Abstract
Ocimum tenuiflorum is a sacred medicinal plant bestowed with multiple health benefits. This plant is traditionally considered an adaptogen. Many scientific studies have indicated the anti-stress potential of Ocimum tenuiflorum but with higher doses. The present study investigated the effects of HolixerTM (a clinically studied standardized Ocimum tenuiflorum extract) on modulating stress using two in vivo models, namely the swim endurance study in mice and forced swim test in rats. In addition, we explored the mechanism of action of HolixerTM on the HPA axis using two in vitro cell-based assays to check for its inhibitory effect on cortisol release and CRF1 receptor antagonistic activity. Ocimum tenuiflorum extract enhanced the swimming time in mice, reduced the stress-induced increase in immobility time, and prevented the increase in corticosterone in rats subjected to the forced swim test. Further, Ocimum tenuiflorum extract inhibited cortisol release and exhibited a significant CRF1 receptor antagonist activity. Thus, Ocimum tenuiflorum extract was found effective in managing stress, and the effect could be due to the inhibition of cortisol release and the antagonistic effect on the CRF1 receptors.
1 Introduction
Stress is an inevitable feeling that everyone undergoes in day-to-day life [1]. Stress is a normal physiological response that helps us to notice unusual or unexpected threats in and around us. Stressful situations either make people vulnerable or increase their capacity to deal with stressful situations depending on the duration of stress and the adaptogenic capacity of people. The term “stress” was first introduced by Hens Selye into the medical world to represent the effects of anything that seriously threatens homeostasis and is stated as “nonspecific response of the body to any demand [2, 3]. Acute stress (short-term extreme situations requiring a fight-or-flight response) is a survival strategy in healthy individuals [4–6]. However, prolonged unmanageable stress called chronic stress leads to many diseases like cardiovascular disease [7], diabetes [8, 9] and depression [8, 10]. Managing chronic stress involves the removal of stressors, gaining more knowledge about the stress, and taking supplements that help reduce the stress. Removing the stressor is not always possible (E.g., work stress). Gaining more knowledge about stress needs more time and effort, which could be a daunting task for many of us in today’s fast-paced world. Intake of supplements to reduce stress is a simple task and could get accomplished by the majority in today’s scenario. Indian tradition has left us with a medicinal plant that could reduce our stress in day-to-day life. Drinking water soaked with the leaves of this plant has been given as a “Prashad” in many Hindu temples to calm the people and make them peaceful during their short visits to these temples.
Ocimum tenuiflorum L. (Synonym: Ocimum sanctum L.) commonly known as Tulsi or Holy Basil is the plant used for managing stress. It is a sacred Indian medicinal plant with longstanding traditional use in multiple Indian systems of medicines such as Ayurveda, Siddha, and Unani [11]. Ocimum tenuiflorum has protected against iron-induced testicular toxicity by counteracting redox imbalance [12] and exhibited better seizure control, memory retention, oxidative stress reduction, and neuronal structure preservation [13]. It showed antistress effects in different animal models [14] and prevented a stress-induced decline in macrophage function [15]. In a human clinical study, the plant effectively managed symptoms of general stress when used at a dose of 1200 per day for six weeks [16]. Various phytochemicals, namely vicenin-2, orientin, cynaroside, betulinic acid, genistein with syringic acid, rosmarinic acid, eugenol, carnosic acid, oleanolic acid, ursolic acid, luteolin, and apigenin were confirmed to be present in Ocimum tenuiflorum [17]. Though available scientific literature indicated the antistress property of different extracts of Ocimum tenuiflorum, there are hardly any studies that evaluated the antistress property of Ocimum tenuiflorum extract at a low dose of ≤50 mg/kg body weight in mice. Therefore, the current study was aimed to evaluate the antistress property and the mechanism of action of a recently developed clinically studied [18] novel Ocimum tenuiflorum extract (HolixerTM) at lower doses.
2 Materials and methods
2.1 Plant material
Ocimum tenuiflorum plant material used in this study was obtained from a cultivated source and authenticated by the National Institute of Science Communication and Information Resources, New Delhi (Voucher number: RD-21872). The plant material was used to prepare a standardized extract of Ocimum tenuiflorum (developed and named HolixerTM by M/s Natural Remedies Pvt. Ltd., Bangalore, India). The Ocimum tenuiflorum extract used in this study is a patent-pending (202241023540) hydroalcoholic extract derived from the leaves-rich aerial parts of Ocimum tenuiflorum. It was manufactured in a good manufacturing practice (GMP)-certified facility and is phytochemically standardized to ≥5%w/w (by High-Performance Liquid Chromatography) of the Ocimum Bioactive Complex. The extract complies with the United States Pharmacopeia (USP) requirements on microbial, heavy metals, residual pesticides, aflatoxins, and residual solvent limits for dietary supplements.
