Abstract
Klinefelter syndrome (KS) mosaicism 47,XXY/46,XX/46,XY is an extremely rare disorder. Mixed connective tissue disorder (MCTD) is a systemic rheumatological disease with overlapping characteristic features of systemic lupus erythematosus (SLE), systemic sclerosis (SSc), polymyositis (PM)/dermatomyositis (DM), and rheumatoid arthritis (RA). It contains a higher titer level of U1-RNP and anti-RNP antibodies. A 50-year-old man was referred to our clinic with gynecomastia, lower extremity rash, persistent fever, arthralgia, muscle weakness, dry eye and mouth, Raynaud’s phenomenon abnormal, and hormone levels. He was a follow-up patient for MCTD. Chromosome analysis of the patient revealed an abnormal karyotype of mos47,XXY/46,XX/46,XY. Fluorescence in situ hybridization (FISH) analysis indicated ish(SRYx1),(DZYx1)(DZX1x2)/ish (SRYx0),(DYZ1x0)(DZX1x2)/ish(SRYx1), (DZYx1)(DZX1x1). Although the prevalence of autoimmune diseases in Klinefelter syndrome is unknown, it is thought that the estimated frequency is higher than men, close levels to that of women. This might be explained by several genes that regulate the function of the immune system located on the X chromosome and the gene dosage mechanism that is the escape of X-inactivation in early embryogenesis for KS development. To the best of our knowledge, this is the first case to report a 47,XXY/46,XX/46,XY Klinefelter syndrome patient with MCTD.
Keywords: Klinefelter syndrome, MCTD, mosaicism, cytogenetic analyses
Introduction
Klinefelter syndrome (KS) is the most frequently observed chromosomal disorder, with an estimated frequency of 1:500 to 1:1,000 in males. Although clinical features of KS have been widely varied, the disease is generally characterized by tall stature, eunuchoid skeleton, narrow shoulders, variable level of intelligence from low to normal IQ, small testes, gynecomastia, and infertility. Laboratory analysis of KS includes lower serum testosterone, higher serum follicle-stimulating hormone (FSH), and luteinizing hormone (LH) levels accompanied by impaired spermatogenesis (Lanfranco et al., 2004).
The most common form of KS is the regular type (47, XXY), which accounts for 80% of all cases. The other common forms of KS are 47,XX and, der(Y), 47,X, der(X), Y,48,XXXY, 48,XXYY, 49,XXXXY, 47,XXY/46,XY mosaicism (Groth et al., 2013; Lanfranco et al., 2004; Radicioni et al., 2010). Although 47,XXY/46,XY mosaicism constitutes 10% of all KS patients, nearly 15 cases with 47 XXY/46, XX mosaicism of KS have been reported, thus far.
KS patients have an increased risk of autoimmune diseases, including multiple sclerosis, acquired hypothyroidism, rheumatoid arthritis (RA), Sjogren’s syndrome (SSs), and systemic lupus erythematosus (SLE) (Fish, 2008; Gleicher & Barad, 2007). Mixed connective tissue disorder (MCTD) is a systemic rheumatological disease with overlapping features of SLE, SSs, polymyositis/dermatomyositis (PM/DM), and RA. It is characterized by higher titer level of U1-RNP and anti-RNP antibodies. Raynaud’s phenomenon (RP), arthralgias, muscle weakness, and swollen hands are the most frequently observed clinical indications of MCTD. The disease is less common in males than in females (Martínez-Barrio et al., 2018; Ortega-Hernandez & Shoenfeld, 2012; Pepmueller, 2016).
Although KS has an increased risk of some autoimmune diseases, the 47, XXY/46,XX/46,XY mosaicism of KS is an infrequent condition. Furthermore, only three MCTD cases have been previously reported in KS. However, no case has been reported with both mosaic KS features and MCTD. To the best of our knowledge, this is the first case to report a 47,XXY/46,XX/46,XY KS patient with MCTD.
Case Summary
Case History and Physical Examination
A 50-year-old man was referred to our clinic with gynecomastia, lower extremity rash, persistent fever, arthralgia, muscle weakness, dry eye and mouth, and RP. He was a follow-up patient for MCTD. In his medical history, it was recorded that physicians performed an angiographic procedure two times this year due to his persistent angina pectoris. His left three fingers were amputated due to cyanosis in 4 months. His physical examination detected a eunuchoid body, sparse axillary hair growth, pubic hair distribution of feminine type, small testes, bilateral gynecomastia, digital ulcers, telangiectasias on his face, and splenomegaly. Written informed consent was obtained from the patient for the publication of this case report.
