Abstract
Breast augmentation is one of the most popular cosmetic surgery procedures in the world and it requires a comprehensive study of the methods performed. As less-invasive techniques are sought, tissue fillers have found its purpose in these procedures. However, it has been revealed that some of them may be associated with serious complications. One of them is the Aquafilling/Los Deline gel. A case report of a female patient who developed unprecedented sequelae after Aquafilling injection—distant migration of the gel in the hand—was presented in this study. The patient underwent total gel removal from the left forearm, arm, and both breasts as well as wound debridement and irrigation. We discovered a canal connecting the left breast to the left forearm, created by a polyacrylamide hydrogel dislocation. It was thoroughly revised using an endoscope. Despite the advantages of tissue fillers such as simplicity of use and less invasiveness, certain complications can occur after injection. Although a few of them have been banned due to these sequelae, new ones continue to appear. Every new product should be examined very carefully before it is introduced to the market.
Keywords: breast augmentation, complications, aquafilling
Introduction
Breast augmentation remains the most popular aesthetic surgical procedure, with 1,862,506 procedures performed worldwide in 2018. Nearly 54% of patients who underwent this type of surgery in 2018 were between the ages of 19 and 34 years. 1 The most popular ones are silicone or saline breast implant insertions; however, tissue fillers for breast augmentation are becoming increasingly popular due to their simplicity of use. They do not require general anesthesia compared with the implant procedure. 2 These injections also serve the purpose of breast shape correction after implant insertion. 3
That said, apart from their advantages, certain sequelae can occur after the injection of tissue fillers. The heterogeneity of these substances makes it difficult to draw conclusions after studying single filler complications for each one of them. Moreover, new ones are constantly being developed.
One such filler is polyacrylamide gel (PAAG), which was invented in Ukraine in the late 1980s. This preparation was banned in most countries after complications associated with PAAG injections were detected, including pain, breast hardening, breast deformity, lumps, gel migration, fistulas and gel leakage. The mean time from injection to complication was 6.1 years. 4
Another tissue filler developed in the Czech Republic was Aquafilling gel (Biomedica. spol, s,r,o, Czech Republic) (the same product was later renamed as Los Deline), which was supposed to consist of a 96 to 98% solution of 0.9% sodium chloride and 2 to 4% described substance as cation co-polyamide. However, at the request of the President of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products in Poland, a chemical analysis of the filler was performed, which revealed the substance to be a polyacrylamide gel. 5 6 Although the United States Food and Drug Administration (USFDA) has not approved its use, it is still used in some countries of the European Union. 7 Incoming reports of complications after Aquafilling injections have made it a major concern regarding aesthetic plastic surgery. 8 9 10 11 It was banned in Poland in 2020; however, several patients were treated in the Department of Plastic Surgery, Medical Center of Postgraduate Education in Warsaw, after serious complications from its injection. Therefore, we present a case report of a patient who developed distinctive as well as unprecedented sequelae after Aquafilling breast augmentation with following literature review.
Case Report
A 35-year-old female patient presented herself to our plastic surgery department on August 6, 2020, with a chief complaint of numbness and pain in her left arm. She also observed a bulge near her left elbow. In January 2018, she underwent Aquafilling breast augmentation with 150 mL injected into each breast. An ultrasound scan revealed an 8 to 12 mm wide subfascial chain of fluid accumulation along the left forearm and arm, entering between the biceps brachii and pectoralis major muscles. Furthermore, the left breast became significantly smaller and slightly deformed. At the distal end of the arm and forearm, where the accumulation of fluid was located, we could observe skin protrusions. We referred her for an MRI of the left breast and arm, as well as CT of the neck, chest and abdomen. Imaging examinations showed gel migration along the left arm and forearm almost to the carpal tunnel ( Fig. 1A and B ). They also found gel in the left axilla and along axillary vessels. We marked the location of the containers on the skin preoperatively using ultrasound examination.
Fig. 1.
