Abstract
Astrocytes are recognized as more important cells than historically thought in synaptic function through the reciprocal exchange of signaling with the neuronal synaptic elements. The idea that astrocytes are active elements in synaptic physiology is conceptualized in the Tripartite Synapse concept. This review article presents and discusses recent representative examples that highlight the heterogeneity of signaling in tripartite synapse function and its consequences on neural network function and animal behavior.
Keywords: Tripartite synapse, Astrocytes, Gliotransmission, Synaptic function, Network function
Introduction
Astrocytes are known to play important homeostatic roles in brain function, providing trophic, structural and metabolic support for neurons [1–3]. They have additionally been shown to display a calcium-based excitability and to be able to act as sensors and modulators of synaptic transmission and plasticity. Through the expression of a wide variety of membrane receptors expressed, astrocytes sense the synaptic activity by responding to different synaptically released neurotransmitters, which generally leads to the elevation of the astrocyte calcium levels. These calcium elevations stimulates the release of gliotransmitters, which acting on neuronal receptors, regulate synaptic transmission and plasticity [4–8]. Thus, in addition to the classical information flow between the pre- and postsynaptic neuronal elements of the synapse, there is a signaling exchange between these neuronal elements and the adjacent astrocytes. This bidirectional communication between astrocytes and the neuronal elements led to the concept of the tripartite synapse, which epitomizes the idea that synaptic function results from the interaction of three synaptic elements, the presynaptic terminal, the postsynaptic cell and the surrounding astrocyte. In this review, we will present and discuss recent paradigmatic examples that highlight the heterogeneity of signaling in tripartite synapse function.
Astrocytes Sense Synaptic Activity
Astrocytes are known to express receptors for a large plethora of neurotransmitters, such as glutamate, GABA, endocannabinoids, dopamine, serotonin, ATP/Adenosine, acetylcholine or opioids [9–12]. Such diversity of receptors illustrates the ability of astrocytes to sense multiple neuronal signals, which can be integrated in a non-linear manner [13] to confer a high variability of the astrocytic responses (Fig. 1). Many of the neurotransmitter receptors expressed by astrocytes are G protein-coupled receptors (GPCRs), which, upon activation, lead to intracellular calcium elevations [14–19]. Gq GPCRs activate phospholipase C that generates diacylglycerol and inositol 1,4,5-trisphosphate (IP3) which, ultimately, induces the release of calcium from the endoplasmic reticulum through activation of IP3 receptors. Type 2 IP3 receptors (IP3R2) have been shown to be the main responsible of the GPCR-mediated calcium mobilization in astrocytes [20–23]. Accordingly, in the IP3R2 knockout mice, which lack IP3R2, astrocyte calcium elevations in the soma are unaffected by neurotransmitters [24]. The relatively small calcium activity observed in restricted regions of the processes have been shown to have a mitochondrial origin [25–27]. In addition to GPCRs-mediated calcium mobilization, ionotropic receptors to glutamate and ATP are involved in astroglial Ca2+-signaling and neuron-glia communication [28–31]. Therefore, the role of ionotropic component of Ca2+ mobilization might help to explain some contradictory results obtained in astrocytic IP3R2 knockout-mice.
Fig. 1.
