First described by Bergstrand in 1930, osteoid osteomas are benign lesions of bone. 1 The cause of osteoid osteoma is unknown, but suggested etiologies include benign neoplastic cells, trauma, and inflammation. Histologically, osteoid osteomas are composed of mature lamellar bone and have a simple karyotype with FOS and FOSB rearrangements on cytogenetic studies. 2 3 This can be utilized as diagnostic markers for osteoblastoma and osteoid osteoma. There is no known potential for malignant transformation. 3 Osteoid osteomas account for approximately 5% of all primary bone lesions and 10% of benign bone tumors. These lesions measure less than 2 cm in diameter and commonly occur between the first and third decades of life, with a male predilection of 3:1. 4 Lesions with a diameter larger than 2 cm are referred to as osteoblastoma, which has an increased incidence in patients with Gardner's syndrome, as they harbor a germline mutation in the APC gene. 2 Lesions have a predilection for long bones, particularly the diaphysis and metaphysis of the femur and tibia. 5 In the upper limb, the phalanges of the hand are the most commonly affected sites. 6
The typical clinical presentation is a dull persistent ache that gradually increases on intensity. The pain tends to be higher at night and is responsive to nonsteroidal anti-inflammatory drugs. Other symptoms include limping or progressive scoliosis with spinal lesions.
Radiological imaging is the standard of reference for diagnosis. Radiography is the first-line imaging modality and displays oval lytic lesions in cortical bone with minimal surrounding reactive sclerosis. 7 Despite the characteristic features of lesions, radiographs can often be inconclusive or normal in the presence of an osteoid osteoma ( Fig. 1 ). Differential diagnostic considerations on radiographs include a Brodie's abscess and/or an osseous stress reaction. 8 Chondroblastoma and clear cell chondrosarcoma are additional differential diagnoses of epiphyseal lesions in pediatric and young adult populations. Computed tomography (CT) is often the most accurate imaging modality, due to the ability to delineate subtle areas of sclerosis around small nidus lesions centrally. Physiological imaging such as bone scintigraphy is highly sensitive for identifying an active nidus, but the specificity is reduced due to the absence of morphological information and broad differential diagnosis of lesions and processes that present with increased radiotracer activity. 9 Single-photon emission CT (SPECT-CT) combines the metabolic information and morphological detail for characterization of lesion size, shape, anatomical location (juxta-articular, intra-articular, diaphyseal, etc.), and the extent of sclerosis. Magnetic resonance imaging (MRI) affords similar diagnostic performance than CT, with the added advantage of the absence of ionizing radiation, which is a mandatory consideration in this often young patient population. 10
Fig. 1.
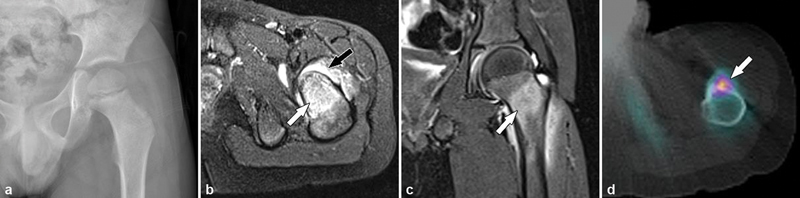
A 16-year-old boy presenting with left hip pain, which was worse at night and relieved with aspirin. ( a ) Anteroposterior pelvic radiograph was normal. The diagnosis of osteoid osteoma can sometimes be made on standard radiographs; however, as in this case, radiographs are often normal. MRI ( b, c ) characteristically demonstrated extensive bone marrow edema (white arrows) around the osteoid osteoma. However, as in this case, a high degree of bone marrow edema may mask the nidus. Note the synovitis with large joint effusion (black arrow) associated with the lesion. In such patients, CT imaging may be more sensitive in identifying a subtle nidus. Tc-99m bone scintigraphy may be useful for identifying a metabolically active lesion; however, SPECT-CT ( d ) is superior in localizing the active nidus ( d , arrow), while the CT component provides morphological information for procedural planning.
