Abstract
Objectives
The objective of this review was to characterize the use of biomarkers of male hypogonadism in childhood and adolescence.
Contents
The hypothalamic-pituitary-gonadal (HPG) axis is active during fetal life and over the first months of postnatal life. The pituitary gland secretes follicle stimulating hormone (FSH) and luteinizing hormone (LH), whereas the testes induce Leydig cells to produce testosterone and insulin-like factor 3 (INSL), and drive Sertoli cells to secrete anti-Müllerian hormone (AMH) and inhibin B. During childhood, serum levels of gonadotropins, testosterone and insulin-like 3 (INSL3) decline to undetectable levels, whereas levels of AMH and inhibin B remain high. During puberty, the production of gonadotropins, testosterone, and INSL3 is reactivated, inhibin B increases, and AMH decreases as a sign of Sertoli cell maturation.
Summary and outlook
Based on our knowledge of the developmental physiology of the HPG axis, these biomarkers can be used in clinical practice to interpret the physiopathology of hypogonadism. Additionally, these markers can have diagnostic value in different forms of hypogonadism that may appear during childhood and adolescence.
Keywords: ambiguous genitalia, cryptorchidism, gonadal dysgenesis, hypergonadotropic hypogonadism, hypogonadotropic hypogonadism, micropenis, sexual development disorders, testicle
Introduction
Hypogonadism in males is typically defined as a testicular failure characterized by androgen deficiency. Although this definition is widely accepted in the endocrinology of adults, it is hardly useful in pediatric patients [1]. To better understand the difficulties that may arise from an inadequate use of this definition of hypogonadism in children and adolescents, it is necessary to consider the developmental physiopathology of the hypothalamic-pituitary-gonadal (HPG) axis.
Developmental physiology of the HPG axis
Testis differentiation occurs by the 6th week of embryonic development (week 8 after last menstrual period (LMP)) before HPG axis function is activated [2]. The seminiferous cords originate from interaction of Sertoli cells, which surround germ cells, whereas Leydig cells appear in interstitial tissue. Sertoli cells secrete Anti-Müllerian hormones (AMH), which cause the regression of paramesonephric ducts or Müllerian ducts (primitive uterus and Fallopian tubes) during the 8th and 9th week of intrauterine life (Figure 1). At this stage, AMH is independent from pituitary gonadotropins, albeit from the second half of gestation it is sensitive to follicle stimulating hormone (FSH) [3]. Sertoli cells also secrete inhibin B, which is stimulated by FSH and controls negative feedback effect on pituitary production of FSH [4, 5]. Leydig cells produce androgens (Figure 1), which cause mesonephric ducts (Wolffian ducts) to develop into epididymis, vas deferens, and seminal vesicles. In addition, androgens induce the differentiation of the urogenital sinus and formation of external genitalia [6]. The synthesis of androgens is activated by human chorionic gonadotropin (hCG) action during the first trimester of gestation, and of pituitary luteinizing hormone (LH) in a later stage. Leydig cells also secrete insulin-like 3 factor (INSL3) which is in conjunction with androgens and induce the descent of the testis into the scrotal sac [7, 8].
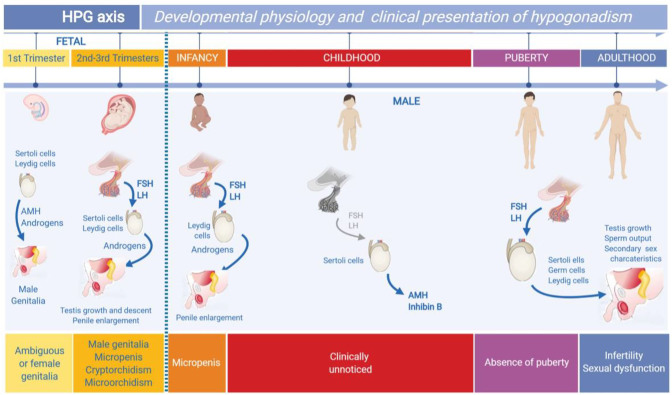
Figure 1:
Ontogeny of the hypothalamic-pituitary-gonadal axis (HPG) in males and impact on the clinical presentation of hypogonadism.
Gonad differentiation occurs during the first trimester of fetal life separately from pituitary gonadotropins. Testicular androgens and the anti-Müllerian (AMH) hormone induce male genital differentiation, and its absence results in female development. Hypogonadism in this period of life induces the development of ambiguous genitalia or female genitalia in XY individuals. In the second and third trimester, androgens induce testicular descent and penile growth. Primary and secondary hypogonadism result in micropenis, microorchidism, and/or cryptorchidism in newborns with male genitalia. During the first days of postnatal life, gonadotropin and androgen secretion is activated. Hypogonadism prevents penile growth. During childhood, gonadotropins and testosterone are generally low or even undetectable. Hypogonadism established in this period is not associated with evident clinical signs and can only be detected by AMH or inhibin B determination. At puberty, the HPG axis reactivates, thereby driving the typical development of secondary sexual characteristics. Hypogonadism can inhibit puberty totally or partially or cause infertility and sexual dysfunction in later stages of life. This Figure was modified using BioRender ({ut1}https://biorender.com/) under the authorization of [1]. © 2019 Elsevier Ltd.
Concentrations of all gonadal axis hormones in blood are low at birth and increase progressively from the first week of life [9]. Levels of gonadotropins, testosterone, and INSL3 are similar to those of adults until the sixth month of life, when they start to decline to low or undetectable levels [10]. High AMH levels persist in childhood, which is indicative of Sertoli cell immaturity [11, 12], whereas inhibin B partially decreases but remains detectable [5]. During childhood, the testicles grow in an unnoticeable wayloffset 4.5mm in clinical terms, being Sertoli cell population the one that most contributes to testes volume [13]. Despite the exposure to high androgen concentrations until 6 months of life, Sertoli cell maturation does not start, as they do not express androgen receptor [14–16]. After the first year of life, androgen receptor expression appears. However, Sertoli cells remain immature in childhood owing to low testosterone levels [17]. Germ cells only proliferate by mitosis but do not enter meiosis, which prevents spermatogenesis.
Pubertal onset is characterized by a reactivation of the gonadotrope, which starts the cyclical production of FSH and LH. FSH induces the proliferation of immature Sertoli cells. Thus, testis volume starts to increase progressively. LH induces Leydig cell secretion of testosterone. Increased levels of intratesticular testosterone induce the maturation of Sertoli cells, which refrain AMH production and stimulate inhibin B secretion [13]. Another characteristic of mature Sertoli cells is their ability to develop the blood-testis barrier (BTB) and functionally sustain adult spermatogenesis [18]. The significant proliferation of germ cells causes a remarkable increase of testes volume.
Biomarkers of the HPG axis
Pituitary hormones: LH and FSH
Gonadotropins LH and FSH share the alpha subunit with pituitary thyrotropin (TSH) and hCG, and owe their specificity to their beta subunit. LH and FSH are secreted by the pituitary gonadotrope in response to the stimulus of gonadotropin-releasing hormone (GnRH) produced by the hypothalamus.