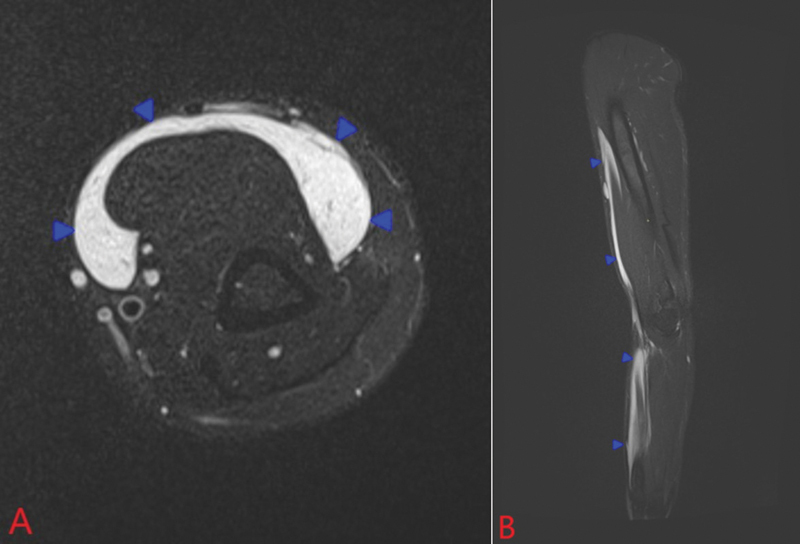
MRI T2-weighted STIR images in the axial ( A ) and sagittal ( B ) planes showing longitudinal subfascial accumulation of fluid (blue arrowheads) surrounding the anterior compartment muscles of the arm and forearm, consistent with migrated Aquafilling gel.
She underwent surgery on October 16, 2020. Under general anesthesia, we made incisions (one at each mark) on the arm and forearm, removed the gel, and took a sterile swab ( Fig. 2 ). These collections were placed in various planes, also including the subcutaneous layer. Most of them were located under the fascia, around and intramuscular to the flexor muscle compartment. We used an endoscope to check the course of the canal, which started just above the carpal tunnel entering just below the fascia, near the flexors, and then passed through the medial side of the arm, along the biceps brachii, and underneath the deltoid muscle entering the axillary pectoralis major muscle ( Fig. 3 ). We rinsed off any remnant gel.
Fig. 2.
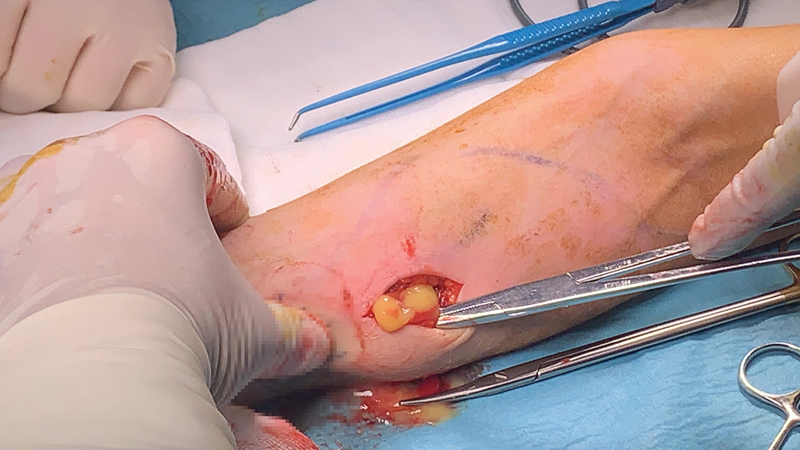
Gel draining through the incision in the left forearm.
Fig. 3.
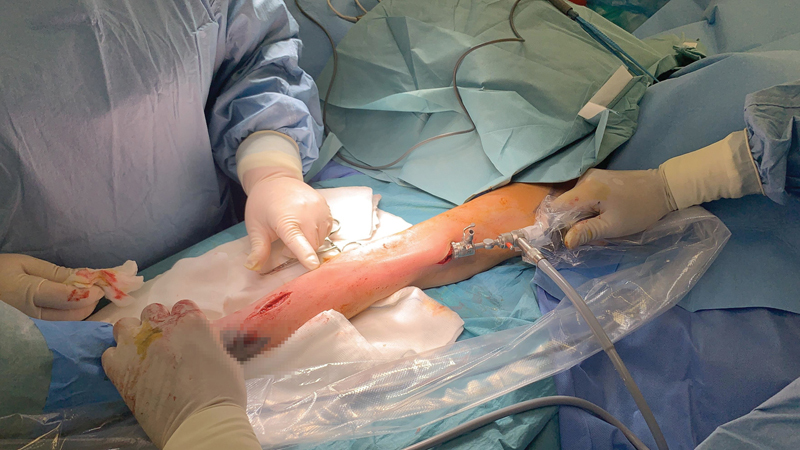
The gel creating a canal along the left forearm and arm. We checked it by endoscopy and rinsed off the remaining gel.