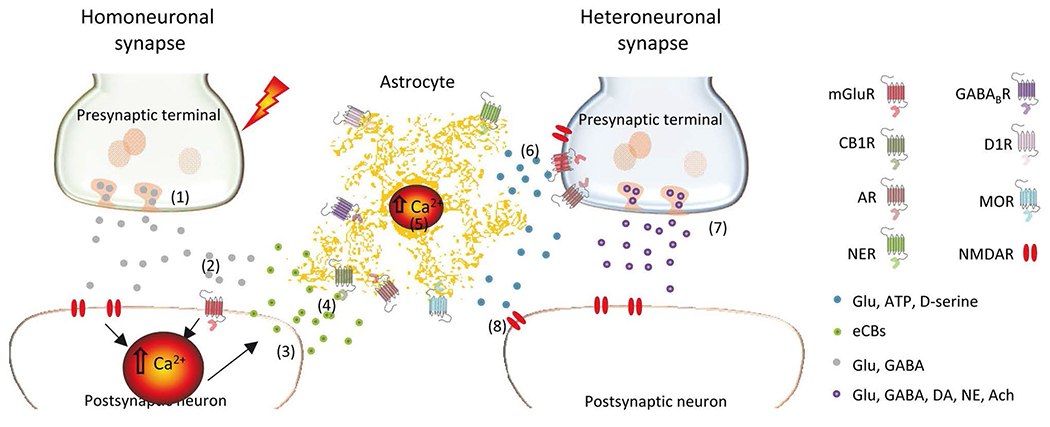
Neuron-astrocyte signaling and lateral communication. (1) the presynaptic terminal releases neurotransmitter. (2) Binding of neurotransmitter to the GPCRs (mGluRs) in the postsynaptic neuron. (3) Increase the postsynaptic calcium via PLC and release of retrograde messenger eCBs which (4) bind to the GPCRs in the astrocyte, CB1R. (5) Induction of calcium release from the ER and astrocyte calcium increase. (6) The exocytosis of endosoms-containing glitransmitters through SNARE complex induces the gliotransmitters release and, in turn, the interaction with GPCRs in the presynaptic terminal of heteroneuronal synapse. (7) Later, it is triggered the modulation of neurotransmitter release and (8) the Induction of slow inward currents dependent on extrasynaptic NMDARs. (Glu glutamate; eCBs endocannabinoids; DA dopamine; NE norepinephrine; Ach acetylcholine)
Notably, activation of Gi/o GPCRs also leads to calcium elevations in astrocytes, although the intracellular signaling pathways activated remains to be fully elucidated (see [32], for a discussion of the potential mechanisms involved). The fact that Gq GPCR activation led to cellular activation of both neurons and astrocytes, whereas the Gi/o GPCR activation led to cellular inhibition in neurons and cellular activation in astrocytes has led to suggest that inhibition is a specific property of neurons and may be fundamentally different between neurons and astrocytes [32].
Astrocytes Regulate Synaptic Transmission and Plasticity Through the Release of Gliotransmitters
The astrocyte calcium signal stimulates the release of gliotransmitters through calciumand SNARE protein-dependent processes, probably involving vesicle exocytosis [8, 33–38]. While astrocytes are known to be able to release different neuroactive substances, glutamate, d-serine and ATP/adenosine are the gliotransmitters most clearly identified [39–42]. The existence of different gliotransmitters that can distinctly impact neurotransmission in different brain areas and circuits represents clear evidence of the heterogeneity of astrocyte-induced synaptic regulation at tripartite synapse.
For example, it has been shown to depress excitatory synaptic transmission through activation of presynaptic A1 receptors in the hippocampus [43, 44] and is also responsible for the developmental regulation of the spike timing-dependent depression in the hippocampus [45]. The synaptic regulation of astrocytic ATP/adenosine has also recently been found in the nucleus accumbens, a key brain area involved in reward and addiction. Astrocytes in this nucleus respond to dopaminergic inputs from the ventral tegmental area with calcium elevations that stimulates ATP/adenosine release the consequent activation of presynaptic A1 receptors and the depression of the excitatory synaptic transmission, which is a crucial synaptic event in brain reward signaling [46].
