Abstract
Background:
Tumors of the fourth ventricle are exceedingly rare; however, such lesions are formidable due to the severe postoperative neurological complications (pNCs) which often occur. The adoption of the telovelar approach over the transvermian was created to supposedly mitigate the pNCs; however, there is a lack of sufficient data supporting this theory.
Methods:
Records from six hospitals were reviewed for patients surgically treated for a single tumor within the 4th ventricle from 2016 to 2022. The pNCs which had 10 or more occurrences among the patients were individually assessed as the dependent variable in a binary logistic regression model against covariates which included the surgical approach.
Results:
This study of 67 patients confirms no significant differences in risk for pNCs between the transvermian and telovelar approach. Rather, multivariate analysis identified neurophysiological monitoring (IONM) as a protective factor for postoperative speech and swallowing defects (odds ratio [OR]: 0.076, 95% confidence interval [CI] 0.011–0.525). Furthermore, intraoperative external ventricular drainage (EVD) was a protective factor for postoperative gait and focal motor defects (OR: 0.075, 95% CI 0.009–0.648) and for postoperative hydrocephalus (OR: 0.020, 95% CI 0.002–0.233). A univariate meta-analysis pooling the present study’s patients and an additional 304 patients from the three additional studies in the literature confirms no significant differences in risk between the transvermian and telovelar approach for pNCs.
Conclusion:
Intraoperative adjuncts including IONM and EVD may play a significant role in the postoperative outcome. Despite the present study’s sample size being a major limitation, the findings may provide great value to neurosurgeons given the scarcity of the current literature.
Keywords: Complications, Fourth ventricle, Telovelar, Transvermian, Tumor

INTRODUCTION
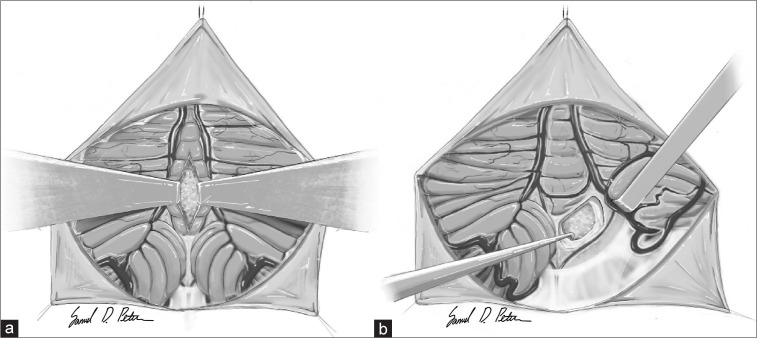
Tumors of the fourth ventricle are exceedingly rare; however, such lesions are considered formidable and pose a significant challenge to most neurosurgeons. Surrounded by vital structures of the brainstem, the available options to surgically approach such lesions are limited, while the risk of complications is high. The present literature shows that the prevalence of postoperative neurological complications (pNCs) following surgical treatment is fairly high. These include cerebellar mutism syndrome (CMS), gait disturbances, cranial nerve defects, and visual impairment as some of the most common and their prevalence rates are listed as follows: 20.5%,[5,8,13,17,20,22] 30.2%,[9,14,20] 21.2%,[9,14,20] and 29%.[9] The traditional surgical approach used for over a century to gain access to the fourth ventricle is the transvermian approach, which involves splitting the inferior half of the cerebellar vermis and retracting the halves laterally [Figure 1a]. In 1992, Matsushima et al. proposed an alternative approach that gave access to the fourth ventricle without harming the vermis through an inferior-superior trajectory through the cerebellomedullary fissure [Figure 1b].[13] The proposed “transcerebellomedullary fissure approach,” now known as the telovelar, was theorized to reduce the risk of several pNCs including CMS which has been strongly associated with vermis splitting.[6,15] In spite of the telovelar approach being less invasive, the transvermian is still commonly used among surgeons today likely due to its lesser technical demand from the surgeon.[20]
Figure 1:
A basic illustration of the midline approach types. (a) The transvermian approach: a midline incision of the inferior half of the cerebellar vermis and retracting the two halves exposing a tumor in the fourth ventricle. (b) The (unilateral) telovelar approach: lateral retraction of the right cerebellar tonsil and opening the tela choroidea exposing a tumor in the fourth ventricle.
The current literature is scarce regarding the risks and benefits of utilizing the telovelar over the transvermian approach and to the best of our knowledge, only three studies exist assessing the two approach types against postoperative complications.[9,14,20] Out of the three studies, only Ferguson et al. found that the telovelar approach significantly reduces the risk of various pNCs however, the transvermian approach still remains widely used. Thus, the authors designed a multicenter study on the matter in hopes to resolve the current controversy. Whether or not a significant difference in risk for pNCs is identified between the approach types, the findings from this study may contribute greatly to the future development of an official guideline for treating fourth ventricle tumors (FVTs) which is needed.
MATERIALS AND METHODS
Data extraction
This study was registered under local research and development protocols, and ethical approval was granted from the institutional review board. Patients diagnosed with a FVT who underwent surgical resection between January 2016 and September 2022 at 6 neurosurgical centers (Gdańsk, Poland; Olsztyn, Poland; Wrocław, Poland; Szczecin, Poland; and 2 in Łódź, Poland) were included in this study. Any patients with additional intracranial tumors located elsewhere were excluded from the study. For each patient, demographic data, risk factors, reasons for treatment, clinical presentation, and tumor characteristics on preoperative imaging were collected. Data regarding the surgical approach were obtained from detailed operative reports. The selection of surgical approach was based on surgeon preference due to the lack of an official guideline and the extent of resection was determined from the review of the postoperative magnetic resonance imaging (MRI) and computed tomography (CT) scans. Gross-total resection was defined as complete tumor resection with no evidence of residual tumor on postoperative MRI or CT. Postoperative neurological outcomes were reviewed using in-patient hospital records and outpatient clinic notes. Both new and worsening deficits were counted as complications. Postoperative neurological function was assessed before discharge.
