Abstract
Background:
While the health effects of air pollution and temperature are widely studied, the molecular effects are poorly understood. Extracellular microRNAs (ex-miRNAs) have the potential to serve as diagnostic or prognostic biomarkers and/or to act as intercellular signaling molecules that mediate the effects of environmental exposures on health outcomes.
Methods:
We examined the relationship between short-term exposure to air pollution and ambient temperature and the ex-miRNA profiles of participants in the Normative Aging Study (NAS) from 1999 to 2015. Our exposures were defined as same-day, two-day, three-day, one-week, two-week, and three-week moving averages of PM2.5, NO2, O3, and temperature which were derived from high-resolution spatio-temporal models. The ex-miRNA profiles of the subjects were obtained during follow-up visits. We analyzed the data using a longitudinal quantile regression model adjusted for individual covariates, batch effects, and time trends. We adjusted for multiple comparisons using a false discovery rate (FDR) correction. Ex-miRNAs that were significantly associated with exposures were further investigated using pathway analyses.
Results:
We found that all the examined exposures were associated with changes in ex-miRNA profiles in our study, particularly PM2.5 which was responsible for most of the statistically significant results. We found 110 statistically significant exposure-outcome relationships that revealed associations with the levels of 52 unique ex-miRNAs. Pathway analyses showed these ex-miRNAs have been linked to target mRNAs, genes, and biological mechanisms that could affect virtually every organ system, and as such may be linked to multiple clinical disease presentations such as cardiovascular disease, respiratory disease, and neurological disease.
Conclusions:
Air pollution and temperature exposures were significantly associated with alterations in the ex-miRNA profiles of NAS subjects with possible biological consequences.
Keywords: air pollution, microRNA, particulate matter, ozone, nitrogen dioxide, ambient temperature
1. Introduction
Studies have linked air pollution and temperature to numerous health outcomes including cardiovascular, respiratory, neurological, and endocrine diseases as well as mortality(Danesh Yazdi et al., 2022, 2021; Di et al., 2017; Dockery et al., 1993; Eze et al., 2015; Klompmaker et al., 2021; Ma et al., 2022; Schinasi et al., 2018; Shi et al., 2020; Wei et al., 2022). The main mechanisms hypothesized to link air pollution and these outcomes are inflammation and oxidative stress(Hajat et al., 2015; Ostro et al., 2014; van Eeden et al., 2005; Viehmann et al., 2015; Zhao et al., 2013). However, there is very limited information on the specific molecular mechanisms that mediate these effects.
One of the ways in which environmental exposures may exert their biological effects is through epigenetic changes, such as alterations in individuals’ microRNA (miRNA) profiles. MiRNAs are short non-coding sequences of RNA, usually about 22 nucleotides in length, that have been shown to play an important role in regulating gene expression, primarily through their role in altering messenger RNA (mRNA) stability and/or translation(Chekulaeva and Filipowicz, 2009; Eulalio et al., 2008; van Rooij, 2011). It stands to reason that any exposure that might change the body’s normal regulatory mechanisms for gene expression could have downstream effects that may even lead to clinical disease presentation. Extracellular miRNA (ex-miRNA), particularly those found in extracellular vesicles (EV-miRNA), are of special interest as they can be used as mechanisms for intercellular communication and biomarkers of illness(Andres et al., 2020; Mori et al., 2019). They are also stable and can be extracted in a non-invasive manner(Mitchell et al., 2008; Mori et al., 2019). As such, they have great potential for use in health outcomes research.
Recent studies have begun to look at the relationship between air pollution and ex-miRNAs more extensively(Chen et al., 2020, 2022; Eckhardt et al., 2022a; Ferrari et al., 2022; Pavanello et al., 2016; Pergoli et al., 2017; Rodosthenous et al., 2016, 2018; Wang et al., 2022). A study among steel workers in Northern Italy found EV miR-302b, miR-300c, and miR-30d to be associated with long-term exposure to particulate matter and particulate matter metals. These EV-miRNAs were also associated with biomarkers of inflammation and coagulation(Pavanello et al., 2016). A previous study found changes in levels of EV miR-150 and miR-155 among patients with coronary artery disease after short-term exposure to ambient ozone(Chen et al., 2020). A pilot study conducted among the participants of the Normative Aging Study found changes in several EV-miRNAs in response to long-term exposure to PM2.5 which were linked to pathways leading to cardiovascular disease (CVD) such as oxidative stress, inflammation, and atherosclerosis(Rodosthenous et al., 2016). Another study in the same population showed effect measure modification of the effect of long-term PM2.5 on systolic blood pressure by EV miR-199a/b and miR-223-3p(Rodosthenous et al., 2018). However, the results of these studies have not always been consistent, and typically, only a few miRNAs are studied at a time with relatively few subjects(Chen et al., 2020; Pavanello et al., 2016; Rodosthenous et al., 2018, 2016). Furthermore, while temperature is reported as a covariate in some of these studies, none report the effects as an exposure of interest. As such, there is a great need for further research in this area, particularly in terms of epidemiological studies using ex-miRNAs, and particularly for short-term exposure studies which are quite sparse.
In this study, we used data from participants in the Normative Aging Study (NAS) to look at the relationship between short-term exposure to air pollution and ambient temperature and changes in the ex-miRNA profiles of NAS subjects from 1999 to 2015. We then used the ex-miRNAs with expression levels that changed significantly over time to identify relevant biological pathways and clinical diseases, particularly cardiovascular disease, respiratory disease, and neurological disease, as these have been studied more extensively in air pollution epidemiology.
2. Material and Methods
2.1. Study Population
Our study population drew from the cohort of men enrolled in the NAS. The details of this population have been published elsewhere(Bell et al., 1966). Briefly, this cohort, established in 1963, consisted of men who used the services of the US Department of Veterans Affairs (VA), lived in the Greater Boston Area, and did not have any chronic conditions. Participants were followed up every three to five years, during which they underwent a number of exams and answered certain questionnaires. The participants in this study included all NAS members who lived in the contiguous United States and whose plasma samples collected between 1999 and 2015 were used to sequence their plasma extracellular miRNA.
The VA Boston Health Care System and Harvard TH Chan School of Public Health Institutional Review Boards approved this study. All participants provided written consent for inclusion in the cohort.
2.2. Exposure Assessment
Our exposures of interest included short-term exposure to the following: fine particulate matter (PM2.5), nitrogen dioxide (NO2), ozone (O3), and mean daily temperature (measured in Kelvin, K).
PM2.5, NO2, and O3 were estimated from ensemble models that used predictions from three machine learning algorithms: a random forest (RF), a gradient boosting machine (GBM), and a neural network (NN) in a geographically weighted generalized additive model (GAM)(Di et al., 2019a, 2019b; Requia et al., 2020). For each pollutant, predictors were extracted from satellite data, land-use data, meteorological data, and chemical transport models and used as input for the machine learning algorithms. The predictions generated by the machine learning algorithms and the ensemble model were validated against measured values from held out monitors using ten-fold cross validation. All three pollutants demonstrated strong values of 0.86, 0.79, and 0.91 for PM2.5, NO2, and O3, respectively. The models estimated daily pollutant levels on a 1 km x 1 km scale from 2000 to 2016 across the contiguous United States(Di et al., 2019a, 2019b; Requia et al., 2020).
For observations prior to 2000, when modeled air pollution data was not available, we used measured values at the Countway Library Supersite to assign air pollution values. First, we regressed modeled pollution levels against Countway air pollution measurements and gridMET temperature data for all the days in the year 2000 (where we have both values) for each individual in the dataset. Then, we extracted those coefficients and used measured values in 1999 from Countway to predict the pollution levels for each individual with visits in 1999. If data was not collected from Countway for any reason, that day and any moving average including that day was considered to be missing.
