Abstract
Background and Objectives
The safety and efficacy of tenecteplase (TNK) in patients with tandem lesion (TL) stroke is unknown. We performed a comparative analysis of TNK and alteplase in patients with TLs.
Methods
We first compared the treatment effect of TNK and alteplase in patients with TLs using individual patient data from the EXTEND-IA TNK trials. We evaluated intracranial reperfusion at initial angiographic assessment and 90-day modified Rankin scale (mRS) with ordinal logistic and Firth regression models. Because 2 key outcomes, mortality and symptomatic intracranial hemorrhage (sICH), were few in number among those who received alteplase in the EXTEND-IA TNK trials, we generated pooled estimates for these outcomes by supplementing trial data with estimates of incidence obtained through a meta-analysis of studies identified in a systematic review. We then calculated unadjusted risk differences to compare the pooled estimates for those receiving alteplase with the incidence observed in the trial among those receiving TNK.
Results
Seventy-one of 483 patients (15%) in the EXTEND-IA TNK trials possessed a TL. In patients with TLs, intracranial reperfusion was observed in 11/56 (20%) of TNK-treated patients vs 1/15 (7%) alteplase-treated patients (adjusted odds ratio 2.19; 95% CI 0.28–17.29). No significant difference in 90-day mRS was observed (adjusted common odds ratio 1.48; 95% CI 0.44–5.00). A pooled study-level proportion of alteplase-associated mortality and sICH was 0.14 (95% CI 0.08–0.21) and 0.09 (95% CI 0.04–0.16), respectively. Compared with a mortality rate of 0.09 (95% CI 0.03–0.20) and an sICH rate of 0.07 (95% CI 0.02–0.17) in TNK-treated patients, no significant difference was observed.
Discussion
Functional outcomes, mortality, and sICH did not significantly differ between patients with TLs treated with TNK and those treated with alteplase.
Classification of Evidence
This study provides Class III evidence that TNK is associated with similar rates of intracranial reperfusion, functional outcome, mortality, and sICH compared with alteplase in patients with acute stroke due to TLs. However, the CIs do not rule out clinically important differences.
Trial Registration Information
clinicaltrials.gov/ct2/show/NCT02388061; clinicaltrials.gov/ct2/show/NCT03340493.
Accounting for 15%–30% of large vessel occlusion (LVO) ischemic strokes, tandem lesions (TLs) in the anterior circulation pose a challenge for stroke physicians and interventionalists.1 The increased technical difficulty of endovascular thrombectomy and the potential need for revascularization of the extracranial internal carotid artery (eICA) increases the risk of procedural complications.1,2 In addition, thrombolysis may be less effective due to a lack of anterograde flow and larger clot burden.3 Compared with stand-alone intracranial occlusions, the presence of TLs are associated with worsened clinical outcomes, and the management approach remains an area of ongoing debate.4,5
With 3 specific genetic alterations that result in prolonged half-life, higher fibrin specificity, and an increased resistance to plasminogen activator inhibitor,6 tenecteplase (TNK) has the potential to improve reperfusion rates and clinical outcomes.7,8 These specific changes result in a higher affinity to fibrin-rich clot, thereby increasing the probability of clot clearance and the ability to administer TNK as a single bolus dose. However, these changes also bring a potential risk of hemorrhagic complications with TNK, specifically in patients with TLs who may require immediate carotid stenting and the administration of periprocedural antithrombotic medications to prevent stent thrombosis.
The efficacy of TNK in patients with TLs in the anterior circulation is unknown. In this study, we aimed to investigate the safety and efficacy of TNK, at 0.40 and 0.25 mg/kg dosing, and perform a comparative analysis against standard dose alteplase in patients with TLs using pooled individual patient data from the EXTEND-IA TNK trials and estimates obtained through a systematic review and meta-analysis of the relevant literature.9,10
Primary research question: What are the differences in the safety and efficacy of TNK vs alteplase in patients with a LVO and concurrent TL?
Methods
Participants
Participants were enrolled in the Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke trial (EXTEND-IA TNK) and the Determining the Optimal Dose of Tenecteplase Before Endovascular Therapy for Ischemic Stroke trial (EXTEND-IA TNK Part 2).9,10 Both studies were multicenter, prospective, randomized trials designed to assess the efficiency of TNK in patients with CT angiography (CTA)–confirmed occlusions of the internal carotid, middle cerebral, or basilar artery. Patients were enrolled within 4.5 hours of symptom onset and received both a thrombolytic and endovascular thrombectomy. In the first study, patients were randomized to open-label TNK (0.25 mg/kg) or alteplase (0.90 mg/kg), and in the second study, patients were randomized to 0.25 or 0.40 mg/kg of open-label TNK. Exclusion criteria for these trials have been previously described.9,10 In this analysis, patients with a basilar artery occlusion were excluded.
