BACKGROUND:
Spinal anesthesia is safe and effective in lumbar surgeries, with numerous advantages over general anesthesia (GA). Nevertheless, 1 major concern preventing the widespread adoption of this anesthetic modality in spine surgeries is the potential for intraprocedural anesthetic failure, resulting in the need to convert to GA intraoperatively.
OBJECTIVE:
To present a novel additional prone dose algorithm for when a first spinal dose fails to achieve the necessary effect.
METHODS:
A total of 422 consecutive patients undergoing simple and complex thoracolumbar surgeries under spinal anesthesia were prospectively enrolled into our database. Data were retrospectively collected through extraction of electronic health records.
RESULTS:
Sixteen of 422 required a second prone dose, of whom 1 refused and was converted to GA preoperatively. After 15 were given a prone dose, only 2 required preoperative conversion to GA. There were no instances of intraoperative conversion to GA. The success rate for spinal anesthesia without the need for conversion rose from 96.4% to 99.5%. In patients who required a second prone dose, there were no instances of spinal headache, deep vein thrombosis, pneumonia, urinary tract infection, urinary retention, readmission within 30 days, acute pain service consult, return to operating room, durotomy, or cerebrospinal fluid on puncture.
CONCLUSION:
Use of an additional prone dose algorithm was able to achieve a 99.5% success rate, and those who received this second dose did not experience any complications or negative operative disadvantages. Further research is needed to investigate which patients are at increased risk of inadequate analgesia with spinal anesthesia.
KEY WORDS: Spinal anesthesia, Prone dose, Algorithm, Lumbar, Spine surgery
ABBREVIATIONS:
- EBL
estimated blood loss
- GA
general anesthesia (GA).
The use of spinal anesthesia in lumbar surgery has been shown to be safe and effective.1 The technique confers numerous clinical advantages over GA including reduced complications, mortality, pain, use of analgesics, hospitalization time, intraoperative blood loss, anesthesia times, operative times, postoperative cognitive dysfunction, cost, and even environmental harm.2-5 Despite this, spinal anesthesia has yet to gain widespread adoption in mainstream practice. One major concern with using spinal anesthesia is the potential for intraprocedural anesthetic failure, resulting in the need to convert to GA intraoperatively. Fortunately, this appears to be rare, with some large series showing a 0% incidence.1,6-8 Preoperative conversion to GA, however, is reported in approximately 4% of cases, when the intrathecal dose fails to achieve the necessary anesthetic effect.9
At our center, we use a novel second prone dose algorithm when a first spinal dose fails to achieve the necessary effect. Here, we describe our experience using this anesthetic algorithm in our cohort of 422 patients undergoing simple and complex thoracolumbar surgeries under spinal anesthesia, as well as analyze its efficacy at reducing the rate of preoperative conversion to GA. We also investigate complication rates with an additional spinal dose. Finally, we examine which patients are more susceptible to difficulty with administration of spinal anesthesia and discuss methods of overcoming these limitations.
METHODS
Between 2017 and 2021, 422 consecutive patients undergoing simple and complex thoracolumbar surgeries under spinal anesthesia were prospectively enrolled into our database. Sixteen patients had an unsuccessful first spinal anesthesia dose requiring either an additional prone dose or conversion to GA, which was compared with 406 patients who did have a successful first dose that achieved adequate analgesia. Surgeries were generally performed with minimally invasive techniques: Fusions were performed through a minimally invasive transforaminal approach using the Wiltse plane, and laminectomies were performed through a unilateral paramedian exposure approach.10 Research was performed in accordance with the principles outlined in the Declaration of Helsinki, and approval for this study was provided by the hospital's institutional review board. Requirement for patient consent was waived for this retrospective study. Data are unavailable because of the need to protect patient privacy. This study was reported in line with the Strengthening The Reporting of Observational studies in Epidemiology (STROBE) checklist.11
Our algorithm for spinal anesthesia administration is illustrated in Figure 1. Lumbar MRI scans are first reviewed to assess anatomy and plan for optimal location of the spinal anesthetic injection, typically 1 to 2 levels above the level of the procedure and below the conus medullaris. For the initial spinal anesthesia administration, the patient is in an upright position leaning forward, and 3 mL of isobaric 0.5% bupivacaine is injected into the intrathecal space. The patient is then positioned prone, prepped, and draped in the 10 to 15 minutes necessary for the anesthetic to take effect. Anesthesia effectiveness is determined by infiltration of subcutaneous local anesthesia at the incision site. If there is no pain during injection, surgery may commence. If there is pain, the level of anesthesia is identified and determined whether to be ascending by rechecking every 1 to 2 minutes. If the level ascends to the surgical site, surgery may then commence. If the level fails to reach the surgical site over an additional 10 minutes, a second 15-mg isobaric bupivacaine dose is administered by the surgical team with the patient in the prone position. Given that patients are typically positioned in lordosis for many surgeries, a position not favorable for lumbar puncture, we have used fluoroscopy when necessary for assistance. The anesthetic level is then checked every 1 to 2 minutes for effect. If the second dose fails to achieve adequate anesthesia within 10 to 15 minutes, the patient is undraped and transitioned to general endotracheal anesthesia in the supine position.