Maximum and minimum daily temperature were obtained from the gridMET dataset (Abatzoglou, 2013). This dataset contains predicted levels of meteorological parameters on a 4 km x 4 km scale from 1999 to 2015. The value for maximum and minimum daily temperature were averaged to obtain the daily mean.
Exposure levels were assigned based on levels at the closest grid-cell centroid to the residential address for both air pollution and temperature exposures.
We calculated several short-term levels for all exposures: same-day exposure, two-, and three- day, one-, two-, and three-week moving averages.
2.3. Outcome Measurement
We obtained blood samples from consenting cohort participants during normal follow-up visits. These blood samples were centrifuged and processed to obtain the plasma. Extracellular RNA was isolated from these plasma samples using the procedure described in Gandhi et al(Gandhi et al., 2017) and sequenced according to a procedure described previously by Srinivasan et al (Srinivasan et al., 2019). The exRNA extraction was performed using the Norgen Plasma/Serum Circulating and Exosomal RNA Purification Kits (Slurry Format). The small RNAseq libraries were made using the NEBNext® Small RNA Library Prep Set for Illumina® (Multiplex Compatible). Sequencing was performed on a HiSeq2500. FASTQ sequencing data were mapped using the ExceRpt small RNA sequencing data analysis pipeline on the Genboree Workbench (http://genboree.org/site/exrna_toolset/). Mapping parameters specified a minimum read length of 15 nucleotides and 0 mismatches allowed, with the rest on default. Samples that had fewer than 10,000 total input reads or low miRNA mapping (<100,000 mapped miRNA reads, ) were dropped during quality control. Further details are available in Eckhardt, et al, 2022(Eckhardt et al., 2022b). MiRNAs were reported as read counts per million. Reads that were not aligned to a single unique miRNA due to sequence similarities were collapsed into a single category that represented multiple miRNAs. Values for individuals who had multiple samples on the same visit day were calculated as the average of the samples. We included miRNAs in our analysis that were detectable in at least 40% of our samples. This resulted in 1508 samples that passed quality control and processing (and were within the study’s geographic limits and follow-up times) and 567 ex-miRNAs that were used in the analyses.
2.4. Covariate Measurement
We included age, body mass index (BMI), highest educational attainment (maximum years), drinking habits (at least two drinks a day: yes or no), smoking habits (current/former smoker: yes or no), pack-years of smoking, diabetes status (physician diagnosed: yes or no), and batch effects (batch pool) as covariates. This information was obtained from questionnaires and physical assessments done during study visits. We also accounted for long-term trends by adding a term which counted the number of days since January 1st, 1995. This date was chosen to pre-date the beginning of the study. We adjusted for seasonality by including sine and cosine functions for day of year.
2.5. Statistical Analysis
Ex-miRNA measurements were generally not normally distributed, and we were interested in how exposures might influence the lower and upper ends of the distribution of ex-miRNA levels as well as the middle. Quantile regression also makes no distributional assumption about the outcome. Therefore, we used a longitudinal quantile regression model to estimate the change in ex-miRNA level in response to air pollution and temperature. This model included fixed effects for the exposures and the covariates and an approximation of a random intercept for each individual. For each ex-miRNA, we looked at the following quantiles as outcomes: 10th percentile, 25th percentile, 50th percentile, 75th percentile, and 90th percentile. We ran separate models for each quantile, exposure duration, and ex-miRNA outcome. The model was as follows:
Where is the quantile of interest for the ex-miRNA of interest in individual in measure is a vector of exposures, is a vector of covariates, is a random intercept approximation by individual, and is the residual. The standard errors of the model were calculated using a bootstrapping approach with 1000 bootstraps. We corrected for multiple comparisons using a false discovery rate (FDR) procedure that accounted for the use of multiple ex-miRNAs as outcomes of interest. An exposure was considered to be significantly associated with an outcome if the adjusted p-value was less than 0.05.
Statistically significant ex-miRNAs were then linked to relevant biological pathways using a Kyoto Encyclopedia for Genes and Genomes (KEGG) Pathway Analysis run through the DIANA-miRPath web server(Vlachos et al., 2015). Significant pathways were identified using an FDR correction at a threshold p-value of 0.05. We also analyzed significant ex-miRNAs and their target mRNAs, genes, biological pathways, and diseases, particularly cardiovascular, respiratory, and neurological disorders using QIAGEN Ingenuity Pathway Analysis microRNA Target Filter(QIAGEN Inc., https://digitalinsights.qiagen.com/IPA)(Krämer et al., 2014).
We also conducted sensitivity analyses by running the raw count data through a Deseq procedure, which includes a normalization procedure and a negative binomial model, using a similar model specification to our main analyses but without the random intercept as that functionality is not supported(Love et al., 2014). Given the longitudinal nature of the majority of our data, this may lead to wider confidence intervals and larger p-values.
All data cleaning and analyses were done in R version 3.6.3. The “rqpd” package was used to conduct the regression analysis(Koenker and Bache, 2014) and “DESeq2” was used to conduct the Deseq procedure(Love et al., 2014).
3. Results
3.1. Baseline Characteristics of Study Population
Our study population used for analysis consisted of 734 individuals with 1508 measurements from 1999 to 2015. The vast majority were white, and the average age was 72.7 years at baseline. At their baseline visit, most participants did not have physician-diagnosed diabetes, were current or former smokers, and did not drink at least two alcohol drinks each day. On average, our population had 15 years of education and a BMI of 28.2 (kg/m2). Over sixty percent of participants had two or more samples analyzed (Table 1). Fourteen observations were dropped from the analyses due to missing covariate data. The number of observations missing exposure data can be found in Table S1.
Table 1.
Demographic Characteristics for Study Population at Baseline (1999–2015)
| N=734 Individuals | ||
|---|---|---|
| Variable | N(%) or Mean (SD) | |
| Age (years) | 72.7 (6.9) | |
| Race | White | 713 (97.1%) |
| Black | 14 (1.9%) | |
| Hispanic White | 5 (0.7%) | |
| Hispanic Black | 1(0.1%) | |
| Diabetes | Yes | 102 (13.9%) |
| No | 632 (86.1%) | |
| Alcohol Consumption (≥2/Day) | Yes | 136 (18.5%) |
| No | 598 (81.5%) | |
| Ever Smoker | No | 224 (30.5%) |
| Yes | 504 (68.7%) | |
| Smoking pack-years | 21.3(26.6) | |
| Education (years) | 15.0 (2.9) | |
| Body Mass Index (kg/m 2 ) | 28.2 (4.1) | |
| Number of Samples | 1 | 274 (37.3%) |
| 2 | 206 (28.1%) | |
| 3 | 196 (26.7%) | |
| 4 | 56 (7.6%) | |
| 5 | 2 (0.3%) | |
3.2. Exposure Distribution
Our exposures of interest included three air pollutants: PM2.5, NO2, and O3, as well as average daily ambient temperature. The distribution of these variables can be seen in Table 2. The median level for the pollutants was 8.11 μg/m3 for PM2.5, 23.97 parts per billion (ppb) for NO2, and 42.18 ppb for O3. These represent fairly low levels of exposure and reflect the reduction in air pollution levels that has occurred over time in the United States. The distribution of temperature reflects the seasonality that is observed in the New England area. The correlation between same-day exposures can be seen in Table 3. There were moderate positive correlations between exposures except between NO2 and O3 which had a smaller negative relationship.
Table 2.
Same-Day Exposure Distribution
| Variable | Minimum | 10th Percentile | 25th Percentile | Mean | Median | 75th Percentile | 90th Percentile |
Maximum |
|---|---|---|---|---|---|---|---|---|
| PM25 (μg/m3) | 0.27 | 3.84 | 5.40 | 9.65 | 8.11 | 11.93 | 17.46 | 56.34 |
| NO2 (ppb) | −0.24* | 9.16 | 15.41 | 25.10 | 23.97 | 34.03 | 42.07 | 76.22 |
| O3 (ppb) | 4.41 | 16.68 | 23.99 | 33.96 | 31.65 | 42.18 | 53.76 | 94.30 |
| Mean Daily Temperature (K) | 257.90 | 273.05 | 278.89 | 285.66 | 286.20 | 292.90 | 297.12 | 305.70 |
Prediction models may occasionally result in negative exposure values.