Histopathological examination can also be used in the diagnosis of osteoid osteomas ( Fig. 2 ). With hematoxylin–eosin staining, there is typically a 1- to 2-cm yellow or red nidus of osteoid and woven bone, which is surrounded by a rim of vascularized, fibrous connective tissue. There may also be sclerotic bone surrounding the lesion. Histologically, osteoid osteomas are similar to osteoblastomas and often differ only by size. 11
Fig. 2.
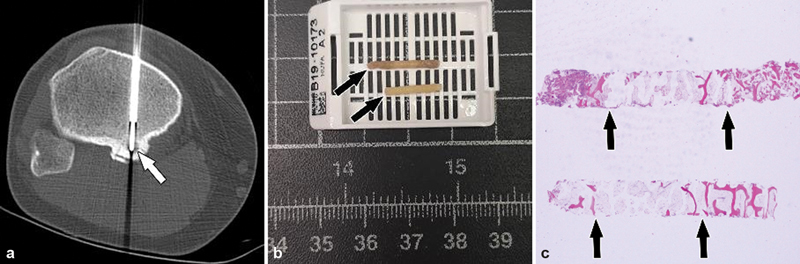
( a ) CT-guided core bone biopsy and radiofrequency ablation of an osteoid osteoma (arrow) in the posterior aspect of the proximal tibia of a young male patient. ( b ) Two core bone biopsy specimens (arrows) were obtained, which under microscopy ( c ) demonstrated a distinct boundary between the nidus and reactive bone (arrows).
Treatment Strategies
The course of osteoid osteomas is long but self-limiting with lesions usually healing over 18 to 24 months. Thus, some osteoid osteomas can be managed conservatively ( Fig. 3 ) with nonsteroidal anti-inflammatory drugs alone 12 ; however, a subset of patients requires invasive treatment if there is insufficient pain control. Surgical resection or curettage treatments have success rates of 88 to 97%, 13 but large resection margins, prolonged hospitalization, need for rehabilitation, and risk of pathological fractures are limitations. Percutaneous thermal ablation treatments, including radiofrequency, cryoablation, and laser ablation, have replaced open surgery as the first-line minimally invasive treatment at many institutions worldwide.
Fig. 3.
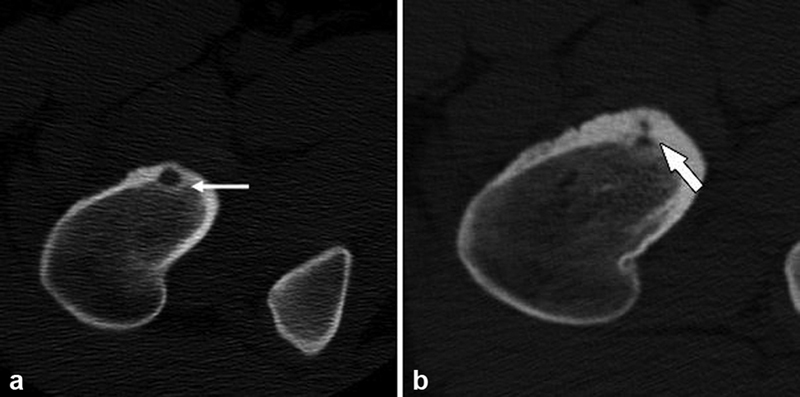
A 17-year-old patient with right hip pain and confirmed proximal femoral osteoid osteoma ( a ) with a lucent nidus (white arrow) who declined percutaneous ablation treatment. One year later, repeat CT imaging ( b ) following conservative treatment with nonsteroidal anti-inflammatory drugs demonstrated radiological healing with involution of the nidus and progressive sclerosis (white arrow). Delayed symptom regression has been reported with osteoid osteoma. 7 14
Numerous studies have shown that radiofrequency ablation treatment has similar success rates to open surgical resection albeit with a significantly reduced risk of complications. 14 15 16 17 During radiofrequency ablation, CT or MRI is used to localize the lesion and plan the needle trajectory ( Fig. 4 ). 18 19 Multiplanar evaluation of 3D CT and MRI data or multiaxial 2D MRI is used to ensure optimal placement of the needle within the nidus. After the skin is prepped and appropriate anesthetic administrated, the trajectory of the needle path is carefully planned to avoid neurovascular damage. An 11- to 14-gauge needle is advanced into the lesion under image guidance.
