Abstract
Background.
Allergic diseases are triggered by signaling through the high-affinity IgE receptor, FcεRI. In both mast cells (MCs) and basophils, FcεRI is a tetrameric receptor complex comprising a ligand-binding α subunit (FcεRIα), a tetraspan β subunit (FcεRIβ, MS4A2) responsible for trafficking and signal amplification, and a signal transducing dimer of single transmembrane γ subunits (FcεRIγ). However, FcεRI also exist as presumed trimeric complexes that lack FcεRIβ and are expressed on several cell types outside the MC and basophil lineages. Despite known differences between humans and mice in the presence of the trimeric FcεRI complex, questions remain as to how it traffics and whether it signals in the absence of FcεRIβ. We have previously reported that targeting FcεRIβ with exon-skipping oligonucleotides eliminates IgE-mediated degranulation in mouse MCs, but equivalent targeting in human MCs was not effective at reducing degranulation.
Results.
Here, we report that the FcεRIβ-like protein MS4A6A exists in human mast cells and compensates for FcεRIβ in FcεRI trafficking and signaling. Human MS4A6A promotes surface expression of FcεRI complexes and facilitates degranulation. MS4A6A and FcεRIβ are encoded by highly related genes within the MS4A gene family that cluster within the human gene loci 11q12-q13, a region linked to allergy and asthma susceptibility.
Conclusions.
Our data suggest the presence of either FcεRIβ or MS4A6A is sufficient for degranulation indicating that MS4A6A could be an elusive FcεRIβ-like protein in human MCs that performs compensatory functions in allergic disease.
Graphical Abstract
Background
Allergic disorders including atopic dermatitis, allergic rhinitis, and asthma affect a significant portion of the global population and have been increasing in prevalence, particularly in industrialized countries over recent decades.1 Worldwide, over 300 million patients suffer from asthma with disease severity being associated with impaired quality of life and morbidity.2 In the United States the mean annual healthcare costs for asthma are estimated to be $3,100 per patient,3 yet the current treatments involving daily high-dose inhaled corticosteroids and long acting β2-adrenoceptor agonists still do not control symptoms for a subset of patients, who are hospitalized more frequently and pose a substantial healthcare burden.4
Mast cells (MCs) are myeloid lineage immune cells which reside in all human tissues including the skin, nasal mucosa, and lungs, and are critically involved in the process of allergic inflammation in these organs.5,6 Upon sensitization to an allergen, TH2 cells induce B cells to produce IgE, which binds to FcεRI, the high-affinity receptor for IgE found on MCs and basophils.7 The FcεRI receptor is a tetrameric complex composed of an α subunit which contains the IgE-binding domain, a β subunit (encoded by the MS4A2 gene) and two γ subunits. The β and γ subunits contain immunoreceptor tyrosine-based activation motif (ITAM) domains involved in signal transduction. Crosslinking of FcεRI-bound IgE molecules by allergen promotes aggregation of FcεRI complexes, whereby Lyn kinase is recruited by the β subunit, to potentiate Syk kinase activation and trans-phosphorylation of the γ subunits to amplify signaling.8 This signaling cascade initiates the influx of extracellular Ca2+ culminating in degranulation9 with the release of preformed granules containing histamine, proteoglycans including heparin, proteases such as tryptase and chymase, and cytokines TNF-α and IL-4. Additionally, eicosanoids including prostaglandins and leukotrienes and cytokines such as IL-3, IL-5 and GM-CSF are generated de novo resulting in the recruitment and activation of eosinophils, neutrophils, and macrophages.10
FcεRIβ, is encoded by the MS4A2 gene, which is a member of the membrane spanning 4A (MS4A) family of genes that encode 4-pass transmembrane (TM) proteins expressed in immune cells with similar topology, but low homology, to tetraspanins.11,12 There are at least 16 MS4A genes in humans clustered around chromosome 11q12-q13, a region linked to allergy and asthma susceptibility.13–15 We identified expression of a truncated isoform of FcεRIβ in MCs, that lacks exon 3, which encodes the 1st and 2nd TM regions of FcεRIβ.16,17 This 1st TM region is critical for trafficking the FcεRI complex to the plasma membrane.18 Therefore, we predicted that alternative splicing of FcεRIβ would result in loss of association with the FcεRI complex, which was later confirmed by using a splice switching oligonucleotide (SSO) approach to induce alternative splicing of FcεRIβ precursor mRNA.19 FcεRIβ SSOs target protein-protein interactions resulting in almost complete loss of surface FcεRI expression in mice. However, FcεRIβ SSOs are less effective in human MCs compared to mouse MCs19 suggesting that a more complex mechanism of FcεRI trafficking exists in human MCs. This lack of translation to human MCs highlights the need to better understand FcεRI complex formation and how trafficking and signaling of the complexes are regulated in each species.
In humans and mice, FcεRI are expressed exclusively in MCs and basophils as tetrameric complexes containing the FcεRIβ subunits.20–23 However, in humans FcεRI also exist as trimeric complexes lacking FcεRIβ which are expressed on several cell types,23–31 whereas mice do not express the trimeric form, at least under non-inflammatory conditions.20,32,33 Therefore, FcεRIβ may be less critical for FcεRI trafficking in humans and trimeric FcεRI could account for the lack of translation of FcεRIβ SSOs that we have observed.19 However, data from studies using transgenic mice with humanized FcεRIα, and targeted disruption of FcεRIβ that generates mice expressing only trimeric FcεRI, demonstrate that truly trimeric FcεRI does not elicit a strong degranulation response or substantial Ca2+ signaling.34 Therefore, our findings that human MCs still degranulate strongly and produce a robust Ca2+ response, even after treatment with SSOs that eliminate full length FcεRIβ expression19 is incompatible with a compensatory mechanism for the trimeric FcεRI complex in this context. Thus we propose that signaling must occur through a different mechanism.
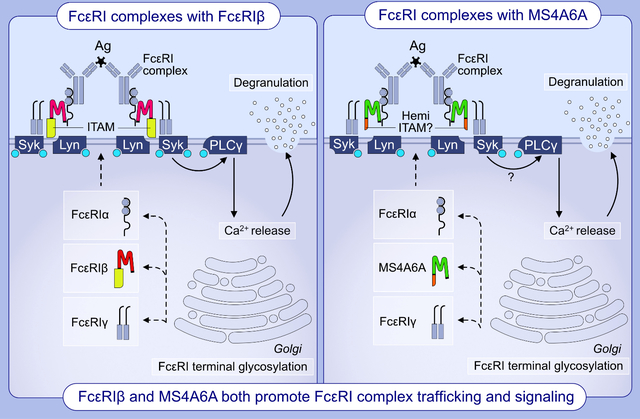
The suggestion that unidentified FcεRIβ-like proteins could exist and function in human FcεRI was proposed as a caveat of seminal experiments characterizing human and mouse FcεRI.35 Given our findings described above with targeted disruption of FcεRIβ, we began to search for potential candidates for an unidentified FcεRIβ-like protein. We have previously reported that human MCs express MS4A4A mRNA and protein, which functions to promote FcεRI and KIT signaling through recruitment of the receptors into lipid raft membrane microdomains.36,37 In theory, MS4A4A could provide such an FcεRIβ-like protein. However, silencing MS4A4A had no significant effect on FcεRI surface expression, and while MS4A4A did promote FcεRI signaling, the data suggest that any interaction between MS4A4A and FcεRI likely occurs at the cell surface37. In this study, we broadened our search to examine other MS4A genes and establish that human MCs also express MS4A6A, which is highly homologous with FcεRIβ. Further, we propose that similarly to the known function of FcεRIβ in trafficking FcεRI to the cell surface, MS4A6A acts to regulate FcεRI trafficking and signaling through an IgE-mediated pathway. Our data suggest that MS4A6A can exhibit redundancy and compensate for FcεRIβ, as it promotes surface expression of FcεRI complexes and triggers signaling, which we predict is mediated through a putative hemi-ITAM domain in the C terminus of the protein (see Graphical Abstract).