Table 3.
Same-Day Exposure Correlations
| Exposures | Mean Daily Temperature (K) | NO2 (ppb) | O3 (ppb) | PM25 (μg/m3) |
|---|---|---|---|---|
| Mean Daily Temperature (K) | 1 | −0.29 | 0.49 | 0.19 |
| NO2 (ppb) | −0.29 | 1 | −0.19 | 0.34 |
| O3 (ppb) | 0.49 | −0.19 | 1 | 0.30 |
| PM25 (μg/m3) | 0.19 | 0.34 | 0.30 | 1 |
3.3. Regression Results
There were a total of 145 significant results, reflecting 110 relationships between our exposures of interest and outcomes over various exposure time windows (Table S1). Of these, 71 were associated with exposure to PM2.5, 19 were due to daily temperature, 10 were attributed to O3, and 10 significant relationships were found with NO2. In our analysis, 34 ex-miRNAs were up-regulated due to exposure and 76 were down-regulated. These results identified associations with 52unique ex-miRNAs. PM2.5 affected the expression profile of 41 unique ex-miRNAs while the non-PM exposures affected17 unique ex-miRNAs.
Figure 1A shows the number of unique ex-miRNAs that were found to be significantly associated with each pollutant and exposure time window. The greatest number of significant relationships was found for exposure to the 2-day, 3-day and 3-week moving averages of PM2.5. For average daily temperature, most of the significant results came from exposures of 3-days or longer, suggesting that longer-term trends in temperature may be important in changes in individual ex-miRNA profiles. Our other exposures and time windows accounted for a smaller portion of the significant results. The significant effects seen with exposure to PM2.5 tended to focus on higher quantiles of the outcome while those for ozone tended to be found in the lower quantiles of the outcome. Mean temperature and NO2 were associated mostly with lower and upper quartiles of ex-miRNAs but not the median (Figure 1B).
Figure 1.
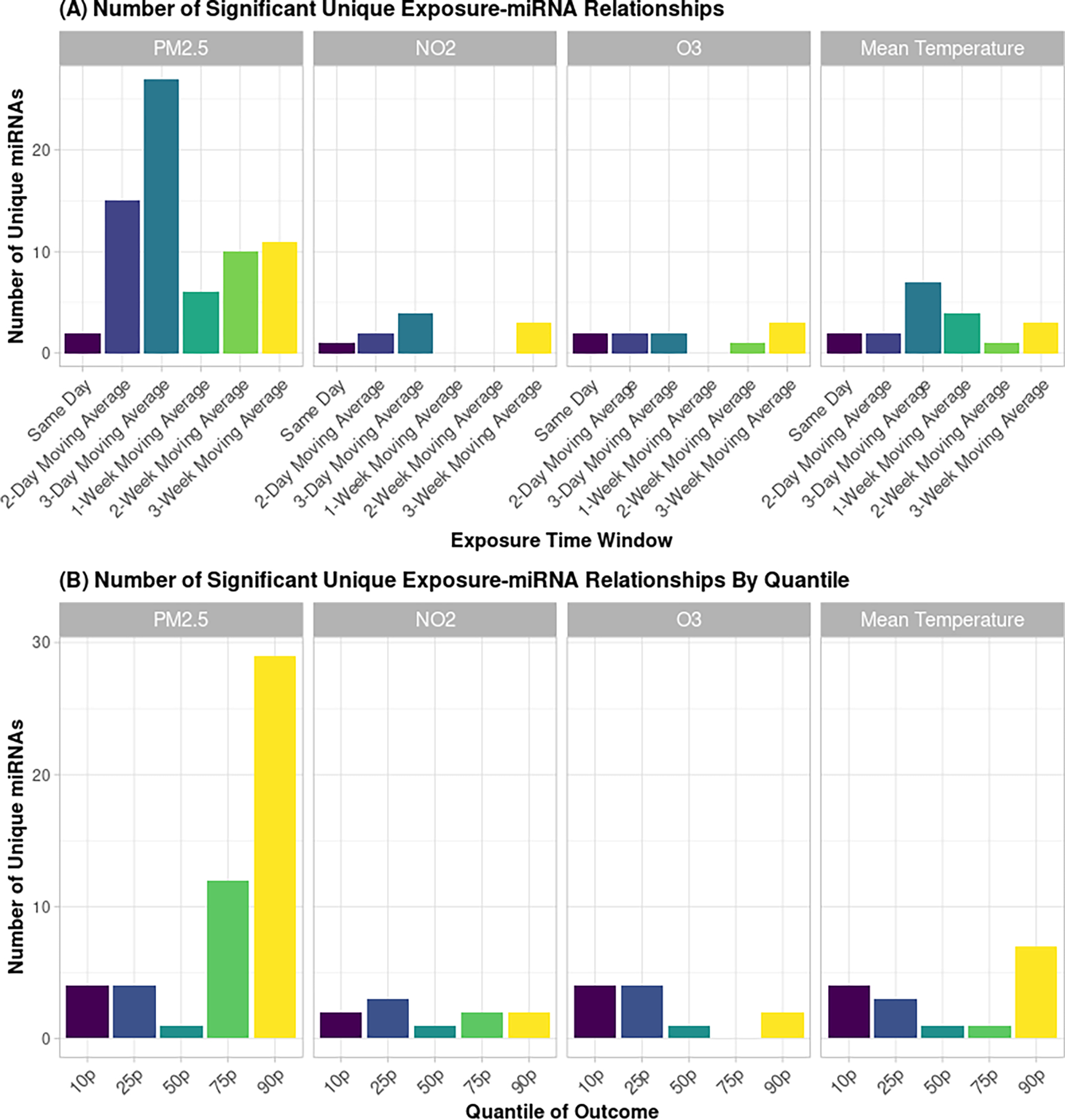
The number of unique miRNAs that were significantly altered after exposure to air pollutants and ambient temperature in each (A) time window and (B) quantile
Figure 2 shows the significant associations between our exposures of interest and ex-miRNAs by time window for those relationships that were significant in at least two quantiles of the outcome. The nine ex-miRNAs identified were miR-1228-5p, miR-1323, miR-140-3p, miR-19b-3p, miR-2115-3p, miR-296-3p, miR-3127-3p, miR-6772-3p, and miR-93-3p. Of these nine, changes in levels of miR-2115-3p and miR-3127-3p were associated with all of our exposures of interest in at least two quantiles of the outcome. Figure 3 shows the direction and magnitude of the significant associations between these ex-miRNAs and all of the exposures of interest. In most but not all of these ex-miRNAs, PM2.5, O3, and NO2 were associated with a down-regulation and increased mean temperature was associated with an up-regulation of ex-miRNA levels.
Figure 2.
