Abstract
Understanding the bioelectrical properties of bone tissue is key to developing new treatment strategies for bone diseases and injuries, as well as improving the design and fabrication of scaffold implants for bone tissue engineering. The bioelectrical properties of bone tissue can be attributed to the interaction of its various cell lineages (osteocyte, osteoblast and osteoclast) with the surrounding extracellular matrix, in the presence of various biomechanical stimuli arising from routine physical activities; and is best described as a combination and overlap of dielectric, piezoelectric, pyroelectric and ferroelectric properties, together with streaming potential and electro‐osmosis. There is close interdependence and interaction of the various electroactive and electrosensitive components of bone tissue, including cell membrane potential, voltage‐gated ion channels, intracellular signaling pathways, and cell surface receptors, together with various matrix components such as collagen, hydroxyapatite, proteoglycans and glycosaminoglycans. It is the remarkably complex web of interactive cross‐talk between the organic and non‐organic components of bone that define its electrophysiological properties, which in turn exerts a profound influence on its metabolism, homeostasis and regeneration in health and disease. This has spurred increasing interest in application of electroactive scaffolds in bone tissue engineering, to recapitulate the natural electrophysiological microenvironment of healthy bone tissue to facilitate bone defect repair.
Keywords: bone, dielectric, electric, ferroelectric, homeostasis, metabolism, piezoelectric, pyroelectric, regeneration
The cellular and extracellular matrix (ECM) components of bone tissue contribute to its electroactive and electrosensitive properties.
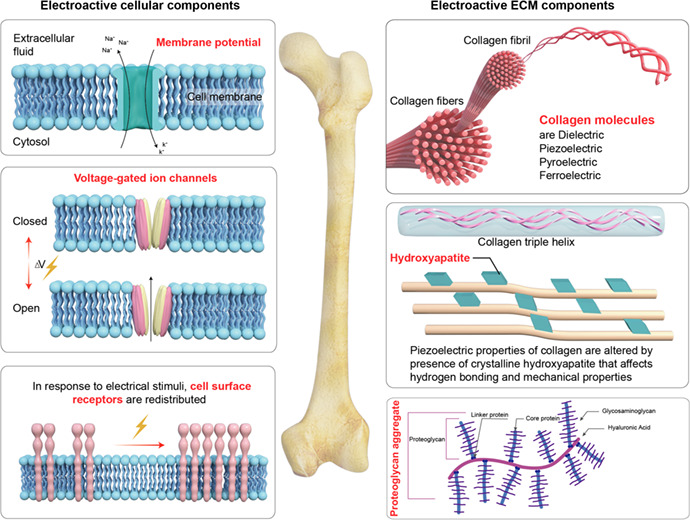
1. INTRODUCTION
Bone is a highly‐vascularized, but hard and rigid tissue that serves to provide structural support, and protect vulnerable soft tissues and organs within the human body. 1 It can occur as either compact or cancellous (trabecular) forms. 2 For the purpose of this review, the soft vascular, hematopoietic and marrow components of bone tissue will not be discussed, and the focus will be on its hard component per se. Similar to most other biological tissues, bone is composed of cells dispersed within extracellular matrix.
There are three major cell types within bone tissue: osteoblasts, osteocytes and osteoclasts. Osteoblasts originate from mesenchymal stromal/stem cells within the bone marrow, and serve to deposit osteoid, which is the organic portion of bone extracellular matrix that has not yet been mineralized 3 ; osteoblasts will subsequently mature into osteocytes within mineralized bone matrix. 4 Osteoclasts are large multi‐nucleated cells involved in bone resorption, and are derived from myeloid precursors within the bone marrow upon stimulation with MCSF (macrophage colony‐stimulating factor) and RANKL (receptor activator of nuclear factor kappa‐Β ligand). 5 It is this delicate antagonistic balance between the bone‐formation activities of osteoblasts versus the bone‐resorption activities of osteoclasts that regulates bone tissue homeostasis and remodeling. 6
Bone tissue has abundant extracellular matrix, consisting of 35% organic matrix, and 65% inorganic mineral matrix by volume. 7 The organic matrix is predominantly made up of collagen type I fibers (90%) that confer tensile strength to bone extracellular matrix via their highly stable triple helical structure. 8 The remainder of the organic matrix is composed of a diverse array of various proteoglycans (i.e. biglycan, lumican, osteoadherin) and glycoproteins (i.e. osteocalcin, osteopontin, osteonectin) that play key structural and mineralization roles in bone tissue. 9 The inorganic mineralized component of bone matrix is composed predominantly of nanocrystalline hydroxyapatite (Ca10 (PO4)6 (OH)2), a double salt of calcium phosphate and calcium hydroxide, together with lesser amounts of magnesium, fluoride and manganese salts. 10 These confer most of the hardness and rigidity to bone tissue. The deposition and incorporation of hydroxyapatite nanocrystals within the network of collagen fibrils, 11 , 12 is in turn responsible for the compressive strength of bone tissue.
The bioelectrical properties of bone tissue can be attributed to the interaction of its various cells lineages (osteocyte, osteoblast and osteoclast) with the bone extracellular matrix (Table 1) in the presence of various biomechanical stimuli arising from routine physical activities; and can be described as a combination of dielectric, piezoelectric, pyroelectric, and ferroelectric properties, together with streaming potential and electro‐osmosis, as presented in Table 2. 13 There is a hierarchical ordering of electrical properties, so that a ferroelectric material also possesses pyroelectric, piezoelectric and dielectric properties. These will be discussed in the following section. Indeed, the electrophysiological properties of bone tissue are key factors in regulating its homeostasis, remodeling and regeneration, and will therefore be the focus of this review.
TABLE 1.
The electroactive and electrosensitive components of bone tissue
| Electroactive/electrosensitive components | Description | Key references |
|---|---|---|
| Cell membrane potential | Estimated strength within the range of 40–500 mV mm−1, maintained by K+ and Na+ ion pumps | Brodwick 41 |
| Voltage‐sensitive ion channels | Ion channels that play mechanosensory and mechanotransduction role in bone tissue, are also sensitive and responsive to electrical stimuli, mediating ionic flux, which in turn deactivates or activates various signaling pathways that play key roles in bone tissue homeostasis, remodeling and regeneration. |
Zhang et al 42 Wright et al 43 McDonald 46 Miyauchi et al 50 |
| Intracellular signaling pathways | Influx of ions within cytosol in response to electrical stimuli can activate calcium‐calmodulin‐calcinueurin‐NFAT, RAS and ERK signaling pathways, which in turn promote transcription of various osteogenic genes |
Winslow et al 52 Zou et al 53 Wu et al 54 Kim et al 55 |
| Cell surface receptors | Some cell surface receptors, such as that for epidermal growth factor (EGF), fibronectin and concanavalin, have been reported to undergo redistribution on the cell membrane, in response to electrical stimuli, which in turn modulates cell adhesion, spreading and migration | Li et al 60 |
| Collagen | Possess dielectric, piezoelectric, pyroelectric and ferrolectric properties |
Williams and Saha 15 Nair et al 24 Lang 33 El Messiery et al 34 |
| Hydroxyapatite | High elastic moduli of hydroxyapatite crystals influence the transmission of mechanical loads on bone, and hence the mechanical response of collagen fibers to tensional or compressive forces, which in turn determines the generation of piezoelectric effect. Additionally, hydroxyapatite restricts the ability of collagen to form hydrogen bonds with water molecules, thereby exerting a profound influence on its bioelectrical properties |
Ahn and Grodzinsky 28 Marzec et al 61 |
| Proteoglycans and glycosminoglycans | Proteoglycans are constituted of a protein core that is covalently attached to highly anionic glycosaminoglycans chain (such as chondrotin sulfate, keratan sulfate, dermatan sulfate, and heparan sulfate) | Song et al 63 |
TABLE 2.