Methods
For more detailed methods, see supplemental material.
Cell Cultures
LAD2 human MCs were cultured as described.36 HLMCs were obtained and cultured as described.38,39 Human umbilical cord blood derived mast cells (CBMCs) were cultured as described.37
Transfection of cells
Transfections were performed as previously described.17
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis
MC activation was performed with a dose-response of streptavidin as for degranulation assays and immunoblotting was carried out as described.17,36
Flow cytometry
Surface receptor expression was assessed using flow cytometry as previously described.17
Ca2+ signaling assay
Changes in cytosolic Ca2+ levels were determined as previously described.17,37
Confocal microscopy
Confocal microscopic imaging was performed as previously described.36
Statistical analysis
For comparison of multiple data sets, one- or two-way ANOVA with Bonferroni’s, Sidak’s, or Tukey’s posttest were used, as appropriate, to determine statistical significance. For pairwise data, Student’s t test was used. p ≤ 0.05 was considered statistically significant.
Results
Alternative splicing of FcεRIβ exhibits less profound effects on surface FcεRI complexes in human MCs compared to mouse MCs.
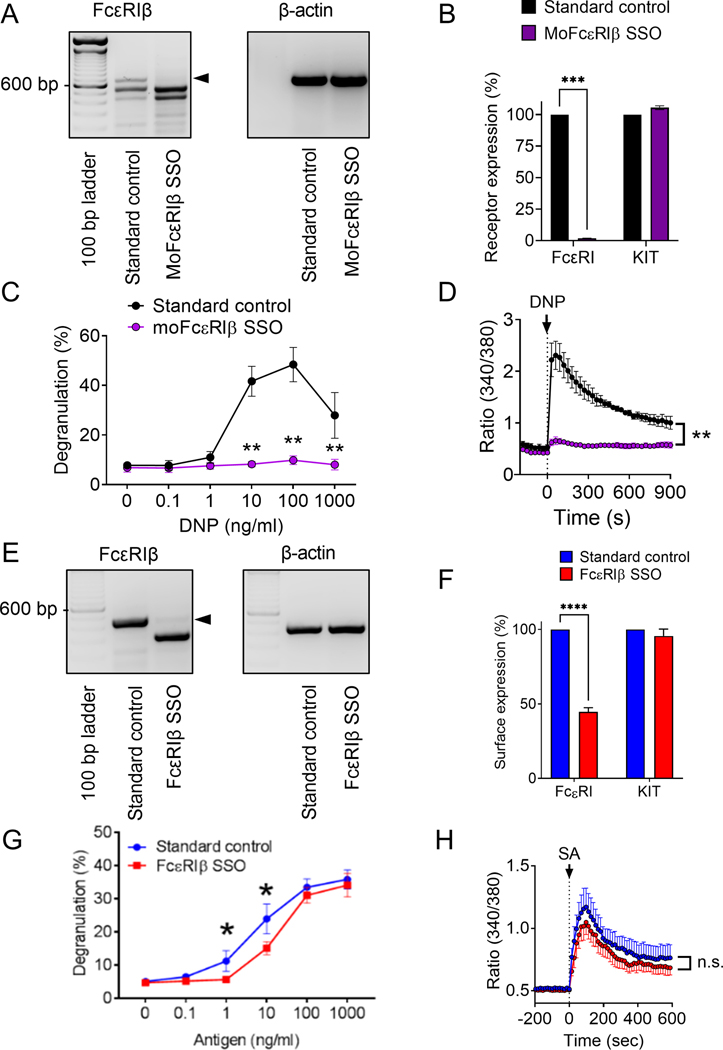
We have identified the expression of a truncated isoform of FcεRIβ in MCs, encoded by mRNA with exon 3 spliced out.16,17 Exon 3 of MS4A2 encodes the 1st and 2nd transmembrane regions of FcεRIβ. Singleton et al. demonstrated that the 1st transmembrane region of FcεRIβ is critical for trafficking the FcεRI complex to the plasma membrane.18 Our prediction was that alternative splicing of FcεRIβ by skipping exon 3 would result in loss of association with the FcεRI complex. Our published studies confirmed this prediction16,17 and we devised SSOs to force alternative FcεRIβ splicing with a view to eliminate FcεRI trafficking to the cell surface. Targeting murine FcεRIβ resulted in efficient exon skipping in mouse bone marrow-derived cultured MCs (BMMCs) (Fig. 1A) and a corresponding elimination of surface FcεRI expression (Fig. 1B).19 FcεRIβ SSO treated BMMCs also became unresponsive to antigen with no evidence of IgE-dependent degranulation (Fig. 1C) or Ca2+ influx (Fig. 1D), but degranulation and Ca2+ influx in response to thapsigargin was unaffected.19
Figure 1: Surface expression of FcεRIα and degranulation of human MCs is not dependent upon the presence of FcεRIβ in the FcεRI receptor complex.
A-D Mouse BMMCs were treated with MoFcεRIβ splice-switching oligonucleotide (SSO). A) Qualitative RT-PCR of FcεRIβ and β-actin. B) Flow cytometric analysis of surface expression of Kit and FcεRIα upon treatment with standard control oligonucleotide (black) and FcεRIβ SSO (purple). C) BMMC degranulation upon stimulation with DNP following treatment with a standard control oligonucleotide and FcεRIβ SSO. D) Ratiometric calcium signaling following stimulant addition at the arrowhead. E-H) LAD2 MCs were treated with FcεRIβ SSO, termed FcεRIβ SSO, at 10 μM to induce skipping of FcεRIβ at exon 3. (E) Qualitative RT-PCR of FcεRIβ showing expression of the full length FcεRIβ variant when treated with a standard control oligonucleotide and the shorter truncated FcεRIβ variant after successful exon skipping; (F) Flow cytometric analysis of surface expression of KIT and FcεRIα upon treatment with standard control oligonucleotide (blue) and FcεRIβ SSO (red); (G) LAD2 cell degranulation upon stimulation with streptavidin (antigen) following treatment with a standard control oligonucleotide and FcεRIβ SSO; (H) Ratiometric calcium signaling following stimulant addition at the arrowhead. Data are the mean ± SEM from three experiments. *P <0.05, **P <0.01, ****P< 0.0001, n.s. = not significant, paired t-test (B & F), or ANOVA with post-test (C, D, G & H).
We have reported that an equivalent SSO that targets the corresponding human exon of FcεRIβ was less effective at reducing degranulation and surface FcεRIα expression, but the reason for the difference in efficacy between species was not clear.19 To rule out a lack efficacy of the human SSO to cause exon skipping, we tested several SSOs that targeted different regions of the exon and identified the most effective exon skipping constructs to study further (Supplemental Fig. S1). Comparable to murine BMMCs (Fig. 1A), FcεRIβ SSOs resulted in efficient exon skipping and formation of the alternatively spliced open reading frame of FcεRIβ that lacked the 135bp exon 3 (Fig. 1E). However, unlike murine MCs where surface FcεRIα was reduced by >98% with FcεRIβ SSO,19 efficient targeting of human FcεRIβ was only partially effective at reducing surface FcεRIα expression with a maximum achievable decrease of 58% in surface receptors with SSO treatment (Fig. 1F). In stark contrast to murine BMMCs, FcεRIβ SSOs gave only a minor attenuation of degranulation in response to IgE crosslinking (Fig. 1G), and the limited inhibitory effect on Ca2+ influx did not reach significance (Fig. 1H). Taken together, these data corroborate our previous studies19 and rule out the potential mechanism of inefficiency of human FcεRIβ SSOs to account for the lack of effect on degranulation and intracellular free Ca2+ when FcεRIβ is targeted in human MCs. These observations suggest a species difference between the dependency for FcεRIβ in FcεRI trafficking and signaling in MCs where even efficient alternative splicing of FcεRIβ is insufficient to fully perturb FcεRI trafficking and has little effect on FcεRI function.
