Abstract
Extracellular vesicles (EVs) are lipid-bound vesicles that originate from the endosomal system or budded off from the plasma membrane. EVs are involved in cell-cell communication via transporting DNA, RNA, and proteins from one cell to another. Tear EVs (tEVs) have been reported in dry eye, Sjӧgren’s Syndrome, and primary open-angle glaucoma. In this study, we sought to investigate the presence of tEVs in relation to keratoconus (KC). Tears were passively collected from the lateral meniscus from 10 healthy (5 males and 5 females) and 9 KC (4 males and 5 females) subjects. Tear samples were processed and analyzed using the ExoView™ R100. Statistical analysis was performed using a Mann-Whitney U non-parametric Student’s t-test. All tEVs, in both Healthy and KC subjects, showed a CD9+ dominant tEV cohort independent of sex. A significant decrease in CD63+/CD9+ and CD63+/CD81+/CD9+ was found in the male KC tEVs (p<0.05), but not in females compared to their healthy counterparts. Neither Healthy nor KC tEVs showed differences in the total number of tEVs, however significant differences were identified between the sexes (p<0.05), with males having a higher number of tEVs. tEVs diameters ranged from 50–200nm, in both Healthy and KC cohorts, with the majority in the 50–80nm range suggesting exosome-dominant cohorts. To our knowledge, this is the first time, to date, that tEVs have been isolated and characterized in KCs. While further studies are warranted, the tEVs differences between KC and Healthy subjects suggest a potential role for tEVs in KC pathogenesis.1
Keywords: Keratoconus, tear, extracellular vesicles, cornea, tetraspanin, phenotype
1. Introduction
Extracellular vesicles (EVs) are membranous structures released from cells originated from the endosomal system or shed from the plasma membrane (Koritzinsky et al. 2017; Batrakova and Kim 2015; van Niel, D’Angelo, and Raposo 2018; Raposo and Stoorvogel 2013; Cocozza et al. 2020). Currently, EVs are divided into three types: microvesicles, apoptotic vesicles, and exosomes (Batrakova and Kim 2015; Cocozza et al. 2020; Akers et al. 2013; O’Brien et al. 2020). Briefly, microvesicles range from 100–1000nm in diameter and are released from cells by shedding of the plasma membrane (Zhang and Yu 2019). Apoptotic vesicles are products of an apoptotic cell disassembly process (Zhang and Yu 2019) with a size range of 1000–5000nm in diameter (Zhang and Yu 2019; Crescitelli et al. 2013). In contrast, exosomes are released from multivesicular bodies with sizes ranging from 50nm to 150nm (Zhang and Yu 2019). Exosomes carry a wide variety of cargos, such as proteins, cell-surface receptors, microRNAs, and mRNAs that aid in vesicle formation and trafficking (Dai et al. 2020; Koritzinsky et al. 2017). Currently, there are three main types of tetraspanins used to identify EVs: CD63, CD81, and CD9 (Mathieu et al. 2021; Huang et al. 2019). Tetraspanins are scaffold proteins that span the membrane four times and is present in all cell types (Ghossoub et al. 2020; Huang et al. 2019). EVs are found in the cornea, and have been reported to assist in corneal epithelium wound healing, however their role in the corneal pathobiology remains elusive (Zieske, Hutcheon, and Guo 2020). All three EV types have been reported in human bodily fluids, including blood, urine, saliva, and tears (Aqrawi et al. 2017; Alberro et al. 2021; Erdbrugger et al. 2021; Li et al. 2020; Akers et al. 2013; de Freitas et al. 2021).
Due to their rich composition in nutrients, metabolic products, water, electrolytes, proteins, lipids, mucins, and defensins (Tiffany 2003; Pieczynski et al. 2021) tear fluids operate as both a delivery and an excretory system directly interacting with and influencing the human cornea (Tiffany 2003). The presence of tear EVs (tEVs) was originally reported by Grigor’eva et al. in 2016 (Grigor’eva et al. 2016) but remains vastly understudied (Pieczynski et al. 2021). In the context of the human cornea, to date, tEVs have been reported in subjects with dry eye, Sjӧgren’s Syndrome, and primary open angled glaucoma (POAG) (Aqrawi et al. 2017; Zhang et al. 2020; Tamkovich et al. 2019). Considering the close proximity of tear fluid to the cornea and the number of diseases/dystrophies that could be affected by the presence of tEVs, clinical studies are lacking.
Keratoconus (KC) is a human cornea disease characterized by significant thinning, bulging, and scarring (Fournie et al. 2013; Asimellis and Kaufman 2022; Lang, Maier, and Reinhard 2021), which can ultimately lead to devastating vision loss if left untreated (Moussa et al. 2017; Jian et al. 2018; Fournie et al. 2013). KC is generally considered as a non-inflammatory, progressive condition that affects approximately 1:400 people worldwide (Sharif et al. 2018; Sharif, Fowler, and Karamichos 2018; Fournie et al. 2013). It typically starts during puberty (Vohra, Tuteja, and Chawla 2022; Santodomingo-Rubido et al. 2022) and affects both sexes and all ethnicities (Santodomingo-Rubido et al. 2022). The tear fluid in those who suffer from KC has been investigated using proteome (Lopez-Lopez et al. 2021; Nishtala et al. 2016; de Almeida Borges et al. 2020; Galvis et al. 2015) and cytokine (Balasubramanian et al. 2012; Fodor et al. 2021; Zhang et al. 2021; Sorkhabi et al. 2015; Galvis et al. 2015) screening, but to date, there are no studies showing the existence of tEVs in those subjects. Our study serves as the first insight into the presence and phenotype of tEVs in KC.
Overall, tEVs are a rich, untapped resource that may hold vast clues to the health status of the individuals producing them. Consequently, it is paramount to determine better understanding both ocular homeostasis and pathology. Understanding their potential role and function in corneal health and disease states is of significant potential interest to researchers and clinicians alike.
2. Materials and Methods:
2.1. Ethics Statement
All experiments described in this study adhered to the Declaration of Helsinki. Prior to inclusion in the study, written consent was obtained from each subject. Institutional Review Board (IRB) approval from Dean McGee Institute (1576837-2) and Aarhus University Hospital (1-10-72-77-14) was received according to the institutional and federal guidelines. All tear samples were de-identified before processing. In addition, all studies were reviewed and approved by the North Texas Regional Institutional Review Board (#2020-030).
2.2. Experimental design
Tears were collected from both healthy and KC subjects. Tear collections were performed using glass capillary tubes, passively collected from the lateral meniscus after inducing tear flow (McKay et al. 2020; Karamichos et al. 2015; Sharif et al. 2019; Priyadarsini et al. 2014). Collected samples were given a unique identification code and were stored in Eppendorf tubes at −20°C.