Search strategy selection criteria
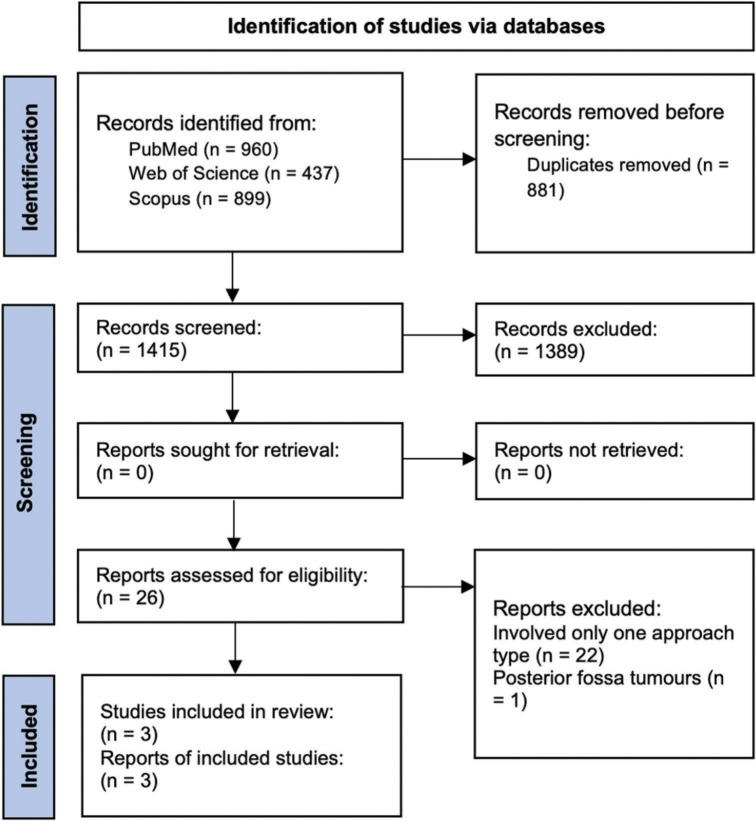
To conduct a meta-analysis, the screening process was performed according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses. PubMed, Web of Science, and Scopus databases were used to retrieve studies from inception to September 2022, without language limits. The following keywords were used in all three databases: (fourth_ventricle* OR fourth_ventricular) AND (tumor* OR tumor*) AND (transvermian OR telovelar OR transcerebellomedullary_fissure OR approach OR treatment). The search was performed independently by two authors (Samuel D. Pettersson and Eduardo Orrego-Gonzalez), and another author (Rafael A. Vega) arbitrated any disagreements on inclusion or exclusion of the studies.
The first phase of screening included assessing the article titles and abstracts for three requirements: written in the English language, involving patients with fourth ventricle tumors, and reporting the telovelar or transvermian approaches. The studies that passed the first screening were reassessed in a second screening phase. The studies were required to provide extractable data. Any studies that involved fewer than ten patients and/or consisted of patients who underwent only one of the approach types were excluded from the study.
Statistical analysis
Given that Ferguson et al.[9] remain as the only study confirming a significant difference between the telovelar and transvermian approaches, several variables of interest for the present study were chosen based on their study to assess the replicability of their univariate and multivariate findings. All intra- and postoperative complications reported in 10 or more patients were individually inputted into a univariate binary logistic regression model as the dependent variable. Patient demographics, tumor characteristics on preoperative diagnostic imaging, and surgical factors were selected as the predictor variables. To identify the variables independently associated with the postoperative complication, all variables with P < 0.25 from the univariate analysis were inputted into a multivariable binary logistic regression model to calculate multivariate-derived odds ratios (ORs).[11] P < 0.05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics version 28.0.1.1 (IBM Corp.).
Regarding the meta-analysis, the approach used was treated as a dichotomous variable and was pooled into an overriding OR and 95% confidence interval (CI) to identify risk factors for each pNCs that were reported at least twice in the literature. If a study failed to provide standard deviations, the value was calculated using standard errors, CIs, t-values, or P-values that relate to the differences between means in two groups.[1] The quality of each extractable study fulfilling the selection criteria was assessed using the modified Newcastle-Ottawa Scale (NOS). A maximum number of 2 points could be given within the comparability category while in the remaining ones, a maximum of 1. A total score of (1) ≥7 indicated high quality, (2) 6–4 moderate quality, and (3) ≤3 low quality. The quality assessment was performed independently by two authors (S.D.P and E.O.) and in the case of any disagreements, the concerned study was discussed and a final decision on the quality rating was made by author R.V. Random-effects models were used and the heterogeneity of the overall OR and MD was calculated using the I2 statistic. Publication bias was assessed by visual inspection of funnel plot asymmetry. P < 0.05 was considered statistically significant. All analyses were performed using Review Manager version 5.4 (Cochrane IMS).
