Abstract
Background and Objectives
Real-time quaking-induced conversion (RT-QuIC) assay detects misfolded α-synuclein (AS) in the skin and CSF of patients with the synucleinopathies Parkinson disease and dementia with Lewy bodies. Isolated REM sleep behavior disorder (IRBD) constitutes the prodromal stage of these synucleinopathies. We aimed to compare the ability of RT-QuIC to identify AS in the skin and CSF of patients with IRBD.
Methods
This was a cross-sectional study where consecutive patients with polysomnographic-confirmed IRBD and age-matched controls without RBD underwent skin biopsy and lumbar puncture the same day. Three-millimeter skin punch biopsies were obtained bilaterally in the cervical region from dorsal C7 and C8 dermatomes and in distal legs. RT-QuIC assessed AS in these 6 skin sites and the CSF.
Results
We recruited 91 patients with IRBD and 41 controls. In the skin, sensitivity to detect AS was 76.9% (95% CI 66.9–85.1), specificity 97.6% (95% CI 87.1–99.9) positive predictive value 98.6% (95% CI 91.0–99.8), negative predictive value 65.6% (95% CI 56.6–73.6), and accuracy 83.3% (95% CI 75.9–89.3). In the CSF, the sensitivity was 75.0% (95% CI 64.6–83.6), the specificity was 97.5% (95% CI 86.8–99.9), the positive predictive value was 98.5% (95% CI 90.5–99.8), the negative predictive value was 63.9% (95% CI 55.2–71.9), and the accuracy was 82.0% (95% CI 74.3–88.3). Results in the skin and CSF samples showed 99.2% agreement. Compared with negative patients, RT-QuIC AS-positive patients had a higher likelihood ratio of prodromal Parkinson disease (p < 0.001) and showed more frequently hyposmia (p < 0.001), dopamine transporter imaging single-photon emission CT deficit (p = 0.002), and orthostatic hypotension (p = 0.014). No severe or moderate adverse effects were reported. There was no difference between the percentage of participants reporting mild adverse events secondary to skin biopsy or lumbar puncture (9.1% vs 17.2%; p = 0.053). One hundred and ten (83%) and 104 (80%) participants, respectively, stated they would accept to undergo skin biopsy and lumbar puncture again for research purposes.
Discussion
Our study in IRBD shows that (1) RT-QuIC detects AS in the skin and CSF with similar high sensitivity, specificity, and agreement, (2) AS RT-QuIC positivity is associated with supportive features and biomarkers of synucleinopathy, and (3) skin punch biopsy and lumbar puncture have comparable mild adverse effects, tolerance, and acceptance. RT-QuIC in the skin or CSF might represent a patient selection strategy for future neuroprotective trials targeting AS in IRBD.
Classification of Evidence
This study provides Class III evidence that RT-QuIC–detected AS in the skin and CSF distinguishes patients with IRBD from controls.
Parkinson disease (PD) and dementia with Lewy bodies (DLB) are characterized by intraneuronal Lewy bodies and Lewy neurites containing pathologic deposits of misfolded α-synuclein (AS) as their main component.1,2 AS plays a key role in the pathophysiology of these synucleinopathies as this protein is thought to be neurotoxic propagating by cell-to-cell transmission in the CNS.3,4
Isolated REM sleep behavior disorder (IRBD), a parasomnia characterized by nightmares and vigorous behaviors during REM sleep,5,6 represents the prodromal stage of the synucleinopathies because most patients with IRBD (1) are eventually diagnosed with PD, DLB, and occasionally with multiple system atrophy (MSA)7,8 and (2) have pathologic AS in the CSF and peripheral organs.9-20 In patients with IRBD, in vivo identification of abnormal AS would not be important only to confirm the presence of prodromal synucleinopathy but also as selection criteria for future neuroprotective trials, particularly when the chosen agent targets AS propagation. It is unclear, however, where is the most reliable site to identify AS in IRBD. Using immunohistochemistry and immunofluorescence, the sensitivity to detect AS in IRBD varies with 25% sensitivity in the colon,9 50%–89% in the salivary glands,10,11 and 55%–82% in the skin.13-17 The skin seems the preferable site for biopsy because the procedure is technically easy and related adverse events are few, mild, and transient.21
Real-time quaking-induced conversion (RT-QuIC) is a novel ultrasensitive method that identifies misfolded proteins in biofluids and tissues.22 In PD and DLB, RT-QuIC and similar seeding aggregation assays detect AS in the CSF and skin with sensitivity and specificity of 85%–100%.23-29 In IRBD, RT-QuIC also detects AS in the CSF with high sensitivity and specificity.18,19,23,25 In addition, the RT-QuIC assay detects AS in the olfactory mucosa with a sensitivity of 44% in IRBD.20 In the current study, we aimed to measure the ability of RT-QuIC to detect AS in the skin of patients with IRBD and compare the results with those obtained simultaneously in the CSF. In IRBD, this information could be useful for patient stratification in future neuroprotective trials using interventions against AS to halt the neurodegenerative process. The primary research question addressed in the study is to determine whether AS can be detected by RT-QuIC with the same ability in the CSF than in the skin in patients with IRBD.
Methods
Participants
We performed a cross-sectional case-control study at the Hospital Clinic de Barcelona (HCB), Spain, and at the Istituto delle Scienze Neurologiche di Bologna (ISNB), Italy, between May 2021 and June 2022. We invited to participate all consecutive patients with polysomnographic-confirmed IRBD who were attending at the HCB during the study period. Patients with IRBD were asked to undergo skin biopsy and lumbar puncture the same morning, in addition to clinical assessments, neurologic examination, and ancillary interventions.