Human MCs express MS4A proteins with high sequence homology to FcεRIβ.
Our data with FcεRIβ SSOs suggests that FcεRIβ is dispensable for FcεRI signaling, and to a lesser degree, trafficking in human MCs. This observation indicates that in human MCs, other compensatory mechanisms may exist. Because the presence of as-yet-unidentified FcεRIβ-like proteins has not been ruled out in prior studies,35 we hypothesized that an FcεRIβ-like protein compensates for FcεRIβ in human MCs and predicted that other members of the gene family that includes FcεRIβ were candidates.
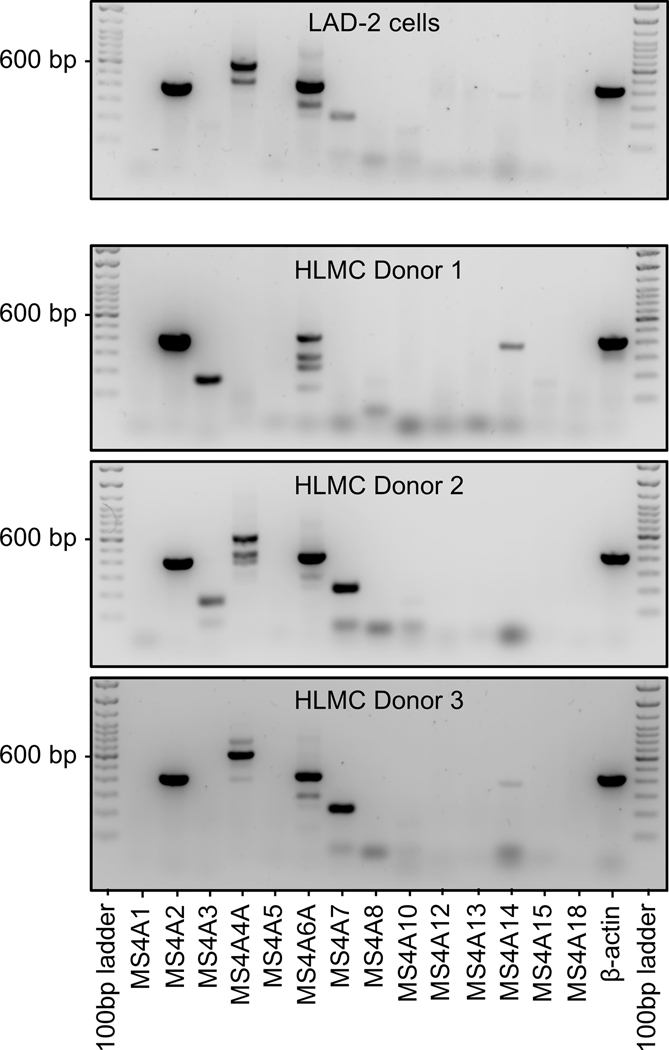
We therefore designed primers to amplify known MS4A family gene members (see Supplemental Methods) and examined expression in human MCs. We utilized the human MC line LAD2, because these cells best represent mature human MCs and have high expression of FcεRI.40 We determined that human LAD2 MCs express MS4A2 (FcεRIβ) and MS4A6A under standard culture conditions (Fig. 2). We also identified weak, but consistent expression of MS4A3 and MS4A7 (Fig. 2). We have reported previously that human LAD2 MCs also express MS4A4A36,37 and we confirm that data in the current study (Fig. 2). RT-PCR for the other known MS4A genes were negative under normal culture conditions and while MS4A3 and MS4A7 were expressed, expression was weak suggesting these genes are unlikely to be the primary candidates for FcεRIβ-like proteins. Examining protein expression for MS4A3 and MS4A7 confirmed the lack of robust expression in LAD-2 cells (Supplemental Fig. S2).
Figure 2: Expression of MS4A gene family members in human mast cells.
RT-PCR of genes in the MS4A family shows human LAD-2 MCs express MS4A2, MS4A4A, MS4A6A and MS4A7 with apparent splice variants of MS4A4A and MS4A6A. Three HLMC examples are shown with varying expression of MS4A family members. HLMC consistently expressed MS4A2, and MS4A6A, with variable expression of MS4A3, MS4A4A, MS4A7 and MS4A14.
In order to confirm expression profiles for MS4A gene proteins in primary human MCs, we examined the expression in human lung MCs (HLMCs). Expression of the comparable MS4A proteins was identified in HLMCs (Fig. 2). However, in contrast to LAD-2 cells, expression of MS4A3 and MS4A7 were strong in some donors and not detected in others (Fig. 2). There was also some variability in expression of MS4A4A in HLMCs (Fig. 2). HLMC consistently expressed FcεRIβ with 11/11 donors expressing FcεRIβ. MS4A6A expression was expressed by 10/11 donors. MS4A4A was expressed in 8/11 donors. MS4A3 was expressed in 2/3 donors and MS4A7 was expressed in 9/11 donors. Protein for full length MS4A3 was confirmed in HLMCs, but full length MS4A7 protein was not confirmed (Supplemental Fig. S2). These data suggest that MS4A2 (FcεRIβ), MS4A4A and MS4A6A are expressed at similar levels and that MS4A4A and MS4A6A proteins are the most likely candidates for an FcεRIβ-like protein in human MCs, because while HLMCs express other MS4A gene variants, LAD-2 cells that signal robustly through the receptor do not express these variants.
