Abstract
Background:
Diagnosing ruptured micro-arteriovenous malformation (AVM) could be difficult using digital subtraction angiography (DSA) in the acute stage, and a repeat DSA is recommended in DSA-negative cases. Arterial spin labeling (ASL) is a useful noninvasive tool for detecting AVM, but the efficacy of a repeat ASL for DSA and ASL-negative ruptured micro-AVM in the acute stage is unclear. Here, we report a case of ruptured micro-AVM that was not detected in the acute stage by ASL but in the chronic stage by ASL.
Case Description:
A 43-year-old man developed right upper-extremity paralysis, and computed tomography (CT) revealed a left frontal lobe hemorrhage. Magnetic resonance imaging, including ASL, CT angiography, and DSA, showed no abnormal findings associated with hemorrhage in the acute stage. The second ASL 93 days after the hemorrhage showed a high signal on the cortical vein of the left frontal lobe and superior sagittal sinus, and subsequent DSA detected a micro-AVM in the left precentral gyrus.
Conclusion:
Repeat ASL is less invasive and useful for detecting micro-AVMs which showed no findings on ASL and DSA in the acute stage.
Keywords: Arterial blood, Arterial spin labeling, Digital subtraction angiography, Hematoma, Micro-arteriovenous malformation

INTRODUCTION
Digital subtraction angiography (DSA) is the gold standard for the definitive diagnosis of arteriovenous malformations (AVM). However, some cases of ruptured AVM with hematoma do not show any findings on DSA in the acute stage.[6,9] Repeat DSA is recommended in DSA-negative cases,[1,10] but there is no consensus regarding the timing and frequency. Arterial spin labeling (ASL) is a magnetic resonance imaging (MRI) technique that can evaluate cerebral blood flow less invasively using magnetically labeled protons in arterial blood as endogenous tracers. ASL has been reported as a useful screening tool for diagnosing cerebral ischemia, epilepsy, hyperperfusion, and arteriovenous shunt (AVS).[2-5,11] In addition, one study previously reported the efficacy of ASL in detecting ruptured micro-AVM.[8] In the case, a high ASL signal was observed in the nidus, and it was thought to reflect pooled blood within the nidus, which was observed by DSA. Here, we report a case of micro-AVM identified by a repeat ASL for the first time in the chronic stage.
CASE REPORT
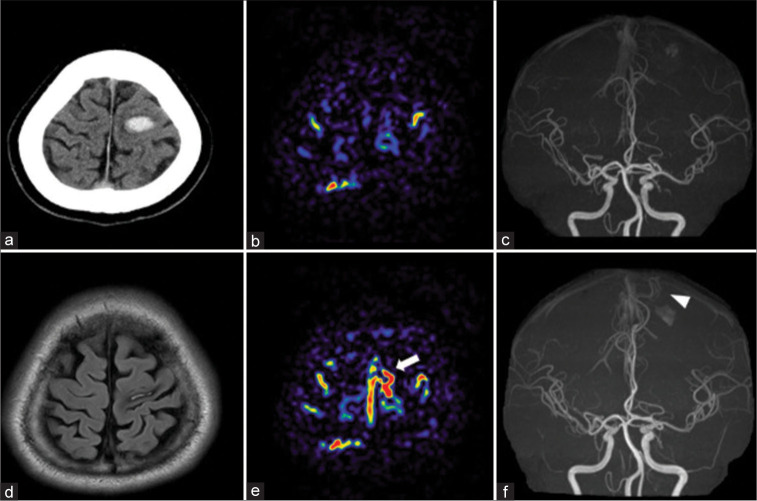
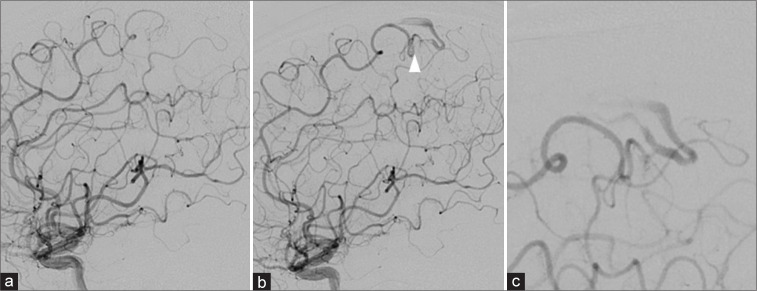
A 43-year-old male with no history of hypertension, hyperlipidemia, or diabetes mellitus was admitted to our hospital with sudden onset of the right upper-extremity paralysis. On admission, computed tomography (CT) revealed left frontal lobe hemorrhage [Figure 1a]. There were no remarkable findings suggesting abnormal vessels that could cause hemorrhage on three-dimensional CT angiography on admission. We conducted DSA the day after onset, but no abnormal findings were observed. MRI (3T, Ingenia 5.7, PHILIPS, USA), including ASL (post-labeling delay: 1200 ms) [Figure 1b] and magnetic resonance angiography (MRA) [Figure 1c] conducted 3 days after onset did not show abnormal vascular structures. Although slight upper-extremity paralysis persisted, activities of daily living were performed independently. We conducted a second MRI 93 days after onset. Fluid-attenuated inversion recovery revealed shrinkage of the hemorrhage [Figure 1d]. The ASL showed a high focal signal on the cortical vein in the left frontal lobe and superior sagittal sinus (SSS) [Figure 1e], and MRA revealed a thicker left posterior internal frontal artery adjacent to the hematoma [Figure 1f], which was not seen in the first ASL and MRA. Highly suspecting AVM, we performed a second DSA 98 days after the onset and detected micro-AVM in the left frontal lobe, which was not observed in the initial DSA [Figures 2a-c]. The micro-AVM was located in the left precentral gyrus and was composed of a 6 mm nidus with a single feeding artery from the left posterior internal frontal artery and a single draining vein arising from the posterior side of the nidus and flowing toward the SSS through the frontal cortical vein where the ASL showed a high signal. We scheduled a stereotactic radiation therapy because the AVM was in the precentral gyrus. This patient provided written informed consent.