FIGURE 1.
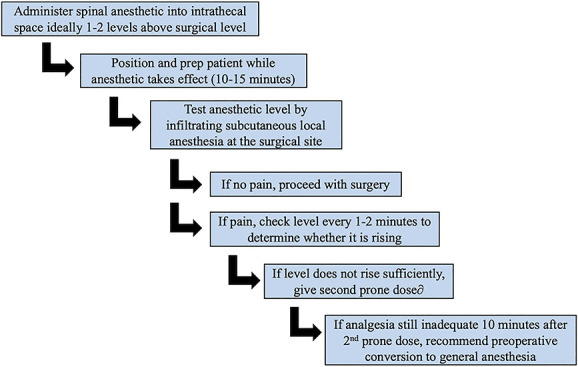
Additional prone dose algorithm after initial administration of spinal anesthetic.
Data were retrospectively collected through extraction of patient electronic health records. Demographic information collected includes age, sex, body mass index (BMI), and American Society of Anesthesiology (ASA) score. Surgical information collected includes whether the operation was a fusion, estimated blood loss, length of hospitalization, operative time, preoperative conversion to GA, intraoperative conversion to GA, and adjunctive intraoperative ketamine use. Perioperative complications recorded include spinal headache, deep vein thrombosis, pneumonia, urinary tract infection, urinary retention, readmission within 30 days, acute pain service consult, return to operating room, durotomy, and intraoperative visualization of the spinal anesthetic puncture.
Statistical analyses were conducted using R version 4.1.1. The Wilcoxon rank sum test, Pearson χ2 test, or Fisher exact test were used for comparison of groups. A P-value of <.05 was considered significant.
RESULTS
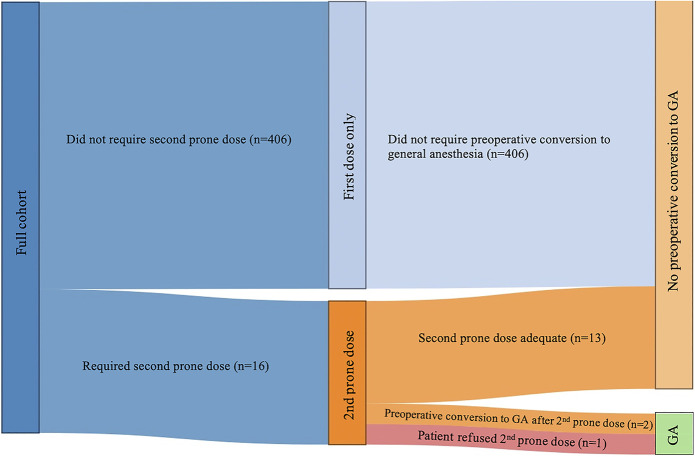
Figure 2 illustrates a Sankey diagram of the flow of patients who required an additional prone dose or were converted to GA. A total of 422 consecutive patients underwent spinal anesthesia for thoracolumbar surgery. Of these, 16 required a second prone dose, representing a 3.8% failure rate of the first spinal injection. One of these patients, however, refused an additional prone dose and underwent preoperative conversion to GA after only 1 dose. Thus, this patient was excluded from subsequent analysis of the algorithm. After being given a second prone dose, 13 of the 15 achieved adequate anesthesia and only 2 required preoperative conversion to GA. There were no instances of intraoperative conversion to GA. Using a second prone dose algorithm, the success rate for spinal anesthesia without the need for preoperative conversion to GA rose from 96.4% (406/421) to 99.5% (419/421).