Summary of the bioelectrical properties of bone tissue
| Electrical properties | Definition | Mechanisms | Key references |
|---|---|---|---|
| Dielectric properties | Ability to display polarization of negative and positive charges upon application of an external electrical field | Separation of hydrogen bonds between collagen and hydroxyapatite (HA), upon application of electrical field |
Ray and Behari 14 Williams and Saha 15 Sierpowska et al 19 Haba et al 20 |
| Piezoelectric properties | Ability to generate an electric field in response to mechanical force | Application of mechanical force causes dipole rearrangement upon sliding of collagen fibers over each other and subsequent separation and polarization of –CO– and –NH– groups, which in turn generates a physiological electrical potential |
Zaszczyńska et al 21 Fukada and Yasuda 22 Lipieca et al 23 Nair et al 24 Kwon and Cho. 25 Bassett and Becker. 26 Tang et al 27 Ahn and Grodzinsky 28 Walsh and Guzelsu 29 |
| Pyroelectric properties | Ability to generate an electrical potential through polarization of negative and positive charges in response to changing temperature | Distortion of collagen triple helical structure with changing temperature, resulting in polarization of charged amino acid residues, thus generating an electrical potential |
Athenstaedt 30 Ravi et al 31 Ramachandran and Kartha 32 Lang 33 |
| Ferroelectric properties | Capacity to exhibit reversible spontaneous polarization and hysteresis loop even in the absence of an electric field, similar to typical ferroelectric domain alignment | Collagen fibers can spontaneously and reversibly change their orientation in different directions, even in the absence of an electric field |
El Messiery et al 34 Hastings et al 35 |
| Streaming potential | Generation of electrical potential by the flow of fluid and ions, driven by mechanical loading of bone | Exposure of bone to mechanical stress via physical activity force the flow of fluids containing charged ions through the canaliculi and pores of bone tissue; it is this flow of ions against the charged bone surface that results in the generation of an electric potential |
Gross and Williams 36 Guzelsu and Walsh 37 Qin et al 38 |
| Electro‐osmosis | Electrically induced flow of fluid through a narrow channel | Generation of endogenous electrical potential in bone (i.e. piezoelectric potential), induces flow of interstitial fluid through the channels and pores of bone tissue (canaliculi, lacunae) | Crolet et al 39 , 40 |
2. BONE IS AN ELECTROACTIVE TISSUE
2.1. Dielectric properties of bone tissue
Dielectric materials refer to electrically insulating or non‐conducting materials that are able to display polarization of negative and positive charges upon application of an external electrical field. The dielectric constant is a measure of the polarizability of a dielectric material upon application of an external electric field. Bone tissue has been demonstrated to exhibit dielectric properties, which arise from the separation of hydrogen bonds between collagen and hydroxyapatite (HA) upon application of an external electrical field. 14 The dielectric behavior of bone tissues is dependent on the moisture content of the sample, as well as the frequency of the applied electric field, 15 , 16 , 17 with measurements on wet bone samples being more physiologically relevant than those on dry bone. The dielectric coefficient of bone tissue has been demonstrated to be closely interrelated with its mineral density and elastic modulus, which implies that the health status and mechanical performance of bone can be evaluated by assessing its dielectric properties. 18 , 19 Indeed, the study of Haba et al 20 utilizing impedance spectroscopy, reported a non‐linear correlation between bone mineral density (BMD) and the dielectric coefficients of the trabecular and sub‐chondral femoral head bone of human patients undergoing hip replacement due to osteoarthritis.
2.2. Piezoelectric properties of bone tissue
Piezoelectric materials refer to materials with the ability to generate an electric field in response to mechanical stress, as a result of linear electromechanical interaction between the electrical and mechanical state in crystalline materials. 21 In piezoelectric crystalline materials, there is an inverse arrangement of the basic repeating unit, and electrical neutrality arises from a perfect balance of positive and negative charges. Mechanical deformation disrupts the inversion symmetry and balance between positive and negative charges, resulting in the generation of electrical potential. 21
The piezoelectric properties of natural bone were first reported in 1957. 22 However, it was not until 2012, that Lipieca et al 23 confirmed the piezoelectric effect of bone at the molecular level via infrared spectroscopy, by showing that collagen fibrils are rich in –CO– and –NH– groups, which are dipoles. Indeed, the piezoelectric properties of bone tissue can be mainly attributed to its abundance of collagen. 24 , 25 The application of mechanical force on bone tissue causes dipole rearrangement as collagen fibers slide over each other, with subsequent separation and polarization of charged groups, which in turn generates a physiological electrical potential (piezoelectric) during routine physical activities such as walking, running and jumping. It had been reported that the polarity of the generated piezoelectric charges is dependent on the direction of mechanical stress or bone deformation, 26 with tension and compression generating positive and negative piezoelectric charges, respectively (Figure 1). The piezoelectric constant (d33) of bone tissue has been reported to lie within a range of 0.7–2.3 pC/N. 27
FIGURE 1.
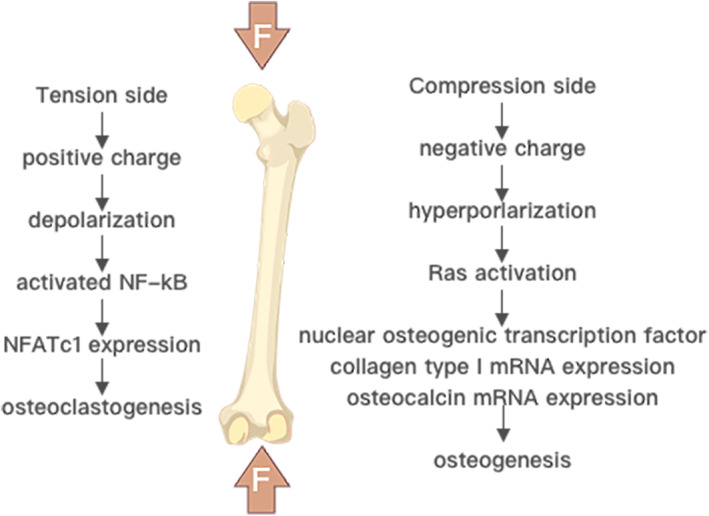
The piezoelectric potential induced by mechanical deformation of bone is negatively charged in areas of bone compression and positively charge in areas of traction. Adapted from Kao et al. 83
The piezoelectric properties of collagen in turn enable bone to be a dynamic tissue capable of responding to mechanical stress (Wolff's law 28 ), via piezoelectric stimuli on endogenous cells within bone tissue such as osteoblasts and osteocytes, as well as through generation of a greater zeta potential via increased surface charge density, which in turn increases streaming potential and electro‐osmosis (Figure 2), ultimately resulting in higher stiffness of bone tissues while decreasing hydraulic permeability. 29
FIGURE 2.
