Abstract
The class B carbapenem-hydrolyzing β-lactamase IND-1 has been characterized for Chryseobacterium indologenes strain 001. With internal primers for the bla gene for IND-1 (blaIND-1) and an internal blaIND-1 probe, PCR amplifications failed, while hybridization results were positive when DNA from another C. indologenes isolate, strain CIP101026, was used as a template. Thus, a blaIND-related gene was cloned from this C. indologenes reference strain. Sequencing of the insert of a recombinant plasmid conferring resistance to carbapenems revealed an open reading frame with a G + C content of 39.9% and coding for a 243-amino-acid preprotein named IND-2. IND-2 shared 80% amino acid identity with IND-1 and had a similar broad-spectrum resistance profile, including resistance to carbapenems. It was classified in functional subgroup 3a of class B carbapenem-hydrolyzing β-lactamases. IND-1 and IND-2, despite their genetic diversity, possessed similar kinetic parameters, except that ceftazidime was hydrolyzed less by IND-2. To obtain the entire blaIND-related gene sequences of eight other C. indologenes isolates, PCR was performed using internal and external primers, followed by inverse PCR techniques. The likely chromosome-mediated metallo-β-lactamases of the 10 C. indologenes isolates were divided into several groups and subgroups. IND-1, IND-2, IND-2a, IND-3, and IND-4 shared 77 to 99% amino acid identity.
The genus Chryseobacterium, defined in 1994 by Vandamme et al., comprises six species, including Chryseobacterium meningosepticum (previously Flavobacterium meningosepticum) and Chryseobacterium indologenes (previously Flavobacterium indologenes), which are the most common clinical species of this genus (29). C. meningosepticum isolates are associated with meningitis in newborns or in immunocompromised patients (4). C. indologenes is responsible mostly for nosocomial infections linked to the use of intravascular devices (10, 28). In 1997, Hsueh et al. reported 36 cases of infections caused by C. indologenes over a 3-year period at the National Taiwan University Hospital (11). These infections were intra-abdominal infections, biliary tract infections, or wound sepsis (11). C. indologenes is also implicated in pneumonia (9). C. indologenes is resistant to nearly all penicillins, except piperacillin, to restricted-spectrum cephalosporins, to aztreonam, and to carbapenems (1). Among β-lactams, only extended-spectrum cephalosporins show in vitro activity against C. indologenes (8). Minocycline and ciprofloxacin seem to be the most effective antibiotics and have been successfully used to treat C. indologenes infections (10).
Recently, two Ambler class B β-lactamases have been characterized for C. meningosepticum, which is also resistant to all β-lactams, including carbapenems (2, 26). The unrelated BlaB and GOB-1 exhibit a broad-spectrum profile and are chromosome encoded. These carbapenem-hydrolyzing β-lactamases (CHβLs) have molecular and biochemical heterogeneity among C. meningosepticum isolates. We have characterized a β-lactamase, IND-1, from C. indologenes strain 001 (1). Since plasmid-mediated CHβLs have been increasingly reported for gram-negative species worldwide (5, 12, 18, 23, 24, 25), it was of interest to identify naturally occurring CHβLs of gram-negative species and to search for their degree of identity with the plasmid-mediated CHβLs. Thus, we performed an extended genetic and biochemical study of β-lactamase-mediated resistance to carbapenems in C. indologenes.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this work are listed in Table 1. C. indologenes 002, 007, 008, and 009 were isolated from bronchoalveolar brush border, blood culture, rectal swab, and biliary liquid drainage, respectively, at the Hôpital de Bicêtre (Le Kremlin-Bicêtre, France) from 1997 to 1999. C. indologenes 003 and 004 were isolated from blood cultures at the Hôpital Robert Debré (Paris, France). C. indologenes 005 was from the Assistance Publique-Hôpitaux de Marseille (Marseille, France). C. indologenes 006 was isolated at the Hôpital de la Pitié Salpêtrière (Paris, France), and the C. indologenes reference strain CIP101026 was from the Pasteur Institute (Paris, France) strain collection. Each C. indologenes isolate was identified according to standard biochemical techniques (19, 29, 31), and their identification was checked at the Center for Bacterial Identification at the Pasteur Institute. These C. indologenes isolates were epidemiologically unrelated, as assessed by pulsed-field gel electrophoresis of XbaI-restricted DNA (data not shown).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH10B | F′ mcrA Δ(mrr-hsdRMS-mrcBC) Φ80ΔlacZDM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK1 rpsL nupG | Gibco BRL |
| E. coli JM109 | endA1 hsdR17 gyrA96 Δ(lac proA) recA1 relA supE44 thi F′ (lacIqlacZΔM15 proAB+ traD36) | 32 |
| In vitro-obtained rifampin-resistant E. coli JM109 | Rifampin resistant | This study |
| C. indologenes CIP101026 | Carbapenem resistant | Pasteur Institute |
| C. indologenes 001 | Carbapenem resistant | 1 |
| C. indologenes 002 | Carbapenem resistant | This study |
| C. indologenes 003 | Carbapenem resistant | This study |
| C. indologenes 004 | Carbapenem resistant | This study |
| C. indologenes 005 | Carbapenem resistant | This study |
| C. indologenes 006 | Carbapenem resistant | This study |
| C. indologenes 007 | Carbapenem resistant | This study |
| C. indologenes 008 | Carbapenem resistant | This study |
| C. indologenes 009 | Carbapenem susceptible | This study |
| Plasmids | ||
| pPCR-Script Amp SK(+) | Ampicillin resistance | Stratagene |
| pPCR-Script Cam SK(+) | Chloramphenicol resistance | Stratagene |
| pBK-CMV phagemid | Neomycin and kanamycin resistance | Stratagene |
| pSO-1 | blaIND-1 in the BamHI site of pBK-CMV | 1 |
| pSO-2 | blaIND-2 in the BamHI site of pBK-CMV | This study |
| pSO-3 | blaIND-3 in the SacI/HindIII site of pBK-CMV | This study |
| pSO-4 | blaIND-4 in the SacI/HindIII site of pBK-CMV | This study |
Escherichia coli DH10B and rifampin-resistant E. coli JM109 were used for cloning and conjugation, respectively (Table 1). All strains were stored at −70°C in Trypticase soy (TS) broth supplemented with 15% glycerol until testing.
