSKCC and IMDC) | LEN+PEM (N=355) | n=117 | SUN (N=357) | n=206 |
| NCT02811861 comparison 2 | "any subsequent therapy" | all risk groups (according to MSKCC and IMDC) | LEN+EVE (N=357) | n=167 | SUN (N=357) | n=206 |
| Other comparisons (in no particular order) | ||||||
| NCT00081614 | "second‐line therapy" | favourable and intermediate risk groups (according to MSKCC) | BEV+ERL (N=51) | n=7 | BEV+PLA (N=53) | n=17 |
| NCT00738530 | "post‐protocol therapy (not limited to second‐line therapy) of patients progressive disease or those in whom trial therapy was discontinued" | all risk groups (according to MSKCC) | IFN+BEV (N=327) | n=180 | IFN+PLA (N=322) | n=202 |
| NCT00609401 | "subsequent therapy (second‐line) at relapse" | all risk groups (according to MSKCC) | SOR+ILN (N=66) | n=49 | SOR (N=62) | n=48 |
| NCT00619268 comparison 3* | "second‐line therapy after study treatment failure" | all risk groups (according to MSKCC) | TEM+BEV (N=88) | n=61 | IFN+BEV (N=41) | n=27 |
| NCT01274273 | "subsequent systemic anticancer therapy following study treatment discontinuation" | favourable and intermediate risk groups according to MSKCC; all risk groups according to IMDC | ILN+IFN+BEV (N=59) | n=50 | ILN+IFN (N=59) | n=46 |
| NCT00719264 | "new cancer therapy after treatment discontinuation" | all risk groups (according to MSKCC) | EVE+BEV (N=182) | n=3 | IFN+BEV (N=183) | n=2 |
| NCT00920816 | "follow‐up systemic therapy after discontinuation of study treatment" | all risk groups (according to MSKCC) | AXI (N=192) | n=29 | SOR (N=96) | n=19 |
| NCT02811861 comparison 3* | "any subsequent therapy" | all risk groups (according to MSKCC and IMDC) | LEN+PEM (N=355) | n=117 | LEN+EVE (N=357) | n=167 |
*In the three‐arm trials, only two comparisons (arm A versus arm C (comparison 1) and arm B versus arm C (comparsion 2)) were reported, so we manually added a third comparison (arm A versus arm B (comparison 3)).
**Three‐arm trial, only data for this comparison (arm A versus arm C) was reported.
***Cross over trial. N represents number of participants who received only first‐line (first‐period) treatment.
As for the interventions that we chose for the Table 1, we found that PEM+AXI (RR 0.72, 95% CI 0.64 to 0.81, low certainty), AVE+AXI (RR 0.61, 95% CI 0.52 to 0.72, low certainty) and NIV+IPI (RR 0.86, 95% CI 0.79 to 0.94, low certainty) may reduce the risk for subsequent therapy, when compared to SUN alone, respectively. We are uncertain whether NIV+CAB (RR 0.57, 95% CI 0.44 to 0.75, very low certainty) and LEN+PEM (RR 0.57, 95% CI 0.48 to 0.68, very low certainty) reduce the risk for subsequent therapy, when compared to SUN alone, respectively. Lastly, we are uncertain whether CAB alone reduces or increases the risk for subsequent therapy (RR 0.93, 95% CI 0.74 to 1.16, very low certainty), when compared to SUN. Comparison data were not available for PAZ alone compared to SUN alone.
Subgroup analyses
We were able to conduct only one subgroup analysis for one outcome. Other subgroup analyses were not possible due to the distribution of characteristics across trials or due to a lack of available subgroup data (see Differences between protocol and review).
Follow‐up time
We conducted a subgroup analysis for the outcome OS in the combined risk groups (total trial population) for the follow‐up times <5 years and ≥ 5 years. For most trials, follow‐up time was estimated from the Kaplan‐Meier‐Curves of the respective effect estimates for OS. Out of 21 trials that were included in the analysis of OS in the total trial population, 14 trials had a follow‐up time for this outcome of <5 years (Jonasch 2010; NCT00081614; NCT00098657/NCT00083889; NCT00334282; NCT00631371; NCT00719264; NCT00738530; NCT00920816; NCT01024920; NCT01108445; NCT01984242; NCT02420821; NCT02761057; NCT02811861), while the remaining seven trials had a follow‐up time of ≥ 5 years (NCT00072046; NCT00420888; NCT00609401; NCT00720941; NCT00979966; NCT02231749; NCT02853331),
In the analysis of trials with a follow‐up of less than five years, the network consisted of three sub‐networks. In subnetwork 1, which included SUN as our main comparator, we did not find notable effects between interventions. We observed moderate heterogeneity in this subnetwork (Q=1.81, df = 1, P = 0.18; I² = 44.6%, Tau² = 0.0284). In the ranking of treatments, LEN+PEM was the best treatment option (P‐score: 0.89) and IFN alone the worst (P‐score: 0.25). These results are similar to those of the main analyses: LEN+PEM was also rated the best treatment option, whereas IFN alone was the second‐worst treatment option. Results for all network comparisons, including the ranking of treatments, are shown in Figure 95 in Appendix 16.
In the analysis of trials with a follow‐up time of five or more years, the network consisted of two sub‐networks. In subnetwork 1, we found evidence suggesting that OS was higher with NIV+IPI (HR 0.69, 95% CI 0.59 to 0.81) and PEM+AXI (HR 0.73, 95% CI 0.60 to 0.81) when compared to SUN alone, respectively. In the ranking of treatments, NIV+IPI was the best treatment option (P‐score: 0.85) and SUN alone was the worst (P‐score: 0.15). Results for all network comparisons, including the ranking of treatments, are shown in Figure 96 in Appendix 16.
Sensitivity analyses
Sensitivity analyses were not possible for every outcome that was planned for (see Differences between protocol and review).
Fixed‐effects
We conducted fixed‐effect NMA for the outcomes OS, SAE and PFS.
For sub‐network 1 of the outcome OS in all risk groups combined, the fixed‐effect model yielded somewhat different results (Figure 97 in Appendix 16). The results from the fixed‐effect model suggested substantially better OS with LEN+PEM, NIV+IPI and PEM+AXI when compared to SUN alone, respectively, and the confidence intervals were more narrow. However, there were no changes in the direction of the effect and there were no changes in the ranking of these three treatments according to their P‐score. Furthermore, in the fixed‐effect model, ATE+BEV was favoured over SUN alone (HR 0.95, 95% CI 0.80 to 1.12), as opposed to the random‐effects model, where SUN alone was favoured over ATE+BEV; hence, the ranking of these two treatments changed according to their P‐score.
For the outcome SAEs (all risk groups combined), the fixed‐effect model yielded somewhat different results (Figure 98 in Appendix 16). Firstly, the ranking and order of treatments slightly changed. Secondly, the direction of effect for the comparison ATE alone versus SUN alone changed, as ATE alone was favoured over SUN alone (HR 0.99, 95% CI 0.71 to 1.36) in the fixed‐effect model. Furthermore, the results suggested a substantially lower risk for SAE with SUN alone when compared to PEM+AXI, LEN+EVE and NIV+IPI, respectively (the direction of effects remained unchanged).
For sub‐network 1 of the outcome PFS in the MSKCC favourable risk group, the fixed‐effect model yielded different results (Figure 99 Appendix 16). Here, AXI alone was favoured over SUN alone (HR 0.95, 95% CI 0.52 to 1.74) as opposed to the result of the random‐effects model, where SUN alone was favoured over AXI alone; hence, the ranking of these two treatments changed. Furthermore, results suggested substantially better PFS with LEN+PEM and LEN+EVE when compared to SUN alone, respectively, and the confidence intervals were more narrow. However, there were no changes in the direction of effects. For subnetwork 1 of the outcome PFS in the MSKCC intermediate and poor risk groups, the fixed‐effect model yielded somewhat different results (Figure 100 in Appendix 16). Results suggested substantially better PFS with LEN+EVE versus SUN alone. For subnetwork 2, the fixed‐effect model yielded slightly different results (Figure 101 in Appendix 16). The fixed‐effect model for subnetwork 1 of the outcome PFS in the IMDC intermediate and poor risk groups yielded only little differences as well (Figure 102 in Appendix 16).
Assumption of proportional hazards
We conducted this sensitivity analysis for the outcome OS (all risk groups combined). Five trials reported that they tested the assumption of proportional hazards, but only four reported that the assumption was also validated (Jonasch 2010; NCT00609401; NCT00920816; NCT02420821). In NCT00334282, the assumption was not validated.
For this sensitivity analysis, the network consisted of two sub‐networks. An analysis was conducted for sub‐network 1; sub‐network 2 contained only one trial, so no further analyses were conducted. For sub‐network 1, the main comparator was SOR alone, and we did not find notable effects between interventions. In the ranking of treatments, the best treatment option was SOR+ILN (P‐score: 0.74), whereas SOR+IFN was the worst option (P‐score: 0.06) (Figure 103 in Appendix 16).
Risk of bias
We conducted sensitivity analyses according to the risk of bias ('low risk of bias' or 'some concerns' versus 'high risk of bias') in the outcomes OS and SAEs in the combined risk groups.
For the outcome OS (all risk groups combined), which included 21 trials, five trials had an overall 'low risk of bias' (NCT00334282; NCT00720941; NCT02231749; NCT02420821; NCT02853331), 12 trials had 'some concerns' (Jonasch 2010; NCT00072046; NCT00081614; NCT00609401; NCT00631371; NCT00719264; NCT00738530; NCT00920816; NCT01024920; NCT01108445; NCT01984242; NCT02761057) and the remaining four trials had a 'high risk of bias' (NCT00098657/NCT00083889; NCT00420888; NCT00979966; NCT02811861). The network of trials at 'low risk of bias' or 'some concerns' consisted of four sub‐networks; analyses were conducted for sub‐networks 1, 2 and 3, while subnetwork 4 contained only one trial. For sub‐network 1, moderate heterogeneity (Q=1.81, df=1, P = 0.18; I²=44.6%, Tau²=0.0284) was observed. We did not find notable effects between interventions in subnetwork 1. In the ranking of treatments, NIV+IPI was the best treatment option (P‐score: 0.81) and EVE alone was the worst option (P‐score: 0.33). Results for all network comparisons, including the ranking of treatments, are shown in Figure 104 in Appendix 16. As for the trials that were at 'high risk of bias', the network was fully connected. We found evidence suggesting substantially better OS with LEM+PEM (HR 0.66, 95% CI 0.49 to 0.88) compared to SUN alone (Figure 105 in Appendix 16). In the ranking of treatments, the best treatment option was LEN+PEM (P‐score: 0.95), whereas NAP+IFN (P‐score: 0.17) was the worst option.
For the outcome SAE (all risk groups combined), which included 22 trials, one trial had an overall 'low risk of bias' (NCT02853331), five trials had overall 'some concerns' (NCT00720941; NCT00920816; NCT00903175; NCT00126594; NCT01984242), and the remaining 16 trials had an overall 'high risk of bias' (NCT00065468; NCT00098657/NCT00083889; NCT00117637; NCT00619268; NCT00631371; NCT00719264; NCT00732914; NCT00738530; NCT00979966; NCT01024920; NCT01108445; NCT01613846; NCT01835158; NCT02231749; NCT02420821; NCT02811861). The network of trials at 'low risk of bias' or 'some concerns' consisted of two sub‐networks. For sub‐network 1, the evidence suggests no difference in the risk for SAE between PAZ alone and SUN alone (HR 0.99, 95% CI 0.87 to 1.14). Instead, we found evidence that suggested a substantially lower risk of SAE with SUN alone, when compared to ATE+BEV (RR 0.58, 95% CI 0.40 to 0.84) and PEM+AXI (RR 0.78, 95% CI 0.65 to 0.93), respectively. In the ranking of treatments, the best treatment option was PAZ alone (P‐score: 0.81), closely followed by SUN alone (P‐score: 0.80) and the worst treatment option was ATE+BEV (P‐score: 0.03). Results for all network comparisons, including the ranking of treatments, are shown in Figure 106 in Appendix 16. As for the trials at 'high risk of bias', the network was fully connected. We did not find notable effects between interventions. In the ranking of treatments, TEM alone was the best treatment option (P‐score: 0.89), whereas LEN+PEM was the worst treatment option (P‐score: 0.17) (Figure 107 in Appendix 16).
Discussion
Summary of main results
The primary objective of this systematic review with network meta‐analysis was to evaluate and compare the benefits and harms of first‐line therapies for adults with advanced renal cell carcinoma (RCC), and thereby produce a clinically relevant ranking of therapies. Secondary objectives were to maintain the currency of the evidence by using a living systematic review approach, as well as to incorporate data from clinical study reports (CSRs).
We identified a total of 55 eligible trials; of these, 36 randomised‐controlled trials (RCTs) were included in quantitative analyses and narrative reporting in this review, with a total of 15,177 participants. In these trials, 22 drugs and 17 different combinations were assessed. The substance sunitinib (SUN) was the main comparator in this review, and also the main comparator in 16 included trials. All trials but one (NCT01064310) were included in the network meta‐analyses. Overall risk of bias was mostly judged high across trials because most were open‐label trials, hindering blinded outcome assessments. Reporting harms especially lacked details about the method of analysis and method of outcome measurement. The certainty in the evidence for all outcomes ranged from moderate to very low. The main outcomes and comparisons are presented in the Table 1, Table 2 and Table 3.
Primary outcomes
Overall survival (OS)
See Table 1, Table 2, and Table 3.
PEM+AXI: We found that this combination probably improves OS, when compared to SUN alone, in the combined groups. However, we were not able to obtain subgroup data per risk group (neither for International Metastatic RCC Database Consortium (IMDC) nor Memorial Sloan Kettering Cancer Center (MSKCC criteria)).
AVE+AXI: Subgroup data according to IMDC risk groups revealed that AVE+AXI may improve OS in the favourable, intermediate and poor risk groups, when compared to SUN alone. We were not able to obtain data for the risk groups according to MSKCC criteria, and neither for all risk groups combined.
NIV+CAB: For the risk groups according to IMDC criteria, we are uncertain whether NIV+CAB improve or decrease OS in the favourable risk groups. However, we found that NIV+CAB probably improve OS in the intermediate and poor risk groups, when compared to SUN alone, respectively. We were not able to obtain data for the risk groups according to MSKCC criteria, and neither for all risk groups combined.
LEN+PEM: We found that in the favourable risk group according to IMDC criteria, there may be little or no difference in OS between LEN+PEM and SUN in the favourable risk groups. As for the MSKCC favourable risk group, we are uncertain whether LEN+PEM improves OS. However, for both the IMDC and MSKCC intermediate and poor risk groups, we found that LEN+PEM probably improves OS, when compared to SUN alone. Looking at all risk groups combined, we found that LEN+PEM may improve OS.
NIV+IPI: For the risk groups according to IMDC criteria, there probably is little or no difference in OS between NIV+IPI and SUN in the favourable risk groups, but NIV+IPI probably improve OS in the intermediate and poor risk groups. We were not able to obtain data for the risk groups according to MSKCC criteria. Looking at all risk groups combined, we found that NIV+IPI probably improve OS.
CAB: We were not able to obtain subgroup data for the favourable risk groups (neither for IMDC nor MSKCC criteria), but found that for the IMDC intermediate and poor risk groups, CAB alone may improve slightly OS, when compared to SUN alone. We were not able to obtain data for the MSKC intermediate and poor risk groups. Looking at all risk groups combined, we are uncertain whether CAB alone improves OS.
PAZ: We found that there is probably little or no difference in OS between PAZ alone and SUN alone in the combined groups. We were not able to obtain subgroup data per risk group.
Quality of life (QoL)
See Table 1, Table 2, and Table 3.
We were not able to obtain subgroup data per risk groups for this outcome. Looking at the combined risk groups, we were also not able to obtain data on quality of life for PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI, and CAB alone. We obtained data for the comparison PAZ alone and SUN alone, where one RCT reported that the mean post‐score of the intervention group (PAZ) was higher than that of the control group (SUN). However, we are uncertain about the evidence we found.
Serious adverse events (SAEs)
See Table 1, Table 2, and Table 3.
We were not able to obtain subgroup data per risk group for this outcome. Looking at the combined risk groups, we were also unable to obtain data on AVE+AXI and NIV+CAB. As for the other substances, we found that PEM+AXI probably increase slightly the risk for SAEs when compared to SUN. Furthermore, we found that LEN+PEM and NIV+IPI probably increase the risk for SAEs. There probably is little or no difference in the risk for SAEs between PAZ alone and SUN alone, and we are uncertain whether CAB alone reduces or increases the risk for SAEs, when compared to SUN alone.
Secondary outcomes
Progression‐free survival (PFS)
See Table 1, Table 2, and Table 3.
PEM+AXI: We found that this combination probably improves slightly PFS, when compared to SUN alone, in the combined groups. However, we were not able to obtain subgroup data per risk group.
AVE+AXI: For the IMDC favourable risk group, we found that AVE+AXI may improve PFS, and for the IMDC intermediate and poor risk groups that AVE+AXI probably improve PFS, when compared to SUN, respectively. We were not able to obtain data for the risk groups according to MSKCC criteria, and neither for all risk groups combined.
NIV+CAB: For the IMDC favourable risk group, NIV+CAB may improve PFS, and for the IMDC intermediate and poor risk groups, NIV+CAB probably improve PFS, when compared to SUN alone, respectively. We were not able to obtain data for the risk groups according to MSKCC criteria, and neither for all risk groups combined.
LEN+PEM: We found that in the IMDC favourable risk groups, LEN+PEM may improve PFS, but we are uncertain whether LEN+PEM improve PFS in the MSKCC favourable risk groups, when compared to SUN alone, respectively. For the IMDC and MSKCC intermediate and poor risk groups, we found that LEN+PEM probably improve PFS, when compared to SUN alone. Looking at the combined risk groups, LEN+PEM probably improve PFS.
NIV+IPI: For the IMDC favourable risk groups we found that NIV+IPI probably reduce PFS, when compared to SUN alone, but there may be little or no difference in PFS between NIV+IPI and SUN in the IMDC intermediate and poor risk groups. We were not able to obtain subgroup data according to MSKCC criteria. Looking at the combined groups, there may be little or no difference between NIV+IPI and SUN in improving PFS.
CAB: For the IMDC intermediate and poor risk groups, we found that CAB alone probably improves PFS when compared to SUN alone. We were not able to obtain data for the other risk groups. Looking at the combined risk groups, we found that CAB alone may improve PFS.
PAZ: We were not able to obtain subgroup data. Looking at the combined risk groups, there probably is little or no difference in PFS between PAZ alone and SUN alone.
Adverse events (AEs)
See Table 1, Table 2, and Table 3.
We were not able to obtain subgroup data per risk group for this outcome. Looking at the combined risk groups; we were also not able to obtain data for PEM+AXI and NIV+IPI. However, we found that there probably is little or no difference in the risk for AEs in AVE+AXI, NIV+CAB and PAZ alone, when compared to SUN alone, respectively. The combination LEN+PEM probably increases slightly the risk for AEs, when compared to SUN alone. Lastly, we are uncertain whether CAB alone reduces or increases the risk for AEs, when compared to SUN.
Number of participants who discontinued treatment due to an AE
We were not able to obtain subgroup data per risk group for this outcome. Looking at the combined risk groups, we observed that AVE+AXI, PAZ alone, CAB alone, PEM+AXI, NIV+CAB, LEN+PEM, and NIV+IPI decrease or increase the risk for study discontinuation due to an AE, when compared to SUN alone, respectively.
Time to initiation of first subsequent therapy
See Table 1, Table 2, and Table 3.
We were not able to analyse this outcome as a time‐to‐event outcome, mainly due to differences in outcome definition and reporting. Therefore, we reported the results narratively. Moreover, we were not able to obtain subgroup data, and also no data for the substance PAZ. For the combined risk groups, we found that PEM+AXI, AVE+AXI and NIV+IPI may reduce the risk for subsequent therapy, when compared to SUN alone, respectively. We are uncertain whether NIV+CAB, NIV+IPI and CAB alone reduce the risk for subsequent therapy, when compared to SUN alone, respectively.
Overall completeness and applicability of evidence
In this systematic review with network meta‐analysis, we included 36 RCTs that assessed first‐line treatments for adults with advanced RCC. All trials but one (NCT00126594) were published as full‐text publications. For 11 trials, we also identified study protocols (some including a statistical analysis plan) that provided detailed information on the study design, participants, methods and outcomes, which informed our risk of bias assessments (NCT00720941; NCT00903175; NCT01030783; NCT01064310; NCT01835158; NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177). For two trials (NCT00334282; NCT00720941), we identified clinical study reports and for another trial (NCT01064310), a scientific result summary was found, which we used as our primary sources for data extraction and risk of bias assessment. In addition, we identified 19 ongoing trials and five trials that are still awaiting classification. Regarding heterogeneity between trials, we determined moderate heterogeneity within the networks for the outcome OS in the analyses for all risk groups combined and for IMDC intermediate and poor risk groups, substantial heterogeneity for PFS in the analysis of the MSKCC favourable risk group, and moderate heterogeneity for PFS in both the MSKCC and IMDC intermediate and poor risk groups. Lastly, we observed substantial heterogeneity for the outcome SAE (all risk groups combined). The heterogeneity probably originates from the slight differences in the included trials with regard to some effect modifiers (for example, regarding histology types or differences in the sites of metastases; and in the analyses of all risk groups combined, also differences with regard to the risk groups). Nevertheless, our included trials remain largely comparable. Unfortunately, subgroup analyses for some of these characteristics were not possible (see Differences between protocol and review), so we were not able to further explore heterogeneity statistically. Looking at the concordance between survival outcomes (OS and PFS) for the interventions presented in the summary of findings tables, we found that overall, across all risk groups, OS and PFS were improved (albeit to different extent) with every treatment, when the treatment was compared to SUN alone. The only instance where a survival outcome was not improved but rather reduced was in the IMDC favourable risk groups, where we found that NIV+IPI probably reduces PFS, when compared to SUN. However, it should be noted that the certainty in the evidence varies across OS and PFS even when we observed improvements. The main reason for this is that for the outcome PFS, some evidence was rated as a high risk of bias, namely when the imaging scans were assessed by unblinded study investigators. In comparison, some studies conducted blinded independent central review to avoid bias due to unblinded outcome assessment (see also Implications for research).
There are several limitations to this review that we want to address. Firstly, the results of the review mostly apply to people with clear cell carcinoma, as most trials in this review included participants with the clear cell type, whereas other types were underrepresented. We aimed to conduct subgroup analyses for the different histology types (clear cell type, papillary type, sarcomatoid type), but this was not possible as in most trials, only participants with clear cell RCC were included, followed by trials in which most participants had clear cell RCC. In addition, we did not have sufficient subgroup data for histology types to perform subgroup analyses. Hence, results should be interpreted with caution. Secondly, regarding the network meta‐analyses of our key interventions of interest, meaning those that we chose for our summary of findings tables, we only had direct evidence from one trial per comparison (except for one comparison, namely CAB alone versus SUN alone in the PFS analysis of the combined risk groups). Due to insufficient data, we were not able to combine direct evidence in pairwise analyses, and we were not able to create so‐called closed loops of direct and indirect evidence. Most interventions of interest were compared to SUN alone in the included trials, but not to each other. Hence, there is a great lack of head‐to‐head comparisons of these interventions. Therefore, there was no additional benefit from network meta‐analyses. Thirdly, reporting of AEs differed between trials; therefore, some trials could not be included in the analyses for this outcome. We were not able to include data on AEs from 18 trials (50% of included trials) due to major reporting differences between studies. At protocol stage, we decided to include the number of participants who experienced at least one event, instead of cumulated events to avoid double counting. Moreover, we were interested in grade 3 or 4 adverse events, and in all‐cause adverse events. However, we found major reporting differences between trials, meaning that half of the included trials reported cumulated grades of severity, cumulated events and/or treatment‐related instead of all‐cause AEs, which made it impossible to use all data for our analyses. As for the individual AEs of interest, reporting of insomnia and depression was sparse, so analysing these specific events was not possible at all. As for the timing of outcome measurement and reporting, all AEs and also SAEs reported were those that occurred during treatment. However, because most trials provided continuous therapy, while others provided therapy for a fixed period of time, the time points of occurrence of AEs and SAEs most likely varied between trials. In addition, for trials with continuous therapy (where therapy was administered until progression, or even beyond progression if a clinical benefit was observed according to the treating clinician), inevitably, there is an increased risk for the occurrence of SAEs. Therefore, results for these outcomes should be interpreted with caution. The same applies to the outcome 'number of participants who discontinued treatment due to an AE'. Fourthly, a total of 22 trials reported the outcome health‐related QoL. However, a total of 25 different scales were used across trials to measure this outcome, so we prioritised scales for assessment in this review. In the end, we prioritised five scales, from four of which data were extractable. However, neither network meta‐analyses nor pairwise meta‐analyses were possible for this outcome, mainly due to a lack of available comparisons within our pre‐specified time points. Fifthly, the outcome Time to First Subsequent Therapy (TFST) was not reported as such (i.e. as time‐to‐event outcome). Instead, trials reported the number of participants who received subsequent anticancer therapy after discontinuation of study treatment. Hence, analyses were not feasible, and we reported results narratively, which should also be interpreted with caution because the definition of this outcome varied across trials and the time point of reporting was unclear. We reported data on this outcome in the SoF table 1, but particularly downgraded by two levels in the domain 'indirectness' due to an indirect measurement of our outcome of interest. Hence, the certainty in the evidence for this outcome ranges from low to very low. Sixthly, we were not able to perform relevant subgroup analyses by sex (male, female), age (< 65 years, > 65 years), prior nephrectomy (yes, no), prior radiotherapy (yes, no), histology type (clear cell, papillary, sarcomatoid), and sites of metastases (lung, bone, liver). It was not possible to differentiate by study (e.g., by analysing and comparing studies with only women against studies with only men, as all studies included both sexes), and there was a great lack of subgroup data. While few studies did report some subgroup data for the outcome OS, potential network or pairwise meta‐analyses would have included no more than two or three studies. Such analyses would not have produced meaningful results. However, we want to stress the importance of assessing the benefits and harms of the different treatments in different subgroups. For example, research has found that immune checkpoint inhibitors seem more effective in men than women; whereas for women, immune checkpoint inhibitors combined with chemotherapy seem more effective than for men (Wang 2019). Additional subgroups that were not considered in this review, but could be assessed in an update of the review, are ethnicity (Nassar 2022) and Eastern Cooperative Oncology Group (ECOG) performance status. Lastly, most trials provided a hazard ratio (HR) for the outcomes OS and PFS, and we were able to include all interventions listed in Table 1 (Description of studies) in our analyses. However, networks were not always fully connected, meaning that only treatments within the same sub‐network could be compared to each other. Moreover, only five trials reported that they tested the assumption of proportional hazards, thereof four reported that the assumption was also validated. For the remaining studies it remains unclear whether the assumption of proportional hazards was tested. If the assumption is not validated, it is unclear which impact this would have on the meta‐analysis.
Risk of bias
We assessed the risk of bias for the total population (i.e. all risk groups combined) for the outcomes OS, PFS, AEs, SAEs and QoL. For OS and PFS, risk of bias was additionally assessed for each risk group (i.e. favourable, intermediate or poor risk group per IMDC or MSKCC). Risk of bias was predominantly judged as 'high risk of bias' or 'some concerns' across most trials and outcomes. The judgement between the total population and the risk groups differed for only one trial and one outcome (NCT00720941). The main reasons for negative judgements were the lack of detailed information about the randomisation process, the blinding of outcome assessors, the method of analysis and the method of outcome measurement. Furthermore, pre‐agreed study protocols and statistical analyses plans (SAPs) were missing for most trials. Only 11 pre‐agreed study protocols were available (NCT00720941; NCT00903175; NCT01030783; NCT01064310; NCT01835158; NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177). Three of these protocols did not include a SAP (NCT00903175; NCT01030783; NCT01835158), and for one protocol the date of finalisation was not reported (NCT01030783). Clinical study reports (CSRs) were available for two trials (NCT00334282; NCT00720941); a scientific result summary was available for one trial (NCT01064310).
Certainty of the evidence
We rated our certainty in the evidence for the outcomes included in the SoF table (OS, QoL, SAEs, PFS, AEs, and TFST).
All risk groups combined
For all outcomes that were analysed in the combined risk groups (OS, QoL, SAEs, PFS, AEs, and TFST (the latter reported narratively)), our certainty in the evidence ranged from moderate to very low. For OS, we downgraded by one level in the domain 'study limitations' due to a high risk of bias in one comparison; by one level for imprecision in two comparisons, because of a wide confidence interval (CI) and the upper CI limit suggested no difference between interventions; by one level for imprecision in one comparison because the upper CI limit suggested no difference between interventions; by one level for imprecision in one comparison because of a wide CI that favoured either of the compared treatments. Lastly, for one comparison, we downgraded by one level for indirectness because in one trial (NCT02761057) seven per cent of the total study population received previous systemic therapy, and by two levels for imprecision because of a very wide CI that included values that favoured either of the compared treatments, and the evidence stemmed from only one trial with 90 participants (NCT02761057). For QoL, the only available evidence was rated as very low because we downgraded by two levels for study limitations due to a high risk of bias, and by two levels for imprecision because of a very wide CI that included values that favoured either of the compared treatments, and because the evidence stemmed from only one trial with four participants analysed (NCT00720941). For SAEs, the certainty in the evidence ranged from moderate to very low. Most evidence was downgraded by one level for study limitations because of a high risk of bias. In one instance, we downgraded by one level for imprecision because of a wide CI that favoured either of the compared treatments; and in another instance, we downgraded by two levels for imprecision because of a very wide CI that included values that favoured either of the compared treatments, and because the evidence stemmed from only one trial with 157 participants (NCT01835158). For PFS, the certainty in the evidence ranged from moderate to low. For most evidence we downgraded by one level for study limitations because of a high risk of bias. For one comparison, we downgraded by one level for indirectness because in one trial (NCT02761057) seven per cent of the total study population received previous systemic therapy. In two comparisons, we downgraded by one level for imprecision because of a wide CI that favoured either of the compared treatments. For AEs, the certainty of the evidence was mostly moderate, except for one comparison that was rated as very low. For all comparisons, we rated down by one level for study limitations due to a high risk of bias. For one comparison, we additionally downgraded by two levels for imprecision because of a wide CI that included values that favoured either of the interventions, and because the evidence stemmed from only one trial with 157 participants (NCT01835158). Lastly, for TFST, the certainty in the evidence ranged from low to very low. For all comparisons, we rated down by two levels for indirectness due to indirect measurement of the outcome of interest. For three comparisons, we rated down by one level for study limitations due to a high risk of bias. For one comparison, we downgraded by two levels for imprecision because of a wide CI that included values that favoured either of the interventions, and because the evidence stemmed from only one trial with 157 participants (NCT01835158).
Favourable risk groups (according to IMDC and MSKCC)
In the IMDC favourable risk groups for OS, our certainty in the evidence ranged from low to very low. We mostly downgraded by one level for study limitations due to a high risk of bias and/or by one level for 'imprecision' when the CI was wide and included values that favoured either of the compared treatments; when the CI was wide and the upper CI limit suggested no difference between interventions; or when the evidence stemmed from only one trial with < 150 participants. In some instances, we downgraded by two levels for imprecision, because of a very wide CI that included values that favoured either of the compared treatments, and/or when the evidence stemmed from only one trial with < 150 participants. For PFS, the evidence ranged from moderate to low. We mostly rated down by one level for study limitations due to a high risk of bias. In one instance, we additionally downgraded by one level for imprecision because of a wide CI, where the upper CI limit suggested no difference between interventions; and in another instance we downgraded by one level for imprecision because the evidence stemmed from only one trial with < 150 participants.
In the MSKCC favourable risk groups for OS, the certainty of the evidence ranged from low to very low. We mostly downgraded by one level for study limitations. In one instance, we additionally downgraded by one level for imprecision because of a wide CI that included values that favoured either of the compared treatments; and in another instance we downgraded by two levels for imprecision because of a very wide CI that included values that favoured either of the compared treatments. For PFS, there was only one comparison, where we rated our certainty in the evidence as very low. We downgraded by one level for study limitations due to a high risk of bias, and by two levels for imprecision because of a very wide CI that included values that favoured either of the compared treatments.
Intermediate and poor risk groups (according to IMDC and MSKCC)
In the IMDC risk groups for OS, our certainty in the evidence ranged from moderate to very low. Most evidence was downgraded by one level for study limitations because of a high risk of bias, and by one level for imprecision because of a wide CI that favoured either of the compared treatments. In one instance, we downgraded by two levels for imprecision because of a very wide CI that included values that favoured either of the compared treatments, and because the evidence stemmed from only one trial with 157 participants. For PFS, the certainty of the evidence ranged from moderate to low. Most evidence was downgraded by one level for study limitations because of a high risk of bias, and by one level for imprecision because of a wide CI that favoured either of the compared treatments. In one instance, we downgraded by one level for imprecision because the evidence stemmed from only one trial with 157 participants.
In the MSKCC risk groups for OS, the certainty of the evidence ranged from moderate to low. The evidence was downgraded by one level for study limitations because of a high risk of bias, and/or by one level for imprecision because of a wide CI that favoured either of the compared treatments. For PFS, the only available comparison was rated as moderate because we downgraded by one level for study limitations due to a high risk of bias.
Potential biases in the review process
A key strength of our review is that it is a very comprehensive review and includes all available treatment options in the first‐line treatment setting for adults with advanced RCC. We explored the effectiveness of all treatment options (where data were available), i.e. the effectiveness of different combinations of substances from different drug categories as well as the effectiveness of individual substances alone.
To prevent potential bias in our review, the important steps in the review development process were conducted by two review authors independently (i.e. study screening and selection, data extraction, and risk of bias assessments). Only the GRADE assessment was conducted by one review author (AAa) first, and then the assessment was independently examined by another review author (VP). Discrepancies were then resolved by discussion. Overall, seven co‐authors (AAa, BB, CH, ET, MG, ND, VP) were involved in the different important steps of the review development. Any conflicts that arose during the review process were resolved by discussion until a consensus was reached, and if necessary, by involving a third review author.
Trials and related publications were identified by a sensitive search strategy developed by an experienced information specialist, and we searched all relevant databases (CENTRAL; MEDLINE; Embase), several trial registries (ISRCTN; EU Clinical Trial Register; ClinicalTrials.gov; WHO ICTRP) as well as conference proceedings of relevant conferences (ASCO; ESMO). We also reviewed other published systematic reviews on first‐line therapies for adults with advanced RCC to make sure that we did not miss any trials. The fact that this is a living systematic review during its development process, with the last search conducted in February 2022, and due to our extensive search strategy, we are confident that we have identified all relevant trials to address the research question of our review. Besides the 36 included trials, we identified an additional 19 ongoing trials that could be included in an update of this review. Methodologically, we followed all current Cochrane guidelines and recommendations in every stage of our review process and are not aware of any deficiencies in our review process. For transparency, we have documented and justified all changes to our methods from the published protocol (Goldkuhle 2020) in the Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
We believe that, currently, our systematic review is the most comprehensive systematic review with network meta‐analyses that explored different treatment options in the first‐line therapy for advanced RCC). However, we identified a number of systematic reviews with meta‐analyses or network meta‐analyses assessing first‐line therapy in advanced RCC. Here, we present the results of those systematic reviews that also conducted network meta‐analyses, and we only assessed the most recent reviews of 2021/2022 due to the rapidly evolving treatment landscape. It should be noted that methodologically, the reviews differ from our review in that, firstly, none of the reviews included data from clinical study reports. Secondly, except for one review (Riaz 2021), none were living systematic reviews. Thirdly, not all reviews conducted a risk of bias and/or GRADE assessment. Lastly, most reviews included only a few selected trials that assessed only a selected number of treatment options. Hence, the reviews did not assess the full range of available treatment options for advanced RCC in the first‐line treatment setting, making our review the most comprehensive. We compared results mainly for the outcomes OS and PFS. As for harms, most reviews reported treatment‐related AEs or treatment‐related discontinuations due to AEs, which are not comparable to our data (we assessed all‐cause AEs). In one review, health‐related quality of life (HRQoL) was assessed, the instruments included being EQ‐5D and FACT‐FKSI Symptom Index.
We primarily compared the results of our review to the results of the most recent reviews published in 2022 (Bosma 2022; Nocera 2022) and to one review from 2021 that is a living systematic review (Riaz 2021). In addition, we present brief summaries of the results of other reviews published in 2021.
Comparison of results of our review to other recent reviews
We identified one living systematic review with network meta‐analyses that includes a total of 14 trials, which are also included in our review (https://rcc.network-meta-analysis.com/RCC.html) (Riaz 2021).
Comparison of results for Overall survival (OS)
The analyses for OS and all risk groups combined showed that LEN+PEM (HR 0.66, 95% CI 0.49 to 0.88), CAB+NIV (HR 0.66, 95% CI 0.50 to 0.87), PEM+AXI (HR 0.68, 95% CI 0.55 to 0.85) and NIV+IPI (HR 0.69, 95% CI 0.59 to 0.81) showed a substantial benefit for OS, compared to SUN. Treatment ranking (according to SUCRA analyses) showed that LEN+PEM (83%) had the highest likelihood of being the preferred treatment option for OS, closely followed by NIV+CAB (82%) and PEM+AXI (80%). In our analysis, we also found that LEN+PEM may improve OS, and in according to our ranking of treatments, it was also the best treatment option (P‐score 0.85). Furthermore, we also found that PEM+AXI and NIV+IPI probably improve OS, when compared to SUN, respectively. We are uncertain whether CAB improves OS, and we did not have evidence for the comparison NIV+CAB versus SUN.
For the favourable risk groups (unclear whether IMDC or MSKCC risk groups were reported in the review), AVE+AXI (HR 0.81, 95% CI 0.34 to 1.94), NIV+CAB (HR 0.84, 95% CI 0.36 to 1.99) and PAZ alone (HR 0.88, 95% CI 0.63 to 1.22) may or may not improve OS when compared to SUN. However, AVE+AXI (SUCRA 63%) had the highest likelihood of being the preferred treatment option, closely followed by PAZ (62%) and NIV+CAB (60%). In our analysis of the IMDC favourable risk group, we found that AVE+AXI may improve OS, and it was also the best treatment option according to the ranking of treatments (P‐score: 0.83). The reviews are also in agreement that there may be little or no difference between LEN+PEM and SUN in improving OS (our result: HR 1.15, 95% CI 0.55 to 2.40; the other reviews' result: HR 1.14, 95% CI 0.55 to 2.38). We are also uncertain about the effect of NIV+CAB. As for the MSKCC favourable risk groups, we are also uncertain about the effect of LEN+PEM, and we found that there may be little or no difference between PAZ and SUN.
For the intermediate and poor risk groups (unclear whether IMDC or MSKCC risk groups were reported in the review), NIV+CAB (HR 0.52, 95% CI 0.28 to 0.98), LEN+PEM (HR 0.61, 95% CI 0.44 to 0.85), PEM+AXI (HR 0.63, 95% CI 0.49 to 0.80) and NIV+IPI (HR 0.65, 95% CI 0.54 to 0.78) showed a substantial benefit in OS, compared to SUN. Treatment ranking showed that NIV+CAB (SUCRA: 82%) had the highest likelihood of being the preferred treatment option, followed by LEN+PEM (SUCRA: 73%). This is similar to our results, where for the IMDC risk groups, we found that NIV+CAB and LEN+PEM probably improve OS, and CAB alone may improves slightly OS, when compared to SUN, respectively. However, there is a difference in the ranking of these two treatments, because we found that LEN+PEM (P‐score: 0.81) was the best treatment option, followed by NIV+CAB (P‐score: 0.74). Furthermore, we also found that NIV+IPI probably improves slightly OS, compared to SUN. For the MSKCC risk groups, we also found that LEN+PEM probably improves OS, and it was the best treatment option according to the ranking of treatments (P‐score: 0.81).
Comparison of results forProgression‐free survival (PFS)
For PFS and for all risk groups combined, LEN+PEM (HR 0.39, 95% CI 0.31 to 0.48), CAB alone (HR 0.48, 95% CI 0.31 to 0.74), NIV+CAB (HR 0.52, 95% CI 0.43 to 0.63), AVE+AXI (HR 0.69, 95% CI 0.58 to 0.83) and PEM+AXI (HR 0.71, 95% CI 0.60 to 0.84) showed a substantial benefit in PFS, compared to SUN. Treatment ranking showed that LEN+PEM (SUCRA 98% CI) had the highest likelihood of being the preferred treatment option. We also found that LEN+PEM probably improve PFS, and PEM+AXI probably improve slightly PFS, when compared to SUN, respectively. We also found that CAB alone may improves PFS. In our ranking of treatments, LEN+PEM (P‐score: 0.98) was also the best treatment option, closely followed by CAB alone (Pscore: 0.92), LEN+EVE (P‐score: 0.87) and PEM+AXI (P‐score: 0.86),
As for the favourable risk groups (unclear whether IMDC or MSKCC risk groups were reported in the review), LEN+PEM (HR 0.40, 95% CI 0.27 to 0.60) and AVE+AXI (HR 0.63, 95% CI 0.40 to 0.99) showed a substantial benefit in PFS, compared to SUN. The combination LEN+PEM had the highest likelihood (96%) of being the preferred treatment option. For the IMDC risk group, we also found that LEN+PEM, NIV+CAB and AVE+AXI probably improve PFS, when compared to SUN, respectively. LEN+PEM was also the best treatment option (P‐score: 0.94). As the MKSCC risk group, we are uncertain whether LEN+PEM improves PFS, when compared to SUN.
For the intermediate and poor risk groups (unclear whether IMDC or MSKCC risk groups were reported in the review), LEN+PEM (HR 0.37, 95% CI 0.28 to 0.49), NIV+CAB (HR 0.47, 95% CI 0.33 to 0.67), CAB alone (HR 0.48, 95% CI 0.31 to 0.74), AVE+AXI (HR 0.65, 95% CI 0.44 to 0.95), PEM+AXI (HR 0.69, 95% CI 0.56 to 0.84) and NIV+IPI (HR 0.74, 95% CI 0.62 to 0.88) showed a substantial benefit in PFS, compared to SUN. Treatment ranking showed that LEN+PEM (SUCRA 95%) has the highest likelihood of being the preferred treatment option. For the IMDC risk groups, we also found that CAB alone, LEN+PEM, AVE+AXI and NIV+CAB probably improve PFS, when compared to SUN, respectively. However, we found that there may be little or no difference in PFS between NIV+IPI and SUN. LEN+PEM was also the best treatment option in our ranking (P‐score: 0.94). For the MSKCC groups, we also found that LEN+PEM probably improve PFS, compared to SUN, and it was also the best treatment option (P‐score: 0.98).
Bosma 2022 included six trials, which we also included (NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177) and assessed OS, PFS, and HRQoL. Harms were also assessed, however, in this review, treatment‐related grade 3–4 AEs and treatment‐related drug discontinuation were assessed, so we did compare these results to ours. Across all risk groups and with regard to OS, results suggested better OS with NIV+CAB (HR 0.60, 95% CrI 0.40 to 0.90), NIV+IPI (HR 0.69, 95% CrI 0.59 to 0.81), PEM+AXI (HR 0.68, 95% CrI 0.55 to 0.84), and LEN+PEM (HR 0.66, 95% CrI 0.49 to 0.88) when compared to SUN, respectively. This is similar to the results of our review, where we also found improvements in OS and PFS with PEM+AXI and LEN+PEM in comparison to SUN. However, we did not have evidence for AVE+AXI and NIV+CAB in the combined risk groups. Looking at the different subgroups, treatment ranking showed that AVE+AXI (65%), PEM+AXI (78%) and LEN+PEM (89%) for the favourable, intermediate and poor risk groups, respectively, had the highest likelihood of being the preferred treatment options in terms of OS. In our review, we found that for the IMDC risk groups, AVE+AXI was the best treatment option for the favourable risk group and LEN+PEM was the best option for the intermediate or poor risk groups. As for the MSKCC risk groups, LEN+EVE was the best option for the favourable risk group and LEN+PEM the best option for the intermediate and poor risk groups.
With regard to PFS, results of the other review suggested better PFS with AVE+AXI (HR 0.69, 95% CrI 0.57 to 0.83), NIV+CAB (HR 0.51, 95% CrI 0.41 to 0.64), PEM+AXI (HR 0.71, 95% CrI 0.60 to 0.84), and LEN+PEM (HR 0.39, 95% CrI 0.32 to 0.48) when compared to SUN, respectively. Treatment ranking (based on SUCRA) revealed that LEN+PEM (99%) had the highest likelihood of being the preferred treatment option for the entire population in terms of PFS. As for the different risk groups, LEN+PEM also had the highest likelihood of being the preferred treatment option for the favourable, intermediate and poor risk groups with a 96%, 98% and 89% likelihood, respectively. We obtained the same result in our review: according to our ranking of treatments, LEN+PEM was the best treatment option across all groups, and both amongst IMDC and MSKCC.
As for HRQoL, analysis of treatment ranking for EQ‐5D showed that LEN+PEM (SUCRA 85%) followed by NIV+CAB (SUCRA 75%) were associated with the highest likelihood of being the preferred treatment. For the Functional Assessment of Cancer Therapy Kidney Cancer Symptom Index (FKSI) questionnaire, NIV+IPI (SUCRA 93%) followed by NIV+CAB (SUCRA 66%) was associated with the highest likelihood of being the preferred treatment option. Unfortunately, an analysis of these treatments on QoL was not feasible in our review.
Nocera 2022 only assessed four RCTs with proven OS benefit relative to SUN, which we also included (NCT02231749; NCT02853331; NCT02811861; NCT03141177). Outcomes included OS, PFS and treatment‐related grade 3+4 AEs; the main comparator being SUN. Results showed that the combination NIV+CAB (P‐score: 0.77), followed by LEN+PEM (P‐score: 0.63), PEM+AXI (P‐score: 0.57) and NIV+IPI (P‐score: 0.53) had the highest likelihood of OS benefit for all risk groups combined. As we did not have results for NIV+CAB in the combined risk groups, the ranking in our review was different: LEN+PEM came first with a P‐score of 0.85, followed by NIV+IPI with a P‐score: 0.83 and then PEM+AXI with a P‐score of 0.78. The results for PFS in the other review suggest that the treatments in the following order showed the highest likelihood of benefit (for all risk groups combined): LEN+PEM (P‐score: 0.99), NIV+CAB (P‐score: 0.76), PEM+AXI (P‐score:0.50), NIV+IPI (P‐score: 0.24). Again, we did not have data for NIV+CAB, so our ranking was different: LEN+PEM was the best option (P‐score: 0.98), PEM+AXI was the fourth‐best option (P‐score: 0.86) and NIV+IPI sixth‐best option (P‐score: 0.71).
Brief summary of results from reviews published in 2021
Cattrini 2021 only assessed immunotherapy and included six trials that we also included (NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177). Outcomes assessed were OS in the total population, OS per IMDC subgroup and grade ⩾3 AEs. The main comparator was SUN. In terms of OS benefit, results showed the highest likelihood (based on SUCRA analyses) for the combinations of NIV+CAB (82%), LEN+PEM (72%), PEM+AXI (68%) and NIV+IPI (56%) being the preferred treatments for all risk groups combined. With regard to the IMDC risk groups, PEM+AXI (78%) had the highest likelihood of being the preferred treatment for the intermediate risk group, and PEM+LEN (74%) the highest for the poor risk group. Contradicting results were shown for the favourable risk group. With regard to toxicity: NIV+IPI (96%), followed by ATE+BEV (87%), SUN (55%) and AVE+AXI (54%) were the preferred options with the highest tolerability.
Liu 2021 included five trials that we also included (NCT01984242; NCT02231749; NCT02420821; NCT02684006; NCT02853331) and assessed immunotherapy treatment options only. For PFS, and compared to SUN, results suggested better PFS with AVE+AXI (HR 0.69, 95% CrI 0.56 to 0.85) and PEM+AXI (HR 0.69, 95 CrI 0.57 to 0.83), followed by NIV+IPI (HR 0.82, 95% CrI 0.68 to 0.99) when compared to SUN, respectively. For OS, and compared to SUN, results suggested better OS with PEM+AXI (HR 0.53, 95% CrI 0.38 to 0.74) and NIV+IPI (HR 0.63, 95% CrI 0.48 to 0.83). However, no data were available for AVE+AXI and ATE alone. As for AEs, ATE alone had a lower risk for AEs (odds ratio (OR) 0.26, 95% CI 0.15 – 0.43), followed by NIV+IPI (OR 0.50, 95% CI 0.39 to 0.64) and ATE+BEV (OR 0.60, 95% CI 0.47 to 0.76) when compared to SUN, respectively.
Mori 2021 included five trials that we also included (NCT00720941; NCT02231749; NCT02420821; NCT02684006; NCT02853331) and assessed OS, PFS and treatment‐related AEs. For OS and for all risk groups combined, results suggested better OS with PEM+AXI (HR 0.85, 95% CrI 0.73 to 0.98) and NIV+IPI (HR 0.86, 95% CrI 0.75 to 0.99), but the upper CI limits suggested no difference to SUN, respectively. PEM+AXI (P‐score 0.80) was the best option based on treatment ranking. For PFS and for all risk groups combined, PEM+AXI (HR 0.86, 95% CrI 0.76 to 0.97) and AVE+AXI (HR 0.85, 95% CrI 0.74 to 0.98) may improve PFS but the upper limit of the CIs suggested no difference. AVE+AXI (P‐score: 0.82) and PEM+AXI (P‐score: 0.80) were the best options based on treatment ranking.
Quhal 2021 assessed immunotherapies only and included six trials that we also included (NCT02231749; NCT02420821; NCT02684006; NCT02811861; NCT02853331; NCT03141177). For OS and for all risk groups combined, PEM+AXI (HR 0.85, 95% CrI 0.73 to 0.98) and NIV+IPI (HR 0.85, 95% CrI 0.76 to 0.95) may lead to better OS, but the upper CI limits suggest no difference to SUN, respectively. According to SUCRA treatment ranking, NIV+CAB had the highest likelihood of providing the maximal OS (P‐score 0.75739). Results of effectiveness were unclear for the favourable risk groups. As for the intermediate and poor risk groups, LEN+PEM (HR 0.71, 95% CI 0.54 to 0.95) and NIV+CAB (HR 0.73, 95% CI 0.55 to 0.97) showed improvements in OS, but the upper CI limits suggested no difference to SUN, respectively. For PFS and for all risk groups combined, results suggested that LEN+PEM (HR 0.66, 95% CrI 0.61 to 0.72), NIV+CAB (HR 0.75, 95% CrI 0.67 to 0.84), AVE+AXI (HR 0.85, 95% CrI 0.75 to 0.96) and PEM+AXI (HR 0.86, 95% CrI 0.76 to 0.97) showed improvements in PFS when compared to SUN, respectively. Treatment ranking according to SUCRA showed that LEN+PEM (P‐score: 0.99) had the highest likelihood of providing the maximal PFS, followed by NIV+CAB (P‐score: 0.82). As for the favourable risk group, LEN+PEM (HR 0.68, 95% CI 0.57 to 0.80) and PEM+AXI (HR 0.82, 95% CI 0.70 to 0.96) suggest improvement in PFS when compared to SUN, respectively. As for the intermediate and poor risk groups, results suggested that LEN+PEM (HR 0.64, 95% CI 0.55 to 0.74), NIV+CAB (HR 0.71, 95% CI 0.61 to 0.82) and AVE+AXI (HR 0.83, 95% CI 0.70 to 0.97) improve PFS when compared to SUN, respectively.
Authors' conclusions
Implications for practice.
The findings of our living systematic review with network meta‐analyses may be an aid to clinicians and people with advanced renal cell carcinoma in decision‐making about treatment options for first‐line therapy. However, most evidence for currently recommended treatment options for the different risk groups stems from direct evidence from one trial only; hence, the results of this review should be interpreted with caution. Furthermore, before a decision is met about a treatment option, the results of all outcomes should be taken into consideration, meaning benefits and harms should be contrasted with one another. Because most networks in the network meta‐analyses in this review are not fully connected, not all treatment combinations could be compared to each other. Furthermore, the main results of our review on the effectiveness of different combination therapies stem from comparisons to SUN alone. Considering the interventions that are currently most recommended (PEM+AXI, AVE+AXI, NIV+CAB, LEN+PEM, NIV+IPI, CAB alone, and PAZ alone) across clinical practice guidelines (NCCN; ESMO; EAU; German S3 guideline) and across the different risk groups, we found the following evidence on survival, harms and quality of life, when each intervention is compared to SUN alone.
Implications for survival, harms and quality of life in the combined risk groups
For both OS and PFS, we are most certain in the evidence for PEM+AXI (probably improve OS, probably improve slightly PFS), LEN+PEM (may improve OS, probably improve PFS), and CAB alone (may improve PFS, but we are uncertain on the evidence for OS). We found that NIV+IPI probably improve OS, but for PFS, there may be little or no difference between NIV+IPI and SUN. For both OS and PFS, we found that there is probably little or no difference between PAZ alone and SUN alone. We did not have evidence for AVE+AXI and NIV+CAB for neither OS nor PFS. It should be highlighted that some of these treatments come with a higher incidence in SAEs and AEs (severity grades 3 and 4). We found that PEM+AXI probably increase slightly, and NIV+IPI and LEN+PEM probably increase the risk for SAEs, when compared to SUN, respectively. We are uncertain whether CAB alone reduces or increases the risk, and there is probably little or no difference in the risk for SAEs between PAZ alone and SUN alone. We did not have evidence for AVE+AXI and NIV+CAB on the comparative risk for SAEs. As for AEs, we found that LEN+PEM probably increase slightly the risk for AEs, and there is probably little or no difference in the risk for AEs between AVE+AXI, NIV+CAB, and PAZ alone, when compared to SUN alone, respectively. We are uncertain in the evidence for CAB alone, and we did not have evidence on PEM+AXI and NIV+IPI. All in all, when making treatment decisions, it should be individually evaluated whether the benefits of some of these interventions in improving OS or PFS outweigh their increased risk for harms. Unfortunately, we had a great lack of evidence on the impact of these interventions on the quality of life of people with advanced RCC.
Implications for survival, harms and quality of life for favourable versus intermediate + poor risk groups (IMDC and MSKCC)
For the favourable risk groups, we were most certain in the evidence for AVE+AXI, which may improve OS and PFS (in the IMDC group). We were not certain in the remaining evidence for OS. As for PFS, we were most certain in the evidence on NIV+CAB and LEN+PEM, namely that each may improve PFS (in the IMDC group), and that NIV+IPI probably reduce PFS (in IMDC group). We are missing evidence on CAB and PAZ for the favourable risk groups. As for the intermediate and poor risk groups, we were most certain in the evidence for LEN+PEM, namely that this combination probably improves OS and PFS (in the IMDC and MSKCC groups). We were also most certain in that NIV+CAB probably improves OS and PFS (in the IMDC groups), and that AVE+AXI probably improve PFS and may improve OS (in the IMDC groups). We are also certain that NIV+IPI probably improves OS (in the IMDC group). There may be little or no difference in PFS (for IMDC groups). There was a great lack of evidence for the MSKCC risk groups. We did not have subgroup data by risk group for harms and quality of life.
Implications for research.
The research field on first‐line therapies for adults with advanced renal cell carcinoma is a very fast evolving field due to the continuously changing treatment landscape that includes newer combinations of targeted therapies and immunotherapies. Hence, we identified 19 currently ongoing trials. However, for those interventions that are currently recommended across the different risk groups, thus far we only found direct evidence from one trial only, respectively. Furthermore, in all trials, these interventions were all compared to SUN. Thus, more trials are needed where these interventions and combinations are compared head‐to‐head, and not only to SUN. In our review, the additional benefit from the network meta‐analytic approach is limited because for most interventions, we could not create so‐called closed loops (involving at least three interventions) of direct and indirect evidence, where each direct comparison of interventions can be supplemented by an indirect comparison.
Regarding the outcome measurement of PFS, more studies are needed that perform blinded independent central reviews (BICR) of imaging scans when assessing PFS. Most studies in this review were not blinded and PFS was assessed presumably by unblinded study investigators, which we assessed as a high risk of bias. In comparison, few studies in this review that were non‐masked conducted BICR to control bias in the outcome measurement, which we assessed as a low risk of bias. In some instances, PFS was assessed both by the unblinded investigators and by BICR, and results were compared by the studies.
In this review, we initially aimed to conduct important subgroup analyses (e.g., by histology type, sex or age), but none were possible due to a great lack of reporting of subgroup data by the primary studies. Assessing the impact of immunotherapies and targeted therapies on different subgroups is essential, for example to understand differences by sex or ethnicity in responding to different therapies. Specifically for RCC, more studies are needed that assess histology types such as the papillary type or the sarcomatoid type. Most participants in this review had clear cell RCC, thus the results of this review are mostly applicable to the clear cell type. A sufficient and thorough analysis of different subgroups could be achieved by analysing individual participant data (IPD) provided by study authors.
Lastly, making study protocols (SPs), statistical analyses plans (SAPs) and clinical study reports (CSRs) publicly available would allow for a more detailed and accurate assessment of studies and data. All SPs, SAPs and CSRs that were found in this review informed data extraction and risk of bias assessments.
History
Protocol first published: Issue 12, 2020
Acknowledgements
We thank Joanne Platt (Information Specialist, Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group) for peer‐reviewing the search strategy (at protocol stage).
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Michael Brown, Cochrane's Editorial Board, Michigan State University College of Human Medicine, USA;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Sam Hinsley, Cochrane Central Editorial Service;
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service;
Copy Editor (copy editing and production): Heather Maxwell;
Peer‐reviewers (provided comments and recommended an editorial decision): Stella O'Brien (consumer review), Nuala Livingstone, Cochrane Evidence Production Methods (methods review), Anne Littlewood, Information Specialist at Cochrane Oral Health (search review). Two additional peer reviewers provided clinical peer review, but chose not to be publicly acknowledged.