Fig. 4.
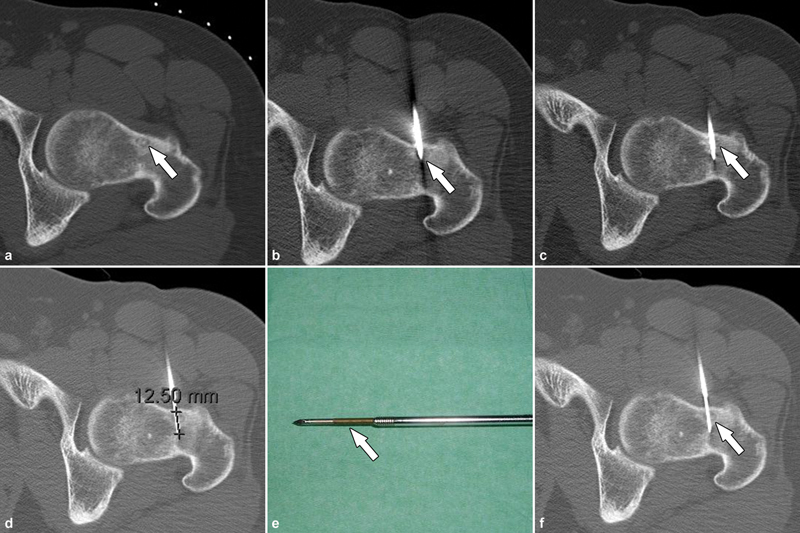
CT-guided percutaneous radiofrequency ablation under general anesthesia in supine position in a 25-year-old male presenting with left hip pain. ( a ) The sclerotic central nidus (arrow) is characteristically surrounded by a hypoattenuating halo and surrounding sclerotic reactive bone. ( b ) A 13-gauge coaxial bone drill system was utilized to penetrate the healthy cortical bone (arrow) to reach the central aspect of nidus ( c ). ( d ) To prevent unintentional supraphysiological heating of adjacent tissues along the tract of the trocar through heat conduction, the cannula with the trocar was partially withdrawn by ≥1 cm, while leaving the electrode tip ( e , arrow) in place before commencing ablation. ( f ) Once the electrode tip was located just beyond the central aspect of the nidus (arrow), ablation was initiated. As in this case, ablation often requires a single cycle lasting 4 minutes at 90 °C, but up to three treatment cycles may be performed depending on the size of the nidus to achieve a satisfactory ablation zone.
In some cases, where there is prominent perilesional sclerotic bone, a drill can be passed over a guide-wire to create a path for the needle to pass across the deep lesion margin. Once the needle is confirmed to be within the nidus, an electrode is passed through a trocar (commonly 14 gauge) into the center of the nidus, adopting a coaxial technique. At this point, the electrode is connected to the radiofrequency generator. 20 The ablation zone can reach up to 90 °C in temperature. A distance of at least 1 cm away from heat-sensitive structures is recommended and thermoprotective measures are implemented. 21 22 MRI-guided procedures offer the use of MR thermometry to monitor the temperature of surrounding tissues. 23 After ablation, patients can usually resume bearing weight on the same day. With a reported complication rate of only 3%, percutaneous thermal ablation is a management option with favorable safety profiles for treating osteoid osteoma and is therefore currently considered the best first-line therapeutic strategy in cases refractory to conservative management. 24 25 Figs. 5 to 7 present three other patients treated with thermal ablation techniques.
Fig. 5.