Dr. Parpura et al., in 1994 described how glutamate released from astrocytes induced neuronal calcium elevation in astrocyte-neuron co-cultures that, however, did not occur in solitary neurons [47]. Moreover, Drs. Parpura and Haydon later described that the astrocytic calcium stimulates glutamate release to modulate adjacent neurons at physiological levels [48]. Furthermore, the modulation of synaptic transmission by astrocytic glutamate has been widely documented in the hippocampus [49–52]. Moreover, it has also been reported that glutamate mediates the spike timing-dependent depression in the barrel cortex [53] and the synaptic potentiation in the dorsal striatum [54]. In nucleus accumbens, metabotropic glutamate receptor 5 (mGluR5) in astrocytes induces Ca2+ elevations with correlated NMDAR-dependent slow inward currents which increase the excitation, raising the astrocytes as potential intermediary in neuronal adaptation [55]. In the hippocampus, the astrocytic glutamate induces a short-term potentiation of the synaptic efficacy through activation of neuronal group I metabotropic glutamate receptors (mGluRs). While endocannabinoids (eCBs) released by neurons lead to direct homosynaptic depression by activating presynaptic type 1 eCB receptors (CB1Rs), they also activate these receptors in astrocytes, leading to the astrocyte-mediated synaptic potentiation of adjacent synapses, a process termed lateral regulation of synaptic transmission [52]. Moreover, astrocytic glutamate is also involved in some forms of synaptic plasticity. Indeed, the astrocyte-induced glutamate-mediated transient potentiation can become long-term potentiation when the nitric oxide is released by postsynaptic neuron [56]. In addition, cholinergic-induced long-term potentiation (LTP) has been shown to be mediated by glutamate released by astrocytes activated by cholinergic inputs [57].
Finally, d-serine, acting as a co-agonist of the N-methyl-d-aspartate receptors (NMDARs), has been found to regulate synaptic transmission and plasticity the hippocampus and barrel cortex [58–60]. In barrel cortex, d-serine improves the cholinergic plasticity induced by whisker stimulation through astrocytic muscarinic acetylcholine receptors (mAchRs) and mediated by NMDARs [58]. In the hippocampus, d-serine has been shown to be crucial for the LTP and object recognition memory task [59, 60]. However, the main source of the d-serine is a matter of debate. While some studies point to the neurons as origin of d-serine, the astrocytes have emerged as main machinery of d-serine release [42, 61]. Nevertheless, further investigations are needed to provide the balanced point of view.
Through binding to neuronal receptors, gliotransmitters have been shown to modulate both excitatory and inhibitory synaptic transmission in many brain areas [43, 46, 50, 52–54, 62–64]. Whether different gliotransmitters are released by different astrocytes or whether single astrocytes can release different gliotransmitters is a relevant question for our understanding of the heterogeneity of both the astrocyte properties and the astrocyte-mediated synaptic regulation. We have recently investigated this issue by stimulating either single interneurons signaling to astrocytes or single astrocytes and monitoring astrocyte-mediated regulation of the excitatory synaptic transmission in the hippocampus. We found that single hippocampal astrocytes can release both glutamate and ATP/adenosine, producing a temporally distinct biphasic regulation of synaptic transmission, which consists in an initial glutamate-mediated synaptic potentiation and a delayed adenosine-mediated synaptic depression [43]. Moreover, the distinct gliotransmitter release was found to be controlled by the neuronal firing activity, suggesting that astrocytes decode neuronal signaling to produce specific regulatory consequences [43].
The heterogeneity of astrocyte-induced synaptic regulation is not only manifested by the release of different gliotransmitters, but also by the same gliotransmitter acting on different neuronal receptors in specific synapses. In the amygdala, astrocytic release of ATP/Adenosine has been shown to distinctly impact excitatory and inhibitory synaptic transmission in neurons of the same amygdala subnucleus, the centromedial amygdala. Astrocyte activation stimulates the release of ATP/adenosine that leads to the A1-mediated potentiation of inhibitory synapses and A2A-mediated depression of excitatory synapses [64]. This differential regulation of synaptic transmission is translated into the decrease of firing rate of neurons of the centromedial amygdala and a decrease of the fear responses of mice subjected to a fear conditioning paradigm. Furthermore, ATP released by astrocytes induces a short-term depression of the inhibitory synaptic transmission through postsynaptic and extrasynaptic GABAAR down regulation in neocortex [65]. In addition, ATP-derived astrocytes down regulates AMPA by P2XRs and induces depression of field potential in CA1 of hippocampus [66] and downregulates NMDARs trafficking in excitatory terminals with an important role in the induction of LTP [67].
