Abstract
Since 1985 microsporidia have been recognized as a cause of emerging infections in humans, mainly in immunocompromised human immunodeficiency virus-positive subjects. As chitin is a basic component of the microsporidian infective stage, the spore, we evaluated in vitro the susceptibility of a human-derived strain of Encephalitozoon hellem to nikkomycin Z, a peptide-nucleoside antibiotic known as a competitive inhibitor of chitin synthase enzymes. Transmission electron microscopy showed that this drug, at 25 μg/ml, reduced the number of parasitic foci by about 35% ± standard deviation after 7 days of culture (P < 0.0001) and induced cell damage of both mature and immature spores and also other sporogonic and merogonic stages. In particular, an irregular outline of the cell shape and an abnormally condensed cytoplasm in meronts and sporonts were documented. Also, the polar tubule and the polaroplast membranes appeared disarrayed in the sporoblast stage. The spore wall showed an enlarged endospore and delaminated exospore. Mature spores had a complete cytoplasmic disorganization and a swollen and delaminated cell wall. No ultrastructural cell damage was observed in uninfected control cultures treated with the drug.
Microsporidia are widespread, obligate intracellular parasites of most vertebrates and invertebrates. In addition to their causing infections in animals (19), there is great interest in studying these microorganisms because some genera and species have been recognized as a cause of opportunistic infections in immunocompromised humans, chiefly human immunodeficiency virus-positive subjects (9). Although albendazole, a benzimidazole derivative, has been found to be effective in vitro and in a series of patients infected by Encephalitozoon spp. to date there are no other drugs that are proved consistently efficacious against microsporidia infecting humans.
The microsporidian spore is the stage of the parasite that contains chitin in the cell wall (2, 3, 4). Chitin provides high resistance to the environment and confers structural rigidity to the infective stage. Chitin is a carbohydrate polymer not present in mammalian cells, and chitin synthesis inhibition should be a target for microsporidian-specific therapy.
Nikkomycin Z (NIK-Z) is a peptide-nucleoside antibiotic that was recovered as a potent competitive inhibitor of chitin synthase enzymes of fungi and insects and has structural similarity to UDP-N-acetylglucosamine (6, 7). In particular NIK- Z has been found to be effective in vitro and in vivo against some extracellular and intracellular fungal pathogens (5, 12, 13, 16). Moreover, this drug is well tolerated and easily degradable (11, 12) and therefore also might be considered as a potential candidate for therapeutic use against microsporidia. We present the results of a preliminary in vitro study on the sensitivity of NIK-Z on a human isolate of Encephalitozoon hellem.
MATERIALS AND METHODS
Culture system.
The study was performed on a E. hellem isolate (PV-5-95) from a subject with asymptomatic microsporidiosis (18). The strain was cocultured in 25-cm2 Corning flasks at 37°C on monolayers of a fetal bovine lung fibroblast (FBLF) cell line which was grown in Eagle minimal essential medium (E-MEM) and supplemented with 10% heat-inactivated fetal calf serum, 1% penicillin-streptomycin solution, and 1% glutamine. The medium was replaced twice a week. Microsporidian spores were periodically harvested from the supernatant by centrifugation at 3,000 × g and stored at +4°C, to provide the spore concentration for the study protocol. About 107 spores were collected from each flask after 4 weeks of infection. Cell monolayers were infected with 100 μl of a suspension of 109 spores/ml.
The control cultures (FBLF plus E. hellem) were microscopically inspected in order to draw the growth curve. The mean counts of parasitic foci/centimeter squared were performed daily for 1 week postinfection by inverted microscope (objective, 16×; Leitz Diavert, Wetzlar, Germany). The mean number of parasitic foci was defined by counting 30 microscopic fields at a magnification of ×160. At the 7th day postinfection, the cultures were fixed for transmission electron microscopy study.
Drug solution.
A 0.1 M stock solution of NIK-Z (Sigma-Aldrich, Milan, Italy) was prepared in E-MEM and diluted in culture medium to give the working solution of 25 μg/ml. The inhibitory effects of NIK-Z were previously studied in vitro within the concentration range from 0.002 to 202 μM on Candida albicans (5) and on Geotrichum candidum and at concentrations ranging from 1 to 100 μM on Mucor plumbeus (21).
