Abstract
Background:
Exposure to traffic-related air pollution (TRAP) has been associated with increased risks of respiratory diseases, but the biological mechanisms are not yet fully elucidated.
Objectives:
Our aim was to evaluate the respiratory responses and explore potential biological mechanisms of TRAP exposure in a randomized crossover trial.
Methods:
We conducted a randomized crossover trial in 56 healthy adults. Each participant was exposed to high- and low-TRAP exposure sessions by walking in a park and down a road with high traffic volume for 4 h in random order. Respiratory symptoms and lung function, including forced expiratory volume in the first second (), forced vital capacity (FVC), the ratio of to FVC, and maximal mid-expiratory flow (MMEF), were measured before and after each exposure session. Markers of 8-isoprostane, tumor necrosis (), and ezrin in exhaled breath condensate (EBC), and surfactant proteins D (SP-D) in serum were also measured. We used linear mixed-effects models to estimate the associations, adjusted for age, sex, body mass index, meteorological condition, and batch (only for biomarkers). Liquid chromatography–mass spectrometry was used to profile the EBC metabolome. Untargeted metabolome-wide association study (MWAS) analysis and pathway enrichment analysis using mummichog were performed to identify critical metabolomic features and pathways associated with TRAP exposure.
Results:
Participants had two to three times higher exposure to traffic-related air pollutants except for fine particulate matter while walking along the road compared with in the park. Compared with the low-TRAP exposure at the park, high-TRAP exposure at the road was associated with a higher score of respiratory symptoms [2.615 (95% CI: 0.605, 4.626), ] and relatively lower lung function indicators [ (95% CI: , ), ] for and (95% CI: , ; ) for MMEF]. Exposure to TRAP was significantly associated with changes in some, but not all, biomarkers, particularly with a (95% CI: 0.297, 0.691; ) increase for serum SP-D and a (95% CI: , ; ) decrease for EBC ezrin. Untargeted MWAS analysis revealed that elevated TRAP exposure was significantly associated with perturbations in 23 and 32 metabolic pathways under positive- and negative-ion modes, respectively. These pathways were most related to inflammatory response, oxidative stress, and energy use metabolism.
Conclusions:
This study suggests that TRAP exposure might lead to lung function impairment and respiratory symptoms. Possible underlying mechanisms include lung epithelial injury, inflammation, oxidative stress, and energy metabolism disorders. https://doi.org/10.1289/EHP11139
Introduction
Respiratory disease is a major contributor to the global burden of disease.1 Ambient air pollution, especially, has been recognized as an important risk factor for respiratory health.2–5 Traffic emission is the major sour ce of urban air pollution and exposure to traffic-related air pollution (TRAP) has been associated with various respiratory diseases worldwide.6–8 However, most existing studies on air pollution and respiratory morbidity and mortality have been observational and thus had limited ability to establish a causal relationship between exposure and outcome.9,10 Many studies have been conducted under controlled-exposure experimental settings (e.g., by using exposure chambers to create TRAP exposure contrast among the participants).11–14 However, most of these studies considered only one or two pollutants. Meanwhile, in reality, TRAP is a mixture of air pollutants and the main components include nitrogen oxides (), carbon monoxide (CO), fine and ultrafine particulate matter [PM in aerodynamic diameter () and UFP], and black carbon (BC).15–17 Two studies in London, UK, have examined respiratory and cardiovascular responses to TRAP by comparing participants’ health measures after walking down a busy road and in a traffic-free area.18,19 However, their findings may not be generalizable in other countries or regions owing to the differences in vehicle fuel composition, levels of exposure, and population characteristics.
The underlying mechanisms of respiratory effects caused by TRAP have not been fully clarified. The metabolomics technique has emerged as a powerful tool to detect molecular changes following perturbations, such as environmental exposures, comprehensively. However, few studies have fully applied this technique to examine the molecular changes with TRAP exposure.20–22 In addition, only one study has considered profiling metabolites in exhaled breath condensate (EBC) samples to explore changes in the respiratory system.23 Therefore, more evidence is warranted.
To address these knowledge gaps, we conducted a randomized, crossover study in Shanghai, China, to examine the associations of the respiratory effects associated with TRAP exposure and to explore the underlying mechanisms by analyzing the metabolome in EBC.
Methods
Study Design and Participants
We initially recruited 69 healthy, nonsmoking college students ( years of age) from the medical campus of Fudan University in Shanghai, China. Students who had no history of self-reported allergy or chronic cardiopulmonary diseases, who had lived on campus for at least 1 y, who had no secondhand smoking exposure in their main indoor environments (i.e., dormitory and office), and who had not used any medication or dietary supplements in the recent 2 months were eligible to enroll. We measured their height and weight for calculating body mass index (BMI) and collected their demographic and medical information through a questionnaire that included age, sex, smoking, history of disease (e.g., cardiorespiratory and allergic diseases, major surgeries), and medication and dietary supplement use in the recent 2 months. The questionnaire survey can be found in the Supplemental Materials, “Basic Information Questionnaire.” In addition, we conducted pulmonary function tests for all participants at enrollment to screen for those with abnormal lung function or inability in test performing. The pulmonary function test was conducted according to the recommendation of the American Thoracic Society/European Respiratory Society (ATS/ERS)24 using a portable spirometer (Jaeger MasterscreenV5.01, CareFusion). Six participants were then excluded because their lung function test results were below the normal range [i.e., the forced expiratory volume in the first second () to forced vital capacity (FVC) ratio was or the predicted was ] and another 7 participants declined to participate. Eventually, 56 participants were included in the trial.
Each participant was required to finish a high-and a low-TRAP exposure session in random orders intermitted by no less than a 14-d washout period between October and December 2019 (Figure S1). For the high-TRAP exposure session, participants were assigned to walk on the sidewalk along North Caoxi Road, which is located in urban Shanghai and has vehicles on the road per day. For the low-exposure session, the participants were led to walk in Century Park following a preassigned walking route away from traffic roadways.25 For feasibility, participants were divided randomly into 14 groups (1–3 persons per group) and each group was arranged to complete the exposure sessions on different days. In each session, participants were asked to walk at a steady pace for 15 min followed by a 30-min rest for 4 h (from 1300 to 1700 hours). To minimize noise exposure, we provided earplugs during each walking session.
To minimize the differential exposure to TRAP prior to each session, all the participants were asked to stay on campus for at least 3 d before each session. To reduce the influence of other potential confounders, all the participants were asked to provide information that might cause short-term systemic inflammation, including disease conditions, use of alcohol, medication and dietary supplements, and passive smoking, in the past 3 d prior to each exposure session by questionnaire (Supplemental Materials, “Information before each exposure session”). Only those who had not taken medication, dietary supplements, consumed alcohol, been exposed to secondhand smoke, or had symptoms of illness within the past 3 d were allowed to participate in the upcoming exposure session. To minimize possible impacts from the diet, we also provided standardized meals and water to all participants on the day of the exposure session.
The study protocol was registered at ClinicalTrials.gov (NCT04153539). The institutional review board of the School of Public Health, Fudan University, approved the study protocol (IRB#2019-07-0768). All participants provided written informed consent at enrollment.
Exposure Measures
During each exposure session, a trained staff member carried a backpack equipped with portable devices to measure real-time air pollution exposures. mass concentration was measured by MicroPEM (RTI International), which is a portable one-stage impactor equipped with an onboard micro-nephelometer. UFP number concentration was measured by NanoTracer (XP; Oxility), which records the number concentrations of particles with a size between and every 10 s using the diffusion charging method. BC concentration was measured using MicroAeth (AE51; AethLabs), which detects the transmission of light at the wavelength through the active area of a filter (referred to as the sensing channel) of aerosol collection. Nitrogen dioxide () and CO were measured by dynamic baseline electrochemical sensors based on electrochemical sensing and pair differential filter technology, respectively (Sapiens PEK Lite A1; Sapiens Environmental Technology Co. Ltd.).26 The meteorological variables, including ambient temperature and relative humidity, were also collected using a HOBO data logger (Onset Computer Corporation). The 4-h average of pollutant concentrations for each exposure session was calculated for the statistical analysis.