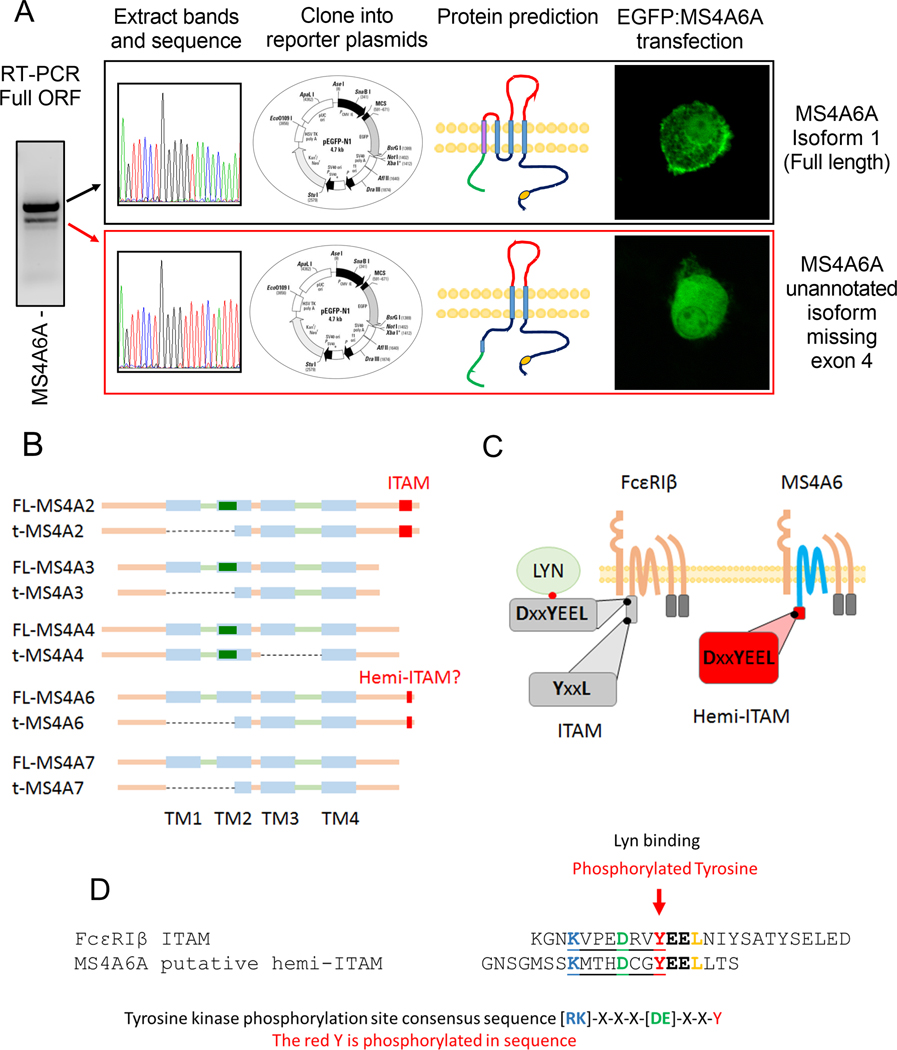
We reported previously that MS4A4A protein functions to potentiate FcεRI signaling, likely through recruitment of the FcεRI complex into lipid rafts following IgE crosslinking.37 This observation demonstrates that MS4A gene family members, other than FcεRIβ, can interact with FcεRI complexes. However, in that study, we did not identify a role for MS4A4A in trafficking of FcεRI to the plasma membrane, or stabilizing the receptor complex, and FcεRI surface expression was not affected by MS4A4A expression.37 Therefore, while MS4A4A protein may interact and regulate FcεRI expression, it is unlikely to perform compensatory roles for FcεRIβ evident in Fig. 1. We therefore progressed to examine MS4A6A. When performing RT-PCR, a double band was evident for MS4A6A (Fig. 3A), which was reminiscent of the bands we have previously reported, that identified the alternative truncation of MS4A2 resulting in truncated FcεRIβ that is incapable of interacting with FcεRI.16,17 We therefore amplified the full open reading frame of MS4A6A (Fig. 3A) extracted both bands, sequenced them and cloned them into pEGFP-N1 expression vectors (Fig. 3A). Sequencing data identified that the two bands of MS4A6A aligned exactly to the FcεRIβ transcripts and the predicted proteins we reported previously (Fig. 3B).16,17 Multiple sequence alignments for MS4A6A and MS4A2 mRNA variants demonstrated that MS4A6A exon 4 directly aligns with MS4A2 exon 3 and translation analysis predicts that the respective exons will encode the 1st and 2nd transmembrane regions for each protein (Fig. 3B, and supplemental Fig. S3). EGFP fusion constructs for full length FcεRIβ and MS4A6A were generated and transfected into LAD2 MCs to examine subcellular distribution of the proteins. We have identified that full length FcεRIβ and truncated FcεRIβ traffic to distinct compartments of the cell with full length FcεRIβ evident in the plasma membrane and truncated FcεRIβ was dispersed in the cytoplasm and in juxtanuclear compartments.16,17 Given the similarity between FcεRIβ variants and MS4A6A variants, we expect a similar localization to FcεRIβ variants for the corresponding MS4A6A variants. Indeed, confocal microscopy demonstrated that full length MS4A6A was evident in the plasma membrane, while truncated MS4A6A was diffusely expressed throughout the cell (Fig. 3A), closely matching FcεRIβ variants.16,17 Furthermore, sequencing of the other MS4A genes expressed in MCs revealed that this truncated region excluding the first two transmembrane regions was conserved among the expressed MS4A family members, with the exception of MS4A4A that contained an exon truncation downstream, corresponding to the 3rd transmembrane region (Fig. 3B) (Supplemental Fig. S4).
Figure 3: MS4A6A is expressed in human MCs and exhibits a similar truncation and putative signaling domain as FcεRIβ.
Amplification of the full open reading frame of the MS4A6A gene for cloning into expression plasmids confirms the presence of a truncated variant; (A) Sequencing and cloning of the two MS4A6A variants into pEGFP-N1 expression vectors provided alignment with the known isoform 1, a 4 pass transmembrane protein evident on the surface within the plasma membrane and an unannotated truncation without the first two transmembrane domains lacking surface expression; (B) Structural comparison of the full length (L) and truncated (S) variants of the MS4A gene family expressed in human mast cells. Potential caveolin-1 binding site depicted as green rectangle; (C) Graphical representation of the ITAM signaling domain of FcεRIβ indicating the tyrosine phosphorylation site where Lyn binds and the putative hemi-ITAM of MS4A6A both located on the Carboxyl-termini; (D) Peptide sequence comparison of the FcεRIβ ITAM and the putative hemi-ITAM of MS4A6A showing consensus sequences for a tyrosine kinase phosphorylation site preceding the Lyn binding site in FcεRIβ and a comparable motif in the putative MS4A6A hemi-ITAM.
Of the MS4A proteins expressed, we identified that MS4A2, MS4A3, and MS4A4A contained a potential caveolin-1 binding site (Fig. 3B, green box), which may indicate their signaling potential within lipid rafts as we predict for MS4A4A.37 FcεRIβ is the only MS4A gene to contain an immunoreceptor tyrosine-based activation motif (ITAM) that is critical for FcεRIβ function.41 However, we identified that MS4A6A contains a putative signaling motif similar to the ITAM in FcεRIβ (Fig. 3C & D). While this domain in MS4A6A does not conform to an ITAM consensus sequence, it does conform to a hemi-ITAM consensus sequence (Y-x-x-L) immediately following a tyrosine kinase phosphorylation site consensus sequence (R/K-x-x-x-D/E-x-x-Y) that would phosphorylate the tyrosine residue within both the FcεRIβ ITAM and MS4A6A hemi-ITAM (Fig. 3D). This region in the C terminal tail of MS4A6A also has very high sequence homology to the Lyn binding site within the C terminal FcεRIβ ITAM (Fig. 3D). Hemi-ITAM signaling is less defined than ITAM signaling. However, the C-type lectin-like receptor 2 (CLEC-2) expressed in platelets contains a hemi-ITAM that recruits Src family kinases and signals through Syk in a PI3K-dependent manner.42,43 Canonical ITAM signaling through FcεRIγ subunits is triggered by Src family kinases that trans-phosphorylate FcεRIγ ITAMs to recruit Syk kinase. In FcεRI signaling, this phosphorylation of FcεRIγ occurs mainly by Lyn kinase recruited to the non-canonical ITAM of FcεRIβ.44 Our prediction analysis proposes the existence of a new pathway that could signal through MS4A6A hemi-ITAM in a similar manner to FcεRIβ.
MS4A6A expression is upregulated by IgE crosslinking and SCF stimulation in human lung mast cells
We also examined expression of the relevant MS4A genes in primary ex vivo human lung MCs (HLMCs) and found that they also expressed MS4A2, MS4A4A, MS4A6A and MS4A7 genes (Fig. 2) (Supplemental Fig. S5A). All donors examined expressed the full length mRNA transcripts of each gene, but expression of truncated variants of the genes, which have the conserved truncation corresponding to the exon encoding the 1st and 2nd transmembrane domains (Fig. 3B), was variable between donors, at least in unstimulated cells. With the focus on MS4A6A and the potential for redundant functions between FcεRIβ and MS4A6A proteins, we examined expression levels of MS4A2 and MS4A6A during MC stimulation with FcεRI loading, crosslinking and activation, in the presence and absence of the MC growth factor SCF, which is known to potentiate FcεRI signaling and MC activation. Interestingly, we found that MS4A2 expression was not significantly altered in any of the conditions, but MS4A6A expression was upregulated when MCs were co-stimulated with SCF and IgE crosslinking and there was a similar trend with IgE loading when SCF was present, although the latter did not reach significance (Supplemental Fig. 5B). Taken together, the expression studies with FcεRI stimuli and SCF suggest that MS4A6A is regulated to a greater degree than FcεRIβ in HLMCs in response to factors that are relevant to allergic inflammation, where MCs may be exposed to persistent allergens and where SCF is present at higher concentrations.45,46
MS4A6A promotes surface FcεRI expression and IgE-dependent degranulation.
