BACKGROUND:
Screening trials before full implantation of a spinal cord stimulation device are recommended by clinical guidelines and regulators, although there is limited evidence for their use. The TRIAL-STIM study showed that a screening trial strategy does not provide superior patient pain outcome at 6-month follow-up compared with not doing a screening trial and that it was not cost-effective.
OBJECTIVE:
To report the long-term follow-up results of the TRIAL-STIM study.
METHODS:
The primary outcome of this pragmatic randomized controlled trial was pain intensity as measured on a numerical rating scale (NRS) and secondary outcomes were the proportion of patients achieving at least 50% and 30% pain relief at 6 months, health-related quality of life, and complication rates.
RESULTS:
Thirty patients allocated to the “Trial Group” (TG) and 36 patients allocated to the “No Trial Group” (NTG) completed outcome assessment at 36-month follow-up. Although there was a reduction in NRS pain and improvements in utility scores from baseline to 36 months in both groups, there was no difference in the primary outcome of pain intensity NRS between TG and NTG (adjusted mean difference: −0.60, 95% CI: −1.83 to 0.63), EuroQol-5 Dimension utility values (adjusted mean difference: −0.02, 95% CI: −0.13 to 0.10), or proportion of pain responders (33% TG vs 31% NTG). No differences were observed between the groups for the likelihood of spinal cord stimulation device explant or reporting an adverse advent up to 36-month follow-up.
CONCLUSION:
The long-term results show no patient outcome benefit in undertaking an SCS screening trial.
KEY WORDS: Randomized controlled trial, Screening trial, Spinal cord stimulation, Neuropathic pain, Long-term follow-up
ABBREVIATIONS:
- AE
adverse event
- CONSORT
Consolidated Standards of Reporting Trials
- EQ-5D
EuroQol-5 Dimension
- EQ-VAS
EuroQol Visual Analog Scale
- FDA
Food and Drug Administration
- IPG
implantable pulse generator
- MCID
minimal clinical important difference
- MD
mean difference
- NRS
numerical rating scale
- NTG
no trial group
- PSPS-T2
persistent spinal pain syndrome type 2
- RCT
randomized controlled trial
- SCS
spinal cord stimulation
- TG
trial group.
The use of spinal cord stimulation (SCS) for inhibition of pain was first described in 1967.1 A number of randomized controlled trials (RCTs) and economic evaluations support the clinical and cost-effectiveness of SCS for neuropathic pain.2-15
Despite many advances in SCS waveforms,16-18 target neural tissue,19,20 and feedback technology,6 common to all RCTs to date is that patients are required to undergo a screening trial to evaluate early response to therapy before full implantation of the SCS device. Screening trials are recommended by clinical guidelines and regulators including the Food and Drug Administration (FDA) in the United States.21-24 Such a screening trial allows patients to experience the sensation generated by SCS and potential pain relief to be achieved. A successful trial has been defined as the patient reporting ≥50% pain relief with stable or reduced pain medications and with stable levels of daily activity.23
Although a successful screening trial should help to identify those patients who would most benefit from SCS and obtain long-term pain relief, there are drawbacks to such a screening trial strategy. Duplication of a clinical procedure at screening and full implant increase patient exposure to infection (because of bacterial colonization of the lead skin exit site). Higher infection rates have been reported for 28-day trials compared with screening trials with shorter durations.25,26
Furthermore, the results from the TRIAL-STIM RCT showed there was no evidence that a screening trial provides superior patient outcomes or is cost-effective compared with not doing a screening trial.27 Qualitative results of the TRIAL-STIM also indicated that the patients were not supportive of the concept of a trial.28
An important limitation of the TRIAL-STIM RCT results was that follow-up was limited to 6 months and therefore too short to evaluate long-term response to SCS. The aim of this report is, therefore, to report the long-term results of the TRIAL-STIM trial.