Subsequently, two incisions were made with one on each breast (starting from the left side) in the inframammary fold ( Fig. 4 ). Most of the gel located under the mammary gland, as well as in the axillary region, was removed along with the inflamed/necrotic part of the large pectoral muscle. Samples of the surrounding tissue and capsula were collected for histopathological examination. In the right breast, the accumulation of fluid was also located below the mammary gland, but was significantly larger ( Fig. 5 ). We irrigated the wounds of both breasts. Redon drainage tubes were inserted—two of them were placed within the arm and one in each breast. The incisions were closed by subcutaneous and intracutaneous sutures.
Fig. 4.
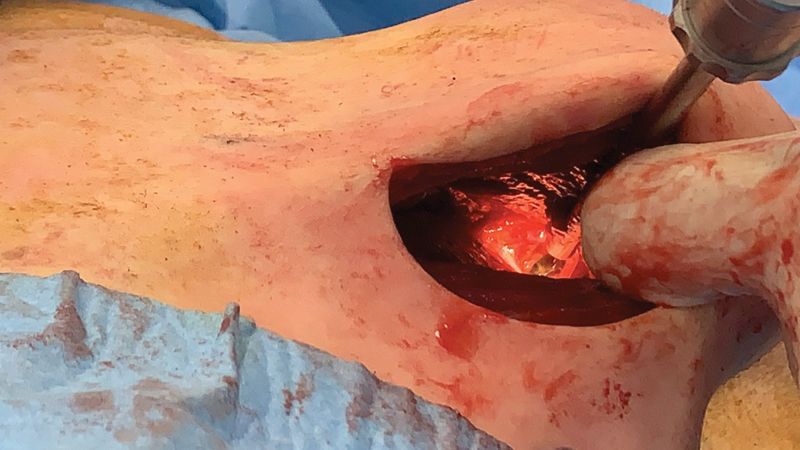
The left breast after removal of the gel as well as necrotic and inflamed tissues. In the upper outer quadrant of the left breast, there is an opening for the canal through the axilla and along the arm almost to the carpal tunnel.
Fig. 5.
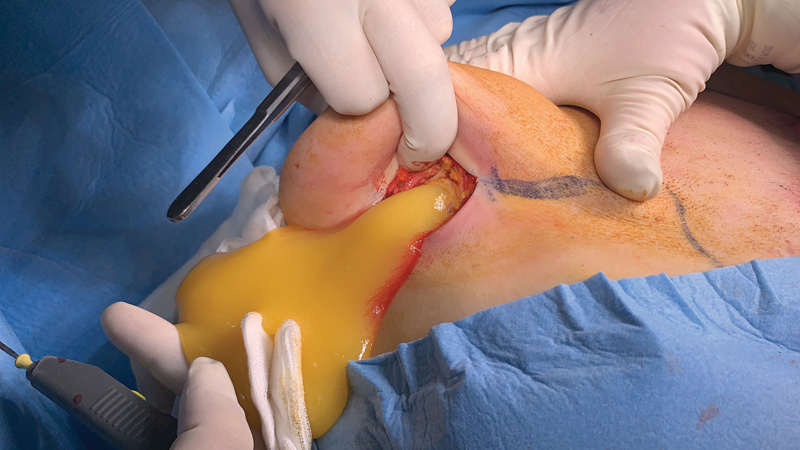
Significant amount of the gel draining through the incision in the right inframammary fold.
The postoperative course was uneventful. She was discharged from the hospital after 3 days. We recommended daily dressing changes with Octenisept cleanser (Schülke & Mayr GmbH, Norderstedt, Germany), as well as metamizole, paracetamol, and ketoprofen, for pain treatment. Moreover, clindamycin was prescribed as a prolonged antibiotic prophylaxis. The patient was seen at 1, 6, and 10 months later. There was no accumulation of fluid in the control ultrasound examination, no pain or other symptoms, and the patient waited for breast implant augmentation.
Discussion
Although silicone or saline implant insertion is extremely popular around the world, many doctors and manufacturers are still looking for a less-invasive method of breast augmentation. It has led to the use of tissue fillers for this purpose as well as the invention of several new ones. Historically, many fillers were used, such as mineral and vegetable oils, paraffin, hyaluronic acid, polyacrylamide hydrogel (PAAG), collagen, hydroxyapatite, and polymethylmethacrylate (PMMA) microspheres. 12 Despite their initial good impressions, most of them were associated with serious complications. We must emphasize that findings relevant to one filler are usually not applicable to all of them.