Age-dependent regulation of synaptic transmission by astrocytes represents an important biological variable that has been relatively understudied and deserves further attention to understand the full impact of astrocytes on brain function. Yet, recent studies have addressed the impact of signaling at tripartite synapse during brain development and aging. During development, critical period of synapse function maturation plays a significant role in the establishment of properly efficient neuronal circuits. The group of Rodriguez-Moreno has elegantly shown that the spike timing-dependent plasticity (STDP) in the hippocampus is temporally regulated. The spike timing-dependent depression shown by young animals become spike timing-dependent potentiation in adult mice, a maturation phenomenon that depends on adenosine of astrocytic origin [45, 68]. In aging, neuron-astrocyte signaling has been found to be largely preserved across the lifespan of mice [69], although the decline in the astrocyte-neuron network has been observed. It has been shown a decrease in the P2X, AMPA and NMDARs-mediated miniature glial synaptic currents in old mice [70] and astrocytic Ca2+ signaling age-related decrease that underlies to the synaptic transmission modulation [71]. Together all these changes define a remodeling of synaptic strength and information processing, contributing to a cognitive impairment. In fact, synaptic plasticity is severely compromised in an Alzheimer’s disease (AD) mouse animal model deficient of astrocyte IP3R2-mediated calcium signal at early stages of the disease, indicating that astrocytes and their calcium signaling play crucial roles in the AD pathology, accelerating the progression of synaptic plasticity dysfunction [69].
Astrocytes Regulate Network Function and Animal Behavior
While much effort has been done to investigate the mechanisms underlying astrocytic regulation of synaptic transmission and plasticity and the specific their specific properties in certain synapses and brain regions, their impact on network function and animal behavior have only been initially explored. While recent reviews provide a comprehensive discussion on these issues (see e.g., [72, 73]), we will describe here some recent specific examples of the contribution of astrocytes to neural network function and behavior.
Network function results not only from the activity of glutamatergic excitatory and GABAergic inhibitory signals but also from the activity of neuromodulators like acetylcholine, dopamine or norepinephrine and cannabinoids. Astrocytes have been recently found to respond to these neuromodulators, suggesting that they can mediate their actions in the control of network activity. For example, in vivo cholinergic-induced regulation of LTP in hippocampus and cortex has been shown to be mediated by astrocytes [57, 58]. Han et al., in 2012 showed that exogenous cannabinoid induces LTD in vivo which depends on astroglial CB1R expression with an impairment in the spatial working memory as consequence of this down regulation [74]. Norepinephrine (NE) also signal to astrocytes, and the NE release associated with locomotor activity enhances the astrocyte calcium signaling as a detector of neuronal activity in different brain areas [75]. Finally, dopamine has been recently shown to activate astrocytes in the nucleus accumbens and regulate glutamatergic excitatory inputs in that region thus mediating the behavior effects of the psychostimulant amphetamine [46].
Cortical network function has been found to be regulated by astrocytes [76–80]. More recently, astrocytes in the somatosensory cortex have been shown to respond with calcium elevations to sensory stimulation in vivo that were associated with cortical gamma activity [81]. Sensory stimuli elicit a surge of neuronal network activity in the gamma range that was followed by a delayed astrocyte activity that dampens the steady-state of this activity. This sensory-evoked gamma activity increase is enhanced in IP3R2 knockout mice, in which astrocyte calcium signaling is impaired, and is decreased by pharmacogenetic stimulation of astrocytes with “designer receptor exclusively activated by designer drugs” (DREADDS), indicating that cortical astrocytes respond to sensory inputs and regulate sensory-evoked neuronal network activity maximizing its dynamic range [81]. Astrocytes in the medial prefrontal cortex, a key region involved in goal-directed behavior, have also found to alter the firing properties of cortical neurons and gamma oscillations by modulating the inhibition/excitation balance in that region [82]. Disrupting the astrocyte signaling in this network activity is manifested as working memory deficits [82].