2.2 Chemicals and reagents
Human adreno-carcinoma (NCI-H295R) cell line and DMEM: F-12 Medium were obtained from ATCC. Corning® ITS+ Universal Culture Supplement Premix and Corning Nu-Serum™ were used. Dimethyl sulfoxide (DMSO), forskolin, antalarmin hydrochloride, and ovine CRF were obtained from Sigma. Cortisol-HTRF Kit was obtained from Cisbio. Human recombinant CHO cell line was obtained from Invitrogen. Carboxy methyl cellulose (HiMedia Laboratories Pvt. Ltd., Mumbai, India) was used in this study.
2.3 Animals
Male Swiss albino mice of age 6–8 weeks were used in the swim endurance test, and male albino Wistar rats aged 6–8 weeks were used in the evaluation of the antistress activity of Ocimum tenuiflorum extract. All animals were maintained in a room at 22°C (±3°C), and relative humidity was kept between 30 and 70%. Artificial light was set to give a cycle of 12 hours light and 12 hours dark. UV-treated water and conventional laboratory rodent diet were offered ad libitum. Animals were adapted to the environment for at least one week before experiments. All the experimental procedures were approved by the Institutional Animal Ethics Committee (IAEC) of Natural Remedies Pvt Ltd, Bangalore, India (Approval no.: IAEC/NR-PCL-02/07/2021 & IAEC/NR-PCL-02/03/2020) and experiments were conducted according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines and in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
2.4 Swim endurance test in mice
The swim endurance test was carried out using male Swiss albino mice aged 6–8 weeks [19, 20]. Animals were divided into four groups of 6 animals each and were housed with three animals per cage during the experiment. The body weight of each animal within a group was within ±20% of the group mean. Group 1 was administered orally with 0.5% w/v carboxy methyl cellulose (CMC) at a 10 ml/kg dose. Ocimum tenuiflorum extract was suspended at a specific concentration in 0.5% CMC and administered orally to groups 2, 3, and 4 at 12.5, 25, and 50 mg/kg body weight per day for ten days. On the 10th day, one hour after administering the test substance, animals were subjected to the swim endurance test. Animals were made to swim in separate porcelain tanks of 30 cm in height and 70 cm in diameter filled with water maintained at 28–30°C. The endurance of each mouse was measured as swimming time recorded from the beginning of the time till exhaustion. A mouse was considered exhausted and rescued when it exhibited a loss of coordinated movements and failed to return to the water surface within a 10-s period to breathe for three consecutive times.
2.5 Antistress activity in the forced swim test
Animals were divided into five groups of 6 animals each and were housed with four animals per cage during the experiment. The body weight of each animal within a group was within ±20% of the group mean. Groups 1 and 2 were administered orally with 0.5% w/v carboxy methyl cellulose (CMC) at a dose of 10 ml/kg body weight for eight days. Ocimum tenuiflorum extract was suspended at a specific concentration in 0.5% CMC and administered orally to groups 3, 4, and 5 at a dose of 12.5, 25, and 50 mg/kg body weight respectively once a day for eight days. On the 8th day, one hour after the administration of the test substance or vehicle, animals of groups 2 to 5 were subjected to the forced swim test [21]. Animals were allowed to swim for 5 minutes in a cylindrical tank constructed of transparent Plexiglas of 28 cm diameter and a height of 55 cm filled with water to a height of 40 cm. Animals were observed for immobility time as soon as they were subjected to the forced swim test. Immobility is characterized by the lack of movement except that which is necessary to keep the animal’s nose above the water level. Blood was collected from the retro-orbital plexus and centrifuged at 4000 g for serum separation, and corticosterone levels were determined in the serum using LC-MS/MS technique.
2.6 Cortisol release assay
2.6.1 Cell culture and treatment
The cortisol release study was carried out to assess the effect of Ocimum tenuiflorum extract on cortisol secretion [22]. Human adreno-carcinoma (NCI-H295R) cells (No. CRL-2128) were grown in 75-cm2 flasks at 37°C with 5% CO2 in DMEM: F-12 Medium (No. 30–2006) containing 1% ITS+ Universal Culture Supplement Premix (No. 354352) and 2.5% Nu-serum supplement (No. 355100). The medium was changed three times a week. Before the start of the experiment, cells were subcultured from 80% confluent stock cultures into 48-well culture plates for 24 hours. After incubation, old media was replaced with fresh media (500 μl/well) containing the test sample (Ocimum tenuiflorum extract) at different non-cytotoxic concentrations from 6.25 μg/ml to 200 μg/ml and forskolin at 10 μM concentration. Dimethyl sulfoxide (DMSO) was used as the vehicle for the test sample and the concentration of DMSO in the test system was not more than 0.4%. After incubating for a period of 48 hours at 37°C in an atmosphere of 5% CO2, cell culture supernatant was removed from each well and frozen at -80°C for cortisol measurement.