Appendices
Appendix 1. Updated search strategy for CENTRAL (for the update search in February 2022)
Cochrane Central Register of Controlled Trials in the Cochrane Library
ID Search
#1 MeSH descriptor: [Carcinoma, Renal Cell] explode all trees
#2 ((collecting duct* or hypernephroid* or nephroid*) NEAR/2 carcinoma*):ti,ab,kw
#3 ((grawitz NEAR/11 tumo?r*) or hypernephroma*):ti,ab,kw
#4 ((renal* or kidney* or nephron*) NEAR/6 (cancer* or neoplasms* or carcinoma* or tumour* or tumor* or adenocarcinoma*)):ti,ab,kw
#5 #1 or #2 or #3 or #4
#6 (advance* or metasta*):ti,ab,kw
#7 #5 and #6
#8 (mRCC or RCC):ti,ab,kw
#9 #7 or #8
#10 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] explode all trees
#11 MeSH descriptor: [Antibodies, Monoclonal, Humanized] explode all trees
#12 MeSH descriptor: [Antibodies, Monoclonal, Humanized] explode all trees
#13 MeSH descriptor: [Antineoplastic Agents] explode all trees
#14 (antibod* near/2 monoclonal*):ti,ab,kw
#15 ((anticancer* or "anti‐cancer*" or anticarcinogen* or antineoplastic* or antitumo?r* or anti‐tumo?r*) NEAR/2 (agent* or drug*)):ti,ab,kw
#16 antineoplastic*:ti,ab,kw
#17 (antibod* NEAR/2 (neoplasm* or tumo?r* or cancer* or antitumor?r*)):ti,ab,kw
#18 #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17
#19 (car NEAR/1 t cell therap*):ti,ab,kw
#20 (targeted NEAR therap*):ti,ab,kw
#21 MeSH descriptor: [Protein Kinase Inhibitors] explode all trees
#22 tyrosine kinase inhibitor*:ti,ab,kw
#23 (tivozanib* or AV‐951 or AV951 or KRN‐951 or KRN951):ti,ab,kw
#24 MeSH descriptor: [Sunitinib] explode all trees
#25 (sunitinib* or "SU‐011248" or SU11248 or sutent):ti,ab,kw
#26 (pazopanib* or GW‐786034 or GW786034 or votrient*):ti,ab,kw
#27 MeSH descriptor: [Sorafenib] explode all trees
#28 (sorafenib* or "bay‐43‐9006" or "bay439006" or "bay‐439006" or "bay‐5459085" or "bay5459085" or "bay‐673472" or "bay673472" or nexavar):ti,ab,kw
#29 MeSH descriptor: [Lapatinib] explode all trees
#30 (lapatinib* or GW‐282974* or GW‐2016 or GW‐572016 or GW2016 or GW572016 or GW282974* or tykerb or tyverb or AZD6094 or AZD‐6094 or HMPL504 or MPL‐504):ti,ab,kw
#31 (savolitinib* or volitinib* or AZD6094 or AZD‐6094 or HMPL504 or HMPL‐504):ti,ab,kw
#32 MeSH descriptor: [Cetuximab] this term only
#33 (cetuximab* or erbitux or c225 or anti‐EGFR monoclonal antibod*):ti,ab,kw
#34 (urelumab* or BMS‐663513 or BMS663513 or anti‐CD137 monoclonal antibod*):ti,ab,kw
#35 (dovitinib* or TKI258 or TKI 258 or chir 258 or chir258):ti,ab,kw
#36 MeSH descriptor: [Axitinib] explode all trees
#37 (axitinib* or AG‐013736 or AG013736 or AG‐13736 or AG‐13736 or inlyta*):ti,ab,kw
#38 (cabozantinib* or XL184 or XL‐184 or cabometyx* or cometriq* or BMS 907351 or BMS907351):ti,ab,kw
#39 (SILA‐9268A or SILA9268A or WY‐090217 or WY090217):ti,ab,kw
#40 cell cycle inhibitor 779:ti,ab,kw
#41 MeSH descriptor: [Erlotinib Hydrochloride] this term only
#42 (Erlotinib* or tarceva* or OSI‐774 or OSI774):ti,ab,kw
#43 #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 #39 or #40 or #41 or #42
#44 mTOR inhibitor*:ti,ab,kw
#45 (apitolisib* or GDC‐0980 or GDC0980 or RG7422 or RG 7422):ti,ab,kw
#46 MeSH descriptor: [Sirolimus] explode all trees
#47 ( sirolimus* or rapamycin* or AY‐22989 or AY22989 or I 2190a or I2190a or cypher or opsiria or perceiva or rapamune):ti,ab,kw
#48 MeSH descriptor: [Everolimus] explode all trees
#49 (everolimus* or rad001 or rad‐001 or sdz‐rad or afinitor* or certican* or zortress):ti,ab,kw
#50 (temsirolimus* or CCI779 or CCI‐779 or rapamune* or torisel*):ti,ab,kw
#51 (tivozanib* or AV‐951 or AV951 or KRN‐951 or KRN951 or KIL‐8951 or KIL8951 or fotivda):ti,ab,kw
#52 #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51
#53 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees
#54 ((angiogenes* or angiogenic* or angiostatic* or angiogenet*) NEAR/2 (antagonist* or inhibitor* or agent*)):ti,ab,kw
#55 ((anti‐angiogenetic* or antiangiogenetic* or anti angiogenes*) NEAR/2 (effect* or agent* or drug*)):ti,ab,kw
#56 (neovascularization* NEAR/2 inhibitor*):ti,ab,kw
#57 (lenvatinib* or E7080 or E‐7080 or "ER‐203492‐00" or "ER‐20349200" or "ER20349200" or "ER203492‐00" or lenvima* or kisplyx):ti,ab,kw
#58 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees
#59 (bevacizumab* or antiVEGF* or rhuMab‐VEGF or ABP‐215 or ABP215 or ainex* or altuzan* or avastin*):ti,ab,kw
#60 #53 or #54 or #55 or #56 or #57 or #58 or #59
#61 MeSH descriptor: [Immunotherapy] explode all trees
#62 immunotherap*:ti,ab,kw
#63 MeSH descriptor: [Interferons] explode all trees
#64 (interfer?on* or cl 884 or cl884):ti,ab,kw
#65 MeSH descriptor: [Interleukins] explode all trees
#66 interleukin*:ti,ab,kw
#67 (atezolizumab* or mpdl3280a or "mpdl‐3280a" or anti‐PDL1 or antiPDL1 or tec?ntriq*):ti,ab,kw
#68 #61 or #62 or #63 or #64 or #65 or #66 or #67
#69 checkpoint inhibitor*:ti,ab,kw
#70 MeSH descriptor: [Nivolumab] explode all trees
#71 (nivolumab* or opdivo* or MDX 1106 or MDX1106 or BMS936558 or BMS‐936558 or CMAB‐819 or CMAB819 or ONO‐4538 or ONO4538):ti,ab,kw
#72 (pembrolizumab* or MK‐3475 or MK3475 or keytruda or lambrolizumab* or sch900475 or sch 900475):ti,ab,kw
#73 (durvalumab* or MEDI4736 or MEDI‐4736 or imfinzi):ti,ab,kw
#74 (tremelimumab* or ticilimumab* or CP‐675 206 or CP‐675206 or CP675206 or CP‐675 or CP675):ti,ab,kw
#75 MeSH descriptor: [Ipilimumab] explode all trees
#76 (ipilimumab* or yervoy* or MDX‐010 or MDX010 or mdx‐ctla‐4 or Anti‐CTLA‐4 MAb or MDX‐101 or MDX101 or BMS‐734016 or BMS34016):ti,ab,kw
#77 (LY2510924 or LY 2510924):ti,ab,kw
#78 #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77
#79 #18 or #43 or #52 or #60 or #68 or #78
#80 #9 and #79
#81 #80 in Trials
Appendix 2. Search strategy for CENTRAL (for all searches up to April 2021)
Cochrane Central Register of Controlled Trials in the Cochrane Library
ID Search
#1 MeSH descriptor: [Carcinoma, Renal Cell] explode all trees
#2 ((collecting duct* or hypernephroid* or nephroid*) NEAR/2 carcinoma*):ti,ab,kw
#3 ((grawitz NEAR/11 tumo?r*) or hypernephroma*).ti,ab,kw
#4 ((renal* or kidney* or nephron*) NEAR/6 (cancer* or neoplasms* or carcinoma* or tumour* or tumor* or adenocarcinoma*)):ti,ab,kw
#5 #1 or #2 or #3 or #4
#6 (advance* or metasta*):ti,ab,kw
#7 #5 and #6
#8 (mRCC or RCC):ti,ab,kw
#9 #7 or #8
#10 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] explode all trees
#11 MeSH descriptor: [Antibodies, Monoclonal, Humanized] explode all trees
#12 MeSH descriptor: [Antibodies, Monoclonal, Humanized] explode all trees
#13 MeSH descriptor: [Antineoplastic Agents] explode all trees
#14 (antibod* near/2 monoclonal*):ti,ab,kw
#15 ((anticancer* or "anti‐cancer*" or anticarcinogen* or antineoplastic* or antitumo?r*) NEAR/2 (agent* or drug*)):ti,ab,kw
#16 antineoplastic*:ti,ab,kw
#17 (antibod* NEAR/2 (neoplasm* or tumo?r* or cancer* or antitumor?r*)):ti,ab,kw
#18 #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17
#19 (car NEAR/1 t cell therap*):ti,ab,kw
#20 (targeted NEAR therap*):ti,ab,kw
#21 MeSH descriptor: [Protein Kinase Inhibitors] explode all trees
#22 tyrosine kinase inhibitor*:ti,ab,kw
#23 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951):ti,ab,kw
#24 MeSH descriptor: [Sunitinib] explode all trees
#25 (sunitinib* or "su 011248" or su11248 or sutent):ti,ab,kw
#26 (pazopanib* or GW 786034 or GW786034 or votrient*):ti,ab,kw
#27 MeSH descriptor: [Sorafenib] explode all trees
#28 (sorafenib* or "bay 43‐9006" or "bay 439006" or "bay 5459085" or "bay5459085" or "bay 673472" or "bay673472" or nexavar):ti,ab,kw
#29 MeSH descriptor: [Lapatinib] explode all trees
#30 (lapatinib* or gw 282974x or gw 2016 or gw2016 or gw 572016 or gw572016 or gw282974x or tykerb or tyverb):ti,ab,kw
#31 (savolitinib* or volitinib*):ti,ab,kw
#32 MeSH descriptor: [Lapatinib] explode all trees
#33 (cetuximab* or erbitux or c225 or anti‐EGFR monoclonal antibod*):ti,ab,kw
#34 (urelumab* or BMS‐663513 or BMS663513 or anti‐CD137 monoclonal antibod*):ti,ab,kw
#35 (dovitinib* or TKI258 or TKI 258 or chir 258 or chir258):ti,ab,kw
#36 MeSH descriptor: [Axitinib] explode all trees
#37 (axitinib* or AG‐013736 or AG013736 or AG‐13736 or AG‐13736 or inlyta*):ti,ab,kw
#38 (cabozantinib* or XL184 or XL 184 or cabometyx* or cometriq* or BMS 907351 or BMS907351):ti,ab,kw
#39 (certican* or cci 779 or cci779 or zortress or AY 22989 or AY22989):ti,ab,kw
#40 (SILA 9268A or SILA9268A or WY‐090217 or WY090217):ti,ab,kw
#41 cell cycle inhibitor 779:ti,ab,kw
#42 MeSH descriptor: [Axitinib] explode all trees
#43 (Erlotinib* or tarceva* or OSI 774 or OSI774):ti,ab,kw
#44 #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43
#45 mTOR inhibitor*:ti,ab,kw
#46 (apitolisib* or GDC‐0980 or GDC0980 or RG7422 or RG 7422):ti,ab,kw
#47 MeSH descriptor: [Sirolimus] explode all trees
#48 (rapamycin* or sirolimus* or ay 22989 or ay22989 or i 2190a or i2190a or cypher or opsiria or perceiva or rapamune):ti,ab,kw
#49 MeSH descriptor: [Everolimus] explode all trees
#50 (everolimus* or "rad 001" or sdz rad or afinitor* or certican*):ti,ab,kw
#51 (temsirolimus* or rapamycin* or "RAD 001" or rapamune* or afinitor* or torisel*):ti,ab,kw
#52 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951 or kil 8951 or kil8951 or fotivda):ti,ab,kw
#53 #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52
#54 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees
#55 ((angiogenes* or angiogenic* or angiostatic* or angiogenet*) NEAR/2 (antagonist* or inhibitor* or agent*)):ti,ab,kw
#56 ((anti‐angiogenetic* or antiangiogenetic* or anti angiogenes*) NEAR/2 (effect* or agent* or drug*)):ti,ab,kw
#57 (neovascularization* NEAR/2 inhibitor*):ti,ab,kw
#58 (lenvatinib* or E7080 or E 7080 or "er 203492‐00" or "er203492‐00" or lenvima* or kisplyx):ti,ab,kw
#59 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees
#60 (bevacizumab* or antiVEGF* or rhuMab‐VEGF or abp 215 or abp215 or ainex* or altuzan* or avastin*):ti,ab,kw
#61 #54 or #55 or #56 or #57 or #58 or #59 or #60
#62 MeSH descriptor: [Immunotherapy] explode all trees
#63 immunotherap*:ti,ab,kw
#64 MeSH descriptor: [Interferons] explode all trees
#65 (interfer?on* or cl 884 or cl884):ti,ab,kw
#66 MeSH descriptor: [Interleukins] explode all trees
#67 interleukin*:ti,ab,kw
#68 (atezolizumab* or mpdl3280a or "mpdl 3280a" or anti‐PDL1 or antiPDL1 or tec?ntriq*):ti,ab,kw
#69 #62 or #63 or #64 or #65 or #66 or #67 or #68
#70 Checkpoint inhibitor*:ti,ab,kw
#71 MeSH descriptor: [Nivolumab] explode all trees
#72 (nivolumab* or opdivo* or MDX 1106 or MDX1106 or bms936558 or bms 936558 or cmab 819 or cmab819 or ono 4538 or ono4538):ti,ab,kw
#73 (pembrolizumab* or MK‐3475 or MK3475 or keytruda or lambrolizumab* or sch900475 or sch 900475):ti,ab,kw
#74 (durvalumab* or MEDI4736 or MEDI 4736 or imfinzi):ti,ab,kw
#75 (tremelimumab* or ticilimumab* or CP‐675 206 or CP‐675206 or CP675206):ti,ab,kw
#76 MeSH descriptor: [Ipilimumab] explode all trees
#77 (ipilimumab* or yervoy* or MDX‐010 or MDX010 or mdx‐ctla‐4 or MDX‐101 or MDX101 or bms 734016 or bms734016):ti,ab,kw
#78 (LY2510924 or LY 2510924):ti,ab,kw
#79 #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78
#80 #18 or #44 or #53 or #61 or #69 or #79
#81 #9 and #80
Appendix 3. Updated search strategy MEDLINE (for the update search in February 2022)
Medline (OVID)
# Searches
1 Carcinoma, Renal Cell/
2 ((collecting duct* or hypernephroid* or nephroid*) adj2 carcinoma*).tw,kf.
3 ((grawitz adj1 tumo?r*) or hypernephroma*).tw,kf.
4 ((renal* or kidney* or nephron*) adj6 (cancer* or neoplasms* or carcinoma* or tumour* or tumor* or adenocarcinoma*)).tw,kf.
5 or/1‐4
6 (advance* or metasta*).tw,kf.
7 5 and 6
8 (mRCC or RCC).tw,kf.
9 7 or 8
10 antineoplastic combined chemotherapy protocols/
11 exp Antibodies, Monoclonal, Humanized/
12 Antineoplastic Agents/ad, ae
13 *Antineoplastic Agents/tu
14 antineoplastic.hw.
15 (antibod* adj2 monoclonal*).tw,kf.
16 ((anticancer* or "anti‐cancer*" or anticarcinogen* or antineoplastic* or antitumo?r*) adj2 (agent* or drug*)).tw,kf.
17 antineoplastic*.tw,kf,nm.
18 (antibod* adj2 (neoplasm* or tumo?r* or cancer* or antitumor?r*)).tw,kf.
19 or/10‐18
20 (car adj1 t cell therap*).tw,kf,nm.
21 (targeted adj therap*).tw,kf.
22 Protein Kinase Inhibitors/
23 tyrosine kinase inhibitor*.tw,kf,nm.
24 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951).tw,kf,nm.
25 sunitinib/
26 (sunitinib* or "su 011248" or su11248 or sutent).tw,kf,nm.
27 (pazopanib* or GW 786034 or GW786034 or votrient*).tw,kf,nm.
28 sorafenib/
29 (sorafenib* or bay 43‐9006 or bay 439006 or bay 5459085 or bay5459085 or bay 673472 or bay673472 or nexavar).tw,kf,nm.
30 Lapatinib/
31 (lapatinib* or GW‐282974* or GW‐2016 or GW‐572016 or GW2016 or GW572016 or GW282974* or tykerb or tyverb or AZD6094 or AZD‐6094 or HMPL504 or HMPL‐504).tw,kf,nm.
32 (savolitinib* or volitinib* or AZD6094 or AZD‐6094 or HMPL504 or HMPL‐504).tw,kf,nm.
33 Cetuximab/
34 (cetuximab* or erbitux or c225 auch c‐225 or anti‐EGFR monoclonal antibod*).tw,kf,nm.
35 (urelumab* or BMS‐663513 or BMS663513 or anti‐CD137 monoclonal antibod*).tw,kf,nm.
36 (dovitinib* or TKI258 or TKI 258 or chir 258 or chir258).tw,kf,nm.
37 Axitinib/
38 (axitinib* or AG‐013736 or AG013736 or AG‐13736 or AG‐13736 or inlyta*).tw,kf,nm.
39 (cabozantinib* or XL184 or XL‐184 or XL184cpd or XL‐184cpd or cabometyx* or cometriq* or BMS‐907351 or BMS907351).tw,kf,nm.
40 (certican* or cci 779 or cci779 or zortress or AY 22989 or AY22989).tw,kf,nm. Rausnehmen!!!
41 (SILA 9268A or SILA9268A or WY‐090217 or WY090217).tw,kf,nm.
42 cell cycle inhibitor 779.tw,kf,nm.
43 Erlotinib Hydrochloride/
44 (Erlotinib* or tarceva* or OSI 774 or OSI774).tw,kf,nm.
45 or/20‐44
46 mTOR inhibitor*.tw,kf.
47 (apitolisib* or GDC‐0980 or GDC0980 or RG7422 or RG 7422).tw,kf,nm.
48 Sirolimus/
49 (rapamycin* or sirolimus* or ay 22989 or ay22989 or i 2190a or i2190a or cypher or opsiria or perceiva or rapamune).tw,kw,nm.
50 Everolimus/
51 (everolimus* or rad001 or rad‐001 or sdz‐rad or afinitor* or certican* or zortress).tw,kf,nm.
52 (temsirolimus* or rapamycin* or CCI779 or CCI‐779 or rapamune* or torisel*).tw,kf,nm.
53 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951 or kil 8951 or kil8951 or fotivda).tw,kf,nm.
54 or/46‐53
55 exp Angiogenesis Inhibitors/
56 ((angiogenes* or angiogenic* or angiostatic* or angiogenet*) adj2 (antagonist* or inhibitor* or agent*)).tw,kf.
57 ((anti‐angiogenetic* or antiangiogenetic* or anti angiogenes*) adj2 (effect* or agent* or drug*)).tw,kf.
58 (neovascularization* adj2 inhibitor*).tw,kf.
59 (lenvatinib* or E7080 or E 7080 or er 203492‐00 or er203492‐00 or lenvima* or kisplyx).tw,kf,nm.
60 Bevacizumab/
61 (bevacizumab* or antiVEGF* or rhuMab‐VEGF or abp 215 or abp215 or ainex* or altuzan* or avastin*).tw,kf,nm.
62 or/55‐61
63 Immunotherapy/
64 immunotherap*.tw,kf.
65 exp Interferons/
66 (interfer?on* or cl 884 or cl884).tw,kf.
67 exp Interleukins/
68 interleukin*.tw,kf,nm.
69 (atezolizumab* or mpdl3280a or mpdl 3280a or RG‐7446 or RG744 or anti‐PDL1 or antiPDL1 or tec?ntriq*).tw,kf.
70 or/63‐69
71 Checkpoint inhibitor*.tw,kf.
72 Nivolumab/
73 (nivolumab* or opdivo* or MDX 1106 or MDX1106 or bms936558 or bms 936558 or cmab 819 or cmab819 or ono 4538 or ono4538).tw,kf,nm.
74 (pembrolizumab* or MK‐3475 or MK3475 or keytruda or lambrolizumab* or sch900475 or sch 900475).tw,kf,nm.
75 (durvalumab* or MEDI4736 or MEDI 4736 or imfinzi).tw,kf,nm.
76 (tremelimumab* or ticilimumab* or CP‐675 206 or CP‐675206 or CP675206 or CP‐675 or CP675).tw,kf,nm.
77 Ipilimumab/
78 (ipilimumab* or yervoy* or MDX‐010 or MDX010 or mdx‐ctla‐4 or Anti‐CTLA‐4 MAb or MDX‐101 or MDX101 or bms 734016 or bms734016).tw,kf,nm.
79 (LY2510924 or LY 2510924).tw,kf,nm.
80 or/71‐79
81 19 or 45 or 54 or 62 or 70 or 80
82 9 and 81
83 randomized controlled trial.pt.
84 controlled clinical trial.pt.
85 randomi?ed.ab.
86 placebo.ab.
87 drug therapy.fs.
88 randomly.ab.
89 trial.ab.
90 groups.ab.
91 or/83‐90
92 exp animals/ not humans/
93 91 not 92
94 clinical trial, phase iii/
95 ("Phase 3" or "phase3" or "phase III" or P3 or "PIII").ti,ab,kw.
96 (94 or 95) not 92
97 93 or 96
98 82 and 97
Appendix 4. Search strategy for MEDLINE (for all searches up to April 2021)
MEDLINE (Ovid)
# Searches
1 CARCINOMA, RENAL CELL/
2 ((collecting duct* or hypernephroid* or nephroid*) adj2 carcinoma*).tw,kf.
3 ((grawitz adj1 tumo?r*) or hypernephroma*).tw,kf.
4 ((renal* or kidney* or nephron*) adj6 (cancer* or neoplasms* or carcinoma* or tumour* or tumor* or adenocarcinoma*)).tw,kf.
5 or/1‐4
6 (advance* or metasta*).tw,kf.
7 5 and 6
8 (mRCC or RCC).tw,kf.
9 7 or 8
10 ANTINEOPLASTIC COMBINED CHEMOTHERAPY PROTOCOLS/
11 exp ANTIBODIES, MONOCLONAL, HUMANIZED/
12 ANTINEOPLASTIC AGENTS/ad, ae
13 *ANTINEOPLASTIC AGENTS/tu
14 antineoplastic.hw.
15 (antibod* adj2 monoclonal*).tw,kf.
16 ((anticancer* or "anti‐cancer*" or anticarcinogen* or antineoplastic* or antitumo?r*) adj2 (agent* or drug*)).tw,kf.
17 antineoplastic*.tw,kf.
18 (antibod* adj2 (neoplasm* or tumo?r* or cancer* or antitumor?r*)).tw,kf.
19 or/10‐18
20 (car adj1 t cell therap*).tw,kf,nm.
21 (targeted adj therap*).tw,kf.
22 PROTEIN KINASE INHIBITORS/
23 tyrosine kinase inhibitor*.tw,kf.
24 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951).tw,kf,nm.
25 SUNITINIB/
26 (sunitinib* or "su 011248" or su11248 or sutent).tw,kf,nm.
27 (pazopanib* or GW 786034 or GW786034 or votrient*).tw,kf,nm.
28 SORAFENIB/
29 (sorafenib* or bay 43‐9006 or bay 439006 or bay 5459085 or bay5459085 or bay 673472 or bay673472 or nexavar).tw,kf,nm.
30 LAPATINIB/
31 (lapatinib* or gw 282974x or gw 2016 or gw2016 or gw 572016 or gw572016 or gw282974x or tykerb or tyverb).tw,kw.
32 (savolitinib* or volitinib*).tw,kf,nm.
33 CETUXIMAB/
34 (cetuximab* or erbitux or c225 or anti‐EGFR monoclonal antibod*).tw,kf,nm.
35 (urelumab* or BMS‐663513 or BMS663513 or anti‐CD137 monoclonal antibod*).tw,kf,nm.
36 (dovitinib* or TKI258 or TKI 258 or chir 258 or chir258).tw,kf,nm.
37 AXITINIB/
38 (axitinib* or AG‐013736 or AG013736 or AG‐13736 or AG‐13736 or inlyta*).tw,kf,nm.
39 (cabozantinib* or XL184 or XL 184 or cabometyx* or cometriq* or BMS 907351 or BMS907351).tw,kf,nm.
40 (certican* or cci 779 or cci779 or zortress or AY 22989 or AY22989).tw,kf,nm.
41 (SILA 9268A or SILA9268A or WY‐090217 or WY090217).tw,kf,nm.
42 cell cycle inhibitor 779.tw,kf,nm.
43 ERLOTINIB HYDROCHLORIDE/
44 (Erlotinib* or tarceva* or OSI 774 or OSI774).tw,kf,nm.
45 or/20‐44
46 mTOR inhibitor*.tw,kf.
47 (apitolisib* or GDC‐0980 or GDC0980 or RG7422 or RG 7422).tw,kf,nm.
48 SIROLIMUS/
49 (rapamycin* or sirolimus* or ay 22989 or ay22989 or i 2190a or i2190a or cypher or opsiria or perceiva or rapamune).tw,kw,nm.
50 EVEROLIMUS/
51 (everolimus* or "rad 001" or sdz rad or afinitor* or certican*).tw,kf,nm.
52 (temsirolimus* or rapamycin* or "RAD 001" or rapamune* or afinitor* or torisel*).tw,kf,nm.
53 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951 or kil 8951 or kil8951 or fotivda).tw,kf,nm.
54 or/46‐53
55 exp ANGIOGENESIS INHIBITORS/
56 ((angiogenes* or angiogenic* or angiostatic* or angiogenet*) adj2 (antagonist* or inhibitor* or agent*)).tw,kf.
57 ((anti‐angiogenetic* or antiangiogenetic* or anti angiogenes*) adj2 (effect* or agent* or drug*)).tw,kf.
58 (neovascularization* adj2 inhibitor*).tw,kf.
59 (lenvatinib* or E7080 or E 7080 or er 203492‐00 or er203492‐00 or lenvima* or kisplyx).tw,kf,nm.
60 BEVACIZUMAB/
61 (bevacizumab* or antiVEGF* or rhuMab‐VEGF or abp 215 or abp215 or ainex* or altuzan* or avastin*).tw,kf,nm.
62 or/55‐61
63 IMMUNOTHERAPY/
64 immunotherap*.tw,kf.
65 exp INTERFERONS/
66 (interfer?on* or cl 884 or cl884).tw,kf.
67 exp INTERLEUKINS/
68 interleukin*.tw,kf,nm.
69 (atezolizumab* or mpdl3280a or mpdl 3280a or anti‐PDL1 or antiPDL1 or tec?ntriq*).tw,kf.
70 or/63‐69
71 Checkpoint inhibitor*.tw,kf.
72 NIVOLUMAB/
73 (nivolumab* or opdivo* or MDX 1106 or MDX1106 or bms936558 or bms 936558 or cmab 819 or cmab819 or ono 4538 or ono4538).tw,kf,nm.
74 (pembrolizumab* or MK‐3475 or MK3475 or keytruda or lambrolizumab* or sch900475 or sch 900475).tw,kf,nm.
75 (durvalumab* or MEDI4736 or MEDI 4736 or imfinzi).tw,kf,nm.
76 (tremelimumab* or ticilimumab* or CP‐675 206 or CP‐675206 or CP675206).tw,kf,nm.
77 IPILIMUMAB/
78 (ipilimumab* or yervoy* or MDX‐010 or MDX010 or mdx‐ctla‐4 or MDX‐101 or MDX101 or bms 734016 or bms734016).tw,kf,nm.
79 (LY2510924 or LY 2510924).tw,kf,nm.
80 or/71‐79
81 19 or 45 or 54 or 62 or 70 or 80
82 9 and 81
83 randomized controlled trial.pt.
84 controlled clinical trial.pt.
85 randomi?ed.ab.
86 placebo.ab.
87 drug therapy.fs.
88 randomly.ab.
89 trial.ab.
90 groups.ab.
91 or/83‐90
92 exp ANIMALS/ not HUMANS/
93 91 not 92
94 CLINICAL TRIAL, PHASE III/
95 ("Phase 3" or "phase3" or "phase III" or P3 or "PIII").ti,ab,kw.
96 (94 or 95) not 92
97 93 or 96
98 82 and 97
99 limit 98 to dt=20200218‐20201025
Appendix 5. Updated search strategy for Embase (for the update search in February 2022)
Embase (Ovid)
# Searches
1 renal cell carcinoma/
2 ((collecting duct* or hypernephroid* or nephroid*) adj2 carcinoma*).tw,kw.
3 ((grawitz adj1 tumo?r*) or hypernephroma*).tw,kw.
4 ((renal* or kidney* or nephron*) adj6 (cancer* or neoplasms* or carcinoma* or tumor* or tumour* or adenocarcinoma*)).tw,kw.
5 or/1‐4
6 (advance* or metasta*).tw,kw.
7 5 and 6
8 (mRCC or RCC).tw,kw.
9 7 or 8
10 exp monoclonal antibody/
11 antineoplastic agent/ae, ad [Adverse Drug Reaction, Drug Administration]
12 *antineoplastic agent/dt [Drug Therapy]
13 antineoplastic.hw.
14 (antibod* adj2 monoclonal*).tw,kw.
15 ((anticancer* or "anti cancer*" or anitcarcinogen* or antitumo?r* or "anti tumo?r*") adj2 (agent* or drug*)).tw,kw.
16 antineoplastic*.tw,kw.
17 (antibod* adj2 (neoplasm* or tumo?r* or cancer* or antitumo?r*)).tw,kw.
18 or/10‐17
19 (car adj1 t cell therap*).tw,kw.
20 "CAR T cell therapy"/
21 chimeric antigen receptor T‐cell/dt
22 protein kinase inhibitor/
23 tyrosine kinase inhibitor*.tw,kw.
24 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951).tw,kw.
25 sunitinib/
26 (sunitinib* or "su 011248" or su11248 or sutent).tw,kw.
27 pazopanib/
28 (pazopanib* or GW 786034 or GW786034 or votrient*).tw,kw.
29 sorafenib/
30 (sorafenib* or bay 43‐9006 or bay 439006 or bay439006 or bay 5459085 or bay5459085 or bay 673472 or bay673472 or nexavar).tw,kw.
31 lapatinib/ or lapatinib plus pazopanib/
32 (lapatinib* or gw 282974x or gw 2016 or gw2016 or gw 572016 or gw572016 or gw282974x or tykerb or tyverb).tw,kw.
33 savolitinib/
34 (volitinib* or savolitinib* or azd 6094 or azd6094 or hmpl 504 or hmpl504).tw,kw.
35 cetuximab/
36 (cetuximab* or c 225 or c225 or erbitux or imc 225 or imc c225 or imc‐c225 or imc225 or imcc 225 or monoclonal antibody C 225).tw,kw.
37 urelumab/
38 (urelumab* or BMS‐663513 or BMS663513 or anti‐CD137 monoclonal antibod*).tw,kw.
39 dovitinib/
40 (dovitinib* or TKI258 or TKI 258 or chir 258 or chir258).tw,kw.
41 axitinib/
42 (axitinib* or AG‐013736 or AG013736 or AG‐13736 or AG‐13736 or inlyta*).tw,kw.
43 cabozantinib/
44 (cabozantinib* or XL184 or XL 184 or cabometyx* or cometriq* or BMS 907351 or BMS907351).tw,kw.
45 (SILA 9268A or SILA9268A or WY‐090217 or WY090217).tw,kw.
46 cell cycle inhibitor 779.tw,kw.
47 erlotinib/
48 (erlotinib* or tarceva* or OSI 774 or OSI774).tw,kw.
49 or/19‐48
50 mTOR inhibitor*.tw,kw.
51 (apitolisib* or GDC‐0980 or GDC0980 or RG7422 or RG 7422).tw,kw.
52 rapamycin/
53 (rapamycin* or sirolimus* or ay 22989 or ay22989 or i 2190a or i2190a or cypher or opsiria or perceiva or rapamune).tw,kw.
54 everolimus/
55 (everolimus* or "rad 001" or rad001 or sdz rad or afinitor* or certican* or zortress).tw,kw.
56 temsirolimus/
57 (temsirolimus* or rapamycin* or CCI779 or CCI‐779 or rapamune* or torisel*).tw,kw.
58 tivozanib/
59 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951 or kil 8951 or kil8951 or fotivda).tw,kw.
60 or/50‐59
61 angiogenesis inhibitor/
62 ((angiogenes* or angiogenic* or angiostatic* or angiogenet*) adj2 (antagonist* or inhibitor* or agent*)).tw,kw.
63 ((anti‐angiogenetic* or antiangiogenetic* or anti angiogenes* or antiangiogenic*) adj2 (effect* or agent* or drug*)).tw,kw.
64 (neovascularization* adj2 inhibitor*).tw,kw.
65 ((neovascularization* or vascularization*) adj2 inhibitor*).tw,kw.
66 lenvatinib/
67 (lenvatinib* or E7080 or E 7080 or er 203492‐00 or er203492‐00 or lenvima* or kisplyx).tw,kw.
68 bevacizumab/
69 (bevacizumab* or antiVEGF* or rhuMab‐VEGF or abp 215 or abp215 or ainex* or altuzan* or avastin*).tw,kw.
70 or/61‐69
71 chimeric antigen receptor immunotherapy/
72 cancer immunotherapy/
73 immunotherap*.tw,kw.
74 interferon/
75 (interfer?on* or cl 884 or cl884).tw,kw.
76 interleukin derivative/
77 interleukin*.tw,kw.
78 atezolizumab/
79 (atezolizumab* or mpdl3280a or mpdl 3280a or anti‐PDL1 or antiPDL1 or tec?ntriq*).tw,kw.
80 checkpoint inhibitor*.tw,kw.
81 nivolumab/
82 (nivolumab* or opdivo* or MDX 1106 or MDX1106 or bms936558 or bms 936558 or cmab 819 or cmab819 or ono 4538 or ono4538).tw,kw.
83 pembrolizumab/
84 (pembrolizumab* or MK‐3475 or MK3475 or keytruda or lambrolizumab* or sch900475 or sch 900475).tw,kw.
85 durvalumab/
86 (durvalumab* or MEDI4736 or MEDI 4736 or imfinzi).tw,kw.
87 ticilimumab/
88 (tremelimumab* or ticilimumab* or CP‐675 206 or CP‐675206 or CP675206).tw,kw.
89 ipilimumab/
90 (ipilimumab* or yervoy* or MDX‐010 or MDX010 or mdx‐ctla‐4 or MDX‐101 or MDX101 or mdx010 or bms 734016 or bms734016).tw,kw.
91 (LY2510924 or LY 2510924).tw,kw.
92 or/71‐91
93 Randomized controlled trial/
94 Controlled clinical study/
95 random*.ti,ab.
96 randomization/
97 intermethod comparison/
98 placebo.ti,ab.
99 (compare or compared or comparison).ti.
100 (open adj label).ti,ab.
101 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
102 double blind procedure/
103 parallel group$1.ti,ab.
104 (crossover or cross over).ti,ab.
105 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
106 (controlled adj7 (study or design or trial)).ti,ab.
107 (volunteer or volunteers).ti,ab.
108 trial.ti.
109 or/93‐108
110 (animal experiment/ or Animal experiment/) not (human experiment/ or human/)
111 109 not 110
112 18 or 49 or 60 or 70 or 92
113 9 and 112 and 111
Appendix 6. Search strategy for Embase (for all searches up to April 2021)
Embase (Ovid)
# Searches
1 RENAL CELL CARCINOMA/
2 ((collecting duct* or hypernephroid* or nephroid*) adj2 carcinoma*).tw,kw.
3 ((grawitz adj1 tumo?r*) or hypernephroma*).tw,kw.
4 ((renal* or kidney* or nephron*) adj6 (cancer* or neoplasms* or carcinoma* or tumor* or tumour* or adenocarcinoma*)).tw,kw.
5 or/1‐4
6 (advance* or metasta*).tw,kw.
7 5 and 6
8 (mRCC or RCC).tw,kw.
9 7 or 8
10 exp MONOCLONAL ANTIBODY/
11 ANTINEOPLASTIC AGENT/ae, ad
12 *antineoplastic agent/dt
13 antineoplastic.hw.
14 (antibod* adj2 monoclonal*).tw,kw.
15 ((anticancer* or "anti cancer*" or anitcarcinogen* or antitumo?r* or anti tumo?r*) adj2 (agent* or drug*)).tw,kw.
16 antineoplastic*.tw,kw.
17 (antibod* adj2 (neoplasm* or tumo?r* or cancer* or antitumo?r*)).tw,kw.
18 or/10‐17
19 (car adj1 t cell therap*).tw,kw.
20 "CAR T cell therapy"/
21 CHIMERIC ANTIGEN RECEPTOR T‐CELL/dt
22 PROTEIN KINASE INHIBITOR/
23 tyrosine kinase inhibitor*.tw,kw.
24 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951).tw,kw.
25 SUNITINIB/
26 (sunitinib* or "su 011248" or su11248 or sutent).tw,kw.
27 PAZOPANIB/
28 (pazopanib* or GW 786034 or GW786034 or votrient*).tw,kw.
29 SORAFENIB/
30 (sorafenib* or bay 43‐9006 or bay 439006 or bay 5459085 or bay5459085 or bay 673472 or bay673472 or nexavar).tw,kw.
31 LAPATINIB/ or LAPATINIB PLUS PAZOPANIB/
32 (lapatinib* or gw 282974x or gw 2016 or gw2016 or gw 572016 or gw572016 or gw282974x or tykerb or tyverb).tw,kw.
33 SAVOLITINIB/
34 (volitinib* or savolitinib* or azd 6094 or azd6094 or hmpl 504 or hmpl504).tw,kw.
35 CETUXIMAB/
36 (cetuximab* or c 225 or c225 or erbitux or imc 225 or imc c225 or imc‐c225 or imc225 or imcc 225 or monoclonal antibody C 225).tw,kw.
37 URELUMAB/
38 (urelumab* or BMS‐663513 or BMS663513 or anti‐CD137 monoclonal antibod*).tw,kw.
39 DOVITINIB/
40 (dovitinib* or TKI258 or TKI 258 or chir 258 or chir258).tw,kw.
41 AXITINIB/
42 (axitinib* or AG‐013736 or AG013736 or AG‐13736 or AG‐13736 or inlyta*).tw,kw.
43 CABOZANTINIB/
44 (cabozantinib* or XL184 or XL 184 or cabometyx* or cometriq* or BMS 907351 or BMS907351).tw,kw.
45 (certican* or cci 779 or cci779 or zortress or AY 22989 or AY22989).tw,kw.
46 (SILA 9268A or SILA9268A or WY‐090217 or WY090217).tw,kw.
47 cell cycle inhibitor 779.tw,kw.
48 ERLOTINIB/
49 (erlotinib* or tarceva* or OSI 774 or OSI774).tw,kw.
50 or/19‐49
51 mTOR inhibitor*.tw,kw.
52 (apitolisib* or GDC‐0980 or GDC0980 or RG7422 or RG 7422).tw,kw.
53 RAPAMYCIN/
54 (rapamycin* or sirolimus* or ay 22989 or ay22989 or i 2190a or i2190a or cypher or opsiria or perceiva or rapamune).tw,kw.
55 EVEROLIMUS/
56 (everolimus* or "rad 001" or rad001 or sdz rad or afinitor* or certican*).tw,kw.
57 TEMSIROLIMUS/
58 (temsirolimus* or cci 779 or cci779 or afinitor* or torisel*).tw,kw.
59 TIVOZANIB/
60 (tivozanib* or AV‐951 or AV951 or KRN 951 or KRN951 or kil 8951 or kil8951 or fotivda).tw,kw.
61 or/51‐60
62 ANGIOGENESIS INHIBITOR/
63 ((angiogenes* or angiogenic* or angiostatic* or angiogenet*) adj2 (antagonist* or inhibitor* or agent*)).tw,kw.
64 ((anti‐angiogenetic* or antiangiogenetic* or anti angiogenes* or antiangiogenic*) adj2 (effect* or agent* or drug*)).tw,kw.
65 (neovascularization* adj2 inhibitor*).tw,kw.
66 ((neovascularization* or vascularization*) adj2 inhibitor*).tw,kw.
67 LENVATINIB/
68 (lenvatinib* or E7080 or E 7080 or er 203492‐00 or er203492‐00 or lenvima* or kisplyx).tw,kw.
69 BEVACIZUMAB/
70 (bevacizumab* or antiVEGF* or rhuMab‐VEGF or abp 215 or abp215 or ainex* or altuzan* or avastin*).tw,kw.
71 or/62‐70
72 CHIMERIC ANTIGEN RECEPTOR IMMUNOTHERAPY/
73 cancer immunotherapy/
74 immunotherap*.tw,kw.
75 INTERFERON/
76 (interfer?on* or cl 884 or cl884).tw,kw.
77 interleukin derivative/
78 interleukin*.tw,kw.
79 ATEZOLIZUMAB/
80 (atezolizumab* or mpdl3280a or mpdl 3280a or anti‐PDL1 or antiPDL1 or tec?ntriq*).tw,kw.
81 checkpoint inhibitor*.tw,kw.
82 NIVOLUMAB/
83 (nivolumab* or opdivo* or MDX 1106 or MDX1106 or bms936558 or bms 936558 or cmab 819 or cmab819 or ono 4538 or ono4538).tw,kw.
84 PEMBROLIZUMAB/
85 (pembrolizumab* or MK‐3475 or MK3475 or keytruda or lambrolizumab* or sch900475 or sch 900475).tw,kw.
86 DURVALUMAB/
87 (durvalumab* or MEDI4736 or MEDI 4736 or imfinzi).tw,kw.
88 TICILIMUMAB/
89 (tremelimumab* or ticilimumab* or CP‐675 206 or CP‐675206 or CP675206).tw,kw.
90 IPILIMUMAB/
91 (ipilimumab* or yervoy* or MDX‐010 or MDX010 or mdx‐ctla‐4 or MDX‐101 or MDX101 or mdx010 or bms 734016 or bms734016).tw,kw.
92 (LY2510924 or LY 2510924).tw,kw.
93 or/72‐92
94 RANDOMIZED CONTROLLED TRIAL/
95 CONTROLLED CLINICAL STUDY/
96 random*.ti,ab.
97 RANDOMIZATION/
98 INTERMETHOD COMPARISON/
99 placebo.ti,ab.
100 (compare or compared or comparison).ti.
101 (open adj label).ti,ab.
102 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
103 DOUBLE BLIND PROCEDURE/
104 parallel group$1.ti,ab.
105 (crossover or cross over).ti,ab.
106 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
107 (controlled adj7 (study or design or trial)).ti,ab.
108 (volunteer or volunteers).ti,ab.
109 trial.ti.
110 or/94‐109
111 (ANIMAL EXPERIMENT/ or ANIMAL EXPERIMENT/) not (HUMAN EXPERIMENT/ or HUMAN/)
112 110 not 111
113 18 or 50 or 61 or 71 or 93
114 9 and 113
115 112 and 114
Appendix 7. Updated search strategy for clinical trial registries (for the update search in February 2022)
ClinicalTrials.gov (https://clinicaltrials.gov/)
expert search
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) AND (axitinib OR cabozantinib OR dovitinib OR erlotinib OR lapatinib OR pazopanib OR savolitinib OR sorafenib OR sunitinib OR bevacizumab OR lenvatinib OR temsirolimus OR everolimus OR nivolumab OR ipilimumab OR pembrolizumab OR lambrolizumab OR atezolizumab OR durvalumab OR tremelimumab OR ticilimumab OR cetuximab OR urelumab OR interferon OR interleukin OR apitolisib OR LY2510924 OR tivozanib OR certican OR "cci 779" OR cci779 OR zortress OR "AY 22989" OR AY22989 OR SILA 9268A OR SILA9268A OR WY‐090217 OR WY090217 OR rapamycin or sirolimus OR "ay 22989" OR ay22989 OR "i 2190a" OR i2190a OR cypher OR opsiria OR perceiva OR rapamune)
Interventional Studies
WHO ICTRP (https://trialsearch.who.int/)
Advanced search
1. Condition: "advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer" Intervention: axitinib OR cabozantinib OR dovitinib OR erlotinib OR lapatinib OR pazopanib OR savolitinib OR sorafenib OR sunitinib Recruitment status: ALL
2. Condition: "advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer" Intervention: bevacizumab OR lenvatinib OR temsirolimus OR everolimus OR nivolumab OR ipilimumab OR pembrolizumab OR lambrolizumab OR atezolizumab Recruitment status: ALL
3. Condition: "advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer" Intervention: durvalumab OR tremelimumab OR ticilimumab OR cetuximab OR urelumab OR interferon OR interleukin OR apitolisib OR LY2510924 OR tivozanib Recruitment status: ALL
4. Condition: "advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer" Intervention: certican* OR "cci 779" OR cci779 OR zortress OR "AY 22989" OR AY22989 OR "SILA 9268A" OR SILA9268A OR "WY‐090217" OR WY090217 Recruitment status: ALL
EU‐clincal trials register (www.clinicaltrialsregister.eu)
1. ("advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer") AND (axitinib OR cabozantinib OR dovitinib OR erlotinib OR lapatinib OR pazopanib OR savolitinib OR sorafenib OR sunitinib)
2. ("advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer") AND (bevacizumab OR lenvatinib OR temsirolimus OR everolimus)
3. ("advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer") AND (nivolumab OR ipilimumab OR pembrolizumab OR lambrolizumab OR atezolizumab)
4. ("advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer") AND (durvalumab OR tremelimumab OR ticilimumab OR cetuximab OR urelumab)
5. ("advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer") AND (interferon OR interleukin OR apitolisib OR LY2510924 OR tivozanib)
6. ("advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer") AND (certican* OR "cci 779" OR cci779 OR zortress OR "AY 22989" OR AY22989)
7. ("advanced renal cell cancer" OR "advanced renal cell carcinoma" OR "metastatic renal cell carcinoma" OR "metastatic renal cell cancer") AND ("SILA 9268A" OR SILA9268A OR "WY‐090217" OR WY090217)
Appendix 8. Search strategy for clinical trial registers (for all searches up to April 2021)
ClinicalTrials.gov (clinicaltrials.gov)
Basic Search Advanced Renal Cell Carcinoma AND axitinib Advanced Renal Cell Carcinoma AND cabozantinib Advanced Renal Cell Carcinoma AND pazopanib Advanced Renal Cell Carcinoma AND sorafenib Advanced Renal Cell Carcinoma AND sunitinib
Advanced search Conditions: Advanced Renal Cell Carcinoma Interventions: axitinib OR cabozantinib OR pazopanib OR sorafenib OR sunitinib 133/ nicht verwendbar !!!!! axitinib OR cabozantinib OR dovitinib OR erlotinib OR lapatinib OR pazopanib OR savolitinib OR sorafenib OR sunitinib bevacizumab OR levantinib temsirolimus OR everolimus nivolumab OR ipilimumab OR pembrolizumab OR Lambrolizumab OR Atezolizumab Durvalumab OR tremelimumab OR ticilimumab OR Cetuximab OR Urelumab Interferon Interleukin Recruitment: All studies Study type: Interventional studies Age: adult, older adult
previous searches in ClincalTrials.gov
Interventional Studies | advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer | axitinib OR cabozantinib OR dovitinib OR erlotinib OR lapatinib OR pazopanib OR savolitinib OR sorafenib OR sunitinib | Adult, Older Adult
Interventional Studies | advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer | bevacizumab OR levantinib | Adult, Older Adult
Interventional Studies | advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer | temsirolimus OR everolimus | Adult, Older Adult
Interventional Studies | advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer | nivolumab OR ipilimumab OR pembrolizumab OR Lambrolizumab OR Atezolizumab | Adult, Older Adult
Interventional Studies | advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer | Durvalumab OR tremelimumab OR ticilimumab OR Cetuximab OR Urelumab | Adult, Older Adult
Interventional Studies | advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer | Interferon | Adult, Older Adult
Interventional Studies | advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer | Interleukin | Adult, Older Adult
WHO ICTRP apps.who.int/trialsearch/AdvSearch.aspx
Advanced search 1. Condition: advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer Intervention: axitinib OR cabozantinib OR dovitinib OR erlotinib OR lapatinib OR pazopanib OR savolitinib OR sorafenib OR sunitinib Recruitment status: ALL 2. Condition: advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer Intervention: bevacizumab OR levantinib Recruitment status: ALL 3. Condition: advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer Intervention: temsirolimus OR everolimus Recruitment status: ALL 4. Condition: advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer Intervention: nivolumab OR ipilimumab OR pembrolizumab OR Lambrolizumab OR Atezolizumab Recruitment status: ALL 5. Condition: advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer Intervention: durvalumab OR tremelimumab OR ticilimumab OR Cetuximab OR urelumab Recruitment status: ALL 6. Condition: advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer Intervention: Interferon Recruitment status: ALL 7. Condition: advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer Intervention: Interleukin Recruitment status: ALL EU‐clincal trials register (www.clinicaltrialsregister.eu)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and (axitinib OR cabozantinib OR dovitinib OR erlotinib OR lapatinib OR pazopanib OR savolitinib OR sorafenib OR sunitinib)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) AND (bevacizumab OR levantinib)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) AND (temsirolimus OR everolimus)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and (nivolumab OR ipilimumab OR pembrolizumab OR Lambrolizumab OR Atezolizumab)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and (durvalumab OR tremelimumab OR ticilimumab OR Cetuximab OR urelumab)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and interferon
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and interleukin
ISRCTN (www.isrctn.com)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and (axitinib OR cabozantinib OR dovitinib OR erlotinib OR lapatinib OR pazopanib OR savolitinib OR sorafenib OR sunitinib)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) AND (bevacizumab OR levantinib)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) AND (temsirolimus OR everolimus)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) AND (nivolumab OR ipilimumab OR pembrolizumab OR Lambrolizumab OR Atezolizumab)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and (durvalumab OR tremelimumab OR ticilimumab OR Cetuximab OR urelumab)
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and interferon
(advanced renal cell cancer OR advanced renal cell carcinoma OR metastatic renal cell carcinoma OR metastatic renal cell cancer) and interleukin
Appendix 9. Risk of bias assessment for the outcome overall survival
| Trial | Risk of bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
|
NCT03141177 IMDC favourable risk group |
Low risk of bias | Interactive Response Technology was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 8 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 1.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 3% of those who received treatment discontinued due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | High risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC risk group) were pre‐specified in the protocol and SAP. However, the time point that produced this numerical result was not pre‐specified. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data and inconsistency with pre‐planned analyses. |
|
NCT03141177 IMDC intermediate risk group |
Low risk of bias | Interactive Response Technology was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 8 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 1.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 3% of those who received treatment discontinued due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | High risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC risk group) were pre‐specified in the protocol and SAP. However, the time point that produced this numerical result was not pre‐specified. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data and inconsistency with pre‐planned analyses. |
|
NCT03141177 IMDC poor risk group |
Low risk of bias | Interactive Response Technology was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 8 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 1.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 3% of those who received treatment discontinued due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | High risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC risk group) were pre‐specified in the protocol and SAP. However, the time point that produced this numerical result was not pre‐specified. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data and inconsistency with pre‐planned analyses. |
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported analyses were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) MSKCC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per MSKCC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) MSKCC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per MSKCC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) MSKCC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per MSKCC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) IMDC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 2 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC risk group) were pre‐specified in the protocol and SAP. | High risk of bas | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) IMDC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 2 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not further explained). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) IMDC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 2 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported analyses were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) MSKCC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per MSKCC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) MSKCC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bais | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not further explained). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per MSKCC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) MSKCC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per MSKCC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) IMDC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 6 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information on about missing outcome data. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) IMDC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 6 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) IMDC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 6 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Low risk of bias | A study protocol with SAP available. All reported subgroup analyses (including analyses per IMDC risk group) were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data. |
|
NCT01108445 Total trial population (combined risk groups) |
Low risk of bias | Participants were randomised in a 1:1 ratio. Randomisation was done under allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant withdrew after consent, but before randomisation and before study drug was assigned. The method of analysis was appropriate (ITT). |
Low risk of bias | All 108 participants were evaluable. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT01392183 Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. No statement about the method of analysis. | Low risk of bias | Detailed flow diagram provided, no indication of loss to follow‐up. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the allocation concealment and method of analysis; missing study protocol and SAP. |
|
NCT00334282 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was blinded: both participants and those delivering the intervention were not aware of assigned interventions. The method of analysis was appropriate (ITT). | Low risk of bias | 1.3% of those who received treatment were lost to follow‐up. No analysis to correct for bias, but numbers are low and probably did not have an effect on the outcome. |
Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Low risk of bias | CSR and study protocol with SAP available. All reported analyses were pre‐specified in the protocol and SAP. | Low risk of bias | Overall judged low risk of bias. |
|
NCT00065468 Comparison 1 (TEM vs. IFN) Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the single‐drug arm and 7 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.9% did not receive the intended interventions and therefore did not have outcome data. 2% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about the allocation concealment; missing study protocol and SAP. |
|
NCT00065468 Comparison 2 (IFN+TEM vs. IFN) Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the combination arm and 7 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. 1.7% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about the allocation concealment; missing study protocol and SAP. |
|
NCT00738530 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 6 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. 4.4% of those who received treatment withdrew consent or were lost to follow‐up before final data cut off for OS analysis. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT00738530 MSKCC favourable risk group |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 28 participants randomised to the experimental arm and 24 participants randomised to the control arm. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 6 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. 4.4% of those who received treatment withdrew consent or were lost to follow‐up before final data cut off for OS analysis. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT00738530 MSKCC intermediate risk group |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 28 participants randomised to the experimental arm and 24 participants randomised to the control arm. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 6 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. 4.4% of those who received treatment withdrew consent or were lost to follow‐up before final data cut off for OS analysis. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT00738530 MSKCC poor risk group |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 28 participants randomised to the experimental arm and 24 participants randomised to the control arm. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 6 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. 4.4% of those who received treatment withdrew consent or were lost to follow‐up before final data cut off for OS analysis. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT00072046 Total trial population (combined risk groups) |
Some concerns | Participants were randomised via a stratified random block design, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 13 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. Of those who received treatment, less than 1% were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement (objective outcome). | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing information about the allocation concealment; missing study protocol and SAP. |
|
NCT00072046 MSKCC favourable risk group |
Some concerns | Participants were randomised via a stratified random block design, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 13 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. Of those who received treatment, less than 1% were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing information about the allocation concealment; missing study protocol and SAP. |
|
NCT00072046 MSKCC intermediate risk group |
Some concerns | Participants were randomised via a stratified random block design, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 13 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. Of those who received treatment, less than 1% were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing information about the allocation concealment; missing study protocol and SAP. |
|
NCT00072046 MSKCC poor risk group |
Some concerns | Participants were randomised via a stratified random block design, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 13 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. Of those who received treatment, less than 1% were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing information about the allocation concealment; missing study protocol and SAP. |
|
NCT00609401 Total trial population (combined risk groups) |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. The method of analysis was appropriate (ITT). | Low risk of bias | 6.3% were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT00081614 Total trial population (only favourable and intermediate risk groups included in the trial) |
Low risk of bias | Interactive voice response service was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. The method of analysis was appropriate (ITT). | Low risk of bias | 1 participant randomised to the experimental arm was lost to follow‐up, which probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
Jonasch 2010 Total trial population (combined risk groups) |
Low risk | Randomisation method appropriate and allocation concealed. No imbalances. | Low risk of bias | No information provided about whether the participants or those delivering the intervention were blinded or not. Only 1 participant randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). |
Low risk | 1 participant did not receive the intended intervention and therefore did not have outcome data. Of those who received treatment, 8.8% came off study before the first 8‐week response assessment. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement (objective outcome). | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT00098657/NCT00083889 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but it is not mentioned who conducted the randomisation or whether it was conducted centrally so that nobody could foresee assignment. However, there were no baseline imbalances that would suggest a problem with randomisation. | Some concerns | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement (objective outcome). | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the randomisation process and allocation concealment; deviations from intended interventions only in the control group; lack of information about missing data; missing study protocol and SAP. |
|
NCT00098657/NCT00083889 MSKCC intermediate risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but it is not mentioned who conducted the randomisation or whether it was conducted centrally so that nobody could foresee assignment. However, there were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Some concerns | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement (objective outcome). | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the randomisation process and allocation concealment; deviations from intended interventions only in the control group; lack of information about missing data; missing study protocol and SAP. |
|
NCT00098657/NCT00083889 MSKCC poor risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but it is not mentioned who conducted the randomisation or whether it was conducted centrally so that nobody could foresee assignment. However, there were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Some concerns | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement (objective outcome). | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the randomisation process and allocation concealment; deviations from intended interventions only in the control group; lack of information about missing data; missing study protocol and SAP. |
|
NCT00920816 Total trial population (combined risk groups) |
Low risk of bias | A centralised registration system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1% did not receive the intended interventions and therefore did not have outcome data. 2.1% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT01024920 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice randomisation system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | 3% did not receive the intended interventions and therefore did not have outcome data. 1 participant randomised to the control arm was lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT00631371 Total trial population (combined risk groups) |
Low risk of bias | A computerised centrally located randomisation system was used. There were no baseline imbalances that would suggest a problem with randomisation. |
Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 7 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 5.5% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT01835158 Total trial population (only intermediate and poor risk groups included in the trial) |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 6 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 4.5% did not receive the intended interventions and therefore did not have outcome data. There is a statement about loss to follow‐up, but not how many. |
Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | Study protocol available with some statistical considerations briefly described, but no separate SAP available to fully check the pre‐planned analyses. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; missing SAP. |
|
NCT02231749 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 11 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.3% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. Final revisions of both done before data cutoff (with extended follow‐up). Analyses were preplanned and reported. | Low risk of bias | Overall judged low risk of bias. |
|
NCT02231749 IMDC favourable risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 11 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.3% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. Final revisions of both done before data cutoff (with extended follow‐up). Analyses were preplanned and reported. | Low risk of bias | Overall judged low risk of bias. |
|
NCT02231749 IMDC intermediate&poor risk groups combined |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned Only 3 participants randomised to the experimental arm and 11 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.3% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. Final revisions of both done before data cutoff (with extended follow‐up). Analyses were preplanned and reported. | Low risk of bias | Overall judged low risk of bias. |
|
NCT01984242 Comparison 1 (ATE vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice/web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 0.3% did not receive the intended interventions and therefore did not have outcome data. 1.5% of those who received treatment were lost to follow‐up. However, these numbers are very low and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT01984242 Comparison 2 (ATE+BEV vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice/web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 0.3% did not receive the intended interventions and therefore did not have outcome data. 3.5% of those who received treatment were lost to follow‐up. However, these numbers are very low and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. |
|
NCT02420821 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk if bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. Less than 1% were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were aware of the assigned intervention, but knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. All reported analyses were pre‐specified in the protocol and SAP. | Low risk of bias | Overall judged low risk of bias. |
|
NCT02684006 IMDC favourable risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 5 participants randomised to the experimental arm and 1 participant randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. Participants without IMDC risk group allocation were excluded from the analysis. |
Some concerns | 1.5% did not receive the intended interventions and therefore did not have outcome data. No information about study flow for the second interim analysis (which is the result considered in this review). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | High risk of bias | Study protocol and SAP available but discrepancies were found between statements in the publications and the SAP about the pre‐specification of subgroup analyses. However, the time point (third interim analysis for OS) was pre‐specified. | High risk of bias | Overall judged high risk of bias due to lack of information about potential losses of follow‐up; lack of information about the subgroup analyses in the study protocol and SAP. |
|
NCT02684006 IMDC intermediate risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 5 participants randomised to the experimental arm and 1 participant randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. unclear. The method of analysis was appropriate. Participants without IMDC risk group allocation were excluded from subgroup analyses. |
Some concerns | 1.5% did not receive the intended interventions and therefore did not have outcome data. No information about study flow for the second interim analysis (which is the result considered in this review). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | High risk of bias | Study protocol and SAP available but discrepancies were found between statements in the publications and the SAP about the pre‐specification of subgroup analyses. However, the time point (third interim analysis for OS) was pre‐specified. | High risk of bias | Overall judged high risk of bias due to lack of information about potential losses of follow‐up; lack of information about the subgroup analyses in the study protocol and SAP. |
|
NCT02684006 IMDC poor risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 5 participants randomised to the experimental arm and 1 participant randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. Participants without IMDC risk group allocation were excluded from subgroup analyses. |
Some concerns | 1.5% did not receive the intended interventions and therefore did not have outcome data. No information about study flow for the second interim analysis (which is the result considered in this review). | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | High risk of bias | Study protocol and SAP available but discrepancies were found between statements in the publications and the SAP about the pre‐specification of subgroup analyses. However, the time point (third interim analysis for OS) was pre‐specified. | High risk of bias | Overall judged high risk of bias due to lack of information about potential losses of follow‐up; lack of information about the subgroup analyses in the study protocol and SAP. |
|
NCT02853331 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system or integrated web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 4 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. No indication of loss to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. All reported analyses were pre‐specified in the protocol and SAP. | Low risk of bias | Overall judged low risk of bias. |
|
NCT00719264 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about the randomisation and allocation concealment; missing study protocol and SAP. |
|
NCT00720941 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 2.9% of those who received treatment were lost to follow‐up. However, these numbers are small and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | CSR and study protocol with SAP available. All reported analyses were pre‐specified in the protocol and SAP. | Low risk of bias | Overall judged low risk of bias. |
|
NCT00720941 MSKCC favourable risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 17 participants randomised to the experimental arm and 21 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. |
Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 2.9% of those who received treatment were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are small and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | High risk of bias | A post‐hoc analysis according to risk group was conducted. | High risk of bias | Overall judged high risk of bias due to post‐hoc analysis. |
|
NCT00720941 MSKCC intermediate risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 17 participants randomised to the experimental arm and 21 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. |
Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 2.9% of those who received treatment were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are small and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | High risk of bias | A post‐hoc analysis according to risk group was conducted. | High risk of bias | Overall judged high risk of bias due to post‐hoc analysis. |
|
NCT00720941 MSKCC poor risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 17 participants randomised to the experimental arm and 21 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. |
Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 2.9% of those who received treatment were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are small and probably did not have an effect on the outcome. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | High risk of bias | A post‐hoc analysis according to risk group was conducted. | High risk of bias | Overall judged high risk of bias due to post‐hoc analysis. |
|
NCT00420888 Total trial population |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; missing study protocol and SAP. |
|
NCT00420888 MSKCC favourable risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; missing study protocol and SAP. |
|
NCT00420888 MSKCC intermediate risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; missing study protocol and SAP. |
|
NCT00420888 IMDC favourable risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. HENG risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; missing study protocol and SAP. |
|
NCT00420888 IMDC intermediate risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed There were no baseline imbalances that would suggest a problem with randomisation. HENG risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; missing study protocol and SAP. |
|
NCT00420888 IMDC poor risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. HENG risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; missing study protocol and SAP. |
|
NCT00979966 Total trial population |
High risk of bias | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were baseline imbalances that could suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. No information whether there were deviations form intended interventions and no information provided about the method of analysis. | High risk of bias | No information whether there was loss to follow‐up. | Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the randomisation, the allocation concealment, the deviations from intended interventions, the method of analysis and missing outcome data; missing study protocol and SAP. |
|
NCT02761057 SWOG Comparison 1 (CAB vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Randomisation was done by the Statistical Center. There were no baseline imbalances that would suggest a problem with randomisation. |
Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). |
Low risk of bias | 4.3% did not receive the intended interventions and therefore did not have outcome data. 2.2% had no protocol treatment. Only 1 participant was lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. |
Low risk of bias | No precise information provided about the outcome assessors, but either way knowledge of intervention received could not have affected outcome measurement. | Some concerns | Study protocol available, but no original SAP. | Some concerns | Overall judged some concerns due to missing SAP. |
Appendix 10. Risk of bias assessment for the outcome quality of life at the end of treatment
| Trial | Risk of bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
|
NCT00720941 Instrument: FACIT‐F Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 90.7% of those randomised did not have evaluable outcome data at the end of treatment. No analysis to correct for missing outcome data. | High risk of bias | QoL is a participant‐reported outcome, therefore outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | CSR and study protocol with SAP available. Scale and time point were prespecified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to the high number of participants without outcome data and the outcome assessors’ awareness of assigned intervention. |
|
NCT00098657/NCT00083889 Instrument:FKSI‐DRS Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information about allocation concealment. However, there were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. 91.6% of those randomised did not have evaluable outcome data the end of treatment. Analysis to correct for missing outcome data were conducted. | High risk of bias | QoL is a participant‐reported outcome, therefore outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias. | Overall judged high risk of bias due to lack of information about the randomisation process and allocation concealment; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00098657/NCT00083889 Instrument: EQ‐5D (VAS) Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information about allocation concealment. However, there were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. 91.4% of those randomised did not have evaluable outcome data the end of treatment. Analysis to correct for missing outcome data were conducted. | High risk of bias | QoL is a participant‐reported outcome, therefore outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias. | Overall judged high risk of bias due to lack of information about the randomisation process and allocation concealment; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00098657/NCT00083889 Instrument: FACT‐G Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information about allocation concealment. However, there were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. 91.7% of those randomised did not have evaluable outcome data the end of treatment. Analysis to correct for missing outcome data were conducted. | High risk of bias | QoL is a participant‐reported outcome, therefore outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias. | Overall judged high risk of bias due to lack of information about the randomisation process and allocation concealment; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT01108445 Instrument: FKSI‐ DRS Total trial population (combined risk groups) |
Low risk of bias | Participants were randomised in a 1:1 ratio. Randomisation was done under allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant withdrew after consent, but before randomisation and before study drug was assigned. No precise information provided about the method of analysis. |
High risk of bias | 43.5% of those randomised did not have evaluable outcome data at the end of treatment. No analysis to correct for bias. | High risk of bias | QoL is a participant‐reported outcome, therefore outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis; high number of participants without outcome data; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00920816 Instrument: FKSI‐ DRS Total trial population (combined risk groups) |
Low risk of bias | A centralised registration system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | HIgh risk of bias | 1% did not receive the intended interventions and therefore did not have outcome data. 60.4% of those randomised did not have evaluable outcome data. No analysis to correct for bias. | High risk of bias | QoL is a participant‐reported outcome, therefore outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to high number of participants without outcome data; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00920816 Instrument: EQ‐5D (VAS) Total trial population (combined risk groups) |
Low risk of bias | A centralised registration system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 1% did not receive the intended interventions and therefore did not have outcome data. 60.8% of those randomised did not have evaluable outcome data. No analysis to correct for bias. | High risk of bias | QoL is a participant‐reported outcome, therefore outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged high risk of bias due to high number of participants without outcome data; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
Appendix 11. Risk of bias assessment for the outcome serious adverse events
| Trial | Risk of bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement[BB1] | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
|
NCT02811861 Comparison 1 (LEN+PEM vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was not appropriate (as‐treated). | Low risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Measurement of SAEs could have differed between intervention groups due to longer follow‐up of the intervention arm. | Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to inappropriate method of analysis; probable differences in outcome measurement between intervention arms. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was not appropriate (as‐treated). | Low risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to inappropriate method of analysis. |
|
NCT00065468 Comparison 1 (TEM+IFN) Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the single‐drug arm and 7 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis (as‐treated is indicated). | Low risk of bias | 1.9% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about allocation concealment, method of analysis and method of outcome measurement; missing study protocol and SAP. |
|
NCT00065468 comparison 2 Comparison 2 (IFN+TEM vs. IFN) Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the combination arm and 7 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis (as‐treated is indicated). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about allocation concealment, method of analysis and method of outcome measurement; missing study protocol and SAP. |
|
NCT01024920 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice randomisation system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. No precise information provided about the method of analysis. | Low risk of bias | 3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis and method of outcome measurement; missing study protocol and SAP. |
|
NCT01835158 Total trial population (only intermediate and poor risk groups included in the trial) |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 6 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis. | Low risk of bias | 4.5% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | Study protocol available with some statistical considerations briefly described, but no separate SAP available to fully check the pre‐planned analyses. | High risk of bias | Overall judged high risk of bias due to lack of information about the method of analysis; missing SAP. |
|
NCT01984242 Comparison 1 (ATE vs. SUN) Total population (combined risk groups) |
Low risk of bias | Interactive voice/web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis, but data for crossed‐over participants were reported separately. We assume participants were analysed as randomised in period 1. | Low risk of bias | 0.3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are very low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about method of outcome measurement; missing study protocol and SAP. |
|
NCT01984242 Comparison 2 (ATE+BEV vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice/web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis, but data for crossed‐over participants were reported separately. We assume participants were analysed as randomised in period 1. | Low risk of bias | 0.3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are very low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about method of outcome measurement; missing study protocol and SAP. |
|
