Abstract
Staphylococcus aureus with intermediate glycopeptide susceptibility (glycopeptide-intermediate S. aureus [GISA]) has been isolated from patients with apparent therapy failures. We studied the killing activity of vancomycin over a range of simulated conventional doses (1 to 1.5 g every 12 h) against three of these GISA strains in an in vitro pharmacodynamic infection model. We also studied the activity of a new glycopeptide (LY333328) at a simulated dose of 3 mg/kg of body weight every 24 h or 5 mg/kg every 24 h, as well as the potential for vancomycin and gentamicin synergy against these GISA strains. Four doses of vancomycin with or without gentamicin or two doses of LY333328 were administered over the 48-h study period. The vancomycin and LY333328 MICs and minimal bactericidal concentrations (MBCs) for the three GISA strains (strains 14379, 992, and Mu50) were 8 and 8 μg/ml and 1 and 2 μg/ml, respectively, for GISA 14379, 6 and 6 μg/ml and 1 and 1 μg/ml, respectively, for GISA 992, and 8 and 12 μg/ml and 2 and 8 μg/ml, respectively, for GISA Mu50. Vancomycin and LY333328 MICs and MBCs were 0.75 and 1.0 μg/ml and 1 and 1 μg/ml, respectively for a vancomycin-susceptible comparator strain (methicillin-resistant S. aureus [MRSA] 494). The addition of albumin to the growth medium increased the LY333328 MICs and MBCs approximately 8- to 16-fold. Vancomycin was bacteriostatic against the three GISA strains at doses of 1, 1.125, and 1.25 g every 12 h. Vancomycin was bactericidal at the dose of 1.5 g every 12 h against all strains; bactericidal activity occurred against the GISA strains at 8- to 10-fold lower ratios of the peak concentration to the MIC and the area under the concentration-time curve from time zero to 24 h (AUC0–24) to the MIC compared to those for the vancomycin-sensitive control strain. Overall, vancomycin activity was significantly correlated with the AUC0–24 (R2 = 0.79; P < 0.001) by multiple stepwise regression analyses. The addition of gentamicin did not significantly affect killing activity against any strain. LY333328 was bactericidal against GISA strains 14379 and 992 and against MRSA 494 only with the 5-mg/kg/day dose simulations. The higher dose of LY333328 also prevented regrowth over the 48-h experiments for all strains tested. Higher doses of vancomycin (1.5 g every 12 h) and LY333328 (5 mg/kg every 24 h) may represent potential treatment options for infections caused by GISA strains.
Infections due to methicillin-resistant Staphylococcus aureus (MRSA) continued to be a significant problem in the 1990s. Vancomycin, a glycopeptide antibiotic, often is the only available treatment option. Although an alarming increase in the prevalence of vancomycin-resistant enterococci has occurred along with the increased use of vancomycin, resistance in clinical strains of staphylococci had remained rare until recently (5–7). The isolation of glycopeptide-intermediate S. aureus (GISA) for which MICs are 8 μg/ml was reported first from Japan in 1997 (7) and soon thereafter from the United States (5, 6). Each GISA strain was recovered from patients who had received extended courses of treatment with vancomycin (7, 13). The expression of this reduced vancomycin susceptibility appears to be heterogeneous (1, 13, 14), and these clinical strains share with laboratory-derived vancomycin-resistant staphylococcal mutants such characteristics as thickened cell walls (3, 10, 23, 25–27), increased levels of production of cell wall precursors (25, 26), decreased levels of autolysis (10, 22, 25, 26), and increased levels of penicillin-binding protein production (13, 19).
The recent isolation of these clinical strains of GISA heightens the importance of testing alternative antimicrobial regimens against these already methicillin-resistant bacteria. Although all patients with GISA infections appear to have failed vancomycin therapy, each had factors that could promote the failure of antimicrobial therapy such as an undebrided wound infection or the presence of an indwelling catheter (5–7, 13, 29). Decreased vancomycin activity has previously been reported for these GISA strains via determination of MICs and minimum bactericidal concentrations (MBCs) and time-kill curves studies and in rabbit endocarditis models (1, 2, 9, 12, 21), but the impact of decreased susceptibility on glycopeptide killing over the range of expected in vivo concentration-time profiles is not known. The objectives of the current investigation were to quantify the killing activities of two glycopeptides (vancomycin and LY333328) at various simulated doses and to determine the potential for gentamicin-vancomycin synergy against three strains of GISA by using an in vitro pharmacodynamic infection model.
MATERIALS AND METHODS
Bacterial strains.
The three GISA strains tested in this investigation were 14379 (William Beaumont Hospital, Royal Oak, Michigan [J. Mitchell, M. Ionescu, D. Farnaz, S. Donabedian, M. B. Perri, L. A. Thal, J. Sunstrum, J. W. Chow, T. Smith, and M. J. Zervos, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LB-14, 1997]), Mu-50 (Juntendo Hospital, Tokyo, Japan [13]), and 992 (Centers for Disease Control and Prevention, Atlanta, Ga.) (5, 29). A clinical strain of MRSA (MRSA 494) was used to compare the activity of the glycopeptides against GISA to that against a glycopeptide-sensitive strain. We have previously evaluated the activity of vancomycin against strain 494 in a similar in vitro infection model (15).
Antimicrobial agents and media.
