Abstract
Background and Objectives
There is growing evidence that bilingualism can induce neuroplasticity and modulate neural efficiency, resulting in greater resistance to neurologic disease. However, whether bilingualism is beneficial to neural health in the presence of epilepsy is unknown. We tested whether bilingual individuals with temporal lobe epilepsy (TLE) have improved whole-brain structural white matter network organization.
Methods
Healthy controls and individuals with TLE recruited from 2 specialized epilepsy centers completed diffusion-weighted MRI and neuropsychological testing as part of an observational cohort study. Whole-brain connectomes were generated via diffusion tractography and analyzed using graph theory. Global analyses compared network integration (path length) and specialization (transitivity) in TLE vs controls and in a 2 (left vs right TLE) × 2 (bilingual vs monolingual) model. Local analyses compared mean local efficiency of predefined frontal-executive and language (i.e., perisylvian) subnetworks. Exploratory correlations examined associations between network organization and neuropsychological performance.
Results
A total of 29 bilingual and 88 monolingual individuals with TLE matched on several demographic and clinical variables and 81 age-matched healthy controls were included. Globally, a significant interaction between language status and side of seizure onset revealed higher network organization in bilinguals compared with monolinguals but only in left TLE (LTLE). Locally, bilinguals with LTLE showed higher efficiency in frontal-executive but not in perisylvian networks compared with LTLE monolinguals. Improved whole-brain network organization was associated with better executive function performance in bilingual but not monolingual LTLE.
Discussion
Higher white matter network organization in bilingual individuals with LTLE suggests a neuromodulatory effect of bilingualism on whole-brain connectivity in epilepsy, providing evidence for neural reserve. This may reflect attenuation of or compensation for epilepsy-related dysfunction of the left hemisphere, potentially driven by increased efficiency of frontal-executive networks that mediate dual-language control. This highlights a potential role of bilingualism as a protective factor in epilepsy, motivating further research across neurologic disorders to define mechanisms and develop interventions.
One factor increasingly studied as a measure of resilience, particularly in neurodegenerative disease, is bilingualism—proficiency and/or active use of 2 or more languages. Extensive literature demonstrates that bilingualism is a unique process that leads to neuroplastic changes in the brain, presumably due to adaptation to the demands of dual-language use. This type of cognitive stimulation has been associated with increased resistance against cognitive aging and neurodegeneration1-5 via mechanisms of cognitive and/or neural reserve.3 Converging studies suggest increased efficiency of frontal-executive control networks in older bilinguals and bilinguals with Alzheimer disease (AD).6-9 Other studies reported bilingualism-related modulation of connectivity within language-specific networks in AD10,11 and in healthy bilinguals.12-14 These findings suggest that the bilingual brain adapts to the demands of dual-language use, similar to a mental gym. Despite a large bilingual population worldwide and a growing number of bilingual individuals in the United States,15 the potential role of bilingualism as a neuroprotective factor in epilepsy has been minimally investigated.
The broad and heterogeneous effects of focal epilepsy on the brain16 have led to the conceptualization of epilepsy as a network-level disorder, often linked to devastating effects on cognition. The significant social and health care burden associated with cognitive dysfunction suggests a need to identify protective factors, such as bilingualism, to help maintain brain health for individuals with epilepsy. A previous study from our laboratory demonstrated that bilinguals with temporal lobe epilepsy (TLE) had comparable executive function but lower white matter integrity compared with monolinguals, providing evidence for cognitive reserve.17 Here, we examine microstructural changes that may help explain function (i.e., neural reserve). Specifically, it is unknown whether the wiring among cortical regions increases due to bilingualism, thereby increasing whole-brain or subnetwork connectome organization in epilepsy.
A shift to understanding epilepsy as a network disorder has been accompanied by advances in quantifying whole-brain network connectivity, which provides the scaffolding for dynamic exchanges of information flow between brain regions.18 A healthy brain network is thought to have an optimal balance of integration and specialization (i.e., high clustering of connections between neighboring brain regions, yet a short path between any 2 distant regions) that allows for rapid transfer and integration of information that supports complex cognitive functions. Previous studies have shown that integration and specialization of structural networks are disrupted in individuals with recurring seizures.19 Network alterations have been associated with degree of cognitive impairment,20-22 disease progression,23 and postsurgical cognition,24 demonstrating clinical relevance of whole-brain network topology in epilepsy.25
As network disruption presents a key target for resilience, the current study investigated the effect of bilingualism on whole-brain structural network organization in TLE. We hypothesized that being bilingual may provide a protective buffer against whole-brain white matter network dysfunction. In addition, we evaluated differences in local efficiency of frontal-executive and perisylvian (i.e., language specific) networks in bilingual and monolingual TLE given that bilingual language control is mediated by the coordination of language and executive control regions,26 and epilepsy can alter language and executive function networks.27 We also tested whether better network organization is related to higher executive function and/or language performance.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board at the University of California (UC) San Diego (UCSD) or UC San Francisco (UCSF). All participants provided written informed consent.
Participant and Inclusion Criteria
Our initial sample had 133 patients with drug-resistant TLE and 84 neurologically healthy controls (HCs). A TLE diagnosis was established by epileptologists, based on video-EEG telemetry, seizure semiology, and neuroimaging. Inclusion criteria consisted of (1) age 18–65 years, (2) estimated premorbid IQ >70, (3) no evidence of large lesions or visible extratemporal pathology on MRI, (4) structural and diffusion-weighted MRI (dMRI) that passed visual quality inspection, and (5) sufficient evidence of bilingualism evidenced at follow-up interview. Sixteen patients were excluded for tumor (n = 3), bilateral seizure focus (n = 7), artifact and/or significant outlier in dMRI data (n = 4), and low bilingual proficiency (n = 2). Three HCs with incomplete demographic information were excluded.