FIGURE 2.
Sankey diagram showing flow of patients who required second prone dose or preoperative conversion to general anesthesia. Not drawn to scale for illustrative purposes. GA, general anesthesia.
Table 1 compares the baseline characteristics of patients requiring a second prone dose to those who did not. The second prone dose cohort had a younger mean age than the single dose cohort, although this did not achieve statistical significance (P-value = .052). Female sex, BMI, ASA score, and history of spine surgery at the same level were balanced between the groups.
TABLE 1.
Baseline Characteristics
| Variable | Successful first dose (n = 406)a | Unsuccessful first dose (n = 16)a | P valueb |
|---|---|---|---|
| Age, y | 63 (14) | 58 (10) | .052 |
| Female | 176 (43%) | 4 (25%) | .15 |
| BMI | 28.6 (5.1) | 26.5 (4.9) | .17 |
| ASA score | 2.57 (0.59) | 2.44 (0.51) | .27 |
| History of spine surgery at same level | 63 (16%) | 2 (12%) | >.99 |
ASA, American Society of Anesthesiology; BMI, body mass index.
Mean (SD); n (%).
Wilcoxon rank sum test; Pearson χ2 test; Fisher exact test.
Table 2 compares surgical characteristics between each group. There was a greater proportion of nonfusion surgeries that required a second prone dose (P-value = .01). As expected, reoperative conversion rates to GA were higher in those who have already received a second prone dose compared with those who only received a single dose of spinal. Estimated blood loss, length of stay, operative time, and adjunctive intraoperative ketamine use were comparable between the groups.
TABLE 2.
Surgical Characteristics
| Variable | Successful first dose (n = 406)a | Unsuccessful first dose (n = 16)a | P valueb |
|---|---|---|---|
| Fusion | 233 (57%) | 4 (25%) | .010 |
| Estimated blood loss | 15 (25) | 15 (22) | .76 |
| Length of stay | 2.00 (1.70) | 1.19 (1.17) | .051 |
| Operative time | 96 (31) | 85 (30) | .18 |
| Preoperative conversion to general anesthesia | 0 (0%) | 3 (19%) | <.001 |
| Adjunctive ketamine use | 5 (1.2%) | 1 (6.2%) | .21 |
n (%); Mean (SD).
Pearson's χ2 test; Wilcoxon rank sum test; Fisher exact test.
Table 3 compares the rates of perioperative complications between the groups. In the patients who had an unsuccessful first dose, there were no instances of spinal headache, deep vein thrombosis, pneumonia, urinary tract infection, urinary retention, readmission within 30 days, acute pain service consult, return to operating room, durotomy, or cerebrospinal fluid (CSF) on puncture.
TABLE 3.
Perioperative Complications
| Variable | Successful first dose (n = 406)a | Unsuccessful first dose (n = 16)a | P valueb |
|---|---|---|---|
| Spinal headache | 5 (1.2%) | 0 (0%) | >.99 |
| Deep vein thrombosis | 0 (0%) | 0 (0%) | >.99 |
| Pneumonia | 3 (0.7%) | 0 (0%) | >.99 |
| Urinary tract infection | 3 (0.7%) | 0 (0%) | >.99 |
| Urinary retention | 18 (4.4%) | 0 (0%) | >.99 |
| Readmission within 30 d | 4 (1.0%) | 0 (0%) | >.99 |
| Acute pain service | 12 (3.0%) | 0 (0%) | >.99 |
| Return to operating room | 1 (0.2%) | 0 (0%) | >.99 |
| Durotomy | 6 (1.5%) | 0 (0%) | >.99 |
| CSF on puncture | 4 (1.0%) | 0 (0%) | >.99 |
CSF, cerebrospinal fluid.
n (%).
Fisher exact test.
DISCUSSION
In this study, we demonstrate that the use of an additional prone dosing algorithm allowed us to extend our success in avoiding preoperative conversion to GA from 96.4% to 99.5%. There were no cases of intraoperative conversion to GA. The patients who required a second prone dose did not experience any other perioperative complications.
