Abstract
Transjugular intrahepatic portosystemic shunt (TIPS) is one of the most technically complex procedures in interventional radiology, the need to connect two veins with variable anatomy, located in two different planes in hard and many times small cirrhotic livers using a needle, can be quite challenging. Despite more than 30 years of performing TIPS, the complex hemodynamics of the portal system are not fully understood, and sometimes unpredictable alterations of the portal flow can lead to serious unexpected complications. The best strategies to prevent TIPS complications are optimal patient selection, meticulous technique, operator experience, and immediate correction of identified adverse events. The purpose of this article is to review the technical complications with TIPS, the unique complications related to the use of stent grafts, and the late complications after the procedure, with emphasis on ways to prevent and treat them.
Keywords: portal hypertension, transjugular intrahepatic portosystemic shunt, complications, interventional radiology
Transjugular intrahepatic portosystemic shunt (TIPS) is one of the most technically complex procedures in interventional radiology, the need to connect two veins with variable anatomy, located in two different planes in hard and many times small cirrhotic livers using a needle, can be quite challenging. Despite more than 30 years of performing TIPS, the complex hemodynamics of the portal system are not fully understood, and sometimes unpredictable alterations of the portal flow can lead to serious unexpected complications. The best strategies to prevent TIPS complications are optimal patient selection, meticulous technique, operator experience, and immediate correction of identified adverse events. The purpose of this article is to review the technical complications with TIPS, the unique complications related to the use of stent grafts, and the late complications after the procedure, with emphasis on ways to prevent and treat them.
Procedural Complications
The overall rate of procedural complications ranges from 10 to 20%. Reported procedural TIPS mortality is less than 2%. Life-threatening complications include hemoperitoneum, hemobilia, liver ischemia, cardiac failure, and sepsis. 1 2 3 Complications can be avoided by careful patient selection and procedural planning. Elective TIPS candidates should be evaluated during a scheduled clinic appointment. Review of the history, recent images, and laboratory exams is mandatory. The most recent MELD and MELD-Na scores should be calculated to assess the expected prognosis. The patient and family should be informed about the potential complications and available alternatives. A careful review of the most recent cross-sectional imaging studies available is very important to understand the anatomy, assess vessel patency, and rule out tumors. A diagnostic echocardiogram is essential in nonurgent cases. Cardiology clearance is highly recommended in patients with history of valvular disease, prior myocardial infarction, pulmonary hypertension, or with any other significant cardiac history. In high-risk patients (MELD ≥ 18. MELD-Na ≥ 15) who are potential transplant candidates, and who will be undergoing an elective TIPS, waiting until the patient is listed for transplantation is recommended. On the day of the procedure, review of any recent signs of decompensation or drastic changes in history or laboratory results is advised. The operator needs to make sure all the equipment for the procedure is available including TIPS sets, balloons, wires, and stents. If a difficult approach is anticipated, the use of intravascular ultrasound (IVUS) or transabdominal US (TAUS) is advised.
Complications with Venous Access
With the use of micropuncture sets and US guidance for jugular vein access, complications with accidental arterial punctures of the carotid, subclavian, or brachiocephalic arteries are unusual. Pneumothorax is also rare. After the procedure, proper hemostasis at puncture site should be confirmed; a perfect TIPS can be ruined by large neck hematoma. Crossing the heart to get into the inferior vena cava (IVC) is usually straightforward but can be sometimes challenging; pay attention to the presence of arrhythmias. Cardiac perforation is exceedingly rare but has been reported and can be potentially fatal. 4 If getting a wire into the IVC is difficult, a second “buddy” wire can be placed in the IVC and be left as a security wire in case the IVC access is lost when trying to select the hepatic vein (HV).
Complication with HV Access
Selecting the HV can be challenging in transplant patients due to the cephalad orientation of the veins after surgical anastomosis. In Budd–Chiari patients, the HV can be occluded, absent, or severely compressed by an enlarged caudate lobe.
In a standard TIPS, the right HV is selected. Access to the inferior accessory right HV should be avoided because punctures from this low location are usually extracapsular and can be associated with hemoperitoneum or puncture of abdominal organs ( Figs. 1 and 2 ). 5 This anatomic variant is present in up to a third of patients and the operator should be able to recognize its presence. Access to this vein may simulate a portal vein (PV) access in the initial fluoroscopic image ( Fig. 3 ).
Fig. 1.
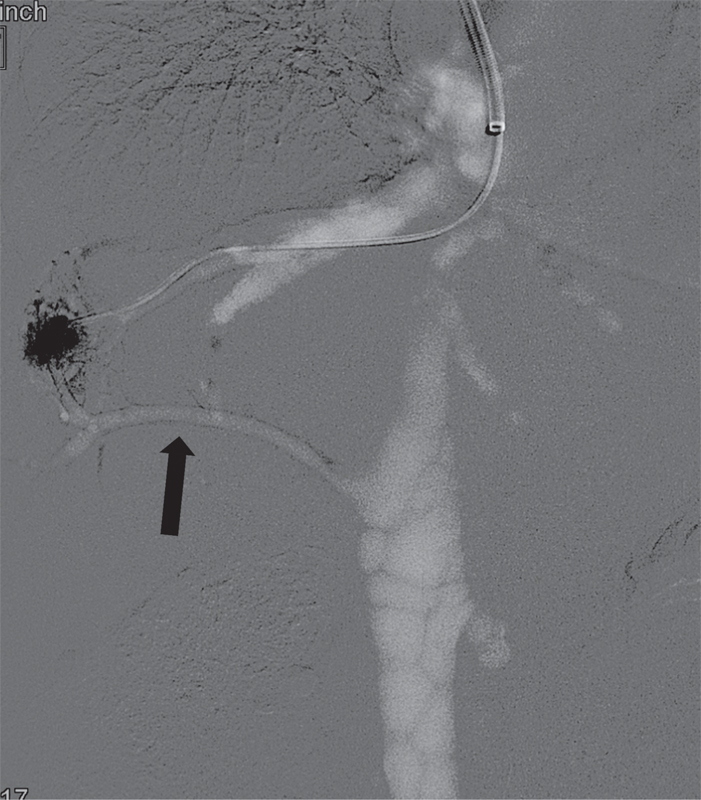
CO 2 venogram shows opacification of the inferior accessory right HV (arrow).
Fig. 2.
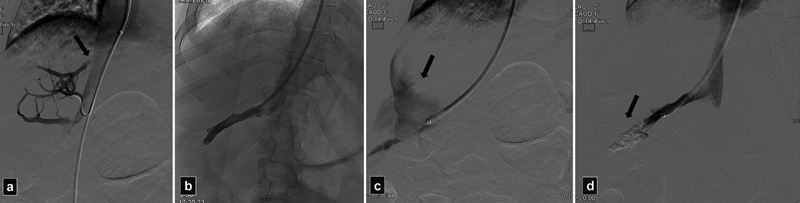
( a ) Venogram shows stenosis of the right HV (arrow). ( b ) The inferior accessory vein was selected for a transjugular liver biopsy. ( c ) After the biopsy patient became hypotensive, venogram shows contrast extravasation into the peritoneum (arrow). ( d ) Venogram after glue embolization shows cast of glue (arrow). Bleeding was controlled but blood transfusion and prolonged hospitalization were required.
Fig. 3.
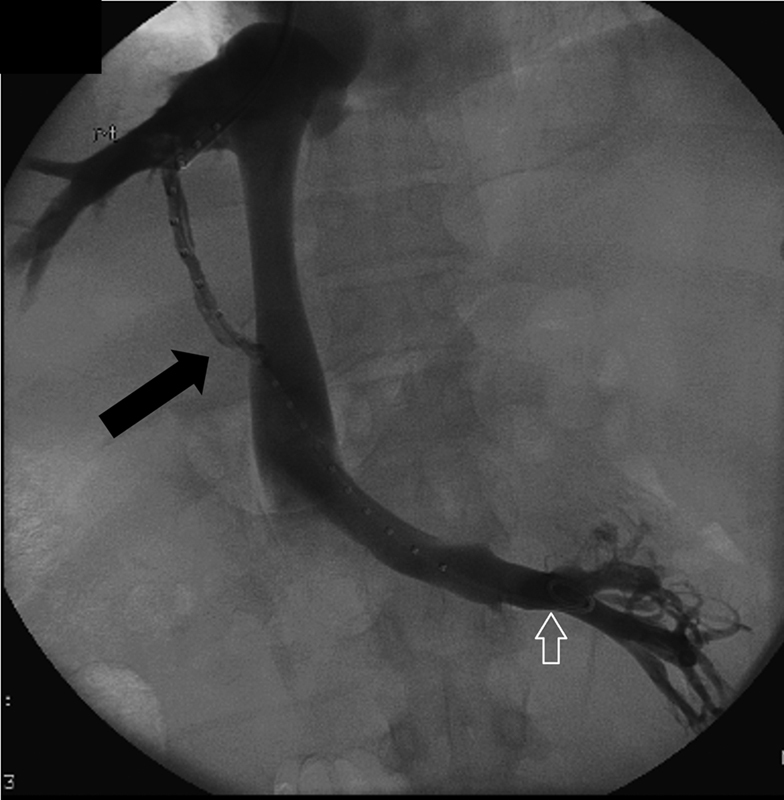
Venogram shows accidental puncture of the inferior accessory right HV (arrow) closely simulating a portal venous access. White arrow—catheter in the left renal vein.
Performing a wedge portogram with contrast or carbon dioxide (CO 2 ) can lead to capsular rupture ( Fig. 4 ). Forceful injections in very peripheral positions should be avoided. Use of an occlusion balloon to obtain the venogram is an excellent option. The volume of CO 2 should be limited to 20 to 50 mL injected at a slow steady rate, avoiding an explosive forceful injection. With the older CO 2 systems that used a drainage bag, air embolism was a possibility if the valves were accidentally left open to room air, but now is rare when using a closed system. Use of CO 2 directly from a source tank should be avoided. Most cases of capsular rupture are self-limited and rarely would require embolization of the distal HV. 6 Excessive amounts of CO 2 can produce an air-lock phenomenon potentially leading to acute cardiovascular collapse ( Fig. 5 ).
Fig. 4.
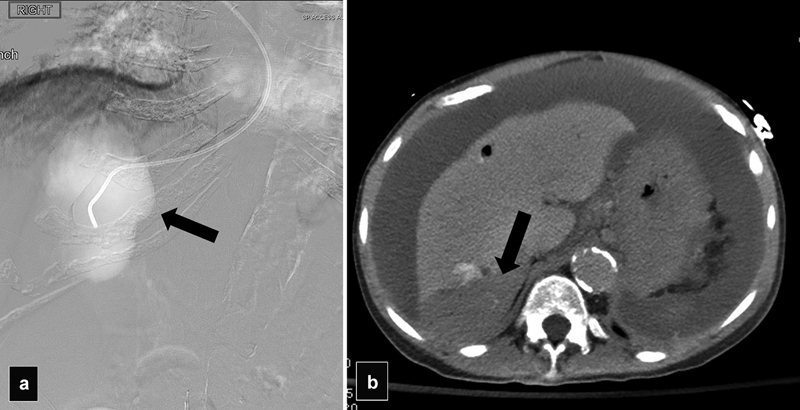
( a ) Wedge portogram with CO 2 demonstrates a localized collection of gas suggestive of subcapsular extravasation (arrow). ( b ) CT shows a subcapsular hematoma (arrow).
Fig. 5.