Language Status Characterization
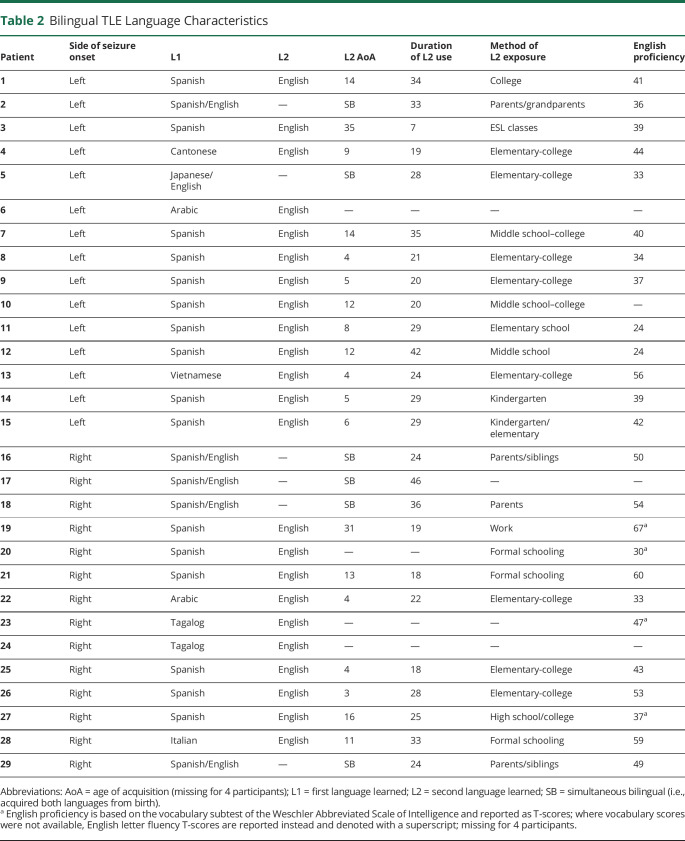
Bilingualism in TLE was established according to the following criteria: participants self-identified as bilingual and responded to a series of questions evaluating proficiency and the amount of active use of the non-English language spoken. All bilingual TLE (B-TLE) participants demonstrated English proficiency and completed a neuropsychological battery in English. Objective English proficiency was measured with the vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence (WASI) or with letter fluency (F-A-S) when WASI was not administered. B-TLEs reported either learning English as their second language (L2; n = 23) or simultaneously learning English and another language before age 6 years (n = 6). Based on these criteria, 15 left TLEs (LTLEs) were classified as bilingual (B-LTLE) and 43 as monolingual (M-LTLE). Fourteen right TLEs (RTLEs) were classified as bilingual (B-RTLE) and 45 as monolingual (M-RTLE). Bilinguals learned English on average at 8.4 years (SD 9.0; range 0–35), typically considered just past the cutoff for early L2 acquisition (conventionally 6–7 years). Bilinguals used both languages for an average of 27.1 years (SD 8.9; range 5–46). Bilingual groups did not differ in age of English acquisition (p = 0.65) or duration of English use (p = 0.76). All B-TLEs received some education in English and were actively immersed in an English environment but used their other language at home. The most common other language was Spanish (n = 21). Although 19 of the HCs reported knowing a language other than English, we were confident in the bilingual status of only 11 (i.e., English as L2), as a detailed proficiency interview was not conducted with HCs.
Image Acquisition
Imaging at UCSD (n TLE = 61; n HC = 47) and UCSF (n TLE = 30) was performed with identical protocols on the same manufacturer and model—GE Discovery MR750 3 T with an 8-channel phased-array head coil. Thirty-six TLEs and 24 HCs were additionally scanned on a GE 1.5 T EXCITE HD scanner at UCSD. Image acquisitions at both 3 T centers included a conventional 3-plane localizer, GE calibration scan, a T1-weighted 3-dimensional structural scan (repetition time [TR] = 8.08 milliseconds, echo time [TE] = 3.16 milliseconds, inversion time [TI] = 600 milliseconds, flip angle = 8°, field of view [FOV] = 256 mm, matrix = 256 × 192, and slice thickness = 1 mm3), and a single-shot pulsed-field gradient spin-echo EPI sequence (TE/TR = 96 milliseconds/17 seconds; FOV = 24 cm, matrix = 128 × 128 × 48; axial). Image acquisition at the 1.5 T scanner included a conventional 3-plane localizer, GE calibration scan, 2 T1-weighted 3D structural scans (TR = 10.7 milliseconds, TE = 3.8 milliseconds, TI = 1,000 milliseconds, flip angle = 8°, bandwidth = 31.25 Hz/pixel, FOV = 256 mm, matrix = 256 × 192, and slice thickness = 1.0 mm), and for dMRI, a single-shot echo-planar imaging with isotropic 2.5 mm voxels (TR = 12.3 seconds, TE = 75.6 milliseconds, flip angle = 90°, FOV = 240 mm, matrix = 96 × 96, slice thickness = 2.5 mm, and partial k-space acquisition). On all scanners, dMRIs were acquired with 30 diffusion gradient directions using a b value of 1,000 s/mm2 with an additional b = 0 volume. For use in nonlinear B0 distortion correction, 2 additional b = 0 volumes were acquired with either forward or reverse phase-encode polarity. Field strength was not significantly associated with graph theory metrics (ps > 0.05). The proportion of patients scanned using 1.5 vs 3 T did not differ by language group or side of seizure onset (p = 0.79).
dMRI Processing, Tractography, and Connectome Generation
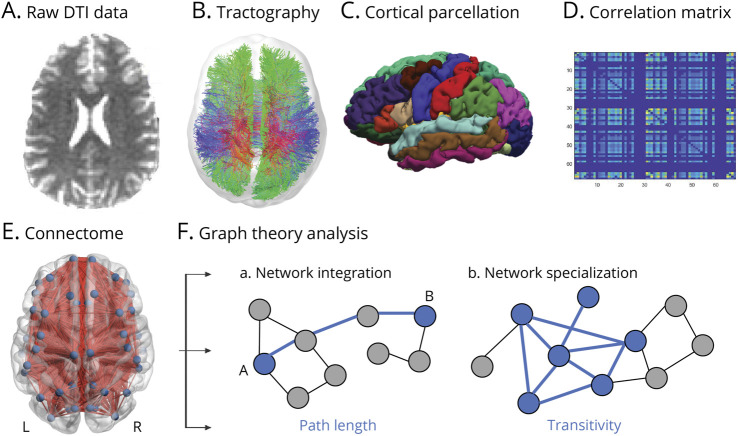
Detailed methods are provided in eAppendix 1 (links.lww.com/WNL/C665). T1-weighted and dMRIs were preprocessed using the multimodal imaging pipeline (Figure 1, A–E).28
(A) dMRI data were corrected for head motion, geometric susceptibility-induced distortions, and eddy current-induced artifacts. Postprocessing was performed using MRtrix3 tools.
(B) Probabilistic global tractography was performed using the default iFOD2 algorithm,29 based on fiber orientation distributions derived from the dMRI signal using constrained spherical deconvolution.30 Tractography parameters were informed by QSIprep's default tractography workflows.31 Streamlines were refined using the anatomically constrained tractography framework using the T1-weighted image.32 Those with anatomically plausible termination such as the gray-white matter interface were kept. Minimum and maximum fiber lengths were set to 30 and 250 mm. Streamlines were dynamically seeded using the SIFT model, until 10 million candidates were found. The SIFT2 algorithm in tcksift2 was used.