2.3. Cohort included
Tear samples were collected from 5 healthy males (HM), 5 healthy females (HF), 4 KC males (KCM), and 5 KC females (KCF) (Table 1). KC subjects included in this study were diagnosed clinically via slit-lamp biomicroscopy and ancillary diagnostic testing, including ultrasound pachymetry and computerized corneal tomography (Pentacam, Oculus Inc., Wetzlar, Germany). Corneal curvature, refractive astigmatism, keratometric astigmatism, best-corrected visual acuity, and corneal thickness measurements were recorded. KC severity was determined using the ABCD grading system (Belin et al. 2020; Dubinsky-Pertzov et al. 2021). Group 1 was the least severe KC group with an anterior radius curvature (ARC), converted to dioptric values, of <48.0D, followed by group 2 with ARC <53.0D, then group 3 with an ARC<55.0D, and finally group 4 being the most severe group with an ARC >55.0D, but no subjects in this presented study met criteria for inclusion in this category. For this cohort, 4 subjects were in Group 1, 4 in Group 2, and 1 in Group 3. The Healthy subjects included had no previous or active ocular or systemic disease(s). Furthermore, HM and HF subjects had no prior or future scheduled ocular surgeries. The average age for the healthy cohort was 55.5±18.69 years old and 32.2±9.17 for KCs (Table 1).
Table 1:
Age, Sex, and KC severity of tear samples collected from KC subjects.
| Healthy | KC | |
|---|---|---|
| Age (years) | ||
| Overall | 55.5 ± 18.69 | 32.2 ±9.17 |
| Male | 61.8 ± 17.21 | 26.8 ± 9.23 |
| Female | 49.4 ± 17.79 | 37.6 ± 5.68 |
| Sex | ||
| Male | 5 | 4 |
| Female | 5 | 5 |
| KC Severity (ABCD system) | ||
| 1: <48D | − | 4 |
| 2: <53D | − | 4 |
| 3: <55D | − | 1 |
| 4: >55D | − | 0 |
2.4. Tear fluid preparation and processing
ExoView kits (EV-TETRA-C, NanoView Biosciences, Boston, MA, USA) were used to analyze the tEVs (Wise et al. 2021; Deng et al. 2022; Samoylenko et al. 2021). Briefly, all tear samples were diluted to optimized 100x Solution B (1 μL tears + 99 μL Solution B). 70 uL of the diluted sample were placed on the chips overnight. The chips included three mouse antibodies targeting human tetraspanin markers (CD9+, CD63+ and CD81+). The next morning, the chips were washed three times. A detection antibody cocktail containing three mouse antibodies, anti-human CD81+ conjugated with Alexfluor 488, CD63+ conjugated with Alexfluor 647, and CD9+ conjugated with Alexfluor 555 and blocking solution, was added to the chips and incubated at room temperature for 1 hour, and subjected to three cycles of washing using a chip washer (CW100, NanoView Biosciences, Boston, MA, USA). The chips were then dried and scanned using the ExoView Scanner software version 3.1 (ExoView R100, NanoView Biosciences, Boston, MA, USA). The total particle counts, immunofluorescence, and sizes of the tEVs were analyzed using the ExoView Analyzer software version 3.1.
2.5. Tetraspanin fingerprinting and Statistical analysis
The tetraspanin fingerprint pie charts for the captured tEVs from each sample showed the breakdown of the tetraspanin phenotype for those particles captured by each wavelength. The colocalized staining is considered an accurate measure of identifying the phenotype of captured EVs (Han et al. 2021) by allowing the total tetraspanin positive cohort to be accurately summed across the different capture type and generating a single detected total value, thus allowing for comparison of both the quantity and type of tEVs in each subject cohort. Our analysis and dataset explored the changes in total tEVs among the KC and Healthy cohorts. Furthermore, we identified and defined KC-specific sub-cohorts, when compared to healthy subjects.
GraphPad Prism 9.2.0 software was used for statistical analysis. Mann-Whitney U nonparametric tests were utilized, and values of p ≤ 0.05 were considered significant. Figures 1, 3 and 5 were plotted with the mean ± standard error of the mean.
Figure 1:
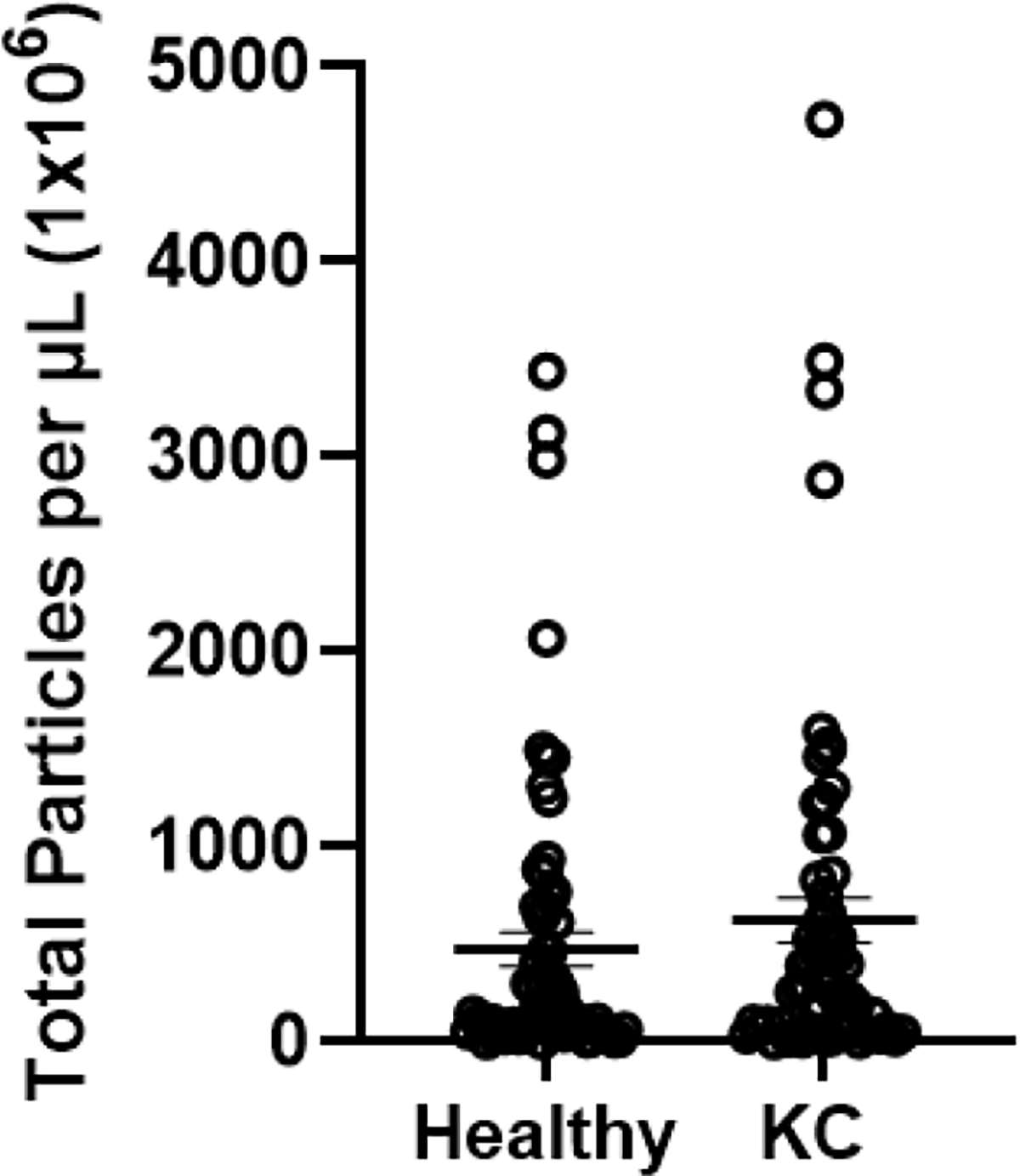
Total tEV counts for healthy and KC subjects. Healthy: n=10, KC: n=9. p=0.3026
Figure 3: Total particle count for Healthy and KC samples.