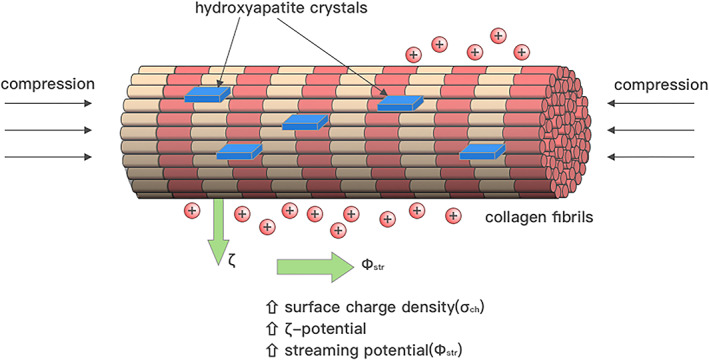
Tissue compression can lead to an increase in the surface charge density of the bone matrix. This in turn increases the zeta potential and streaming potential, as well as electro‐osmosis, all of which contributes to a stronger endogenous electric field. Adapted from Tikhonova et al. 112
2.3. Pyroelectric properties of bone tissue
Pyroelectric materials refer to materials that can generate an electric field through polarization of negative and positive charges in response to changing temperature. Bone tissue has been demonstrated to exhibit pyroelectric properties, which is attributed to its abundance of collagen fibers. 30 , 31 It is hypothesized that changes in temperature cause distortion in the triple helical structure of collagen, resulting in polarization of its charged amino acid residues, thus generating a temporary voltage and hence electric field. 32 Lang et al 33 reported that the pyroelectric coefficient of human femur is approximately 0.0036 ± 0.0021 μC/m2K, within a temperature range of 25–60°C. Nevertheless, it must be noted that the study of Lang et al 33 was based on dry bone samples, and it is doubtful that pyroelectric properties are relevant to living bone tissues, in which temperature is maintained close to 37°C in the human body. Even with fever, the relatively small temperature increase is highly unlikely to lead to appreciable pyroelectric effects.
2.4. Ferroelectric properties of bone tissue
Ferroelectric materials refer to materials that exhibit reversible spontaneous polarization and hysteresis loop even in the absence of an electric field, similar to typical ferroelectric domain alignment. In bone tissue, the collagen fibers can spontaneously and reversibly change their orientation in different directions, even in the absence of an electric field. 34 The existence of permanent dipoles and hysteresis loops within bone structure has been confirmed by the study of Hastings et al, 35 which also reported remnant polarization (Pr = 0.00068 μC/cm2) in bone tissue. The fact that collagen fibers within bone tissue can spontaneously change their polarization, even in the absence of an external electric field, is thus indicative of the ferroelectric properties of bone. 34 , 35
2.5. Streaming potential and electro‐osmosis in bone tissue
Streaming potential refers to generation of electrical potential by the flow of fluid and ions driven by mechanical loading of bone. 36 , 37 Exposure of bone to mechanical stress via physical activity forces the flow of fluids containing charged ions through the canaliculi and pores of bone tissue, and it is this flow of ions against the charged bone surface that results in the generation of an electric field, which is termed streaming potential, 38 as illustrated in Figure 3. Electro‐osmosis refers to the flow of interstitial fluid through the channels and pores of bone tissue (canaliculi, lacunae), induced by endogenous electrical potential, for example generation of piezoelectric potential by physical activities. 39 , 40 This in turn exerts a profound influence on bone remodeling and regeneration. 39 , 40
FIGURE 3.
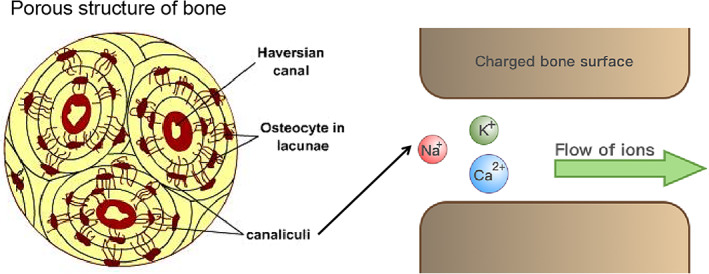
Schematic illustration depicting how the flow of fluids containing charged ions against the charged bone surface results in the generation of an electric field, which is termed streaming potential.
3. ELECTROACTIVE AND ELECTROSENSITIVE COMPONENTS OF BONE TISSUE
3.1. Cells
3.1.1. Cell membrane potential
Within bone tissues, there is an electric field around endogenous cells such as osteoblasts, osteocytes and osteoclasts due to transmembrane potential. 41 This arises from differences in ionic concentrations inside and outside the cell (i.e. K+, Na+, Ca2+), as a result of the action of ion pumps, with an estimated strength within the range of 40–500 mV mm−1. 41 Changes in transmembrane potential can be effected in response to electrical or mechanical stimuli, via voltage‐gated and mechanoresponsive ion channels respectively, which could in turn alter cell metabolism, as well as regulate various signaling pathways that control biological processes such as cell migration, adhesion, proliferation and differentiation, which in turn exert a wider effect on bone tissue homeostasis and remodeling. 42
3.1.2. Voltage‐sensitive ion channels
There exist a diverse array of Ca2+, Na+, K+ and Cl− ion channels within bone tissue that play key roles in homeostasis, remodeling and regeneration, many of which are known to be voltage sensitive and responsive to electrical stimuli. 41 These include Piezo1/2 channels, NMDA receptors, TRP family channels, voltage‐sensitive calcium channels (VSCCs), purinergic receptors, Ca2+ release‐activated Ca2+ channels (CRACs), calcium and voltage‐dependent big conductance potassium (BKCa) channels, small conductance channels, calcium‐activated potassium (SKCa) channels, TREK2 potassium channels, and epithelial sodium channels (ENaCs). 42 Although most of these play mechanosensory and mechanotransduction roles in bone tissue, they are also sensitive and responsive to electrical stimuli, resulting in ionic flux, which in turn deactivates or activates various signaling pathways that play key roles in bone tissue homeostasis, remodeling and regeneration.
Of particular interest is the role of voltage‐gated Ca2+ ion channels in regulating osteogenesis, osteoblast and osteoclast functions, as well as bone regeneration. It was reported that voltage‐gated Ca2+ channels 43 , 44 , 45 , 46 , 47 mediated an influx of Ca2+ ions into osteoblasts upon application of electrical stimuli, which in turn enhanced osteogenic differentiation via calmodulin‐mediated upregulation of transforming growth factor‐beta 1 (TGF‐beta1). 48 Although electrical stimuli can also trigger the activation of voltage‐gated Na+, K+ and Cl− channels, enhanced osteogenesis is thought to be mediated predominantly by voltage‐gated Ca2+ channels, as shown by the study Zhang et al 49 ; who observed that the pro‐osteogenic effects of electrical stimuli were completely nullified by specific inhibitors of voltage‐gated Ca2+ channels, whereas only a slight detrimental effect was observed with inhibitors of voltage‐gated Na+, K+ and Cl− channels.