METHODS
Study Design
TRIAL-STIM was a multicenter, single-blind, parallel 2 group randomized trial (ISRCTN, ISRCTN60778781). The study was conducted across 3 participating sites in the United Kingdom: South Tees Hospitals NHS Foundation Trust (The James Cook University Hospital), Basildon and Thurrock University Hospitals NHS Foundation Trust, and Leeds Teaching Hospitals NHS Trust. The full study protocol has been published previously.29 The study was approved by the UK Health Research Authority North East Research Ethics Committee (17/NE/0056). The trial was conducted and reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines.30
Study Participants
Participants were eligible for SCS if they met the following criteria according to NHS guidance (NICE TA159)21: neuropathic pain of intensity of at least 5 on a numerical rating scale (NRS); had endured pain for longer than 6 months despite receiving suitable conservative medical and surgical management; had undergone a satisfactory multidisciplinary assessment by a team with experience in providing SCS; and had the capacity to provide informed consent. Full eligibility criteria have been previously reported.27,29
Randomization and Masking
Participants were allocated (1:1) to 1 of 2 groups: trial group (TG) with a screening trial followed by SCS implantation in light of the screening trial result, or no trial group (NTG) with a strategy of SCS implantation only. Patients were randomized to groups through a password-protected web-based system developed and maintained by the Exeter Clinical Trials Unit. The allocation was stratified by center, and minimized on patient age (≥65 or <65 years), sex, and presence of persistent spinal pain syndrome type 2 (PSPS‐T2). The methods for randomization and masking have been previously described.27,29
Procedures
Type of device and stimulation were not restricted, and devices from 4 major SCS manufacturers were used.
Screening Trial and Implantation Strategy (TG)
Patients who were randomly assigned to the TG arm underwent a screening trial that involved passing an external or an internalized tunneled SCS lead, or leads, to an external stimulator in accordance with the center's standard operating procedure.29 Considering the RCTs3,31 listed in the NICE TA15921 clinical evidence section along with international guidelines,23 a screening trial was defined as successful when a patient obtained ≥50% pain relief and satisfactory on table paresthesia coverage (≥80%) of the pain area, observed a reduction in pain medications or reported improvement in quality of life and function, and successful location of leads at anatomic target for paresthesia-free therapies. Patients who did not have a successful screening trial did not receive an implant but all patients were followed up. Patients who had a successful trial had the implantation of the implantable pulse generator (IPG) on a separate occasion.
Implantation-Only Strategy (NTG)
In the NTG group, all patients received a permanent implant in one surgery when the following was observed: satisfactory on table paresthesia coverage (≥80%) of the pain area, no dislike of sensations,32 and satisfactory anatomic lead location for paresthesia-free devices.
Outcomes
The primary outcome was pain intensity assessed on the NRS.33 Secondary outcomes assessed at 36-month follow-up were the proportion of patients achieving at least 50% and 30% pain relief as measured on the NRS,33 health-related quality of life using the EuroQol-5 Dimension (EQ-5D)-5L tool,34 and complication rates.
Statistical Analysis
The sample size calculation has been described elsewhere.27,29 The mean time to follow-up was 37.3 (range 36-40 months) and 37.1 (range 36-40 months) for the TG and NTG groups, respectively. The follow-up time is subsequently referred to as 36-month follow-up.
The primary analysis compared primary and secondary outcomes between randomized groups including patients who completed the 36-month follow-up assessment.27 No imputation of missing data is conducted for the analyses at 36 months to avoid making assumptions over long-term follow-up periods. A complete case assessment was considered to be more appropriate than conducting an intention-to-treat analysis with or without data imputation at 36 months to avoid making assumptions about missing data over long-term follow-up periods.
Linear regression methods with adjustment for baseline outcome scores and stratification/minimization variables were used to compare continuous outcomes. Logistic regression analysis adjusting for stratification/minimization variables were used to compare binary outcomes.
All analyses were performed using STATA v14.0 (StataCorp LP).