Laboratory Findings
The patient’s hemogram and biochemical tests were normal. Serological findings, hormone levels, and thrombophilia test results are shown in Table 1. The other test results are as follows: growth hormone, 0.637 ng/mL (0.03–2.47); somatomedin-C levels, 45.91 ng/mL (55–248); anti-TPO, 8.17 IU/mL (0–34); and anti-Tg, 25.21 IU/mL (0–115). Doppler ultrasonography revealed that his right testicle was 0.7 mL and his left testicle was 0.3 mL. The spleen measured was 192 mm × 60 mm in abdominal ultrasonography, and the liver size was 146 mm. Furthermore, ultrasonography revealed a cystic area behind the posterior half of the urinary bladder. It was assessed as compatible with a Mullerian ductus cyst. Echocardiography revealed that the left ventricle ejection fraction was 55% (>52), and the aortic and mitral valve maximum gradients were 12 and 4 mmHg, respectively. These findings are compatible with increased left atrium size and a mild degree of mitral deficiency. DEXA analysis showed osteopenic lesions on the right femur and lumbar vertebrae. Barret’s esophagus was detected in samples taken from the esophagus during the endoscopic procedure.
Table 1.
The Comparison of Previously Reported Cases of Klinefelter Syndrome With MCTD and Our Case with 47,XXY/46,XX/47 XY
| Case 1 | Case 2 | Case 3 | Our Case | |
|---|---|---|---|---|
| Author | Ishihara et al. | Takeuchi et al. | Kasten et al. | Kalayci Yigin et al. |
| Year | 1994 | 1999 | 2005 | 2020 |
| Ethnicity | Asian (Japanese) | Asian (Japanese) | Caucasian | Caucasian |
| Karyotype | 47, XXY | 47, XXY | 47, XXY | 47,XXY/46,XX/47 XY |
| Overlapping connective tissue disorder | Sjogren’s syndrome | Sjogren’s syndrome | Sjogren’s syndrome | Sjogren’s syndrome |
| Age at the onset of MCTD | 28 | 58 | 43 | 25 |
| Clinical characteristics | ||||
| Height | 162 cm | 162 cm | 190 cm | 172 cm |
| Weight | N/A | 53 kg | 80 kg | 76 kg |
| Gynecomastia | + | + | + | + |
| Raynaud’s phenomenon | + | + | + | + |
| Joint abnormalities | + | + | + | + |
| Pulmonary system abnormalities | + | + | + | + |
| Intellectual level | Normal | Normal | Normal | Normal |
| Hematological abnormalities | + | Mild anemia | Mild anemia | Mild anemia |
| Thrombophilic event | ||||
| FV Leiden | N/A | N/A | – | Heterozygote |
| MTHFR C677T | N/A | N/A | – | Heterozygote |
| MTHFR A1298C | N/A | N/A | – | Heterozygote |
| Factor XIII | N/A | N/A | – | Heterozygote |
| Ovotesticular dysfunction | N/A | N/A | N/A | Yes |
| Serological findings | ||||
| Rheumatoid factor | – | 5 IU/mL | 70 IU/mL | 54.5 IU/mL Cut off index 14 < |
| Antinuclear antibody | +1:2560 homogeneous speckled | +homogeneous speckled | 1/5,120 | (+) |