In the case of osteoclasts, however, it was reported that the influx of Ca2+ ions mediated by voltage‐gated Ca2+ channels upon exposure to electrical stimuli resulted in cytoskeletal changes (disruption of actin ring formation) that impeded formation of the osteoclast‐specific adhesion structure – the podosome, which in turn inhibited the bone resorption activity of these cells. 50 , 51
3.1.3. Intracellular signaling pathways
Electrical stimuli such as piezoelectric potential generated through physical activities, trigger the opening of voltage‐gated Ca2+ channels on cell membranes, thus leading to an influx of Ca2+ ions within the cytosol. The increased level of intracellular Ca2+ levels activates calmodulin, causing it to bind and activate calcineurin, which in turn dephosphorylates nuclear factor of activated T cells (NFAT), enabling its translocation to the cell nuclei. 52 Subsequently, NFAT then facilitates the transcription of various pro‐osteogenic genes such as bone morphogenetic proteins (BMPs) and transforming growth factor β (TGF‐β), which are responsible for upregulating bone extracellular matrix production, as well as the synthesis of various proteins and growth factors involved in bone metabolism, homeostasis and growth. 53 , 54 Indeed, upregulation of TGF‐β expression induced by electrical stimuli via the calcium/calmodulin signaling pathway, appears to play a key role in bone defect healing and regeneration. 48 Besides the calcineurin‐calmodulin‐NFAT signaling cascade, elevated intracellular Ca2+ levels can also activate the Ras and extracellular signal‐related protein kinase (ERK) signaling pathways that promote transcription of pro‐osteogenic genes such as Runx2. 55 , 56 , 57 Additionally, elevated intracellular Ca2+ levels can promote cell adhesion by facilitating the binding of integrin to fibronectin, 58 as well as promoting cell migration by activating gelsolin to release more actin. 59
3.1.4. Cell surface receptors
Cell surface receptors for epidermal growth factor (EGF), fibronectin and concanavalin, which are transmembrane proteins connected to cytoskeletal actin, have been reported to undergo redistribution on the cell membrane, in response to electrical stimuli, which in turn modulates cell adhesion, spreading and migration. 60
3.2. Extracellular matrix
3.2.1. Collagen
As mentioned earlier, collagen (particularly Type I collagen) is highly abundant in bone extracellular matrix, and the dielectric, piezoelectric, pyroelectric and ferroelectric properties of bone tissue can be mainly attributed to the unique structural properties of collagen fibers. To briefly summarize, the dielectric properties of bone arise from the separation of hydrogen bonds between collagen and hydroxyapatite (HA) upon application of an external electrical field, 14 while piezoelectric properties are generated by polarization of –CO– and –NH– groups upon sliding of collagen fibers over each other, when mechanical force is applied. 24 , 25 , 26 The pyroelectric properties of bone are due to distortion in the triple helical structure of collagen in response to changing temperature, resulting in polarization of its charged amino acid residues, thus generating a temporary voltage and hence electric field. 33 Finally, the ferroelectric properties of bone are due to the ability of collagen fibers to spontaneously and reversibly change their orientation in different directions, in response to electric and magnetic stimuli, similar to typical ferroelectric domain alignment. 34 , 35
3.2.2. Hydroxyapatite
Although the bioelectrical properties of bone can be attributed mainly to collagen, the inorganic mineralized component of bone tissue, in particular hydroxyapatite, also contribute significantly to its bioelectrical properties. There are two major ways by which hydroxyapatite can influence the bioelectrical properties of bone tissue. Firstly, the high elastic moduli of hydroxyapatite crystals influence the transmission of mechanical loads on bone, and hence the mechanical response of collagen fibers to tensional or compressive forces, which in turn determines the generation of piezoelectric effect. 28 Secondly, hydroxyapatite restricts the ability of collagen to form hydrogen bonds with water molecules, thereby exerting a profound influence on its bioelectrical properties. 61 It is well known that there are significant differences in the dielectric and piezoelectric properties of wet versus dry bone samples. 15 , 62
3.2.3. Proteoglycans and glycosminoglycans
Proteoglycans are glycosylated proteins, which mean that they have a protein core that is covalently attached to highly anionic glycosaminoglycan chains (such as chondrotin sulfate, keratan sulfate, dermatan sulfate, and heparan sulfate). 63 The major proteoglycans in bone tissue include biglycan, lumican, and osteoadherin. The abundance of negative charges present on glycosaminoglycan chains enable sequestration of Ca2+ ions and various bioactive growth factors, as well as contributing to the overall negative charge of bone tissue, which is the reason why bone regeneration and healing is promoted when the natural electrical microenvironment at the defect site is recapitulated by negatively charged scaffold implants. 64 , 65
4. ELECTROPHYSIOLOGICAL PROPERTIES OF BONE TISSUE DURING DEFECT HEALING AND REGENERATION
Bone defect healing is a complex physiological process involving hemorrhage, coagulation, inflammation, angiogenesis, cell migration, and progressive tissue remodeling, which has been described in detail in several excellent reviews. 66 , 67 , 68 The focus here will be on how the electrophysiological properties of bone tissue changes during injury, and how these in turn influence bone defect healing and regeneration. Injury of bone tissue is associated with reduced electrical potential at the defect site. For small defects below critical size, the local electrophysiological microenvironment of the defect site is often quickly restored by the formation of periosteum‐like tissue. 69 , 70 Consequently, restoration of the electrophysiological microenvironment by periosteum‐like tissue exerts a galvanotactic effect that recruits cells from surrounding endogenous tissues into the defect site. 71 The cell recruitment mechanism involves surface charges attracting ions, which enhance cell attachment and protein adsorption via ionic charge interactions. 72 , 73 The larger the defect (still below critical size), the longer it will take for periosteum‐like tissue to form, thus delaying electrophysiological microenvironment recovery, which in turn slows down the healing process. In the case of defects exceeding critical size, the healing capacity is completely lost because of failure of periosteum tissues to form at the defect site; hence the need for implantation of an electroactive scaffold in situ to aid bone defect healing by recapitulating the electrophysiological environment of healthy bone tissue, 74 , 75 as illustrated in Figure 4.
FIGURE 4.
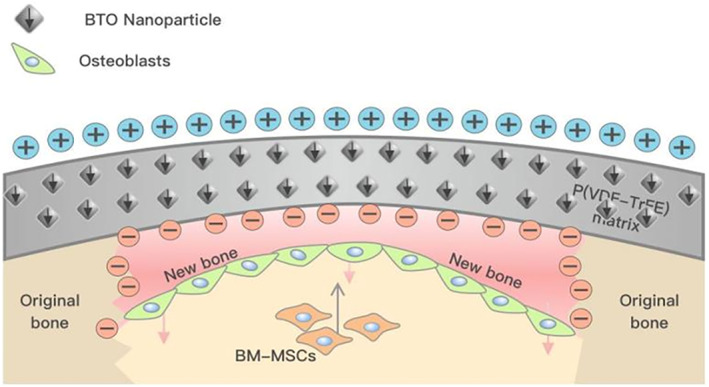
Restoration of the physiological electrical potential at the defect site with an electroactive scaffold can facilitate bone repair and regeneration. Adapted from Zhang et al. 71
More recently, some studies have suggested that the electrophysiological properties of bone can also modulate the inflammatory response at the defect site, which in turn influences the healing and regeneration process. 76 , 77 It has been reported that an electrical field could regulate macrophage migration, phagocytic activity and cytokine production. 76 , 77 In a recently published study, Dai et al 78 demonstrated that recapitulation of the physiological electrical microenvironment at bone defects with polarized nanocomposite membranes promotes the transformation of pro‐inflammatory M1 macrophages to the pro‐healing M2 phenotype, thereby facilitating bone repair and regeneration. It can thus be hypothesized that for smaller less than critical‐sized bone defects, rapid restoration of the electrical microenvironment via formation of periosteum‐like tissue is likely to mitigate inflammation at the defect site through promoting polarization of macrophages to the M2 phenotype, thus facilitating bone healing and regeneration.
5. ELECTROACTIVE SCAFFOLDS PROMOTE BONE REGENERATION BY SIMULATING AND RECAPITULATING THE BIOELECTRICAL PROPERTIES OF HEALTHY BONE TISSUE
As previously discussed in section 4, bone defects arising from traumatic injuries compromise the electrophysiological properties of bone tissue. Hence the implantation of electroactive biomaterials is a biomimetic strategy to promote bone healing and regeneration by simulating and recapitulating the natural bioelectrical properties of healthy bone tissue. Currently to date, there have already been several excellent reviews that have provided a comprehensive overview and critical analysis of electroactive scaffolds in bone tissue engineering. 74 , 75 Hence, only a brief overview and summary will be provided here.