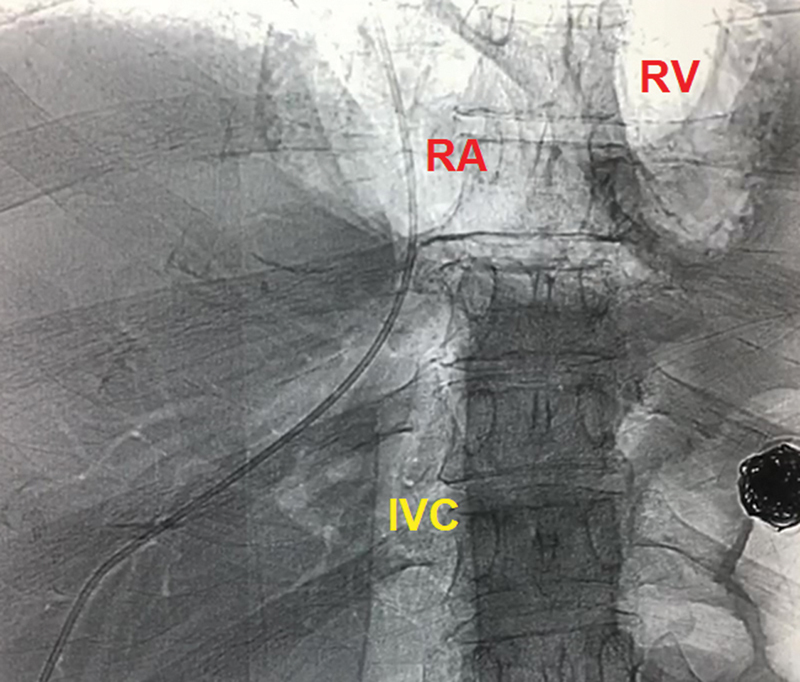
Radiograph shows gas filling the IVC and the cardiac chambers by an excessive amount of CO 2 that was injected during a wedge venogram. Patient had a sudden cardiovascular collapse but was successfully resuscitated and recovered without major sequelae. (Case courtesy of Clayton Trimmer, DO, Dallas, Texas.)
Complications with Portal Vein Access
The most challenging, time-consuming, and riskier step during TIPS is PV access. Multiple punctures can lead to life-threatening bleeding complications.
Recommendations to decrease the number of transparenchymal punctures include the following:
Know where you are : In most cases, a standard TIPS is created between the right HV and the right PV. The right HV catheter reaches a lateral position near the abdominal wall. The arrow of the metallic TIPS cannula is seen pointing posterior and laterally. The puncture is directed anteriorly and medially to select the right PV or the portal bifurcation. In a middle HV access, the catheter can be seen more medially in the liver. With hypertrophy of the caudate lobe, the middle HV can be seen more lateral simulating a right HV. The arrow of the metallic cannula is seen pointing anteriorly and laterally. A puncture from the middle HV is directed posteriorly. One of the main causes of TIPS failure is not recognizing the selected HV. A steep oblique or lateral fluoroscopic view can also help determine the HV selected. The catheter or needle is seen in an anterior position in the middle HV, or posterior in the right HV. 5
Avoid punctures that are too deep : Experience would teach you that you need to perform a rapid and short trust with the needle to be able to pierce through a cirrhotic liver parenchyma; slow soft needle advancement is discouraged because this maneuver may just “tent” the wall of the PV without piercing it. Deep, low, long punctures are discouraged as these are often extra capsular.
Alternatives : If too many punctures are performed unsuccessfully, TAUS may be useful as it provides a noninvasive, fast, and unexpensive guidance; this is an underutilized method because it is operator dependent and limited in obese patients and requires practice. 7 Prepping and draping the right upper abdomen in advance allows to perform the liver scan in a sterile fashion. IVUS is also a useful method to help decrease the number of punctures. 8 9 Other alternatives include further bending the needle to reach a different portion of the vein or selecting a different HV. Accessing the PV using a percutaneous approach is a well-established technique to help guide the initial puncture into the PV that is especially useful in pediatric patients. Many other alternatives have been described but are beyond of the scope of this article.
Although there is no significant difference in complications when using a 19-G needle or a 16-G puncture needle, 10 in smaller patients or those with severe coagulopathies, using a 21-G needle (Cook Medical, Bloomington, IN) can significantly decrease the incidence of bleeding complications. Once access into PV is obtained with a 21-G needle, a 0.018-in wire is advanced into the superior mesenteric or splenic vein and the system may be upsized to a 0.035-in system using one of the many available coaxial support systems.
Hemoperitoneum and Liver Hematomas
Puncture of the liver capsule occurs in up to 33% of patients, with intraperitoneal hemorrhage in only 1 to 2% of cases. 11 Small livers such as those patients with advanced cirrhosis and those in children are at higher risk of extracapsular punctures. In patients with a large volume of ascites, draining the fluid before the procedure may help decrease the mobility of the liver during the punctures. Proper planning and use of adjunct imaging guidance (IVUS or TAUS) decreases the incidence of hemoperitoneum. With IVUS guidance, the incidence of extracapsular punctures is around 9%. 8
If acute hemoperitoneum is identified during TIPS, a PV venogram should be performed followed by a hepatic angiogram with embolization of any bleeding vessels ( Fig. 6 ). As the flow of the PV is decompressed with the TIPS, most of the PV branch injuries will stop spontaneously. A portogram with an occlusion balloon may help better delineate any potential injures ( Fig. 6 ).
Fig. 6.
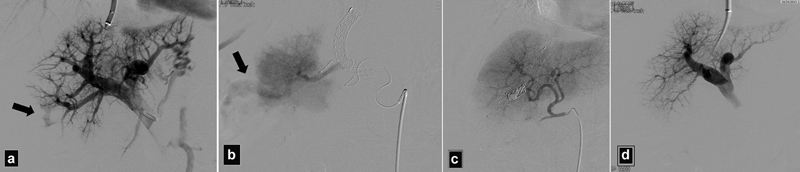
( a ) Portogram in a patient who became hypotensive after PV access shows active extravasation into the peritoneum (arrow). ( b ) DSA angiogram shows active extravasation from the right hepatic artery (arrow). ( c ) Angiogram after coil embolization shows control of the bleeding. ( d ) Portogram using an occlusion balloon shows no active extravasation from the portal branches.
A rare but very serious complication is massive intraperitoneal bleeding after balloon dilation of the TIPS. 12 Although the PV bifurcation is extrahepatic in around 48% of cases, most of the time punctures near or at the bifurcation are safe. Very low punctures below the portal bifurcation, like the ones that are performed for direct intrahepatic shunts (DIPS) or mesocaval shunts, can lead to rapid exsanguination and need to be corrected immediately. If contrast extravasation is seen, the dilatation balloon is kept inflated while the stent graft is rapidly deployed ( Figs. 7 and 8 ).
Fig. 7.
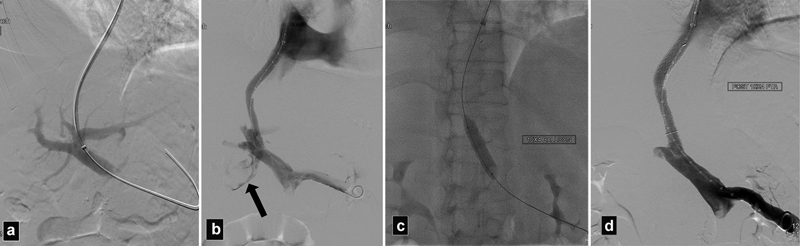
( a ) Portogram shows access into the left portal vein. ( b ) Portogram after Viatorr stent deployment shows extravasation of contrast into the peritoneum (arrow). Patient became hypotensive. ( c ) Radiograph shows balloon inflated to tamponade the bleeding. ( d ) Portogram after prolonged balloon inflation demonstrates no further extravasation of contrast. In this particular case, the portal vein was probably injured after the deployment of the uncovered portion of the stent graft.
Fig. 8.
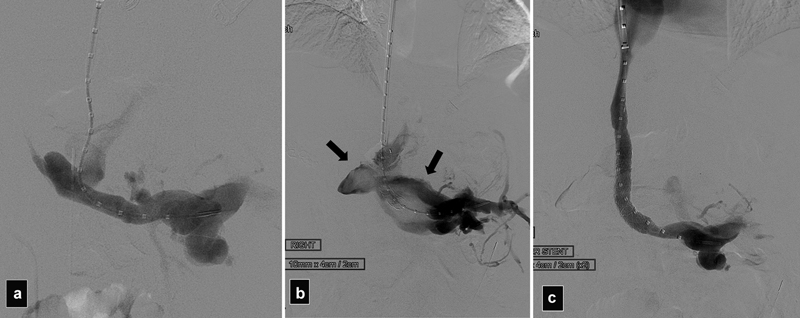
( a ) Portogram after creation of a DIPS in a patient with portal vein thrombosis. ( b ) After deployment of the stent graft, massive extravasation of contrast into the peritoneum was seen (arrows), patient was hypotensive. ( c ) Portogram after additional stent graft placement shows no further extravasation. In this case, the first stent was too short and did not reach the IVC resulting in the bleeding.
Intraparenchymal bleeding can occur with formation of hematomas that can lead to biliary or portal compression; hematomas rarely become infected and require drainage. 13 Subcapsular hematomas can also occur after the wedge venography or the needle punctures ( Fig. 4 ). Observation with serial check of the hemoglobin and hematocrit levels with blood transfusion is needed. In some cases, hepatic angiograms with embolization are required.
Hepatic Artery Injuries
Arterial injuries during TIPS creation have a reported incidence of 6%, with less than 2% having clinical significance. 3 14 An arterial access into a branch of the hepatic artery is usually recognized due to sharp angulation of the wire to the left and then toward the midline when the wire reaches to aorta. In cases of a replaced right hepatic artery, the course of the wire can closely simulate the course of the PV ( Fig. 9 ). Arterial access is further recognized when contrast is injected. Balloon dilation of a tract with the hepatic artery can have disastrous consequences. In patients with PV thrombosis, the hepatic artery can further hypertrophy and accidental arterial access more easily obtained even when using IVUS ( Fig. 10 ). In most cases, the arterial access can be abandoned without further interventions, but occasionally complications of arterial punctures can occur and include arteriovenous fistulas, pseudoaneurysms with active bleeding and intraperitoneal hemorrhage, arterial dissection or occlusion, and arteriobiliary fistula. 15 Arterioportal fistulas could worsen the preexisting portal hypertension. 13 Embolization of the hepatic artery can be performed if the lesion is in a small peripheral branch or if significant bleeding occurred ( Fig. 6 ). Embolization of larger branches of the hepatic artery can lead to liver infarcts and/or liver failure as the liver circulation is heavily dependent on the hepatic artery in cirrhotic patients. Arterial flow is even more important after a TIPS where the portal flow is diverted away from the liver by the portosystemic shunt. Rarely, placement of a stent graft to treat a hepatic artery injury is a viable alternative to preserve the arterial circulation.
Fig. 9.
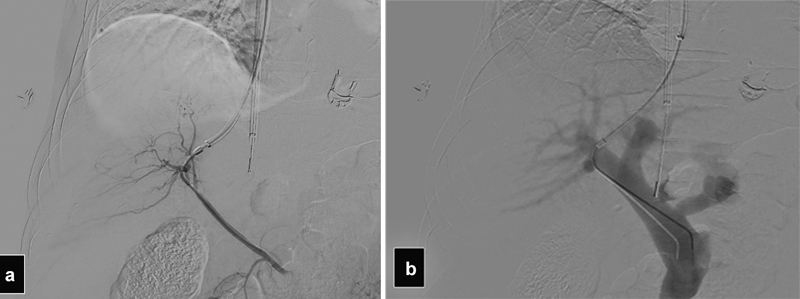
( a ) Angiogram shows accidental access into a replaced right hepatic artery. ( b ) The access was abandoned and a new access into the right portal vein obtained. Note the similar course of the catheter in both films.
Fig. 10.
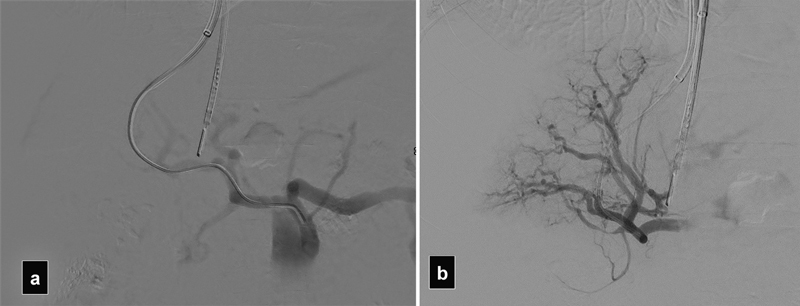
( a and b ) DSA shows accidental access into the hepatic artery obtained in a patient with chronic portal vein thrombosis. Note the hypertrophied hepatic artery branches.