RESULTS
A total of 79 patients adhered to the selection criteria. Out of the 79, a total of 12 were excluded as these patients underwent a non-midline approach (a trans-cerebellar approach) due to the tumor having invaded up to the surface of the cerebellar hemisphere. Thus, 67 patients were included in the study. About 47.8% of the included patients were female and 44.8% were pediatrics (<18-years-old) [Table 1]. The overall mean age of the patients was 26.6 ± 22.2 years. Regarding preoperative symptoms, headaches (50.7%), dizziness (41.8%), nausea and vomiting (38.8%), gait difficulties (23.9%), and visual changes (9.0%) were the most common. Only four patients had cranial nerve deficits at presentation.
Table 1:
Patient demographics, preoperative symptomology, and surgical factors.
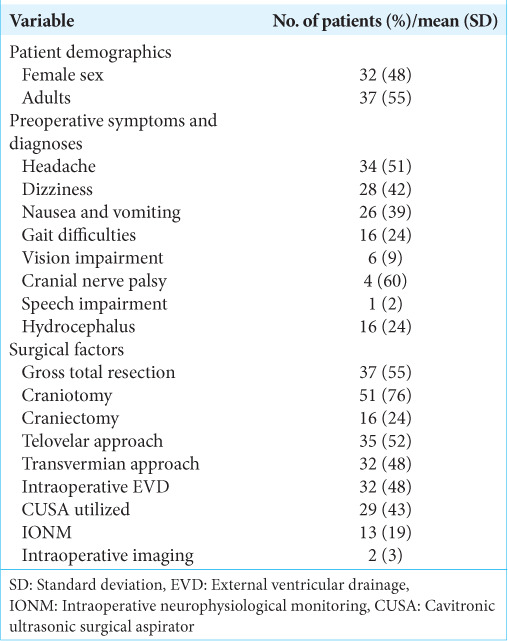
All surgical procedures began with exposing the posterior fossa through a craniotomy (76.1%) or craniectomy (23.9%), following the telovelar (52.2%) or transvermian approach (47.8%) to expose the fourth ventricle. An external ventricular drain was placed prior to the operation in almost half the patients (47.8%) and intraoperative neurophysiological monitoring (IONM) was performed in 19.4% of the procedures. Despite gross total resection being the goal for each patient, such a result was only achieved in 55.2% of the patients.
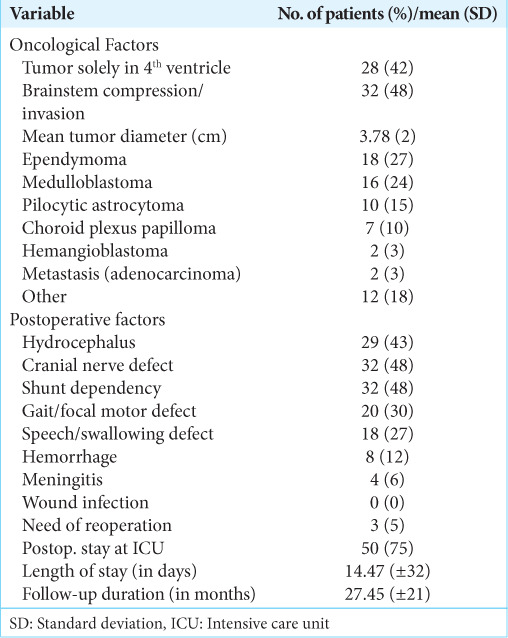
The tumor type was reported postoperative with the most common being medulloblastoma (23.9%), ependymoma (26.9%), pilocytic astrocytoma (14.9%), and choroid plexus papilloma (10.4%) [Table 2]. The mean maximal tumor diameter was 3.78 ± 1.49 cm. Common postoperative complications included hydrocephalus (43.3%), cranial nerve defects (47.7%), gait/focal motor defects (29.9%), and speech/swallowing defects (26.9%). The mean length of stay was 14.47 ± 32.20 days and 74.6% of patients were admitted to an intensive care unit. The follow-up period among each patient varied greatly, thus the mean and standard deviation was 27.45 ± 20.45 months.
Table 2:
Tumor histology and postoperative morbidity in 67 patients with fourth ventricle tumors.
Risk factors for postoperative neurological complications
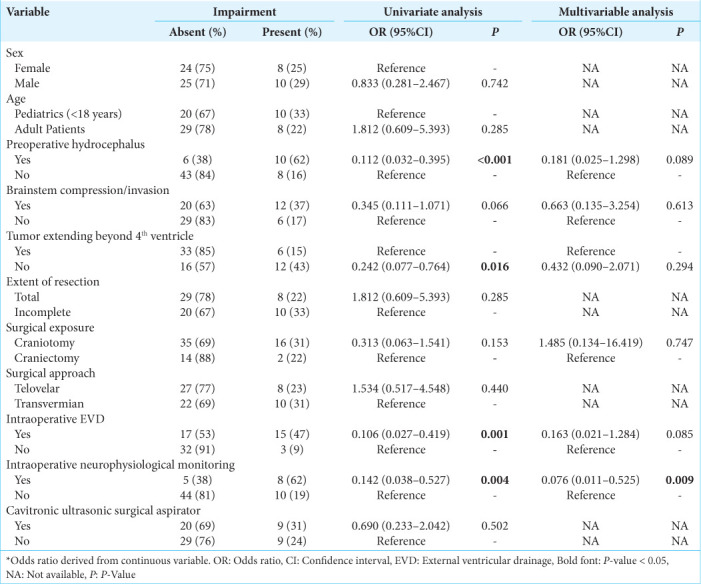
Four pNCs had 10 or more occurrences which allowed them to be inputted into regression analysis. For postoperative cranial nerve defects, neither the univariate nor multivariate analysis yielded no statistically significant predictors [Supplementary Table 1]. Regarding postoperative speech/swallowing defects, univariate analysis confirmed preoperative hydrocephalus, tumor extending beyond the fourth ventricle, complete resection, intraoperative external ventricular drainage (EVD), and IONM as significant predictors [Table 3]. When accounting for these factors including brainstem compression and surgical exposure which surpassed below the threshold of P = 0.25 in univariate analysis, multivariate analysis confirmed IONM as an independent predictor (OR: 0.076, 95% CI 0.011–0.525; P = 0.009) [Table 3].