Evaluation of drug effect.
The drug solution was added to the culture medium of both infected and control flasks at the 16th h and was replaced every 24 h. A count of cells showing parasitic foci per centimeter squared was performed microscopically, and the mean number of infected cells was obtained from six experiments.
To evaluate the infectivity rate of the E. hellem spores obtained from the NIK-Z-treated cultures, spores were harvested every day and counted. In addition, these spores were adjusted to a concentration of 109 spores/ml and inoculated onto fresh FBLF to determine if a new infection would take place.
Microscopical observations were performed every day until 10 days postinfection. Each pharmacological test was repeated six times, and each mean point was determined from six replicates.
Statistical analysis.
Repeated-measure analysis of variance (ANOVA) was used to test for statistically significant changes in number of foci over time and between groups; difference between two groups at one specific time was evaluated by the Mann-Whitney U test. We used Bonferroni's correction (17) to adjust the observed significance level to the number of multiple comparisons. All tests were two tailed. Analyses were performed with the statistics package SPSSPC (1998 release).
RESULTS
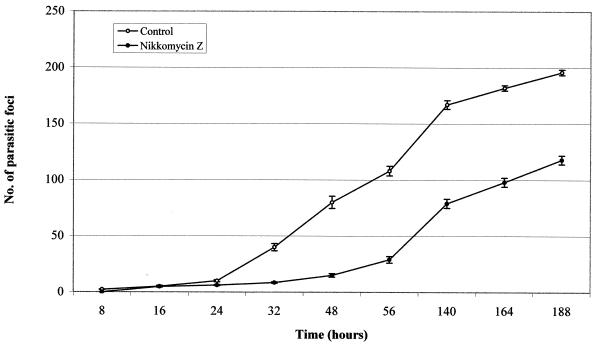
The treatment with NIK-Z (25 μg/ml) significantly inhibited the in vitro rate of infection in FBLF monolayers parasitized with E. hellem. The presence of parasitic foci was observed from 8 h to 7 days posttreatment. The percentage of treated infected cells never rose above 30%, whereas the number of infected cells in the control cultures increased to about 70% (Fig. 1). ANOVA by repeated measures showed that effects of both treatment and time were significant (treatment, F1,10 = 1,269.84 [P = 0.000]; time, F8,80 = 928.73 [P = 0.000]). There were significant interactions between treatment and time (F8,80 = 89.45 [P = 0.000]). For all evaluated time frames, except the comparisons at 16 and 24 h, the differences between the two groups were significant (P < 0.0055 for the differences to be significant after Bonferroni's correction) (Table 1).
FIG. 1.
Kinetics of E. hellem grown in FBLF cells, after treatment with NIK-Z (number of spores added, 100 μl of a suspension of 109 spores/ml; drug concentration, 25 μg/ml). Each point represents the mean number ± standard error of parasitic foci in 30 microscopic fields (magnification, ×160) in six experiments. Bars indicate standard error. These data cross index to those reported in Table 1.
TABLE 1.
Decrease of infective potentiality in E. hellem treated with NIK-Z
| Time (h) | Mean infective potentiality ± SDa
|
Z | P | |
|---|---|---|---|---|
| Controls | Treated spores | |||
| 8 | 2.2 ± 0.4 | 0 ± 0 | 3.21 | 0.0013b |
| 16 | 5.0 ± 2.1 | 4.8 ± 1.5 | 0.0 | NSc |
| 24 | 1.0 ± 2.3 | 6.2 ± 1.2 | 2.58 | 0.0099 (NS) |
| 32 | 40 ± 7.9 | 8.5 ± 1.5 | 2.89 | 0.0038b |
| 48 | 80 ± 13 | 15 ± 3.9 | 2.88 | 0.0039b |
| 56 | 108 ± 10 | 29 ± 7.1 | 2.89 | 0.0039b |
| 140 | 167 ± 9.5 | 79 ± 9.9 | 2.88 | 0.0039b |
| 164 | 182 ± 5.9 | 98 ± 9.9 | 2.88 | 0.0039b |
| 188 | 196 ± 6 | 118 ± 9.6 | 2.88 | 0.0039b |
Comparison between number of parasitic foci in treated and control samples (Mann-Whitney U test).