TL Definition
All patients received noncontrast CT imaging, CT perfusion imaging, and a CTA on initial assessment. In this analysis, we defined TLs as a combination of eICA pathology (ipsilateral stenosis >70% or occlusion) and intracranial LVO, based on the EASI Pilot trial and the ongoing EASI-TOC trial (NCT04261478).11 TLs were first assessed on baseline CTA and confirmed with digital subtraction angiography (DSA) by a clinician blinded to treatment allocation. In both trials, the type (carotid stenting, angioplasty, aspiration, no intervention) and timing of carotid intervention, in addition to any posttreatment medications, were determined and administered by the treating team. Posttreatment medications were defined in our analysis as any relevant antiplatelet or anticoagulant medication administered during the carotid procedure or within the first 24 hours after procedure completion.
Clinical and Imaging Outcomes
After randomization and treatment, all patients received either a CT or an MRI at 24 hours and serial clinical assessments up to 90 days poststroke. Both clinical and imaging outcome assessments were performed by clinicians blinded to treatment allocation. Clinical outcomes evaluated in this study include disability level at 90 days through an ordinal analysis of the modified Rankin scale (mRS), freedom from disability (defined as an mRS of 0–1 or no change from baseline at 90 days), functional independence (defined as an mRS of 0–2 or no change from baseline at 90 days), and all-cause mortality. Imaging outcomes assessed in this analysis include final extended treatment in cerebral ischemia (eTICI) scores, rates of successful intracranial reperfusion at initial angiographic assessment, and symptomatic intracranial hemorrhage (sICH, defined as subarachnoid hemorrhage associated with clinical symptoms or a parenchymal hematoma type 2 combined with at least a 4-point increase in the NIH Stroke Scale [NIHSS]).12 Angiographic reperfusion was scored prethrombectomy and postthrombectomy using the eTICI scale. Hemorrhage outcomes were determined with standardized imaging assessment by a central adjudication committee, blinded to treatment allocation.
Statistical Analysis
Our analysis was performed in 2 stages:
A post hoc assessment of the EXTEND-IA TNK data
A systematic review and meta-analysis of proportions (specifically looking at mortality and sICH, see further for detailed methodology) designed to expand our study population and provide a more representative estimate of event rates, given the small sample of alteplase-treated patients in the EXTEND-IA TNK trials.
Individual patient data were pooled across both studies and across the TNK dose groups. The Fisher exact test, Mann-Whitney U test, Kruskal-Wallis test, and analysis of variance were used as appropriate when evaluating baseline patient characteristics. We first compared the treatment effects of TNK (0.40 and 0.25 mg/kg dosing pooled) and 0.90 mg/kg of alteplase in patients with TLs. To assess for any potential treatment-by-TL interactions, we also estimated the treatment effect of TNK and alteplase in non-TL patients (as described earlier) and included a multiplicative treatment-by-TL interaction term. For 90-day mRS, we constructed ordinal logistic regression models to estimate common odds ratios (ORs), adjusting for individual study, baseline NIHSS, age, and time from symptom onset to arterial puncture (selected a priori, mRS 5–6 merged together). For successful intracranial reperfusion at initial angiographic assessment, freedom from disability, functional independence, and mortality, we constructed Firth logistic regression models to estimate ORs, adjusting for the aforementioned variables. These estimates are presented with respective 95% CIs using 0.90 mg/kg of alteplase as the reference. All reported p values for interaction are 2-sided, with p < 0.05 regarded as indicative of statistical significance, with no multiplicity correction. For all ordinal logistic regression models, the proportional odds assumption was assessed using the Brant test.
Analysis of Mortality and sICH in Patients With TLs: Systematic Review and Meta-analysis
When we initially compared the effectiveness of alteplase and TNK among patients in the EXTEND-IA TNK trials, we found low event rates for mortality and sICH in patients treated with alteplase. This raised the concern of bias in our findings. As such, we chose to supplement outcome data from the EXTEND-IA TNK trials with data collected through a systematic review of the relevant literature. We then performed a study-level meta-analysis of proportions, comparing mortality and sICH in patients with TLs treated with TNK vs alteplase.
The systematic review was conducted using recommendations from the Cochrane Handbook for Systematic Reviews,13 and findings were prepared with guidance from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.14 We conducted a comprehensive search of MEDLINE and EMBASE, from inception until February 2022. Supplementary searches included scanning the reference list of included studies and reviews identified through the primary search. Included studies were randomized controlled trials or observational cohort studies that enrolled adults experiencing an acute LVO stroke with a concurrent occlusion or stenosis of the extracranial carotid artery (TL) requiring treatment with endovascular clot retrieval ± an IV thrombolytic. We did not have any restrictions for outcomes reported, but our analysis was specifically focused on mortality and sICH outcomes. For thrombolytic use, we limited our study selection to either alteplase or TNK use. Studies that were excluded from our review included those involving pediatric patients, acute stroke patients without an LVO, patients with LVO without a concurrent TL, and patients treated with thrombolytic only or treated with prestent retriever era devices. If a study included patients with TLs who were treated with clot retrieval with or without thrombolytic, but the results of the combined therapy cohort (clot retrieval and thrombolytic) were not presented in an adequate detail, then this study was excluded. Case reports, case series, and abstract only data were excluded. We had no restrictions on country of study, ethnicity, sex, socioeconomic status of study populations, healthcare location of research, or language of the published study. A detailed search strategy using keywords and Medical Subject Headings terms is summarized in eTable 1 (links.lww.com/WNL/C681). A 2-stage screening was performed by first assessing both abstracts and titles for potentially relevant articles and then performing full-text screening. Screening and full-text review was conducted using Covidence Systematic Review software (Covidence, Melbourne, Australia).