Table 4 compares the spinal anesthesia failure rates requiring conversion to GA in other published series of lumbar surgeries. Letchuman et al12 described a series of 15 decompression and lumbar fusion surgeries under spinal anesthesia, reporting a 7% intraoperative conversion to GA. It is unclear whether this switch happened before an incision or during the middle of surgery. Kolcun et al13 reported a series of endoscopic transforaminal lumbar interbody fusions under spinal anesthesia, in which 4 of 100 cases (4%) required conversion to GA. Again, it is unclear whether this was pre-incision or post-incision. Pierce et al9 described a series of 361 spinal anesthesia patients undergoing laminectomies and diskectomies, reporting 14 conversions to general, all before an incision being made. Eleven of the 14 were converted after prone positioning. Walcott et al6 studied a sample of 81 spinal anesthesia patients undergoing diskectomies and posterior decompression of which 2 intraoperative conversions to GA were recorded. Lessing et al14 conducted a study on 56 spinal anesthesia patients all age 70 years or older who underwent lumbar decompression and fusion procedures, of which there were no reported conversions to GA. Singeisen et al15 reported a series of 368 spinal anesthesia patients who underwent decompression, diskectomy, and fusions in the prone position with 7 confirmed conversions to GA. West et al16 described 34 patients undergoing lumbar diskectomy, laminectomy, and fusions under spinal anesthesia of which none required conversion to GA.
TABLE 4.
Previously Published Rates of Failure and Conversion to General Anesthesia in Lumbar Surgeries
We also attempted to understand what types of patients were more susceptible to failed spinal doses. Patients who required a second prone dose in our cohort were younger, occurred more in nonfusion surgeries, and had higher rates of eventual conversion to GA. We cannot conclusively explain why age and nonfusion surgeries showed a difference, although the higher rates of conversion in the second dose group was expected as the selection of patients who have already failed the initial spinal dose greatly increases the chances of including patients with underlying pathologies preventing the successful administration of spinal anesthesia. The potential reasons for failed spinal anesthesia are numerous and multifaceted. At the operator level, there could be an inability to obtain CSF and enter the correct space because of incorrect needle insertion of poor patient positioning or abnormal patient anatomy.17 Even the appearance of clear fluid at the needle hub may sometimes not confirm successful lumbar puncture, as it enters a congenital arachnoid cyst or a Tarlov cyst that mimics an initial “flow” of CSF but does not allow for intrathecal spread of anesthetic.17,18 The spinal needle may be inadvertently withdrawn during injection resulting in partial epidural administration. Operator error, however, would not well-explain the finding that younger patients are more likely to need an additional dose. Similarly, spinal anesthesia is always induced under direct supervision of an attending anesthesiologist. Although the procedure may be performed by a nurse anesthetist or resident, an unsuccessful lumbar puncture will result in attending aide. Thus, operator error is likely not a substantial contributing factor.
Intrathecal spread of anesthetic may be prevented by anatomic abnormalities such as kyphosis or scoliosis or rarely by the formation of complete septae by spinal ligaments. However, these abnormalities are common in older patients and, therefore, also unlikely to be the causative issue. The size of the thecal sac and volume of CSF present are considerations as a large lumbar cistern may prevent the analgesic from reaching an effective concentration. A case study by Spiegel et al19 presented a patient whose exceptionally large intrathecal volume, as measured by MRI, was the likely cause of a failed spinal anesthesia attempt. Another study by Wang et al20 used the cross-sectional area of the dural sac acquired using ultrasound to effectively dose spinal anesthesia for patients undergoing transurethral prostate resection. These studies show that size of the dural sac may have serious implications in the effectiveness of spinal anesthesia and is likely a major causative factor of first dose failure in our manuscript. This theory is consistent with the younger age of involved patients as they have less facet and ligamentous hypertrophy. It is also consistent with the lower rate of second dose administration in fusion patients as they would be likely to have a more degenerative spine and smaller lumbar cistern. This is an active topic of further research at this time.