Generally, electroactive bone tissue engineering scaffolds can be broadly classified into four distinct types: (i) piezoelectric scaffolds, (ii) electroconductive scaffolds, (iii) electrostimulation scaffolds with implantable energy harvesters and (iv) electroresponsive scaffolds, each of which will be briefly described in turn. Piezoelectric scaffolds are capable of generating electrical stimuli in response to mechanical loading or deformation of the scaffold. 79 , 80 Their main advantage is that pro‐osteogenic electrical stimuli can be generated without requiring an external power source; they are instead produced by natural bodily movement and routine physical activity. By contrast, electroconductive scaffolds promote osteogenesis and bone regeneration not by generation of electrical stimuli, but by enabling electron transport at the cell‐substrate interface, which in turn facilitates cell‐substrate interaction, cross‐talk, and intercellular communication. 81 , 82 Electrostimulation scaffolds with implantable energy harvesters harness various potential sources of energy within the human body to generate pro‐osteogenic electrical stimuli, for example, mechanical motion 83 , 84 , 85 and electrochemical energy. 86 , 87 Electroresponsive scaffolds, on the other hand, do not produce electrical stimuli, but instead respond to electrical stimuli, to effect drug release 88 , 89 or to induce changes in cellular function. 90
Electroactive scaffolds are known to promote bone defect healing and regeneration via five major mechanisms that involve (i) enhancement of osteogenesis, (ii) enhancement of angiogenesis, (iii) mitigation of inflammation, (iv) suppression of osteoclastogenesis, and (v) anti‐bacterial effects, each of which will be briefly described in turn. The enhancement of osteogenesis by electroactive scaffolds is thought to be effected by promoting the clustering of focal adhesions (FAs), which in turn trigger the mechanotransduction signaling axis to drive osteogenic differentiation via YAP/TAZ signaling. 91 , 92 Additionally, electroactive scaffolds can also elevate intracellular levels of Ca2+ ions via modulation of voltage‐gated calcium channels 93 or connexin 43, 94 which in turn promote osteogenic differentiation via the calcineurin/NFAT signaling pathway 52 or the protein kinase C (PKC) signaling pathway. 95 Enhancement of angiogenesis by electroactive scaffolds may involve activation of multiple signaling pathways by electrical stimuli, which include the VEGF/VEGFR, 96 ERK/MAPK, 97 PI3K‐Akt and Rho‐ROCK, 98 Akt, Erk1/2, and JNK 99 signaling pathways. Although suppression of osteoclastogenesis by electroactive scaffolds has been reported, 100 , 101 , 102 the underlying mechanisms are still unclear. Another mechanism by which electroactive scaffolds promote bone healing and regeneration is by mitigating inflammation by enhancing polarization of macrophages to the pro‐healing M2 phenotype, 78 , 103 which involves the AKT2‐IRF5/HIF‐1α 78 and RhoA/ROCK 103 signaling pathways. Finally, electroactive scaffolds can also promote bone defect healing and regeneration by exerting an anti‐bacterial effect through electropermeabilization of bacterial membrane, 104 increased generation of ROS 105 and disruption of the respiratory chain on the bacterial membrane. 106
Currently, the overwhelming majority of animal studies on the use of electroactive scaffolds to promote bone regeneration have focused on traumatic injury in young healthy animals, of which there are many excellent reviews. 74 , 75 , 107 , 108 , 109 What is lacking in the scientific literature are studies on using electroactive scaffolds to facilitate bone healing under degenerative conditions due to disease pathology, such as type II diabetes and osteoporosis. To date, there are only two known studies that have utilized electroactive scaffolds to promote bone healing under diabetic conditions, 78 , 103 which are characterized by increased inflammation 110 and bone resorption. 111 In the study of Dai et al, 78 it was demonstrated that hyperglycemic conditions under type II diabetic conditions aggravated the inflammatory action of macrophages, which impeded bone regeneration in a rat bone defect model. Inflammation was mitigated by covering the bone defect with a polarized BaTiO3/P(VDF‐TrFE) nanocomposite membrane that promoted transition of macrophages from the pro‐inflammatory M1 phenotype to the pro‐healing M2 phenotype. A favorable osteoimmunomodulatory environment was thus created by the implanted electroactive nanocomposite membrane, which was conducive to bone defect healing. Similar results from the study of Li et al 103 were reported, which demonstrated that an electroactive scaffold composed of polydopamine‐mediated graphene oxide (PGO) and hydroxyapatite nanoparticle (PHA)‐incorporated conductive alginate/gelatin (AG), could promote periodontal bone healing in a diabetic rat model, by facilitating M1 to M2 transition of macrophage phenotype. In the future, more animal models of various human diseases that lead to bone degenerative conditions are needed for pre‐clinical investigations of how electroactive scaffold can promote bone healing under pathological conditions.
6. CONCLUSIONS
The dielectric, piezoelectric, pyroelectric and ferroelectric properties, and the streaming potential and electro‐osmosis of bone tissue can be attributed to close interdependence and interaction of its various electroactive and electrosensitive components, including cell membrane potential, voltage‐gated ion channels, intracellular signaling pathways, cell surface receptors, and various matrix components such as collagen, hydroxypapatite, proteoglycans and glycosaminoglycans. These various interactions and cross‐talk, which define the electrophysiological properties of bone tissue, in turn exert a profound influence on its metabolism, homeostasis and regeneration in health and disease. Hence, a deeper understanding of the bioelectrical properties of bone tissue could therefore provide cues for new therapeutic strategies in bone tissue engineering and orthopedic surgery. For this purpose, animal models of various human diseases that lead to degenerative bone conditions would be particularly useful for studying how disease pathology affects the bioelectrical properties of bone tissues, and could facilitate the development of new treatment modalities to enhance bone healing via recapitulating the bioelectrical properties of healthy bone.
AUTHOR CONTRIBUTIONS
BCH and YB wrote most of the review article. XL, YM and YL wrote certain sections of the review article. XZ and XD conceived the idea behind the review article and provided guidance and supervision.
CONFLICT OF INTEREST
None. Xuehui Zhang is an Editorial Board member of AMEM and a co‐author of this article. To minimize bias, he was excluded from all editorial decision‐making related to the acceptance of this article for publication.
ACKNOWLEDGMENT
The authors gratefully acknowledge support for this work from funding bodies.
Heng BC, Bai Y, Li X, et al. The bioelectrical properties of bone tissue. Anim Models Exp Med. 2023;6:120‐130. doi: 10.1002/ame2.12300
Boon Chin Heng and Yunyang Bai have equal contributions.