We collected information regarding study design and enrollment criteria, baseline patient characteristics, and details of the intervention used in each study. Baseline patient characteristics included age, sex, definition of TL, and details of the intervention used. Detailed outcome data were collected wherever possible. The definition of each outcome was determined by the individual study. Study design, patient characteristics, and outcome definitions were summarized in tabular format. Risk of bias assessments of randomized controlled trials and nonrandomized observational studies were performed using the Cochrane Collaboration tool and the Newcastle-Ottawa Assessment scale, respectively. Results were summarized in tabular format.
We assessed study characteristics and study design to ensure that there would be enough similarity between the included studies to allow for reliable data pooling. For both TNK and alteplase treatments, respectively, we intended to pool study-level proportion estimates of participants achieving predefined outcomes using a DerSimonian-Laird model, with the estimate of heterogeneity taken from the inverse-variance fixed-effect model, as implemented in the metaprop Stata command.15 Outcome effect estimates from the selected studies were to be pooled with data from the primary studies (the EXTEND-IA TNK trials) where available and were visually represented with forest plots of each individual study proportion alongside the pooled study-level proportion. To ensure all studies were to be retained in the analysis, including studies with zero events, a Freeman-Tukey double arcsine transformation was to be used. If outside studies could not be identified for a specific thrombolytic, then pooled data from the EXTEND-IA TNK trials were to be presented as an unadjusted proportion with exact binomial (Clopper-Pearson) 95% CIs. We then estimated the difference between TNK and alteplase treatment as unadjusted risk differences. Statistical analysis was performed using SPSS software, version 27.0 (IBM, Armonk, NY) and Stata software, version 17 (StataCorp, College Station, TX).
Standard Protocol Approvals, Registrations, and Patient Consents
Local research ethics board approval was obtained at all EXTEND-IA TNK enrolling sites. Written informed consent was obtained from the participant or a legal representative before enrollment, except in jurisdictions allowing deferral of consent for emergency treatment, in which case consent was obtained at a later time point to continue participation. Because we did not seek individual patient data from the studies selected in our search, local ethics board approval was not required for the systematic review and meta-analysis. Each study selected from our systematic review reported approval from their local research ethics boards.
Data Availability
The authors declare that supporting data and methodological detail are available within the article and online-only supplement. Data that support the findings of this study can be obtained from the study authors on reasonable request.
Results
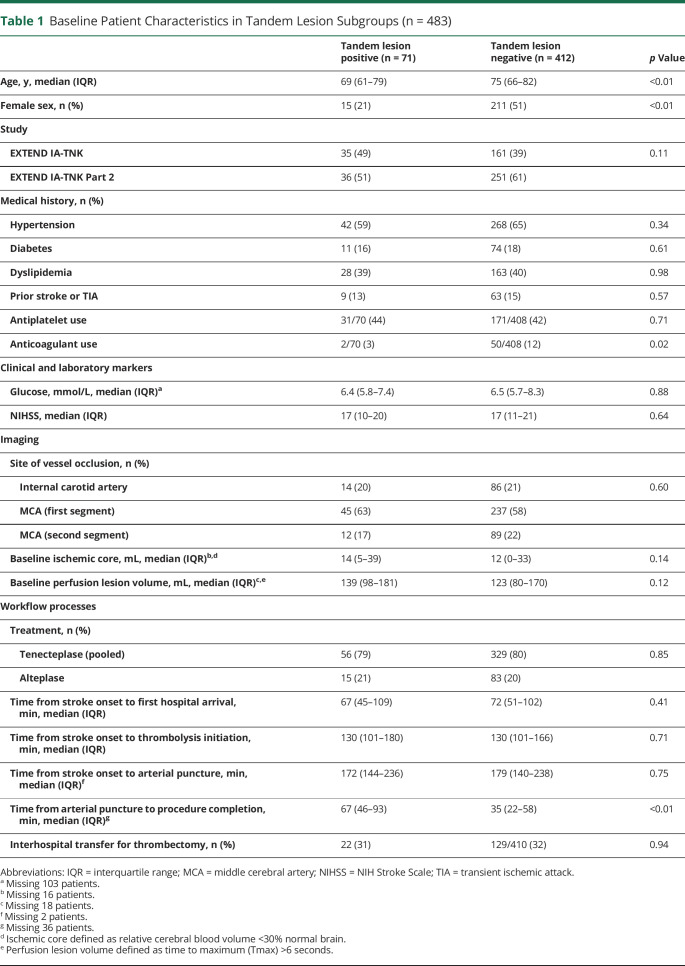
Of 502 patients recruited in the 2 trials, 483 with an anterior circulation occlusion were included in the primary analysis, after exclusion of 19 patients with a basilar artery occlusion (eFigure 1, links.lww.com/WNL/C681). Seventy-six suspected TLs were identified on initial CTA, of which 71/483 (15%) were confirmed, after exclusion of 2 lesions deemed on DSA to be pseudo-occlusions and 3 lesions with carotid stenosis <70%. Baseline patient characteristics, stratified by TL status, are outlined in Table 1. Patients with TLs were significantly younger and more often male. A lower proportion of anticoagulant use was observed in this cohort and the times from arterial puncture to procedural completion were notably longer. Baseline characteristics of patients with TLs did not differ between the 2 pooled trials (eTable 2). sICH was observed in 11 of 483 patients (3 events of clinical subarachnoid hemorrhage and 8 events of PH2 with an NIHSS increase of 4 or more). Patients with TLs exhibited a numerically higher proportion of sICH (4/71 [6%] vs 7/412 [2%], p = 0.05) and parenchymal hematoma (6/71 [9%] vs 14/412 [3%], p = 0.06) and a lower proportion of freedom from disability (29/71 [41%] vs 207/412 [50%], p = 0.15).