Biliary Punctures and Biliary Obstruction
Accidental punctures of the bile ducts and gallbladder can occur in up to 5% of patients and usually will not require further interventions. 13 15 Punctures of the central hepatic ducts near the hilum are not too uncommon during TIPS as they are located next to the PV branches ( Fig. 11 ). Biliary dilatation significantly increases the risk of biliary punctures and is considered a relative contraindication to TIPS. Biliary punctures can lead to hemobilia, cholangitis, or early stent occlusion. The use of covered stents has significantly decreased the incidence of TIPS failure due to stent stenosis and occlusion caused by pseudo intimal hyperplasia secondary to small bile duct leaks. 16 In the era of bare metal stents, cases of fistulas between a bile duct and the HV or the PV leading to recurrent bacteremia with gram-negative organism and anemia were reported. 17 18 These complications are rare with the use of stent grafts. Biliary dilatation after TIPS is a rarely reported clinically significant complication; however, in follow-up images, it is not that rare to see isolated segmental dilatation after TIPS, especially after using stent grafts. 5 19 20 21 The postulated mechanism for biliary obstruction is an external compression of the bile ducts by the stent; however, actual perforation of the common bile duct by the stent has been reported. 22 Biliary dilatation eventually leads to atrophy of the affected segment. Very rarely the stagnant bile can get infected and lead to recurrent cholangitis and bacteremia that may require endoscopic or percutaneous biliary drainage ( Fig. 12 ). Biloma formation after TIPS has also been reported that have required percutaneous drainage if infected ( Fig. 13 ). 23
Fig. 11.
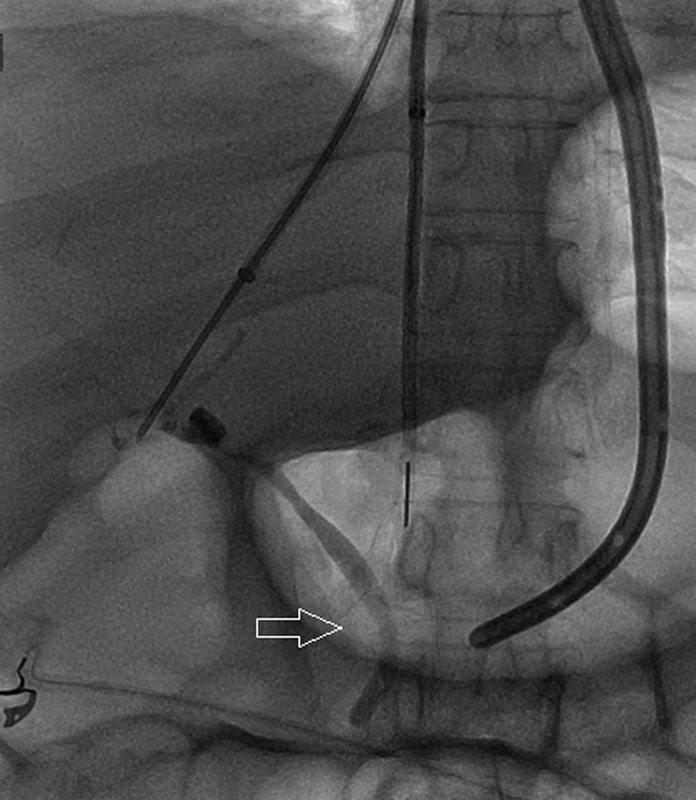
Radiograph shows accidental opacification of the biliary ducts (arrow).
Fig. 12.
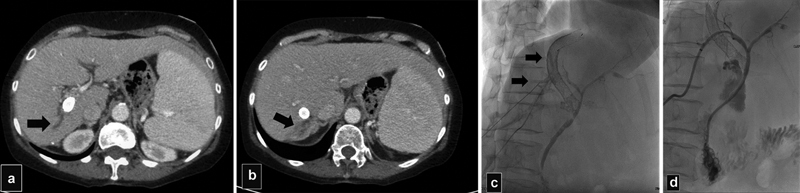
( a and b ) In a patient with primary biliary cirrhosis and a TIPS for recurrent GI bleeding that developed recurrent E. coli bacteremia for last 8 months. CT scan shows area of focal biliary dilatation (arrow) and segmental atrophy posterior to the TIPS stent. ERCP was unable to visualize intrahepatic area. ( c ) Percutaneous biliary cholangiogram shows focal dilation of segmental branches in segment 6 (arrows) produced by external compression by the stent. ( d ) An internal/external biliary drain was placed with later placement of a plastic stent with resolution of the bacteremia.
Fig. 13.
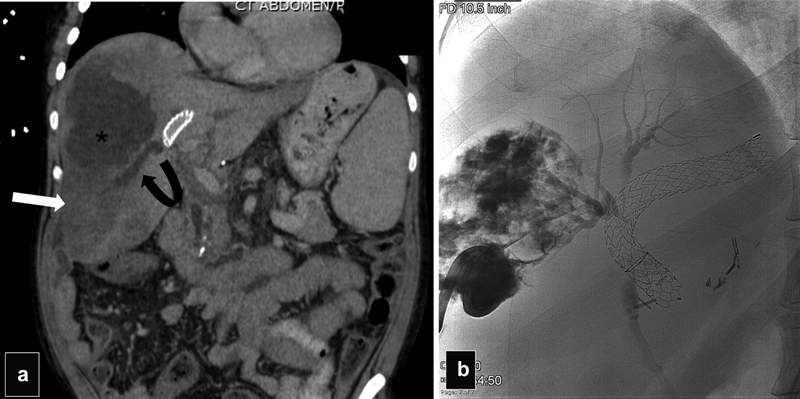
Patient with liver transplant who underwent TIPS for variceal bleeding presented with sepsis 1 month after the procedure. ( a ) Coronal CT scan shows a liver infarct (arrow), a biloma (asterisk), and thrombosis of the right portal vein (curved arrow) ( b ) Abscessogram after drainage of a large biloma shows communication with right biliary ducts that are compressed by the TIPS.
Puncture of Extrahepatic Organs
Puncture of the gallbladder occurs in less than 10% of cases mostly with no major sequela. Rarely, significant bleeding inside the gallbladder could result in acute cholecystitis if the cystic duct gets occluded, and also bile leakage can occur if the wall of the gallbladder is damaged. 13 Puncture of the right kidney can occur in less than 1.5% and may lead to pericapsular hematomas or hematuria that is usually self-limited. Puncture of the bowel may increase the risk of infection. 5 23 Occasionally, the lymphatic system is opacified and is recognized by the serpiginous configuration of small slow-flowing channels ( Fig. 14 ).
Fig. 14.
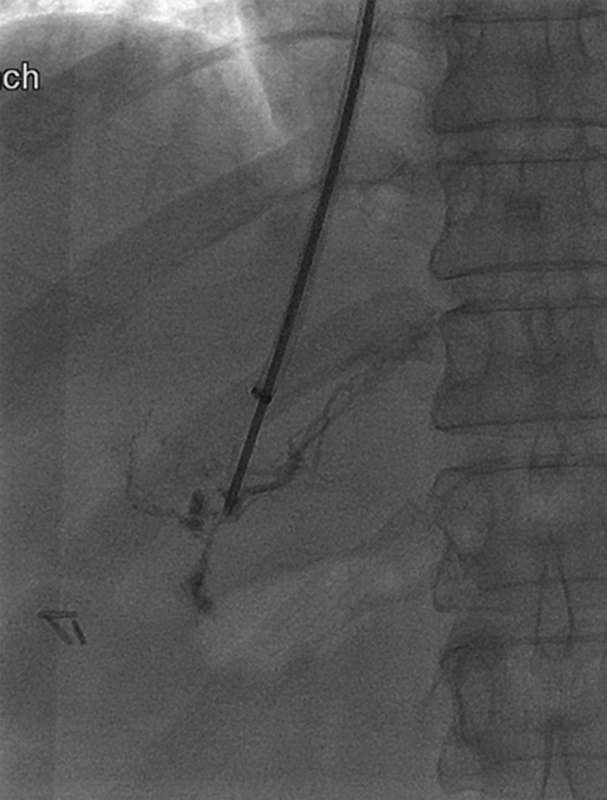
Radiograph shows opacification of the liver lymphatics.
Unique Complications with the Use of Stent Grafts
The use of the Viatorr stent graft (Gore Medical, Flagstaff, AZ) revolutionized the role of TIPS in the management of many portal hypertension complications due to proven increased primary and secondary patency of TIPS; however, its unique design can lead to some special complications.
Issues with loading the stent : Inadequate advancement of the loader sheath into the 10-Fr Flexor sheath (Cook, Bloomington, IN) may result in partial deployment of the uncovered portion inside the sheath potentially leading to the stent getting stuck near the valve requiring replacement of a very expensive stent.
Issues with stent length and deployment : The ideal position of the cephalic portion of the Viatorr is at or within 1 cm of HV-IVC confluence. Precise measurement using a calibrated catheter is always recommended. Stents that are too short inside the HV tend to have early restenosis that may lead to stent dysfunction or thrombosis ( Fig. 15 ). Stents that are too long may end up misplaced inside the IVC, or in the right atrium (RA) ( Fig. 16 ). Heart perforation with hemopericardium, arrhythmias, and valvular damage has been reported with stents that are placed too deep in the RA and were more frequent with the Wallstent due to its sharp edges. 24 25 26 27 These severe heart complications are very rare with the use of a fully covered stent. Stents too deep in the RA or the IVC could interfere with caval cross-clamping during liver transplantation.
Fig. 15.
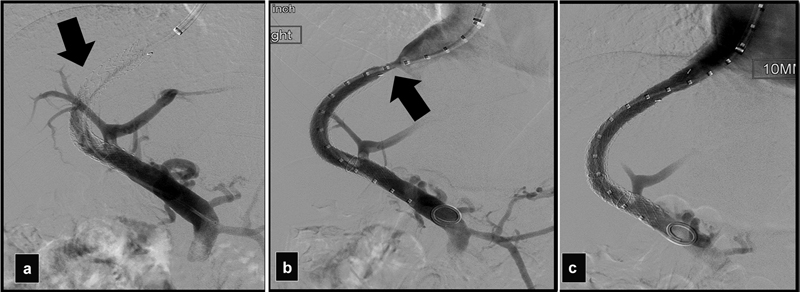
A stent that was too short resulted in occlusion of the TIPS. ( a ) Portogram shows occluded shunt (arrow). ( b ) Portogram after balloon thrombectomy shows a stenosis at the HV (arrow). ( c ) Portogram after another Viatorr placed more proximally shows patent shunt.
Fig. 16.
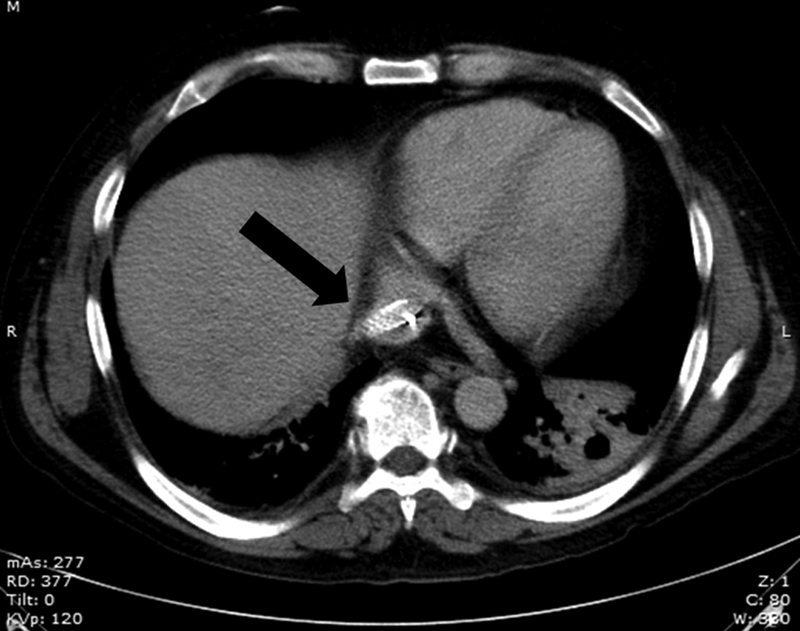
Axial CT shows a stent that was extending into the RA (arrow).