Concluding Remarks
Since the decade of 1990, accumulating evidence of new roles of astrocytes transformed the idea of synapse function, establishing the tripartite synapsis concept that changed the view of classical neuron-neuron communication to include astrocytes as additional important underlying network activity and brain function through the concerted signaling with neurons.
Funding
This work was supported by National Institute of Neurological Disorders and Stroke [Grant No. R01NS097312] and National Institute on Drug Abuse [Grant No. R01DA048822]. National Institute of Mental Health [Grant No R01MH119355].
References
- 1.Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E et al. (2011) Role of astrocytes in brain function and disease. Toxicol Pathol 39:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasile F, Dossi E, Rouach N (2017) Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct 222:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halassa MM, Fellin T, Haydon PG (2007) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13:54–63 [DOI] [PubMed] [Google Scholar]
- 5.Perea G, Araque A (2010) GLIA modulates synaptic transmission. Brain Res Rev 63:93–102 [DOI] [PubMed] [Google Scholar]
- 6.Santello M, Calì C, Bezzi P (2012) Gliotransmission and the tripartite synapse. Adv Exp Med Biol 970:307–331. 10.1007/978-3-7091-0932-8_14 [DOI] [PubMed] [Google Scholar]
- 7.Araque A, Carmignoto G, Haydon PG et al. (2014) Gliotransmitters travel in time and space. Neuron 81:728–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savtchouk I, Volterra A (2018) Gliotransmission: beyond black-and-white. J Neurosci 38:14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durkee CA, Araque A (2019) Diversity and specificity of astrocyte-neuron communication. Neuroscience 396:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corkrum M, Rothwell PE, Thomas MJ et al. (2019) Opioid-mediated astrocyte-neuron signaling in the nucleus accumbens. Cells 8:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parpura V, Verkhratsky A (2013) Astroglial amino acid-based transmitter receptors. Amino Acids 44:1151–1158 [DOI] [PubMed] [Google Scholar]
- 12.Verkhratsky A, Rodríguez JJ, Parpura V (2012) Neurotransmitters and integration in neuronal-astroglial networks. Neurochem Res 37:2326–2338 [DOI] [PubMed] [Google Scholar]
- 13.Perea G, Araque A (2005) Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci 25:2192–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zorec R, Araque A, Carmignoto G et al. (2012) Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 10.1042/AN20110061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khakh BS, McCarthy KD (2015) Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb Perspect Biol 7:a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigetomi E, Patel S, Khakh BS (2016) Probing the complexities of astrocyte calcium signaling. Trends Cell Biol 26:300–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra-Gomes S, Sousa N, Pinto L, Oliveira JF (2018) Functional roles of astrocyte calcium elevations: from synapses to behavior. Front Cell Neurosci 11:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malarkey EB, Ni Y, Parpura V (2008) Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56:821–835 [DOI] [PubMed] [Google Scholar]
- 19.Innocenti B, Parpura V, Haydon PG (2000) Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci 20:1800–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal A, Wu PH, Hughes EG et al. (2017) Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93:587–605.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariotti L, Losi G, Sessolo M et al. (2016) The inhibitory neurotransmitter GABA evokes long-lasting Ca2+ oscillations in cortical astrocytes. Glia 64:363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharp AH, Nucifora FC, Blondel O et al. (1999) Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol 406:207–220 [PubMed] [Google Scholar]
- 23.Holtzclaw LA, Pandhit S, Bare DJ et al. (2002) Astrocytes in adult rat brain express type 2 inositol 1,4,5-trisphosphate receptors. Glia 39:69–84 [DOI] [PubMed] [Google Scholar]
- 24.Petravicz J, Fiacco TA, McCarthy KD (2008) Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 28:4967–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan R, Huang BS, Venugopal S et al. (2015) Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci 18:708–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stobart JL, Ferrari KD, Barrett MJP et al. (2018) Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 98:726–735.e4 [DOI] [PubMed] [Google Scholar]