Vancomycin (lot 35H040425) and gentamicin (lot 96H0975) were purchased commercially (Sigma Chemical Company, St. Louis, Mo.). LY333328 (lot 103 RM6) was supplied by Eli Lilly & Company (Indianapolis, Ind.). Mueller-Hinton broth (Difco, Detroit, Mich.) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) (SMHB) was used for broth susceptibility testing and in all in vitro infection models. Human albumin (lot 00769-00006; Swiss Red Cross, Berne, Switzerland) was added to SMHB at a concentration of 4 gm/dl for LY333328 susceptibility testing and for the infection models since this drug's activity can be significantly affected by the presence of protein (18). Tryptic soy agar (TSA, Difco) plates were used to determine colony counts.
In vitro susceptibility testing and FIC indices.
Microdilution MICs and MBCs for vancomycin, LY333328, and gentamicin were determined with a standard inoculum of 5 × 105 CFU/ml according to the guidelines of the National Committee for Clinical Laboratory Standards (20). Fractional inhibitory concentrations (FICs) were determined by the checkerboard technique with an inoculum of 5 × 105 CFU/ml. The FIC index for each combination was computed by the following equation: FIC index = (MIC of drug A alone/MIC of drug A in the combination) + (MIC of drug B alone/MIC of drug B in the combination). Synergy, additivity, indifference, and antagonism were defined as FIC indices of ≤0.5, 0.5 to 1.0, 1 to 4, and ≥4, respectively (11).
Time-kill curve synergy studies.
Combinations of the two glycopeptides with gentamicin at 1/2×, 1×, and 2× the MICs were tested for synergistic, additive, or antagonistic activity. Synergy was defined as ≥2 log10 CFU/ml greater killing compared to the level of killing of the most active compound alone, additivity was defined as <2 log10 CFU/ml greater killing, and antagonism was defined as decreased killing activity compared to the killing activity of the least active compound. All time-kill curve studies were performed in triplicate with a starting inoculum of 106 CFU/ml. Two to three colonies from an overnight growth of organism on TSA plates were added to normal saline, and the density was adjusted as necessary to produce a 0.5 McFarland suspension of organisms which was subsequently diluted 1:10 with SMHB. A 0.2-ml volume of this suspension was added to 1.7 ml of SMHB plus 0.1 ml of antibiotic stock solution in a 24-well tissue culture plate (final volume, 2.0 ml per well). Samples (0.1 ml) were removed from each well at 0, 4, 8, and 24 h, diluted appropriately in cold 0.9% sodium chloride, placed on TSA with an autoplate spiral dispenser (model 3000; Spiral Bioscience, Frederick, Md.), and incubated at 35°C for 24 to 48 h. The bacterial inoculum (log10 CFU per milliliter) was determined with a laser bacteria colony counter (model 500A; Spiral System Instruments, Inc., Frederick, Md.). We have determined that these methods have a limit of detection of 2.5 log10 CFU/ml (1, 12).
In vitro pharmacodynamic infection model.
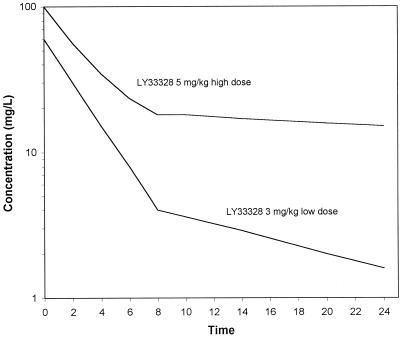
The in vitro infection model has been described previously (17) and consisted of a 250-ml one-compartment glass chamber with ports for the addition and removal of SMHB, injection of antibiotics, and removal of samples. Prior to each experiment, colonies from an overnight growth of bacteria on TSA were added to SMHB as necessary to obtain a suspension of 108 CFU/ml. A volume of 2.5 ml of this suspension was added to the infection models to produce a starting inoculum of 106 CFU/ml. Fresh stock solutions of vancomycin, LY333328, and gentamicin were prepared on the first day of the experiment and were stored at 2 to 8°C between dose administration times. Vancomycin was administered every 12 h for four doses to simulate the typical peak and trough concentrations in serum obtained from doses of 1, 1.125, 1.25, and 1.5 g (33). LY333328 was administered every 24 h for two doses to simulate the expected peak and trough concentrations obtained from (i) a dosage of 3-mg/kg of body weight in humans (60 and 1.5 μg/ml) or (ii) a loading dose of 5 mg/kg followed by a maintenance dose of 4 mg/kg (100 and 15 μg/ml) (Fig. 1). These concentration-time profiles were based on pharmacokinetic data for humans from preliminary phase I trials (M. Zeckel, personal communication, 1998). Gentamicin was administered every 12 h for four doses to simulate peak and trough concentrations of approximately 8 and 1 μg/ml (32) in combination with vancomycin at 1 g every 12 h. Antibiotics were administered into the infection model over 1 min. A peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Il.) was used to displace antibiotic-containing medium with fresh SMHB to simulate the terminal half-lives of vancomycin (6 h) and gentamicin (3 h) (32, 33). Previous investigations by our group have shown that the loss of bacteria during medium displacement from the infection model does not significantly affect the evaluation of antibiotic killing (17). Two-compartment elimination pharmacokinetics were simulated for LY333328 (an alpha half-life of 2 h and a beta half-life of 12 h [low dose] or 18 h [high dose]) by adjusting the pump flow rates at the appropriate time from one monoexponential elimination rate to another monoexponential elimination rate. During administration of regimens containing vancomycin with gentamicin, the central compartment elimination rate was set for the drug with the shortest half-life (gentamicin) and vancomycin was administered into a supplemental chamber to maintain its longer half-life (4). The infection models were placed in water baths and were maintained at 37°C for the entire 48-h study period. Each experimental regimen was repeated two to six times to study reproducibility.