Antimicrobial agents and MIC determinations.
The antimicrobial agents used in this study have been described elsewhere (21). MICs were determined by an agar dilution technique with Mueller-Hinton agar (Sanofi-Diagnostics Pasteur, Marnes-la-Coquette, France) and an inoculum of 104 CFU per spot. The plates were incubated at 35°C for 18 h before MIC determinations (16).
Cloning experiments and analysis of recombinant plasmids.
Genomic DNA was extracted as described previously (3). Fragments from Sau3AI partially digested genomic DNA from C. indologenes CIP101026 were cloned into the pBK-CMV phagemid (Ozyme, Saint Quentin-en-Yvelines, France) and expressed in E. coli DH10B as described previously (3). Antibiotic-resistant colonies were selected on amoxicillin (30 μg/ml)- and kanamycin (30 μg/ml)-containing TS agar plates.
Recombinant plasmid DNA was obtained from 100-ml TS broth cultures grown overnight in the presence of amoxicillin (30 μg/ml) at 37°C. Plasmid DNAs were recovered by using Qiagen (Courtaboeuf, France) columns before restriction digest analyses.
Conjugation assays and plasmid content.
Direct transfer of resistance markers into in vitro-obtained rifampin-resistant E. coli JM109 was attempted by liquid and solid conjugation assays (21). Transconjugants were selected on TS agar plates containing rifampin (200 μg/ml) and amoxicillin (30 μg/ml). Plasmid DNA extraction of C. indologenes isolates was attempted by using methods reported previously (2).
Hybridization experiments and PCR strategy.
The electrophoresis gel containing SspI-restricted genomic DNAs of C. indologenes isolates was transferred on a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Les Ullis, France) by the Southern method (22), and the transferred DNAs were UV cross-linked (Stratalinker; Stratagene) (21). The probe made from a PCR-generated 695-bp internal fragment of the bla gene for IND-1 (blaIND-1) was labeled with an ECL nonradioactive labeling and detection kit (Amersham Pharmacia Biotech).
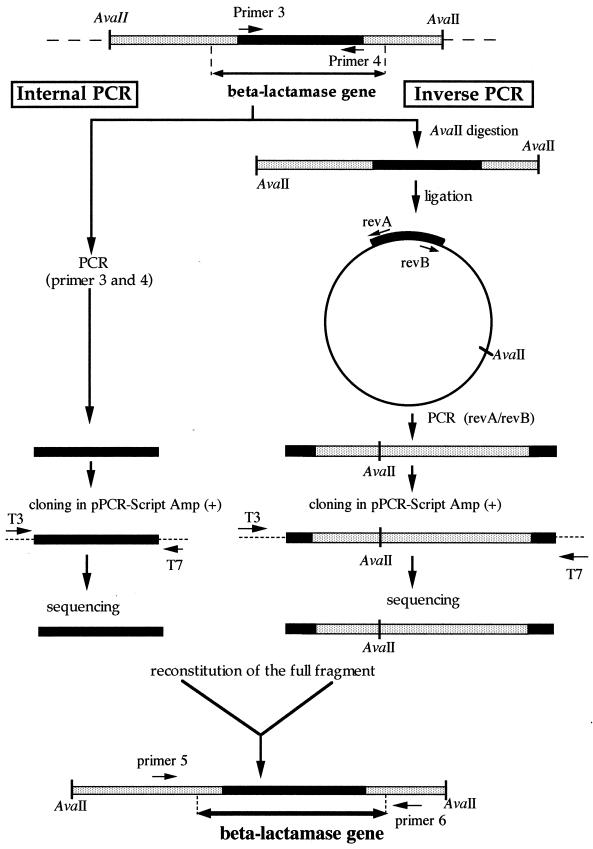
In order to amplify IND-like β-lactamase genes from other C. indologenes isolates, we designed primers based on the external regions of blaIND-2: primer 1, 5′-GGTTTGCATATCTATCTGCC-3′; and primer 2, 5′-ATCCAAAGAGAGGCTGGAGT-3′. PCR products were obtained for only four C. indologenes isolates. Thus, we designed other primers based on the conserved regions of blaIND-1 and blaIND-2 (primer 3, 5′-GCCCAGGTTAAAGATTTTGTAAT-3′; and primer 4, 5′-CATGGCCACCGCCTTTCCATTC-3′) to characterize the other IND-like β-lactamase genes (Fig. 1).
FIG. 1.
Strategies of direct PCR and IPCR. Black bars represent the internal PCR product sequence obtained with primers 3 and 4. Grey bars represent known sequences of IPCR products. revA and revB (or revC and revD for C. indologenes 009) represent primers used for amplification of flanking regions. T3 and T7 primers were used for sequencing the IPCR products. Primers 5 and 6 were used for cloning the entire blaIND-3 gene into pPCR-Script Cam SK(+) and into pBK-CMV. Primers 7 and 8 (same positions as primers 5 and 6, respectively) were used for cloning the blaIND-4 gene. Double-headed arrows represent the entire blaIND-related gene fragment.