Table 3:
Univariate and multivariable binary logistic regression assessing risk factors for postoperative speech/swallowing defects.
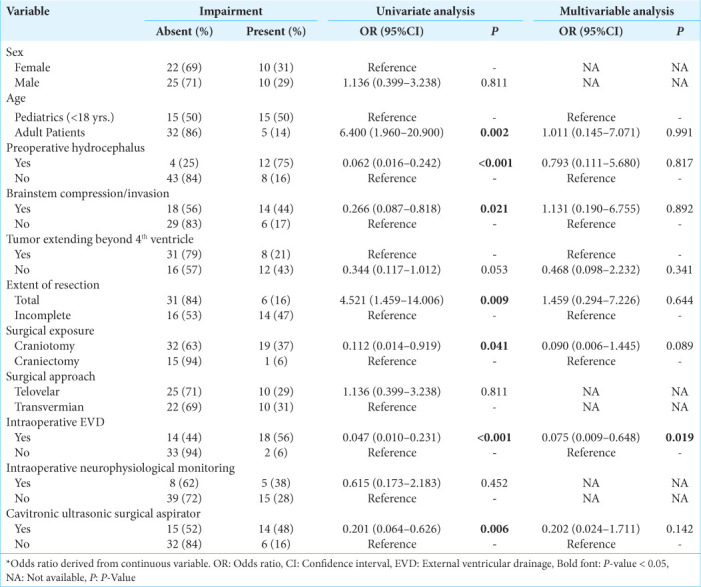
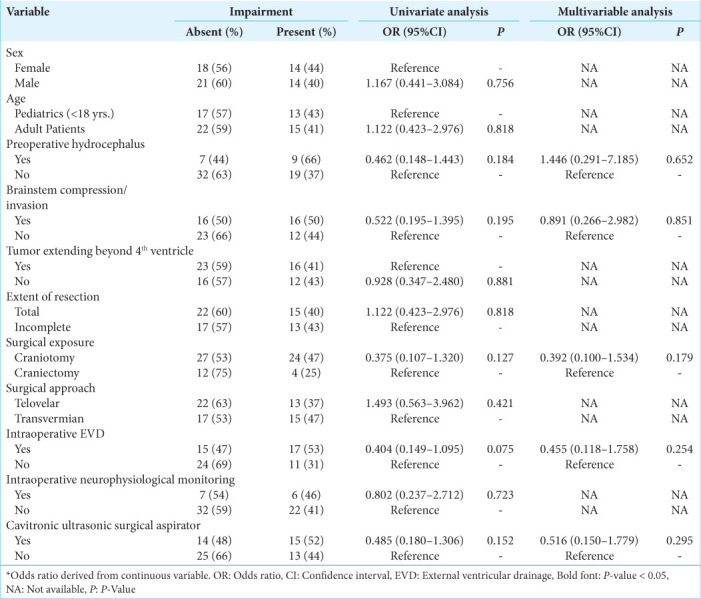
For postoperative gait/focal motor defects, univariate analysis confirmed adult age, preoperative hydrocephalus, brainstem compression, complete resection, surgical exposure, intraoperative EVD, and using a cavitronic ultrasonic surgical aspirator (CUSA) as significant predictors [Table 4]. When accounting for these factors including a tumor extending beyond the fourth ventricle, multivariate analysis confirmed intraoperative EVD as an independent predictor (OR: 0.075, 95% CI 0.009–0.648; P = 0.019) [Table 4].
Table 4:
Univariate and multivariable binary logistic regression assessing risk factors for postoperative gait/focal motor defect.
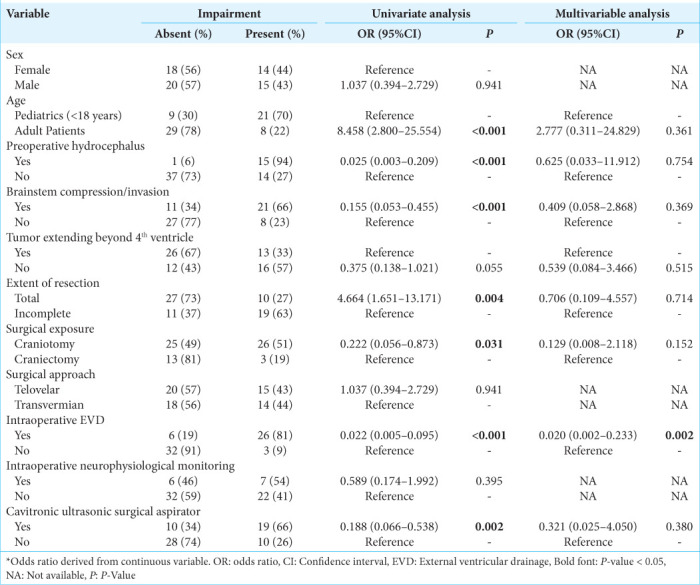
For postoperative hydrocephalus, univariate analysis confirmed adult age, preoperative hydrocephalus, brainstem compression, complete resection, surgical exposure, intraoperative EVD, and using a CUSA as significant predictors [Table 5]. When accounting for these factors including tumors extending beyond the fourth ventricle, multivariate analysis confirmed intraoperative EVD as an independent predictor (OR: 0.020, 95% CI 0.002–0.233; P = 0.002) [Table 5].
