Abstract
We investigated the activity of LY333328 alone and combined with gentamicin, both in vitro and in a rabbit model of experimental endocarditis, against the susceptible strain Enterococcus faecalis JH2-2 and its two glycopeptide-resistant transconjugants, BM4316 (VanA) and BM4275 (VanB). MICs of LY333328 and gentamicin were 2 and 16 μg/ml, respectively, for the three strains. In vitro, LY333328 alone was bactericidal at 24 h against JH2-2 at a concentration of 2 μg/ml and against BM4316 and BM4275 at a concentration of 30 μg/ml. The combination of LY333328 and gentamicin (4 μg/ml) was synergistic and bactericidal after 24 h of incubation against the three strains at LY333328 concentrations of 2 μg/ml for JH2-2 and 8 μg/ml for BM4275 and BM4316. The combination of LY333328 and gentamicin was the only regimen demonstrating in vitro bactericidal activity against BM4316. In vivo, intravenous treatment with LY333328 alone, providing peak and trough serum levels of 83.3 ± 1.3 and 3.8 ± 0.2 μg/ml, respectively, was inactive against BM4316 and BM4275 and selected mutants resistant to LY333328 in half of the rabbits infected with the VanA-type strain (MICs, 8 to 20 μg/ml). However, the LY333328-gentamicin combination was active against the three strains and prevented the emergence of mutants resistant to both components of the combination. We conclude that the LY333328-gentamicin combination might be of interest for the treatment of enterococcal infections, particularly against VanA-type strains.
In recent years, enterococci have become significant nosocomial pathogens and now represent the second leading cause of nosocomial infections in the United States (17). A major reason for their spread in the hospital environment is their ability to resist most of the available antibiotics, including β-lactams, aminoglycosides, and glycopeptides, through intrinsic and/or acquired mechanisms of resistance (14, 21). Vancomycin-resistant enterococci have emerged since 1989 and have rapidly increased, being responsible for severe hospital outbreaks (4, 11, 21). In 1998, almost 15% of enterococci isolated in intensive care units in the United States exhibited vancomycin resistance (13). The lack of uniformly effective antimicrobial therapy for patients infected with glycopeptide-resistant enterococci has led to new therapeutic proposals.
LY333328 is a semisynthetic carbohydrate-modified glycopeptide derivative that interacts directly with bacterial proteins involved in the transglycosylation step of cell wall biosynthesis. LY333328 has demonstrated excellent in vitro concentration-dependent activity against vancomycin-susceptible and -resistant enterococci (16, 18, 23). We previously showed that the activity of intramuscular (i.m.) LY333328 against experimental Enterococcus faecalis endocarditis was limited compared to that observed in vitro (16). This discrepancy could be explained, in part, by insufficient serum LY333328 concentrations achieved with the i.m. route. In order to investigate whether serum levels were the major factor limiting the in vivo activity of LY333328, we studied the potential benefit of an intravenous (i.v.) route of administration of LY333328, which may provide higher serum drug levels, in experimental endocarditis due to E. faecalis strains susceptible or resistant to glycopeptides. In addition, we evaluated the activity of LY333328-gentamicin combinations in vitro and in the same animal model in terms of bactericidal activity and prevention of in vivo emergence of mutants resistant to one or both components of the combinations.
(This work was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September 1999 [abstract 1020].)
MATERIALS AND METHODS
Bacterial strains.
E. faecalis JH2-2 is susceptible to glycopeptides and β-lactams and is intrinsically resistant to low levels of aminoglycosides (7). E. faecalis BM4275 harbors a 250-kb chromosomal vanB element conferring VanB-type resistance and was obtained by conjugal transfer of vancomycin resistance from clinical isolate Enterococcus faecium BM4120 to JH2-2 (9, 15). E. faecalis BM4316 harbors a 50-kb vanA element that confers VanA-type resistance and was obtained by conjugal transfer from clinical isolate E. faecium HM1074 to JH2-2 (1, 16). All cultures and antibiotic susceptibility testing were performed in brain heart infusion (BHI) broth or agar (Difco Laboratories, Detroit, Mich.) at 37°C.
In vitro susceptibility testing and selection of mutants.