After an initial denaturation step (5 min at 95°C), 42 cycles of amplification were performed as follows: denaturation at 95°C for 1 min, annealing at 53°C for 1 min, and DNA extension at 72°C for 1 min. A terminal DNA extension step was also performed at 72°C for 10 min. After sequencing of the PCR products, 580-bp-long sequences were characterized. In order to obtain the entire sequences of the β-lactamase genes, the inverse PCR (IPCR) technique was used (20). This technique enabled us to amplify, by PCR, flanking regions of a DNA fragment. Primers in inverted orientation with regard to the gene were used to amplify the restricted DNA fragment, which had been previously circularized by ligation (Fig. 1). Briefly, 4 μg of genomic DNA was restricted by AvaII (known to be absent from the blaIND-1 and blaIND-2 coding sequences). Since an AvaII restriction site was present in another blaIND-like gene (later identified as blaIND-4), PstI was used in IPCR for C. indologenes 009. After heat inactivation of the restriction enzyme, T4 DNA ligase was added to the reaction mixture and incubation was performed at 4°C overnight. IPCR was performed using the degenerate primers revA (5′-CCAYGGGACRTCAAATAAGAC-3′) and revB (5′-CWGCMACYGAYCTKGGATATA-3′) (where Y was C or T, R was A or G, W was A or T, M was A or C, and K was G or T), designed by comparison of blaIND-1 and blaIND-2 sequences and exhibiting orientations opposite those of primers 3 and 4, respectively (Fig. 1). Since IPCR with revA and revB failed, primers revC (5′-CTTTGCCGTCAAAAACTCCG-3′) and revD (5′-GCTAATGTAGAACAATGGCC-3′) were used in IPCR for C. indologenes 009. A 40-cycle amplification protocol was used, each cycle consisting of a denaturation step at 95°C for 1 min, a primer annealing step at 53°C, and an elongation step at 72°C for 3 min; 3 U of Taq polymerase was added per tube. The 1.4- or 1.7-kb IPCR products were purified, cloned into pPCR-Script Amp SK(+) as described by the manufacturer (Stratagene), and sequenced using T3 and T7 universal primers. DNA sequences adjacent to the AvaII (or PstI) restriction site in the cloned IPCR product were added to the PCR products obtained with primers 3 and 4 in order to deduce the entire blaIND-like gene sequences from five C. indologenes isolates.
In order to establish a valid comparison of MICs of β-lactams for E. coli DH10B harboring either blaIND-1, blaIND-2, blaIND-3, or blaIND-4, PCR products of blaIND-3 from C. indologenes 005, obtained using external primers 5 (5′-CCCAGCAAGTCCTAACTTTAATTAC-3′) and 6 (5′-CTAGTTACCTAGAGATAGCACG-3′), and of blaIND-4 from C. indologenes 009, obtained using external primers 7 (5′-TTATGAGGAAAAATGTTAGG-3′) and 8 (5′-GAACAGTTAATAGAAAAGCGGG-3′), were cloned first into pPCR-Script Cam SK(+) (Stratagene) and then into pBK-CMV and transformed back into E. coli DH10B by electroporation.
DNA sequencing and protein analysis.
Once the blaIND-like gene sequences were reconstituted using the above-described PCR strategy, a series of external primers (primers 1 and 2, 5 and 6, and 7 and 8) were used to obtain a PCR fragment for each C. indologenes strain as a template; the fragments were sequenced directly using an Applied Biosystems sequencer (ABI 373). Sequencing was also performed for the inserts of recombinant plasmids pSO-2, pSO3, and pSO4. The nucleotide and deduced protein sequences were analyzed with software available over the Internet from the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov) and from Pedro's BioMolecular Research Tools website (http://www.fmi.ch/biology/research_tools.htlm).
Multiple nucleotide or protein sequence alignments were carried out using the program ClustalW, available over the Internet from the University of Cambridge website (http://www2.ebi.ac.uk/clustalw). Among the Ambler class B β-lactamases, nine were compared to IND-like β-lactamases: GOB-1 and BlaB from C. meningosepticum (2, 26), VIM-1 and VIM-2 from Pseudomonas aeruginosa (12, 23), CphA from Aeromonas hydrophila (14), IMP-1 from Serratia marcescens (18), CcrA from Bacteroides fragilis (25), B-II from Bacillus cereus (13), and L-1 from Stenotrophomonas maltophilia (30).
β-Lactamase purification.
A culture of E. coli DH10B(pSO-2) was grown overnight at 37°C in 6 liters of TS broth containing kanamycin (30 μg/ml) and amoxicillin (30 μg/ml). Bacterial suspensions were pelleted, resuspended in 60 ml of 100 mM phosphate buffer (pH 6.0), disrupted by sonification (three times at 50 W for 30 s each time using a Vibra Cell 75022 Phospholyser; Bioblock, Illkirch, France), and centrifuged for 1 h at 48,000 × g and 4°C. Nucleic acids were precipitated by the addition of 0.2 M spermin (7% [vol/vol]; Sigma, Saint-Quentin Fallavier, France) overnight at 4°C. This suspension was ultracentrifuged at 100,000 × g for 1 h at 4°C. Similar unpurified β-lactamase extracts were obtained from 10-ml cultures of C. indologenes isolates and subsequently resuspended in 0.5 ml of sodium phosphate buffer.