Table 5:
Univariate and multivariable binary logistic regression assessing risk factors for postoperative hydrocephalus.
Meta-analysis
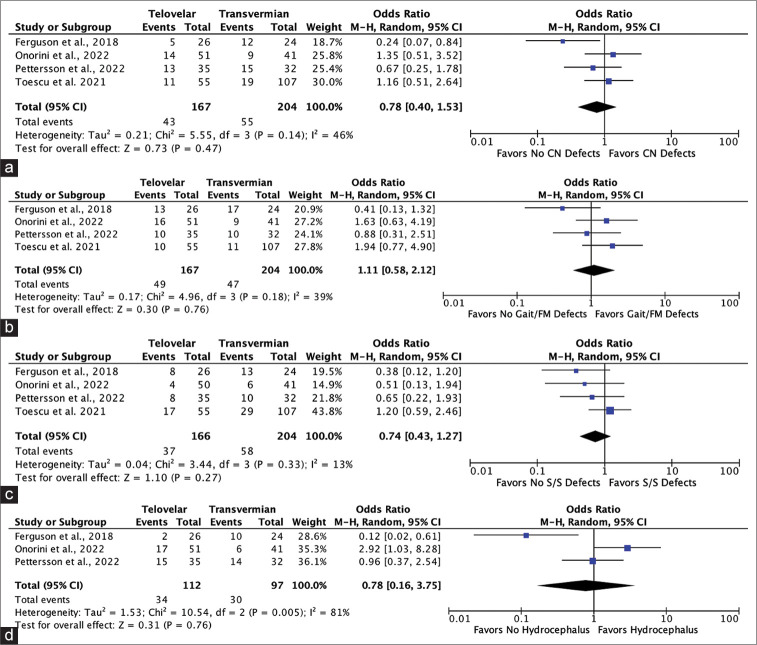
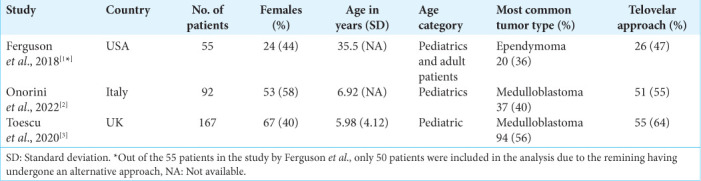
A total of 1415 unduplicated records were identified. Titles and abstracts were assessed for relevance and 1389 records were removed. Out of the remaining 26 records, 22 involved only one surgical approach type, and 1 did not involve solely fourth ventricle tumors [Supplementary Figure 1]. A total of three studies from the literature contributing a maximum of 304 patients were included in this meta-analysis as well as the 67 patients from the present study. All were retrospective cohorts with two rated as high quality and one as moderate [Supplementary Table 2]. Baseline characteristics of the three studies are shown in Supplementary Table 3. Our pooled random effects analysis on the various postoperative complications reported in the literature confirms no significant differences between the midline approach types and neurological complications [Figures 2a-d].
Figure 2:
Forest plots showing no significant risk between utilizing the telovelar over the transvermian for (a) postoperative cranial nerve defects, (b) gait/focal motor defects, (c) postoperative speech/ swallowing defects, or (d) postoperative hydrocephalus following fourth ventricle tumor resection. CN: Cranial nerve, FM: Focal motor, S/S: Speech/swallowing, CI: Confidence interval, M-H: Mantel-haenszel, P: P-value, df: degrees of freedom, Z: Cohen’s D effect size.
DISCUSSION
The optimal way to approach tumors of the fourth ventricle is controversial. Furthermore, given the rarity of these tumors, the available studies on methods of management are scarce which has resulted in the overall treatment of such tumors being heavily opinion-based in regard to the various adjuncts one can utilize including neurophysiologic monitoring and intraoperative imaging. The telovelar approach has been shown to become the more popular choice over the past decades from our experiences and from the results from Toescu et al.[20] However, the literature lacks evidence that postoperative outcomes are superior when utilizing the less invasive method. Both approach types offer excellent exposure of the fourth ventricular floor, however, the transvermian approach provides a slightly greater working angle in the sagittal plane and thus, a better exposure of the midline superior half of the roof of the fourth ventricle including the fastigium. The main disadvantage is the limited lateral exposure unless a part of the vermis or the tonsils is removed. On the other hand, the telovelar approach offers great access to the lateral recess including the foramen of Luschka without the need to remove any neural tissue.[7,19] However, the limited working angle makes the approach technically challenging due to the superior medullary velum providing a narrow angle to the fourth ventricle. One can increase the working angle, but a C1 laminectomy is required to do so, which can possibly increase postoperative morbidity.