The MICs of LY333328 (Eli Lilly France, Saint-Cloud, France), vancomycin (Eli Lilly France), teicoplanin (Aventis, Levallois-Perret, France), and gentamicin (Panpharma, Fougères, France) were determined by the method of Steers et al. (20) with 105 CFU per spot on BHI agar after 24 h of incubation. For time-kill curves, exponentially growing E. faecalis cells were diluted in glass tubes containing 10 ml of BHI broth to obtain 106 CFU/ml and incubated with LY333328 (2, 8, or 30 μg/ml), vancomycin (30 μg/ml), teicoplanin (30 μg/ml), or gentamicin (4 μg/ml), alone or in combination. Aliquots (0.1 ml) were taken after 0, 3, 6, and 24 h of incubation and plated on BHI agar after serial dilutions to enumerate the surviving bacteria after 24 h of incubation. The lower limit of detection was 1 log10 CFU/ml. A bactericidal effect was defined as a decrease of ≥3 log10 CFU/ml between the initial inoculum and the bacterial count at 24 h. A synergistic effect was defined as a decrease of ≥2 log10 CFU/ml between the combination and its most active constituent after 24 h, and the number of surviving organisms in the presence of the combination had to be ≥2 log10 CFU/ml below the starting inoculum. At least one of the drugs had to be present in a concentration which did not affect the growth curve of the test organism when used alone. As previously shown, antibiotic carryover did not interfere with bacterial counts at the concentrations of LY333328 tested (16). For each strain, time-kill curves were performed in three independent experiments, showing very similar results.
Selection of spontaneous LY333328- or gentamicin-resistant mutants was performed by plating 0.1 ml of an overnight culture of JH2-2, BM4275, or BM4316 on agar containing LY333328 at four times the MIC, gentamicin at two times the MIC, or neither antibiotic. These antibiotic concentrations were the lowest concentrations allowing the selection of resistant mutants (data not shown). MICs for colonies growing on antibiotic-containing agar were determined to confirm the selection of resistance. Mutation frequencies were determined by dividing the number of CFU obtained on selective media by the number of CFU obtained on media devoid of antibiotic after 48 h of incubation.
Experimental endocarditis.
Aortic endocarditis was induced in female New Zealand White rabbits (2.2 to 2.5 kg) by insertion of a polyethylene catheter through the right carotid artery into the left ventricle, as previously described (11). Twenty-four hours after catheter insertion, each rabbit was inoculated by the ear vein with 108 CFU of E. faecalis JH2-2, BM4275, or BM4316 in 1 ml of 0.9% NaCl. The catheter was left in place throughout the experiment. Forty-eight hours after inoculation, animals received 20 mg of LY333328/kg of body weight (in a final concentration of 12.5 mg of 5% dextrose/ml) by the ear vein through an i.v. infusion over 5 min once daily (o.d.) or 3 mg of gentamicin/kg i.m. twice daily (b.i.d.), or the combination of the two drugs. The o.d. rate of administration of LY333328 was chosen since preliminary clinical studies showed that the LY333328 half-life is long, ranging from 132 to 356 h in healthy men (J. Chien, D. Allerheiligen, D. Phillips, B. Cerimele, and H. R. Thomasson, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A55, p. 18, 1998), thus allowing a single administration per day. Animals were treated for 5 days and sacrificed 12 h after the last injection of gentamicin or 24 h after the last injection of LY333328 by i.v. injection of pentobarbital. Control animals were left untreated and sacrificed at the same time as treated animals (“end-of-therapy” controls). In addition, one group of animals was killed at the beginning of the treatment (“start-of-therapy” controls) to evaluate the bacterial counts and to search for the presence of mutants resistant to LY333328 or to gentamicin before therapy. At the time of sacrifice, the heart was removed and the chambers on the left side were examined to confirm vegetative endocarditis. For each rabbit, vegetations were excised, pooled, weighed, and homogenized in 1 ml of sterile distilled water. Vegetation homogenates were plated on agar after serial dilutions to count surviving bacteria and on agar containing LY333328 at four times the MIC for treatment with LY333328, gentamicin at two times the MIC for treatment with gentamicin, or both selective media for treatment with the combination to enumerate mutants after 48 h of incubation. The MICs of LY333328 and gentamicin for the bacteria recovered from the selective media were determined. Results were expressed as log10 CFU per gram of vegetation. For a better evaluation of the activity of LY333328 alone or in combination, the results of a teicoplanin regimen (20 mg/kg b.i.d. for 5 days after a loading dose of 40 mg/kg) against the three strains, as well as vancomycin (50 mg/kg b.i.d) and amoxicillin (50 mg/kg four times a day) 5-day regimens against susceptible strain E. faecalis JH2-2, which we previously reported (7, 9, 16), were included in the results. These regimens have been previously shown to lead to peak and trough serum antibiotic levels of 28 ± 10 and 0.8 ± 0.5 μg/ml for amoxicillin (7), 57 ± 5.5 and 7.0 ± 1.5 μg/ml for vancomycin, and 63 ± 23 and 25 ± 10 μg/ml for teicoplanin (3), respectively.