NCT00719264 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment; 1 participant randomised to the experimental arm had no post baseline safety assessment. No precise information provided about the method of analysis. | Low risk of bias | 1.1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process, allocation concealment, method of analysis and method of outcome measurement; missing study protocol and SAP. |
|
NCT00720941 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Some concerns | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was not appropriate (as‐treated), but this probably did not have an effect on the outcome as there is evidence that participants actually received the assigned intervention. |
Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are small and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Low risk of bias | CSR and study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | Some concerns | Overall judged some concerns due to inappropriate method of analysis. |
|
NCT00732914 Total trial population (combined risk groups) |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 5 participants randomised to the experimental arm and 7 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis, but data for first period reported separately. We assume participants in first period received their allocated intervention. | Low risk of bias | 3.3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No information provided about outcome assessors and method of measuring SAEs. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about outcome assessors and method of outcome measurement; missing study protocol and SAP. |
|
NCT01613846 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 6 participants randomised to the one experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | 2.9% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No information provided about outcome assessors and method of measuring SAEs. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process, allocation concealment, outcome assessors and method of outcome measurement; missing study protocol and SAP. |
|
NCT00738530 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 2 participants from the control arm and 6 participants from the intervention arm did not receive any treatment. The method of analysis was not appropriate (as‐treated). | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs and outcome assessors. However, we assume SAEs were assessed by the investigators and this was a double‐blind study. Furthermore, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to inappropriate method of analysis; lack of information about method of outcome measurement; missing study protocol and SAP. |
|
NCT00117637 NCT00117637 Total trial population (combined risk groups) |
High risk of bias | Participants were randomised in a 1:1 ratio, but no information provided about the allocation concealment. There were some baseline imbalances that could suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. No precise information provided about the method of analysis, but data for crossed‐over participants were reported separately. We assume participants were analysed as randomised in period 1. | Low risk of bias | No indication of loss to follow‐up. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the allocation concealment and method of outcome measurement; baseline imbalances; missing study protocol and SAP. |
|
NCT00098657/NCT00083889 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information about allocation concealment. However, there were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 15 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis. | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias. | Overall judged high risk of bias due to lack of information about the randomisation process, allocation concealment, method of analysis and method of outcome measurement; missing study protocol and SAP. |
|
NCT01108445 Total trial population (combined risk groups) |
Low risk of bias | Participants were randomised in a 1:1 ratio. Randomisation was done under allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant withdrew after consent, but before randomisation and before study drug was assigned. No precise information provided about the method of analysis. |
Low risk of bias | All 108 participants were evaluable. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis and method of outcome measurement; missing study protocol and SAP. |
|
NCT00903175 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis, but data for crossed‐over participants were reported separately. We assume participants were analysed as randomised in period 1. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | Study protocol available with some statistical methods described, but no separate SAP available to fully check the pre‐planned analyses. | Some concerns | Overall judged some concerns due to missing SAP. |
|
NCT00619268 Comparison 1 (BEV+TEM vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | A computerised centrally located randomisation system was Used. There were no baseline imbalances that would suggest a problem with randomisation. |
High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis. |
Low risk of bias | Only 1 participant did not receive the intended intervention and therefore did not have outcome data. | High risk of bias | No information provided about outcome assessors and method of measuring SAEs. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis, outcome assessors and method of outcome measurement; missing study protocol and SAP. |
|
NCT00619268 Comparison 2 (BEV+IFN vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | A computerised centrally located randomisation system was Used. There were no baseline imbalances that would suggest a problem with randomisation. |
High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis. |
Low risk of bias | Only 1 participant did not receive the intended intervention and therefore did not have outcome data. | High risk of bias | No information provided about outcome assessors and method of measuring SAEs. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis, outcome assessors and method of outcome measurement; missing study protocol and SAP. |
|
NCT00631371 Total trial population (combined risk groups) |
Low risk of bias | A computerised centrally located randomisation system was used. There were no baseline imbalances that would suggest a problem with randomisation. |
High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 7 participants randomised to the experimental arm did not receive any treatment. No precise information provided about the method of analysis. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore, did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis; missing study protocol and SAP. |
|
NCT02231749 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm, and 11 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis. | Low risk of bias | 1.3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are very low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis. |
|
NCT02420821 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 15 participants randomised to the control arm did not receive any treatment. Conflicting information about method of analysis in the protocol. | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to conflicting information about method of analysis. |
|
NCT02853331 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system or integrated web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 4 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | Low risk of bias | Overall judged low risk of bias. |
|
NCT00920816 Total trial population (combined risk groups) |
Low risk of bias | A centralised registration system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. No precise information provided about the method of analysis, but data for first period reported separately. We assume participants in first period received their allocated intervention. | Low risk of bias | 1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about method of outcome measurement; missing study protocol and SAP. |
|
NCT00979966 Total trial population (combined risk groups) |
High risk of bias | Participants were randomised in a 1:1 ratio, but no information provided about who conducted randomisation and whether allocation was concealed. There were baseline imbalances that could suggest a problem with randomisation. Small study population. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. No information whether there were deviations form intended interventions and no information provided about the method of analysis. | Low risk of bias | No indication of loss to follow‐up. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process, allocation concealment, deviations from intended interventions, method of analysis and method of outcome measurement; baseline imbalances; missing study protocol and SAP. |
|
NCT00126594 Total trial population (combined risk groups) |
Some concerns | No information provided about randomisation process and allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. The method of analysis was appropriate. | Low risk of bias | No indication of loss to follow‐up. | Some concerns | No information provided about method of measuring SAEs. Outcome assessors were not blinded. However, a standardised definition of SAEs was used and included objective outcome events. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about randomisation process, allocation concealment, method of outcome measurement; missing study protocol and SAP. |
Appendix 12. Risk of bias assessment for the outcome progression‐free survival
| Trial | Risk of bias | ||||||||||||||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||||||||||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | ||||||||||||
|
NCT03141177 IMDC favourable risk group |
Low risk of bias | Interactive Response Technology was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 8 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 1.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 3% of those who received treatment discontinued due to “other” reasons (not explained further). | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent central review committee. | High risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. However, the time point that produced this result was not pre‐specified in the protocol. The results of the final PFS analysis were already reported in a previous publication. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data and inconsistency with the protocol regarding the time point of analyses and reporting. | |||||||||||
|
NCT03141177 IMDC intermediate risk group |
Low risk of bias | Interactive Response Technology was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 8 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 1.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 3% of those who received treatment discontinued due to “other” reasons (not explained further). | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent central review committee. | High risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. However, the time point that produced this result was not pre‐specified in the protocol. The results of the final PFS analysis were already reported in a previous publication. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data and inconsistency with the protocol regarding the time point of analyses and reporting. | |||||||||||
|
NCT03141177 IMDC poor risk group |
Low risk of bias | Interactive Response Technology was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 8 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 1.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 3% of those who received treatment discontinued due to “other” reasons (not explained further). | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent central review committee. | High risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. However, the time point that produced this result was not pre‐specified in the protocol. The results of the final PFS analysis were already reported in a previous publication. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data and inconsistency with the protocol regarding the time point of analyses and reporting. | |||||||||||
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisations. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. All reported analyses were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) MSKCC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not further explained). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) MSKCC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) MSKCC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not further explained). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) IMDC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 2 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) IMDC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 2 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 1 (LEN+PEM vs.SUN) IMDC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 2 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. All reported analyses were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) MSKCC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) MSKCC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) MSKCC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) IMDC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 6 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) IMDC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 6 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) IMDC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 6 participants randomised to the experimental arm and 4 participants randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without IMDC risk group allocation were excluded from subgroup analyses. | High risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. 2% discontinued treatment due to “other” reasons (not explained further). | High risk of bias | Independent review was conducted but no statement whether it was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | A study protocol with SAP available. The subgroup analyses according to IMDC risk group were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; the outcome assessors’ probable awareness of the assigned interventions. | |||||||||||
|
NCT01108445 Total trial population (combined risk groups) |
Low risk of bias | Participants were randomised in a 1:1 ratio. Randomisation was done under allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant withdrew after consent, but before randomisation and before study drug was assigned. The method of analysis was appropriate (ITT). |
Low risk of bias | All 108 participants were evaluable. | High risk of bias | Scans were read by a trained radiologist, but no information whether the person was blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ probable awareness of the assigned interventions; missing study protocol and SAP. | |||||||||||
|
NCT01108445 MSKCC favourable risk group |
Low risk of bias | Participants were randomised in a 1:1 ratio. Randomisation was done under allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant withdrew after consent, but before randomisation and before study drug was assigned. The method of analysis was appropriate (ITT). |
Low risk of bias | All 108 participants were evaluable. | High risk of bias | Scans were read by a trained radiologist, but no information whether the person was blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ probable awareness of the assigned interventions; missing study protocol and SAP. | |||||||||||
|
NCT01108445 MSKCC intermediate risk group |
Low risk of bias | Participants were randomised in a 1:1 ratio. Randomisation was done under allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant withdrew after consent, but before randomisation and before study drug was assigned enrolment and randomisation). The method of analysis was appropriate (ITT). |
Low risk of bias | All 108 participants were evaluable | High risk of bias | Scans were read by a trained radiologist, but no information whether the person was blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ probable awareness of the assigned interventions; missing study protocol and SAP. | |||||||||||
|
NCT01108445 MSKCC poor risk group |
Low risk of bias | Participants were randomised in a 1:1 ratio. Randomisation was done under allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant withdrew after consent, but before randomisation and before study drug was assigned. The method of analysis was appropriate (ITT). |
Low risk of bias | All 108 participants were evaluable | High risk of bias | Scans were read by a trained radiologist, but no information whether the person was blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ probable awareness of the assigned interventions; missing study protocol and SAP. | |||||||||||
|
NCT01392183 Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. No statement about the method of analysis. | Low risk of bias | Detailed flow diagram provided, no indication of loss to follow‐up. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. The radiographic response was assessed by blinded radiologists. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the allocation concealment and method of analysis; missing study protocol and SAP. | |||||||||||
|
NCT00334282 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was blinded: both participants and those delivering the intervention were not aware of assigned interventions. The method of analysis was appropriate (ITT). | Low risk of bias | 1.3% of those who received treatment were lost to follow‐up. No analysis to correct for bias, but numbers are low and probably did not have an effect on the outcome. |
Low risk of bias | Outcome assessors were not aware of the assigned intervention. All imaging scans were evaluated by an independent imaging‐review committee (IRC) blinded to study treatment. | Low risk of bias | CSR and study protocol with SAP available. All reported analyses were pre‐specified in the protocol and SAP. | Low risk of bias | Overall judged low risk of bias. | |||||||||||
|
NCT01030783 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | No information provided about loss to follow‐up. 2.3% discontinued due to “other” reasons (not explained further). | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent radiology review. | Some concerns | No SAP available. Study protocol available, but unclear whether it was finalized before unblinded outcome data were available. | High risk of bias | Overall judged high risk of bias due to lack of information about missing outcome data; missing study protocol and SAP. | |||||||||||
|
NCT00065468 Comparison 1 (TEM vs. IFN) Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised, but no information provided about allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the single‐drug arm and 7 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.9% did not receive the intended interventions and therefore did not have outcome data. 2% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent central review. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about allocation concealment; missing study protocol and SAP. | |||||||||||
|
NCT00065468 Comparison 2 (TEM+IFN vs. IFN) Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised, but no information provided about allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the combination arm and 7 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. 1.7% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent central review. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to lack of information about allocation concealment; missing study protocol and SAP. | |||||||||||
|
NCT00738530 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 6 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. 3.9% of those who received treatment withdrew consent or were lost to follow‐up before interim data cut. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00738530 MSKCC favourable risk group |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 28 participants randomised to the experimental arm and 24 participants randomised to the control arm. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 6 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. 3.9% of those who received treatment withdrew consent or were lost to follow‐up before interim data cut. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00738530 MSKCC intermediate risk group |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 28 participants randomised to the experimental arm and 24 participants randomised to the control arm. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 6 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. 3.9% of those who received treatment withdrew consent or were lost to follow‐up before interim data cut. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00738530 MSKCC poor risk group |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 28 participants randomised to the experimental arm and 24 participants randomised to the control arm. | Low risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 6 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. 3.9% of those who received treatment withdrew consent or were lost to follow‐up before interim data cut. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00072046 Total trial population (combined risk groups) |
Some concerns | Participants were randomised via a stratified random block design, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 13 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received the intervention were lost to follow‐up. These numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention and knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the allocation concealment; the assessors’ awareness of assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00081614 (only favourable and intermediate risk groups included in the trial) |
Low risk of bias | Interactive voice response service was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. No information provided about the method of analysis. | Low risk of bias | 1 participant randomised to the experimental arm was lost to follow‐up, which probably did not have an effect on the outcome. | High risk of bias | No precise information provided about the outcome assessors. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the method of analysis; the outcome assessors’ probable awareness of the assigned interventions; missing study protocol and SAP. | |||||||||||
|
NCT00117637 Total trial population (combined risk groups) |
High risk of bias | Participants were randomised in a 1:1 ratio, but no information provided about the allocation concealment. There were some baseline imbalances that could suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. The method of analysis was appropriate (ITT). | Low risk of bias | 1.1% randomised to the control arm were lost to follow‐up. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS assessed by blinded independent radiological review. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the allocation concealment; baseline imbalances; missing study protocol and SAP. | |||||||||||
|
Jonasch 2010 Total trial population (combined risk groups) |
Low risk of bias | Randomisation method appropriate and allocation concealed. No imbalances. | Low risk of bias | No information provided about whether the participants or those delivering the intervention were blinded or not. Only 1 participant randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). |
Low risk of bias | 1 participant did not receive the intended intervention and therefore did not have outcome data. Of those who received treatment, 8.8% came off study before the first 8‐week response assessment. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No information whether the investigator (outcome assessor) was blinded. We can only assume no (not blinded) because investigator assessment was compared to blinded review of 20 participants’ scans. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about blinding of outcome assessor; missing study protocol and SAP. | |||||||||||
|
NCT00098657/NCT00083889 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information about allocation concealment. However, there were no baseline imbalances that would suggest a problem with randomisation. | Some concerns | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by blinded independent central review. | Some concerns | No study protocol or SAP available. | High risk of bias. | Overall judged high risk of bias due to lack of information about the randomisation process and allocation concealment; deviations from intended interventions only in the control group; lack of information about missing data; missing study protocol or SAP. | |||||||||||
|
NCT00732914 Total trial population (combined risk groups) |
Low risk of bias | Randomszation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 5 participants randomised to the experimental arm and 7 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 3.3% did not receive the intended interventions and therefore, did not have outcome data. 4.5% of those who received treatment discontinued due to “other” reasons, including (but not limited to) loss to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention and knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to the assessors’ awareness of assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00732914 MSKCC favourable risk group |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 8 participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 5 participants randomised to the experimental arm and 7 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | 3.3% did not receive the intended interventions and therefore, did not have outcome data. 4.5% of those who received treatment discontinued due to “other” reasons, including (but not limited to) loss to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention and knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to the assessors’ awareness of assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00732914 MSKCC intermediate risk group |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 8 participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 5 participants randomised to the experimental arm and 7 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | 3.3% did not receive the intended interventions and therefore, did not have outcome data. 4.5% of those who received treatment discontinued due to “other” reasons, including (but not limited to) loss to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention and knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due the assessors’ awareness of assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00920816 Total trial population (combined risk groups) |
Low risk of bias | A centralized registration system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1% did not receive the intended interventions and therefore did not have outcome data. 2.1% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a masked independent review committee. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00920816 MSKCC favourable risk group |
Low risk of bias | A centralized registration system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 7 participants randomised to the experimental arm and 1 participant randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). However, 8 participants for whom MSKCC risk group was not available were still included in the analysis and allocated to the intermediate/poor risk group. |
Low risk of bias | 1% did not receive the intended interventions and therefore did not have outcome data. 2.1% of those who received treatment were lost to follow‐up. Unclear how many of these were assigned to the favourable risk group. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a masked independent review committee. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00920816 MSKCC intermediate+poor risk groups combined |
Low risk of bias | A centralized registration system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 7 participants randomised to the experimental arm and 1 participant randomised to the control arm | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). However, 8 participants for whom MSKCC risk group was not available were still included in the analysis and allocated to the intermediate/poor risk group. |
Low risk of bias | 1% did not receive the intended interventions and therefore did not have outcome data. 2.1% of those who received treatment were lost to follow‐up. Unclear how many of these were assigned to the intermediate/poor risk group. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a masked independent review committee. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT01024920 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice randomisation system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | 3% did not receive the intended interventions and therefore did not have outcome data. 1 participant randomised to the control arm was lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to the assessors’ awareness of assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00631371 Total trial population (combined risk groups) |
Low risk of bias | A computerized centrally located randomisation system was used. There were no baseline imbalances that would suggest a problem with randomisation. |
Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 7 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 5.5% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. An independent blinded assessment was conducted. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00631371 MSKCC favourable risk groups |
Low risk of bias | A computerized centrally located randomisation system was used. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. |
Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 7 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 5.5% of those who received treatment were lost to follow‐up. Unclear how many of these were assigned to the favourable risk group. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. An independent blinded assessment was conducted. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00631371 MSKCC intermediate risk groups |
Low risk of bias | A computerized centrally located randomisation system was used. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. |
Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 7 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 5.5% of those who received treatment were lost to follow‐up. Unclear how many of these were assigned to the intermediate risk group. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. An independent blinded assessment was conducted. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT00631371 MSKCC poor risk groups |
Low risk of bias | A computerized centrally located randomisation system was used. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. |
Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 7 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 5.5% of those who received treatment were lost to follow‐up. Unclear how many of these were assigned to the poor risk group. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. An independent blinded assessment was conducted. | Some concerns | No study protocol or SAP available. | Some concerns | Overall judged some concerns due to missing study protocol and SAP. | |||||||||||
|
NCT01835158 Total trial population (only intermediate and poor risk groups included in the trial) |
Low risk of bias | Randomszation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 6 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 4.5% did not receive the intended interventions and therefore did not have outcome data. 16% of those who received treatment had missing radiographic images or were unevaluable for tumour response assessments, but there is evidence that the result was not biased by missing outcome data. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent radiology review committee. | Some concerns | Study protocol available with some statistical considerations briefly described, but no separate SAP available to fully check the pre‐planned analyses. | Some concerns | Overall judged some concerns due to missing SAP. | |||||||||||
|
NCT01835158 IMDC intermediate risk group |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 6 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 4.5% did not receive the intended interventions and therefore did not have outcome data. 16% of those who received treatment had missing radiographic images or were unevaluable for tumour response assessments, but there is evidence that the result was not biased by missing outcome data. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent radiology review committee. | Some concerns | Study protocol available with some statistical considerations briefly described, but no separate SAP available to fully check the pre‐planned analyses. | Some concerns | Overall judged some concerns due to missing SAP. | |||||||||||
|
NCT01835158 IMDC poor risk group |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 6 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 4.5% did not receive the intended interventions and therefore did not have outcome data. 16% of those who received treatment had missing radiographic images or were unevaluable for tumour response assessments, but there is evidence that the result was not biased by missing outcome data. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent radiology review committee. | Some concerns | Study protocol available with some statistical considerations briefly described, but no separate SAP available to fully check the pre‐planned analyses. | Some concerns | Overall judged some concerns due to missing SAP. | |||||||||||
|
NCT02231749 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 11 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.3% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No information whether the independent radiological review committee was blinded. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. Final revisions of both done before data cutoff (with extended follow‐up). Analyses were preplanned and reported. | High risk of bias | Overall judged high risk of bias due to lack of information about the outcome assessor and blinding to outcome assessment. | |||||||||||
|
NCT02231749 IMDC favourable risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 11 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.3% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome | High risk of bias | No information whether the independent radiological review committee. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. Final revisions of both done before data cutoff (with extended follow‐up). Analyses were preplanned and reported. | High risk of bias | Overall judged high risk of bias due to lack of information about the outcome assessor and blinding to outcome assessment. | |||||||||||
|
NCT02231749 IMDC intermediate and poor risk groups combined |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 11 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 1.3% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome | High risk of bias | No information whether the independent radiological review committee. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. Final revisions of both done before data cutoff (with extended follow‐up). Analyses were preplanned and reported. | High risk of bias | Overall judged high risk of bias due to lack of information about the outcome assessor and blinding to outcome assessment. |
|||||||||||
|
NCT00720941 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomszation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 2.9% of those who received treatment were lost to follow‐up. However, these numbers are small and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. PFS was assessed by a blinded independent review committee. | Low risk of bias | CSR and study protocol with SAP available. All reported analyses were pre‐specified in the protocol and SAP. | Low risk of bias | Overall judged low risk of bias. | |||||||||||
|
NCT01984242 Comparison 1 (ATE vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice/web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 0.3% did not receive the intended interventions and therefore did not have outcome data. 1.5% of those who received treatment were lost to follow‐up. These numbers are very low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about whether the outcome assessors were blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about blinding of outcome assessor; missing study protocol and SAP. | |||||||||||
|
NCT01984242 Comparison 2 (ATE+BEV vs. SUN) Total trial population (combined all risk groups) |
Low risk of bias | Interactive voice/web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 0.3% did not receive the intended interventions and therefore did not have outcome data. 3.5% of those who received treatment were lost to follow‐up. These numbers are very low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about whether the outcome assessors were blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about blinding of outcome assessor; missing study protocol and SAP. | |||||||||||
|
NCT02420821 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | The investigators were the outcome assessors and they were not blinded to treatment allocation. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. All reported analyses were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of blinding of the outcome assessors. | |||||||||||
|
NCT02420821 MSKCC favourable risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. Unclear to which risk groups they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about the outcome assessors. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. All reported subgroup analyses were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of blinding of the outcome assessors. | |||||||||||
|
NCT02420821 MSKCC intermediate risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. Unclear to which risk groups they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about the outcome assessors. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. All reported subgroup analyses were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of blinding of the outcome assessors. | |||||||||||
|
NCT02420821 MSKCC poor risk group |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 15 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. Unclear to which risk groups they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about the outcome assessors. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | Study protocol and SAP available. All reported subgroup analyses were pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to lack of blinding of the outcome assessors. | |||||||||||
|
NCT02684006 IMDC favourable risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 5 participants randomised to the experimental arm and 1 participant randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. Participants without IMDC risk group allocation were excluded from the analysis. |
Some concerns | 1.5% did not receive the intended interventions and therefore did not have outcome data. No information about study flow for the second interim analysis (which is the result considered in this review). | Low risk of bias | Outcome assessors were not aware of the assigned intervention. A blinded independent central review was conducted. | High risk of bias | Study protocol and SAP available but discrepancies were found between statements in the publications and the SAP about the pre‐specification of subgroup analyses. Also the time point that produced this result was not pre‐specified in the protocol (final PFS analysis was already reported). | High risk of bias | Overall judged high risk of bias due to lack of information about potential losses of follow‐up; lack of information about the subgroup analyses in the study protocol and SAP. | |||||||||||
|
NCT02684006 IMDC intermediate risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 5 participants randomised to the experimental arm and 1 participant randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. Participants without IMDC risk group allocation were excluded from subgroup analyses. |
Some concerns | 1.5% did not receive the intended interventions and therefore did not have outcome data. No information about study flow for the second interim analysis (which is the result considered in this review). | Low risk of bias | Outcome assessors were not aware of the assigned intervention. A blinded independent central review was conducted. | High risk of bias | Study protocol and SAP available but discrepancies were found between statements in the publications and the SAP about the pre‐specification of subgroup analyses. Also the time point that produced this result was not pre‐specified in the protocol (final PFS analysis was already reported). | High risk of bias | Overall judged high risk of bias due to lack of information about potential losses of follow‐up; lack of information about the subgroup analyses in the study protocol and SAP. | |||||||||||
|
NCT02684006 IMDC poor risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. IMDC risk group was not available for 5 participants randomised to the experimental arm and 1 participant randomised to the control arm. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. Participants without IMDC risk group allocation were excluded from subgroup analyses. |
Some concerns | 1.5% did not receive the intended interventions and therefore did not have outcome data. No information about study flow for the second interim analysis (which is the result considered in this review). | Low risk of bias | Outcome assessors were not aware of the assigned intervention. A blinded independent central review was conducted. | High risk of bias | Study protocol and SAP available but discrepancies were found between statements in the publications and the SAP about the pre‐specification of subgroup analyses. Also the time point that produced this result was not pre‐specified in the protocol (final PFS analysis was already reported). | High risk of bias | Overall judged high risk of bias due to lack of information about potential losses of follow‐up; lack of information about the subgroup analyses in the study protocol and SAP. | |||||||||||
|
NCT02853331 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system or integrated web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 4 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. No indication of loss to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. A blinded independent central review was conducted. | High risk of bias | Final PFS analysis was already reported in a previous publication. The timing of analysis (which is the time point for the final OS analysis) for this numerical result of PFS was not pre‐specified in the protocol. | High risk of bias | Overall judged high risk of bias due to the analysis time point not being pre‐specified. | |||||||||||
|
NCT00719264 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. Less than 1% of those who received treatment were lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No information provided about whether the outcome assessors were blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the randomisation and allocation concealment; the outcome assessors’ probable awareness of the assigned interventions; missing study protocol and SAP. | |||||||||||
|
NCT00420888 Total trial population (only favourable and intermediate risk groups included in the trial) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted randomisation and whether allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | High risk of bias | No precise information provided about the outcome assessors (including lack of information about blinding). Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; the outcome assessors’ probable awareness of the assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00420888 MSKCC favourable risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted randomisation and whether allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | High risk of bias | No precise information provided about the outcome assessors (including lack of information about blinding). Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; the outcome assessors’ probable awareness of the assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00420888 MSKCC intermediate risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted randomisation and whether allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | High risk of bias | No precise information provided about the outcome assessors (including lack of information about blinding). Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; the outcome assessors’ probable awareness of the assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00420888 IMDC favourable risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. HENG risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | High risk of bias | No precise information provided about the outcome assessors (including lack of information about blinding). Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; the outcome assessors’ probable awareness of the assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00420888 IMDC intermediate risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. HENG risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | High risk of bias | No precise information provided about the outcome assessors (including lack of information about blinding). Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; the outcome assessors’ probable awareness of the assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00420888 IMDC poor risk group |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. HENG risk group was available for all randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants did not receive any treatment. The method of analysis was appropriate (ITT). |
High risk of bias | 1.5% did not receive the assigned interventions and therefore did not have outcome data. No information whether there was loss to follow‐up. | High risk of bias | No precise information provided about the outcome assessors (including lack of information about blinding). Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomisation process and allocation concealment; missing outcome data; the outcome assessors’ probable awareness of the assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT00979966 Total trial population (combined risk groups) |
High risk of bias | Participants were randomised in a 1:1 ratio, but no information provided about who conducted randomisation and whether allocation was concealed. There were baseline imbalances that could suggest a problem with randomisation. Small study population. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. No information whether there were deviations form intended interventions and no information provided about the method of analysis. | High risk of bias | No information about loss to follow‐up. | High risk of bias | No precise information provided about who assessed the outcome and whether they were blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the randomisation, the allocation concealment, the deviations from intended interventions, the method of analysis and missing outcome data; the outcome assessors’ probable awareness of the assigned interventions; missing study protocol and SAP. | |||||||||||
|
NCT00903175 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 1 participant randomised to the control arm was lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Study protocol available with some statistical methods described, but no separate SAP available to fully check the pre‐planned analyses. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ awareness of the assigned intervention; missing SAP. | |||||||||||
|
NCT00903175 MSKCC favourable risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 2 randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 1 participant randomised to the control arm was lost to follow‐up. Unclear to which risk group they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Study protocol available with some statistical methods described, but no separate SAP available to fully check the pre‐planned analyses.. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ awareness of the assigned intervention; missing SAP. | |||||||||||
|
NCT00903175 MSKCC intermediate risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 2 randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 1 participant randomised to the control arm was lost to follow‐up. Unclear to which risk groups they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Study protocol available with some statistical methods described, but no separate SAP available to fully check the pre‐planned analyses. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ awareness of the assigned intervention; missing SAP. | |||||||||||
|
NCT00903175 MSKCC poor risk group |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was not available for 2 randomised participants. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). Participants without MSKCC risk group allocation were excluded from subgroup analyses. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. 1 participant randomised to the control arm was lost to follow‐up. Unclear to which risk groups they were assigned to. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Study protocol available with some statistical methods described, but no separate SAP available to fully check the pre‐planned analyses.. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ awareness of the assigned intervention; missing SAP. | |||||||||||
|
NCT01481870 Total trial population (only favourable and intermediate risk groups included) |
Low risk of bias | Participants were randomised in a 1:1 ratio. The assignment was obtained at enrolment by the investigator via the Internet. There were no baseline imbalances that would suggest a problem with randomisation. |
Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 1 participant randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 3.2% did not receive the intended interventions and therefore did not have outcome data. 22% of those who received treatment did not have outcome data. | High risk of bias | No information provided about who assessed the outcome and whether they were blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to high number of participants with missing outcome data; the outcome assessors’ probable awareness of the assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT01481870 MSKCC favourable risk group |
Low risk of bias | Participants were randomised in a 1:1 ratio. The assignment was obtained at enrolment by the investigator via the Internet There were no baseline imbalances that would suggest a problem with randomisation. MSKCC risk group was available for all randomised participants. |
Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 1 participant randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). | High risk of bias | 3.2% did not receive the intended interventions and therefore did not have outcome data. 22% of those who received treatment did not have outcome data. | High risk of bias | No information provided about who assessed the outcome and whether they were blinded. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to high number of participants with missing outcome data; the outcome assessors’ probable awareness of the assigned intervention; missing study protocol and SAP. | |||||||||||
|
NCT02761057 Comparison 1 (CAB vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | The randomisation was done by the Study Center. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate (ITT). |
Low risk of bias | 4.3% did not receive the intended interventions and therefore did not have outcome data. 2.2% had no protocol treatment. Only 1 participant was lost to follow‐up. However, these numbers are low and probably did not have an effect on the outcome. |
High risk of bias | No precise information provided about method of measuring PFS. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Some concerns | Study protocol available, but no original SAP. | High risk of bias | Overall judged high risk of bias due to lack of information about method of outcome measurement; the outcome assessors’ awareness of the assigned intervention; missing SAP. | |||||||||||
Appendix 13. Risk of bias assessment for the outcome adverse events
| Trial | Risk of bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
|
NCT03141177 Total trial population (combined all risk groups) |
Low risk of bias | Interactive Response Technology was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 8 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | 1.7% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Adverse events (AEs) were reported by the participants and the investigator was responsible for detecting, documenting and reporting events. Both were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ awareness of assigned intervention. |
|
NCT02811861 Comparison 1 (LEN+PEM vs. SUN) Total trial population (all combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was not appropriate (as‐treated). | Low risk of bias | 2.8% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | AEs were most likely reported by the participants and assessed by the investigator. Both were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to inappropriate method of analysis and the outcome assessors’ awareness of assigned intervention. |
|
NCT02811861 Comparison 2 (LEN+EVE vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the experimental arm and 17 participants randomised to the control arm did not receive any treatment. The method of analysis was not appropriate (as‐treated). | Low risk of bias | 2.7% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | AEs were most likely reported by the participants and assessed by the investigator. Both were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to inappropriate method of analysis and the outcome assessors’ awareness of assigned intervention. |
|
NCT00065468 Comparison 1 (TEM vs. IFN) Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the single‐drug arm and 7 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis (as‐treated is indicated). | Low risk of bias | 1.9% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to the National Cancer Institute Common Toxicity Criteria (NCICTC), version 3. Measurement of AEs could have differed between intervention groups due to differences in number of visits to the healthcare provider. AEs were assessed by the participants who were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about allocation concealment, method of analysis and method of outcome measurement; probable differences in outcome measurement between intervention arms; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00065468 comparison 2 Comparison 2 (IFN+TEM vs. IFN) Total trial population (only intermediate and poor risk groups included in the trial) |
Some concerns | Participants were randomised, but no information provided about the allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the combination arm and 7 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis (as‐treated is indicated). | Low risk of bias | 2.2% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 3. AEs were assessed by the participants who were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about allocation concealment, method of analysis and method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00081614 Total trial population (only favourable and intermediate risk groups included in the trial) |
Low risk of bias | Interactive voice response service was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. No information provided about the method of analysis. | Low risk of bias | Only 3 randomised did not have outcome data and 1 from the control group was lost to follow‐up. | Some concerns | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 3. Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk due to lack of information about method of analysis and method of outcome measurement; missing study protocol and SAP. |
|
NCT01024920 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice randomisation system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. No precise information provided about the method of analysis. | Low risk of bias | 3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 3. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis and method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT01835158 Total trial population (only intermediate and poor risk groups included in the trial) |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 6 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis. | Low risk of bias | 4.5% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 4. AEs were assessed by the participants and the investigator. Both were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Study protocol available with some statistical considerations briefly described, but no separate SAP available to fully check the pre‐planned analyses. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis and method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing SAP. |
|
NCT01984242 Comparison 1 (ATE vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice/web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis, but data for crossed‐over participants were reported separately. We assume participants were analyzed as randomised in period 1. | Low risk of bias | 0.3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are very low and probably did not have an effect on the outcome. | High risk of bias | No information provided about method of measuring AEs. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT01984242 Comparison 2 (ATE+BEV vs. SUN) Total trial population (combined risk groups) |
Low risk of bias | Interactive voice/web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis, but data for crossed‐over participants were reported separately. We assume participants were analyzed as randomised in period 1. | Low risk of bias | 0.3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are very low and probably did not have an effect on the outcome. | High risk of bias | No information provided about method of measuring AEs. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT02684006 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 8 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | 1.5% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 4.03. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Study protocol and SAP available. However, the exact time point of outcome measurement is unclear. | High risk of bias | Overall judged high risk of bias due to lack of information about method and time point of outcome measurement; the outcome assessors’ awareness of assigned intervention. |
|
NCT00719264 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm and 2 participants randomised to the control arm did not receive any treatment; 1 participant randomised to the experimental arm had no post baseline safety assessment. No precise information provided about the method of analysis. | Low risk of bias | 1.1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 3. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomiz sation process, allocation concealment, method of analysis and method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00720941 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Some concerns | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was not appropriate (as‐treated), but this probably did not have an effect on the outcome as there is evidence that participants actually received the assigned intervention. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are small and probably did not have an effect on the outcome. | High risk of bias | AEs were most likely reported by the participants. The investigator and site staff were responsible for detecting, documenting and reporting events. All were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Low risk of bias | CSR and study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to inappropriate method of analysis and the outcome assessors’ awareness of assigned intervention. |
|
NCT00732914 Total trial population (combined risk groups) |
Low risk of bias | Randomisation was performed centrally. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 5 participants randomised to the experimental arm and 7 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis, but data for first period reported separately. We assume participants in first period received their allocated intervention. | Low risk of bias | 3.3% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 3. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about the method of outcome measurement; outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT01613846 Total trial population (combined risk groups) |
Some concerns | Participants were randomised in a 1:1 ratio, but no information provided about who conducted the randomisation and whether the allocation was concealed. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 6 participants randomised to the experimental arm and 5 participants randomised to the control arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | 2.9% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 4.03. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about randomistion process, allocation concealment and method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT02420821 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice and web response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm and 15 participants randomised to the control arm did not receive any treatment. Conflicting information about method of analysis in the protocol. | Low risk of bias | 2% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Adverse events were reported by the participants and/or study personnel was responsible for detecting, documenting and reporting events. Both were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Low risk of bias | A study protocol with SAP available. Safety analysis was pre‐specified in the protocol and SAP. | High risk of bias | Overall judged high risk of bias due to conflicting information about method of analysis; the outcome assessors’ awareness of assigned intervention. |
|
NCT00920816 Total trial population (combined risk groups) |
Low risk of bias | A centralized registration system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 3 participants randomised to the experimental arm did not receive any treatment. The method of analysis was appropriate. | Low risk of bias | 1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT01030783 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 1 participant randomised to the experimental arm did not receive any treatment. The method of analysis was not appropriate (as‐treated). | Low risk of bias | 0.2% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are very low and probably did not have an effect on the outcome. | High risk of bias | Measurement of AEs could have differed between intervention groups due to differences in number of visits to the healthcare provider. AEs were assessed by the participants and the investigator. Both were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No SAP available. Study protocol available, but unclear whether it was finalized before unblinded outcome data were available. | High risk of bias | Overall judged high risk of bias due to inappropriate method of analysis; probable differences in outcome measurement between intervention arms; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00738530 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice recognition system was used for randomisation. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was double‐blind: both participants and those delivering the intervention were not aware of assigned interventions. Only 2 participants from the control arm and 6 participants from the intervention arm did not receive any treatment. The method of analysis was not appropriate (as‐treated). | Low risk of bias | 1.2% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | Low risk of bias | Outcome assessors were not aware of the assigned intervention. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to inappropriate method of analysis; missing study protocol and SAP. |
|
NCT01274273 Total trial population (combined risk groups) |
Low risk of bias | Participants were randomised in a 1:1 ratio. Allocation was probably controlled by an external unit. There were no baseline imbalances that would suggest a problem with randomszation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. No information provided about the method of analysis. | Low risk of bias | We assume all participants received the intended interventions. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 3. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis and method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT01108445 Total trial population (combined risk groups) |
Low risk of bias | Participants were randomised in a 1:1 ratio. Randomisation was done under allocation concealment. There were no baseline imbalances that would suggest a problem with randomisation. | High risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned intervention. Only 1 participant withdrew after consent, but before randomisation and before study drug was assigned. No precise information provided about the method of analysis. |
Low risk of bias | All 108 participants were evaluable. | High risk of bias | No precise information provided about method of measuring AEs. However, AEs were defined according to NCICTC, version 4. Outcome assessors were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No study protocol or SAP available. | High risk of bias | Overall judged high risk of bias due to lack of information about method of analysis and method of outcome measurement; the outcome assessors’ awareness of assigned intervention; missing study protocol and SAP. |
|
NCT00903175 Total trial population (combined risk groups) |
Low risk of bias | Interactive voice response system was used for randomsation. There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | The study was open‐label: both participants and those delivering the intervention were aware of assigned interventions. Only 2 participants randomised to the control arm did not receive any treatment. No precise information provided about the method of analysis, but data for crossed‐over participants were reported separately. We assume participants were analyzed as randomised in period 1. | Low risk of bias | Less than 1% did not receive the intended interventions and therefore did not have outcome data. However, these numbers are low and probably did not have an effect on the outcome. | High risk of bias | AEs were assessed by the participants who were aware of the assigned intervention. Knowledge of intervention received could have affected outcome measurement. |
Some concerns | Study protocol available with some statistical methods described, but no separate SAP available to fully check the pre‐planned analyses. | High risk of bias | Overall judged high risk of bias due to the outcome assessors’ awareness of assigned intervention; missing SAP. |
Appendix 14. Additional figures (risk of bias summary plots)
1. Summary plot for OS for all risk groups combined: Figure 53
53.
Summary plot for OS for all risk groups combined
2. Summary plot for OS per MSKCC favourable, intermediate, poor risk, respectively: Figure 54
54.
Summary plot for OS per MSKCC favourable, intermediate, poor risk, respectively
3. Summary plot for OS per IMDC favourable, intermediate, poor risk, respectively: Figure 55
55.
Summary plot for OS per IMDC favourable, intermediate, poor risk, repsectively
4. Summary plot for QoL for all risk groups combined: Figure 56
56.
Summary plot for QoL for all risk groups combined
5. Summary plot for SAEs for all risk groups combined: Figure 57
57.
Summary plot for SAEs for all risk groups combined
6. Summary plot for PFS (all risk groups combined): Figure 58
58.
Summary plot for PFS (all risk groups combined)
7. Summary plot for PFS per MSKCC favourable, intermediate, poor risk, respectively: Figure 59
59.
Summary plot for PFS per MSKCC favourable, intermediate, poor risk, respectively
8. Summary plot for PFS per IMDC favourable, intermediate, poor risk, respectively: Figure 60
60.
Summary plot for PFS per IMDC favourable, intermediate, poor risk, respectively
9. Summary plot for all‐cause grade 3 or 4 AEs for all risk groups combined: Figure 61
61.
Summary plot for all‐cause grade 3 or 4 AEs for all risk groups combined
Appendix 15. Additional figures (main analyses of OS, SAEs, PFS, AEs, and Number of participants who discontinued study treatment due to an AE)
1. Pairwise comparison for OS (all risk groups combined): Figure 62
62.
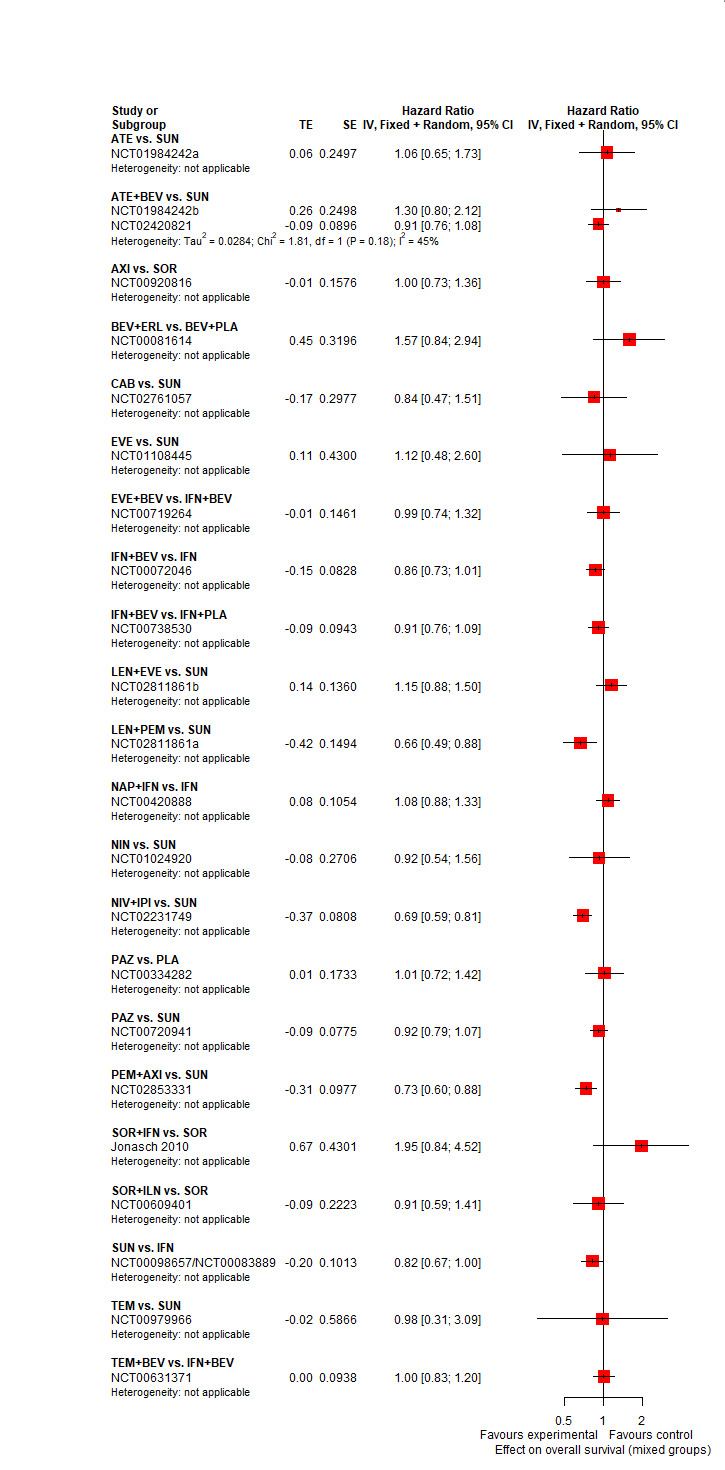
Pairwise comparison for OS (all risk groups combined)
2. Pairwise comparison for OS (MSKCC favourable): Figure 63
63.
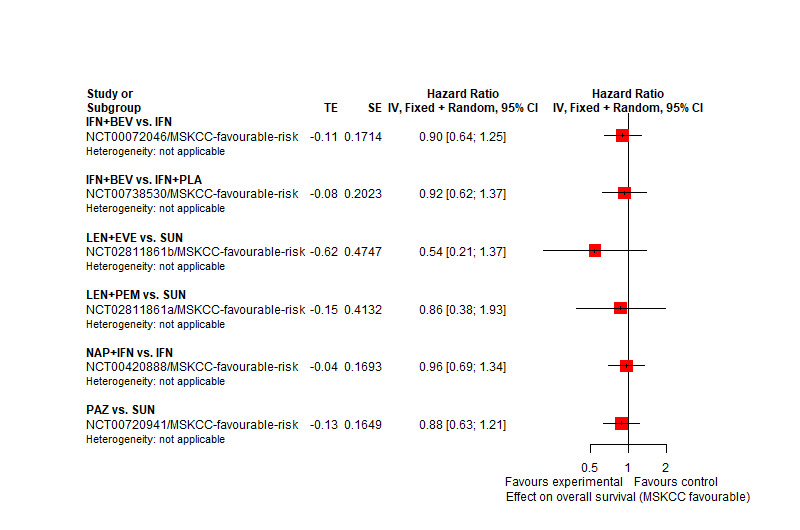
Pairwise comparison for OS (MSKCC favourable)
3. Pairwise comparison for OS (IMDC favourable): Figure 64
64.
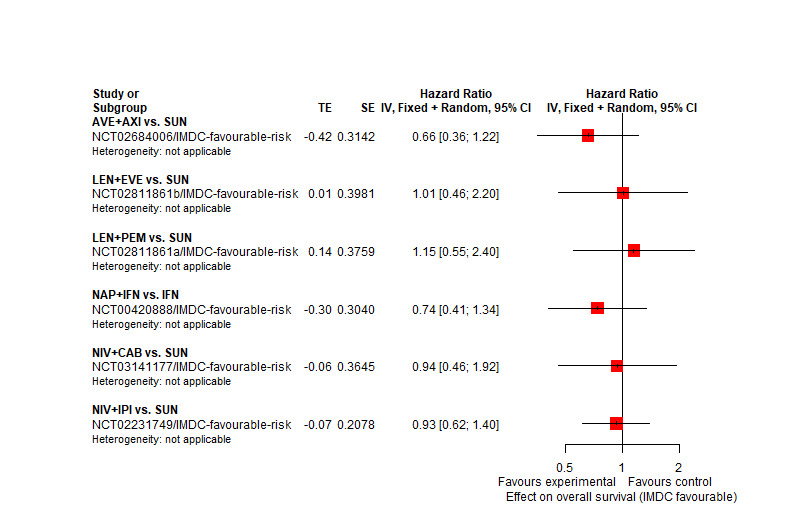
Pairwise comparison for OS (IMDC favourable)
4. Pairwise comparison for OS (MSKCC intermediate, poor): Figure 65
65.
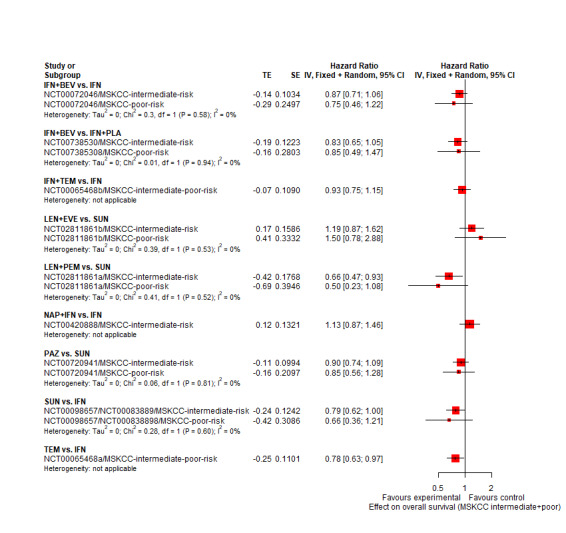
Pairwise comparison for OS (MSKCC intermediate, poor)
5. Pairwise comparison for OS (IMDC intermediate, poor): Figure 66
66.
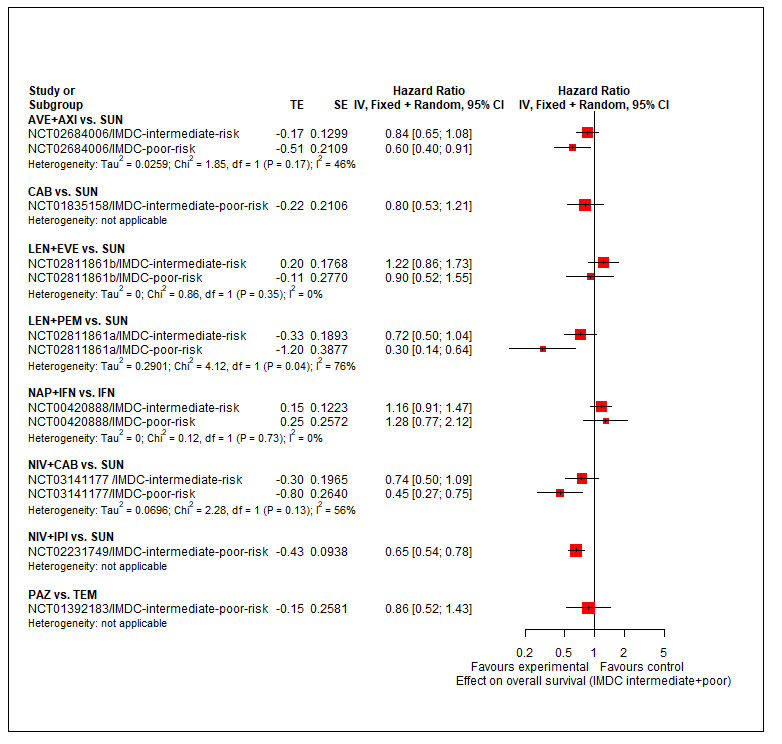
Pairwise comparison for OS (IMDC intermediate, poor)
6. Pairwise comparison for SAEs (all risk groups combined): Figure 67
67.
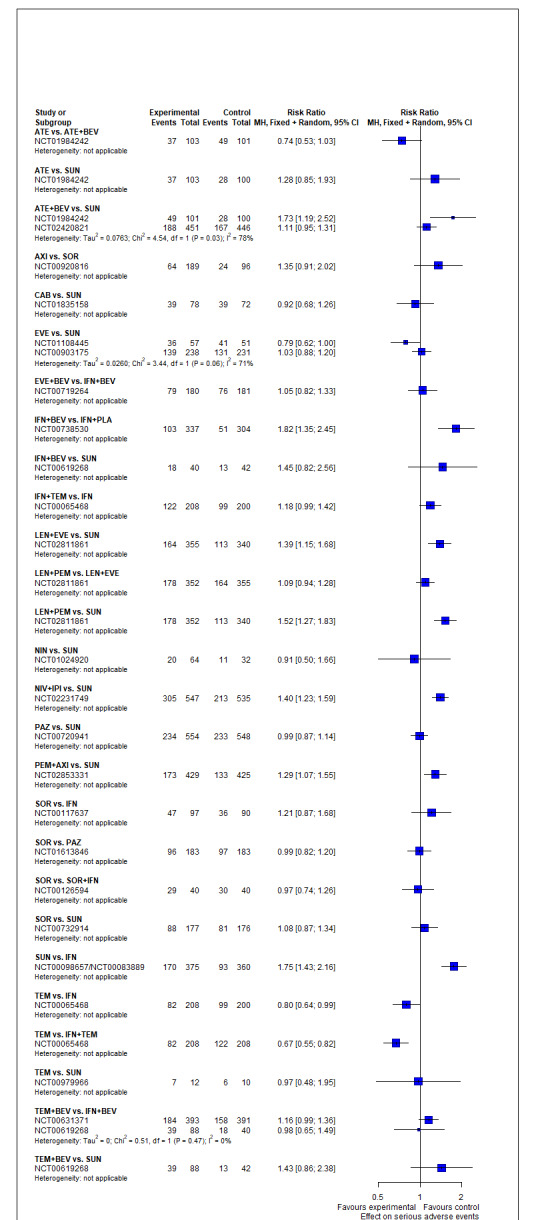
Pairwise comparison for SAEs (all risk groups combined)
7. Forest plot of splitting direct and indirect evidence for SAE (all risk groups combined): Figure 68
68.
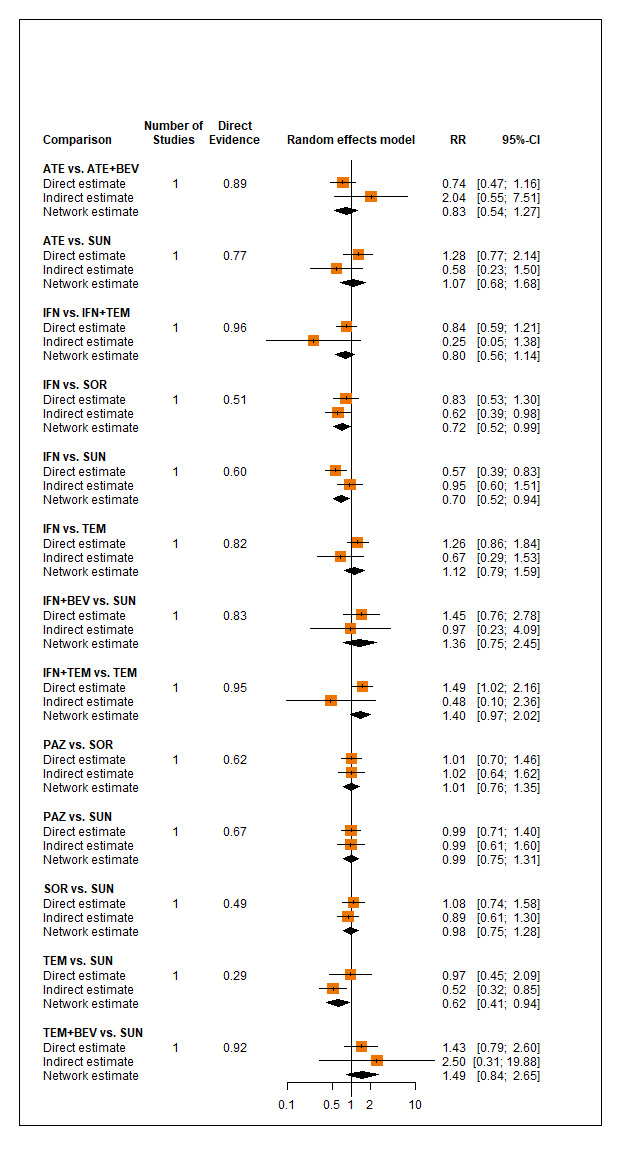
Forest plot of splitting direct and indirect evidence for SAE (all risk groups combined)
8. Net heat plot for SAEs (all risk groups combined) : Figure 69
69.
Net heat plot for SAEs (all risk groups combined)
9. Pairwise comparison for PFS (all risk groups combined): Figure 70
70.
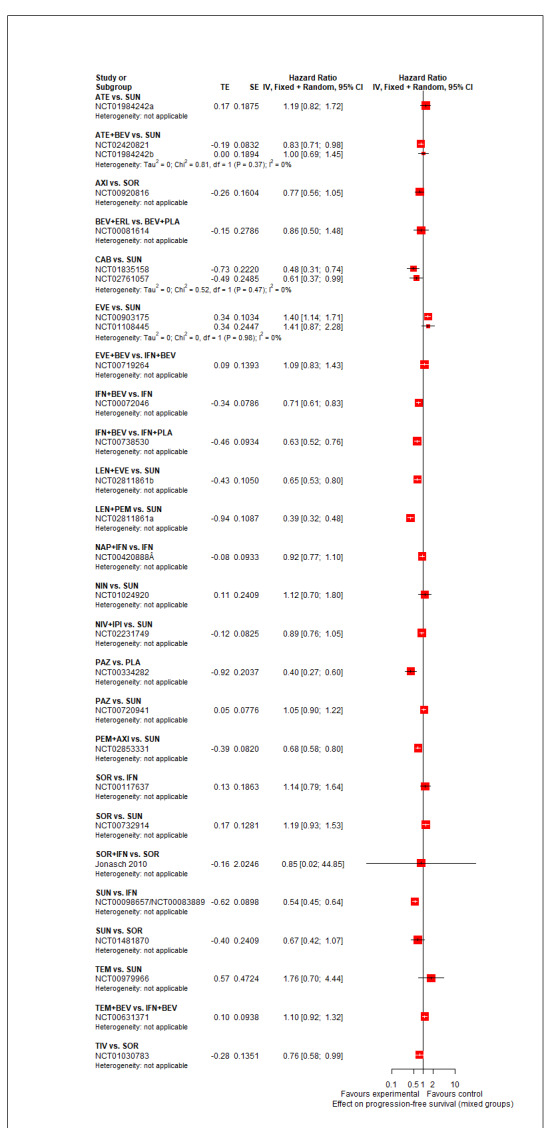
Pairwise comparison for PFS (all risk groups combined)
10. Forest plot of splitting direct and indirect evidence for PFS (all risk groups combined): Figure 71
71.
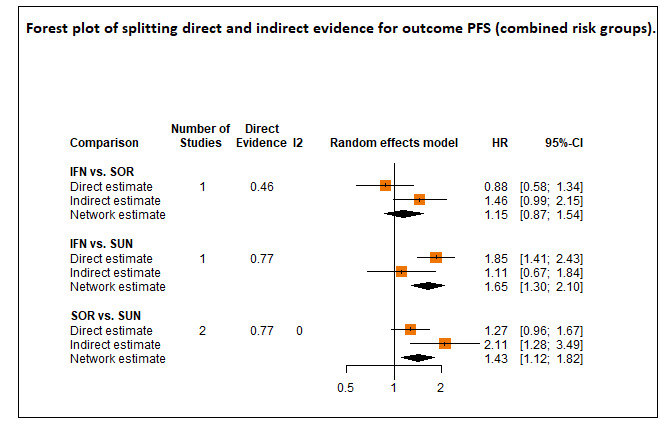
Forest plot of splitting direct and indirect evidence for PFS (all rsik groups combined)
11. Net heat plot for PFS (all risk groups combined): Figure 72
72.
Net heat plot for PFS (all risk groups combined)
12. Pairwise comparison for PFS (MSKCC favourable): Figure 73
73.

Pairwise comparison for PFS (MSKCC favourable)
13. Pairwise comparison for PFS (IMDC favourable):Figure 74
74.
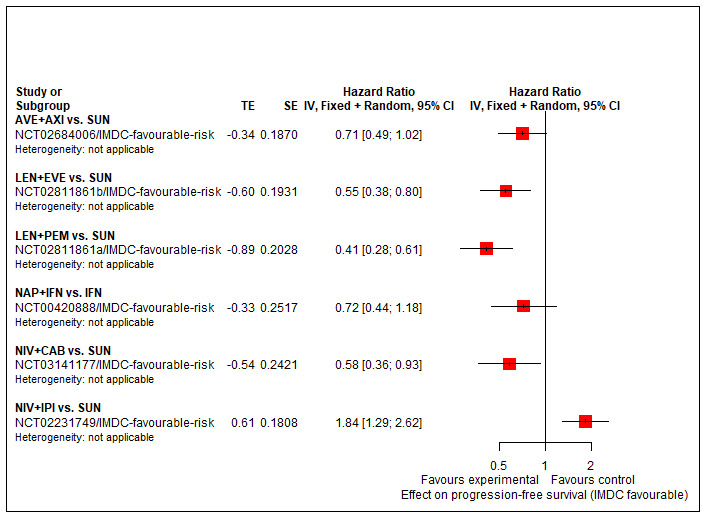
Pairwise comparison for PFS (IMDC favourable)
14. Pairwise comparison for PFS (MSKCC intermediate, poor): Figure 75
75.
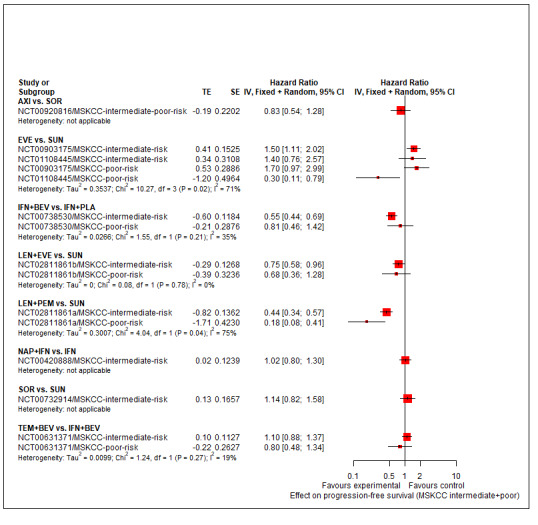
Pairwise comparison for PFS (MSKCC intermediate, poor)
15. Pairwise comparison for PFS (IMDC intermediate, poor): Figure 76
76.
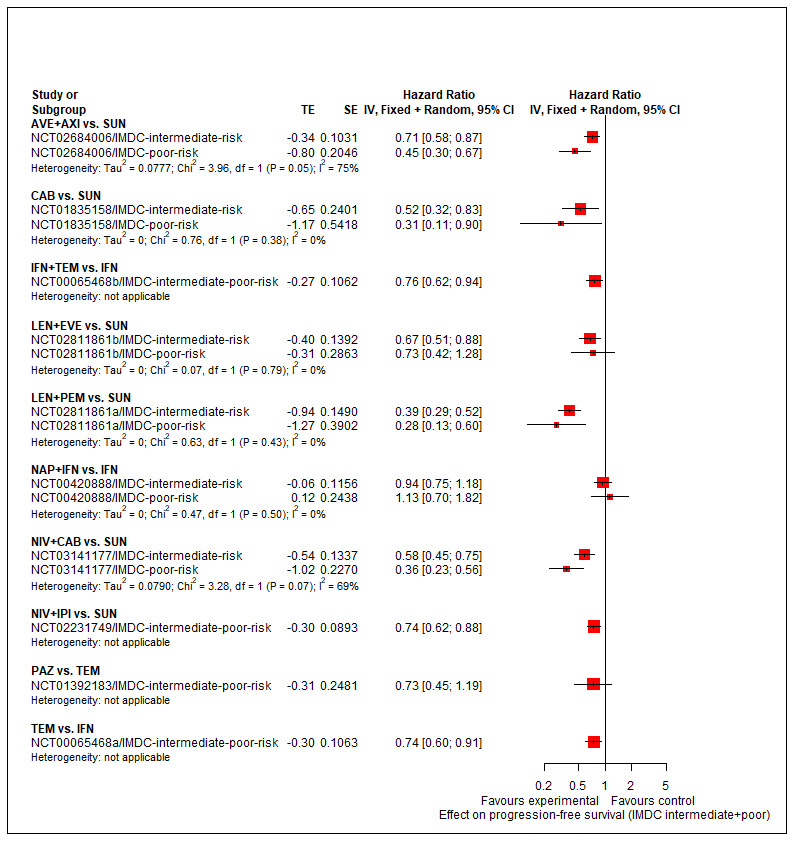
Pairwise comparison for PFS (IMDC intermediate, poor)
13. Pairwise comparison for all‐cause AEs (all risk groups combined): Figure 77
77.
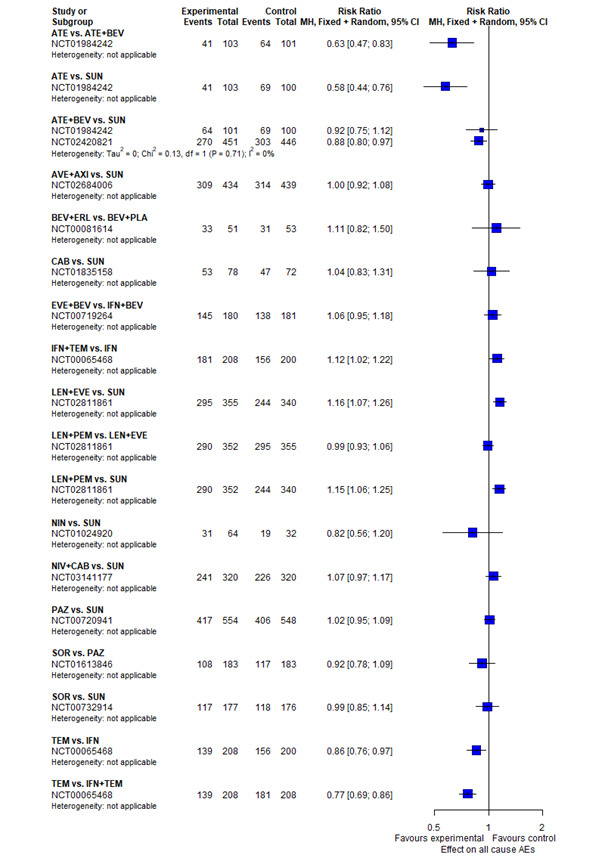
Pairwise comparison for all‐cause AEs (all risk groups combined)
16. Forest plot of splitting direct and indirect evidence for all‐cause grade 3or 4 AEs (all risk groups combined): Figure 78
78.
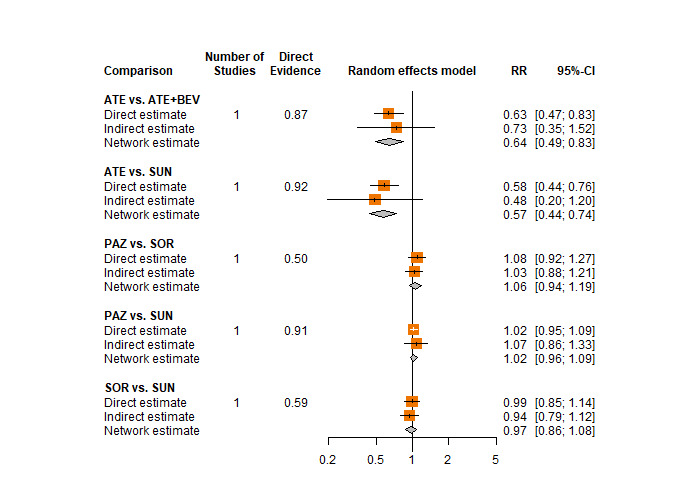
Forest plot of splitting direct and indirect evidence for all‐cause grade 3or 4 AEs (all risk groups combined)
17. Net heat plot for all‐cause AEs (all risk groups combined): Figure 79
79.
Net heat plot for all‐cause AEs (all risk groups combined)
18. Pairwise comparison for AE hand‐foot‐syndrome (all risk groups combined): Figure 80
80.