Luteinizing hormone
LH binds to luteinizing hormone choriogonadotropin receptor (LHCGR), which is stimulated by LH and hCG and is present in Leydig cell membranes. LH stimulates testicular steroidogenesis resulting in an increase in circulating testosterone concentrations. LH has a trophic effect on Leydig cells, thereby inducing their proliferation (hyperplasia) and stimulating INSL3 secretion [19, 20]. Decreased levels of LH induce Leydig cell dedifferentiation into mesenchymal precursors and a reduction of androgen and INSL3 levels after the 3 to 6 month period of postnatal activation, which is typically known as "mini-puberty" [10, 13, 21]. During puberty, which generally begins at any point from the ages of 9 to 14 [22], LH stimulates the proliferation of Leydig cells and the production of androgens and INSL3.
Circulating LH levels are very low during the first postnatal hours [23] and increase during the first week of life [9] to remain at similar levels to those of puberty until 3 to 6 months of life [10]. Then, serum LH declines to non-detectable levels by a series of well-known mechanisms and remains stable until pubertal onset (Figure 2). During puberty, LH is secreted in a pulsatile fashion at 90 min intervals first during the night and later the whole day [24]. Circulating LH levels increase progressively during puberty following Tanner stages [12]. As it occurs with other gonadal axis hormones, LH levels must be determined based on Tanner stage [25] rather than age. This is due to considerable inter-individual variability in the age of pubertal onset and end in the general population [22].
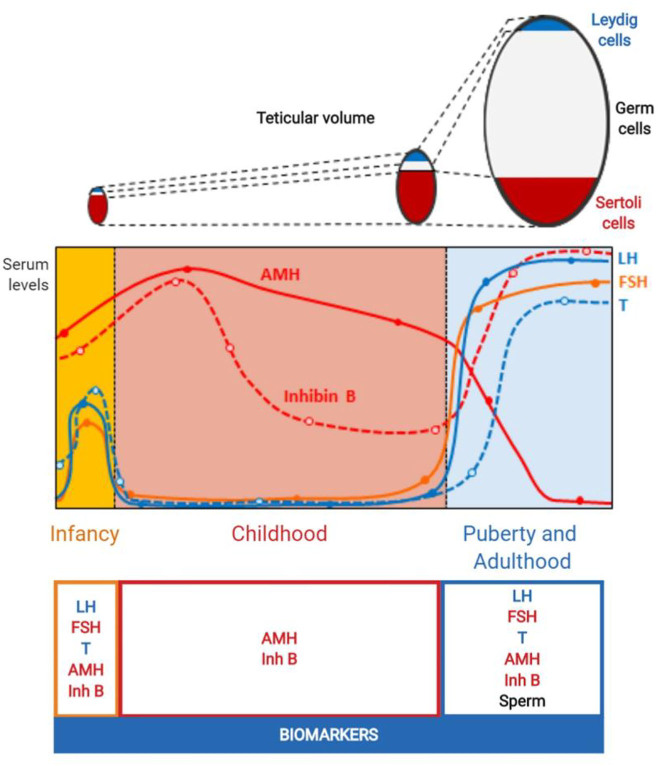
Figure 2:
Ontogeny of the evolution of testicular volume from birth to adulthood. Seminiferous cords (Sertoli cells + germ cells) are the main component of the testes. From birth and during the prepubertal period (i. e., until 9–14, Tanner stage 1), the volume of seminiferous cords is determined by Sertoli cells, whereas germ cell proliferation determines testicular volume during puberty (i. e., Tanner stages 2 to 5) (adult spermatogenesis). This Figure was modified using BioRender (https://biorender.com/) under the authorization of [1]. © 2019 Elsevier Ltd.
Follicle stimulating hormone
FSH binds to its specific receptor follicle stimulating hormone receptor (FSHR), which is expressed in Sertoli cell membranes. FSH stimulates immature Sertoli cell proliferation (from fetal life until the onset of puberty) and determines testes size in this stage of life. FSH stimulates the secretion of AMH [26] and inhibin B [27]. As LH, serum FSH is low at birth [23] and progressively increases during the first week of life [9]. In males, FSH concentrations are slightly lower than those of LH in "mini-puberty" [12]. During childhood, FSH also declines, but not as steeply as LH (Figure 2). As a result, circulating FSH levels in this stage of life exceed those of LH [12, 28].
Testicular hormones
Testicles contain two cell populations with endocrine function: Sertoli cells and Leydig cells. Endocrine activity of Leydig cells is clinically informative during postnatal activation or "mini-puberty" and puberty, whereas Sertoli cell activity is more informative in childhood.
Testosterone
Circulating levels of testosterone and LH undergo the same variations (Figure 2) i. e., they are low at birth [23] and progressively increase during the first month of life [9]. Although it is recommended that determination of steroid levels be performed by immunoassay [29], other steroids may be unspecifically detected during the first two and three weeks of life; therefore, extraction is required prior to determination to avoid overestimation [9]. From 3 to 6 months of life, circulating testosterone in plasma decreases to undetectable levels, and increases in Tanner stage 2 or 3 [12]. During childhood, estimation of the functional activity of Leydig cells can be based on circulating testosterone levels after hCG stimulation (2 to 3 IM injections of 1,500 to 2,500 IU at 48 h intervals [30].
Insulin-like 3 or Insulin-like 3 factorTesticular secretion of INSL3 is similar to that of testosterone [10, 13, 21]. However, INSL3 only reflects the long-term trophic effect of gonadotropins on Leydig cells, and INSL3 determination is not informative after acute hCG stimulation [19].
Anti-Müllerian hormoneAMH is a distinctive marker of prepuberal Sertoli cell population (Figure 2). Serum AMH decreases at birth and increases during the first weeks of life [9] to peak at 2–3 years of age, being 100-fold higher in males [11, 12, 31]. This is of clinical relevance, as samples from males require dilution for AMH concentrations to be within the range of detection of the immunoassays currently used in clinical laboratories.
Although basal AMH production is not dependent on gonadotropins [32], FSH stimulates testicular AMH secretion [26, 33–35]. In turn, increased testosterone concentrations inhibit AMH secretion [18, 36]. However, the increase of circulating androgen levels induced by medication is not enough to inhibit AMH [37]. Similarly, in males younger than 1 year, testosterone does not inhibit AMH production, as Sertoli cells do not express the androgen receptor in this stage of life [14, 38].
Inhibin B
Inhibins are dimeric proteins secreted by the gonads [39] with two isoforms with the same alpha subunit but distinct beta subunits. Inhibin B, which is complexed with a beta-B subunit, is the only form of inhibin with physiological relevance in males [40, 41]. In men, inhibin B is secreted in high amounts by Sertoli cells [4] and its production is stimulated by FSH [27, 34]. At the same time, inhibin B is the main inhibitor of FSH secretion from the pituitary gland. Levels of FSH are very elevated in patients with a depressed secretion or loss of inhibin B as in anorchia. However, during childhood, FSH may not be elevated [28], which is indicative of the hypothalamic–pituitary latency (gonadotrope) during that period of life.
Levels of inhibin B increase during the first weeks of life [9] reaching adult concentrations at 2 years of life [42, 43]. From 3 years of age, levels of inhibin B decrease slightly, but remain detectable (Figure 2) and higher than in girls. At puberty, inhibin B levels rise to a peak at Tanner stage 2 or 3 [4, 43, 44]. Thereafter, levels of inhibin B reflect Sertoli cell activity and interaction with germ cells.