Antibiotic concentrations.
For determination of serum LY333328 concentrations, blood of three uninfected rabbits was sampled 0.5, 1, 3, 6, 12, and 24 h after a 5-min i.v. infusion of 20 mg/kg. LY333328 was assayed by a validated method (5) according to which a solid-phase extraction followed by high-performance liquid chromatography with fluorescence detection is used, as previously reported (8). Quality control samples (at three concentrations) were assayed with the study samples. Accuracy of the quality control samples ranged from 100.3 to 101.7%, and precision ranged from 0.16 to 0.50%. The lower limit of detection was 2 μg/ml. The area under the curve (AUC) for LY333328 was calculated by the trapezoidal rule. Gentamicin concentrations in serum were measured in uninfected rabbits 0.5 and 12 h after a single i.m. injection of 3 mg of gentamicin/kg by fluorescence polarization immunoassay (AxSYM system; Abbott Diagnostics, Rungis, France).
Statistics.
All the results were expressed as means ± standard deviations. Comparisons of bacterial counts in the vegetations of rabbits treated with the various regimens were performed by analysis of variance followed, when significant, by the Scheffe test for multiple comparisons (19). A P value of <0.05 was considered significant.
RESULTS
Susceptibility tests.
All three studied strains were susceptible to LY333328, with an LY333328 MIC of 2 μg/ml, despite acquired resistance to vancomycin and teicoplanin in BM4316 (VanA) and to vancomycin in BM4275 (VanB) (Table 1). The three strains exhibited similar intrinsic low levels of resistance to gentamicin, as shown by MICs of gentamicin of 16 μg/ml (Table 1).
TABLE 1.
MICs of glycopeptides and gentamicin for the study strains of E. faecalis
| Drug | MIC (μg/ml) for E. faecalis:
|
||
|---|---|---|---|
| JH2-2 (susceptible) | BM4275 (VanB) | BM4316 (VanA) | |
| LY333328 | 2 | 2 | 2 |
| Vancomycin | 2 | 128 | 256 |
| Teicoplanin | 1 | 1 | 64 |
| Gentamicin | 16 | 16 | 16 |
In vitro bactericidal activity.
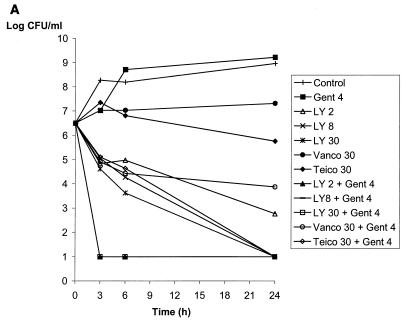
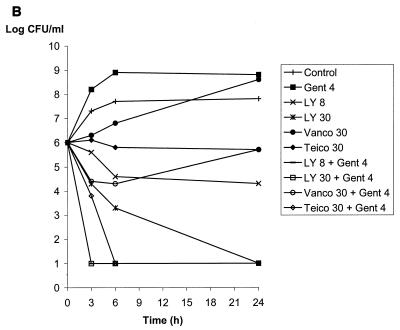
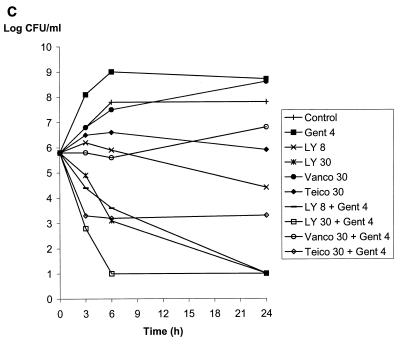
Vancomycin or teicoplanin alone at 30 μg/ml and gentamicin at 4 μg/ml did not reduce the bacterial counts at 24 h of JH2-2 (susceptible), BM4275 (VanB), and BM4316 (VanA), as shown in Fig. 1 and previously reported (16). In contrast, LY333328 alone was bactericidal at 24 h against JH2-2 at a concentration of 2 μg/ml and against BM4275 and BM4316 at a concentration of 30 μg/ml (Fig. 1). The vancomycin-gentamicin combination was active against susceptible strain JH2-2 (Fig. 1A) but did not reduce the bacterial counts of strains BM4275 and BM4316, which are resistant to vancomycin (Fig. 1B and C). Similarly, the teicoplanin-gentamicin combination was bactericidal at 24 h against strains JH2-2 and BM4275, which are