FIG. 1.
Concentration-time profiles for the high (5 mg/kg) and low (3 mg/kg) doses of LY333328 as simulated in the in vitro infection models.
Pharmacodynamic analyses.
Samples (0.5 ml) were removed from each infection model at 0, 0.5, 1, 2, 4, 8, 24, 28, 32, and 48 h. Each sample was diluted in cold 0.9% sodium chloride, and bacterial counts were determined either as described above in the section on time-kill curve studies (for vancomycin and gentamicin regimens) or by plating 20 μl from appropriately diluted samples in triplicate onto TSA plates and incubating the plates at 37°C for 24 h (for LY333328 model samples). The latter method had a limit of detection of 2.0 log10 CFU/ml. Average colony counts (in log10 CFU per milliliter) in the infection models were plotted versus time to generate time-kill curves. The reductions in the log10 CFU per milliliter over 48 h were determined and compared between regimens and to assess the regimens for synergy, additivity, or antagonism. Bactericidal activity was defined as a persistent ≥3-log10 CFU/ml reduction from the starting inoculum at the 48-h time point. For each regimen, the area under the time-kill curve (AUKC) was also calculated by using the trapezoidal rule.
Pharmacokinetic analyses.
Calculations of the values for the pharmacokinetic parameters were based on antibiotic concentration data obtained from samples after the first vancomycin dose (0, 0.5, 1, 2 4, 8, and 12 h). Peak and/or trough concentrations were obtained for the remaining three doses over the 48-h experiment. The infection model data were not accepted unless these peak and trough concentrations remained within ca. 25% of the expected concentrations. Samples (0.5 ml) from the infection models were stored at −70°C until analysis (no later than 1 month from the sampling date). Vancomycin and gentamicin concentrations were determined by a fluorescence polarization immunoassay (TDx; Abbott Diagnostics), which had sensitivities of 2.0 μg/ml for vancomycin and 0.27 μg/ml for gentamicin and interday coefficients of variation of <12% for vancomycin and <11% for gentamicin (15). We have previously shown that the concentrations in control samples prepared in SMHB vary by <10% compared to those in the serum control standards (15). Concentrations of LY333328 were determined by microbioassay with Mueller-Hinton agar with 5% horse blood and a clinical strain of Streptococcus pneumoniae (strain R852) as the indicator organism. Standards (prepared in SMHB with 4 g of human albumin per dl) and samples were placed in triplicate in the agar wells and were allowed to diffuse into the agar for 18 to 24 h. A concentrated suspension of the indicator organism (ca. 108 CFU/ml) was applied to the agar, and the plates were incubated in candle jars at 37°C for 24 h. The bioassay had a limit of detection of 0.1 μg/ml, had inter- and intraday coefficients of variation of <2.2% for the low- dose (0.1 μg/ml) and high-dose (100 μg/ml) standards, and was linear (R2 > 0.98) over the range of concentrations tested. All pharmacokinetic parameters were calculated by using RStrip software (Micromath, Salt Lake City, Utah). Mean pharmacodynamic indices (ratio of the peak concentration to the MIC [peak:MIC], the ratio of the area under the concentration-time curve [AUC] from time zero to 24 h [AUC0–24] to the MIC [AUC0–24:MIC], the ratio of the trough concentration to the MIC [trough:MIC], and the time above the MIC) were computed by integrating the raw pharmacokinetic data with the MIC data for the appropriate organism.
Statistical analyses.
The colony counts at 48 h were compared between groups by analysis of variance with Tukey's test for multiple comparisons. Relationships between antimicrobial effect (quantified either by the residual bacterial inoculum at 48 h or by the AUKC) and pharmacokinetic and pharmacodynamic indices (peak concentration, peak:MIC, trough concentration, trough:MIC, AUC, and AUC:MIC) were examined by multivariate and stepwise linear regressions. The time above the MIC was not examined, as this parameter was 100% of the dosing interval for all regimens studied. Additionally, the relationship between pharmacokinetic and pharmacodynamic indices and the bacterial inoculum at 48 h was examined by using a sigmoidal Emax model (three-parameter Hill equation), log10 CFU per milliliter at 48 h = (Emax · PIS)/(PIS50% + PIS), where Emax represents the maximal antimicrobial effect, PI represents the pharmacokinetic and pharmacodynamic variable of interest, PI50% is the value of the pharmacokinetic and pharmacodynamic variable of interest that produced 50% of Emax, and S is an exponent that describes the degree of sigmoidicity. For all comparisons, a P value of ≤0.05 indicated statistical significance. All analyses were performed with SigmaPlot (version 5.00) and/or SigmaStat (version 2.03; SPSS, Inc., Chicago, Ill.) software.
RESULTS
Susceptibility testing, FIC indices, and time-kill curve studies.