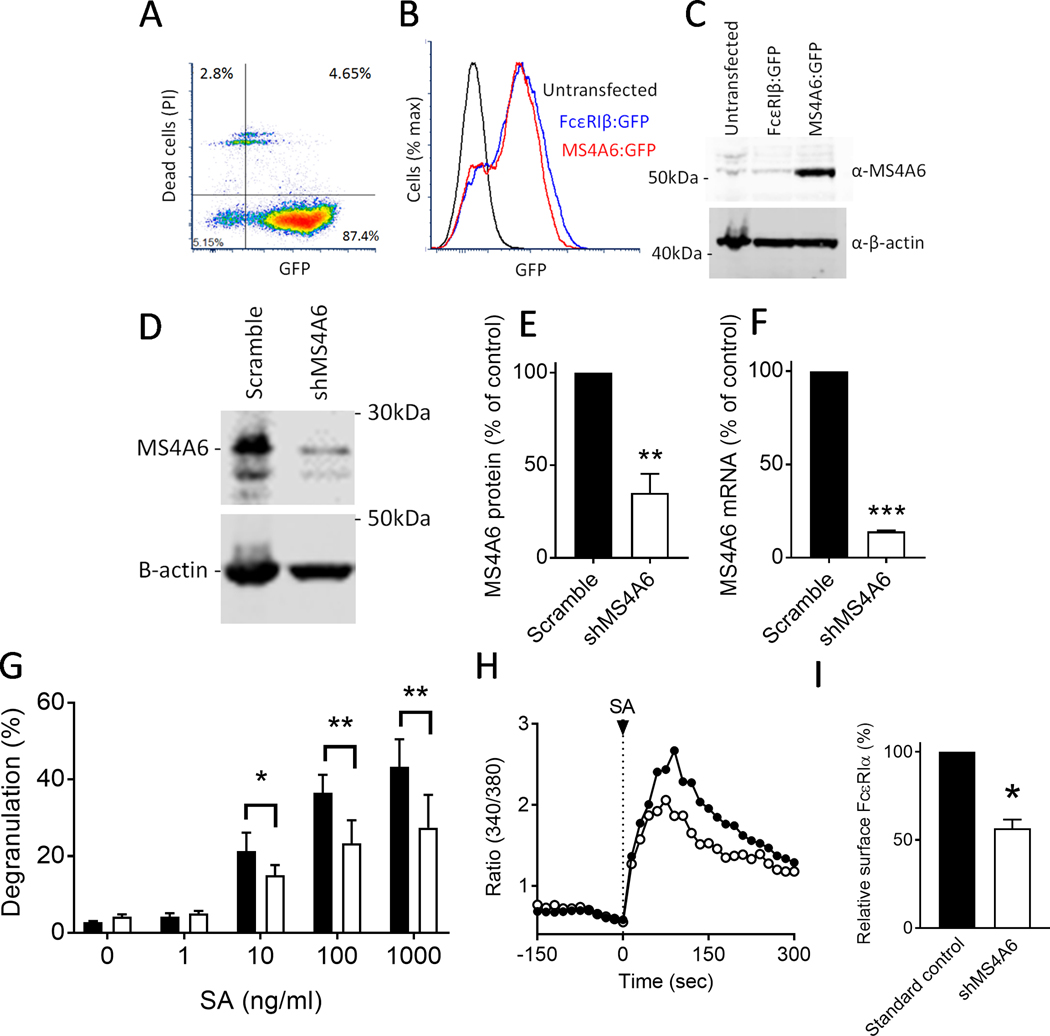
Due to the highly homologous sequence of MS4A6A and FcεRIβ and the similar localization within the cell for the alternative isoforms, we predicted that MS4A6A could traffic FcεRI to the plasma membrane and act as an FcεRIβ-like protein, exhibiting redundancy between the two proteins. We began to test this hypothesis by validating an antibody for MS4A6A and utilizing gene targeting using shRNA and lentiviral delivery as we have performed for other MS4A proteins (2, 26). We performed transfections with the EGFP fusion constructs for full length FcεRIβ and MS4A6A, that we generated from cloning in HLMCs (Fig. 3), into LAD2 cells and assessed expression with flow cytometry (Fig. 4A & B). Transfection efficiency with EGFP constructs was >90% with >85% of transfected cells remaining viable (Fig. 4A). EGFP expression for MS4A6A and FcεRIβ were also comparable in terms of efficiency and level of expression (Fig. 4B). Following transfection, LAD2 cells were lysed and antibodies for MS4A6A were tested against the transfected lysates. We identified an antibody that recognized MS4A6A, and not the highly homologous FcεRIβ (Fig. 4C).
Figure 4: Knockdown of MS4A6A partially reduces degranulation and surface expression of FcεRIα.
A-D LAD2 cells were used to validate an MS4A6A antibody to determine transfection and knockdown efficiency. (A) LAD2 MCs transfected with an EGFP-MS4A6A fusion construct confirms high viability of GFP-positive cells by flow cytometry; (B) Transfection of EGFP-FcεRIβ and EGFP-MS4A6A into LAD2 MCs shows high GFP expression and transfection efficiency, which was comparable between the two constructs when compared to untransfected cells by flow cytometry; (C) Validation of an MS4A6A antibody using Western blots of cell lysates from transfected LAD2 MCs shows selectivity of the Ab for cells transfected with MS4A6A-GFP over FcεRIβ-GFP. The predicted weight of MS4A6A is 26 kDa, and GFP is 25 kDa. (D) Western blotting with the validated antibody confirms lentivirus knockdown of natively expressed MS4A6A with shRNA (predicted weight is 26 kDa); (E) Lentivirus knockdown with shMS4A6A significantly reduces protein expression by >60% compared to scramble; (F) qRT-PCR shows mRNA expression of MS4A6A is reduced by >80% after lentivirus knockdown with shMS4A6A compared to scramble; (G) Streptavidin-induced degranulation of LAD2 MCs treated with biotinylated IgE is reduced upon knockdown of MS4A6A (white) compared to scramble control (black); (H) Ratiometric calcium signaling after streptavidin stimulation at arrow shows a reduced Ca2+ response in MC with shMS4A6A knockdown (white) compared to scramble control (black). (I) Flow cytometric analysis shows surface expression of FcεRIα is significantly reduced upon shMS4A6A knockdown (white) compared to scramble control (black). Data are the mean±SEM from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, paired t-test (E), (F) & (I), or ANOVA with post-test (G).
We used this antibody to validate MS4A6A knockdown using lentivirus shRNA against MS4A6A and established that knockdown of MS4A6A at the protein level was >60% (Fig. 4D & E) and >80% at the mRNA level (Fig. 4F). We confirmed that knockdown of MS4A6A does not result in reduced mRNA expression for FcεRIβ (Supplemental Fig. S6). Having established knockdown, we then examined degranulation and found that MS4A6A knockdown modestly reduced IgE-dependent degranulation (Fig. 4G) and Ca2+ influx (Fig. 4H) in LAD2 MCs. We also assessed surface FcεRIα as a measure of trafficking and found that surface FcεRIα expression was reduced by approximately 40% with knockdown of MS4A6A (Fig. 4I). Taken together, these data suggest that MS4A6A functions, to some degree, in FcεRI trafficking and signaling and provides a potential candidate for an FcεRIβ-like protein.
Full-length MS4A6A promotes FcεRI trafficking and exhibits redundancy with FcεRIβ.