RESULTS
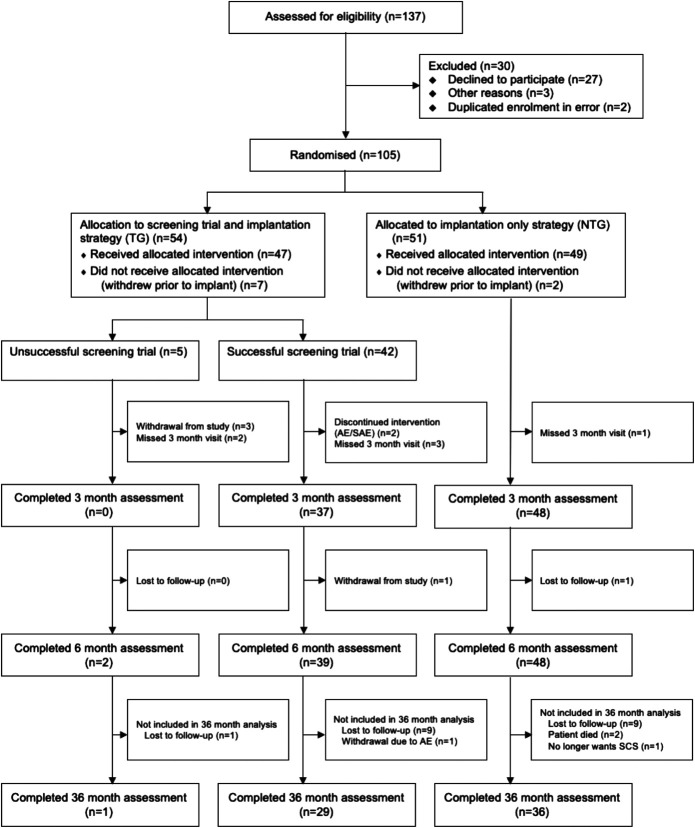
From June 2017 to September 2018, 137 patients were assessed for eligibility, with 105 patients proceeding to enrolment. Of the 105 participants, 54 were randomized to the screening TG. Seven of the patients in TG withdrew before the screening trial; of the remaining 47, an unsuccessful screening trial was observed for 5 (11%) patients and 42 (89%) patients had a successful screening trial and were implanted with an SCS system. Forty-nine of the 51 NTG patients received an SCS implant. Thirty TG patients and 36 NTG patients completed the follow-up at 36 months (Figure). Loss to follow-up in all instances was due to failure to reach the patients via telephone (maximum of 3 attempts), email, and letter.
FIGURE.
Trial profile. AEs, adverse event; NTG, no trial group; SAE, serious adverse event; TG, trial group.
The only difference between the TG and NTG groups' baseline characteristics and outcome ratings was the duration of pain, which was slightly longer in the NTG group. Participants in the study had a mean NRS pain score of 7.5, an average age of 50.4 years, and fairly equal sex representation. The most frequent primary diagnoses in both groups were PSPS-T2 (52% TG, 55% NTG) and radiculopathy (15% TG, 23% NTG).
At 36-month follow-up, there was no difference in the primary outcome of clinic-assessed NRS score between TG and NTG (adjusted mean difference: −0.60, 95% CI: −1.83 to 0.63; see Table 1). Considering a minimal clinical important difference (MCID) of 2 in the NRS,35,36 or recently suggested MCID ranges from 0.9 to 2.7,37 there were no clinically relevant differences in pain intensity at 36 months or change from baseline between TG and NTG groups. There was evidence of clinically significant reductions in mean NRS pain from baseline to 36 months for both TG (7.03-4.63) and NTG (7.58-5.47).
TABLE 1.
Complete Case Analysis of Primary and Secondary Continuous Outcomes
| Measure | Time Point | TG (N = 30) | NTG (N = 36) | Unadjusted mean difference (95%CI)a | Adjusted mean difference (95%CI)a,b | ||
|---|---|---|---|---|---|---|---|
| Meana | SDa | Meana | SDa | ||||
| NRS | Baseline | 7.03 | 1.10 | 7.58 | 1.23 | −0.55 (−1.12 to 0.02) | – |
| 36-mo follow-up | 4.63 | 2.48 | 5.47 | 2.37 | −0.84 (−2.04 to 0.36) | −0.60 (−1.83 to 0.63) | |
| Change from baseline at 36 mos | −2.40 | 2.40 | −2.11 | 2.55 | −0.29 (−1.54 to 0.96) | −0.23 (−1.47 to 1.00) | |
| EQ-5D | Baseline | 0.39 | 0.20 | 0.31 | 0.23 | 0.08 (−0.02 to 0.19) | – |
| 36-mo follow-up | 0.58 | 0.20 | 0.55 | 0.26 | 0.03 (−0.08 to 0.15) | −0.02 (−0.13 to 0.10) | |
| Change from baseline at 36 months | 0.19 | 0.22 | 0.24 | 0.31 | −0.05 (−0.19 to 0.08) | −0.06 (−0.20 to 0.08) | |
| EQ-VAS | Baseline | 48.32 | 20.10 | 54.08 | 21.77 | −5.76 (−16.11 to 4.59) | – |
| 36-mo follow-up | 53.87 | 20.64 | 59.29 | 22.79 | −5.41 (−16.16 to 5.33) | −4.14 (−14.90 to 6.61) | |
| Change from baseline at 36 mo | 5.55 | 27.84 | 5.20 | 22.94 | 0.35 (−12.15 to 12.84) | −0.15 (−13.12 to 12.80) | |
EQ-5D, EuroQol-5 Dimension; EQ-VAS, EuroQol Visual Analog Scale; NRS, numerical rating scale; NTG, no trial group; TG, trial group.