Significant P value after Bonferroni's correction.
NS, not significant.
No ultrastructural damages were seen in the host cells (FBLF) treated with NIK-Z during the experimental time schedule (188 h) (Table 2).
TABLE 2.
Reduction of the infective potentiality of residual E. hellem spores collected in cultures previously treated for 188 h with NIK-Z
| Time (days) | Mean infective potentiality ± SDa
|
Z | Pb | |
|---|---|---|---|---|
| Controls | Treated spores | |||
| 2 | 78 ± 12 | 0 ± 0 | 3.08 | 0.0021 |
| 4 | 138 ± 8.2 | 0 ± 0 | 3.08 | 0.0021 |
| 6 | 172 ± 13 | 0 ± 0 | 3.08 | 0.0021 |
| 7 | 198 ± 17 | 0.5 ± 0.8 | 2.93 | 0.0033 |
| 8 | 211 ± 13 | 4.2 ± 1.7 | 2.88 | 0.0039 |
| 10 | 215 ± 10 | 9.5 ± 1.9 | 2.89 | 0.0038 |
Comparisons were between the number of parasitic foci in treated and control samples (Mann-Whitney U test).
All P values are after Bonferroni's correction.
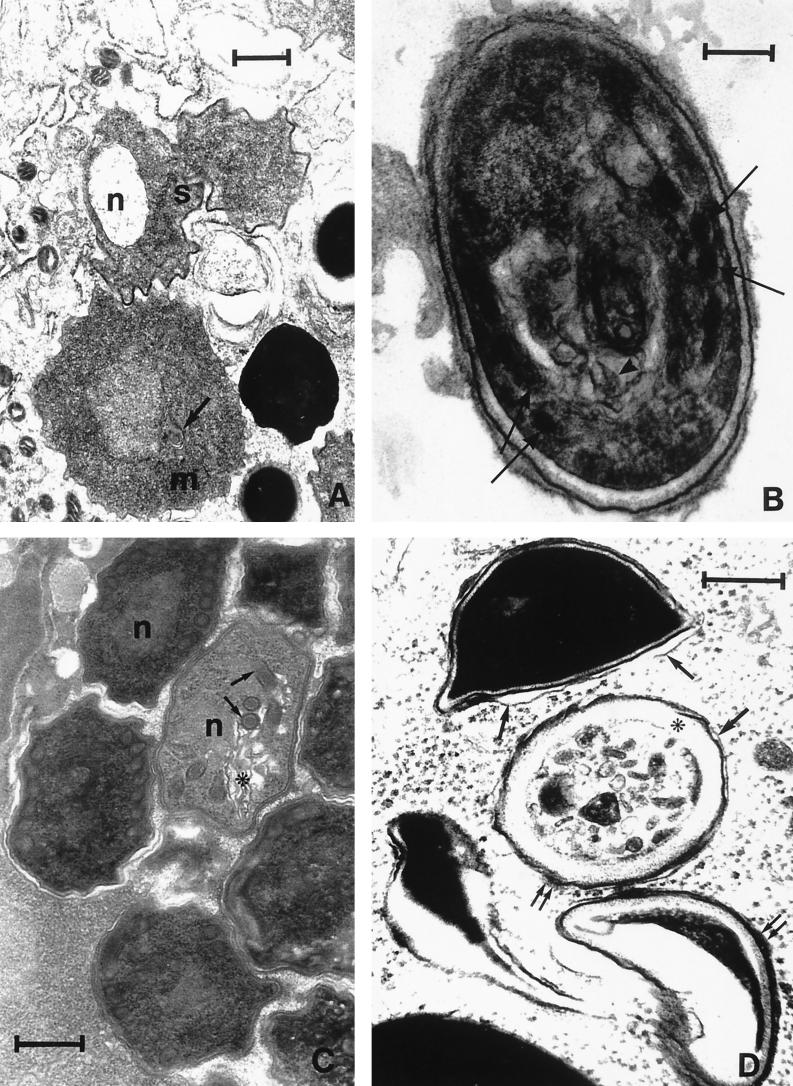
The transmission electron microscopy study showed cell damage in both merogonic and sporogonic stages. In particular, we documented an irregular outline of the cell shape and an abnormally condensed cytoplasm in meronts and sporonts (Fig. 2A), whereas the polar tubule and the polaroplast membranes appeared disarrayed in the sporoblast stage (Fig. 2B). The nucleus, the polar coils, and the polaroplast membranes of the treated spores were also disarrayed, and the cytoplasm appeared vacuolated and misshapen. The spore wall was abnormally shaped, with an enlarged endospore and delaminated exospore (Fig. 2C). Mature spores were too severely damaged and distorted, with a complete cytoplasmic disorganization and a swollen and delaminated cell wall (Fig. 2D). In addition to these ultrastructurally abnormal features, the parasites were often seen surrounded by host cell cytoplasm, as a consequence of the disrupted limiting membrane of the parasitophorous vacuole.
FIG. 2.
