Abstract
Complications of overshunting, including hepatic encephalopathy and hepatic insufficiency, remain prevalent following transjugular intrahepatic portosystemic shunt (TIPS) creation. Smaller diameter TIPS may reduce the risk of overshunting, but the use of smaller stents must be weighed against the risk of undershunting and persistent or recurrent hemorrhage, ascites, and other complications of portal hypertension. This article explores the question of optimal shunt diameter by examining outcomes for smaller diameter TIPS stent-grafts (<10 mm), underdilated stent-grafts, and variable diameter stent-grafts.
Keywords: TIPS, small diameter, controlled expansion, interventional radiology, portal hypertension
Transjugular intrahepatic portosystemic shunt (TIPS) creation is a mainstay in the treatment paradigm for complications of portal hypertension, particularly variceal bleeding and refractory ascites. 1 2 3 4 5 Important strides have been made to improve patency since the early TIPS studies using bare metal stents. However, post-TIPS hepatic encephalopathy (HE) remains prevalent with 1-year incidence rates of 21 to 57%. 1 3 6 7 8 9 Although multifactorial in nature, HE depends on the degree of portosystemic shunting and hepatic dysfunction. Poiseuille's law describes the relationship between portosystemic flow and stent dimensions, including diameter to the fourth power and length ( Fig. 1 ). For the interventional radiologist, prescribing a stent diameter and target portosystemic pressure gradient (PSPG) is necessary to balance adequate portal decompression with the risk of HE, hepatic insufficiency, and right heart failure ( Fig. 1 ).
Fig. 1.
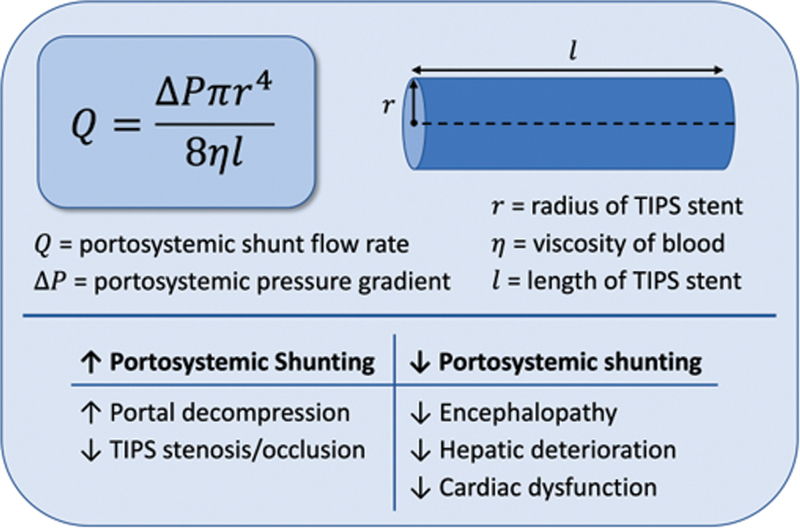
Poiseuille's equation applied to a transjugular intrahepatic portosystemic shunt (TIPS) stent with clinical considerations for degree of portosystemic shunting.
Initial considerations of TIPS stent diameter were derived from surgical portosystemic shunt data. Compared to total portal decompression with a large-diameter portocaval shunt (16–mm end-to-side anastomosis or graft), smaller and partial portocaval shunts (8-mm side-to-side H-graft) demonstrated higher residual PSPG but significantly lower rates of HE, with acceptably low risk of recurrent variceal bleeding and acceptable long-term patency. 10 11 12 13 As surgical care evolved, smaller diameter surgical partial portocaval shunts became the standard of care.