The MICs and MBCs for strains 14379, Mu50, 992, and 494 are summarized in Table 1. LY333328 MICs and MBCs in SMHB were slightly higher for Mu50 than for 14379, 992, and 494. Both 14379 and Mu50 were gentamicin resistant; 992 and 494 were gentamicin sensitive. Addition of 4 g of albumin per dl to SMHB minimally affected the MIC and MBC for 494, but the GISA MICs and MBCs for the GISA strains all became four to eight times higher. The FICs of vancomycin or LY333328 plus gentamicin for 14379, Mu50, 992, and 494 were 1.5 and 0.3, 2 and 2, 2 and 2, and 1.0 and 0.75, respectively. The combination of gentamicin with vancomycin or LY333328 in test tube killing curve studies provided additive or synergistic activity against the three GISA strains and against the vancomycin-sensitive MRSA strain at 1/2× the MIC only. The concentrations of vancomycin or LY333328 ≥1× the MIC alone provided killing activity that was at or near the limits of detection at 24 h.
TABLE 1.
MICs and MBCs for GISA strains 14379, Mu50, and 992
| Organism | MIC/MBC (μg/ml)
|
|||
|---|---|---|---|---|
| Vancomycin |
LY333328
|
Gentamicin | ||
| SMHB | Albumina | |||
| 14379 | 8 /8 | 1 /2 | 4 /8 | 64 />256 |
| Mu50 | 8 /12 | 2 /8 | 16 /16 | 128 /128 |
| 992 | 6 /6 | 1 /1 | 16 /16 | 0.25 /0.5 |
| 494 (control) | 0.75 /1 | 0.5–1 /2–4 | 0.5 /4 | 1 /1 |
SMHB with 4 g of human albumin per dl.
In vitro infection models: pharmacokinetics and pharmacodynamics.
The values obtained for the pharmacokinetic parameters are listed in Table 2. The values for the pharmacodynamic parameters used in the regression analyses were calculated by dividing the pharmacokinetic data shown in Fig. 2 by the MICs for the individual strains (8, 8, 6, and 0.75 μg/ml for 14379, Mu50, 992, and MRSA 494, respectively). All calculated values for the pharmacodynamic parameters were significantly lower for the GISA strains than for MRSA strain 494 (data not shown). For both regimens of LY333328, the peak:MIC and AUC0–24:MIC ratios were significantly higher for GISA 14379 than for GISA 992 and Mu50.
TABLE 2.
Values of pharmacokinetic parameters obtained with infection modelsa
| Regimen | Peak concn (μg/ml) | Trough concn (μg/ml) | Alpha half-life (h) | Beta half-life (h) | AUC0–24 (μg · h/ml) |
|---|---|---|---|---|---|
| Vancomycin, 1 g every 12 h | 35.0 ± 4.8 | 8.4 ± 1.8 | NDb | 6.2 ± 1.0 | 464 ± 52 |
| Vancomycin, 1.125 g every 12 h | 52.7 ± 3.0 | 15.5 ± 2.1 | ND | 6.2 ± 0.3 | 678 ± 59 |
| Vancomycin, 1.25 g every 12 h | 59.0 ± 5.7 | 17.1 ± 2.0 | ND | 6.7 ± 1.0 | 804 ± 32 |
| Vancomycin, 1.5 g every 12 h | 66.7 ± 4.6 | 18.6 ± 1.1 | ND | 6.3 ± 0.6 | 885 ± 42 |
| LY333328, low dose | 57.1 ± 9.9 | 1.2 ± 0.3 | 1.8 ± 0.2 | 9.1 ± 1.6 | 192 ± 31 |
| LY333328, high dose | 97.2 ± 10.9 | 11.6 ± 2.0 | 1.4 ± 0.2 | 19.5 ± 2.8 | 612 ± 80 |
| Gentamicin every 12 h | 8.0 ± 0.8 | 0.7 ± 0.2 | ND | 3.1 ± 0.4 | 65.1 ± 6.9 |
Values are means ± standard deviations.
ND, not determined.
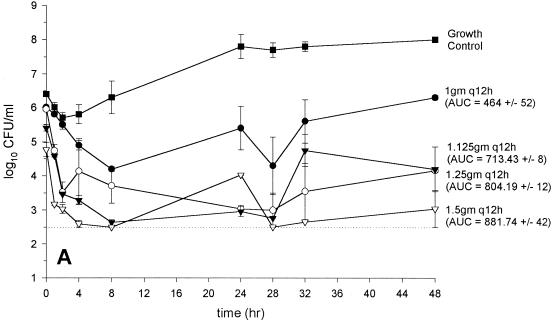
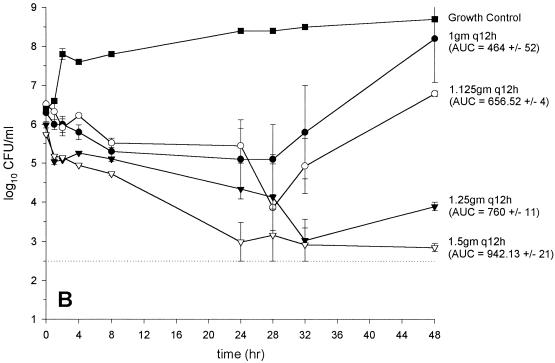
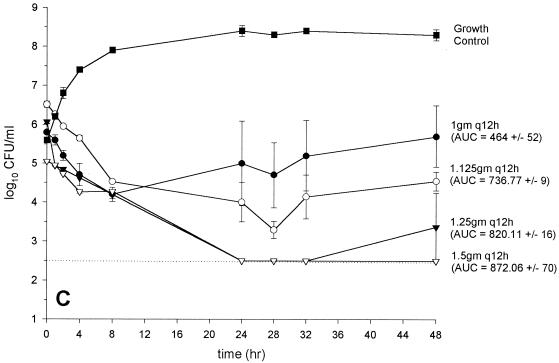
FIG. 2.