Means, standard deviation, and unadjusted and adjusted mean difference between groups calculated based on available data (complete case analysis). No imputation of missing data was performed.
Adjusted for baseline value of the measure (36-month follow-up data only), sex, age (≥65 vs < 65 years), presence of PSPS‐T2 (yes or no), and site.
Improvements were seen in EQ-5D-5L and EuroQol Visual Analog Scale (EQ-VAS) from baseline to 36 months in both groups. There was no evidence of difference between TG and NTG groups for EQ-5D utility scores (adjusted mean difference: −0.02, 95% CI: −0.13 to 0.10). The mean change from baseline at 36-month follow-up for EQ-5D utility scores observed was around 0.2 for both TG and NTG groups. Considering a MCID of 0.074 for the EQ-5D utility scores,38 there were no clinically significant differences between the TG and NTG at 36 months or change from baseline. Clinically significant improvements in EQ-5D utility scores were observed at 36 months for both TG (0.19) and NTG (0.24). Similarly, for EQ-VAS, there were no significant differences between TG and NTG groups (adjusted mean difference: −4.14, 95% CI: −14.90 to 6.61).
The proportion of patients who were responders considering a ≥50% (33% TG; 31% NTG) or ≥30% (53% TG; 42% NTG) reduction in pain from baseline were not statistically different between the TG and NTG groups (Table 2).
TABLE 2.
Responders Per Group According to Level of Pain Reduction (Complete Case Analysis at 36-Month Follow-up)
| Measure | TG (N = 30) | NTG (N = 36) | Unadjusted Odds ratio (95%CI)a |
Adjusted Odds ratio (95% CI)a,b |
||
|---|---|---|---|---|---|---|
| Reduction in NRS ≥ 50% | Yes: n (%) | 10 (33%) | 11 (31%) | 1.14 (0.35-3.63) | 1.33 (0.42-4.23) | |
| No: n (%) | 20 (67%) | 25 (69%) | ||||
| Reduction in NRS ≥ 30% | Yes: n (%) | 16 (53%) | 15 (42%) | 1.60 (0.54-4.76) | 1.75 (0.61-5.03) | |
| No: n (%) | 14 (47%) | 21 (58%) | ||||
NRS, numerical rating scale; NTG, no trial group; TG, trial group.
Risk ratio between groups calculated based on available data (complete case analysis). No imputation of missing data was performed.
Adjusted for baseline value of NRS, sex, age (≥65 vs < 65 years), presence PSPS-T2 of (yes or no), and site.
Considering a MCID of 2 in the NRS,35,36 there were no clinically relevant differences in pain intensity at 36 months or change from baseline between TG and NTG groups according to type of SCS programming (Table 3). Similarly, there were no clinically relevant differences according to type of SCS programming when considering the overall trial population.
TABLE 3.
Pain Intensity Per Group According to SCS Waveform (Complete Case Analysis at 36-Month Follow-up)
| SCS Waveform | TG (N = 30)a | NTG (n = 36) | Total (n = 66)a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| NRS at 36-month follow-up | |||||||||
| Paresthesia stimulation | 11 | 3.45 | 2.98 | 17 | 4.91 | 2.12 | 28 | 4.34 | 2.55 |
| HF stimulation | 9 | 5.11 | 1.36 | 14 | 6.11 | 2.51 | 23 | 5.72 | 2.16 |
| Burst stimulation | 9 | 5.11 | 2.15 | 5 | 5.60 | 2.79 | 14 | 5.29 | 2.30 |
| Change from baseline in NRS at 36 months | |||||||||
| Paresthesia stimulation | 11 | −3.18 | 2.99 | 17 | −2.56 | 2.73 | 28 | −2.80 | 2.80 |
| HF stimulation | 9 | −1.89 | 2.15 | 14 | −1.50 | 2.37 | 23 | −1.87 | 2.18 |
| Burst stimulation | 9 | −2.44 | 1.81 | 5 | −2.30 | 2.59 | 14 | −2.04 | 2.22 |
HF, high-frequency; NRS, numerical rating scale; NTG, no trial group; SCS, spinal cord stimulation; TG, trial group.
Programming method missing for 1 patient in the TG group.