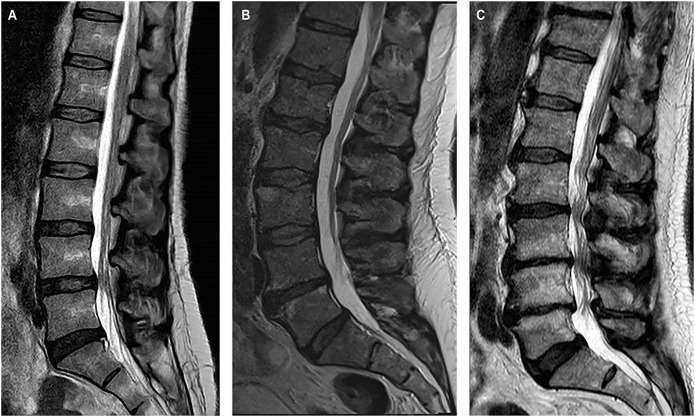
Three patients required preoperative conversion to GA: 2 after a second prone dose failed to provide adequate analgesia and 1 who refused a second dose. Patient 1 was a 49-year-old female with a BMI of 19.7, ASA score of 2, and received a L4-L5 transforaminal lumbar interbody fusion for spinal stenosis and spondylolisthesis. The operation took 97 minutes, and she stayed for 2 days in the hospital before discharge. There were no complications. Patient 2 was a 56-year-old male who also received a second prone dose and required preoperative conversion to GA. He has a BMI of 30.27, ASA score of 3, and received a L4-L5 microdiskectomy for recurrent disk herniation. The operation took 88 minutes, and he stayed for 1 day in the hospital. There were no complications. Patient 3 was a 69-year-old male who required preoperative conversion to GA before the second dose. He has a BMI of 28.7, ASA score of 2, and received a L2-L3 far lateral microdiskectomy for a disk herniation. The operation took 89 minutes, and he left the same day from the hospital. There were no complications. Imaging for all 3 patients is shown in Figure 3.
FIGURE 3.
Preoperative mid-sagittal T2-weighted lumbar MRI scans for patients who required preoperative conversion to general anesthesia. A, 49-year-old woman who received an additional prone dose. 1. B, 56-year-old man who also received an additional prone dose. C, 69-year-old man who received preoperative conversion to general anesthesia before an additional prone dose.
There are several considerations to keep in mind while implementing our additional prone dose algorithm. Patients should always be first counseled about the steps involved in spinal anesthesia, and the possibilities for requiring a second dose while prone as well as for ultimately converting to GA. Before administering the first dose of anesthetic, lumbar MRI scans are always reviewed for any abnormal anatomy. At our institution, the anesthesiologist administers the first dose of spinal anesthetic while the patient is sitting upright, and the surgeon provides the second as needed when the patient is positioned prone and already prepped for surgery. The patient is never sedated with additional intravenous anesthetics until adequate anesthesia at the surgical site is confirmed. This allows for consistent feedback regarding the anesthetic effect. Then, the decision to administer a second prone dose is always the surgeon's judgement call in communication with the anesthesiologist and the patient. Conversion to GA also requires agreement between the patient and the surgical and anesthetic teams. We use a timeline of longer than 10 minutes postadministration of a second dose of spinal anesthetic as the benchmark for failure. Intraoperatively, ketamine may also be a useful adjunct in cases of unrecognized incomplete anesthesia or if anesthesia begins to wear off late in the case. Finally, we often induce mild sedation with intravenous propofol, ketamine, dexmedetomidine, fentanyl, or midazolam. However, it is advisable to withhold sedatives in high-risk patients for polypharmacy in risk of postoperative cognitive dysfunction and delirium.
Limitations
There are several limitations in our study. The data in this study were analyzed retrospectively and suffer from the potential for missing or incomplete data. Furthermore, we did not compare 1 cohort using this additional prone dose algorithm with another cohort that did not. However, those that did receive a second prone dose would have otherwise required conversion to GA. In addition, a greater number of patients who had an unsuccessful first dose of spinal anesthesia are needed to strengthen statistical power, especially in the case of assessing complication rates. Finally, although an attending physician was present for all spinal blocks, several different anesthesia providers were involved with varying levels of expertise which could have led to failure of spinal anesthesia.
CONCLUSION
With the use of a second prone dose algorithm, spinal anesthesia can achieve a 99.3% success rate defined as the avoidance of preoperative conversion to GA. Patients in our cohort who received a second prone dose did not have any complications or negative operative disadvantages. Further research is needed to investigate which patients are at increased risk of inadequate analgesia with spinal anesthesia.
Acknowledgments
Statisticians at DataLab, Tufts University School of Medicine.
Contributor Information
Andy Y. Wang, Email: andy.wang@tufts.edu.