Male hypogonadism
Male hypogonadism in adults has been defined as [1] a testicular dysfunction reflected in androgen deficiency with or without impaired sperm production [45]. Based on the developmental physiology described above, all male infants and children would meet the criteria for hypogonadism, as they do not produce testosterone or sperm. However, Sertoli cells are active during childhood, thereby inducing a slight testicular growth (Figure 2) and the production of AMH [46] and inhibin B [42, 43]. Determination of Sertoli cells is useful as an indicator of testicular function in the pediatric population. For a definition of male hypogonadism to be applicable to children, diagnosis of a diminished testicular function should be established taking the testicular function expected for the age of the patient as a reference, which may involve Sertoli cells (AMH, inhibin B), Leydig cells (testosterone, INSL3), and/or germ-cells [47].
Considering this principle, male hypogonadism should not only be classified based on the constituent of the HPG axis primarily affected, but also on the period of life and the testicular population primarily affected (Tables 1 and 2).
Table 1:
Fetal-onset male hypogonadism.
| Genitalia | Childhood | Puberty-Adulthood | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LH | FSH | T | AMH | Inh B | LH | FSH | T | AMH | Inh B | Sperm. | |||
| Primary hypogonadism (Testicular) | Generalized gonadal failure | ||||||||||||
| Gonadal dysgenesis | Female or ambiguous | N-H | N-H | L-ND | L-ND | L-ND | H | H | L-ND | L-ND | L-ND | Azoosp. | |
| Testicular regression syndrome Testicular torsion |
Micropenis Empty scrotum |
N-H | N-H | L-ND | L-ND | L-ND | H | H | L-ND | L-ND | L-ND | Azoosp. | |
| Klinefelter syndrome, XX Male | Male | N | N | N | N | N | H | H | N-L | L-ND | L-ND | Azoosp. | |
| Dissociated gonadal failure | |||||||||||||
| Leydig cells | |||||||||||||
| Hypoplasia/aplasia Steroidogenic defects |
Female or hypovirilized | N-H | N | L-ND | N-H | N | H | H | L-ND | N-H | L-ND | Azoosp. | |
| INSL3 mutations | Cryptorchidism | N | N | N | N | N | N | N-H | N | N-L | Oligosp. | ||
| Sertoli cells | |||||||||||||
| FSH-R mutations | Small testicles | N | N | N | L | L | N | H | N | L | L | Oligosp. | |
| AMH mutations | PMDS | N | N | N | ND | N | N | N | N | ND | N | N | |
| Secondary hypogonadism (Central) | Generalized gonadal failure | ||||||||||||
| Multiple pituitary hormone deficiency | Micropenis, cryptorchidism | L | L | L | L | L | L | L | L | L | L | Oligosp./azoosp. | |
| Isolated central hypogonadism | Micropenis, cryptorchidism | L | L | L | L | L | L | L | L | L | L | Oligosp./azoosp. | |
| Dissociated gonadal failure | |||||||||||||
| Multiple pituitary hormone deficiency | Small testicles | N | L | N | L | L | N | L | N | L | |||
| Isolated central hypogonadism: TAC3 or TACR3 mutations |
Micropenis, cryptorchidism | L | N | L | N | N | L | N | L | L | Oligosp./azoosp. | ||
| LHβ mutations | Micropenis, cryptorchidism | L | N | L | N | N | L | H | L | H | Oligosp./azoosp. | ||
| FSHβ mutations | Small testicles | N | L | N | H | L | N | Oligosp./azoosp. | |||||
| Dual hypogonadism (Combined) | Generalized gonadal failure | ||||||||||||
| Prader-Willi syndrome X-linked congenital adrenal hypoplasia |
Micropenis, cryptorchidism | L-N | L-N | L-N | L | L | N | N | L | L | L | Oligosp./azoosp. | |
L, N, H: Low, Normal, High with respect to reference range for age in males ND: non-detectable. Male hypogonadism: an extended classification based on a developmental, endocrine physiology-based approach. Andrology. 2013;1(1):3–16. © 2012 American Society of Andrology and European Academy of Andrology.
Table 2:
Postnatal-onset male hypogonadism.
| Childhood | Puberty-Adulthood | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LH | FSH | T | AMH | Inh B | LH | FSH | T | AMH | Inh B | Spermatogenesis | ||
| Primary hypogonadism (Testicular) | Generalized gonadal failure | |||||||||||
| Orchitis Testicular torsion or trauma |
N | . | L-ND | L-ND | L-ND | L | L | L-ND | L-ND | L-ND | Oligosp./azoosp. | |
| Down's syndrome | N-H | N-H | L-N | L-N | L-N | L | L | L | L | L | Azoosp. | |
| Varicocele | N | N | N | N | N | N-H | N | L-N | N | N | Teratozoosp./asthenozoosp. | |
| Chronic diseases: Granulomatous disease, amyloidosis, cystic fibrosis, kidney failure |
L | L | L-ND | L-ND | L-ND | Oligosp./azoosp. | ||||||
| Late-onset hypogonadism | Not applicable | N-H | N-H | L | L | |||||||
| Dissociated gonadal failure | ||||||||||||
| Chromosome Y deletions: AZF
Genetic mutations: CILD1, USP9Y |
N | N | N | N | N | N | A | N | L | Oligosp./azoosp. | ||
| Chemotherapy Abdomino-pelvic radiotherapy |
N | N | N-L | N-L | N-H | H | N-L | L | Oligosp./azoosp. | |||
| Drug therapies: Spironolactone, ketoconazole |
N | N | N-L | N-H | N-H | L | Oligosp. | |||||
| Secondary hypogonadism (Central) | Generalized gonadal failure | |||||||||||
| Pituitary and CNS damage: Tumors, histiocytosis, trauma, etc. |
L | L | L | L | L | L | L | L | L | L | Oligosp./azoosp. | |
| Functional central hypogonadism: Chronic diseases, drug/alcohol abuse, etc., |
L-N | L-N | L | L | Oligosp./azoosp. | |||||||
| Dual hypogonadism (Combined) | Generalized gonadal failure | |||||||||||
| Brain radiotherapy + chemotherapy Lead poisoning Marihuana use Total body irradiation |
L-N | L-N | L-N | L | L | L-N | L-N | L | L | L | Oligosp./azoosp. | |
L, N, H: Low, Normal, High with respect to reference range for age in males ND: non-detectable. Male hypogonadism: an extended classification based on a developmental, endocrine physiology-based approach. Andrology. 2013;1(1):3–16. © 2012 American Society of Andrology and European Academy of Andrology.
Primary (“Hypergonadotropic”), secondary (“Hypogonadotropic”) or dual hypogonadism
Hypogonadism may be caused by a primary defect in the hypothalamus or pituitary gland or an abnormality in the gonads. In rare cases, both, the hypothalamic–pituitary axis and the testes present a primary defect, which originates dual or combined hypogonadism [47].