For comparisons, a control group was recruited at the HCB to undergo skin biopsy and lumbar puncture the same morning. Controls were healthy individuals and consecutive individuals with sleep disorders not associated with the development of synucleinopathies (e.g., obstructive sleep apnea and insomnia). In controls, IRBD was excluded by clinical history and video-polysomnography. Controls reported no motor or cognitive complaints, and their neurologic examination was normal.
Exclusion criteria for all participants were skin disorders, coagulation abnormalities, and anticoagulant use. In the present study, we excluded the 52 patients with IRBD and 40 controls who participated in our previous work where we assessed AS in the CSF by RT-QuIC.18
Standard Protocol Approvals, Registrations, and Patient Consents
The ethical committees of the HCB (HCB/2020/1400) and Area Vasta Emilia Centro (EM1173-2021-20226) approved this study, and participants gave written informed consent.
Clinical and Ancillary Assessments
Two hours before skin biopsy and lumbar puncture were performed, patients with IRBD underwent a comprehensive assessment where the presence of prodromal PD markers and risk factors were evaluated, as previously described.18,30 In brief, we assessed motor function using the Movement Disorders Society Unified Parkinson's Disease Rating Scale Part III (MDS-UPDRS-III),31 cognition with the Montreal Cognitive Assessment (MoCA) test,32,33 and smell with the 40-item University of Pennsylvania Smell Identification Test (UPSIT-40).34,35 Orthostatic hypotension,36 constipation,37 depression,38 and hypersomnia39 were evaluated using diagnostic criteria and scales. Within 4 weeks before or after skin biopsy and lumbar puncture, patients underwent dopamine transporter imaging single-photon emission CT (DAT-SPECT) (Symbia Intevo 16; Siemens Medical System, Erlangen, Germany) as previously described40 according to European Association of Nuclear Medicine practice guidelines.41 With all this information, the likelihood ratio of prodromal PD was calculated in each patient according to published criteria.30 Prodromal PD was defined as probability ≥80%.30 Controls in whom RT-QuIC showed AS positivity in the skin or CSF were asked to undergo the same extensive clinical and ancillary assessment where the likelihood ratio of prodromal PD was also calculated.
Procedures
In each participant, six 3-mm skin punch biopsies were obtained bilaterally, after intradermal anesthesia, in the dorsal cervical region at C7 and C8 dermatomes and in distal legs 10 cm above the external malleolus, according to guidelines.21 Lumbar puncture was performed immediately after skin biopsy under local anesthesia, according to guidelines.18,42 Skin samples and CSF were stored at −80°C until shipped on dry ice to the ISNB for RT-QuIC analysis. All skin biopsies and lumbar punctures were performed by 2 experienced neurologists (A.M.-L. and G.M.).
One week after skin biopsy and lumbar puncture, participants were contacted by telephone and were asked whether they had experienced adverse effects (and which) from each procedure. We also asked them whether they would accept to undergo skin biopsy and lumbar puncture again for future research projects.
RT-QuIC Analysis
At the ISNB, RT-QuIC assay was performed blinded to participant category and the exact localization of each skin sample. Skin samples were processed as previously reported.28,43 Briefly, after thawing, skin samples were washed 3 times in cold 1X phosphate-buffered saline (PBS) and homogenized at 1% (wt/vol) with a gentleMACS Octo Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany) in cold 1X PBS supplemented with Complete Protease Inhibitor Mixture (Roche, Basel, Switzerland). Skin homogenates were then centrifuged at 1,000g for 10 minutes at 4°C, and supernatants were stored at −80°C until use.
RT-QuIC was performed as previously reported.25,28 Briefly, black 96-well plates with a clear bottom (Nalgene Nunc International, Rochester, NY) were preloaded with six 0.8-mm silica beads (OPS Diagnostics, Lebanon, NJ) per well. CSF and skin homogenate samples were thawed and vortexed before use. For the CSF assay, 15 μL of CSF was added as seed to 85 μL of buffer containing 40 mM phosphate buffer, pH 8.0, 170 mM NaCl, 10 mM thioflavin-T, 0.0015% sodium dodecyl sulfate, and 0.1 g/L recombinant α-synuclein filtered with a 100-kDa-molecular-weight cutoff filter (Amicon centrifugal filters; Merck Millipore, Burlington, MA). For the skin assay, 2 μL of skin homogenate at 10−2 final dilution was added to 98 μL of reaction mix containing 40 mM phosphate buffer, pH 8.0, 170 mM NaCl, 10 mM thioflavin-T, and 0.1 g/L of recombinant AS filtered as above. The recombinant wild-type α-synuclein used for both skin and CSF RT-QuIC was produced in house, as described.25 After loading, the plate was sealed with a plate sealer film (Nalgene Nunc International) and incubated into a FLUOstar Omega (BMG LABTECH, Ortenberg, Germany) plate reader at 42°C with intermittent double-orbital shaking at 400 rpm for 1 minute, followed by a 1-minute rest. Thioflavin-T fluorescence measurements were taken every 45 minutes using 450-nm excitation and 480-nm emission filters. To overcome the intrinsic plate-to-plate variability and batch-to-batch variations of AS seeding activity, we normalized the relative fluorescent units for every time point for the median of the maximum values reached by the positive control replicates during the 30-hour run in each experimental plate and expressed them as a percentage. Samples ran in quadruplicates and deemed positive if at least 2 of the 4 replicates gave a fluorescence signal higher than the chosen threshold cutoff value. The latter was set as the 30% of the median of the maximum values reached by the positive control replicates during the 30-hour run. Samples giving a positive signal in a single replicate were rerun up to 3 times and eventually deemed negative unless giving a definite positive response (e.g., at least 2 of 4).