Precise placement of the transition between the uncovered and covered portion can be challenging in cases of very tortuous tracts or when the PV is very small. Inadequate tension, much angulated access tracts, or very small PVs may result in having the false impression that the radiopaque gold marker is at the parenchyma/PV junction, when in reality the device is still too deep inside the PV ( Fig. 17 ). Deployment of the covered portion inside the main portal could be associated with thrombosis/stenosis of the main PV or its main branches and eventual liver ischemia due to the complete diversion of the portal flow ( Fig. 18 ). A stent that is too deep inside the main PV could also interfere with a future liver transplant. Excessive tension during deployment may result in the uncovered portion getting deployed inside the liver parenchyma increasing the risk of stent restenosis.
Fig. 17.
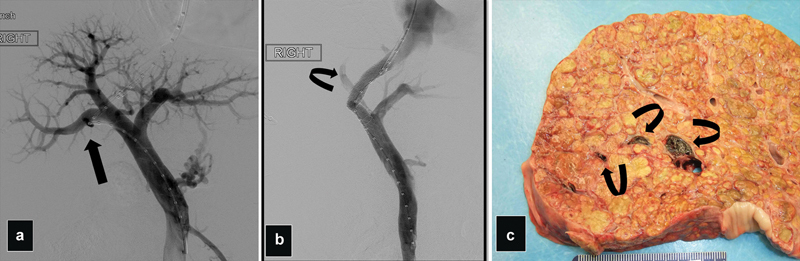
( a ) Portogram shows severe angulation in the approach into the right PV (arrow). ( b ) Portogram after Viatorr deployment shows around 2–3 cm of the covered portion misplaced inside the right PV causing occlusion of the posterior PV and decrease flow in the anterior right PV (curve arrow). ( c ) Fulminant liver failure developed 2 days after TIPS. Necropsy photograph shows a cirrhotic liver with thrombus in the right PV branches (arrows).
Fig. 18.
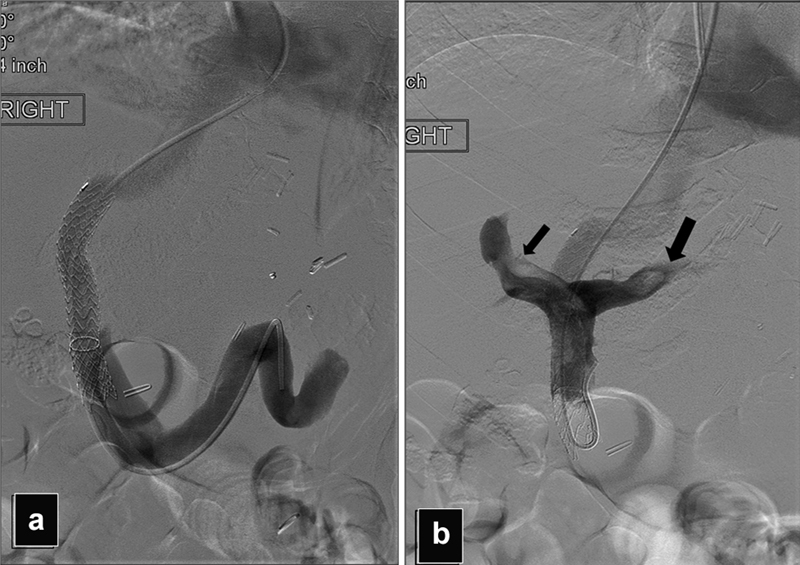
Acute liver failure after the TIPS. ( a ) Portogram shows covered portion of the Viatorr misplaced too deep into the main portal vein causing poor flow in the intraparenchymal portal vein branches. ( b ) Portogram using a reverse curve catheter shows partial thrombosis of the right and left portal vein branches (arrows).
Stent and Coil Migration
Stent migration is more frequent with covered than with bare metal stents. 13 Proximal stent migration can occur more frequently than distal stent migration into the PV. Stent migration into the heart may rarely occur during stent revisions when overlapping stents are placed near the IVC. The most common mechanisms for stent migration include using undersize stents, using short and/or incompletely overlapped stents, moving the stent with a partially deflated angioplasty balloon, or when advancing the sheath through the shunt and/or operator errors. Stent retrieval from the heart is technically challenging but possible ( Fig. 19 ). Special care needs to be taken to prevent damage of the valves or cardiac perforation during removal; the use of general anesthesia and transesophageal echocardiogram can be very helpful to prevent major complications. Open heart surgery may be required in some cases. Trying to remove a migrated or misplaced stent from the main PV may be very difficult and traumatic and many times, it is better to leave the stent in that position than trying to remove it.
Fig. 19.
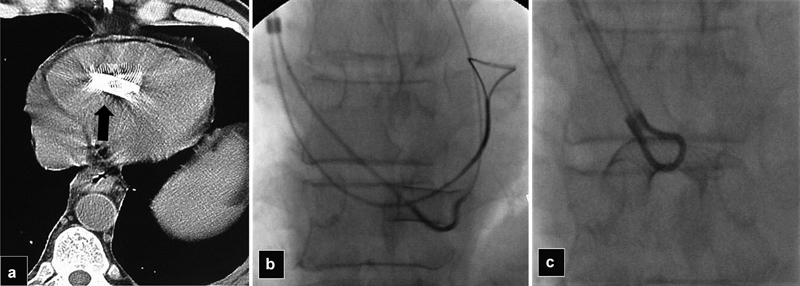
( a ) CT scan shows a Wallstent (arrow) that migrated into the right ventricle after TIPS revision with placement of overlapping stents. ( b ) Because of the sharp ends of a Wallstent, manipulation of the stent may result in cardiac perforation. Radiograph shows a SOS catheter passed under the body of the stent and the wire was snared. ( c ) The stent was folded and retrieved.
Coil migration can occur during embolization of gastric and esophageal varices. Using oversized coils can result in migration into the main PV. Undersized coils can migrate through large portosystemic shunts into the lungs. Coil retrieval is recommended ( Fig. 20 ). Pulmonary embolization of glue can occur during variceal embolization; smaller amounts of glue usually are well tolerated and cause no symptoms. Embolization of large amounts of glue or sclerosing agents into the lungs could lead to serious complications.
Fig. 20.
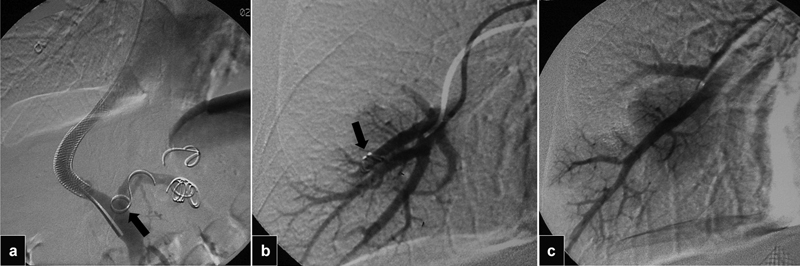
( a ) Portogram shows an oversized coil partially extending into the main portal vein (arrow). ( b ) Pulmonary angiogram shows that coil (arrow) later migrated into the right lung. ( c ) Angiogram after successful snare retrieval of the coil shows patent branches.
Postprocedural Complications
Portal or mesenteric vein dissection is very rare and it could lead to pseudoaneurysm formation or portal/mesenteric vein thrombosis; most of the time dissections are self-limited if produced by the wire only. 15 Perforation of distal mesenteric veins can occur if the wire is advanced distally into small peripheral branches, and keeping the wire at the main splenic or main mesenteric vein is recommended. Perforation of venous branches with the wire is usually not associated with significant bleeding, but perforation with the larger sheaths can lead to significant hemorrhage. This serious complication can occur when advancing larger sheaths inside the PV for stent graft deployment or inside varices to deploy vascular plugs.
Intravascular hemolysis occurs in less than 10% of patients with the use of bare metal stents; it is more infrequent with the use of stent grafts. This rare complication is usually self-limited and rarely requires treatment.
Microbial Seeding and Infection
TIPS infection (tipsitis or endotipsitis) is another rare complication that occurs in less than 1% of patients and is associated with persistent bacteremia without any other identifiable source of infection and usually seen with vegetations or thrombus within the TIPS. 28 Treatment consists of prolonged antibiotics. Removing the clots from the shunt with dedicated cultures could help treat this rare condition. Most cases have been reported with the use of bare stents, but it can also occur with stent grafts. The use of broad-spectrum prophylactic antibiotics is recommended during the initial procedure. Potential sources of infection include the biliary tree, gallbladder, the intestine, and ascitic fluid in cases of untreated bacterial peritonitis. In some reported cases of bacteremia related to biliary fistulas, the use of stent grafts has been successful in treating this complication. 29 Liver abscesses can also occur that can be related to infected bilomas, hematomas, or rarely as a complication of liver infarction ( Figs. 13 and 21 ). 30 Percutaneous drainage is usually required to resolve the infection.
Fig. 21.
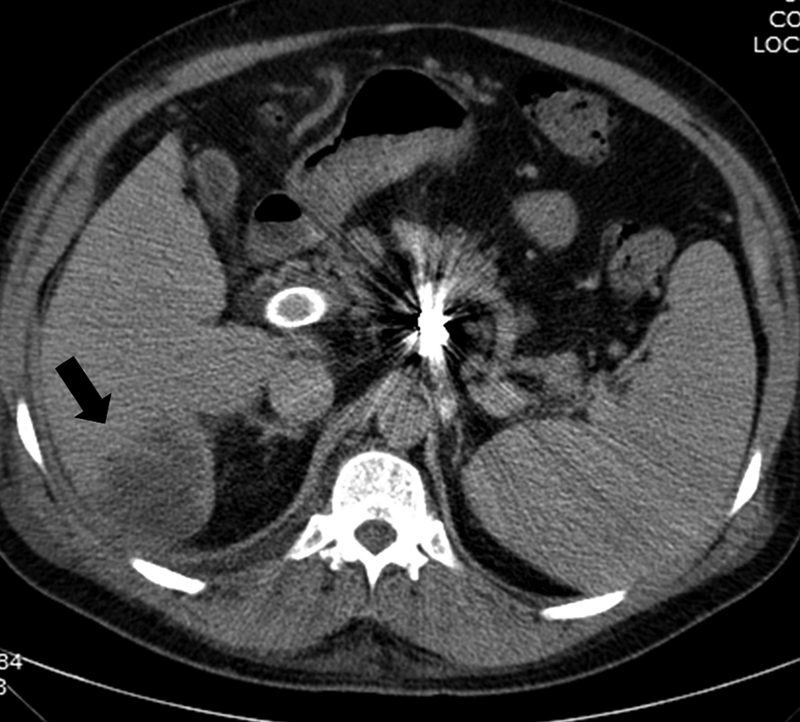
Axial CT scan in a patient with TIPS for portal vein thrombosis presenting with high fevers and bacteremia shows hypodense area in the right lobe (arrow); percutaneous drained was then performed confirming hepatic abscess.
Hepatic encephalopathy (HE) is one of the most recognized frequent and feared complications after TIPS: this topic is covered in a separate article of this volume.