The telovelar and transvermian approaches offer their own unique advantages to different regions of the fourth ventricle. Thus, not all fourth ventricle tumors are ideal candidates for the telovelar approach when compared to the transvermian, and vice versa. The increasing trend shown by Toescu et al.[20] in the adoption of the telovelar over the transvermian is questionable as it is driven by a lack of scientific backing. A possible cause of the trend may be due to the fact that given the incredibly scarce articles on treating tumors of the fourth ventricle; neurosurgeons may be incorporating the results from the more abundant studies assessing posterior fossa tumors into their decision-making process for the surgical approach. Among the posterior fossa studies, vermis incision has been shown to be a strong risk factor for specifically postoperative CMS (pCMS).[15] However, surgeons must be aware that vermis incision as a significant risk factor for pCMS from the posterior fossa studies cannot be interpreted as the telovelar being a significant protective factor, since among posterior fossa tumors, very few are found within the fourth ventricle. A recent review article assessing risk factors for pCMS from studies involving posterior fossa tumors had made the erroneous interpretation after reporting their forest plot which compared vermis incision against no incision, to rather a vermis incision against the telovelar approach.[4] Intuitively, invading neural tissue should not be conducted if avoidable. However, no evidence exists showing that incising specifically the inferior half of the cerebellar vermis is independently associated with any pNCs other than by Ferguson et al.[9] The purpose of the present study was to further provide more reliable data to neurosurgeons in order to allow decision-making to be based on datasets involving fourth ventricle tumors only. Our pooled analysis which includes 372 patients harboring tumors of the fourth ventricle further strengthens the neurosurgical community’s confidence that a difference between the two approach types for pNCs is likely non-significant.
Methods of mitigating the risk for pNCs
Despite no significant difference in risk between utilizing the telovelar over the transvermian approach, the postoperative complication rates remain concerningly high. Thus, it is critical to discuss the additional factors identified by the present study which were shown to independently mitigate the risks for pNCs as such a discussion currently does not exist in the literature.
IONM
The use of IONM is an opinion-based decision from the surgeon and thus, each clinic varies in the frequency of the adjunct’s incorporation into the surgery. Choosing to use IONM will require a longer operating room preparation time and adds additional challenges for the surgeon and the anesthesia team. Factors such as maintaining the body core temperature above 36.5°C for sufficient amplitudes from motor evoked potentials, keeping the electrodes fixed to their structures throughout the procedure, and having to use an intravenous anesthetic rather than an inhaled are some of the many added challenges. Therefore, this is likely the reason why only 33% of the patients from Ferguson et al.[9] and 19.4% of our patients had IONM. Our multivariate analysis identified that IONM substantially reduces the risk for speech/swallowing defects (OR: 0.076; P = 0.009) which does not come as a surprise. Speech/swallowing are controlled by the vagus and glossopharyngeal nerves which can be monitored by placing electrodes onto the vocal cords, trapezius muscles, and sternocleidomastoid muscles. The nuclei of the vagus and glossopharyngeal nerves reside at the floor of the fourth ventricle making the nerves at great risk for permanent damage during the resection of fourth ventricle tumors. At our institutions, complete resection is always the end-goal. However, IONM can play a critical role in identifying whether a greater resection margin is safety possible or would rather lead to neurological damage. Thus, our findings suggest that the use of IONM should be greatly considered before operating on future patients with tumors of the fourth ventricle.
EVD
Performing EVD before surgery is controversial. Older studies from the 70’s and 80’s found that preoperative EVD was overall positive as it was shown to be associated with the lower mortality rates, less deterioration, and immediate resolution of papilledema when compared to no EVD.[2,16] However, the recent literature has raised a concern on EVD in general as shunt infection has been now well reported which ranges a prevalence of 1.2–19%.[3] Another reason why preoperative EVD is controversial is due to the fact that preoperative hydrocephalus has been shown to resolve on its own among 60–90% of pediatric and 96% of adult patients after the resection of a posterior fossa tumor.[12,14,18] Therefore, preoperative EVD is not often justified by many surgeons. However, some patients will have persistent hydrocephalus postoperatively and studies investigating predictors for identifying such patients do exist. For pediatrics, the Canadian Preoperative Prediction Rule for Hydrocephalus (CPPRH) is a scoring system for pediatrics that can be used to identify children who are at a great risk for developing persistent hydrocephalus.[10] For adult patients, Won et al. developed a similar grading system in 2017.[21] According to our multivariate analysis identifying pre/intraoperative EVD as a protective factor for postoperative gait and focal motor defects (OR: 0.075; P = 0.019) and for postoperative hydrocephalus (OR: 0.020; P = 0.002), we recommend surgeons to utilize the CPPRH and Won et al.’s[21] risk scoring systems to help with identifying patients who are ideal candidates for the preoperative drainage to mitigate the risks for pNCs.
Limitations
The present study has several limitations. Aside from being retrospective in nature, the sample size is small given the rarity of fourth ventricle tumors. Therefore, having the room to control the patients who had any of the pNCs before surgery to increase the quality of our results could not be done without sacrificing the ability to analyze risk factors for the few pNCs which had lower occurrences. Furthermore, pediatrics and adult patients were mixed in our analysis and performing subgroup analyses for the two age groups would have been ideal to yield stronger results. However, the limited sample size prevented the ability to perform such an analysis. It is also worth to point out that brainstem compression/ invasion, although non-significant, yields an OR indicating the variable as a protective factor for two of the pNCs in multivariate analysis. This, however, does not make intuitive sense and may suggest that a confounder could be affecting our analysis. To improve on the risks of bias in the present study, we encourage future investigators with a larger cohort of patients to perform a propensity score analysis to reduce selection bias as it is the optimal method to carry out such a study. A minimum sample size of 200 patients would be required. Regarding our multivariate analysis, three independent protective factors for various pNCs were identified, however, given their wide CIs, we advise specialists to interpret the results with caution as the regression models may be overfitted. Overall, we encourage future investigators to improve upon the present study by accounting for its limitations, and by assessing the replicability of the significant findings.