Table 1.
Baseline Patient Characteristics in Tandem Lesion Subgroups (n = 483)
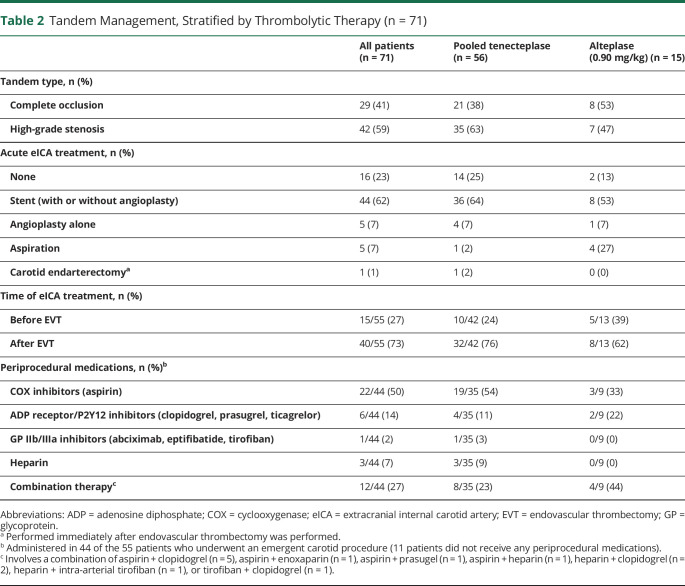
All 71 patients with TLs received thrombolysis before initiation of the angiographic procedure: 56 (79%) patients received TNK and 15 (21%) patients received alteplase. In 5 patients, the TLs could not be crossed with instrumentation. Fifty-five (77%) lesions were treated during the acute phase of the patient's care: 44 (62%) patients underwent carotid artery stenting (± angioplasty), 5 (7%) underwent angioplasty alone, and 5 (7%) had their lesions aspirated. One patient was treated with an immediate carotid endarterectomy after completion of endovascular thrombectomy. Of the 71 TLs, 42 (59%) were classified as high-grade stenosis and 29 (41%) were complete occlusions. Stenting was more frequent in those with a high-grade stenosis (eTable 3, links.lww.com/WNL/C681). No major difference in thrombolytic allocation was observed. Failure to pass the lesion rates and time metrics were similar between the 2 groups. A higher rate of mortality in patients treated with a complete occlusion was observed (high-grade stenosis: 2/42 [5%] vs complete occlusion 6/29 [21%]).
Periprocedural carotid artery management, stratified by thrombolytic medication, is summarized in Table 2, and a detailed list of all tandem patients and their management is provided in eTable 4 (links.lww.com/WNL/C681). Fifteen of the 55 (27%) patients required treatment of the carotid artery lesion before initiating intracranial endovascular thrombectomy. No major differences in treatment approach or clinical outcomes were observed in those patients who required treatment prethrombectomy compared with those whose carotid artery was treated postthrombectomy (eTable 5).
Table 2.