Shunt diameter (range: 6–10 mm) inversely correlates with post-TIPS creation PSPG, 14 and each 1-mm Hg decrease in PSPG is estimated to increase the risk of HE by approximately 20%. 15 For esophageal variceal bleeding, a hemodynamic target of PSPG <12 mm Hg is recommended by current guidelines 4 since associated bleeding was seen most commonly with PSPG ≥ 12 mm Hg. 16 17 18 Alternatively, a 50% reduction in PSPG from pre-TIPS baseline is also espoused by some investigators. Studies note a mortality decrease when PSPG was decreased to <12 mm Hg or by 20% from baseline through medication, TIPS, or surgical shunt. 18 19 The hemodynamic targets for gastric varices and recurrent ascites are proposed to be lower gradients, but remain controversial and need further investigation. 4 In addition, autonomic activity, volume status, general anesthesia, recent oral intake, and use of beta blockade or somatostatin may result in considerable uncertainty, variability, or inaccuracy of measured PSPG.
Early use of bare stents generally focused on stents with diameters of 10 to 14 mm, in part because of the expected pseudointimal hyperplasia, in part because of the expected recoil, which resulted in a smaller TIPS diameter than the nominal stent diameter. The early use of stent-grafts was likewise focused on these sizes, and the first Food and Drug Administration (FDA)-approved device was available in 8-, 10-, and 12-mm models (Viatorr TIPS stent graft [VTS]; W. L. Gore, Flagstaff, AZ). Given high reported rates of post-TIPS HE and the near elimination of pseudointimal hyperplasia and recoil by using impermeable polymers and more robust stents, several studies explored the safety and efficacy of smaller diameter 8-mm stents compared with standard 10-mm stent-grafts (Viatorr; or Fluency; Bard, Murray Hill, NJ). Table 1 summarizes the overall incidences for reported outcomes, including post-TIPS PSPG, HE, variceal bleeding, and the need for large-volume paracentesis (LVP).
Table 1. Studies comparing 8-mm stents to 10-mm stents.
| Study group | Stent size | n | Population | Pre-HE | Post-PSPG (mm Hg) | Post-HE | Post-variceal bleeding | Post-LVP |
|---|---|---|---|---|---|---|---|---|
| Riggio et al 9 | 8 mm | 22 | Variceal bleeding, refractory ascites | 27% | 8.9 ± 2.7 a | 50% | 8% | 60% a |
| 10 mm | 23 | Variceal bleeding, refractory ascites | 13% | 6.5 ± 2.7 a | 48% | 0% | 7% a | |
| Miraglia et al 20 | 8 mm | 111 | Refractory ascites | 32% | 7.5 ± 2.6 a | 41% | 3% | 58% a |
| 10 mm | 60 | Refractory ascites | 33% | 6.6 ± 3.4 a | 44% | 10% | 31% a | |
| Wang et al 21 | 8 mm | 64 | Variceal bleeding | NR | 8.2 ± 3.0 | 30% a | 20% | – |
| 10 mm | 63 | Variceal bleeding | NR | 7.4 ± 3.0 | 61% a | 16% | – | |
| Trebicka et al 23 | 8 mm | 41 | Variceal bleeding, refractory ascites | 27% | NR | NR | NR | NR |
| 10 mm b | 41 | Variceal bleeding, refractory ascites | 34% | NR | NR | NR | NR | |
| Luo et al 22 | 8 mm | 32 | Variceal bleeding | 0% | 9.2 ± 3.5 | 25% a | 31% | – |
| 10 mm | 32 | Variceal bleeding | 0% | 7.4 ± 3.7 | 47% a | 15% | – |
Abbreviations: PSPG, portosystemic pressure gradient; HE, hepatic encephalopathy; LVP, large-volume paracentesis; NR, not reported.
Note: Data indicate overall incidence of clinical outcome unless otherwise specified.
Reported statistical significance between 8- and 10-mm groups.
Subgroup included patients with under-dilated 10-mm stent.
Current techniques and devices allow an interventionalist to prescribe a diameter of TIPS for better control of shunting and more precise balancing between the risks of clinical failure and HE. This article reviews the techniques and outcome data for small diameter, underdilated, and variable diameter TIPS stents.