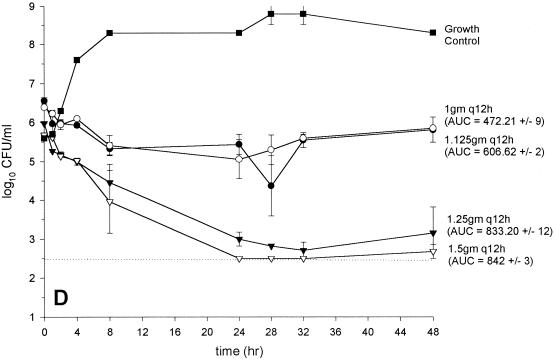
Activity of vancomycin versus GISA 14379 (MIC and MBC, 8 and 8 μg/ml, respectively) (A), GISA Mu50 (MIC and MBC, 8 and 12 μg/ml, respectively) (B), GISA 992 (MIC and MBC, 6 and 6 μg/ml, respectively) (C), and (D) MRSA 494 control (MIC and MBC, 0.75 and 1 μg/ml, respectively) (D). (●, 1 g every 12 h; ○, 1.125 g every 12 h; ▾, 1.25 g every 12 h; ▿, 1.5 g every 12 h; ▪, growth control). The dashed line indicates the reliable limit of detection (2.5 log10 CFU/ml).
In vitro infection models: vancomycin monotherapy regimens.
The results from the regimens with vancomycin administered every 12 h are depicted in Fig. 2A to D. Colony counts, at 48 h were significantly higher for Mu50 than for all other strains for the regimens of 1 and 1.125 g every 12 h. Vancomycin at 1.25 g every 12 h produced similar inocula at 48 h for all strains tested (range, 3.5 to 4.2 log10 CFU/ml). Vancomycin at 1.5 g every 12 h was bactericidal against all GISA strains tested. There were no elevations in vancomycin microtiter MICs or MBCs for any of the bacterial colonies obtained at 48 h. Colony counts at 48 h were not significantly different from those for the most potent monotherapy regimen for the vancomycin (1 g every 12 h) plus gentamicin combination regimens (data not shown).
Pharmacodynamic predictors of vancomycin activity.
When pharmacokinetic and pharmacodynamic data for all strains tested were analyzed by simple linear regression, three parameters significantly correlated with the bacterial inoculum at 48 h: peak concentration (R2 = 0.66; P < 0.0001), trough concentration (R2 = 0.41; P = 0.0047), and AUC0–24 (R2 = 0.78; P < 0.0001). However, the AUC0–24 was the only parameter that significantly correlated with the bacterial inoculum at 48 h by both forward and backward stepwise regression analyses (R2 = 0.79; P < 0.001). The analyses were similar for the correlation of AUKC with the pharmacokinetic parameters (data not shown). No significant correlation of the pharmacodynamic indices with the inoculum at 48 h existed, a finding that related to the similar activities of vancomycin at 1.25 and 1.5 g every 12 h against the GISA strains and the vancomycin-sensitive control strain 494 (Fig. 2A to D). Accordingly, the bactericidal activity of vancomycin (defined by a reduction of the starting inoculum by ≥3 log10 CFU/ml and predicted by sigmoidal Emax modeling) occurred at substantially (ca. 8- to 10-fold) lower peak:MIC, trough:MIC, and AUC:MIC ratios for the GISA strains than for the vancomycin-sensitive strain (Table 3).
TABLE 3.
Minimum vancomycin parmacodynamic indices producing bactericidal activity for GISA strains and a vancomycin-sensitive, methicillin-resistant control strain in in vitro infection modelsa
| Strain | Peak:MIC | Trough:MIC | AUC0–24:MIC |
|---|---|---|---|
| GISA | 10.0 | 3.1 | 134 |
| MRSA (control) | 85.8 | 24.8 | 1,113 |
The values listed represent the pharmacodynamic index value that corresponds with a bacterial inoculum at 48 h of 3 log10 CFU/ml. These values were calculated from the three-parameter Hill equation that described the relationship between the given pharmacodynamic index and the bacterial inoculum at 48 h.
In vitro infection models: LY333328 regimens.