Electron micrographs of E. hellem in FBLF cells treated with NIK-Z (25 μg/ml) for 48 h. (A) Meronts (m) and sporonts (s) showing irregular outline of cell shape: membrane bundles (arrow) and abnormally condensed cytoplasm were observed in the meront and an empty nucleus (n) in the sporont. (Bar = 0.6 μm.) (B) Sporoblast showing irregular cell outline, disarrayed polar coils (arrows), and the polaroplast membranes (arrowhead). (Bar = 0.43 μm.) (C) “Young” spore showing nucleus (n), polar coils (arrows), and disarrayed polaroplast membranes (∗). The cytoplasm appears vacuolated and misshapen. The exospore is delaminated. (Bar = 0.25 μm.) (D) Mature spores are severely damaged and greatly distorted. Note the complete disruption of the cytoplasmic organization. The endospore (∗) appears extremely enlarged, and the exospore appears delaminated (arrows) and swollen (double arrows). (Bar = 0.37.)
On the contrary, no damage was seen in control cultures treated with NIK-Z but not infected with microsporidia.
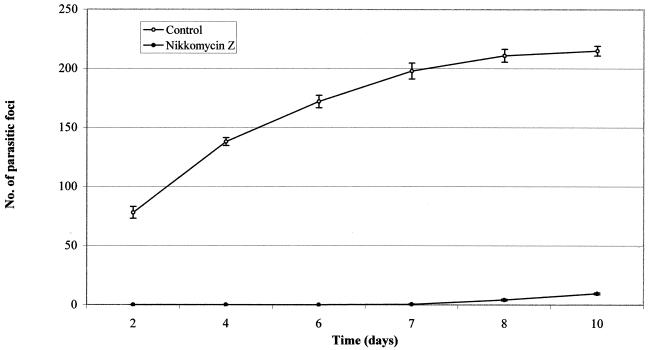
Finally, spores collected in the supernatant of drug-treated cultures after 1 week of incubation also demonstrated a dramatic decrease in infectivity: fewer parasitic foci were detected after 7 days in monolayers infected with treated parasites than in the control parasites (Fig. 3). ANOVA by repeated measures showed that effects of both treatment and time were significant (treatment, F1,10 = 3415.84 [P = 0.000]; time, F5,50 = 138.3 [P = 0.000]). In addition there were significant interactions between treatment and time (F5,50 = 105.3 [P = 0.000]). In all the time intervals considered, the two groups were statistically different (P < 0.0083 for the differences to be significant after Bonferroni's correction) (Table 2).
FIG. 3.
Reinfection of FBLF cells with E. hellem spores previously treated with NIK-Z (number of spores added, 100 μl of a suspension of 109 spores/ml). Each point represents the mean number ± standard error of parasitic foci in 30 microscopic fields (magnification, ×160) in six experiments. Bars indicate standard error. These data cross-index to those reported in Table 2.