PV and HV Thrombosis
PV and HV thrombosis are common imaging findings after TIPS that are mostly unreported ( Figs. 22 and 23 ). In a retrospective study by our group of 423 patients of which 138 had cross-sectional imaging within 1 year after TIPS, PV or HV thrombosis was seen in cross-sectional imaging in 63.0% of patients. Most of the affected veins were in right portal system at the lobar or segmental areas, while the left PV was affected only in 7% of patients. The presence of venous thrombosis was not associated with worse outcomes unless thrombosis of the main PV occurred. 31 Rarely thrombosis of the HV may create hepatic congestion with a Budd–Chiari type of syndrome or lead to segmental ischemia. 32 Uncommonly, TIPS can lead to IVC thrombosis. 13 Thrombosis of the hepatic and/or PV branches is probably due to a combination of trauma during the access of the branch, partial blockage by the graft, and/or alterations of the flow dynamics after TIPS. Contrast-enhanced imaging is recommended in patients who develop severe elevations of the liver enzymes, severe abdominal pain, and/or fevers after the procedure, to rule out any complications related to venous thrombosis.
Fig. 22.
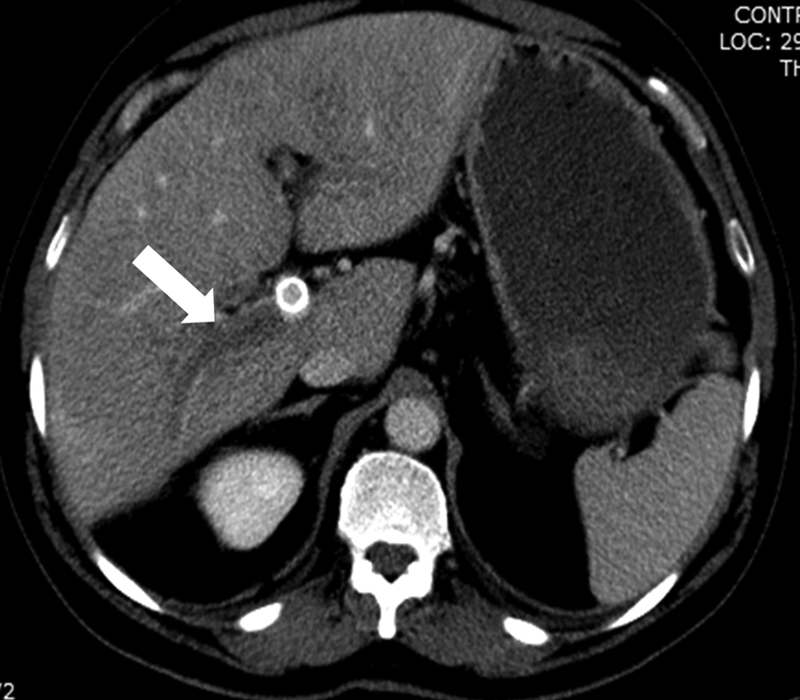
Axial CT with contrast obtained 2 weeks after TIPS shows thrombosis of a right posterior portal branch (arrow).
Fig. 23.
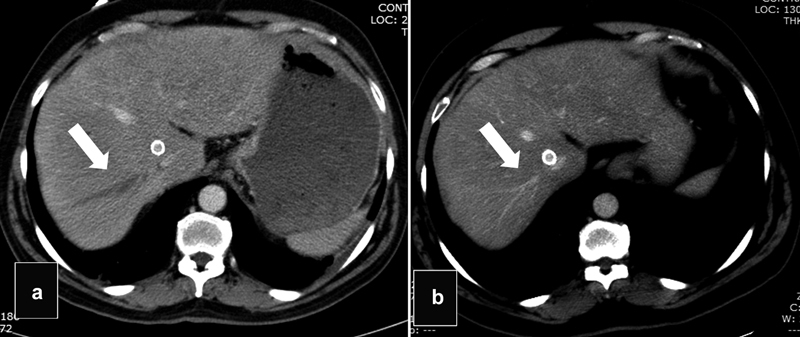
( a ) Axial CT scan shows thrombosis of the right HV (arrow). ( b ) Axial scan 3 months later shows spontaneous recanalization of the right HV (arrow).
Liver Infarcts
Liver infarction is seen in less than 2.5% of all patients and around 9% of all those patients who get imaging after TIPS for different reasons. 33 Liver infarction was associated with two times the risk of death and acute liver failure (ALF) in one study. 33 Risk factors included PSG < 5 mm Hg, arterial injuries, PV, and/or HV thrombosis. Infarction is seen as wedge-shape areas or hypoperfusion that are recognized only if the patients get cross-sectional imaging after the procedure ( Fig. 24 ). Most of the infarcts are located in the posterior segments near the areas of the needle punctures and stent placement. Patients may present with no symptoms or with elevation of liver enzymes, ALF, and rarely with formation of bilomas. 30
Fig. 24.
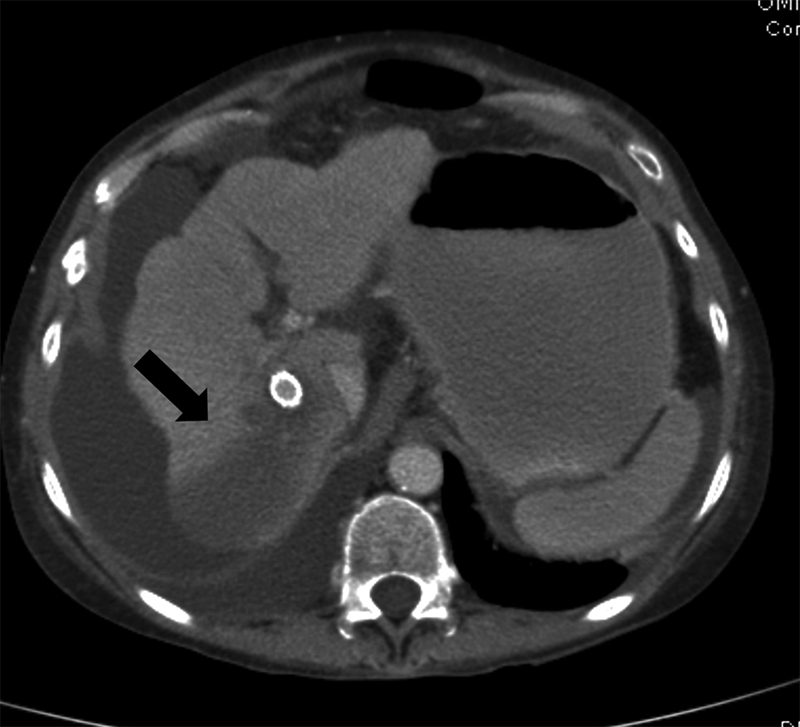
Axial CT scan in a patient with marked elevation of the liver enzymes and abdominal pain shows a large hypodense area corresponding to a liver infarction (arrow).
TIPS Thrombosis
TIPS thrombosis and restenosis used to be a frequent problem with the use of uncovered stents. 16 With the use of polytetrafluoroethylene (ePTFE)-covered stents, the long-term patency of shunts has significantly increased with 20 to 30% incidence of TIPS dysfunction at 2 years. 13 34 Risk factors for stent thrombosis include stents that are placed too short into the HV, stents that are underdilated, or creation of very tortuous tracts 35 36 ( Figs. 15 and 25 ). Stents that are reduced for the treatment of HE can develop thrombosis. 37 Large competing shunts and large gastric or esophageal varices can divert the flow away from the TIPS and may cause shunt thrombosis and most require embolization to redirect the flow into the TIPS. Hypercoagulable patients with Budd–Chiari syndrome are particularly at risk for TIPS thrombosis and most of them required lifelong anticoagulation ( Fig. 26 ). Tumoral thrombosis of the TIPS and the PV can also occur in patients with advanced HCC and must be differentiated from bland thrombus before restoration of the shunt flow.
Fig. 25.
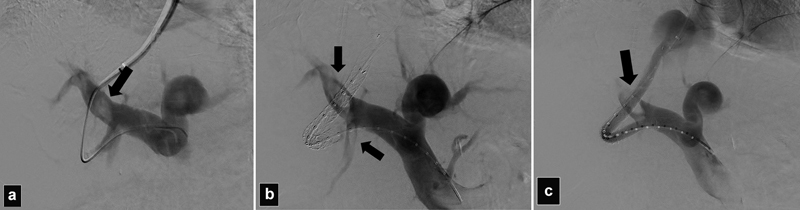
( a ) Portogram shows a very tortuous access into a low right posterior portal vein branch (arrow). ( b ) Acute thrombosis TIPS occurred during the procedure. ( c ) After successful declotting, the shunt is patent. In both b and c , note the associated partial thrombosis of the right portal branches (arrows).
Fig. 26.
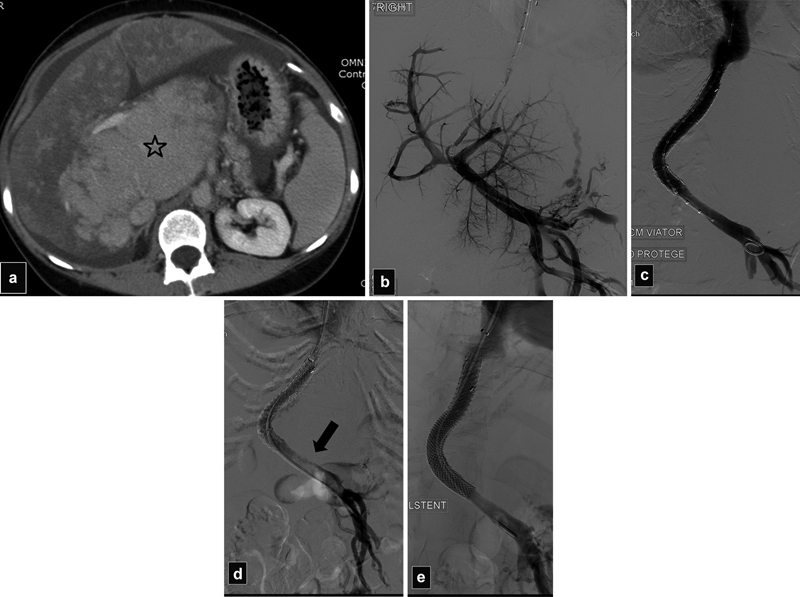
( a ) CT scan shows severe hypertrophy of the caudate lobe (star) and mottled enhancement of the liver in a patient with Budd–Chiari secondary to a hypercoagulable syndrome. ( b ) Initial portogram shows patent portal vein. ( c ) After TIPS with overlapping stents, the portal vein is patent. ( d ) Portogram the day after TIPS shows acute thrombosis of the main portal vein (arrow). ( e ) Portogram after declotting and additional stent placement into the main portal vein shows patent shunt.
Stent thrombosis is usually treated with balloon dilatation and restenting. Crossing a chronically occluded stent can be very challenging; techniques to improve success include using the stiffer cannula of the TIPS set to increase support and occasionally puncturing the occluded stent percutaneously with a Chiba needle then passing a wire that is snared via the jugular approach to get access into the stent ( Fig. 27 ). Occasionally, a parallel TIPS may be required if recanalization of an occluded TIPS is unsuccessful. 15 Paradoxical embolization with stroke has been reported after TIPS in patients with patent foramen ovale. 38
Fig. 27.