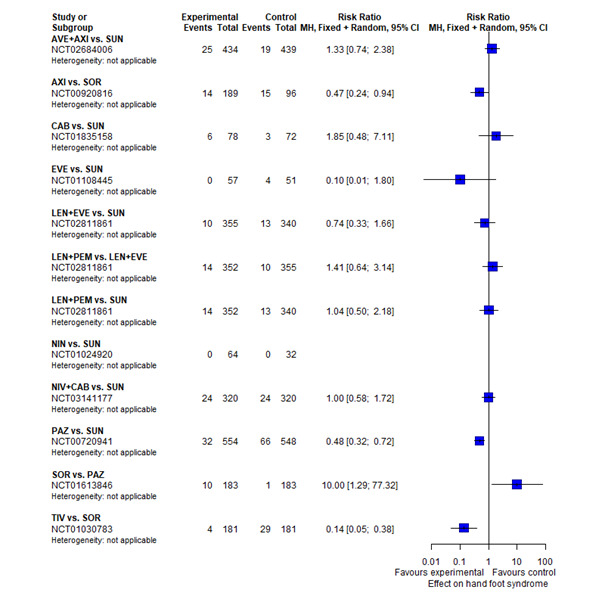
Pairwise comparison for AE hand‐foot‐syndrome (all risk groups combined)
19. Pairwise comparison for AE fatigue (all risk groups combined): Figure 81
81.
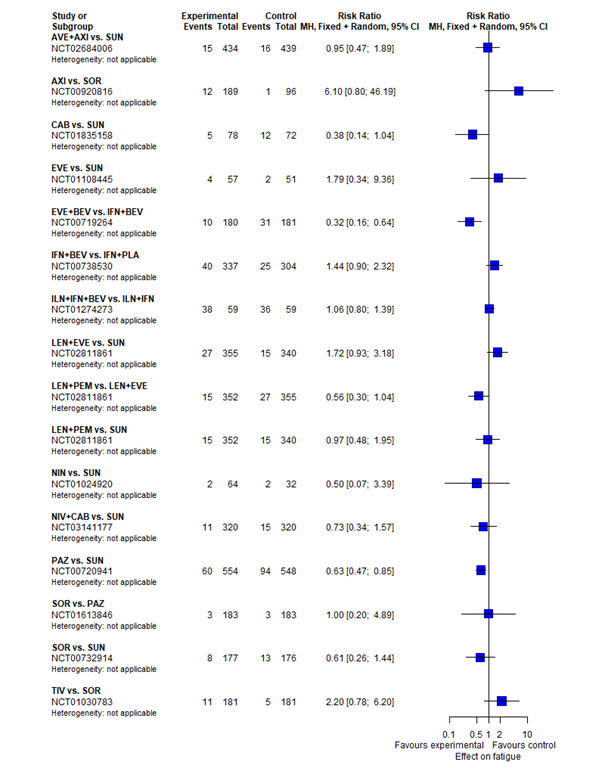
Pairwise comparison for AE fatigue (all risk groups combined)
20. Pairwise comparison for AE diarrhoea (all risk groups combined): Figure 82
82.
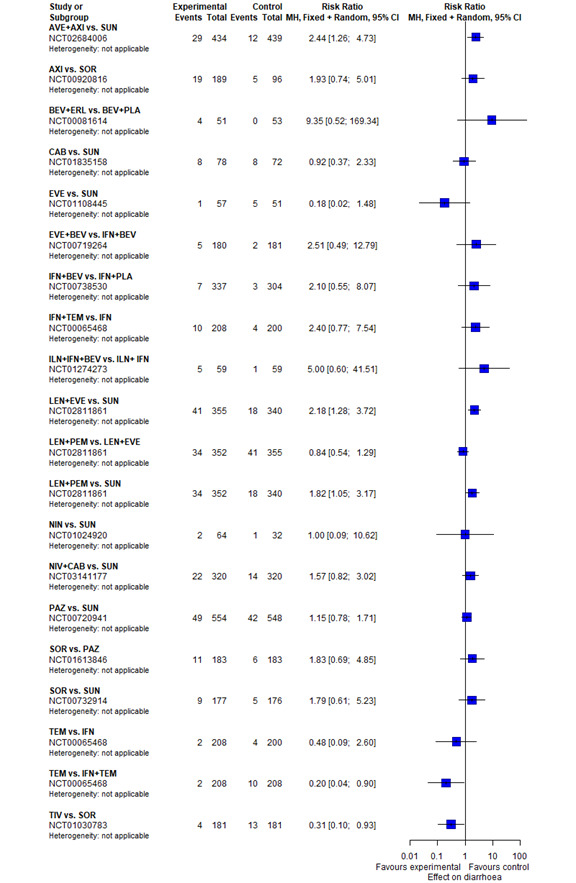
Pairwise comparison for AE diarrhoea (all risk groups combined)
21.Forest plot of splitting direct and indirect evidence for AE diarrhoea (all risk groups combined): Figure 83
83.
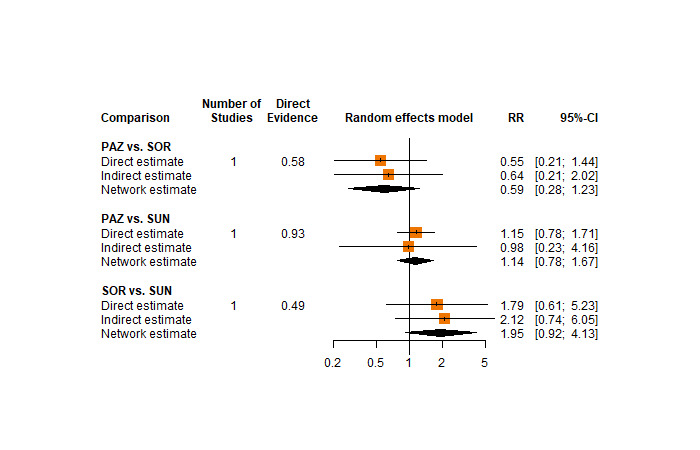
Forest plot of splitting direct and indirect evidence for AE diarrhoea (all risk groups combined)
22. Net heat plot for AE diarrhoea (all risk groups combined): Figure 84
84.
Net heat plot for AE diarrhoea (all risk groups combined)
23. Pairwise comparison for AE vomiting (all risk groups combined): Figure 85
85.
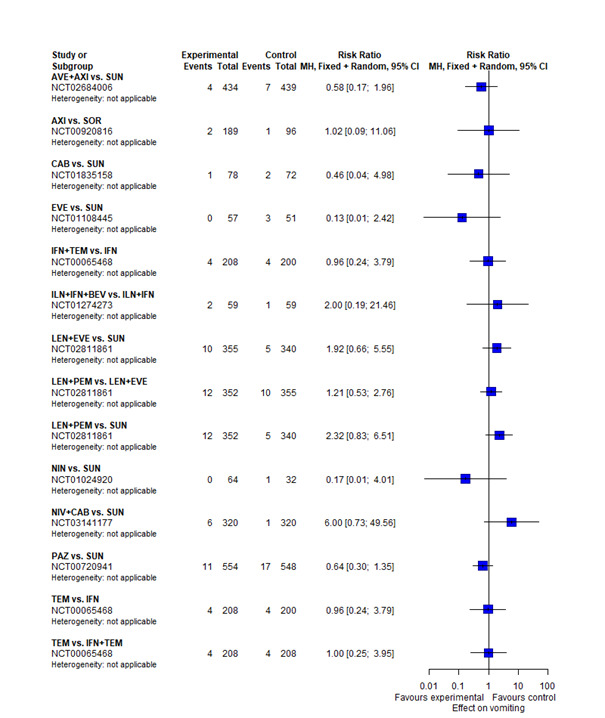
Pairwise comparison for AE vomiting (all risk groups combined)
24. Pairwise comparison for AE loss of appetite (all risk groups combined): Figure 86
86.
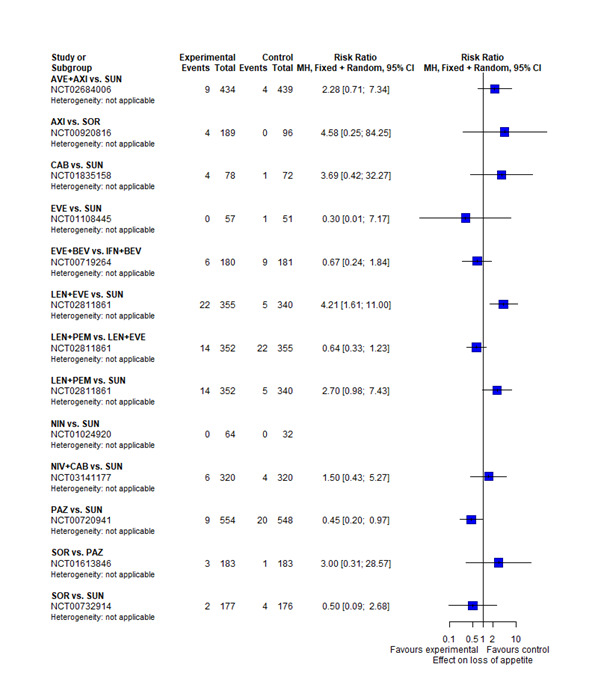
Pairwise comparison for AE loss of appetite (all risk groups combined)
25. Forest plot of splitting direct and indirect evidence for AE loss of appetite (all risk groups combined): Figure 87
87.
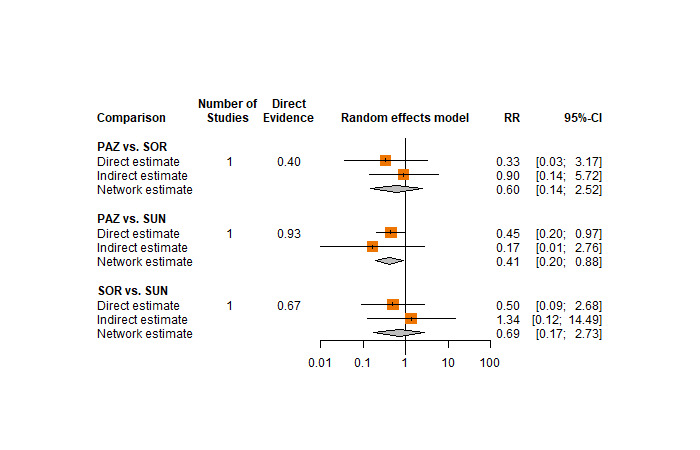
Forest plot of splitting direct and indirect evidencefor AE loss of appetite (all risk groups combined)
26. Net heat plot for AE loss of appetite (all risk groups combined): Figure 88
88.
Net heat plot for AE loss of appetite (all risk groups combined)
27. Pairwise comparison for AE weight loss (all risk groups combined): Figure 89
89.
Pairwise comparison for AE weight loss (all risk groups combined)
28. Pairwise comparison for AE stomatitis (all risk groups combined): Figure 90
90.
Pairwise comparison for AE stomatitis (all risk groups combined)
29. Forest plot of splitting direct and indirect evidence for AE stomatitis (all risk groups combined): Figure 91
91.
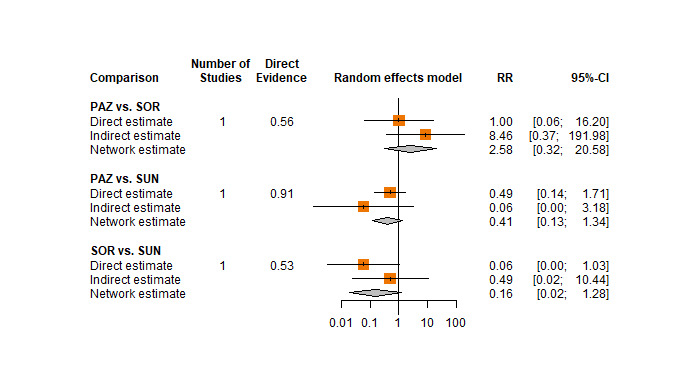
Forest plot of splitting direct and indirect evidence for AE stomatitis (all risk groups combined)
30. Net heat plot for AE stomatitis (all risk groups combined): Figure 92
92.
Net heat plot for AE stomatitis (all risk groups combined)
31. Pairwise comparison for AE mucosal inflammation (all risk groups combined): Figure 93
93.

Pairwise comparison for AE mucosal inflammation (all risk groups combined)
32. Pairwise comparison for Number of participants who discontinued treatment due to an AE (all risk groups combined): Figure 94
94.
Pairwise comparison for the outcome Number of participants who discontinued treatment due to an AE (combined risk groups). Any two treatments are connected by a line when there is at least one trial comparing the two treatments.
Appendix 16. Additional figures (subgroup and sensitivity analyses)
1. Follow‐up time <5 years for OS (all risk groups combined): Figure 95
95.
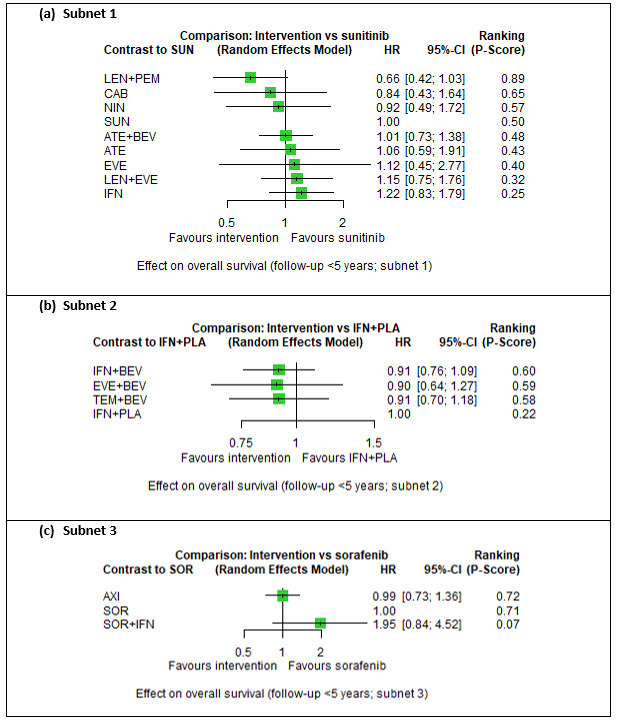
Follow‐up time <5 years for OS (all risk groups combined)
2. Follow‐up time 5 years or more for OS (all risk groups combined): Figure 96
96.
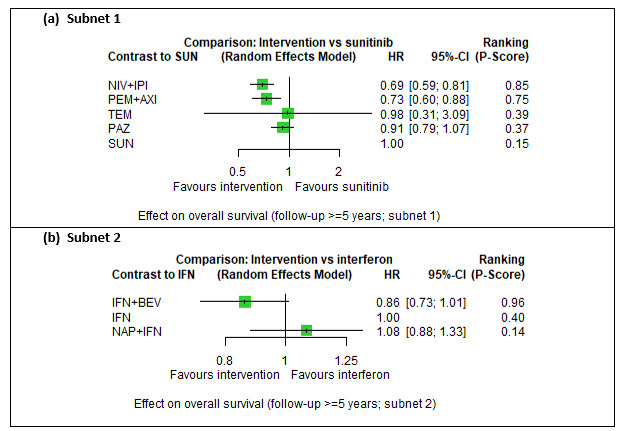
Follow‐up time 5 years or more for OS (all risk groups combined)
3. Fixed‐effect model for OS (all risk groups combined): Figure 97
97.
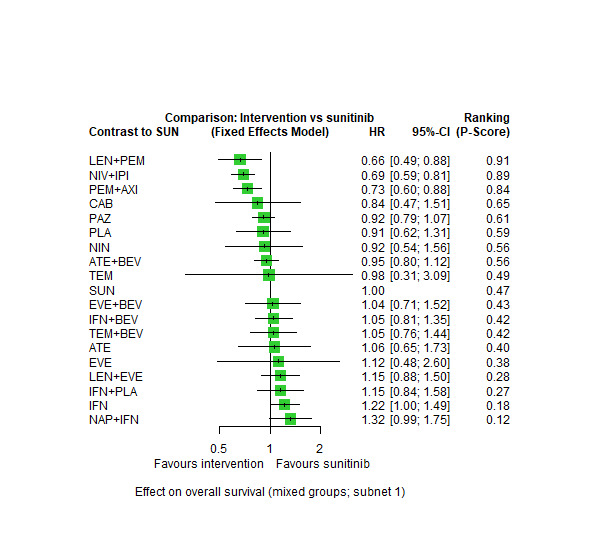
Fixed‐effect model for OS (all risk groups combined)
4. Fixed‐effect model for SAE (all risk groups combined): Figure 98
98.
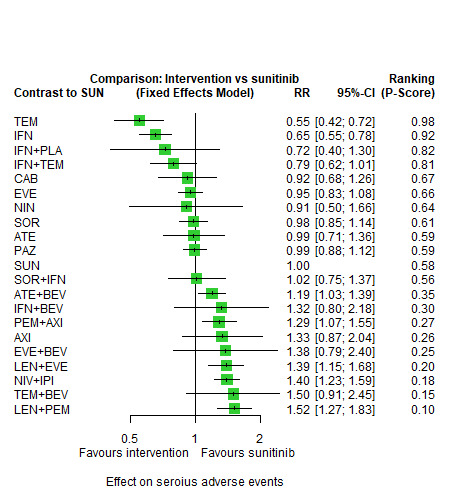
Fixed‐effect model for SAE (all risk groups combined)
5. Fixed‐effect model for PFS (MSKCC favourable ‐ subnet 1): Figure 99
99.
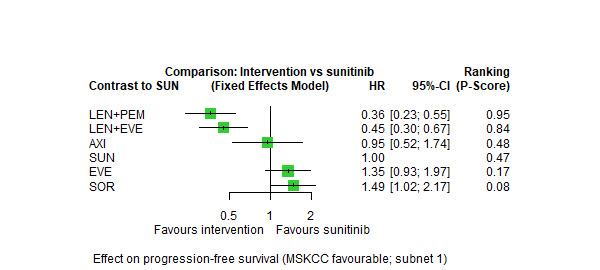
Fixed‐effect model for PFS (MSKCC favourable ‐ subnet 1)
6. Fixed‐effect model for PFS (MSKCC intermediate, poor ‐ subnet 1): Figure 100
100.
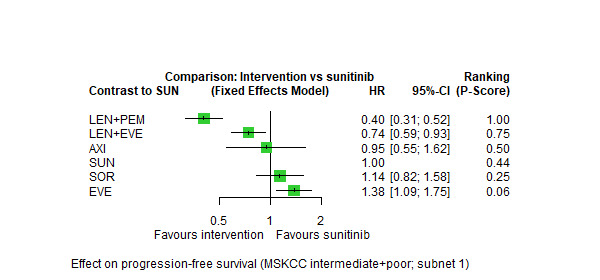
Fixed‐effect model for PFS (MSKCC intermediate, poor ‐ subnet 1)
7. Fixed‐effect model for PFS (MSKCC intermediate, poor ‐ subnet 2): Figure 101
101.
Fixed‐effect model for PFS (MSKCC intermediate, poor ‐ subnet 2)
8. Fixed‐effect model for PFS (IMDC intermediate, poor ‐ subnet 1): Figure 102
102.
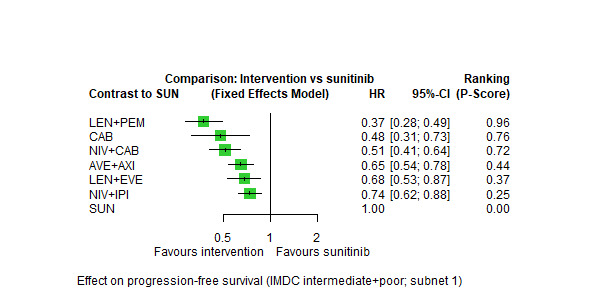
Fixed‐effect model for PFS (IMDC intermediate, poor ‐ subnet 1)
9. Validation of the PH assumption for the outcome OS (all risk groups combined): Figure 103
103.
Validation of the PH assumption for the outcome OS (all risk groups combined)
10. Sensitivity analysis for the outcome OS (all risk groups combined) with trials at 'low risk of bias' or 'some concerns': Figure 104
104.
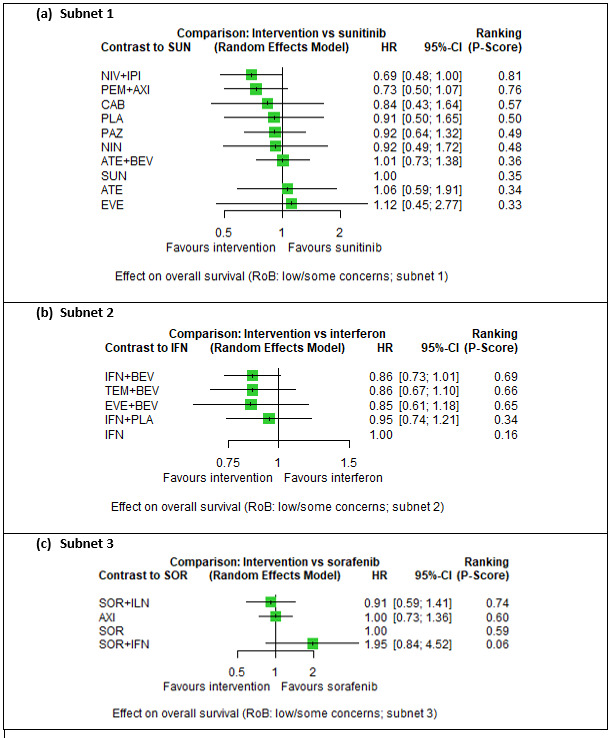
Sensitivity analysis for the outcome OS (all risk groups combined) with trials at 'low risk of bias' or 'some concerns'
11. Sensitivity analysis for the outcome OS (all risk groups combined) with trials at 'high risk of bias': Figure 105
105.
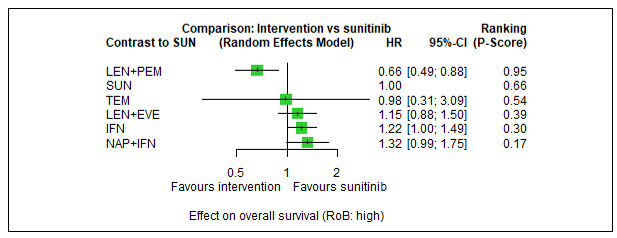
Sensitivity analysis for the outcome OS (all risk groups combined) with trials at 'high risk of bias'
12. Sensitivity analysis for the outcome SAE (all risk groups combined) with trials at 'low risk of bias' or 'some concerns': Figure 106
106.
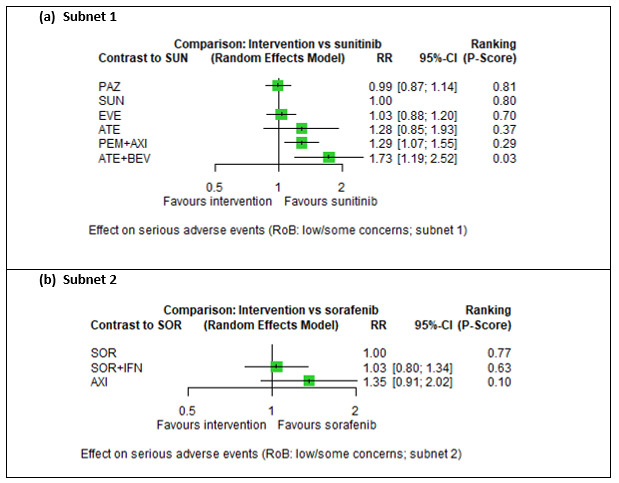
Sensitivity analysis for the outcome SAE (all risk groups combined) with trials at 'low risk of bias' or 'some concerns'
13. Sensitivity analysis for the outcome SAE (all risk groups combined) with trials at 'high risk of bias': Figure 107
107.
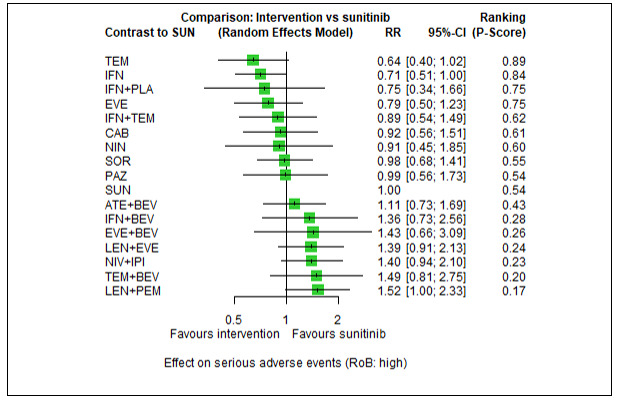
Sensitivity analysis for the outcome SAE (all risk groups combined) with trials at 'high risk of bias'
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jonasch 2010.
| Study characteristics | ||
| Methods |
Study name: ‐ (NCT also not available) Study design: randomised, phase II trial Blinding: no information Study dates: June 24, 2005 ‐ June 18, 2007 (date of enrolment) Date of data cut‐off: not reported Location:USA.; type of centre: cancer centre (1 study location) Cross‐over study or cross over permitted: not per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Sample size: N = 80 Age, median in years (range): experimental arm: 60.7 (43‐81), control arm: 62.4 (45‐83) Sex (m/f): experimental arm: 29/11, control arm: 32/8 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 40): Sorafenib 400 mg (oral, twice/day) + Interferon alfa (0.5 MIU, subcutaneous injection, twice/day) Control arm (n = 40): Sorafenib, 400mg (oral, twice/day) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Outcomes relevant to this review but not reported: QoL; TFST; number of participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): ORR |
|
| Notes |
Funding sources: National Cancer Institute’s Cancer Therapy Evaluation Program Conflict of interest disclosures: "Supported by the National Cancer Institute’s Cancer Therapy Evaluation Program." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00065468.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: randomised, phase III (three‐arm trial) Blinding: none, open‐label Study dates: July 2003 – April 2005 (date of enrolment) Date of data cut‐off: exact date not reported. The results presented and used in this review are of the second interim analysis. Location: 25 countries (Argentina, Australia, Canada, Czech Republic, Former Serbia and Montenegro Germany, Greece, Hungary, Italy, Latvia, Lithuania, Mexico, the Netherlands, Poland, Russian Federation, Serbia, Slovakia, South Africa, Spain, Sweden, Taiwan, Turkey, Ukraine, UK, USA; types of centres: cancer centres, hospitals, university hospitals (153 study locations) Cross‐over study or cross‐over permitted: no |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 626 Age, median in years (range): experimental arm I: 60 (23‐86), experimental arm II arm: 58 (32‐81), control arm: 59 (32‐82) Sex (m/f): experimental arm I: 148/59, experimental arm II: 139/70, control arm: 145/65 Prognostic factors:
|
|
| Interventions |
Experimental arm I (n = 207): Interferon alfa, 3 MIU (1st week), 9 MIU (2nd week), 18 MIU (thereafter) (subcutaneous injection, three times/week) Experimental arm II (n = 209): Temsirolimus (25 mg, intravenous, once/week) Control arm (n = 210): Interferon alfa 3 MIU (1st week), 6 MIU (thereafter) (subcutaneous injection, three times/week) + Temsirolimus (15 mg, intravenous, once/week) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST; number of participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): objective response (OR); participants with clinical benefit; duration of response (DR); time to treatment failure (TTF); quality‐adjusted time without symptoms or toxicity (Q‐TWIST) |
|
| Notes |
Funding sources: Pfizer Declarations of Interests: Quote: "Dr. Hudes reports receiving consulting and lecture fees from Pfizer Pharmaceuticals and consulting fees from Wyeth Pharmaceuticals; Drs. Carducci and Motzer, consulting fees from Wyeth Pharmaceuticals; Dr. Dutcher, consulting and lecture fees from Novartis, Chiron, Bayer, and Onyx Pharmaceuticals, consulting fees from Wyeth Pharmaceuticals, lecture fees from Pfizer Pharmaceuticals, and research grants from Bayer, Chiron, Genentech, Pfizer, and Wyeth Pharmaceuticals; Dr. Figlin, consulting and lecture fees and research grants from Wyeth Pharmaceuticals; Dr. Kapoor, consulting fees and research grants from Wyeth Pharmaceuticals and research grants from Bayer Pharmaceuticals; Dr. McDermott, consulting fees from Bayer, Onyx, and Genentech Pharmaceuticals and lecture fees from Novartis Pharmaceuticals; and Dr. Schmidt‐Wolf, symposium support fees from Wyeth Pharmaceuticals. Mr. O’Toole, Ms. Lustgarten, and Dr. Moore report being full‐time employees of Wyeth Pharmaceuticals. No other potential conflict of interest relevant to this article was reported." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00072046.
| Study characteristics | ||
| Methods |
Study name: CALGB 90206 Study design: randomised, phase III Blinding: none, open‐label Study dates: October 2003 – November 2012 (date of enrolment) Date of data cut‐off: March 24, 2009 (for OS), not reported for PFS Location: 2 countries (Canada, USA.), types of centres: cancer centres, medical centres, hospitals (493 study locations) Cross‐over study or cross over permitted: no |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 732 Age (years, median with range): experimental arm: 61 (56 to 70), control arm: 62 (55 to 70) Sex (m/f): experimental arm: 269/100, control arm: 239/124 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 369): Bevacizumab (10 mg/kg, intravenous), Interferon alfa (9 MIU, subcutaneous injection) Control arm (n = 363): Interferon alfa (9 MIU, subcutaneous injection) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, SAE, TFST, number of participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): objective response rate (ORR) |
|
| Notes |
Funding sources: Walter M. Stadler, Genentech; Daniel A. Vaena, Genentech; Janice Dutcher, Novartis, Genentech, Pfizer, sponsor: Alliance for Clinical Trials in Oncology, collaborators: National Cancer Institute (NCI) & NCIC Clinical Trials Group Declaration of Interest: Quote: "Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. (...) For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00081614.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: randomised, parallel, placebo‐controlled phase II trial Blinding: double‐blind (investigator and participants) Study dates: March 2004 ‐ July 2005 Date of data cut‐off: not reported Location: 1 country (USA), types of centres: cancer centres, medical centres, hospitals/clinics, university hospitals (20 study locations) Cross‐over study or cross over permitted: not a cross‐over study; no information whether cross over was permitted |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 104 Age (median (years, range)): experimental arm: 66 (38‐86), control arm: 61 (35‐78) Sex (m/f): experimental arm: male: 33/18, control arm: 40/13 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 51): bevacizumab (10 mg/kg, intravenous) + erlotinib (150 mg, oral, daily) Control arm (n = 53): bevacizumab (10 mg/kg, intravenous) + Placebo (150 mg, oral, daily) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, SAE, TFST, number of participants who discontinued treatment Other outcomes (not relevant to this review): ORR |
|
| Notes |
Funding sources: Ronald M. Bukowski, Bayer/Onyx, Genentech, Wyeth, Pfizer, Amgen; Robert A. Figlin, Genentech; Janice P. Dutcher, Bayer, Pfizer, Wyeth, Chiron/Novartis, Idera, Genentech; David F. McDermott, Genentech, Pfizer, Bayer/Onyx Declarations of Interests: Quote; "Although all authors completed the disclosure declaration, the following authors or their immediate family members indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00098657/NCT00083889.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: randomised, phase III trial Blinding: none, open‐label Study dates: August 2004 – October 2005 (date of randomisation) Date of data cut‐off: November 2005 Location: 11 countries (Australia, Brazil, Canada, France, Germany, Italy, Poland, Russian Federation, Spain, U.K., USA), types of centres: cancer centres, medical centres, university hospitals (124 study locations) Cross‐over study or cross over permitted: not a cross‐over study per design, but cross over to sunitinib was permitted in case of disease progression |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample Size: N = 750 Age (years, median with range): experimental arm: 62 (27‐87), control arm: 59 (34‐85) Sex (M/F): experimental arm: 267/108, control arm: 269/106 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 375): Sunitinib (50mg, oral, once/day) Control arm (n = 375): Interferon alfa (3 MIU (1st week), 6 MIU (2nd week), and 9 MIU (thereafter), subcutaneous injection, thrice/week) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): objective response (OR), time to tumour progression (TTP), duration of response (DR), laboratory results, pharmacokinetics |
|
| Notes |
Funding sources: Pfizer Declarations of Interests: Quote: "Dr. Motzer reports receiving research grants from Pfizer and Genentech, consulting fees from Wyeth, and lecture fees from Bayer Pharmaceuticals; Dr. Hutson, consulting and lecture fees from Pfizer, Bayer Pharmaceuticals, and Onyx Pharmaceuticals; Dr. Michaelson, consulting fees from Pfizer and Wyeth Pharma‐ ceuticals and lecture fees from Pfizer; Dr. Bukowski, research grants from Pfizer, Bayer Pharmaceuticals, Genentech, Genzyme, and Bristol‐Myers Squibb and consulting and lecture fees from Pfizer, Bayer Pharmaceuticals, Onyx Pharmaceuticals, and Genentech; Dr. Rixe, consulting and lecture fees from Pfizer; Dr. Oudard, consulting and lecture fees from Pfizer; Dr. Negrier, consulting fees from Pfizer and Bayer Pharmaceuticals; and Dr. Figlin, research grants from Pfizer, consulting fees from Pfizer and Onyx Pharmaceuticals, and lecture fees from Pfizer and Bayer Pharmaceuticals. Ms. Kim and Drs. Chen, Bycott, and Baum report being full‐time employees of Pfizer and having equity ownership in the company. No other potential conflict of interest relevant to this article was reported." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00117637.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: randomised, phase II trial Blinding: none, open‐label Study dates: June 28, 2005 ‐ September 30, 2005 (date of randomisation) Date of data cut‐ off: not reported Location: 7 countries* (France, Germany, Poland, Russian Federation, Ukraine, UK, USA), types of centres: not reported Cross‐over study or cross over permitted: yes, cross over trial** *discrepancies between information provided in the publication and information provided on ct.gov; we included information from ct.gov. **For cross‐over trials, we only extracted data from the first period. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 189 Age (median in years (range)): experimental arm: 62 (34‐78), control arm: 62.5 (18‐80) Sex (m/f): experimental arm: 65/32, control arm: 52/40 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 97): Sorafenib (400mg, oral, twice/day) Control arm (n = 92): Interferona alfa (9 MIU, subcutaneous injection, thrice/week) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: OS, TFST Other outcomes (not relevant to this review): response duration, OR, DCR, CR |
|
| Notes |
Funding sources: Thomas E. Hutson, Bayer/Onyx, Pfizer Inc, Wyeth; MichaelStaehler, Bayer Healthcare, Pfizer Inc, Roche, Novartis, Wyeth; DavidCella, Bayer Healthcare; Ronald Bukowski, Bayer Healthcare, Wyeth,Novartis Declarations of interests: quote: "Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00126594.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: randomised, parallel assignment, phase II (three‐arm trial) Blinding: none, open‐label Study dates: June, 2005 ‐ August, 2013 Date of data cut‐off: not reported Location: 1 country (USA), type of centre: cancer centre (1 study location) Cross‐over study or cross over permitted: not per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
More inclusion and exclusion criteria on CT.gov. Sample size: N = 80 Age (median in years (range)): experimental arm: 60.7 (43‐81), control arm: 62.4 (45‐83) Sex (m/f): experimental arm: 29/11, control arm: 32/8 Prognostic factors:
|
|
| Interventions |
Experimental arm: sorafenib (400 mg, oral, twice/day) Control arm: sorafenib (400 mg, oral, twice/day) and recombinant interferon alfa‐2b (0.5 MIU, subcutaneous injection, twice/day) |
|
| Outcomes |
Primary outcome(s) ‐ Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST, number of participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): ORR, DoR |
|
| Notes |
Funding sources: National Cancer Institute (NCI), M.D. Anderson Cancer Center Declarations of Interests: not found Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00334282.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: randomised, placebo‐controlled trial, phase III Blinding: quadruple (participant, care provider, investigator, outcomes assessor) Study dates: April 2006 – April 2007 (date of enrolment) Date of data cut‐off: March 15, 2010 (final analysis of OS and updated safety data), May 23, 2008 (final PFS analysis) Location: 25 countries (Argentina, Australia, Austria, Brazil, Chile, China, Czech Republic, Estonia, Greece, Hong Kong, India, Ireland, Italy, Republic of Korea, Latvia, Lithuania, Mexico, New Zealand, Pakistan, Poland, Russian Federation, Slovakia, Tunisia, Ukraine, UK.), types of centres: (100 study locations) Cross‐over study or cross over permitted: not per design, but cross over was permitted from placebo to pazopanib |
|
| Participants |
Inclusion criteria:
Or,
Exclusion criteria:
Sample size: N=233 treatment‐naive participants Age (years, median with range): experimental arm: 65 (25‐80), control arm: 60 (25‐81) Sex (m/f): experimental arm: 61/19, control arm: 109/36 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 155): Pazopanib (800 mg, oral, once/day) Control arm (n = 78): Placebo (800mg, oral, once/day) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: AE in first‐line participants, TFST, number of (first‐line) participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): DoR, CR, ORR |
|
| Notes |
Funding sources: GlaxoSmithKline Declarations of Interests: Quote: "No potential conflict of interest." stated by EL. Clinical study report available: yes Study protocol available: yes Statistical analysis plan available: yes *Trial included both participants who have received prior treatment and participants who are treatment‐naive. Results are reported separately for the treatment‐naive participants in the publication. Hence, all data reported in this review refers to the treatment‐naive group of participants only. |
|
NCT00420888.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: randomised, parallel‐group trial, phase II/ III Blinding: none, open‐label Study dates: May 2007 ‐ October 2010 (date of randomisation) Date of data cut‐off: not reported Location: 5 countries (Bulgaria, Romania, Russian Federation, Ukraine, UK), types of centres: hospitals, urology clinics, cancer centres, research centres (51 study locations) Cross‐over study or cross over permitted: not per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Sample size: N = 513 Age, mean in years (standard deviation (SD)): experimental arm: 58 (25‐79), control arm: 57 (19‐83) Sex (m/f): experimental arm: 183/77), control arm: 183/70 prognostic factors:
|
|
| Interventions |
Experimental arm (n=253): naptumomab (15 mg/kg, intravenous, once/day) + IFN‐alfa (9 MIU, subcutaneous injection, thrice/week) Control arm (n = 260): IFN‐alfa (9 MIU, subcutaneous injection, thrice/week) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: quality of life (QoL), serious adverse events (SAEs), time to first subsequent therapy (TFST), number of participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): response rate (RR); immunological response to treatment in participants receiving naptumomab; pharmacokinetics |
|
| Notes |
Funding sources: GlaxoSmithKline, Novartis, Pfizer and Bayer Declarations of interests: "R.E. Hawkins reports receiving commercial research grants from Glaxo‐SmithKline, Novartis, and Pfizer; speakers bureau honoraria from Bristol‐ Meyers Squibb, GlaxoSmithKline, Novartis, and Pfizer; and is a consultant/ advisory board member for Pfizer. G. Hedlund, G. Forsberg, and O. Nordle have ownership interest (including patents) in Active Biotech. T. Eisen is an employee of AstraZeneca; reports receiving commercial research grants from Bayer, GlaxoSmithKline, and Pfizer and other research grants from AstraZeneca; has ownership interest (including patents) in AstraZeneca; and is a consultant/ advisory board member for Aveo, Bayer, Bristol‐Meyers Squibb, GlaxoSmithKline, Immatics, Novartis, and Pfizer. No potential conflicts of interest were disclosed by the other authors." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00609401.
| Study characteristics | ||
| Methods |
Study name: ROSORC Study design: randomised, phase II Blinding: none, open‐label Study dates: October 2006 ‐ February 2008 (date of enrolment) Date of data cut‐off: September 30, 2012 (for OS) Location: 1 country (Italy), types of centres: not reported, but multicentre study Cross‐over study or cross over permitted: not a cross‐over study; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 128 Age (years, median with range): experimental arm: 64 (57‐69), control arm: 62 (52‐69) Sex (m/f): experimental arm: 52/14, control arm: 43/19 Prognostic factors:
|
|
| Interventions | Experimental arm (n = 66): sorafenib (400 mg, oral, twice/day) + IL‐2 (3 MIU, subcutaneous injection, 5 days/week)Control arm (n = 62): Sorafenib (400mg, oral, twice/day) | |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL,TFST, number of participants who discontinued treatment Other outcomes (not relevant to this review): ORR |
|
| Notes |
Funding sources: editorial assistance for this manuscript was provided by Dragonfly Editorial, funded by Bayer HealthCare. This study was supported in part by Bayer HealthCare. Declarations of Interests: Quote: "The authors have declared no conflicts of interest." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00619268.
| Study characteristics | ||
| Methods |
Study name: TORAVA Study design: randomised, phase II (three‐arm trial) Blinding: none, open‐label Study dates: March 3, 2008 ‐ May 6, 2009 (date of randomisation) Date of data cut‐off: not reported Location: 1 country (France), types of centres: cancer centres/institutes, hospitals, university hospitals (29 study locations) Cross‐over study or cross over permitted: not a cross‐over study per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 171 Age (years, median with range): experimental arm: 62.0 (33‐83), control arm I: 61.2 (33‐83), control arm II: 61.9 (40‐79) Sex (m,f) : experimental arm: 65/23, control arm I: 32/10, Group 3: 27/14 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 88): Temsirolimus (25mg, intravenous, once/week) + Bevacizumab (10mg/kg, intravenous, every 2 weeks) Control arm I (n = 42): Sunitinib (50mg, oral, daily) Control arm II (n=41): Bevacizumab (10mg/kg, intravenous, every 2 weeks) + IFN‐alpha‐2a (9 MIU, subcutaneous injection, thrice/week) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): ORR, DoR, PFR |
|
| Notes |
Funding sources: French Ministry of Health and Wyeth Pharmaceuticals, Centre Leon Berard Declarations of Interests: Quote "SN has received honoraria from Novartis, Wyeth, Pfizer, GlaxoSmithKline, and Roche; and has received research funding from Wyeth, Roche, and Novartis. DP has received honoraria from Bayer, Eli Lilly, and Roche. J‐OB has received honoraria from Amgen and is a consultant with Novartis. LG and BL have received honoraria from Novartis. BE has received honoraria from Bayer, Roche, Pfizer, Genentech, Novartis, GlaxoSmithKline, and Aveo; and is a consultant with Bayer, Pfizer, and Roche. All other authors declared no conflicts of interest." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00631371.
| Study characteristics | ||
| Methods |
Study name: INTORACT Study design: randomised, phase III Blinding: none, open‐label Study dates: April 10, 2008 ‐ October 19, 2010 (date of randomisation) Date of data cut‐off: April 19, 2012 Location: 30 countries (Argentina, Australia, Belgium, Brazil, Canada, Chile, Colombia, Czech Republic, France, Germany, Hong Kong, Hungary, India, Italy, Republic of Korea, Malaysia, Mexico, Netherlands, Poland, Portugal, Russian Federation, Serbia, Singapore, Slovakia, South Africa, Spain, Taiwan, Ukraine, UK., USA), types of centres: hospitals, university hospitals, cancer centres, research centres (172 study locations) Cross‐ over study or cross over permitted: not per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 791 Age (years, median with range): experimental arm: 59 (22‐87), control arm: 58 (23‐81) Sex (m/f, %): experimental arm: 286/114, control arm: 270/121 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 400): Bevacizumab (10 mg/kg, intravenous, every two weeks) + Temsirolimus (25mg, intravenous, once/week) Control arm (n = 391): Bevacizumab (10 mg/kg, intravenous, every two weeks) + IFN‐alfa (9 MIU, subcutaneous, three times/ week) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): ORR |
|
| Notes |
Funding Sources: Brian I. Rini, Pfizer Declarations of Interests: Quote: "Although all authors completed the disclosure declaration, the following author(s) and/or an author’s immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article (...)For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00719264.
| Study characteristics | ||
| Methods |
Study name: RECORD‐2 Study design: a two‐arm, RCT, phase II Blinding: none, open‐label Date of enrolment/randomisation: not reported Date of data cut‐off: December 31, 2011 (for PFS); August 30, 2012 (for OS and safety) Location: 21 countries (Belgium, Brazil, Czech Republic, Egypt, France, Germany, Hong Kong, Hungary, Italy, Republic of Korea, the Netherlands, Russian Federation, Singapore, South Africa, Spain, Switzerland, Taiwan, Thailand, Turkey, UK, USA.) types of centres: (108 study locations) Cross‐over study or cross over permitted: not per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N=365 Age, Mean (years, SD): experimental arm: 60.71 (10.6) , control arm: 59.9 (10.3) Sex, m/f): experimental arm: 138/44, control arm: 131/52 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 182): everolimus (10 mg, daily) + bevacizumab (10 mg/kg, every two weeks) Control arm (n= 183): IFN, dose escalated from 3 MIU during week 1, 6 MIU during week 2, and 9 MIU during week 3 of treatment and subsequently (if tolerated), 3 times per week plus intravenous bevacizumab 10 mg/kg every 2 weeks |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: PFS, TFST Other outcomes (not relevant to this review): DoE, disease related symptoms, RDD, best OR |
|
| Notes |
Funding sources: Novartis Pharmaceuticals. No grant numbers applied. Declarations of Interests: Quote: "All remaining authors have declared no conflicts of interest." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00720941.
| Study characteristics | ||
| Methods |
Study name: COMPARZ Study design: a two‐arm, randomised, phase III Blinding: none, open‐label Date of enrolment/randomisation: August 2008 ‐ September 2011 Date of data cut‐off: 21st May 2012 (PFS), 30th Sep. 2013 (for OS, AE and SAE) Location: 14 countries (Australia, Canada, China, Germany, Ireland, Italy, Japan, Republic of Korea, the Netherlands, Spain, Sweden, Taiwan, UK, USA), types of centres: not reported (227 study locations) Cross‐over study or cross over permitted: not per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N=1110 Age, continuous mean (years, SD): Group 1: 60.9 (10.89), Group 2: 61.2 (10.98) Sex (m/f): Group 1: 398/159, Group 2: 415/138 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 557): Pazopanib (800 mg, oral, once/day) Control arm (n = 553): Sunitinib (50 mg, oral, once/day) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST, Safety (AE, SAE) Other outcomes (not relevant to this review): laboratory results, MRU, CTSQ, SQLQ, FKSI‐ 19 scale, FACIT‐F scale, DoR |
|
| Notes |
Funding sources: supported by GlaxoSmithKline Pharmaceuticals and Novartis Pharmaceuticals Declarations of Interests: not reported Clinical study report available: yes Study protocol available: yes Statistical analysis plan available: yes |
|
NCT00732914.
| Study characteristics | ||
| Methods |
Study name: SWITCH Study design: a two‐arm, randomised, phase III Blinding: none, open‐label Study dates: February 2009 ‐ December 2011 (date of randomisation) Date of data cut‐off: August 15, 2013 (primary analysis) (data used in this review); January 14, 2014 (post‐hoc analysis of OS) Location: 1 country (Germany), types of centres: urology clinic (1 study location) Cross‐over study or cross over permitted: yes, cross‐over study* *For cross over trials we only extracted data from the first period. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Excluded therapies and medications, previous and concomitant:
Sample size: N =365 Median age (years, range): experimental arm: 63 (39‐84), control arm: 65 (40‐83) Sex (m/f): experimental arm: 139/43, control arm: 135/48 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 182): Sorafenib (400 mg, oral, twice/day) Control arm ( n =183): Sunitinib (50 mg, oral, once/day) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: first‐line OS, QoL, TFST Other outcomes (not relevant to this review): OS (after second‐line treatment), time to progression (TTP), DCR (disease control rate), cardiotoxicity *First‐line refers to first‐line treatment (i.e. period 1 in this cross‐over study). |
|
| Notes |
Funding sources: Bayer, Pfizer, and Novartis quote: :"The SWITCH trial was sponsored by the German Cancer Society (DKG) with a financial grant from Bayer HealthCare. The Main Association of Austrian Social Security Institutions also supported the study. The specific role of the sponsors was in the design and conduct of the study. Bayer HealthCare also funded medical writing support for the preparation of this article." Declarations of Interests: Quote: "Christian Eichelberg certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (...)." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00738530.
| Study characteristics | ||
| Methods |
Study name: AVOREN Study design: randomised, parallel, placebo‐controlled phase III Blinding: the study was planned as a double‐blind trial, but was unblinded after a protocol amendment: quote: "An interim analysis of overall survival was prespecified after 250 deaths. On the basis of new second‐line therapies that became available while the trial was in progress, which could have confounded analyses of overall survival data, we agreed with regulatory agencies that the preplanned final analysis of progression‐free survival would be acceptable for regulatory submission." The protocol was amended to allow the study to be unblinded at this point. Study dates: between June 2004 and October 2005 (date of enrolment) Date of data cut‐off: September 8, 2006 for final analysis of PFS (data used in this review) and interim analysis of OS; cutoff for final analysis of OS (data used in this review) was September 2008 Location: 18 countries (Australia, Belgium, Czech Republic, Finland, France, Germany, Hungary, Israel, Italy, the Netherlands, Norway, Poland, Russian Federation, Singapore, Spain, Switzerland, Taiwan, UK., types of centres: hospitals, cancer centres (104 study locations) Cross‐over study or cross over permitted: not per design, but cross over from the control group to receive bevacizumab was recommended for participants who had not progressed |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 649 Age (years, median with range): experimental arm: 61 (30‐82), control arm: 60 (18‐81) Sex (m/f): experimental arm: 222/10, control arm: 234/88 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 327): Bevacizumab (10mg/kg, intravenous, every two weeks + IFN‐alfa (9 MIU, subcutaneous injection, thrice/week) Control arm (n = 322): Placebo + IFN‐a (9 MIU, subcutaneous injection, thrice/week) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST Other outcomes (not relevant to this review): ORR, OR, CR, TTF, TTP |
|
| Notes |
Funding sources: Alain Ravaud, F. Hoffmann‐La Roche, GlaxoSmithKline Declarations of Interests: Quote: "Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article(...), please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT00903175.
| Study characteristics | ||
| Methods |
Study name: RECORD‐3 Study design: RCT, phase II Blinding: none, open‐label Study Dates: from October 2009 to June 2011 date of enrolment) Date of data cut‐off: September 3, 2012 (primary analysis) Location: 19 countries (Argentina, Australia, Brazil, Canada, Denmark, France, Germany, Hong Kong, Italy, Republic of Korea, Mexico, the Netherlands, Peru, Spain, Taiwan, Thailand, Turkey, UK, USA), types of centres: cancer centres/institutes, hospitals, (84 study locations) Cross‐over study: yes* *For cross‐over trials, we extracted data on the first period only. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N=471 Age (median in years, (range): experimental arm: 62 (20‐89), control arm: 62 (29‐84) Sex (m/f): experimental arm: 166/72, control arm: 176/57 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 238): everolimus (10 mg, oral, daily) Control arm (n = 233): sunitinib (50 mg, oral, daily) |
|
| Outcomes |
Primary outcome(s)
Secondary Outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): EORTC, FKSI‐DRS Risk Score, DoR, ORR |
|
| Notes |
Funding sources: Robert J. Motzer, Novartis, Pfizer, GlaxoSmithKline; Carlos H. Barrios, Novartis; Thomas Cosgriff, Novartis; Thomas W. Flaig, Amgen, Bayer AG/Onyx Pharmaceuticals, Genentech, GlaxoSmithKline, Novartis, Pfizer, ZymoGenetics; Ray Page, Pfizer; J. Thaddeus Beck, Novartis; Jennifer Knox, Pfizer, Novartis Declarations of Interests: Quote: "Although all authors completed the disclosure declaration, the following author(s) and/or an author’s immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article (...), (...)please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts ofInterest section in Information for Contributors." Clinical study report available: no Study protocol available: yes Statistical analysis plan available: yes |
|
NCT00920816.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: a two‐arm RCT, phase III Blinding: none, open‐label Study dates: August 25 2009 – July 27 2012 Date of data cut‐off: July 27, 2012 (for PFS) and December 18, 2014 (for OS) Location: 14 countries (Bosnia and Herzegovina, Bulgaria, Chile, China, India, Malaysia, Mexico, Philippines, Romania, Russian Federation, South Africa, Taiwan, Ukraine, U.S.A.), types of centres: cancer centres, medical centres, hospitals, university hospitals (125 study locations) Cross‐over study or cross over permitted: no |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 288 Age, mean (range): experimental arm: 58 (23‐83), control arm: 58 (20‐77) Sex (m/f): experimental arm: 134/58, Group 0: 74/22 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 192): axitinib (5 mg, oral, twice/day) Control arm (n = 96): sorafenib (400 mg, oral, twice/day) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): OS and PFS in second‐line participants, DoR, OR |
|
| Notes |
Funding sources: AVEO, Bayer, GlaxoSmithKline, Novartis, and Pfizer Declarations of Interests: Quote: "Angel H. Bair, Brad Rosbrook, and Glen I. Andrews are employees of and own stock in Pfizer. Nicholas J. Vogelzang has served on a speakers bureau for Pfizer. The remaining authors have stated that they have no conflicts of interest." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no *Data of treatment‐naive participants was extracted for this review. |
|
NCT00979966.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: RCT, parallel assignment, phase II Blinding: none, open‐label Study dates: July 2009 ‐ July 2012 Date of data cut‐off: not reported Location: 1 country (Germany), types of centres: clinics, university hospitals (14 study locations) Cross‐over study or cross over permitted: not per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
More inclusion and exclusion criteria on CT.gov. Sample size: N = 22 Age, mean (range): experimental arm: 57.4 (29‐85), control arm: 64.8 (46‐80) Sex (m/f): experimental arm: 8/4, control arm: 8/2 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 12): temsirolimus (25 mg, intravenous, once/week) Control arm (n = 10): sunitinib (50 mg, oral, once/day) |
|
| Outcomes |
Primary outcome(s) ‐ Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): OR, TTP, CR/PR, RB |
|
| Notes |
Funding sources: This study was supported by a grant from Pfizer Germany. Declarations of Interests: Full details provided in Bergmann (2020). Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT01024920.
| Study characteristics | ||
| Methods |
Study name: ‐ Study design: two‐arm RCT, phase II Blinding: none, open‐label Study dates: 11 March 2010 ‐ 14 December 2010 (date of enrolment) Date of data cut‐off: 21 February, 2014 Location: 5 countries (Hungary, Poland, Romania, Ukraine, UK), types of centres: cancer centres, hospitals, university hospitals, research centres (15 study locations) Cross‐over study or cross over permitted: not per design; not reported whether cross over was permitted at some point (e.g. upon progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N=96 Age median (yrs, range): experimental arm: 62 (42‐86), control arm: 58 (29‐79) Sex (m/f): experimental arm: 44/2, control arm: 22/10 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 64): nintedanib (200 mg, oral, twice/day) Control arm (n = 32): sunitinib (50 mg, oral, once/day) Treatment was four weeks on treatment, two weeks off treatment (one cycle = six weeks). |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST Other outcomes (not relevant to this review): TTP, TTF, OR |
|
| Notes |
Funding sources: Quote: "Pfizer, Bayer and AstraZeneca, and travel funding for a conference from Novartis. MM and YS have received research funding from Boehringer Ingelheim. RJJ has received research funding from Bristol‐Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer and Roche. Funding for this study was provided by Boehringer Ingelheim." Declaration of Interest: Quote: "TE is a part‐time employee (from 1 September 2014) of AstraZeneca and owns stock or other ownership interest in the company, and has had a consulting or advisory role and received honoraria from Bayer, Pfizer, AVEO Oncology and GlaxoSmithKline. RJJ has received honoraria from Bristol‐Myers Squibb, GlaxoSmithKline, Novartis, Pfizer and Roche. A‐BL, GT and HD are all employees of Boehringer Ingelheim. IB and NM report no conflicts of interest." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT01030783.
| Study characteristics | ||
| Methods |
Study name: TIVO‐I Study design: randomised, parallel, phase III trial Blinding: none, open‐label Study dates: February ‐ August 2010 (date of randomisation) Date of data cut‐off: December 15, 2011 Location: 16 countries (Argentina, Bulgaria, Canada, Chile, Czech Republic, France, Hungary, India, Italy, Poland, Romania, Russian Federation, Serbia, Ukraine, U.K., U.S.A.), types of centres: not reported (86 study locations) Cross‐over study: not per design, but cross over was permitted upon progression (from sorafenib to pazopanib) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 362 treatment‐naive participants Age (years, median with range): experimental arm: 59 (23‐83), control arm: 59 (23‐83) Sex (m/f): experimental arm: 185/75, control arm: 189/68 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 181 treatment‐naive participants): tivozanib (1.5 mg, oral, once/day) ‐ treatment cycle (four weeks): three weeks on treatment, one week off treatment Control arm (n = 181 treatment‐naive participants): sorafenib (400 mg, oral, twice/day) ‐ treatment cycle was four weeks on treatment |
|
| Outcomes |
Primary Outcome(s)
Secondary Outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): ORR, DoR, pharmacokinetics, health outcome measurements |
|
| Notes |
Funding sources: Robert J. Motzer, AVEO Pharmaceuticals, Pfizer, GlaxoSmithKline; Dmitry Nosov, Bayer Pharmaceuticals, Pfizer, GlaxoSmithKline; Timothy Eisen, Bayer Pharmaceuticals, Pfizer, GlaxoSmithKline; Vladimir Lesovoy, Pfizer; Anna Alyasova, AVEO Pharmaceuticals, Astellas Pharma; David Cella, AVEO Pharmaceuticals; Thomas E. Hutson, Pfizer, AVEO Pharmaceuticals, Novartis, GlaxoSmithKline Declaration of Interest: Quote: "Although all authors completed the disclosure declaration, the following author(s) and/or an author’s immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. (...)For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts ofInterest section in Information for Contributors." Clinical study report available: no Study protocol available: yes Statistical analysis plan available: yes *Seperate data were reported for treatment‐naive participants; hence, we extracted the data for analyses only on the treatment‐naive. |
|
NCT01064310.
| Study characteristics | ||
| Methods |
Study name: PISCES Study design: randomised, phase III b Blinding: quadruple (participant, care provider, investigator, outcomes assessor) Study dates: May 2010 – October 2011 Date of data cut‐off: not reported Location: 5 countries (Finland, France, Germany, Italy, UK), types of centres: not reported (51 study locations) Cross‐over study or cross over permitted: yes, cross‐over study* *For cross‐over studies, we only extracted data on period 1. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N=169* (*) One patient was randomly assigned in error, and no data were entered into the study for this patient; data were available for 168 participants. Age (years, median): experimental arm: 64, control arm: 62 Sex (male/f): experimental arm: 61/3, control arm: 52/10 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 86): pazopanib (800 mg, oral, once/day) ‐ 10 weeks on treatment, followed by 2 weeks wash‐out Control arm (n = 82): sunitinib (50 mg, oral, once/day) ‐ 4 weeks on treatment, 2 weeks placebo, followed by another 4 weeks on treatment |
|
| Outcomes |
Primary outcome(s) ‐ Secondary outcome(s)
Relevant to this review but not reported: PFS, OS, TFST Other outcomes (not relevant to this review): number of participants with preference for pazopanib versus sunitinib as assessed by the Patient Preference Questionnaire (PPQ), (BL), dose reduction, dose modification |
|
| Notes |
Funding sources: Novartis Pharmaceuticals, Camillo Porta, Bayer Schering Pharma, Novartis, Pfizer; Petri Bono, GlaxoSmithKline, Pfizer; Thomas Powles, GlaxoSmithKline, Pfizer; Tim Eisen, Bayer AG, Pfizer, GlaxoSmithKline; Robert Hawkins, Pfizer, GlaxoSmithKline; David Cella, GlaxoSmithKline, Pfizer, AVEO Pharmaceuticals Declaration of Interest: Quote: "Although all authors completed the disclosure declaration, the following author(s) and/or an author’s immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. (...)For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors." Clinical study report available: only a 'scientific result summary' available Study protocol available: yes Statistical analysis plan available: yes |
|
NCT01108445.
| Study characteristics | ||
| Methods |
Study name: ASPEN Study design: a two‐arm RCT, phase II Blinding: none, open‐label Study dates: Between Sept 23, 2010, and Oct 28, 2013 (date of randomisation) Date of data cut‐off: December, 2014 (database lock) Location: 3 countries (Canada, UK, USA), types of centres: medical centres/agencies, clinics, hospitals, (18 study locations) Cross‐over study or cross over permitted: not a cross‐over study per design, but cross over was permitted upon progression (from sunitinib to everolimus) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 131 participants signed consent. 22 were screen failures. 1 withdrew consent prior to being randomised. 108 participants were randomised. Age (years, median with range): experimental arm: 64 (29‐90), control arm: 59 (24‐100) Sex (m/f): experimental arm: 44/13, control arm: 37/14 Prognostic factors:
|
|
| Interventions |
Experimental arm (n= 57): everolimus (10 mg, oral, once/day) Control arm (n= 51): sunitinib (50 mg, oral, once/day) Treatment was four weeks on treatment, two weeks off treatment (one cycle = six weeks). |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): Time‐to‐new metastatic disease, DoR, tumour shrinkage, clinical benefit rate, SD, ORR |
|
| Notes |
Funding: Novartis and Pfizer Declaration of Interest: Quote: "AJA and DJG reports grants from Novartis and Pfizer during the conduct of the study; grants and personal fees from Dendreon, Sanofi ‐Aventis, Bayer, Medivation/Astellas, and Janssen, outside the submitted work. DJG also reports grants from Innocrin and Exelixis and personal fees from BMS and Janssen. TE is an employee of AstraZeneca and reports grants from AstraZeneca, personal fees from Novartis, Roche, BMS, and AVEO, grants from Bayer, grants and personal fees from Pfizer, GSK, personal fees and grant to institution from Astellas, outside the submitted work. JAG reports grants and personal fees from Pfizer and Novartis, during the conduct of the study; grants and personal fees from Bayer and Medivation/Astellas, and personal fees from Sanofi‐Aventis, outside the submitted work. TFL reports grants from Novartis and Pfizer, during the conduct of the study; grants from Abbott, Abraxis, Acceleron, Amgen, AstraZeneca, Biovex, and Cerulean, Eisai, Eli Lilly grants and personal fees from Argos and Aveo, Bristol‐Myers Squibb, Celgene, GlaxoSmithKline, Hoffman‐La Roche, Immatics, Merck, Roche, Synta, Threshold, Tracon, EMD Serono, Millennium, and Schering‐Plough, personal fees from Genentech, and grants and personal fees from Novartis, Pfizer, Prometheus, and Wyeth, outside the submitted work. CKK reports personal fees from Pfizer, Novartis, BMS, and Sanofi‐Aventis, outside the submitted work. UNV reports grants and personal fees from Novartis and Pfizer, outside the submitted work. CWR reports personal fees from Pfizer and Genentech, research grant to institution from Onyx, outside the submitted work. RJJ reports grants from Pfizer and Novartis, during the conduct of the study; grants and personal fees from Pfizer, and grants, personal fees, and non‐financial support from Novartis and GSK, outside the submitted work. WMS reports grants and personal fees from Pfizer, outside the submitted work. LMP reports personal fees from Pfizer and Novartis. SH, SB, JP, REH, JDH, IP, AP, CML, and SO declare no competing interests." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT01274273.
| Study characteristics | ||
| Methods |
Study name: DARENCA Study design: a two‐arm, RCT, phase II Blinding: none, open‐label Study dates: 26 October 2009 ‐ 21 November 2014 (date of randomisation) Date of data cut‐off: 31 May 2017 (final analysis) Location: 1 country (Denmark), type of centre: university hospitals (2 study locations) Cross‐over study or cross over permitted: no |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 118 Age, Mean (years, range): experimental arm: 58 (28‐70), control arm: 55 (37‐69) Sex (m/f): experimental arm: 46/13, control arm:: 47/12 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 59): interleukin‐2 (2.4 MIU/m2, subcutaneous, twice/day, 5 days per week) + interferon (3 MIU, subcutaneous, once/day, 5 days/week) + bevacizumab (10 mg/kg, intravenous, every 2 weeks) Control arm (n = 59): interleukin‐2 (2.4 MIU/m2, subcutaneous, twice/day, 5 days per week) + interferon (3 MIU, subcutaneous, once/day, 5 days/week) All cytokines were administered over 4‐week cycles for up to a maximum of 9 cycles (i.e., 9 months): |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST, SAEs Other outcomes (not relevant to this review): NED, biomarkers in imaging and biopsies, surgical resection, TTF, TTP, DoR, ORR (CR/PR), tolerability, TTF |
|
| Notes |
Funding sources: Quote: "This study was supported financially by Roche and Novartis and BEV was provided by Roche." Declarations of Interests: "No potential conflict of interest was reported by the authors. Roche provided BEV. Roche and Novartis did not have access to data." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT01392183.