DISCUSSION
The working hypothesis of the research, confirmed by our preliminary data, was that the exposure to inhibitors of chitin synthase would affect the chitin component of the microsporidian cell wall and, if chitin indeed has a role in sporogenesis, that this would affect the spore viability and infectivity.
Studies on the molecular phylogeny of the microsporidia demonstrated that these microorganisms display a large evolutionary distance from most eukaryotes and relegate them closer to the fungi (23). One of the mean features that strengthens the relationship between microsporidia and fungi is the presence of chitin and trehalose (2, 4, 15, 22).
In this report we first demonstrated the effect of NIK-Z as a competitive inhibitor of chitin synthase on morphogenesis of various stages of an E. hellem microsporidian strain. In fact, in monolayers treated with NIK-Z at dose of 25 μg/ml, for 48 h of incubation, we observed that a low percentage of spores was able to infect new FBLF cultures but at an infectivity rate lower than that observed in cultures inoculated with nontreated spores.
Moreover, on the basis of our study, we demonstrated NIK-Z is not directly toxic to the host cell monolayers. Our data agree with the results reported by others on fungal pathogens treated with the same drug. The inhibitory effect was related to several factors, such as nutrient concentration in the culture medium (21), the different affinity for the chitinases (5, 10), the uptake rate and the affinity for the cellular transport system, and the peptidase of the infected cells (8).
Unlike several fungal species, microsporidia are obligate intracellular pathogens. Therefore, the effective concentration of the NIK-Z in these protozoa was influenced also by the drug transport across the plasma membrane and by the peptide transport system of the infected cells. The drug effectiveness might be negatively affected by the peptidases of the host cell fibroblasts.
Nevertheless, our results indicate that NIK-Z was unable to induce complete damage of all microsporidia within host cells, so that a residual number of spores escaped drug effect, even if their infectivity was partially inhibited. In fact, parasitic foci in FBLF cells infected with drug-treated spores were observed only after several days of incubation, and their percentage was significantly lower than those of controls.
In conclusion, our preliminary results seem to indicate that NIK-Z could be a candidate drug for the treatment of some microsporidial infections, such as encephalitozoonosis. However, a more extensive study of the drug kinetics and of other strains of the same and other related species (Encephalitozoon cuniculi, Encephalitozoon intestinalis) is required. Our results also indicate the need to test this drug on these protozoa in combination with other chemotherapeutic agents, namely, azole derivatives, on the basis of recent successfully performed studies against some chitinous fungi (1, 14, 20).
ACKNOWLEDGMENTS
This work was partially supported by the 2nd National AIDS Project (AIDS-related opportunistic infections and TBC), grant 50B.35, and MURST 1998–2000 from the Italian Ministry of Health and of Scientific Research, Rome, Italy.
We thank C. Tinelli, Biometric Unit, IRCCS Policlinico San Matteo, Pavia, Italy, and P. Galeotti, Department of Animal Biology, University of Pavia, for the statistical analysis of the study results.