Tandem Management, Stratified by Thrombolytic Therapy (n = 71)
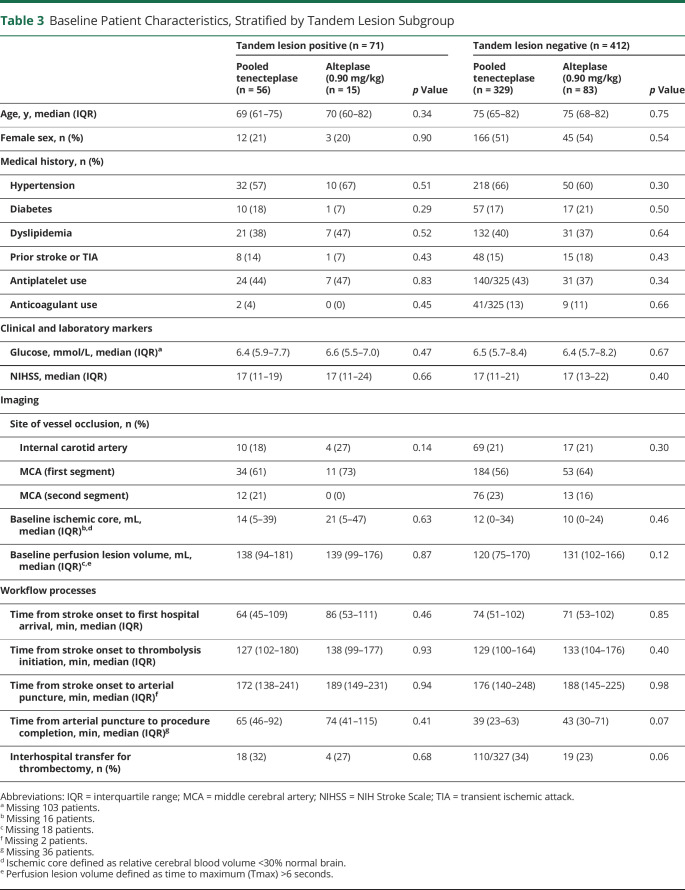
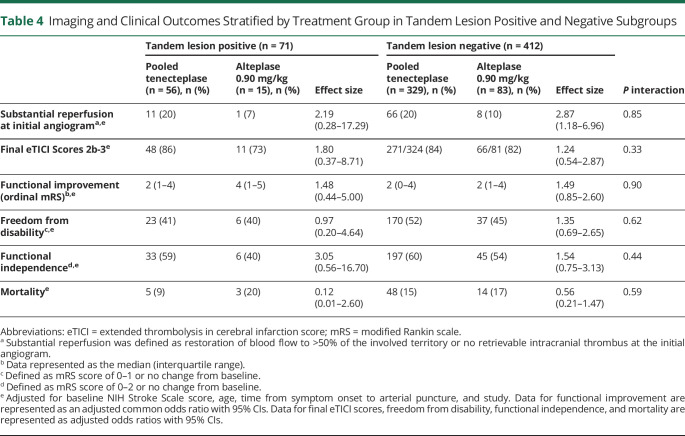
In exploratory univariable analysis, no major differences in baseline patient characteristics were observed between treatment arms within TL and non-TL subgroups (Table 3). In patients with TLs, reperfusion of the intracranial target at initial angiographic assessment was observed in 11/56 (20%) TNK-treated patients and 1/15 (7%) patients treated with alteplase (Table 4). Similarly, in the non-TL subgroup, reperfusion at initial angiographic assessment was observed in 66/329 (20%) TNK-treated patients compared with 8/83 (10%) alteplase-treated patients (p interaction = 0.85). Rates of successful reperfusion (eTICI 2b-3) postprocedure in the TL subgroup were 86% (TNK) vs 73% (alteplase) and within the non-TL subgroup were 84% (TNK) vs 82% (alteplase), p interaction = 0.33. Clinical outcomes of patients with TLs and non-TL patients stratified by thrombolytic therapy and 90-day mRS distributions are summarized in Table 4 and shown in Figure 1, respectively. We did not observe differences in functional outcomes between TNK and alteplase in either patients with TLs or non-TL patients. Among patients with TLs, sICH occurred in 7% (4/56) of TNK-treated patients and 0% (0/15) alteplase-treated patients compared with 2% (6/329, TNK) and 1% (1/83, alteplase) in non-TL patients. Of the 4 patients with TLs with an sICH, 2 received 0.40 mg/kg of TNK and 2 received 0.25 mg/kg of TNK. Three of the 4 patients were stented, with 1 patient experiencing an arterial perforation during the procedure, and 1 patient underwent a CEA immediately after endovascular thrombectomy, receiving periprocedural heparin (eTable 4, links.lww.com/WNL/C681).
Table 3.
Baseline Patient Characteristics, Stratified by Tandem Lesion Subgroup
Table 4.
Imaging and Clinical Outcomes Stratified by Treatment Group in Tandem Lesion Positive and Negative Subgroups
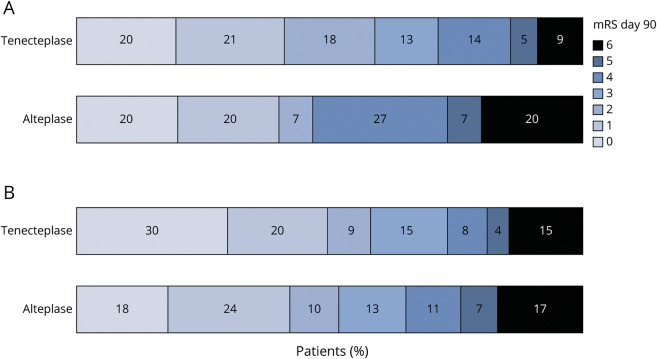
Figure 1. mRS Scores at 90 Days Stratified by Treatment in Patients With a Tandem Lesion (A) and Without a Tandem Lesion (B).
(A) No significant differences were observed between tenecteplase (pooled analysis of 0.25 and 0.40 mg/kg dosing) and alteplase (acOR 1.48 95% CI 0.44–5.00), adjusting for baseline NIH Stroke Scale, age, time from symptom onset to arterial puncture, and study. (B) No significant differences were observed between tenecteplase (pooled analysis of 0.25 mg/kg and 0.40 mg/kg dosing) and alteplase (acOR 1.49 95% CI 0.85–2.60), adjusting for baseline NIH Stroke Scale, age, time from symptom onset to arterial puncture, and study. acOR = adjusted common odds radio; mRS = modified Rankin scale.