We attributed an RT-QuIC score based on the number of positive wells and compared the score distribution among skin sites. The scores were defined as follows: 1.00 = 4/4 positive wells; 0.75 = 3/4 positive wells; 0.50 = 2/4 positive wells; 0.25 = 1/4 positive wells; and 0.0 = 0/4 positive wells. We also measured the time needed by the fluorescence signal to reach the threshold (LAG), and we calculated a LAG score as follows: 1.0 = LAG <18 hours; 0.75 = LAG 18–24 hours; and 0.50 = LAG >24 hours and compared the distribution of the LAG score between the skin sites and between skin and CSF.
Statistical Analysis
Data are reported as mean, SD, number, and percentage. We calculated the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy, including 95% CIs, for detection of AS in the skin and CSF. The Wilson method was used to compute CIs. For the skin, participants were categorized as AS positive if at least 1 of the 6 skin samples was positive for AS. In each participant, the CSF was classified as either AS positive or AS negative. RT-QuIC fluorescence responses were analyzed and plotted using GraphPad Prism 9.0 for Windows. Differences between groups were assessed using the χ2 test, Fisher exact test, Student t test, and Mann-Whitney U test, as appropriate. We calculated the overall percentage agreement (the proportion of patients with IRBD in whom the skin biopsy and the lumbar puncture gave the same positive or negative outcome for the presence of AS) between skin biopsy and lumbar puncture. Analyses were performed with SPSS version 25.0 for Windows (SPSS, Inc., Chicago, IL). p Values of less than 0.05 were significant.
Data Availability
Data from this study are available from the corresponding authors on request, if reasonable.
Results
Of the 134 eligible patients who had routine visits at the HCB during the study period, 91 agreed to participate, 33 declined, and 10 were excluded because of anticoagulant therapy. In total, 91 patients and 41 controls participated in the study. Patients included 70 (76.9%) men and 21 (23.1%) women with a mean age of 69.6 ± 8.4 years, and a mean interval between IRBD diagnosis and this study of 3.5 ± 3.8 years. Controls were 24 (58.5%) men and 17 (41.5%) women with a mean age of 69.8 ± 8.1 years. Patients and controls were matched for age (p = 0.887), with male predominance in patients (p = 0.035). Of the 132 enrolled participants (91 patients and 41 controls), 88 (96.7%) patients and 40 (97.6%) controls underwent both skin biopsy and lumbar puncture. Three (3.3%) patients and 1 (2.4%) control accepted only skin biopsy.
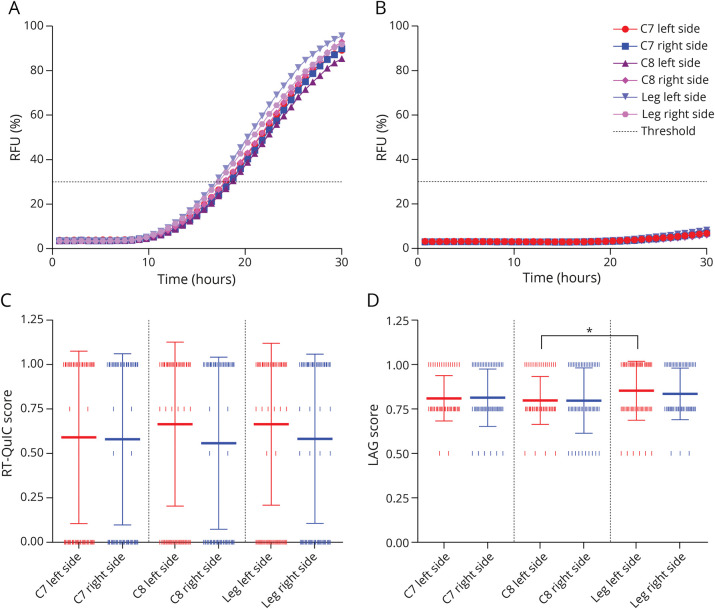
In the skin, RT-QuIC was AS positive in 70 (76.9%) of 91 patients and in 1 (2.4%) of 41 controls, resulting in a sensitivity of 76.9% (95% CI 66.9–85.1), specificity of 97.6% (95% CI 87.1–99.9), positive predictive value of 98.6% (95% CI 91.0–99.8), negative predictive value of 65.6% (95% CI 56.6–73.6), and accuracy of 83.3% (95% CI 75.9–89.3). The kinetics and overall results of AS RT-QuIC in the skin of patients and controls are shown in Figure 1, A and B.
Figure 1. Kinetics of α-Synuclein RT-QuIC Reactions in the Skin of Patients With IRBD.