Primary hypogonadism
In adult medicine, primary hypogonadism (testicular or peripheral) is known as "hypergonadotropic" [1, 45] and is characterized by a primary defect in the testis. Deficient inhibin B and testosterone production reduces the negative feedback effect on the HPG axis, which results in an increased production of gonadotropins. This phenomenon does not always occur resulting in LH and FSH dissociation. Some examples of primary hypogonadism include Klinefelter syndrome, Testicular regression syndrome (TRS), and orchitis, to name a few.
Secondary hypogonadism
Secondary hypogonadism (hypothalamus–pituitary or central) is known as "hypogonadotropic" in adult medicine [1, 45] and is characterized by a primary defect in the hypothalamus or the pituitary gland. Impaired production of LH and FSH prevents the normal development of Leydig cells and seminiferous cords (Sertoli cells and germ cells). The developmental physiology of the HPG axis makes the diagnosis of these conditions challenging. LH and FSH dissociation may also occur. Examples of secondary hypogonadism include Kallmann syndrome, multihormonal pituitary hormone deficiency, and pituitary hormone deficiency after central nervous system surgery, to name a few.
Dual hypogonadism
There are rare conditions where both, the HPG axis and the gonads present primary damage. In contrast with primary and secondary hypogonadism, impairment of all testicular cell populations is concomitant and not secondary. Dual hypogonadism conditions include Prader–Willi syndrome and gonadal failure in oncologic patients treated with chemotherapy and cranial radiotherapy, among others.
Generalized or dissociated gonadal failure
Generalized hypogonadism
In these cases, all testicular cell populations exhibit primary damage, concentrations are decreased, and germ-cell production is impaired. Examples include testicular dysgenesis and Kallmann syndrome (isolated hypogonadotropic hypogonadism with hyposmia).
Dissociated hypogonadism
Dissociated hypogonadism is characterized by primary damage in a specific testicular cell population. In the short or long term, the production of other populations becomes impaired at different degrees. Some examples include Leydig cell hypoplasia secondary to LHCG-R mutations, FSH deficiency for FSH beta-subunit gene mutations involving Sertoli cells, and post-chemotherapy gonadal failure, which primarily damages germ cells.
Hypogonadism of fetal, childhood, pubertal or adult onset
The clinical manifestations of hypogonadism are dependent on the period of life where failure occurs. Fetal hypogonadism established during the first trimester causes a disorder of sex development (DSD), which manifests in the form of ambiguous or female genitalia at birth [48]. Gonadal dysgenesis is an example of generalized fetal hypogonadism, whereas Leydig cell hypoplasia is a dissociated form. Central hypogonadism does not result in genital ambiguity, as Leydig cell function during the first trimester of gestation is not dependent on pituitary gonadotropins, but on placental hCG. Hypogonadism occurred from the second trimester of fetal life, be it testicular, central or dual, results in micropenis or cryptorchidism in males without genital ambiguity [49–51]. As the HPG axis remains active for the first 3–6 months of postnatal life [9, 10], this period represents a window of opportunity to establish a diagnosis of hypogonadism [49–51].
Hypogonadism diagnosed in childhood may remain unnoticed. This is due to the fact that HPG activity decreases during childhood. For the condition to be diagnosed, suspicion or active screening is required (i. e., by baseline AMH or inhibin B determination, or measuring testosterone levels in response to hCG-induced stimulation). Otherwise, diagnosis will be delayed until puberty [52].
At puberty, male hypogonadism is characterized by the absence or interruption of normal pubertal development [22, 25]. As a result of androgen deficiency, secondary sex characteristics do not appear, i. e., body proportions typically are eunuchoid (upper/lower body proportion <1, with a span exceeding 6 cm), deepening of the voice is compromised, bone maturation is delayed, and testicular volume does not increase, which indicates disturbed spermatogenesis.
Hypogonadism established in adulthood is characterized by decreased libido, impotence and oligozoospermia [45]. Men of an older age may develop a mild androgen deficiency known as late-onset hypogonadism [53], which has similar symptoms to those of hypogonadism in young men.
Clinical utility of HPG axis biomarkers in childhood and adolescence
From birth to 3–6 months of life
During this period, the HPG is active, and all hormones are informative.
Newborns with ambiguous or female genitalia
In newborns with ambiguous genitalia, the causes of a DSD must be investigated. In patients with a 46,XY karyotype, the cause may be gonadal dysgenesis or generalized fetal‐onset primary hypogonadism established in the first trimester of gestation. These patients generally exhibit very low levels of AMH, inhibin B, testosterone and INSL3, whereas gonadotropins are elevated [48, 51, 54]. Imaging studies demonstrate the presence of uterus and fallopian tubes due to AMH deficiency. When genital ambiguity co-occurs with the absence of Müllerian structures, the cause may be dissociated primary fetal hypogonadism with a specific failure of the Leydigian sector. The causes may be Leydig cell hypoplasia secondary to LHCG-R mutations or an abnormality in the proteins involved in testicular steroidogenesis [55]. Differential diagnosis from gonadal dysgenesis is based on the presence of low testosterone levels, high LH levels, and AMH levels within normal range for males [56]. Hypogonadism is excluded when testosterone and AMH concentrations are high. Then, the cause of DSD may be insensitivity to androgens secondary to androgen-receptor mutations, a deficient DHT production in peripheral tissues secondary to 5α-reductase mutations, or a non-endocrine cause [48, 54, 57]. In patients with sex chromosome anomalies (i. e., deletions of the short arm of the Y chromosome; 45,X/46,XY or other mosaicisms involving the presence of the Y-chromosome), the cause of the DSD is gonadal dysgenesis.
A rare form of DSD 46,XY is persistent Müllerian duct syndrome (PMDS), which is characterized by cryptorchidism and fully-developed male genitals. Gonadotropins and testosterone are within the normal range for males, whereas AMH is very low or undetectable when the cause is an AMH mutation, and normal in AMHR2 mutations [58]. The first case corresponds to dissociated primary fetal hypogonadism specifically affecting Sertoli cells. The second case corresponds to peripheral resistance to AMH, with the absence of hypogonadism.
In newborns with karyotype 46,XX, genital ambiguity occurs as the result of excess suprarenal (i. e., congenital suprarenal hyperplasia) [59] or placental androgen production (aromatase deficiency) [60]. These patients have ovaries, and AMH and inhibin B concentrations are within the normal range for females [56]. Nevertheless, genital ambiguity may be secondary to testicular tissue development in the form of ovotestis or dysgenetic testis [61]. In the two first cases, AMH and testosterone are generally in an intermediate point between normal ranges for males and females, whereas gonadotropins can be elevated or even within normal range in the presence of functional ovarian tissue. Cases have been reported of males born with karyotype 46,XX and normal male genitalia. The detection of these cases is based on discordance with an eventual karyotype developed during gestation. These patients exhibit normal HPG axis hormone levels for males until puberty, as described below.
Newborns with micropenis, cryptorchidism, and/or micro-orchidism
Micropenis, cryptorchidism and/or micro-orchidism are signs of HPG axis failure. This type of fetal hypogonadism establishes from the second trimester of gestation after male genital differentiation has started. Co-occurrence of low levels of LH, FSH, testosterone, INSL3, AMH, and inhibin B is highly suggestive of central fetal hypogonadism (hypogonadotropin) affecting all HPG axis sectors [34, 51, 62–64]. However, these low levels may also be due to a generalized primary testicular failure from the second trimester of gestation. TRS is characterized by undetactable levels of testicular hormones with elevated gonadotropin concentrations [51, 65].