Systematic Review and Meta-analysis of Mortality and sICH
Among the 2,349 records retrieved, title and abstract screening narrowed our search to 55 articles (eFigure 2, links.lww.com/WNL/C681). Through full-text screening, we identified 7 studies where we could pool the effect estimates of mortality and sICH at the study level.16-22 The selected 7 studies (eTable 6) were observational cohorts of acute stroke patients with an LVO and TL who were treated with endovascular thrombectomy ± IV alteplase. Although randomized data and studies of TNK were searched for, no additional data were found. Most studies were retrospective analyses of prospectively collected observational data. TLs were defined as an occlusion of the eICA or stenosis, ranging from 50% to 99%. In 5 of the 7 studies, sICH was defined using the European Cooperative Acute Stroke Study criteria. All studies selected for the meta-analysis used alteplase. We did not identify any study that used TNK and reported on sICH or mortality outcomes. As such, TNK-associated sICH and mortality outcomes were reported from the EXTEND-IA TNK trials as unadjusted proportions with binomial (Clopper-Pearson) 95% CIs.
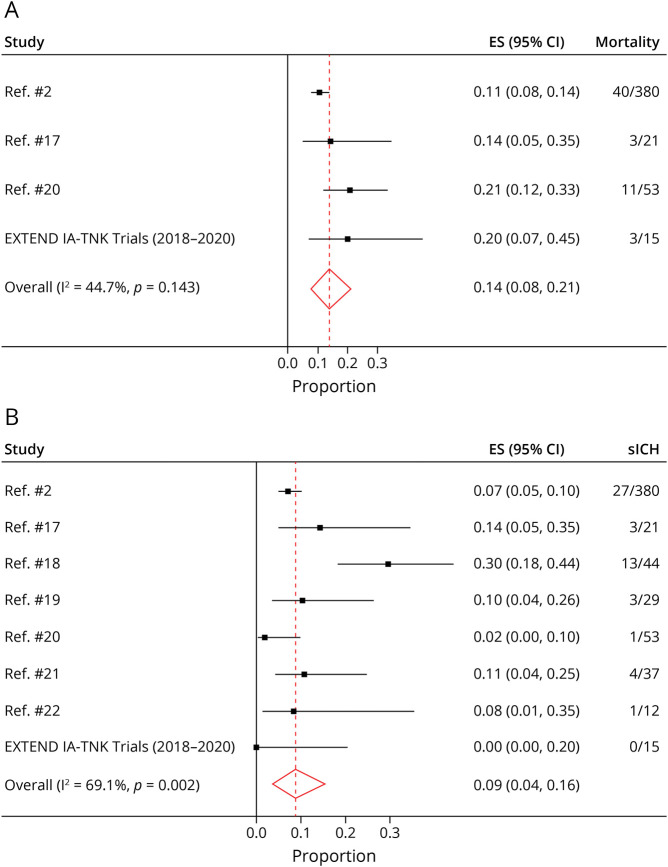
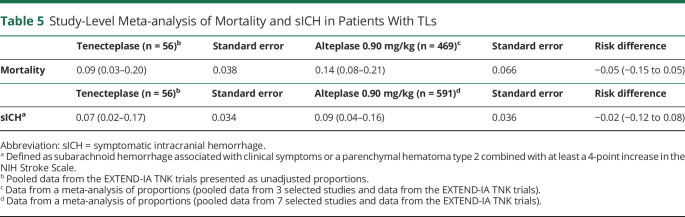
The pooled study-level proportion of alteplase-associated mortality and sICH was 0.14 (95% CI 0.08–0.21, Figure 2A) and 0.09 (95% CI 0.04–0.16, Figure 2B), respectively. Compared with a mortality rate of 0.09 (95% CI 0.03–0.20) and an sICH rate of 0.07 (95% CI 0.02–0.17) in TNK-treated patients from the EXTEND-IA TNK trials, no statistically significant difference in mortality or sICH rates were observed (Table 5). These results remained consistent in a sensitivity analysis where TNK sICH rates were compared with the alteplase data from the 7 selected observational studies alone (EXTEND-IA TNK data excluded; eTable 7, links.lww.com/WNL/C681). We also did not observe a difference in sICH rates between TNK and alteplase-treated patients in the non-TL group (risk difference 0.01; 95% CI −0.02 to 0.04).
Figure 2. Study-Level Meta-analysis of Proportions of Mortality (A) and Symptomatic Intracranial Hemorrhage (B) in Patients With Tandem Lesions Treated With IV Alteplase.
ES = effect size.
Table 5.
Study-Level Meta-analysis of Mortality and sICH in Patients With TLs
Classification of Evidence
This study provides Class III evidence that TNK is associated with similar rates of intracranial reperfusion, functional outcome, mortality, and sICH compared with alteplase, in patients with acute stroke due to TLs. However, the CIs do not rule out clinically important differences.