| Anti-RNP | Anti-RNP (+) |
Anti-RNP (+) |
Anti U1-RNP (+) | Anti U1-RNP (+) |
| Anti-Sm | (−) | (−) | (−) | 1.7 U/mL a |
| Anti-dsDNA(IgG) | (+) | (−) | (−) | 5.28 IU/mL a |
| Anti-SSA | 1:64 | (−) | (−) | 4.43 IU/mL a |
| Anti-SSB | 1:1 | (−) | (−) | 1.19 IU/mL a |
| Ant-CCP | N/A | N/A | N/A | 5.28 IU/mL a |
| Anti-Jo1 | N/A | N/A | N/A | 3.26 IU/mL a |
| Antiphospholipid IgG | N/A | N/A | N/A | 3.09 IU/mL a |
| Antiphospholipid IgM | N/A | N/A | N/A | 0.5 IU/mL a |
| Laboratory analysis | ||||
| AZFa,b,c deletion | N/A | N/A | N/A | (−) |
| Total protein | 9.4 g/dL | 7.5 g/dL | N/A | 5.8 g/dL |
| Albumin | 2.8 g/dL | 4.4 g/dL | N/A | 3.45 g/dL |
| Globin | 6.6 g/dL | N/A | N/A | NA |
| Hormonal status | ||||
| FSH | 28.9 mIU/mL (2.9–8.2) |
91.9 mIU/mL (2.9–8.2) |
20.6 mIU/mL (1.0–8.0) |
21.21 mIU/mL (1.6–12.4) |
| LH | 13.5 mIU/mL (1.8–5.2) |
42.1 mIU/mL (1.8–5.2) | 23.8 mIU/mL (2.0–12.0) |
33.34 mIU/mL (0.8–6) |
| Total testosterone | 3.1 ng/mL (3.8–9.9) |
14.5 ng/mL (25–110) |
2.0 ng/mL (2.8–8.0) |
53.25 ng/dL (55–248) |
| Estradiol | <20 pg/mL | N/A | N/A | (?) |
| GH | N/A | 2.22 ng/mL (<0.42) |
N/A | 0.637 ng/mL (0.03–2.47) |
| Somatomedin-C | N/A | N/A | N/A | 45.91 ng/mL (55–248) |
| PRL | N/A | 24.6 ng/mL (1.7–10) |
N/A | 24 ng/mL (4.1–15.2) |
| DHEA-SO4 | 344 ng/mL (400–3,500) |
296 ng/mL (400–3,500) |
||
Note. MCTD = mixed connective tissue disorder; FSH = follicle-stimulating hormone; LH: luteinizing hormone; GH: growth hormone; PRL: prolactine; DHEA-SO4: dehydroepiandrosterone sulfate.
Negative <12 IU/mL, borderline 12 to 18 IU/mL, positive >18 IU/mL.
Cytogenetic and Molecular Cytogenetics Analysis
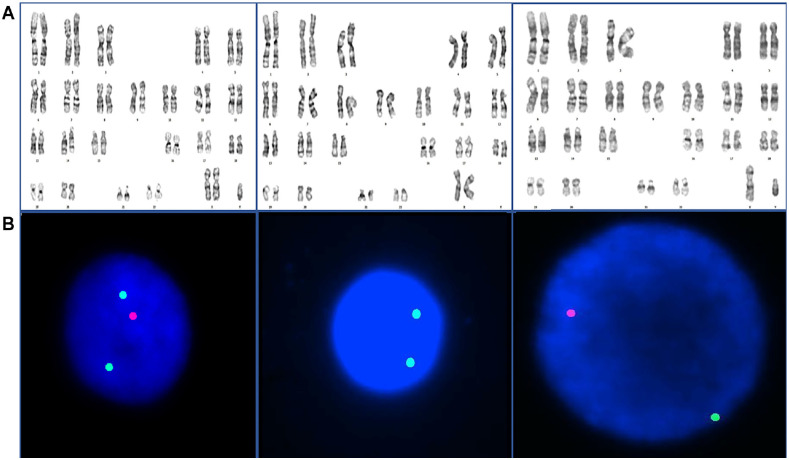
Peripheral venous blood was taken from the patient for chromosome and fluorescence in situ hybridization (FISH) analysis with heparin and a 72-hr lymphocyte culture was performed at 37°C in phytohemagglutinin-induced cell culture. Metaphase preparations were obtained after the culture was stained using the GTG banding method (G-bands by Tripsin using Giemsa) and karyotype analyses were described according to the International System for Human Cytogenetic Nomenclature. Overall, 100 metaphases were analyzed. Chromosomal analysis of the patient revealed mos47, XXY[85]/46,XX[4]/46, XY[13] (Figure 1A).
Figure 1.
Results of Karyotype and FISH Analysis in Mosaic KS Patient. (A) Conventional Cytogenetic Analysis Showed a 47,XXY/46,XX/46,XY and (B) Interphase FISH Detected XXY Signals in 92% of Cells Analyzed and XX Signals in 5%, and XY Signals in the Remaining 3%
FISH: fluorescence in situ hybridization; KS: Klinefelter syndrome.