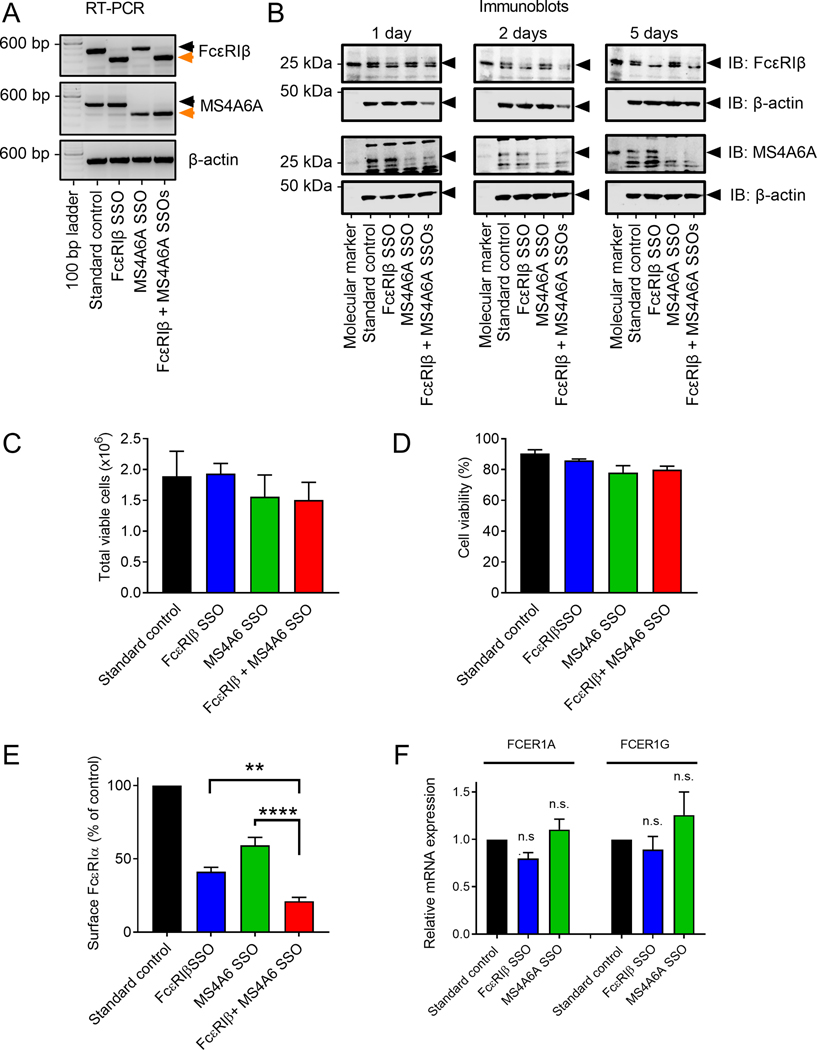
The highly conserved splicing of the 1st and 2nd transmembrane regions of FcεRIβ, encoded by exon 3 of FcεRIβ, and the corresponding exon 4 of MS4A6A, may be critical for the role of MS4A6A in FcεRI function and perhaps the first transmembrane region of both proteins can bind to the FcεRI complex. We therefore used the SSO method that we used for exon 3 of FcεRIβ,19 to specifically remove exon 4 encoding the 1st and 2nd transmembrane regions of MS4A6A (Fig. 5A). Despite the high sequence homology between FcεRIβ and MS4A6A, the splicing target site sequences were distinct and SSOs could be designed to specifically target each precursor mRNA. Highly efficient and specific exon skipping for each transcript was achieved without any off-target effects on the other mRNA transcripts (Fig. 5A). In addition, a combination of both FcεRIβ and MS4A6A constructs targeted both transcripts with equal efficacy (Fig. 5A). We confirmed that the SSOs targeting the pre-mRNA resulted in loss of full length variants of FcεRIβ and MS4A6A at the protein level (Fig. 5B). The resulting reduction in protein for both FcεRIβ and MS4A6A was evident by 24 hours, but efficacy increased at 2 days and no full length protein was visible after 5 days (Fig. 5B). Neither FcεRIβ, nor MS4A6A SSOs alone, or in combination significantly affected LAD2 cell proliferation (Fig. 5C) or survival (Fig. 5D) over the course of the experiments (5 days).
Figure 5: FcεRIβ and MS4A6A exhibit redundancy in FcεRIα surface expression.
A-F Following the same approach as with FcεRIβ, splice switching oligonucleotides (SSO) were successfully employed to eliminate the expression of the full length variant of MS4A6A and splicing was switched to the truncated form. Exon skipping was rapidly and selectively achieved in both LAD2 MCs and primary human MCs derived from umbilical cord blood (CMBCs) and lung (HLMCs). (A) Selective and efficient exon skipping of both FcεRIβ and MS4A6A shown by RT-PCR of LAD2 MCs; (B) Western blot data demonstrating that full length FcεRIβ and MS4A6A proteins are reduced after exon skipping (arrows) after 24 hours (left panels), 48 hours (middle panels) and 5 days (right panels). B actin was used as a loading control; (C) Total number of viable LAD2 cells after treatment with a standard control, FcεRIβ and MS4A6A SSOs for exon skipping; (D) The percentage of viable cells after SSO treatment shows little effect; (E) Flow cytometric analysis of surface expression of FcεRIα in LAD2 cells treated with SSOs for individual and combined exon skipping; (F) QRT-PCR of FcεRI α and γ subunits expression in LAD2 cells treated with FcεRIβ or MS4A6 SSOs. Black bars represent standard control oligonucleotide. Blue bars represent FcεRIβ SSO. Green bars represent MS4A6A SSO. Red bars represent FcεRIβ + MS4A6A SSOs. Data are the mean±SEM from at least three independent experiments. **P < 0.01, **** P < 0.0001, n.s. = not significant, ANOVA with post-test.
Analysis of surface FcεRIα expression with SSOs targeting either FcεRIβ or MS4A6A individually, or in combination, reduced surface FcεRIα expression. SSOs targeting FcεRIβ reduced FcεRI surface expression by ~60%, and MS4A6A SSOs reduced surface FcεRI expression by ~40% (Fig. 5E), which is in agreement with the knockdown data for MS4A6A (Fig. 4I). Combined FcεRIβ and MS4A6A SSOs had an additive effect reducing FcεRI surface expression by >80% (Fig. 5E). Quantitative RT-PCR for the FcεRI subunits, FcεRIα and FcεRIγ, revealed that mRNA for both subunits were not reduced (Fig. 5F) suggesting that the reduction in surface expression of FcεRIα was due to altered trafficking rather than downregulation of FcεRI subunit gene expression.
Both full-length MS4A6A and FcεRIβ promote FcεRI function and exhibit redundancy.