All devices were tested for comparability and calibrated for data quality control. We conducted side-by-side tests on devices for each air pollutant measurement under laboratory conditions to evaluate the reproducibility within units in a scenario without any major indoor pollutant sources. For each type of device, all the units were placed on a table at the center of the lab () and set to run for a continuous 24 h with a fixed data acquirement interval (i.e., 5 min for NanoTracer, 1 min for MicroPEM, 5 min for MicroAeth, and 1 min for Sapiens PEK Lite). We kept all the windows and doors closed to minimize potential influence from airflow disturbance. We conducted tests for comparability of devices three times in total, that is, before the trial (September 2019), at the middle of the trial (November 2019), and at the end of the trial (December 2019). To reduce the exposure misclassification, two units showing the best interconsistency in the comparability tests were used in the trial (Figures S2–S4).
Health Measurements
Respiratory symptoms.
We used a questionnaire adapted from the Swedish Performance Evaluation System (SPES)27 to measure the degree of respiratory symptoms. This questionnaire requires participants to use a scale of 0–5 to rate their self-perceived severity of 14 respiratory tract symptoms, including irritation of the nose, itchy nose, dry nose, burning nose, stuffy nose, running nose, itchy throat, irritation throat, swelling throat, burning throat, urge to cough, pressure on the chest, oppression to breath, and expectoration. A higher score indicates greater symptom severity, with a score of 0 referring to no symptoms and a score of 5 referring to a severe symptom. The total respiratory symptom score was then calculated by summing up the scores across the 14 symptoms. The questionnaires were completed half an hour before and immediately after each exposure session.
Lung function test.
Lung function was measured before and after each exposure session in a lab located on campus using a portable spirometer (Jaeger MasterscreenV5.01; CareFusion) under the instruction of the same trained operator. The lung function test was conducted according to the recommendation of the ATS/ERS.24 Specifically, participants were instructed to make three attempts intermitted by at least 3 min and a qualified measure should have at least two acceptable and reproducible test results from all attempts. The measures of lung function included in this study were , an indicator of airway obstruction; FVC, an indicator of airway restriction; and maximal mid-expiratory flow (MMEF), an indicator of small airway function. We also calculated the ratio of and FVC () to examine airway obstruction.
Sample collection and analysis.
EBC and peripheral blood samples were collected before and after each exposure session within 1 h in a lab after returning to the campus. All participants were required to refrain from eating or drinking for at least 2 h before the testing and specimen collection. The EBC was collected by trained staff using an exhaled breath condensate collection system (Erich Jaeger GmbH) following the ATS/ERS recommended guidelines.28 Participants were asked to rinse their mouths before sample collection to avoid saliva contamination. During the sample collection, participants were instructed to breathe into the collection system using tidal breathing to ensure that the volume of EBC was reproducible within individuals. The collection system runs at to condense water vapor in the exhaled breath. All EBC samples were stored at within 2 h after collection. Blood samples () were collected by a professional medical staff member (a clinical nurse) using the serum separator tube (BD Vacutainer SST II advance) and were processed for serum extraction immediately after collection and then stored at until analysis.
Biomarkers in the EBC and blood samples collected before and after each exposure session were quantified using an enzyme linked immunosorbent assay (ELISA) according to the operating manual provided by the manufacturers. Specifically, serum surfactant proteins D (SP-D) (DSFPD0; R&D Systems) and EBC ezrin (SEB297Hu; Cloud Clone Corp.) are indicators of lung epithelial injury, whereas EBC 8-isoprostane (516351; Cayman Chemical) and tumor necrosis () (HSTA00E; R&D Systems) are markers of airway oxidative stress and inflammation, respectively. All samples collected from the same participant were processed in the same batch. All samples were analyzed twice according to the protocol of the manufacturer and a blank well was set in each batch and all samples (including the blank and standard controls). The measured value of each well was subtracted by the average value of the blank controls. Then means of two duplicated samples were used as the final measurement results in the statistical analysis. The coefficients of variation (CVs, %) for SP-D, 8-isoprostane, ezrin, and detection were all . The limits of detection (LODs) for SP-D, 8-isoprostane, ezrin, and detection were , , , and , respectively. Half of the LOD was used to replace those measurements below the LOD. Eventually, only measurements of two samples on ezrin and were below the LOD and were replaced.
EBC samples collected after each exposure session were used for metabolomic analysis in ultra-high performance liquid chromatography–mass spectrometry (UHPLC-MS/MS) (Vanquish; Thermo Fisher Scientific; and Orbitrap MS; Thermo). The detailed description on LC-MS/MS analysis can be found elsewhere.29 In brief, LC-MS/MS analyses were performed using an UHPLC system (Vanquish; Thermo Fisher Scientific) with a UPLC BEH Amide column (, ) coupled to a Q Exactive HF-X (QE HFX) mass spectrometer (Orbitrap MS; Thermo). The QE HFX mass spectrometer was used owing to its ability to acquire MS/MS spectra in information-dependent acquisition mode in the control of the acquisition software (Xcalibur; Thermo). To validate the quality of measurements, quality control samples were generated by mixing aliquots of all samples with equal volume. The same pretreatment method was applied for quality control samples and samples to be tested. Pooled quality control samples were inserted in every several samples to assess the repeatability of the instrument. ProteoWizard was adopted to convert the raw data into the mzXML format and processed with a XCMS-based program for peak extraction. We excluded the features with missing values . Half of the minimum value was used to replace each of the missing values. All data were Pareto-scaled before statistical analysis.
Statistical Analysis
We first calculated the differences in respiratory system symptom scores, lung function measures, and concentrations of biomarkers (i.e., EBC ezrin, EBC 8-isoprostane, EBC , and serum SP-D) before and after each exposure session (i.e., adjusting for the baseline). Then we applied linear mixed-effect (LME) models to estimate the associations of TRAP exposure with changes in each of the aforementioned health measures. Specifically, TRAP exposure was first fitted as a binary indicator of exposure session (0 for low-exposure session and 1 for high-exposure session) in the model to compare the difference of changes in these measures under the two exposure scenarios. In addition, we fitted separate models with continuous variables of air pollutants (UFP, BC, , , or CO) at each exposure session (i.e., averages of pollutants concentrations over the 4-h exposure session) as the exposure of interest. For all models, we adjusted for demographic characteristics [i.e., age (continuous variable), sex (binary variable), and BMI (continuous variable)] to control for the potential heterogeneity between individuals, and meteorological conditions [i.e., temperature (continuous variable) and relative humidity (continuous variable)] to control for the potential heterogeneity between days. For biomarkers, we additionally adjusted for batch number in the fixed effect terms. A random intercept for each participant was also added into the model to account for within-participant correlations.19,30 Last, we fitted nonlinear models for each pollutant using a natural spline function, adjusting for the same set of covariates. The likelihood ratio test was used to examine for possible nonlinear exposure–response relationships, and the results suggested no statistically significant nonlinear exposure–response relationship (Table S1). All analyses were implemented in R software (version 3.4.4; R Development Core Team) using the package lme4. Effect estimates for the biomarkers were expressed as mean absolute changes with 95% confidence intervals (CIs) associated with TRAP exposure and per interquartile range (IQR) increase of air pollutants.
The untargeted metabolome-wide association study (MWAS) was conducted for EBC metabolomics. We obtained the mass-to-charge ratio (m/z), retention time, and ion intensity for each detected metabolic feature. Features that were detected in of the samples were excluded. Ion intensities of the remaining features were log-transformed for normalization and were then added into separate LME models as dependent variables to examine their associations with TRAP exposure (binary) and with each pollutant (continuous),31 adjusting for the same set of covariates. The Benjamini–Hochberg false discovery rate (FDR) was calculated to control for multiple comparisons, and an FDR of was considered statistically significant.