Michelle Olmos, Email: Michelle.Olmos@tufts.edu.
Tameem Ahsan, Email: Tameem.Ahsan@tufts.edu.
Matthew Kanter, Email: Matthew.Kanter@tufts.edu.
Penny Liu, Email: pliu@tuftsmedicalcenter.org.
Konstantin Balonov, Email: kbalonov@tuftsmedicalcenter.org.
Ron I. Riesenburger, Email: rriesenburger@tuftsmedicalcenter.org.
Funding
Andy Y. Wang was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number TL1TR002546. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Breton JM, Ludwig CG, Yang MJ, et al. Spinal anesthesia in contemporary and complex lumbar spine surgery: experience with 343 cases. J Neurosurg Spine. 2021;36(4):534-541. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Roman RJ, Govindarajan V, Bryant JP, Wang MY. Spinal anesthesia in awake surgical procedures of the lumbar spine: a systematic review and meta-analysis of 3709 patients. Neurosurg Focus. 2021;51(6):E7. [DOI] [PubMed] [Google Scholar]
- 3.Patil H, Garg N, Navakar D, Banabokade L. Lumbar spine surgeries under spinal anesthesia in high-risk patients: a retrospective analysis. World Neurosurg. 2019:S1878-8750(19)30117-2. [DOI] [PubMed] [Google Scholar]
- 4.Ehsani R, Djalali Motlagh S, Zaman B, Sehat Kashani S, Ghodraty MR. Effect of General versus spinal anesthesia on postoperative delirium and early cognitive dysfunction in elderly patients. Anesth Pain Med. 2020;10(4):e101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng T, Zhong Z, Meng L. Impact of spinal anaesthesia vs. general anaesthesia on peri-operative outcome in lumbar spine surgery: a systematic review and meta-analysis of randomised, controlled trials. Anaesthesia. 2017;72(3):391-401. [DOI] [PubMed] [Google Scholar]
- 6.Walcott BP, Khanna A, Yanamadala V, Coumans JV, Peterfreund RA. Cost analysis of spinal and general anesthesia for the surgical treatment of lumbar spondylosis. J Clin Neurosci. 2015;22(3):539-543. [DOI] [PubMed] [Google Scholar]
- 7.Li ZZ, Zhao HL, Cao Z, Shang WL, Hou SX. [Technical notes and clinical efficacy analysis of full-endoscopic thoracic discectomy via transforaminal approach]. Zhonghua Yi Xue Za Zhi. 2020;100(4):279-285. [DOI] [PubMed] [Google Scholar]
- 8.Wang MY, Chang P, Grossman J. Development of an enhanced recovery after surgery (ERAS) approach for lumbar spinal fusion. J Neurosurg Spine. 2017;26(4):411-418. [DOI] [PubMed] [Google Scholar]
- 9.Pierce JT, Kositratna G, Attiah MA, et al. Efficiency of spinal anesthesia versus general anesthesia for lumbar spinal surgery: a retrospective analysis of 544 patients. Local Reg Anesth. 2017;10:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tataryn Z, Alkhalili K, Kryzanski JT. Hydrodissection of Wiltse's plane to facilitate exposure during minimally invasive transforaminal lumbar interbody fusion. Cureus. 2017;9(11):e1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. [DOI] [PubMed] [Google Scholar]
- 12.Letchuman V, Agarwal N, Mummaneni VP, et al. Awake spinal surgery: simplifying the learning curve with a patient selection algorithm. Neurosurg Focus. 2021;51(6):E2. [DOI] [PubMed] [Google Scholar]
- 13.Kolcun JPG, Brusko GD, Basil GW, Epstein R, Wang MY. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurg Focus. 2019;46(4):E14. [DOI] [PubMed] [Google Scholar]
- 14.Lessing NL, Edwards CC, 2nd, Brown CH, 4th, et al. Spinal anesthesia in elderly patients undergoing lumbar spine surgery. Orthopedics. 2017;40(2):e317-e322. [DOI] [PubMed] [Google Scholar]
- 15.Singeisen H, Hodel D, Schindler C, Frey K, Eichenberger U, Hausmann ON. Signifikant kürzere Anästhesiezeit bei lumbaler Wirbelsäulenchirurgie: prozessanalytischer Vergleich von Spinalanästhesie und Intubationsnarkose [Significantly shorter anesthesia time for surgery of the lumbar spine: process analytical comparison of spinal anesthesia and intubation narcosis]. Anaesthesist. 2013;62(8):632-638. [DOI] [PubMed] [Google Scholar]
- 16.West JL, De Biase G, Bydon M, et al. What is the learning curve for lumbar spine surgery under spinal anesthesia? World Neurosurg. 2022;158:e310-e316. [DOI] [PubMed] [Google Scholar]