Figure 1:
(a) Computed tomography (CT) on admission. Magnetic resonance imaging (b and c) 3 days and (d-f) 93 days after onset (a) CT: Hematoma in the left primary motor cortex. (b) Arterial spin labeling (ASL): No obvious high signal around the hematoma. (c) Magnetic resonance angiography (MRA): No abnormal vessel around the hematoma (d) Flow-attenuated inverted recovery: Shrinkage of hematoma. (e) ASL: High focal signal on the convexity of the left frontal lobe and suprasagittal sinus (white arrow), which was not seen in previous ASL. (f) MRA showing a newly depicted vessel (white arrowhead) flowing in the direction of the hematoma.
Figure 2:
Digital subtraction angiography (DSA). (a) 1 day after onset (b) 93 days after onset (a) abnormal vessel and early venous filling were not observed. (b) Micro-arteriovenous malformation (AVM) (white arrowhead) was observed, which was not seen on DSA 5 days after onset. (c) Enlarged micro-AVM image. The micro-AVM comprised a 6 mm nidus with a single feeding of a branch of the left anterior parietal artery. A draining vein arose from the posterior side of the nidus and flowed toward the superior sagittal sinus through the cortex vein on the frontal lobe.
DISCUSSION
In ASL, arterial blood is magnetically labeled at the cervical segment, and brain images are acquired following a short delay to allow the labeled arterial blood to flow through the cerebral arteries and into the capillary bed.[12,14] Under normal conditions, labeled blood is not detected within the venous structures, as the capillary transit time is longer than the T1 delay time of the labeled arterial spins.[12,14] In the presence of an AVS, such as an AVM or dural arteriovenous fistula (DAVF), the transit time of the labeled blood is shortened due to the lack of a capillary bed. Consequently, ASL signals can be detected within the draining system, suggesting a diagnosis of intracranial AVS.[7,13] This venous signal on ASL has been reportedly useful for diagnosing DAVF or small AVM, with a diagnostic sensitivity as high as 78%.[9] In the present case, no findings were observed in acute-stage ASL. This was consistent with acute-stage DSA, which showed no evidence of AVS. However, in the chronic stage, a high ASL signal was observed in the cortical vein and SSS for the first time, and the high ASL signal was believed to indicate an AVS, corroborated by chronic stage DSA.
According to the previous reports, acute-stage DSA may not reveal a vascular malformation in some cases of ruptured AVM with hematoma.[6,9] Several mechanisms, such as slow blood flow within the nidus, intralesional thrombosis, post-hemorrhagic vascular spasm, or compression of the nidus by the hematoma, have been suggested as factors for the absence of AVM visualization in the acute stage.[6,9] In the present case, only chronic-stage ASL and DSA detected micro-AVMs. This could be because shrinkage of the hematoma over time affected the revascularization of the micro-AVM in the chronic stage. Angiography should be repeated in patients with intracranial hemorrhage without a specific cause and whose initial angiography is negative or unclear about AVS.[1,10] However, DSA has invasive aspects, including radiation exposure, catheter use, and injection of contrast media. Furthermore, the appropriate timing and frequency of additional DSA are still unclear; hence, less-invasive screening tools are needed. In the present case, chronic-stage ASL demonstrated a high signal on drainage systems, which was not seen in the acute stage, and subsequent DSA detected micro-AVM. Repeat ASL is expected to be a useful screening tool for determining the timing of repeat DSA because of its less invasive nature.