From 6 months to pubertal age
In this period of life, gonadotropins, testosterone, and INSL3 are uninformative, whereas Sertoli cells are of greater clinical utility.
If the condition is congenital but diagnosis was delayed, identifying the cause of the problem may be challenging. Low levels of AMH and inhibin B are indicative of Sertoli cell deficiency. However, it is difficult to establish whether the disorder is secondary to a primary testicular failure or a HPG axis failure. Gonadotropins may normalize during childhood in patients with primary hypogonadism (dysgenetic DSD or DSD caused by Leydig cell dysfunction, TRS or anorchidism). Otherwise said, primary male hypogonadism is not always "hypergonadotropic" at prepubertal age [28]. These patients show normal testosterone levels (i. e., undetectable), unless an hCG stimulation test is performed to determine the presence of functional Leydig cells. Undetectable levels of AMH and inhibin B are confirmatory of anorchidism.
In children without a perinatal history of micropenis, the probability of fetal hypogonadism is lower. Finding is generally incidental and occurs during evaluation of cryptorchidism, torsion, testicular trauma or oncologic treatments that may affect gonadal function. Again, gonadotropins and basal testosterone have poor diagnostic value, as gonadotropins do not increase during childhood, which is the period in which gonadal damage occurs (from 6 months of life) [28]. In patients without palpable gonads, detectable levels of AMH [66] or inhibin B [67] guarantees the presence of ectopic gonads, and testosterone levels increase after a hCG stimulation test [66]. Low levels of AMH [52, 66, 68, 69] or inhibin B [67, 68] are indicative of an abnormal testicular function. Cases have been reported of children with monorchidism with normal AMH and inhibin B values [70]. Circulating levels of INSL3 are not of clinical utility in this age group [71].
At pubertal age
The absence of signs of pubertal development is suggestive of androgen deficiency. Although this abnormality can be secondary to primary hypogonadism, a testicular failure rarely affects the Leydig population, thereby inhibiting androgen secretion completely. In primary hypogonadism, the structures most frequently affected is the tubular sector, which translates into a small testicular volume [72]. Examples of this condition include Klinefelter syndrome [73, 74], XX males [75] and patients receiving chemotherapy [69]. These patients generally exhibit normal circulating levels of testicular hormones and gonadotropins until Tanner stage 3 of pubertal development. Then, primary hypogonadism becomes "hypergonadotropic".
Most frequently, the absence of pubertal development can be due to an HPG axis failure in the form of congenital or acquired central hypogonadism, a delayed reactivation of the HPG axis, or simple delayed puberty [22]. Differential diagnosis is challenging. Once general causes such as acute or chronic systemic diseases have been excluded, circulating levels of HPG-axis hormones are not necessarily informative. Gonadotropin concentrations at prepubertal levels are not of utility in differential diagnosis of central hypogonadism and simple delayed puberty, and GnRH [76] (or analogs) stimulation tests are required [77]. The presence of other pituitary deficiencies facilitates diagnosis, as they are indicative of gonadotropin deficiency. Testosterone and INSL3 remain at prepubertal levels and are not useful to distinguish central hypogonadism from simple delayed puberty [78]. In contrast, diagnosis is confirmed by AMH and inhibin B levels, as they are lower in patients with central hypogonadism as compared to those with simple delayed puberty [78, 79].
As in primary hypogonadism, central hypogonadism can affect all cell populations (i. e., generalized) or initially affect a single sector of the HPG axis. Examples of dissociated central hypogonadism include tachykinin Precursor 3 (TAC3) and tachykinin receptor 3 (TACR3) mutations [80] and LH beta sub-unit mutations [81], which manifest in the form of low LH levels and normal FSH levels. Other examples are FSH beta subunit mutations [82], which are associated with a decline in FSH and LH production and normal androgen levels.
Dual hypogonadism is characterized by concomitant HPG axis and gonad dysfunction. This condition can be congenital of which hypogonadism is a late manifestation, as in the case of Prader–Willi syndrome [83, 84] and delayed-onset X-linked adrenal hypoplasia congenita due to dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1 (DAX-1) gene mutations [85]. Dual hypogonadism can also be acquired as in the case of patients exposed to chemotherapy, which primarily affects the testes, and cranial radiotherapy, which affects the hypothalamus. Although gonadal hormone levels are low, gonadotropins do not increase. In other words, these conditions mimic eugonadotropic hypogonadism.
Conclusions
Hypogonadism may have a fetal or postnatal origin and has different clinical manifestations according to the period of life in which it is established. This condition may affect any functional testicular component, or initially involve a single component, thereby resulting in specific clinical and biochemical manifestations. Primary damage generally occurs to the gonads or the HPG axis and rarely affects the two systems concomitantly. Gonadotropins and androgens are useful biomarkers in newborns and pubertal males, whereas AMH and inhibin B are more informative in childhood.
Footnotes
Research funding: None declared.
Author contributions: The author has accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: R.A. Rey receives honoraria under a Beckman-Coulter agreement for the development of an AMH ELISA kit. Dr. Rey also receives honoraria from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) for technologic services requiring the use of an AMH ELISA kit.