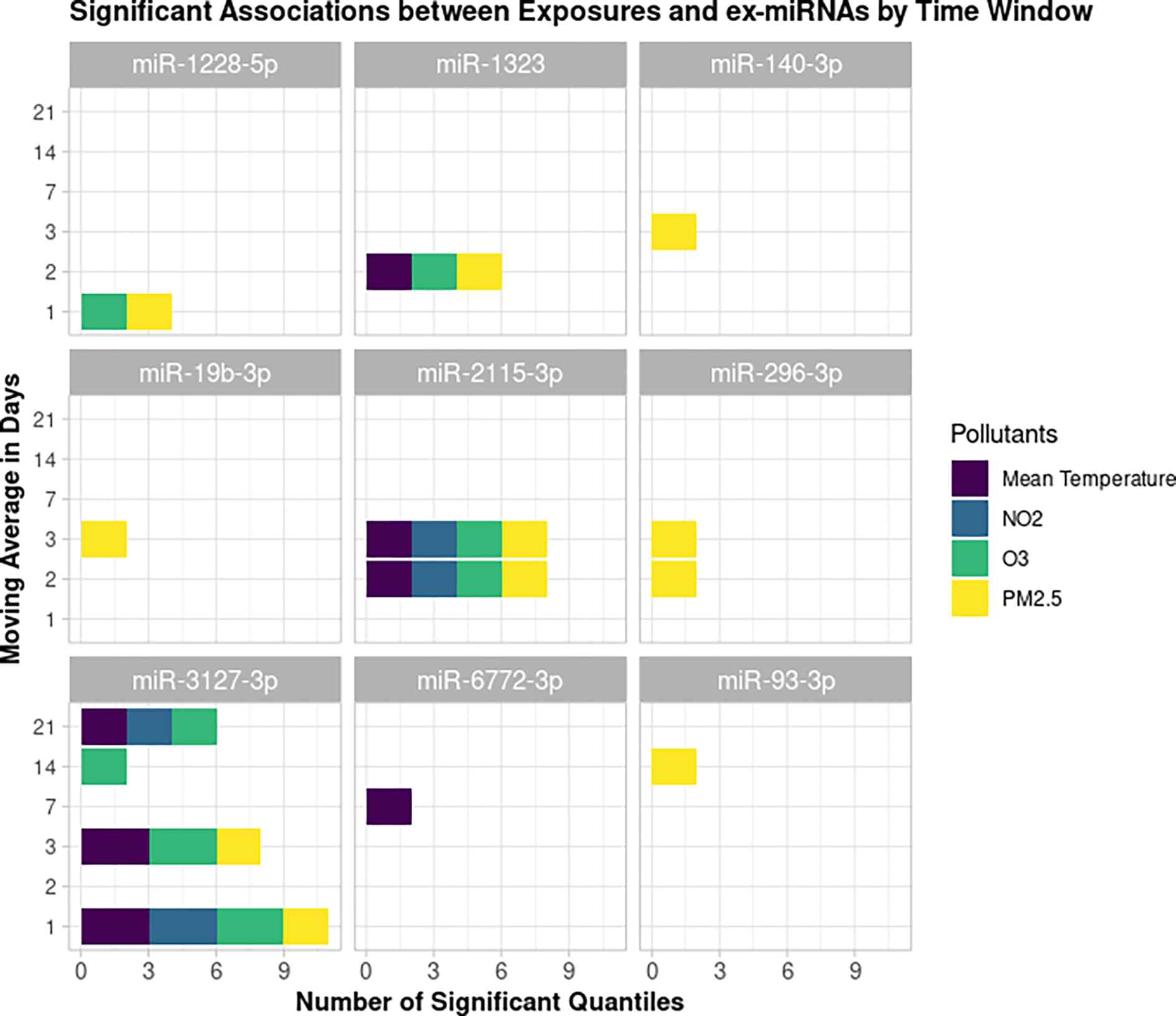
Significant associations between exposure and ex-miRNAs by exposure time window which were significant in at least two quantiles
Figure 3.
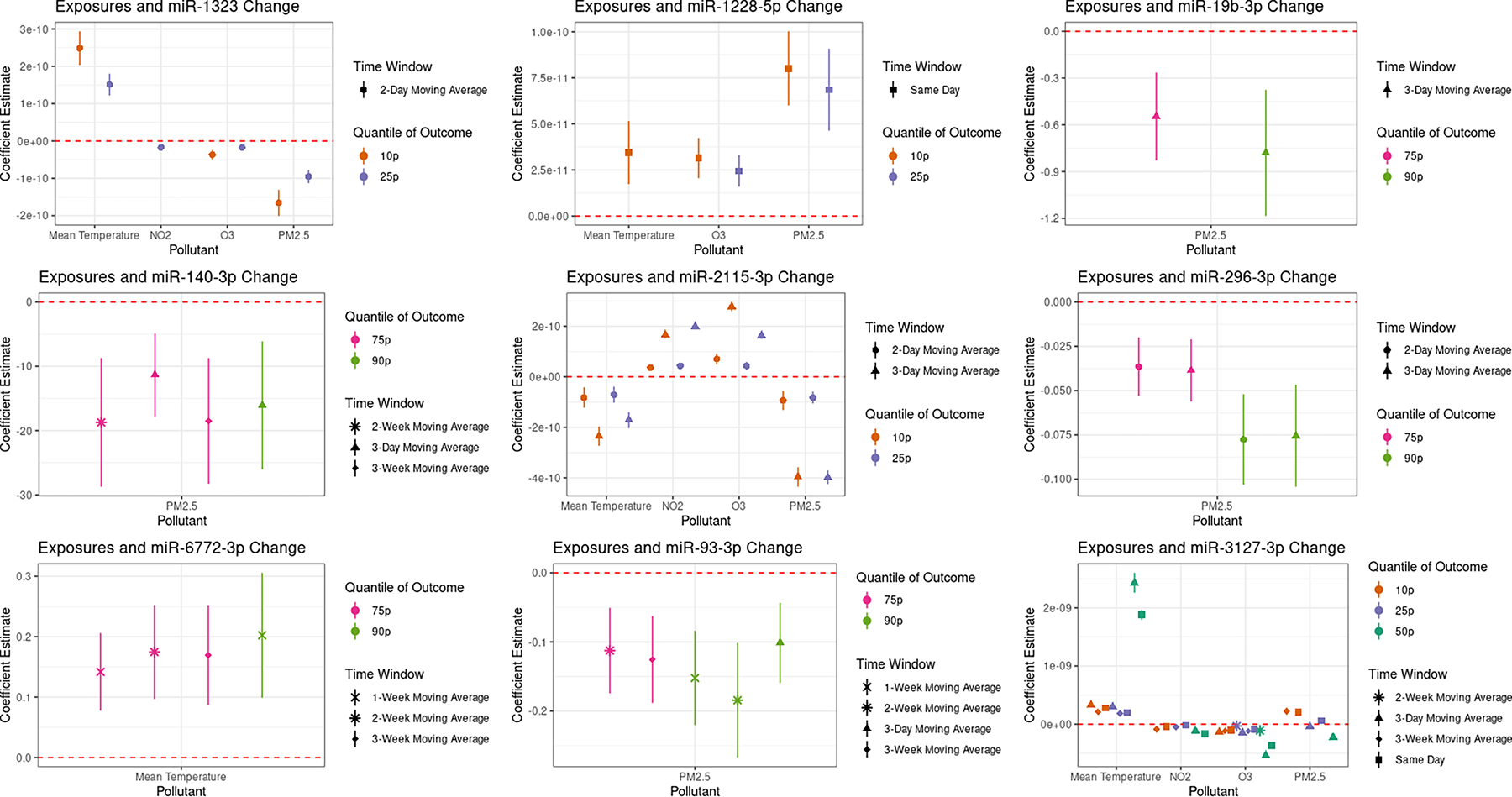
Significant changes in outcome quantile levels in response to exposures among ex-miRNAs which had at least two significant associations for the same exposure and in the same time window
3.4. Pathway Analysis
KEGG Pathway Analysis showed that most of the ex-miRNAs we found to have significantly changed in relation with exposure to air pollution and temperature were associated with genes that affected biological pathways primarily associated with the development of a variety of cancers, infectious disease, but also related to inflammation and other major types of adverse outcomes as they affect fundamental cell functions and communication. The full results can be seen in Tables S3–S8. Table 4 lists the significant KEGG pathways that were relevant based on the ex-miRNAs found to be associated with air pollution and ambient temperature. Two of the pathways, “fatty acid biosynthesis” and “fatty acid metabolism”, were found to be significantly associated with ex-miRNAs associated with exposure in every time window we studied.