susceptible to teicoplanin (Fig. 1A and B), whereas the activity of the combination against teicoplanin-resistant strain BM4316 was less pronounced (reduction of 2.5 log10 CFU/ml after 24 h of incubation) (Fig. 1C). Combinations of LY333328 at 8 μg/ml and gentamicin were bactericidal at 24 h against the three strains. Of note, combinations including LY333328 were the only combinations that were bactericidal against BM4316 (Fig. 1C). A synergistic effect between LY333328 and gentamicin could be seen at 24 h for the three strains at LY333328 concentrations of 2 μg/ml for JH2-2 (Fig. 1A) and 8 μg/ml for BM4275 and BM4316 (Fig. 1B and C). At higher concentrations of LY333328, synergy could not be observed because of the bactericidal activity of LY333328 alone. The rate of killing of LY333328 at 8 μg/ml in combination with gentamicin was more pronounced for JH2-2 and BM4275 than for BM4316. At a higher concentration of LY333328 (30 μg/ml), the rate of bactericidal activity of the combination against BM4316 was markedly increased.
FIG. 1.
Bactericidal activity of LY333328 at 2, 8, and 30 μg/ml (LY 2, LY 8, and LY 30, respectively), vancomycin at 30 μg/ml (Vanco 30), teicoplanin at 30 μg/ml (Teico 30), and gentamicin at 4 μg/ml (Gent 4), alone or in combinations, against susceptible strain JH2-2 (A), VanB-type strain BM4275 (B), and VanA-type strain BM4316 (C).
Experimental endocarditis.
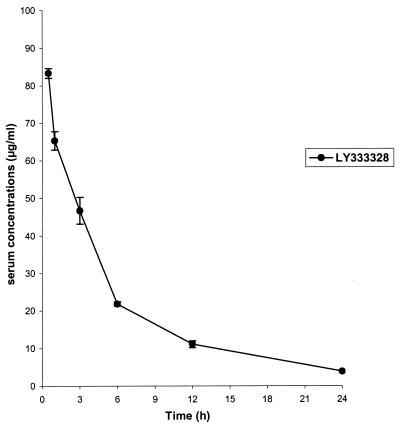
Mean antibiotic serum LY333328 levels after a single injection of 20 mg/kg i.v. are shown in Fig. 2. Peak (0.5 h after injection) and trough (24 h after injection) serum LY333328 concentrations were 83.3 ± 1.3 and 3.8 ± 0.2 μg/ml, respectively, and the AUC was 460.8 ± 10.4 μg · h/ml. Peak and trough serum gentamicin concentrations were 7.0 ± 1.3 and <0.2 μg/ml, respectively.
FIG. 2.
Serum LY333328 levels (means ± standard deviations) 0.5, 1, 3, 6, 12, and 24 h after a single injection of 20 mg of LY333328/kg i.v. to three uninfected rabbits.
As shown in Table 2, LY333328 alone was poorly active since mean bacterial counts in the vegetations of rabbits infected with any of the three strains after a 5-day treatment ranged between 7.9 and 8.1 log10 CFU/g of vegetation. In contrast, mean bacterial counts in vegetations from rabbits treated with the LY333328-gentamicin combination ranged between 6.1 and 6.8 log10 CFU/g of vegetation. A comparison of bacterial counts in rabbits treated with the combination and those of start-of-therapy controls showed that the combination reduced bacterial counts in the vegetations, although this reduction was not statistically significant (Table 2). Compared to those for end-of-therapy controls, the bacterial counts for JH2-2 and the VanA-type strain were reduced by a statistically significant amount (P < 0.05), but not those for the VanB-type strain (P = 0.06).
TABLE 2.