Article Note: The original submission can be found here: https://doi.org/10.1515/almed-2019-0043
References
- 1.Grinspon RP, Freire AV, Rey RA. Hypogonadism in pediatric Health: adult medicine concepts fail. Trends Endocrinol Metabol. 2019;30:879–90. doi: 10.1016/j.tem.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Casoni F, Malone SA, Belle M, Luzzati F, Collier F, Allet C, et al. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent foetal brains. Development. 2016;143:3969–81. doi: 10.1242/dev.139444. [DOI] [PubMed] [Google Scholar]
- 3.Edelsztein NY, Grinspon RP, Schteingart HF, Rey RA. Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testes of children and adolescents with disorders of the gonadal axis. Int J Pediatr Endocrinol. 2016;2016:20. doi: 10.1186/s13633-016-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson AM. Inhibin B in the assessment of seminiferous tubular function. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14:389–97. doi: 10.1053/beem.2000.0086. [DOI] [PubMed] [Google Scholar]
- 5.Bergadá I, Bergadá C, Campo S. Role of inhibins in childhood and puberty. J Pediatr Endocrinol Metab. 2001;14:343–53. doi: 10.1515/JPEM.2001.14.4.343. [DOI] [PubMed] [Google Scholar]
- 6.Makela JA, Koskenniemi JJ, Virtanen HE, Toppari J. Testes development. Endocr Rev. 2019;40:857–905. doi: 10.1210/er.2018-00140. [DOI] [PubMed] [Google Scholar]
- 7.Ivell R, Hartung S. The molecular basis of cryptorchidism. Mol Hum Reprod. 2003;9:175–81. doi: 10.1093/molehr/gag025. [DOI] [PubMed] [Google Scholar]
- 8.Klonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testes descent and external genitalia development. Dev Biol. 2004;270:1–18. doi: 10.1016/j.ydbio.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Bergadá I, Milani C, Bedecarrás P, Andreone L, Ropelato MG, Gottlieb S, et al. Time course of the serum gonadotropin surge, inhibins, and anti-Mullerian hormone in normal newborn males during the first month of life. J Clin Endocrinol Metab. 2006;91:4092–8. doi: 10.1210/jc.2006-1079. [DOI] [PubMed] [Google Scholar]
- 10.Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy, minipuberty. Horm Res Paediatr. 2014;82:73–80. doi: 10.1159/000362414. [DOI] [PubMed] [Google Scholar]
- 11.Aksglæde L, Sorensen K, Boas M, Mouritsen A, Hagen CP, Jensen RB, et al. Changes in anti-Mullerian hormone (AMH) throughout the life span: a population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J Clin Endocrinol Metab. 2010;95:5357–64. doi: 10.1210/jc.2010-1207. [DOI] [PubMed] [Google Scholar]
- 12.Grinspon RP, Bedecarrás P, Ballerini MG, Iñíguez G, Rocha A, Mantovani Rodrigues Resende EA, et al. Early onset of primary hypogonadism revealed by serum anti-Müllerian hormone determination during infancy and childhood in trisomy 21. Int J Androl. 2011;34:e487–98. doi: 10.1111/j.1365-2605.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 13.Rey RA. Mini-puberty and true puberty: differences in testicular function. Ann Endocrinol. 2014;75:58–63. doi: 10.1016/j.ando.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Chemes HE, Rey RA, Nistal M, Regadera J, Musse M, Gonzalez-Peramato P, et al. Physiological androgen insensitivity of the foetal, neonatal, and early infantile testes is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J Clin Endocrinol Metab. 2008;93:4408–12. doi: 10.1210/jc.2008-0915. [DOI] [PubMed] [Google Scholar]
- 15.Berensztein EB, Baquedano MS, Gonzalez CR, Saraco NI, Rodriguez J, Ponzio R, et al. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testes. Pediatr Res. 2006;60:740–4. doi: 10.1203/01.pdr.0000246072.04663.bb. [DOI] [PubMed] [Google Scholar]
- 16.Boukari K, Meduri G, Brailly-Tabard S, Guibourdenche J, Ciampi ML, Massin N, et al. Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testes development. J Clin Endocrinol Metab. 2009;94:1818–25. doi: 10.1210/jc.2008-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rey RA, Musse M, Venara M, Chemes HE. Ontogeny of the androgen receptor expression in the foetal and postnatal testes: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc Res Tech. 2009;72:787–95. doi: 10.1002/jemt.20754. [DOI] [PubMed] [Google Scholar]
- 18.Edelsztein NY, Rey RA. Importance of the androgen receptor signaling in gene transactivation and transrepression for pubertal maturation of the testes. Cells. 2019;8:861. doi: 10.3390/cells8080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bay K, Andersson AM. Human testicular insulin-like factor 3: in relation to development, reproductive hormones and andrological disorders. Int J Androl. 2011;34:97–109. doi: 10.1111/j.1365-2605.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- 20.Ivell R, Wade JD, Anand-Ivell R. INSL3 as a biomarker of Leydig cell functionality. Biol Reprod. 2013;88:147. doi: 10.1095/biolreprod.113.108969. [DOI] [PubMed] [Google Scholar]
- 21.Bay K, Hartung S, Ivell R, Schumacher M, Jurgensen D, Jorgensen N, et al. Insulin-like factor 3 serum levels in 135 normal men and 85 men with testicular disorders: relationship to the luteinizing hormone-testosterone axis. J Clin Endocrinol Metab. 2005;90:3410–18. doi: 10.1210/jc.2004-2257. [DOI] [PubMed] [Google Scholar]
- 22.Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366:443–53. doi: 10.1056/NEJMcp1109290. [DOI] [PubMed] [Google Scholar]
- 23.Corbier P, Dehennin L, Castanier M, Mebazaa A, Edwards DA, Roffi J. Sex differences in serum luteinizing hormone and testosterone in the human neonate during the first few hours after birth. J Clin Endocrinol Metab. 1990;71:1344–8. doi: 10.1210/jcem-71-5-1344. [DOI] [PubMed] [Google Scholar]
- 24.Liu JH, Patel B, Collins G. Central causes of amenorrhea. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, editors. Endotext. South Dartmouth (MA): MDText.com, Inc; 2000. [Google Scholar]
- 25.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urrutia M, Grinspon RP, Rey RA. Comparing the role of anti-Mullerian hormone as a marker of FSH action in male and female fertility. Expet Rev Endocrinol Metabol. 2019;14:203–14. doi: 10.1080/17446651.2019.1590197. [DOI] [PubMed] [Google Scholar]
- 27.Raivio T, Toppari J, Perheentupa A, McNeilly AS, Dunkel L. Treatment of prepubertal gonadotrophin-deficient boys with recombinant human follicle-stimulating hormone. Lancet. 1997;350:263–4. doi: 10.1016/s0140-6736(05)62227-1. [DOI] [PubMed] [Google Scholar]
- 28.Grinspon RP, Ropelato MG, Bedecarrás P, Loreti N, Ballerini MG, Gottlieb S, et al. Gonadotrophin secretion pattern in anorchid boys from birth to pubertal age: pathophysiological aspects and diagnostic usefulness. Clin Endocrinol. 2012;76:698–705. doi: 10.1111/j.1365-2265.2011.04297.x. [DOI] [PubMed] [Google Scholar]
- 29.Taylor AE, Keevil B, Huhtaniemi IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol. 2015;173:D1–12. doi: 10.1530/eje-15-0338. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed SF, Keir L, McNeilly J, Galloway P, O'Toole S, Wallace AM. The concordance between serum anti-Mullerian hormone and testosterone concentrations depends on duration of hCG stimulation in boys undergoing investigation of gonadal function. Clin Endocrinol. 2010;72:814–9. doi: 10.1111/j.1365-2265.2009.03724.x. [DOI] [PubMed] [Google Scholar]