Table 4.
KEGG Pathways Associated with All Exposure Time Windows*
| Fatty acid biosynthesis | Hippo signaling pathway |
| Fatty acid metabolism | Colorectal cancer |
| Prion diseases | Prostate cancer |
| ECM-receptor interaction | Pancreatic cancer |
| Proteoglycans in cancer | Transcriptional misregulation in cancer |
| p53 signaling pathway | Adherens junction |
| Hepatitis B | Cell cycle |
| Pathways in cancer | Oocyte meiosis |
| Glioma | Protein processing in endoplasmic reticulum |
| Viral carcinogenesis | Signaling pathways regulating pluripotency of stem cells |
| Steroid biosynthesis | Arrhythmogenic right ventricular cardiomyopathy (ARVC) |
| Chronic myeloid leukemia | RNA transport |
| mRNA surveillance pathway | Epstein-Barr virus infection |
| Bladder cancer | Melanoma |
| FoxO signaling pathway | Small cell lung cancer |
| Thyroid hormone signaling pathway | Ubiquitin mediated proteolysis |
| Focal adhesion | Shigellosis |
| Lysine degradation | RNA degradation |
| Bacterial invasion of epithelial cells | MicroRNAs in cancer |
| Renal cell carcinoma | Purine metabolism |
| TGF-beta signaling pathway | Glutathione metabolism |
3.5. Ingenuity Pathway Analysis
We used Ingenuity Pathway Analysis (IPA) microRNA Target Filter to find relationships between individual significantly affected ex-miRNAs and relevant mRNAs, genes, pathways, and clinical diseases. The table of full results from the IPA is available in Table S9. From our full list of significant ex-miRNAs, 17 could be linked to 496 unique mRNAs in relationships that had been experimentally validated. The ex-miRNAs we found to be significantly associated with short-term exposure to air pollution and ambient temperature were linked through their predicted mRNA targets with a host of pathways, which in turn are associated with numerous adverse health outcomes, such as cancer, cardiovascular disease, endocrine disorders, dermatological disease, respiratory disease, auditory disease, connective tissue disease, and neurological disease.
Of the previous nine ex-miRNAs mentioned which were significantly associated with at least one exposure and one time window in two quantiles, four, miR-140-3p, miR-296-3p, miR-93-3p, miR-19b-3p, were identified in the IPA with several mRNA targets which could then be linked to numerous disease states, including but not limited to those that can be seen in Figure 4.
Figure 4.
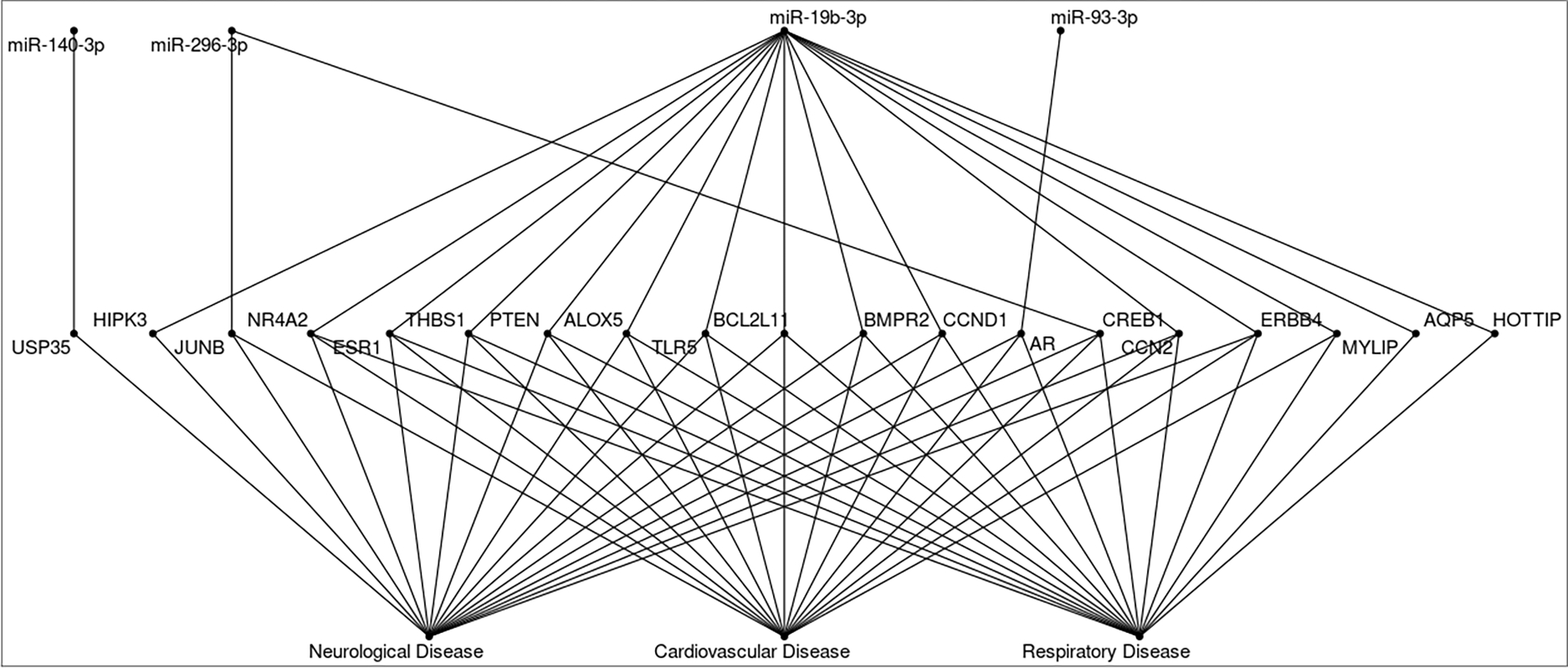
IPA microRNA Target Filter Results for select ex-miRNAs
3.6. Sensitivity Analysis
We also ran a Deseq analysis for each exposure time window as a sensitivity analysis. The full results can be found in Table S10. Deseq analysis revealed 103 significant exposure-outcome associations with 70 unique ex-miRNAs. Most of these significant associations were between ozone and the ex-miRNA and occurred during the three-week moving average exposure time window. There were ten ex-miRNAs that were significant both in the main analyses and in the Deseq analysis, namely: miR-145-5p, miR140-3p, miR-127-3p, miR-16-5p, miR-19b-3p, miR-15b-5p, miR-15a-5p, miR-181a-3p, miR-93-5p, and miR-17-5p. For four of these, miR-140-3p, miR-17-5p, miR-181a-3p, and miR-93-5p, the associations were with the same exposure. In each of those cases, the direction of association was consistent across both analyses, even when they were significant in different time windows.
4. Discussion
In this study, we identified relationships between short-term exposure to air pollution and temperature and repeated measures of ex-miRNA expression among participants of the Normative Aging Study between 1999 and 2015 using a longitudinal quantile regression approach. We looked at changes among hundreds of sequenced ex-miRNAs. We found a number of significant relationships, mostly from exposure to fine particulate matter (PM2.5) after adjusting for other pollutants and covariates. Future research could explore longer-term moving averages of air pollutants and temperature. Both KEGG analysis and IPA showed that the ex-miRNAs found to be significantly associated with air pollution were involved in a variety of pathways and could have broad system-level effects including pathways involving cancer, cell communication, and cell cycle changes. In addition, we found that PM2.5 tended to have a greater effect on the 75th and 90th percentile of the ex-miRNA levels than on the median level, suggesting that the impact on health may be underestimated by focusing on mean or median changes. Mean ambient temperature, O3, and NO2, on the other hand, had the most significant relationships at the lower and upper quantiles of outcome.