To identify the underlying biological pathways related to TRAP exposure, we used the mummichog pathway analysis (version 1.0.10; Python) for pathway enrichment analysis. Different from the traditional targeted metabolomic analysis strategy, the mummichog approach uses algorithms that leverage known metabolic pathways and networks to predict the function of each metabolite without identifying the metabolites a priori.32 This method has been commonly used in previous studies for predicting network activity from untargeted metabolomic analyses.31,33–35 We applied the mummichog approach to screen for the features that differ significantly between high-and low-TRAP exposure sessions or that were associated with air pollutants, under positive- and negative-ion modes, respectively. We applied Fisher’s exact test (FET) as an enrichment test of metabolic features on pathways, adjusting for type I error based on a method by Berriz et al.36 An adjusted from FET was considered statistically significant. Metabolite features associated with enriched metabolic pathways were then annotated in the human metabolome database (HMDB), the METLIN database, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.37
Results
Descriptive Statistics
As shown in Table 1, the mean (SD) of the age and BMI of participants were 23.5 y and , respectively. Among the included 56 participants, 25 were males. No participant reported alcohol, medication and dietary supplement use, or exposure to smoking during the study period. During the road exposure session (i.e., high-TRAP exposure), the concentrations of UFP, BC, , and CO were approximately two to three times higher than in the park exposure session (i.e., low-TRAP exposure), whereas concentration showed a relatively smaller difference, with only a 40% higher exposure in the road session compared with the park exposure session. Temperature and relative humidity were comparable in both sessions (Table 2). Spearman rank-correlation coefficients (r) between the five air pollutants ranged from 0.25 to 0.87 (Table S2).
Table 1.
Demographic characteristics of participants () in a randomized controlled trial of exposure to traffic-related air pollution in Shanghai, China, 2019.
| Variables | or (%) |
|---|---|
| Sex | |
| Male | 25 (44.6) |
| Female | 31 (55.4) |
| Age (y) | |
| BMI () | |
Note: BMI, body mass index; SD, standard deviation.
Table 2.
Exposure conditions in high (road)- and low (park)-traffic-related air pollution (TRAP) exposure sessions for adults participating in a randomized crossover trial in China.
| Variable | Group | Mean | SD | Min | P25 | P50 | P75 | Max |
|---|---|---|---|---|---|---|---|---|
| UFP () | Road | 33,467 | 6,678 | 24,555 | 28,651 | 31,608 | 35,564 | 49,885 |
| Park | 14,996 | 4,549 | 8,123 | 11,695 | 14,603 | 17,488 | 24,460 | |
| BC () | Road | 4 | 1 | 3 | 4 | 4 | 5 | 9 |
| Park | 2 | 0 | 1 | 1 | 1 | 2 | 3 | |
| (ppb) | Road | 44 | 9 | 23 | 39 | 45 | 49 | 59 |
| Park | 14 | 3 | 8 | 11 | 14 | 15 | 21 | |
| CO (ppb) | Road | 948 | 196 | 676 | 804 | 928 | 1,053 | 1,521 |
| Park | 333 | 156 | 41 | 234 | 296 | 450 | 654 | |
| () | Road | 27 | 19 | 11 | 17 | 22 | 26 | 98 |
| Park | 19 | 9 | 6 | 13 | 18 | 20 | 56 | |
| Temperature (°C) | Road | 22 | 4 | 12 | 20 | 22 | 24 | 26 |
| Park | 22 | 3 | 13 | 21 | 22 | 24 | 25 | |
| Relative humidity (%) | Road | 42 | 10 | 25 | 38 | 41 | 46 | 69 |
| Park | 49 | 13 | 31 | 40 | 46 | 54 | 79 |
Note: BC, black carbon; CO, carbon monoxide; IQR, interquartile range; max, maximum; min, minimum; , nitrogen dioxide; P25, 25th percentile; P50, 50th percentile; P75, 75th percentile; , fine particulate matter; SD, standard deviation; UFP, ultrafine particles.
Respiratory Symptoms in Relation to TRAP Exposure
Overall, total respiratory symptom scores before the road and park exposure sessions were similar ( for the road exposure session and for the park exposure session, respectively; Table S3). At the end of each session, the mean total symptom score increased by 3.21 for the road exposure session; the scores for dry nose, itchy throat, and urge to cough had the largest increases. However, no notable increase in total score was found for the park exposure session. Table 3 shows changes in the total score of respiratory symptoms related to TRAP and individual pollutants. Compared with changes in respiratory symptom scores before and after the park exposure session, we observed an increase of 2.615 (95% CI: 0.605, 4.626; ) in the changes in symptoms scores for the road exposure session. In addition, we found larger changes of symptom scores were associated with exposure to UFP (IQR: ; score difference per IQR increase: 1.793; 95% CI: 0.113, 3.474; ), BC (IQR: ; score per IQR increase difference: 2.917; 95% CI: 0.873, 4.960; ), and (IQR: ; score difference per IQR increase: 3.360; 95% CI: 1.252, 5.467; ) than with exposure to CO (IQR: ; score difference per IQR increase: 1.413; 95% CI: , 3.436; ) and (IQR: ; score difference per IQR increase: 0.503; 95% CI: , 1.301; ), respectively.
Table 3.
Baseline-adjusted mean changes (95% confidence intervals) in total respiratory symptom score, lung function measures, and biomarkers associated with exposure sessions (road vs. park, adults) and with per IQR increase of air pollutants in a randomized crossover trial in China.
| TRAP [park(0)/road(1)] | UFP (IQR: ) | BC (IQR: ) | (IQR: ) | CO (IQR: ) | (IQR: ) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | |
| Total symptoms score | 2.615 (0.605, 4.626) | 1.793 (0.113, 3.474) | 2.917 (0.873, 4.960) | 3.360 (1.252, 5.467) | 1.413 (, 3.436) | 0.503 (, 1.301) | ||||||
| Lung function | ||||||||||||
| FVC (L) | (, 0.009) | (, 0.037) | (, 0.054) | (, 0.036) | (, 0.062) | 0.018 (, 0.041) | ||||||
| (L) | (, ) | (, 0.017) | (, 0.023) | (, ) | (, 0.042) | 0.017 (, 0.044) | ||||||
| /FVC | (, 0.148) | (, 0.231) | (, 0.116) | (, ) | (, 0.222) | (, 0.117) | ||||||
| MMEF (L/s) | (, ) | (, 0.057) | (, 0.012) | (, ) | (, 0.053) | (, 0.066) | ||||||
| Biomarkers | ||||||||||||
| Serum SP-D (ng/mL) | 0.494 (0.297, 0.691) | 0.311 (0.126, 0.496) | 0.476 (0.273, 0.680) | 0.498 (0.280, 0.715) | 0.381 (0.164, 0.597) | 0.070 (, 0.171) | ||||||
| EBC ezrin (ng/mL) | (, ) | (, ) | (, ) | (, ) | (, ) | (, 0.008) | ||||||
| EBC 8-isoprostane (pg/mL) | 0.190 (, 0.385) | 0.171 (0.008, 0.334) | 0.191 (, 0.383) | 0.205 (0.005, 0.405) | 0.219 (0.030, 0.409) | 0.070 (, 0.151) | ||||||
| EBC (pg/mL) | 0.007 (, 0.023) | 0.004 (, 0.017) | 0.003 (, 0.018) | 0.009 (, 0.024) | 0.011 (, 0.027) | 0.003 (, 0.010) | ||||||
Note: All the linear mixed-effect models were adjusted for age, sex, body mass index, batch number (only for biomarkers), and a random intercept for each participant and the previous 4-h moving average of temperature and relative humidity. IQR were calculated based on data measured at both high-and low-exposure sessions. BC, black carbon; CI, confidence interval; CO, carbon monoxide; EBC, exhaled breath condensate; , forced expiratory volume in the first second; FVC, forced vital capacity; IQR, interquartile range; MMEF, maximal mid-expiratory flow; , nitrogen dioxide; , fine particulate matter; SP-D, surfactant proteins D; , tumor necrosis ; TRAP, traffic-related air pollution; UFP, ultrafine particles.