- 27.Reyes RC, Parpura V (2008) Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci 28:9682–9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalo U (2006) NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci 26:2673–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton N, Vayro S, Kirchhoff F et al. (2008) Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia 56:734–749 [DOI] [PubMed] [Google Scholar]
- 30.Palygin O, Lalo U, Pankratov Y (2011) Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br J Pharmacol 163:1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalo U, Bogdanov A, Pankratov Y (2019) Age- and experience-related plasticity of ATP-mediated signaling in the neocortex. Front Cell Neurosci 13:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durkee CA, Covelo A, Lines J et al. (2019) G i/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 67:1076–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni Y, Malarkey EB, Parpura V (2007) Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem 103:1273–1284 [DOI] [PubMed] [Google Scholar]
- 34.Parpura V, Zorec R (2010) Gliotransmission: exocytotic release from astrocytes. Brain Res Rev 63:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agulhon C, Petravicz J, McMullen AB et al. (2008) What is the role of astrocyte calcium in neurophysiology? Neuron 59:932–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araque A, Li N, Doyle RT, Haydon PG (2000) SNARE protein-dependent glutamate release from astrocytes. J Neurosci 20:666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bezzi P, Gundersen V, Galbete JL et al. (2004) Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7:613–620 [DOI] [PubMed] [Google Scholar]
- 38.Schwarz Y, Zhao N, Kirchhoff F, Bruns D (2017) Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways. Nat Neurosci 20:1529–1539 [DOI] [PubMed] [Google Scholar]
- 39.Ni Y, Parpura V (2009) Dual regulation of Ca 2+ -dependent glutamate release from astrocytes: vesicular glutamate transporters and cytosolic glutamate levels. Glia 57:1296–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubišić V, Parpura V (2017) Two modes of enteric gliotransmission differentially affect gut physiology. Glia 65:699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montana V, Ni Y, Sunjara V et al. (2004) Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci 24:2633–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henneberger C, Papouin T, Oliet SHR, Rusakov DA (2010) Long-term potentiation depends on release of d-serine from astrocytes. Nature 463:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Covelo A, Araque A (2018) Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife 7:e32237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serrano A (2006) GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci 26:5370–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pérez-Rodríguez M, Arroyo-García LE, Prius-Mengual J et al. (2019) Adenosine receptor-mediated developmental loss of spike timing-dependent depression in the hippocampus. Cereb Cortex 29:3266–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corkrum M, Covelo A, Lines J et al. (2020) Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. Neuron 105:1036–1047.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parpura V, Basarsky TA, Liu F et al. (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747 [DOI] [PubMed] [Google Scholar]
- 48.Parpura V, Haydon PG (2000) Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA 97:8629–8634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang J, Jiang L, Goldman SA, Nedergaard M (1998) Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1:683–692 [DOI] [PubMed] [Google Scholar]
- 50.Perea G, Gómez R, Mederos S et al. (2016) Activity-dependent switch of gabaergic inhibition into glutamatergic excitation in astrocyte-neuron networks. Elife 5:e20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jourdain P, Bergersen LH, Bhaukaurally K et al. (2007) Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10:331–339 [DOI] [PubMed] [Google Scholar]
- 52.Navarrete M, Araque A (2010) Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68:113–126 [DOI] [PubMed] [Google Scholar]
- 53.Min R, Nevian T (2012) Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci 15:746–753 [DOI] [PubMed] [Google Scholar]
- 54.Martín R, Bajo-Grañeras R, Moratalla R et al. (2015) Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–734 [DOI] [PubMed] [Google Scholar]
- 55.D’Ascenzo M, Fellin T, Terunuma M et al. (2007) mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA 104:1995–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gómez-Gonzalo M, Navarrete M, Perea G et al. (2015) Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb Cortex 25:3699–3712 [DOI] [PubMed] [Google Scholar]