This study adds to the growing literature looking at the relationship between environmental air pollution and ex-miRNA profiles. A pilot study of EV-associated miRNAs in this population found only miR-30d-5p to be significantly associated with short-term exposure to PM2.5, which we did not find to be significantly altered in our study; however, the sample size in the pilot was much smaller, a different ex-miRNA extraction method (Qiagen miRNeasy®) was used, and a different miRNA measurement method (NanoString nCounter®) was used (Rodosthenous et al., 2016). A study among coronary artery disease patients looking at short-term exposure to PM2.5 and O3 and EV-miRNA in plasma found significant effects only for exposure to O3 and EV miR-150 and miR-155, both of which were up-regulated with no significant effect on EV-miR-21, miR-126, and miR-146(Chen et al., 2020). We did not find significant effects for miR-150 or miR-155. However, we did find down-regulation for ex-miR-21-3p and ex-miR-146a-5p in response to PM2.5 exposure, which is consistent with this study, though it was statistically non-significant. In a study conducted among the participants of the Susceptibility to Particle Health Effects, miRNAs and Exosomes (SPHERE) cohort, PM10 was found to be associated with downregulation of plasma EV-miRNAs: miR-218-5p, miR-99b-5p, let-7c-5p, miR-331-3p, miR-185-5p, miR-642-5p, miR-106a-5p, miR-143-3p, and miR-652-3p. They also found that five of these EV-miRNAs (let-7c-5p, miR-331-3p, miR-185-5p, miR106a-5p, and miR-652-3p) significantly mediated the relationship between PM10 exposure and fibrinogen levels, which plays a role in coagulation(Pergoli et al., 2017). We did not find significant effects between air pollution and miR-106a-5p, miR-185-5p, miR-143-3p, miR-218-5p, miR-331-3p, or miR-99b-5p and we found let-7c-5p to be upregulated in response to PM2.5 exposure. We did, however, find significant downregulation of miR-652-3p associated with exposure to PM2.5. A study conducted among steel plant workers in northern Italy looking at short-term exposures to metal-rich particulate matter found significant upregulation for plasma microvesicle miRNAs miR-128 and miR-302c(Bollati et al., 2015). We did not find a significant relationship between air pollution and miR-128. In an experimental crossover study conducted in England comparing short-term exposure to traffic-related air pollution (TRAP) and plasma circulating miRNA, multiple miRNAs were found to be associated with higher exposure to pollution: miR-133a-3p, miR-193b-3p, miR-1224-5p, miR-433-3p, miR-145-5p, miR-27a-5p, miR-580-3p, miR-3127-5p, and miR-6716-3p(Krauskopf et al., 2018). We did not find a significant relationship with miR-133a-3p, miR-1224-5p, miR-433-3p, miR-27a-5p, and any air pollutants. We did find a significant relationship between PM2.5 and miR-145-5p and in both studies the level of miR-145-5p was downregulated. miR-3127-5p was also significantly upregulated in both studies. Furthermore, we found several common KEGG pathways including those relating to cancer, cellular processes, and signal transduction(Krauskopf et al., 2018). A similar study also looking at TRAP, this time in Barcelona, among a selection of the participants in the TAPAS II cohort, found circulating miR-28-3p, miR-222-3p, miR-146-5p, miR-30b-5p/30c-5p, miR-320a-3p/320b/320c/320d/320e to be positively associated with increased pollution and miR-532-5p, miR-192-5p/215-5p, miR-144-3p, and miR-425-5p to be negatively correlated with increased pollution(Krauskopf et al., 2019). We did not find significant associations with miR-28-3p, miR-222-3p, miR-30b-5p, miR-30c-5p, miR-320a, miR-320b,miR-320c, miR-320d, miR-320e, miR-532-5p, miR-192-5p, miR-215-5p, andmiR-144-3p. We did find that exposure to PM2.5 was associated with a downregulation of miR-146a-5p and miR-425-5p.
Theiologyical pathways we found in the KEGG analysis have been linked to numerous health conditions. Several specifically impacted a variety of cancers and infectious diseases. Others could be linked to the functions of multiple organ systems. The Hippo signaling pathway is involved in cardiac development, cardiomyocyte hypertrophy and apoptosis, cardiomyocyte autophagy, heart regeneration, and angiogenesis(Zhou and Zhao, 2018). It has also been linked to gliomas(Masliantsev et al., 2021), Huntington’s Disease (Mueller et al., 2018), and neurodegeneration(Sahu and Mondal, 2020). It is also thought to be important in the development of idiopathic pulmonary fibrosis(Gokey et al., 2018; Sun et al., 2021). Fatty acid metabolism influences neuroinflammation(Bogie et al., 2020) and cardiac pathology(Lopaschuk et al., 2010). Protein misfolding in the endoplasmic reticulum has been linked to neurodegenerative conditions and inflammation(Wang and Kaufman, 2016). FoxO signaling pathways may play a role in ischemic heart disease, diabetic cardiomyopathy, and myocardial hypertrophy(Xin et al., 2017). Lysine degradation has been associated with type 2 diabetes(Razquin et al., 2019). These pathways are fundamental and often have affects at a cellular level.
Our study had some limitations. The cohort studied was fairly homogenous; all subjects were male and almost all were white. This limits the generalizability of the results. However, as a cohort of elderly individuals, they are at particular risk of the adverse effects of air pollution and temperature. It also eases comparability of results within the group in terms of their ex-miRNA profiles. While we did have a larger sample size than most previous literature in this area, it might still be possible that we did not have enough power to detect the effects of our exposures for some of the ex-miRNAs we studied. We were also not able to assess all of our sequenced ex-miRNAs as they were found to be non-detectable in a large portion of the samples. This limits our ability to look at the full range of ex-miRNAs. A larger sample size would resolve this problem. We also did not assess hemolysis as part of our processing procedure. Moreover, our model assumed no residual or unmeasured confounding, which cannot be verified. Our study has also several strengths. We used ex-miRNAs as our outcome of interest which could serve as biomarkers of disease(Eckhardt et al., 2022b). We used high-resolution spatiotemporal models to assign exposure and looked at ambient temperature as an exposure of interest, which has not been done in previous epidemiological studies looking at extracellular miRNA. We used a long-established cohort with extensive follow-up. We had a rich history on the participants and were able to use follow-up visits for those with multiple samples to provide time-varying confounders. We could also account for the longitudinal nature of the data by using a method which approximated a random intercept. Our quantile regression accounted for the potential non-normal distribution of the residuals and allowed us to look at multiple quantiles. Quantile regression is also robust to outliers and makes no distributional assumption about the ex-miRNAs. Finally, we also used multiple databases to identify relevant miRNA-mRNA-gene interactions and biological pathways/diseases.
5. Conclusions
Short-term exposure to air pollution and temperature were associated with alterations in the ex-miRNA profiles of elderly men in the Normative Aging Study. The ex-miRNAs identified were associated with mRNAs and genes which are involved in pathways that affect numerous health outcomes. The results of this study add to the literature which look at the molecular effects of exposure to environmental factors such as air pollution and ambient temperature.
Supplementary Material
Acknowledgements:
This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Funding: This work was supported by grant R01ES027747 from the National Institute of Environmental Health Sciences.
Footnotes
Declaration of Competing Interests: Dr. Feiby L. Nassan is a current employee and a shareholder of Biogen, Cambridge, MA. The original work on this study at Harvard T. H. Chan School of Public Health (HSPH), however, pre-dated the current employment. This manuscript does not mention any Biogen products or any of the disease states that Biogen is actively doing research in (to the coauthor’s knowledge). Dr. Joel D. Schwartz has appeared as an expert witness on behalf of the US Department of Justice in cases involving violations of the Clean Air Act.