Since the primary 6-month end point, there were 2 explants of the SCS device. One explant in TG because the device was not switching on; a new IPG has been scheduled for implant. There was one explant in NTG because of pain at the IPG site. Between six- and 36-month follow-ups, there were 3 adverse events (AEs) corresponding to fractured leads for 2 patients (1 TG and 1 NTG) and 1 patient in TG reporting problems charging the device; none of these AEs resulted in device explant. Overall (ie, during the 36-month study period, which included 2 additional device explants in TG because of implant-related wound infections), the likelihood of having an explant was not significantly different between TG (3 explants from a total of 54 patients) and NTG groups (1 explant from a total of 51 patients; unadjusted odds ratio 0.34, 95% CI 0.03-3.4). Similarly, no difference was observed for the likelihood of reporting an AE (TG [14 out of 54 patients]; NTG [12 out of 51 patients]; odds ratio 0.88, 95% CI 0.36-2.14).
DISCUSSION
The results of this study demonstrate that SCS screening trials do not provide superior patient outcomes in the long-term when compared with not doing a screening trial. These findings support the initial conclusions at the 6-month primary end point.27 The results further support that the patients' preference to undergo an SCS implant as a single-stage procedure28 may not be detrimental to obtain benefits from SCS. It is also noteworthy that at 36- to 40-month follow-up, we observed no difference in the number of explants between the groups.
Clinically important reductions in pain intensity and improvements in health-related quality of life were observed at 36-month follow-up for both groups, confirming the long-term effectiveness of SCS. Previous long-term follow-ups of RCTs have reported similar effects of SCS for patients with neuropathic pain.39,40 Although superior to a MCID of 2 points in the NRS,35,36 the pain reduction obtained by patients in our study is less than previously reported. RCT samples are usually highly selected with frequent exclusion of participants with a higher risk profile, and therefore, less representative of clinical practice.41,42 TRIAL-STIM was a pragmatic RCT, the eligibility criteria used reflects clinical practice in the United Kingdom and is in line with NICE guidance for SCS.21 Therefore, we consider that our study provides an overall more realistic representation of the impact of SCS in clinical practice than RCTs that adopt eligibility criteria not reflective of clinical practice. Although it may be argued that the smaller effect of SCS reflects poor patient selection, it is useful to note that a multidisciplinary assessment was part of our inclusion criteria and that this included a psychological assessment at all 3 centers. Furthermore, the low rate of AEs reported in the study compares favorably to other RCTs and supports the expertise of the implanters involved.
Although our study was not powered for a comparison between the 3 waveforms used in this study (paresthesia stimulation, high frequency at 10 kHz, and burst), we did not observe clinically relevant differences between these waveforms at 6 or at 36 months. Discussion considering a smaller MCID is presented in Supplementary Material 1, http://links.lww.com/NEU/D381.
Parallel and crossover RCTs have previously reported no differences in effectiveness when comparing different paresthesia-free frequencies,43 high frequency (10 kHz) vs paresthesia-inducing stimulation,44 high frequency (5 kHz) vs placebo,45 and burst vs different frequencies (ie, 40, 500, and 1200 Hz).46 However, superior pain reduction has been observed for tonic subthreshold stimulation at 500 Hz vs burst or placebo,47 high frequency (10 kHz) vs paresthesia-inducing stimulation,17 burst vs tonic stimulation,16 or 5882 Hz vs 1200 Hz, 3030 Hz, and sham.48 Not only different study settings and population characteristics, but also program settings, number of leads, and electrode contacts, or how sham stimulation was enabled may all contribute to the discrepancy in the results reported. Methodological and reporting deficiencies in trials of SCS have been previously highlighted.49,50 Development of SPIRIT and CONSORT extensions specific to implantable neurostimulation devices may potentially lead to improved reporting and transparency of clinical trial protocols and reports while facilitating identification of possible reasons for discrepant findings.51
Strengths
We provide a long-term follow-up of the only RCT to date that evaluated the clinical utility of SCS screening trials. Similar to the TRIAL-STIM RCT, the follow-up assessment was independent from industry funding. Few RCTs of SCS are fully independent of industry and none report results to 36 months. We observed some loss to follow-up; however, this was not substantial, considering that 77% of the participants that completed the primary end point at 6 months completed the follow-up at 36 months.