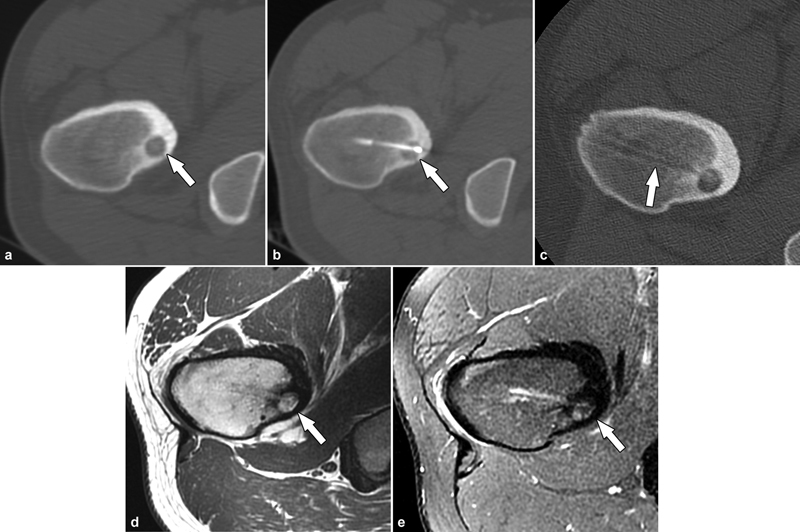
Percutaneous radiofrequency ablation of an osteoid osteoma in an 18-year-old patient. ( a ) Axial CT demonstrated the lucent nidus of the osteoid osteoma in the proximal femur (arrow) with surrounding reactive sclerosis. ( b ) Successful radiofrequency ablation of the nidus (arrow). ( c ) Final CT images demonstrated the needle track and the bony tunnel (arrow). Follow-up MRI at 12 months utilizing axial T1-weighted ( d ) and STIR ( e ) MR images demonstrated healing of the osteoid osteoma (arrows) and resolution of the bone marrow edema. The patient was pain-free postprocedure.
Fig. 7.
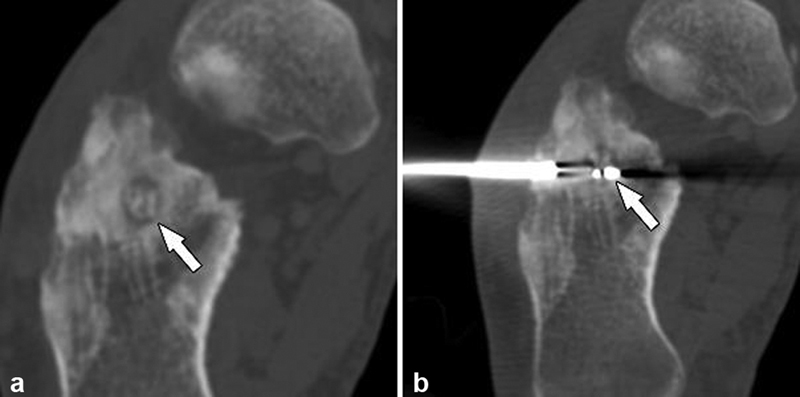
Final patient presentation with an osteoid osteoma in the calcaneus subjacent to the subtalar joint ( a , arrow) with ablation probe placement through a lateral approach ( b , arrow).
Fig. 6.
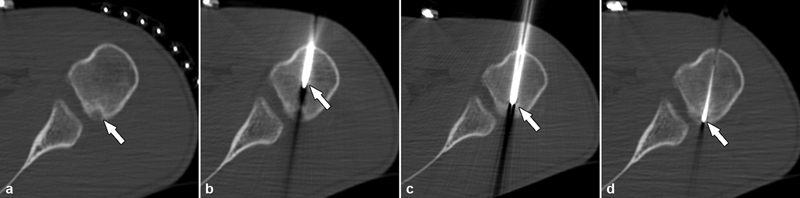
Percutaneous radiofrequency ablation of an intra-articular osteoid osteoma in a 24-year-old male. ( a ) Axial CT image demonstrated an osteoid osteoma within the cortex of the proximal humerus, abutting the articular surface of the humeral head at the inferior margin of the glenohumeral joint (arrow). ( b ) The trocar (arrow) was advanced to reach the lesion. After removing the trocar obturator ( c , arrow), the electrode ( d , arrow) was inserted to commence the ablation process. Intra-articular lesions are often associated with substantial synovitis, pain, and motion restriction as in this case where thermoprotective measures were not adopted; the patient developed painful synovitis requiring morphine. Joint distension with dextrose 5% can be used to provide thermoprotection during radiofrequency ablation with continuous administration to distant cartilage and neurovascular bundles away from the ablation zone. Intra-articular injection of local anesthetic and steroids may be beneficial to reduce postprocedural pain.