CONCLUSION
Ruptured micro-AVMs might be misdiagnosed during acute-stage examinations. Repeat ASL is less invasive and useful for detecting micro-AVMs which showed no findings on ASL and DSA in the acute stage.
Footnotes
How to cite this article: Kochi R, Suzuki Y, Yamazaki H, Aikawa T, Endo H, Tominaga T. Efficacy of repeat arterial spin labeling for angiogram-negative ruptured micro-arteriovenous malformation: A case report. Surg Neurol Int 2023;14:119.
Contributor Information
Ryuzaburo Kochi, Email: ryuzaburo0618@hotmail.co.jp.
Yasuhiro Suzuki, Email: yasuhiro@nsg.med.tohoku.ac.jp.
Hiroshi Yamazaki, Email: yamazaki.hiroshi@twmu.ac.jp.
Takashi Aikawa, Email: aikawa.takashi.0@gmail.com.
Hidenori Endo, Email: hideendo@gmail.com.
Teiji Tominaga, Email: tomi@nsg.med.tohoku.ac.jp.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Alen JF, Lagares A, Paredes I, Campollo J, Navia P, Ramos A, et al. Cerebral microarteriovenous malformations: A series of 28 cases. J Neurosurg. 2013;119:594–602. doi: 10.3171/2013.4.JNS121740. [DOI] [PubMed] [Google Scholar]
- 2.Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, Part 1: Technique and artifacts. AJNR Am J Neuroradiol. 2008;29:1228–34. doi: 10.3174/ajnr.A1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, Part 2: Hypoperfusion patterns. AJNR Am J Neuroradiol. 2008;29:1235–41. doi: 10.3174/ajnr.A1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, Part 3: Hyperperfusion patterns. AJNR Am J Neuroradiol. 2008;29:1428–35. doi: 10.3174/ajnr.A1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Detre JA, Alsop DC, Vives LR, Maccotta L, Teener JW, Raps EC. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology. 1998;50:633–41. doi: 10.1212/wnl.50.3.633. [DOI] [PubMed] [Google Scholar]
- 6.Hino A, Fujimoto M, Yamaki T, Iwamoto Y, Katsumori T. Value of repeat angiography in patients with spontaneous subcortical hemorrhage. Stroke. 1998;29:2517–21. doi: 10.1161/01.str.29.12.2517. [DOI] [PubMed] [Google Scholar]
- 7.Hodel J, Leclerc X, Kalsoum E, Zuber M, Tamazyan R, Benadjaoud MA, et al. Intracranial arteriovenous shunting: Detection with arterial spin-labeling and susceptibility-weighted imaging combined. AJNR Am J Neuroradiol. 2017;38:71–6. doi: 10.3174/ajnr.A4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochi R, Endo H, Uchida H, Kawaguchi T, Omodaka S, Matsumoto Y, et al. Efficacy of arterial spin labeling for detection of the ruptured micro-arteriovenous malformation: Illustrative cases. J Neurosurg Case Lessons. 2022;3:CASE21597. doi: 10.3171/CASE21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le TT, Fischbein NJ, Andre JB, Wijman C, Rosenberg J, Zaharchuk G. Identification of venous signal on arterial spin labeling improves diagnosis of dural arteriovenous fistulas and small arteriovenous malformations. AJNR Am J Neuroradiol. 2012;33:61–8. doi: 10.3174/ajnr.A2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogilvy CS, Heros RC, Ojemann RG, New PF. Angiographically occult arteriovenous malformations. J Neurosurg. 1988;69:350–5. doi: 10.3171/jns.1988.69.3.0350. [DOI] [PubMed] [Google Scholar]
- 11.Shimogawa T, Morioka T, Sayama T, Haga S, Kanazawa Y, Murao K. The initial use of arterial spin labeling perfusion and diffusion-weighted magnetic resonance images in the diagnosis of nonconvulsive partial status epileptics. Epilepsy Res. 2017;129:162–73. doi: 10.1016/j.eplepsyres.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: Clinical applications in the brain. J Magn Reson Imaging. 2015;41:1165–80. doi: 10.1002/jmri.24751. [DOI] [PubMed] [Google Scholar]
- 13.Wolf RL, Wang J, Detre JA, Zager EL, Hurst RW. Arteriovenous shunt visualization in arteriovenous malformations with arterial spin-labeling MR imaging. AJNR Am J Neuroradiol. 2008;29:681–7. doi: 10.3174/ajnr.A0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaharchuk G. Arterial spin-labeled perfusion imaging in acute ischemic stroke. Stroke. 2014;45:1202–7. doi: 10.1161/STROKEAHA.113.003612. [DOI] [PMC free article] [PubMed] [Google Scholar]