(C) Cortical parcellations derived from FreeSurfer (Desikan-Killiany, 68 regions of interest [ROIs]) were used to determine connectome edge weights and were defined as the sum of SIFT2 weights of all streamlines connecting a pair of nodes. Connectome edges were scaled by the inverse of the 2 node volumes.
(D) Undirected, binary graph matrices were created at multiple density levels.33 Each ROI constituted a node in the network with each connection edge defined by traced fiber connections between nodes. Each density level was defined as the threshold at which the number of edges in the graph equaled from 10% to 65% of total possible edges. These were selected in concordance with similar methods with an equivalent number of ROIs.34
(E) This panel depicts a whole-brain structural connectome imposed on a standard brain template.
Figure 1. Illustration of White Matter Connectome Generation and Graph Theory Analysis.
(A) Raw diffusion-weighted MRI data were preprocessed and registered to a T1 space. (B) Probabilistic whole-brain tractography was used to construct white matter fiber tracts connecting different brain regions (shown here is tractography from 1 participant for visualization). (C) Data were analyzed in a parcellated anatomic space with regions of interest (ROIs) selected from the Desikan-Killiany atlas, which were registered to each participant's diffusion tensor imaging space. (D) White matter connectivity matrices were generated by assessing pairwise connections between each pair of ROIs and input into a 2-dimensional matrix (shown here is an individual connectome) where each row and column represent an ROI, and the corresponding color is the strength of the structural connectivity between that pair of ROIs. (E) Network render of a structural connectome, which is equivalent to brain graphs, with ROIs representing nodes and white matter connections representing edges. For visualization, a matrix averaged across participants is imposed on a standard brain template using the BrainNet Viewer. (F) Connectivity matrices were analyzed with graph theory analysis to extract 2 global metrics of interest that reflect brain network organization—path length (a measure of network integration) and transitivity (a normalized clustering coefficient; a measure of network specialization).
Global Graph Theory Metrics
Connectivity matrices were analyzed with the Brain Connectivity Toolbox33 to yield 2 global graph theory metrics of interest (F) that reflect key organizational principles of the brain—network specialization (measured by transitivity) and network integration (measured by path length)—and are among the most commonly investigated and frequently aberrant in epilepsy.19 We used 55 different density levels to calculate a single area under the curve for the resulting receiver operating characteristic curves for each participant for each measure.
Transitivity (or a normalized mean clustering coefficient) is defined as the ratio of the number of estimated connections among its first-degree neighbors to the number of all possible connections, ranging from 0 (no first-degree neighbors of a node link to each other) and 1 (all first-degree neighbors of the node also link to each other), which reflects the degree to which nodes tend to cluster together (higher = neighbors of a node are more densely interconnected). We chose transitivity instead of clustering coefficient because clustering coefficient is normalized individually for each node and may be disproportionately influenced by nodes with a low degree.33
Path length is defined as the minimum number of intermediate nodes needed to connect any 2 nodes. Networks with short path lengths indicate a higher global capacity of exchanging information across the whole network (lower = any 2 ROIs can be reached in a smaller number of steps).
Subnetwork Analysis
Mean local efficiency—that is, the average of global efficiency constrained to only the local neighbors of specific nodes—was computed for the following 2 subnetworks.
Frontal-Executive
Considerable research demonstrates neural overlap across language and executive control in bilinguals, for example, in the dorsolateral prefrontal and anterior cingulate regions.26 These regions have also been shown to be connected more efficiently in aging bilinguals or those with AD.9,11 Thus, we averaged the following bilateral frontal-executive ROIs: caudal middle frontal gyrus, caudal anterior cingulate gyrus, and rostral anterior cingulate gyrus.
Perisylvian
Given evidence that bilingualism modulates network efficiency12 and structural plasticity within language regions35 or tracts that connect them,36 the following ROIs were averaged: left inferior frontal gyrus (pars opercularis, pars triangularis, and pars orbitalis), left middle temporal gyrus, left supramarginal gyrus, left inferior parietal lobe, and bilateral superior temporal gyrus.
Neuropsychological Measures
A subset of participants (n = 86) completed (1) WASI Block Design, a measure of perceptual reasoning, (2) Delis-Kaplan Executive Function System Category Fluency, a measure of semantic fluency, and (3) Trail Making Test-B, a timed task of nonverbal task switching (i.e., higher score = worse performance).
Statistical Analysis
Fisher exact or χ2 tests and independent samples t tests or 1-way Kruskal-Wallis H analysis of variance (ANOVA) were used to examine group differences in demographic and clinical variables. Multivariate ANOVAs were used to compare graph metrics between TLE vs HC, and in a 2 (L- vs RTLE) × 2 (bilingual vs monolingual) model, with Bonferroni correction of p = 0.025. Q-Q plots were acceptable, and assumptions were met (i.e., graph metrics were normally distributed, homogeneity of variance-covariance, equality of variances, and no significant outliers). Group differences in local efficiency of subnetworks were assessed with a 2 (frontal-executive vs perisylvian) × 2 (bilingual vs monolingual) ANOVA. Simple main effects using the pooled variance were performed for significant interactions. Spearman bivariate correlations (limited to patient groups that showed significant modulation of graph metrics) tested associations between neuropsychological performance and graph metrics. Correlations between groups were compared using 95% CIs and Fisher z test.
Data Availability
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.
Results
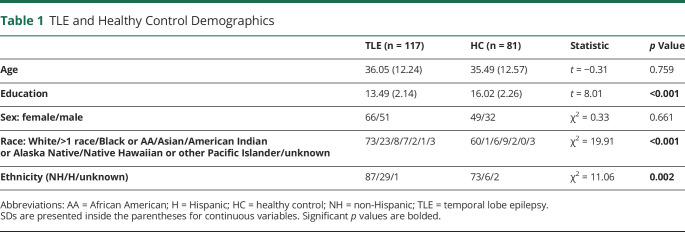
Demographic and Clinical Variables
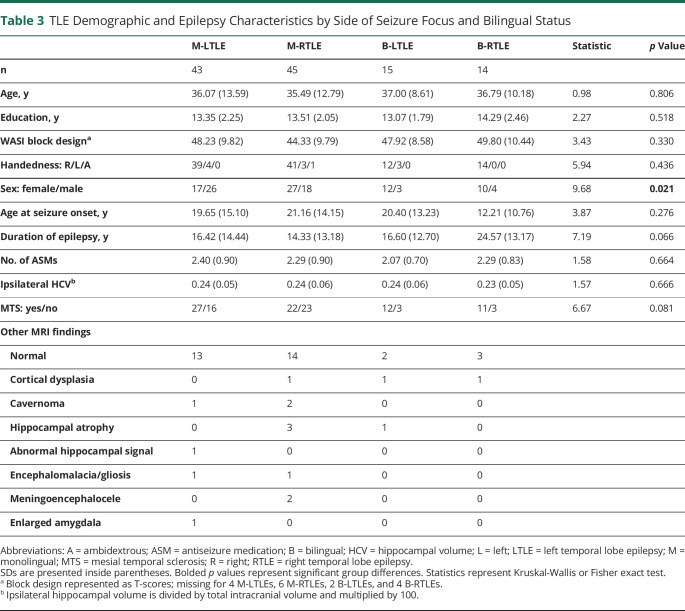
Our final sample included 117 TLE (n = 88 monolingual; n = 29 bilingual) and 81 HC (Table 1). Table 2 shows detailed language characteristics for B-TLE. Table 3 presents demographic and epilepsy-related variables for TLE (eTables 1 and 2, links.lww.com/WNL/C665, present this information by site). TLE groups did not significantly differ in age, education, handedness, perceptual reasoning, age at seizure onset, or number of medications. The distribution of sex significantly differed across groups such that M-LTLE had a lower proportion of female participants compared with B-LTLE (p = 0.015) and to a lesser extent compared with M-RTLE (p = 0.060). The distribution of mesial temporal sclerosis (MTS) showed a marginally significant difference across groups such that M-RTLE had a lower proportion of MTS compared with B-LTLE (p = 0.041). Bilinguals generally had a higher proportion of MTS than monolinguals, although not significantly. Other detailed MRI findings are presented in Table 3.