Limitations
Analysis of cost-effectiveness at 36 months was not conducted as the length of recall required between assessments (ie, 30 months) would limit interpretation of the results (see Supplementary Material 2, http://links.lww.com/NEU/D381). Previously, it had been suggested that the use of screening trials could be cost-saving if at least 20% of patients had an unsuccessful trial.52 Recent evidence from a budget impact analysis evaluating the costs or savings of conducting screening trials suggests that this figure may be closer to 14.4%,53 an unsuccessful trial failure rate approximately two-fold of previous rates observed in UK clinical practice.54
Practice Implications
Screening trials continue to be recommended by clinical guidelines and required by regulators. The FDA recently issued a letter to healthcare providers reminding of the importance of conducting a screening trial to confirm satisfactory pain relief before implanting a SCS device.55 Our findings suggest that the utility of screening trials in identifying long-term responders to SCS may be limited. We consider that patient selection with support from a multidisciplinary team may identify suitable patients who will obtain satisfactory pain relief with SCS in the long term based on pain reduction and limited number of device explants in the NTG group. An e-health tool has been recently developed to assist the assessment of the appropriateness of a patient for referral and selection for SCS, considering clinical and psychosocial factors.56 A study of 483 consecutive patients in 12 experienced European centers showed a strong correlation between the retrospective application of the e-tool panel recommendations and SCS trial and treatment outcome.57 Further prospective research is ongoing to evaluate the predictive value in e-tool-naive centers that are blinded to the e-tool panel recommendations.
We do not suggest that screening trials should be eradicated, but they should not be made mandatory. An informed decision on whether to perform a screening trial should be based on professional judgment and patient preferences, considering the advantages and disadvantages of screening trials.
CONCLUSION
The results of this long-term follow-up demonstrate that there is no patient outcome benefit in undertaking a SCS screening trial.
Footnotes
Supplemental digital content is available for this article at neurosurgery-online.com.
Contributor Information
Sam Eldabe, Email: seldabe@mac.com.
Sarah Nevitt, Email: Sarah.Nevitt@liverpool.ac.uk, lrig@liverpool.ac.uk.
Sara Griffiths, Email: sara.griffiths4@nhs.net.
Ashish Gulve, Email: ashishgulve@icloud.com.
Simon Thomson, Email: simon.thomson1@gmail.com.
Ganesan Baranidharan, Email: g.baranidharan@nhs.net.
Rachel Houten, Email: Rachel.Houten@liverpool.ac.uk.
Morag Brookes, Email: morag.brookes1@nhs.net.
Anu Kansal, Email: a.kansal@nhs.net.
Jenny Earle, Email: jennyearle@phonecoop.coop.
Jill Bell, Email: jillgoody1964@icloud.com.
Rui V. Duarte, Email: Rod.Taylor@glasgow.ac.uk.
Funding
The TRIAL-STIM study was funded by the National Institute for Health Research Research for Patient Benefit (RfPB) programme (Project Number: PB-PG-0815-20028). This follow-up assessment was funded by the NIHR Policy Research Programme (PRP; Project Number: NIHR201444). The funding source had no role in the study design, data collection, data analysis, interpretation of data, writing of the manuscript, approval, or decision to submit the manuscript for publication. The views expressed in this publication are those of the authors and do not necessarily reflect those of the PRP programme, NIHR, NHS, or the Health and Social Care.
Disclosures
Dr Duarte has received consultancy fees from Boston Scientific Corp, Mainstay Medical, Medtronic Ltd and Saluda Medical. Dr Eldabe has received consultancy fees from Medtronic Ltd, Mainstay Medical, and Boston Scientific Corp. He has received department research funding from the National Institute of Health Research, Medtronic Ltd, Boston Scientific, and Saluda Medical. Dr Gulve has received honoraria for consulting as well as advisory board meetings for Nevro Corp, Boston Scientific Corp, and Abbott. Dr Thomson has received consultancy fees from Boston Scientific Corp, Mainstay Medical and Saluda Medical. Dr Baranidharan is a consultant for Medtronic, Boston Scientific, and Saluda Medical, and has a consulting agreement and is on the advisory board for Nevro Corp, Nalu Medical Inc, Abbott, and Boston Scientific Corp. Dr Taylor has received consultancy fees from Medtronic Ltd, Nevro Corp, and Saluda Medical. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplemental Digital Content
Supplementary Material 1. Consideration of a smaller minimally clinical important difference (MCID).Supplementary Material 2. Cost-effectiveness analysis considerations.