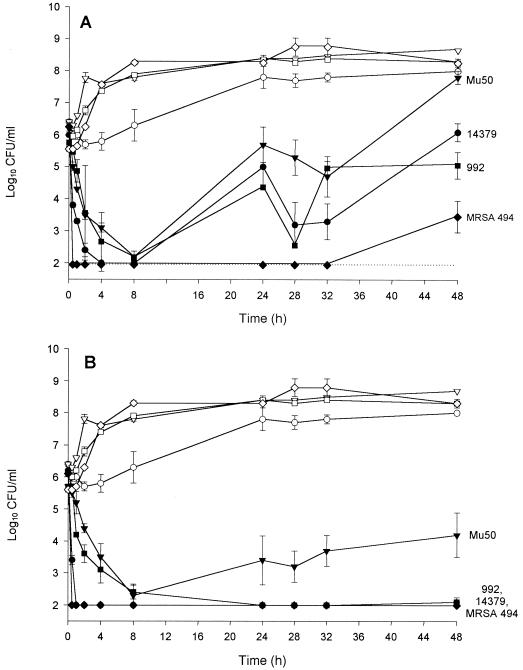
The results from the LY333328 infection models are shown in Fig. 3. The low-dose (Fig. 3A) and high-dose (Fig. 3B) regimens both produced initially rapid killing activity against all strains. Regrowth occurred at the 24-h time points for all GISA strains for the low-dose LY333328 infection models. The effects of the second low doses of LY333328 were substantially decreased, and colony counts at 48 h were similar to or higher than the starting inocula. Colony counts for MRSA 494 remained below the limits of detection from 0.5 h until the 48-h time point, when slight regrowth occurred. Administration of LY333328 as the high-dose regimen resulted in bactericidal activity and suppression of colony counts to just at or below the limits of detection throughout the 48-h experiments for strains 14379, 992, and 494. Mu50 was the only strain that regrew during the high-dose experiments, but the colony counts between 24 and 48 h were all significantly lower than those obtained with the low-dose regimen.
FIG. 3.
Activities of the low-dose (A) and high-dose (B) LY333328 regimens against the three strains of GISA and the control strain of MRSA in the in vitro infection models (●, 14379; ▾, Mu50; ▪, 992; ♦, MRSA 494; growth controls for each strain are represented by the corresponding unfilled symbols). The dashed line indicates the reliable limit of detection (2 log10 CFU/ml).
LY333328 MICs and MBCs (in SMHB) were retested for colonies obtained from the low-dose LY333328 regrowth time points. The MICs and MBCs for 992 increased to 2 and 4 μg/ml, respectively, at 32 h and 8 and 16 μg/ml, respectively, at 48 h. The MICs for Mu50 remained the same (2 μg/ml), but the MBC increased to 16 μg/ml at 24 h and 32 μg/ml at 48 h. Vancomycin MICs and MBCs for these bacteria also increased to 16 and 32 μg/ml, respectively. The LY333328 MICs and MBCs for 14379 and 494 (at 48 h) increased to 2 and 16 μg/ml and 2 and 2 μg/ml, respectively. These elevations in MICs and MBCs were not stable; after three passes on antibiotic-free TSA plates, the MICs and MBCs decreased to 1 and 4 μg/ml, 2 and 8 μg/ml and 2 and 8 μg/ml for 14379, 992, and Mu50, respectively.
DISCUSSION
Vancomycin's slower killing activity versus the rates of killing for beta-lactam antibiotics, its inadequate penetration into some infected tissue sites, and the compromised health status of patients receiving vancomycin are some factors postulated to cause therapeutic failures of or slow responses to vancomycin therapy (8, 15, 28). The recent clinical reports of MRSA with decreased vancomycin susceptibility indicate that the failures of therapy against MRSA soon will be related directly to vancomycin resistance.
Decreased vancomycin susceptibility has decreased the rate (1, 12) and extent (10, 13) of vancomycin killing activity in test tube killing curve studies and has also resulted in significantly decreased vancomycin activity in rabbit models of endocarditis (2, 9, 21). In our infection models, vancomycin at 1 or 1.125 g every 12h had significantly less killing activity against GISA strain Mu50 than against the other two GISA strains and the vancomycin-sensitive strain. However, the simulated dosing regimens of 1.25 and 1.5 g every 12 h produced bactericidal activity against all three GISA strains as well as the vancomycin-sensitive control strain. As vancomycin has been administered to hospitalized patients at higher doses that produce trough concentrations in excess of 20 μg/ml without significant adverse events (including nephrotoxicity), these dose regimens might be useful for the treatment (or prevention) of GISA infections until other agents become available (16, 19; M. J. Rybak, D. M. Cappelletty, M. Ruffing, R. C. Mercier, H. H. Houlihan, M. E. Klepser, and D. P. Levine, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-46, 1997).
In our infection models, the values of the pharmacodynamic indices that correlated with bactericidal activity were from 8 to 10 times lower for the GISA strains tested than for the vancomycin-susceptible strain. These data agree with the results from a recent study that evaluated the pharmacokinetic and pharmacodynamic indices for vancomycin-susceptible and -intermediate S. aureus strains in the neutropenic mouse thigh infection model (M. Dudley, D. Griffith, E. Corcoran, C. Liu, K. Sorensen, V. Tembe, D. Cotter, S. Chamberland, and S. Chen. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2031, 1999). In that study, the AUC:MIC and peak:MIC ratios that produced 50% maximal effect were from 4 to 15 times lower for two GISA strains than for three vancomycin-sensitive strains. The exact reasons for observations of similar vancomycin activity at lower pharmacodynamic index values are unknown, but the heterogeneous expression of reduced vancomycin susceptibility may play at least some role (1).