Table 1.
TLE and Healthy Control Demographics
Table 2.
Bilingual TLE Language Characteristics
Table 3.
TLE Demographic and Epilepsy Characteristics by Side of Seizure Focus and Bilingual Status
Global Graph Theory Metrics
TLE vs HC
To replicate previous work and understand the effects of TLE on network organization in our sample, we first examined global graph metrics in TLE vs HC. The omnibus group effect was significant (F(2,195) = 5.01; p = 0.008; ηp2 = 0.05; Wilks Λ = 0.95) such that TLE had (1) lower transitivity than HC (F(1,198) = 8.56; p = 0.004; ηp2 = 0.04; TLE: mean 45.58; 95% CI 45.40–45.77; HC: mean 46.01; 95% CI 45.79–46.23) and (2) longer path length than HC (F(1,198) = 8.79; p = 0.003; ηp2 = 0.04; TLE: mean 69.78; 95% CI 69.57–69.98; HC: mean 69.29; 95% CI 69.05–69.54). Group differences remained significant after controlling for age, education, and sex (ps = 0.007 and 0.013, for transitivity and path length, respectively).
Language Status by Side of Seizure Onset
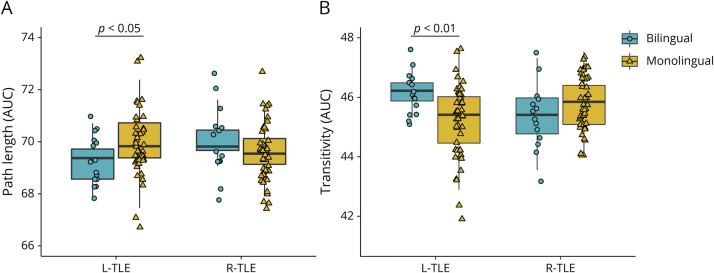
Figure 2 plots individual data. There was a significant language status by side of seizure onset interaction of a medium effect size (F(2,112) = 5.46; p = 0.005; ηp2 = 0.09; Wilks Λ = 0.91) and no significant main effects. The interaction was significant for each metric (transitivity: F(2,116) = 11.01; p = 0.001; ηp2 = 0.09; path length: F(1,116) = 5.35; p = 0.022; ηp2 = 0.05). For transitivity, the effect of bilingualism was present only in LTLE (F(1,113) = 10.57; p = 0.002; ηp2 = 0.09) and not RTLE (p = 0.147) such that B-LTLE had higher transitivity than M-LTLE (B-LTLE: mean 46.19; 95% CI 45.68–46.71; M-LTLE: mean 45.21; 95% CI 44.91–45.52). The effect of bilingualism on path length was present in LTLE (F(1,113) = 4.79; p = 0.031; ηp2 = 0.04) and not RTLE (p = 0.276) such that B-LTLE had shorter path length than M-LTLE (B-LTLE: mean 69.27; 95% CI 68.68–69.85; M-LTLE: mean 70.02; 95% CI 69.67–70.37). Direct comparison between B- and M-LTLE suggested differences of a large effect size (Cohen d = 0.89 and 0.63, for transitivity and path length, respectively). We also tested whether bilingual HC differed from monolingual HC by case-control matching M-HC on age, education, and sex to (1) 19 B-HC and (2) 11 B-HC whose bilingual status we were more confident in. Neither analysis revealed a trend toward group differences (ps > 0.05) (eAppendix and eFigure 1, links.lww.com/WNL/C665).
Figure 2. Network Integration (A) and Specialization (B) in Bilinguals and Monolinguals With L-TLE vs R-TLE.
The effect of bilingualism on network organization was dependent on side of seizure focus such that bilingual LTLE showed increased network organization relative to monolingual LTLE. Plotted are AUC of path length and transitivity separately by side of seizure focus (L- vs R-TLE) and language status (bilingual vs monolingual). Boxplot denotes the median (bold bar), first and third quartiles (box limits), and ±1.5 times the interquartile range (whiskers). Bilingual and monolingual TLEs are coded as circles and triangles, respectively. AUC = area under the curve; LTLE = left TLE; RTLE = right TLE; TLE = temporal lobe epilepsy.
Control Analyses
The 2-way omnibus interaction between language status and side of seizure onset remained significant and of the same effect size when individually controlling for variables not closely matched across groups or variables that may affect network organization—age, sex, MTS, and duration of epilepsy (all ps < 0.05; ηp2 = 0.08–0.09). Excluding 6 simultaneous bilinguals (2 B-LTLE; 4 B-RTLE) did not change the interaction (p = 0.013; ηp2 = 0.08). We repeated the analysis with clustering coefficient as it may be a more commonly reported global metric—the interaction remained significant with a similar effect size (p = 0.008; ηp2 = 0.08).
Site and Scanner Effects
The main findings were consistent and of the same effect size when (1) examining only the main site (UCSD; n = 87), (2) including site as a covariate and (3) examining 3T participants scanned on an identical scanner and protocol (eAppendix 1 and eFigure 2, links.lww.com/WNL/C665).
Additional Atlas Comparison
We reanalyzed the data using a functionally derived atlas, the HCP Glasser 360 (eFigure 3, links.lww.com/WNL/C665). The 2-way interaction was observed across each global metric (ps < 0.05; ηp2 = 0.04), with a similar pattern of results as reported above (see eAppendix 1).