Mean normalized fluorescence emission for each skin site in patients with IRBD (A) and controls (B). Bars indicating the SEM were omitted to improve the readability. (C) Distribution in the number of RT-QuIC–positive replicates among skin sites. We attributed an RT-QuIC score based on the number of positive wells and compared the score distribution among skin sites (see Methods for score calculation). (D) Distribution of the LAG (time to threshold) score (see Methods for score calculation) across skin sites. Both leg sites showed a shorter LAG than cervical sites, but only the difference between left C8 and left leg was significant (*p ≤ 0.05). The RT-QuIC and LAG scores are plotted as mean ± SD. The vertical pipes indicate individual values. The red and blue lines in (C) and (D) indicate the skin site from the left and right sides, respectively. Panels (C) and (D) include only data from patients with IRBD. IRBD = isolated REM sleep behavior disorder; RFU = relative fluorescence units; RT-QuIC = real-time quaking-induced conversion.
In the CSF, RT-QuIC was AS positive in 66 (75.0%) of 88 patients and in 1 (2.5%) of 40 controls, giving a sensitivity of 75.0% (95% CI 64.6–83.6), specificity of 97.5% (95% CI 86.8–99.9), positive predictive value of 98.5% (95% CI 90.5–99.8), negative predictive value of 63.9% (95% CI 55.2–71.9), and accuracy of 82.0% (95% CI 74.3–88.3).
Compared with AS-negative patients, AS-positive patients were older, had a higher likelihood of prodromal PD, and showed more frequently abnormal DAT-SPECT, orthostatic hypotension, and hyposmia. Depression was more common in AS-negative patients (Table 1). AS-negative patients were taking antidepressants more frequently than AS-positive patients (85.7% vs 30%, p < 0.001).
Table 1.
Comparisons Between Patients With IRBD With α-Synuclein RT-QuIC Positivity and Negativity
One (2.4%) of the 41 controls showed AS positivity in the 6 skin sites and in the CSF. She was an 81-year-old woman with sleep apnea who had a low prodromal PD probability of 5.9%. She had hyposmia (UPSIT-40 score of 14) but lacked DAT-SPECT deficit, parkinsonian signs, memory complaints, orthostatic hypotension, constipation, depression, and hypersomnia. The MoCA test score was 26 points (normal), the MDS-UPDRS-III score was 2 points, and the brain MRI was unremarkable.
A total of 128 participants (88 patients and 40 controls) underwent both procedures. Among the 88 patients, 67 (52.3%) were AS positive in both skin and CSF, 1 (0.8%) was positive in the skin but negative in CSF, and none (0%) was negative in the skin but positive in CSF. Among the 40 controls, only 1 (2.5) was AS positive in both skin and CSF. The overall agreement between AS RT-QuIC result in the skin and CSF was 99.2%, 98.9% in patients with IRBD and 100% in controls.
Twelve (13.2%) patients with IRBD had coexistent mild cognitive impairment (MCI), and 10 (83.3%) of them showed AS positivity. Compared with the 79 (86.8%) patients with IRBD without MCI, those with MCI were older, had lower UPSIT and MoCA test scores, and showed higher PD prodromal probability (eTable 1, links.lww.com/WNL/C689).
Table 2 and Figure 1 summarize AS RT-QuIC results in the skin of the patients. Among the 70 patients with AS positivity in the skin, left C7 was positive in 78.6%, right C7 in 78.6%, left C8 in 88.6%, right C8 in 75.7%, left leg in 90.0%, and right leg in 80.0%. In 48.5% of the AS-positive patients, AS was identified in all 6 sites. Two 3-site combinations (both legs + left C7 and both legs + left C8) detected 100% AS skin positivity in the AS-positive patients. The percentage of patients with positivity in the cervical area was similar to the patients with positivity in the legs (74.7% vs 75.8%; p = 0.86) (eTable 2, links.lww.com/WNL/C689). Most positive skin samples gave a 4-of-4 response irrespectively to the site. There were no significant differences in the kinetics of the fluorescence response, measured by the LAG time among most skin sites and between each skin site and the CSF in positive patients (Figure 2). As the only exception, the left leg showed lower mean LAG time than the left C8 site (p = 0.02) (Figure 1D).
Table 2.
α-Synuclein RT-QuIC Results in the Skin and CSF of Patients and Controls
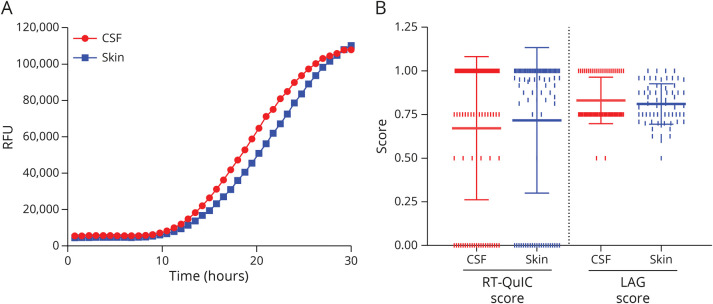
Figure 2. Comparison of AS Seeding Activity in the Skin and CSF of Patients With IRBD.
Comparison of α-synuclein RT-QuIC kinetics of fluorescence emission between the skin (mean of all positive sites) and CSF. Bars indicating the SEM were omitted to improve the readability. (B) Comparison of the number of positive replicates (RT-QuIC score, left) and LAG times (LAG score, right) between the skin (mean of all positive sites) and CSF. Only participants with CSF and at least 1 RT-QuIC positive skin site were evaluated (n = 87). The RT-QuIC and LAG scores are plotted as mean ± SD (see Methods for score calculation). The vertical pipes indicate individual values. AS = misfolded α-synuclein; IRBD = isolated REM sleep behavior disorder; RFU = relative fluorescence units; RT-QuIC = real-time quaking-induced conversion.