| Study characteristics | ||
| Methods |
Study name: TemPa Study design: a two‐arm, RCT, phase II Blinding: none, open‐label Study dates: November 2012 ‐ June 2017 (date of enrolment) Date of data cut‐off: not reported Location: 1 country (USA), type of centre: cancer centre (1 study location) Cross‐over study or cross over permitted: yes, cross‐over study* *For cross‐over studies, only data on period 1 were extracted for analyses. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N=69 Age (median, range (years)): experimental arm: 61 (42‐80), control arm: 61 (37‐74) Sex (m/f): experimental arm: 24/11, control arm: 28/6 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 35 ): temsirolimus (25 mg, intravenous, once/week) Control arm( = 34): pazopanib (800 mg, oral, once/day) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: SAEs, QoL, TFST Other outcomes (not relevant to this review): ‐ |
|
| Notes |
Funding sources: Novartis Pharmaceuticals and in part by the Cancer Center Support Grant (NCI Grant P30 CA016672) Declarations of Interests: Quote; "Nizar M. Tannir certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Tannir has received grant funding and personal fees for consultancy from Pfizer and Novartis. Karam has received personal fees for consultancy from Pfizer. Wood has received grant funding from Pfizer. Zurita has received grant funding and personal fees for consultancy from Pfizer, and grant funding from Novartis." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT01481870.
| Study characteristics | ||
| Methods |
Study name: CROSS‐J‐RCC Study design: randomised, phase III Blinding: none, open‐ label Study dates: February 2010 ‐ July 2012 Date of data cut‐off: June 30, 2015 Location: 1 country (Japan), types of centres: unclear, according to the text: 39 institutions: according to CT.gov: 1 location Crossing‐over study or cross over permitted: yes, cross‐over study* *For cross‐over studies, only data on period 1 were extracted for analyses. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 124, 120 participants were evaluated Age (years, median with range): experimental arm: 67 (41‐79), control arm: 66 (44‐79) Sex (m/f): experimental arm: 46/11, control arm: 53/10 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 60): sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on treatment, 2 weeks off treatment (1 cycle = 6 weeks) Control arm (n = 64): sorafenib (400 mg, oral, twice/week) ‐ no breaks |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, SAEs TFST Other outcomes (not relevant to this review): ‐ |
|
| Notes |
Funding sources: Quote: "The present study was supported in part by the Japanese Urological Research Network and Pfizer." Declaration of Interest: "Y.T. has received grants and lecture and advisory fees from Novartis, Japan; Ono, and Astellas, grants and lecture fees from Astellas and Pfizer, Japan lecture fees from Bristol‐Myers Squibb, Japan and grants from Takeda, Japan. T. Kondo has received lecture fees from Intuitive Surgical, Novartis, Ono, and Pfizer. W.O. has received grants form Nipuro and Takeda and lecture fees from Astellas, Bristol‐Myers Squibb, Ono, and Pfizer. J.T. has received lecture fees from Pfizer. M.T. has received lecture fees from Pfizer. H.M. has received grants and lecture fees from Janssen, grants from Kyowa‐Hakko‐Kirin, Japan;Merck biopharma, Japan; Sharp & Dohme, Takeda Terumo, and Toyo Steel. R.S. has received lecture fees from Intuitive Surgical and Novartis and a grant from Astellas. T.S. has received grants and lecture fees from Astellas and Takeda and lecture fees from Novartis. N. Shinohara has received grants and lecture fees from Astellas and Ono, lecture fees from Bayer, and lecture fees from Bayer, GlaxoSmithKline, Pfizer, and Takeda. M.O. has received grants and lecture fees from Pfizer and lecture fees from Bayer, Bristol‐Myers Squibb, Novartis, and Ono. H.K. has received grant and lecture fees from Pfizer and grants from Astellas, Ono, Sanofi, Japan; Taiho, and Takeda. The remaining authors declare that they have no competing interests." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT01613846.
| Study characteristics | ||
| Methods |
Study name: SWITCH II Study design: a two‐arm, RCT, phase III Blinding: none, open‐label Study dates: June 14th, 2012 ‐ November 14th, 2016 Date of data cut‐off: not reported Location: 3 countries (Austria, Germany, the Netherlands), types of centres: cancer centres/clinics, hospitals, university hospitals (72 study locations) Cross‐over study or cross over permitted: yes, cross‐over study* *For cross‐over trials, we extracted data on period 1 only. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 377 Age, median (years (range)): experimental arm: 68 (31‐84), control arm: 68 (26‐86) Sex (m/f, %): experimental arm: 136/53 (72/28), control arm: 137/51 (73/27) Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 189): sorafenib (400 mg, oral, twice/day) Control arm (n = 188): pazopanib (800 mg, oral, once/day) |
|
| Outcomes |
Primary outcome(s) ‐ Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): PFS in second‐line treatment, DCR (CR,PR,SD, RECIST), biomarker programme, time to treatment failure, TTP |
|
| Notes |
Funding sources: Quote: "The SWITCH II trial was sponsored by the Technical University of Munich, Germany with financial grants from Bayer HealthCare GmbH and Novartis GmbH. Bayer HealthCare GmbH and Novartis GmbH were not involved in the trial concept and design. The Association for Urologic Oncology (AUO) of the German Cancer Society supported this study (AN 33/11) as well as the main Association of Austrian Social Security Institutions. The specific role of the sponsors was in the design and conduct of the study." Declaration of Interest: Quote:"It is certified that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following:" Margitta Retz, Janssen‐Cilag, Martin Bögemann, Marc‐Oliver Grimm, Maria De Santis, Christian Bolenz, Carsten Bokemeyer, Jürgen E. Geschwend. No conflicts of Interest declare: Uwe Zimmermann, Lothar Müller, Christian Leiber, Dogu Teber, Manfred Wirth, Aart Becker, Jan Lehmann, Robbert van Alphen, Martin Indorf, Melanie Frank. Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT01835158.
| Study characteristics | ||
| Methods |
Study name: CABOSUN Study design: a two‐arm, RCT, phase II Blinding: none, open‐label Study dates: July 9, 2013 to April 6, 2015 (date of randomisation) Date of data cut‐off: July 01, 2017 (for OS), September 15, 2016 (for PFS per IRC) Location: 1 country (USA), types of centres: cancer centres/clinics, medical centres, hospitals (488 study locations) Cross‐over study or cross over permitted: no |
|
| Participants |
Inclusion criteria:
Sample size: N = 157 Age (years, median with range): experimental arm: 63 (56‐69) , control arm: 64 (57‐71) Sex (m/f): experimental arm: 66/13, control arm: 57/21 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 79): cabozantinib (60 mg, oral, once/day) Control arm (n = 78): sunitinib (50 mg, oral, once/day) A treatment cycle was defined as 6 weeks in both study groups (4 weeks on treatment, 2 weeks off). |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST Other outcomes (not relevant to this review): ORR |
|
| Notes |
Funding sources:Quote: "The study was designed by the Alliance for Clinical Trials in Oncology, endorsed by the ECOGeAmerican College of Radiology Imaging Network Group and approved by the Cancer Therapy Evaluation Program of the National Cancer Institute part of the National Institutes of Health (the funder)." Declaration of Interest: "TKC reports personal fees for an advisory/consulting role from Pfizer, GlaxoSmithKline, Novartis, Merck, Bayer, Eisai, Roche, Prometheus Labs Inc., Foundation Medicine Inc., Bristol‐Myers Squibb, and research funding from Pfizer, GlaxoSmithKline, Novartis, Bristol‐Myers Squibb, Merck, Exelixis Inc., Roche, AstraZeneca, Tracon and Peloton. MDM reports attendance at advisory boards for Pfizer and Exelixis, Inc., outside the submitted work. OH reports relevant financial activities outside the submitted work, and participation at an advisory board for Pfizer. MJM reports attendance at advisory boards for Bayer, Astellas and Progenics, personal fees and research support from Progenics, and research support from Endocyte, outside the submitted work. DRF reports research support from Seattle Genetics and Novartis, outside the submitted work. DG reports personal fees from Dendreon, Novartis, Sanofi, Bayer, Medivation, Biopharm, Axess Oncology, Exelixis, Inc., Pfizer, GlaxoSmithKline, Astellas Pharma, Innocrin Pharma, Bristol‐Myers Squibb, Genentech, Janssen, Acceleron Pharma, Celgene,Merk Sharp & Dohme, and Myovant Sciences, Inc, and research funding from Dendreon, Novartis, Bayer, Exelixis, Inc., Pfizer, Astellas Pharma, Innocrin Pharma, Bristol‐Myers Squibb, Genentech, Janssen, Millennium, Acerta Pharma, outside the submitted work. CH, MM and CS are the employees of Exelixis, Inc. All other authors declare no competing interests." Clinical study report available: no Study protocol available: yes Statistical analysis plan available: no |
|
NCT01984242.
| Study characteristics | ||
| Methods |
Study name: IMmotion150 Study design: a two‐arm, RCT, phase II (three‐arm trial) Blinding: none, open‐label Study dates: 8 January 2014 to 16 March 2015 (date of enrolment) Date of data cut‐off: 17 October 2016 (clinical cutoff date) Location: 9 countries (Czech Republic, France, Germany, Italy, Poland, Romania, Spain, UK, USA), types of centres: cancer centres, medical centres, hospitals, research institutions (45 study locations) Cross‐over study or cross over permitted: not per design, but participants enrolled in atezolizumab (except EU participants) or sunitinib group could crossover to receive atezolizumab and bevacizumab combination therapy in case of disease progression |
|
| Participants |
Inclusion criteria:
Exclusion criteria: Disease‐Specific Exclusions:
Sample size: N=305 Age, median (years, range): experimental arm I: 62 (32‐88), experimental arm II: 61 (27‐81), control arm: 61 (25‐85) Sex (m/f): experimental arm I: 74/27, experimental arm II: 77/26, control arm: 79/22 Prognostic factors:
|
|
| Interventions |
Experimental arm I (n = 101): atezolizumab (1200 mg, intravenous, every three weeks) + Bevacizumab (15 mg/kg, intravenous, every three weeks) Experimental arm II (n = 103): atezolizumab (1200 mg, intravenous, every three weeks) Control arm (n = 101): sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on treatment, 2 weeks off treatment (1 cycle ‐ 6 weeks) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST, QoL Other outcomes (not relevant to this review): OS and PFS in different subgroups, pharmacokinetics of study drugs, laboratory parameters, disease progression, DoR,OR (CR, PR), number of deaths, DP |
|
| Notes |
Funding sources: Quote; Prometheus Laboratories. M.B.A., Roche/Genentech, Novartis, Pfizer, Eisai, and Exelixis, F. Hoffmann‐La Roche, AG (...) Declaration of Interest: "D.F.M. reports a consulting/advisory role for Bristol‐Myers Squibb, Merck, Roche/ Genentech, Pfizer, Exelixis, Novartis, Eisai, X4 Pharmaceuticals, and Array BioPharma (...). J.A.R., J.H., T.H., C. Suárez, and R.D. have nothing to disclose." Clinical study report available: no Study protocol available: no Statistical analysis plan available: no |
|
NCT02231749.
| Study characteristics | ||
| Methods |
Study name: Checkmate 214 Study design: a two‐arm RCT, phase III Blinding: none, open‐label Study dates: October 16, 2014 ‐ February 23, 2016 (date of randomisation) Date of data cut‐off: For OS and PFS: 25 February 2020 (Albiges 2020); for QoL: August 7, 2017 (Cella 2019) Location: 28 countries (Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, Colombia, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Israel, Italy, Japan, Republic of Korea, Mexico, the Netherlands, Poland, Spain, Sweden, Taiwan, Turkey, UK USA), types of centres: not reported (190 study locations) Cross‐over study or cross over permitted: not per design, but cross over was permitted from the control arm to the experimental arm for intermediate and poor risk participants |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N=1096 Age, median (years, range): experimental arm: 62 (26‐85), control arm: 62 (21‐85) Sex (m/f): experimental arm: 413/137, control arm: 395/151 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 550): nivolumab (3 mg/kg, intravenous) + ipilimumab (1 mg/kg, intravenous), every 3 weeks for four doses (induction phase) followed by nivolumab montherapy (maintenance therapy) Control arm (n = 546): sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on treatment, 2 weeks off treatment (1 cycle = 6 weeks) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL Other outcomes (not relevant to this review): ORR |
|
| Notes |
Funding sources: Bristol‐Myers Squibb and ONO Pharmaceutical and grants and personal fees from Pfizer, Novartis, Eisai, Exelixis, and Genentech/Roche (...). Declarations of Interests: "PS, VN, and BR declare no competing interests." For a more detailed description please refer to the publication. Clinical study report available: no Study protocol available: yes Statistical analysis plan available: yes |
|
NCT02420821.
| Study characteristics | ||
| Methods |
Study name: IMmotion 151 Study design: a two‐arm, RCT, phase III Blinding: none, open‐label Study dates: May 20, 2015 ‐ Oct 12, 2016 (date of enrolment) Date of data cut‐off: September 29, 2017 (PFS; first interim analysis); August 13, 2018 (second interim analysis); safety and OS data were updated at the cutoff date February 14, 2020 (final analysis) Location: 21 countries (Australia, Bosnia and Herzegovina, Brazil, Canada, Czech Republic, Denmark, France, Germany, Italy, Japan, Republic of Korea, Mexico, Poland, Russian Federation, Singapore, Spain, Taiwan, Thailand, Turkey, UK USA), types of centres: cancer centres/institutes, medical centres, hospitals, university hospitals (154 study locations) Cross‐over study or cross over permitted: no |
|
| Participants |
Inclusion criteria:
Exclusion criteria: Disease‐specific exclusions:
General medical exclusions:
Sample size: N=915 Age, median (years, range): experimental arm: 62 (56‐69), control arm: 60 (54‐66) Sex (m/f): experimental arm: 317/137, control arm: 352/109 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 454): atezolizumab (1200 mg, intravenous) + bevacizumab (15 mg/kg, intravenous), once every 3 weeks Control arm (n= 461): sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on treatment, 2 weeks off treatment (1 cycle = 6 weeks) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST Other outcomes (not relevant to this review): OS and PFS in different subgroups, pharmacokinetics, laboratory parameters (ATA), PD, DoR (OR, CR, PR), PD |
|
| Notes |
Funding sources: Hoffmann–La Roche Ltd and Genentech Inc. Declaration of Interest: quote: "CSu, FP, and BMell have nothing to disclose." For a detailed description please refer to publication. Clinical study report available: no Study protocol available: yes Statistical analysis plan available: yes |
|
NCT02684006.
| Study characteristics | ||
| Methods |
Study name: JAVELIN Study design: a two‐arm, RCT, phase III Blinding: none, open‐label Study dates: March 29, 2016 ‐ December 19, 2017 (date of randomisation) Date of data cut‐off: June 20, 2018 (for safety); exact dates for OS and PFS not reported, but data for PFS was reported and extracted from the second interim analysis for OS, and OS data were reported/extracted from the third interim analysis (longest follow‐up available for both outcomes) Location: 21 countries (Australia, Austria, Belgium, Canada, Denmark, France, Germany, Hungary, Israel, Italy, Japan, Republic of Korea, Mexico, the Netherlands, New Zealand, Romania, Russian Federation, Spain, Sweden, UK, USA), types of centres: hospitals, university hospitals, medical centres, centres/institutes (280 study locations) Cross‐over study or cross over permitted: no |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N = 886 Age, median (years, range): experimental arm: 62 (29‐83),control arm: 61 (27‐88) Sex (m/f): experimental arm: 316/126 (71.5/28.5), control arm: 344/100 (77.5/22.5) Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 442): avelumab (10 mg/kg, intravenous, every 2 weeks) + axitinib ( mg, oral, twice/day) Control arm (n = 444): sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on, 2 weeks off (1 cycle = 6 weeks) |
|
| Outcomes |
Primary outcome(s) ‐ Secondary outcome(s)
Relevant to this review but not reported: QoL Other outcomes (not relevant to this review): OS and PFS in different subgroups, TTF, VAS, DR, TTR, DC, OR, biomarker status, laboratory parameters (ADA), pharmacokinetics |
|
| Notes |
Funding sources: Pfizer and Merck Declaration of Interest: Disclosure forms provided by the authors are available at NEJM.org. Clinical study report available: no Study protocol available: yes Statistical analysis plan available: yes |
|
NCT02761057.
| Study characteristics | ||
| Methods |
Study name: SWOG 1500 Study design: RCT, phase II Blinding: none, open‐label Study dates: April 5, 2016 ‐ Dec 15, 2019 (date of randomisation) Date of data cut‐off: October 16, 2020 (for first analysis); exact date for updated analyses not reported Location: 2 countries (Canada, U.S.A.), types of centres: cancer centres, medical centres, hospitals, (597 study locations) Cross‐over study or cross over permitted: not per design; not reported whether cross over was allowed at some point (e.g. at progression) |
|
| Participants |
Inclusion / exclusion criteria:
More inclusion & exclusion criteria on CT.gov. Sample size: N=147 Age, median (years, range): 66 (58‐75) (across all participants) Sex (m/f): 112 females; 35 males (across all participants) Prognostic factors:
|
|
| Interventions |
Experimentarl arm I (N = 46): sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on treatment, 2 weeks off treatment (1 cycle = 6 weeks) Experimental arm II (N = 44): cabozantinib (60 mg, oral, once/day) Experimental arm III (N = 28): crizotinib (250 mg, oral, twice/day) Experimental arm IIII (N = 29): savolitinib (600 mg, oral, once/day) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST Other outcomes (not relevant to this review): ORR |
|
| Notes |
Funding sources: National Institutes of Health and National Cancer Institute. Declaration of Interest: yes Clinical study report available: no Study protocol available: yes Statistical analysis plan available: Other: *7% of the study population received one prior line of systemic therapy (excluding VEGF‐directed or MET‐directed drugs). |
|
NCT02811861.
| Study characteristics | ||
| Methods |
Study name: CLEAR Study design: RCT, phase III (three‐arm trial) Blinding: none, open‐label Study dates: October 13, 2007 ‐ July 24, 2019 (date of randomisation) Date of data cut‐off: August 28, 2020 (for final analysis of PFS and interim analysis of OS) Location: 20 countries (Australia, Austria, Belgium, Canada, Czech Republic, France, Germany, Greece, Ireland, Israel, Italy, Japan, Republic of Korea, the Netherlands, Poland, Russian Federation, Spain, Switzerland, UK USA), types of centres: hospitals, university hospitals, medial centres, cancer centres (183 study locations) Cross‐over study or cross over permitted: not a cross‐over study; not reported whether cross over was permitted at some point (e.g. after progression) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
More exclusion criteria on CT.gov. Sample size: N = 1069 Age, median (years, range): experimental arm I: 62 (32‐86), experimental arm II: 64 (34‐88), control arm: 61 (29‐82) Sex (m/f): experimental arm I: 266/91, experimental arm II: 255/100, control arm: 275/82 Prognostic factors:
|
|
| Interventions |
Experimental arm I (n = 355): lenvatinib (18 mg, oral, once/day) + Everolimus (5mg, oral, once/day) Experimental arm II (n = 352): lenvatinib (20 mg, oral, once/day), Pembrolizumab (200mg, intravenous, every 3 weeks) Control arm (n=340): Sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on treatment, 2 weeks off treatment (1 cycle = 6 weeks) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): PFS on next‐line therapy, ORR, TTF, AUC, time of clearance |
|
| Notes |
Funding sources: Eisai and Merck Sharp and Dohme Declaration of Interest: Disclosure forms provided by the authors are available at NEJM.org. Clinical study report available: no Study protocol available: yes Statistical analysis plan available: yes |
|
NCT02853331.
| Study characteristics | ||
| Methods |
Study name: KEYNOTE‐ 426 Study design: a two‐arm, RCT, phase III Blinding: none, open‐label Study dates: Oct 24, 2016 ‐ Jan 24, 2018 (date of randomisation) Date of data cut‐off: exact dates for OS and PFS not reported, but data for both outcomes was extracted at the longest follow‐up available (median 42.8 months). Data for OS subgroups were available for a shorter follow‐up (median 30.6 months) Location: 16 countries (not reported), types of centres: hospitals, cancer centres (129 study locations) Cross‐over study or cross over permitted: no |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: N=861 Age, median (years, range): experimental arm: 62 (55‐68), control arm: 61 (53‐68) Sex (m/f): experimental arm: 308/124, control arm: 320/109 Prognostic factors:
|
|
| Interventions |
Experimental arm (n = 432): pembrolizumab (200 mg, intravenous, every 3 weeks for up to 35 cycles) + sxitinib (5 mg, oral, twice/day) Control arm (n = 429): sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on treatment, 2 weeks off treatment (1 cycle = 6 weeks) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST, QoL scale (reporting is planned) Other outcomes (not relevant to this review): TTD, DoR, ORR, DCR |
|
| Notes |
Funding sources: Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc. Declaration of Interest: For a very detailed description please refer to the publication. Clinical study report available: no Study protocol available: yes Statistical analysis plan available: yes |
|
NCT03141177.
| Study characteristics | ||
| Methods |
Study name: CheckMate 9ER Study design: RCT, phase III Blinding: none, open‐label Study Dates: September 2017 ‐ May 2019 (date of randomisation) Date of data cut‐off: March 30, 2020 (for safety); exact dates for OS and PFS not reported, but we extracted data for the longest follow‐up time available (median 23.5 months) Location: 18 countries, (Argentina, Australia, Brazil, Chile, Czech Republic, Germany, Greece, Israel, Italy, Japan, Mexico, Poland, Romania, Russia, Spain, Turkey, UK, USA), types of centres: not reported (only "local institutions") Cross‐over study or cross over permitted: no |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Statement on CT.gov: "Other protocol defined inclusion/exclusion criteria could apply." Sample size: N=651 Age, median (years, range): experimental arm: 62 (29‐90), control arm: 61 (28‐86) Sex (m/f, (%)): experimental arm: 249/74 (77.1/22.9), control arm: 232/96 (70.7/29.3) Prognostic factors:
|
|
| Interventions |
Experimental group (n = 323): nivolumab (240 mg, intravenous, every 2 weeks) + cabozantinib (40 mg, oral, once/day) Control group (n =3 28): sunitinib (50 mg, oral, once/day) ‐ treatment was 4 weeks on treatment, 2 weeks off treatment (1 cycle = 6 weeks) |
|
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST Other outcomes (not relevant to this review): laboratory values, ORR |
|
| Notes |
Funding sources: Bristol‐Myers Squibb and others Declarations of Interests:Quote: "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org." Clinical study report available: not yet Study protocol available: yes Statistical analysis plan available: yes |
|
AEs: adverse events; ; v; CNS: central nervous system; CT: computed tomography; DCR: disease control rate; DR: duration of response; ECOG: Eastern Cooperative Oncology Group; ITT: intention‐to‐treat; KPS: Karnofsky Performance Status LDH: lactate dehydrogenase; MRI: magnetic resonance imaging; ORR: objective response rate; mTOR: mammalian target of rapamycin; OS: overall survival ; PFS: progression‐free survival ; QoL: quality of life; RCC: renal cell carcinoma; RR: response rate; SAEs: serious adverse events; SD: standard deviation; TTP: time to progression; VEGF: Vascular endothelial growth factor.
NCT not available for this study.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aass 2005 | Irrelevant interventions (interferon alfa‐2a with/without 13‐cis‐retinoic acid). |
| Abdel 2018 | Wrong study design (single‐arm) and wrong patient population (recurrent/refractory). |
| Adler 1987 | Irrelevant interventions (hormono‐immuno‐ versus hormonotherapy). |
| Amin 2018 | CheckMate 016 study. Wrong study design (non‐randomised). |
| Amin 2018a | CheckMate 016 study. Wrong study design (non‐randomised). |
| Atkins 1991 | Irrelevant comparison (interferon vs. interleukin). |
| Atkins 1993 | Irrelevant comparison (interferon vs. interleukin). |
| Atzpodien 1997 | Irrelevant comparison (interleukin vs. interferon and 5‐fluorouracil). |
| Atzpodien 1997a | Irrelevant comparison (interleukin vs. interferon and 5‐fluorouracil). |
| Atzpodien 1999 | Irrelevant comparison (13‐cis‐retinoic acid, IFN‐alpha, IL‐2 and chemotherapy). |
| Atzpodien 2001 | Irrelevant comparison (interferon+interleuking and 5‐FU versus tamoxifen). |
| Atzpodien 2004 | Irrelevant comparison (interleukin vs. interferon). |
| Atzpodien 2006 | Irrelevant comparison (interleukin‐2/interferon‐alpha2a/13‐retinoic acid‐based chemoimmunotherapy). |
| Barrios 2009 | Wrong study design (single‐group assignment). |
| Beaumont 2009 | RECORD‐1 study. Second‐line treatment. |
| Beaumont 2011 | RECORD‐1 study. Second‐line treatment. |
| Berg 1998 | Irrelevant comparison (interleukin versus interferon alpha‐2A). |
| Bex 2017 | Irrelevant intervention (nephrectomy). |
| Boccardo 1998 | Irrelevant comparison (interleukin versus interferon alpha‐2A). |
| Bracarda 2007 | Wrong study design (same intervention, different schedules). |
| Buckley 2019 | PRISM study. Dose‐finding study. |
| Cella 2016 | Checkmate025 study. Second‐line treatment. |
| Choueiri 2017 | Prior therapy allowed (more than 10% of patients). |
| Choueiri 2020 | Prior therapy allowed (more than 10% of patients). |
| Choueiri 2020a | Prior therapy allowed (more than 10% of patients). |
| Cirkel 2016 | Wrong study design (rotating treatments). |
| Cirkel 2017 | ROPETAR study. Wrong study design (rotating treatments). |
| Climent 2020 | ROPETAR study. Wrong study design (rotating treatments). |
| Cole 2003 | Irrelevant comparison (interleukin versus interferon alpha‐2A). |
| Collinson 2012 | STAR Trial. Irrelevant comparison (sunitinib; temporary cessation versus continuation). |
| Collinson 2018 | PRISM. Dose‐finding study. |
| Colomba 2021 | Wrong study design (single‐group assignment). |
| Conter 2013 | Wrong study design (dose‐finding study). |
| de Mulder 1991 | Irrelevant comparison (interferon versus interleukin). |
| Demirci 1999 | Irrelevant interventions (vinblastine and interferon alpha with 5‐flourouracil). |
| Dexeus 1988 | Irrelevant comparison (chemotherapy versus interferon). |
| Dexeus 1989 | Irrelevant comparison (chemotherapy versus interferon). |
| DRKS00010309 2016 | TAURUS. Terminated study. |
| Dubois 1997 | Irrelevant comparator (p75 tumour necrosis factor receptor immunoglobulin G chimera). |
| Eisen 2019 | Irrelevant intervention (adjuvant therapy). |
| Elhilali 2000 | Irrelevant comparison (interferon vs. placebo). |
| Epaillard 2020 | Wrong study design (biomarker‐driven trial). |
| Escudier 2005 | Irrelevant comparator. |
| Euctr 2006‐003429‐95‐ES | Wrong study design (non‐randomised). |
| Euctr2006‐002851‐33‐AT | Ended prematurely. |
| Euctr2006‐005751‐16‐NL | Study ended prematurely. |
| Euctr2007‐002556‐41‐AT | Irrelevant comparison (trivax (cancer vaccine) with sunitinib versus sunitinib alone). |
| EUCTR2008‐002667‐13‐DE 2008 | Adjuvant setting. |
| Euctr2012‐001730‐33‐ES | Study ended prematurely. |
| Euctr2015‐002133‐22‐FR | Irrelevant intervention(s). |
| Euctr2018‐001495‐38‐FR | Study ended prematurely. |
| Feldman 2020 | Wrong study design (cohort study). |
| Feldman 2020a | Wrong study design (cohort study). |
| Figlin 1998 | Irrelevant comparison (CD8(+) tumour‐infiltrating lymphocytes in combination with recombinant interleukin‐2). |
| Figlin 1999 | Irrelevant comparison (CD8(+) tumor‐infiltrating lymphocytes in combination with recombinant interleukin‐2). |
| Figlin 2014 | ADAPT trial. Terminated (no results). |
| Figlin 2014a | ADAPT trial. Terminated (no results). |
| Figlin 2017 | ADAPT trial. Terminated (no results). |
| Figlin 2018 | ADAPT trial. Terminated (no results). |
| Figlin 2020 | ADAPT trial. Terminated (no results). |
| Flaherty 2015 | Prior therapy allowed. |
| Foon 1988 | Irrelevant comparison (interferon alpha(2B)‐interferon/gamma‐ interferon or the combination). |
| Fossa 1989 | Irrelevant comparison (recombinant interferon‐alpha with or without vinblastine). |
| Fossa 1992 | Irrelevant comparison (recombinant interferon‐alpha with or without vinblastine). |
| Gao 2017 | Prior therapy allowed. |
| Gao 2019 | Prior therapy allowed. |
| Gedye 2021 | Wrong study design (single‐group assignment). |
| Ghiorghiu 2018 | More than 10% received prior therapy. |
| Gleave 1997 | Irrelevant comparison (interferon gamma‐1b injection versus placebo). |
| Gleave 1997a | Irrelevant comparison (interferon gamma‐1b injection versus placebo). |
| Gleave 1998 | Irrelevant comparison (interferon gamma‐1b injection versus placebo). |
| Gore 2008 | Irrelevant comparison (interferon‐a (IFN), interleukin‐2 (IL2) and 5‐fluorouracil (5FU) vs IFN alone). |
| Gore 2010 | Irrelevant comparison (interferon‐a (IFN), interleukin‐2 (IL2) and 5‐fluorouracil (5FU) vs IFN alone). |
| Gruenwald 2020 | Irrelevant intervention (behavioral intervention, concomitant coaching). |
| Haas 2016 | Wrong intervention (adjuvant therapy). |
| Hainsworth 2015 | Irrelevant comparator (CXCR4 inhibitor LY2510924). |
| Hainsworth 2016 | Irrelevant comparator (CXCR4 inhibitor LY2510924). |
| Han 2002 | Irrelevant comparison (interleukin‐2 versus subcutaneous interleukin‐2/interferon). |
| Harima 1990 | Irrelevant comparison (interferon‐alpha (IFN) plus fluoropyrimidine (FP) and IFN alone). |
| Henriksson 1998 | Irrelevant comparison (tamoxifen vs interleukin 2, alpha‐interferon (leucocyte) and tamoxifen). |
| Hutson 2006 | Wrong study design (discontinuation design) and wrong patient population (recurrent). |
| Hutson 2021 | CheckMate 920 trial. Wrong study design (non‐randomised). |
| ISRCTN95351638 | PRISM. Dose‐finding study. |
| Jager 2005 | Second‐line therapy. |
| Jayson 1998 | Irrelevant comparison (interleukin 2 and interleukin 2‐interferon alpha). |
| Jeon 1999 | Wrong study design (cohort study). |
| JPRN‐JapicCTI‐122014 | Prior therapy allowed. |
| JPRN‐jRCTs031180024 | Irrelevant comparison (nivolumab combined with image‐guided three dimensional beam‐convergent and extremely hypofractionated radiotherapy). |
| JPRN‐UMIN000001995 | Prior therapy allowed. |
| Kinouchi 2004 | Irrelevant comparison (interferon‐alpha (IFN) versus IFN + cimetidine). |
| Kinouchi 2006 | Irrelevant comparison (interferon‐alpha (IFN) versus IFN + cimetidine). |
| Larkin 2019 | KEYNOTE‐427. Wrong study design (non‐randomised). |
| Law 1995 | Irrelevant comparison (interleukin‐2 with or without lymphokine‐ activated killer cells). |
| Lee 2020 | KEYNOTE‐427. Wrong study design (non‐randomised). |
| Lee 2021 | Wrong study design (cohort study). |
| Lindskog 2020 | Irrelevant comparison (ilixadencel plus sunitinib versus sunitinib alone). |
| Lissoni 1993 | Irrelevant comparison (interleukin‐2 subcutaneous immunotherapy versus interleukin‐2 plus interferon‐alpha). |
| Liu 2012 | Irrelevant comparison (autologous CIK cell immunotherapy versus interleukin‐2 treatment combination with IFN‐α‐2a). |
| Lummen 1996 | Irrelevant comparison (interferon‐gamma versus interleukin‐2 and interferon‐alpha2b). |
| Madhusudan 2004 | Irrelevant comparison (interferon alpha alone or in combination with thalidomide). |
| McDermott 2001 | Irrelevant comparison (interleukin‐2 versus interleuking + interferon). |
| McDermott 2005 | Irrelevant comparison (interleukin‐2 versus interleukin + interferon). |
| McDermott 2013 | BEST trial. Prior therapy allowed. |
| McDermott 2020 | KEYNOTE‐427. Wrong study design (non‐randomised). |
| Mickisch 2001 | Irrelevant comparison (radical nephrectomy plus interferon‐alfa‐based immunotherapy compared with interferon alfa alone). |
| Minasian 1993 | Wrong study design (cohort study). |
| Molina 2009 | RECORD‐1 study. Prior therapy allowed. |
| Motzer 2001 | Irrelevant comparison (interleukin‐12 versus interferon‐alpha2a). |
| Mulders 2012 | Prior therapy (50% of patients). |
| Naglieri 1998 | Irrelevant comparison (interleukin‐2 (IL‐2) and interferon‐alpha immunotherapy versus an IL‐2 and 4‐epirubicin immuno‐chemotherapy). |
| NCT00002737 | Irrelevant comparison (interferon alfa plus isotretinoin versus interferon alfa alone). |
| NCT00005966 | Irrelevant comparison (interferon‐alfa2b alone versus interferon‐alfa2b plus thalidomide). |
| NCT00019539 | Irrelvant comparison (bevacizumab versus thalidomide) and prior therapy allowed. |
| NCT00027664 | Irrelevant comparison (interferon alfa with thalidomide versus interferon alfa alone). |
| NCT00053820 | Irrelevant comparison (interleukin‐2 and fluorouracil versus interferon alfa alone). |
| NCT00073307 | Second‐line therapy. |
| NCT00100906 | Irrelevant interventions and dose‐finding study. |
| NCT00378703 | One line of prior therapy was allowed. |
| NCT00416871 | Irrelevant comparison (interleukin infusion versus interleukin by injection). |
| NCT00467025 | Irrelevant intervention (AMG386). |
| NCT00491738 | SABRE‐R study. Terminated (no results). |
| NCT00709995 | Phase II not conducted. |
| NCT00835978 | Dose‐finding study. |
| NCT00873236 | Study ended prematurely. |
| NCT01164228 | Irrelevant comparison (sunitinib with or without gemcitabine hydrochloride). |
| NCT01223027 | Second‐line treatment. |
| NCT01408004 | Wrong study design (efficacy of rotating regimen). |
| NCT01444807 | Wrong study design (single‐group assignment). |
| NCT01616186 | Study withdrawn. |
| NCT01664182 | Second‐line therapy. |
| NCT01673386 | Study terminated (no results). |
| NCT01727089 | Second‐line therapy. |
| NCT01727336 | Second‐line therapy. |
| NCT01793636 | Second‐line therapy. |
| NCT02014636 | Phase II (randomised part) not conducted. |
| NCT02127710 | Wrong study design (single‐group assignment). |
| NCT02667886 | Second‐line therapy. |
| NCT02724020 | Second‐line therapy. |
| NCT02960906 | Wrong‐study design (BIOmarker‐driven trial). |
| NCT03035630 | Study terminated (no results). |
| NCT03092856 | Second‐line therapy. |
| NCT03095040 | Second‐line therapy. |
| NCT03173560 | Dose‐finding study. |
| NCT03501381 | Second‐line therapy. |
| NCT03595124 | Second‐line therapy. |
| NCT03829111 | Irrelevant intervention (probiotics in addition to nivolumab and ipilimumab). |
| NCT04195750 | Second‐line therapy. |
| NCT04300140 | Second‐line therapy. |
| Negrier 1996 | Irrelevant comparison (interleukin‐2 versus interferon alfa). |
| Negrier 1997 | Irrelevant comparison (interleukin‐2 and interferon with or without fluorouracil). |
| Negrier 1998 | Irrelevant comparison (interleukin‐2 versus interferon alfa). |
| Negrier 2000 | Irrelevant comparison (interleukin‐2 and interferon with or without fluorouracil). |
| Negrier 2006 | Irrelevant comparison (intravenous interleukin versus subcutaneous interleukin). |
| Negrier 2007 | Irrelevant comparison (medroxyprogesterone with interferon alfa‐2a or interleukin 2 versus a combination of both). |
| Negrier 2008 | Irrelevant comparison (intravenous interleukin versus subcutaneous interleukin). |
| Nosov 2010 | Wrong study design (randomised discontinuation trial). |
| Nosov 2012 | Wrong study design (randomised discontinuation trial). |
| Pal 2015 | Second‐line therapy. |
| Pal 2021a | Second‐line therapy. |
| Passalacqua 2010 | Irrelevant comparison (interleukin‐2 versus interferon‐alpha). |
| Plimack 2015 | Wrong study design (dose‐finding study). |
| Pyrhonen 1995 | Irrelevant comparison (interferon alfa‐2a with vinblastine versus vinblastine alone). |
| Pyrhonen 1996 | Irrelevant comparison (interferon alfa‐2a with vinblastine versus vinblastine alone). |
| Pyrhonen 1999 | Irrelevant comparison (interferon alfa‐2a with vinblastine versus vinblastine alone). |
| Ravaud 2006 | Full‐text not available; inclusion criteria unclear. |
| Ravaud 2016 | Adjuvant therapy. |
| Rexer 2017 | Terminated study. |
| Richards 1977 | Irrelevant interventions (chemotherapy). |
| Rini 2011 | Irrelevant comparator (AMG 386). |
| Rini 2012 | Irrelevant comparator (AMG 386). |
| Rodriguez‐Vida 2020 | Terminated study (no results). |
| Rpcec 2017 | Irrelevant comparison (HeberFERON intravenous versus HeberFERON subcutaneous). |
| Sternberg 2013 | Wrong study design (cohort study) and wrong study population (refractory). |
| Szarek 2021 | Second‐line treatment. |
| Tannir 2016 | Terminated study. |
| Taylor 2020 | Wrong study design (single‐group assignment) and wrong patient population (selected solid Tumours). |
| Taylor 2020a | Wrong study design (single‐group assignment) and wrong patient population (selected solid Tumours). |
| Thiam 2010 | RECORD‐1 study. Second‐line treatment. |
| Trump 2004 | Irrelevant comparison (subcutaneous interferon alfa‐2a, subcutaneous interleukin‐2 and intravenous fluorouracil versus oral 13‐cis‐retinoic acid versus IFN‐alpha‐2a and vinblastine.). |
| Twardowski 2015 | Prior therapy allowed (more than 10% with prior therapy). |
| Twardowski 2017 | Prior therapy allowed (more than 10% with prior therapy). |
| Verzoni 2018 | Irrelevant comparator (cytoreductive nephrectomy). |
| Voss 2015 | Wrong study design (single‐group assignment). |
| Voss 2019 | Second‐line therapy (1–3 prior therapy lines). |
| Witte 1995 | Irrelevant comparison (interleukin versus interferon). |
| Wood 2013 | ADAPT study. Terminated (no results). |
| Wright 2020 | Second‐line therapy. |
| Yang 2002 | Prior therapy (majority of patients). |
| Yang 2003 | Prior therapy (majority of patients). |
| Zhou 2016 | Prior chemotherapy (13% of patients). |
| Zhou 2019 | Prior chemotherapy (13% of patients). |
Characteristics of studies awaiting classification [ordered by study ID]
Liu 2017.
| Methods |
Study type: randomised, parallel trial Blinding: no information* Study dates: no information* Cross‐over study: no Status: no information* |
| Participants |
Estimated enrolment: N = 56 was the final sample size Risk groups: no information* Inclusion criteria: no information*. Included were patients with diagnosed advanced renal cell carcinoma. Exclusion criteria: no information* |
| Interventions |
Experimental arm: sunitinib Control arm: Interleukin‐ 2 combined with interferon‐alpha treatment |
| Outcomes |
Primary outcome(s)
Secondary outcome(s) ‐ Outcomes relevant to this review but not to be assessed: AEs/SAEs, QoL, TFST, number of patients who discontinued treatment due to an AE Other outcomes to be assessed (not relevant to this review): ‐ |
| Notes | *Only abstract available, no further information present so far |
NCT01217931.
| Methods |
Study type: randomised, phase II study Blinding: no, open‐label Study dates: January 2011 ‐ January 2022 (final data collection date for primary outcome measures) Cross‐over study: sequential two‐agent assessment Status: active, not recruiting (as of May 4, 2022) |
| Participants |
Estimated enrolment: 240 Risk groups: no information Inclusion criteria:
Exclusion criteria:
More exclusion criteria on CT.gov. |
| Interventions | Group 1: Pazopanib + possible Bevacizumab Group 2: Pazopanib + possible Everolimus Group 3: Everolimus + possible Bevacizumab Group 4: Everolimus + possible Pazopanib Group 5: Bevacizumab + possible Pazopanib Group 6: Bevacizumab + possible Everolimus |
| Outcomes |
Primary outcome(s) ‐ Secondary outcome(s) ‐ Outcomes relevant to this review but not to be assessed: PS, PFS, TFST, AE, SAE, QoL, number of participants who discontinued treatment due to an AE Other outcomes to be assessed (not relevant to this review): time to overall treatment failure |
| Notes | Prior systemic therapy:quote: "participants must not have received any prior targeted therapy (anti‐VEGF agents or mTOR inhibitors), including adjuvant therapy, and must not have received any prior chemotherapy for mRCC. However, participants who had received prior immunotherapy, such as cytokines or vaccines, are permitted to enroll." ‐‐> Awaiting results to check number of participants with prior immunotherapy (if any), and whether results are reported separately for the treatment‐naive participants. Funding sources: M.D. Anderson Cancer Center, Novartis |
NCT01688973.
| Methods |
Study type: interventional, randomised, parallel assignment, phase II Blinding: no, open‐label Accrual period: August 20, 2012 ‐ April 30, 2017 Cross‐over study: no Status: completed (as of January 3, 2019) |
| Participants |
Estimated enrolment: N =55 Risk groups: no information Inclusion criteria:
More inclusion criteria on CT.gov. Exclusion criteria: not reported Sample size: N = 55, 50 participants were analysed Age, median (years, range): experimental arm: 63.6 (22.8‐81.9), control arm: 62.1 (20.3 ‐ 76.1) Sex (m/f, (%)): experimental arm: 15/10 (60/40), control arm: 19/6 (76/24) Prognostic factors:
|
| Interventions |
Experimental arm (n = 25): tivantinib orally twice daily and erlotinib hydrochloride orally once daily on days 1‐28 Control arm (n = 25): tivantinib orally twice daily on days 1‐28 |
| Outcomes |
Primary outcome(s) ‐ Secondary outcome(s)
Relevant to this review but not listed on CT.gov: OS, TFST, QoL, number of participants who discontinued treatment Other outcomes (not relevant to this review): response rate |
| Notes | Previous therapy: participants may have received prior therapy (see inclusion criteria). Awaiting results to check whether results for treatment‐naive participants are reported separately. |
NCT01829841.
| Methods |
Study type: RCT, parallel assignment, phase II Blinding: unclear, "double‐blind" stated in the title, but "none (open‐label)" in the description on CT.gov Study dates: May 2011‐ May 2016 (actual study completion period) Countries: multicentre Cross‐over study: no Status: completed (as of May 3, 2018) |
| Participants |
Estimated enrolment: N = 150 Risk groups: no information Inclusion criteria:
Exclusion criteria:
Age, median (years, range): 18‐75 Sex (m/f, (%)): all sexes are eligible More inclusion criteria on CT.gov. |
| Interventions |
Experimental arm: Famitinib (Famitinib 25 mg once daily orally) Control arm: Sunitinib (Sunitinib 50 mg orally once daily) |
| Outcomes |
Primary outcome(s) ‐ Secondary outcome(s)
Relevant to this review but not to be assessed: TFST, SAE, number of participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): ORR, DCR, body vitals, laboratory parameters |
| Notes |
Funding sources: Jiangsu HengRui Medicine Co., Ltd.Cancer Institute and Hospital, Chinese Academy of Medical Sciences Previous therapy: may include participants in second‐line therapy (see inclusion criteria). Awaiting results to check whether results for treatment‐naive participants are reported separately. |
NCT03541902.
| Methods |
Study type: interventional, randomised, parallel assignment, phase II Blinding: no, open‐label Accrual period: May 15, 2018 ‐ July 31, 2022 (estimated study completion date) Countries: multicenter (N = 3 in the USA) Cross‐over study: no Status: active, not recruiting (as of June 1st, 2022) |
| Participants |
Estimated enrolment: N = 84 Risk groups: no information Inclusion criteria:
Exclusion criteria:
More inclusion and exclusion criteria on CT.gov. |
| Interventions |
Experimental arm: cabozantinib orally once daily on days 1‐42) Control arm: sunitinib malate (orally once daily on days 1‐28 |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: SAE, TFST, participants who discontinued treatment due to an AE, QoL Other outcomes (not relevant to this review): ORR |
| Notes |
Funding sources: M.D. Anderson Cancer CenterExelixisNational Cancer Institute (NCI) Previous therapy: participants may have received prior therapy (see inclusion criteria). Awaiting results to check whether results for treatment‐naive participants are reported separately. |
AEs: adverse events; CT: computed tomography; ECOG: Eastern Cooperative Oncology Group (ECOG);MRI: magnetic resonance imaging; mTOR: mechanistic target of rapamycin ; OS: overall survival ; PFS: progression‐free survival ; QoL: quality of life; RCC: renal cell carcinoma; SAEs: serious adverse events.
Characteristics of ongoing studies [ordered by study ID]
EUCTR2008‐000928‐71‐IT.
| Study name | ‐ |
| Methods |
Study type: RCT, phase II Blinding: no, open‐label Accrual period: no information Country: Italy Cross‐over study: no Status: ongoing |
| Participants |
Estimated enrolment: no information Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Experimental arm: Temsirolimus+Interferon‐alpha Control arm: Temsirolimus monotherapy |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST, participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): ORR, TTP |
| Starting date | No information |
| Contact information | ‐ |
| Notes | Funding source: Gruppo Oncologico Italiano Di Ricerca |
NCT02210117.
| Study name | ‐ |
| Methods |
Study type: RCT, early phase I, parallel assignment Blinding: no, open‐label Accrual period: November 25, 2014 ‐ May 21, 2020 (final data collection date for primary outcome measure)) Countries: no information Cross‐over study: no Status: active, not recruiting (as of March 23, 2020) |
| Participants |
Estimated enrolment: N=105 Inclusion criteria:
Exclusion criteria:
More inclusion and exclusion criteria on CT.gov. |
| Interventions |
Experimental arm I: Nivolumab + Bevacizumab + surgery Experimental arm II: Nivolumab + Ipilimumab + surgery Control arm: Nivolumab + surgery |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST, participants who discontinued treatment due to an AE, safety (AEs/SAEs) Other outcomes (not relevant to this review): ORR, DoR, immunological changes in tumour tissue and peripheral blood |
| Starting date | 25.11.2014 |
| Contact information | Padmanee Sharma (M.D. Anderson Cancer Center) |
| Notes | Funding sources: M.D. Anderson Cancer Center, National Cancer Institute (NCI) |
NCT02996110.
| Study name | FRACTION‐RCC |
| Methods |
Study type: RCT, phase II, parallel assignment Blinding: no, open‐label Accrual period: January 17, 2017‐ January 18, 2023 (estimated study completion date) Countries: multicentre (35 study centres) Cross‐over study: no Status: Active, not recruiting (as of March 9, 2022) |
| Participants |
Estimated enrolment: N=200 Inclusion criteria:
Exclusion criteria:
Other protocol defined inclusion/exclusion criteria could apply |
| Interventions |
Experimental arm I: Nivolumab + Relatlimab Experimental arm II: Nivolumab + BMS‐986205 Experimental arm III: Nivolumab + BMS‐813160 Control arm: Nivolumab + Ipilimumab |
| Outcomes |
Primary outcome(s):
Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR, DoR |
| Starting date | 17.01.2017 |
| Contact information | Bristol‐Myers Squibb |
| Notes | Funding source: Bristol‐Myers Squibb |
NCT03075423.
| Study name | SUNIFORECAST |
| Methods |
Study type: RCT, phase II, parallel assignment Blinding: no, open‐label Accrual period: November 1, 2017 ‐ December 31, 2023 (estimated study completion date) Countries: international (Belgium, Czechia, France, Germany, the Netherlands, Spain, the UK), multicentre (40 study locations) Cross‐over study: no Status: Recruiting (as of February 23, 2022) |
| Participants |
Estimated enrolment: N = 306 Inclusion criteria:
Exclusion criteria:
More inclusion & exclusion criteria on CT.gov. |
| Interventions |
Experimental arm: Iplilmumab + Nivolumab Control arm: Sunitinib |
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
Relevant to this review but not reported: TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR |
| Starting date | 01.11.2017 |
| Contact information | Lothar Bergmann, MD; Nicola Goekbuget, MD |
| Notes | Funding sources: Nicola Goekbuget |
NCT03260894.
| Study name | KEYNOTE‐679/ECHO‐302 |
| Methods |
Study type: RCT, phase III Blinding: no, open‐label Accrual period: December 7, 2017 ‐ February 8, 2022 (estimated study completion date) Countries: multicentre (140 study locations) Cross‐over study: no Status: Active, not recruiting (as of February 28, 2022) |
| Participants |
Estimated enrolment: N = 129 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Experimental arm: pembrolizumab + epacadostat Control arm: sunitinib or Pazopanib |
| Outcomes |
Primary outcome(s): Secondary outcome(s):
Relevant to this review but not reported: SAEs, OS, PFS, QoL, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR |
| Starting date | 07.12.2017 |
| Contact information | Mark Jones, MD |
| Notes | Funding sources: Incyte Corporation, Merck Sharp & Dohme Corp. |
NCT03592472.
| Study name | ‐ |
| Methods |
Study type: RCT, phase III Blinding: Double‐blind (participant, investigator) Accrual period: July 17, 2018 ‐ June 30, 2022 (estimated study completion date) Countries: multicentre study (38 locations in the US, China, Italy, Korea, Poland, Spain) Cross‐over study: yes Status:‐ Recruiting (as of May 12, 2021) |
| Participants |
Estimated enrolment: N = 413 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Experimental arm: pazopanib plus abexinostat Control arm: pazopanib plus placebo |
| Outcomes |
Primary outcome(s): Secondary outcome(s):
Relevant to this review but not reported: TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR, DOR |
| Starting date | July 17, 2018 |
| Contact information | ‐ |
| Notes | Funding source: Xynomic Pharmaceuticals, Inc. |
NCT03729245.
| Study name | BEMPEG |
| Methods |
Study type: RCT, phase III, parallel assignment Blinding: no, open‐label Accrual period: December 18, 2018 ‐ June 2024 (estimated study completion date) Countries: 116 study locations Cross‐over study: no Status: active, not recruiting (as of April 4, 2022) |
| Participants |
Estimated enrolment: N=623 Inclusion criteria:
Exclusion criteria:
Additional protocol defined inclusion/exclusion criteria and exceptions apply |
| Interventions |
Experimental arm: Bempegaldesleukin + Nivolumab Control arm: Sunitinib or Cabozantinib |
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
Relevant to this review but not reported: SAEs, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR, changes in cancer‐ related symptoms |
| Starting date | 18.12.2018 |
| Contact information | |
| Notes | Funding sources: Nektar Therapeutics, Bristol‐Myers Squib |
NCT03793166.
| Study name | PDIGREE |
| Methods |
Study type: RCT, phase III, parallel assignment Blinding: no, open‐label Accrual period: May 9, 2019 ‐ April 9, 2022 (estimated study completion date) Countries: multicentre, 804 study locations Cross‐over study: no Status: Recruiting (as of June 3, 2022) |
| Participants |
Estimated enrolment: N=1046 Inclusion criteria:
Exclusion criteria:
More inclusion criteria on CT.gov. |
| Interventions |
Experimental arm: nivolumab + cabozantinib Control arm: nivolumab + ipilimumab |
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
Relevant to this review but not reported: QoL, SAEs, TFST Other outcomes (not relevant to this review): CR, OR |
| Starting date | 09.05.2019 |
| Contact information | Tian Zhang |
| Notes | Funding sources: National Cancer Institute (NCI) |
NCT03873402.
| Study name | ‐ |
| Methods |
Study type: RCT, parallel assignment, phase IIIB Blinding: quadruple blinding (participant, care provider, investigator, outcomes assessor) Accrual period: April 29, 2019 ‐ April 19, 2025 (estimated study completion date) Countries: multicentre (80 study locations) Cross‐over study: no Status: active, not recruiting (as of February 10, 2022) |
| Participants |
Estimated enrolment: N=418 Inclusion criteria:
Exclusion criteria:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm I: Nivolumab Experimental arm II: Ipilimumab Control arm: Ipilimumab placebo |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR, DCR, DoR, TTR, clinical laboratory results |
| Starting date | April 29, 2019 |
| Contact information | ‐ |
| Notes | Funding source: Bristol‐Myers Squibb |
NCT03937219.
| Study name | COSMIC‐313 |
| Methods |
Study type: RCT, parallel assignment, phase III Blinding: yes, double‐ blind Accrual period: June 25, 2019 ‐ March 2025 (estimated study completion date) Countries: multicentre (167 study locations) Cross‐over study: no Status: Active, not recruiting (as of March 10, 2022) |
| Participants |
Estimated enrolment: N = 840 Inclusion criteria:
Exclusion criteria:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm: cabozantinib + nivolumab + ipilimumab (4 doses) followed by cabozantinib + nivolumab Control arm: Cabozantinib‐matched placebo + nivolumab + ipilimumab (4 doses) followed by cabozantinib‐matched placebo + nivolumab |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: QoL, AEs/SAEs, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ‐ |
| Starting date | June 25, 2019 |
| Contact information | ‐ |
| Notes | Funding source: Exelixis |
NCT04090710.
| Study name | CYTOSHRINK |
| Methods |
Study type: RCT, parallel assignment, phase II Blinding: no, open‐label Accrual period: January 29, 2020 ‐ December 31, 2023 Countries: international (Australia, Canada), multicentre (7 study locations) Cross‐over study: no Status: Recruiting (as of March 31, 2022) |
| Participants |
Estimated enrolment: N=78 Inclusion criteria:
Exclusion criteria:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm: Radiation: SBRT + ipilimumab/nivolumab Control arm: ipilimumab/ nivolumab |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Relevant to this review but not reported: TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR, drug tolerability, stool microbiome, blood immune signature changes |
| Starting date | January 29, 2020 |
| Contact information | Ontario Clinical Oncology Group (OCOG) |
| Notes | Funding source: Ontario Clinical Oncology Group (OCOG) |
NCT04203901.
| Study name | ‐ |
| Methods |
Study type: RCT, phase IIb Blinding: no, open‐label Accrual period: July 22, 2020 ‐ March, 2022 (estimated study completion date) Countries: national (USA), single‐ centre (Texas) Cross‐over study: no Status: Recruiting (as of Februrary 11, 2022) |
| Participants |
Estimated enrolment: N=120 Inclusion criteria:
Exclusion criteria:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm: CMN‐001 and Nivolumab+Ipilimumab (1st line therapy), Lenvatinib + Everolimus (2nd line therapy after progression) Control arm: Nivolumab+Ipilimumab (1st line therapy), Lenvatinib + Everolimus (2nd line therapy after progression) |
| Outcomes |
Primary outcome(s)
Secondary Outcome(s)
Relevant to this review but not reported: QoL, SAEs, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): tumour response |
| Starting date | July 22, 2020 |
| Contact information | CoImmune; Mark DeBenedette, PhD |
| Notes | Funding source: CoImmune |
NCT04394975.
| Study name | ‐ |
| Methods |
Study type: RCT, sequential assignment, phase III Blinding: no, open‐label Accrual period: August 20, 2020 ‐ June 30, 2023 estimated study completion date) Countries: multicentre Cross‐over study: no Status: Recruiting (as of January 21, 2021) |
| Participants |
Estimated enrolment: N= 380 Inclusion criteria:
Exclusion criteria: participants with any of the following conditions will not be included in the study:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm: axitinib Control arm: sunitinib |
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
Relevant to this review but not reported: QoL, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR, DoR, DCR, biomarkers, incidence and grade of AEs and SAEs related to study drugs |
| Starting date | August 20, 2020 |
| Contact information | Shanghai Junshi Bioscience Co., Ltd., Fugui Wang |
| Notes | Funding source: Shanghai Junshi Bioscience Co., Ltd. |
NCT04523272.
| Study name | ‐ |
| Methods |
Study type: RCT, parallel assignment, phase III Blinding: no, open‐label Accrual period: August 25, 2020 ‐ June 2023 Countries: multicentre (26 study locations) Cross‐over study: no Status: Recruiting (as of September 10, 2020) |
| Participants |
Estimated enrolment: N = 418 Inclusion criteria:
Exclusion criteria:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm: TQB2450 + anlotinib Control arm: sunitinib mMalate capsules |
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
Relevant to this review but not reported: QoL, AEs/SAEs, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): DCR, DoR, |
| Starting date | August 25, 2020 |
| Contact information | Jun Guo, Doctor |
| Notes | Funding source: Chia Tai Tianqing Pharmaceutical Group Co., Ltd |
NCT04540705.
| Study name | PIVOT IO 011 |
| Methods |
Study type: RCT, parallel assignment, phase 1/2 study Blinding: no, open‐label Accrual period: September 11, 2020 ‐ January 17, 2026 (estimated study completion date) Countries: international (5 countries: Brazil, Argentina, USA, Spain, Canada, ), multicentre Cross‐over study: no Status: active, not recruiting (as of May 18, 2022) |
| Participants |
Estimated enrolment: N = 250 Inclusion criteria:
Exclusion criteria:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm I: part 1A (part 1): nivolumab + bempegaldesleukin + bxitinib Experimental arm II: part 1B (part 1): nivolumab + bempegaldesleukin + cabozantinib Experimental arm III: arm A (part 2): nivolumab + bempegaldesleukin + cabozantinib Control arm: arm B (part 2): nivolumab + cabozantinib |
| Outcomes |
Primary outcome(s) :
Secondary outcome(s) :
Relevant to this review but not reported: QoL, TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): DLTs, laboratory results, ORR |
| Starting date | September 11, 2020 |
| Contact information | Bristol‐Myers Squibb |
| Notes | Funding sources: Bristol‐Myers Squibb |
NCT04736706.
| Study name | ‐ |
| Methods |
Study type: RCT, parallel assignment, phase III Blinding: no, open‐label Accrual period: April 14, 2021 ‐ October 29, 2026 (estimated study completion date) Countries: international (USA, Australia, Chile, Czechia, Denmark, Finland, Guatemala, Hungary, Korea, Norway, Poland, Russia, Spain, Sweden, Taiwan, Turkey, Ukraine), multicentre (92 study locations) Cross‐over study: no Status: Recruiting (as of May 31, 2022) |
| Participants |
Estimated enrolment: N = 1431 Inclusion criteria:
Exclusion criteria:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm I: pembrolizumab + belzutifan + lenvatinib Experimental arm II: pembrolizumab/quavonlimab + lenvatinib Control arm: pembrolizumab + lenvatinib |
| Outcomes |
Primary outcome(s) :
Secondary outcome(s):
Relevant to this review but not reported: QoL, TFST, SAEs Other outcomes (not relevant to this review): ORR, DoR |
| Starting date | April 14, 2021 |
| Contact information | Merck Sharp & Dohme Corp. |
| Notes | Funding sources: Merck Sharp & Dohme Corp., Eisai Inc. |
NCT05043090.
| Study name | SAMETA |
| Methods |
Study type: RCT, phase III, parallel assignment Blinding: no, open‐label Accrual period: October 28, 2021 ‐ June 9, 2025 (estimated study completion date) Countries: multicentre (172 locations in the USA, Argentina, Australia, Brazil, Canada, Chile, Czech Republic, France, Germany, China, India, Isreal, Italy, Korea, Mexico, the Netherlands, Poland, Romania, Russia, Singapore, Spain, Taiwan, Turkey, Ukraine, the UK) Cross‐over study: no Status: recruiting (as of May 17, 2022) |
| Participants |
Estimated enrolment: N=220 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Experimental arm I: savolitinib + durvalumab Experimental arm II: durvalumab Control arm: sunitinib |
| Outcomes |
Primary outcome(s) :
Secondary outcome(s):
Relevant to this review but not reported: TFST, AEs, SAEs, number of participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): ORR, DoR, DCR |
| Starting date | October 28, 2021 |
| Contact information | Toni Choueiri, Dana‐Farber Cancer Institute |
| Notes | Funding source: AstraZeneca |
NCT05096390.
| Study name | PAXIPEM |
| Methods |
Study type: RCT, phase II, parallel assignment Blinding: no, open‐label Accrual period: December 2021 ‐ December 2025 (estimated study completion date) Countries: multicentre (11 locations in France) Cross‐over study: no Status: Not yet recruiting (as of October 27, 2021) |
| Participants |
Estimated enrolment: N = 72 Inclusion criteria:
Exclusion criteria:
More inclusion & exclusion criteria on CT.gov. |
| Interventions |
Experimental arm: axitinib + pembrolizumab Control arm: axitinib monotherapy |
| Outcomes |
Primary outcome(s) :
Secondary outcome(s):
Relevant to this review but not reported: TFST, QoL, number of participants who discontinued treatment due to an AE Other outcomes (not relevant to this review): DoR, BoR |
| Starting date | December 2021 |
| Contact information | Sylvie Negrier |
| Notes | Funding source: Centre Leon Berard |
UMIN 000012522.
| Study name | ESCAPE |
| Methods |
Study type: RCT, phase III, parallel assignment Blinding: no, open‐label Accrual period: no information Countries: national (Japan) Cross‐over study: no information Status: recruiting |
| Participants |
Estimated enrolment: N = 144 Inclusion criteria:
Exclusion criteria:
*Other protocol‐defined inclusion/exclusion criteria apply |
| Interventions |
Experimental arm: cytokines (IL‐2+ IFN) as 1st‐line followed by 2nd‐line axitinib Control arm: sunitinib as 1st‐line followed by 2nd‐line axitinib |
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
Relevant to this review but not reported: TFST, number of patients who discontinued treatment Other outcomes (not relevant to this review): ORR, TTF, DCR |
| Starting date | ‐ |
| Contact information | Kanazawa University Hospital |
| Notes | Funding sources: Innovative Clinical Research Center, Kanazawa University Hospital |
AEs: adverse events CNS: central nervous system’; DVT: deep vein thrombosis; MRI: magnetic resonance imaging; mTOR: mechanistic target of rapamycin ; OS: overall survival ; PFS: progression‐free survival ; QoL: quality of life; RCC: renal cell carcinoma; RRCT: randomised controlled trial; SAEs: serious adverse events.
Differences between protocol and review
We made some changes to the methods that were pre‐specified in the protocol for this review (Goldkuhle 2020).
Criteria for considering studies in this review
Types of interventions
In the protocol for this review, we pre‐specified that we would create two networks with interventions, one for the favourable risk group, and one combined for the intermediate and poor risk groups. During the conduct of this review we found that trials reported data for the different risk groups according to different criteria, i.e. risk groups either according to the IMDC or the MSKCC criteria. In some trials, data were even reported for both criteria. Together with the clinical experts on this review, we decided to analyse data not only separately for the different risk groups (i.e. favourable vs. intermediate and poor risk), but also separately by the different criteria (i.e. IMDC or MSKCC), as these should be looked at separately. In addition, as many trials reported both data for the separate risk groups and all risk groups combined (which we called the 'total trial population'), we also decided to conduct one overall analysis for each outcome with all risk groups combined. This was particularly the case for the safety outcomes, as no subgroup data according to risk group were reported for these. For the outcomes OS and PFS, we were able to extract data for both the total trial population (i.e., all risk groups combined) and separately according to the IMDC favourable/intermediate/poor risk groups and the MSKCC favourable/intermediate/poor risk groups.
Types of outcome measures
In the protocol we stated that we will extract all individual AEs reported in the trials as well as the frequency of the specific AEs. However, this was not feasible for this review, but will be considered in an update of this review.
We planned to analyse the outcome TFST as a time‐to‐event outcome. However, none of the included trials reported this outcome as a time‐to‐event outcome. We planned to analyse such outcomes as dichotomous outcomes if time‐to‐event analyses were not possible. Hence, we extracted the number of participants who received subsequent anticancer therapy after discontinuation of trial therapy. However, reporting between trials was heterogenous, for example in terms of the definition of this outcome and the timing of reporting (participants received different lengths of therapy, and it was unclear at which time point therapy stopped), so we refrained from pooling data and reported the results narratively in tabular form instead. Hence, we also reported this outcome narratively in the 'Summary of Findings' table.