Local Efficiency of Subnetworks in LTLE
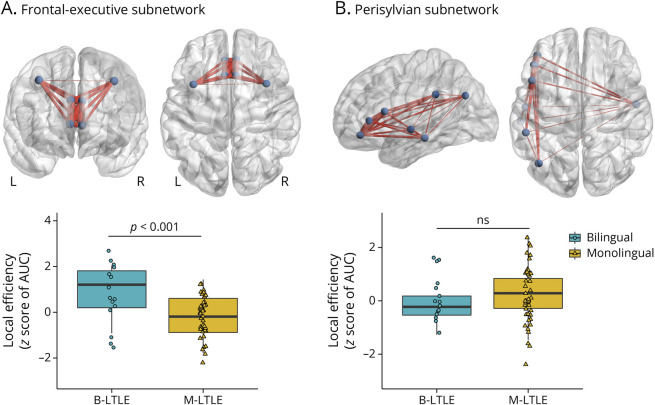
In a secondary analysis, we examined whether improved whole-brain network organization in B-LTLE is related to local efficiency of subnetworks underlying language and/or executive control. A model with z scores (relative to HC) of local efficiency showed a robust subnetwork by language status interaction of a large effect size (F(1,56) = 12.35; p < 0.001; ηp2 = 0.18). The interaction revealed that B-LTLE had higher mean local efficiency than M-LTLE in the frontal-executive subnetwork (F(1,56) = 14.18; p < 0.001; ηp2 = 0.20), with no group difference in the perisylvian subnetwork (p = 0.319) (Figure 3). See eAppendix 1 (links.lww.com/WNL/C665) for the HCP atlas analysis.
Figure 3. Local Efficiency of Predefined (A) Frontal-Executive and (B) Perisylvian Subnetworks in B-LTLE and M-LTLE.
A significant interaction between subnetwork and language status such that B-LTLE had higher frontal-executive, but not perisylvian, local efficiency relative to M-LTLE. Panel A visualizes nodes that comprised the frontal-executive subnetwork in coronal and axial views. Panel B visualizes nodes that comprised the perisylvian (i.e., language) subnetwork in sagittal and axial views. Plotted are z scores (relative to healthy controls) of the mean subnetwork efficiency. B-LTLE and M-LTLEs are coded as circles and triangles, respectively. AUC = area under the curve; B-LTLE = bilingual left temporal lobe epilepsy; M-LTLE = monolingual left temporal lobe epilepsy.
MTS Subsample Analysis
We repeated the analyses in 68 TLEs with lesion evidence of only MTS (see eAppendix 1 and eFigure 4, links.lww.com/WNL/C665). The overall 2-way interaction remained significant across global metrics (p < 0.005; ηp2 = 0.16) such that B-LTLE had increased network organization relative to M-LTLE. Local efficiency of subnetworks also revealed a consistent interaction (p < 0.001; ηp2 = 0.31) such that B-LTLE had increased frontal-executive but not perisylvian sub network efficiency relative to M-LTLE, suggesting that findings are independent of lesion differences between groups.
Correlations Between Cognition and Network Organization
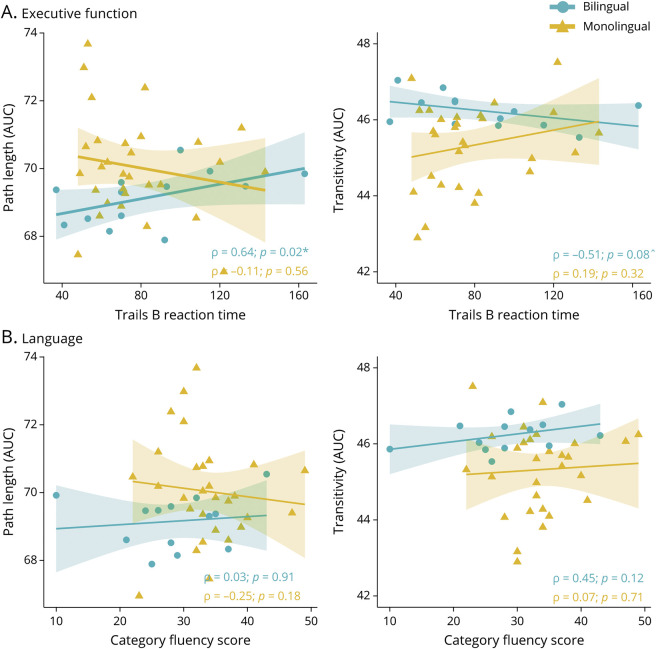
Because B-LTLE was the only group evidencing modulation of graph theory measures, we examined whether increased network organization in B-LTLE was associated with higher executive function and/or language performance, and whether these associations differed from M-LTLE (Figure 4). In B-LTLE, better Trails B performance was significantly associated with shorter path length (ρ(13) = 0.64; p = 0.020; 95% CI 0.14–0.88) and with higher transitivity, although this did not reach significance (ρ(13) = -.51; p = 0.076; 95% CI −0.83 to 0.06). Category fluency was not associated with either metric in B-LTLE (ps > 0.05). No associations were significant in M-LTLE (ps > 0.05). Comparing correlations between network organization and Trails B performance for B- vs M-LTLE revealed a reliable group difference (path length: difference 0.75; 95% CI 0.13–1.17; transitivity: difference −0.70; 95% CI −1.15 to −0.02). Correlations with subnetwork efficiency were not significant in either group (ps > 0.05).
Figure 4. Correlations Between Global Network Integration (Path Length) and Specialization (Transitivity) and (A) Executive Function and (B) Language Performance in LTLE.
Spearman bivariate correlations between executive function measured by Trails B (panel A) and language function measured by category fluency (panel B) and (left panels) and (right panels) for 13 bilinguals and 30 monolinguals with LTLE. Significant correlations are denoted with *, and marginally significant correlations are denoted with ^. Bilingual and monolingual LTLEs are coded as circles and triangles, respectively. AUC = area under the curve; LTLE = left temporal lobe epilepsy.
Discussion
An exciting discovery is that bilingualism may serve as a neuroprotective factor in neurologic disease.3,37 There is converging evidence of delayed diagnosis of dementia in bilinguals relative to monolinguals by an average of 4–5 years2,4,5 (but see reference 38), as well as neuroprotective effects of bilingualism in primary progressive aphasia39 and stroke.40 However, little is known about the underlying neurobiological mechanisms. There are strong reasons to believe that bilingualism can act as reserve in other neurologic disorders41 including epilepsy. We previously demonstrated increased cognitive reserve in bilinguals with TLE.17 The current study builds on this and shows that bilingualism may help retain a well-organized global information transfer between highly interconnected regions. These findings are important given reports that focal epilepsy decreases efficiency of whole-brain networks.19
Our work demonstrated that bilingualism increased network organization relative to monolingualism, with this increase evident only in individuals with a left-sided seizure focus. That is, bilinguals with LTLE showed a pattern of network integration and specialization that most closely resembled that of HC. Second, B-LTLE had increased local efficiency of frontal-executive but not language-related networks compared with M-LTLE. Third, better network organization was related to better executive functioning in B-LTLE, whereas the same pattern was not observed in M-LTLE. Together, these findings suggest that bilingualism may attenuate and/or compensate for network inefficiency in individuals with a left hemisphere seizure focus, likely related to increased efficiency of frontal-executive networks and task-switching performance modulated by dual-language experience.