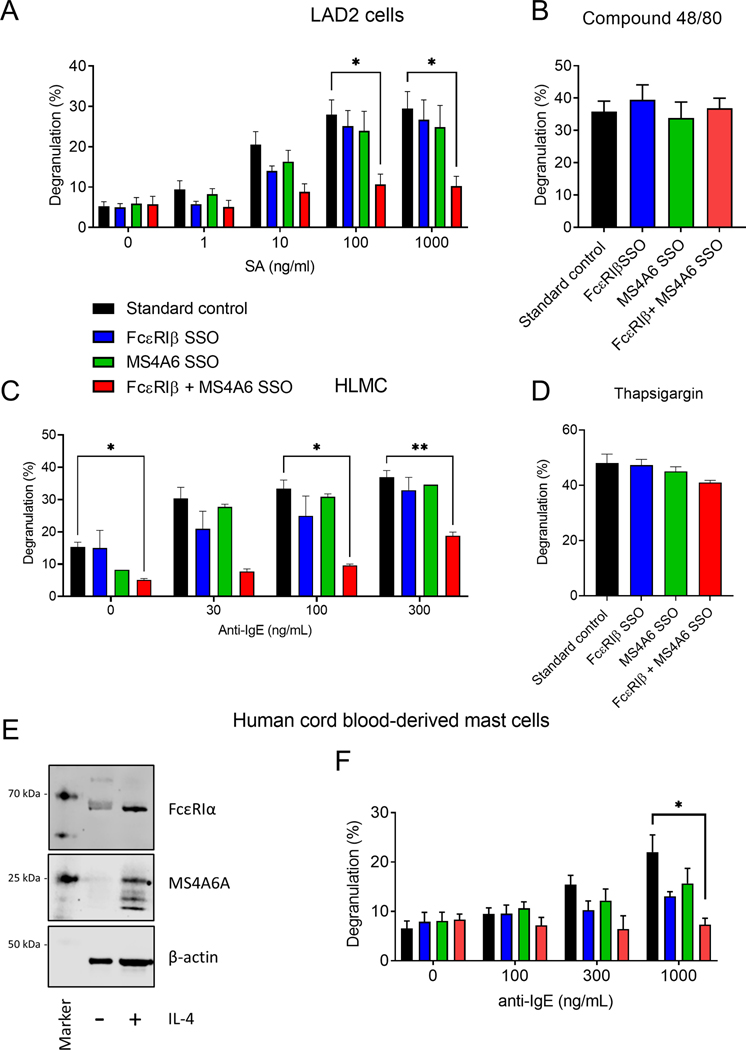
We next assessed degranulation in response to IgE-crosslinking with SSOs for FcεRIβ or MS4A6A alone and in combination. Exon skipping FcεRIβ or MS4A6A alone had only a minor effect on LAD2 MC degranulation, which did not reach significance in these experiments (Fig. 6A). However, a combination of FcεRIβ and MS4A6A SSOs markedly inhibited IgE-dependent degranulation (Fig. 6A), while no condition affected compound 48/80 induced degranulation suggesting specificity to FcεRI activation (Fig. 6B). We next confirmed the compensatory role for MS4A6A on FcεRIβ function using primary HLMCs, where comparable results in IgE-dependent degranulation were seen (Fig. 6C & D). Finally, we also confirmed a conserved function for MS4A6A in primary CBMCs. We have shown previously that IL-4 stimulation of CBMCs is required to upregulate surface FcεRIα expression and enable degranulation.37 We therefore examined MS4A6A and FcεRIα expression with and without IL-4 stimulation for 7 days and found that MS4A6A expression could be induced in human CMBCs upon exposure to IL-4 and this was associated with an upregulation of FcεRIα expression (Fig. 6E). In addition, the function of MS4A6A in degranulation was conserved in CBMCs (Fig. 6F) and across all mast cell types tested strongly suggesting biological redundancy between FcεRIβ and MS4A6A.
Figure 6: FcεRIβ and MS4A6A exhibit redundancy in IgE-dependent human mast cell degranulation.
(A) Dose-responsive degranulation of LAD2 cells stimulated with streptavidin following treatment with standard control oligonucleotide (black), FcεRIβ SSO (blue), MS4A6A SSO (green), and combined FcεRIβ + MS4A6A SSOs; (B) LAD2 cells treated with SSOs degranulate in response to stimulation with Compound 48/80 through an IgE-independent mechanism; (C) Dose-responsive degranulation of HLMCs stimulated with α-IgE following treatment with standard control oligonucleotide (black), FcεRIβ SSO (blue), MS4A6A SSO (green), and combined FcεRIβ + MS4A6A SSOs; (D) HLMC cells treated with SSOs degranulate in response to stimulation with thapsigargin through an IgE-independent mechanism. (E) Western blot analysis of MS4A6A and FcεRIα expression with and without IL-4 treatment for 7 days. Β-actin was used as a loading control. (F) Dose-responsive degranulation of CBMCs stimulated with α-IgE following treatment with standard control oligonucleotide (black), FcεRIβ SSO (blue), MS4A6A SSO (green), and combined FcεRIβ + MS4A6A SSOs (red). Data are the mean±SEM from at least three independent experiments. *P < 0.05, **P < 0.01, ANOVA with post-test.
MS4A6A and FcεRIβ trigger distinct downstream Syk signaling.
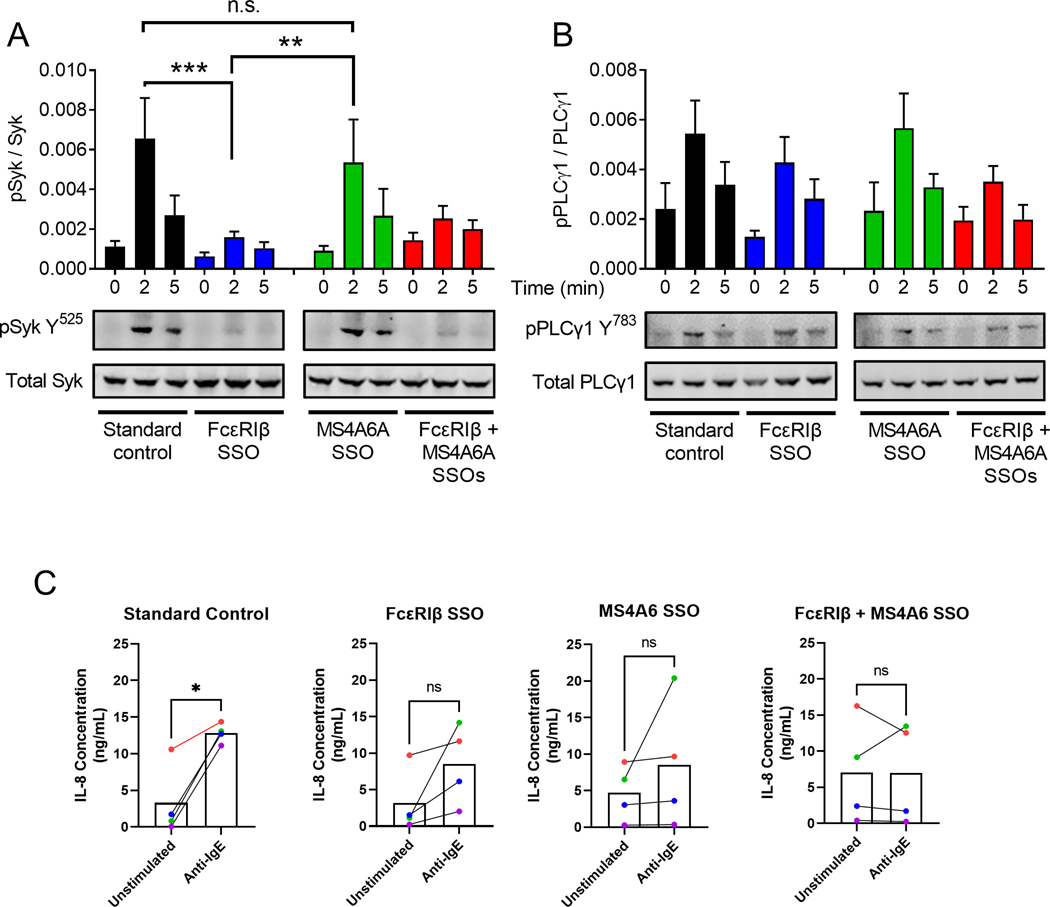
We next assessed whether FcεRIβ and MS4A6A exhibited complete redundancy and resulted in comparable signaling proximally to FcεRI. Crosslinking FcεRI promotes Syk kinase activation and trans-phosphorylation of the γ subunits to amplify signaling.8 We, therefore, examined the activating phosphorylation of Syk kinase at Tyrosine 525 that drives downstream signaling. Interestingly, we found that phosphorylation of this tyrosine residue required FcεRIβ and not MS4A6A (Fig. 7A). However, despite a lack of phosphorylation at Tyrosine 525 when FcεRIβ was not expressed, the downstream signal of the activating tyrosine residue 783 of PLCγ1 remained intact (Fig. 7B). These data suggest that while FcεRIβ drives phosphorylation of Tyrosine 525 of Syk and MS4A6A does not, both proteins are able to promote activation of PLCγ1 and thus drive downstream Ca2+ flux and degranulation. Detailed study of the signaling downstream of FcεRIβ and MS4A6A is required to establish exactly how each protein participates in signaling and examination of other phosphorylation events in Syk kinase are needed. However, these initial studies suggest that each protein promotes a distinct downstream phosphorylation response and thus could drive differential functional outcomes downstream of IgE signals.