8- versus 10-mm Diameter TIPS
A randomized controlled trial by Riggio et al comparing 8- versus 10-mm first-generation VTS in a mixed cohort of variceal bleeding and refractory ascites was terminated early at 45 patients due to high rates of portal HTN complications in the 8-mm group. 9 The 1-year probability of freedom from portal HTN complications (variceal bleeding or refractory ascites) was 83% in the 10-mm group versus 42% in the 8-mm group. No difference in post-TIPS HE was observed, possibly due to small sample size and early study termination. Similarly, in a retrospective cohort study of 171 patients with refractory ascites, Miraglia et al demonstrated comparable rates of HE but a significantly higher need for paracentesis in the 8-mm group. 20 Both studies reported significantly higher post-PSPG with the use of 8-mm stent-grafts ( Table 1 ).
In contrast, in a randomized controlled trial of 82 patients receiving TIPS for variceal bleeding, Wang et al concluded that compared with 10-mm stent-grafts, 8-mm stent-grafts resulted in comparable rates of recurrent variceal bleeding with a significantly lower risk of HE (2-year incidence of 27 vs. 43%), comparable TIPS dysfunction (2-year incidence of 16 vs. 16%), and significantly better hepatic function. 21 Luo et al confirmed similar findings in a retrospective propensity-score–matched cohort of 62 patients with TIPS placement for variceal bleeding. In the 8-mm group, the risk of HE was significantly lower, whereas cumulative 1- and 3-year rates of variceal bleeding were not significantly higher. 22
While none of these studies reported a difference in overall survival, Trebicka et al reported improved transplant-free survival in patients who underwent TIPS creation using 8-mm stent-grafts when propensity matched for age, Model for End-Stage Liver Disease (MELD) score, and serum bilirubin. 23 However, the matched groups still differed by significantly lower Child-Pugh class and a higher proportion of TIPS indication of variceal bleeding in the 8-mm group.
These studies arrived at significantly different conclusions in different patient populations: two studies favored 8-mm stent-grafts in Chinese populations with variceal bleeding and two studies favored 10-mm stent-grafts in Italian populations with predominantly refractory ascites. Additional sources of heterogeneity included degree of liver dysfunction, differences in technical approach including concomitant variceal embolization, follow-up protocol and duration, and definition of outcomes. A meta-analysis of these studies concluded that 8-mm stent-grafts result in a significantly lower risk of HE, similar risk of variceal rebleeding, higher rate of stent dysfunction, and improved 1 - and 3-year overall survival. 24
Smaller than 8-mm Diameter TIPS
A few studies have investigated smaller diameter stents underdilated to less than 8 mm ( Table 2 ). Schepis et al demonstrated promising safety and efficacy of small underdilated stents (6 or 7 mm) compared to nominally dilated stents (8 or 10 mm) for refractory ascites and variceal bleeding in a prospective, nonrandomized study. 14 Stent diameter, 6 to 10 mm, inversely correlated with post-PSPG and percent change in PSPG. 14 While only half of underdilated stent-grafts (6–7 mm) reached the target post-PSPG of <12 mm Hg indicated for variceal bleeding, underdilated stents demonstrated comparable 1-year probability of remaining free from LVP (75 vs. 79%), no cases of recurrent variceal bleeding, comparable frequency of suspected TIPS dysfunction (11 vs. 14%), and significantly lower post-HE (27 vs. 54%). These findings were further confirmed in a prospective validation group of patients with stent-grafts underdilated to only 6 mm. 14
Table 2. Studies comparing underdilated <8-mm stents to nominally dilated ≥8 mm.