REFERENCES
- 1.Andriole V T. Current and future antifungal therapy: new targets for antifungal agents. J Antimicrob Chemother. 1999;44:151–162. doi: 10.1093/jac/44.2.151. [DOI] [PubMed] [Google Scholar]
- 2.Bigliardi E, Selmi M G, Lupetti P, Corona S, Gatti S, Scaglia M, Sacchi L. Microsporidian spore wall. Ultrastructural findings on Encephalitozoon hellem exospore. J Eukaryot Microbiol. 1996;43:181–186. doi: 10.1111/j.1550-7408.1996.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Bigliardi E, Gatti S, Sacchi L. Ultrastructure of microsporidian spore wall: the Encephalitozoon cuniculi exospore. Ital J Zool. 1997;64:1–5. [Google Scholar]
- 4.Canning E U, Hollister W S. In vitro and in vivo investigations of human microsporidia. J Protozool. 1991;38:631–635. [PubMed] [Google Scholar]
- 5.Chapman T, Kinsman O, Houston J. Chitin biosynthesis in Candida albicans grown in vitro and in vivo and its inhibition by nikkomycin Z. Antimicrob Agents Chemother. 1992;36:1909–1914. doi: 10.1128/aac.36.9.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen E. Chitin biochemistry: synthesis and inhibition. Annu Rev Entomol. 1987;32:71–93. [Google Scholar]
- 7.Debono M, Gordee R S. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 8.Decker H, Zahner H, Heitsch H, Konig W A, Fiedler H P. Structure-activity relationships of the nikkomycins. J Gen Microbiol. 1991;137:1805–1813. doi: 10.1099/00221287-137-8-1805. [DOI] [PubMed] [Google Scholar]
- 9.Franzen C, Muller A. Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia. Clin Microbiol Rev. 1999;12:243–285. doi: 10.1128/cmr.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaughran J P, Lai M H, Kirsch D R, Silverman S J. Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isozyme Chs3 in vivo. J Bacteriol. 1994;176:5857–5860. doi: 10.1128/jb.176.18.5857-5860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graybill J R, Najvar L K, Bocanegra R, Hector R F, Luther M F. Efficacy of nikkomycin Z in the treatment of murine histoplasmosis. Antimicrob Agents Chemother. 1998;42:2371–2374. doi: 10.1128/aac.42.9.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hector R F, Zimmer B L, Pappagianis D. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob Agents Chemother. 1990;34:587–593. doi: 10.1128/aac.34.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hector R F. Compounds active against cell walls of medically important fungi. Clin Microbiol Rev. 1993;6:1–21. doi: 10.1128/cmr.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hector R F, Schaller K. Positive interaction of nikkomycins and azoles against Candida albicans in vitro and in vivo. Antimicrob Agents Chemother. 1992;36:1284–1289. doi: 10.1128/aac.36.6.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeling P J, Doolittle W F. Evidence that eukaryotic triosephosphate isomerase is of alpha-proteobacterial origin. Proc Natl Acad Sci USA. 1997;94:1270–1275. doi: 10.1073/pnas.94.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payne J V, Barrett-Bee K J, Shallow D A. Peptide substrates rapidly modulate expression of dipeptide and oligopeptide permeases in Candida albicans. FEMS Microbiol Lett. 1991;79:15–20. doi: 10.1016/0378-1097(91)90519-g. [DOI] [PubMed] [Google Scholar]
- 17.Rice W R. Analyzing table of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 18.Scaglia M, Gatti S, Sacchi L, Corona S, Chichino G, Bernuzzi A M, Barbarini G, Croppo G P, Da Silva A, Pieniazek N J, Visvesvara G S. Asymptomatic respiratory tract microsporidiosis due to Encephalitozoon hellem in three patients with AIDS. Clin Infect Dis. 1998;26:174–176. doi: 10.1086/516264. [DOI] [PubMed] [Google Scholar]
- 19.Shadduck J A, Storts R, Adams L G. Selected examples of emerging and reemerging infectious diseases in animals. ASM News. 1996;62:586–588. [Google Scholar]
- 20.Tariq V N, Scott E M, McCain N E. Use of decimal assay for additivity to demonstrate synergy in pair combination of econazole, nikkomycin Z, and ibuprofen against Candida albicans in vitro. Antimicrob Agents Chemother. 1995;12:2615–2619. doi: 10.1128/aac.39.12.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tariq V N, Devlin P L. Sensitivity of fungi to Nikkomycin Z. Fung Gen Biol. 1996;20:4–11. doi: 10.1006/fgbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 22.Vavra J. Structure of the microsporidia. In: Bulla L A Jr, Cheng T C, editors. Comparative pathology. New York, N.Y: Plenum Press; 1976. pp. 1–85. [Google Scholar]
- 23.Weiss L M, Vossbrinck C R. Molecular biology, molecular phylogeny, and molecular diagnostic approaches to the microsporidia. In: Wittner M, editor. The microsporidia and microsporidiosis. Washington, D.C.: American Society for Microbiology; 1999. pp. 129–171. [Google Scholar]