2.6.2 Cortisol assay
Cortisol concentration in the cell supernatant was determined using a cortisol-HTRF Kit [No. 64CRTPEB]. The assay was performed in triplicate. Briefly, 10 μL of the vehicle or sample was added to a 384 well plate. Cortisol-d2 (5 μL) and anti-cortisol-cryptate (5 μL) were added to all the wells except negative control for which 5 μL of detection buffer was used instead of the Cortisol-d2 reagent. The plate was sealed with a plate sealer and incubated for 2 h at room temperature in the dark. Reading was taken in a microplate reader (PHERAstar) at two wavelengths (665 and 620 nm). Percentage inhibition of cortisol by the test sample was calculated by considering the cortisol released by forskolin as 100%.
2.7 Antagonist effect in human CRF1 receptor
Ocimum tenuiflorum extract was tested for its antagonistic effect on CRF1 receptor [22, 23]. Before the treatment with test substances, human recombinant (CHO) cells (10,000 cells per well) were plated on 384-well, black-wall, clear-bottom assay plate containing growth medium and incubated for 20 h at 37°C and 5% CO2. After incubation, the cells were treated with Ocimum tenuiflorum extract at various non-cytotoxic concentrations ranging from 6.25 to 200 μg/ml for 30 minutes, followed by 30 minutes of incubation with ovine CRF (15 nM) at 37°C. DMSO was used as the solvent for the test substance, and cells treated only with the vehicle were used to get the control response. Antalarmin hydrochloride, a standard antagonist to the CRF1 receptor was used as the positive control. Levels of cAMP were determined in triplicate wells using HTRF detection method. Control (untreated but stimulated with CRF) cAMP level was considered a 100% response.
2.8 Statistical analysis
Data obtained in the studies were analyzed using one-way ANOVA followed by the Bonferroni method as the post–hoc test. In the case of heterogeneous data, the Dunnett T3 method was used after transformation. All values were reported as mean ± SD. Results were considered statistically significant if the p-value was less than 0.05.
3 Results
3.1 Swim endurance capacity of Ocimum tenuiflorum extract
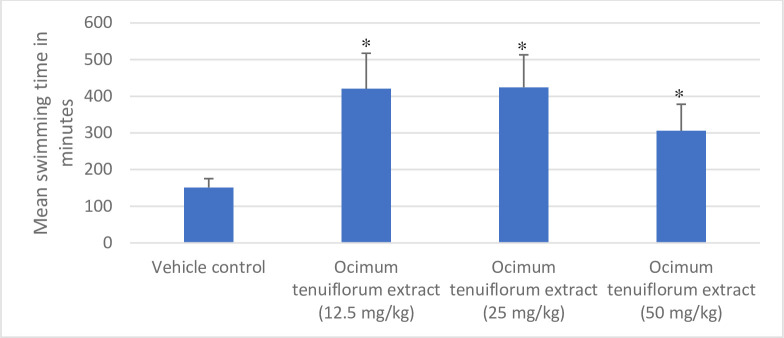
Results of the swim endurance test in mice are shown in Fig 1. Ocimum tenuiflorum extract at all the tested doses (12.5, 25, and 50 mg/kg) significantly enhanced the endurance capacity of mice compared to vehicle control mice measured in terms of the swimming time till exhaustion. Ocimum tenuiflorum extract at 12.5 mg/kg and 25 mg/kg bw showed maximum endurance enhancement in mice compared to the 50 mg/kg dose.
Fig 1. Effect of Ocimum tenuiflorum extract on swimming time in swim endurance study in mice.
Ocimum tenuiflorum extract at 12.5, 25 and 50 mg/kg were evaluated using swim endurance test in mice. Swimming time till exhaustion was noted for each mouse and results presented as mean ± SD (n = 6). *p<0.05 compared to vehicle control. Higher the swimming time, better the endurance capacity of animals.
3.2 Antistress effect of Ocimum tenuiflorum extract