References
- Abatzoglou JT, 2013. Development of gridded surface meteorological data for ecological applications and modelling. Int. J. Climatol. 33, 121–131. 10.1002/joc.3413 [DOI] [Google Scholar]
- Andres J, Smith LC, Murray A, Jin Y, Businaro R, Laskin JD, Laskin DL, 2020. Role of extracellular vesicles in cell-cell communication and inflammation following exposure to pulmonary toxicants. Cytokine Growth Factor Rev. 51, 12–18. 10.1016/j.cytogfr.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell B, Rose CL, Damon A, 1966. The Veterans Administration Longitudinal Study of Healthy Aging. Gerontologist 6, 179–184. 10.1093/geront/6.4.179 [DOI] [PubMed] [Google Scholar]
- Bogie JFJ, Haidar M, Kooij G, Hendriks JJA, 2020. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv. Drug Deliv. Rev. 159, 198–213. 10.1016/j.addr.2020.01.004 [DOI] [PubMed] [Google Scholar]
- Bollati V, Angelici L, Rizzo G, Pergoli L, Rota F, Hoxha M, Nordio F, Bonzini M, Tarantini L, Cantone L, Pesatori AC, Apostoli P, Baccarelli AA, Bertazzi PA, 2015. Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J. Appl. Toxicol. 35, 59–67. 10.1002/jat.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekulaeva M, Filipowicz W, 2009. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr. Opin. Cell Biol. 21, 452–460. 10.1016/j.ceb.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Chen H, Smith GJ, Masood S, Tong H, 2022. Extracellular MicroRNAs as Putative Biomarkers of Air Pollution Exposure BT - Biomarkers in Toxicology, in: Patel VB., Preedy VR., Rajendram R. (Eds.),. Springer International Publishing, Cham, pp. 1–24. 10.1007/978-3-030-87225-0_28-1 [DOI] [Google Scholar]
- Chen H, Xu Y, Rappold A, Diaz-Sanchez D, Tong H, 2020. Effects of ambient ozone exposure on circulating extracellular vehicle microRNA levels in coronary artery disease patients. J. Toxicol. Environ. Health. A 83, 351–362. 10.1080/15287394.2020.1762814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh Yazdi M, Wang Y, Di Q, Wei Y, Requia WJ, Shi L, Sabath MB, Dominici F, Coull BA, Evans JS, Koutrakis P, Schwartz JD, 2021. Long-Term Association of Air Pollution and Hospital Admissions Among Medicare Participants Using a Doubly Robust Additive Model. Circulation 143, 1584–1596. 10.1161/CIRCULATIONAHA.120.050252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh Yazdi M, Wei Y, Di Q, Requia WJ, Shi L, Sabath MB, Dominici F, Schwartz J, 2022. The effect of long-term exposure to air pollution and seasonal temperature on hospital admissions with cardiovascular and respiratory disease in the United States: A difference-in-differences analysis. Sci. Total Environ. 843, 156855. 10.1016/j.scitotenv.2022.156855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, Sabath MB, Choirat C, Koutrakis P, Lyapustin A, Wang Y, Mickley LJ, Schwartz J, 2019a. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ. Int. 130, 104909. 10.1016/j.envint.2019.104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern RF, Kelly JT, Sabath MB, Choirat C, Koutrakis P, Lyapustin A, Wang Y, Mickley LJ, Schwartz J, 2019b. Assessing NO2 Concentration and Model Uncertainty with High Spatiotemporal Resolution across the Contiguous United States Using Ensemble Model Averaging. Environ. Sci. Technol. 10.1021/acs.est.9b03358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang, Yan, Zanobetti A., Wang, Yun, Koutrakis P, Choirat C, Dominici F, Schwartz JD., 2017. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 376, 2513–2522. 10.1056/NEJMoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE, 1993. An Association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 329, 1753–1759. 10.1056/NEJM199312093292401 [DOI] [PubMed] [Google Scholar]
- Eckhardt CM, Baccarelli AA, Wu H, 2022a. Environmental Exposures and Extracellular Vesicles: Indicators of Systemic Effects and Human Disease. Curr. Environ. Heal. reports 9, 465–476. 10.1007/s40572-022-00357-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt CM, Gambazza S, Bloomquist TR, De Hoff P, Vuppala A, Vokonas PS, Litonjua AA, Sparrow D, Parvez F, Laurent LC, Schwartz J, Baccarelli AA, Wu H, 2022b. Extracellular Vesicle-Encapsulated microRNAs as Novel Biomarkers of Lung Health. Am. J. Respir. Crit. Care Med. 10.1164/rccm.202109-2208OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Huntzinger E, Izaurralde E, 2008. Getting to the Root of miRNA-Mediated Gene Silencing. Cell 132, 9–14. 10.1016/j.cell.2007.12.024 [DOI] [PubMed] [Google Scholar]
- Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Kunzli N, Schikowski T, Probst-Hensch NM, 2015. Association between Ambient Air Pollution and Diabetes Mellitus in Europe and North America: Systematic Review and Meta-Analysis. Environ. Health Perspect. 123, 381–389. 10.1289/ehp.1307823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari L, Iodice S, Cantone L, Solazzo G, Dioni L, Hoxha M, Vicenzi M, Mozzoni P, Bergamaschi E, Persico N, Bollati V, 2022. Extracellular vesicles and their miRNA contents counterbalance the pro-inflammatory effect of air pollution during physiological pregnancy: A focus on Syncytin-1 positive vesicles. Environ. Int. 169, 107502. 10.1016/j.envint.2022.107502 [DOI] [PubMed] [Google Scholar]
- Gandhi R, Laurent LC, Sood AK, Filant J, Nejad P, Paul A, Srinivasan S, 2017. Isolation of exosomal RNA from serum or plasma using the Norgen BioTek Plasma/Serum circulating and exosomal RNA purification mini kit. Protoc. Exch. 10.1038/protex.2017.078 [DOI] [Google Scholar]
- Gokey JJ, Sridharan A, Xu Y, Green J, Carraro G, Stripp BR, Perl A-KT, Whitsett JA, 2018. Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI insight 3. 10.1172/jci.insight.98738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, Vedal S, Kaufman JD, 2015. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation a repeat-measures analysis in the multi-ethnic study of atherosclerosis (MESA). Epidemiology 26, 310–320. 10.1097/eDe.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompmaker JO, Hart JE, James P, Sabath MB, Wu X, Zanobetti A, Dominici F, Laden F, 2021. Air pollution and cardiovascular disease hospitalization – Are associations modified by greenness, temperature and humidity? Environ. Int. 156, 106715. 10.1016/j.envint.2021.106715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenker RW, Bache SH, 2014. rqpd: Regression Quantiles for Panel Data. [Google Scholar]
- Krämer A, Green J, Pollard JJ, Tugendreich S, 2014. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530. 10.1093/bioinformatics/btt703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauskopf J, Caiment F, van Veldhoven K, Chadeau-Hyam M, Sinharay R, Chung KF, Cullinan P, Collins P, Barratt B, Kelly FJ, Vermeulen R, Vineis P, de Kok TM, Kleinjans JC, 2018. The human circulating miRNome reflects multiple organ disease risks in association with short-term exposure to traffic-related air pollution. Environ. Int. 113, 26–34. 10.1016/j.envint.2018.01.014 [DOI] [PubMed] [Google Scholar]