The β-lactamase extract from E. coli DH10B(pSO-2) was filtered through a 0.45-μm-pore-size filter (Millipore) prior to being loaded onto a preequilibrated S-Sepharose column (Amersham Pharmacia Biotech). The enzyme recovered in the flowthrough was dialyzed overnight at 4°C against 20 mM Tris-HCl buffer (pH 8.0). The enzyme fraction was then loaded onto a preequilibrated Q-Sepharose column (Amersham Pharmacia Biotech). The enzyme was eluted with a linear NaCl gradient (0 to 1 M) in Tris-HCl buffer (pH 8.0). The β-lactamase was eluted at a concentration of 350 mM NaCl. The fraction containing the β-lactamase activity was dialyzed overnight against 100 mM phosphate buffer (pH 7.0) containing 50 μM ZnCl2. The specific activities of the β-lactamase extract and of the β-lactamase purified from E. coli DH10B(pSO-2) were compared using 100 μM imipenem as the substrate as described previously (2).
N-terminal sequencing and IEF analysis.
In order to determine the site for cleavage of the mature protein for IND-2 β-lactamase, the purified enzyme was submitted to Edman analysis as described previously (2) but with the following modifications in the protein transfer technique. The buffer consisted of 10 mM 3-cyclohexylamino-propane sulfonic acid (CAPS) (pH 11)–10% (vol/vol) methanol, and the transfer was carried out for 30 min at 50 V.
A purified enzyme preparation from a culture of E. coli DH10B(pSO-2) and extracts from cultures of 10 C. indologenes isolates were subjected to analytical isoelectric focusing (IEF) on a pH 3.5 to 9.5 Ampholine polyacrylamide gel (Ampholine PAG plate; Amersham Pharmacia Biotech) for 90 min at 1,500 V, 50 mA, and 30 W. The focused β-lactamases were detected by overlaying the gel with a 0.2% (wt/vol) starch agar gel containing 1% (wt/vol) benzylpenicillin in 100 mM phosphate buffer (pH 7.0) as described previously (2, 14). The pI values were determined and compared to those of known β-lactamases.
Kinetic measurements and identification of relative molecular mass.
The relative molecular mass of the β-lactamase IND-2 from E. coli DH10B(pSO-2) was estimated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis analysis. Purified enzyme and marker proteins were boiled for 5 min in a 1% SDS–3% β-mercaptoethanol solution and then subjected to electrophoresis on a 12% polyacrylamide gel (25 mA, 4 h) (3). Renaturation of β-lactamase activity after denaturing electrophoresis and revelation by overlaying the gel with 1 mM nitrocefin were performed as described previously (14).
Purified β-lactamase was used for kinetic measurements performed at 30°C with 100 mM phosphate buffer (pH 7.0) supplemented with 50 μM ZnCl2 (1). The rates of hydrolysis were determined with an ULTROSPEC 2000 UV spectrophotometer (Amersham Pharmacia Biotech) and analyzed by computer with SWIFT II software (Amersham Pharmacia Biotech). Km and kcat values were determined by analyzing β-lactam hydrolysis under initial rate conditions using the Eadie-Hoffstee linearization of the Michaelis-Menten equation as described previously (7).
Various concentrations of EDTA or clavulanic acid were preincubated with the enzyme for 10 min at 30°C before the rate of imipenem hydrolysis was tested. Then, the 50% inhibitory concentration was determined for each inhibitor.
Induction experiments and specific activity.
The inducibility of CHβL expression in C. indologenes clinical isolates was examined as described previously (22) with cefoxitin (5 μg/ml) or imipenem (1 μg/ml) as the inducer. Hydrolysis measurements were recorded with imipenem as the substrate. The total protein content was measured with bovine serum albumin as the standard (Bio-Rad DC protein assay kit).
Specific activities with benzylpenicillin, imipenem, or meropenem as substrates were determined for culture extracts from E. coli DH10B harboring either pSO-2 or pSO-4 in order to compare the efficacies of carbapenem hydrolysis by the β-lactamases IND-2 and IND-4.
Nucleotide sequence accession numbers.
The IND-like nucleotide and deduced amino acid sequences reported in this work will appear in the GenBank/EMBL database under accessions no. AF219127 to AF219135.
RESULTS AND DISCUSSION
Identification of the β-lactamase IND-2 and comparison with IND-1.
Several species belonging to the Chryseobacterium genus express a CHβL. Among them, C. meningosepticum expresses two nonrelated CHβLs, BlaB and GOB-1, with genetic and biochemical heterogeneity (2). Recently, a CHβL, IND-1, from C. indologenes strain 001 was characterized (1). This enzyme shares 43% amino acid identity with the most related metallo-β-lactamase, BlaB, from C. meningosepticum, its gene being very likely located on the chromosome. In order to investigate the distribution of blaIND-1 among several C. indologenes isolates, we performed PCR amplification and hybridization experiments. Negative PCR results indicated the likely absence of the blaIND-1 gene among the tested C. indologenes isolates, except for C. indologenes 001. However, positive hybridization results obtained with a blaIND-1 internal probe revealed the presence of blaIND-related genes in all C. indologenes isolates studied (data not shown).