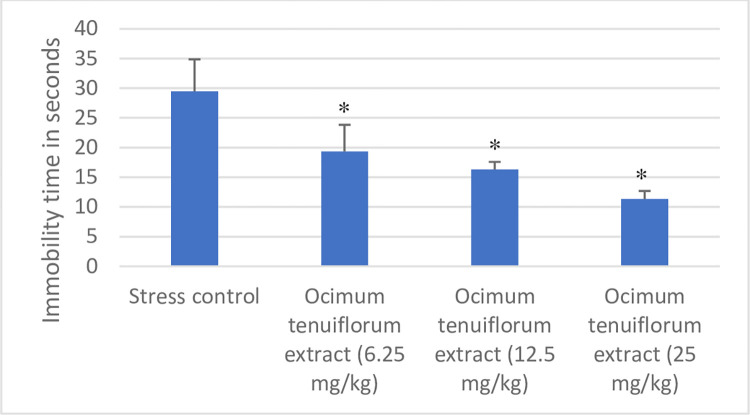
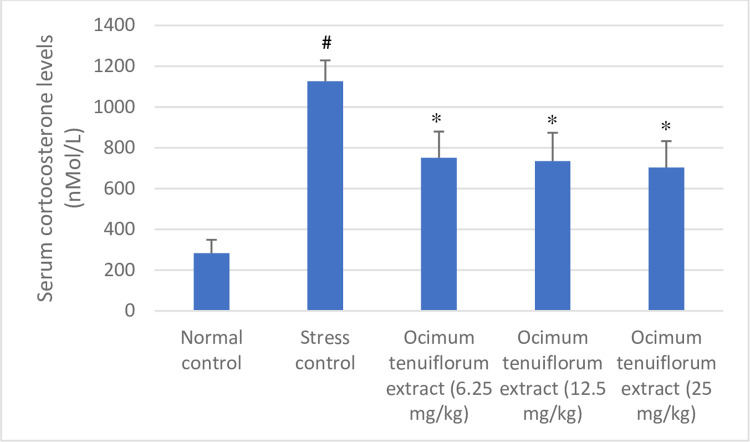
The antistress effect of Ocimum tenuiflorum extract was studied using the forced swim test model. In addition to the immobility time, the serum corticosterone level was measured to understand the effect of Ocimum tenuiflorum extract on the stress hormone in rats. Results of Ocimum tenuiflorum extract in the forced swim test using rats are shown in Figs 2 and 3. Rats that got stressed on account of exposure to forced swimming tests and are unable to manage the forced swimming stress remain immobile for a longer time compared to rats that can manage the stress induced by forced swim test. Ocimum tenuiflorum extract showed a significant dose-dependent decrease in the immobility time from 6.25 mg/kg to 25 mg/kg dose compared to stress control rats indicating the ability of the extract to effectively manage the forced swim test stress. In addition, rats treated with Ocimum tenuiflorum extract successfully prevented the increase in serum level of corticosterone despite the stressful environment indicating its effectiveness.
Fig 2. Effect of Ocimum tenuiflorum extract on immobility time of rats subjected to forced swim test.
Ocimum tenuiflorum extract at 6.5, 12.5, and 25 mg/kg doses were evaluated in the forced swim test. The immobility time of animals during a 5-minute forced swim test was noted for each animal. Results are presented as mean ± SD (n = 6). *p<0.05 compared to stress control. Lesser the immobility time, the higher the antistress effect.
Fig 3. Effect of Ocimum tenuiflorum extract on serum corticosterone level of rats subjected to forced swim test.
Ocimum tenuiflorum extract at 6.5, 12.5, and 25 mg/kg doses were evaluated in the forced swim test. Serum corticosterone levels were analyzed using the LCMS/MS technique. Results are presented as mean ± SD (n = 6). #p<0.05 compared to normal control. *p<0.05 compared to stress control. The lesser the serum corticosterone levels better the antistress effect.
3.3 Cortisol release assay
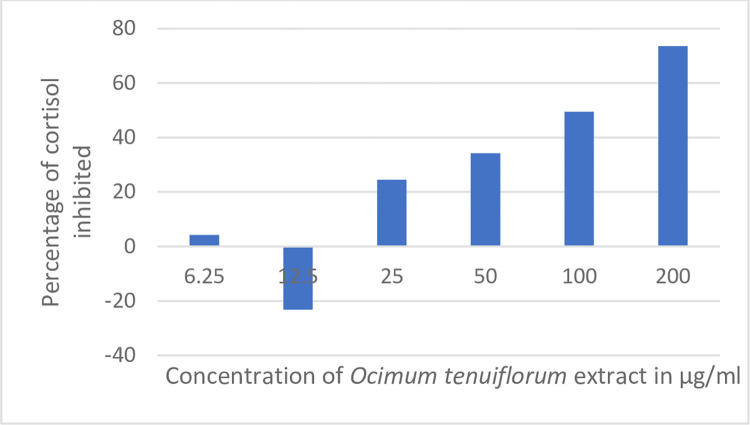
Ocimum tenuiflorum was tested for its effect on forskolin-induced release of cortisol. Results of the effect of Ocimum tenuiflorum extract on cortisol are shown in Fig 4. Ocimum tenuiflorum showed a concentration-dependent reduction in the cortisol levels from 25 to 100 μg/ml. About 49.5% of cortisol was inhibited at 100 μg/ml concentration and 73.6% at 200 μg/ml concentration indicating the appreciable stress managing potential of Ocimum tenuiflorum extract.
Fig 4. Effect of Ocimum tenuiflorum on the release of cortisol.
Ocimum tenuiflorum extract at different non-cytotoxic concentrations was tested in the forskolin-induced cortisol release assay using human adreno-carcinoma (NCI-H295R) cells. The cortisol released by the cells was analyzed using a cortisol-HTRF kit, and the percentage of cortisol inhibited by the test substance is shown. The higher the percentage of cortisol inhibited, the better the activity.