Peripheral blood was subjected to a short-term culture for 72 hr to obtain metaphase spreads for standard cytogenetic banding analyses (Verma & Babu, 1989). For FISH analysis, the prepared slides were dehydrated in 70%, 85%, and 100% ethanol (5 min each) and allowed to air dry. A probe mix was applied to the preparations. The preparations were denatured for 1 to 5 min at 73°C±1°C and incubated overnight at 37°C in a moist chamber. Post-hybridization washes were carried out 3 times (5 min each) in 2× SSC/50% formamide and twice in 2× SSC (5 min each) at 40°C. The slides were counterstained with propidium iodide or 4,6-diamidine, 2-phenylindole dihydrochloride (DAPI) solution. They were examined with fluorescence microscopy (Carl Zeiss, Germany).
FISH analysis indicated ish(SRYx1),(DZYx1)(DZX1x2)[92/100]/ish (SRYx0),(DYZ1x0)(DZX1x2)[5/100]/ ish(SRYx1), (DZYx1)(DZX1x1)[3/100] (Figure 1B).
Molecular Analysis
The peripheral blood sample was obtained using an EDTA tube, and genomic DNA isolation was performed according to standard techniques (Wizard Genomic DNA; Promega, Madison, WI, USA). For automatic DNA fragment analysis, ST multiplex amplification, including the AMXY marker and 5-dye fluorescent system, was used with electrophoresis (Chr. X: 104bp, Chr. Y: 109bp; Xp22.1, Yp11.2). Samples were evaluated using a genetic analyzer (Applied Biosystems 3500 ABI PRISM®). The Evaluated STS regions were sY82, sY83, sY84, sY86, sY88, and sY1065 for AZFa region; sY105, sY121, sY127, sY134, sY143, and sY153 for AZFb region; sY1191, sY1291, sY254, and sY255 for the AZFc regions. The ZFX/ZFY regions and terminal sY160 regions were used as an internal control. These regions were amplified and fragmented and analyzed. Y-microdeletion analysis has not detected any deletions in the previously explained SRY, AZFa, AZFb, and AZFc regions. Segregation analyses could not be performed because the parents were not living.
Discussion
As individuals with KS are phenotypically normal at birth, physicians rarely diagnose individuals during this period. Approximately 10% of KS patients can be diagnosed in the prepubertal period, and 25% are diagnosed during their lifetime. Due to the mild and variable phenotype, some individuals cannot even be diagnosed for life (Berglund et al., 2019). Many KS patients experience infertility, the primary clinical manifestation of KS that signals a referral for medical testing (Eberl et al., 2005). However, the mosaic form of KS is not characterized by infertility, as seen in our patient with four children, two sons and two girls, and a normal Y-microdeletion analysis.
Autoimmune diseases (ADs) are complex heterogeneous disorders with diverse factors influencing pathogenesis, such as genes, the immune system, and the environment. Although ADs are frequently seen in the general population, the prevalence of ADs in KS is unknown. A few hypotheses attempt to explain the mechanism of ADs in KS. One proposes an escape from X-inactivation and gene dosage (Seminog et al., 2015). Due to the gene dosage effect, X-overexpression may cause an increased gene dosage, leading to susceptibility to AD (Fujimoto et al., 2015). Another hypothesis poses that the genes responsible for controlling immune regulation are located in X chromosomes (Seminog et al., 2015). Many studies in the literature showed overexpression of genes in the X-chromosomes and ADs (Golks et al., 2007; Jacob et al., 2009; Kaufman et al., 2013; Shen et al., 2010). The last common hypothesis explains how ADs are more common in females than in males due to a potential association between high estrogen levels and reduced T-cell immune response.
MCTD is an AD that manifests in a mixture of SS, SLE, and RA (Ortega-Hernandez & Shoenfeld, 2012). Three previously reported cases involved a regular type KS with MCTD; all three subjects suffered from Raynaud’s phenomenon, as well as pulmonary, hematologic, and rheumatological manifestations with serological findings (Table 1) (Ishihara et al., 1994). Although SS-A and SS-B were negative in our patient, the other manifestations meet the American-European consensus criteria for Sjogren’s syndrome (Fazaa et al., 2014).
KS patients have a high risk of malignancy and thromboembolic conditions, and their symptoms should be monitored accordingly (Erkal et al., 2018). Because KS patients may develop ovarian and testicular tissues within the same gonad or separately, KS patients should undergo a physical examination or ultrasonography. In addition, the results of these exams could reveal reverse gonadal tissue or its remnants, both of which might be predisposed to malignancy. Therefore, early diagnosis and surgical procedures are vital in managing KS patients (Talreja et al., 2015).