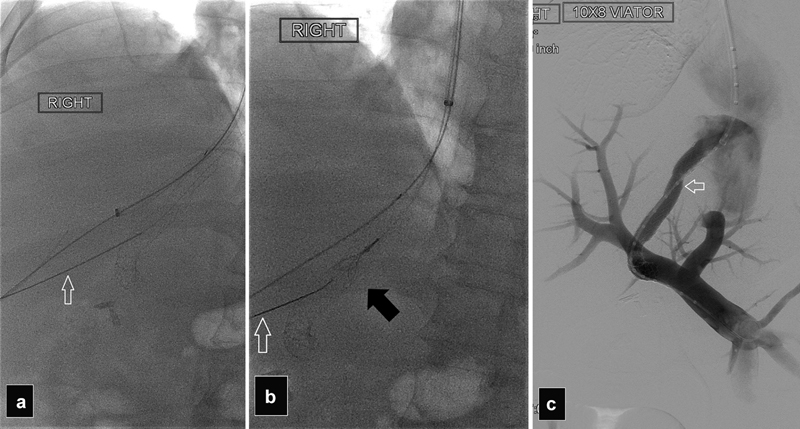
( a and b ) After unsuccessful TIPS recanalization using a jugular approach, radiographs show percutaneous puncture of an occluded TIPS with a Chiba needle (white arrows) with a snare (black arrow) trapping the wire. ( c ) Portogram after TIPS revision with additional stent placement shows patent TIPS (arrow)
Liver Failure after TIPS
ALF, also referred as acute on chronic liver failure, can be a devastating complication after TIPS. ALF typical presentation consists of marked elevation in liver enzymes, severe coagulopathy, and severe HE. Transient elevation of the liver enzymes is very common after TIPS with an acute (within 7 days) two- to threefold increase in bilirubin and transaminases that usually resolve within 2 weeks. 39 40 However, marked elevation of the liver enzymes might be an indicator of irreversible liver injury and liver failure. The definition of ALF is not well established. Gaba and Lakhoo defined ALF as a threefold bilirubin and/or twofold INR elevation compared with baseline within 30 days of procedure, excluding other identifiable causes for the observed alterations (such as biliary obstruction or suspected biliary vascular fistula). Associated clinical outcomes were classified as prolonged hospitalization/increase in care level (grade 1), TIPS reduction or liver transplantation (grade 2), or death (grade 3). In 270 patients, abnormal elevation of LFTs and INR was a common finding after TIPS with up to 29% showing greater than threefold bilirubin and greater than twofold INR postprocedure. ALF had an overall incidence of 20%, grade 1 (10%), 2 (3%), and 3 (8%). Bilirubin levels increased to at least triple the baseline value in ∼50% of dying patient's versus only 20% of surviving patients. 41 In another study, bilirubin was found to be an independent predictor of 30-day mortality after TIPS with a 40% increased risk of death for each 1 mg/dL increase above 3.0 mg/dL. 42
A study of 216 patients with low pre-TIPS MELD score (≤12) by Luca et al found that the incidence of liver failure within 3 months was 9.3% with a poor prognosis; two-thirds of cases progressed to death or required liver transplantation during the 1st year. Refractory ascites, low hemoglobin, and platelet count, MELD 11 or 12 versus ≤10, and Child–Pugh score >7 carried a higher risk of developing ALF after TIPS. There was no correlation between stent diameter and decrease in portosystemic pressure gradient (PSG). 43
The potential mechanisms of ALF after TIPS are variable and probably multifactorial and include excessive shunting of the portal flow away from the liver, damage of the hepatic artery after the punctures, compression of the artery by the metallic stent, thrombosis of the PV, and/or HV. Contrast-enhanced images in patients who develop ALF may help elucidate the source if it is related to vessel thrombosis, infarction, or other potential causes that could be recognized in contrast-enhanced imaging. 13
Patients at high risk for ALF include Child–Pugh score >12, MELD score >18, Emory score >3, or an APACHE II score >18. High bilirubin, high PSG, low albumin, sarcopenia, and refractory ascites are independent factors associated with higher mortality after TIPS within 1 year. 43 44 45 46 47 Liver volume was not associated with ALF in one retrospective study. 48
The issue of the final PSG and adverse outcomes is controversial. A relative reduction of PSG after TIPS by 20 to 50% has been recommended with a target of < 12 mm Hg for variceal bleeding control, while for patients with refractory ascites, an ideal target of 5 to 8 mm Hg has been suggested, but this value may be difficult to achieve in clinical practice, as the final PSG after a TIPS is not always predictable. 40 49 Quality improvement guidelines of the American Society of Interventional Radiology recommend that the PSG after TIPS should not be less than 5 mm Hg as an excessive decrease in the PSG may lead to liver ischemia and/or HE. This threshold was also recommended by other groups. 24 40 50
Treatment of ALF after TIPS is mostly supportive with extensive workup to rule out gastrointestinal bleeding, sepsis, and electrolyte imbalance which need treatment. Hypothyroidism can rarely be a cause of hyperammonemia in cirrhotic patients but could be the source of refractory HE. 44 Shunt reduction should be considered early before irreversible liver failure develops. Shunt closure is also an option if reduction does not work. Liver transplantation is oftentimes the only option to treat this complication and that can be otherwise rapidly fatal.
Renal Function Complications
Renal dysfunction is a common and serious problem in patients with cirrhosis and is significantly associated with increased rates of morbidity and mortality. Hepatorenal syndrome (HRS) has been classified as type I when it is a rapid (< 2 weeks) deterioration of the renal function with doubling of serum creatinine to > 2.5 mg/dL, or a creatinine clearance of < 20 mL/min. 51 Type I HRS has a very poor prognosis; 80% of patients die within 2 weeks, and only 10% survive longer than 3 months. 52 Type II HRS is defined by a slow deterioration of renal function with a serum creatinine level > 1.5 mg/dL and/or creatinine clearance < 40 mL/min, and it has a better prognosis.
Early report on TIPS on patients with impaired renal function showed that 16% developed acute kidney injury (AKI) as defined by a 0.3 mg/dL increase in serum creatinine within 48 hours of the procedure and it was tough to be related to the use of intravenous contrast material for the procedure. 53 Other factors that can be associated with AKI after TIPS include hypotension, bleeding, use of nephrotoxic medications, and preexisting chronic kidney disease (CKD). Studies have shown that TIPS may improve HRS especially the type II. 54 55 56 Limited series on TIPS in patients with stage 4 or 5 CKD have shown improvement in the ascites in 85% and control of variceal bleeding in 90%. 57 CKD markedly increases the risk of HE after TIPS, especially in patients who are already on dialysis. 57 58
The use of TIPS on patients who are on dialysis already has been described in limited series, very careful management of the fluids and blood pressure before and after the procedure with close coordination with nephrology is crucial to prevent fluid redistribution issues after the TIPS. 58
In patients who develop worsening renal function after TIPS, initiation of hemodialysis may be required while the patients await liver transplantation. 51 Increased creatinine (≥1.5 mg/dL) 15 to 40 days after the procedure haven been associated with increased mortality compared with patients with normal creatinine at that time period. 56
Right Heart Failure and Pulmonary Hypertension after TIPS
TIPS leads to a sudden increase in cardiac preload and output that can rapidly worsen the hyperdynamic circulatory state of patients with cirrhosis. The large-volume blood shift from the splanchnic to the systemic circulation results in a sudden but mostly transitory increase in right heart pressures and cardiac output. 59 60 The cardiac output increases by 22%, systemic vascular resistance decreases by 26%, the right atrial pressure (RAP) may increase by 50%, and mean pulmonary artery pressure (mPAP) by 40%. 61
TIPS is contraindicated in patients with right heart failure and severe pulmonary hypertension, defined as mPAP > 45 mm Hg at right heart catheterization and pulmonary capillary wedged pressure (PCWP) ≤15 mm Hg or systolic PAP > 50 mm Hg at echocardiography. In theory, TIPS can be performed in cases of mild PAH (25–35 mm Hg) according to a recent consensus. 62 For cases of moderate pulmonary hypertension with mPAP between 35 and 45 mm Hg, TIPS could be considered mainly for patients with variceal bleeding but with great caution for elective patients with refractory ascites. 11 Severe cardiac valvular insufficiency and severe aortic stenosis are also contraindicators for the procedure.
It is estimated that as many as 50% of end-stage liver disease patients undergoing liver transplantation show signs of cardiac dysfunction with 7 to 21% of patients dying from heart failure in the posttransplantation period. 11 63 The recent rise in cases with nonalcoholic fatty liver disease (NAFLD) needing TIPS makes the issue of cardiac decompensation even more relevant, as these patients commonly have multiple associated comorbidities such as obesity, diabetes, hypertension, high cholesterol, and high incidence of renal and cardiac disease. 64
Studies have found a close correlation between the RAP and portal pressures > 25 mm Hg before TIPS with developing symptomatic heart failure after the procedure. 65 66 Other risk factors include the presence of previous congestive heart failure, preexisting cirrhotic cardiomyopathy (CCM), prolonged QTc interval, left atrial dilatation, an elevated pre-TIPS BNP > 40 pg/mL, and the presence of severe aortic stenosis.
CCM is characterized by decreased cardiac function with altered diastolic relaxation, electrophysiological abnormalities with blunted contractile responsiveness to stress. Most of the time CCM is a silent condition but can become symptomatic under physical or pharmacological stress. CCM can be difficult to diagnose, as patients can have a normal left ventricular ejection fraction as rest but have abnormal responses to exercise, sodium load, or orthostatism. 11 Cirrhotic patients with refractory ascites and severe diastolic dysfunction may not only respond to TIPS but may have shorter survival than those with normal diastolic function. 11 40 67 Other studies, however, have found no correlation between diastolic dysfunction and post-TIPS survival or cardiac failure after the procedure. 40 65 68 69
Most of the time, acute heart failure after TIPS can be managed with aggressive medical treatment including aggressive diuresis. In some rare cases, a life-threatening acute pulmonary edema or severe right heat failure can develop and TIPS reduction or closure may be required ( Fig. 28 ). 37 70 In earlier studies, the risk of cardiac failure after TIPS has been cited at 12.5% in patients without previous cardiac issues, and up to 39% in patients with known structural heart disease, with a very high risk (up to 80%) in patients with aortic stenosis. 11 69 Other more recent studies cite the incidence of symptomatic heart failure to be <1% with good outcomes with aggressive medical management. 65
Fig. 28.
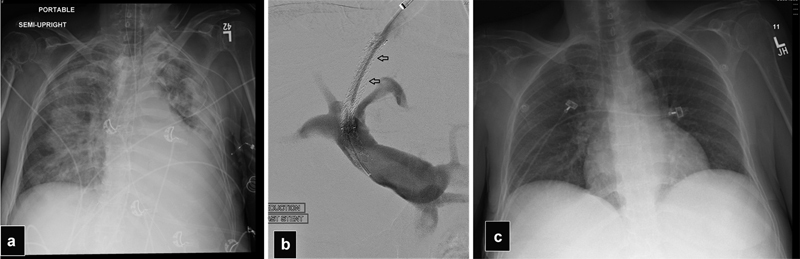
( a ) Frontal radiograph in a patient with a normal left ventricle function that developed severe right heart failure with pulmonary edema 3 days after TIPS. ( b ) Portogram after TIPS was reduced with a partially expanded balloon-expandable stent graft inside the Viatorr. Notice the narrow column of contrast in the TIPS (arrows). ( c ) Frontal radiograph after TIPS reduction shows resolution of the pulmonary edema.
Hernia Incarceration
Many patients with refractory ascites develop umbilical and less frequently inguinal hernias. The rapid and sustained ascitic fluid decompression after TIPS could increase the risk of hernia incarceration that may require urgent surgical repair. Incarcerated hernias after TIPS have been reported with incidence varying from 12 to 25% with the majority requiring emergent surgical repair with increased morbidity and mortality due to the potential for associated small bowel obstruction and perforation. 71 72 73 Most of the episodes of incarceration occur in the first 2 to 3 months after the procedure. If the ascites is controlled, elective surgical repair of the hernia should be performed, and the patients need to be educated about the risk and clinical presentation of this complication.
Radiation Injury
TIPS procedures can be very long, especially if the PV is thrombosed, and complex recanalization procedures are needed. The constant use of fluoroscopy and the need to use a high radiation dose because of the anatomic region in patients, who are frequently obese and/or have severe ascites, can lead to significant radiation exposure to the patients and the operators. TIPS is probably the procedure that gives the most radiation in interventional radiology with ∼8% of cases having accumulated a dose greater than 5 Gy. 74 75 The reported rate of radiation skin burn with TIPS is 0.1%. 5 In an internal review at our institution of 1,276 cases performed between 2018 and 2019, 15 out of 44 procedures that had a radiation dose >5 Gy were TIPS. High body mass index (BMI) was identified as significant risk factor for high radiation dose. Only one patient had a reported transient skin burn.