REFERENCES
- 1. Girón J, Kerstner E, Medeiros T, et al. Biomaterials for bone regeneration: an orthopedic and dentistry overview. Braz J Med Biol Res. 2021;54(9):e11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oftadeh R, Perez‐Viloria M, Villa‐Camacho JC, Vaziri A, Nazarian A. Biomechanics and mechanobiology of trabecular bone: a review. J Biomech Eng. 2015;137(1):0108021‐01080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henry JP, Bordoni B. Histology, Osteoblasts. StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 4. Palumbo C, Ferretti M. The osteocyte: from “prisoner” to “orchestrator”. J Funct Morphol Kinesiol. 2021;6(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonald MM, Kim AS, Mulholland BS, Rauner M. New insights into osteoclast biology. JBMR Plus. 2021;5(9):e10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast‐osteoclast communication and bone homeostasis. Cells. 2020;9(9):2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khonsary SA. Guyton and hall: textbook of medical physiology. Surg Neurol Int. 2017;8:275. [Google Scholar]
- 8. Gautieri A, Vesentini S, Redaelli A, Buehler MJ. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett. 2011. Feb 9;11(2):757‐766. [DOI] [PubMed] [Google Scholar]
- 9. Camozzi V, Vescini F, Luisetto G, Moro L. Bone organic matrix components: their roles in skeletal physiology. J Endocrinol Invest. 2010;33(7 Suppl):13‐15. [PubMed] [Google Scholar]
- 10. Ulian G, Moro D, Valdrè G. Hydroxylapatite and related minerals in bone and dental tissues: structural, spectroscopic and mechanical properties from a computational perspective. Biomolecules. 2021;11(5):728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiner S, Traub W. Organization of hydroxyapatite crystals within collagen fibrils. FEBS Lett. 1986;206(2):262‐266. [DOI] [PubMed] [Google Scholar]
- 12. Fang W, Ping H, Huang Y, et al. Growth of mineralized collagen films by oriented calcium fluoride nanocrystal assembly with enhanced cell proliferation. J Mater Chem B. 2021;9(33):6668‐6677. [DOI] [PubMed] [Google Scholar]
- 13. Singh S, Saha S. Electrical properties of bone. A review. Clin Orthop Relat Res. 1984;186:249‐271. [PubMed] [Google Scholar]
- 14. Ray S, Behari J. Electrical conduction in bone in frequency range 0.4‐1.3 GHz. Biomater Med Devices Artif Organs. 1986;14(3–4):153‐165. [DOI] [PubMed] [Google Scholar]
- 15. Williams PA, Saha S. The electrical and dielectric properties of human bone tissue and their relationship with density and bone mineral content. Ann Biomed Eng. 1996;24(2):222‐233. [DOI] [PubMed] [Google Scholar]
- 16. Amin B, Shahzad A, O'Halloran M, Elahi MA. Microwave bone imaging: a preliminary investigation on numerical bone phantoms for bone health monitoring. Sensors (Basel). 2020;20(21):6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amin B, Elahi MA, Shahzad A, Porter E, O'Halloran M. A review of the dielectric properties of the bone for low frequency medical technologies. Biomed Phys Eng Express. 2019;5(2):1‐10. [DOI] [PubMed] [Google Scholar]
- 18. Amin B, Shahzad A, Farina L, et al. Dielectric characterization of diseased human trabecular bones at microwave frequency. Med Eng Phys. 2020;78:21‐28. [DOI] [PubMed] [Google Scholar]
- 19. Sierpowska J, Töyräs J, Hakulinen MA, Saarakkala S, Jurvelin JS, Lappalainen R. Electrical and dielectric properties of bovine trabecular bone‐‐relationships with mechanical properties and mineral density. Phys Med Biol. 2003;48(6):775‐786. [DOI] [PubMed] [Google Scholar]
- 20. Haba Y, Wurm A, Köckerling M, Schick C, Mittelmeier W, Bader R. Characterization of human cancellous and subchondral bone with respect to electro physical properties and bone mineral density by means of impedance spectroscopy. Med Eng Phys. 2017;45:34‐41. [DOI] [PubMed] [Google Scholar]
- 21. Zaszczyńska A, Gradys A, Sajkiewicz P. Progress in the applications of smart piezoelectric materials for medical devices. Polymers (Basel). 2020;12(11):2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukada E, Yasuda I. On the piezoelectric effect of bone. J Physical Soc Japan. 1957;12:1158‐1162. [Google Scholar]
- 23. Lipiec E, Kowalska J, Wiechech A, Zielinski PM, Kwiatek WM, Iwaniec M. Infrared spectroscopy in molecular study of the piezoelectric effect in pig’s shin bone. Acta Phys pol. 2012;121:16‐21. [Google Scholar]
- 24. Nair M, Calahorra Y, Kar‐Narayan S, Best SM, Cameron RE. Self‐assembly of collagen bundles and enhanced piezoelectricity induced by chemical crosslinking. Nanoscale. 2019;11(32):15120‐15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwon J, Cho H. Piezoelectric heterogeneity in collagen type I fibrils quantitatively characterized by piezoresponse force microscopy. ACS Biomater Sci Eng. 2020;6(12):6680‐6689. [DOI] [PubMed] [Google Scholar]
- 26. Ca B, Ro B. Generation of electric potentials by bone in response to mechanical stress. Science. 1962;137(3535):1063‐1064. [DOI] [PubMed] [Google Scholar]
- 27. Tang Y, Wu C, Wu Z, Hu L, Zhang W, Zhao K. Fabrication and in vitro biological properties of piezoelectric bioceramics for bone regeneration. Sci Rep. 2017;7:43360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahn AC, Grodzinsky AJ. Relevance of collagen piezoelectricity to “Wolff's law”: a critical review. Med Eng Phys. 2009;31(7):733‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walsh WR, Guzelsu N. Ion concentration effects on bone streaming potentials and zeta potentials. Biomaterials. 1993;14(5):331‐336. [DOI] [PubMed] [Google Scholar]
- 30. Athenstaedt H. Permanent longitudinal electric polarization and pyroelectric behaviour of collagenous structures and nervous tissue in man and other vertebrates. Nature. 1970;228(5274):830‐834. [DOI] [PubMed] [Google Scholar]
- 31. Ravi HK, Simona F, Hulliger J, Cascella M. Molecular origin of piezo‐ and pyroelectric properties in collagen investigated by molecular dynamics simulations. J Phys Chem B. 2012;116(6):1901‐1907. [DOI] [PubMed] [Google Scholar]
- 32. Gn R, Kartha G. Structure of collagen. Nature. 1954;174(4423):269‐270. [DOI] [PubMed] [Google Scholar]
- 33. Lang SB. Pyroelectric effect in bone and tendon. Nature. 1966;212:704‐705. [Google Scholar]
- 34. El Messiery MA, Hastings GW, Rakowski S. Ferro‐electricity of dry cortical bone. J Biomed Eng. 1979;1(1):63‐65. [DOI] [PubMed] [Google Scholar]
- 35. Hastings GW, ElMessiery MA, Rakowski S. Mechano‐electrical properties of bone. Biomaterials. 1981;2(4):225‐233. [DOI] [PubMed] [Google Scholar]
- 36. Gross D, Williams WS. Streaming potential and the electromechanical response of physiologically‐moist bone. J Biomech. 1982;15(4):277‐295. [DOI] [PubMed] [Google Scholar]
- 37. Guzelsu N, Walsh WR. Streaming potential of intact wet bone. J Biomech. 1990;23(7):673‐685. [DOI] [PubMed] [Google Scholar]
- 38. Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng. 2002;30(5):693‐702. [DOI] [PubMed] [Google Scholar]
- 39. Crolet JM, Racilă M, Marguier A, Placide O. Osteosynthesis by electro‐osmosis. A numerical simulation. Rec Res Med Biol Biosci. 2013;8:39‐44. [Google Scholar]
- 40. Crolet JM, Racilă M, Marguier A, Placide O. Electro osmosis and bone remodeling – a numerical simulation. Int J Biol Biomed Eng. 2014;8:21‐26. [Google Scholar]
- 41. Brodwick MS. Ion channels: membrane potential‐dependent ion channels in cell membrane. Science. 1983;222(4628):1115‐1116. [DOI] [PubMed] [Google Scholar]