TRAP Exposure and Lung Function
We found no notable differences in lung function measures before the road and park exposure sessions (Table 4). Compared with those measured before the exposure sessions, we observed lower FVC, , and MMEF in our participants after the road exposure session, whereas no drastic changes were found between all lung function measures before and after the park exposure session. After adjusting for all covariates in LME models, we found TRAP exposure was associated with lower and MMEF (Table 3). For example, compared with lung function changes in the park exposure session, and MMEF decreased by (95% CI: , ; ) and (95% CI: , ; ) after the road exposure session.
Table 4.
Lung function measures and airway biomarkers () before and after each exposure session (road and park) for adults participating in a randomized crossover trial in Shanghai, China.
| Road | Park | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Lung function | ||||
| FVC (L) | ||||
| (L) | ||||
| MMEF (L/s) | ||||
| Biomarkers | ||||
| Serum SP-D (ng/mL) | ||||
| EBC ezrin (ng/mL) | ||||
| EBC 8-isoprostane (pg/mL) | ||||
| EBC (pg/mL) | ||||
Note: EBC, exhaled breath condensate; , forced expiratory volume in the first second; FVC, forced vital capacity; MMEF, maximal mid-expiratory flow; SD, standard deviation; SP-D, surfactant proteins D; , tumor necrosis .
In addition, among specific air pollutants, we found that was significantly associated with a decline in three of the four measures of lung function. For example, an IQR () increase of was associated with a (95% CI: , 0.036; ), (95% CI: , ; ), 0.878 (95% CI: , ; ), and (95% CI: , ; ) decrease in FVC, , , and MMEF, respectively. We also found most effect estimates of other air pollutants on lung function measures were negative; however, their associations were suggestive or null. For example, an IQR () increase of UFP was associated with a (95% CI: , 0.037; ), (95% CI: , 0.017; ), 0.396 (95% CI: , 0.231; ), and (95% CI: , 0.057; ) decreases in FVC, , , and MMEF, respectively.
TRAP Exposure and Airway Biomarkers
Table 3 shows the baseline-adjusted mean changes of serum SP-D, EBC ezrin, 8-isoprostane, and associated with exposure sessions and specific air pollutants. Compared with the park exposure session, we found participants had lower EBC ezrin (; 95% CI: , ; ) and higher EBC 8-isoprostane (; 95% CI: , 0.385; ), and serum SP-D (; 95% CI: 0.297, 0.691; ), respectively, after the road exposure session. Consistently, UFP, BC, , and CO exposures were all associated with higher serum SP-D (, , , and , respectively) and EBC 8-isoprostane (, , , and , respectively) and lower EBC ezrin (, , , and , respectively). However, no significant changes were found in related to the exposure sessions and specific air pollutants. In addition, the associations between biomarkers and exposure showed null.
TRAP Exposure and Airway Metabolome
In total, we detected 2,305 and 2,098 metabolite features from positive- and negative-ion modes, respectively. Among them, 936 (positive mode) and 945 (negative mode) metabolic features, respectively, were identified as differential metabolic features between the road and park exposure sessions. The mummichog pathway analysis further identified perturbations in 23 and 32 metabolic pathways based on these features under the positive- and negative-ion modes, respectively (Figure 1; Table S4). These enriched metabolic pathways were closely related to the systemic inflammatory response (e.g., arginine and proline metabolism, and leukotriene metabolism), oxidative stress (e.g., linoleate metabolism, purine metabolism, methionine and cysteine metabolism, and vitamin E metabolism), and energy metabolism [e.g., tricarboxylic acid (TCA) cycle].
Figure 1.
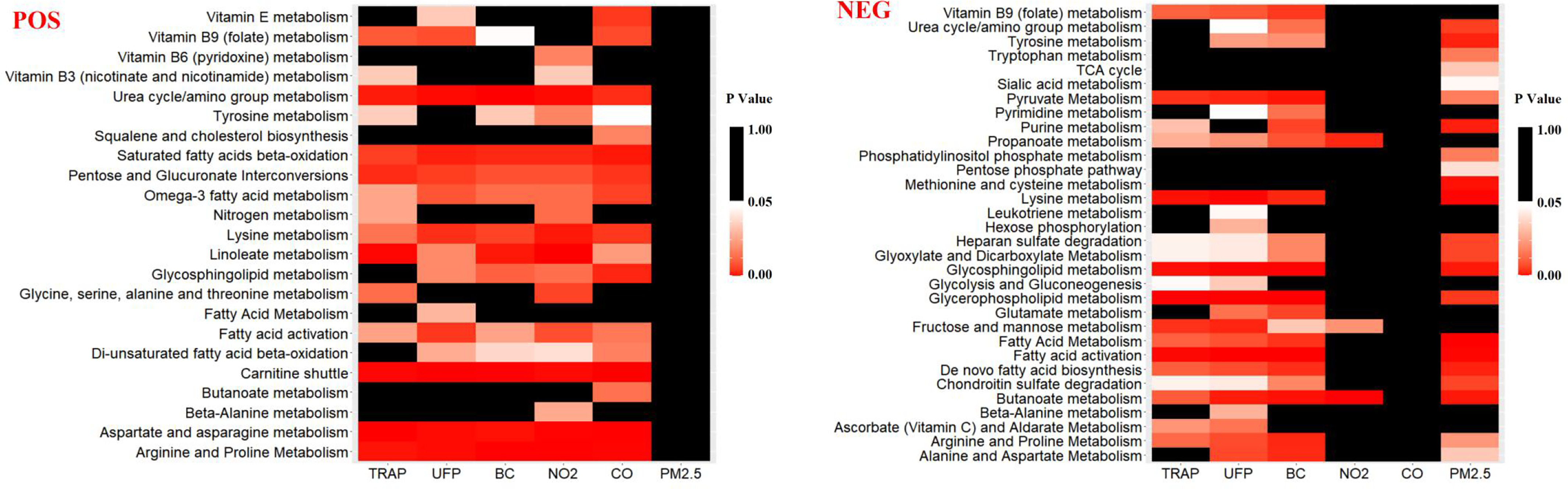
The identified metabolic pathways associated with TRAP and individual air pollutants in positive-ionization mode (POS) and negative-ionization mode (NEG) based on the untargeted metabolome-wide association study (MWAS) conducted for exhaled breath condensate (EBC) metabolomics in a randomized crossover trial in China ( adults). TRAP exposure was fitted as a binary indicator of exposure (low/high); individual pollutants were modeled as continuous variables. The linear mixed-effect models and the mummichog pathway analysis (version 1.0.10; Python) were applied for pathway enrichment analysis. Fisher’s exact test (FET) as an enrichment test of metabolic features on pathways was applied, and an adjusted from FET was considered statistically significant. -Values are shown in Table S4. Note: BC, black carbon; CO, carbon monoxide; , nitrogen dioxide; , fine particulate matter; TCA, tricarboxylic acid; TRAP, traffic-related air pollution; UFP, ultrafine particles.
Among these enriched metabolic pathways, 156 and 129 unique metabolic features were associated with TRAP air pollutants in the positive- and negative-ionization modes, respectively. Based on the enriched metabolic pathways, we further confirmed 27 of them were associated with TRAP (Table 5). Most of these features were endogenous metabolites related to inflammatory, oxidative stress, and energy metabolism. For example, we found metabolites related to inflammation and oxidative stress, including leukotriene , 13-hydroxyoctadecadienoic acid, hypoxanthine, uric acid, and l-arginine, increased after the road exposure session and with individual pollutants. Meanwhile, metabolites that were suggested to have anti-inflammation and anti-oxidative stress properties (e.g., ) were found to be reduced after the road exposure session. Moreover, metabolites related to energy metabolism (e.g., fumarate, succinate, d-glucose, lactate, pyruvate) were also found to increase after the road exposure session and with higher UFP and BC concentrations.
Table 5.