In patients with IRBD, the number of positive RT-QuIC reactions was higher in the CSF (75.0%) than in each individual skin site (left C7 in 60.4%, p = 0.04; right C7 in 60.4%, p = 0.04; left C8 in 68.1%, p = 0.31; right C8 in 58.2%, p = 0.02; left distal leg in 69.2%, p = 0.39; and right distal leg in 61.5%, p = 0.05) (Table 2). In contrast, skin samples showed a higher prevalence of 4 out of 4 positive replicates than the CSF (Table 2), suggesting that the apparent discrepancy might be related to a heterogeneous distribution of AS in the skin.
Aiming to compare the AS seeding activity between skin and CSF in each individual who tested positive by RT-QuIC, we created an RT-QuIC score accounting for both the number of positive replicates in positive samples and the negative sites. By applying this score, we found no differences between the skin and CSF (Figure 2B). This result was in line with the similar LAG time detected in the CSF and skin (Figure 2B).
All participants were contacted 1 week after the procedures. No severe or moderate adverse effects were reported. Complications related to skin biopsy were noted by 12 (9.1%) participants and consisted of pain (n = 8), wound infection (n = 3), and dysesthesias in 1 foot (n = 1). Complications related to lumbar puncture were reported by 22 (17.2%) participants and consisted of headache (n = 17), back pain (n = 7), and dizziness (n = 2). All these complications were minor and transient. The percentage of participants who reported complications secondary to skin biopsy was not different to the percentage of participants who reported complications secondary to lumbar puncture (9.1% vs 17.2%, p = 0.053). One hundred ten (83.3%) participants stated that they would accept undergoing skin biopsy again, and 103 (80.5%) would accept another lumbar puncture.
Classification of Evidence
This study provides Class III evidence that RT-QuIC–detected AS in the skin and CSF distinguishes patients with IRBD from controls.
Discussion
In this cross-sectional case-control study, we applied the RT-QuIC assay to identify AS in the skin and CSF of a cohort of consecutive patients with IRBD. We found that RT-QuIC identified AS with similar high sensitivity, specificity, and accuracy in the skin as in the CSF. The overall percentage agreement was 99%, indicating that RT-QuIC detects and excludes AS with the same ability in the skin as in the CSF. Skin punch biopsy and lumbar puncture shared similar optimal safety and tolerability. Taken together, our study indicates that skin biopsy and lumbar puncture are comparable procedures for identifying AS in IRBD using RT-QuIC.
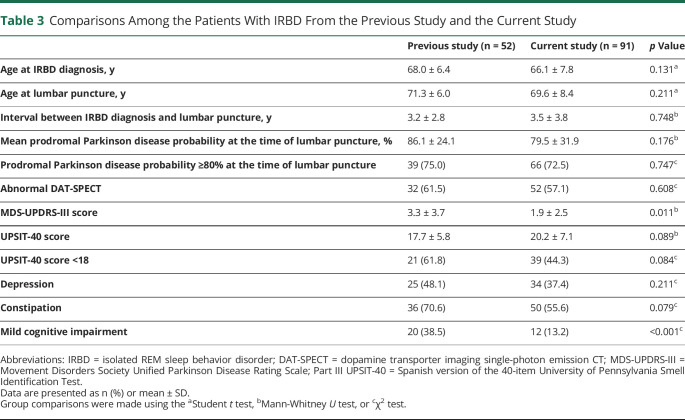
We found that in patients, the sensitivity to identify AS in the CSF was 75%. This percentage is lower than the one observed in a previous study in a group of different 52 patients with IRBD also from the HCB, where the sensitivity was 90%.18 One possible explanation for this difference might be the use of different RT-QuIC protocols, including the recombinant protein. Methodological RT-QuIC differences may also explain the increased specificity in the current study compared with the previous work (98% vs 90%). Alternatively, sensitivity differences between the 2 studies could be explained by patients' selection criteria and patients' characteristics. In the current study, we recruited all consecutive eligible individuals with IRBD attending our institution regardless of their clinical characteristics. In contrast, there was a selection bias in the previous study as we enrolled a subgroup of 20 (38.5%) patients with IRBD with comorbid MCI. The percentage of patients with IRBD with coexistent MCI was higher in the previous study (38.5% vs 13.2%; p < 0.001). In both studies, most patients with IRBD with MCI were CSF AS positive (19 of 20 patients in the previous and 10 of 12 in the current study). In individuals recruited in memory units with MCI, the sensitivity to detect AS in the CSF is 100% when coexisting RBD is documented by video-polysomnography.44 In addition, our previous work included patients with more mild parkinsonian signs than in the current study (Table 3). Taken together, the enriched IRBD cohort recruited in the previous study showing more cognitive and motor deficits may explain higher CSF AS positivity than in the current IRBD cohort. Of note, in 3 different studies (that included much smaller IRBD cohorts ranging from 3 to 18 patients), the sensitivity to detect AS in CSF by RT-QuIC varied from 39% to 100%.19,23,25
Table 3.