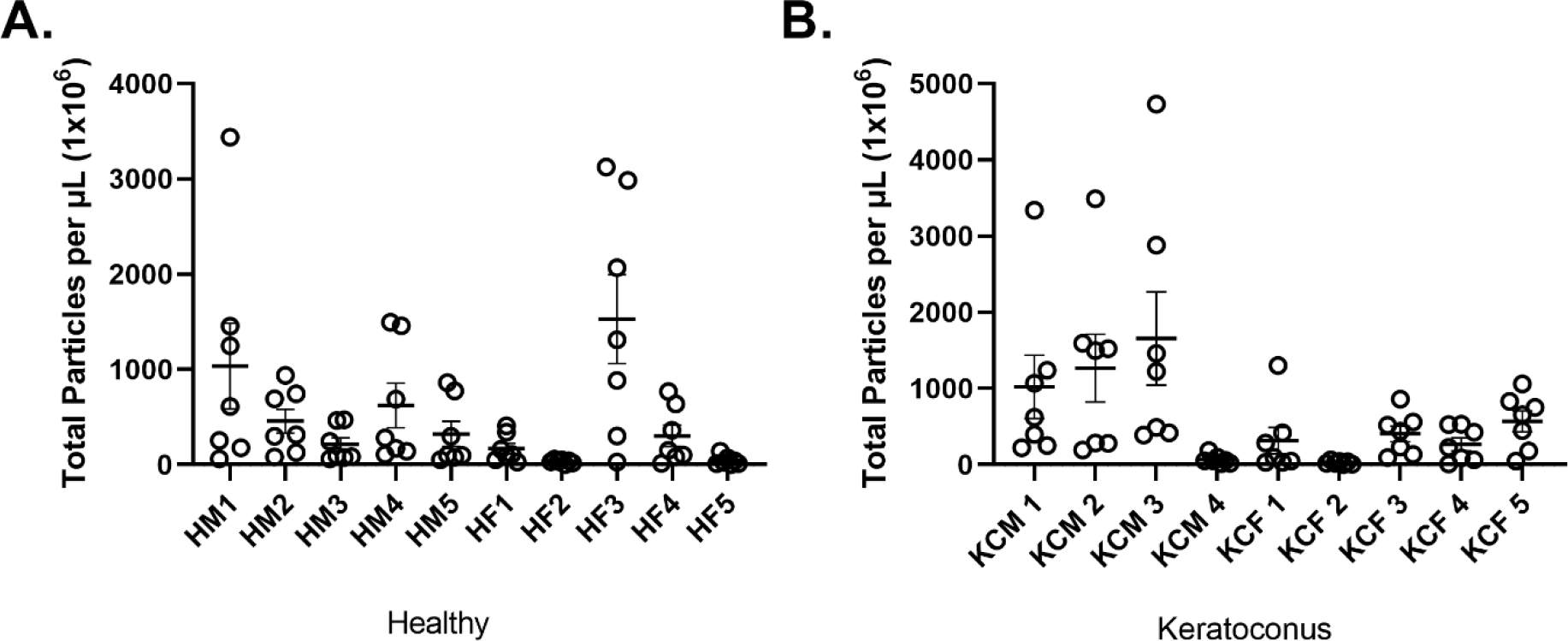
A) Total particle count for each healthy sample. B) Total particle count for each KC samples.
Figure 5: Total Particle count for Male vs Female samples.
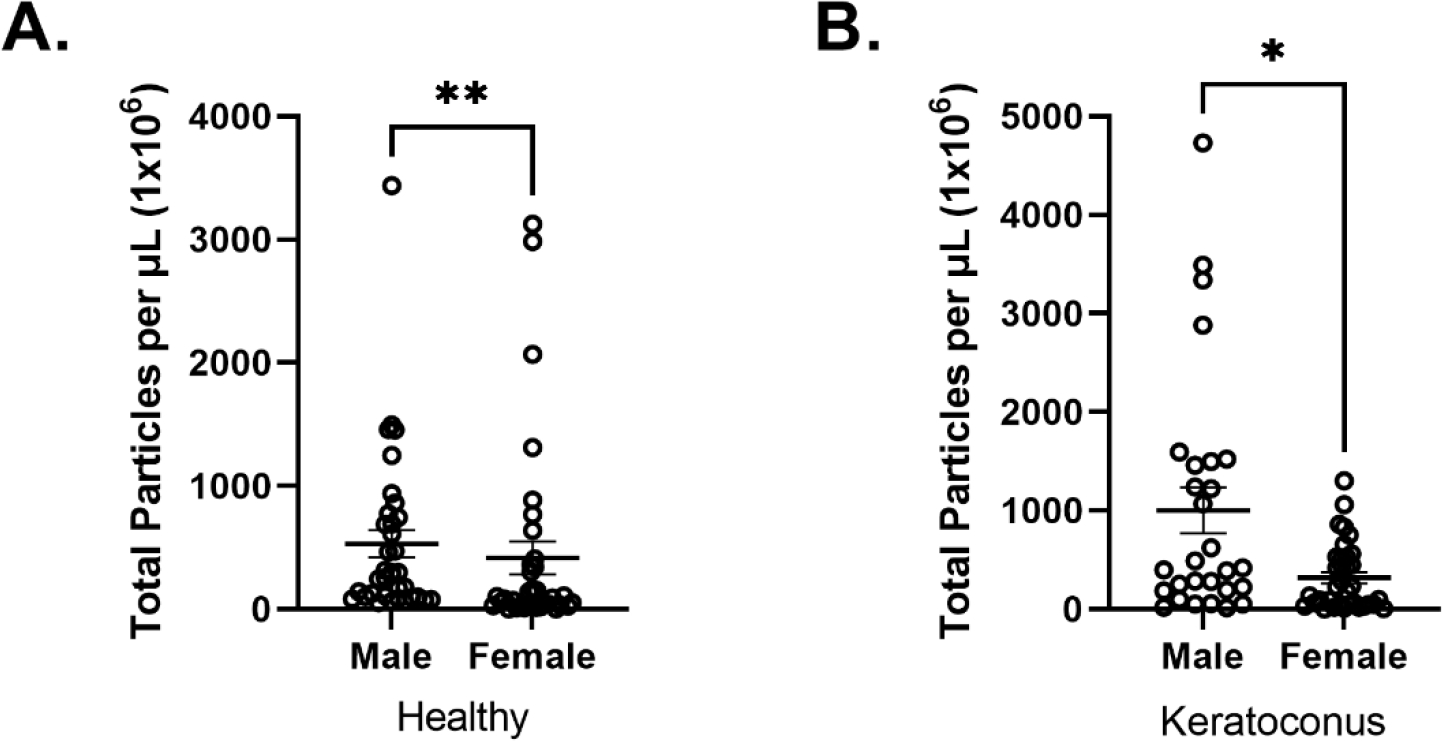
A) Total particle count for HM vs HF with a significance of **p<0.01. B) Total particle count for KCM vs KCF with a significant difference of *p<0.05.