- 57.Navarrete M, Perea G, de Sevilla DF et al. (2012) Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol 10:e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takata N, Mishima T, Hisatsune C et al. (2011) Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31:18155–18165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robin LM, Oliveira da Cruz JF, Langlais VC et al. (2018) Astroglial CB1 receptors determine synaptic d-serine availability to enable recognition memory. Neuron 98:935–944.e5 [DOI] [PubMed] [Google Scholar]
- 60.Shigetomi E, Jackson-Weaver O, Huckstepp RT et al. (2013) TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive d-serine release. J Neurosci 33:10143–10153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papouin T, Henneberger C, Rusakov DA, Oliet SHR (2017) Astroglial versus neuronal d-serine: fact checking. Trends Neurosci 40:517–520 [DOI] [PubMed] [Google Scholar]
- 62.Andersson M, Blomstrand F, Hanse E (2007) Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol 585:843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perea G, Yang A, Boyden ES, Sur M (2014) Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat Commun 5:3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin-Fernandez M, Jamison S, Robin LM et al. (2017) Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci 20:1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lalo U, Palygin O, Rasooli-Nejad S et al. (2014) Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol 12:e1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pougnet JT, Toulme E, Martinez A et al. (2014) ATP P2X receptors downregulate AMPA receptor trafficking and postsynaptic efficacy in hippocampal neurons. Neuron 83:417–430 [DOI] [PubMed] [Google Scholar]
- 67.Lalo U, Palygin O, Verkhratsky A et al. (2016) ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD-95 multi-protein complex. Sci Rep 6:33609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falcón-Moya R, Pérez-Rodríguez M, Prius-Mengual J et al. (2020) Astrocyte-mediated switch in spike timing-dependent plasticity during hippocampal development. Nat Commun 11:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gómez-Gonzalo M, Martin-Fernandez M, Martínez-Murillo R et al. (2017) Neuron-astrocyte signaling is preserved in the aging brain. Glia 65:569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lalo U, Palygin O, North RA et al. (2011) Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell 10:392–402 [DOI] [PubMed] [Google Scholar]
- 71.Lalo U, Bogdanov A, Pankratov Y (2018) Diversity of astroglial effects on aging- and experience-related cortical metaplasticity. Front Mol Neurosci 11:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oliveira JF, Sardinha VM, Guerra-Gomes S et al. (2015) Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci 38:535–549 [DOI] [PubMed] [Google Scholar]
- 73.Kofuji P, Araque A (2020) G-protein-coupled receptors in astrocyte-neuron communication. Neuroscience S0306–4522:30177–30179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han J, Kesner P, Metna-Laurent M et al. (2012) Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148:1039–1050 [DOI] [PubMed] [Google Scholar]
- 75.Paukert M, Agarwal A, Cha J et al. (2014) Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82:1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fellin T, Halassa MM, Terunuma M et al. (2009) Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci USA 106:15037–15042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poskanzer KE, Yuste R (2011) Astrocytic regulation of cortical UP states. Proc Natl Acad Sci USA 108:18453–18458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HS, Ghetti A, Pinto-Duarte A et al. (2014) Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci USA 111:E3343–E3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poskanzer KE, Yuste R (2016) Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci USA 113:E2675–E2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sardinha VM, Guerra-Gomes S, Caetano I et al. (2017) Astrocytic signaling supports hippocampal–prefrontal theta synchronization and cognitive function. Glia 65:1944–1960 [DOI] [PubMed] [Google Scholar]
- 81.Lines J, Martin ED, Kofuji P et al. (2020) Astrocytes modulate sensory-evoked neuronal network activity. Nat Commun 11:3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mederos S, Sánchez-Puelles C, Esparza J et al. (2021) GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat Neurosci 24:82–92 [DOI] [PubMed] [Google Scholar]