REFERENCES
- 1.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46(4):489-491. [PubMed] [Google Scholar]
- 2.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-106; discussion 106-107. [DOI] [PubMed] [Google Scholar]
- 3.Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. [DOI] [PubMed] [Google Scholar]
- 4.de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155(11):2426-2431. [DOI] [PubMed] [Google Scholar]
- 5.Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. 2014;37(11):3016-3024. [DOI] [PubMed] [Google Scholar]
- 6.Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123-134. [DOI] [PubMed] [Google Scholar]
- 7.Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. 2021;78(6):687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte RV, Nevitt S, McNicol E, et al. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain. 2020;161(1):24-35. [DOI] [PubMed] [Google Scholar]
- 9.Duarte RV, Nevitt S, Maden M, et al. Spinal cord stimulation for the management of painful diabetic neuropathy: a systematic review and meta-analysis of individual patient and aggregate data. Pain. 2021;162(11):2635-2643. [DOI] [PubMed] [Google Scholar]
- 10.Kemler MA, Furnée CA. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology. 2002;59(8):1203-1209. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RJ, Taylor RS. Spinal cord stimulation for failed back surgery syndrome: a decision-analytic model and cost-effectiveness analysis. Int J Technol Assess Health Care. 2005;21(3):351-358. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RS, Bentley A, Campbell B, Murphy K. High-frequency 10 kHz spinal cord stimulation for chronic back and leg pain: cost-consequence and cost-effectiveness analyses. Clin J Pain. 2020;36(11):852-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor RS, Ryan J, O'Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010;26(6):463-469. [DOI] [PubMed] [Google Scholar]
- 14.Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess. 2009;13(17):iii. ix-x, 1-154. [DOI] [PubMed] [Google Scholar]
- 15.Niyomsri S, Duarte RV, Eldabe S, et al. A systematic review of economic evaluations reporting the cost-effectiveness of spinal cord stimulation. Value in Health. 2020;23(5):656-665. [DOI] [PubMed] [Google Scholar]
- 16.Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with burst (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56-66. [DOI] [PubMed] [Google Scholar]
- 17.Kapural L, Yu C, Doust MW, et al. Novel 10-kHz High-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851-860. [DOI] [PubMed] [Google Scholar]
- 18.Sweet J, Badjatiya A, Tan D, Miller J. Paresthesia-free high-density spinal cord stimulation for postlaminectomy syndrome in a prescreened population: a prospective case series. Neuromodulation. 2016;19(3):260-267. [DOI] [PubMed] [Google Scholar]
- 19.Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eldabe S, Copley S, Gulve A, et al. A prospective long-term follow-up of dorsal root ganglion stimulation for the management of chronic intractable pain. Pain. 2022;163(4):702-710. [DOI] [PubMed] [Google Scholar]
- 21.NICE. Technology Appraisal Guidance [TA159]: Spinal Cord Stimulation for Chronic Pain of Neuropathic or Ischaemic Origin. London: National Institute for Health and Care Excellence; 2008. [Google Scholar]
- 22.Camberlin C, San Miguel L, Smit Y, Post P, Gerkens S, De Laet C. Neuromodulation for the management of chronic pain: implanted spinal cord stimulators and intrathecal analgesic delivery pumps. In Health Technology Assessment (HTA). Brussels: Belgian Health Care Knowledge Centre (KCE); 2012. [Google Scholar]
- 23.Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17(6):515-550; discussion 550. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services. National Coverage Determination (NCD) for Electrical Nerve Stimulators (160.7); 2020. Accessed 12 February 2020. https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=240&ncdver=1&bc=AAAAQAAAAAAA&. [Google Scholar]
- 25.Rigoard P, Basu S, Desai M, et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain. 2019;160(6):1410-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North R, Desai MJ, Vangeneugden J, et al. Postoperative infections associated with prolonged spinal cord stimulation trial duration (PROMISE RCT). Neuromodulation. 2020;23(5):620-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldabe S, Duarte RV, Gulve A, et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost-effectiveness (TRIAL-STIM)? A randomised controlled trial. Pain. 2020;161(12):2820-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chadwick R, McNaughton R, Eldabe S, et al. To trial or not to trial before spinal cord stimulation for chronic neuropathic pain: the patients' view from the TRIAL-STIM randomized controlled trial. Neuromodulation. 2021;24(3):459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eldabe S, Gulve A, Thomson S, et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost-effectiveness? (TRIAL-STIM Study): study protocol for a randomised controlled trial. Trials. 2018;19(1):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clin Res Ed). 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemler MA, Barendse GA, van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):618-624. [DOI] [PubMed] [Google Scholar]