| Study group | Stent size | n | Population | Pre-HE | Post-PSPG (mm Hg) | Post-HE | Post-variceal bleeding | Post-LVP |
|---|---|---|---|---|---|---|---|---|
| Schepis et al 14 | 6–7 mm | 42 | Variceal bleeding, refractory ascites | 5% | 11.3 ± 3.7 | 26% a | 0% | 75% b |
| 8–10 mm | 53 | Variceal bleeding, refractory ascites | 8% | 10.5 ± 5.2 | 53% a | 0% | 79% b | |
| Liu et al 25 | 6 mm | 73 | Variceal bleeding | NR c | 11.0 ± 3.8 | 11% a | 10% | – |
| 8 mm | 61 | Variceal bleeding | NR c | 10.6 ± 3.8 | 30% a | 10% | – | |
| Yao et al 30 | 6 mm | 33 | Variceal bleeding | NR | 11.1 ± 3.8 | 70% d | 9% | – |
| 8 mm | 33 | Variceal bleeding | NR | 10.8 ± 3.7 | 79% d | 18% | – |
Abbreviations: HE, hepatic encephalopathy; LVP, large volume paracentesis; NR, not reported; PSPG, portosystemic pressure gradient.
Note: Data indicate overall incidence of clinical outcome unless otherwise specified.
Reported statistical significance between 8- and 10-mm groups.
Outcome reported is 1-year freedom from LVP.
Not specifically reported, though study used an exclusion criterion of HE within 3 months of transjugular intrahepatic portosystemic shunt.
Outcome reported is cumulative freedom from overt HE.
Similar findings were observed in a retrospective case–control study of 134 patients investigating 8-mm self-expanding stent-grafts underdilated to 6 mm versus nominally dilated 8-mm stent-grafts to treat variceal bleeding. 25 Only 70% of underdilated stent-grafts and 75% of nominally dilated stent-grafts achieved target PSPG <12 mm Hg; however, the remainder of patients achieved ≥50% reduction in PSPG. Underdilated stent-grafts demonstrated significantly lower HE, comparable rates of recurrent variceal bleeding, and comparable rates of TIPS dysfunction (11 vs. 13%). Although the mean diameter of underdilated stent-grafts increased spontaneously from 6.0 to 8.0 mm at 3 months, observed improvement in HE may be attributed to higher frequency of post-HE in the early months after TIPS creation when underdilated stents had not fully self-expanded. 8
Passive Dilation
Passive dilation of an underdilated self-expanding stent occurs when the intrinsic radial force of the stent gradually overcomes the stiffness of the surrounding cirrhotic liver parenchyma. It was initially believed that the stiffness of the cirrhotic liver parenchyma and portal vein wall would prevent passive dilation of the nitinol-framed VTS. However, this has been clearly demonstrated to be false. For a 10-mm stent-graft underdilated to 8 mm, self-dilation to ≥9 mm has been observed in nearly all patients on follow-up CT and three-dimensional (3D) ultrasonography for legacy VTS stents as well as for bare metal stents. 26 27 28 29 A prospective 3D ultrasonography study demonstrated a small degree of initial recoil and reduction in stent cross-sectional area immediately after initial placement, followed by significant expansion at 1 and 6 weeks. 28
Data for self-expansion of stent-grafts underdilated to <8 mm is limited. Using 8-mm VTS devices, Schepis et al observed only one case of self-expansion. 14 With a 10-mm VTS, 74% of stents dilated to 6 mm and 32% of stents dilated to 7 mm underwent self-expansion. 14 These results may be explained by higher intrinsic radial force for a 10-mm stent dilated to 6 mm (36% of nominal luminal area) compared to an 8-mm stent dilated to 6 mm (56% of nominal luminal area). However, one group has since observed passive dilation to nominal diameter with other 8-mm covered stents (Fluency). 25 30 Since passive dilation will result in increased portosystemic shunting and a potentially higher risk of HE and cardiac dysfunction, underdilation is no longer recommended by current guidelines. 4 5
Controlled Expansion Stents
Recognizing the need to adjust TIPS stent diameters reliably to as small as 6 mm, techniques to externally constrain a TIPS were implemented, using a balloon-mounted stent (fixed or expandable diameter) deployed within the hepatic parenchymal tract followed by a VTS. 31 32 In 2017, a new controlled expansion stent-graft (Viatorr TIPS Endoprosthesis with Controlled Expansion [VCX]; W. L. Gore) was developed as a single-stent solution to allow adjustable stent diameters without passive dilation. The VCX is fitted with a polymeric sleeve that constrains the lined portion of the stent-graft to a nominal diameter of 8 mm, but can be balloon expanded up to 10 mm. 33 VCX stent-grafts dilated to 8 mm demonstrated stable diameter in vivo at 6 months 34 and 12 months without passive dilation. 35