Our findings point to a neuroprotective effect of bilingualism on network integration and specialization in TLE. A likely mechanism for these results is neural reserve. Neural (or brain) reserve is conceptualized as neurobiological hardware (e.g., number of neurons or synapses), allowing some individuals to better cope with pathology.42 Bilingualism may act as consistent training for the brain due to a complex process of managing 2 simultaneous languages that are always coactive. This invokes a widespread language control network, requiring activation of the spoken language, inhibition of the other language, monitoring to prevent cross-language interference, and switching between languages. This process may strengthen general executive control networks43 and may lead to greater overall efficiency, particularly when these networks are affected by TLE. This idea is supported by increased neural efficiency in older bilinguals, evidenced by lower activation of regions supporting executive function,6 increased connectivity within related networks,7 and more preserved cortical thickness of these regions and white matter tracts that connect them.8,36,44 Our novel findings suggest that bilingualism may reinforce regions affected by epilepsy as well as the white matter connections among them, leading to better organized global information transfer between highly interconnected regions.
The epileptogenic focus is often lateralized, whereas neurodegeneration (e.g., in dementia) is typically bilateral. This unique aspect of epilepsy interacted with bilingualism in that we observed enhanced global network organization in bilinguals with LTLE only. Although it is not fully clear why bilingualism was only protective in LTLE, a possible explanation is that dual-language use is only protective in the face of disruption to the left side of the brain, demonstrating the interaction between language networks and disease. Dysfunction of the left hemisphere may invoke increased compensatory processes in the bilingual brain, potentially drawing on resources from the contralateral hemisphere and/or in nonlanguage regions. We previously reported that language was more frequently reorganized to the right hemisphere in bilinguals with LTLE,45 suggesting that neuroplasticity related to bilingualism is most pronounced when the left hemisphere is affected. Another possible contributor to the bilingualism effect in LTLE only could be related to B-RTLE having a worse epilepsy phenotype, evidenced by approximately a decade longer duration of epilepsy and higher proportion of MTS. However, we observed the same interaction when we controlled for these factors.
In subnetwork comparisons, B-LTLE showed higher efficiency within the bilateral frontal-executive but not the primarily left-lateralized perisylvian regions compared with M-LTLE. An epilepsy-specific speculation is that left hemisphere seizures may reduce perisylvian network efficiency, with bilinguals relying more on networks outside of the typical perisylvian network to handle dual-language demands more efficiently. This interpretation is consistent with several studies that reported increased functional and/or metabolic connectivity in bilinguals compared with monolinguals with AD in frontoparietal/executive networks.7,9,11 A more transdiagnostic explanation is a mechanism of executive control compensation in the presence of bilingualism. This explanation fits with the prevailing view that the greatest benefits of bilingualism in dementia tend to be observed in executive control rather than in language or in medial temporal lobe networks directly affected by AD.37,46,47
In B-LTLE, a modulated whole-brain network organization was associated with better task switching performance—a relationship not present in M-LTLE or in language performance. This lends additional support to the proposal that executive function is the domain that may be the most affected by dual-language experience and provides a functional correlate to whole-brain white matter network topology. Furthermore, this suggests that the efficacy of neural reserve varies across patients in a way which we hypothesize could be due to specific features of each individual's bilingual experience. Although we do not have sensitive measures available to ground this hypothesis, we believe that this should motivate prospective research on the mechanisms of neural resilience and bilingualism.
Much work remains to be done to elucidate bilingualism's protective mechanisms and its potential for intervention in neurologic disease.41 Notably, our sample of bilingual TLEs was relatively small, requiring future replication. Given the cross-sectional design, it is unknown whether increased network organization in B-LTLE stems from preserved network organization (i.e., an attenuating effect) or a reparation of decreased network organization as a result of chronic seizures. In addition, although we propose neural reserve as the most likely mechanism, a recent concept of brain maintenance could apply42—a reduced development of pathology over time based on genetics or lifestyle experiences.42 That is, ongoing bilingual language use may have attenuated epilepsy-related reduction of network organization. Although we did not observe increased network organization in healthy bilingual controls—suggesting that a neuroprotective effect of bilingualism may only be present in the face of neurologic disruption—future inclusion of a better-characterized bilingual control group will help to disentangle the effects of disease and bilingualism. In addition, it is possible that unmeasured lifestyle factors other than language experience may have differed between groups. For example, it is well accepted that occupational history, diet, physical exercise, and cognitive and social stimulation are proxies for cognitive reserve42 and should be examined in future studies along with bilingualism. On a methodological level, although we replicated our global findings across 2 commonly used atlases, it is possible that results could differ when other atlases are used. Therefore, future studies should further test the reproducibility of graph theory metrics across atlases.
Importantly, bilingualism is not a dichotomous phenomenon. It is increasingly recognized that neuroanatomic differences are shaped by factors such as proficiency, age of L2 acquisition (i.e., learned early and simultaneously with L1 vs much later than L1), amount of language exposure, frequency of language switching, and typological similarity between languages.48 Two recent graph theory studies with healthy adults found that several of these bilingual language factors modulated whole-brain and regional network organization.13,14 In addition, bilingualism's effects on the brain likely follow a dynamic nonlinear pattern. This is outlined in a number of models including the Dynamic Restructuring Model (DRM49), which proposes a specific time course for the effect of bilingualism on structural neuroplasticity. Notably, almost all our B-LTLEs used their L2 for 2 decades or more, likely having reached a more efficient level of language control. As this was a retrospective study, we had limited variability in duration and amount of L2 use to fully test the DRM. Future studies would benefit from detailed exploration of bilingual language factors to deconstruct bilingualism and understand how these factors influence network organization in TLE.
Finally, longitudinal studies that examine the interplay between when L2 is learned relative to when seizures begin would be helpful for understanding the time course of bilingualism-related neuroplasticity. This could have clinical implications for prevention and early intervention for network dysfunction. In addition, although the majority of studies that reported delayed onset of dementia included lifelong bilinguals, even late L2 learning (i.e., past childhood) has been associated with increased white matter integrity,35,44 which invites the question of whether immersion and/or L2 learning in monolinguals with epilepsy can also attenuate network abnormalities.