Comparisons Among the Patients With IRBD From the Previous Study and the Current Study
In the present and previous study,18 patients with AS positivity showed more frequently supportive clinical features and markers of synucleinopathy (abnormal DAT-SPECT, hyposmia, and dysautonomia) than patients with AS negativity. This finding suggests that AS-positive patients have a higher burden of pathology in the nervous system than those with AS negativity. Longitudinal follow-up of our patients will elucidate whether those with AS positivity will be diagnosed earlier with an overt synucleinopathy than those with AS negativity, as we previously observed.18
We found that 25% of the patients were AS negative in the skin and CSF. We do not know the exact reason for this observation. One possibility is that some of these cases represent a very early stage of prodromal synucleinopathy in whom AS cannot be detected yet by RT-QuIC, as they still showed markers of neurodegeneration (e.g., 29% of the AS-negative patients had DAT-SPECT deficit, Table 2). The frequent use of antidepressants in AS-negative patients might indicate that these medications triggered an early RBD presentation in participants with less advanced neurodegenerative process. Another explanation is that some AS-negative patients might represent prodromal MSA because RT-QuIC on the CSF detects AS with much lower sensitivity in MSA than in PD and DLB.25,45,46 This is particularly true for the RT-QuIC assay used in the present study.25 Alternatively, some patients with IRBD might not have a synucleinopathy, as the absence of AS has been reported in few postmortem neuropathologic cases with RBD.47 In AS-negative patients, follow-up with serial clinical and ancillary tests, including longitudinal RT-QuIC assessment, is necessary to elucidate their significance.
Only 1 control showed AS positivity in the skin and CSF. She was an 81-year-old woman with low prodromal PD probability. She had hyposmia but lacked other biomarkers of neurodegeneration. In controls, AS positivity by RT-QuIC is not rare,18,20 and longitudinal follow-up of these individuals will elucidate whether they develop neurologic symptomatology with time.
Skin punch biopsy is a minimally invasive technique associated with minor discomfort and complications. The procedure is easy to perform, fast, well tolerated, and inexpensive and requires no suture.21 Skin punch biopsy identifies abnormal proteins such as the abnormal prion protein and AS.13-17,43 In patients with PD, DLB, and IRBD, there are no official guidelines indicating the number and sites where the skin punches should be performed to obtain the highest sensitivity to detect AS. In the present study, 3 site combinations (both distal legs and left C7 or C8) yielded the same sensitivity as the 6 sites that were analyzed. Multicenter studies with larger cohorts are warranted to confirm this finding.
Lumbar puncture is also a minimally invasive procedure that is rapid, easy to perform, associated with minor complications, and does not require stitches.42 Lumbar puncture is increasingly performed in memory units to detect Alzheimer disease biomarkers.48 Similarly, AS in the CSF is detected by RT-QuIC in patients with prodromal and manifest synucleinopathies.18,19,23-26 An advantage of lumbar puncture is that it allows obtaining much more CSF than required for 1 study and permits storing extra biofluid for additional investigations.
In our study, skin biopsy and lumbar puncture showed similar sensitivity and specificity to detect AS, and their agreement was very high. These observations indicate that in IRBD, the pathophysiologic process involves the CNS and the peripheral autonomic system in the skin simultaneously. Feasibility, safety, and tolerability were similar between skin biopsy and lumbar puncture, and most participants would repeat any of these 2 procedures. Taken together, our study shows that skin punch biopsy and lumbar puncture are 2 comparable approaches to identify AS by RT-QuIC in IRBD.
The olfactory mucosa is another location where RT-QuIC can detect AS. The procedure is easy and less aggressive than lumbar puncture and skin biopsy and does not require anesthesia. Moreover, nasal samples from the olfactory mucosa can be obtained in individuals taking anticoagulants. In 1 study in IRBD, sensitivity to detect AS in the olfactory mucosa was 44%, and specificity was 90%.20
RT-QuIC is a seed aggregation and amplification assay, which constituted an important advance for the diagnosis of sporadic Creutzfeldt-Jakob disease, as it detects the misfolded prion protein in the CSF and skin with an accuracy greater than 90%.43,49 The aggregation properties of the pathologic prion protein are shared by AS, a protein that constitutes the pathologic hallmark of PD, DLB, and MSA. In living patients with PD and DLB, RT-QuIC detects AS in the CSF and in the skin with high sensitivity and specificity of 90%–100%.25-28 In 1 recent study involving patients with PD and DLB undergoing skin biopsy and lumbar puncture, RT-QuIC showed similar high diagnostic accuracy in both specimens (97% in the skin and 99% in the CSF).28 Although RT-QuIC has not been incorporated into the diagnostic criteria of PD1 and DLB,2 this assay differentiates these synucleinopathies from those neurodegenerative diseases without AS brain pathology such as Alzheimer disease, frontotemporal dementia, and progressive supranuclear palsy.23-25 RT-QuIC is also able to detect CSF AS in the prodromal phase of the synucleinopathies in individuals with MCI due to Lewy body disease45 and with pure autonomic failure.25 IRBD also represents prodromal synucleinopathy,5-8 and our present study documents that RT-QuIC frequently detects AS simultaneously in the CSF and skin of individuals with IRBD.
Because abnormal AS is thought to be neurotoxic and one of the drivers of neurologic symptomatology,3,4 research has been focused on altering AS aggregation and propagation in the CNS. The IRBD population constitutes an ideal target to participate in neuroprotective trials with interventions to stop or halt AS propagation. Our current study could help with patient stratification by excluding those with AS negativity if the intervention is directed against AS. However, the implementation of lumbar puncture and skin biopsy to detect AS would exclude participants rejecting to undergo these procedures and those with coagulation abnormalities and anticoagulant use, raising ethical problems.