Use of the synergistic combination of beta-lactam or glycopeptide agents with aminoglycosides has been recommended to improve the response to therapy in patients with certain staphylococcal infections such as endocarditis (15). Testing of the FIC for gentamicin plus vancomycin indicated additivity or indifference against all GISA strains evaluated in this study. However, in the in vitro infection models, the combination did not significantly improve the killing activity compared with that of the most potent antimicrobial regimen alone. Modest (but nonsignificant) improvements in activity were observed only for GISA Mu50 (at 48 h the inoculum was 5.0 ± 1.7 log10 CFU/ml for vancomycin plus gentamicin, whereas it was 7.8 ± 1.2 log10 CFU/ml for vancomycin alone). Similar reductions in vegetation density were observed for vancomycin combined with amikacin against GISA Mu50 in a rabbit model of endocarditis (2). Although arbekacin combined with vancomycin appeared to help resolve the infection in the Japanese patient with the Mu50 GISA infection (7, 13), the combination of vancomycin with an aminoglycoside does not appear to provide consistent additivity and/or synergy against the GISA strains isolated so far.
LY333328 is a glycopeptide antibiotic that is currently in phase II clinical studies and that has potent activity against gram-positive bacteria including methicillin-susceptible S. aureus, MRSA, and vancomycin-resistant enterococci (18, 34). Although resistance to one glycopeptide appears to decrease susceptibility to other glycopeptides (3, 24, 30, 31), the LY333328 MICs and MBCs were only slightly higher for the GISA strains than for the vancomycin-sensitive MRSA strains and were within the range of the MICs at which 90% of isolates are inhibited reported for MRSA in recent studies (34). The addition of albumin to the test medium substantially increased the MICs and MBCs for all of the GISA strains, while it only slightly increased the MICs and MBCs for the vancomycin-sensitive MRSA strain. The decreases in GISA susceptibility to LY333328 in the presence of albumin were most apparent during the low-dose infection model simulations. Dramatic regrowth occurred for all GISA isolates by 24 h, even though rapid killing to the limits of detection occurred with the first dose. Regrowth also occurred for the vancomycin-sensitive strain, but was detected only at 48 h. The decrease in LY333328 concentrations to subinhibitory levels 4 h after administration of the dose (Fig. 1) likely contributed to the regrowth of the GISA strains. The selection of more resistant subpopulations occurred over the 48-h experiment and probably was related to the decreased killing effects observed with the second LY333328 dose. The resistant subpopulations became more prevalent with longer times of exposure to LY333328, an observation supported by previous glycopeptide resistance selection studies (10, 14, 26). It should be noted that this resistance was not stable to serial passages on antibiotic-free agar.
The high doses of LY333328 caused initial killing similar to that caused by the low doses but completely suppressed regrowth for strains 14379, 992, and 494. The times above the MIC for these strains were 24, 15, and 24 h. Even though the time above the MIC was also 15 h for Mu50, this GISA strain regrew, but to a much lesser extent than it did in the low-dose models. It is possible that this strain (which, of the three GISA strains tested, has the lowest baseline susceptibility to LY333328) requires continuous concentrations greater than 1× the MIC for adequate suppression of regrowth.
In conclusion, we determined that vancomycin activity was similar against all three GISA strains and a vancomycin-sensitive MRSA strain when administered as simulated doses of 1.25 or 1.5 g every 12 h in an in vitro pharmacodynamic infection model. In addition, it appears that the AUC::MIC was correlated with killing for the GISA strains tested. The reasons for similar antimicrobial effects against isolates with eightfold lower susceptibility are unknown and should be studied further. The combination of vancomycin with gentamicin did not provide synergistic activity, but these results could be strain dependent. LY333328 administered as a 5-mg/kg dose provided potent killing and durable regrowth suppression against two of the three GISA strains tested. Further study of this glycopeptide against GISA appears to be warranted.
ACKNOWLEDGMENTS
This study was supported by a grant from Eli Lilly & Co.
We thank Keiichi Hiramatsu, Marcus Zervos, and Fred C. Tenover for supplying the strains of vancomycin-intermediate S. aureus.