Discussion
The prevalence of TLs reported in the EXTEND-IA TNK trials was 15%. Baseline characteristics in this pooled individual patient data cohort were similar to past descriptions of patients with TLs studied in prospectively collected registries.1,2 Specifically, the TL population tended to be younger and more likely to be male. While the time from puncture to procedural completion was notably longer in this cohort, most patients (40/55, 72%) received treatment of the extracranial carotid artery lesion after endovascular thrombectomy. In the minority of patients where the extracranial carotid artery lesion was treated first, the primary reason provided by the treating team for the extracranial first approach was initial difficulty passing through the internal carotid artery to reach the intracranial target. The rate of carotid stenting in this cohort was similar to prior studies.1
Notably, the periprocedural medications used after carotid artery treatment varied widely, both for the type of medication used and the timing of medication commencement. Periprocedural medication choice and timing were at the discretion of the treating physician, and our results further highlight the lack of real-world management consensus between clinicians. Aspirin monotherapy was administered in a number of cases (Table 1). However, the dose of aspirin, route of administration, timing, and use in combination with other antithrombotic therapies was heterogeneous (eTable 4, links.lww.com/WNL/C681). It is important to note that IV aspirin, used in a number of cases, is not readily available in regions such as North America. Given this variability in practice, determining whether periprocedural medical therapy was associated with outcomes or hemorrhagic complications were not possible. Further investigations in larger datasets will be required, and ongoing clinical trials such as EASI-TOC (NCT04261478), which implement standardized antiplatelet regimens, will be highly informative.
It is postulated that the presence of a TL can reduce the effectiveness of a thrombolytic medication due to the reduction of cerebral blood flow to the intracranial target.3 We did not observe this in our study because the rates of substantial intracranial reperfusion at initial assessment were 20% in both TL and non-TL cohorts when treated with TNK, compared with 8%–10% in those treated with alteplase with no evidence of statistical interaction by TL status. The difference in recanalization rates between the 2 treatment arms among patients with TLs was not statistically significant, but this may be due to the comparisons being underpowered. Rates of successful recanalization after thrombectomy were similar in both TNK-treated and alteplase-treated groups. Further investigations of prethrombectomy intracranial reperfusion in subgroups based on TL type (stenosis vs occlusion) are also warranted. Due to limited sample size, this was not possible in our analysis, albeit we did not observe differences in prethrombectomy intracranial reperfusion between patients who presented with high-grade stenosis compared with patients with a complete occlusion (eTable 3, links.lww.com/WNL/C681).
Functional outcomes did not differ between treatment groups in the EXTEND IA-TNK trials, but there was a numerical trend favoring TNK (Figure 1A). The 7% incidence of sICH with TNK in this study is comparable with data in alteplase-treated patients with TLs from previously published literature (Table 5). The number of patients with TLs who received alteplase in the EXTEND-IA TNK trials was small (n = 15), thereby highlighting the benefits of using data from the systematic review to better evaluate low event rate variables such as mortality and sICH. However, there are several limitations in our analytical approach. First, the studies involved in the meta-analyses were all observational cohorts (eTable 6, links.lww.com/WNL/C681); we did not identify any randomized controlled trials. Sample size varied from study to study, and with the exception of Anadani et al.,16 the sample sizes of most selected studies were less than 50 patients. While most studies scored well in the patient and outcome selection sections of the Newcastle Ottawa Risk of Bias Assessment, cohort heterogeneity between studies was still observed. Clot retrieval time windows varied between 4.5 and 16 hours from symptom onset, and the percent stenosis also varied, with some studies requiring >90% stenosis and others requiring just 50%.18,22 Furthermore, because this assessment was unadjusted, we were not able to take into account other factors that influence sICH or mortality, such as baseline ischemic volume, number of thrombectomy attempts, and time to reperfusion metrics. With this in mind, our results relating specifically to sICH and mortality are hypothesis generating and will require further validation. Refined investigations of functional outcomes in this particular patient population, through individual patient-level data pooling, should become more feasible in the coming years because ongoing randomized controlled trials that assess TNK and alteplase are completed (e.g., ETERNAL-LVO [NCT04454788], ATTEST-2 [NCT02814409], AcT [NCT03889249]23).
In a study of 2 randomized controlled trials and a systematic review of the literature, functional outcomes, mortality, and sICH did not significantly differ between the thrombolytic therapies used before the start of endovascular clot retrieval in patients with TLs, noting that these patients tended to be younger and male. TNK seems both safe and effective, but further investigation of this novel thrombolytic within this specific patient cohort is warranted.
Glossary
- CTA
CT angiography
- DSA
digital subtraction angiography
- eICA
extracranial internal carotid artery
- eTICI
extended treatment in cerebral ischemia
- LVO
large vessel occlusion
- mRS
modified Rankin scale
- NIHSS
NIH Stroke Scale
- sICH
symptomatic intracranial hemorrhage
- TL
tandem lesion
- TNK
tenecteplase
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Study Funding
This study did not receive study-specific funding. The EXTEND-IA TNK trials received funding from the National Health and Medical Research Council Australia (GNT1113352, GNT1111972). V. Yogendrakumar is supported by a Canadian Institute of Health Research Fellowship Award, a University of Melbourne Research Scholarship, and a Detweiler Travelling Fellowship.