Miraglia et al reported early experience with the VCX for the treatment of variceal bleeding and refractory ascites in 75 patients with 100% successful placement rate and no periprocedural (<24 hours) adverse events. 34 Dilated to 8 mm, 92% of patients reached the target PSPG <12 mm Hg, 80% of patients with TIPS indication of refractory ascites had resolved ascites or reduction in paracentesis frequency, and 95% of patients with TIPS indication of variceal bleeding had no recurrent variceal bleeding in a mean follow-up of 6 months. Post-TIPS HE occurred in 22% and TIPS dysfunction requiring revision occurred in 10%. Stent-graft diameter of 8-mm was retained on serial in vivo measurements within 6 months of placement.
In a single-center retrospective study of 33 patients, post-TIPS HE was observed in 61% of patients receiving VCX for refractory ascites and variceal bleeding. 36 However, 33% of patients had HE prior to TIPS placement and only 82% received post-TIPS lactulose or rifaximin. Mean post-PSPG of 6 mm Hg was slightly lower compared to prior studies utilizing 8-mm stents ( Table 1 ).
In a prospective case–control study comparing VCX dilated to 8- versus 10-mm VTS underdilated to 8 mm, VCX did not significantly increase in stent diameter and exhibited a nonsignificant increase in PSPG at 1 week compared to immediately postprocedure (9.4 vs. 10.4 mm Hg). 35 Outcomes were more favorable in the VCX group: lower incidence of HE (23 vs. 54%), lower need for LVP (11 vs. 21%), lower rate of hospitalization for heart failure (2 vs. 15%), similar rate of variceal bleeding (0 vs. 2%), and improved 1-year survival (15 vs. 30%). Pre-TIPS MELD and use of VCX were significant predictors of improved survival in multivariate analysis.
Future Directions
Controlled expansion technology permits operators to fine-tune and sequentially increase the stent-graft diameter confidently and reliably to the desired degree of portosystemic shunting, expanding options to personalize a TIPS to indication and comorbidities. However, there is a need to critically reevaluate the hemodynamic targets for TIPS creation.
A threshold of PSPG <12 mm Hg was proposed from observations that variceal bleeding occurred only with a gradient ≥12 mm Hg. 16 17 18 This cutoff was validated in early TIPS studies with bare metal stents where all patients with recurrent variceal bleeding and ascites had baseline gradients ≥12 mm Hg. 37 With sequential dilation of a VCX, 92% of patients achieved PSPG <12 mm Hg at 8 mm. 34 Similarly with an externally constrained and incrementally dilatable TIPS stent-graft, Cui et al demonstrated 100% of patients achieved PSPG <12 mm Hg with stent diameters of 6 to 8 mm. 32 In contrast, bare metal stents prone to tissue prolapse through interstices and elastic recoil required dilation up to 12 mm to achieve PSPG <12 mm Hg. 37 38 Over the long term, in patients whose TIPS were created using bare metal stents, PSPG increased over time 37 owing to reductions in stent diameter from pseudo-intimal hyperplasia 39 —a limitation that has been largely eliminated by the use of covered stents. 6 These findings suggested that a smaller diameter covered stent may be equivalent to a larger diameter uncovered stent. 40
A relative hemodynamic target of >50% reduction in PSPG, rather than an absolute target, has demonstrated comparable safety and efficacy for variceal bleeding 38 and refractory ascites 41 when using bare metal stents. A relative reduction is more likely to control for variability in PSPG measurements arising from interoperator technique, vasoactive drugs, volume status, autonomic status, and type of anesthesia. Relative PSPG reduction is now recognized in current guidelines. 4 5 Using covered stents, Schepis et al demonstrated that only 50% of stents underdilated to 6 to 7 mm achieved PSPG <12 mm Hg; however, PSPG was reduced on average by 50%, yielding a lower incidence of HE and comparable rates of variceal bleeding and TIPS dysfunction. 14 These results suggest that tolerating PSPG >12 mm Hg while still achieving a significant relative PSPG reduction may maintain efficacy. Moreover, early data in a small cohort of patients with immediate PSPG ≥12 mm Hg demonstrated comparable rates of HE, variceal bleeding, TIPS dysfunction, and survival compared to PSPG <12 mm Hg. 42 The embolization or sclerotherapy of varices and other natural shunts introduces further uncertainty by increasing portal pressure and promoting hepatopetal flow, while decreasing the risk of recurrent hemorrhage. The adoption of balloon-occluded retrograde transvenous obliteration of varices and its variants may preclude the necessity for the creation of TIPS in some eligible patients.