In conclusion, our findings suggest that bilingualism is relevant to brain health in epilepsy. We provide evidence of a neuroprotective role of bilingualism on white matter network organization in LTLE and suggest that this may be related to the integrity of frontal-executive networks. Enhanced network organization in bilingual individuals with LTLE may be explained by the phenomenon of neural reserve. As some epilepsies represent progressive disorders, potentially leading to reduced structural integrity over time,50 it will be important to test whether bilingualism may slow the progression of network dysfunction in epilepsy and/or whether it may lead to more efficient recovery of networks after surgery. Our preliminary findings support future studies examining bilingualism as an important resilience factor and testing of interventions aimed at increasing bilingual usage in individuals with neurologic disease.
Acknowledgment
The authors thank Jonathan Rodriguez, Sanam Lalani, and Alice Ballerini for helpful discussion, Anna Christina Macari, Daniel Asay, and Jun Rao for assistance with data collection and processing, and the patients of the UCSD Epilepsy Center for their participation and contribution to science. Some of the study data were collected and managed using REDCap, a secure web platform for building and managing online databases and surveys. The REDCap software system provided by the UCSD Clinical and Translational Research Center is supported by Award Number UL1TR001442 from the National Center for Research Resources.
Glossary
- 3D
3 dimensional
- AD
Alzheimer disease
- ANOVA
analysis of variance
- B-LTLE
bilingual LTLE
- B-RTLE
bilingual RTLE
- dMRI
diffusion-weighted MRI
- DRM
dynamic restructuring model
- FOV
field of view
- HC
healthy control
- LTLE
left TLE
- M-LTLE
monolingual LTLE
- M-RTLE
monolingual RTLE
- MTS
mesial temporal sclerosis
- ROI
region of interest
- RTLE
right TLE
- TE
echo time
- TI
inversion time
- TLE
temporal lobe epilepsy
- TR
repetition time
- UC
University of California
- UCSD
UC San Diego
- UCSF
UC San Francisco
- WASI
Wechsler Abbreviated Scale of Intelligence
Appendix. Authors
Footnotes
Editorial, page 847
Study Funding
This research was supported by funding from the National Institute of Neurological Disorders and Stroke (NINDS) (R01NS124585 and R01NS122827) to C.R.M. A.S. was supported by an F32 from the NINDS (1F32NS119285) and APA Society for Clinical Neuropsychology Early Career Pilot Study Award. E.K. was supported by a K-award from the NINDS (K01NS124831). A.R. was supported by an F31 from the NINDS (1F31NS111883-01). M.P. was supported by the National Institute on Deafness and Other Communication Disorders (K01 DC016904).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Alladi S, Bak TH, Duggirala V, et al. Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology. 2013;81(22):1938-1944. doi: 10.1212/01.wnl.0000436620.33155.a4. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JAE, Hawrylewicz K, Grundy JG. Does bilingualism protect against dementia? A meta-analysis. Psychon Bull Rev. 2020;27(5):952-965. doi: 10.3758/s13423-020-01736-5. [DOI] [PubMed] [Google Scholar]
- 3.Berkes M, Bialystok E. Bilingualism as a contributor to cognitive reserve: what it can do and what it cannot do. Am J Alzheimers Dis Other Demen. 2022;37:15333175221091416. doi: 10.1177/15333175221091417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brini S, Sohrabi HR, Hebert JJ, et al. Bilingualism is associated with a delayed onset of dementia but not with a lower risk of developing it: a systematic review with meta-analyses. Neuropsychol Rev. 2020;30:1-24. doi: 10.1007/s11065-020-09426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulavicius AM, Mizzaci CC, Tavares DRB, et al. Bilingualism for delaying the onset of Alzheimer's disease: a systematic review and meta-analysis. Eur Geriatr Med. 2020;11(4):651-658. doi: 10.1007/s41999-020-00326-x. [DOI] [PubMed] [Google Scholar]
- 6.Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J Neurosci. 2013;33(2):387-396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grady CL, Luk G, Craik FIM, Bialystok E. Brain network activity in monolingual and bilingual older adults. Neuropsychologia. 2015;66:170-181. doi: 10.1016/j.neuropsychologia.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luk G, Bialystok E, Craik FIM, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci. 2011;31(46):16808-16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perani D, Farsad M, Ballarini T, et al. The impact of bilingualism on brain reserve and metabolic connectivity in Alzheimer's dementia. Proc Natl Acad Sci USA. 2017;114(7):1690-1695. doi: 10.1073/pnas.1610909114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowoll ME, Degen C, Gorenc L, et al. Bilingualism as a contributor to cognitive reserve? Evidence from cerebral glucose metabolism in mild cognitive impairment and Alzheimer's disease. Front Psychiatry. 2016;7:62. doi: 10.3389/fpsyt.2016.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sala A, Malpetti M, Farsad M, et al. Lifelong bilingualism and mechanisms of neuroprotection in Alzheimer dementia. Hum Brain Mapp. 2022;43(2):581-592. doi: 10.1002/hbm.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Pentón L, Pérez Fernández A, Iturria-Medina Y, Gillon-Dowens M, Carreiras M. Anatomical connectivity changes in the bilingual brain. Neuroimage. 2014;84:495-504. doi: 10.1016/j.neuroimage.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 13.Fedeli D, Del Maschio N, Sulpizio S, Rothman J, Abutalebi J. The bilingual structural connectome: dual-language experiential factors modulate distinct cerebral networks. Brain Lang. 2021;220:104978. doi: 10.1016/j.bandl.2021.104978. [DOI] [PubMed] [Google Scholar]
- 14.Sulpizio S, Del Maschio N, Del Mauro G, Fedeli D, Abutalebi J. Bilingualism as a gradient measure modulates functional connectivity of language and control networks. Neuroimage. 2020;205:116306. doi: 10.1016/j.neuroimage.2019.116306. [DOI] [PubMed] [Google Scholar]
- 15.Zeigler K, Camarota SA. 67.3 Million in the United States Spoke a Foreign Language at Home in 2018. Center for Immigration Studies; 2019:1-7. [Google Scholar]
- 16.Tavakol S, Royer J, Lowe AJ, et al. Neuroimaging and connectomics of drug-resistant epilepsy at multiple scales: from focal lesions to macroscale networks. Epilepsia. 2019;60(4):593-604. doi: 10.1111/epi.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes A, Paul BM, Marshall A, et al. Does bilingualism increase brain or cognitive reserve in patients with temporal lobe epilepsy? Epilepsia. 2018;59(5):1037-1047. doi: 10.1111/epi.14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 2015;50:162-170. doi: 10.1016/j.yebeh.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Slinger G, Otte WM, Braun KPJ, van Diessen E. An updated systematic review and meta-analysis of brain network organization in focal epilepsy: looking back and forth. Neurosci Biobehavioral Rev. 2022;132:211-223. doi: 10.1016/j.neubiorev.2021.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Larivière S, Bernasconi A, Bernasconi N, Bernhardt BC. Connectome biomarkers of drug-resistant epilepsy. Epilepsia. 2021;62(1):6-24. doi: 10.1111/epi.16753. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Cruces R, Bernhardt BC, Concha L. Multidimensional associations between cognition and connectome organization in temporal lobe epilepsy. Neuroimage. 2020;213:116706. doi: 10.1016/j.neuroimage.2020.116706. [DOI] [PubMed] [Google Scholar]