- 32.Eldabe S, Raphael J, Thomson S, et al. The effectiveness and cost-effectiveness of spinal cord stimulation for refractory angina (RASCAL study): study protocol for a pilot randomized controlled trial. Trials. 2013;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. [DOI] [PubMed] [Google Scholar]
- 34.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. [DOI] [PubMed] [Google Scholar]
- 36.Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol. 2018;101:87-106.e102. [DOI] [PubMed] [Google Scholar]
- 37.Sabourin S, Tram J, Sheldon BL, Pilitsis JG. Defining minimal clinically important differences in pain and disability outcomes of patients with chronic pain treated with spinal cord stimulation. J Neurosurg Spine. Published online ahead of print June 4, 2021. DOI: 10.3171/2020.11.SPINE201431. [DOI] [PubMed] [Google Scholar]
- 38.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523-1532. [DOI] [PubMed] [Google Scholar]
- 39.Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762-770; discussion 770. [DOI] [PubMed] [Google Scholar]
- 40.Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):667-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hariton E, Locascio JJ. Randomised controlled trials—the gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 2018;125(13):1716-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson SJ, Tavakkolizadeh M, Love-Jones S, et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial. Neuromodulation. 2018;21(1):67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Andres J, Monsalve-Dolz V, Fabregat-Cid G, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med. 2017;18(12):2401-2421. [DOI] [PubMed] [Google Scholar]
- 45.Perruchoud C, Eldabe S, Batterham AM, et al. Analgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled study. Neuromodulation. 2013;16(4):363-369; discussion 369. [DOI] [PubMed] [Google Scholar]
- 46.Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJ. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain. 2017;21(3):507-519. [DOI] [PubMed] [Google Scholar]
- 47.Eldabe S, Duarte R, Gulve A, et al. Analgesic efficacy of “burst” and tonic (500 Hz) spinal cord stimulation patterns: a randomized placebo-controlled crossover study. Neuromodulation. 2021;24(3):471-478. [DOI] [PubMed] [Google Scholar]
- 48.Al-Kaisy A, Palmisani S, Pang D, et al. Prospective, randomized, sham-control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS frequency study). Neuromodulation. 2018;21(5):457-465. [DOI] [PubMed] [Google Scholar]
- 49.Duarte RV, McNicol E, Colloca L, Taylor RS, North RB, Eldabe S. Randomized placebo-/sham-controlled trials of spinal cord stimulation: a systematic review and methodological appraisal. Neuromodulation. 2020;23(1):10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNicol E, Ferguson M, Bungay K, et al. Systematic review of research methods and reporting quality of randomized clinical trials of spinal cord stimulation for pain. J Pain. 2021;22(2):127-142. [DOI] [PubMed] [Google Scholar]
- 51.Duarte RV, Bresnahan R, Copley S, et al. Reporting guidelines for clinical trial protocols and reports of implantable neurostimulation devices: protocol for the SPIRIT-iNeurostim and CONSORT-iNeurostim extensions. Neuromodulation. Published online ahead of print November 17, 2021. DOI: 10.1016/j.neurom.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Duarte RV, Thomson S. Trial versus no trial of spinal cord stimulation for chronic neuropathic pain: cost analysis in United Kingdom National Health Service. Neuromodulation. 2019;22(2):208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duarte RV, Houten R, Nevitt S, et al. Screening trials of spinal cord stimulation for neuropathic pain in England—a budget impact analysis. Front Pain Res. 2022;3:974904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomson SJ, Kruglov D, Duarte RV. A spinal cord stimulation service review from a single centre using a single manufacturer over a 7.5 year follow-up period. Neuromodulation. 2017;20(6):589-599. [DOI] [PubMed] [Google Scholar]
- 55.U.S. Food and Drug Administration. Conduct a Trial Stimulation Period Before Implanting a Spinal Cord Stimulator (SCS) - Letter to Health Care Providers; 2020. https://www.fda.gov/medical-devices/letters-health-care-providers/conduct-trial-stimulation-period-implanting-spinal-cord-stimulator-scs-letter-health-care-providers. Accessed December 2021. [Google Scholar]
- 56.Thomson S, Huygen F, Prangnell S, et al. Appropriate referral and selection of patients with chronic pain for spinal cord stimulation: European consensus recommendations and e-health tool. Eur J Pain. 2020;24(6):1169-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomson S, Huygen F, Prangnell S, et al. Applicability and validity of an e-health tool for the appropriate referral and selection of patients with chronic pain for spinal cord stimulation: results from a European retrospective study. Neuromodulation. Published online ahead of print January 21, 2022. DOI: 10.1016/j.neurom.2021.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Consideration of a smaller minimally clinical important difference (MCID).Supplementary Material 2. Cost-effectiveness analysis considerations.