Other areas of research include bidirectionally adjustable diameter devices to allow for reduction of diameter if needed. Selective shunting by creation of extrahepatic splenorenal or splenocaval shunts, mimicking surgical splenorenal shunts, can reduce PSPG while maintaining hepatopetal flow from the intestines, the main source of toxins responsible for causing HE.
In conclusion, a widening array of devices and techniques is available to the interventionalist to offer personalized treatment of complications of portal hypertension. Variable expansion stent-grafts offer more intraprocedural options to tailor stent size, and may facilitate future studies on optimization of hemodynamic targets for portosystemic shunts.
Footnotes
Conflict of Interest A.N.G. has no conflict of interest. D.Y.S. is the editor-in-chief of Journal of Vascular and Interventional Radiology and serves on Data Safety Monitoring Board for W. L. Gore, unrelated to the current article. D.R. has no conflict of interest.
References
- 1.Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group . García-Pagán J C, Caca K, Bureau C. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Abraldes J G, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(01):310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 3.Bureau C, Thabut D, Oberti F. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152(01):157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Baveno VII Faculty . de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022;76(04):959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Advancing Liver Therapeutic Approaches (ALTA) Consortium . Boike J R, Thornburg B G, Asrani S K. North American Practice-Based Recommendations for transjugular intrahepatic portosystemic shunts in portal hypertension. Clin Gastroenterol Hepatol. 2022;20(08):1636–1.662E39. doi: 10.1016/j.cgh.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bureau C, Garcia-Pagan J C, Otal P. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126(02):469–475. doi: 10.1053/j.gastro.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Riggio O, Angeloni S, Salvatori F M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103(11):2738–2746. doi: 10.1111/j.1572-0241.2008.02102.x. [DOI] [PubMed] [Google Scholar]
- 8.Mamiya Y, Kanazawa H, Kimura Y. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Hepatol Res. 2004;30(03):162–168. doi: 10.1016/j.hepres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Riggio O, Ridola L, Angeloni S. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53(02):267–272. doi: 10.1016/j.jhep.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Sarfeh I J, Rypins E B, Mason G R. A systematic appraisal of portacaval H-graft diameters. Clinical and hemodynamic perspectives. Ann Surg. 1986;204(04):356–363. doi: 10.1097/00000658-198610000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarfeh I J, Rypins E B. Partial versus total portacaval shunt in alcoholic cirrhosis. Results of a prospective, randomized clinical trial. Ann Surg. 1994;219(04):353–361. doi: 10.1097/00000658-199404000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins J C, Ong M J, Rypins E B, Sarfeh I J.Partial portacaval shunt for variceal hemorrhage: longitudinal analysis of effectiveness Arch Surg 199813306590–592., discussion 592–594 [DOI] [PubMed] [Google Scholar]
- 13.Rosemurgy A S, Serafini F M, Zervos E E, Goode S E. Small-diameter prosthetic H-graft portacaval shunt: definitive therapy for variceal bleeding. J Gastrointest Surg. 1998;2(06):585–591. doi: 10.1016/s1091-255x(98)80061-9. [DOI] [PubMed] [Google Scholar]