Another thermal ablation technique for managing osteoid osteoma is interstitial laser ablation (ILA), which is widely used in treating thyroid nodules, liver cancer, renal cell carcinoma, and other diseases. 26 Laser ablation involves tissue coagulation, vaporization, and degradation through the heat produced by the tissue that absorbs the laser. 18 The initial planning of the procedure is similar to radiofrequency ablation. Using CT or MRI guidance, imaging is used initially to locate the lesion and plan the needle trajectory. A coaxial needle technique is typically used to target the lesion. The optical laser fibers are then passed through a cannula and activated. The laser is activated at multiple sites to ensure adequate destruction of tissue. ILA has been shown to have a high efficacy of over 99% in some studies 27 and a very low incidence of adverse effects (1%).
Other thermoablation techniques include cryoablation and high-intensity focused ultrasound (HIFU). 28 Cryoablation involves the use of a liquid–gas; usually, argon, which flows, circulates through the tip of the cryoprobe in a closed system to form an ice ball during freezing cycles, 19 29 30 which is then followed by the thawing of the ice through similarly circulating helium gas. Complete osteoid osteoma cell death is achieved with temperatures of less than −40 °C. 31 32 This method has been used successfully to treat osteoid osteomas and osteoblastomas. 28 However, multiple probes may be required to target the larger nidus of osteoblastoma. 33
Transcutaneous HIFU describes the delivery of 4 to 400 MHz of ultrasound waves via an external probe to deliver 65 to 85 °C thermal ablation to osteoid osteomas, resulting in coagulative necrosis with both pain palliation and locoregional neoplastic cell death. MRI-guided HIFU has been applied to bladder, prostate, and uterine tumors, and may be successful in treating osteoid osteomas as well. 34 35
In conclusion, osteoid osteoma is a benign bone lesion that can cause pain of varying intensities over a prolonged period in predominantly young patients. When conservative treatment fails, radiofrequency ablation, ILA, and cryoablation are safe and effective modalities for osteoid osteomas and preferred over open surgery in many institutions. Radiofrequency ablation has the broadest scientific body of evidence. 34 36 37 38 39 40 41 Transcutaneous HIFU is an emerging technique with promising but early results. 42
References
- 1.Bergstrand H. Uuber Eine Eigenartige, Wahrscheinlich Bisher Nicht Beschriebene Osteoblastische Krankheit in Den Langen Knochen Der Hand Und Des Fusses. Acta Radiol. 1930;11(06):596–613. [Google Scholar]
- 2.Franceschini N, Lam S W, Cleton-Jansen A M, Bovée J VMG. What's new in bone forming tumours of the skeleton? Virchows Arch. 2020;476(01):147–157. doi: 10.1007/s00428-019-02683-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fittall M W, Mifsud W, Pillay N. Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun. 2018;9(01):2150. doi: 10.1038/s41467-018-04530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward W G, Eckardt J J, Shayestehfar S, Mirra J, Grogan T, Oppenheim W. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop Relat Res. 1993;291(291):229–235. [PubMed] [Google Scholar]
- 5.Barei D P, Moreau G, Scarborough M T, Neel M D. Percutaneous radiofrequency ablation of osteoid osteoma. Clin Orthop Relat Res. 2000;373(373):115–124. doi: 10.1097/00003086-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Kitsoulis P, Mantellos G, Vlychou M. Osteoid osteoma. Acta Orthop Belg. 2006;72(02):119–125. [PubMed] [Google Scholar]
- 7.Chai J W, Hong S H, Choi J Y. Radiologic diagnosis of osteoid osteoma: from simple to challenging findings. Radiographics. 2010;30(03):737–749. doi: 10.1148/rg.303095120. [DOI] [PubMed] [Google Scholar]