For long procedures, avoid using steep oblique projections. If possible, reposition the beam entrance site to avoid irradiation of the same part of the skin. Other known measures of radiation protection include trying to perform the procedure in the low fluoroscopy mode and avoiding magnification, using collimators, digital magnification, last image hold, trying not to do many digital subtraction angiography runs, and keeping the image intensifier close to the patient. Many of the newer fluoroscopic equipment have digital software that allows to significantly decrease the radiation dose while keeping adequate imaging quality. Scheduling TIPS in the newer rooms, if available, is another way to prevent significant radiation exposure to the patients and operators.
Some patients may have increased radiosensitivity due to autoimmune conditions, connective tissue, or genetic disorder (ataxia telangiectasia, Fanconi anemia; Fig. 29 ). Diabetes mellitus and hyperthyroidism also can increase radiosensitivity. 76
Fig. 29.
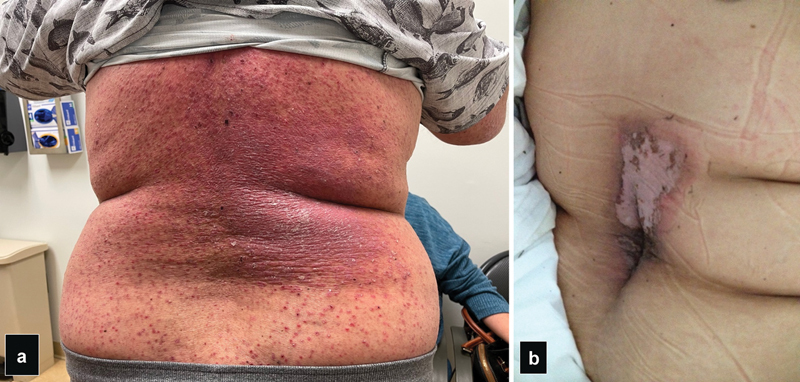
Photographs of two patients with radiation injuries after TIPS. ( a ) Radiation-induced dermatitis after 5 Gy TIPS. Patient had seborrheic dermatitis predisposing him to the radiation injury. ( b ) Radiation injury after 7 Gy TIPS showing desquamation of the skin in a square area. Obesity was a major contributing factor in both patients.
If the skin dose exceeded 5 Gy, it is recommended that the patients are notified of possible skin reactions that are usually manifested as transient erythema in the first 2 weeks, followed by erythema and epilation 2 to 8 weeks after exposure. For skin doses over 10 Gy, dry or moist desquamation can occur with prolonged erythema and permanent epilation, while for doses over 15 Gy, ulcerations that may require surgical interventions and dermal atrophy can occur. It is very important that the patients are notified of these possible skin changes as they may seek medical care by other specialties, including dermatologist, who may not be aware of these injuries leading to the wrong diagnosis and treatments.
Conclusions
TIPS has revolutionized the treatment of many of the portal hypertension complications and has mostly replaced surgically created portosystemic shunts. TIPS procedures are expected to increase with the recent rise of NAFLD in the general population. A variety of complications can occur during and after the procedure that can lead to severe morbidity and mortality. Adequate patient selection, careful technique with the use of adjunct imaging guidance techniques such as IVUS and TAUS, and early interventions to correct any treatable complications are all important steps to prevent and treat adverse outcomes with this complex and remarkable procedure.
References
- 1.Barton R E, Rosch I, Saxon R R, Lakin P C, Petersen B D, Keller F S. TIPS: short-and long-term results: a survey of 1750 patients. Semin Intervent Radiol. 1995;12:364–367. [Google Scholar]
- 2.Freedman A M, Sanyal A J, Tisnado J. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics. 1993;13(06):1185–1210. doi: 10.1148/radiographics.13.6.8290720. [DOI] [PubMed] [Google Scholar]
- 3.Gaba R C, Khiatani V L, Knuttinen M G. Comprehensive review of TIPS technical complications and how to avoid them. AJR Am J Roentgenol. 2011;196(03):675–685. doi: 10.2214/AJR.10.4819. [DOI] [PubMed] [Google Scholar]
- 4.McCowan T C, Hummel M M, Schmucker T, Goertzen T C, Culp W C, Habbe T G. Cardiac perforation and tamponade during transjugular intrahepatic portosystemic shunt placement. Cardiovasc Intervent Radiol. 2000;23(04):298–300. doi: 10.1007/s002700010072. [DOI] [PubMed] [Google Scholar]
- 5.Shah R P, Sze D Y. Complications during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(01):61–73. doi: 10.1053/j.tvir.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Semba C P, Saperstein L, Nyman U, Dake M D. Hepatic laceration from wedged venography performed before transjugular intrahepatic portosystemic shunt placement. J Vasc Interv Radiol. 1996;7(01):143–146. doi: 10.1016/s1051-0443(96)70751-0. [DOI] [PubMed] [Google Scholar]
- 7.Tavare A N, Wigham A, Hadjivassilou A. Use of transabdominal ultrasound-guided transjugular portal vein puncture on radiation dose in transjugular intrahepatic portosystemic shunt formation. Diagn Interv Radiol. 2017;23(03):206–210. doi: 10.5152/dir.2016.15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai A K, Andring B, Faulconer N. Utility of intravascular US-guided portal vein access during transjugular intrahepatic portosystemic shunt creation: retrospective comparison with conventional technique in 109 patients. J Vasc Interv Radiol. 2016;27(08):1154–1159. doi: 10.1016/j.jvir.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Farsad K, Kaufman J A. Novel image guidance techniques for portal vein targeting during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(01):10–20. doi: 10.1053/j.tvir.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Cannataci C, Cimo' B, Mamone G. Portal vein puncture-related complications during transjugular intrahepatic portosystemic shunt creation: Colapinto needle set vs Rösch-Uchida needle set. Radiol Med (Torino) 2021;126(11):1487–1495. doi: 10.1007/s11547-021-01404-1. [DOI] [PubMed] [Google Scholar]
- 11.Vizzutti F, Schepis F, Arena U. Transjugular intrahepatic portosystemic shunt (TIPS): current indications and strategies to improve the outcomes. Intern Emerg Med. 2020;15(01):37–48. doi: 10.1007/s11739-019-02252-8. [DOI] [PubMed] [Google Scholar]
- 12.Saxon R R, Keller F S. Technical aspects of accessing the portal vein during the TIPS procedure. J Vasc Interv Radiol. 1997;8(05):733–744. doi: 10.1016/s1051-0443(97)70655-9. [DOI] [PubMed] [Google Scholar]
- 13.Mamone G, Milazzo M, Di Piazza A. Transjugular intrahepatic portosystemic shunt (TIPS) complications: what diagnostic radiologists should know. Abdom Radiol (NY) 2022;47(12):4254–4270. doi: 10.1007/s00261-022-03685-0. [DOI] [PubMed] [Google Scholar]
- 14.Haskal Z J, Cope C, Shlansky-Goldberg R D. Transjugular intrahepatic portosystemic shunt-related arterial injuries: prospective comparison of large- and small-gauge needle systems. J Vasc Interv Radiol. 1995;6(06):911–915. doi: 10.1016/s1051-0443(95)71211-8. [DOI] [PubMed] [Google Scholar]
- 15.Patel R K, Chandel K, Tripathy T P, Mukund A. Complications of transjugular intrahepatic portosystemic shunt (TIPS) in the era of the stent graft - What the interventionists need to know? Eur J Radiol. 2021;144:109986. doi: 10.1016/j.ejrad.2021.109986. [DOI] [PubMed] [Google Scholar]
- 16.Saxon R R, Mendel-Hartvig J, Corless C L. Bile duct injury as a major cause of stenosis and occlusion in transjugular intrahepatic portosystemic shunts: comparative histopathologic analysis in humans and swine. J Vasc Interv Radiol. 1996;7(04):487–497. doi: 10.1016/s1051-0443(96)70789-3. [DOI] [PubMed] [Google Scholar]
- 17.Jawaid Q, Saeed Z A, Di Bisceglie A M. Biliary-venous fistula complicating transjugular intrahepatic portosystemic shunt presenting with recurrent bacteremia, jaundice, anemia and fever. Am J Transplant. 2003;3(12):1604–1607. doi: 10.1046/j.1600-6135.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 18.Willner I R, El-Sakr R, Werkman R F, Taylor W Z, Riely C A. A fistula from the portal vein to the bile duct: an unusual complication of transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 1998;93(10):1952–1955. doi: 10.1111/j.1572-0241.1998.00553.x. [DOI] [PubMed] [Google Scholar]
- 19.Korrapati P, Bidari K, Komanduri S. Biliary obstruction after transjugular intrahepatic portosystemic shunt placement in a patient with Budd-Chiari syndrome. ACG Case Rep J. 2015;2(02):101–103. doi: 10.14309/crj.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duller D, Kniepeiss D, Lackner C. Biliary obstruction as a complication of transjugular intrahepatic portosystemic shunt. Liver Transpl. 2009;15(05):556–557. doi: 10.1002/lt.21608. [DOI] [PubMed] [Google Scholar]
- 21.Bucher J N, Hollenbach M, Strocka S. Segmental intrahepatic cholestasis as a technical complication of the transjugular intrahepatic porto-systemic shunt. World J Gastroenterol. 2019;25(43):6430–6439. doi: 10.3748/wjg.v25.i43.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel F, Bick B. Biliary obstruction after transjugular intrahepatic portosystemic shunt placement. ACG Case Rep J. 2021;8(06):e00618. doi: 10.14309/crj.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehmood F, Khalid A, Frager S. Perihepatic biloma in a non-cirrhotic patient after transjugular intrahepatic portosystemic shunt (TIPS) Cureus. 2022;14(03):e23399. doi: 10.7759/cureus.23399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Society of Interventional Radiology Standards of Practice Committee . Dariushnia S R, Haskal Z J, Midia M. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2016;27(01):1–7. doi: 10.1016/j.jvir.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Prahlow J A, O'Bryant T J, Barnard J J. Cardiac perforation due to Wallstent embolization: a fatal complication of the transjugular intrahepatic portosystemic shunt procedure. Radiology. 1997;205(01):170–172. doi: 10.1148/radiology.205.1.9314980. [DOI] [PubMed] [Google Scholar]
- 26.Ripamonti R, Ferral H, Alonzo M, Patel N H. Transjugular intrahepatic portosystemic shunt-related complications and practical solutions. Semin Intervent Radiol. 2006;23(02):165–176. doi: 10.1055/s-2006-941447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi P H, Mao J, Slater K. Atrial septal perforation from TIPS stent migration. J Vasc Interv Radiol. 2004;15(06):629–632. doi: 10.1097/01.rvi.0000130165.74003.f0. [DOI] [PubMed] [Google Scholar]
- 28.Mizrahi M, Adar T, Shouval D, Bloom A I, Shibolet O. Endotipsitis-persistent infection of transjugular intrahepatic portosystemic shunt: pathogenesis, clinical features and management. Liver Int. 2010;30(02):175–183. doi: 10.1111/j.1478-3231.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 29.Suhocki P V, Smith A D, Tendler D A, Sexton D J. Treatment of TIPS/biliary fistula-related endotipsitis with a covered stent. J Vasc Interv Radiol. 2008;19(06):937–939. doi: 10.1016/j.jvir.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Lopera J E, Katabathina V, Bosworth B. Segmental liver ischemia/infarction after elective transjugular intrahepatic portosystemic shunt creation: clinical outcomes in 10 patients. J Vasc Interv Radiol. 2015;26(06):835–841. doi: 10.1016/j.jvir.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 31.Mandal P, O'Donnell B P, Smith E R. Portal and hepatic vein thrombosis after transjugular intrahepatic portosystemic shunt: incidence in follow-up imaging and clinical implications. Int J Gastrointest Interv. 2022;11:18–23. [Google Scholar]
- 32.Bureau C, Otal P, Chabbert V, Péron J M, Rousseau H, Vinel J P. Segmental liver ischemia after TIPS procedure using a new PTFE-covered stent. Hepatology. 2002;36(06):1554. doi: 10.1053/jhep.2002.35449. [DOI] [PubMed] [Google Scholar]