- 31.Hagen CP, Aksglæde L, Sorensen K, Main KM, Boas M, Cleemann L, et al. Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95:5003–10. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- 32.Lasala C, Carré-Eusèbe D, Picard JY, Rey R. Subcellular and molecular mechanisms regulating anti-Müllerian hormone gene expression in mammalian and nonmammalian species. DNA Cell Biol. 2004;23:572–85. doi: 10.1089/dna.2004.23.572. [DOI] [PubMed] [Google Scholar]
- 33.Young J, Chanson P, Salenave S, Noel M, Brailly S, O'Flaherty M, et al. Testicular anti-mullerian hormone secretion is stimulated by recombinant human FSH in patients with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:724–8. doi: 10.1210/jc.2004-0542. [DOI] [PubMed] [Google Scholar]
- 34.Bougnères P, François M, Pantalone L, Rodrigue D, Bouvattier C, Demesteere E, et al. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. J Clin Endocrinol Metab. 2008;93:2202–5. doi: 10.1210/jc.2008-0121. [DOI] [PubMed] [Google Scholar]
- 35.Grinspon RP, Urrutia M, Rey RA. Male central hypogonadism in paediatrics – the relevance of follicle-stimulating hormone and Sertoli cell markers. Eur Endocrinol. 2018;14:67–71. doi: 10.17925/ee.2018.14.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edelsztein NY, Racine C, di Clemente N, Schteingart HF, Rey RA. Androgens downregulate anti-Mullerian hormone promoter activity in the Sertoli cell through the androgen receptor and intact SF1 sites. Biol Reprod. 2018;99:1303–12. doi: 10.1093/biolre/ioy152. [DOI] [PubMed] [Google Scholar]
- 37.Young J, Rey R, Couzinet B, Chanson P, Josso N, Schaison G. Antimüllerian hormone in patients with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1999;84:2696–9. doi: 10.1210/jcem.84.8.5972. [DOI] [PubMed] [Google Scholar]
- 38.Grinspon RP, Andreone L, Bedecarrás P, Ropelato MG, Rey RA, Campo SM, et al. Male central precocious puberty: serum profile of anti-mullerian hormone and inhibin B before, during, and after treatment with GnRH analogue. Internet J Endocrinol. 2013;2013:823064. doi: 10.1155/2013/823064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger H. Remembrance: the story of inhibin--the Melbourne version. Endocrinology. 1992;131:1585–6. doi: 10.1210/endo.131.4.1396302. [DOI] [PubMed] [Google Scholar]
- 40.Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, McNeilly AS, et al. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81:3341–5. doi: 10.1210/jcem.81.9.8784094. [DOI] [PubMed] [Google Scholar]
- 41.Illingworth PJ, Groome NP, Byrd W, Rainey WE, McNeilly AS, Mather JP, Bremner WJ. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab. 1996;81:1321–5. doi: 10.1210/jcem.81.4.8636325. [DOI] [PubMed] [Google Scholar]
- 42.Bergadá I, Rojas G, Ropelato MG, Ayuso S, Bergadá C, Campo SM. Sexual dimorphism in circulating monomeric and dimeric inhibins in normal boys and girls from birth to puberty. Clin Endocrinol. 1999;51:455–60. doi: 10.1046/j.1365-2265.1999.00814.x. [DOI] [PubMed] [Google Scholar]
- 43.Andersson AM, Skakkebæk NE. Serum inhibin B levels during male childhood and puberty. Mol Cell Endocrinol. 2001;180:103–7. doi: 10.1016/S0303-7207(01)00520-2. [DOI] [PubMed] [Google Scholar]
- 44.Trigo RV, Bergadá I, Rey R, Ballerini MG, Bedecarrás P, Bergadá C, et al. Altered serum profile of inhibin B, Pro-alphaC and anti-Mullerian hormone in prepubertal and pubertal boys with varicocele. Clin Endocrinol. 2004;60:758–64. doi: 10.1111/j.1365-2265.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- 45.Salonia A, Rastrelli G, Hackett G, Seminara SB, Huhtaniemi IT, Rey RA, et al. Paediatric and adult-onset male hypogonadism. Nat Rev Dis Prim. 2019;5:38. doi: 10.1038/s41572-019-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Josso N, Rey RA, Picard JY. Anti-müllerian hormone: a valuable addition to the toolbox of the pediatric endocrinologist. Internet J Endocrinol. 2013;2013:674105. doi: 10.1155/2013/674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rey RA, Grinspon RP, Gottlieb S, Pasqualini T, Knoblovits P, Aszpis S, et al. Male hypogonadism: an extended classification based on a developmental, endocrine physiology-based approach. Andrology. 2013;1:3–16. doi: 10.1111/j.2047-2927.2012.00008.x. [DOI] [PubMed] [Google Scholar]
- 48.Rey RA, Grinspon RP. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract Res Clin Endocrinol Metabol. 2011;25:221–38. doi: 10.1016/j.beem.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab. 2005;90:3122–7. doi: 10.1210/jc.2004-2465. [DOI] [PubMed] [Google Scholar]
- 50.Bouvattier C, Maione L, Bouligand J, Dode C, Guiochon-Mantel A, Young J. Neonatal gonadotropin therapy in male congenital hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2012;8:172–82. doi: 10.1038/nrendo.2011.164. [DOI] [PubMed] [Google Scholar]
- 51.Grinspon RP, Loreti N, Braslavsky D, Valeri C, Schteingart H, Ballerini MG, et al. Spreading the clinical window for diagnosing foetal-onset hypogonadism in boys. Front Endocrinol. 2014;5:51–4. doi: 10.3389/fendo.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grinspon RP, Gottlieb S, Bedecarras P, Rey RA. Anti-Müllerian hormone and testicular function in prepubertal boys with cryptorchidism. Front Endocrinol. 2018;9:181. doi: 10.3389/fendo.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Androl. 2009;32:1–10. doi: 10.1111/j.1365-2605.2008.00924.x. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015) Clin Endocrinol. 2016;84:771–88. doi: 10.1111/cen.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendonça BB, Costa EM, Belgorosky A, Rivarola MA, Domenice S. 46,XY DSD due to impaired androgen production. Best Pract Res Clin Endocrinol Metabol. 2010;24:243–62. doi: 10.1016/j.beem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Freire AV, Grinspon RP, Rey RA. Importance of serum testicular protein hormone measurement in the assessment of disorders of sex development. Sex Dev. 2018;12:30–40. doi: 10.1159/000479572. [DOI] [PubMed] [Google Scholar]
- 57.Grinspon RP, Rey RA. When hormone defects cannot explain it: malformative disorders of sex development. Birth Defects Res C Embryo Today. 2014;102:359–73. doi: 10.1002/bdrc.21086. [DOI] [PubMed] [Google Scholar]
- 58.Picard JY, Cate RL, Racine C, Josso N. The persistent mullerian duct syndrome: an update based upon a personal experience of 157 cases. Sex Dev. 2017;11:109–25. doi: 10.1159/000475516. [DOI] [PubMed] [Google Scholar]
- 59.Turcu AF, Auchus RJ. Adrenal steroidogenesis and congenital adrenal hyperplasia. Endocrinol Metab Clin N Am. 2015;44:275–96. doi: 10.1016/j.ecl.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belgorosky A, Guercio G, Pepe C, Saraco N, Rivarola MA. Genetic and clinical spectrum of aromatase deficiency in infancy, childhood and adolescence. Horm Res Paediatr. 2009;72:321–30. doi: 10.1159/000249159. [DOI] [PubMed] [Google Scholar]