Our study has limitations. First, this is a cross-sectional study without clinical follow-up data to confirm whether those patients with AS positivity develop an overt clinical synucleinopathy earlier than those with AS negativity, as reported in our previous study.18 Second, the study did not include serial skin biopsies and lumbar punctures to determine whether AS negativity may evolve into AS positivity with time, as previously observed.18,19 Third, we did not evaluate the presence of AS in the skin by immunofluorescence or in other organs such as the salivary glands by immunohistochemistry because the objective of our study was to compare the ability of the RT-QuIC assay to detect AS in 2 different biospecimens. Fourth, we performed 6 skin punches on each participant in the neck and legs. It is possible that if we had biopsied more sites in other regions (e.g., thigh and forearm), some of the AS-negative patients would have been changed into AS positive because of a possible patchy pathologic distribution of AS in the skin. However, in our study, only 1 patient was positive in the skin and negative in the CSF. Fifth, patients and controls were not sex matched. Nevertheless, we are not aware that sex may influence AS deposition in the nervous system. Finally, postmortem neuropathology was not available.
Our work has strengths. First, data were collected prospectively in consecutive patients with IRBD without any selection bias. Second, we included a large cohort of 91 patients considering the complexity of obtaining skin and CSF samples in a single individual at the same time, particularly in an underdiagnosed disorder such as IRBD.50 Moreover, none of the 91 patients from the current study participated in our previous work.18 Third, video-polysomnography was performed to diagnose RBD in all patients and to exclude this parasomnia in all controls. Fourth, all patients and the AS-positive control were well characterized at the time of skin biopsy and lumbar puncture. In all these individuals, we assessed a comprehensive series of biomarkers of neurodegeneration including dopaminergic deficit by DAT-SPECT and hyposmia by a smell test. This approach allowed us to calculate their likelihood ratio for prodromal PD. Fifth, skin biopsy and lumbar puncture were performed on the same day to evaluate their agreement to detect AS without any temporal limitation. Sixth, the RT-QuIC analyses were performed in a blinded fashion where investigators were masked to participant category and clinical data. Finally, the applied RT-QuIC protocol has shown high accuracy in distinguishing patients with PD and DLB from controls using skin and CSF samples in large cohorts that included patients with neuropathologic confirmation.25,28,44 In summary, RT-QuIC detection of AS either in the skin or in the CSF is a highly sensitive and specific biomarker of the prodromal stage of the synucleinopathies that could be used for patient stratification in future neuroprotective trials in IRBD.
Glossary
- AS
misfolded α-synuclein
- DAT-SPECT
dopamine transporter imaging single-photon emission CT
- DLB
dementia with Lewy bodies
- HCB
Hospital Clinic de Barcelona
- IRBD
isolated REM sleep behavior disorder
- ISNB
Istituto delle Scienze Neurologiche di Bologna
- MCI
mild cognitive impairment
- MDS-UPDRS-III
Movement Disorders Society Unified Parkinson's Disease Rating Scale
- MoCA
Montreal Cognitive Assessment
- MSA
multiple system atrophy
- PBS
phosphate-buffered saline
- PD
Parkinson disease
- RT-QuIC
real-time quaking-induced conversion
- UPSIT-40
40-item University of Pennsylvania Smell Identification Test
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This work was supported by grants given by Sociedad Española de Sueño (SES) and IKEA, by the Italian Ministry of Health (Ricerca Corrente), and by Fondazione Carisbo. G. Mayà was funded by Contracte de Recerca Clínic-La Pedrera, granted by Hospital Clínic de Barcelona.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30(12):1591-1601. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recasens A, Ulusoy A, Kahle PJ, Di Monte DA, Dehay B. In vivo models of alpha-synuclein transmission and propagation. Cell Tissue Res. 2018;373(1):183-193. [DOI] [PubMed] [Google Scholar]
- 4.Hansen C, Li JY. Beyond α-synuclein transfer: pathology propagation in Parkinson's disease. Trends Mol Med. 2012;18(5):248-255. [DOI] [PubMed] [Google Scholar]
- 5.Iranzo A, Santamaria J, Tolosa E. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol. 2016;15(4):405-419. [DOI] [PubMed] [Google Scholar]
- 6.Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration- an update. Nat Rev Neurol. 2018;14(1):40-55. [DOI] [PubMed] [Google Scholar]
- 7.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12(5):443-453. [DOI] [PubMed] [Google Scholar]
- 8.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprenger FS, Stefanova N, Gelpi E, et al. Enteric nervous system α-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology. 2015;85(20):1761-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilas D, Iranzo A, Tolosa E, et al. Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2016;15(7):708-718. [DOI] [PubMed] [Google Scholar]
- 11.Iranzo A, Borrego S, Vilaseca I, et al. α-Synuclein aggregates in labial salivary glands of idiopathic rapid eye movement sleep behavior disorder. Sleep. 2018;41(8). doi: 10.1093/sleep/zsy101 [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Arcos A, Vilaseca I, Aldecoa I, et al. Alpha-synuclein aggregates in the parotid gland of idiopathic REM sleep behavior disorder. Sleep Med. 2018;52:14-17. [DOI] [PubMed] [Google Scholar]
- 13.Antelmi E, Donadio V, Incensi A, Plazzi G, Liguori R. Skin nerve phosphorylated α-synuclein deposits in idiopathic REM sleep behavior disorder. Neurology. 2017;88(22):2128-2131. [DOI] [PubMed] [Google Scholar]
- 14.Doppler K, Jentschke HM, Schulmeyer L, et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol. 2017;133(4):535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doppler K, Mammadova S, Kuzkina A, et al. Association between probable REM sleep behavior disorder and increased dermal alpha-synuclein deposition in Parkinson's disease. Parkinsonism Relat Disord. 2022;99:58-61. [DOI] [PubMed] [Google Scholar]