Activity of vancomycin, teicoplanin, amoxicillin, and LY333328 alone or combined with gentamicin for 5 days in rabbits with aortic endocarditis due to E. faecalis
| Regimend | Mean log10 CFU/g of vegetation ± SD (no. of animals with mutantse/total) for E. faecalis:
|
||
|---|---|---|---|
| JH2-2 (susceptible) | BM4275 (VanB type) | BM4316 (VanA type) | |
| Start-of-therapy controls | 8.3 ± 1.2 (0/13) | 7.7 ± 0.8 (0/4) | 6.4 ± 1.6 (0/7) |
| End-of-therapy controls | 9.8 ± 1.0 (—/12) | 8.1 ± 1.3 (—/7) | 8.8 ± 1.0 (—/10) |
| VM 50 mg/kg b.i.d.f | 7.3 ± 1.1 (—/6)b | NDg | ND |
| TE 20 mg/kg b.i.d.af | 6.5 ± 0.7 (—/9)c | 7.4 ± 1.9 (—/7) | 7.9 ± 1.5 (—/5) |
| AMX 50 mg/kg q.i.d.f | 7.7 ± 0.8 (—/8) | ND | ND |
| LY 20 mg/kg i.v. o.d. | 8.0 ± 1.5 (0/8) | 8.1 ± 0.6 (0/7) | 7.9 ± 1.5 (3/6) |
| Gent 3 mg/kg i.m. b.i.d. | 8.4 ± 1.0 (2/9) | 6.8 ± 2.3 (3/7) | 8.8 ± 1.0 (4/8) |
| LY + Gent | 6.8 ± 2.0 (0/7)c | 6.5 ± 1.6 (0/8) | 6.1 ± 1.6 (0/6)b |
After a loading dose of 40 mg/kg.
P < 0.05 versus end-of-therapy controls.
P < 0.01 versus end-of-therapy controls.
VM, vancomycin; TE, teicoplanin; AMX, amoxicillin; Gent, gentamicin; LY, LY333328; q.i.d., four times a day.
—, mutants were selected on agar containing LY333328 at four times the MIC and/or gentamicin at two times the MIC.
Results previously published (7, 9, 16) and provided here for comparison.
ND, Not done.
Selection of LY333328- and gentamicin-resistant mutants in vitro and in vivo.
No mutants resistant to LY333328 were obtained by plating JH2-2 on LY333328-containing agar. Spontaneous LY333328-resistant BM4275 and BM4316 mutants were obtained at frequencies of 10−7 on agar containing four times the MIC of LY333328. These mutants were stable after three subcultures on antibiotic-free agar. MICs for these mutants ranged between four and eight times the MIC for the parental strain. Spontaneous gentamicin-resistant mutants were recovered at frequencies of 10−7 for the three strains. MICs for these mutants ranged between two and six times the MIC for the parental strain, as previously observed (9).
In vivo, no LY333328- or gentamicin-resistant mutants were recovered from animals sacrificed at the beginning of the treatment. LY333328 alone selected mutants resistant to this drug in three of six rabbits infected with VanA-type strain BM4316 (Table 2). A comparison of counts of bacteria growing on agar containing four times the MIC of LY333328 and on antibiotic-free agar at the time of sacrifice showed that the proportions of mutants in the vegetations of these three rabbits ranged from 6 × 10−4 to 2 × 10−1. MICs of LY333328 for these mutants ranged between 8 and 20 μg/ml, i.e., between 4 and 10 times the MIC of LY333328 for the parental strain, BM4316. No mutants were detected in the case of infection with JH2-2 or VanB-type strain BM4275.
JH2-2, BM4275, and BM4316 mutants resistant to gentamicin were recovered in two of nine, three of seven, and four of eight rabbits, respectively, after the 5-day gentamicin monotherapy (Table 2) and represented 4 × 10−6 to 1 × 10−1 of the surviving bacteria. MICs for the gentamicin-resistant mutants were increased two- to sixfold in comparison to those for the parental strain, as previously described (9).
For the three strains, the combination of LY333328 and gentamicin prevented the emergence of mutants resistant to one or both components of the combination.
DISCUSSION
We previously observed a potent in vitro bactericidal activity of LY333328 against the susceptible strain E. faecalis JH2-2 and its two glycopeptide-resistant transconjugants, BM4281 and BM4316, contrasting with a limited in vivo activity of the drug after i.m. administration in the rabbit model of endocarditis (16). Two factors could account for this discrepancy: a high rate of protein binding of LY333328 (>99%), reducing the bactericidal activity of the drug in the presence of rabbit serum, and the heterogeneous pattern of diffusion of the drug into the vegetations (16). In addition, we hypothesized that the i.m. route of administration of LY333328 could be an additional limiting factor since serum levels obtained in rabbits after i.m. injection ranged between 18 and 10 μg/ml, which corresponded to an unbound concentration of LY333328 of <0.2 μg/ml, whereas bactericidal activity was achieved in broth with concentrations of 8 to 30 μg of LY333328/ml, according to the strain studied (16).