- 8.Boscainos P J, Cousins G R, Kulshreshtha R, Oliver T B, Papagelopoulos P J. Osteoid osteoma. Orthopedics. 2013;36(10):792–800. doi: 10.3928/01477447-20130920-10. [DOI] [PubMed] [Google Scholar]
- 9.Cazzato R L, Garnon J, De Marini P. French multidisciplinary approach for the treatment of MSK tumors. Semin Musculoskelet Radiol. 2020;24(03):310–322. doi: 10.1055/s-0040-1710052. [DOI] [PubMed] [Google Scholar]
- 10.Dalili D, Isaac A, Bazzocchi A. Interventional techniques for bone and musculoskeletal soft tissue tumors: current practices and future directions - part I. ablation. Semin Musculoskelet Radiol. 2020;24(06):692–709. doi: 10.1055/s-0040-1719103. [DOI] [PubMed] [Google Scholar]
- 11.Zileli M, Cagli S, Basdemir G, Ersahin Y. Osteoid osteomas and osteoblastomas of the spine. Neurosurg Focus. 2003;15(05):E5. [PubMed] [Google Scholar]
- 12.Aiba H, Hayashi K, Inatani H. Conservative treatment for patients with osteoid osteoma: a case series. Anticancer Res. 2014;34(07):3721–3725. [PubMed] [Google Scholar]
- 13.Park J H, Pahk K, Kim S, Lee S H, Song S H, Choe J G. Radionuclide imaging in the diagnosis of osteoid osteoma. Oncol Lett. 2015;10(02):1131–1134. doi: 10.3892/ol.2015.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kransdorf M J, Stull M A, Gilkey F W, Moser R P., Jr Osteoid osteoma. Radiographics. 1991;11(04):671–696. doi: 10.1148/radiographics.11.4.1887121. [DOI] [PubMed] [Google Scholar]
- 15.Levine E, Neff J R. Dynamic computed tomography scanning of benign bone lesions: preliminary results. Skeletal Radiol. 1983;9(04):238–245. doi: 10.1007/BF00354124. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal D I, Springfield D S, Gebhardt M C, Rosenberg A E, Mankin H J. Osteoid osteoma: percutaneous radio-frequency ablation. Radiology. 1995;197(02):451–454. doi: 10.1148/radiology.197.2.7480692. [DOI] [PubMed] [Google Scholar]
- 17.Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol. 2005;56(03):403–408. doi: 10.1016/j.ejrad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Kaul D, Bonhomme O, Schwabe P, Gebauer B, Streitparth F. Osteoid osteoma with a multicentric nidus: interstitial laser ablation under MRI guidance. Case Rep Orthop. 2013;2013:254825. doi: 10.1155/2013/254825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sequeiros R B, Sinikumpu J J, Ojala R, Järvinen J, Fritz J. Pediatric musculoskeletal interventional MRI. Top Magn Reson Imaging. 2018;27(01):39–44. doi: 10.1097/RMR.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 20.Jankharia B, Burute N. Percutaneous radiofrequency ablation for osteoid osteoma: how we do it. Indian J Radiol Imaging. 2009;19(01):36–42. doi: 10.4103/0971-3026.44523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecigne R, Garnon J, Cazzato R L. Transforaminal insertion of a thermocouple on the posterior vertebral wall combined with hydrodissection during lumbar spinal radiofrequency ablation. AJNR Am J Neuroradiol. 2019;40(10):1786–1790. doi: 10.3174/ajnr.A6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cazzato R L, Palussière J, Auloge P. Complications following percutaneous image-guided radiofrequency ablation of bone tumors: A 10-year dual-center experience. Radiology. 2020;296(01):227–235. doi: 10.1148/radiol.2020191905. [DOI] [PubMed] [Google Scholar]
- 23.Garnon J, Cazzato R L, Caudrelier J. Adjunctive thermoprotection during percutaneous thermal ablation procedures: review of current techniques. Cardiovasc Intervent Radiol. 2019;42(03):344–357. doi: 10.1007/s00270-018-2089-7. [DOI] [PubMed] [Google Scholar]
- 24.Lindner N J, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wörtler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br. 2001;83(03):391–396. doi: 10.1302/0301-620x.83b3.11679. [DOI] [PubMed] [Google Scholar]