- 14.Schepis F, Vizzutti F, Garcia-Tsao G. Under-dilated TIPS associate with efficacy and reduced encephalopathy in a prospective, non-randomized study of patients with cirrhosis. Clin Gastroenterol Hepatol. 2018;16(07):1153–1.162E10. doi: 10.1016/j.cgh.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Yao J, Zuo L, An G. Risk factors for hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with hepatocellular carcinoma and portal hypertension. J Gastrointestin Liver Dis. 2015;24(03):301–307. doi: 10.15403/jgld.2014.1121.243.yao. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Tsao G, Groszmann R J, Fisher R L, Conn H O, Atterbury C E, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5(03):419–424. doi: 10.1002/hep.1840050313. [DOI] [PubMed] [Google Scholar]
- 17.Groszmann R J, Bosch J, Grace N D. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99(05):1401–1407. doi: 10.1016/0016-5085(90)91168-6. [DOI] [PubMed] [Google Scholar]
- 18.Bosch J, García-Pagán J C.Prevention of variceal rebleeding Lancet 2003361(9361):952–954. [DOI] [PubMed] [Google Scholar]
- 19.D'Amico G, Garcia-Pagan J C, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131(05):1611–1624. doi: 10.1053/j.gastro.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Miraglia R, Maruzzelli L, Tuzzolino F, Petridis I, D'Amico M, Luca A. Transjugular intrahepatic portosystemic shunts in patients with cirrhosis with refractory ascites: comparison of clinical outcomes by using 8- and 10-mm PTFE-covered stents. Radiology. 2017;284(01):281–288. doi: 10.1148/radiol.2017161644. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Lv Y, Bai M. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol. 2017;67(03):508–516. doi: 10.1016/j.jhep.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Luo X, Wang X, Zhu Y. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with expanded polytetrafluoroethylene-covered stent-grafts: 8-mm versus 10-mm. Cardiovasc Intervent Radiol. 2019;42(05):737–743. doi: 10.1007/s00270-019-02162-4. [DOI] [PubMed] [Google Scholar]
- 23.Trebicka J, Bastgen D, Byrtus J. Smaller-diameter covered transjugular intrahepatic portosystemic shunt stents are associated with increased survival. Clin Gastroenterol Hepatol. 2019;17(13):2793–27990. doi: 10.1016/j.cgh.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 24.Huang Z, Yao Q, Zhu J. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPS) created using covered stents of different diameters: a systematic review and meta-analysis. Diagn Interv Imaging. 2021;102(05):279–285. doi: 10.1016/j.diii.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Ma J, Zhou C. Potential benefits of underdilation of 8-mm covered stent in transjugular intrahepatic portosystemic shunt creation. Clin Transl Gastroenterol. 2021;12(06):e00376. doi: 10.14309/ctg.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaba R C, Parvinian A, Minocha J. Should transjugular intrahepatic portosystemic shunt stent grafts be underdilated? J Vasc Interv Radiol. 2015;26(03):382–387. doi: 10.1016/j.jvir.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Pieper C C, Sprinkart A M, Nadal J. Postinterventional passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stents. J Vasc Interv Radiol. 2015;26(03):388–394. doi: 10.1016/j.jvir.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Pieper C C, Jansen C, Meyer C. Prospective evaluation of passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stent grafts - a three-dimensional sonography study. J Vasc Interv Radiol. 2017;28(01):117–125. doi: 10.1016/j.jvir.