- 61.Grinspon RP, Rey RA. Molecular characterization of XX maleness. Int J Mol Sci. 2019;20:6089. doi: 10.3390/ijms20236089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trabado S, Maione L, Bry-Gauillard H, Affres H, Salenave S, Sarfati J, et al. Insulin-like peptide 3 (INSL3) in men with congenital hypogonadotropic hypogonadism/Kallmann syndrome and effects of different modalities of hormonal treatment: a single-center study of 281 patients. J Clin Endocrinol Metab. 2014;99:E268–75. doi: 10.1210/jc.2013-2288. [DOI] [PubMed] [Google Scholar]
- 63.Braslavsky D, Grinspon RP, Ballerini MG, Bedecarrás P, Loreti N, Bastida G, et al. Hypogonadotropic hypogonadism in infants with congenital hypopituitarism: a challenge to diagnose at an early stage. Horm Res Paediatr. 2015;84:289–97. doi: 10.1159/000439051. [DOI] [PubMed] [Google Scholar]
- 64.Lambert AS, Bougnères P. Growth and descent of the testes in infants with hypogonadotropic hypogonadism receiving subcutaneous gonadotropin infusion. Int J Pediatr Endocrinol. 2016;2016:13. doi: 10.1186/s13633-016-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McElreavey K, Jorgensen A, Eozenou C, Merel T, Bignon-Topalovic J, Tan DS, et al. Pathogenic variants in the DEAH-box RNA helicase DHX37 are a frequent cause of 46,XY gonadal dysgenesis and 46,XY testicular regression syndrome. Genet Med. 2019;22:150–9. doi: 10.1038/s41436-019-0606-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee MM, Donahoe PK, Silverman BL, Hasegawa T, Hasegawa Y, Gustafson ML, et al. Measurements of serum Müllerian inhibiting substance in the evaluation of children with nonpalpable gonads. N Engl J Med. 1997;336:1480–6. doi: 10.1056/NEJM199705223362102. [DOI] [PubMed] [Google Scholar]
- 67.Hildorf S, Dong L, Thorup J, Clasen-Linde E, Andersen CY, Cortes D. Sertoli cell number correlates with serum inhibin B in infant cryptorchid boys. Sex Dev. 2019;13:74–82. doi: 10.1159/000497374. [DOI] [PubMed] [Google Scholar]
- 68.Hamdi SM, Almont T, Galinier P, Mieusset R, Thonneau P. Altered secretion of Sertoli cells hormones in 2-year-old prepubertal cryptorchid boys: a cross-sectional study. Andrology. 2017;5:783–9. doi: 10.1111/andr.12373. [DOI] [PubMed] [Google Scholar]
- 69.Grinspon RP, Arozarena M, Prada S, Bargman G, Sanzone M, Morales Bazurto M, et al. Safety of standardised treatments for haematologic malignancies as regards to testicular endocrine function in children and teenagers. Hum Reprod. 2019;34:2480–94. doi: 10.1093/humrep/dez216. [DOI] [PubMed] [Google Scholar]
- 70.Grinspon RP, Habib C, Bedecarrás P, Gottlieb S, Rey RA. Compensatory function of the remaining testes is dissociated in boys and adolescents with monorchidism. Eur J Endocrinol. 2016;174:399–407. doi: 10.1530/eje-15-0938. [DOI] [PubMed] [Google Scholar]
- 71.van Brakel J, de Muinck Keizer-Schrama S, Hazebroek FWJ, Dohle GR, de Jong FH. INSL3 and AMH in patients with previously congenital or acquired undescended testes. J Pediatr Surg. 2017;52:1327–31. doi: 10.1016/j.jpedsurg.2017.03.064. [DOI] [PubMed] [Google Scholar]
- 72.Ladjouze A, Donaldson M. Primary gonadal failure. Best Pract Res Clin Endocrinol Metabol. 2019;33:101295. doi: 10.1016/j.beem.2019.101295. [DOI] [PubMed] [Google Scholar]
- 73.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet. 2004;364:273–83. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- 74.Bastida MG, Rey RA, Bergadá I, Bedecarrás P, Andreone L, del Rey G, et al. Establishment of testicular endocrine function impairment during childhood and puberty in boys with Klinefelter syndrome. Clin Endocrinol. 2007;67:863–70. doi: 10.1111/j.1365-2265.2007.02977.x. [DOI] [PubMed] [Google Scholar]
- 75.Aksglæde L, Skakkebæk NE, Juul A. Abnormal sex chromosome constitution and longitudinal growth: serum levels of insulin-like growth factor (IGF)-I, IGF binding protein-3, luteinizing hormone, and testosterone in 109 males with 47,XXY, 47,XYY, or sex-determining region of the Y chromosome (SRY)-positive 46,XX karyotypes. J Clin Endocrinol Metab. 2008;93:169–76. doi: 10.1210/jc.2007-1426. [DOI] [PubMed] [Google Scholar]
- 76.Grinspon RP, Ropelato MG, Gottlieb S, Keselman A, Martinez A, Ballerini MG, et al. Basal follicle-stimulating hormone and peak gonadotropin levels after gonadotropin-releasing hormone infusion show high diagnostic accuracy in boys with suspicion of hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2010;95:2811–18. doi: 10.1210/jc.2009-2732. [DOI] [PubMed] [Google Scholar]
- 77.Resende EA, Lara BH, Reis JD, Ferreira BP, Pereira GA, Borges MF. Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab. 2007;92:1424–9. doi: 10.1210/jc.2006-1569. [DOI] [PubMed] [Google Scholar]
- 78.Rohayem J, Nieschlag E, Kliesch S, Zitzmann M. Inhibin B, AMH, but not INSL3, IGF1 or DHEAS support differentiation between constitutional delay of growth and puberty and hypogonadotropic hypogonadism. Andrology. 2015;3:882–7. doi: 10.1111/andr.12088. [DOI] [PubMed] [Google Scholar]
- 79.Adan L, Lechevalier P, Couto-Silva AC, Boissan M, Trivin C, Brailly-Tabard S, et al. Plasma inhibin B and antimullerian hormone concentrations in boys: discriminating between congenital hypogonadotropic hypogonadism and constitutional pubertal delay. Med SciMonit. 2010;16:CR511–CR517. [PubMed] [Google Scholar]
- 80.Francou B, Bouligand J, Voican A, Amazit L, Trabado S, Fagart J, et al. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PLoS One. 2011;6:e25614. doi: 10.1371/journal.pone.0025614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lofrano-Porto A, Barra GB, Giacomini LA, Nascimento PP, Latronico AC, Casulari LA, et al. Luteinizing hormone beta mutation and hypogonadism in men and women. N Engl J Med. 2007;357:897–904. doi: 10.1056/nejmoa071999. [DOI] [PubMed] [Google Scholar]
- 82.Layman LC, Porto ALA, Xie J, da Motta LACR, da Motta LDC, Weiser W, et al. FSHβ gene mutations in a female with partial breast development and a male sibling with normal puberty and azoospermia. J Clin Endocrinol Metab. 2002;87:3702–7. doi: 10.1210/jcem.87.8.8724. [DOI] [PubMed] [Google Scholar]
- 83.Hirsch HJ, Eldar-Geva T, Bennaroch F, Pollak Y, Gross-Tsur V. Sexual dichotomy of gonadal function in Prader-Willi syndrome from early infancy through the fourth decade. Hum Reprod. 2015;30:2587–96. doi: 10.1093/humrep/dev213. [DOI] [PubMed] [Google Scholar]
- 84.Eiholzer U, l'Allemand D, Rousson V, Schlumpf M, Gasser T, Girard J, et al. Hypothalamic and gonadal components of hypogonadism in boys with prader-labhart- willi syndrome. J Clin Endocrinol Metab. 2006;91:892–8. doi: 10.1210/jc.2005-0902. [DOI] [PubMed] [Google Scholar]
- 85.Bergadá I, Andreone L, Bedecarrás P, Ropelato MG, Copelli S, Laissue P, et al. Seminiferous tubule function in delayed-onset X-linked adrenal hypoplasia congenita associated with incomplete hypogonadotrophic hypogonadism. Clin Endocrinol. 2008;68:240–6. doi: 10.1111/j.1365-2265.2007.03026.x. [DOI] [PubMed] [Google Scholar]