- 22.Reyes A, Kaestner E, Bahrami N, et al. Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology. 2019;92(17):e1957-e1968. doi: 10.1212/wnl.0000000000007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex. 2011;21(9):2147-2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- 24.Doucet GE, Rider R, Taylor N, et al. Presurgery resting-state local graph-theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia. 2015;56(4):517-526. doi: 10.1111/epi.12936. [DOI] [PubMed] [Google Scholar]
- 25.Davis KA, Morgan VL. Network analyses in epilepsy: are nodes and edges ready for clinical translation? Neurology. 2021;96(5):195-196. doi: 10.1212/WNL.0000000000011316. [DOI] [PubMed] [Google Scholar]
- 26.Abutalebi J, Green D. Bilingual language production: the neurocognition of language representation and control. J Neurolinguist. 2007;20(3):242-275. doi: 10.1016/j.jneuroling.2006.10.003. [DOI] [Google Scholar]
- 27.Stasenko A, Lin C, Bonilha L, Bernhardt BC, McDonald CR. Neurobehavioral and clinical comorbidities in epilepsy: the role of white matter network disruption. Neuroscientist. 2022;2022:10738584221076133. doi: 10.1177/10738584221076133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagler DJ, Hatton S, Cornejo MD, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202:116091. doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tournier JD, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions [online]. Proc Intl Soc Mag Reson Med. 2010;18:1670. semanticscholar.org/paper/Improved-probabilistic-streamlines-tractography-by-Tournier-Calamante/b4ffcb9ec889a8a68bffc46387a96b78a50ef94a. Accessed June 28, 2022. [Google Scholar]
- 30.Dhollander T, Connelly A. A novel iterative approach to reap the benefits of multi-tissue CSD from just single-shell (+b=0) diffusion MRI data. 24th International Society of Magnetic Resonance in Medicine; Singapore; 2016; Volume: 24, 3010.
- 31.Cieslak M, Cook PA, He X, et al. QSIPrep: an integrative platform for preprocessing and reconstructing diffusion MRI data. Nat Methods. 2021;18:775-778. nature.com/articles/s41592-021-01185-5. Accessed June 28, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith RE, Tournier JD, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012;62(3):1924-1938. doi: 10.1016/j.neuroimage.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059-1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Struck AF, Boly M, Hwang G, et al. Regional and global resting-state functional MR connectivity in temporal lobe epilepsy: results from the Epilepsy Connectome Project. Epilepsy Behav. 2021;117:107841. doi: 10.1016/j.yebeh.2021.107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li P, Legault J, Litcofsky KA. Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex. 2014;58:301-324. doi: 10.1016/j.cortex.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JAE, Grundy JG, De Frutos J, Barker RM, Grady C, Bialystok E. Effects of bilingualism on white matter integrity in older adults. Neuroimage. 2018;167:143-150. doi: 10.1016/j.neuroimage.2017.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallo F, DeLuca V, Prystauka Y, Voits T, Rothman J, Abutalebi J. Bilingualism and aging: implications for (delaying) neurocognitive decline. Front Hum Neurosci. 2022;16:819105. doi: 10.3389/fnhum.2022.819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zahodne LB, Schofield PW, Farrell MT, Stern Y, Manly JJ. Bilingualism does not alter cognitive decline or dementia risk among Spanish-speaking immigrants. Neuropsychology. 2014;28(2):238-246. doi: 10.1037/neu0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Leon J, Grasso SM, Welch A, et al. Effects of bilingualism on age at onset in two clinical Alzheimer's disease variants. Alzheimers Dement. 2020;16(12):1704-1713. doi: 10.1002/alz.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alladi S, Bak TH, Mekala S, et al. Impact of bilingualism on cognitive outcome after stroke. Stroke. 2016;47(1):258-261. doi: 10.1161/STROKEAHA.115.010418. [DOI] [PubMed] [Google Scholar]
- 41.Voits T, Pliatsikas C, Robson H, Rothman J. Beyond Alzheimer's disease: can bilingualism be a more generalized protective factor in neurodegeneration? Neuropsychologia. 2020;147:107593. doi: 10.1016/j.neuropsychologia.2020.107593. [DOI] [PubMed] [Google Scholar]
- 42.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305-1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green DW. Mental control of the bilingual lexico-semantic system. Bilingualism. 1998;1(2):67-81. doi: 10.1017/S1366728998000133. [DOI] [Google Scholar]
- 44.Pliatsikas C, Moschopoulou E, Saddy JD. The effects of bilingualism on the white matter structure of the brain. Proc Natl Acad Sci USA. 2015;112(5):1334-1337. doi: 10.1073/pnas.1414183112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stasenko A, Schadler A, Kaestner E, et al. Can bilingualism increase neuroplasticity of language networks in epilepsy? Epilepsy Res. 2022;182:106893. doi: 10.1016/j.eplepsyres.2022.106893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gold BT. Lifelong bilingualism and neural reserve against Alzheimer's disease: a review of findings and potential mechanisms. Behav Brain Res. 2015;281:9-15. doi: 10.1016/j.bbr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smirnov DS, Stasenko A, Salmon DP, Galasko D, Brewer JB, Gollan TH. Distinct structural correlates of the dominant and nondominant languages in bilinguals with Alzheimer's disease (AD). Neuropsychologia. 2019;132:107131. doi: 10.1016/j.neuropsychologia.2019.107131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Połczyńska MM, Bookheimer SY. General principles governing the amount of neuroanatomical overlap between languages in bilinguals. Neurosci Biobehav Rev. 2021;130:1-14. doi: 10.1016/j.neubiorev.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pliatsikas C. Understanding structural plasticity in the bilingual brain: the Dynamic Restructuring Model. Bilingualism. 2020;23(2):459-471. doi: 10.1017/s1366728919000130. [DOI] [Google Scholar]
- 50.Galovic M, de Tisi J, McEvoy AW, et al. Resective surgery prevents progressive cortical thinning in temporal lobe epilepsy. Brain. 2020;143(11):3262-3272. doi: 10.1093/brain/awaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.