Although we extracted and estimated some data for the outcome QoL, pooling data were not feasible. Even after combining all available data for the different time points, only one time point (long‐term, 1 year after initiation of treatment) would have been feasible for network meta‐analyses. However, for this time period ("long‐term"), the individual time points also varied between two and four years, so pooling these different time points in a combined "long‐term" analysis would not have produced meaningful results. For the other time points, comparisons including SUN were sparse (only two or three trials per time point) and only when combining different scales; so again, an analysis would have not delivered meaningful results. Conducting pairwise meta‐analyses was also not possible because each comparison was reported by one trial only. Hence, we decided to report results for this outcome narratively in this review.
Data collection and analysis
Assessment of publication bias
Creating a 'comparison‐adjusted' funnel plot was not feasible for this review because the individual effects are centred on the 1 to maintain the same reference line. That is, for each comparison, the pooled effect is used as the reference. This resulted in the effect being centred on the 1 for all comparisons consisting of only one trial. Since we often had one trial for each comparison in our analyses, there are barely any trials outside the reference line, so the funnel plot could not provide meaningful results.
Data synthesis
Certainty of the evidence
In the protocol we stated that we will use GRADEpro GDT for the GRADE assessment. However, as the software cannot be used for network meta‐analyses, and because we did not conduct any traditional pairwise meta‐analyses, we were not able to use this software. Instead, we created our own table in Excel where we conducted and recorded our GRADE assessments (judgements and explanations).
Summary of findings table
In the protocol we had stated that we will create two networks (one for the favourable risk group and one for the intermediate and poor risk groups) and thus present one summary of findings (SoF) table each. However, as we now have an additional network per outcome for the combined risk groups, we provided three SoF tables: one for the total trial population (i.e. all risk groups combined); one for the favourable risk group (results presented separately for IMDC and MSKCC); and one for the intermediate and poor risk groups (results presented separately for IMDC and MSKCC).
Furthermore, we stated that we will use CINeMA to present the SoF table. However, we decided to create a SoF table manually in a format applicable to the PICO of this review with network meta‐analyses. Lastly, we did not calculate NNT/NNH as planned in the protocol of this review, for simplicity reasons, as we already present absolute effect numbers for every network treatment estimate in the SoF tables.
Living systematic review considerations
At protocol stage, we proposed an approach for updating this review. However, due to restricted funding there are currently no plans for an update.
We had proposed the following approach.
Following the approaches proposed by Cochrane, whenever new evidence (i.e. studies, data, or other information) relevant to the review is identified, we will extract the data and assess risk of bias as appropriate. We will wait until the accumulating evidence changes one or more of the following components of the review before incorporating it and republishing the review.
The findings for one or more of the primary outcomes change either in the size of the point estimate or the direction of effects, or both.
The credibility (e.g. GRADE rating) of one or more primary outcomes.
New settings, populations, interventions, comparisons, or outcomes are studied.
Furthermore, following these suggestions, we will not use formal sequential meta‐analysis approaches for updated (network) meta‐analyses.
In order to inform our readers on any changes in the review and its conclusions, including the search results and all additional evidence, we plan to update the status information of the review, for example when new searches are undertaken. Once we identify an additional trial or other substantial information with direct relevance to the review conclusions, we will republish the review with a new citation.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses on administration routes and different dosages was not feasible because administration routes did not differ between trials for the individual drugs, and there were only little differences in the dosages for a few drugs administered in the trials. Particularly our main comparator, sunitinib, was administered the same way in all trials and the dosage was administered across trials. For more details see Table 1. Interventions in the included trials in Description of studies. Furthermore, subgroup analyses for a follow‐up time of less than one year was also not possible as no trial had such a short follow‐up time.
In addition, it was not possible to conduct the following pre‐specified subgroup analyses for the outcomes OS, SAE and QoL, the main reasons being that either subgroup data were not available or because trials could not be grouped according to the pre‐specified characteristics. Only for the outcome OS, we were indeed able to extract some subgroup data according to age, sex and prior nephrectomy. However, the analyses would have included no more than two or three trials, respectively, so no meaningful results would have been created.
age (< 65 versus > 65), as all trials included participants over and under the age of 65, and insufficient subgroup data were reported.
sex (male versus female), as all trials included both men and women, and insufficient subgroup data were reported.
nephrectomy (yes versus no), as in all trials but one, most participants had previously received a nephrectomy (full or partial), or a nephrectomy was allowed based on the inclusion criteria and no further information was provided about how many participants actually had a prior nephrectomy.
radiotherapy (yes versus no), because in most trials, most participants had received previous radiotherapy, or radiotherapy was "allowed" based on the inclusion criteria and no further information was provided about how many participants actually had previously received radiotherapy.
histology type (clear cell type, papillary type, sarcomatoid type), because in most trials, only participants with clear cell RCC were included, followed by trials in which most participants had clear cell RCC. Only in three trials it was clearly indicated that mostly participants with non‐clear cell (papillary type) RCC were included.
site of metastases (lung, bone, liver), as most trials reported several metastatic sites, so we could not group these trials. Only few trials reported only one metastatic site, and few trials included advanced metastatic RCC but without reporting the sites of the metastases.
Sensitivity analysis
In addition to our primary outcomes (OS, SAE), we also conducted sensitivity analyses using the fixed‐effect model for the outcome PFS. As we did not analyse data for the outcome QoL, there is no such sensitivity analysis for this outcome.
For time‐to‐event outcomes, we had planned to conduct sensitivity analyses to explore the robustness of findings in case we had to use variable techniques to reconstruct HR from primary trial reports. However, as reconstruction of the HR was either not necessary or not possible, such sensitivity analyses were not conducted.
We also planned to conduct sensitivity analyses on quality components (overall low risk of bias or some concerns versus overall high risk of bias). This was not possible for the outcome QoL as all trials were at high risk of bias for this outcome.
Sensitivity analyses for completed but not published trials was not possible because only one trial in the included trials for analyses was not published in a publication (except for a retrospective analysis of a subpopulation of the total trial population, which was not of interest for this review). Some outcome data were published on the trial registry, which we incorporated into this review.
Lastly, sensitivity analyses on the influence of trial design (blinded trial, unblinded (open‐label)) was also not feasible, as 32 of 36 trials were open‐label (i.e. non‐blinded) trials. One trial did not report whether it was blinded or not, and of those three trials that were blinded, one was not included in any analyses, so no meaningful results would have been produced with a sensitivity analysis of the remaining two trials only.
Contributions of authors
AAa: review development, including screening and study selection, data extraction, risk of bias assessment, GRADE assessment, interpretation and writing of results, developing the draft
BB: review development, including screening and study selection, data extraction, risk of bias assessment, assisted in writing the draft (i.e. description of studies and bias assessments)
AAb: conducted the statistical analyses, proofread the draft
IM: designed the search strategies and conducted all searches, proofread the draft
VP: screening and study selection, provided methodological expertise on NMA, assisted in the GRADE assessment, proofread the draft
ET: data extraction, risk of bias assessment, proofread the draft
CH: searching for CSRs, data extraction for the characteristics of included studies, proofread the draft
ND: data extraction, risk of bias assessment, proofread the draft
MG: screening and study selection, searching for CSRs, provided methodological expertise, proofread the draft
PM: provided clinical expertise, proofread the draft
PD: provided methodological and clinical expertise
AH: provided clinical expertise
NS: provided methodological and content expertise, proofread the draft
Sources of support
Internal sources
-
University Hospital Cologne, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Germany, Germany
Provision of the offices, including technical equipment
External sources
-
Bundesministerium für Bildung und Forschung (BMBF) (German Federal Ministry of of Education and Research), Germany
Grant number: 01KG1901
Declarations of interest
AAa: The grant by the German Federal Ministry of Education and Research does not lead to a conflict of interest. She is editor at Cochrane, but was not involved in the editorial process for this review.
BB: none known.
AAb: The grant by the German Federal Ministry of Education and Research does not lead to a conflict of interest. She is editor at Cochrane, but was not involved in the editorial process for this review.
IM: none known. She is information specialist for Cochrane Haematology, but was not involved in the editorial process for this review.
VP: none known.
ET: none known.
CH: none known.
ND: none known.
PM: none known.
PD: none known; he is Co‐ordinating Editor of Cochrane Urology, but was not involved in the editorial process for this review.
MG: none known.
AH: speaker honorary and research grant for renal cell carcinoma by BMS; however, this does not lead to a conflict of interest.
NS: none known; she is Co‐ordinating Editor of Cochrane Haematology, but was not involved in the editorial process for this review.
New
References
References to studies included in this review
Jonasch 2010 {published data only}
- Jonasch E, Corn P, Asche RG. Randomized phase II study of sorafenib with or without low-dose IFN in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology. Conference: ASCO Annual Meeting 2007;25(18 Suppl):Abstract 5104. [Google Scholar]
- Jonasch E, Corn P, Pagliaro LC, Warneke CL, Johnson MM, Tamboli P, et al. Upfront, randomized, phase 2 trial of sorafenib versus sorafenib and low-dose interferon alfa in patients with advanced renal cell carcinoma: clinical and biomarker analysis. Cancer 2010;116(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00065468 {published data only}
- Atkins MB, Hidalgo M, Stadler W, Logan T, Dutcher JP, Hudes G, et al. A randomized double-blind phase 2 study of intravenous CCI-779 administered weekly to patients with advanced renal cell carcinoma. In: Proceedings of the American Society of Clinical Oncology. Vol. 21 (Pt 1). 2002:10a, Abstract 36. [ClinicalTrials.gov: NCT00065468]
- Bellmunt J, Szczylik C, Feingold J, Strahs A, Berkenblit A. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Annals of Oncology 2008;19(8):1387-92. [ClinicalTrials.gov: NCT00065468] [DOI] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. A phase 3, randomized, 3-arm study of temsirolimus (TEMSR) or interferon-alpha (IFN) or the combination of TEMSR + IFN in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma (adv RCC). Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2006;24(18S):LBA4. [ClinicalTrials.gov: NCT00065468] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New England Journal of Medicine 2007;356(22):2271-81. [ClinicalTrials.gov: NCT00065468] [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Pullenayegum E, Alemao E, Strahs A, Purvis J. Evaluation of adverse event-related hospitalizations in patients with advanced renal cell carcinoma on treatment with temsirolimus or interferon-alfa: results from a phase 3 randomized trial. European Journal of Cancer, Supplement 2009;7(2-3):441. [ClinicalTrials.gov: NCT00065468] [Google Scholar]
NCT00072046 {published data only}
- Halabi S, Rini BI, Stadler WM, Small EJ. Use of progression-free survival (PFS) to predict overall survival (OS) in patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology. Conference: ASCO Annual Meeting 2010;28(15 Suppl 1):4525-4525. [ClinicalTrials.gov: NCT00072046] [Google Scholar]
- Melichar B, Bracarda S, Bellmunt J, Ravaud A, Negrier S, Jethwa S, et al. First-line bevacizumab + reduced-dose interferon-alpha2a in patients (pts) with metastatic renal cell carcinoma (mRCC): An update on overall survival. European Journal of Cancer, Supplement 2009;7(2-3):430. [ClinicalTrials.gov: NCT00072046] [Google Scholar]
- Nixon AB, Halabi S, Liu Y, Starr MD, Brady JC, Shterev I, et al. Predictive biomarkers of overall survival in patients with metastatic renal cell carcinoma treated with IFNalpha +/- bevacizumab: results from CALGB 90206 (alliance). Clinical Cancer Research 2021;OF1 - OF8:29. [ClinicalTrials.gov: NCT00072046] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Halabi S, Rosenberg J, Stadler WM, Vaena DA, Atkins JN, et al. Bevacizumab plus interferon-alpha versus interferon-alpha monotherapy in patients with metastatic renal cell carcinoma: results of overall survival for CALGB 90206. Journal of Clinical Oncology 2009;27(15S Pt 1):239. [ClinicalTrials.gov: NCT00072046] [Google Scholar]
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. Journal of Clinical Oncology 2010;28(13):2137-43. [ClinicalTrials.gov: NCT00072046] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. Journal of Clinical Oncology 2008;26(33):5422-8. [ClinicalTrials.gov: NCT00072046] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00081614 {published data only}
- Bukowski RM, Kabbinavar F, Figlin RA, Flaherty K, Srinivas S, Vaishampayan U, et al. Bevacizumab with or without erlotinib in metastatic renal cell carcinoma (RCC). Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2006;24(18S):4523. [ClinicalTrials.gov: NCT00081614] [Google Scholar]
- Bukowski RM, Kabbinavar FF, Figlin RA, Flaherty K, Srinivas S, Vaishampayan U, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. Journal of Clinical Oncology 2007;25(29):4536-41. [ClinicalTrials.gov: NCT00081614] [DOI] [PubMed] [Google Scholar]
NCT00098657/NCT00083889 {published data only}
- Blagoev KB, Wilkerson J, Stein WD, Motzer RJ, Bates SE, Fojo AT. Effect of sunitinib (Su) administration on post-treatment survival in patients with metastatic renal cell carcinoma (mRCC) treated on the upfront randomized phase III trial of sunitinib or interferon alfa (IFN). Journal of Clinical Oncology. Conference: ASCO Annual Meeting 2011;29(15 Suppl 1):4634-4634. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Bushmakin A, Cappelleri JC, Korytowsky B, Sandin R, Matczak E, Cella D. Sunitinib (Su) dosing schedule and data collection timepoints: Impact on quality of life (QoL) outcomes in metastatic renal cell carcinoma (mRCC). Annals of Oncology 2012;9:ix269. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Charbonneau C, Kim ST, Li JZ, Motzer RJ. Quality of life (QoL) with sunitinib versus interferon-alfa (IFN) as first-line therapy in patients with metastatic renal cell carcinoma (mRCC): final results. Journal of Clinical Oncology 2009;27(15S Pt I):330. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Castellano D, Muro XG, Perez-Gracia JL, Gonzalez-Larriba JL, Abrio MV, Ruiz MA, et al. Patient-reported outcomes in a phase III, randomized study of sunitinib versus interferon-{alpha} as first-line systemic therapy for patients with metastatic renal cell carcinoma in a European population. Annals of Oncology 2009;20(11):1803-12. [ClinicalTrials.gov: NCT00098657/NCT00083889] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Bushmakin AG, Cappelleri JC, Charbonneau C, Li JZ, Kim ST, et al. Health-related quality of life (HrQoL) in metastatic renal cell carcinoma (mRCC) patients receiving sunitinib (Su) or interferon (IFN)-alfa in a randomized phase III trial: updated geographic analysis. Annals of Oncology 2009;19(Suppl 8):189. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Cella D, Bushmakin AG, Cappelleri JC, Charbonneau C, Sandin R, Negrier S, et al. Comparison of health-related quality of life (HrQoL) in patients reporting the same adverse event (AE) fatigue on different treatments. European Journal of Cancer 2011;47:233. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Cella D, Li JZ, Cappelleri JC, Bushmakin A, Charbonneau C, Kim ST, et al. Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: results from a phase III randomized trial. Journal of Clinical Oncology 2008;26(22):3763-9. [ClinicalTrials.gov: NCT00098657/NCT00083889] [DOI] [PubMed] [Google Scholar]
- Cella D, Michaelson MD, Bushmakin AG, Cappelleri JC, Charbonneau C, Kim ST, et al. Final quality of life (QoL) results with geographical analysis for sunitinib versus interferon-alfa, as first-line therapy in patients with metastatic renal cell carcinoma (mRCC). Value in Health 2009;12(7):A283. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Cella D, Michaelson MD, Cappelleri JC, Bushmakin AG, Charbonneau C, Kim ST, et al. Quality of life (QoL) with sunitinib versus interferon-alfa (IFN-alpha) as first-line therapy in patients with metastatic renal cell carcinoma (mRCC): Final results. Journal of Clinical Oncology 2009;1:6529. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Doehn C. Sunitinib vs interferon-alpha for the treatment of metastatic renal cell carcinoma.. Onkologe 2007;13(9):862-4. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Figlin RA, Hutson TE, Tomczak P, Michaelson MD, Bjarnason GA, Lin X, et al. Characterizing prior nephrectomy patients with metastatic renal cell carcinoma (mRCC) treated with first-line sunitinib versus interferon-alfa. European Journal of Cancer 2013;2:S644-5. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Figlin RA, Hutson TE, Tomczak P, Michelson MD, Bukowski RM, Negrier S, et al. Overall survival with sunitinib versus interferon (IFN)-alfa as first-line treatment of metastatic renal cell carcinoma (mRCC). Journal of clinical oncology: ASCO annual meeting proceedings 2008;26(15S Pt 1):256. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27(22):3584-90. [ClinicalTrials.gov: NCT00098657/NCT00083889] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Phase III randomized trial of sunitinib malate (SU11248) versus interferon-alfa (IFN-{alpha}) as first-line systemic therapy for patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2006;24(18S):LBA3. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New England Journal of Medicine 2007;356(2):115-24. [ClinicalTrials.gov: NCT00098657/NCT00083889] [DOI] [PubMed] [Google Scholar]
- Negrier S, Figlin RA, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, et al. Overall survival with sunitinib versus interferon (IFN)-alfa as first-line treatment of metastatic renal cell carcinoma (mRCC). Annals of Oncology 2008;19 (S8):viii190. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Patel PH, Schwartz LH, Baum MS, Motzer RJ. Superior efficacy of sunitinib compared with interferon as first-line therapy in patients with metastatic renal cell carcinoma. American Journal of Hematology/Oncology 2007;6(5):260-4. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Patil S, Figlin RA, Hutson TE, Michaelson D, Negrier S, Kim ST, et al. TWiST analysis to estimate overall benefit for metastatic renal cell carcinoma (mRCC) patients (pts) treated in a phase III trial of sunitinib versus interferon-alfa (IFN-alpha). Journal of Clinical Oncology 2010;28(15 Suppl 1):4594-4594. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Patil S, Figlin RA, Hutson TE, Michaelson MD, Negrier S, Kim ST, et al. Q-TWiST analysis to estimate overall benefit for patients with metastatic renal cell carcinoma treated in a phase III trial of sunitinib vs interferon-alpha. British Journal of Cancer 2012;106(10):1587-90. [ClinicalTrials.gov: NCT00098657/NCT00083889] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering LM, Pyle L, Larkin JM. Sunitinib is superior to interferon alpha with respect to quality of life for patients with renal cell carcinoma. Nature Clinical Practice Oncology 2009;6(1):6-7. [ClinicalTrials.gov: NCT00098657/NCT00083889] [DOI] [PubMed] [Google Scholar]
- Reddy K, Bukowski RM. Phase III study of sunitinib malate (SU11248) versus interferon-α as first-line treatment in patients with metastatic renal cell carcinoma. Clinical Genitourinary Cancer 2006;5(1):23-5. [ClinicalTrials.gov: NCT00098657/NCT00083889] [DOI] [PubMed] [Google Scholar]
- Smith AD, Zhang H, Souza F, Roda M, Penman A. Predicting survival in metastatic RCC on sunitinib using MASS criteria: evaluation of a large multicentered prospective phase III trial. Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):407. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Szczylik C, Negrier S, Oudard S, Figlin RA, Hutson TE, Tomczak P, et al. Overall survival with sunitinib versus interferon-alfa (IFN) as first-line treatment for metastatic renal cell carcinoma (mRCC). European Urology, Supplements 2009;8(4):182. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
- Yang S, Hudes G, DeSouza P, Alemao E, Strahs A, Purvis J. Evaluation of quality of life in patients with advanced renal cell carcinoma treated with temsirolimus vs interferon-alfa: results from a phase III randomized trial. European Journal of Cancer, Supplement 2009;7(2-3):433. [ClinicalTrials.gov: NCT00098657/NCT00083889] [Google Scholar]
NCT00117637 {published data only}
- Escudier B, Szczylik C, Demkow T, Staehler M, Rolland F, Negrier S, et al. Randomized phase II trial of the multi-kinase inhibitor sorafenib versus interferon (IFN) in treatment-naive patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2006;24(18S):4501. [ClinicalTrials.gov: NCT00117637] [DOI] [PubMed] [Google Scholar]
- Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27(8):1280-9. [ClinicalTrials.gov: NCT00117637] [DOI] [PubMed] [Google Scholar]
- Escudier. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27(13):2305. [ClinicalTrials.gov: NCT00117637] [DOI] [PubMed] [Google Scholar]
- Felsch M, Zaim S, Dicken V, Lehmacher W, Scheuring UJ. Comparison of central and local serial CT assessments of metastatic renal cell carcinoma patients in a clinical phase IIB study. Acta Radiologica 2017;58(2):249-55. [ClinicalTrials.gov: NCT00117637] [DOI] [PubMed] [Google Scholar]
- Szczylik C, Demkow T, Staehler M, Rolland F, Negrier S, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon in patients with advanced renal cell carcinoma: final results. Journal of Clinical Oncology: ASCO annual meeting proceedings 2007;25(18 Suppl):Abstract 5025. [ClinicalTrials.gov: NCT00117637] [DOI] [PubMed] [Google Scholar]
NCT00126594 {published data only}
- Sorafenib tosylate with or without recombinant interferon alpha-2b in treating patients with metastatic kidney cancer. https://clinicaltrials.gov/ct2/show/NCT00126594. [ClinicalTrials.gov: NCT00126594]
NCT00334282 {published and unpublished data}
- Cella D, Pickard AS, Duh MS, Guerin A, Mishagina N, Antras L, et al. Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomised phase III trial. European Journal of Cancer 2012;48(3):311-23. [ClinicalTrials.gov: NCT00334282] [DOI] [PubMed] [Google Scholar]
- Pickard AS, Cella D, Duh MS, Guerin A, Mishagina N, Antras L, et al. Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomized phase III trial. In: Journal of Clinical Oncology. Vol. 29. 2011. [ClinicalTrials.gov: NCT00334282] [DOI] [PubMed]
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. Journal of Clinical Oncology 2010;28(6):1061-8. [ClinicalTrials.gov: NCT00334282] [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Hawkins RE, Szczylik C, Davis ID, Wagstaff J, McCann L, et al. A randomized, double-blind phase III study (VEG105192) of pazopanib (paz) versus placebo (pbo) in patients with advanced/metastatic renal cell carcinoma (mRCC): updated safety results. Journal of Clinical Oncology 2011;29(7 Suppl 1):313. [ClinicalTrials.gov: NCT00334282] [Google Scholar]
- Sternberg CN, Hawkins RE, Szczylik C, Davis ID, Wagstaff J, McCann L, et al. Randomized, double-blind phase III study of pazopanib in patients with advanced/metastatic renal cell carcinoma (mRCC): final overall survival (OS) results. Annals of Oncology 2010;8:viii10. [ClinicalTrials.gov: NCT00334282] [Google Scholar]
- Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. European Journal of Cancer 2013;49(6):1287-96. [ClinicalTrials.gov: NCT00334282] [DOI] [PubMed] [Google Scholar]
NCT00420888 {published data only}
- Eisen T, Hedlund G, Forsberg G, Nordle O, Hawkins R. Baseline biomarker trend analysis of a randomized phase 2/3 study of naptumomab estafenatox plus IFN-a vs IFN-a in advanced renal cell carcinoma. European Journal of Cancer 2013;2:S645. [ClinicalTrials.gov: NCT00420888] [Google Scholar]
- Elkord E, Burt DJ, Sundstedt A, Nordle Ö, Hedlund G, Hawkins RE. Immunological response and overall survival in a subset of advanced renal cell carcinoma patients from a randomized phase 2/3 study of naptumomab estafenatox plus IFN-α versus IFN-α. Oncotarget 2015;6(6):4428-39. [ClinicalTrials.gov: NCT00420888] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RE, Gore M, Shparyk Y, Bondar V, Gladkov O, Ganev T, et al. A randomized phase II/III study of naptumomab estafenatox + IFNα versus IFNα in renal cell carcinoma: final analysis with baseline biomarker subgroup and trend analysis. Clinical Cancer Research 2016;22(13):3172-81. [ClinicalTrials.gov: NCT00420888] [DOI] [PubMed] [Google Scholar]
- NCT00420888. ABR-217620/Naptumomab Estafenatox With Interferon-alpha (IFN-alpha) Compared to IFN-alpha Alone in Patients With Advanced Renal Cell Carcinoma. clinicaltrials.gov/show/NCT00420888 first received 11 January 2007. [ClinicalTrials.gov: NCT00420888]
NCT00609401 {published data only}
- Procopio G, Verzoni E, Bracarda S, Conti G, Guadalupi V, Ortega C, et al. Subgroup analysis and updated results of a randomized study comparing sorafenib plus interleukin-2 versus sorafenib alone as first-line treatment in metastatic renal cell carcinoma. Anticancer Research 2010;30(4):1461-2. [ClinicalTrials.gov: NCT00609401] [Google Scholar]
- Procopio G, Verzoni E, Bracarda S, Ricci S, Miceli R, Bertolini A, et al. A randomized, prospective, phase 2 study, with sorafenib (So) and interleukin-2 (IL-2) versus So alone as first line treatment in advanced renal cell cancer (RCC): ROSORC trial. European Journal of Cancer 2009;7(2-3):425. [ClinicalTrials.gov: NCT00609401] [Google Scholar]
- Procopio G, Verzoni E, Bracarda S, Ricci S, Ridolfi L, Sacco C, et al. Overall survival (OS) of sorafenib (So) plus interleukin-2 (IL-2) versus So alone in patients with treatment-naive metastatic renal cell carcinoma (mRCC): final update of the ROSORC trial. Journal of Clinical Oncology 2013;31(6 Suppl 1):356. [ClinicalTrials.gov: NCT00609401] [Google Scholar]
- Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. A randomized, open label, prospective study comparing the association between sorafenib (So) and interleukin-2 (IL-2) versus So alone in advanced untreated renal cell cancer (RCC): ROSORC trial. Journal of Clinical Oncology 2009;27(15S Pt 1):258. [ClinicalTrials.gov: NCT00609401] [Google Scholar]
- Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. Overall survival for sorafenib plus interleukin-2 compared with sorafenib alone in metastatic renal cell carcinoma (mRCC): final results of the ROSORC trial. Annals of Oncology 2013;24(12):2967-71. [ClinicalTrials.gov: NCT00609401] [DOI] [PubMed] [Google Scholar]
- Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. British Journal of Cancer 2011;104(8):1256-61. [ClinicalTrials.gov: NCT00609401] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. Subgroup analysis and updated results of the randomized study comparing sorafenib (So) plus interleukin-2 versus So alone as first-line treatment in metastatic renal cell carcinoma. Journal of Clinical Oncology: ASCO annual meeting proceedings 2010;28(15 Suppl):Abstract 4589. [ClinicalTrials.gov: NCT00609401] [Google Scholar]
- Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, Porta C, et al. Proof of concept of correlation between progression-free survival (PFS) and overall survival (OS) in treatment-naive patients with advanced renal-cell carcinoma (mRCC): final results of a randomized phase II study comparing sorafenib plus interleukin-2 (IL-2) versus sorafenib alone: the ROSORC trial. Journal of Clinical Oncology 2013;31(15 Suppl 1):e15589. [ClinicalTrials.gov: NCT00609401] [Google Scholar]
NCT00619268 {published data only}
- Bay JO, Negrier S, Perol D, Gravis G, Chevreau C, Delva R, et al. Updated results on long-term overall survival (OS) of the French randomized phase II trial TORAVA in metastatic renal cell carcinoma (mRCC) patients. Journal of Clinical Oncology 2012;30(15 Suppl 1):4625. [ClinicalTrials.gov: NCT00619268] [Google Scholar]
- Escudier BJ, Negrier S, Gravis G, Chevreau C, Delva R, Bay J, et al. Can the combination of temsirolimus and bevacizumab improve the treatment of metastatic renal cell carcinoma? Results of the randomized TORAVA phase II trial. Journal of Clinical Oncology: ASCO annual meeting proceedings 2010;28(15 Suppl):Abstract 4516. [ClinicalTrials.gov: NCT00619268] [Google Scholar]
- Negrier S, Gravis G, Perol D, Chevreau C, Delva R, Bay JO, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncology 2011;12(7):673-80. [ClinicalTrials.gov: NCT00619268] [DOI] [PubMed] [Google Scholar]
NCT00631371 {published data only}
- Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer A, Escudier B. Randomized phase IIIb trial of temsirolimus and bevacizumab versus interferon and bevacizumab in metastatic renal cell carcinoma: results from intoract. Annals of Oncology 2012;9:ix14. [ClinicalTrials.gov: NCT00631371] [DOI] [PubMed] [Google Scholar]
- Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. Journal of Clinical Oncology 2014;32(8):752-9. [ClinicalTrials.gov: NCT00631371] [DOI] [PubMed] [Google Scholar]
NCT00719264 {published data only}
- Ravaud A, Bajetta E, Kay AC, Pelov D, Hollaender N, Rouyrre N, et al. Everolimus with bevacizumab versus interferon alfa-2a plus bevacizumab as first-line therapy in patients with metastatic clear cell renal cell carcinoma. In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 28. 2010. [ClinicalTrials.gov: NCT00719264]
- Ravaud A, Barrios CH, Alekseev B, Tay MH, Agarwala SS, Yalcin S, et al. RECORD-2: phase II randomized study of everolimus and bevacizumab versus interferon alpha-2a and bevacizumab as first-line therapy in patients with metastatic renal cell carcinoma. Annals of Oncology 2015;26(7):1378-84. [ClinicalTrials.gov: NCT00719264] [DOI] [PubMed] [Google Scholar]
- Ravaud A, Barrios CH, Alekseev BY, Tay MH, Agarwala SS, Yalcin S, et al. Randomized phase II study of first-line everolimus plus bevacizumab (E+B) versus interferon alpha-2a plus bevacizumab (I+B) in patients (pts) with metastatic renal cell carcinoma (mRCC): Record-2 final overall survival (OS) and safety results. Journal of Clinical Oncology: ASCO annual meeting proceedings 2013;31(15 Suppl 1):4576. [ClinicalTrials.gov: NCT00719264] [Google Scholar]
NCT00720941 {published and unpublished data}
- Beaumont JL, Diaz J, Deen KC, McCann L, Powles T, Hackshaw MD, et al. Q-TWiST analysis of patients with metastatic renal cell carcinoma (mRCC) randomized to pazopanib (PAZ) or sunitinib (SUN). Journal of Clinical Oncology: ASCO annual meeting proceedings 2014;32(15 Suppl 1):4581. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Bjarnason GA, Kollmannsberger C, Ahmad Q, Dezzani L, Elmeliegy M, Han J, et al. Effects of pazopanib (PAZ) and sunitinib (SUN) dose modification on safety and efficacy in patients with metastatic renal cell carcinoma (mRCC) from COMPARZ. Kidney cancer 2018;2:S18. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Bjarnason GA, Kollmannsberger CK, Ahmad Q, Dezzani L, Elmeliegy M, Han J, et al. Effects of pazopanib (PAZ) and sunitinib (SUN) dose modification on safety and efficacy in patients with metastatic renal cell carcinoma (mRCC) from COMPARZ. Journal of Clinical Oncology 2017;35(15 Suppl 1):4574. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Cella D, Hackshaw MD, Diaz J, Huang C, Deen KC, Crescenzo R, et al. Quality of life (QoL) among patients with renal cell carcinoma (RCC) treated with pazopanib versus sunitinib in the COMPARZ study. Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):346. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Guo J, Jin J, Huang Y, Wang JW, Lim HY, Uemura H, et al. Comparison of PFS and safety for Asian compared to North American and European populations in the phase III trial of pazopanib versus sunitinib in patients with treatment-naive RCC (COMPARZ). Journal of Clinical Oncology 2013;31(6 Suppl 1):366. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Guo J, Jin J, Oya M, Uemura H, Takahashi S, Tatsugami K, et al. Safety of pazopanib and sunitinib in treatment-naive patients with metastatic renal cell carcinoma: Asian versus non-Asian subgroup analysis of the COMPARZ trial. Journal of Hematology and Oncology 2018;11(1):69. [ClinicalTrials.gov: NCT00720941] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T, Xu CF, Choueiri TK, Deen KC, Xue Z, Bartlett-Pandite AN, et al. Association of hyperbilirubinemia in pazopanib- or sunitinib-treated patients in COMPARZ with UGT1A1 polymorphisms. Journal of Clinical Oncology: ASCO annual meeting proceedings 2013;31(15 Suppl):4569. [ClinicalTrials.gov: NCT00720941] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattan MW, Sternberg CN, Mehmud F, Bhatt K, Chakrabarti D, McCann L, et al. Development and validation of a prognostic nomogram for progression-free and overall survival in patients with advanced renal cell carcinoma (aRCC) treated with pazopanib. Journal of Clinical Oncology. Conference 2014;32(4 Suppl 1):405. [ClinicalTrials.gov: NCT00720941] [DOI] [PubMed] [Google Scholar]
- Motzer R, Hutson TE, Reeves J, Hawkins R, Guo J, Nathan P, et al. Randomized, open-label, phase III trial of pazopanib versus sunitinib in first-line treatment of patients with metastatic renal cell carcinoma (mRCC): results of the COMPARZ trial. Annals of Oncology 2012;9:ix13. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. New England Journal of Medicine 2013;369(8):722-31. [ClinicalTrials.gov: NCT00720941] [DOI] [PubMed] [Google Scholar]
- Pond GR, Dietrich M, Grunwald V. Prognostic ability of early tumor shrinkage on overall survival (OS) in metastatic renal cell carcinoma (mRCC)-a validation study. Annals of Oncology 2016;27 Suppl 6:VI283. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Sheng X, Jin J, He Z, Huang Y, Zhou A, Wang J, et al. Pazopanib versus sunitinib in Chinese patients with locally advanced or metastatic renal cell carcinoma: pooled subgroup analysis from the randomized, COMPARZ studies. BMC Cancer 2020;20(1):219. [ClinicalTrials.gov: NCT00720941] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X, Jin J, He Z, Huang Y, Zhou AP, Wang J, et al. Efficacy and safety of pazopanib (PAZ) versus sunitinib (SUN) in patients (pts) with locally advanced or metastatic renal cell carcinoma (RCC): a pooled China subgroup analysis from COMPARZ studies. Journal of Clinical Oncology 2018;36(15):e16588. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Tannir NM, Porta C, Grunwald V, Choueiri TK, Ahmad QI, Carrasco-Alfonso MJ, et al. Long-term response and time to response to pazopanib (PAZ) and sunitinib (SUN) in metastatic renal cell carcinoma (mRCC): COMPARZ subanalysis. Kidney Cancer 2018;2 Suppl 1:25-6. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
- Xu CF, Johnson T, Choueiri TK, Deen KC, Xue Z, Spraggs CF, et al. Association of IL8 polymorphisms with overall survival in patients with renal cell carcinoma in COMPARZ (pazopanib versus sunitinib phase III study). Journal of Clinical Oncology: ASCO annual meeting proceedings 2013;31(15 Suppl 1):4519. [ClinicalTrials.gov: NCT00720941] [Google Scholar]
NCT00732914 {published data only}
- Eichelberg C, Vervenne WL, De Santis M, Fischer von Weikersthal L, Goebell PJ, Lerchenmüller C, et al. SWITCH: a randomised, sequential, open-label study to evaluate the efficacy and safety of sorafenib-sunitinib versus sunitinib-sorafenib in the treatment of metastatic renal cell cancer. European Urology 2015;68(5):837-47. [ClinicalTrials.gov: NCT00732914] [DOI] [PubMed] [Google Scholar]
- Fischer Von Weikersthal L, Goebell PJ, De Santis M, Lerchenmuller C, Zimmermann U, Bos MMEM, et al. SWITCH: A randomized sequential open-label study to evaluate efficacy and safety of sorafenib (So) / sunitinib (Su) versus Su/So in the treatment of metastatic renal cell cancer (mRCC). Oncology Research and Treatment 2014;5:5. [ClinicalTrials.gov: NCT00732914] [DOI] [PubMed] [Google Scholar]
- Fischer Von Weikersthal L, Vervenne WL, Goebell PJ, Eichelberg C, Freier W, De Santis M, et al. Phase III randomized sequential open-label study to evaluate efficacy and safety of sorafenib (So) followed by sunitinib (Su) versus sunitinib followed by sorafenib in patients with advanced / metastatic renal cell carcinoma without prior systemic therapy (SWITCH Study)-Safety interim analysis results. Onkologie 2012;6:237. [ClinicalTrials.gov: NCT00732914] [Google Scholar]
- Goebell PJ, Vervenne W, De Santis M, Von Weikersthal LF, Lerchenmuller CA, Zimmermann U, et al. Subgroup analyses of a randomized sequential open-label study (SWITCH) to evaluate efficacy and safety of sorafenib (So)/sunitinib (Su) versus Su/So in the treatment of metastatic renal cell cancer (mRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2014;32(15 Suppl 1):4567. [ClinicalTrials.gov: NCT00732914] [Google Scholar]
- Michel MS, Vervenne W, De Santis M, Von Weikersthal LF, Goebell PJ, Lerchenmueller J, et al. SWITCH: a randomized sequential open-label study to evaluate efficacy and safety of sorafenib (So)/sunitinib (Su) versus Su/So in the treatment of metastatic renal cell cancer (mRCC). Journal of Clinical Oncology 2014;32(4 Suppl 1):393. [ClinicalTrials.gov: NCT00732914] [Google Scholar]
- Michel MS, Vervenne W, Goebell PJ, Von Weikersthal LF, Freier W, De Santis M, et al. Phase III randomized sequential open-label study to evaluate efficacy and safety of sorafenib (So) followed by sunitinib (Su) versus sunitinib followed by sorafenib in patients with advanced/metastatic renal cell carcinoma without prior systemic therapy (SWITCH Study): safety interim analysis results. Journal of Clinical Oncology 2012;30(15 Suppl 1):4539. [ClinicalTrials.gov: NCT00732914] [Google Scholar]
- Retz M, Bedke J, Bögemann M, Grimm MO, Zimmermann U, Müller L, et al. SWITCH II: phase III randomized, sequential, open-label study to evaluate the efficacy and safety of sorafenib-pazopanib versus pazopanib-sorafenib in the treatment of advanced or metastatic renal cell carcinoma (AUO AN 33/11). European Journal of Cancer 2019;107:37-45. [ClinicalTrials.gov: NCT00732914] [DOI] [PubMed] [Google Scholar]
NCT00738530 {published data only}
- Bajetta E, Ravaud A, Bracarda S, Negrier S, Szczylik C, Bellmunt Molins J, et al. Efficacy and safety of first-line bevacizumab (BEV) plus interferon-a2a (IFN) in patients (pts) >65 years with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2008;26(15S pt I):273. [ClinicalTrials.gov: NCT00738530] [Google Scholar]
- Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. Journal of Clinical Oncology 2010;28(13):2144-50. [ClinicalTrials.gov: NCT00738530] [DOI] [PubMed] [Google Scholar]
- Escudier B, Bellmunt J, Negrier S, Melichar B, Bracarda S, Ravaud A, et al. Final results of the phase III, randomized, double-blind AVOREN trial of first-line bevacizumab (BEV) + interferon alfa-2a (IFN) in metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2009;27(15S Pt 1):239. [ClinicalTrials.gov: NCT00738530] [Google Scholar]
- Escudier B, Koralewski P, Pluzanska A, Ravaud A, Bracarda S, Szczylik C, et al. A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-a2a vs placebo/interferon-α2a as first-line therapy in metastatic renal cell carcinoma. Journal of Clinical Oncology: ASCO annual meeting proceedings 2007;25(18 Suppl):Abstract 3. [ClinicalTrials.gov: NCT00738530] [Google Scholar]
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370(9605):2103-11. [ClinicalTrials.gov: NCT00738530] [DOI] [PubMed] [Google Scholar]
- Sommerauer M, Doehn C. Bevacizumab plus interferon alfa-2a in the treatment of metastatic renal cell carcinoma: a randomized, double-blind phase III study. Onkologe 2008;14(5):535-537. [ClinicalTrials.gov: NCT00738530] [Google Scholar]
NCT00903175 {published data only}
- Hsieh J, Chen D, Wang P, Chen Y, Marker M, Patel P, et al. Differential overall survival (OS) results in RECORD-3 study based on three distinct mRCC molecular subgroups classified by BAP1 and/or PBRM1 mutations. Journal of Clinical Oncology 2016;34(2 Suppl 1):561. [ClinicalTrials.gov: NCT00738530] [Google Scholar]
- Hsieh J, Chen D, Wang P, Chen YB, Mahtab M, Pate P, et al. Differential overall survival results in RECORD-3 study based on three distinct clear cell metastatic renal cell carcinoma molecular subgroups classified by BAP1 and/ or PBRM1 mutations. BJU International 2016;118 Suppl 5:10. [ClinicalTrials.gov: NCT00738530] [Google Scholar]
- Hsieh JJ, Chen D, Wang PI, Chen YB, Redzematovic A, Marker M, et al. Identification of efficacy biomarkers in a large metastatic renal cell carcinoma (mRCC) cohort through next-generation sequencing (NGS): results from RECORD-3. BJU International 2015;5:13. [ClinicalTrials.gov: NCT00738530] [Google Scholar]
- Hsieh JJ, Chen D, Wang PI, Marker M, Redzematovic A, Chen YB, et al. Genomic biomarkers of a randomized trial comparing first-line everolimus and sunitinib in patients with metastatic renal cell carcinoma. European Urology 2017;71(3):405-14. [ClinicalTrials.gov: NCT00738530] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JJ, Barrios CH, Kim TM, Cosgriff T, Srimuninnimit V, Pittman KB, et al. Final overall survival analysis for the RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC (mRCC). Journal of Clinical Oncology 2015;33(15 Suppl 1):4554. [ClinicalTrials.gov: NCT00738530] [Google Scholar]
- Knox JJ, Kay AC, Schiff E, Hollaender N, Rouyrre N, Ravaud A, et al. First-line everolimus followed by second-line sunitinib versus the opposite treatment sequence in patients with metastatic renal cell carcinoma (mRCC). In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 28. 2010. [ClinicalTrials.gov: NCT00738530]
- Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff T, Harker WG, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2014;32(25):2765-72. [ClinicalTrials.gov: NCT00738530] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff T, Harker WG, et al. Record-3: phase II randomized trial comparing sequential first-line everolimus (EVE) and second-line sunitinib (SUN) versus first-line SUN and second-line EVE in patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2013;31(15 Suppl 1):4504. [ClinicalTrials.gov: NCT00738530] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00920816 {published data only}
- Hutson TE, Al-Shukri S, Stus VP, Lipatov ON, Shparyk Y, Bair AH, et al. Axitinib versus sorafenib in first-line metastatic renal cell carcinoma: overall survival from a randomized phase III trial. Clinical Genitourinary Cancer 2017;15(1):72-6. [ClinicalTrials.gov: NCT00920816] [DOI] [PubMed] [Google Scholar]
- Hutson TE, Al-Shukri S, Stus VP, Lipatov ON, Shparyk Y, Bair AH, et al. Overall survival analysis from a randomised phase III trial of axitinib vs sorafenib as first-line therapy in patients with metastatic renal cell carcinoma. European Journal of Cancer 2015;51:476-77. [ClinicalTrials.gov: NCT00920816] [Google Scholar]
- Hutson TE, Gallardo J, Lesovoy V, Al-Shukri S, Stus V, Bair AH, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2013;31(6 Suppl 1):1287-94. [ClinicalTrials.gov: NCT00920816] [DOI] [PubMed] [Google Scholar]
- Sheng X, Bi F, Ren X, Cheng Y, Wang J, Rosbrook B, et al. First-line axitinib versus sorafenib in Asian patients with metastatic renal cell carcinoma (mRCC): subgroup analysis of data from a phase III trial. Annals of Oncology 2017;28:x82. [ClinicalTrials.gov: NCT00920816] [DOI] [PubMed] [Google Scholar]
- Sheng X. First-line axitinib versus sorafenib in Asian patients with metastatic renal cell carcinoma: exploratory subgroup analyses of phase III data. Future Oncology 2019;15(1):3267-77. [ClinicalTrials.gov: NCT00920816] [DOI] [PubMed] [Google Scholar]
NCT00979966 {published data only}
- Bergmann L, Grunwald V, Maute L, Grimm MO, Weikert S, Schleicher J, et al. A prospective randomized phase-II trial with temsirolimus vs. sunitinib in non-clear renal cell carcinoma. A study of the CESAR central european society for anticancer drug research-EWIV and interdisciplinary renal cell carcinoma group of the German cancer society (IAGN). European Journal of Cancer 2015;3:517. [ClinicalTrials.gov: NCT00979966] [DOI] [PubMed] [Google Scholar]
- Bergmann L, Grunwald V, Maute L, Grimm MO, Weikert S, Schleicher J, et al. A randomized phase IIa trial with temsirolimus versus sunitinib in advanced non-clear cell renal cell carcinoma: an intergroup study of the CESAR Central European Society for Anticancer Drug Research-EWIV and the Interdisciplinary Working Group on Renal Cell Cancer (IAGN) of the German Cancer Society. Oncology Research and Treatment 2020;43(7-8):333-9. [ClinicalTrials.gov: NCT00979966] [DOI] [PubMed] [Google Scholar]
NCT01024920 {published data only}
- Eisen T, Loembe AB, Shparyk Y, MacLeod N, Jones RJ, Mazurkiewicz M, et al. A randomised, phase II study of nintedanib or sunitinib in previously untreated patients with advanced renal cell cancer: 3-year results. British Journal of Cancer 2015;113(8):1140-7. [ClinicalTrials.gov: NCT01024920] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen T, Shparyk Y, Jones R, MacLeod NJ, Temple G, Finnigan H, et al. Phase II efficacy and safety study of nintedanib versus sunitinib in previously untreated renal cell carcinoma (RCC) patients. Journal of Clinical Oncology 2013;31(15 Suppl 1):4506. [ClinicalTrials.gov: NCT01024920] [Google Scholar]
- Eisen T, Shparyk Y, MacLeod NJ, Wallenstein G, Temple G, Khder Y, et al. Effects of nintedanib (BIBF 1120) on QTc interval in previously untreated patients with renal cell cancer (RCC): results from an open-label, phase II study. In: Journal of Clinical Oncology (Conference). Vol. 30. 2012. [ClinicalTrials.gov: NCT01024920]
- Eisen T, Shparyk Y, Macleod N, Jones R, Wallenstein G, Temple G, et al. Effect of small angiokinase inhibitor nintedanib (BIBF 1120) on QT interval in patients with previously untreated, advanced renal cell cancer in an open-label, phase II study. Investigational New Drugs 2013;31(5):1283-93. [ClinicalTrials.gov: NCT01024920] [DOI] [PubMed] [Google Scholar]
NCT01030783 {published data only}
- Cella D, Ivanescu C, Skaltsa K, Casamayor M, Strahs AL, Esteves B, et al. Treatment benefit of tivozanib hydrochloride versus sorafenib on health-related quality of life (HRQoL) among patients (pts) with advanced/metastatic renal cell carcinoma (mRCC): TIVO-1 study results. Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):355. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Eisen TQG, Sternberg CN, Tomczak P, Harza M, Jinga V, Esteves B, et al. Detailed comparison of the safety of tivozanib versus sorafenib in patients with advanced/metastatic renal cell carcinoma (mRCC) from a phase 3 trial. Annals of Oncology 2012;9:ix262-3. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Hutson T, Nosov D, Tomczak P, Bondarenko I, Lipatov ON, Sternberg CN, et al. Tivozanib vs sorafenib targeted therapy for advanced renal cell carcinoma: final results of a phase III trial (901) and efficacy results of a 2nd line tivozanib extension study (902). Journal of Clinical Oncology: ASCO annual meeting proceedings 2015;33(15 Suppl 1):4557. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Hutson TE, Nosov D, Eisen T, Lipatov ON, Tomczak P, Alyasova A, et al. Subgroup analyses of a phase III trial comparing tivozanib hydrochloride versus sorafenib as initial targeted therapy for patients (pts) with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2013;31(6 Suppl 1):354. [ClinicalTrials.gov: NCT01030783] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson TE, Nosov D, Harza M, Esteves B, Strahs AL, Berkenblit A, et al. Fewer dose adjustments with tivozanib vs. sorafenib in the phase III TIVO-1 study in advanced renal cell carcinoma (RCC). Urologe 2013;52(1 Suppl 1):89-90. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Hutson TE, Nosov D, Harza M, Esteves B, Strahs AL, Berkenblit A, et al. Rates of dose adjustment in patients treated with tivozanib versus sorafenib in the phase III TIVO-1 study. Journal of Clinical Oncology: ASCO annual meeting proceedings 2013;31(15 Suppl 1):4564. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Mehta A, Sonpavde G, Escudier B. Tivozanib for the treatment of renal cell carcinoma: results and implications of the TIVO-1 trial. Future Oncology 2014;10(11):1819-26. [ClinicalTrials.gov: NCT01030783] [DOI] [PubMed] [Google Scholar]
- Motzer R, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a phase III randomized, open-label, multicenter trial. BJU International 2012;2:14. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Motzer R, Nosov D, Eisen T, Lipatov O, Tomczak P, Lyulko O, et al. Tivozanib hydrochloride versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from the phase III randomized, open-label, multicenter TIVO-1 trial. European Urology, Supplements 2012;11(5):185. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Motzer RJ, Eisen T, Hutson TE, Szczylik C, Krygowski M, Strahs AL, et al. Overall survival results from a phase III study of tivozanib hydrochloride versus sorafenib in patients with renal cell carcinoma. Journal of Clinical Oncology 2013;31(6 Suppl 1):350. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. Journal of Clinical Oncology 2013;31(30):3791-9. [ClinicalTrials.gov: NCT01030783] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V. Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a phase III randomized, open-label, multicenter trial. In: Clinical Advances in Hematology & Oncology. Vol. 10. 2012:4-6. [ClinicalTrials.gov: NCT01030783] [DOI] [PMC free article] [PubMed]
- Motzer RJ, Nosov D, Tomczak P, Berkenblit A, Esteves B, Strahs AL, et al. Efficacy and safety data from patients with advanced renal cell cancer treated with tivozanib hydrochloride after progression on sorafenib. Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):364. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Sternberg CN, Eisen T, Tomczak P, Strahs AL, Esteves B, Berkenblit A, et al. Tivozanib in patients treatment-naive for mRCC in the TIVO-1 study. Der Urologe 2013;52(1 Suppl 1):89. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
- Sternberg CN, Eisen T, Tomczak P, Strahs AL, Esteves B, Berkenblit A, et al. Tivozanib in patients treatment-naive for metastatic renal cell carcinoma: a subset analysis of the phase III TIVO-1 study. Journal of Clinical Oncology 2013;31(15 Suppl 1):4513. [ClinicalTrials.gov: NCT01030783] [Google Scholar]
NCT01064310 {published and unpublished data}
- Cella D, Kaiser K, Beaumont J, Diaz J, McCann L, Mehmud F, et al. Quality of life (QoL) among renal cell carcinoma (RCC) patients in a randomized double blind cross-over patient preference study of pazopanib (P) versus sunitinib (S). Annals of Oncology 2012;9:ix261-2. [ClinicalTrials.gov: NCT01064310] [Google Scholar]
- Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. Journal of Clinical Oncology 2014;32(14):1412-8. [DOI] [PubMed] [Google Scholar]
- Lai JS, Beaumont JL, Diaz J, Khan S, Cella D. Validation of a short questionnaire to measure symptoms and functional limitations associated with hand-foot syndrome and mucositis in patients with metastatic renal cell carcinoma. Cancer 2016;122(2):287-95. [DOI] [PubMed] [Google Scholar]
NCT01108445 {published data only}
- Armstrong AJ, Broderick S, Eisen T, Stadler WM, Jones RJ, Garcia JA, et al. Final clinical results of a randomized phase II international trial of everolimus vs. Sunitinib in patients with metastatic non-clear cell renal cell carcinoma (ASPEN). Journal of Clinical Oncology 2015;33(15 Suppl 1):4507. [ClinicalTrials.gov: NCT01064310] [Google Scholar]
- Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncology 2016;17(3):378-88. [ClinicalTrials.gov: NCT01064310] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AJ, Halabi S, Eisen T, Stadler WM, Jones RR, Vaishampayan UN, et al. ASPEN: a randomized phase II trial of everolimus versus sunitinib in patients with metastatic non-clear cell renal cell carcinoma. In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 31. 2013. [ClinicalTrials.gov: NCT01064310]
- Armstrong AJ, Nixon AB, Carmack A, Eisen T, Stadler WM, Jones RJ, et al. Angiokines associated with outcomes after sunitinib or everolimus treatment in patients with non-clear cell renal cell carcinoma. Clinical Cancer Research 2021;16:16. [ClinicalTrials.gov: NCT01064310] [DOI] [PubMed] [Google Scholar]
NCT01274273 {published data only}
- Donskov F, Jensen NV, Smidt-Hansen T, Brondum L, Geertsen PF. A randomized phase II trial of interleukin-2/interferon-alpha plus bevacizumab versus interleukin-2/interferon-alpha in metastatic renal cell carcinoma (mRCC): results from the Danish renal cancer group (DARENCA) study 1. Journal of Clinical Oncology 2016;34(15 Suppl):4563. [ClinicalTrials.gov: NCT01274273] [DOI] [PubMed] [Google Scholar]
- Donskov F, Jensen NV, Smidt-Hansen T, Brøndum L, Geertsen P. A randomized phase II trial of interleukin-2 and interferon-α plus bevacizumab versus interleukin-2 and interferon-α in metastatic renal-cell carcinoma (mRCC): results from the Danish renal cancer group (DaRenCa) study-1. Acta Oncologica 2018;57(5):589-94. [ClinicalTrials.gov: NCT01274273] [DOI] [PubMed] [Google Scholar]
NCT01392183 {published data only}
- Rini BI, Gruenwald V, Fishman MN, Melichar B, Ueda T, Bair AH, et al. Axitinib with or without dose titration for first-line metastatic renal cell carcinoma (mRCC): unblinded results from a randomized phase II study. In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 31. 2013. [ClinicalTrials.gov: NCT01392183]
- Rini BI, Melichar B, Ueda T, Fishman MN, Pithavala Y, Chen Y, et al. Randomised phase II trial of axitinib with or without dose titration in first-line metastatic renal cell carcinoma (mRCC): efficacy and pharmacokinetic (PK) analyses. European Journal of Cancer 2013;2:S657. [ClinicalTrials.gov: NCT01392183] [Google Scholar]
- Tannir NM, Msaouel P, Ross JA, Devine CE, Chandramohan A, Gonzalez GM, et al. Temsirolimus versus pazopanib (TemPa) in patients with advanced clear-cell renal cell carcinoma and poor-risk features: a randomized phase II trial. European Urology Oncology 2019;3(5):687-694. [ClinicalTrials.gov: NCT01392183] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannir NM, Msaouel P, Ross JA, Devine CE, Chandramohan A, Gonzalez GM, et al. Temsirolimus versus pazopanib (TemPa) in patients with advanced clear-cell renal cell carcinoma and poor-risk features: a randomized phase II trial. European Urology Oncology 2020;3(5):687-94. [ClinicalTrials.gov: NCT01392183] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannir NM, Msaouel P, Ross JA, Devine CE, Chandramohan A, Gonzalez GM, et al. Temsirolimus versus pazopanib (TemPa) in patients with advanced clear-cell renal cell carcinoma and poor-risk features: a randomized phase II trial. European Urology Oncology 2020;3(5):687-94. [ClinicalTrials.gov: NCT01392183] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannir NM, Ross JA, Devine CE, Chandramohan A, Wang X, Lim ZD, et al. A randomized phase II trial of pazopanib (PAZ) versus temsirolimus (TEM) in patients (PTS) with advanced clear-cell renal cell carcinoma (aCCRCC) of intermediate and poor-risk (the TemPa trial). Journal of Clinical Oncology 2018;36(6 Suppl 1):583. [ClinicalTrials.gov: NCT01392183] [Google Scholar]
NCT01481870 {published data only}
- Tomita Y, Naito S, Sassa N, Takahashi A, Kondo T, Koie T, et al. Sunitinib versus sorafenib as first-line therapy followed by sorefenib and sunitinib for patients with metastatic renal cell carcinoma (RCC) with clear cell histology: a multicenter randomized trial, CROSS-J-RCC. Journal of Clinical Oncology 2017;35(6):469. [ClinicalTrials.gov: NCT01481870] [Google Scholar]
- Tomita Y, Naito S, Sassa N, Takahashi A, Kondo T, Koie T, et al. Sunitinib versus sorafenib as first-line therapy for patients with metastatic renal cell carcinoma with favorable or intermediate MSKCC risk factors: a multicenter randomized trial, CROSS-J-RCC. Journal of Clinical Oncology 2014;32(4 Suppl 1):502. [ClinicalTrials.gov: NCT01481870] [Google Scholar]
- Tomita Y, Naito S, Sassa N, Takahashi A, Kondo T, Koie T, et al. Sunitinib versus sorafenib as initial targeted therapy for mCC-RCC with favorable/intermediate risk: multicenter randomized trial CROSS-J-RCC. Clinical Genitourinary Cancer 2020;18(4):e374-85. [ClinicalTrials.gov: NCT01481870] [DOI] [PubMed] [Google Scholar]
NCT01613846 {published data only}
- Gschwend J, Beeker A, De Santis M, Brehmer B, Zaiss MR, Schirrmacher-Memmel S, et al. Phase III randomized sequential open-label study to evaluate the efficacy and safety of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the treatment of advanced/metastatic renal cell carcinoma (SWITCH-2 study). In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 31. 2013. [ClinicalTrials.gov: NCT01613846]
- Retz M, Bedke J, Bogemann M, Grimm MO, Zimmermann U, Muller L, et al. SWITCH II: phase III randomized, sequential, open-label study to evaluate the efficacy and safety of sorafenib-pazopanib versus pazopanib-sorafenib in the treatment of advanced or metastatic renal cell carcinoma (AUO AN 33/11). European Journal of Cancer 2019;107:37-45. [ClinicalTrials.gov: NCT01613846] [DOI] [PubMed] [Google Scholar]
- Retz M, Bedke J, Herrmann E, Grimm MO, Zimmermann U, Muller L, et al. Phase III randomized, sequential, open-label study to evaluate the efficacy and safety of sorafenib-pazopanib versus pazopanib-sorafenib in the treatment of metastatic renal cell carcinoma (SWITCH-II). Annals of Oncology 2017;28 Suppl 5:v295. [ClinicalTrials.gov: NCT01613846] [DOI] [PubMed] [Google Scholar]
- Rexer H. First line therapy of advanced or metastasized renal cell carcinoma: open, randomized phase III sequence study to examine the effectiveness and tolerance of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the first-line treatment of patients with advanced or metastasized renal cell carcinoma (SWITCH-2-AN 33/11).. Urologe - Ausgabe A 2012;51(5):724-6. [ClinicalTrials.gov: NCT01613846] [DOI] [PubMed] [Google Scholar]
- Rexer H. First-line therapy of advanced or metastasized renal cell carcinoma: phase III, open, randomized sequence study to examine efficacy and tolerance of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the first-line treatment of patients with advanced or metastasized renal cell carcinoma (SWITCH-2 - AN 33/11). Der Urologe 2014;53(5):735-8. [ClinicalTrials.gov: NCT01613846] [DOI] [PubMed] [Google Scholar]
- Schirrmacher-Memmel S, Retz M, De Santis M, Brehmer B, Zaiss M, Beeker A, et al. Phase III randomized sequential open-label study to evaluate the efficacy and safety of sorafenib followed by pazopanib vs pazopanib followed by sorafenib in the treatment of advanced / metastatic renal cell carcinoma-design of the SWITCH-2 Study. Onkologie 2013;7:75. [ClinicalTrials.gov: NCT01613846] [Google Scholar]
NCT01835158 {published data only}
- Alba SO, Jose MB, Silvia FC. Cabozantinib for the treatment of advanced renal cell carcinoma in treatment-naive adults. European Journal of Clinical Pharmacy 2019;21(3):160-3. [ClinicalTrials.gov: NCT01835158] [Google Scholar]
- Chen RC, Choueiri TK, Feuilly M, Meng J, Lister J, Marteau F, et al. Quality-adjusted survival with first-line cabozantinib or sunitinib for advanced renal cell carcinoma in the CABOSUN randomized clinical trial (Alliance). Cancer 2020;126(24):5311-18. [ClinicalTrials.gov: NCT01835158] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RC, Feuilly M, Meng J, Lister J, Marteau F, Morris MJ, et al. Quality-adjusted time without symptoms or toxicity (Q-TWiST): analysis of cabozantinib (Cabo) vs sunitinib (Sun) in patients with advanced renal cell carcinoma (aRCC) of intermediate or poor risk (Alliance A031203). Journal of Clinical Oncology 2018;36(15 Suppl 1):4556. [ClinicalTrials.gov: NCT01835158] [Google Scholar]
- Choueiri TK, Halabi S, Sanford B, Hahn O, Michaelson MD, Walsh M, et al. CABOzantinib versus SUNitinib (CABOSUN) as initial targeted therapy for patients with metastatic renal cell carcinoma (mRCC) of poor and intermediate risk groups: results from ALLIANCE A031203 trial. Annals of Oncology 2016;27 Suppl 6:vi566. [ClinicalTrials.gov: NCT01835158] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. Journal of Clinical Oncology 2017;35(6):591-7. [ClinicalTrials.gov: NCT01835158] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri TK, Hessel C, Halabi S, Sanford B, Hahn O, Michaelson MD, et al. Progression-free survival (PFS) by independent review and updated overall survival (OS) results from Alliance A031203 trial (CABOSUN): cabozantinib versus sunitinib as initial targeted therapy for patients (pts) with metastatic renal cell carcinoma (mRCC). Annals of Oncology 2017;28 Suppl 5:v623. [ClinicalTrials.gov: NCT01835158] [Google Scholar]
- Choueiri TK, Hessel C, Halabi S, Sanford B, Michaelson MD, Hahn O, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. European Journal of Cancer 2018;94:115-25. [ClinicalTrials.gov: NCT01835158] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DR, Feuilly M, Meng J, Lister J, Marteau F, Morris MJ, et al. ECOG score analysis as a proxy for healthrelated quality of life assessment in patients with poor or intermediate risk metastatic renal cell carcinoma from the CABOSUN trial (Alliance A031203). In: Journal of Clinical Oncology (Conference). Vol. 36. 2018. [ClinicalTrials.gov: NCT01835158]
- George DJ, Hessel C, Halabi S, Michaelson MD, Hahn O, Walsh M, et al. Cabozantinib versus sunitinib for untreated patients with advanced renal cell carcinoma of intermediate or poor risk: subgroup analysis of the Alliance A031203 CABOSUN trial. Oncologist 2019;24(11):1497-1501. [ClinicalTrials.gov: NCT01835158] [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DJ, Hessel C, Halabi S, Sanford BL, Dror Michaelson M, Hahn OM, et al. Cabozantinib versus sunitinib for previously untreated patients with advanced renal cell carcinoma (RCC) of intermediate or poor risk: subgroup analysis of progression-free survival (PFS) and objective response rate (ORR) in the Alliance A031203 CABOSUN trial. Journal of Clinical Oncology 2018;36(6 Suppl 1):582. [ClinicalTrials.gov: NCT01835158] [Google Scholar]
NCT01984242 {published data only}
- Atkins MB, McDermott DF, Powles T, Motzer RJ, Rini BI, Fong L, et al. IMmotion150: a phase II trial in untreated metastatic renal cell carcinoma (mRCC) patients (pts) of atezolizumab (atezo) and bevacizumab (bev) vs and following atezo or sunitinib (sun). Journal of Clinical Oncology 2017;35(15):4505. [ClinicalTrials.gov: NCT01984242] [Google Scholar]
- Grunwald V, McDermott DF, Atkins M, Motzer R, Rini B, Escudier B, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) vs sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). Oncology Research and Treatment 2017;40 Suppl 3:113-4. [ClinicalTrials.gov: NCT01984242] [Google Scholar]