In the present study, we investigated whether an i.v. route of administration of LY333328, providing higher serum levels, would increase the in vivo activity of the drug. Again, we observed an excellent in vitro bactericidal activity of LY333328 alone against the glycopeptide-susceptible and -resistant strains (Table 1 and Fig. 1). However, in vivo results indicated that, although both mean peak serum levels (≈83 μg/ml) and AUC (≈461 μg · h/ml) obtained after i.v. perfusion were much higher than those obtained with the i.m. route (mean peak serum level, ≈16 μg/ml; AUC, ≈305 μg · h/ml, calculated from data in reference 16), the level of activity of i.v. LY333328 alone was comparable to that observed with the i.m. route (Table 2). Thus, increasing peak serum LY333328 levels and AUC did not improve the in vivo activity of the drug. In contrast, animals receiving LY333328 i.v. o.d. had serum levels below those achieved with the b.i.d. i.m. route during approximately the last 10 h of the dosing regimen. In addition, trough serum levels were lower than those obtained after i.m. administration (3.82 ± 0.23 versus 10.4 ± 0.9 μg/ml) (16) and below the concentrations required for in vitro bactericidal activity of the drug against the glycopeptide-resistant strains (Fig. 1). Therefore, LY333328 activity seems to be more time dependent than dose dependent. This pharmacokinetic and pharmacodynamic profiles combined with a high protein binding (>99%) and a heterogeneous pattern of diffusion in cardiac vegetations may result in subinhibitory concentrations of LY333328 in the vegetations during the second part of the dosing regimen, which may explain the limited activity of the drug and which may facilitate the emergence of LY333328-resistant mutants. Thus, optimizing trough serum levels rather than AUC would be mandatory to achieve maximal killing. This would be of course more easily achievable in humans than in animals because of the more-prolonged elimination half-life in humans (Chien et al., 38th ICAAC).
Mutants resistant to LY333328 were recovered after treatment with LY333328 alone in three of six rabbits infected with VanA-type strain BM4316. The numbers of resistant mutants in these rabbits ranged from 330 to 55,000 CFU per animal. Since each animal was inoculated initially with 108 bacteria with a spontaneous rate of mutations to LY333328 of 10−7 in vitro, generating approximately 10 mutants per animal, the number of LY333328-resistant mutants increased between 33- and 5,500-fold during therapy with LY333328 alone. The increase in the number of mutants between the inoculation and the sacrifice and the fact that no LY333328-resistant mutants were recovered from animals sacrificed at the beginning of therapy suggest that these mutants emerged in vivo under the selective pressure of LY333328. The levels of LY333328 resistance in these mutants were moderate (MICs, ≤20 μg/ml), similar to the low level of resistance of mutants mediated by genes of the vanA cluster in enterococci that were constructed in vitro (2). We do not think that the emergence of LY333328-resistant mutants was responsible for the low activity of LY333328 against BM4316, since the drug was also poorly active against JH2-2 and BM4275 despite the absence of selection of resistant mutants in these strains. In addition, derivatives of BM4316 resistant to LY333328 constituted only 6 × 10−4 to 2 × 10−1 of the entire population of surviving bacteria, meaning that the majority of the bacteria remained susceptible to LY333328. However, it can be speculated that a more prolonged course of LY333328 therapy may lead to a complete replacement of the bacterial population in favor of LY333328-resistant mutants, which may account for therapeutic failures. To our knowledge, in vivo selection of LY333328-resistant mutants under treatment with LY333328 had never been reported before. Of note, an LY333328-dependent strain of VanA-type E. faecalis has been isolated from a blood culture of a patient who had received multiple courses of either vancomycin or teicoplanin (22).
Mutants resistant to LY333328 were not observed in the case of infection due to VanB-type strain BM4275 (Table 2). However, this does not preclude the possibility that LY333328-resistant mutants derived from VanB-type strains might be selected in vivo. Indeed, low-level LY333328 resistance was recently observed in vitro in strains harboring mutations in the vanSB sensor genes of the vanB cluster (2).