- 16.Miglis MG, Zitser J, Schneider L, et al. Cutaneous α-synuclein is correlated with autonomic impairment in isolated rapid eye movement sleep behavior disorder. Sleep. 2021;44(12):zsab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Qassabi A, Tsao TS, Racolta A, et al. Immunohistochemical detection of synuclein pathology in skin in idiopathic rapid eye movement sleep behavior disorder and parkinsonism. Mov Disord. 2021;36(4):895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iranzo A, Fairfoul G, Ayudhaya ACN, et al. Cerebrospinal fluid α-synuclein detection by RT-QuIC in patients with isolated REM sleep behavior disorder. Lancet Neurol. 2021;20(3):203-210. [DOI] [PubMed] [Google Scholar]
- 19.Poggiolini I, Gupta V, Lawton M, et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain 2022;145(2):584-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefani A, Iranzo A, Holzknecht E, et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain. 2021;144(4):1118-1126. [DOI] [PubMed] [Google Scholar]
- 21.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(7):903-912, e44-e49. [DOI] [PubMed] [Google Scholar]
- 22.Candelise N, Baiardi S, Franceschini A, Rossi M, Parchi P. Towards an improved early diagnosis of neurodegenerative diseases: the emerging role of in vitro conversion assays for protein amyloids. Acta Neuropathol Commun. 2020;8(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairfoul G, McGuire LI, Pal S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol. 2016;3(10):812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groveman BR, Orru CD, Hughson AG, et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun. 2018;6(1):7. doi: 10.1186/s40478-018-0508-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi M, Candelise N, Baiardi S, et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020;140(1):49-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bongianni M, Ladogana A, Capaldi S, et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann Clin Transl Neurol. 2019;6(10):2120-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manne S, Kondru N, Jin H, et al. Blinded RT-QuIC analysis of α-Synuclein biomarker in skin tissue from Parkinson's disease patients. Mov Disord. 2020;35(12):2230-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mammana A, Baiardi S, Quadalti C, et al. RT-QuIC detection of pathological α-synuclein in skin punches of patients with Lewy body disease. Mov Disord. 2021;36(9):2173-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang UJ, Boehme AK, Fairfoul G, et al. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson's disease. Mov Disord. 2019;34(4):536-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB. Update of the MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2019;34(10):1464-1470. [DOI] [PubMed] [Google Scholar]
- 31.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. [DOI] [PubMed] [Google Scholar]
- 32.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 33.Delgado C, Araneda A, Behrens MI. Validation of the Spanish-language version of the Montreal Cognitive Assessment test in adults older than 60 years. Neurologia. 2019;34(6):376-385. [DOI] [PubMed] [Google Scholar]
- 34.Doty RL. The Smell Identification TestTM Administration Manual, 3rd ed. Sensonics, Inc.; 1995. [Google Scholar]
- 35.Iranzo A, Serradell M, Vilaseca I, et al. Longitudinal assessment of olfactory function in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2013;19(6):600-604. [DOI] [PubMed] [Google Scholar]
- 36.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69-72. [DOI] [PubMed] [Google Scholar]
- 37.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. [DOI] [PubMed] [Google Scholar]
- 38.Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 39.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. [DOI] [PubMed] [Google Scholar]
- 40.Iranzo A, Santamaría J, Valldeoriola F, et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder Ann Neurol. 2017;82(3):419-428. [DOI] [PubMed] [Google Scholar]
- 41.Morbelli S, Esposito G, Arbizu J, et al. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging. 2020;47(8):1885-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mammana A, Baiardi S, Rossi M, et al. Detection of prions in skin punch biopsies of Creutzfeldt-Jakob disease patients.Ann Clin Transl Neurol. 2020;7(4):559-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi M, Baiardi S, Teunissen CE, et al. Diagnostic value of the CSF α-synuclein real-time quaking-induced conversion assay at the prodromal MCI stage of dementia with Lewy bodies. Neurology. 2021;97(9):e930-e940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quadalti C, Calandra-Buonaura G, Baiardi S, et al. Neurofilament light chain and α-synuclein RT-QuIC as differential diagnostic biomarkers in parkinsonisms and related syndromes. NPJ Parkinsons Dis. 2021;7(1):93. doi: 10.1038/s41531-021-00232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Compta Y, Painous C, Soto M, et al. Combined CSF α-SYN RT-QuIC, CSF NFL and midbrain-pons planimetry in degenerative parkinsonisms: from bedside to bench, and back again. Parkinsonism Relat Disord. 2022;99:33-41. [DOI] [PubMed] [Google Scholar]
- 47.Boeve BF, Silber MH, Ferman TJ, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013;14(8):754-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alcolea D, Martínez-Lage P, Izagirre A, et al. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer disease: a multicenter study in Spain. J Alzheimers Dis. 2014;39(4):719-726. [DOI] [PubMed] [Google Scholar]
- 49.McGuire LI, Peden AH, Orrú CD, et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 2012;72(2):278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pujol M, Pujol J, Alonso T, et al. Idiopathic REM sleep behavior disorder in the elderly Spanish community: a primary care center study with a two-stage design using video-polysomnography. Sleep Med. 2017;40:116-121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study are available from the corresponding authors on request, if reasonable.