- 25.de Berg J C, Pattynama P MT, Obermann W R, Bode P J, Vielvoye G J, Taminiau A HM.Percutaneous computed-tomography-guided thermocoagulation for osteoid osteomas Lancet 1995346(8971):350–351. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Kang J, Golas B J, Yeung V W, Madoff D C. Minimally invasive local therapies for liver cancer. Cancer Biol Med. 2014;11(04):217–236. doi: 10.7497/j.issn.2095-3941.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangi A, Alizadeh H, Wong L, Buy X, Dietemann J L, Roy C. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology. 2007;242(01):293–301. doi: 10.1148/radiol.2421041404. [DOI] [PubMed] [Google Scholar]
- 28.Cazzato R L, Auloge P, Dalili D. Percutaneous image-guided cryoablation of osteoblastoma. AJR Am J Roentgenol. 2019;213(05):1157–1162. doi: 10.2214/AJR.19.21390. [DOI] [PubMed] [Google Scholar]
- 29.Whitmore M J, Hawkins C M, Prologo J D.Cryoablation of osteoid osteoma in the pediatric and adolescent population J Vasc Interv Radiol 20162702232–237., quiz 238 [DOI] [PubMed] [Google Scholar]
- 30.Dalili D, Isaac A, Roshidi A, Åström G, Fritz J. Image-guided sports medicine and musculoskeletal tumor interventions: a patient-centered model. Semin Musculoskelet Radiol. 2020;24(03):290–309. doi: 10.1055/s-0040-1710065. [DOI] [PubMed] [Google Scholar]
- 31.Rybak L D. Fire and ice: thermal ablation of musculoskeletal tumors. Radiol Clin North Am. 2009;47(03):455–469. doi: 10.1016/j.rcl.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Gage A A, Baust J G. Adenine Press; 2004. Cryosurgery for Tumors - A Clinical Overview; pp. 187–199. [DOI] [PubMed] [Google Scholar]
- 33.Fritz J, Sonnow L, Morris C D. Adjuvant MRI-guided percutaneous cryoablation treatment for aneurysmal bone cyst. Skeletal Radiol. 2019;48(07):1149–1153. doi: 10.1007/s00256-018-3115-1. [DOI] [PubMed] [Google Scholar]
- 34.Napoli A, Bazzocchi A, Scipione R. Noninvasive therapy for osteoid osteoma: a prospective developmental study with MR Imaging-guided high-intensity focused ultrasound. Radiology. 2017;285(01):186–196. doi: 10.1148/radiol.2017162680. [DOI] [PubMed] [Google Scholar]
- 35.Yeo S Y, Elevelt A, Donato K. Bone metastasis treatment using magnetic resonance-guided high intensity focused ultrasound. Bone. 2015;81:513–523. doi: 10.1016/j.bone.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Napoli A, Anzidei M, Marincola B C. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics. 2013;33(06):1555–1568. doi: 10.1148/rg.336125162. [DOI] [PubMed] [Google Scholar]
- 37.Bazzocchi A, Napoli A, Sacconi B.MRI-guided focused ultrasound surgery in musculoskeletal diseases: the hot topics Br J Radiol 201689(1057):2.0150358E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masciocchi C, Zugaro L, Arrigoni F. Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: a propensity score matching study. Eur Radiol. 2016;26(08):2472–2481. doi: 10.1007/s00330-015-4111-7. [DOI] [PubMed] [Google Scholar]
- 39.Niazi G E, Basha M AA, Elsharkawi W FA, Zaitoun M MA. Computed tomography-guided radiofrequency ablation of osteoid osteoma in atypical sites: efficacy and safety in a large case series. Acad Radiol. 2021;28(02):68–76. doi: 10.1016/j.acra.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 40.Costanzo A, Sandri A, Regis D.CT-guided radiofrequency ablation of osteoid osteoma using a multi-tined expandable electrode system Acta Biomed 201788(4S):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beyer T, van Rijswijk C SP, Villagrán J M. European multicentre study on technical success and long-term clinical outcome of radiofrequency ablation for the treatment of spinal osteoid osteomas and osteoblastomas. Neuroradiology. 2019;61(08):935–942. doi: 10.1007/s00234-019-02226-9. [DOI] [PubMed] [Google Scholar]
- 42.Cazzato R L, Garnon J, Koch G.Musculoskeletal interventional oncology: current and future practices Br J Radiol 202093(1115):2.0200465E7. 10.1259/bjr.20200465 [DOI] [PMC free article] [PubMed] [Google Scholar]