We cloned the blaIND-like gene from C. indologenes CIP101026, which showed a hybridization pattern different from that of C. indologenes 001 (data not shown). Only one recombinant E. coli DH10B clone was obtained after electroporation of ligation products of Sau3AI partially restricted genomic DNA of C. indologenes CIP101026 into phagemid pBK-CMV, followed by selection on amoxicillin-containing plates. The recombinant plasmid (pSO-2) possessed a 1.5-kb insert and expressed a β-lactam resistance phenotype similar to that of E. coli DH10B(pSO-1), expressing β-lactamase IND-1.
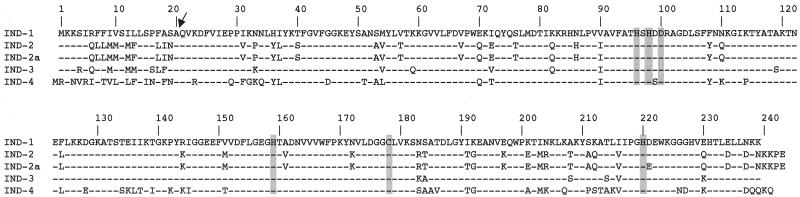
Sequencing of the insert revealed an open reading frame of 729 bp, from bp 477 to 1,205, encoding a putative 243-amino-acid preprotein named IND-2 (Fig. 2). A putative cleavage site was found between alanine and glutamine at positions 20 and 21, respectively (17). This site was confirmed, since Edman analysis (six cycles) determined the N-terminal sequence of the purified IND-2 β-lactamase from cultured E. coli DH10B(pSO-2) cells to be QVKD (Fig. 2). No sequence typical of a class 1 integron and no open reading frame likely coding for a regulatory protein were found within the 1.5-kb insert. Amino acid sequence analysis revealed a G+C content of 39.9%, similar to that found for other C. indologenes genes, as recorded in the EMBL sequence database, indicating the likely chromosome location of blaIND-2. A comparison of the IND-2 amino acid sequence with that of IND-1 revealed only 80% amino acid identity (83% without the leader peptide sequence), showing the heterogeneity of CHβLs in C. indologenes, like that found in C. meningosepticum (2).
FIG. 2.
Amino acid sequence comparison of the IND-like β-lactamases of the 10 C. indologenes isolates. Broken lines indicate identical amino acids. IND-1 was from C. indologenes 001; IND-2 was from C. indologenes CIP101026, 002, and 003; IND-2a was from C. indologenes 004; IND-3 was from C. indologenes 005, 006, 007, and 008; and IND-4 was from C. indologenes 009. Shading correspond to conserved residues in CHβLs (25). Numbering is according to that for the IND-1 β-lactamase. The arrow indicates the peptide leader cleavage site determined for IND-2 by N-terminal sequence analysis of E. coli DH10B(pSO-2) cultures.
Despite relatively low amino acid identity between β-lactamases IND-2 and IND-1, the amino acids that may be involved in the catalytic site of these metalloenzymes were identical (Fig. 2).
The MICs of β-lactams for C. indologenes CIP101026 (IND-2) showed that it was resistant or had reduced susceptibility to most β-lactams tested, including carbapenems, but not to piperacillin and cefepime (Table 2). E. coli DH10B(pSO-2) showed a broad-spectrum profile of resistance, with resistance to aminopenicillins and to early cephalosporins. E. coli DH10B(pSO-2) also showed reduced susceptibility to ureidopenicillins, cephalosporins, and carbapenems but remained fully susceptible to cefepime, aztreonam, and moxalactam. The addition of clavulanic acid to amoxicillin or tazobactam to piperacillin did not modify the MICs (data not shown). Despite the sequence heterogeneity of the CHβLs IND-1 and IND-2, the MICs of β-lactams for C. indologenes 001 and CIP101026 on the one hand and for E. coli DH10B(pSO-1) and E. coli DH10B(pSO-2) on the other hand remained similar (Table 2). However, E. coli DH10B(pSO-1) was slightly less susceptible to ceftazidime than E. coli DH10B(pSO-2). The level of resistance to carbapenems remained low in E. coli, as previously described for other CHβLs, confirming the major role of the permeability coefficient for each β-lactam in gram-negative bacteria (15).
TABLE 2.
MICs of β-lactams for several C. indologenes isolates, E. coli DH10B harboring recombinant plasmids, and E. coli DH10B (reference strain)a
| β-Lactam | MIC (μg/ml) for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C. indologenes 001 | C. indologenes CIP101026 | C. indologenes 005 | C. indologenes 009 | E. coli DH10B(pSO-1) | E. coli DH10B(pSO-2) | E. coli DH10B(pSO-3) | E. coli DH10B(pSO-4) | E. coli DH10B | |
| Amoxicillin | 256 | 256 | 256 | 256 | >512 | >512 | >512 | >512 | 2 |
| Ticarcillin | 256 | 512 | 512 | 512 | >512 | >512 | >512 | >512 | 2 |
| Piperacillin | 16 | 16 | 8 | 8 | 64 | 64 | 64 | 64 | 1 |
| Cephalothin | 256 | 256 | 128 | 128 | 128 | 128 | 128 | 128 | 4 |
| Cefepime | 1 | 2 | 0.5 | 1 | 0.06 | 0.06 | 0.06 | 0.06 | 0.03 |
| Cefoxitin | 8 | 16 | 8 | 16 | 16 | 16 | 16 | 16 | 1 |
| Ceftazidime | 32 | 8 | 8 | 8 | 8 | 4 | 4 | 8 | 0.12 |
| Cefotaxime | 64 | 32 | 32 | 32 | 16 | 16 | 8 | 16 | 0.12 |
| Aztreonam | >128 | >128 | >128 | >128 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Imipenem | 32 | 64 | 32 | 4 | 2 | 2 | 1 | 2 | 0.06 |
| Meropenem | 32 | 32 | 32 | 16 | 0.5 | 0.5 | 0.5 | 0.5 | 0.06 |
C. indologenes 001 and E. coli DH10B(pSO-1) produced IND-1, C. indologenes CIP101026 and E. coli DH10B(pSO-2) produced IND-2, C. indologenes 005 and E. coli DH10B(pSO-3) produced IND-3, and C. indologenes 009 and E. coli DH10B(pSO-4) produced IND-4.