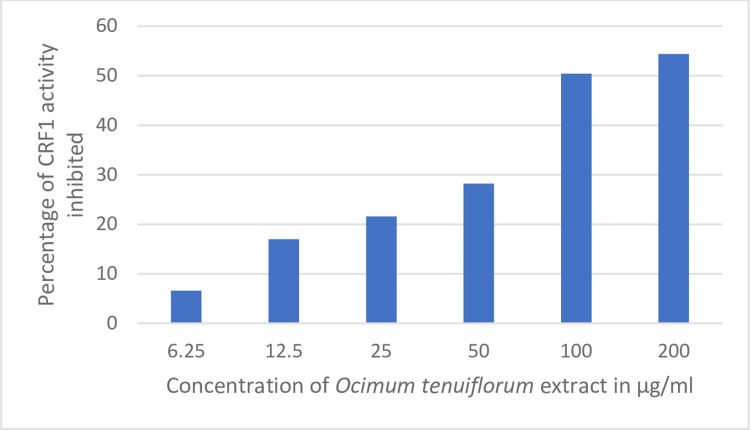
3.4 CRF1 receptor assay
The CRF1 receptor assay was conducted to see if the cortisol inhibition property of Ocimum tenuiflorum could be mediated through antagonism at the CRF1 receptor. Results of the CRF1 activity are shown in Fig 5. Ocimum tenuiflorum extract inhibited the CRF1 activity by 50.4% at 100 μg/ml. This antagonistic effect of Ocimum tenuiflorum extract against the CRF1 receptor could partly be responsible for the cortisol inhibition property of Ocimum tenuiflorum extract.
Fig 5. Antagonistic effect of Ocimum tenuiflorum extract with CRF1 receptor.
Ocimum tenuiflorum extract at different non-cytotoxic concentrations was tested using human recombinant (CHO) cells containing CRF1 receptors. The antagonistic effect was assessed by analyzing the levels of cAMP using a HTRF detection method. Reduction in the levels of cAMP is presented as the percentage of CRF1 activity inhibited. The higher the percentage of CRF1 activity inhibited, the better the antagonistic effect.
4 Discussion
Multiple scientific studies indicate the usefulness of cortisol as a biomarker of stress in humans [24–26]. Chronic stress affects the functions of the nervous system; immune system; cardiovascular system; gastrointestinal system; and endocrine system [27]. Cortisol is a stress hormone secreted by the human body in response to stress; homeostatic regulatory mechanisms maintain cortisol levels within a narrow range; the hypothalamus-pituitary-adrenal axis (HPA) regulates the release of cortisol. During stressful situations, the HPA axis gets activated and releases the corticotrophin-releasing hormone (CRH) from the hypothalamus. The CRH helps in the release of adrenocorticotrophic hormone (ACTH) by binding with the corticotrophin-releasing hormone receptor 1 (CRF1) of the anterior pituitary gland, and the ACTH stimulates the adrenal glands to secrete cortisol helping the body to combat stress [28, 29]. When cortisol level in the blood reaches a particular threshold, it inhibits the release of CRH from the hypothalamus and is called the negative feedback mechanism. Cortisol provides more energy to the body by increasing the blood glucose level and is used to combat stress. Cortisol increases blood glucose levels by breaking down the protein and fat stores. Thus, the HPA axis protects the body during stressful situations and is a normal physiological function. However, long-term repeated activation of the HPA-axis results in the dysfunction of the negative feedback mechanism leading to increased cortisol levels in the blood, and a higher level of blood cortisol for a prolonged duration in people with chronic stress may lead to obesity [30], poor immunity [31], diabetes [30], cardiovascular disease [32–34], osteoporosis [35], poor memory [36], and depression [36]. Thus, cortisol is considered a primary target of substances that reduce chronic stress, and reducing cortisol levels to a near-normal level is the goal of reducing the risk of subsequent health implications.
Ocimum tenuiflorum is an adaptogenic herb with multiple health benefits. Therefore, we evaluated its stress managing properties using swim endurance test in mice, and forced swim test in rats, cortisol release assay in vitro and CRF1 antagonistic effect in vitro. Several studies have already shown the antistress property of Ocimum tenuiflorum. Ethanol extract from the leaves of Ocimum tenuiflorum showed antistress effects in both acute stress and chronic unpredictable stress models at a dose of 200 mg/kg body weight in rats. The extract was reported to have significantly prevented the stress induced increase in plasma corticosterone levels [37].
The phytochemical constituents that are analysed as part of Ocimum Bioactive Complex (patent-pending), including rosmarinic acid and luteolin, was hypothesized to contribute to the antistress effect of HolixerTM. The individual phytoconstituents of OBC have been well researched for its various pharmacological activity, in particular the anti-stress effect. Rosmarinic acid has been reported to reduce plasma corticosterone levels and increase the expression of hippocampal mineralocorticoid receptors, glucocorticoid receptors and brain-derived neurotrophic factor in the hippocampus of rats induced with HIV-1 viral protein tat injection and subsequent repetitive restrain stress [38]. Rosmarinic acid has also been reported to reduce maternal stress induced corticosterone increase [39], chronic unpredictable stress induced increase in corticosterone [40] and reverse stress induced changes of SUV39H1, Mkp-1, and BDNF expression in the mice hippocampus [41]. Luteolin-7-O-glucuronide, another constituent of OBC, has been found to reduce blood corticosterone in a mouse sleep deprivation model, wherein the compound improved depression-like and stress coping behaviours [42].