In this study, we focus on Aquafilling gel, which is commonly used for breast augmentation in the European Union. It is a relatively new product (invented in 2005) and, therefore, its long-term performance may not yet be assessed. However, complications associated with this filler have been reported to be occurring. 2 8 9 10 11 13 These articles address sequelae such as asymmetric scattering of the gel through the fibroglandular tissue, infiltration of the pectoralis major muscle, pain and migration of the gel into the breast, under the greater pectoral muscle, to the axilla, and even to the abdominal wall. In our case report, we describe an additional complication—gel migration into the arm and forearm. This is not the first gel filler migration report; however, according to our knowledge, it is the first case of such distal hand migration. It seems that the filler moves along the path of least resistance; however, due to its invasiveness it can cross the fascia as well.
There is ample evidence indicating that Aquafilling gel causes inflammatory responses in the surrounding tissues. 7 Complications usually occur several years after the injection. 14 We suspect that in this case, the short period of time between gel migration and operation assisted us in an uneventful postoperative course. However, macroscopic and microscopic changes in the breast tissue are usually dramatic, with some kind of necrotic and inflammatory changes having skeletonized or obstructed vessels. The spread of Aquafilling is unpredictable and associated with a diverse intraoperative view. It may be limited by a capsule as well as infiltrate the surrounding tissue. Therefore, in our opinion a modification of gel consistency cannot prevent the spread of the product. Furthermore, injection of this filler can even lead to infection and sepsis. 14 There are concerns about its impact on the human body cells as well as its toxicity.
Moreover, we lack data regarding sequelae, such as breast cancer and precancerous lesions, which have been reported after the injection of other fillers. 15 Acrylamide has been associated with neurotoxicity, genotoxicity, and carcinogenicity. However, regarding polyacrylamide, this issue is not clearly defined. 16 17 In addition, tissue fillers can interfere with cancer screening examinations. 18
Over the course of the history of tissue fillers, numerous complications have been reported, some of which were noticed only after a long period of time. 19 Complications following Aquafilling gel injection usually appear a few years later. 14 On this basis, the risk of similar scenarios should be evaluated in every case after Aquafilling injection.
The question of how to manage asymptomatic patients remains unanswered. Polyacrylamide hydrogel injections may cause breast deformities; however, their radical removal (if possible) often results in the need for corrective procedures. 20 In contrast, complications associated with tissue filler injections, once they occur, are very difficult to treat. 21 In our opinion, it is only a matter of time before a setback occurs and we recommend surgical removal of this gel in every patient.
Conclusion
Numerous tissue fillers have been invented in the course of time. Some of them, due to their toxicity and complications, were or should have been banned. However, prior to that decision, they have usually caused considerable damage. To avoid a repetition of such events, new fillers should be thoroughly examined as medicinal products before they are placed on the market. This study is an alert from us to plastic surgeons and other doctors engaging in aesthetic surgery. In our opinion, Aquafilling/Los Deline should be prohibited in every country and for every indication.
Conflict of Interest The authors declare that they have no conflict of interest. The article has not been published or presented howsoever before. The participant has consented to the submission of the case report to the journal. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' Contributions
Piotr Gierej: study design, data interpretation, and writing of the manuscript. Marcin Radziszewski: collection of data, data analysis, and writing of the manuscript. Piotr Miłoński: collection of data and data interpretation. Bartłomiej Noszczyk: study design and writing of the manuscript.
References
- 1.ISAPS International survey on aesthetic/cosmetic procedures performed in 2018Accessed March 7, 2022 from:https://www.isaps.org/wp-content/uploads/2020/10/ISAPS-Global-Survey-Results-2018-1.pdf
- 2.Jung B K, Yun I S, Kim Y S, Roh T S. Complication of AQUAfilling ® gel injection for breast augmentation: case report of one case and review of literature . Aesthetic Plast Surg. 2018;42(05):1252–1256. doi: 10.1007/s00266-018-1107-0. [DOI] [PubMed] [Google Scholar]