Annotated exhaled breath concentrate (EBC) metabolite features associated with high (road)- and low (park)-exposure sessions and with air pollutants in a randomized crossover study of traffic-related air pollution exposure, adults, Shanghai, China.
| m/z | Chemical identity | ESI | Pathway | Estimated associations with TRAP |
|---|---|---|---|---|
| 173.0041 | Hypoxanthine | Purine metabolism | TRAP (); UFP (/); BC (/); (/); CO (/); (/) | |
| 149.0093 | Uric acid | Purine metabolism | TRAP (); UFP (/); BC (); (/); CO (/); (/) | |
| 169.0173 | Methionine | Methionine and cysteine metabolism | TRAP (); UFP (); BC (); (/); CO (/); (/) | |
| 157.1084 | l-Arginine | Arginine and proline metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 194.1149 | Citrulline | Arginine and proline metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 104.0709 | Glutamate | Arginine and proline metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 86.0604 | 5-Oxoproline | Aspartate and asparagine metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 224.1281 | Glutathione | Glutathione metabolism; aspartate and asparagine metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 415.2809 | 11′-Carboxy--tocotrienol | Vitamin E metabolism | TRAP (/); UFP (); BC (/); (/); CO (); (/) | |
| 154.1226 | 13′-Carboxy--tocopherol | Vitamin E metabolism | TRAP (/); UFP (); BC (/); (/); CO (); (/) | |
| 152.1069 | 13′-Carboxy--tocotrienol | Vitamin E metabolism | TRAP (/); UFP (); BC (/); (/); CO (); (/) | |
| 225.0254 | l-Lysine | Lysine metabolism | TRAP (); UFP (); BC (/); (/); CO (/); (/) | |
| 148.0757 | l-Phenylalanine | Tyrosine metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 105.0427 | l-Serine | Glycine, serine, alanine, and threonine metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 90.0554 | l-Alanine | Glycine, serine, alanine, and threonine metabolism | TRAP (); UFP (); BC (); (); CO (/); (/) | |
| 194.9275 | Fumarate | TCA cycle | TRAP (); UFP (); BC (); (/); CO (/); () | |
| 117.0189 | Succinate | TCA cycle | TRAP (); UFP (); BC (); (/); CO (/); (/) | |
| 89.0238 | d-Glucose | Glycolysis and gluconeogenesis | TRAP (); UFP (); BC (); (/); CO (/); (/) | |
| 91.0215 | Lactate | Glycolysis and gluconeogenesis | TRAP (); UFP (); BC (); (/); CO (/); (/) | |
| 103.0031 | Pyruvate | Glycolysis and gluconeogenesis | TRAP (); UFP (); BC (); (/); CO (/); (/) | |
| 351.2212 | Leukotriene | Leukotriene metabolism | TRAP (/); UFP (); BC (/); (/); CO (/); (/) | |
| 337.237 | 10,11-Dihydro-leukotriene | Leukotriene metabolism | TRAP (/); UFP (); BC (/); (/); CO (/); (/) | |
| 245.226 | Linoleate | Linoleate metabolism; fatty acid activation | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 168.1131 | 13-HPODE | Linoleate metabolism | TRAP (); UFP (/); BC (/); (/); CO (); (/) | |
| 261.2209 | 13-HODE | Linoleate metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 259.2034 | 13-OxoODE | Linoleate metabolism | TRAP (); UFP (); BC (); (); CO (); (/) | |
| 301.2159 | Linoleate metabolism; fatty acid activation | TRAP (); UFP(/); BC(/); (); CO(/); (/) |
Note: For the positive-ion mode, the following adducts were considered: , , , , , , , , , , . For the negative-ion mode, the following adducts were considered: , , , , , , , , . /, An association (effect) of the metabolites related to the pollutant or TRAPs is not identified in pathway analysis; 13-HODE, 13-hydroxyoctadecadienoic acid; 13-HPODE, 13-hydroperxyoctadecadienoic acid; 13-OxoODE, 13-oxooctadecadienoic acid; BC, black carbon; Br, bromine; CO, carbon monoxide; ESI, electron spray ionization; H, hydrogen; K, potassium; Na, sodium; , nitrogen dioxide; O, oxygen; , fine particulate matter; TCA, tricarboxylic acid; TRAP, traffic-related air pollution; UFP, ultrafine particles.
Discussion
In this study, we conducted a randomized crossover trial to explore respiratory responses related to TRAP exposure among healthy adults. After comparing changes before and after the road and park exposure sessions, we found TRAP exposure was associated with higher respiratory symptom scores and lower lung function. Further, biomarkers related to lung epithelial injury, airway inflammation, and oxidative stress increased after exposure to TRAP. Metabolomics analysis in EBC samples identified metabolic signals and pathways closely related to inflammation, oxidative stress, and energy metabolism (Figure 2). We also found UFP, BC, , and CO, but not , were significantly associated with changes in respiratory health markers, and had stronger associations with changes in respiratory symptom scores and lung function measures.
Figure 2.
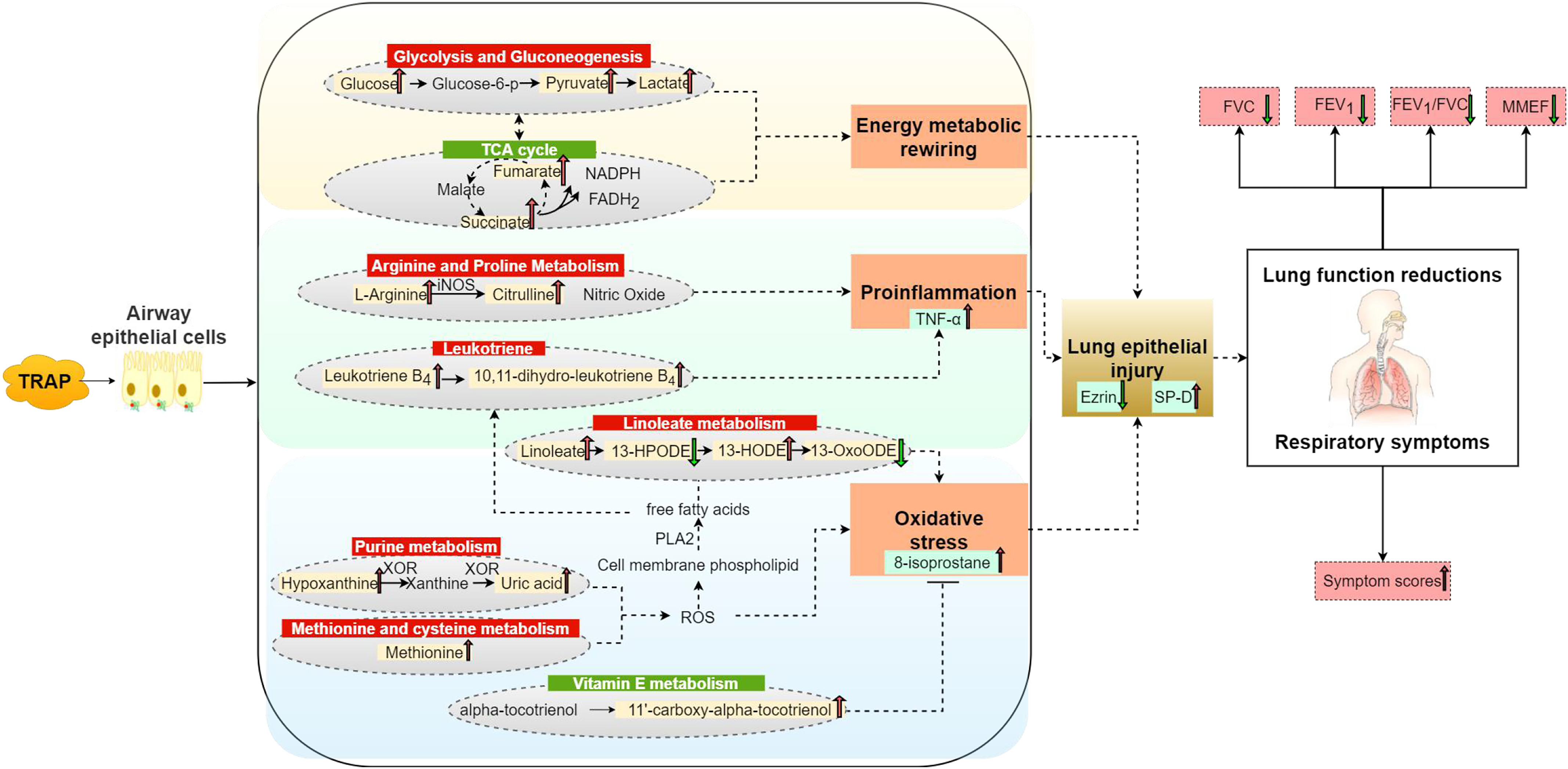
Possible mechanisms underlying the respiratory effects of TRAP exposure identified in this randomized crossover study in China ( adults). Note: 13-HODE, 13-hydroxyoctadecadienoic acid; 13-HPODE, 13-hydroperxyoctadecadienoic acid; 13-OxoODE, 13-oxooctadecadienoic acid; EBC, exhaled breath condensate; , reduced form of flavin adenine dinucleotide; , forced expiratory volume in the first second; FVC, forced vital capacity; iNOS, inducible nitric oxide synthase; MMEF, maximal mid-expiratory flow; NADPH, reduced form of nicotinamide adenine dinucleotide phosphate; p, phosphate; PLA2, phospholipase A2; ROS, reactive oxygen species; SP-D, surfactant proteins D; TCA, tricarboxylic acid; , tumor necrosis ; TRAP, traffic-related air pollution; XOR, xanthine oxidoreductase.