CONCLUSION
The present study confirms no significant differences in risks for pNCs between the telovelar and transvermian approaches to tumors of the fourth ventricle. Rather, the intraoperative adjuncts including neurophysiological monitoring and EVD may play a significant role in the postoperative outcome. Since both the telovelar and transvermian approach offer their own unique advantages to tumors situated in various locations of the fourth ventricle, our findings suggest that surgeons should use the surgical approach which offers the greatest access to the specific target in the fourth ventricle, as forcing one’s entry into regions of the ventricle which are not easily accessible with one approach when compared to the latter may rather risk damaging the adjacent structures, and possibly, increase the risk for neurological complications.
Footnotes
How to cite this article: Pettersson SD, Jabbar R, Popławska M, Och A, Orrego-Gonzalez E, Klepinowski T, et al. Telovelar versus transvermian approach to tumors of the fourth ventricle and their impact on postoperative neurological complications: A multicenter study. Surg Neurol Int 2023;14:124.
Contributor Information
Samuel D. Pettersson, Email: samueldpettersson@gumed.edu.pl.
Redwan Jabbar, Email: rredwanbakal@gmail.com.
Mirosława Popławska, Email: poplawska.em@gmail.com.
Aleksander Och, Email: aleksoch7@gmail.com.
Eduardo Orrego-Gonzalez, Email: eorregog@bidmc.harvard.edu.
Tomasz Klepinowski, Email: tomasz.klepinowski@pum.edu.pl.
Michał Krakowiak, Email: michalkrakowiak@gumed.edu.pl.
Leszek Sagan, Email: leszekm.sagan@gmail.com.
Maciej Radek, Email: maciej.radek@umed.lodz.pl.
Krzysztof Zakrzewski, Email: krzysztof.zakrzewski@iczmp.edu.pl.
Emilia Nowoslawska, Email: enowos@poczta.onet.pl.
Katarzyna Kwiecien, Email: kkwiecien92@gmail.com.
Paulina Skrzypkowska, Email: paulinawika2259@gmail.com.
Tomasz Szmuda, Email: tomasz.szmuda@gumed.edu.pl.
Grzegorz Miękisiak, Email: gmiekisiak@gmail.com.
Rafael A. Vega, Email: rvega@bidmc.harvard.edu.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY FIGURE
Supplementary Figure 1:
PRISMA flowchart of the scientific literature search and study selection. Data added to the PRISMA template (from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71) under the terms of the Creative Commons Attribution License. n: number of references
SUPPLEMENTARY TABLES
Supplementary Table 1:
Univariate and multivariate binary logistic regression assessing risk factors for postoperative cranial nerve defects among the 67 patients.
Supplementary Table 2:
The included studies and their quality rating using the Newcastle -Ottawa Scale.
Supplementary Table 3:
Baseline characteristics of studies collected from the literature and included in meta-analysis.
REFERENCES
- 1.7.7.3.2 Obtaining Standard Deviations from Standard Errors and Confidence Intervals for Group Means. Available from: https://www.handbook-5-1.cochrane.org/chapter_7/7_7_3_2_obtaining_standard_deviations_from_standard_errors_and.htm [Last accessed on 2022 Sep 25]
- 2.Albright L, Reigel DH. Management of hydrocephalus secondary to posterior fossa tumors. J Neurosurg. 1977;46:52–5. doi: 10.3171/jns.1977.46.1.0052. [DOI] [PubMed] [Google Scholar]
- 3.Anania P, Battaglini D, Balestrino A, D’Andrea A, Prior A, Ceraudo M, et al. The role of external ventricular drainage for the management of posterior cranial fossa tumours: A systematic review. Neurosurg Rev. 2020;44:1243–53. doi: 10.1007/s10143-020-01325-z. [DOI] [PubMed] [Google Scholar]
- 4.Ashida R, Nazar N, Edwards R, Teo M. Cerebellar Mutism syndrome: An overview of the pathophysiology in relation to the cerebrocerebellar anatomy, risk factors, potential treatments, and outcomes. World Neurosurg. 2021;153:63–74. doi: 10.1016/j.wneu.2021.06.065. [DOI] [PubMed] [Google Scholar]
- 5.Atallah A, Rady MR, Kamal HM, El-Mansy N, Alsawy MF, Hegazy A, et al. Telovelar approach to pediatric fourth ventricle tumors: Feasibility and outcome. Turk Neurosurg. 2019;29:497–505. doi: 10.5137/1019-5149.JTN.24078-18.3. [DOI] [PubMed] [Google Scholar]
- 6.Cobourn K, Marayati F, Tsering D, Ayers O, Myseros JS, Magge SN, et al. Cerebellar Mutism syndrome: Current approaches to minimize risk for CMS. Childs Nerv Syst. 2020;36:1171–9. doi: 10.1007/s00381-019-04240-x. [DOI] [PubMed] [Google Scholar]
- 7.Deshmukh VR, Figueiredo EG, Deshmukh P, Crawford NR, Preul MC, Spetzler RF. Quantification and comparison of telovelar and transvermian approaches to the fourth ventricle. Neurosurgery. 2006;58(4 Suppl 2):ONS-202–6. doi: 10.1227/01.NEU.0000207373.26614.BF. discussion ONS-206-7. [DOI] [PubMed] [Google Scholar]