Mutants resistant to gentamicin were selected in rabbits infected with JH2-2, BM4275, and BM4316 and treated with gentamicin alone. Although the level of resistance of these mutants is moderate, we previously showed in the same experimental model that the emergence of these mutants could be responsible for therapeutic failures (9).
Combinations of LY333328 and gentamicin were synergistic and rapidly bactericidal in vitro against the three strains, whatever their phenotype of resistance to glycopeptides. It is important to emphasize that the study strains had a low level of resistance to gentamicin and that this result would not be applicable to enterococcal strains exhibiting a high level of resistance to aminoglycosides. The observation of a synergistic effect between these two components against glycopeptide-resistant enterococci is consistent with previous in vitro results (12, 23). In vivo, the combinations of LY333328 and gentamicin were active, although less intensively than in vitro, and prevented the emergence of mutants resistant to LY333328 and/or to gentamicin, regardless of the phenotype of resistance to glycopeptides of the strain. We previously studied the conditions of emergence of mutants resistant to the combinations of gentamicin and glycopeptides (vancomycin and teicoplanin) in VanB-type strains in vitro and in vivo and showed that therapy with vancomycin and gentamicin selected mutants resistant to the vancomycin-gentamicin combination. These mutants had acquired resistance to gentamicin in addition to their resistance to vancomycin. Resistance to both components of the combination was thus required for resistance to the combination. The combination of teicoplanin and gentamicin remained active against the VanB-type strains and prevented the emergence of mutants resistant to one or both components of the combination since simultaneous acquisition of resistance to both components of the combination, a combination of two rare events, could not be observed in vivo (9). Similarly, we can speculate that mutants resistant to one or both components of the combination of LY333328 and gentamicin were not observed in the present study since the strains that were inoculated into rabbits were susceptible to LY333328 and displayed a low level of resistance to gentamicin. However, whether prior treatment with a glycopeptide alone, which may select LY333328-resistant mutants, would compromise the efficacy of the LY333328-gentamicin combination remains to be investigated.
The conclusion of this study and of our previous work (16) is that monotherapy with LY333328 for the treatment of tissue infections due to glycopeptide-resistant E. faecalis suffers from several limiting factors: high protein binding, which reduces the bactericidal activity of LY333328, heterogeneous diffusion of the drug into the infected site, and selection of LY333328-resistant mutants derived from VanA-type strains. Limited antibacterial activity and emergence of resistance are major limiting factors for the use of LY333328 as a single agent for high-inoculum infections in which bactericidal activity is important for cure. The present study demonstrates that the combination of LY333328 with gentamicin increases the bactericidal activity of LY333328 and prevents the emergence of mutants resistant to LY333328 or to gentamicin, whatever the phenotype of resistance to glycopeptides of the strain.
ACKNOWLEDGMENTS
This work was supported by Eli Lilly Laboratories, Saint-Cloud, France. Agnès Lefort was supported by l'Académie Nationale de Médecine.
We thank Patrice Courvalin, Unité des Agents Bactériens, Institut Pasteur, who kindly provided the strains of E. faecalis, and Sophie Dautrey, Laboratoire de Toxicologie, Hôpital Bichat, for technical assistance.