Specific activity before and after purification showed a purification factor of 420-fold for IND-2 from E. coli DH10B(pSO-2) cultures. The specific activity of the purified enzyme was 129 μmol/min/mg of protein with imipenem as a substrate, and the relative molecular mass of the purified enzyme was estimated by SDS-polyacrylamide gel electrophoresis to be 31 kDa. β-Lactamase activity with a pI of 8.8 was detected in E. coli DH10B(pSO-2), and a similar pI was found in E. coli DH10B(pSO-1). A second and weaker β-lactamase activity with a pI of 7.2 was also detected and likely reflects protein degradation during extraction, as described previously (1). β-Lactamase IND-2 showed a broad-spectrum profile of hydrolysis (Table 3). The kinetic parameters of purified β-lactamase IND-2 revealed strong activities against benzylpenicillin and piperacillin. Ampicillin, cephalothin, cefotaxime, and imipenem were also effectively hydrolyzed by IND-2, but ceftazidime and cefepime were poor substrates for this CHβL. Fifty percent inhibitory concentrations obtained with imipenem as a substrate showed that IND-2 activity was inhibited by EDTA (0.5 μM) but not by clavulanic acid (>100 μM), like other class B β-lactamases. The broad hydrolysis spectrum of this CHβL enabled us to classify IND-2 in functional subgroup 3a (5, 6).
TABLE 3.
Kinetic parameters of β-lactam antibiotics for the purified CHβL IND-2 compared to those available for IND-1 (1)a
| Substrate | IND-2
|
IND-1
|
||||||
|---|---|---|---|---|---|---|---|---|
| Km (μM) | kcat (s−1) | kcat/Km (μM−1 · s−1) | Vmax rel | Vmax/Kmb | Km (μM) | Vmax rel | Vmax/Kmb | |
| Ampicillin | 95 | 70 | 0.7 | 22 | 15 | 363 | 33 | 2.4 |
| Benzylpenicillin | 70 | 320 | 4.6 | 100 | 100 | 26.4 | 100 | 100 |
| Cefepime | 2,200 | 1 | <0.001 | 0.1 | <0.01 | — | — | — |
| Cefotaxime | 10 | 9 | 0.9 | 2.8 | 19 | 60 | 19 | 8.7 |
| Cefoxitin | 40 | 2 | 0.05 | 0.8 | 1.4 | 30 | 1.6 | 1.3 |
| Ceftazidime | 440 | 2 | 0.005 | 0.7 | 0.1 | 765 | 15 | 0.5 |
| Cephalothin | 16 | 19 | 1.2 | 6 | 24 | 79 | 47 | 16 |
| Imipenem | 170 | 100 | 0.6 | 30 | 12 | 198 | 90 | 12 |
| Meropenem | 920 | 170 | 0.2 | 53 | 4 | 845 | 105 | 3.3 |
| Piperacillin | 210 | 430 | 2 | 130 | 43 | 365 | 255 | 18.4 |
Standard deviations were within 10%. —, not done.
Relative to the value for benzylpenicillin, which was set at 100.
In order to establish a valid comparison between the kinetic parameters of IND-1 and IND-2, the values for the maximum rate of metabolism (Vmax), relative to that for benzylpenicillin (Vmax rel), for IND-2 were added in the table (Table 3). Hydrolysis efficacy did not vary significantly for both CHβLs, especially for carbapenems. However, the Vmax/Km value of IND-1 for ceftazidime (relative to that for benzylpenicillin) was fivefold higher than that of IND-2, whereas this value of IND-2 for ampicillin was sixfold higher than that of IND-1. Thus, amino acid sequence changes in these CHβLs may be linked to variability in their biochemical properties.
Identification of other IND-like β-lactamases.
PCR products were obtained from C. indologenes isolates CIP101026, 002, 003, and 004 and sequenced by using external primers 1 and 2. Therefore, in order to characterize other blaIND-related genes, partial nucleotide sequences of blaIND-related genes from five other C. indologenes isolates were obtained using PCR amplification of a 580-bp blaIND-like internal fragment and PCR primers 3 and 4 (Fig. 1). IPCR was then performed in order to obtain the entire β-lactamase gene sequence and to characterize flanking regions from the five other C. indologenes isolates, 005, 006, 007, 008, and 009. Sequencing of internal PCR and IPCR products enabled us to obtain the entire nucleotide sequences of all the blaIND-related genes. A comparison of the deduced amino acid sequences of these genes enabled us to determine four groups of IND-like β-lactamases (Fig. 2). C. indologenes 001 produced IND-1; C. indologenes CIP101026, 002, and 003 produced IND-2; C. indologenes 004 produced an IND-2 variant, named IND-2a; C. indologenes 005, 006, 007, and 008 produced an IND-like β-lactamase, named IND-3, sharing 91% amino acid identity with IND-1 (94% if the leader peptide sequence was excluded); and C. indologenes 009, which was susceptible to carbapenems, produced an IND-related β-lactamase, named IND-4, displaying 72% amino acid identity with IND-1 (77% if the leader peptide sequence was excluded). None of the IND-like sequences shared more than 43% amino acid identity with the most closely related CHβL, BlaB, as described for IND-1 β-lactamase. β-Lactamases IND-1, IND-2, IND-3, and IND-4 could be classified on a molecular basis in subclass B1 (24). Within a given IND-1-like gene group (IND-1, IND-2, IND-3, and IND-4), the DNA sequences surrounding the β-lactamase gene were identical, whereas they were different from one group to another. Thus, IND-like genes of a given group lie within a specific genetic context, indicating possible subspecies within C. indologenes.