- 17.Fettes PD, Jansson JR, Wildsmith JA. Failed spinal anaesthesia: mechanisms, management, and prevention. Br J Anaesth. 2009;102(6):739-748. [DOI] [PubMed] [Google Scholar]
- 18.Popham PA. Anatomical causes of failed spinal anaesthesia may be commoner than thought. Br J Anaesth. 2009;103(3):459; author reply 459. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel JE, Hess P. Large intrathecal volume: a cause of true failed spinal anesthesia. J Anesth. 2007;21(3):399-402. [DOI] [PubMed] [Google Scholar]
- 20.Wang WB, Sun AJ, Yu HP, Dong JC, Xu H. Dural sac cross-sectional area is a highly effective parameter for spinal anesthesia in geriatric patients undergoing transurethral resection of the prostate: a prospective, double blinded, randomized study. BMC Anesthesiol. 2020;20(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
COMMENTS
The authors provide a well written manuscript that highlights that a second intrathecal dose of isobaric bupivacaine given in the prone position by the surgeon prevented conversion to general anesthesia in 13 of 15 spinal surgery patients (87%) who failed to achieve adequate analgesia with an initial intrathecal dose administered by the anesthesia team in the sitting position. Patients receiving this second dose did not have a significantly higher rate of complications than those receiving only 1 dose. We commend the authors for providing evidence regarding the safety and efficacy of this simple salvage protocol. It may be the case that risks of converting to general anesthesia outweigh the risks of attempting a second intrathecal dose.
There are several other take home points in this paper. Patients can be counseled prior to surgery that a second intrathecal dose (and patience from the surgeon, anesthesiologist, and patient) may be required. It is interesting that younger patients were more likely to require a second dose. Although the authors hypothesize that this may be due to larger lumbar cistern volumes in younger patients, they also point out that the reasons for inadequate spinal anesthesia are “numerous and multifaceted.”
In addition to other recent reports,1a-3a this paper is an important study that advances the application of spinal anesthesia to spinal surgery.
Timothy Chryssikos
Praveen Mummaneni
San Franciscio, California, USA
- 1a.Kai-Hong Chan A, Choy W, Miller CA, Robinson LC, Mummaneni PV. A novel technique for awake, minimally invasive transforaminal lumbar interbody fusion: technical note. Neurosurg Focus. 2019;46(4):E16. [DOI] [PubMed] [Google Scholar]
- 2a.Letchuman V, Agarwal N, Mummaneni VP, et al. Pearls and pitfalls of awake spine surgery: a simplified patient-selection algorithm. World Neurosurg. 2022;161:154-155. [DOI] [PubMed] [Google Scholar]
- 3a.Letchuman V, Agarwal N, Mummaneni VP, et al. Awake spinal surgery: simplifying the learning curve with a patient selection algorithm. Neurosurg Focus. 2021;51(6):E2. [DOI] [PubMed] [Google Scholar]
The authors describe their experience using an additional prone spinal anesthesia dose for when a first spinal dose fails to achieve the necessary effect in patients undergoing thoracolumbar surgeries. The success rate for spinal anesthesia without the need for conversion rose from 96.4% to 99.5%. There is a growing interest in spinal anesthesia for lumbar spine surgery, especially within enhanced recovery after surgery protocols. Recent studies have shown patient-centered benefits of spinal anesthesia for spine surgery, including decreased postoperative fatigue, faster postoperative mobilization and better pain control. In the current study only 15 patients were given a prone dose, and 2 required preoperative conversion to general anesthesia. A greater number of patients who had an unsuccessful first dose of spinal anesthesia is needed to better assess the complication rates of this technique. Further research is also needed to investigate which patients are at increased risk of inadequate analgesia with spinal anesthesia.
Gaetano De Biase
Alfredo Quiñones-Hinojosa
Jacksonville, Florida, USA