3. Results:
3.1. Healthy vs. KC
Figure 1 shows that the overall tetraspanin tEV counts were increased by 1.31 fold in KCs, without reaching the significance threshold (p=0.3026). Among the specific sub-cohorts, those with fold increases greater than the total were two of the mono-tetraspanin cohorts, CD81+ and CD9+, as shown in Figure 2A–B. These tEVs displayed only a single tetraspanin type with CD63+ down 1.13 fold, CD81+ up 3.77 fold, and CD9+ up 1.54 fold. The only multi-tetraspanin cohort greater than the total was CD81+/CD9+ at 1.79 fold. There was a significant decrease in CD63+/CD9+ (p=0.0228) when comparing the total healthy and total KC tEVs, as shown in Figure 2B.
Figure 2: Phenotypes of tEVs isolated from Healthy and KC samples.

A) Phenotype of Healthy tEVs. B) Phenotype of KC tEVs. CD63+/CD9+ is significantly decreased between healthy and KC subjects. *p<0.05
3.2. Tear EVs are Predominantly CD9+ Positive and Uniform in Size
The tetraspanin fingerprint results for all tEVs showed a similar phenotype, with the largest cohort of particles being CD9+ and the second largest being CD63+/CD9+ colocalized. When comparing the healthy against KC tEVs, results were consistent within each group as 70–84% of the total captured tEV particles contained CD9+ (includes CD9+, CD63+/CD9+, CD81+/CD9+, and CD63+/CD81+/CD9+), 36–53% contain CD63+ (includes CD63+, CD63+/CD81+, CD63+/CD9+and CD63+/CD81+/CD9+) and 27–32% contain CD81+ (includes CD81+, CD63+/CD81+, CD81+/CD9+, CD63+/CD81+/CD9+). Data indicates that tEVs have a consistent CD9+ dominant phenotype. Strong colocalization of CD81+/CD9+ (13–17%) and CD63+/CD9+ (17–29%) was also found, but less frequently. Furthermore, CD81+ and CD63+/CD81+ tEVs comprised 8–17% of the total tEVs when not colocalized with CD9+ as shown in Table 2. This general phenotype persisted across all tEVs, as shown in Figure 3A–B. The HM tEVs showed counts that were more spread out compared to HF tEVs whose counts were more grouped/closer together. The same was true between KCM and KCF tEVs. Overall, total tEV counts in the healthy cohort appeared to be grouped/closer together than the KC tEVs, as shown in Figure 3A–B.
Table 2:
Percentage of particles found in each phenotypic category.
| HF | HM | KCF | KCM | |
|---|---|---|---|---|
| CD63+ | 14% | 8% | 17% | 10% |
| CD81+ | 6% | 3% | 3% | 5% |
| CD9+ | 28% | 36% | 34% | 41% |
| CD63+/CD81+ | 10% | 5% | 5% | 6% |
| CD63+/CD9+ | 26% | 29% | 22% | 17% |
| CD81+/CD9+ | 13% | 14% | 14% | 17% |
| CD63+/CD81+/CD9+ | 3% | 5% | 5% | 3% |
HF = Healthy Female; HM = Healthy Male;
KCF = Keratoconus female; KCM = Keratoconus Male
The overall size of the tEVs was uniform with less frequent larger particles, as shown in Figure 4A–C. Most of the tEVs for healthy and KC samples ranged in sizes of 50–80nm, suggesting heavy exosome presence. Regarding sex dependence, HMs had more tEVs above 80nm in diameter, while HFs had less measuring above 80nm. Interestingly, such differences were not seen in the KC cohort, where KCMs and KCFs showed an even size distribution of tEVs.
Figure 4: Particle sizes.
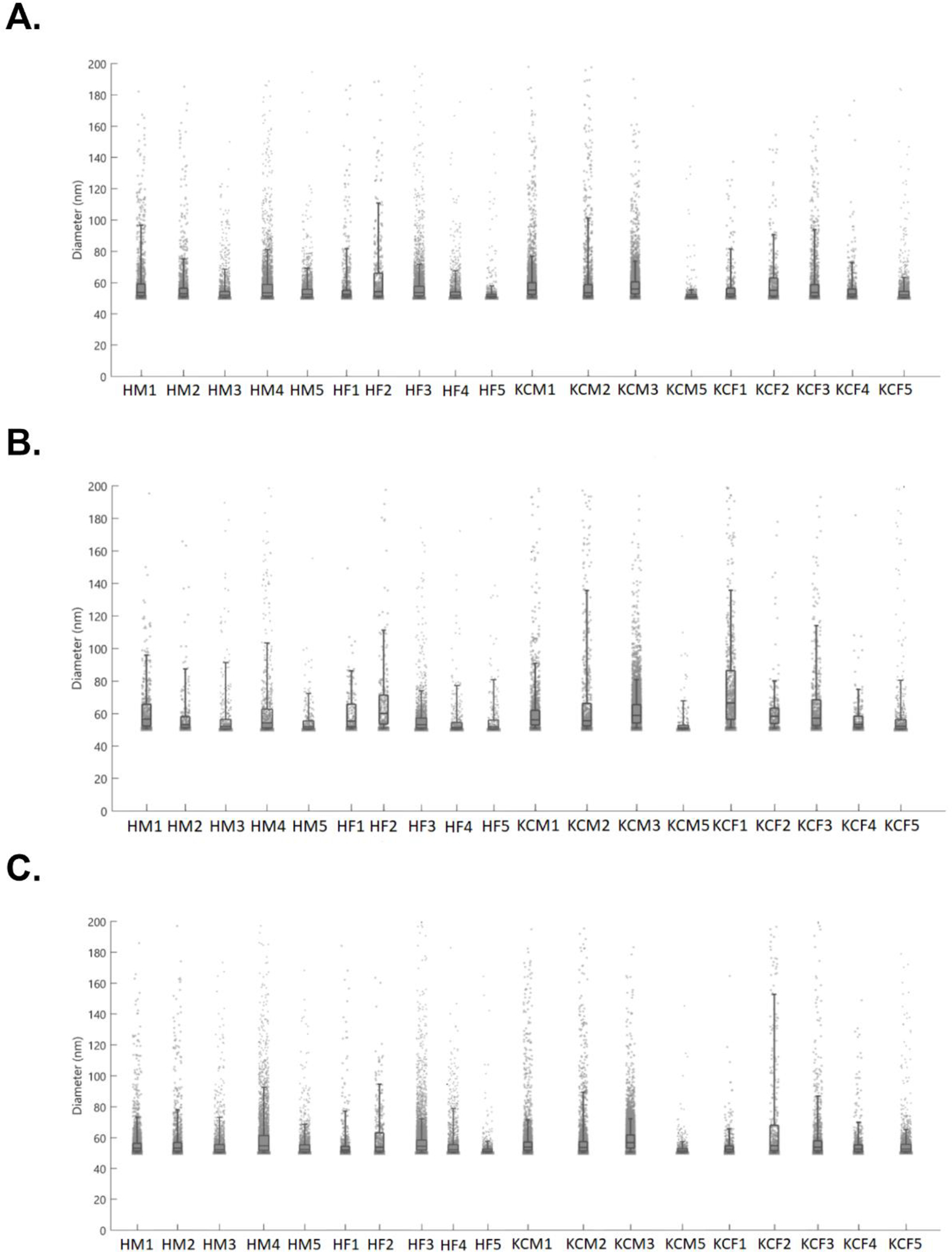
Particle sizes were counted between the cutoff ranges of 50nm-200nm. A) Sizes of particles present when fluoresced at the CD63+ wavelength (647). B) Particles sizes present when fluoresced at CD81+ wavelength (488). C) Sizes of particles present when fluoresced at the CD9+ wavelength (555).