From a molecular point of view, these four main groups of CHβLs showed extensive heterogeneity of amino acid sequences. However, all of the six conserved amino acid residues for metallo-β-lactamases were conserved in the IND-related β-lactamases. Only one amino acid substitution (Asp99Ser) was located within the active site of β-lactamase IND-4 (Fig. 2). A serine residue at this position is also found in CHβL IMP-1, originally identified in S. marscescens (18).
Cloning of the PCR products of the entire blaIND-3 and blaIND-4 sequences into pBK-CMV and electroporation of the obtained plasmids, pSO-3 and pSO-4, into E. coli DH10B were performed in order to compare the MICs of β-lactams conferred by the four groups of CHβLs (IND-1, IND-2, IND-3, and IND-4).
A β-lactamase activity with a pI of 8.4 to 9 was detected in the C. indologenes isolates by IEF. A similar pI of ca. 8.8 was detected for cultures of E. coli DH10B harboring recombinant plasmids pSO-1 (IND-1), pSO-2 (IND-2), pSO-3 (IND-3), and pSO-4 (IND-4). No evidence of β-lactamase inducibility was detected for C. indologenes isolates by a spectrophotometric assay with imipenem or cefoxitin as an inducer and imipenem as a substrate for CHβL.
The MICs of β-lactams for the 10 C. indologenes isolates were similar (within a two-dilution range), except for C. indologenes 009, which was fully susceptible to imipenem (Table 2). The four β-lactamase groups expressed in E. coli DH10B, including E. coli DH10B(pSO-4), conferred similar MICs (within a two-dilution range) (Table 2). In order to explain this surprising result, we determined the specific activity of a β-lactamase extract from E. coli DH10B harboring either pSO-2 or pSO-4 with benzylpenicillin, imipenem, or meropenem as a substrate. The ratio of specific activity with imipenem as a substrate divided by the specific activity with benzylpenicillin as a substrate was sevenfold lower for E. coli DH10B(pSO-4), expressing IND-4, than for E. coli DH10B(pSO-2), expressing IND-2. Similarly, the ratio of the specific activity of meropenem divided by that of benzylpenicillin was 2.5-fold lower for E. coli DH10B(pSO-4) than for E. coli DH10B(pSO-2). Further investigations are necessary to evaluate the role of the amino acid changes observed for IND-4 in the decreased hydrolysis of carbapenems.
A recent study has reported the major role of permeability in the activity of β-lactams against gram-negative bacteria that produce group 3 β-lactamases (15). Therefore, susceptibility to imipenem in parental strain C. indologenes 009 (IND-4) could be due to a low level of carbapenem hydrolysis by IND-4, together with an increased permeability coefficient for carbapenems.
Conclusion.
IND-1-like sequences in C. indologenes, like L-1 from S. maltophilia (27), BlaB and GOB-1 from C. meningosepticum (2), B-II from B. cereus, or CcrA from B. fragilis (25), confirmed the variability of chromosome-located class B β-lactamases within a bacterial species. As opposed to C. meningosepticum, C. indologenes may possess only one CHβL. No pI values other than those of the CHβLs were detected in C. indologenes isolates (data not shown). Thus, the naturally occurring resistance to monobactams, such as aztreonam, observed in C. indologenes may not be linked to β-lactamase.
The origin of the metallo-β-lactamases recently identified as being integron located in gram-negative species (IMP-1, VIM-1, and VIM-2) remains unknown. None of them is related to the CHβLs described in this report. Cloning and expression of IND-like sequences in E. coli DH10B conferred only slightly decreased susceptibility to carbapenems. Thus, if expressed in gram-negative clinical isolates, the CHβLs of C. indologenes would be difficult to detect on the basis of the sole carbapenem resistance pattern.
ACKNOWLEDGMENTS
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES; grant JE-2227), Université Paris XI, Paris, France.
We thank Edouard Bingen, Michel Drancourt, Vincent Jarlier, and Didier Raoult for gifts of C. indologenes clinical isolates.