An ethanol extract of leaves of Ocimum sanctum was reported to prevent the noise-stress induced increase in corticosterone in Wistar rats at an intraperitoneal dose of 100 mg/kg body weight [43]. When restraint-stressed rats, were evaluated in elevated plus maze test, tail suspension test, and forced swimming test after treatment with ethanol extract of Ocimum tenuiflorum leaves, 200 mg/kg body weight dose had better antistress effects [44]. Aqueous extract of Ocimum tenuiflorum leaves at a dose of 100 mg/kg body weight has shown antistress effects in rats subjected to restraint stress [14, 45]. Aqueous leaf extract of Ocimum tenuiflorum was found to reduce stress-induced gastric ulcers in rats at a dose of 100 and 200 mg/kg body weight [46]. The antistress activity was also reported for the methanol extract of the whole plant of Ocimum tenuiflorum extract at doses from 50 to 200 mg/kg body weight in rats subjected to chronic variable stress [22]. The same extract was found to show CRF1 antagonist activity of 63% at 100 μg/ml concentration and cortisol release inhibition of 89% at 100 μg/ml concentration. Methanol extract of the roots of Ocimum sanctum was reported to increase swimming time in mice at an intraperitoneal dose of 400 mg/kg body weight [47]. Though there are many studies available on the antistress activity of Ocimum tenuiflorum, no animal studies are available on the extract at lower doses from 6.25 mg/kg to 50 mg/kg body weight in rats or mice.
In line with previously reported studies of Ocimum tenuiflorum, this study confirmed the antistress potential of Ocimum tenuiflorum but at lower doses in mice and rats, which substantiates the improved efficacy of the extract used in this study. The antistress activity was also substantiated by its stress hormone lowering effect observed in the cortisol release assay and antagonism at the CRF1 receptor. The results presented in this study serve as a plausible mechanism of the stress management property of the Ocimum tenuiflorum extract (HolixerTM) observed in the recent 8-week randomized double-blind placebo-controlled study [18]. In the clinical study, the Ocimum tenuiflorum extract (HolixerTM) significantly reduced stress as measured by the Perceived Stress Scale (PSS), improved sleep as measured by the Athens Insomnia Scale (AIS), and reduced hair cortisol in adults experiencing stress at a daily dose of 250 mg/day.
This study also has few limitations. We could have subjected the animals to chronic stress paradigm before subjecting them to forced swim test that might have produced additional data in chronic stressful condition. Also, adaptogenic biomarkers such as molecular chaperons (e.g., Hsp70), stress-activated c-Jun N-terminal protein kinase (JNK1), Forkhead box O (FoxO) transcription factor, and nitric oxide (NO) would have been a value addition.
5 Conclusion
Overall, the results demonstrated that the standardized extract of Ocimum tenuiflorum exhibits antistress benefits at a very low dose as evident in the two in vivo studies and its antistress activity could be due to the inhibition of cortisol release and antagonistic effect on the CRF1 receptors thereby regulating the HPA axis. The antistress effect of HolixerTM at lower dose has been confirmed in the recent clinical study in subjects experiencing stress.
Supporting information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
All the authors are employed by M/s Natural Remedies Pvt Ltd, India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fink G. Stress: Concepts, Definition and History. In Reference Module Inneuroscience and Biobehavioral Psychology, Elsevier Inc;2017. 1–9. [Google Scholar]
- 2.Tan SY, Yip A. Hans Selye (1907–1982): Founder of the stress theory. Singapore Med J. 2018;59(4):170–171. doi: 10.11622/smedj.2018043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1: 607–28. doi: 10.1146/annurev.clinpsy.1.102803.144141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bracha HS. Freeze, flight, fight, fright, faint: adaptationist perspectives on the acute stress response spectrum. CNS Spectrums.2004; 9(9): 679–685. doi: 10.1017/s1092852900001954 [DOI] [PubMed] [Google Scholar]
- 5.Dragoş D, Tănăsescu MD. The effect of stress on the defense systems. J Med Life.2010; 3(1): 10–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. 2015; 22(1–2): 6–19. doi: 10.1159/000362736 [DOI] [PubMed] [Google Scholar]
- 7.Golbidi S, Frisbee JC, Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am J Physiol Heart Circ. 2015; 308(12): H1476–98. doi: 10.1152/ajpheart.00859.2014 [DOI] [PubMed] [Google Scholar]