- 3.Shin J H, Suh J S, Yang S G. Correcting shape and size using temporary filler after breast augmentation with silicone implants. Arch Aesthetic Plast Surg. 2015;21:124–126. [Google Scholar]
- 4.Unukovych D, Khrapach V, Wickman M. Polyacrylamide gel injections for breast augmentation: management of complications in 106 patients, a multicenter study. World J Surg. 2012;36(04):695–701. doi: 10.1007/s00268-011-1273-6. [DOI] [PubMed] [Google Scholar]
- 5.Roh T S. Position statement of Korean Academic Society of Aesthetic and reconstructive breast surgery: concerning the use of Aquafilling® for breast augmentation. Arch Aesthetic Plast Surg. 2016;22(01):45–46. [Google Scholar]
- 6.Decision of the President of the Office for Registration of Medicinal Products Medical Devices and Biocidal Products in Poland, January 2020, chemical analysis performed by a team from Institute of Polymer and Dye Technology, Faculty of Chemistry, Lodz University of Technology, Poland. Accessed March 7, 2022 from:http://urpl.gov.pl/pl/decyzja-w-sprawie-wycofania-z-obrotu-i-z-u%C5%BCywania-hydrofilowego-%C5%BCelu-do-endoprotetyki-mi%C4%99kkich
- 7.Chalcarz M, Żurawski J. Injection of Aquafilling ® for breast augmentation causes inflammatory responses independent of visible symptoms . Aesthetic Plast Surg. 2021;45(02):481–490. doi: 10.1007/s00266-020-01949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arslan G, Celik L, Atasoy M M, Celik L, Cubuk R. Complication of non-US guided procedure of aquafilling breast gel. Med Ultrason. 2017;19(02):236–237. doi: 10.11152/mu-1021. [DOI] [PubMed] [Google Scholar]
- 9.Ozcan U A, Ulus S, Kucukcelebi A. Breast augmentation with Aquafilling: complications and radiologic features of two cases. Eur J Plast Surg. 2019;42:405–408. [Google Scholar]
- 10.Son M J, Ko K H, Jung H K, Koh J E, Park A Y. Complications and radiologic features of breast augmentation via injection of aquafilling gel. J Ultrasound Med. 2018;37(07):1835–1839. doi: 10.1002/jum.14527. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Chang H, Park J U. Complication of ruptured poly implant Prothèse ® breast implants combined with AQUAfilling ® gel injection: a case report and literature review . Aesthetic Plast Surg. 2019;43(01):46–52. doi: 10.1007/s00266-018-1242-7. [DOI] [PubMed] [Google Scholar]
- 12.Amin S P, Marmur E S, Goldberg D J.Complications from injectable polyacrylamide gel, a new nonbiodegradable soft tissue filler Dermatol Surg 200430(12 Pt 2):1507–1509. [DOI] [PubMed] [Google Scholar]
- 13.Nomoto S, Hirakawa K, Ogawa R. Safety of copolyamide filler injection for breast augmentation. Plast Reconstr Surg Glob Open. 2021;9(02):e3296. doi: 10.1097/GOX.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Namgoong S, Kim H K, Hwang Y. Clinical experience with treatment of aquafilling filler-associated complications: a retrospective study of 146 cases. Aesthetic Plast Surg. 2020;44(06):1997–2007. doi: 10.1007/s00266-020-01889-7. [DOI] [PubMed] [Google Scholar]
- 15.Okubo M, Hyakusoku H, Kanno K, Fumiiri M. Complications after injection mammaplasty. Aesthetic Plast Surg. 1992;16(02):181–187. doi: 10.1007/BF00450611. [DOI] [PubMed] [Google Scholar]
- 16.Dearfield K L, Douglas G R, Ehling U H, Moore M M, Sega G A, Brusick D J.Acrylamide: a review of its genotoxicity and an assessment of heritable genetic risk Mutat Res 1995330(1-2):71–99. [DOI] [PubMed] [Google Scholar]
- 17.Smith E A, Oehme F W. Acrylamide and polyacrylamide: a review of production, use, environmental fate and neurotoxicity. Rev Environ Health. 1991;9(04):215–228. doi: 10.1515/reveh.1991.9.4.215. [DOI] [PubMed] [Google Scholar]
- 18.Lin W C, Hsu G C, Hsu Y C. A late complication of augmentation mammoplasty by polyacrylamide hydrogel injection: ultrasound and magnetic resonance imaging findings of huge galactocele formation in a puerperal woman with pathological correlation. Breast J. 2008;14(06):584–587. doi: 10.1111/j.1524-4741.2008.00652.x. [DOI] [PubMed] [Google Scholar]
- 19.Peters W, Fornasier V. Complications from injectable materials used for breast augmentation. Can J Plast Surg. 2009;17(03):89–96. doi: 10.1177/229255030901700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patlazhan G, Unukovych D, Pshenisnov K. Breast reconstruction and treatment algorithm for patients with complications after polyacrylamide gel injections: a 10-year experience. Aesthetic Plast Surg. 2013;37(02):312–320. doi: 10.1007/s00266-012-0045-5. [DOI] [PubMed] [Google Scholar]
- 21.Ono S, Ogawa R, Hyakusoku H. Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plast Reconstr Surg. 2010;126(04):1349–1357. doi: 10.1097/PRS.0b013e3181ead122. [DOI] [PubMed] [Google Scholar]