Respiratory Symptoms and Lung Function Changes Related to TRAP Exposure
Previous epidemiological studies have found that exposure to TRAP was associated with respiratory symptoms.38–40 However, those studies were mostly observational and focused on vulnerable populations (e.g., children, asthmatics) in Europe. Therefore, it may not be feasible to directly compare their results with our findings. A previous randomized, crossover trial in the UK assessed the effects on respiratory and cardiovascular responses of TRAP exposure among healthy elderly and elderly with chronic lung or heart disease, and it found short-term exposure to TRAP was associated with symptoms in the respiratory tract (e.g., cough, sputum, wheeze) and lower lung function.18 Consistent with our results, that UK study also illustrated that the benefits of walking exercise on lung function might be offset by TRAP exposure among adults.18
Similarly, we found a significant decrease in lung function following acute TRAP exposure. These lung function changes indicate airway obstruction (manifested by a significant decrease in and no significant change in FVC) and small airway changes (manifested by a significant decrease in MMEF). MMEF has been considered a sensitive physiological marker of small airway function decline.41 Therefore, the observed declined MMEF associated with TRAP exposure suggests TRAP exposure might be associated with early pathophysiological impairment of small airways among healthy adults.42
Potential Biological Mechanisms of TRAP-Related Respiratory Effect
We found significant changes of serum SP-D and EBC ezrin associated with TRAP exposure. Both serum SP-D and EBC ezrin are biomarkers of airway epithelial damage. SP-D regulate airway surface tension.43,44 Previous studies in humans have suggested SP-D were inversely associated with lung function.45,46 Ezrin is related to the integrity of the airway epithelial barrier by maintaining the normal morphology and intercellular adhesion of epithelial cells.47,48 We hypothesize that the increase of serum SP-D and decrease of EBC ezrin after the road exposure session suggests that TRAP exposure might compromise the alveolar epithelial integrity and impair airway epithelial lining, which can further impair lung function and lead to respiratory symptoms.
Our airway metabolomic analysis showed that TRAP exposure was associated with increased metabolites in oxidative stress pathways, including hypoxanthine, uric acid, linoleate, 13-hydroxyoctadecadienoic acid (13-HODE), and methionine. Hypoxanthine was oxidized to xanthine and uric acid by xanthine oxidase, and this process could produce reactive oxygen species (ROS).49 ROS can further activate lipid oxidation phospholipase A2, which then hydrolyzes phospholipids to polyunsaturated free fatty acids (e.g., linoleic acid).50 Linoleic acid can be converted into 13-hydroperoxyoctadecadenoic acid (13-HPODE) and 13-HODE by lipoxygenases, and the latter is a potential biomarker for oxidative stress-affecting lipids.50 In this study, we also consistently found that TRAP exposure was associated with higher levels of EBC 8-isoprostane, a biomarker of oxidative stress,51 further supporting the elevated oxidative stress associated with TRAP exposure.
In addition, our data also linked TRAP exposure with two inflammation-related pathways, the leukotriene metabolism and the arginine and proline metabolism. Consistently, we found higher levels of EBC leukotriene (a lipid molecule with pro-inflammatory properties) and a slight increase of EBC after exposure to high levels of TRAP, indicating that TRAP exposure can increase airway inflammation.52
We also observed higher EBC glucose, pyruvate, lactate, succinate, and fumarate after exposure to TRAP. These metabolites are related to energy metabolism, which is essential to cell function and survival. More specifically, the observed increases in these metabolites might suggest up-regulated energy metabolism and activated anaerobic glycolysis in airway epithelial cells. It is possible that energy metabolic rewiring is a potential pathway leading to airway epithelial injury. Consistent with our findings, a recent animal study also found an increased metabolic rearrangement from the TCA cycle to glycolysis as a manifestation of metabolic alteration in lung tissue after exposure.53
Pollutant-Specific Associations with the Outcomes
We found that concentration had a smaller difference between the road and park exposure sessions compared with the other pollutants, which is consistent with previous findings.18,54 Unlike the other pollutants, can be from both local sources and regional transportation.55 In this study, traffic is the main factor contributing to the differential levels of exposure between the road and park sessions. Therefore, when regional transportation dominates, the differences in concentrations contributed by the local sources may be masked by the background level.56
Among all the pollutants considered in the analysis, appeared to have the strongest associations with lung function, especially with decreased MMEF, suggesting may impair small airway function. Unlike the other water-soluble gaseous pollutants, hydrolyzes slowly in the airway and can reach bronchioles and alveoli and cause mucous edema in the small airways.57–59 In addition, we found BC and UFP were associated with lung function decline, which was consistent with previous findings in London.18,19 We found BC and UFP were also associated with changes of biomarkers and metabolic pathways related to oxidative stress and inflammation. Previous studies have reported similar associations of these two pollutants from traffic sources with oxidative stress and inflammation in the respiratory system.60,61 We did not observe significant associations of with any outcome measures, which is in line with prior findings based on the same study design.18,19 One possible reason for the null associations of might be that the difference in exposure levels between the road and the park groups was small.
Strengths and Limitations
The randomized crossover trial design of this study can effectively minimize the possibility of unmeasured confounding. Moreover, the combination of the subclinical indicators and the omics technique allowed us to comprehensively investigate the global molecular responses of the respiratory system related to TRAP exposure. Moreover, our metabolomic analysis was based on the EBC samples, which can reflect the local changes in the respiratory tract after exposure to TRAP.
Our study also has several limitations. First, although we controlled for multiple confounders through the randomized crossover study design,62,63 residual confounding was still possible under the complex real-life environment. For example, without blinding, participants may differentially report respiratory symptoms between these two exposure sessions. In addition, although we considered only participants who lived on the same campus for at least 1 y to reduce the influence of long-term air pollution exposure, early life air pollution exposure (e.g., exposure during childhood) may have long-term impacts on lung function. Second, all outcome measurements were conducted only once right after each exposure session. Therefore, the delayed effects of TRAP exposure on respiratory health were not considered. Finally, although the effects of the single traffic-related pollutant were examined, our ability to identify the independent effects of each pollutant was limited by the strong correlation (Spearman ) among these pollutants. Only healthy young adults were included in this study; therefore, our findings may not be generalizable to other susceptible populations with preexisting conditions.
Conclusion
In this randomized crossover study, we found short-term exposure to TRAP exposure was associated with respiratory symptoms and airway impairment. Airway biomarkers and EBC metabolomic analysis suggested TRAP exposure can lead to lung epithelial injury, airway inflammation, oxidative stress, and energy metabolism disturbance. These findings provide evidence for the adverse respiratory health effects of TRAP exposure and insights into the potential biological mechanisms.
Supplementary Material
Acknowledgments
The study was supported by the Ministry of Science and Technology of the People’s Republic of China [2016YFC0206202 (to J.C.)], the National Natural Science Foundation of China [92043301 (to H.K.), 91843302 (to H.K.), 82030103 (to H.K.), 91543114 (to J.C.), and 81502774 (to J.C.)], the Shanghai 3-y Public Health Action Plan [GWV-10.1-XK11 (to J.C.)], the Shanghai Post-Qi-Ming-Xing Plan [22YF1446000 (to X.D.)], and the National Institute of Environmental Health Sciences [P30009089 (to S.N.C.)].