- 8.Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2017; 1391(1): 20–34. doi: 10.1111/nyas.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma VK, Singh TG. Chronic stress and diabetes mellitus: Curr Diabetes Rev. 2020;16(6): 546–556. 10.2174/1573399815666191111152248 [DOI] [PubMed] [Google Scholar]
- 10.Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J Affect Disord. 2018: 233. 45–67. doi: 10.1016/j.jad.2017.09.052 [DOI] [PubMed] [Google Scholar]
- 11.Godhwani S, Godhwani JL, Vyas DS. Ocimum sanctum—a preliminary study evaluating its immunoregulatory profile in albino rats. J Ethnopharmacol. 1988;24(2–3): 193–198. doi: 10.1016/0378-8741(88)90151-1 [DOI] [PubMed] [Google Scholar]
- 12.Olofinsan KA, Salau VF, Erukainure OL, Islam MS. Ocimum tenuiflorum mitigates iron-induced testicular toxicity via modulation of redox imbalance, cholinergic and purinergic dysfunctions, and glucose metabolizing enzymes activities. Andrologia. 2021; 53(9): e14179. doi: 10.1111/and.14179 [DOI] [PubMed] [Google Scholar]
- 13.Sarangi SC, Pattnaik SS, Katyal J, Kaleekal T, Dinda AK. An interaction study of Ocimum sanctum L. and levetiracetam in pentylenetetrazole kindling model of epilepsy. J Ethnopharmacol. 2020; 249: 112389. doi: 10.1016/j.jep.2019.112389 [DOI] [PubMed] [Google Scholar]
- 14.Tabassum I, Siddiqui ZN, Rizvi SJ. Effects of Ocimum sanctum and Camellia sinensis on stress-induced anxiety and depression in male albino Rattus norvegicus. Indian J Pharmacol. 2010; 42(5): 283–288. doi: 10.4103/0253-7613.70108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharya SK, Rathi N, Mahajan P, Tripathi AK, Paudel KR, Rauniar GP, et al. Effect of Ocimum sanctum, ascorbic acid, and verapamil on macrophage function and oxidative stress in mice exposed to cocaine. Indian J Pharmacol. 2009; 41(3): 134–139. doi: 10.4103/0253-7613.55210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena RC, Singh R, Kumar P, Negi MP, Saxena VS, Geetharani P, et al. Efficacy of an Extract of Ocimum tenuiflorum (OciBest) in the Management of General Stress: A Double-Blind, Placebo-Controlled Study. Evid Based Complementary Altern Med. 2012;894509. 10.1155/2012/894509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girme A, Bhoj P, Saste G, Pawar S, Mirgal A, Raut D, et al. Development and Validation of RP-HPLC Method for Vicenin-2, Orientin, Cynaroside, Betulinic Acid, Genistein, and Major Eight Bioactive Constituents with LC-ESI-MS/MS Profiling in Ocimum Genus. J AOAC Int. 2021; 104(6): 1634–1651. doi: 10.1093/jaoacint/qsab067 [DOI] [PubMed] [Google Scholar]
- 18.Lopresti AL, Smith SJ, Metse AP, Drummond PD. A randomized, double-blind, placebo-controlled trial investigating the effects of an Ocimum tenuiflorum (Holy Basil) extract (HolixerTM) on stress, mood, and sleep in adults experiencing stress. Front nutr. 2022; 9: 965130. doi: 10.3389/fnut.2022.965130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizunoya W, Oyaizu S, Ishihara K, Fushiki T. Protocol for measuring the endurance capacity of mice in an adjustable-current swimming pool. Biosci Biotechnol Biochem. 2002; 66(5): 1133–1136. https://academic.oup.com/bbb/article/66/5/1133/5945034 doi: 10.1271/bbb.66.1133 [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Nath R, Lata A, Singh SP, Kohli RP, Bhargava KP. Withania Somnifera (Ashwagandha), a rejuvenating herbal drug which enhances survival during stress (an adaptogen). Int J Crude Drug Res. 1982; 20(1): 29–35. 10.3109/13880208209083282. [DOI] [Google Scholar]
- 21.Ittiyavirah S, Anurenj DA. Adaptogenic studies of acetone extract of Musa paradisiaca L. fruit peels in albino Wistar rats. Int J Nutr Pharmacol Neurol Dis. 2014; 4: 88–94. [Google Scholar]
- 22.Richard EJ, Illuri R, Bethapudi B, Anandhakumar S, Bhaskar A, Chinampudur Velusami C, et al. Anti-stress Activity of Ocimum sanctum: Possible Effects on Hypothalamic-Pituitary-Adrenal Axis. Phytother Res. 2016;30(5): 805–814. doi: 10.1002/ptr.5584 [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA. 1993; 90(19): 8967–8971. doi: 10.1073/pnas.90.19.8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Reports. 2015; 48(4): 209–216. doi: 10.5483/bmbrep.2015.48.4.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegrist J, Li J. Work stress and altered biomarkers: A synthesis of findings based on the effort-reward imbalance model. Int J Environ Res Public Health. 2017; 14(11). doi: 10.3390/ijerph14111373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steckl AJ, Ray P. Stress biomarkers in biological fluids and their point-of-use detection. ACS Sensors. 2018; 3(10): 2025–2044. doi: 10.1021/acssensors.8b00726 [DOI] [PubMed] [Google Scholar]