- 33.Tuifua T S, Partovi S, Remer E M. Assessment of clinical outcomes, clinical manifestations, and risk factors for hepatic infarction after transjugular intrahepatic portosystemic shunt placement (TIPS): a retrospective comparative study. Cardiovasc Intervent Radiol. 2022;45(10):1512–1523. doi: 10.1007/s00270-022-03219-7. [DOI] [PubMed] [Google Scholar]
- 34.Bureau C, Garcia Pagan J C, Layrargues G P. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27(06):742–747. doi: 10.1111/j.1478-3231.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 35.Klasen-Sansone J, Bode J, Lanzman R S. TIPS geometry influences patency. Z Gastroenterol. 2015;53(01):28–32. doi: 10.1055/s-0034-1385430. [DOI] [PubMed] [Google Scholar]
- 36.Fanelli F, Salvatori F M, Corona M. Stent graft in TIPS: technical and procedural aspects. Radiol Med (Torino) 2006;111(05):709–723. doi: 10.1007/s11547-006-0068-6. [DOI] [PubMed] [Google Scholar]
- 37.Joseph A S, Sandhu B, Khalil A, Lopera J. Transjugular portosystemic shunt reductions: a retrospective single-center experience. J Vasc Interv Radiol. 2019;30(06):876–884. doi: 10.1016/j.jvir.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 38.Vizzutti F, Rega L, Arena U. Paradoxical embolization in TIPS: take a closer look to the heart. Ann Hepatol. 2015;14(01):127–131. [PubMed] [Google Scholar]
- 39.Casadaban L C, Parvinian A, Couture P M. Characterization of liver function parameter alterations after transjugular intrahepatic portosystemic shunt creation and association with early mortality. AJR Am J Roentgenol. 2014;203(06):1363–1370. doi: 10.2214/AJR.13.12232. [DOI] [PubMed] [Google Scholar]
- 40.Rajesh S, George T, Philips C A. Transjugular intrahepatic portosystemic shunt in cirrhosis: An exhaustive critical update. World J Gastroenterol. 2020;26(37):5561–5596. doi: 10.3748/wjg.v26.i37.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaba R C, Lakhoo J. What constitutes liver failure after transjugular intrahepatic portosystemic shunt creation? A proposed definition and grading system. Ann Hepatol. 2016;15(02):230–235. doi: 10.5604/16652681.1193719. [DOI] [PubMed] [Google Scholar]
- 42.Rajan D K, Haskal Z J, Clark T W.Serum bilirubin and early mortality after transjugular intrahepatic portosystemic shunts: results of a multivariate analysis J Vasc Interv Radiol 200213(2, Pt 1):155–161. [DOI] [PubMed] [Google Scholar]
- 43.Luca A, Miraglia R, Maruzzelli L, D'Amico M, Tuzzolino F. Early liver failure after transjugular intrahepatic portosystemic shunt in patients with cirrhosis with model for end-stage liver disease score of 12 or less: incidence, outcome, and prognostic factors. Radiology. 2016;280(02):622–629. doi: 10.1148/radiol.2016151625. [DOI] [PubMed] [Google Scholar]
- 44.Verma R, Jain N, Arora A, Gamangatti S, Chaturvedi S. Beyond MELD predictors of post TIPSS acute liver failure the lesson learned. Indian J Radiol Imaging. 2021;31(03):618–622. doi: 10.1055/s-0041-1736403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ascha M, Abuqayyas S, Hanouneh I. Predictors of mortality after transjugular portosystemic shunt. World J Hepatol. 2016;8(11):520–529. doi: 10.4254/wjh.v8.i11.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Praktiknjo M, Clees C, Pigliacelli A. Sarcopenia is associated with development of acute-on-chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol. 2019;10(04):e00025. doi: 10.14309/ctg.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ronald J, Bozdogan E, Zaki I H. Relative sarcopenia with excess adiposity predicts survival after transjugular intrahepatic portosystemic shunt creation. AJR Am J Roentgenol. 2020;214(01):200–205. doi: 10.2214/AJR.19.21655. [DOI] [PubMed] [Google Scholar]
- 48.Lopera J, Speeg K, Young C. Effect of liver volume in morbidity and mortality after elective transjugular intrahepatic portosystemic shunt. Gastrointest Intervent. 2014;3(02):93–97. [Google Scholar]
- 49.Rössle M, Siegerstetter V, Olschewski M, Ochs A, Berger E, Haag K. How much reduction in portal pressure is necessary to prevent variceal rebleeding? A longitudinal study in 225 patients with transjugular intrahepatic portosystemic shunts. Am J Gastroenterol. 2001;96(12):3379–3383. doi: 10.1111/j.1572-0241.2001.05340.x. [DOI] [PubMed] [Google Scholar]
- 50.Chung H H, Razavi M K, Sze D Y. Portosystemic pressure gradient during transjugular intrahepatic portosystemic shunt with Viatorr stent graft: What is the critical low threshold to avoid medically uncontrolled low pressure gradient related complications? J Gastroenterol Hepatol. 2008;23(01):95–101. doi: 10.1111/j.1440-1746.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- 51.Chutaputti A. Management of refractory ascites and hepatorenal syndrome. J Gastroenterol Hepatol. 2002;17(04):456–461. doi: 10.1046/j.1440-1746.2002.02724.x. [DOI] [PubMed] [Google Scholar]
- 52.Wong F, Blendis L, Fernandez-Esparrach G. Therapy for hepatorenal syndrome. Gastroenterology. 1998;115(02):503–504. doi: 10.1016/s0016-5085(98)70225-2. [DOI] [PubMed] [Google Scholar]
- 53.Danziger J, Thummalakunta L, Nelson R, Faintuch S. The risk of acute kidney injury with transjugular intrahepatic portosystemic shunts. J Nephrol. 2015;28(06):725–728. doi: 10.1007/s40620-015-0187-z. [DOI] [PubMed] [Google Scholar]
- 54.Anderson C L, Saad W E, Kalagher S D. Effect of transjugular intrahepatic portosystemic shunt placement on renal function: a 7-year, single-center experience. J Vasc Interv Radiol. 2010;21(09):1370–1376. doi: 10.1016/j.jvir.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Brensing K A, Textor J, Perz J. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47(02):288–295. doi: 10.1136/gut.47.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lang M, Lang A L, Tsui B Q. Renal-function change after transjugular intra-hepatic portosystemic shunt placement and its relationship with survival: a single-center experience. Gastroenterol Rep (Oxf) 2020;9(04):306–312. doi: 10.1093/gastro/goaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lakhoo J, Gunasekaran S S, Lokken R P. Does advanced chronic kidney disease impact transjugular intrahepatic portosystemic shunt efficacy and safety? Acta Gastroenterol Belg. 2017;80(02):243–248. [PubMed] [Google Scholar]
- 58.Haskal Z J, Radhakrishnan J. Transjugular intrahepatic portosystemic shunts in hemodialysis-dependent patients and patients with advanced renal insufficiency: safety, caution, and encephalopathy. J Vasc Interv Radiol. 2008;19(04):516–520. doi: 10.1016/j.jvir.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Filì D, Falletta C, Luca A. Circulatory response to volume expansion and transjugular intrahepatic portosystemic shunt in refractory ascites: relationship with diastolic dysfunction. Dig Liver Dis. 2015;47(12):1052–1058. doi: 10.1016/j.dld.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Baiges A, Garcia-Pagán J C. Predicting heart failure after TIPS: still more questions than answers. Hepatology. 2019;70(06):1889–1891. doi: 10.1002/hep.30948. [DOI] [PubMed] [Google Scholar]
- 61.Busk T M, Bendtsen F, Henriksen J H. Effects of transjugular intrahepatic portosystemic shunt (TIPS) on blood volume distribution in patients with cirrhosis. Dig Liver Dis. 2017;49(12):1353–1359. doi: 10.1016/j.dld.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 62.AISF TIPS Special Conference . Fagiuoli S, Bruno R, Debernardi Venon W. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49(02):121–137. doi: 10.1016/j.dld.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Myers R P, Lee S S.Cirrhotic cardiomyopathy and liver transplantation Liver Transpl 20006(4, Suppl 1):S44–S52. [DOI] [PubMed] [Google Scholar]
- 64.Manguso G, Vignone A, Merli M. Hemodynamic evaluation of the right heart-pulmonary circulation unit in patients candidate to transjugular intrahepatic portosystemic shunt. J Clin Med. 2022;11(02):461. doi: 10.3390/jcm11020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modha K, Kapoor B, Lopez R, Sands M J, Carey W. Symptomatic heart failure after transjugular intrahepatic portosystemic shunt placement: incidence, outcomes, and predictors. Cardiovasc Intervent Radiol. 2018;41(04):564–571. doi: 10.1007/s00270-017-1848-1. [DOI] [PubMed] [Google Scholar]
- 66.Parvinian A, Bui J T, Knuttinen M G, Minocha J, Gaba R C. Right atrial pressure may impact early survival of patients undergoing transjugular intrahepatic portosystemic shunt creation. Ann Hepatol. 2014;13(04):411–419. [PubMed] [Google Scholar]
- 67.Cazzaniga M, Salerno F, Pagnozzi G. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut. 2007;56(06):869–875. doi: 10.1136/gut.2006.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Armstrong M J, Gohar F, Dhaliwal A. Diastolic dysfunction on echocardiography does not predict survival after transjugular intrahepatic portosystemic stent-shunt in patients with cirrhosis. Aliment Pharmacol Ther. 2019;49(06):797–806. doi: 10.1111/apt.15164. [DOI] [PubMed] [Google Scholar]
- 69.Billey C, Billet S, Robic M A. A prospective study identifying predictive factors of cardiac decompensation after transjugular intrahepatic portosystemic shunt: the Toulouse algorithm. Hepatology. 2019;70(06):1928–1941. doi: 10.1002/hep.30934. [DOI] [PubMed] [Google Scholar]
- 70.Sarwar A, Esparaz A M, Chakrala N. Efficacy of TIPS reduction for refractory hepatic encephalopathy, right heart failure, and liver dysfunction. AJR Am J Roentgenol. 2021;216(05):1267–1272. doi: 10.2214/AJR.19.22497. [DOI] [PubMed] [Google Scholar]
- 71.Smith M T, Rase B, Woods A. Risk of hernia incarceration following transjugular intrahepatic portosystemic shunt placement. J Vasc Interv Radiol. 2014;25(01):58–62. doi: 10.1016/j.jvir.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Young S, Larson L, Bermudez J. Evaluation of the frequency and factors predictive of hernia incarceration following transjugular intrahepatic portosystemic shunt placement. Clin Radiol. 2021;76(04):287–293. doi: 10.1016/j.crad.2020.12.014. [DOI] [PubMed] [Google Scholar]
- 73.McDaniel C, Bell R, Farha N. Risk of hernia-related complications after transjugular intrahepatic portosystemic shunt creation in patients with pre-existing ventral abdominal hernias: 15-year experience at a quaternary medical center. BMJ Open Gastroenterol. 2022;9(01):e000876. doi: 10.1136/bmjgast-2022-000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.RAD-IR Study . Miller D L, Balter S, Cole P E. Radiation doses in interventional radiology procedures: the RAD-IR study: Part I: Overall measures of dose. J Vasc Interv Radiol. 2003;14(06):711–727. doi: 10.1097/01.rvi.0000079980.80153.4b. [DOI] [PubMed] [Google Scholar]
- 75.Miller D L, Balter S, Cole P E. Radiation doses in interventional radiology procedures: the RAD-IR study: Part II: Skin dose. J Vasc Interv Radiol. 2003;14(08):977–990. doi: 10.1097/01.rvi.0000084601.43811.cb. [DOI] [PubMed] [Google Scholar]
- 76.Rehani M M, Miller D L, Baliyan V. High-dose fluoroscopically guided procedures in patients: radiation management recommendations for interventionalists. Cardiovasc Intervent Radiol. 2021;44(06):849–856. doi: 10.1007/s00270-020-02703-2. [DOI] [PubMed] [Google Scholar]