REFERENCES
- 1.Bellais S, Léotard S, Poirel L, Naas T, Nordmann P. Molecular characterization of a carbapenem-hydrolyzing β-lactamase from Chryseobacterium (Flavobacterium) indologenes. FEMS Microbiol Lett. 1999;171:127–132. doi: 10.1111/j.1574-6968.1999.tb13422.x. [DOI] [PubMed] [Google Scholar]
- 2.Bellais S, Aubert D, Naas T, Nordmann P. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing β-lactamases in Chryseobacterium meningosepticum. Antimicrob Agents Chemother. 2000;44:1878–1886. doi: 10.1128/aac.44.7.1878-1886.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellais S, Poirel L, Naas T, Girlich D, Nordmann P. Genetic-biochemical analysis and distribution of the Ambler class A β-lactamase CME-2, responsible for extended-spectrum cephalosporin resistance in Chryseobacterium (Flavobacterium) meningosepticum. Antimicrob Agents Chemother. 2000;44:1–9. doi: 10.1128/aac.44.1.1-9.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch K C, Nadarajah R, Jacobs R. Chryseobacterium meningosepticum: an emerging pathogen among immunocompromised adults. Medicine. 1997;76:30–41. doi: 10.1097/00005792-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bush K. Metallo-beta-lactamases: a class apart. Clin Infect Dis. 1998;27(Suppl. 1):S48–S53. doi: 10.1086/514922. [DOI] [PubMed] [Google Scholar]
- 6.Bush K, Jacoby G A. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornish-Bowden A. Fundamentals of enzyme kinetics. Seattle, Wash: Portland Press, Inc.; 1995. Graphs of the Michaelis-Menten equation; pp. 30–37. [Google Scholar]
- 8.Fraser S L, Jorgensen J H. Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob Agents Chemother. 1997;41:2738–2741. doi: 10.1128/aac.41.12.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsueh P R, Hsiue T R, Wu J J, Teng L J, Ho S W, Hsieh W C, Luh K T. Flavobacterium indologenes bacteremia: clinical and microbiological characteristics. Clin Infect Dis. 1996;23:550–555. doi: 10.1093/clinids/23.3.550. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh P R, Teng L J, Ho S W, Hsieh W C, Luh K T. Clinical and microbiological characteristics of Flavobacterium indologenes infections associated with indwelling devices. J Clin Microbiol. 1996;34:1908–1913. doi: 10.1128/jcm.34.8.1908-1913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsueh P R, Teng L J, Yang P C, Ho S W, Hsieh W C, Luh K T. Increasing incidence of nosocomial Chryseobacterium indologenes infections in Taiwan. Eur J Clin Microbiol Infect Dis. 1997;16:568–574. doi: 10.1007/BF02447918. [DOI] [PubMed] [Google Scholar]
- 12.Lauretti L, Riccio M L, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini G M. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999;43:1584–1590. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim H M, Pene J J, Shaw R. Cloning, nucleotide sequence, and expression of the Bacillus cereus 5/B/6 β-lactamase II structural gene. J Bacteriol. 1988;170:2873–2878. doi: 10.1128/jb.170.6.2873-2878.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massidda O, Rossolini G M, Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among metallo-β-lactamases. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumura N, Minami S, Watanabe Y, Iyobe S, Mitsuhashi S. Role of permeability in the activities of β-lactams against gram-negative bacteria which produce a group 3 β-lactamase. Antimicrob Agents Chemother. 1999;43:2084–2086. doi: 10.1128/aac.43.8.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. NCCLS document M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Nielsen H, Engelbrecht J, Brunak S, Von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickett M J. Methods for identification of Flavobacterium. J Clin Microbiol. 1989;27:2309–2315. doi: 10.1128/jcm.27.10.2309-2315.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirel L, Aubin J T, Gautheret A, Malet I, Huraux J M, Agut H. Use of inverse polymerase chain reaction to characterize a novel human herpesvirus 7 isolate. J Virol Methods. 1997;64:197–203. doi: 10.1016/s0166-0934(96)02167-2. [DOI] [PubMed] [Google Scholar]
- 21.Poirel L, Guibert M, Bellais S, Naas T, Nordmann P. Integron- and carbenicillinase-mediated reduced susceptibility to amoxicillin-clavulanic acid in isolates of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 from French patients. Antimicrob Agents Chemother. 1999;43:1098–1104. doi: 10.1128/aac.43.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poirel L, Guibert M, Girlich D, Naas T, Nordmann P. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob Agents Chemother. 1999;43:769–776. doi: 10.1128/aac.43.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirel L, Naas T, Nicolas D, Collet L, Bellais S, Cavallo J-D, Nordmann P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother. 2000;44:891–897. doi: 10.1128/aac.44.4.891-897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen B A, Gluzman Y, Tally F P. Cloning and sequencing of the class B β-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990;34:1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossolini G M, Franceschini N, Riccio M L, Mercuri P S, Perilli M, Galleni M, Frère J M, Amicosante G. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. Biochem J. 1998;332:145–152. doi: 10.1042/bj3320145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanschagrin F, Dufresne J, Levesque R C. Molecular heterogeneity of the L-1 metallo-β-lactamase family from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1998;42:1245–1248. doi: 10.1128/aac.42.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegman-Igra Y, Schwartz D, Soferman G, Konforti N. Flavobacterium group IIb bacteremia: report of a case and review of Flavobacterium infections. Med Microbiol Immunol. 1987;176:103–111. doi: 10.1007/BF00200682. [DOI] [PubMed] [Google Scholar]
- 29.Vandamme P, Bernardet J F, Segers P, Kersters K, Holmes B. New perspectives in the classification of the flavobacteria: description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int J Syst Bacteriol. 1994;44:827–831. [Google Scholar]
- 30.Walsh T R, Hall L, Assinder S J, Nichols W W, Cartwright S J, MacGowan A P, Bennett P M. Sequence analysis of the L-1 metallo β-lactamase from Xanthomonas maltophilia. Biochim Biophys Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 31.Yabuuchi E, Hashimoto Y, Ezaki T, Ido Y, Takeuchi N. Genotypic differentiation of Flavobacterium indologenes Yabuuchi et al. 1983 from Flavobacterium gleum Holmes et al. 1984. Microbiol Immunol. 1990;34:73–76. doi: 10.1111/j.1348-0421.1990.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 32.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and hosts strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]