REFERENCES
- 1.Aeschlimann J R, Hershberger E, Rybak M J. Analysis of vancomycin population susceptibility profiles, killing activity, and postantibiotic effect against vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1914–1918. doi: 10.1128/aac.43.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backo M, Gaenger E, Burkart A, Chai Y L, Bayer A S. Treatment of experimental staphylococcal endocarditis due to a strain with reduced susceptibility in vitro to vancomycin: efficacy of ampicillin-sulbactam. Antimicrob Agents Chemother. 1999;43:2565–2568. doi: 10.1128/aac.43.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biavasco F, Giovanetti E, Montanari M P, Lupidi R, Varaldo P. Development of in-vitro resistance to glycopeptides: assessment in staphylococci of different species. J Antimicrob Chemother. 1991;27:71–79. doi: 10.1093/jac/27.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Blaser J. In vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother. 1985;25:57–68. doi: 10.1093/jac/15.suppl_a.125. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb Mortal Wkly Rep. 1997;46:813–815. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb Mortal Wkly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Reduced susceptibility of Staphylococcus aureus to vancomycin—Japan, 1996. Morb Mortal Wkly Rep. 1997;46:624–635. [PubMed] [Google Scholar]
- 8.Chuard C, Vaudaux P, Waldvogel F A, Lew D P. Susceptibility of Staphylococcus aureus growing on fibronectin-coated surfaces to bactericidal antibiotics. Antimicrob Agents Chemother. 1993;37:625–632. doi: 10.1128/aac.37.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Climo M W, Patron R L, Archer G L. Combinations of vancomycin and β-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob Agents Chemother. 1999;43:1747–1753. doi: 10.1128/aac.43.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daum R S, Gupta S, Sabbagh R, Milewski W M. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomycin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J Infect Dis. 1992;166:1066–1072. doi: 10.1093/infdis/166.5.1066. [DOI] [PubMed] [Google Scholar]
- 11.Eliopoulos G M, Moellering R C. Antimicrobial combinations. In: Lorain V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 330–396. [Google Scholar]
- 12.Hershberger E, Aeschlimann J R, Moldovan T, Rybak M J. Evaluation of bactericidal activities of LY333328, vancomycin, teicoplanin, ampicillin-sulbactam, trovafloxacin, and RP59500 alone or in combination with rifampin or gentamicin against different strains of vancomycin-intermediate Staphylococcus aureus by time-kill curve methods. Antimicrob Agents Chemother. 1999;43:717–721. doi: 10.1128/aac.43.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 15.Houlihan H H, Mercier R C, Rybak M J. Pharmacodynamics of vancomycin alone or in combination with gentamicin at various dosing intervals against methicillin-resistant Staphylococcus aureus-infected fibrin-platelet clots in an in vitro infection model. Antimicrob Agents Chemother. 1997;41:2497–2501. doi: 10.1128/aac.41.11.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James J K, Palmer S M, Levine D P, Rybak M J. Comparison of conventional dosing versus continuous infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother. 1996;40:696–700. doi: 10.1128/aac.40.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath B J, Lamp K C, Rybak M J. Pharmacodynamic effects of extended dosing intervals of imipenem alone and in combination with amikacin against Pseudomonas aeruginosa in an in vitro model. Antimicrob Agents Chemother. 1993;37:1931–1937. doi: 10.1128/aac.37.9.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercier R C, Houlihan H H, Rybak M J. Pharmacodynamic evaluation of a new glycopeptide, LY333328, and in vitro activity against Staphylococcus aureus and Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:1307–1312. doi: 10.1128/aac.41.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreira B, Boyle-Vavra S, deJonge B L M, Daum R S. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1788–1793. doi: 10.1128/aac.41.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. Approved standard. NCCLS document M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 21.Patron R L, Climo M W, Goldstein B P, Archer G L. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 1999;43:1754–1755. doi: 10.1128/aac.43.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeltz R F, Singh V K, Schmidt J L, Batten M A, Baranyk C S, Nadakakavukaren M J, Jayaswal R K, Wilkinson B J. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob Agents Chemother. 2000;44:294–303. doi: 10.1128/aac.44.2.294-303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanyal D, Johnson R C, George R C, Edwards R, Greenwood D. In-vitro characteristics of glycopeptide resistant strains of Staphylococcus epidermidis isolated from patients on CAPD. J Antimicrob Chemother. 1993;32:267–278. doi: 10.1093/jac/32.2.267. [DOI] [PubMed] [Google Scholar]
- 24.Schlaes D M, Schlaes J H. Teicoplanin selects for Staphylococcus aureus that is resistant to vancomycin. Clin Infect Dis. 1995;20:1071–1073. doi: 10.1093/clinids/20.4.1071. [DOI] [PubMed] [Google Scholar]
- 25.Sieradski K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieradski K, Tomasz A. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical strains of staphylococci. Antimicrob Agents Chemother. 1998;42:100–107. doi: 10.1128/aac.42.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieradski K, Tomasz A. Low-level teicoplanin resistance and heteroresistance to vancomycin. Ann Intern Med. 1998;128:245. doi: 10.7326/0003-4819-128-3-199802010-00021. [DOI] [PubMed] [Google Scholar]
- 28.Small P M, Chambers H F. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34:1227–1231. doi: 10.1128/aac.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith T L, Pearson M L, Wilcox K R, Cruz C, Lancaster M V, Robinson-Dunn B, Tenover F C, Zervos M J, Band J D, White E, Jarvis W R. Emergence of vancomycin resistance in Staphylococcus aureus. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 30.Watanakunakorn C. In-vitro induction of resistance in coagulase-negative staphylococci to vancomycin and teicoplanin. J Antimicrob Chemother. 1988;22:321–324. doi: 10.1093/jac/22.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Watanakunakorn C. In-vitro selection of resistance of Staphylococcus aureus to teicoplanin and vancomycin. J Antimicrob Chemother. 1990;25:69–72. doi: 10.1093/jac/25.1.69. [DOI] [PubMed] [Google Scholar]
- 32.Winter, M. E. Aminoglycosides. In M. A. Koda-Kimble and L. Y. Young (ed.), Basic clinical pharmacokinetics, 2nd ed. Applied Therapeutics, Inc., Vancouver, Wash.
- 33.Winter, M. E. Vancomycin. In M. A. Koda-Kimble and L. Y. Young (ed.), Basic clinical pharmacokinetics, 2nd ed. Applied Therapeutics, Inc., Vancouver, Wash.
- 34.Zeckel M L, Preston D A, Allen B S. In vitro activities of LY333328 and comparative agents against nosocomial gram-positive pathogens collected in a 1997 global surveillance study. Antimicrob Agents Chemother. 2000;44:1370–1374. doi: 10.1128/aac.44.5.1370-1374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]