- 27.Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: A review. EXCLI Journal. 2017; 16: 1057–1072. doi: 10.17179/excli2017-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Cruz-Cano E. Association between FKBP5 and CRHR1 genes with suicidal behavior: A systematic review. Behav Brain Res. 2017;317: 46–61. doi: 10.1016/j.bbr.2016.09.032 [DOI] [PubMed] [Google Scholar]
- 29.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004; 130(3): 355–391. doi: 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- 30.Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017; 83: 25–41. doi: 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009; 23(8): 1148–1155. doi: 10.1016/j.bbi.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 32.Chrousos GP. The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med. 2004; 117(3): 204–207. doi: 10.1016/j.amjmed.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 33.Hamer M, Steptoe A. Cortisol responses to mental stress and incident hypertension in healthy men and women. J Clin Endocrinol Metab. 2012; 97(1): E29–34. doi: 10.1210/jc.2011-2132 [DOI] [PubMed] [Google Scholar]
- 34.Wirtz PH, von Känel R, Emini L, Ruedisueli K, Groessbauer S, Maercker. Evidence for altered hypothalamus-pituitary-adrenal axis functioning in systemic hypertension: blunted cortisol response to awakening and lower negative feedback sensitivity. Psychoneuroendocrinology.2007;32(5):430–436. 10.1016/j.psyneuen.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 35.Dineen R, Behan LA, Kelleher G, Hannon MJ, Brady JJ, Rogers B, et al. The contribution of serum cortisone and glucocorticoid metabolites to detrimental bone health in patients receiving hydrocortisone therapy. BMC Endocr Disord. 2020; 20(1): 154. doi: 10.1186/s12902-020-00633-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: relation to cognitive functioning. Stress.2006;9(3): 143–152. doi: 10.1080/10253890600965674 [DOI] [PubMed] [Google Scholar]
- 37.Gupta P, Yadav DK, Siripurapu KB, Palit G, Maurya R. Constituents of Ocimum sanctum with antistress activity. J Nat Prod. 2007; 70(9): 1410–1416. doi: 10.1021/np0700164 [DOI] [PubMed] [Google Scholar]
- 38.Makhathini KB, Mabandla MV, Daniels WMU. Rosmarinic acid reverses the deleterious effects of repetitive stress and tat protein. Behav Brain Res. 2018; 353: 203–209. doi: 10.1016/j.bbr.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 39.Verma H, Bhattacharjee A, Shivavedi N, Nayak PK. Evaluation of rosmarinic acid against myocardial infarction in maternally separated rats. Naunyn-Schmiedeb. Arch Pharmacol. 2022b; 395(10): 1189–1207. doi: 10.1007/s00210-022-02273-9 Epub 2022 Jul 25. . [DOI] [PubMed] [Google Scholar]
- 40.Verma H, Shivavedi N, Tej GNVC, Kumar M, Nayak PK. Prophylactic administration of rosmarinic acid ameliorates depression-associated cardiac abnormalities in Wistar rats: Evidence of serotonergic, oxidative, and inflammatory pathways. J Biochem Mol Toxicol. 2022a; 36(10): e23160. doi: 10.1002/jbt.23160 Epub 2022 Jul 15. [DOI] [PubMed] [Google Scholar]
- 41.Lee JE, Park SY, Han PL. Aging-Dependent Downregulation of SUV39H1 Histone Methyltransferase Increases Susceptibility to Stress-Induced Depressive Behavior. Mol Neurobiol. 2021; 58(12): 6427–6442. doi: 10.1007/s12035-021-02529-0 Epub 2021 Sep 18. . [DOI] [PubMed] [Google Scholar]
- 42.Ryu D, Jee HJ, Kim SY, Hwang SH, Pil GB, Jung YS. Luteolin-7-O-Glucuronide Improves Depression-like and Stress Coping Behaviors in Sleep Deprivation Stress Model by Activation of the BDNF Signaling. Nutrients. 2022; 14(16): 3314. doi: 10.3390/nu14163314 ; PMCID: PMC9412559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Archana R, Namasivayam A. Effect of Ocimum sanctum on noise induced changes in neutrophil functions. J Ethnopharmacol. 2000; 73(1–2): 81–85. doi: 10.1016/s0378-8741(00)00281-6 [DOI] [PubMed] [Google Scholar]
- 44.Jo KJ, Nam GH, Park YS, Kawk HW, Kim JT, Choi WH, et al. Evaluation of stress-related behavioral and biological activity of Ocimum sanctum extract in rats. Biotechnol Bioprocess Eng. 2020; 25(2): 170–180. 10.1007/s12257-019-0365-2 [DOI] [Google Scholar]
- 45.Kumar RS, Nayak S, Karnataka M. Effect of Ocimum sanctum (Linn.) extract on restraint stress induced behavioral deficits. Pharmacologyonline. 2007; 404: 394–404. [Google Scholar]
- 46.Vaseem A, Subhani G, Afshan K, Ali M, Khan MA, Rumana MT. Effect of Ocimum sanctum Linn. in stress induced gastric ulcers in rats. Int J Med Res Health Sci. 2015; 4(3): 506. 10.5958/2319-5886.2015.00098.3 [DOI] [Google Scholar]
- 47.Maity TK, Mandal SC, Saha BP, Pal M. Effect of Ocimum sanctum roots extract on swimming performance in mice. Phytother Res. 2000; 14(2): 120–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.