- 42. Zhang K, Liu X, Wang L, et al. The mechanosensory and mechanotransductive processes mediated by ion channels and the impact on bone metabolism: a systematic review. Arch Biochem Biophys. 2021;711:109020. [DOI] [PubMed] [Google Scholar]
- 43. Wright CS, Robling AG, Farach‐Carson MC, Thompson WR. Skeletal functions of voltage sensitive calcium channels. Curr Osteoporos Rep. 2021;19(2):206‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang Z, Yue Z, Ma X, Xu Z. Calcium homeostasis: a potential vicious cycle of bone metastasis in breast cancers. Front Oncol. 2020;10:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blair HC, Schlesinger PH, Huang CL, Zaidi M. Calcium signalling and calcium transport in bone disease. Subcell Biochem. 2007;45:539‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McDonald F. Ion channels in osteoblasts: a story of two intracellular organelles. Surgeon. 2004;2(2):63‐69. [DOI] [PubMed] [Google Scholar]
- 47. Brown EM, Chattopadhyay N, Yano S. Calcium‐sensing receptors in bone cells. J Musculoskelet Neuronal Interact. 2004;4(4):412‐413. [PubMed] [Google Scholar]
- 48. Zhuang H, Wang W, Seldes RM, Tahernia AD, Fan H, Brighton CT. Electrical stimulation induces the level of TGF‐beta1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem Biophys Res Commun. 1997;237(2):225‐229. [DOI] [PubMed] [Google Scholar]
- 49. Zhang J, Li M, Kang ET, Neoh KG. Electrical stimulation of adipose‐derived mesenchymal stem cells in conductive scaffolds and the roles of voltage‐gated ion channels. Acta Biomater. 2016;32:46‐56. [DOI] [PubMed] [Google Scholar]
- 50. Miyauchi A, Hruska KA, Greenfield EM, et al. Osteoclast cytosolic calcium, regulated by voltage‐gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J Cell Biol. 1990;111(6 Pt 1):2543‐2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hammerland LG, Parihar AS, Nemeth EF, Sanguinetti MC. Voltage‐activated potassium currents of rabbit osteoclasts: effects of extracellular calcium. Am J Physiol. 1994;267(4 Pt 1):C1103‐C1111. [DOI] [PubMed] [Google Scholar]
- 52. Winslow MM, Pan M, Starbuck M, et al. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell. 2006;10:771‐782. [DOI] [PubMed] [Google Scholar]
- 53. Zou ML, Chen ZH, Teng YY, et al. The Smad dependent TGF‐beta and BMP signaling pathway in bone remodeling and therapies. Front Mol Biosci. 2021;8:593310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu M, Chen G, Li YP. TGF‐β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim YM, Lim HM, Lee EC, Ki GE, Seo YK. Synergistic effect of electromagnetic fields and nanomagnetic particles on osteogenesis through calcium channels and p‐ERK signaling. J Orthop Res. 2021;39(8):1633‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Viti F, Landini M, Mezzelani A, Petecchia L, Milanesi L, Scaglione S. Osteogenic differentiation of MSC through calcium signaling activation: Transcriptomics and functional analysis. PLoS One. 2016;11(2):e0148173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee W, Eo SR, Choi JH, Kim YM, Nam MH, Seo YK. The osteogenic differentiation of human dental pulp stem cells through G0/G1 arrest and the p‐ERK/Runx‐2 pathway by sonic vibration. Int J Mol Sci. 2021;22(18):10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou Z, Li W, He T, Qian L, Tan G, Ning C. Polarization of an electroactive functional film on titanium for inducing osteogenic differentiation. Sci Rep. 2016;6:35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mycielska ME, Djamgoz MB. Cellular mechanisms of direct‐current electric field effects: galvanotaxis and metastatic disease. J Cell Sci. 2004;117(Pt 9):1631‐1639. [DOI] [PubMed] [Google Scholar]
- 60. Li X, Kolega J. Effects of direct current electric fields on cell migration and Actin filament distribution in bovine vascular endothelial cells. J Vasc Res. 2002;39(5):391‐404. [DOI] [PubMed] [Google Scholar]
- 61. Marzec E, Kubisz L, Jaroszyk F. Dielectric studies of proton transport in air‐dried fully calcified and decalcified bone. Int J Biol Macromol. 1996;18(1–2):27‐31. [DOI] [PubMed] [Google Scholar]
- 62. Otter M, Shoenung J, Williams WS. Evidence for different sources of stress‐generated potentials in wet and dry bone. J Orthop Res. 1985;3(3):321‐324. [DOI] [PubMed] [Google Scholar]
- 63. Song Y, Zhang F, Linhardt RJ. Analysis of the glycosaminoglycan chains of proteoglycans. J Histochem Cytochem. 2021;69(2):121‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Olthof MGL, Kempen DHR, Liu X, et al. Effect of biomaterial electrical charge on bone morphogenetic protein‐2‐induced in vivo bone formation. Tissue Eng Part A. 2019;25(13–14):1037‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dadsetan M, Giuliani M, Wanivenhaus F, Brett Runge M, Charlesworth JE, Yaszemski MJ. Incorporation of phosphate group modulates bone cell attachment and differentiation on oligo(polyethylene glycol) fumarate hydrogel. Acta Biomater. 2012;8(4):1430‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Papachristou DJ, Georgopoulos S, Giannoudis PV, Panagiotopoulos E. Insights into the cellular and molecular mechanisms that govern the fracture‐healing process: a narrative review. J Clin Med. 2021;10(16):3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu G, Zhang T, Chen M, et al. Bone physiological microenvironment and healing mechanism: basis for future bone‐tissue engineering scaffolds. Bioact Mater. 2021;6(11):4110‐4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Maruyama M, Rhee C, Utsunomiya T, et al. Modulation of the inflammatory response and bone healing. Front Endocrinol (Lausanne). 2020;11:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thrivikraman G, Lee PS, Hess R, Haenchen V, Basu B, Scharnweber D. Interplay of substrate conductivity, cellular microenvironment, and pulsatile electrical stimulation toward osteogenesis of human mesenchymal stem cells in vitro. ACS Appl Mater Interfaces. 2015;7(41):23015‐23028. [DOI] [PubMed] [Google Scholar]
- 70. Lim HL, Chuang JC, Tran T, Aung A, Arya G, Varghese S. Dynamic electromechanical hydrogel matrices for stem cell culture. Adv Funct Mater. 2011;21(1):55‐63. doi: 10.1002/adfm.201001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang X, Zhang C, Lin Y, et al. Nanocomposite membranes enhance bone regeneration through restoring physiological electric microenvironment. ACS Nano. 2016;10(8):7279‐7286. [DOI] [PubMed] [Google Scholar]
- 72. Vila M, Cicuéndez M, Sánchez‐Marcos J, et al. Electrical stimuli to increase cell proliferation on carbon nanotubes/mesoporous silica composites for drug delivery. J Biomed Mater Res A. 2013;101(1):213‐221. [DOI] [PubMed] [Google Scholar]
- 73. Gupta KK, Kundan A, Mishra PK, et al. Polycaprolactone composites with TiO2 for potential nanobiomaterials: tunable properties using different phases. Phys Chem Chem Phys. 2012;14(37):12844‐12853. [DOI] [PubMed] [Google Scholar]
- 74. Zheng T, Huang Y, Zhang X, Cai Q, Deng X, Yang X. Mimicking the electrophysiological microenvironment of bone tissue using electroactive materials to promote its regeneration. J Mater Chem B. 2020;8(45):10221‐10256. [DOI] [PubMed] [Google Scholar]