- 8.Eissa EM. The role of the telovelar approach in fourth ventricular surgery: A new perspective. Turk Neurosurg. 2018;28:523–9. doi: 10.5137/1019-5149.JTN.21209-17.1. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson SD, Levine NB, Suki D, Tsung AJ, Lang FF, Sawaya R, et al. The surgical treatment of tumors of the fourth ventricle: A single-institution experience. J Neurosurg. 2017;128:339–51. doi: 10.3171/2016.11.JNS161167. [DOI] [PubMed] [Google Scholar]
- 10.Foreman P, McClugage S, 3rd, Naftel R, Griessenauer CJ, Ditty BJ, Agee BS, et al. Validation and modification of a predictive model of postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr. 2013;12:220–6. doi: 10.3171/2013.5.PEDS1371. [DOI] [PubMed] [Google Scholar]
- 11.Hosmer DW, Lemeshow S, Sturdivant RX. 3rd ed. United States: Wiley; 2013. Applied Logistic Regression; pp. 1–510. [Google Scholar]
- 12.Marx S, Reinfelder M, Matthes M, Schroeder HW, Baldauf J. Frequency and treatment of hydrocephalus prior to and after posterior fossa tumor surgery in adult patients. Acta Neurochi (Wien) 2018;160:1063–71. doi: 10.1007/s00701-018-3496-x. [DOI] [PubMed] [Google Scholar]
- 13.Matsushima T, Fukui M, Inoue T, Natori Y, Baba T, Fujii K. Microsurgical and magnetic resonance imaging anatomy of the cerebello-medullary fissure and its application during fourth ventricle surgery. Neurosurgery. 1992;30:325–30. doi: 10.1227/00006123-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Onorini N, Spennato P, Orlando V, Savoia F, Calì C, Russo C, et al. The clinical and prognostic impact of the choice of surgical approach to fourth ventricular tumors in a single-center, single-surgeon cohort of 92 consecutive pediatric patients. Front Oncol. 2022;12:821738. doi: 10.3389/fonc.2022.821738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettersson SD, Kitlinski M, Miekisiak G, Ali S, Krakowiak M, Szmuda T. Risk factors for postoperative cerebellar Mutism syndrome in pediatric patients: A systematic review and meta-analysis. J Neurosurg Pediatr. 2021;29:467–75. doi: 10.3171/2021.11.PEDS21445. [DOI] [PubMed] [Google Scholar]
- 16.Raimondi AJ, Tomita T. Hydrocephalus and infratentorial tumors: Incidence, clinical picture, and treatment. J Neurosurg. 1981;55:174–82. doi: 10.3171/jns.1981.55.2.0174. [DOI] [PubMed] [Google Scholar]
- 17.Rajesh BJ, Rao BR, Menon G, Abraham M, Easwer HV, Nair S. Telovelar approach: Technical issues for large fourth ventricle tumors. Childs Nerv Syst. 2007;23:555–8. doi: 10.1007/s00381-006-0295-0. [DOI] [PubMed] [Google Scholar]
- 18.Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C. Endoscopic third ventriculostomy: The best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Childs Nerv Syst. 2008;24:1405–12. doi: 10.1007/s00381-008-0699-0. [DOI] [PubMed] [Google Scholar]
- 19.Tanriover N, Ulm AJ, Rhoton AL, Jr, Yasuda A. Comparison of the transvermian and telovelar approaches to the fourth ventricle. J Neurosurg. 2004;101:484–98. doi: 10.3171/jns.2004.101.3.0484. [DOI] [PubMed] [Google Scholar]
- 20.Toescu SM, Samarth G, Horsfall HL, Issitt R, Margetts B, Phipps KP, et al. Fourth ventricle tumors in children: Complications and influence of surgical approach. J Neurosurg Pediatr. 2020;27:52–61. doi: 10.3171/2020.6.PEDS2089. [DOI] [PubMed] [Google Scholar]
- 21.Won SY, Gessler F, Dubinski D, Eibach M, Behmanesh B, Herrmann E, et al. A novel grading system for the prediction of the need for cerebrospinal fluid drainage following posterior fossa tumor surgery. J Neurosurg. 2019;132:296–305. doi: 10.3171/2018.8.JNS181005. [DOI] [PubMed] [Google Scholar]
- 22.Zaheer SN, Wood M. Experiences with the telovelar approach to fourth ventricular tumors in children. Pediatr Neurosurg. 2010;46:340–3. doi: 10.1159/000321539. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Ferguson SD, Levine NB, Suki D, Tsung AJ, Lang FF, Sawaya R, et al. The surgical treatment of tumors of the fourth ventricle: A single-institution experience. J Neurosurg. 2017;128:339–51. doi: 10.3171/2016.11.JNS161167. [DOI] [PubMed] [Google Scholar]
- 2.Onorini N, Spennato P, Orlando V, Savoia F, Calì C, Russo C, et al. The clinical and prognostic impact of the choice of surgical approach to fourth ventricular tumors in a single-center, single-surgeon cohort of 92 consecutive pediatric patients. Front Oncol. 2022;12:821738. doi: 10.3389/fonc.2022.821738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toescu SM, Samarth G, Horsfall HL, Issitt R, Margetts B, Phipps KP, et al. Fourth ventricle tumors in children: Complications and influence of surgical approach. J Neurosurg Pediatr. 2020;27:52–61. doi: 10.3171/2020.6.PEDS2089. [DOI] [PubMed] [Google Scholar]