- McDermott DF, Atkins MB, Motzer RJ, Rini BI, Escudier BJ, Fong L, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). Journal of Clinical Oncology 2017;35(6):431. [ClinicalTrials.gov: NCT01984242] [Google Scholar]
- McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nature Medicine 2018;24(6):749-57. [ClinicalTrials.gov: NCT01984242] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Powles T, McDermott D, Rini B, Motzer R, Atkins M, et al. IMmotion150: novel radiological endpoints and updated data from a randomized phase II trial investigating atezolizumab with or without bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma. Kidney Cancer 2018;2 Suppl 1:S22-3. [ClinicalTrials.gov: NCT01984242] [Google Scholar]
- Pal SK, McDermott DF, Atkins MB, Escudier B, Rini BI, Motzer RJ, et al. Patient-reported outcomes (PROs) in IMmotion150: atezolizumab (atezo) alone or with bevacizumab (bev) versus sunitinib (sun) in first-line metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2019;37(15 Suppl):4515. [ClinicalTrials.gov: NCT01984242] [Google Scholar]
- Pal SK, McDermott DF, Atkins MB, Escudier B, Rini BI, Motzer RJ, et al. Patient-reported outcomes in a phase 2 study comparing atezolizumab alone or with bevacizumab vs sunitinib in previously untreated metastatic renal cell carcinoma. BJU International 2020;126(1):73-82. [ClinicalTrials.gov: NCT01984242] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Atkins MB, Escudier B, Motzer RJ, Rini BI, Fong L, et al. Efficacy and safety of atezolizumab plus bevacizumab following disease progression on atezolizumab or sunitinib monotherapy in patients with metastatic renal cell carcinoma in IMmotion150: a randomized phase 2 clinical trial. European Urology 2021;79(5):665-73. [ClinicalTrials.gov: NCT01984242] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, McDermott DF, Rini B, Motzer RJ, Atkins MB, Fong L, et al. IMmotion150: novel radiological endpoints and updated data from a randomized phase II trial investigating atezolizumab (atezo) with or without bevacizumab (bev) vs sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC). Annals of Oncology 2017;28(Suppl 5):v624. [ClinicalTrials.gov: NCT01984242] [Google Scholar]
NCT02231749 {published data only}
- Albiges L, Tannir N, Burotto M, McDermott DF, Plimack ER, Barthelemy P, et al. Nivolumab + ipilimumab (N+I) vs sunitinib (S) for first-line treatment of advanced renal cell carcinoma (aRCC) in CheckMate 214: 4-year follow-up and subgroup analysis of patients (pts) without nephrectomy. Annals of Oncology 2020;31 Suppl 4:S559-60. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. First-line nivolumab plus ipilimumab versus sunitinib in patients without nephrectomy and with an evaluable primary renal tumor in the CheckMate 214 trial. European Urology 2021;81(3):266-271. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020;5(6):e001079. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Camarero J, Hennik PV, Bolstad B, Sommerfelt Gronvold M, Syvertsen C, et al. European Medicines Agency extension of indication to include the combination immunotherapy cancer drug treatment with nivolumab (Opdivo) and ipilimumab (Yervoy) for adults with intermediate/poor-risk advanced renal cell carcinoma. ESMO Open 2020;5(6):e000798. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchoux S, Négrier S, Peretti C, Malcolm B, May J, Marié L, et al. PCN214 cost-effectiveness analysis of nivolumab in combination with ipilimumab versus sunitinib for the first-line treatment of intermediate- to poor-risk advanced renal cell carcinoma in France. In: Value in Health. Vol. 22. 2019:S477. [ClinicalTrials.gov: NCT02231749]
- Cakar E, May J, Malcolm B, Gooden KM, Klijn S. PCN421 stability of lifetime overall survival estimates of nivolumab+ipilimumab IN first-line advanced/metastatic intermediate- or poor-risk renal cell carcinoma. In: Value in Health. Vol. 22. 2019:S517-8. [ClinicalTrials.gov: NCT02231749]
- Cella D, Escudier B, Ivanescu C, Mauer M, Lord-Bessen J, Gooden K. Quality of life in previously untreated patients with advanced renal cell carcinoma (aRCC) in CheckMate 214: updated results. Annals of Oncology 2019;30 Suppl 5:v383-4. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Cella D, Escudier B, Ivanescu C, Maurer M, Lord-Bessen J, Gooden K. Quality of life in previously untreated patients with advanced renal cell carcinoma (aRCC) in CheckMate 214: updated results. Kidney Cancer 2020;4 Suppl 1:S49-50. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Cella D, Escudier B, Saggi SS, Blum S, Ejzykowicz F, Ivanescu C. Time to deterioration in quality of life in previously untreated patients with advanced renal cell carcinoma (aRCC) in CheckMate 214. Annals of Oncology 2020;31 Suppl 4:S562. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Cella D, Grunwald V, Escudier B, Hammers HJ, George S, Nathan P, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncology 2019;20(2):297-310. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Grunwald V, Escudier B, Hammers HJ, George S, Nathan P, et al. Quality of life in patients with advanced renal cell carcinoma in the randomized, open-label CheckMate 214 trial. Journal of Clinical Oncology 2018;36(15):3073. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Characterization of response to nivolumab plus ipilimumab (N1I) or sunitinib (S) in patients (Pts) with previously untreated advanced renal cell carcinoma (aRCC): CheckMate 214. Annals of Oncology 2018;29:viii309-10. [ClinicalTrials.gov: NCT02231749]
- Choueiri TK, Stwalley B, Huo S, May JR, Malcolm B, Nickel K, et al. Comparison of long-term survival and cost-effectiveness (CE) of first-line (1L) treatment options in advanced renal cell carcinoma (aRCC) with intermediate or poor (I/P) prognostic risk. Annals of Oncology 2020;31 Suppl 4:S563-4. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Correction to Lancet Oncol 2019; 20: 1370–85 (The Lancet Oncology (2019) 20(10) (1370–1385), (S1470204519304139), (10.1016/S1470-2045(19)30413-9)). Lancet Oncology 2020;21(6):e304. [ClinicalTrials.gov: NCT02231749] [DOI] [PubMed]
- Correction to Lancet Oncology 2019; 20: 1370-85 (The Lancet Oncology (2019) 20(10) (1370-1385), (S1470204519304139), (10.1016/S1470-2045(19)30413-9)). Lancet Oncology 2020;21(6):e304. [ClinicalTrials.gov: NCT02231749]
- Correction to nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial (The Lancet Oncology (2019) 20(10) (1370-1385), (S1470204519304139), (10.1016/S1470-2045(19)30413-9)). Lancet Oncology 2019;20(10):e559. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed]
- Correction to nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial (The Lancet Oncology (2019) 20(10) (1370-1385), (S1470204519304139), (10.1016/S1470-2045(19)30413-9)). Lancet Oncology 2019;20(10):e559. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed]
- Correction to nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial (The Lancet Oncology (2019) 20(10) (1370–1385), (S1470204519304139), (10.1016/S1470-2045(19)30413-9)). Lancet Oncology 2019;20(10):e559. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed]
- Correction: survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. Journal for Immunotherapy of Cancer 2021;9(5). [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed]
- Di Rienzo P, Zarrelli L, Mennini FS, Marcellusi A, Bini C, Malcolm B et al. PCN170 cost-effectiveness of nivolumab in combination with ipilimumab compared to sunitinib in the first-line treatment of advanced or metastatic intermediate- or poor-risk renal cell carcinoma in Italy. In: Value in Health. Vol. 22. 2019:S469. [ClinicalTrials.gov: NCT02231749]
- Escudier B, Motzer RJ, Tannir NM, Porta C, Tomita Y, Maurer MA, et al. Efficacy of nivolumab plus ipilimumab according to number of IMDC risk factors in CheckMate 214. European Urology 2019;13:13. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Motzer RJ, Tannir NM, Porta C, Tomita Y, Maurer MA, et al. Efficacy of nivolumab plus ipilimumab according to number of IMDC risk factors in CheckMate 214. European Urology 2020;77(4):449-53. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Tannir NM, McDermott DF, Frontera OA, Melichar B, Plimack ER, et al. CheckMate 214: efficacy and safety of nivolumab 1 ipilimumab (N1I) v sunitinib (S) for treatment-naïve advanced or metastatic renal cell carcinoma (mRCC), including IMDC risk and PD-L1 expression subgroups. Annals of Oncology 2017;28:v621-2. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- First-line nivolumab plus ipilimumab is associated with lower costs per responder versus sunitinib among patients with advanced renal cell carcinoma. Journal for Immunotherapy of Cancer 2018;6. [ClinicalTrials.gov: NCT02231749]
- George S, Betts K, Yang S, Du E, Johansen J, Rao S, et al. Nivolumab plus ipilimumab is associated with lower number needed to treat compared with sunitinib for preventing death in advanced renal cell carcinoma. In: Journal for ImmunoTherapy of Cancer. Conference: 33rd Annual Meeting and Pre Conference Programs of the Society for Immunotherapy of Cancer, SITC. Vol. 6 Suppl 1. 2018. [ClinicalTrials.gov: NCT02231749]
- Grimm MO, McDermott DF, Choueiri TK, Motzer RJ, Frontera OA, George S, et al. CheckMate 214 post hoc analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma (aRCC) with sarcomatoid features (sRCC). Oncology Research and Treatment 2020;43:70-1. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Gruenwald V, Cella D, Escudier BJ, Hammers HJ, George S, Nathan P, et al. Quality of life in patients with advanced renal cell carcinoma in the randomized, open-label CheckMate 214 trial. Oncology Research and Treatment 2018;41 Suppl 4:42. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Gruenwald V, Choueiri T, Rini B, Powles T, George S, Grimm MO, et al. Association between depth of response and overall survival: exploratory analysis in patients with previously untreated advanced renal cell carcinoma (aRCC) in CheckMate 214. Kidney Cancer 2020;4:S5-6. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Gruenwald V, Choueiri TK, Rini BI, Powles T, George S, Grimm MO, et al. Association between depth of response (DepOR) and overall survival (OS): exploratory analysis of nivolumab + ipilimumab (N+I) vs sunitinib (S) in patients with previously untreated advanced renal cell carcinoma (aRCC) in CheckMate 214. Oncology Research and Treatment 2020;43:74-5. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Gruenwald V, Choueiri TK, Rini BI, Powles T, George S, Grimm MO, et al. Association between depth of response and overall survival: exploratory analysis in patients with previously untreated advanced renal cell carcinoma (aRCC) in CheckMate 214. Annals of Oncology 2019;30 Suppl 5:v382-3. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Hammers H, Sternberg C, McDermott DF, Larkin J, Ravaud A, Rini B, et al. A phase 3, randomized, openlabel study of nivolumab combined with ipilimumab versus sunitinib monotherapy in subjects with previously untreated metastatic renal cell carcinoma. BJU International 2014;114:9. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Hammers HJ, Plimack ER, Sternberg C, McDermott DF, Larkin JM, Ravaud A, et al. CheckMate 214: a phase III, randomized, open-label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in patients with previously untreated metastatic renal cell carcinoma. In: Journal of Clinical Oncology. Meeting Abstract | 2015 ASCO Annual Meeting I. Vol. 33. 2015. [ClinicalTrials.gov: NCT02231749]
- Jena R. CheckMate 214 trial: immune checkpoint regulators for advanced renal cell carcinoma. Indian Journal of Urology 2020;36(3):231-3. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhdari K, Young KC, Berling M, Gooden KM, Holdgate O. PCN240 evaluating partitioned survival model and response-based modeling approaches for use in cost-effectiveness analysis: estimating and validating survival outcomes. In: Value in Health. Vol. 22. 2019:S101-2. [ClinicalTrials.gov: NCT02231749]
- McDermott DF, Choueiri TK, Motzer RJ, Aren OR, George S, Powles T, et al. CheckMate 214 post-hoc analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features. Journal of Clinical Oncology 2019;37(15):4513. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- McDermott DF, Rini BI, Motzer RJ, Tannir NM, Escudier B, Kollmannsberger CK, et al. Treatment-free interval (TFI) following discontinuation of first-line nivolumab plus ipilimumab (N1I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC): CheckMate 214 analysis. Annals of Oncology 2018;29 Suppl 8:viii309. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- McDermott DF, Rini BI, Motzer RJ, Tannir NM, Escudier B, Kollmannsberger CK, et al. Treatment-free survival (TFS) after discontinuation of first-line nivolumab (NIVO) plus ipilimumab (IPI) or sunitinib (SUN) in intention-to-treat (ITT) and IMDC favorable-risk patients (pts) with advanced renal cell carcinoma (aRCC) from CheckMate 214. Journal of Clinical Oncology 2019;37(7):564. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Motzer RJ, Choueiri TK, May J, Kwon Y, Rusibamayila N, Botteman M, et al. Long-term trend of qualityadjusted time without symptoms or toxicities (Q-TWiST) of nivolumab+ipilimumab (N+I) versus sunitinib (SUN) for the firstline treatment of advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology 2021;39(15 Suppl):6568. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Motzer RJ, Choueiri TK, McDermott DF, Powles T, Yao J, Ammar R, et al. Biomarker analyses from the phase III CheckMate 214 trial of nivolumab plus ipilimumab (N+I) or sunitinib (S) in advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2020;38(15 Suppl):5009. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Motzer RJ, Escudier B, McDermott DF, Aren Frontera O, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. Journal for Immunotherapy of Cancer 2020;8(2):e000891. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Rini BI, McDermott DF, Aren Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncology 2019;20(10):1370-85. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New England Journal of Medicine 2018;378(14):1277-90. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Plimack ER, et al. Nivolumab + ipilimumab (N+I) vs sunitinib (S) for treatment-naive advanced or metastatic renal cell carcinoma (aRCC): results from CheckMate 214, including overall survival by subgroups. In: Journal for ImmunoTherapy of Cancer. Vol. 5. 2017. [ClinicalTrials.gov: NCT02231749]
- NCT02231749. Metastatic renal cell carcinoma (mRCC) patients treated with sunitinib as first-line therapy. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-13004016 first received 24 July 2013. [ClinicalTrials.gov: NCT02231749]
- Oniangue-Ndza C, Gooden KM, May J, Malcolm B, Du EX, Yin L, et al. PCN111 comparison of adverse event costs of nivolumab+ipilimumab versus sunitinib for previously untreated intermediate-/poor-risk advanced renal cell carcinoma in Switzerland. Value in Health 2019;22 Suppl 3:S457. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Paly V, George S, Youn JH, Borrill J, Ejzykowicz F, May JR, et al. POSC321 estimating long-term survivorship rates for previously untreated intermediate or poor (I/P) risk advanced renal cell carcinoma (aRCC) patients treated with nivolumab plus ipilimumab (NIVO+IPI): analyses from the CheckMate 214 trial. Value in Health 2022;25(1 Suppl):S210. [Google Scholar]
- Polanco Sanchez C, Gooden KM, May J, Malcolm B, Du EX, Yin L, et al. PCN115 comparison of adverse event costs of nivolumab+ipilimumab versus sunitinib for previously untreated intermediate-/poor-risk advanced renal cell carcinoma in Spain. Value in Health 2019;22 Suppl 3:S458. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Regan MM, Atkins MB, Powles T, Werner L, Mantia C, Yang S, et al. Treatment-free survival, with and without toxicity, as a novel outcome applied to immuno-oncology agents in advanced renal cell carcinoma. Annals of Oncology 2019;30 Suppl 5:v393-4. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Regan MM, Atkins MB, Powles T, Werner L, Mantia C, Yang S, et al. Treatment-free survival, with and without toxicity, as a novel outcome applied to immuno-oncology agents in advanced renal cell carcinoma. Annals of Oncology 2019;30:v393-4. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Regan MM, Jegede OA, Mantia C, Powles T, Werner L, Huo S, et al. Treatment-free survival, with and without toxicity, after immuno-oncology vs targeted therapy for advanced renal cell carcinoma (aRCC): 42-month results of CheckMate 214. Annals of Oncology 2020;31 Suppl 4:S561. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Regan MM, Jegede OA, Mantia CM, Powles T, Werner L, Motzer RJ, et al. Treatment-free survival after immune checkpoint inhibitor therapy versus targeted therapy for advanced renal cell carcinoma: 42-month results of the CheckMate 214 trial. Clinical Cancer Research 2021;27(24):6687-6695. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexer H. Therapy of untreated local advanced or metastatic renal cell carcinoma: phase III, randomized, open-label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in subjects with previously untreated, local advanced or metastatic renal cell carcinoma (CheckMate 214 - AN 36/15 of the AUO).. Urologe 2015;54(10):1443-5. [ClinicalTrials.gov: NCT02231749] [DOI] [PubMed] [Google Scholar]
- Rini BI, Tannir NM, Escudier B, McDermott DF, Grimm MO, Porta C, et al. Characterization of response to nivolumab plus ipilimumab (N1I) or sunitinib (S) in patients (pts) with previously untreated advanced renal cell carcinoma (aRCC): CheckMate 214. Annals of Oncology 2018;29 Suppl 8:viii309-10. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Rizzo A, Mollica V, Massari F. Re: Nizar M. Tannir, Sabina Signoretti, Toni K. Choueiri, et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res. In press. https://doi.org/10.1158/1078-0432.ccr-20-2063. European Urology Oncology 2020;3(6):804-5. [ClinicalTrials.gov: NCT02231749] [DOI] [PubMed] [Google Scholar]
- Schmidinger M, Shariat SF, Fajkovic H. Dual immune checkpoint inhibition in metastatic renal cell carcinoma: editorial re.: nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced RCC: extended 4-year follow-up of the phase III CheckMate 214 trial. Esmo Open 2021;6(1):100035. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannir NM, Frontera OA, Hammers HJ, Carducci MA, McDermott DF, Salman P, et al. Thirty-month follow-up of the phase III CheckMate 214 trial of first-line nivolumab + ipilimumab (N+I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology 2019;37(7):547. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Tannir NM, Hammers HJ, Amin A, Grimm MO, Rini BI, Mekan S, et al. Characterization of the benefit-risk profile of nivolumab + ipilimumab (N+I) v sunitinib (S) for treatment-naive advanced renal cell carcinoma (aRCC; CheckMate 214). Journal of Clinical Oncology. Conference 2018;36(6 Suppl 1):686. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Tannir NM, McDermott DF, Escudier B, Hammers HJ, Aren OR, Plimack ER, et al. Overall survival and independent review of response in CheckMate 214 with 42-month follow-up: first-line nivolumab + ipilimumab (N+I) versus sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology 2020;38(6):609. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Tannir NM, Motzer RJ, Albiges L, Plimack ER, George S, Powles T, et al. Patterns of progression in patients treated with nivolumab plus ipilimumab (NIVO+IPI) versus sunitinib (SUN) for first-line treatment of advanced renal cell carcinoma (aRCC) in CheckMate 214. Journal of Clinical Oncology. Conference 2021;39(6 Suppl):313. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Tannir NM, Motzer RJ, Plimack ER, McDermott DF, Barthelemy P, Porta C, et al. Outcomes in patients (pts) with advanced renal cell carcinoma (aRCC) who discontinued (DC) first-line nivolumab + ipilimumab (N+I) or sunitinib (S) due to treatment-related adverse events (TRAEs) in CheckMate 214. Journal of Clinical Oncology 2019;37(7):581. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Tannir NM, Signoretti S, Choueiri TK, McDermott DF, Motzer RJ, Flaifel A, et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clinical Cancer Research 2020;27(1):78–86. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannir NM, Signoretti S, Choueiri TK, McDermott DF, Motzer RJ, Flaifel A, et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clinical Cancer Research 2021;27(1):78-86. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Kondo T, Kimura G, Inoue T, Wakumoto Y, Yao M, et al. Nivolumab plus ipilimumab versus sunitinib in previously untreated advanced renal-cell carcinoma: analysis of Japanese patients in CheckMate 214 with extended follow-up. Japanese Journal of Clinical Oncology 2019;50(1):12-9. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Kondo T, Kimura G, Inoue T, Wakumoto Y, Yao M, et al. Nivolumab plus ipilimumab versus sunitinib in previously untreated advanced renal-cell carcinoma: analysis of Japanese patients in CheckMate 214 with extended follow-up. Japanese Journal of Clinical Oncology 2020;50(1):12-9. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas C, Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, et al. Nivolumab + ipilimumab (N+I) vs sunitinib (S) for treatment-naive advanced or metastatic renal cell carcinoma (aRCC): results from CheckMate 214, including overall survival by subgroups. Journal of Oncology Pharmacy Practice 2018;24(5 Suppl 1):17-8. [ClinicalTrials.gov: NCT02231749] [Google Scholar]
- Wan X, Zhang Y, Tan C, Zeng X, Peng L. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncology 2019;5(4):491-6. [ClinicalTrials.gov: NCT02231749] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02420821 {published data only}
- Atkins MB, Rini BI, Motzer RJ, Powles T, McDermott DF, Suarez C, et al. Patient-reported outcomes from the phase III randomized IMmotion151 trial: atezolizumab bevacizumab versus sunitinib in treatment-naive metastatic renal cell carcinoma. Clinical Cancer Research 2020;26(11):2506-14. [ClinicalTrials.gov: NCT02420821] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Motzer RJ, Rini BI, Powles T, McDermott DF, Suarez C, et al. Patient-reported outcomes (PROs) in IMmotion151: atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun) in treatment (tx) naive metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2018;36(15):4511. [ClinicalTrials.gov: NCT02420821] [Google Scholar]
- Grullich C, Motzer RJ, Powles T, Atkins M, Escudier BJ, McDermott D, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). Oncology Research and Treatment 2018;41 Suppl 4:41-2. [ClinicalTrials.gov: NCT02420821] [Google Scholar]
- Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Alekseev BY, et al. Final overall survival and molecular analysis in IMmotion151, apPhase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. In: JAMA Oncology. Vol. 23. 2021:23. [ClinicalTrials.gov: NCT02420821] [DOI] [PMC free article] [PubMed]
- Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2018;36(6):578. [ClinicalTrials.gov: NCT02420821] [Google Scholar]
- Rexer H, Doehn C. First-line treatment for advanced renal cell carcinoma: a phase 3, open-label, randomized study of atezolizumab (Anti-PD-L1-Antibody) in combination with bevacizumab versus sunitinib in patients with untreated advanced renal cell carcinoma ("IMmotion") - AN 37/15 der AUO. Urologe 2016;55(9):1242-3. [ClinicalTrials.gov: NCT02420821] [DOI] [PubMed] [Google Scholar]
- Rini BI, Atkins MB, Escudier B, Powles T, McDermott DF, Alekseev BY, et al. IMmotion151: updated overall survival (OS) and exploratory analysis of the association of gene expression and clinical outcomes with atezolizumab plus bevacizumab vs sunitinib in patients with locally advanced or metastatic renal cell carcinoma (mRCC). In: Cancer Research. Conference: AACR Annual Meeting. Vol. 81. 2021. [ClinicalTrials.gov: NCT02420821]
- Rini BI, Motzer RJ, Powles T, McDermott DF, Escudier B, Donskov F, et al. Atezolizumab (atezo) + bevacizumab (bev) versus sunitinib (sun) in pts with untreated metastatic renal cell carcinoma (mRCC) and sarcomatoid (sarc) histology: iMmotion151 subgroup analysis. Journal of Clinical Oncology 2019;37(15):4512. [ClinicalTrials.gov: NCT02420821] [Google Scholar]
- Rini BI, Motzer RJ, Powles T, McDermott DF, Escudier B, Donskov F, et al. Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: a prespecified subgroup analysis of the IMmotion151 clinical trial. European Urology 2020;79(5):659-662. [ClinicalTrials.gov: NCT02420821] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019;393(10189):2404-15. [ClinicalTrials.gov: NCT02420821] [DOI] [PubMed] [Google Scholar]
NCT02684006 {published data only}
- Albiges L, Rini BI, Haanen JB, Motzer RJ, Kollmannsberger CK, Negrier S, et al. Primary renal tumour shrinkage in patients (pts) who did not undergo upfront cytoreductive nephrectomy (uCN): subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab 1 axitinib (A 1 Ax) vs sunitinib (S) for advanced renal cell carcinoma (aRCC). Annals of Oncology 2019;30 Suppl 5:v359-60. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Bilen MA, Rini BI, Voss MH, Larkin J, Haanen JB, Albiges L, et al. Association of neutrophil-to-lymphocyte ratio with efficacy of first-line avelumab plus axitinib vs. sunitinib in patients with advanced renal cell carcinoma enrolled in the phase 3 JAVELIN renal 101 trial. Clinical Cancer Research 2021;17:17. [ClinicalTrials.gov: NCT02684006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri TK, Larkin JM, Pal SK, Motzer RJ, Venugopal B, Alekseev BY, et al. Efficacy and biomarker analysis of patients (pts) with advanced renal cell carcinoma (aRCC) with sarcomatoid histology (sRCC): subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab plus axitinib (A + Ax) vs sunitinib (S). Annals of Oncology 2019;30 Suppl 5:v361. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Choueiri TK, Motzer RJ, Campbell MT, Alekseev BY, Uemura M, Kollmannsberger CK, et al. Subgroup analysis from JAVELIN renal 101: outcomes for avelumab plus axitinib (A + Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology 2019;37(7):544. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, et al. Updated efficacy results from the JAVELIN renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Annals of Oncology 2020;31(8):1030-1039. [ClinicalTrials.gov: NCT02684006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri TK, Rini BI, Cosgriff T, Miyake H, Vogelzang NJ, Kannourakis G, et al. JAVELIN renal 101: a phase 3 study of avelumab in combination with axitinib vs sunitinib as first-line treatment for patients with advanced renal cell carcinoma (aRCC). BJU International 2016;118 Suppl 5:29-30. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Choueiri TK, Rini BI, Larkin JM, Bjarnason GA, Gravis G, Gurney H, et al. Avelumab plus axitinib vs sunitinib as first-line treatment of advanced renal cell carcinoma: phase 3 study (JAVELIN renal 101). Journal of Clinical Oncology 2017;35(15). [ClinicalTrials.gov: NCT02684006]
- Haanen J. Efficacy of avelumab + axitinib vs sunitinib by IMDC risk group in advanced renal cell carcinoma (aRCC): extended follow-up results from JAVELIN renal 101. Asia-Pacific Journal of Clinical Oncology 2021;17Suppl 7:51. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Haanen JB, Larkin J, Choueiri TK, Albiges L, Rini BI, Atkins MB, et al. Efficacy of avelumab + axitinib (A + Ax) versus sunitinib (S) by IMDC risk group in advanced renal cell carcinoma (aRCC): extended follow-up results from JAVELIN renal 101. Journal of Clinical Oncology 2021;39(15 Suppl):4574. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Jonasch E, Hasanov E, Motzer RJ, Hariharan S, Choueiri TK, Huang B, et al. Evaluation of brain metastasis in JAVELIN renal 101: efficacy of avelumab + axitinib (A+Ax) versus sunitinib (S). Journal of Clinical Oncology 2020;38(6):687. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Motzer RJ, Choueiri T, Larkin J, Albiges L, Haanen JB, Schmidinger M, et al. Phase 3 study of avelumab in combination with axitinib versus sunitinib as first-line treatment for patients with advanced renal cell carcinoma (aRCC). Annals of Oncology 2016;27(6):vi293. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New England Journal of Medicine 2019;380(12):1103-15. [ClinicalTrials.gov: NCT02684006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Penkov K, Haanen JB, Rini BI, Albiges L, Campbell MT, et al. JAVELIN renal 101: a randomized, phase III study of avelumab + axitinib vs sunitinib as first-line treatment of advanced renal cell carcinoma (aRCC). Annals of Oncology 2018;29 Suppl 8:viii724. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN renal 101 trial. Nature Medicine 2020;26(11):1733-41. [ClinicalTrials.gov: NCT02684006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Larkin J, Venugopal B, Haanen JB, Kanayama HO, Eto M, et al. Association of c-reactive protein (CRP) with efficacy of avelumab + axitinib (A + Ax) in advanced renal cell carcinoma (aRCC): long-term follow-up results from JAVELIN renal 101. Journal of Clinical Oncology 2021;39(15 Suppl):4548. [ClinicalTrials.gov: NCT02684006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Motzer RJ, Choueiri TK, Rini BI, Miyake H, Oya M, et al. Efficacy of avelumab plus axitinib (A + Ax) versus sunitinib (S) by number of IMDC risk factors and tumor sites at baseline in advanced renal cell carcinoma (aRCC): extended follow-up results from JAVELIN renal 101. Journal of Clinical Oncology. Conference 2021;39(6 Suppl):302. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Tomita Y, Motzer RJ, Choueiri TK, Rini BI, Miyake H, Uemura H, et al. Efficacy and safety of avelumab plus axitinib (A + Ax) versus sunitinib (S) in elderly patients with advanced renal cell carcinoma (aRCC): extended follow-up results from JAVELIN renal 101. Journal of Clinical Oncology. Conference 2021;39(6 Suppl):301. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Uemura M, Tomita Y, Miyake H, Hatakeyama S, Kanayama HO, Numakura K, et al. Avelumab plus axitinib vs sunitinib for advanced renal cell carcinoma: Japanese subgroup analysis from JAVELIN renal 101. Cancer Science 2019;28:28. [ClinicalTrials.gov: NCT02684006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Tomita Y, Miyake H, Hatakeyama S, Kanayama HO, Numakura K, et al. Avelumab plus axitinib vs sunitinib for advanced renal cell carcinoma: Japanese subgroup analysis from JAVELIN renal 101. Cancer Science 2020;111(3):907-23. [ClinicalTrials.gov: NCT02684006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Tomita Y, Miyake H, Hatakeyama S, Kanayama HO, Numakura K, et al. Randomized phase III trial of avelumab + axitinib vs sunitinib as firstline treatment for advanced renal cell carcinoma: JAVELIN renal 101 Japanese subgroup analysis. Annals of Oncology 2019;30 Suppl 5:v386-7. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
- Voss MH, Motzer RJ, Albiges L, Rini BI, Schmidinger M, Larkin J, et al. Depth of response (DEPOR) analysis and correlation with clinical outcomes from JAVELIN renal 101. Oncology Research and Treatment 2020;43:225. [ClinicalTrials.gov: NCT02684006] [Google Scholar]
NCT02761057 {published data only}
- NCT02761057. Cabozantinib s-malate, crizotinib, volitinib, or sunitinib malate in treating patients with locally advanced or metastatic kidney cancer. clinicaltrials.gov/show/NCT02761057 first received 4 May 2016. [ClinicalTrials.gov: NCT02761057]
- Pal SK, Tangen C, Thompson IM, Balzer-Haas N, George DJ, Heng DY, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 2021;397(10275):695-703. [ClinicalTrials.gov: NCT02761057] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal SK, Tangen C, Thompson IM, Haas NB, George DJ, Heng DY, et al. Sunitinib versus cabozantinib, crizotinib or savolitinib in metastatic papillary renal cell carcinoma (pRCC): results from the randomized phase II SWOG 1500 study. Journal of Clinical Oncology 2021;39(6 Suppl):270. [ClinicalTrials.gov: NCT02761057] [Google Scholar]
- Pal SK, Tangen C, Thompson IM, Haas NB, George DJ, Heng DY, et al. Sunitinib versus cabozantinib, crizotinib or savolitinib in metastatic papillary renal cell carcinoma (pRCC): results from the randomized phase II SWOG 1500 study. Journal of Clinical Oncology 2021;39(6 Suppl):270. [ClinicalTrials.gov: NCT02761057] [Google Scholar]
- Pal SK, Tangen CM, Thompson IM, Shuch BM, Haas NB, George DJ, et al. A randomized, phase II efficacy assessment of multiple MET kinase inhibitors in metastatic papillary renal carcinoma (PRCC): SWOG S1500. In: Journal of Clinical Oncology (Conference). Vol. 35. 2017. [ClinicalTrials.gov: NCT02761057]
NCT02811861 {published data only}
- Choueiri TK, Eto M, Kopyltsov E, Rha SY, Porta CG, Motzer R, et al. Phase III CLEAR trial in advanced renal cell carcinoma (aRCC): outcomes in subgroups and toxicity update. Annals of Oncology 2021;32 Suppl 5:S683-5. [ClinicalTrials.gov: NCT02811861] [Google Scholar]
- Gruenwald V, Powles T, Choueiri TK, Hutson TE, Porta C, Eto M, et al. Lenvatinib plus everolimus or pembrolizumab versus sunitinib in advanced renal cell carcinoma: study design and rationale. Future Oncology 2019;15(9):929-41. [ClinicalTrials.gov: NCT02811861] [DOI] [PubMed] [Google Scholar]
- Gruenwald V, Powles T, Kopyltsov E, Kozlov V, Alonso Gordoa T, Eto M, et al. Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): depth of response and efficacy for selected subgroups in the lenvatinib (L) + pembrolizumab (P) and sunitinib (S) treatment arms. Oncology Research and Treatment 2021;44 Suppl 2:25. [ClinicalTrials.gov: NCT02811861] [Google Scholar]
- Gruenwald V, Powles T, Kopyltsov E, Kozlov V, Gordoa TA, Eto M, et al. Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): depth of response and efficacy for selected subgroups in the lenvatinib (LEN) + pembrolizumab (PEMBRO) and sunitinib (SUN) treatment arms. Journal of Clinical Oncology 2021;39(15 Suppl):4560. [ClinicalTrials.gov: NCT02811861] [Google Scholar]
- Hutson TE, Choueiri TK, Motzer RJ, Rha SY, Alyasova A, Merchan JR, et al. Post hoc analysis of the CLEAR study in advanced renal cell carcinoma (RCC): effect of subsequent therapy on survival outcomes in the lenvatinib (LEN) + everolimus (EVE) versus sunitinib (SUN) treatment arms. Journal of Clinical Oncology 2021;39(15 Suppl):4562. [ClinicalTrials.gov: NCT02811861] [Google Scholar]
- Motzer RJ, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. New England Journal of Medicine 2021;13:13. [ClinicalTrials.gov: NCT02811861] [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Gruenwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase 3 trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab versus sunitinib alone in first-line treatment of patients with metastatic renal cell carcinoma. In: Journal of Clinical Oncology (Conference). Vol. 36. 2018. [ClinicalTrials.gov: NCT02811861]
- Motzer RJ, Gruenwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase 3 trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab vs sunitinib alone in first-line treatment of patients with metastatic renal cell carcinoma. Kidney Cancer 2018;2 Suppl 1:S2-3. [ClinicalTrials.gov: NCT02811861] [Google Scholar]
- Motzer RJ, Gruenwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase III trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab vs sunitinib alone in first-line treatment of patients (pts) with metastatic renal cell carcinoma (RCC). In: Journal of Clinical Oncology (Conference). Vol. 35. 2017. [ClinicalTrials.gov: NCT02811861]
- Motzer RJ, Gruenwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase III trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab vs sunitinib alone in first-line treatment of patients with metastatic renal cell carcinoma (RCC). Asia-Pacific Journal of Clinical Oncology 2017;13:169. [ClinicalTrials.gov: NCT02811861] [Google Scholar]
- Motzer RJ, Porta C, Alekseev B, Rha SY, Choueiri TK, Mendez-Vidal MJ, et al. Health-related quality-of-life (HRQoL) analysis from the phase 3 CLEAR trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) for patients (pts) with advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology 2021;39(15 Suppl):4502. [ClinicalTrials.gov: NCT02811861] [Google Scholar]
- Motzer RJ, Porta C, Eto M, Powles T, Gruenwald V, Hutson TE, et al. Phase 3 trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) monotherapy as a first-line treatment for patients (pts) with advanced renal cell carcinoma (RCC) (CLEAR study). Journal of Clinical Oncology 2021;39(6 Suppl):269. [ClinicalTrials.gov: NCT02811861] [Google Scholar]
NCT02853331 {published data only}
- Correction to Lancet Oncol 2020; 21: 1563–73 (The Lancet Oncology (2020) 21(12) (1563–1573), (S1470204520304368), (10.1016/S1470-2045(20)30436-8)). Lancet Oncology 2020;21(12):e553. [ClinicalTrials.gov: NCT02853331]
- Gafanov R, Powles TB, Bedke J, Stus V, Waddell TS, Nosov D, et al. Subsequent therapy following pembrolizumab + axitinib or sunitinib treatment for advanced renal cell carcinoma (RCC) in the phase III KEYNOTE-426 study. Annals of Oncology 2021;32 Suppl 5:S694. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Kondoh CN, Bae WK, Tamada S, Matsubara N, Lee HJ, Mizuno R, et al. Pembrolizumab plus axitinib (pembro + axi) vs sunitinib in metastatic renal cell carcinoma (mRCC) outcomes of the KEYNOTE-426 study in patients from eastern Asia. Annals of Oncology 2020;31(Suppl 6):1319. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Plimack ER, Powles T, Bedke J, Pouliot F, Stus V, Waddell T, et al. Outcomes for patients in the pembrolizumab+axitinib arm with advanced renal cell carcinoma (RCC) who completed two years of treatment in the phase III KEYNOTE-426 study. Journal of Clinical Oncology 2021;39(6 Suppl):327. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Plimack ER, Rini BI, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy formetastatic renal cell carcinoma: outcomes in the combined IMDC intermediate-/poor-risk and sarcomatoid subgroups of the phase 3 Keynote-426 study. Asia-Pacific Journal of Clinical Oncology 2019;15:33-4. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Plimack ER, Rini BI, Stus V, Gafanov R, Waddell T, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced renal cell carcinoma (RCC): updated analysis of KEYNOTE-426. Journal of Clinical Oncology 2020;38(15):5001. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Powles T, Plimack ER, Soulieres D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncology 2020;21(12):1563-73. [ClinicalTrials.gov: NCT02853331] [DOI] [PubMed] [Google Scholar]
- Powles T, Plimack ER, Stus V, Gafanov RA, Hawkins RE, Nosov D, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for locally advanced or metastatic renal cell carcinoma (mRCC): phase III KEYNOTE-426 study. Journal of Clinical Oncology 2019;37(7):543. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Rexer H, Bedke J. First-line therapy in advanced renal cell carcinoma: a randomized, open-label phase III study evaluating the efficacy and safety of pembrolizumab (MK-3475) in combination with axitinib compared to sunitinib monotherapy as first-line treatment for locally advanced or metastatic renal cell carcinoma (mRCC) (Keynote-426) - AN 39/16 of the AUO. Urologe 2017;56(3):385-6. [ClinicalTrials.gov: NCT02853331] [DOI] [PubMed] [Google Scholar]
- Rini BI, Atkins MB, Plimack ER, Soulieres D, McDermott RS, Bedke J, et al. Characterization and management of treatment-emergent hepatic toxicity in patients with advanced renal cell carcinoma receiving first-line pembrolizumab plus axitinib. Results from the KEYNOTE-426 trial. European Urology Oncology 2021;5(2):225-234. [ClinicalTrials.gov: NCT02853331] [DOI] [PubMed] [Google Scholar]
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. Journal of Clinical Oncology 2019;37(15 Suppl):4500. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New England Journal of Medicine 2019;380(12):1116-27. [ClinicalTrials.gov: NCT02853331] [DOI] [PubMed] [Google Scholar]
- Rini BI, Plimack ER, Stus V, Waddell T, Gafanov R, Pouliot F, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. Journal of Clinical Oncology 2021;39(15 Suppl):4500. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Rini BI, Powles T, Chen M, Puhlmann M, Atkins MB. Phase 3 KEYNOTE-426 trial: pembrolizumab (pembro) plus axitinib versus sunitinib alone in treatment-naive advanced/metastatic renal cell carcinoma (mRCC). In: Journal of Clinical Oncology (Conference). Vol. 35. 2017. [ClinicalTrials.gov: NCT02853331]
- Rini BI, Powles T, Chen M, Song Y, Puhlmann M, Atkins MB. KEYNOTE-426: randomized phase III study of pembrolizumab in combination with axitinib versus sunitinib monotherapy in treatment-naive advanced/metastatic renal cell carcinoma (mRCC). In: Journal for ImmunoTherapy of Cancer. Conference: 31st Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer, SITC. Vol. 4 Suppl 1. 2016. [ClinicalTrials.gov: NCT02853331]
- Soulieres D, Plimack ER, Rini BI, Stus V, Gafanov R, Hawkins R, et al. Pembrolizumab plus axitinib vs sunitinib as first-line therapy for metastatic renal cell carcinoma: outcomes by IMDC risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. Kidney Cancer 2020;4 Suppl 1:40. [ClinicalTrials.gov: NCT02853331] [Google Scholar]
- Tamada S, Kondoh C, Matsubara N, Mizuno R, Kimura G, Anai S, et al. Pembrolizumab plus axitinib versus sunitinib in metastatic renal cell carcinoma: outcomes of Japanese patients enrolled in the randomized, phase III, open-label KEYNOTE-426 study. International Journal of Clinical Oncology 2021;27(1):154-64. [ClinicalTrials.gov: NCT02853331] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada S, Kondoh C, Matsubara N, Mizuno R, Kimura G, Anai S, et al. Pembrolizumab plus axitinib versus sunitinib in metastatic renal cell carcinoma: outcomes of Japanese patients enrolled in the randomized, phase III, open-label KEYNOTE-426 study. International Journal of Clinical Oncology 2022;27(1):154-64. [ClinicalTrials.gov: NCT02853331] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhang T, Wan N, Liang Z, Li J, Chen X, et al. Cost-effectiveness of pembrolizumab plus axitinib as first-line therapy for advanced renal cell carcinoma. Immunotherapy 2020;12(17):1237-46. [ClinicalTrials.gov: NCT02853331] [DOI] [PubMed] [Google Scholar]
NCT03141177 {published data only}
- Apolo AB, Powles T, Burotto M, Bourlon MT, Hsieh JJ, Basso U, et al. Nivolumab plus cabozantinib (N+C) versus sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. Journal of Clinical Oncology 2021;39(15 Suppl):4553. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
- Basso U, Powles T, Burotto M, Bourlon MT, Hsieh JJ, Shah AY, et al. Nivolumab plus cabozantinib (N+C) vs sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. Tumori Journal 2021;107(2 Suppl):42-3. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
- Bedke J, Powles T, Burotto M, Bourlon MT, Hsieh JJ, Basso U, et al. Nivolumab plus cabozantinib (N+C) vs sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. Oncology Research and Treatment 2021;44 Suppl 2:24-5. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
- Cella D, Choueiri TK, Blum SI, Ejzykowicz F, Hamilton M, Zhang J, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma (aRCC) treated with first-line nivolumab plus cabozantinib versus sunitinib: the CheckMate 9ER trial. Journal of Clinical Oncology 2021;39(6 Suppl):285. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
- Cella D, Motzer RJ, Suarez C, Blum SI, Ejzykowicz F, Hamilton M, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncology 2022;23(2):292-303. [ClinicalTrials.gov: NCT03141177] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri TK, Apolo AB, Powles T, Escudier B, Aren OR, Shah A, et al. A phase 3, randomized, open-label study of nivolumab combined with cabozantinib vs sunitinib in patients with previously untreated advanced or metastatic renal cell carcinoma (RCC; CheckMate 9ER). In: Journal of Clinical Oncology. Meeting Abstract | 2018 ASCO Annual Meeting I. Vol. 36. 2018. [ClinicalTrials.gov: NCT03141177]
- Choueiri TK, Powles T, Burotto M, Bourlon MT, Zurawski B, Oyervides Juarez VM, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase III CheckMate 9ER trial. Annals of Oncology 2020;31 Suppl 4:1159. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
- Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. New England Journal of Medicine 2021;384:829-41. [ClinicalTrials.gov: NCT03141177] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kfoury M, Oing C. ESMO20 YO for YO: highlights on metastatic renal cell carcinoma-the CheckMate-9ER trial. ESMO Open 2021;6(1):100025. [ClinicalTrials.gov: NCT03141177] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro G. Nivolumab/cabozantinib combo impresses as frontline RCC treatment: combination reduces risk of disease progression or death by 49% versus sunitinib. Urology Times 2020;48(11):32. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
- Motzer RJ, Choueiri TK, Powles T, Burotto M, Bourlon MT, Hsieh JJ, et al. Nivolumab + cabozantinib (NIVO+CABO) versus sunitinib (SUN) for advanced renal cell carcinoma (aRCC): outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. Journal of Clinical Oncology 2021;39(6 Suppl):308. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
- Oing C, Bokemeyer C. Increasing combination possibilities for first-line treatment of metastatic clear cell renal cell carcinoma with nivolumab plus cabozantinib: Results of the CheckMate-9ER trial.. Onkologe 2021;27(2):168-71. [ClinicalTrials.gov: NCT03141177] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pook D, Powles T, Burotto M, Bourlon MT, Hsieh JJ, Basso U, et al. Nivolumab plus cabozantinib (N+C) vs sunitinib for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. Asia-Pacific Journal of Clinical Oncology 2021;17 Suppl 9:131. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
- Shah AY, Motzer RJ, Apolo AB, Powles T, Escudier B, Zhang J, et al. Cabozantinib (C) exposureresponse (ER) analysis for the phase 3 CheckMate 9ER (CM 9ER) trial of nivolumab plus cabozantinib (N+C) versus sunitinib (S) in first-line advanced renal cell carcinoma (1L aRCC). Journal of Clinical Oncology 2021;39(15 Suppl):4561. [ClinicalTrials.gov: NCT03141177] [Google Scholar]
References to studies excluded from this review
Aass 2005 {published data only}
- Aass N, De Mulder PH, Mickisch GH, Mulders P, Oosterom AT, Poppel H, et al. Randomized phase II/III trial of interferon alfa-2a with and without 13-cis-retinoic acid in patients with progressive metastatic renal cell carcinoma: the European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group (EORTC 30951). Journal of Clinical Oncology 2005;23(18):4172-8. [DOI] [PubMed] [Google Scholar]
Abdel 2018 {published data only}
- Abdel Hakim AM, Nouira Y, Taran R, Amokrane D, Ghosn M, Larbaoui B, et al. Everolimus as first-line or after cytokine therapy in patients with metastatic recurrent and/or unresectable renal cell carcinoma (RCC) (EVERMORE). Annals of Oncology 2018;29 Suppl 8:viii316. [Google Scholar]
Adler 1987 {published data only}
- Adler A, Gillon G, Lurie H, Shaham J, Loven D, Shachter Y, et al. Active specific immunotherapy of renal cell carcinoma patients: a prospective randomized study of hormono-immuno- versus hormonotherapy. Preliminary report of immunological and clinical aspects. Journal of Biological Response Modifiers 1987;6(6):610-24. [PubMed] [Google Scholar]
Amin 2018 {published data only}
- Amin A, Plimack ER, Ernstoff MS, Lewis LD, Bauer TM, McDermott DF, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. Journal for ImmunoTherapy of Cancer 2018;6(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amin 2018a {published data only}
- Amin A, Plimack ER, Lewis LD, Ernstoff MS, Bauer TM, McDermott DF, et al. Updated results from a phase I study of nivolumab in combination with sunitinib or pazopanib in metastatic renal cell carcinoma: the CheckMate 016 study. Kidney Cancer 2018;2 Suppl 1:S45-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 1991 {published data only}
Atkins 1993 {published data only}
- Atkins MB, Sparano J, Fisher RI, Weiss GR, Margolin KA, Fink KI, et al. Randomized phase II trial of high-dose interleukin-2 either alone or in combination with interferon alfa-2b in advanced renal cell carcinoma. Journal of Clinical Oncology 1993;11(4):661-70. [DOI] [PubMed] [Google Scholar]
Atzpodien 1997 {published data only}
- Atzpodien J, Kirchner H, Franzke A, Wandert T, Probst M, Buer J, et al. Results of a randomized clinical trial comparing SC interleukin-2, SC alpha-2a-interferon, and IV bolus 5-fluorouracil against oral tamoxifen in progressive metastatic renal cell carcinoma patients. In: Proceedings of The American Society of Clinical Oncology. Vol. 16. 1997:326a, Abstract 1164.
Atzpodien 1997a {published data only}
- Atzpodien J, Kirchner H, Franzke A. A randomized clinical trial comparing sc interleukin-2, sc alpha-2a-interferon, and iv bolus 5-flurouracil against oral tamoxifen in progressive metastatic renal cell carcinoma patients. European Journal of Cancer 1997;33:S8, A151. [Google Scholar]
Atzpodien 1999 {published data only}
- Atzpodien J, Kirchner H, Bergmann L, Oberneder R, Jonas U, Ganser A. 13cis-retinoic acid, IFN-alpha, IL-2 and chemotherapy in advanced renal cell carcinoma: results of a prospectively randomized trial of the German Cooperative Renal Carcinoma Immunotherapy Group (DGCIN). In: Proceedings of The American Society of Clinical Oncology. Vol. 18. 1999:448a, Abstract 1727.
Atzpodien 2001 {published data only}
- Atzpodien J, Kirchner H, Illiger HJ, Metzner B, Ukena D, Schott H, et al. IL-2 in combination with IFN- alpha and 5-FU versus tamoxifen in metastatic renal cell carcinoma: long-term results of a controlled randomized clinical trial. British Journal Of Cancer 2001;85(8):1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atzpodien 2004 {published data only}
- Atzpodien J, Kirchner H, Jonas U, Bergmann L, Schott H, Heynemann H, et al. Interleukin-2- and interferon alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Journal of Clinical Oncology 2004;22(7):1188-94. [DOI] [PubMed] [Google Scholar]
Atzpodien 2006 {published data only}
- Atzpodien J, Kirchner H, Rebmann U, Soder M, Gertenbach U, Siebels M, et al. Interleukin-2/interferon-alpha2a/13-retinoic acid-based chemoimmunotherapy in advanced renal cell carcinoma: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). British Journal Of Cancer 2006;95(4):463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrios 2009 {published data only}
- Barrios CH, Hernandez-Barajas D, Brown MP, Lee SH, Fein L, Liu JH, et al. Phase II trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma (mRCC): preliminary results. European Journal of Cancer, Supplement 2009;7(2-3):429-30. [DOI] [PubMed] [Google Scholar]
Beaumont 2009 {published data only}
- Beaumont J, Cella D, Hutson T, Bracarda S, Grunwald V, Thompson J, et al. Patient-reported outcomes in a randomized trial of everolimus with metastatic renal cell carcinoma patients. Journal of Clinical Oncology 2009;1:e17516. [Google Scholar]
Beaumont 2011 {published data only}
- Beaumont JL, Butt Z, Baladi J, Motzer RJ, Haas T, Hollaender N, et al. Patient-reported outcomes in a phase III study of everolimus versus placebo in patients with metastatic carcinoma of the kidney that has progressed on vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy. Oncologist 2011;16(5):632-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 1998 {published data only}
- Berg WJ, Bukowski R, Thompson JA, Nemunaitis J, Murphy B, Ellerhorst J, et al. A randomized phase II trial of recombinant human interleukin-12 (IL-12) versus interferon alpha-2A (IFN) in advanced renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. Vol. 17. 1998:318a, Abstract 1226.
Bex 2017 {published data only}
- Bex A, Mulders P, Jewett MA, Wagstaff J, Van Velthoven R, Laguna Pes PM, et al. Immediate versus deferred cytoreductive nephrectomy (CN) in patients with synchronous metastatic renal cell carcinoma (mRCC) receiving sunitinib (EORTC 30073 SURTIME). Annals of Oncology 2017;28 Suppl 5:v622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boccardo 1998 {published data only}
- Boccardo F, Rubagotti A, Canobbio L, Galligioni E, Sorio R, Lucenti A, et al. Interleukin-2, interferon-alpha and interleukin-2 plus interferon-alpha in renal cell carcinoma. A randomized phase II trial. Tumori Journal 1998;84(5):534-9. [DOI] [PubMed] [Google Scholar]
Bracarda 2007 {published data only}
- Bracarda S, Porta C, Boni C. Randomized prospective phase II trial of two schedules of sorafenib daily and interferon-a2a in metastatic renal cell carcinoma (RAPSODY): GOIRC Study 0681. Journal Of Clinical Oncology 2007;25 Suppl:Abstract 5100. [Google Scholar]
Buckley 2019 {published data only}
- Buckley HL, Collinson FJ, Ainsworth G, Poad H, Flanagan L, Katona E et al. PRISM protocol: a randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. BMC Cancer 2019;19(1):1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cella 2016 {published data only}
- Cella D, Gruenwald V, Nathan PD, Doan J, Dastani H, Taylor F, et al. Quality of life (QoL) and overall survival (OS) in patients (pts) with advanced clear-cell renal cell carcinoma (aRCC) treated with nivolumab (NIVI) vs. everolimus (EVE) in the phase III CheckMate 025 study. Journal of Clinical Oncology: ASCO annual meeting proceedings 2016;34(15 Suppl):4549. [Google Scholar]
Choueiri 2017 {published data only}
- Choueiri TK, Jakacki R, Ghiorghiu D, Haddad V, Kohlmann A, Frigault MM, et al. Savolitinib versus sunitinib in patients with MET-driven, unresectable and locally advanced or metastatic papillary renal cell carcinoma: SAVOIR, a randomised, phase III trial. Annals of Oncology 2017;28:v328. [Google Scholar]
Choueiri 2020 {published data only}
- Choueiri TK, Heng DY, Lee JL, Cancel M, Verheijen RB, Mellemgaard A, et al. Efficacy of savolitinib vs sunitinib in patients with MET -driven papillary renal cell carcinoma: the SAVOIR phase 3 randomized clinical trial. JAMA Oncology 2020;6:1247-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choueiri 2020a {published data only}
- Choueiri TK, Heng DY, Lee JL, Cancel M, Verheijen RB, Mellemgaard A, et al. SAVOIR: a phase III study of savolitinib versus sunitinib in pts with MET-driven papillary renal cell carcinoma (PRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2020;38(15 Suppl):5002. [Google Scholar]
Cirkel 2016 {published data only}
- Cirkel GA, Hamberg P, Sleijfer S, Loosveld O, Dercksen W, Los M, et al. A randomized phase II study to compare the efficacy of upfront bi-monthly rotations between pazopanib (PAZ) and everolimus (EVE) versus sequential treatment of first-line PAZ and second-line EVE until progression in patients with metastatic clear cell renal cell cancer (ccRCC) (ROPETAR trial). Journal of Clinical Oncology 2016;34(15):4550. [Google Scholar]
Cirkel 2017 {published data only}
- Cirkel GA, Hamberg P, Sleijfer S, Loosveld OJ, Dercksen MW, Los M, et al. Alternating treatment with pazopanib and everolimus vs continuous pazopanib to delay disease progression in patients with metastatic clear cell renal cell cancer: the ROPETAR randomized clinical trial. JAMA Oncology 2017;3(4):501-8. [DOI] [PubMed] [Google Scholar]
Climent 2020 {published data only}
- Climent Duran MA, Basterretxea L, Sanchez Escribano R, Llabres E, Heras Lopez L, Zambrana F, et al. Pilot study of cabozantinib efficacy, safety and tolerability in metastatic renal carcinoma in aged fragile patients. Annals of Oncology 2020;31 Suppl 4:S608. [Google Scholar]
Cole 2003 {published data only}
- Cole BF, McDermott D, Parker R, Youmans A, Connolly C, Ernstoff M, et al. The impact of treatmen with high-dose interleukin-2 (HD IL-2) or subcutaneous (SC) IL-2/interferon alfa-2b (IFN) on quality of life (QoL) in patients with metastatic renal cell carcinoma (mRCC). Proceedings of The American Society of Clinical Oncology 2003.
Collinson 2012 {published data only}
- Collinson FJ, Gregory WM, McCabe C, Howard H, Lowe C, Potrata D, et al. The STAR trial protocol: a randomised multi-stage phase II/III study of sunitinib comparing temporary cessation with allowing continuation, at the time of maximal radiological response, in the first-line treatment of locally advanced/metastatic renal cancer. BMC Cancer 2012;12:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collinson 2018 {published data only}
- Collinson F, Brown S, Buckley H, Ainsworth G, Howard H, Poad H, et al. PRISM: a randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. Annals of Oncology 2018;29 Suppl 8:viii331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colomba 2021 {published data only}
- Colomba E, Flippot R, Dalban C, Negrier S, Chevreau C, Gravis G, et al. Association of statins and nivolumab activity in patients with metastatic renal cell carcinoma (mRCC): results from the phase II nivoren-GETUG AFU 26 trial. Journal of Clinical Oncology. Conference 2021;39(6 Suppl):359. [Google Scholar]
Conter 2013 {published data only}
- Conter HJ, Wood CG, Matin SF, Tamboli P, Millikan RE, Jonasch E, et al. Ten-year follow-up of patients (pts) with metastatic renal cell carcinoma (mRCC) treated with interferon alfa-2b (IFN) as first-line therapy: results from a randomized trial. Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):365. [Google Scholar]
Demirci 1999 {published data only}
- Demirci D, Tatlisen A, Ekmekcioglu O, Orskiran G, Gulmez I. Comparison of vinblastine and interferon alpha with 5-flourouracil treatment in metastatic renal cell carcinoma: a preliminary report. Erciyes Tip Dergisi 1999;21(2):111-6. [Google Scholar]
de Mulder 1991 {published data only}
- Mulder PH, Debruyne FM, Oosterom A, Bouffioux C, Vermeylen K, Sylvester R. EORTC randomized phase II study of recombinant interferon alpha and recombinant interferon alpha and gamma in advanced renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. Vol. 10. 1991:166, Abstract 528.
Dexeus 1988 {published data only}
- Dexeus F, Logothetis C, Chong C, Sella A, Finn L. Phase III study in metastatic renal cell carcinoma comparing combination chemotherapy versus interferon alternating with combination chemotherapy. In: Proceedings of The American Society of Clinical Oncology. Vol. 7. 1988:131, Abstract 507.
Dexeus 1989 {published data only}
- Dexeus FH, Logothetis CJ, Sella A, Finn L. Interferon alternating with chemotherapy for patients with metastatic renal cell carcinoma. American Journal of Clinical Oncology 1989;12(4):350-4. [DOI] [PubMed] [Google Scholar]
DRKS00010309 2016 {published data only}
- DRKS00010309. A phase 2 randomized, double-blind, crossover, controlled, multi-center subject preference study of tivozanib hydrochloride versus sunitinib in the treatment of subjects with metastatic renal cell carcinoma. drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00010309 2016.
Dubois 1997 {published data only}
- Dubois JS, Trehu EG, Mier JW, Shapiro L, Epstein M, Klempner M, et al. Randomized placebo-controlled clinical trial of high-dose interleukin-2 in combination with a soluble p75 tumor necrosis factor receptor immunoglobulin G chimera in patients with advanced melanoma and renal cell carcinoma. Journal of Clinical Oncology 1997;15(3):1052-62. [DOI] [PubMed] [Google Scholar]
Eisen 2019 {published data only}
- Eisen TQ, Frangou E, Smith B, Ritchie A, Kaplan RS, Oza B, et al. Primary efficacy analysis results from the SORCE trial (RE05): adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: an international, randomised double-blind phase III trial led by the MRC CTU at UCL. Annals of Oncology 2019;30:v891-2. [Google Scholar]
Elhilali 2000 {published data only}
- Elhilali MM, Gleave M, Fradet Y, Davis I, Venner P, Saad F, et al. Placebo-associated remissions in a multicentre, randomized, double-blind trial of interferon gamma-1b for the treatment of metastatic renal cell carcinoma. BJU International 2000;86(6):613-8. [DOI] [PubMed] [Google Scholar]
Epaillard 2020 {published data only}
- Epaillard N, Simonaggio A, Elaidi R, Azzouz F, Brachychenko E, Thibault C, et al. BIONIKK: a phase 2 biomarker driven trial with nivolumab and ipilimumab or VEGFR tyrosine kinase inhibitor (TKI) in naïve metastatic kidney cancer. Bulletin du Cancer 2020;107(5):eS22-7. [DOI] [PubMed] [Google Scholar]
Escudier 2005 {published data only}
- Escudier B, Szczylik C, Eisen T, Stadler WM, Schwartz B, Shan M, et al. Randomized phase III trial of the raf kinase and VEGFR inhibitor sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC). In: Annual meeting proceedings of The American Society of Clinical Oncology. Vol. 23. 2005:380.
Euctr2006‐002851‐33‐AT {published data only}
- Schmidinger M. Sorafenib and bevacizumab as first- line treatment in patients with advanced renal cell carcinoma. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-002851-33-AT 2006.
Euctr 2006‐003429‐95‐ES {published data only}
- EUCTR2006-003429-95-ES. Treatment for renal cancer. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-003429-95-ES 2012.
Euctr2006‐005751‐16‐NL {published data only}
- EUCTR2006-005751-16-NL. An open label, dose escalation safety and tolerability trial of the combination of s.c. recombinant human IL-21 (rIL-21) and sunitinib (phase 1) followed by an open label stratified randomized 2-arm trial of rIL-21 plus sunitinib versus sunitinib alone (phase 2a) in subjects with stage IV renal cell carcinoma. Trial phase: 1/2a. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-005751-16-NL 2007.
Euctr2007‐002556‐41‐AT {published data only}
- EUCTR2007-002556-41-AT. TWIST- randomized prospective phase II study of temsirolimus with or without low-dose interferon alpha in metastatic non-clear renal cell carcinoma- GOIRC STUDY 02/2008 - TWIST. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007%E2%80%90002556%E2%80%9041%E2%80%90AT 2008.
EUCTR2008‐002667‐13‐DE 2008 {published data only}
- EUCTR2008-002667-13-DE. A single-center controlled pilot study to investigate pre- and perioperative therapy with Sorafenib (Nexavar®) in patients who are candidates for a curative surgery of renal cell cancer (PREST = preoperative Sorafenib Therapy in RCC) - PREST. https://www.clinicaltrialsregister.eu/ctr-search/trial/2008-002667-13/ 2008.
Euctr2012‐001730‐33‐ES {published data only}
- EUCTR2012-001730-33-ES. A phase 2 randomized, double-blind, crossover, controlled, multi-center, subject preference study of tivozanib hydrochloride versus sunitinib in the treatment of subjects with metastatic renal cell carcinoma. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-001730-33-ES 2012.
Euctr2015‐002133‐22‐FR {published data only}
- EUCTR2015-002133-22-FR. Study of the drug substances MLN0128 and MLN0128+MLN1117 compared with everolimus in the treatment of patients with metastatic clear cell renal carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-002133-22-FR 2017.
Euctr2018‐001495‐38‐FR {published data only}
- EUCTR2018-001495-38-FR. A randomized, phase 3, double-blind, placebo-controlled study of Pazopanib with or without abexinostat in patients with locally advanced or metastatic renal cell carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001495-38-FR 2018.
Feldman 2020 {published data only}
- Feldman DR, Ged Y, Lee CH, Knezevic A, Molina AM, Chen YB, et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: final results from a phase II trial. Cancer 2020;126(24):5247-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman 2020a {published data only}
- Feldman DR, Ged Y, Lee CH, Knezevic A, Molina AM, Chen YB, et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: final results from a phase II trial. Cancer 2020;126(24):5247-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figlin 1998 {published data only}
- Figlin RA, Thompson JA, Roudet C, Lange P, Belldegrun A. Multi-center randomized placebo controlled phase II/III trial of CD8(+) tumor infiltrating lymphocyte therapy (CD8(+) TIL)/recombinant interleukin-2 (IL-2) in metastatic renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. Vol. 17. 1998:318a, Abstract 1225.
Figlin 1999 {published data only}
- Figlin RA, Thompson JA, Bukowski RM, Vogelzang NJ, Novick AC, Lange P, et al. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. Journal of Clinical Oncology 1999;17(8):2521-9. [DOI] [PubMed] [Google Scholar]
Figlin 2014 {published data only}
- Figlin RA, Wood CG. Patient identification and eligibility insights in the synchronous mRCC population: an update from the ongoing ADAPT* phase 3 study experience. In: Journal of Clinical Oncology (Conference). Vol. 33. 2014.
Figlin 2014a {published data only}
- Figlin RA, Wood CG. Enrollment insights in the synchronous mRCC population: an update from the ongoing ADAPT* phase 3 study experience. In: Journal of Clinical Oncology: ASCO annual meeting proceedings. Vol. 32. 2014.