- 75. Jacob J, More N, Kalia K, Kapusetti G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regen. 2018;38:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Oliveira KMC, Barker JH, Berezikov E, et al. Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci Rep. 2019;9(1):11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li C, Levin M, Kaplan DL. Bioelectric modulation of macrophage polarization. Sci Rep. 2016;6:21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dai X, Heng BC, Bai Y, et al. Restoration of electrical microenvironment enhances bone regeneration under diabetic conditions by modulating macrophage polarization. Bioact Mater. 2020;6(7):2029‐2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Khare D, Basu B, Dubey AK. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials. 2020;258:120280. [DOI] [PubMed] [Google Scholar]
- 80. Jacob J, More N, Kalia K, Kapusetti G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regener. 2018. Feb;27(38):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sikorski P. Electroconductive scaffolds for tissue engineering applications. Biomater Sci. 2020;8(20):5583‐5588. [DOI] [PubMed] [Google Scholar]
- 82. Alizadeh P, Soltani M, Tutar R, et al. Use of electroconductive biomaterials for engineering tissues by 3D printing and 3D bioprinting. Essays Biochem. 2021;65(3):441‐466. [DOI] [PubMed] [Google Scholar]
- 83. Kao FC, Chiu PY, Tsai TT, Lin ZH. The application of nanogenerators and piezoelectricity in osteogenesis. Sci Technol Adv Mater. 2019;20(1):1103‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li G, Zhu Q, Wang B, et al. Rejuvenation of senescent bone marrow mesenchymal stromal cells by pulsed triboelectric stimulation. Adv Sci (Weinh). 2021;8(18):e2100964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zurbuchen A, Pfenniger A, Stahel A, et al. Energy harvesting from the beating heart by a mass imbalance oscillation generator. Ann Biomed Eng. 2013;41(1):131‐141. [DOI] [PubMed] [Google Scholar]
- 86. Haque SU, Duteanu N, Ciocan S, Nasar A, Inamuddin. A review: evolution of enzymatic biofuel cells. J Environ Manage. 2021;298:113483. [DOI] [PubMed] [Google Scholar]
- 87. Mercier PP, Lysaght AC, Bandyopadhyay S, Chandrakasan AP, Stankovic KM. Energy extraction from the biologic battery in the inner ear. Nat Biotechnol. 2012;30(12):1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Irivisoot S, Pareta R, Webster TJ. Electrically controlled drug release from nanostructured polypyrrole coated on titanium. Nanotechnology. 2011;22(8):085101. [DOI] [PubMed] [Google Scholar]
- 89. Kiaee G, Mostafalu P, Samandari M, Sonkusale S. A pH‐mediated electronic wound dressing for controlled drug delivery. Adv Healthc Mater. 2018;7(18):e1800396. [DOI] [PubMed] [Google Scholar]
- 90. Zhang L, Wang Z, Das J, et al. Potential‐responsive surfaces for manipulation of cell adhesion, release, and differentiation. Angew Chem Int Ed Engl. 2019;58(41):14519‐14523. [DOI] [PubMed] [Google Scholar]
- 91. Raic A, Friedrich F, Kratzer D, Bieback K, Lahann J, Lee‐Thedieck C. Potential of electrospun cationic BSA fibers to guide osteogenic MSC differentiation via surface charge and fibrous topography. Sci Rep. 2019;9(1):20003. doi: 10.1038/s41598-019-56508-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shen S, He X, Chen X, Dong L, Cheng K, Weng W. Enhanced osteogenic differentiation of mesenchymal stem cells on P(VDF‐TrFE) layer coated microelectrodes. J Biomed Mater Res B Appl Biomater. 2021;109:2227‐2236. doi: 10.1002/jbm.b.34884 [DOI] [PubMed] [Google Scholar]
- 93. Brighton CT, Wang W, Seldes R, Zhang G, Pollack SR. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83(10):1514‐1523. [DOI] [PubMed] [Google Scholar]
- 94. Park J, Mazare A, Schneider H, von der Mark K, Fischer MJM, Schmuki P. Electric field‐induced osteogenic differentiation on TiO2 nanotubular layer. Tissue Eng Part C Methods. 2016;22(8):809‐821. [DOI] [PubMed] [Google Scholar]
- 95. Liu J, Someren E, Mentink A, et al. The effect of PKC activation and inhibition on osteogenic differentiation of human mesenchymal stem cells. Eng Regener Med. 2010;4:329‐339. [DOI] [PubMed] [Google Scholar]
- 96. Junior FJH, Bagne L, Meneghetti DH, et al. Electrical stimulation: complementary therapy to improve the performance of grafts in bone defects? J Biomed Mater Res B Appl Biomater. 2019;107(4):924‐932. [DOI] [PubMed] [Google Scholar]
- 97. Sheikh AQ, Taghian T, Hemingway B, Cho H, Kogan AB, Narmoneva DA. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low‐amplitude electric field. J R Soc Interface. 2013;10(78):20120548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre‐angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117(Pt 3):397‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen Y, Ye L, Guan L, et al. Physiological electric field works via the VEGF receptor to stimulate neovessel formation of vascular endothelial cells in a 3D environment. Biol Open. 2018;7(9):bio035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Itoh S, Nakamura S, Kobayashi T, Shinomiya K, Yamashita K, Itoh S. Effect of electrical polarization of hydroxyapatite ceramics on new bone formation. Calcif Tissue Int. 2006;78(3):133‐142. [DOI] [PubMed] [Google Scholar]
- 101. Itoh S, Nakamura S, Nakamura M, Shinomiya K, Yamashita K. Enhanced bone regeneration by electrical polarization of hydroxyapatite. Artif Organs. 2006;30(11):863‐869. [DOI] [PubMed] [Google Scholar]
- 102. Itoh S, Nakamura S, Nakamura M, Shinomiya K, Yamashita K. Enhanced bone ingrowth into hydroxyapatite with interconnected pores by electrical polarization. Biomaterials. 2006;27(32):5572‐5579. [DOI] [PubMed] [Google Scholar]
- 103. Li Y, Yang L, Hou Y, et al. Polydopamine‐mediated graphene oxide and nanohydroxyapatite‐incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact Mater. 2022;18:213‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Korem M, Goldberg NS, Cahan A, Cohen MJ, Nissenbaum I, Moses AE. Clinically applicable irreversible electroporation for eradication of micro‐organisms. Lett Appl Microbiol. 2018;67(1):15‐21. [DOI] [PubMed] [Google Scholar]
- 105. Jeong J, Kim C, Yoon J. The effect of electrode material on the generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Res. 2009;43(4):895‐901. [DOI] [PubMed] [Google Scholar]
- 106. Wang G, Jin W, Qasim AM, et al. Antibacterial effects of titanium embedded with silver nanoparticles based on electron‐transfer‐induced reactive oxygen species. Biomaterials. 2017;124:25‐34. [DOI] [PubMed] [Google Scholar]
- 107. Tandon B, Blaker JJ, Cartmell SH. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018;73:1‐20. [DOI] [PubMed] [Google Scholar]
- 108. Dai X, Yao X, Zhang W, et al. The osteogenic role of barium titanate/polylactic acid piezoelectric composite membranes as guiding membranes for bone tissue regeneration. Int J Nanomedicine. 2022;17:4339‐4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barbosa F, Ferreira FC, Silva JC. Piezoelectric electrospun fibrous scaffolds for bone, articular cartilage and osteochondral tissue engineering. Int J Mol Sci. 2022;23(6):2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shen X, Shen X, Li B, et al. Abnormal macrophage polarization impedes the healing of diabetes‐associated tooth sockets. Bone. 2021;143:115618. [DOI] [PubMed] [Google Scholar]
- 111. Ye X, Jiang J, Yang J, Yan W, Jiang L, Chen Y. Specnuezhenide suppresses diabetes‐induced bone loss by inhibiting RANKL‐induced osteoclastogenesis. Acta Biochim Biophys Sin (Shanghai). 2022;54(8):1080‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tikhonova SA, Evdokimov PV, Filippov YY, et al. Electro‐ and Magnetoactive materials in medicine: a review of existing and potential areas of application. Inorg Mater. 2020;56:1319‐1337. [Google Scholar]