- Krauskopf J, van Veldhoven K, Chadeau-Hyam M, Vermeulen R, Carrasco-Turigas G, Nieuwenhuijsen M, Vineis P, de Kok TM, Kleinjans JC, 2019. Short-term exposure to traffic-related air pollution reveals a compound-specific circulating miRNA profile indicating multiple disease risks. Environ. Int. 128, 193–200. 10.1016/j.envint.2019.04.063 [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC, 2010. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 90, 207–258. 10.1152/physrev.00015.2009 [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Danesh Yazdi M, Schwartz J, Réquia WJ, Di Q, Wei Y, Chang HH, Vaccarino V, Liu P, Shi L, 2022. Long-term air pollution exposure and incident stroke in American older adults: A national cohort study. Glob. Epidemiol. 4, 100073. 10.1016/j.gloepi.2022.100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliantsev K, Karayan-Tapon L, Guichet P-O, 2021. Hippo Signaling Pathway in Gliomas. Cells 10. 10.3390/cells10010184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M, 2008. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. 105, 10513–10518. 10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR, 2019. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 30, 656–673. 10.1016/j.cmet.2019.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KA, Glajch KE, Huizenga MN, Wilson RA, Granucci EJ, Dios AM, Tousley AR, Iuliano M, Weisman E, LaQuaglia MJ, DiFiglia M, Kegel-Gleason K, Vakili K, Sadri-Vakili G, 2018. Hippo Signaling Pathway Dysregulation in Human Huntington’s Disease Brain and Neuronal Stem Cells. Sci. Rep. 8, 11355. 10.1038/s41598-018-29319-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Malig B, Broadwin R, Basu R, Gold EB, Bromberger JT, Derby C, Feinstein S, Greendale GA, Jackson EA, Kravitz HM, Matthews KA, Sternfeld B, Tomey K, Green RR, Green R, 2014. Chronic PM2.5 exposure and inflammation: Determining sensitive subgroups in mid-life women. Environ. Res. 132, 168–175. 10.1016/j.envres.2014.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, Bonzini M, Angelici L, Motta V, Pergoli L, Hoxha M, Cantone L, Pesatori AC, Apostoli P, Tripodi A, Baccarelli A, Bollati V, 2016. Extracellular vesicle-driven information mediates the long-term effects of particulate matter exposure on coagulation and inflammation pathways. Toxicol. Lett. 259, 143–150. 10.1016/j.toxlet.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergoli L, Cantone L, Favero C, Angelici L, Iodice S, Pinatel E, Hoxha M, Dioni L, Letizia M, Albetti B, Tarantini L, Rota F, Bertazzi PA, Tirelli AS, Dolo V, Cattaneo A, Vigna L, Battaglia C, Carugno M, Bonzini M, Pesatori AC, Bollati V, 2017. Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation. Part. Fibre Toxicol. 14, 32. 10.1186/s12989-017-0214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razquin C, Ruiz-Canela M, Clish CB, Li J, Toledo E, Dennis C, Liang L, Salas-Huetos A, Pierce KA, Guasch-Ferré M, Corella D, Ros E, Estruch R, Gómez-Gracia E, Fitó M, Lapetra J, Romaguera D, Alonso-Gómez A, Serra-Majem L, Salas-Salvadó J, Hu FB, Martínez-González MA, 2019. Lysine pathway metabolites and the risk of type 2 diabetes and cardiovascular disease in the PREDIMED study: results from two case-cohort studies. Cardiovasc. Diabetol. 18, 151. 10.1186/s12933-019-0958-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requia W, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, Sulprizio M, Amini H, Shi L, Schwartz J, 2020. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ. Sci. Technol. 54, 11037–11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodosthenous RS, Coull BA, Lu Q, Vokonas PS, Schwartz JD, Baccarelli AA, 2016. Ambient particulate matter and microRNAs in extracellular vesicles: a pilot study of older individuals. Part. Fibre Toxicol. 13, 13. 10.1186/s12989-016-0121-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodosthenous RS, Kloog I, Colicino E, Zhong J, Herrera LA, Vokonas P, Schwartz J, Baccarelli AA, Prada D, 2018. Extracellular vesicle-enriched microRNAs interact in the association between long-term particulate matter and blood pressure in elderly men. Environ. Res. 167, 640–649. 10.1016/j.envres.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu MR, Mondal AC, 2020. The emerging role of Hippo signaling in neurodegeneration. J. Neurosci. Res. 98, 796–814. 10.1002/jnr.24551 [DOI] [PubMed] [Google Scholar]
- Schinasi LH, Benmarhnia T, De Roos AJ, 2018. Modification of the association between high ambient temperature and health by urban microclimate indicators: A systematic review and meta-analysis. Environ. Res. 161, 168–180. 10.1016/j.envres.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Shi L, Wu X, Danesh Yazdi M, Braun D, Abu Awad Y, Wei Y, Liu P, Di Q, Wang Y, Schwartz J, Dominici F, Kioumourtzoglou M-A, Zanobetti A, 2020. Long-term effects of PM2·5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet. Heal. 10.1016/S2542-5196(20)30227-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Yeri A, Cheah PS, Chung A, Danielson K, De Hoff P, Filant J, Laurent CD, Laurent LD, Magee R, Moeller C, Murthy VL, Nejad P, Paul A, Rigoutsos I, Rodosthenous R, Shah RV, Simonson B, To C, Wong D, Yan IK, Zhang X, Balaj L, Breakefield XO, Daaboul G, Gandhi R, Lapidus J, Londin E, Patel T, Raffai RL, Sood AK, Alexander RP, Das S, Laurent LC, 2019. Small RNA Sequencing across Diverse Biofluids Identifies Optimal Methods for exRNA Isolation. Cell 177, 446–462.e16. 10.1016/j.cell.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Sun Y, Feng Z, Kang X, Yang W, Wang Y, Luo Y, 2021. New insights into the Hippo/YAP pathway in idiopathic pulmonary fibrosis. Pharmacol. Res. 169, 105635. 10.1016/j.phrs.2021.105635 [DOI] [PubMed] [Google Scholar]
- van Eeden SF, Yeung A, Quinlam K, Hogg JC, 2005. Systemic Response to Ambient Particulate Matter. Proc. Am. Thorac. Soc. 2, 61–67. 10.1513/pats.200406-035MS [DOI] [PubMed] [Google Scholar]
- van Rooij E, 2011. The Art of MicroRNA Research. Circ. Res. 108, 219–234. 10.1161/CIRCRESAHA.110.227496 [DOI] [PubMed] [Google Scholar]
- Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Möhlenkamp S, Nonnemacher M, Jakobs H, Erbel R, Jöckel K-H, Hoffmann B, 2015. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup. Environ. Med. 72, 656 LP–663. 10.1136/oemed-2014-102800 [DOI] [PubMed] [Google Scholar]
- Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG, 2015. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 43, W460–6. 10.1093/nar/gkv403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang T, Rui W, Xie J, Xie Y, Zhang X, Guan L, Li G, Lei Z, Schiffelers RM, Sluijter JPG, Xiao J, 2022. Extracellular vesicles enclosed-miR-421 suppresses air pollution (PM(2.5) )-induced cardiac dysfunction via ACE2 signalling. J. Extracell. vesicles 11, e12222. 10.1002/jev2.12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Kaufman RJ, 2016. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335. 10.1038/nature17041 [DOI] [PubMed] [Google Scholar]
- Wei Y, Qiu X, Sabath MB, Danesh Yazdi M, Yin K, Li L, Peralta AA, Wang C, Koutrakis P, Zanobetti A, Dominici F, Schwartz JD, 2022. Air Pollutants and Asthma Hospitalization in the Medicaid Population. Am. J. Respir. Crit. Care Med. 10.1164/rccm.202107-1596OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Ma Z, Jiang S, Wang D, Fan C, Di S, Hu W, Li T, She J, Yang Y, 2017. FOXOs in the impaired heart: New therapeutic targets for cardiac diseases. Biochim. Biophys. Acta - Mol. Basis Dis. 1863, 486–498. 10.1016/j.bbadis.2016.11.023 [DOI] [PubMed] [Google Scholar]
- Zhao J, Gao Z, Tian Z, Xie Y, Xin F, Jiang R, Kan H, Song W, 2013. The biological effects of individual-level PM(2.5) exposure on systemic immunity and inflammatory response in traffic policemen. Occup. Environ. Med. 70, 426–31. 10.1136/oemed-2012-100864 [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhao M, 2018. How Hippo Signaling Pathway Modulates Cardiovascular Development and Diseases. J. Immunol. Res. 2018, 3696914. 10.1155/2018/3696914 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