References
- 1.Li X, Cao X, Guo M, Xie M, Liu X. 2020. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the Global Burden of Disease Study 2017. BMJ 368:m234, PMID: , 10.1136/bmj.m234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorpe A, Harrison RM. 2008. Sources and properties of non-exhaust particulate matter from road traffic: a review. Sci Total Environ 400(1–3):270–282, PMID: , 10.1016/j.scitotenv.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Mills IC, Atkinson RW, Kang S, Walton H, Anderson HR. 2015. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open 5(5):e006946, PMID: , 10.1136/bmjopen-2014-006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pujades-Rodríguez M, Lewis S, McKeever T, Britton J, Venn A. 2009. Effect of living close to a main road on asthma, allergy, lung function and chronic obstructive pulmonary disease. Occup Environ Med 66(10):679–684, PMID: , 10.1136/oem.2008.043885. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Li F, Gao W. 2017. Traffic-related air pollution and lung cancer: a meta-analysis. Thorac Cancer 8(5):546, PMID: , 10.1111/1759-7714.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. 2017. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int 100:1–31, PMID: , 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Laumbach RJ, Kipen HM. 2012. Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. J Allergy Clin Immunol 129(1):3–11, PMID: , 10.1016/j.jaci.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akopian AN, Fanick ER, Brooks EG. 2016. TRP channels and traffic-related environmental pollution-induced pulmonary disease. Semin Immunopathol 38(3):331–338, PMID: , 10.1007/s00281-016-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuvolone D, Della Maggiore R, Maio S, Fresco R, Baldacci S, Carrozzi L, et al. 2011. Geographical information system and environmental epidemiology: a cross-sectional spatial analysis of the effects of traffic-related air pollution on population respiratory health. Environ Health 10:12, PMID: , 10.1186/1476-069X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sears CG, Braun JM, Ryan PH, Xu Y, Werner EF, Lanphear BP, et al. 2018. The association of traffic-related air and noise pollution with maternal blood pressure and hypertensive disorders of pregnancy in the HOME study cohort. Environ Int 121(pt 1):574–581, PMID: , 10.1016/j.envint.2018.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muala A, Sehlstedt M, Bion A, Osterlund C, Bosson JA, Behndig AF, et al. 2014. Assessment of the capacity of vehicle cabin air inlet filters to reduce diesel exhaust-induced symptoms in human volunteers. Environ Health 13(1):16, PMID: , 10.1186/1476-069X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laumbach RJ, Kipen HM, Kelly-McNeil K, Zhang J, Zhang L, Lioy PJ, et al. 2011. Sickness response symptoms among healthy volunteers after controlled exposures to diesel exhaust and psychological stress. Environ Health Perspect 119(7):945–950, PMID: , 10.1289/ehp.1002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedl MA, Diaz-Sanchez D, Linn WS, Gong H Jr, Clark KW, Effros RM, et al. 2012. Allergic inflammation in the human lower respiratory tract affected by exposure to diesel exhaust. Res Rep Health Eff Inst 165:5–43, PMID: . [PubMed] [Google Scholar]
- 14.Xu Y, Barregard L, Nielsen J, Gudmundsson A, Wierzbicka A, Axmon A, et al. 2013. Effects of diesel exposure on lung function and inflammation biomarkers from airway and peripheral blood of healthy volunteers in a chamber study. Part Fibre Toxicol 10:60, PMID: , 10.1186/1743-8977-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linn WS, Shamoo DA, Anderson KR, Peng RC, Avol EL, Hackney JD. 1994. Effects of prolonged, repeated exposure to ozone, sulfuric acid, and their combination in healthy and asthmatic volunteers. Am J Respir Crit Care Med 150(2):431–440, PMID: , 10.1164/ajrccm.150.2.8049826. [DOI] [PubMed] [Google Scholar]
- 16.Andersen MHG, Frederiksen M, Saber AT, Wils RS, Fonseca AS, Koponen IK, et al. 2019. Health effects of exposure to diesel exhaust in diesel-powered trains. Part Fibre Toxicol 16(1):21, PMID: , 10.1186/s12989-019-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmingsen JG, Rissler J, Lykkesfeldt J, Sallsten G, Kristiansen J, Møller PP, et al. 2015. Controlled exposure to particulate matter from urban street air is associated with decreased vasodilation and heart rate variability in overweight and older adults. Part Fibre Toxicol 12:6, PMID: , 10.1186/s12989-015-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinharay R, Gong J, Barratt B, Ohman-Strickland P, Ernst S, Kelly FJ, et al. 2018. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants older than 60 years with chronic lung or heart disease and age-matched healthy controls: a randomised, crossover study. Lancet 391(10118):339–349, PMID: , 10.1016/S0140-6736(17)32643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, et al. 2007. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med 357(23):2348–2358, PMID: , 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 20.Ladva CN, Golan R, Liang D, Greenwald R, Walker DI, Uppal K, et al. 2018. Particulate metal exposures induce plasma metabolome changes in a commuter panel study. PLoS One 13(9):e0203468, PMID: , 10.1371/journal.pone.0203468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, et al. 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environ Int 120:145–154, PMID: , 10.1016/j.envint.2018.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Veldhoven K, Kiss A, Keski-Rahkonen P, Robinot N, Scalbert A, Cullinan P, et al. 2019. Impact of short-term traffic-related air pollution on the metabolome—results from two metabolome-wide experimental studies. Environ Int 123:124–131, PMID: , 10.1016/j.envint.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladva CN, Golan R, Greenwald R, Yu T, Sarnat SE, Flanders WD, et al. 2017. Metabolomic profiles of plasma, exhaled breath condensate, and saliva are correlated with potential for air toxics detection. J Breath Res 12(1):016008, PMID: , 10.1088/1752-7163/aa863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. 2005. Standardisation of spirometry. Eur Respir J 26(2):319–338, PMID: , 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Du X, Zhang Q, Jiang Y, Li H, Zhu X, Zhang Y, et al. 2022. Dynamic molecular choreography induced by traffic exposure: a randomized, crossover trial using multi-omics profiling. J Hazard Mater 424(pt A):127359, PMID: , 10.1016/j.jhazmat.2021.127359. [DOI] [PubMed] [Google Scholar]
- 26.Zong H, Brimblecombe P, Sun L, Wei P, Ho KF, Zhang Q, et al. 2021. Reducing the influence of environmental factors on performance of a diffusion-based personal exposure kit. Sensors (Basel) 21(14):4637, PMID: , 10.3390/s21144637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller JU, Bruckner T, Triebig G. 2013. Exposure study to examine chemosensory effects of formaldehyde on hyposensitive and hypersensitive males. Int Arch Occup Environ Health 86(1):107–117, PMID: , 10.1007/s00420-012-0745-9. [DOI] [PubMed] [Google Scholar]