REFERENCES
- 1.Arthur M, Depardieu F, Gerbaud G, Galimand M, Leclercq R, Courvalin P. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546 and related elements in the absence of induction. J Bacteriol. 1997;179:97–106. doi: 10.1128/jb.179.1.97-106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur M, Depardieu F, Reynolds P, Courvalin P. Moderate-level resistance to glycopeptide LY333328 mediated by genes of the vanA and vanB clusters in enterococci. Antimicrob Agents Chemother. 1999;43:1875–1880. doi: 10.1128/aac.43.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslangul E, Baptista M, Fantin B, Depardieu F, Arthur M, Courvalin P, Carbon C. Selection of glycopeptide-resistant mutants of VanB-type Enterococcus faecalis BM4281 in vitro and in experimental endocarditis. J Infect Dis. 1997;175:598–605. doi: 10.1093/infdis/175.3.598. [DOI] [PubMed] [Google Scholar]
- 4.Boyce J M, Opal S M, Chow J W, Zervos M J, Potter-Bynoe G, Sherman C B, Romulo R L, Fortna S, Medeiros A A. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32:1148–1153. doi: 10.1128/jcm.32.5.1148-1153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inman E L. Determination of vancomycin related substances by gradient high-performance liquid chromatography. J Chromatogr. 1987;410:363–372. doi: 10.1016/s0021-9673(00)90066-9. [DOI] [PubMed] [Google Scholar]
- 6.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Join-Lambert O, Mainardi J L, Cuvelier C, Dautrey S, Farinotti R, Fantin B, Carbon C. Critical importance of in vivo amoxicillin and cefotaxime concentrations for synergy in treatment of experimental Enterococcus faecalis endocarditis. Antimicrob Agents Chemother. 1998;42:468–470. doi: 10.1128/aac.42.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaatz G W, Seo S M, Aeschlimann J R, Houlihan H H, Mercier R C, Rybak M J. Efficacy of LY333328 against experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1998;42:981–983. doi: 10.1128/aac.42.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefort A, Baptista M, Fantin B, Depardieu F, Arthur M, Carbon C, Courvalin P. Two-step acquisition of resistance to the teicoplanin-gentamicin combination by VanB-type Enterococcus faecalis in vitro and in experimental endocarditis. Antimicrob Agents Chemother. 1999;43:476–482. doi: 10.1128/aac.43.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefort A, Fantin B. Rabbit model of bacterial endocarditis. In: Zak O, Sande M, editors. Handbook of animal models of infection. London, United Kingdom: Academic Press; 1999. pp. 611–617. [Google Scholar]
- 11.Livornese L L, Jr, Dias S, Samel C, Romanowski B, Taylor S, May P, Pitsakis P, Woods G, Kaye D, Levison M E, Johnson C. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992;117:112–116. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- 12.Mercier R C, Penzak S R, Rybak M J. In vitro activities of an investigational quinolone, glycylcycline, glycopeptide, streptogramin, and oxazolidinone tested alone and in combinations against vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:2573–2575. doi: 10.1128/aac.41.11.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moellering R C., Jr Vancomycin-resistant enterococci. Clin Infect Dis. 1998;26:1196–1199. doi: 10.1086/520283. [DOI] [PubMed] [Google Scholar]
- 14.Murray B E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintiliani R, Jr, Courvalin P. Conjugal transfer of the vancomycin resistance determinant vanB between enterococci involves the movement of large genetic elements from chromosome to chromosome. FEMS Microbiol Lett. 1994;119:359–364. doi: 10.1111/j.1574-6968.1994.tb06913.x. [DOI] [PubMed] [Google Scholar]
- 16.Saleh-Mghir A, Lefort A, Petegnief Y, Dautrey S, Vallois J M, Le Guludec D, Carbon C, Fantin B. Activity and diffusion of LY333328 in experimental endocarditis due to vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1999;43:115–120. doi: 10.1128/aac.43.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 18.Schwalbe R S, McIntosh A C, Qaiyumi S, Johnson J A, Johnson R J, Furness K M, Holloway W J, Steele-Moore L. In vitro activity of LY333328, an investigational glycopeptide antibiotic, against enterococci and staphylococci. Antimicrob Agents Chemother. 1996;40:2416–2419. doi: 10.1128/aac.40.10.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steel R G D, Tovrie J H. Multiple comparisons. In: Napier C, Maisel J W, editors. Principles and procedures of statistics: a biometrical approach. New York, N.Y: McGraw-Hill; 1980. pp. 172–194. [Google Scholar]
- 20.Steers E, Foltz E L, Graves B S, Riden J. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot Chemother (Basel) 1959;9:307–311. [PubMed] [Google Scholar]
- 21.Tenover F C, Gaynes R. Dissemination of vancomycin-resistant enterococci in the United States. In: Brun-Buisson C, Eliopoulos G, Leclercq R, editors. Bacterial resistance to glycopeptides. Paris, France: Flammarion Medecine-Sciences; 1998. pp. 101–110. [Google Scholar]
- 22.Wilson P, Koshy C, Minassian M. An LY333328-dependent strain of Enterococcus faecalis isolated from a blood culture. J Antimicrob Chemother. 1998;42:406–407. doi: 10.1093/jac/42.3.406. [DOI] [PubMed] [Google Scholar]
- 23.Zelenitsky S A, Booker B, Laing N, Karlowsky J A, Hoban D J, Zhanel G G. Synergy of an investigational glycopeptide, LY333328, with once-daily gentamicin against vancomycin-resistant Enterococcus faecium in a multiple-dose, in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1999;43:592–597. doi: 10.1128/aac.43.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]