3.3. Sex-Based Differences are Revealed in Total EV Counts
Figure 5A–B describes sex-dependent differences in the total tEVs. In healthy, the total tetraspanin tEVs detected were 1.27 fold higher in HMs than HFs (p=0.0046) (Fig. 5A). Similarly, in KCs, the total tetraspanin tEVs were 3.16 fold higher in KCMs than KCFs (p=0.0122) (Fig. 5B). Despite the differences between the sexes in tEV counts, the total number of tEVs was still increased in KCM and KCF with the KCMs having a fold increase of 1.89 and the KCFs having a fold increase of 0.76 when compared to their healthy counterparts.
When examining the sex differences in the KCs, CD81+/CD9+ were upregulated compared to healthy (Figure 6A–D). The CD81+ cohort also showed an increase of 2.06 fold for HF and KCF and 4.81 fold for HM and KCM when stratified by sex. CD63+/CD9+ (p=0.0317) and CD63+/CD81+/CD9+ (p=0.0476) had a significant decrease between the HMs and KCMs, as shown in Figure 6D.
Figure 6: Phenotypes of tEVs isolated from Healthy and KC samples.
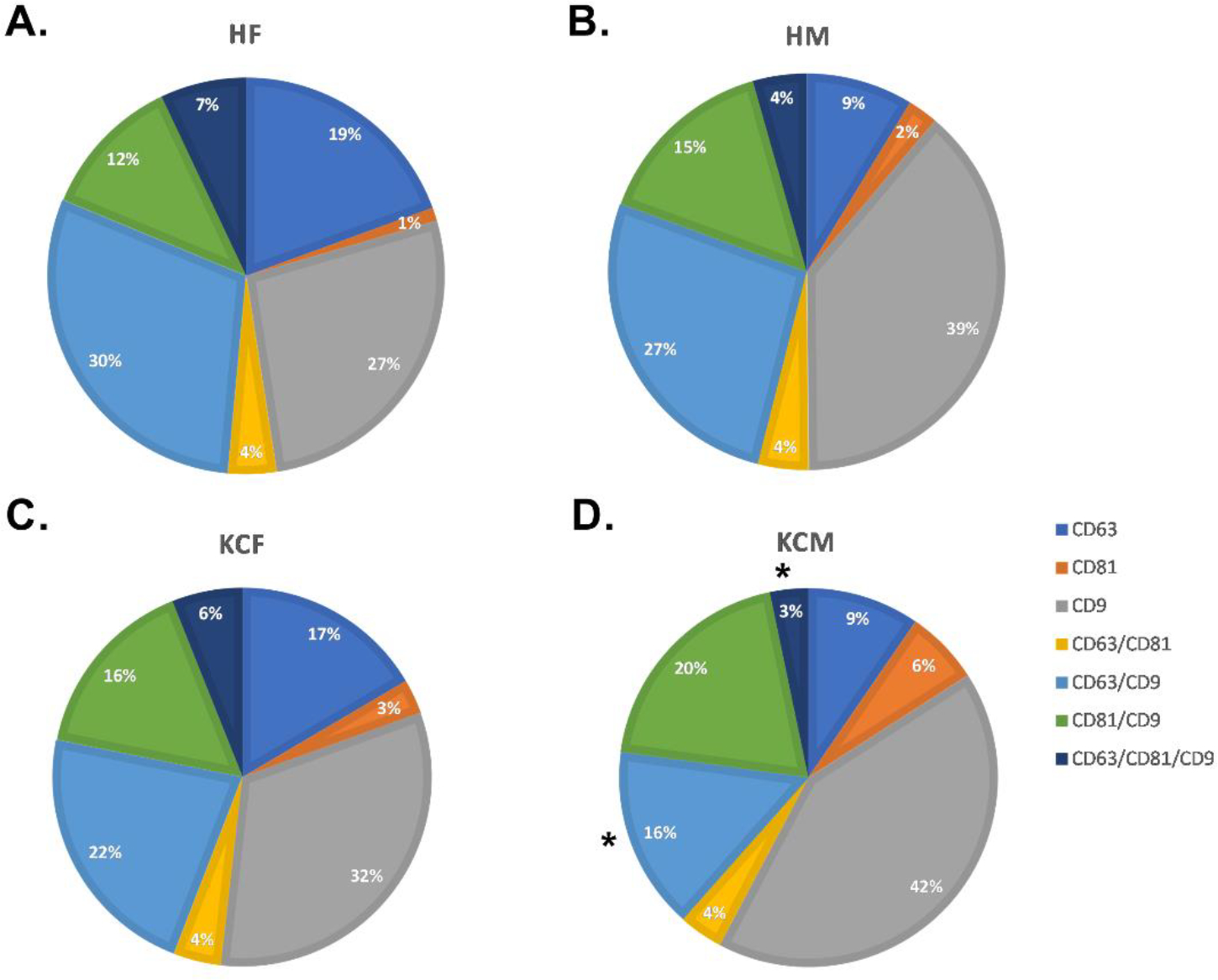
A) Phenotype of HF tEVs. B) Phenotype of HM tEVs. C) Phenotype of KCF tEVs. D) Phenotype of KCM tEVs with a significant decrease in CD63+ and CD63+/CD9+ when compared to the overall KC. *p<0.05
These data revealed subtle changes in the makeup of tEVs within the different cohorts. Further, while tEVs general phenotype seems conserved between sexes, males showed higher counts of tEVs.
4. Discussion
Our study represents the first report of tEVs isolated from KC subjects. KC is primarily considered a bilateral disease of the cornea (Asimellis and Kaufman 2022) with multifactorial onset (de Azevedo Magalhaes, Goncalves, and Gatinel 2021; Bykhovskaya and Rabinowitz 2021; Nishtala et al. 2016) which include: the interaction of hormonal (Karamichos et al. 2021; Escandon et al. 2022; McKay, Priyadarsini, and Karamichos 2022), genetic (Gordon-Shaag et al. 2015; Ates, Estes, and Liu 2021; Wheeler et al. 2012), environmental (Gordon-Shaag et al. 2015), and biochemical factors (Mohammadpour, Heidari, and Hashemi 2018). A simple mechanical action, such as eye-rubbing, is also speculated to increase the risk of KC onset (Nishtala et al. 2016). Down syndrome and Leber’s congenital amaurosis have also been reported to have a high incidence of KC, attributed to the eye rubbing habits associated with the two disorders (Rabinowitz 1998). Currently, treatment options for KC remain limited and include non-surgical treatments, such as glasses and contact lenses, and surgical interventions such as corneal crosslinking, intrastromal corneal ring segments, and keratoplasty (Seitz et al. 2021) (corneal transplantation). Unfortunately, significant drawbacks still exist with these options, as well as an economic burden on patients and the healthcare system.