Figlin 2017 {published data only}
- Figlin R, Nicolette C, Tannir N, Tykodi SS, Chen D, Master V, et al. Interim analysis of the phase 3 ADAPT trial evaluating rocapuldencel-T (AGS-003), an individualized immunotherapy for the treatment of newly-diagnosed patients with metastatic renal cell carcinoma (mRCC). Annals of Oncology 2017;28 Suppl 5:v404. [Google Scholar]
Figlin 2018 {published data only}
- Figlin RA, Wood CG, Gamble AH, Plachco A, Norris MS, Horvatinovich JM, et al. Interim data analysis of the ADAPT trial using the modified intent to treat (mITT) population re-evaluates Rocapuldencel-T for clinical benefit over standard of care. Journal of Clinical Oncology: ASCO annual meeting proceedings 2018;36(15 Suppl 1):4557. [Google Scholar]
Figlin 2020 {published data only}
- Figlin RA, Tannir NM, Uzzo RG, Tykodi SS, Chen DY, Master V, et al. Results of the ADAPT phase 3 study of rocapuldencel-t in combination with sunitinib as first-line therapy in patients with metastatic renal cell carcinoma. Clinical Cancer Research 2020;26(10):2327-35. [DOI] [PubMed] [Google Scholar]
Flaherty 2015 {published data only}
- Flaherty KT, Manola JB, Pins M, McDermott DF, Atkins MB, Dutcher JJ, et al. BEST: a randomized phase II study of vascular endothelial growth factor, RAF kinase, and mammalian target of rapamycin combination targeted therapy with bevacizumab, sorafenib, and temsirolimus in advanced renal cell carcinoma - a trial of the ECOG-ACRIN cancer research group (E2804). Journal of Clinical Oncology 2015;33(21):2384-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foon 1988 {published data only}
- Foon K, Doroshow J, Bonnem E, Fefer A, Graham S, Grosh B, et al. A prospective randomized trial of alpha(2B)-interferon/gamma- interferon or the combination of advanced metastatic renal cell carcinoma. Journal of Biological Response Modifiers 1988;7(6):540-5. [PubMed] [Google Scholar]
Fossa 1989 {published data only}
- Fossa SD, Raabe N, Moe B. Recombinant interferon-alpha with or without vinblastine in metastatic renal carcinoma. Results of a randomised phase II study. British Journal of Urology 1989;64(5):468-71. [DOI] [PubMed] [Google Scholar]
Fossa 1992 {published data only}
- Fossa SD, Martinelli G, Otto U, Schneider G, Wander H, Oberling F, et al. Recombinant interferon alfa-2a with or without vinblastine in metastatic renal cell carcinoma: results of a European multi-center phase III study. Annals of Oncology 1992;3(4):301-5. [DOI] [PubMed] [Google Scholar]
Gao 2017 {published data only}
- Gao J, Karam JA, Wood CG, Matin S, Ahrar K, Jonasch E, et al. Clinical activity, immune and molecular correlates of nivolumab vs. nivolumab plus bevacizumab vs nivolumab plus ipilimumab in metastatic renal cell carcinoma. Cancer Research 2017;77(13 Suppl):CT083. [Google Scholar]
Gao 2019 {published data only}
- Gao J, Karam JA, Tannir NM, Campbell MT, Tidwell RS, Ahrar K, et al. A pilot randomized study evaluating nivolumab (nivo) or nivo + bevacizumab (bev) or nivo + ipilimumab (ipi) in patients with metastatic renal cell carcinoma (MRCC) eligible for cytoreductive nephrectomy, metastasectomy or post-treatment biopsy (Bx). Journal of Clinical Oncology 2019;37(15):4501. [Google Scholar]
Gedye 2021 {published data only}
- Gedye C, Joshi AJ, Zhang AY, Martin AJ, Joshua AM, Harris CA, et al. Denosumab and pembrolizumab in clear cell renal carcinoma (KEYPAD): a phase II trial (ANZUP1601). In: Journal of Clinical Oncology (Conference). Vol. 39. 2021.
Ghiorghiu 2018 {published data only}
- Ghiorghiu D, Jakacki R, Haddad V, Kohlmann A, Frigault MM, Ottesen L, et al. Savolitinib versus sunitinib in patients with MET-driven, unresectable and locally advanced or metastatic papillary renal cell carcinoma: SAVOIR, a randomised, phase III trial. Kidney Cancer 2018;2 Suppl 1:S33. [Google Scholar]
Gleave 1997 {published data only}
- Gleave M, Elhilai M, Fradet Y, Davis I, Venner P, Saad F, et al. A multicenter randomized, double blind trial of actimmune(r) interferon gamma-1b injection versus placebo for the treatment of metastatic renal cell carcinoma. In: Proceedings of the Annual Meeting of the American Society for Clinical Oncology. 1997.
Gleave 1997a {published data only}
- Gleave M, Elhilali M, Fradet Y. A phase III randomized double-blind placebo-controlled multicenter trial comparing interferon gamma to placebo in patients with metastatic renal cell carcinoma. Journal of Urology 1997;157:A1274. [Google Scholar]
Gleave 1998 {published data only}
- Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, et al. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. Canadian urologic oncology group. New England Journal of Medicine 1998;338(18):1265-71. [DOI] [PubMed] [Google Scholar]
Gore 2008 {published data only}
- Gore ME. Interferon-a (IFN), interleukin-2 (IL2) and 5-fluorouracil (5FU) vs IFN alone in patients with metastatic renal cell carcinoma (mRCC): results of the randomised MRC/EORTC RE04 trial. Journal of Clinical Oncology 2008;26(15 Pt 1):259. [Google Scholar]
Gore 2010 {published data only}
- Gore ME, Griffin CL, Hancock B, Patel PM, Pyle L, Aitchison M, et al. Interferon alfa-2a versus combination therapy with interferon alfa-2a, interleukin-2, and fluorouracil in patients with untreated metastatic renal cell carcinoma (MRC RE04/EORTC GU 30012): an open-label randomised trial. Lancet 2010;375(9715):641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruenwald 2020 {published data only}
- Gruenwald V, Ivanyi P, Jacobasch L, Zahn MO, Hubner A, Von Weikersthal LF, et al. A phase III study testing the role of proactive coaching on patient reported outcome in advanced or metastatic renal cell carcinoma treated with sunitinib or a combination of axitinib + checkpoint inhibitor (CPI) in first line therapy (PREPARE). Oncology Research and Treatment 2020;43:224. [Google Scholar]
Haas 2016 {published data only}
- Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387(10032):2008-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hainsworth 2015 {published data only}
- Hainsworth JD, Mace JR, Reeves JA, Crane EJ, Hamid O, Stille JR, et al. Randomized phase II study of sunitinib + CXCR4 inhibitor LY2510924 versus sunitinib alone in first-line treatment of patients with metastatic renal cell carcinoma. Journal of Clinical Oncology: ASCO annual meeting proceedings 2015;33(15 Suppl 1):4547. [Google Scholar]
Hainsworth 2016 {published data only}
- Hainsworth JD, Reeves JA, Mace JR, Crane EJ, Hamid O, Stille JR, et al. A randomized, open-label phase 2 study of the CXCR4 inhibitor LY2510924 in combination with sunitinib versus sunitinib alone in patients with metastatic renal cell carcinoma (RCC). Targeted Oncology 2016;11(5):643-53. [DOI] [PubMed] [Google Scholar]
Han 2002 {published data only}
- Han KR, Pantuck AJ, Belldegrun AS. A randomized phase III trial of high dose interleukin-2 versus subcutaneous interleukin-2/interferon. Current Urology Reports 2002;3(1):11-2. [DOI] [PubMed] [Google Scholar]
Harima 1990 {published data only}
- Harima M, Yasumoto R, Asakawa M, Horii A, Nishio S, Sakamoto W, et al. Comparison of efficacy between interferon-alpha (IFN) plus fluoropyrimidine (FP) and IFN alone in advanced renal cell cancer (RCC): preliminary results. Journal of Cancer Research and Clinical Oncology 1990;116:533. [Google Scholar]
Henriksson 1998 {published data only}
- Henriksson R, Nilsson S, Colleen S, Wersall P, Helsing M, Zimmerman R, et al. Survival in renal cell carcinoma - a randomized evaluation of tamoxifen vs interleukin 2, alpha-interferon (leucocyte) and tamoxifen. British Journal of Cancer 1998;77(8):1311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutson 2006 {published data only}
- Hutson TE, Bukowski RM. A phase II study of GW786034 using a randomized discontinuation design in subjects with locally recurrent or metastatic clear-cell renal cell carcinoma. Clinical Genitourinary Cancer 2006;4(4):296-8. [DOI] [PubMed] [Google Scholar]
Hutson 2021 {published data only}
- Hutson TE, Carthon BC, Yorio J, Babu S, McKean HA, Percent IJ, et al. Safety and efficacy outcomes with nivolumab plus ipilimumab in patients with advanced renal cell carcinoma and low Karnofsky performance status: results from the CheckMate 920 trial. In: Journal of Clinical Oncology. Conference. Vol. 39. 2021.
ISRCTN95351638 {published data only}
- Collinson F, Brown S, Buckley H, Ainsworth G, Howard H, Poad H, et al. PRISM: a randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. Annals of Oncology 2018;29 Suppl 8:viii331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2017-001476-33-GB. A randomised phase II trial comparing two different ways of combining nivolumab and ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-001476-33-GB 2018.
Jager 2005 {published data only}
- Jager E, Heinzer H, Grimm MO, Krause S, Scheuring U, Siebels M. Randomized phase III trial of the multiple kinase inhibitor sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC). In: Onkologie. Vol. 28 Suppl 3. 2005:1–275.
Jayson 1998 {published data only}
- Jayson GC, Middleton M, Lee SM, Ashcroft L, Thatcher N. A randomized phase II trial of interleukin 2 and interleukin 2-interferon alpha in advanced renal cancer. British Journal of Cancer 1998;78(3):366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeon 1999 {published data only}
- Jeon SH, Chang SG. The effect of immunotherapy based on interferon - alpha in advanced renal cell carcinoma. Journal of the Korean Cancer Association 1999;31(5):986-94. [Google Scholar]
JPRN‐JapicCTI‐122014 {published data only}
- JPRN-JapicCTI-122014. A Phase 3 Study of ONO-4538/BMS-936558. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-122014 2012.
JPRN‐jRCTs031180024 {published data only}
- JPRN-jRCTs031180024. NIVOIGERCC. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs031180024 2018.
JPRN‐UMIN000001995 {published data only}
- JPRN-UMIN000001995. Randomized parallel group phase II clinical study of sorafenib/interferon combination therapy and sunitinib monotherapy for advanced renal cell carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000001995 2009.
Kinouchi 2004 {published data only}
- Kinouchi T, Sakamoto J, Tsukamoto T, Akaza H, Kubota J, Ozono S, et al. Prospective randomized trial of natural interferon-alpha (IFN) versus IFN + cimetidine in advanced renal cell carcinoma with pulmonary metastases. Journal of Clinical Oncology 2004;22(14 Suppl):4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinouchi 2006 {published data only}
- Kinouchi T, Sakamoto J, Tsukamoto T, Akaza H, Kubota Y, Ozono S, et al. Prospective randomized trial of natural interferon-alpha versus natural interferon-alpha plus cimetidine in advanced renal cell carcinoma with pulmonary metastasis. Journal of Cancer Research and Clinical Oncology 2006;132(8):499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larkin 2019 {published data only}
- Larkin JM, Tykodi SS, Donskov F, Lee JL, Szczylik C, Malik J, et al. First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): updated follow-up for KEYNOTE-427 cohort A. Annals of Oncology 2019;30 Suppl 5:v381-2. [Google Scholar]
Law 1995 {published data only}
- Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O'Connell M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine- activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995;76(5):824-32. [DOI] [PubMed] [Google Scholar]
Lee 2020 {published data only}
- Lee JL, Ziobro M, Suarez C, Langiewitz P, Matveev VB, Wiechno P, et al. First-line pembrolizumab (pembro) monotherapy in advanced non-clear cell renal cell carcinoma (nccRCC): updated follow-up for KEYNOTE-427 cohort B. Journal of Clinical Oncology: ASCO annual meeting proceedings 2020;38(15):5034. [Google Scholar]
Lee 2021 {published data only}
- Lee CH, Voss MH, Carlo MI, Chen YB, Reznik E, Knezevic A, et al. Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: results of a phase 2 trial. Journal of Clinical Oncology: ASCO annual meeting proceedings 2021;39(15 Suppl):4509. [Google Scholar]
Lindskog 2020 {published data only}
- Lindskog M, Laurell A, Kjellman A, Melichar B, Niezabitowski J, Maroto P, et al. A randomized phase II study with ilixadencel, a cell-based immune primer, plus sunitinib versus sunitinib alone in synchronous metastatic renal cell carcinoma. Journal of Clinical Oncology 2020;38(5):11. [Google Scholar]
Lissoni 1993 {published data only}
- Lissoni P, Barni S, Ardizzoia A, Andres M, Scardino E, Cardellini P, et al. A randomized study of low-dose interleukin-2 subcutaneous immunotherapy versus interleukin-2 plus interferon-alpha as first line therapy for metastatic renal cell carcinoma. Tumori 1993;79(6):397-400. [DOI] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clinical Cancer Research 2012;18(6):1751-9. [DOI] [PubMed] [Google Scholar]
Lummen 1996 {published data only}
- Lummen G, Goepel M, Mollhoff S, Hinke A, Otto T, Rubben H. Phase II study of interferon-gamma versus interleukin-2 and interferon-alpha2b in metastatic renal cell carcinoma. Journal of Urology 1996;155(2):455-8. [PubMed] [Google Scholar]
Madhusudan 2004 {published data only}
- Madhusudan S, Protheroe A, Vasey P, Patel P, Selby P, Altman D, et al. A randomized phase II study of interferon alpha alone or in combination with thalidomide in metastatic renal cancer. Journal of Clinical Oncology 2004;22(14 Suppl):4742. [Google Scholar]
McDermott 2001 {published data only}
- McDermott D, Flaherty L, Clark J, Weiss G, Logan T, Gordon M, et al. A randomized phase III trial of high-dose interleukin-2 (HD IL2) versus subcutaneous (SC) Il2/interferon (IFN) in patients with metastatic renal cell carcinoma (RCC). In: Proceedings of The American Society of Clinical Oncology. Vol. 20 Pt 1. 2001:172a, Abstract 685. [DOI] [PubMed]
McDermott 2005 {published data only}
- McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2005;23(1):133-41. [DOI] [PubMed] [Google Scholar]
McDermott 2013 {published data only}
- McDermott DF, Manola J, Pins M, Flaherty KT, Atkins MB, Dutcher JP, et al. The BEST trial (E2804): a randomized phase II study of VEGF, RAF kinase, and mTOR combination targeted therapy (CTT) with bevacizumab (bev), sorafenib (sor), and temsirolimus (tem) in advanced renal cell carcinoma (RCC). Journal of Clinical Oncology. Conference 2013;31(6 Suppl 1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDermott 2020 {published data only}
- McDermott DF, Lee JL, Bjarnason GA, Larkin JM, Gafanov R, Kochenderfer MD, et al. First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): updated follow-up for KEYNOTE-427 cohort A. Journal of Clinical Oncology: ASCO annual meeting proceedings 2020;38(15):5069. [Google Scholar]
Mickisch 2001 {published data only}
- Mickisch GH, Garin A, Poppel H, Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001;358(9286):966-70. [DOI] [PubMed] [Google Scholar]
Minasian 1993 {published data only}
- Minasian LM, Motzer RJ, Gluck L, Mazumdar M, Vlamis V, Krown SE. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. Journal of Clinical Oncology 1993;11(7):1368-75. [DOI] [PubMed] [Google Scholar]
Molina 2009 {published data only}
- Molina AM, Ginsberg M, Sweeney S, Myrie J, Motzer R J. Efficacy and safety of everolimus treatment in metastatic renal cell carcinoma. American Journal of Hematology/Oncology 2009;8(4):166-169. [Google Scholar]
Motzer 2001 {published data only}
- Motzer RJ, Rakhit A, Thompson JA, Nemunaitis J, Murphy BA, Ellerhorst J, et al. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha2a for patients with advanced renal cell carcinoma. Journal of Interferon and Cytokine Research 2001;21(4):257-63. [DOI] [PubMed] [Google Scholar]
Mulders 2012 {published data only}
- Mulders P, Hawkins R, Nathan P, Jong I, Osanto S, Porfiri E, et al. Cediranib monotherapy in patients with advanced renal cell carcinoma: results of a randomised phase II study. European Journal of Cancer 2012;48(4):527-37. [DOI] [PubMed] [Google Scholar]
Naglieri 1998 {published data only}
- Naglieri E, Gebbia V, Durini E, Lelli G, Abbate I, Selvaggi FP, et al. Standard interleukin-2 (IL-2) and interferon-alpha immunotherapy versus an IL-2 and 4-epirubicin immuno-chemotherapeutic association in metastatic renal cell carcinoma. Anticancer Research 1998;18(3B):2021-6. [PubMed] [Google Scholar]
NCT00002737 {published data only}
- NCT00002737. Interferon alfa with or without isotretinoin in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT00002737 2004.
NCT00005966 {published data only}
- NCT00005966. Interferon alfa-2b with or without thalidomide in treating patients with metastatic or unresectable kidney cancer. clinicaltrials.gov/show/NCT00005966 2003.
NCT00019539 {published data only}
- NCT00019539. Monoclonal antibody therapy in treating patients with advanced kidney cancer. clinicaltrials.gov/show/NCT00019539 2009.
NCT00027664 {published data only}
- NCT00027664. Interferon alfa with or without thalidomide in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT00027664 2003.
NCT00053820 {published data only}
- NCT00053820. Interferon alfa with or without interleukin-2 and fluorouracil in treating patients with advanced metastatic kidney cancer. clinicaltrials.gov/show/NCT00053820 2003.
NCT00073307 {published data only}
- Eisen T, Bukowski RM, Staehler M, Szczylik C, Oudard S, Stadler WM, et al. Randomized phase III trial of sorafenib in advanced renal cell carcinoma (RCC): impact of crossover on survival. Journal of Clinical Oncology: ASCO annual meeting proceedings 2006;24(18S):4524. [Google Scholar]
- Negrier S, Jager E, Porta C, McDermott D, Moore M, Bellmunt J, et al. Efficacy and safety of sorafenib in patients with advanced renal cell carcinoma with and without prior cytokine therapy, a subanalysis of TARGET. Medical Oncology 2010;27(3):899-906. [DOI] [PubMed] [Google Scholar]
NCT00100906 {published data only}
- NCT00100906. Sequential ATRA then IL-2 for modulation of dendritic cells and treatment of metastatic renal cell cancer. clinicaltrials.gov/show/NCT00100906 2005.
NCT00378703 {published data only}
- NCT00378703. Bevacizumab, sorafenib tosylate, and temsirolimus in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT00378703 2006.
NCT00416871 {published data only}
- NCT00416871. Interleukin-2 and interferon in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT00416871 2006.
NCT00467025 {published data only}
- Inc Amgen. A randomized, double blinded, multi-center study 2 study to estimate the efficacy and evaluate the safety and tolerability of sorafenib in combination with AMG 386 or placebo in subjects with metas. https://clinicaltrials.gov/ct2/show/NCT00467025.
NCT00491738 {published data only}
- NCT00491738. A study evaluating the efficacy and safety of sunitinib with or without bevacizumab in first-line patients with metastatic renal cell cancer (SABRE-R). clinicaltrials.gov/show/NCT00491738 2007.
NCT00709995 {published data only}
- NCT00709995. A study for participants with metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT00709995 2008.
NCT00835978 {published data only}
- Rini BI, Gruenwald V, Fishman MN, Melichar B, Ueda T, Karlov P, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. Clinical Advances in Hematology and Oncology 2012;10(9):6-8. [Google Scholar]
- Rini BI, Melichar B, Fishman MN, Oya M, Pithavala YK, Chen Y et al. Axitinib dose titration: analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Annals of Oncology 2015;26(7):1372-7. [DOI] [PubMed] [Google Scholar]
- Rini BI, Melichar B, Ueda T, Grünwald V, Fishman MN, Arranz JA, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncology 2013;14(12):1233-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Tomita Y, Melichar B, Ueda T, Gruenwald V, Fishman MN, et al. Overall survival analysis from a randomized phase II study of axitinib with or without dose titration in first-line metastatic renal cell carcinoma. Clinical Genitourinary Cancer 2016;14(6):499-503. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Uemura H, Oya M, Shinohara N, Habuchi T, Fujii Y, et al. Patients with metastatic renal cell carcinoma who benefit from axitinib dose titration: analysis from a randomised, double-blind phase II study. BMC Cancer 2019;19(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00873236 {published data only}
- EUCTR2008-006414-19-GB. Dynamic contrast enhanced MRI (DCE-MRI) assessment of the vascular changes induced with bevacizumab alone and in combination with interferon-a in patients with advanced renal cell carcinoma. - DCE-MRI changes in tumour vaculature with Bevacizumab and Interferon in advanced RCC. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008-006414-19-GB 2009.
NCT01164228 {published data only}
- NCT01164228. Sunitinib malate with or without gemcitabine hydrochloride in treating patients with advanced kidney cancer that cannot be removed by surgery. clinicaltrials.gov/show/NCT01164228 2010.
NCT01223027 {published data only}
- NCT01223027. Study of dovitinib versus sorafenib in patients with metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT01223027 2010.
NCT01408004 {published data only}
- NCT01408004. Rotating pazopanib and everolimus to avoid resistance. clinicaltrials.gov/show/NCT01408004 2011.
NCT01444807 {published data only}
- NCT01444807. Evaluate the efficacy of sorafenib in renal cell carcinoma patients after a radical resection of the metastases. clinicaltrials.gov/show/NCT01444807 2011.
NCT01616186 {published data only}
- NCT01616186. Everolimus/sorafenib or sunitinib in patients with metastatic renal cell carcinoma (RCC). clinicaltrials.gov/show/NCT01616186 2012.
NCT01664182 {published data only}
- NCT01664182. Trebananib with or without bevacizumab, pazopanib hydrochloride, sorafenib tosylate, or sunitinib malate in treating patients with advanced kidney cancer. clinicaltrials.gov/show/NCT01664182 2012.
NCT01673386 {published data only}
- NCT01673386. A subject treatment preference study of tivozanib hydrochloride versus sunitinib in subjects with metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT01673386 2012.
NCT01727089 {published data only}
- NCT01727089. Bevacizumab with or without TRC105 in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT01727089 2012.
NCT01727336 {published data only}
- NCT01727336. Study of dalantercept and axitinib in patients with advanced renal cell carcinoma. clinicaltrials.gov/show/NCT01727336 2012.
NCT01793636 {published data only}
- NCT01793636. A study comparing AZD2014 vs everolimus in patients with metastatic renal cancer. clinicaltrials.gov/show/NCT01793636 2013.
NCT02014636 {published data only}
- NCT02014636. Safety and efficacy study of pazopanib and MK 3475 in advanced renal cell carcinoma (RCC; KEYNOTE-018). clinicaltrials.gov/show/NCT02014636 2013.
NCT02127710 {published data only}
- EUCTR2014-001858-41-ES. This is a study designed to evaluate the effectiveness and safety of AZD6094 in patients with papillary renal cell cancer. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-001858-41-ES 2015.
NCT02667886 {published data only}
- NCT02667886. Trial of X4P-001 in patients with advanced renal cell carcinoma. clinicaltrials.gov/show/NCT02667886 2016.
NCT02724020 {published data only}
- NCT02724020. MLN0128 and MLN0128 + MLN1117 compared with everolimus in the treatment of adults with advanced or metastatic clear-cell renal cell carcinoma. clinicaltrials.gov/show/NCT02724020 2016.
NCT02960906 {published data only}
- Epaillard N, Simonaggio A, Elaidi R, Azzouz F, Braychenko E, Thibault C et al. A BIOmarker driven trial with nivolumab and ipilimumab or VEGFR tKi in naive metastatic kidney cancer (BIONIKK). clinicaltrials.gov/ct2/show/NCT02960906. [DOI] [PubMed]
NCT03035630 {published data only}
- NCT03035630. Sunitinib followed by avelumab or the reverse for metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT03035630 2017.
NCT03092856 {published data only}
- NCT03092856. Axitinib with or without anti-OX40 antibody PF-04518600 in treating patients with metastatic kidney cancer. clinicaltrials.gov/show/NCT03092856 2017.
NCT03095040 {published data only}
- NCT03095040. CM082 combined with everolimus in chinese patients with metastatic renal cell carcinoma. clinicaltrials.gov/show/NCT03095040 2017.
NCT03173560 {published data only}
- NCT03173560. Trial to assess safety and efficacy of lenvatinib (18 mg vs. 14 mg) in combination with everolimus in participants with renal cell carcinoma. clinicaltrials.gov/ct2/show/NCT03173560 2017.
NCT03501381 {published data only}
- NCT03501381. High dose IL 2 and entinostat in RCC. clinicaltrials.gov/show/NCT03501381 2018.
NCT03595124 {published data only}
- NCT03595124. Axitinib and nivolumab in treating participants with unresectable or metastatic TFE/translocation renal cell carcinoma. clinicaltrials.gov/show/NCT03595124 2018.
NCT03829111 {published data only}
- NCT03829111. CBM588, nivolumab, and ipilimumab in treating patients with stage IV or advanced kidney cancer. clinicaltrials.gov/show/NCT03829111 2019.
NCT04195750 {published data only}
- NCT04195750. A study of MK-6482 versus everolimus in participants with advanced renal cell carcinoma (MK-6482-005). clinicaltrials.gov/show/NCT04195750 2019.
NCT04300140 {published data only}
- NCT04300140. Efficacy and safety study of AVB-S6-500 in patients with clear cell renal cell carcinoma. clinicaltrials.gov/show/NCT04300140 2020.
Negrier 1996 {published data only}
- Negrier S, Escudier B, Lasset C, Savary J, Douillard JY, Chevreau C, et al. The FNCLCC Crecy trial: interleukin 2 (IL2) + interferon (IFN) is the optimal treatment to induce responses in metastatic renal cell carcinoma (mRCC). In: Proceedings of The American Society of Clinical Oncology. Vol. 15. 1996:248, Abstract 629.
Negrier 1997 {published data only}
- Negrier S, Escudier B, Douillard JY, Lesimple T, Rossi JF, Viens P, et al. Randomized study of interleukin-2 and interferon with or without 5-FU (FUCY study) in metastatic renal cell carcinoma. In: Proceedings of The American Society of Clinical Oncology. Vol. 16. 1997:326a, Abstract 1161.
Negrier 1998 {published data only}
- Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. New England Journal of Medicine 1998;338(18):1272-8. [DOI] [PubMed] [Google Scholar]
Negrier 2000 {published data only}
- Negrier S, Caty A, Lesimple T, Douillard JY, Escudier B, Rossi JF, et al. Treatment of patients with metastatic renal carcinoma with a combination of subcutaneous interleukin-2 and interferon alfa with or without fluorouracil. Groupe Francais d'Immunotherapie, Federation Nationale des Centres de Lutte Contre le Cancer. Journal of Clinical Oncology 2000;18(24):4009-15. [DOI] [PubMed] [Google Scholar]
Negrier 2006 {published data only}
- Negrier S, Perol D, Ravaud A, Bay JO, Oudard S, Fargeot P, et al. Is intravenous (iv) IL2 superior to subcutaneous (sc) IL2 in good prognosis patients (pts) with metastatic renal cell carcinoma (mRCC) receiving a combination of IL2 and alpha interferon (IFN)? Results of the prospective randomized PERCY duo trial. Journal of Clinical Oncology: ASCO annual meeting proceedings 2006;24(18 Suppl):4536. [Google Scholar]
Negrier 2007 {published data only}
- Negrier S, Perol D, Ravaud A, Chevreau C, Bay JO, Delva R, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer 2007;110(11):2468-77. [DOI] [PubMed] [Google Scholar]
Negrier 2008 {published data only}
- Negrier S, Perol D, Ravaud A, Bay JO, Oudard S, Chabaud S, et al. Randomized study of intravenous versus subcutaneous interleukin-2, and IFNalpha in patients with good prognosis metastatic renal cancer. Clinical Cancer Research 2008;14(18):5907-12. [DOI] [PubMed] [Google Scholar]
Nosov 2010 {published data only}
- Nosov DA, Bhargava P, Esteves B, Al-Adhami M, Lipatov O, Lyulko AA, et al. Phase 2 randomized discontinuation trial (RDT) of tivozanib in patients with renal cell carcinoma (RCC): results in patients randomized to tivozanib vs. placebo. Annals of Oncology 2010;8:viii271. [Google Scholar]
Nosov 2012 {published data only}
- Nosov DA, Bhargava P, Esteves B, Strahs AL, Lipatov ON, Lyulko AA, et al. Final analysis of the phase 2 randomized discontinuation trial of tivozanib (AV-951) versus placebo in patients with renal cell carcinoma. Journal of Clinical Oncology 2012;29(15 Suppl):4550. [DOI] [PubMed] [Google Scholar]
Pal 2015 {published data only}
- Pal S, Azad A, Bhatia S, Drabkin H, Costello B, Sarantopoulos J, et al. P0125 a phase 1/2 trial of BNC105P with everolimus in metastatic renal cell carcinoma. Clinical Cancer Research 2015;21(15):3420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pal 2021a {published data only}
- Pal SK, Puente J, Heng DY, Glen H, Koralewski P, Stroyakovskiy D, et al. Phase II trial of lenvatinib (LEN) at two starting doses + everolimus (EVE) in patients (pts) with renal cell carcinoma (RCC): results by independent imaging review (IIR) and prior immune checkpoint inhibition (ICI). Journal of Clinical Oncology 2021;39(6 Suppl):307. [Google Scholar]
Passalacqua 2010 {published data only}
- Passalacqua R, Buzio C, Buti S, Porta C, Labianca R, Pezzuolo D, et al. Phase III, randomised, multicentre trial of maintenance immunotherapy with low-dose interleukin-2 and interferon-alpha for metastatic renal cell cancer. Cancer Immunology, Immunotherapy 2010;59(4):553-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plimack 2015 {published data only}
- Plimack ER, Hammers HJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, et al. Updated survival results from a randomized, doseranging phase II study of nivolumab (NIVO) in metastatic renal cell carcinoma (MRCC). Journal of Clinical Oncology 2015;33(15 Suppl):4553. [Google Scholar]
Pyrhonen 1995 {published data only}
- Pyrhonen S, Salminen E, Lehtonen T, Nurmi M, Tammela T, Juusela H, et al. Recombinant interferon alfa-2a (IFN) with vinblastine vs vinblastine alone in advanced renal cell carcinoma (RCC). A phase III study. In: Proceedings of The American Society of Clinical Oncology. Vol. 14. 1995:253, Abstract 686.
Pyrhonen 1996 {published data only}
- Pyrhonen S, Salminen E, Lehtonen T, Nurmi M, Tammela T, Juusela H, et al. Recombinant interferon alfa-2a with vinblastine vs vinblastine alone in advanced renal cell carcinoma: a phase III study. In: Proceedings of The American Society of Clinical Oncology. Vol. 15. 1996:244, Abstract 614.
Pyrhonen 1999 {published data only}
- Pyrhonen S, Salminen E, Ruutu M, Lehtonen T, Nurmi M, Tammela T, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. Journal of Clinical Oncology 1999;17(9):2859-67. [DOI] [PubMed] [Google Scholar]
Ravaud 2006 {published data only}
- Ravaud A, Gardner J, Hawkins R, Maase H, Zantl N, Harper P, et al. Efficacy of lapatinib in patients with high tumor EGFR expression: results of a phase III trial in advanced renal cell carcinoma (RCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2006 2006;24(18 Suppl):4502. [Google Scholar]
Ravaud 2016 {published data only}
- Ravaud A, Motzer RJ, Pandha HS, Staehler M, George D, Pantuck AJ, et al. PR phase III trial of sunitinib (SU) vs placebo (PBO) as adjuvant treatment for high-risk renal cell carcinoma (RCC) after nephrectomy (S-TRAC). Annals of Oncology 2016;27(6):1-36. [Google Scholar]
Rexer 2017 {published data only}
- Rexer H, Steiner T, Gruenwald V. First-line therapy in advanced renal cell carcinoma: a randomized phase II study to examine early switch of tyrosine kinase inhibitors to nivolumab compared to continued tyrosine kinase inhibitor therapy in patients with advanced or metastatic renal cell carcinoma and stable disease after three months of treatment (NIVOSWITCH)-AN 38/15 of the AUO.. Urologe 2017;56(4):509-11. [DOI] [PubMed] [Google Scholar]
Richards 1977 {published data only}
- Richards F, Muss HB, White DR, Cooper MR, Spurr CL. CCNU, bleomycin, and methylprednisolone with or without adriamycin in renal cel carcinoma: a randomized trial. Cancer Treatment Reports 1977;61(8):1591-3. [PubMed] [Google Scholar]
Rini 2011 {published data only}
- Rini BI, Szczylik C, Tannir NM, Koralewski P, Tomczak P, Deptala A, et al. AMG 386 in combination with sorafenib in patients (pts) with metastatic renal cell cancer (mRCC): a randomized, double-blind, placebo-controlled, phase II study. Journal of Clinical Oncology. Conference 2011;29(7 Suppl 1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rini 2012 {published data only}
- Rini B, Szczylik C, Tannir NM, Koralewski P, Tomczak P, Deptala A, et al. AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney: a randomized, double-blind, placebo-controlled, phase 2 study. Cancer 2012;118(24):6152-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez‐Vida 2020 {published data only}
- Rodriguez-Vida A, Bamias A, Esteban E, Saez MI, Lopez-Brea M, Castellano D, et al. Randomised phase II study comparing alternating cycles of sunitinib and everolimus vs standard sequential administration in first-line metastatic renal carcinoma (SUNRISES study). BJU international 2020;126(5):559-67. [DOI] [PubMed] [Google Scholar]
Rpcec 2017 {published data only}
- RPCEC00000229. HEBERFERON in renal cell carcinoma. who.int/trialsearch/Trial2.aspx?TrialID=RPCEC00000229 2017.
Sternberg 2013 {published data only}
- Sternberg C, Bracarda S, Carteni G, Lo Re G, Ruggeri EM, Masini C. Sunitinib expanded-access trial in metastatic renal cell carcinoma (mRCC) - final Italian results. European Journal of Cancer 2013;49:S644. [Google Scholar]
Szarek 2021 {published data only}
- Szarek M, Needle MN, Rini BI, Pal SK, McDermott DF, Atkins MB, et al. Q-TWiST analysis of tivozanib versus sorafenib in patients with advanced renal cell carcinoma in the TIVO-3 study. Clinical Genitourinary Cancer 2021;19(5):468.e1-468.e5. [DOI] [PubMed] [Google Scholar]
Tannir 2016 {published data only}
- Tannir NM, Jonasch E, Albiges L, Altinmakas E, Ng CS, Matin SF, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. European Urology 2016;69(5):866-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2020 {published data only}
- Taylor MH, Lee CH, Makker V, Rasco D, Dutcus CE, Wu J, et al. Phase Ib/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. Journal of Clinical Oncology 2020;38(11):1154-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2020a {published data only}
- Taylor MH, Lee CH, Makker V, Rasco D, Ductus CE, Wu J etal. Erratum: phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. Journal of Clinical Oncology 2020;38(23):2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thiam 2010 {published data only}
- Thiam R, Cuenod CA, Fournier LS, Lamuraglia M, Medioni J, Barascout B, et al. Determination of a new recist threshold using everolimus treatment in metastatic renal cell carcinoma: evaluation from the RECORD-1 study. Annals of Oncology 2010;8:viii74-5. [Google Scholar]
Trump 2004 {published data only}
- Atzpodien J, Kirchner H, Jonas U, Bergmann L, Schott H, Heynemann H, et al. Interleukin-2- and interferon alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the german cooperative renal carcinoma chemoimmunotherapy group (DGCIN). Journal of Clinical Oncology 2004;22(7):1188-94. [DOI] [PubMed] [Google Scholar]
Twardowski 2015 {published data only}
- Twardowski P, Plets M, Plimack ER, Agarwal N, Tangen CM, Vogelzang NJ, et al. SWOG 1107: parallel (randomized) phase II evaluation of tivantinib (ARQ-197) and tivantinib in combination with erlotinib in patients (pts) with papillary renal cell carcinoma (pRCC). Journal of Clinical Oncology: ASCO annual meeting proceedings 2015;33(15 Suppl 1):4523. [Google Scholar]
Twardowski 2017 {published data only}
- Twardowski PW, Tangen CM, Wu X, Plets MR, Plimack ER, Agarwal N, et al. Parallel (randomized) phase II evaluation of tivantinib (ARQ197) and tivantinib in combination with erlotinib in papillary renal cell carcinoma: SWOG S1107. Kidney Cancer 2017;1(2):123-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verzoni 2018 {published data only}
- Verzoni E, Ratta R, Grassi P, Salvioni R, Stagni S, Montone R, et al. TARIBO trial: targeted therapy with or without nephrectomy in metastatic renal cell carcinoma: liquid biopsy for biomarkers discovery. Tumori 2018;104(5):401-5. [DOI] [PubMed] [Google Scholar]
Voss 2015 {published data only}
- Voss MH, Chen Y, Chaim J, Coskey DT, Woo K, Redzematovic A, et al. A phase II trial of everolimus (E) and bevacizumab (B) in advanced non-clear cell renal cell cancer (ncRCC) to show efficacy in patients (pts) with papillary features. Journal of Clinical Oncology: ASCO annual meeting proceedings 2015;33(15 Suppl 1):4522. [Google Scholar]
Voss 2019 {published data only}
- Voss MH, Azad AA, Hansen AR, Gray JE, Welsh SJ, Achour I, et al. Results from a randomised phase I/II trial evaluating the safety and antitumour activity of anti-PD-1 (MEDI0680)/anti-PD-L1 (durvalumab) vs anti-PD-1 (nivolumab) alone in metastatic clear cell renal cell carcinoma (ccRCC). Annals of Oncology 2019;30:v516. [Google Scholar]
Witte 1995 {published data only}
- Witte RS, Leong T, Emstoff MS, Krigel RL, Oken MM, Harris J, et al. A phase II study of interleukin-2 with and without beta-interferon in the treatment of advanced renal cell carcinoma. Investigational New Drugs 1995;13(3):241-7. [DOI] [PubMed] [Google Scholar]
Wood 2013 {published data only}
- Wood GC, Figlin RA. ADAPT: an ongoing international phase 3 randomized trial of autologous dendritic cell immunotherapy (AGS 003) plus standard treatment in advanced renal cell carcinoma (RCC). Journal of Clinical Oncology 2013;32(4 Suppl):449. [Google Scholar]
Wright 2020 {published data only}
- Wright KM. Final data analysis supports tivozanib as superior treatment for patients with RCC. Oncology 2020;34(7):257. [PubMed] [Google Scholar]
Yang 2002 {published data only}
- Yang JC, Haworth L, Steinberg SM, Rosenberg SA, Novotny W. A randomized double-blind placebo-controlled trial of bevacizumab (anti-VEGF antibody) demonstrating a prolongation in time to progression in patients with metastatic renal cancer. Proceedings of The American Society of Clinical Oncology 2002;21 Pt 1:5a, Abstract 15.
Yang 2003 {published data only}
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. New England Journal of Medicine 2003;349(5):427-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2016 {published data only}
- Zhou AP, Ma J, Bai Y, Song Y, Li H, Xie X, et al. Anlotinib versus sunitinib as first line treatment for metastatic renal cell carcinoma (mRCC): preliminary results from a randomized phase II clinical trial. Journal of Clinical Oncology. Conference 2016;34(15 Suppl):4565. [Google Scholar]
Zhou 2019 {published data only}
- Zhou A, Bai Y, Song Y, Luo H, Ren X, Wang X, et al. Anlotinib versus sunitinib as first‐line treatment for metastatic renal cell carcinoma: a randomized phase II clinical trial. Oncologist 2019;24(8):e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Liu 2017 {published data only}
- Liu C, Wang X, Mo, J, Yang H, Liu H, Liao H. Efficacy of sunitinib in the treatment of advanced renal cellcarcinoma and its effect on survival time [Chinese]. Anti-Tumor Pharmacy 2017;7(2):195-9. [Google Scholar]
NCT01217931 {published data only}
- Sequential Two-agent Assessment in Renal Cell Carcinoma Therapy: The START Trial. https://www.clinicaltrials.gov/ct2/show/NCT01217931.
NCT01688973 {published data only}
- Tivantinib with or without erlotinib hydrochloride in treating patients eith metastatic or locally advanced kidney cancer that cannot be removed by surgery. https://www.clinicaltrials.gov/ct2/show/NCT01688973.
NCT01829841 {published data only}
- A Study of famitinib in patients with advanced metastatic renal cell cancer. https://www.clinicaltrials.gov/ct2/show/NCT01829841.
NCT03541902 {published data only}
- Cabozantinib or sunitinib malate in treating participants with metastatic variant histology renal cell carcinoma. https://www.clinicaltrials.gov/ct2/show/NCT03541902.
References to ongoing studies
EUCTR2008‐000928‐71‐IT {published data only}
- EUCTR2007-002556-41-AT. A randomized, open-label, 2-arm, multicentre, phase II study to evaluate the safety and efficacy of trivax, a dendritic cell-based interleukin-12 secreting autologous cancer vaccine, in combination with sunitinib compared with sunitinib alone as first line treatment for patients with metastatic renal-cell carcinoma (mRCC). - Decavarec study TRX 1.0. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-002556-41-AT 2008.
- EUCTR2008-000928-71-IT. TWIST. Randomized prospective phase II study of temsirolimus with or without low-dose interferon alpha in metastatic non-clear renal cell carcinoma: GOIRC study 02/2008 - TWIST. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2008-000928-71-IT 2008.
- EUCTR2008-000928-71-IT. TWIST. Randomized prospective phase II study of temsirolimus with or without low-dose interferon alpha in metastatic non-clear renal cell carcinoma: GOIRC study 02/2008 - TWIST. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008-000928-71-IT 2008.
NCT02210117 {published data only}
- Nivolumab with or without bevacizumab or ipilimumab before surgery in treating patients with metastatic kidney cancer that can be removed by surgery. clinicaltrials.gov/ct2/show/NCT02210117 2014. [ClinicalTrials.gov: NCT02210117]
NCT02996110 {published data only}
- A study to test combination treatments in people with advanced renal cell carcinoma (FRACTION-RCC). clinicaltrials.gov/ct2/show/NCT02996110 2016. [ClinicalTrials.gov: NCT02996110]
NCT03075423 {published data only}
- Ahrens M, Escudier B, Boleti E, Grimm MO, Goupil MG, Barthelemy P, et al. A randomized phase II study of nivolumab plus ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (suniforecast). Oncology Research and Treatment 2020;43 Suppl 1:226. [ClinicalTrials.gov: NCT03075423] [Google Scholar]
- Ahrens M, Escudier B, Haanen JB, Boleti E, Goupil MG, Grimm MO, et al. A randomized phase II study of nivolumab plus ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (SUNIFORECAST). In: Journal of Clinical Oncology. Meeting Abstract | 2021 ASCO Annual Meeting I. Vol. 39. 2021. [ClinicalTrials.gov: NCT03075423]
- Ahrens M, Escudier B, Haanen JB, Boleti E, Gross Goupil M, Grimm MO, et al. An ongoing, randomized phase II study of Nivolumab plus Ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (SUNIFORECAST). Oncology Research and Treatment 2021;44 Suppl 2:74-5. [ClinicalTrials.gov: NCT03075423] [Google Scholar]
- Ahrens M, Scheich S, Gokbuget N, Boleti E, Grunwald V, Escudier B, et al. A randomised phase II study of nivolumab plus ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (SUNIFORECAST). Oncology Research and Treatment 2018;41:73. [ClinicalTrials.gov: NCT03075423] [Google Scholar]
- Rexer H, Bergmann L, Steiner T. A phase 2, randomized, open-label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in subjects with previously untreated and advanced (unresectable or metastatic) non-clear cell renal cell carcinoma - SUNNIFORECAST. Aktuelle Urologie 2020;51(3):236-8. [ClinicalTrials.gov: NCT03075423] [DOI] [PubMed] [Google Scholar]
- Rexer H, Steiner T, Bergmann L. Nivolumab combined with ipilimumab versus sunitinib monotherapy-SUNNIFORECAST AN 41/16 of the AUO: a phase 2, randomized, open-label study in subjects with previously untreated and advanced (unresectable or metastatic) non-clear cell renal cell carcinoma.. Urologe 2017;56(6):802-3. [ClinicalTrials.gov: NCT03075423] [DOI] [PubMed] [Google Scholar]
NCT03260894 {published data only}
- NCT03260894. Pembrolizumab (MK-3475) plus epacadostat vs standard of care in mRCC (KEYNOTE-679/ECHO-302). clinicaltrials.gov/ct2/show/NCT03260894 2017. [ClinicalTrials.gov: NCT03260894]
NCT03592472 {published data only}
- Aggarwal RR, Thomas S, Hauke RJ, Nordquist LT, Munster PN. RENAVIV: a randomized phase III, double-blind, placebo-controlled study of pazopanib with or without abexinostat in patients with locally advanced or metastatic renal cell carcinoma. In: Journal of Clinical Oncology (Conference). Vol. 37. 2019. [ClinicalTrials.gov: NCT03592472]
NCT03729245 {published data only}
- NCT03729245. A study of bempegaldesleukin (NKTR-214: BEMPEG) in combination with nivolumab compared with the investigator's choice of a tyrosine kinase inhibitor (TKI) therapy (either sunitinib or cabozantinib monotherapy) for advanced metastatic renal cell carcinoma (RCC). clinicaltrials.gov/ct2/show/NCT03729245 2018. [ClinicalTrials.gov: NCT03729245]
- Tannir NM, Agarwal N, Pal SK, Cho DC, Formiga M, Guo J, et al. PIVOT-09: a phase III randomized open-label study of bempegaldesleukin (NKTR-214) plus nivolumab versus sunitinib or cabozantinib (investigator's choice) in patients with previously untreated advanced renal cell carcinoma (RCC). In: Journal of Clinical Oncology. Meeting Abstract | 2020 Genitourinary Cancers Symposium. Vol. 38. 2020. [ClinicalTrials.gov: NCT03729245]
NCT03793166 {published data only}
- Zhang T, Ballman K, Choudhury A, Chen R, Watt C, Wen Y, et al. PDIGREE: an adaptive phase 3 trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). Kidney Cancer 2020;4:S39. [ClinicalTrials.gov: NCT03793166] [Google Scholar]
- Zhang T, Ballman KV, Choudhury AD, Chen RC, Watt C, Wen Y, et al. PDIGREE: an adaptive phase 3 trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). In: Journal of Clinical Oncology. Meeting Abstract | 2021 Genitourinary Cancers Symposium. Vol. 37. 2019. [ClinicalTrials.gov: NCT03793166]
- Zhang T, Ballman KV, Choudhury AD, Chen RC, Watt C, Wen Y, et al. PDIGREE: an adaptive phase III trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). In: Journal of Clinical Oncology (Conference). Vol. 39. 2021. [ClinicalTrials.gov: NCT03793166]
NCT03873402 {published data only}
- An immunotherapy study of nivolumab plus ipilimumab versus nivolumab alone in participants with advanced kidney cancer. clinicaltrials.gov/show/NCT03873402 2019. [ClinicalTrials.gov: NCT03873402]
- NCT03873402. A study of nivolumab combined with ipilimumab versus nivolumab alone in participants with advanced kidney cancer. clinicaltrials.gov/ct2/show/NCT03873402 2019. [ClinicalTrials.gov: NCT03873402]
NCT03937219 {published data only}
- Choueiri T, Albiges L, Powles T, Geng A, Mohamed N, Wang F, et al. A phase 3 study (COSMIC-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced renal cell carcinoma of intermediate or poor risk. European Urology Open Science 2020;21Suppl 3:S188. [ClinicalTrials.gov: NCT03937219] [Google Scholar]
- Choueiri T, Albiges L, Powles T, Mohamed N, Wang F, Motzer R. A phase 3 study (COSMIC-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced renal cell carcinoma of intermediate or poor risk. Journal for Immunotherapy of Cancer 2020;8(3 Suppl):A209. [ClinicalTrials.gov: NCT03937219] [Google Scholar]
- Choueiri TK, Scheffold C, Wang F, Powles T, Motzer RJ. A phase 3 study (COSMIC-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced or metastatic renal cell carcinoma (RCC) of intermediate or poor risk. Kidney Cancer 2020;4 Suppl 1:S2-3. [ClinicalTrials.gov: NCT03937219] [Google Scholar]
- De Giorgi U, Choueiri TK, Albiges L, Powles T, Scheffold C, Wang F, et al. A phase 3 study (Cosmic-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced renal cell carcinoma of intermediate or poor risk. Tumori 2020;106(2 Suppl):152. [ClinicalTrials.gov: NCT03937219] [Google Scholar]
NCT04090710 {published data only}
- SBRT with combination ipilimumab/nivolumab for metastatic kidney cancer (CYTOSHRINK). clinicaltrials.gov/ct2/show/NCT04090710 2019. [ClinicalTrials.gov: NCT04090710]
NCT04203901 {published data only}
- Dendritic cell immunotherapy plus standard treatment of advanced renal cell carcinoma. clinicaltrials.gov/ct2/show/NCT04203901 2019. [ClinicalTrials.gov: NCT04203901]
NCT04394975 {published data only}
- Study to evaluate the efficacy and safety of toripalimab in combination with axitinib versus sunitinib monotherapy in advanced renal cell cancer. clinicaltrials.gov/ct2/show/NCT04394975 2020. [ClinicalTrials.gov: NCT04394975]
NCT04523272 {published data only}
- A study of TQB2450 injection combined with anlotinib hydrochloride capsule versus sunitinib in subjects with advanced renal cancer. clinicaltrials.gov/ct2/show/NCT04523272 2020. [ClinicalTrials.gov: NCT04523272]
NCT04540705 {published data only}
- A study to compare bempegaldesleukin (BEMPEG: NKTR-214) combined with nivolumab and tyrosine kinase inhibitor (TKI) to nivolumab and TKI alone in participants with previously untreated kidney cancer that is advanced or has spread (PIVOT IO 011). clinicaltrials.gov/ct2/show/NCT04540705 2020. [ClinicalTrials.gov: NCT04540705]
NCT04736706 {published data only}
- Choueiri T, Plimack E, Powles T, Voss M, Gurney H, Silverman R, et al. Phase 3 study of pembrolizumab + belzutifan + lenvatinib or pembrolizumab/quavonlimab + lenvatinib versus pembrolizumab + lenvatinib as first-line treatment for advanced renal cell carcinoma. Journal for ImmunoTherapy of Cancer 2021;9(2 Suppl):A447. [ClinicalTrials.gov: NCT04736706] [Google Scholar]
- EUCTR2020-002216-52-CZ. An open-label, randomized phase 3 study of immune and targeted combination therapies, as first-line treatment in participants with advanced clear cell renal cell carcinoma (ccRCC). trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-002216-52-CZ 2021. [ClinicalTrials.gov: NCT04736706]
- NCT04736706. A study of pembrolizumab (MK-3475) in combination with belzutifan (MK-6482) and lenvatinib (MK-7902), or pembrolizumab/quavonlimab (MK-1308A) in combination with lenvatinib, versus pembrolizumab and lenvatinib, for treatment of advanced clear cell renal cell carcinoma (MK-6482-012). clinicaltrials.gov/ct2/show/NCT04736706 2021. [ClinicalTrials.gov: NCT04736706]
- Rini BI, Plimack ER, Powles TB, Voss MH, Gurney HP, Silverman R, et al. 717TiP randomized, open-label, 3-arm phase III study comparing MK-1308A + lenvatinib and pembrolizumab (pembro) + belzutifan + lenvatinib versus pembro + lenvatinib as first-line (1L) treatment for advanced clear cell renal cell carcinoma (ccRCC). Annals of Oncology 2021;32 Suppl 5:S722-3. [ClinicalTrials.gov: NCT04736706] [Google Scholar]
NCT05043090 {published data only}
- EUCTR2021-000336-55-ES. A clinical trial to compare the effectiveness of savolitinib plus durvalumab relative to sunitinib for the treatment of renal cell cancer. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-000336-55-ES 2021.
- NCT05043090. Savolitinib plus Durvalumab versus sunitinib and Durvalumab monotherapy in MET-Driven, unresectable and locally advanced or metastatic PRCC. clinicaltrials.gov/ct2/show/NCT05043090 2021.
NCT05096390 {published data only}
- Axitinib +/- Pembrolizumab in first line treatment of mPRCC. clinicaltrials.gov/ct2/show/NCT05096390 2021. [ClinicalTrials.gov: NCT05096390]
UMIN 000012522 {published data only}
- Kadono Y, Konaka H, Izumi K, Anai S, Fujimoto K, Ishibashi K, et al. Efficacy and safety of cytokines versus first-line sunitinib and second-line axitinib for patients with metastatic renal cell carcinoma (ESCAPE study): a study protocol for phase III randomized sequential open-label study. Contemporary Clinical Trials Communications 2019;15:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMIN000012522. Efficacy and safety of cytokines versus sunitinib as first-line followed by second-line axitinib in the treatment of patients with metastatic renal cell carcinoma: a phase III randomized sequential open-label study. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014557 2013.
Additional references
Aguiar 2018
- Aguiar P, Costa de Padua T, Noia Barreto CM, Del Giglio A. Treatment of metastatic renal cell carcinoma: latest evidence and ongoing challenges. Clinical Medicine Insights: Urology 2018;11:1-7. [Google Scholar]
Aldin 2023
- Aldin A. First-line therapy for adults with advanced renal cell carcinoma - supplementary file. Open Science Framework 2023. [DOI: 10.17605/OSF.IO/HXBG5] [DOI] [PMC free article] [PubMed]
American Cancer Society 2022
- Cancer Facts & Figures 2022. Atlanta: American Cancer Society 2022.
ASCO 2021
- American Society of Clinical Oncology. Kidney cancer: introduction. https://www.cancer.net/cancer-types/kidney-cancer/introduction#:~:text=Renal%20cell%20carcinoma%20is%20the,filtration%20units%20in%20each%20kidney. (accessed 21 December 2022).
ASCO 2022
- American Society of Clinical Oncology. Kidney Cancer: Statistics. https://www.cancer.net/cancer-types/kidney-cancer/statistics (accessed 21 December 2022).
Bosma 2022
- Bosma NA, Warkentin MT, Gan CL, Karim S, Heng DY, Brenner DR, et al. Efficacy and safety of first-line systemic therapy for metastatic renal cell aarcinoma: a systematic review and network meta-analysis. European Urology Open Science 2022;37:14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brierley 2016
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th edition. Oxford: John Wiley & Sons, 2016. [Google Scholar]
Capitanio 2019
- Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. European Urology 2019;75(1):74-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cattrini 2021
- Cattrini C, Messina C, Airoldi C, Buti S, Roviello G, Mennitto A, et al. Is there a preferred first-line therapy for metastatic renal cell carcinoma? A network meta-analysis. Therapeutic Advances in Urology 2021;13:17562872211053189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLOS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2019
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Choueiri 2017b
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. New England Journal of Medicine 2017;376(4):354-66. [DOI] [PubMed] [Google Scholar]
CINeMA [Computer program]
- CINeMA [Confidence in Network Meta-Analysis]. Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al, Version (accessed prior to 22 October 2020). The University of Bern, The Campbell Collaboration, The Cochrane Collaboration, 2020. cinema.ispm.unibe.ch/.
Coppin 2004
- Coppin C, Porzsolt F, Autenrieth M, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD001425. [DOI: 10.1002/14651858.CD001425] [DOI] [PubMed] [Google Scholar]
Dabestani 2016
- Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World Journal of Urology 2016;34(8):1081-6. [DOI] [PubMed] [Google Scholar]
de Groot 2018
- Groot S, Redekop WK, Versteegh MM, Sleijfer S, Oosterwijk E, Kiemeney LA, et al. Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Quality of Life Research 2018;27(1):115-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane 2022. Available from www.training.cochrane.org/handbook 2022.
Delahunt 2019
- Delahunt B, Eble JN, Egevad L, Samaratunga H. Grading of renal cell carcinoma. Histopathology 2019;74(1):4-17. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. [DOI] [PubMed] [Google Scholar]
Edwards 2018
- Edwards SJ, Wakefield V, Cain P, Karner C, Kew K, Bacelar M, et al. Axitinib, cabozantinib, everolimus, nivolumab, sunitinib and best supportive care in previously treated renal cell carcinoma: a systematic review and economic evaluation. Health Technology Assessment 2018;22(6):1-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith DG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 2014
- Elliott JH, Turner T, Clavisi O, Thomas J, Higgins JP, Mavergames C, et al. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLOS Medicine 2014;11(2):e1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Escudier 2019
- Escudier B, Porta C, Schmidinger M, Rioux-Leclerq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2019;30(5):706-20. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [DOI] [PubMed] [Google Scholar]
Garner 2016
- Garner P, Hopewell S, Chandler J, MacLehose H, Schunemann HJ, Akl EA, et al. When and how to update systematic reviews: consensus and checklist. BMJ 2016;354:i3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 9 22 October 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). gradepro.org.
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: Assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. [DOI] [PubMed] [Google Scholar]
Harshman 2014
- Harshman LC, Drake CG, Choueiri TK. PD-1 blockade in renal cell carcinoma: to equilibrium and beyond. Cancer Immunology Research 2014;2(12):1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Higgins 2019a
- Higgins JP, Eldridge S, Li T. Chapter 23: including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Higgins 2019b
- Higgins JP, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Higgins 2019c
- Higgins JP, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 16: special topics in statistics. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Keir 2007
- Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Annual Review of Immunology 2007;19(3):309-14. [DOI] [PubMed] [Google Scholar]
Krahn 2013
- Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Medical Research Methodology 2013;13(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Leitlinienprogramm Onkologie
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF). Diagnostics, therapy and aftercare of renal cell carcinoma - Longversion 3.0 [Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms - Langversion 3.0]. https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Nierenzellkarzinom/Version_3/LL_Nierenzellkarzinom_Langversion_3.0.pdf.
Li 2019
- Li T, Higgins JP, Deeks JJ. Chapter 5: collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Liu 2021
- Liu Z, Chen Y, Wei Z, He Y, Wang J, Mu X, et al. Comparative efficacy and safety of immunotherapy in the first-line treatment of metastatic renal cell carcinoma: a systematic review and network meta-analysis. Annals of Palliative Medicine 2021;10(3):2805-14. [DOI] [PubMed] [Google Scholar]
Ljungberg 2022
- Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. European Urology 2022;82(4):399–410. [DOI] [PubMed] [Google Scholar]
Lopez‐Beltran 2009
- Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 update on the classification of renal epithelial tumors in adults. International Journal of Urology 2009;16(5):432-43. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI] [PubMed] [Google Scholar]
Mori 2021
- Mori K, Mostafaei H, Miura N, Karakiewicz PI, Luzzago S, Schmidinger M, et al. Systemic therapy for metastatic renal cell carcinoma in the first-line setting: a systematic review and network meta-analysis. Cancer Immunology, Immunotherapy 2021;70(2):265-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Motzer 2022
- Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, et al. Kidney cancer, version 3.2022 - NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2022;20(1):71-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nassar 2022
- Nassar AH, Adib E, Abou Alaiwi S, El Zarif T, Groha S, Akl, EW, et al. Ancestry-driven recalibration of tumor mutational burden and disparate clinical outcomes in response to immune checkpoint inhibitors. Cancer Cell 2022;40(10):1161-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nocera 2022
- Nocera L, Karakiewicz P I, Wenzel M, Tian Z, Shariat S F, Saad F, et al. Clinical outcomes and adverse events after first-line.treatment in metastatic renal cell carcinoma: a systematic review and network meta-analysis. Journal of Urology 2022;207(1):16-24. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Powles 2021
- Powles T, Albiges L, Bex A, Grünwald V, Porta C, Procopio G, et al. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Annals of Oncology 2021;32(12):1511-9. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI: 10.1136/bmj.g5630] [DOI] [PubMed] [Google Scholar]
Qin 2018
- Qin F, Yu H, Xu CR, Chen HH, Bai JL. Safety of axitinib and sorafenib monotherapy for patients with renal cell carcinoma: a meta-analysis. Journal of Biomedical Research 2018;32(1):30-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quhal 2021
- Quhal F, Mori K, Bruchbacher A, Resch I, Mostafaei H, Pradere B, et al. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. European Urology Oncology 2021;4(5):755-65. [DOI] [PubMed] [Google Scholar]
R Core Team 2019 [Computer program]
- R: A language and environment for statistical computing. R Core Team 2019. Vienna, Austria: R Foundation for Statistical Computing, 2019. Available at www.R-project.org.
Riaz 2021
- Riaz IB, He H, Ryu AJ, Siddiqi R, Naqvi SA, Yao Y, et al. A living, interactive systematic review and network meta-analysis of first-line treatment of metastatic renal cell carcinoma. European Urology 2021;80(6):712-23. [DOI] [PubMed] [Google Scholar]
Rini 2009
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009;373(9669):1119-32. [DOI] [PubMed] [Google Scholar]
Robert Koch Institute 2021
- Robert Koch Institute. Cancer in Germany in 2017/2018 [Krebs in Deutschland für 2017/2018]. https://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_Germany/cancer_chapters_2017_2018/cancer_germany_2017_2018.pdf?__blob=publicationFile 2021.
Rücker 2012
- Rücker G. Network meta-analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3(4):312-24. [DOI] [PubMed] [Google Scholar]
Rücker 2014
- Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Statistics in Medicine 2014;33(25):4353-69. [DOI] [PubMed] [Google Scholar]
Rücker 2015
- Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Medical Research Methodology 2015;15(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rücker 2019
- Rücker G, Krahn U, König J, Efthimiou O, Schwarzer G. netmeta: Network meta-analysis using frequentist methods. R package version 1.10. CRAN.R-project.org/package=netmeta 2019.
Sabatini 2006
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nature Reviews Cancer 2006;6(9):729-34. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLOS One 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez‐Gastaldo 2017
- Sanchez-Gastaldo A, Kempf E, Gonzalez del Alba A, Duran I. Systemic treatment of renal cell cancer: a comprehensive review. Cancer Treatment Reviews 2017;60:77-89. [DOI] [PubMed] [Google Scholar]
Scelo 2018
- Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. Journal of Clinical Oncology 2018;36(36):3574-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarzer 2007
- Schwarzer G. meta: an R package for meta-analysis. R News 2007;7(3):40-5. [Google Scholar]
Schwarzer 2015
- Schwarzer G, Carpenter JR, Rücker G. Chapter 8: network meta-analysis. In: Meta-Analysis With R. Springer International Publishing Switzerland, 2015. [Google Scholar]
Schünemann 2019
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. training.cochrane.org/handbook 2019.
Siegel 2022
- Siegel RL, Miller KD, Fuchs H, Jemal A. Cancer statistics 2022. CA: A Cancer Journal for Clinicians 2022;72(1):7-33. [DOI] [PubMed] [Google Scholar]
Skoetz 2019
- Skoetz N, Goldkuhle M, Weigl A, Dwan K, Labonte V, Dahm P, et al. Methodological review showed correct absolute effect size estimates for time-to-event outcomes in less than one-third of cancer-related systematic reviews. Journal of Clinical Epidemiology 2019;108:1-9. [DOI] [PubMed] [Google Scholar]
Skoetz 2020
- Skoetz N, Goldkuhle M, Dalen EC, Akl EA, Trivella M, Mustafa R, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Sun 2011
- Sun M, Thuret R, Abdollah F, Lughezzani G, Schmitges J, Tian Z, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. European Urology 2011;59(1):135-41. [DOI] [PubMed] [Google Scholar]
Swallow 2018
- Swallow E, Messali A, Ghate S, McDonald E, Duchesneau E, Perez JR. The additional costs per month of progression-free survival and overall survival: an economic model comparing everolimus with cabozantinib, nivolumab, and axitinib for second-line treatment of metastatic renal cell carcinoma. Journal of Managed Care & Specialty Pharmacy 2018;24(4):335-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thekdi 2015
- Thekdi SM, Milbury K, Spelman A, Wei Q, Wood C, Matin SF, et al. Posttraumatic stress and depressive symptoms in renal cell carcinoma: association with quality of life and utility of single-item distress screening. Psycho-Oncology 2015;24(11):1477-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tudur 2001
- Tudur C, Williamson PR, Khan S, Best LY. The value of the aggregate data approach in meta-analysis with time-to-event outcomes. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2001;164(2):357-70. [Google Scholar]
UICC 2017
- Union for International Cancer Control (UICC). TNM Classification of Malignant Tumours. 8th edition. Wiley-Blackwell, 2017. [Google Scholar]
United States Congress 2007
- United States Congress 2007. Food and Drug Administration Amendments Act (FDAAA) of 2007: public law no 110-85. govinfo.gov/content/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf.
Unverzagt 2017
- Unverzagt S, Moldenhauer I, Nothacker M, Rossmeissl D, Hadjinicolaou AV, Peinemann F, et al. Immunotherapy for metastatic renal cell carcinoma. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD011673. [DOI: 10.1002/14651858.CD011673] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2019
- Wang S, Cowley LA, Liu X-S. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules 2019;24(18):3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warren 2018
- Warren AY, Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies. World Journal of Urology 2018;36(12):1913-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012;3(2):111-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Medical Association
- World Medical Association. World Medical Association Declaration of Helsinki - ethical principles for medical research involving human subjects. Journal of the American Medical Association 2013;310(20):2191-4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Goldkuhle 2020
- Goldkuhle M, Aldin A, Jakob T, Adams A, Monsef I, Heidenreich A, et al. First‐line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD013798. [DOI: 10.1002/14651858.CD013798] [DOI] [PMC free article] [PubMed] [Google Scholar]