Our patient had Mullerian cysts. Generally, Mullerian cysts are accepted as benign tumors; however, a malign transformation of Mullerian cysts is reported in the literature. One study notes a roughly 2.5- to 3.5-fold increase in hematological malignancy, leukemia, and lymphoma risk in KS patients (Ji et al., 2016). Both low-androgen levels and FV Leiden mutations are highly predisposed to thromboembolic and cardiac events. Androgen therapy may have a favorable outcome on thrombophilia and MCTD (Chang et al., 2020; Hussein et al., 2020). Due to our patient’s mild anemia and unexplained high level of leukocyte levels, we performed JAK2 and BCR/ABL real-time PCR analysis, and they were normal. However, we suggested a routine follow-up due to our patient’s increased risk of malignancy.
To conclude, although the prevalence of ADs in KS is unknown, it is thought that the prevalence of ADs in KS is more likely in men whose estradiol and testosterone levels are similar to those found in women; the estimated frequency is higher than in men, close levels to that of women (Seminog et al., 2015). This might be explained by several genes that regulate the function of the immune system located on the X chromosome and the gene dosage mechanism that escapes X-inactivation during early embryogenesis for KS development. To the best of our knowledge, our case is the first case report of 47,XXY/46,XX/46,XY with MCTD.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Written informed consent was obtained from the patient to publish this case report.
ORCID iD: Mehmet Seven  https://orcid.org/0000-0001-7878-2039
https://orcid.org/0000-0001-7878-2039
References
- Berglund A., Viuff M. H., Skakkebæk A., Chang S., Stochholm K., Gravholt C. H. (2019). Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47,XXX and 47,XYY syndrome: A nationwide cohort study. Orphanet Journal of Rare Diseases, 14(1), Article 16. 10.1186/s13023-018-0976-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Christiansen C. F., Bojesen A., Juul S., Münster A. M. B., Gravholt C. H. (2020). Klinefelter syndrome and testosterone treatment: A national cohort study on thrombosis risk. Endocrine Connections, 9(1), 34–43. 10.1530/EC-19-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl M. M., Baer M. R., Mahoney M. C., Sait S. N. J., Block A. M. W., Farrell C. D. (2005). Unsuspected Klinefelter syndrome diagnosed during oncologic evaluation: A case series. Journal of the American Board of Family Practice, 18(2), 132–139. 10.3122/jabfm.18.2.132 [DOI] [PubMed] [Google Scholar]
- Erkal B., Kalayci Yigin A., Palanduz S., Dasdemir S., Seven M. (2018). The effect of PAI-1 gene variants and PAI-1 plasma levels on development of thrombophilia in patients with Klinefelter syndrome. American Journal of Men’s Health, 12(6), 2152–2156. 10.1177/1557988318801158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazaa A., Bourcier T., Chatelus E., Sordet C., Theulin A., Sibilia J., Gottenberg J.-E. (2014). Classification criteria and treatment modalities in primary Sjögren’s syndrome. Expert Review of Clinical Immunology, 10(4), 543–551. 10.1586/1744666X.2014.897230 [DOI] [PubMed] [Google Scholar]
- Fish E. N. (2008). The X-files in immunity: Sex-based differences predispose immune responses. Nature Reviews. Immunology, 8(9), 737–744. 10.1038/nri2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M., Ikeda K., Nakamura T., Iwamoto T., Furuta S., Nakajima H. (2015). Development of mixed connective tissue disease and Sjögren’s syndrome in a patient with trisomy X. Lupus, 24(11), 1217–1220. 10.1177/0961203315580873 [DOI] [PubMed] [Google Scholar]
- Gleicher N., Barad D. H. (2007). Gender as risk factor for autoimmune diseases. Journal of Autoimmunity, 28(1), 1–6. 10.1016/j.jaut.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Golks A., Tran T. T. T., Goetschy J. F., Guerini D. (2007). Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO Journal, 26(20), 4368–4379. 10.1038/sj.emboj.7601845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth K. A., Skakkebæk A., Høst C., Gravholt C. H., Bojesen A. (2013). Klinefelter syndrome: A clinical update. Journal of Clinical Endocrinology and Metabolism, 98(1), 20–30. 10.1210/jc.2012-2382 [DOI] [PubMed] [Google Scholar]