Currently, genetic research is being conducted to discover and confirm potential genes associated with KC. The genes that are known to be involved in the development of KC include Superoxide dismutase 1 (SOD1), Visual system homeobox (VSX1), Dedicator of cytokinesis 9 (DOCK9), lysyl oxidase (LOX), type IV collagen alpha 3 (COL4A3), and type IV collagen alpha 4 (COL4A4) (Wheeler et al. 2012; Barrientez et al. 2019; Burdon and Vincent 2013; Saravani et al. 2015). Not only is KC influenced by genetic variations, but hormones are also known to enhance the risk of KC onset. In 2019, our group showed downregulation of Estrone and Estriol and significant upregulation of adrenal-derived DHEA-S in KC vs. healthy patients (Karamichos et al. 2021). More recently, we reported sex biases between Estrone/Estriol and sex hormone receptors in the KC corneal stroma (Escandon et al. 2022). The thyroid hormone, Thyroxine, has also been reported to be present in higher levels in KC vs. healthy tears (McKay, Priyadarsini, and Karamichos 2022; Gatzioufas and Thanos 2008). Additionally, due to hormonal changes, pregnancy has been reported to be a risk factor for KC onset and progression.
Biomechanics are also considered critical to KC pathobiology. KC is known to disorient the collagen fibrils, reducing the cross-links between them and decreasing the number of lamellae found in the cornea, leading to significant instability (Karamichos et al. 2021). Markers for corneal biomechanics, such as corneal hysteresis (CH) and corneal resistance factor (CRF) that are commonly used for defining corneal biomechanics, are also altered in KC subjects (Karamichos et al. 2021). Overall, the use of tears in KC studies provides an easily-accessible, minimally-invasive, and cost-effective resource that can be utilized to understand the disease better. Recent studies on KC tear fluid have revealed an increase in systemic levels of pro-inflammatory factors (McKay et al. 2020) and have shown significant differences in biochemical factors (Shetty et al. 2020). Tear fluid has also aided in determining biomarkers specific to KC, such as Prolactin-Induced Protein (PIP) (Sharif et al. 2019). Proteomics and metabolomics have been utilized, by many, revealing a large number of proteins and metabolites significantly altered in KCs when compared to healthy subjects, including hormones (Lopez-Lopez et al. 2021), immunoglobins (McKay et al. 2020), and metabolites (Karamichos et al. 2015).
Due to the EV biogenesis process, either from the endosomal system or from being shed from the plasma membrane, EVs have different proteins located on their surface (van Niel, D’Angelo, and Raposo 2018; Colombo, Raposo, and Thery 2014). The three tetraspanins, CD63+, CD81+, and CD9+, each play their unique roles in the cell. CD63+ is associated with the endosomal system, which produces exosomes and assists in membrane transport, fusion, and protein kinase signaling, and also aids in releasing EVs from the cell (Yanatori et al. 2021). CD81+ plays a role in membrane organization, protein trafficking, and cell-cell interactions in the immune system (Vences-Catalan et al. 2017) and helps regulate adaptive and innate immune suppression (Vences-Catalan et al. 2017). CD9+ plays a role in cell adhesion, motility, growth, differentiation, and in the immune system as well (Lorico et al. 2021). Studies have shown that all three tetraspanins found on human corneal tissue-derived EVs can aid in corneal wound healing (Samaeekia et al. 2018; Shojaati et al. 2019). In this study, we identified for the first time, significant decreases in the CD63+/CD9+ and CD63+/CD81+/CD9+ KC tEVs, possibly indicating a reduction in exosome release from the endosomal system and reduced immune response ability.
EVs have been thoroughly investigated throughout the body, especially in cancer research (Zieske, Hutcheon, and Guo 2020; Dai et al. 2020). On the other hand, tEVs research is an area that is severely understudied, with only a few studies published in the last decade. Recently, tEV research was conducted on subjects diagnosed with Sjӧgren’s Syndrome and dry eye (Aqrawi et al. 2017). Studies conducted using tear samples from Sjӧgren’s Syndrome subjects reported no significant differences in the number of tEVs isolated from the diseased compared to healthy subjects (Aqrawi et al. 2017), similar to our findings with KC tEVs. A group studying EVs in dry eye discovered a significant difference in the expression profiles of low-molecular-weight proteins and peptides between healthy subjects and patients diagnosed with dry eye (Zhang et al. 2020). Our findings suggest that not only is there a difference between healthy and KC patients, but there is also a sex difference between males and females with different numbers of particles from tears. However, much larger cohorts are needed both in Healthy and in KCs in order to accurately determine such differences. Further, the connection/tie (if any) between tEVs and the EVs found within the cornea tissue is unknown and should be investigated. Overall, our study provides a glimpse of what the characteristics of tEVs are in KC, and shines a spotlight on their existence. Future studies will focus on executing a statistically powered study
5. Conclusion
The novel findings of this study provide considerable insights into KC related to tEVs.
Among all body fluids, tears present a highly advantageous and favorable entity for biomarker studies, mainly due to their proximity to the cornea. Tear sample collection is a non-invasive and economic modality with minimal clinical harm/risk to the patients, and can prove highly impactful when investigating tEVs. Depending on what future clinical studies observe, tEVs could serve as a potential executant for KC, and remains an area of interest for researchers and clinicians alike.
Highlights:
For the first time, tear extracellular vesicles were identified, isolated and characterized in keratoconus subjects.
Tear extracellular vesicles, from keratoconus subjects, exhibited a distinct phenotype, when compared to their healthy counterparts.
Tear extracellular vesicles from male keratoconus subjects showed higher total counts.
Acknowledgements:
We would like to thank the NIH/NEI for the support (EY030028-S1, DK), (EY023242 and EY031631, YL)
Footnotes
Declarations of Interest:
None
EVs: Extracellular vesicles, KC: Keratoconus, tEVs: tear extracellular vesicles, HM: Healthy Males HF: Healthy Females, KCM: Keratoconus Males, KCF: Keratoconus Females, ARC: Anterior radius curvature.