Disclosure
V. Yogendrakumar, L. Churilov, and P.J. Mitchell reports no disclosures relevant to the manuscript. T.J. Kleinig receives educational meeting supports from Boehringer Ingelheim. N. Yassi reports no disclosures relevant to the manuscript. V. Thijs receives personal compensation from Pfizer, Boehringer Ingelheim, Medtronic, BMS, Bayer, Allergan, Amgen, Biotronik and Abbott. D. Shah has received speakers honoraria from Boehringer Ingelheim and Bayer. P. Bailey, H.M. Dewey, P.M.C. Choi, A. Ma, and T. Wijeratne report no disclosures relevant to the manuscript. C. Garcia-Esperon received funding from Boehringer Ingelheim and Bayer for conference travel. G. Cloud, R.V Chandra, D.J. Cordato, B. Yan, and G. Sharma report no disclosures relevant to the manuscript. M. Parsons is on the Global Metalyse (TNK) Advisory Board for Boehringer Ingelheim. G.A. Donnan, S.M. Davis, B.C.V. Campbell, T. Wu, and P. Desmond report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Feil K, Herzberg M, Dorn F, et al. Tandem lesions in anterior circulation stroke: analysis of the German stroke registry-endovascular treatment. Stroke. 2021;52(4):1265-1275. [DOI] [PubMed] [Google Scholar]
- 2.Anadani M, Marnat G, Consoli A, et al. Endovascular therapy of anterior circulation tandem occlusions: pooled analysis from the TITAN and ETIS Registries. Stroke. 2021; 52(10):3097-3105. [DOI] [PubMed] [Google Scholar]
- 3.Kim YS, Garami Z, Mikulik R, Molina CA, Alexandrov AV. Early recanalization rates and clinical outcomes in patients with tandem internal carotid artery/middle cerebral artery occlusion and isolated middle cerebral artery occlusion. Stroke. 2005;36(4):869-871. [DOI] [PubMed] [Google Scholar]
- 4.Jacquin G, Poppe AY, Labrie M, et al. Lack of consensus among stroke experts on the optimal management of patients with acute tandem occlusion. Stroke. 2019;50(5):1254-1256. [DOI] [PubMed] [Google Scholar]
- 5.Poppe AY, Jacquin G, Roy D, Stapf C, Derex L. Tandem carotid lesions in acute ischemic stroke: mechanisms, therapeutic challenges, and future directions. AJNR Am J Neuroradiol. 2020;41(7):1142-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logallo N, Kvistad CE, Thomassen L. Therapeutic potential of tenecteplase in the management of acute ischemic stroke. CNS Drugs. 2015;29(10):811-818. [DOI] [PubMed] [Google Scholar]
- 7.Thomas GR, Thibodeaux H, Errett CJ, et al. A long-half-life and fibrin-specific form of tissue plasminogen activator in rabbit models of embolic stroke and peripheral bleeding. Stroke. 1994;25(10):2072-2078. [DOI] [PubMed] [Google Scholar]
- 8.Tanswell P, Modi N, Combs D, Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41(15):1229-1245. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poppe AY, Jacquin G, Stapf C, et al. A randomized pilot study of patients with tandem carotid lesions undergoing thrombectomy. J Neuroradiol. 2020;47(6):416-420. [DOI] [PubMed] [Google Scholar]
- 12.von Kummer R, Broderick JP, Campbell BCV, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version: 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. handbook.cochrane.org. [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(1):b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anadani M, Marnat G, Consoli A, et al. Endovascular therapy with or without intravenous thrombolysis in acute stroke with tandem occlusion. J Neurointervent Surg. 2022;14(4):314-320. [DOI] [PubMed] [Google Scholar]
- 17.Fernández Menéndez S, Murias Quintana E, Vega Valdés P, et al. Efficacy and safety of endovascular treatment in acute tandem carotid occlusions: analysis of a single-center cohort. Cerebrovasc Dis Extra. 2020;10(2):50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Ros V, Scaggiante J, Sallustio F, et al. Carotid stenting and mechanical thrombectomy in patients with acute ischemic stroke and tandem occlusions: antithrombotic treatment and functional outcome. AJNR Am J Neuroradiol. 2020;41(11):2088-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SE, Choi DS, Baek HJ, et al. Endovascular therapy of acute ischemic stroke related to tandem occlusion: comparison of occlusion and severe stenosis of the proximal cervical internal carotid artery. Br J Radiol. 2019;92(1093):20180051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bücke P, Aguilar Pérez M, AlMatter M, Hellstern V, Bazner H, Henkes H. Functional outcome and safety of intracranial thrombectomy after emergent extracranial stenting in acute ischemic stroke due to tandem occlusions. Front Neurol. 2018;9:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallustio F, Motta C, Koch G, et al. Endovascular stroke treatment of acute tandem occlusion: a single-center experience. J Vasc Intervent Radiol. 2017;28(4):543-549. [DOI] [PubMed] [Google Scholar]
- 22.de Lucena AF, de Castro-Afonso LH, Monsignore LM, et al. Carotid artery stenting in the context of endovascular treatment of acute ischemic stroke. Arq Neuropsiquiatr. 2016;74(3):212-218. [DOI] [PubMed] [Google Scholar]
- 23.Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161-169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that supporting data and methodological detail are available within the article and online-only supplement. Data that support the findings of this study can be obtained from the study authors on reasonable request.