- Hussein T. M., Abd Elmoaty Elneily D., Mohamed Abdelfattah Elsayed F., El-Attar L. M. (2020). Genetic risk factors for venous thromboembolism among infertile men with Klinefelter syndrome. Journal of Clinical and Translational Endocrinology, 20, Article 100228. 10.1016/j.jcte.2020.100228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K., Yoshimura M., Nakao H., Kanakura Y., Kanayama Y., Matsuzawa Y. (1994). T cell abnormalities in mixed connective tissue disease complicated with Klinefelter’s syndrome. Internal Medicine, 33(11), 714–717. 10.2169/internalmedicine.33.714 [DOI] [PubMed] [Google Scholar]
- Jacob C. O., Zhu J., Armstrong D. L., Yan M., Han J., Zhou X. J., Thomas J. A., Reiff A., Myones B. L., Ojwang J. O., Kaufman K. M., Klein-Gitelman M., McCurdy D., Wagner-Weiner L., Silverman E., Ziegler J., Kelly J. A., Merrill J. T., Harley J. B., Mohan C. (2009). Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. Proceedings of the National Academy of Sciences of the United States of America, 106(15), 6256–6261. 10.1073/pnas.0901181106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Zöller B., Sundquist J., Sundquist K. (2016). Risk of solid tumors and hematological malignancy in persons with Turner and Klinefelter syndromes: A national cohort study. International Journal of Cancer, 139(4), 754–758. 10.1002/ijc.30126 [DOI] [PubMed] [Google Scholar]
- Kaufman K. M., Zhao J., Kelly J. A., Hughes T., Adler A., Sanchez E., Ojwang J. O., Langefeld C. D., Ziegler J. T., Williams A. H., Comeau M. E., Marion M. C., Glenn S. B., Cantor R. M., Grossman J. M., Hahn B. H., Song Y. W., Yu C. Y., James J. A., Tsao B. P. (2013). Fine mapping of Xq28: Both MECP2 and IRAK1 contribute to risk for systemic lupus erythematosus in multiple ancestral groups. Annals of the Rheumatic Diseases, 72(3), 437–444. 10.1136/annrheumdis-2012-201851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco F., Kamischke A., Zitzmann M., Nieschlag P. E. (2004). Klinefelter’s syndrome. Lancet, 364(9430), 273–283. 10.1016/S0140-6736(04)16678-6 [DOI] [PubMed] [Google Scholar]
- Martínez-Barrio J., Valor L., López-Longo F. J. (2018). Facts and controversies in mixed connective tissue disease. Medicina Clinica, 150(1), 26–32. 10.1016/j.medcli.2017.06.066 [DOI] [PubMed] [Google Scholar]
- Ortega-Hernandez O. D., Shoenfeld Y. (2012). Mixed connective tissue disease: An overview of clinical manifestations, diagnosis and treatment. Best Practice and Research: Clinical Rheumatology, 26(1), 61–72. 10.1016/j.berh.2012.01.009 [DOI] [PubMed] [Google Scholar]
- Pepmueller P. H. (2016). Undifferentiated connective tissue disease, mixed connective tissue disease, and overlap syndromes in rheumatology. Missouri Medicine, 113(2), 136–140. [PMC free article] [PubMed] [Google Scholar]
- Radicioni A. F., Ferlin A., Balercia G., Pasquali D., Vignozzi L., Maggi M., Foresta C., Lenzi A. (2010). Consensus statement on diagnosis and clinical management of Klinefelter syndrome. Journal of Endocrinological Investigation, 33(11), 839–850. 10.1007/BF03350351 [DOI] [PubMed] [Google Scholar]
- Seminog O. O., Seminog A. B., Yeates D., Goldacre M. J. (2015). Associations between Klinefelter’s syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity, 48(2), 125–128. 10.3109/08916934.2014.968918 [DOI] [PubMed] [Google Scholar]
- Shen N., Fu Q., Deng Y., Qian X., Zhao J., Kaufman K. M., Wu Y. L., Yu C. Y., Tang Y., Chen J. Y., Yang W., Wong M., Kawasaki A., Tsuchiya N., Sumida T., Kawaguchi Y., Howe H. S., Mok M. Y., Bang S. Y., Tsao B. P. (2010). Sex-specific association of X-linked toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proceedings of the National Academy of Sciences of the United States of America, 107(36), 15838–15843. 10.1073/pnas.1001337107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talreja S., Banerjee I., Yadav S. S., Tomar V. (2015). A rare case of lateral ovotesticular disorder with Klinefelter syndrome mosaicism 46, XX/47, XXY: An unusual presentation. Urology Annals, 7(4), 520–523. 10.4103/0974-7796.164855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R. S., Babu A. (1989). Human chromosomes: Manual of basic techniques. Pergamon Press. [Google Scholar]