References:
- Akers JC, Gonda D, Kim R, Carter BS, and Chen CC. 2013. ‘Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies’, J Neurooncol, 113: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberro A, Iparraguirre L, Fernandes A, and Otaegui D. 2021. ‘Extracellular Vesicles in Blood: Sources, Effects, and Applications’, Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqrawi LA, Galtung HK, Vestad B, Ovstebo R, Thiede B, Rusthen S, Young A, Guerreiro EM, Utheim TP, Chen X, Utheim OA, Palm O, and Jensen JL. 2017. ‘Identification of potential saliva and tear biomarkers in primary Sjogren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis’, Arthritis Res Ther, 19: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimellis G, and Kaufman EJ. 2022. ‘Keratoconus.’ in, StatPearls (Treasure Island (FL)). [Google Scholar]
- Ates KM, Estes AJ, and Liu Y. 2021. ‘Potential underlying genetic associations between keratoconus and diabetes mellitus’, Adv Ophthalmol Pract Res, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian SA, Mohan S, Pye DC, and Willcox MD. 2012. ‘Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus’, Acta Ophthalmol, 90: e303–9. [DOI] [PubMed] [Google Scholar]
- Barrientez B, Nicholas SE, Whelchel A, Sharif R, Hjortdal J, and Karamichos D. 2019. ‘Corneal injury: Clinical and molecular aspects’, Exp Eye Res, 186: 107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, and Kim MS. 2015. ‘Using exosomes, naturally-equipped nanocarriers, for drug delivery’, J Control Release, 219: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin MW, Kundu G, Shetty N, Gupta K, Mullick R, and Thakur P. 2020. ‘ABCD: A new classification for keratoconus’, Indian J Ophthalmol, 68: 2831–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon KP, and Vincent AL. 2013. ‘Insights into keratoconus from a genetic perspective’, Clin Exp Optom, 96: 146–54. [DOI] [PubMed] [Google Scholar]
- Bykhovskaya Y, and Rabinowitz YS. 2021. ‘Update on the genetics of keratoconus’, Exp Eye Res, 202: 108398. [DOI] [PubMed] [Google Scholar]
- Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, and Thery C. 2020. ‘SnapShot: Extracellular Vesicles’, Cell, 182: 262–62 e1. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, and Thery C. 2014. ‘Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles’, Annu Rev Cell Dev Biol, 30: 255–89. [DOI] [PubMed] [Google Scholar]
- Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, Buzas EI, and Lotvall J. 2013. ‘Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes’, J Extracell Vesicles, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, and Jiang Y. 2020. ‘Exosomes: key players in cancer and potential therapeutic strategy’, Signal Transduct Target Ther, 5: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Borges D, Alborghetti MR, Franco Paes Leme A, Ramos Domingues R, Duarte B, Veiga M, Trindade Ferrer M, Viana Wanzeler AC, Leite Arieta CE, and Alves M. 2020. ‘Tear proteomic profile in three distinct ocular surface diseases: keratoconus, pterygium, and dry eye related to graft-versus-host disease’, Clin Proteomics, 17: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azevedo Magalhaes O, Goncalves MC, and Gatinel D. 2021. ‘The role of environment in the pathogenesis of keratoconus’, Curr Opin Ophthalmol, 32: 379–84. [DOI] [PubMed] [Google Scholar]
- de Freitas RCC, Hirata RDC, Hirata MH, and Aikawa E. 2021. ‘Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases’, Biomolecules, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Ratri A, Deighan C, Daaboul G, Geiger PC, and Christenson LK. 2022. ‘Single-Particle Interferometric Reflectance Imaging Characterization of Individual Extracellular Vesicles and Population Dynamics’, J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky-Pertzov B, Reinhardt O, Gazit I, Or L, Hecht I, Pras E, and Einan-Lifshitz A. 2021. ‘The ABCD Keratoconus Grading System-A Useful Tool to Estimate Keratoconus Progression in the Pediatric Population’, Cornea, 40: 1322–29. [DOI] [PubMed] [Google Scholar]
- Erdbrugger U, Blijdorp CJ, Bijnsdorp IV, Borras FE, Burger D, Bussolati B, Byrd JB, Clayton A, Dear JW, Falcon-Perez JM, Grange C, Hill AF, Holthofer H, Hoorn EJ, Jenster G, Jimenez CR, Junker K, Klein J, Knepper MA, Koritzinsky EH, Luther JM, Lenassi M, Leivo J, Mertens I, Musante L, Oeyen E, Puhka M, van Royen ME, Sanchez C, Soekmadji C, Thongboonkerd V, van Steijn V, Verhaegh G, Webber JP, Witwer K, Yuen PST, Zheng L, Llorente A, and Martens-Uzunova ES. 2021. ‘Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles’, J Extracell Vesicles, 10: e12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escandon P, Nicholas SE, Cunningham RL, Murphy DA, Riaz KM, and Karamichos D. 2022. ‘The Role of Estriol and Estrone in Keratoconic Stromal Sex Hormone Receptors’, Int J Mol Sci, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor M, Vitalyos G, Losonczy G, Hassan Z, Pasztor D, Gogolak P, and Kolozsvari BL. 2021. ‘Tear Mediators NGF along with IL-13 Predict Keratoconus Progression’, Ocul Immunol Inflamm, 29: 1090–101. [DOI] [PubMed] [Google Scholar]
- Fournie P, Touboul D, Arne JL, Colin J, and Malecaze F. 2013. ‘[Keratoconus]’, J Fr Ophtalmol, 36: 618–26. [DOI] [PubMed] [Google Scholar]
- Galvis V, Sherwin T, Tello A, Merayo J, Barrera R, and Acera A. 2015. ‘Keratoconus: an inflammatory disorder?’, Eye (Lond), 29: 843–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzioufas Z, and Thanos S. 2008. ‘Acute keratoconus induced by hypothyroxinemia during pregnancy’, J Endocrinol Invest, 31: 262–6. [DOI] [PubMed] [Google Scholar]
- Ghossoub R, Chery M, Audebert S, Leblanc R, Egea-Jimenez AL, Lembo F, Mammar S, Le Dez F, Camoin L, Borg JP, Rubinstein E, David G, and Zimmermann P. 2020. ‘Tetraspanin-6 negatively regulates exosome production’, Proc Natl Acad Sci U S A, 117: 5913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Shaag A, Millodot M, Shneor E, and Liu Y. 2015. ‘The genetic and environmental factors for keratoconus’, Biomed Res Int, 2015: 795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigor’eva AE, Tamkovich SN, Eremina AV, Tupikin AE, Kabilov MR, Chernykh VV, Vlassov VV, Laktionov PP, and Ryabchikova EI. 2016. ‘[Characteristics of exosomes andmicroparticles discovered in human tears]’, Biomed Khim, 62: 99–106. [DOI] [PubMed] [Google Scholar]
- Han C, Kang H, Yi J, Kang M, Lee H, Kwon Y, Jung J, Lee J, and Park J. 2021. ‘Single-vesicle imaging and co-localization analysis for tetraspanin profiling of individual extracellular vesicles’, J Extracell Vesicles, 10: e12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zucker B, Zhang S, Elias S, Zhu Y, Chen H, Ding T, Li Y, Sun Y, Lou J, Kozlov MM, and Yu L. 2019. ‘Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains’, Nat Cell Biol, 21: 991–1002. [DOI] [PubMed] [Google Scholar]
- Jian W, Shen Y, Chen Y, Tian M, and Zhou X. 2018. ‘Ocular dimensions of the Chinese adolescents with keratoconus’, BMC Ophthalmol, 18: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Escandon P, Vasini B, Nicholas SE, Van L, Dang DH, Cunningham RL, and Riaz KM. 2021. ‘Anterior pituitary, sex hormones, and keratoconus: Beyond traditional targets’, Prog Retin Eye Res: 101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Zieske JD, Sejersen H, Sarker-Nag A, Asara JM, and Hjortdal J. 2015. ‘Tear metabolite changes in keratoconus’, Exp Eye Res, 132: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koritzinsky EH, Street JM, Star RA, and Yuen PS. 2017. ‘Quantification of Exosomes’, J Cell Physiol, 232: 1587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SJ, Maier P, and Reinhard T. 2021. ‘[Crosslinking and Keratoconus]’, Klin Monbl Augenheilkd, 238: 733–47. [DOI] [PubMed] [Google Scholar]