- 28.Horváth I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, et al. 2005. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 26(3):523–548, PMID: , 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 29.Gu M, Pan H, Yuan Y, Zhou X, Chen L, Wang X, et al. 2022. Sera metabolomics characterization of patients at different stages in Wuhan identifies critical biomarkers of COVID-19. Front Cell Infect Microbiol 12:882661, PMID: , 10.3389/fcimb.2022.882661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J, Lin Z, Chen R, Wang C, Yang C, Cai J, et al. 2017. Cardiovascular benefits of wearing particulate-filtering respirators: a randomized crossover trial. Environ Health Perspect 125(2):175–180, PMID: , 10.1289/EHP73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang D, Ladva CN, Golan R, Yu T, Walker DI, Sarnat SE, et al. 2019. Perturbations of the arginine metabolome following exposures to traffic-related air pollution in a panel of commuters with and without asthma. Environ Int 127:503–513, PMID: , 10.1016/j.envint.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SZ, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, et al. 2013. Predicting network activity from high throughput metabolomics. PLoS Comput Biol 9(7):e1003123, PMID: , 10.1371/journal.pcbi.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellissimo MP, Cai Q, Ziegler TR, Liu KH, Tran PH, Vos MB, et al. 2019. Plasma high-resolution metabolomics differentiates adults with normal weight obesity from lean individuals. Obesity (Silver Spring) 27(11):1729–1737, PMID: , 10.1002/oby.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell SL, Uppal K, Williamson SM, Liu K, Burgess LG, Tran V, et al. 2018. The carnitine shuttle pathway is altered in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 59(12):4978–4985, PMID: , 10.1167/iovs.18-25137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Yang T, Walker DI, Thomas DC, Qiu C, Chatzi L, et al. 2020. Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environ Int 145:106091, PMID: , 10.1016/j.envint.2020.106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berriz GF, King OD, Bryant B, Sander C, Roth FP. 2003. Characterizing gene sets with FuncAssociate. Bioinformatics 19(18):2502–2504, PMID: , 10.1093/bioinformatics/btg363. [DOI] [PubMed] [Google Scholar]
- 37.Uppal K, Walker DI, Liu K, Li S, Go YM, Jones DP. 2016. Computational metabolomics: a framework for the million metabolome. Chem Res Toxicol 29(12):1956–1975, PMID: , 10.1021/acs.chemrestox.6b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Migliore E, Berti G, Galassi C, Pearce N, Forastiere F, Calabrese R, et al. 2009. Respiratory symptoms in children living near busy roads and their relationship to vehicular traffic: results of an Italian multicenter study (SIDRIA 2). Environ Health 8:27, PMID: , 10.1186/1476-069X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranzi A, Porta D, Badaloni C, Cesaroni G, Lauriola P, Davoli M, et al. 2014. Exposure to air pollution and respiratory symptoms during the first 7 years of life in an Italian birth cohort. Occup Environ Med 71(6):430–436, PMID: , 10.1136/oemed-2013-101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penttinen P, Timonen KL, Tiittanen P, Mirme A, Ruuskanen J, Pekkanen J. 2001. Ultrafine particles in urban air and respiratory health among adult asthmatics. Eur Respir J 17(3):428–435, PMID: , 10.1183/09031936.01.17304280. [DOI] [PubMed] [Google Scholar]
- 41.Stockley JA, Ismail AM, Hughes SM, Edgar R, Stockley RA, Sapey E. 2017. Maximal mid-expiratory flow detects early lung disease in α1-antitrypsin deficiency. Eur Respir J 49(3):1602055, PMID: , 10.1183/13993003.02055-2016. [DOI] [PubMed] [Google Scholar]
- 42.Havet A, Hulo S, Cuny D, Riant M, Occelli F, Cherot-Kornobis N, et al. 2020. Residential exposure to outdoor air pollution and adult lung function, with focus on small airway obstruction. Environ Res 183:109161, PMID: , 10.1016/j.envres.2020.109161. [DOI] [PubMed] [Google Scholar]
- 43.Reid KB. 1998. Interactions of surfactant protein D with pathogens, allergens and phagocytes. Biochim Biophys Acta 1408(2–3):290–295, PMID: , 10.1016/s0925-4439(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 44.Mackay RMA, Grainge CL, Lau LC, Barber C, Clark HW, Howarth PH. 2016. Airway surfactant protein D deficiency in adults with severe asthma. Chest 149(5):1165–1172, PMID: , 10.1016/j.chest.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benfante A, Battaglia S, Scichilone N. 2016. Serum surfactant protein D as a marker of asthma severity. Chest 150(2):473–474, PMID: , 10.1016/j.chest.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 46.Benfante A, Battaglia S, Principe S, Di Mitri C, Paternó A, Spatafora M, et al. 2016. Asthmatics with high levels of serum surfactant protein D have more severe disease. Eur Respir J 47(6):1864–1867, PMID: , 10.1183/13993003.02142-2015. [DOI] [PubMed] [Google Scholar]
- 47.Fehon RG, McClatchey AI, Bretscher A. 2010. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11(4):276–287, PMID: , 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia M, Yan X, Jiang X, Wu Y, Xu J, Meng Y, et al. 2019. Ezrin, a membrane cytoskeleton cross-linker protein, as a marker of epithelial damage in asthma. Am J Respir Crit Care Med 199(4):496–507, PMID: , 10.1164/rccm.201802-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida S, Kurajoh M, Fukumoto S, Murase T, Nakamura T, Yoshida H, et al. 2020. Association of plasma xanthine oxidoreductase activity with blood pressure affected by oxidative stress level: MedCity21 health examination registry. Sci Rep 10(1):4437, PMID: , 10.1038/s41598-020-61463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, et al. 2013. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 154(1):213–227, PMID: , 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CJ, Yang NH, Liou SH, Lee HL. 2010. Fast quantification of the exhaled breath condensate of oxidative stress 8-iso-prostaglandin F2α using on-line solid-phase extraction coupled with liquid chromatography/electrospray ionization mass spectrometry. Talanta 82(4):1434–1438, PMID: , 10.1016/j.talanta.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. 1980. Leukotriene-B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 286(5770):264–265, PMID: , 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 53.Jin X, Su H, Ding G, Sun Z, Li Z. 2019. Exposure to ambient fine particles causes abnormal energy metabolism and ATP decrease in lung tissues. Chemosphere 224:29–38, PMID: , 10.1016/j.chemosphere.2019.02.116. [DOI] [PubMed] [Google Scholar]
- 54.Karner AA, Eisinger DS, Niemeier DA. 2010. Near-roadway air quality: synthesizing the findings from real-world data. Environ Sci Technol 44(14):5334–5344, PMID: , 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang K, Zhou L, Fu Q, Yan L, Morawska L, Jayaratne R, et al. 2020. Sources and vertical distribution of PM2.5 over Shanghai during the winter of 2017. Sci Total Environ 706:135683, PMID: , 10.1016/j.scitotenv.2019.135683. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y, Huang L, Li J, Ying Q, Zhang H, Liu X, et al. 2018. Sources of particulate matter in China: insights from source apportionment studies published in 1987–2017. Environ Int 115:343–357, PMID: , 10.1016/j.envint.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Zhang ZL, Wang J, Lu WJ. 2018. Exposure to nitrogen dioxide and chronic obstructive pulmonary disease (COPD) in adults: a systematic review and meta-analysis. Environ Sci Pollut Res Int 25(15):15133–15145, PMID: , 10.1007/s11356-018-1629-7. [DOI] [PubMed] [Google Scholar]
- 58.Ji X, Han M, Yun Y, Li G, Sang N. 2015. Acute nitrogen dioxide (NO2) exposure enhances airway inflammation via modulating Th1/Th2 differentiation and activating JAK-STAT pathway. Chemosphere 120:722–728, PMID: , 10.1016/j.chemosphere.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 59.Han M, Ji XT, Li GK, Sang N. 2017. NO2 inhalation enhances asthma susceptibility in a rat model. Environ Sci Pollut Res Int 24(36):27843–27854, PMID: , 10.1007/s11356-017-0402-7. [DOI] [PubMed] [Google Scholar]
- 60.De Prins S, Dons E, Van Poppel M, Int Panis L, Van de Mieroop E, Nelen V, et al. 2014. Airway oxidative stress and inflammation markers in exhaled breath from children are linked with exposure to black carbon. Environ Int 73:440–446, PMID: , 10.1016/j.envint.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Traboulsi H, Guerrina N, Iu M, Maysinger D, Ariya P, Baglole CJ. 2017. Inhaled pollutants: the molecular scene behind respiratory and systemic diseases associated with ultrafine particulate matter. Int J Mol Sci 18(2):243, PMID: , 10.3390/ijms18020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim CY, In J. 2019. Randomization in clinical studies. Korean J Anesthesiol 72(3):221–232, PMID: , 10.4097/kja.19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhide A, Shah PS, Acharya G. 2018. A simplified guide to randomized controlled trials. Acta Obstet Gynecol Scand 97(4):380–387, PMID: , 10.1111/aogs.13309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