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Hsu M C, Weber C N, Stavropoulos S W. Passive expansion of sub-maximally dilated transjugular intrahepatic portosystemic shunts and assessment of clinical outcomes. World J Hepatol. 2017;9(12):603–612. doi: 10.4254/wjh.v9.i12.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao W, Liu J C, Wu Y J. Effect of underdilated transjugular intrahepatic portosystemic shunt on prognosis in patients with prior splenectomy: a propensity score-matched case-control study. Abdom Radiol (NY) 2022;47(10):3615–3627. doi: 10.1007/s00261-022-03600-7. [DOI] [PubMed] [Google Scholar]
- 31.Farsad K, Kolbeck K J, Keller F S, Barton R E, Kaufman J A. Primary creation of an externally constrained TIPS: a technique to control reduction of the portosystemic gradient. AJR Am J Roentgenol. 2015;204(04):868–871. doi: 10.2214/AJR.14.13104. [DOI] [PubMed] [Google Scholar]
- 32.Cui J, Smolinski S E, Liu F, Xu D, Dulaimy K, Irani Z. Incrementally expandable transjugular intrahepatic portosystemic shunts: single-center experience. AJR Am J Roentgenol. 2018;210(02):438–446. doi: 10.2214/AJR.17.18222. [DOI] [PubMed] [Google Scholar]
- 33.Trieu H, Lee E W. A new and improved transjugular intrahepatic portosystemic shunt (TIPS) stent graft: controlled expansion. Int J Gastrointest Interv. 2018;7(01):18–20. [Google Scholar]
- 34.Miraglia R, Maruzzelli L, Di Piazza A. Transjugular intrahepatic portosystemic shunt using the New Gore Viatorr controlled expansion endoprosthesis: prospective, single-center, preliminary experience. Cardiovasc Intervent Radiol. 2019;42(01):78–86. doi: 10.1007/s00270-018-2040-y. [DOI] [PubMed] [Google Scholar]
- 35.Praktiknjo M, Abu-Omar J, Chang J. Controlled underdilation using novel VIATORR® controlled expansion stents improves survival after transjugular intrahepatic portosystemic shunt implantation. JHEP Rep. 2021;3(03):100264. doi: 10.1016/j.jhepr.2021.100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloster M L, Ren A, Shah K Y. High incidence of hepatic encephalopathy after Viatorr controlled expansion transjugular intrahepatic portosystemic shunt creation. Dig Dis Sci. 2021;66(11):4058–4062. doi: 10.1007/s10620-020-06716-2. [DOI] [PubMed] [Google Scholar]
- 37.Casado M, Bosch J, García-Pagán J C. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114(06):1296–1303. doi: 10.1016/s0016-5085(98)70436-6. [DOI] [PubMed] [Google Scholar]
- 38.Rössle M, Siegerstetter V, Olschewski M, Ochs A, Berger E, Haag K. How much reduction in portal pressure is necessary to prevent variceal rebleeding? A longitudinal study in 225 patients with transjugular intrahepatic portosystemic shunts. Am J Gastroenterol. 2001;96(12):3379–3383. doi: 10.1111/j.1572-0241.2001.05340.x. [DOI] [PubMed] [Google Scholar]
- 39.Ducoin H, El-Khoury J, Rousseau H. Histopathologic analysis of transjugular intrahepatic portosystemic shunts. Hepatology. 1997;25(05):1064–1069. doi: 10.1002/hep.510250503. [DOI] [PubMed] [Google Scholar]
- 40.Bosch J. Small diameter shunts should lead to safe expansion of the use of TIPS. J Hepatol. 2021;74(01):230–234. doi: 10.1016/j.jhep.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Thalheimer U, Leandro G, Samonakis D N. TIPS for refractory ascites: a single-centre experience. J Gastroenterol. 2009;44(10):1089–1095. doi: 10.1007/s00535-009-0099-6. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Luo X, Yang L. Achieving an effective pressure reduction after TIPS: the need for a new target. J Hepatol. 2021;75(01):246–248. doi: 10.1016/j.jhep.2021.02.010. [DOI] [PubMed] [Google Scholar]