- Li J, Guan X, Fan Z, Ching LM, Li Y, Wang X, Cao WM, and Liu DX. 2020. ‘Non-Invasive Biomarkers for Early Detection of Breast Cancer’, Cancers (Basel), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez M, Regueiro U, Bravo SB, Chantada-Vazquez MDP, Varela-Fernandez R, Avila-Gomez P, Hervella P, and Lema I. 2021. ‘Tear Proteomics in Keratoconus: A Quantitative SWATH-MS Analysis’, Invest Ophthalmol Vis Sci, 62: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorico A, Lorico-Rappa M, Karbanova J, Corbeil D, and Pizzorno G. 2021. ‘CD9, a tetraspanin target for cancer therapy?’, Exp Biol Med (Maywood), 246: 1121–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M, Nevo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, Rubinstein E, Boncompain G, Perez F, and Thery C. 2021. ‘Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9’, Nat Commun, 12: 4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TB, Priyadarsini S, and Karamichos D. 2022. ‘Sex Hormones, Growth Hormone, and the Cornea’, Cells, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TB, Serjersen H, Hjortdal J, Zieske JD, and Karamichos D. 2020. ‘Characterization of Tear Immunoglobulins in a Small-Cohort of Keratoconus Patients’, Sci Rep, 10: 9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour M, Heidari Z, and Hashemi H. 2018. ‘Updates on Managements for Keratoconus’, J Curr Ophthalmol, 30: 110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa S, Grabner G, Ruckhofer J, Dietrich M, and Reitsamer H. 2017. ‘Genetics in Keratoconus - What is New?’, Open Ophthalmol J, 11: 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishtala K, Pahuja N, Shetty R, Nuijts RM, and Ghosh A. 2016. ‘Tear biomarkers for keratoconus’, Eye Vis (Lond), 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien K, Breyne K, Ughetto S, Laurent LC, and Breakefield XO. 2020. ‘RNA delivery by extracellular vesicles in mammalian cells and its applications’, Nat Rev Mol Cell Biol, 21: 585–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieczynski J, Szulc U, Harazna J, Szulc A, and Kiewisz J. 2021. ‘Tear fluid collection methods: Review of current techniques’, Eur J Ophthalmol, 31: 2245–51. [DOI] [PubMed] [Google Scholar]
- Priyadarsini S, Hjortdal J, Sarker-Nag A, Sejersen H, Asara JM, and Karamichos D. 2014. ‘Gross cystic disease fluid protein-15/prolactin-inducible protein as a biomarker for keratoconus disease’, PLoS One, 9: e113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz YS 1998. ‘Keratoconus’, Surv Ophthalmol, 42: 297–319. [DOI] [PubMed] [Google Scholar]
- Raposo G, and Stoorvogel W. 2013. ‘Extracellular vesicles: exosomes, microvesicles, and friends’, J Cell Biol, 200: 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, Eslani M, and Djalilian AR. 2018. ‘Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing’, Invest Ophthalmol Vis Sci, 59: 5194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoylenko A, Kogler M, Zhyvolozhnyi A, Makieieva O, Bart G, Andoh SS, Roussey M, Vainio SJ, and Hiltunen J. 2021. ‘Time-gated Raman spectroscopy and proteomics analyses of hypoxic and normoxic renal carcinoma extracellular vesicles’, Sci Rep, 11: 19594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, and Wolffsohn JS. 2022. ‘Keratoconus: An updated review’, Cont Lens Anterior Eye: 101559. [DOI] [PubMed] [Google Scholar]
- Saravani R, Hasanian-Langroudi F, Validad MH, Yari D, Bahari G, Faramarzi M, Khateri M, and Bahadoram S. 2015. ‘Evaluation of possible relationship between COL4A4 gene polymorphisms and risk of keratoconus’, Cornea, 34: 318–22. [DOI] [PubMed] [Google Scholar]
- Seitz B, Daas L, Hamon L, Xanthopoulou K, Goebels S, Spira-Eppig C, Razafimino S, Szentmary N, Langenbucher A, and Flockerzi E. 2021. ‘[Erratum to: Stage-appropriate treatment of keratoconus]’, Ophthalmologe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R, Bak-Nielsen S, Hjortdal J, and Karamichos D. 2018. ‘Pathogenesis of Keratoconus: The intriguing therapeutic potential of Prolactin-inducible protein’, Prog Retin Eye Res, 67: 150–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R, Bak-Nielsen S, Sejersen H, Ding K, Hjortdal J, and Karamichos D. 2019. ‘Prolactin-Induced Protein is a novel biomarker for Keratoconus’, Exp Eye Res, 179: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R, Fowler B, and Karamichos D. 2018. ‘Collagen cross-linking impact on keratoconus extracellular matrix’, PLoS One, 13: e0200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty R, D’Souza S, Khamar P, Ghosh A, Nuijts Rmma, and Sethu S 2020. ‘Biochemical Markers and Alterations in Keratoconus’, Asia Pac J Ophthalmol (Phila), 9: 533–40. [DOI] [PubMed] [Google Scholar]
- Shojaati G, Khandaker I, Funderburgh ML, Mann MM, Basu R, Stolz DB, Geary ML, Dos Santos A, Deng SX, and Funderburgh JL. 2019. ‘Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA’, Stem Cells Transl Med, 8: 1192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkhabi R, Ghorbanihaghjo A, Taheri N, and Ahoor MH. 2015. ‘Tear film inflammatory mediators in patients with keratoconus’, Int Ophthalmol, 35: 467–72. [DOI] [PubMed] [Google Scholar]
- Tamkovich S, Grigor’eva A, Eremina A, Tupikin A, Kabilov M, Chernykh V, Vlassov V, and Ryabchikova E. 2019. ‘What information can be obtained from the tears of a patient with primary open angle glaucoma?’, Clin Chim Acta, 495: 529–37. [DOI] [PubMed] [Google Scholar]
- Tiffany JM 2003. ‘Tears in health and disease’, Eye (Lond), 17: 923–6. [DOI] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, and Raposo G. 2018. ‘Shedding light on the cell biology of extracellular vesicles’, Nat Rev Mol Cell Biol, 19: 213–28. [DOI] [PubMed] [Google Scholar]
- Vences-Catalan F, Duault C, Kuo CC, Rajapaksa R, Levy R, and Levy S. 2017. ‘CD81 as a tumor target’, Biochem Soc Trans, 45: 531–35. [DOI] [PubMed] [Google Scholar]
- Vohra V, Tuteja S, and Chawla H. 2022. ‘Collagen Cross Linking For Keratoconus.’ in, StatPearls (Treasure Island (FL)). [PubMed] [Google Scholar]
- Wheeler J, Hauser MA, Afshari NA, Allingham RR, and Liu Y. 2012. ‘The Genetics of Keratoconus: A Review’, Reprod Syst Sex Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Neviani P, Riwaldt S, Corydon TJ, Wehland M, Braun M, Kruger M, Infanger M, and Grimm D. 2021. ‘Changes in Exosome Release in Thyroid Cancer Cells after Prolonged Exposure to Real Microgravity in Space’, Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanatori I, Richardson DR, Dhekne HS, Toyokuni S, and Kishi F. 2021. ‘CD63 is regulated by iron via the IRE-IRP system and is important for ferritin secretion by extracellular vesicles’, Blood, 138: 1490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cao X, Liu Y, Wang P, and Li X. 2021. ‘Tear Levels of Inflammatory Cytokines in Keratoconus: A Meta-Analysis of Case-Control and Cross-Sectional Studies’, Biomed Res Int, 2021: 6628923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, and Yu D. 2019. ‘Exosomes in cancer development, metastasis, and immunity’, Biochim Biophys Acta Rev Cancer, 1871: 455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Hu L, Ma H, Ni F, Liu F, and Chen H. 2020. ‘Detection of Tear Components Using Matrix-Assisted Laser Desorption Ionization/Time-of-Flight Mass Spectrometry for Rapid Dry Eye Diagnosis’, J Proteome Res, 19: 3644–51. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Hutcheon AEK, and Guo X. 2020. ‘Extracellular Vesicles and Cell-Cell Communication in the Cornea’, Anat Rec (Hoboken), 303: 1727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