Figure 7: FcεRIβ and MS4A6A promote differential phosphorylation of Syk, but not PLCγ1.
(A) Western blot analysis and quantification of phosphorylated Syk (Y525) corrected for total Syk using dual colour analysis with Licor Odyssey imaging. (B) Western blot analysis and quantification of phosphorylated PLCγ1 (Y783) corrected for total PLCγ1. (A-B) Black bars represent standard control oligonucleotide. Blue bars represent FcεRIβ SSO. Green bars represent MS4A6A SSO. Red bars represent FcεRIβ + MS4A6A SSOs. (C) IL-8 ELISA data from HLMC challenged with anti-IgE (1000 ng/mL). Each colour represents a different HLMC donor. The same donors were used for each SSO condition. Data are the mean±SEM from at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ANOVA with post-test (A, B) or paired two tailed t-test (C).
Both MS4A6A and FcεRIβ proteins contribute to IL-8 production in HLMCs.
Given the differential effects of FcεRIβ and MS4A6A on Syk phosphorylation, we next examined whether the proteins differentially regulated cytokine release in HLMCs. We challenged four HLMC donors with or without anti-IgE (1000 ng/mL) and measure release of IL-8 into the supernatant. Each donor was paired across conditions and color coded in the graphs (Fig. 7C). With the standard control treatment, IL-8 release was significantly induced, but treatment with either FcεRIβ or MS4A6A SSOs reduced IL-8 release, which was more evident with double SSO treatment (Fig. 7C). Therefore, these data suggest that IL-8 release follows a similar pattern to that of degranulation.
Conclusions
Taken together, our data suggest that FcεRIβ and MS4A6A exhibit at least partial redundancy in both trafficking and signaling of FcεRI in human MCs. Positive evolutionary selective pressure could explain this redundancy given FcεRI is critical for the immunological protection against parasitic and other infections. However, because surface FcεRI expression is completely abolished in mouse MCs treated with FcεRIβ SSOs, the presence and potential for redundancy of an orthologous Ms4a6 protein in the murine species remains to be investigated. Although further studies are needed to elucidate the IgE-dependent signaling pathways triggered by MS4A6A, MS4A6A contains a potential hemi-ITAM making it unique within the MS4A family and we predict that the hemi-ITAM of MS4A6A confers signaling potential. Therefore, altered ratios of FcεRIβ and MS4A6A within FcεRI complexes could act to fine-tune MC responsiveness in allergic individuals and may trigger differential downstream pathways. Additionally, aberrant expression of one or both proteins might also contribute to the widely varied allergic phenotypes seen in humans, as well as explain discrepancies in the response to common treatments. Further, while LAD-2 cells did not express high levels of other MS4A proteins, enabling us to elucidate the role of MS4A6A in these cells, other MS4A family proteins are expressed in HLMCs and we have not ruled out roles for these proteins in FcεRI function. It is exciting to postulate that the presence of different MS4A proteins in FcεRI complexes could contribute to heterogeneous FcεRI complexes that could regulate differential downstream effects of IgE crosslinking. However, further studies are required to establish any roles for other MS4A family proteins in FcεRI trafficking and signaling and these studies must be tightly controlled with expression analyses in each donor to establish which proteins are expressed.
Of particular interest, with regard to MS4A6A and a potential role in allergy and asthma, the MS4A gene family in humans are clustered around chromosome 11q12-q13, a region previously linked to allergy and asthma susceptibility.13–15 This linkage gained interest and, due to FcεRIβ function in FcεRI, MS4A2 was considered as a viable candidate gene for an association with asthma. However, the clinical benefits of targeting FcεRIβ are not clear and the association of MS4A2 polymorphisms with allergy and asthma is not consistent.47 In addition, attempts to associate polymorphisms in MS4A2 with functional consequences using transfection of cDNA failed to alter FcεRIβ protein function.48,49 While our current study does not help to elucidate roles for the MS4A2 polymorphisms in FcεRI function, it does highlight that the linkage of 11q12-q13 gene loci with asthma and allergy could involve polymorphisms in other highly related genes that could result in complex phenotypes, thus highlighting the need for other candidate genes to be explored.
The identification of the MS4A6A protein and its similar role to FcεRIβ provides an additional, previously unexplored therapeutic target for allergic diseases such as asthma and atopy. While MS4A6A expression in highly allergic as compared to non-allergic individuals remains to be investigated, aberrant expression could serve as further proof of a compelling therapeutic target. Antisense oligonucleotide therapy is an emerging treatment modality that has already been employed for a variety of ophthalmic,50 respiratory,51 and neurodegenerative52 conditions. As such, the success of our in vitro utilization of SSO technology to significantly reduce surface FcεRI expression and MC degranulation indicates its potential for translation to in vivo treatment of allergic diseases. However, it is also important to note that targeting MS4A6A therapeutically poses a more difficult target than FcεRIβ, because very little is known about MS4A6A function and linkage of MS4A6A with Alzheimer’s disease indicates that MS4A6A may be involved in pathways that affect cognitive function.53–56 Therefore, further detailed study of MS4A6A and the pathways that are regulated by the protein are critical to further understand the biology of MS4A6A and how that biology relates to immunology and neurobiology.
In conclusion, we have identified a previously uncharacterized member of the MS4A family, MS4A6A that plays an analogous role to FcεRIβ in the overall function of human MCs. The gene encoding MS4A6A is within the same gene family cluster as that of FcεRIβ, and both are located in a region previously linked to allergy and asthma susceptibility.13
Supplementary Material
We identify that MS4A6A is expressed in human mast cells and has high sequence homology to FcεRIβ.
We show that MS4A6A contains a putative hemi-ITAM that may function similarly to the FcεRIβ ITAM.
We demonstrate that MS4A6A and FcεRIβ perform at least partially redundant roles in FcεRI complex trafficking and function.
Acknowledgements
We thank Hailey Jones and Sydney Joyner for technical assistance.
Funding:
Research was supported by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Award Number R01AI143985, Department of Molecular Biomedical Sciences, College of Veterinary Medicine, start-up funds, Institutional National Research Service Award from the Office of the Director of National Institutes of Health, NIH T32OD011130.
Abbreviations:
- Ag
antigen
- Ca2+
calcium ion
- FcεRI
high-affinity IgE receptor
- ITAM
immunoreceptor tyrosine-based activation motif
- Lyn
Src family tyrosine kinase
- MS4A6A
membrane spanning 4-domains A6A
- PLC
phospholipase C
- Syk
spleen associated tyrosine kinase
Footnotes
Conflict of interest disclosure: G.C. has filed a patent application related to the research reported in this study. An exclusive licensing agreement has been granted to Hoth Therapeutics for this technology. G.C. has research support from Hoth Therapeutics for a project related to the research reported in this publication and also serves on their Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by NC State University in accordance with its policy on objectivity in research. The remaining authors declare no conflicts of interest.
References
- 1.Brozek G, Lawson J, Szumilas D, Zejda J. Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: Four repeated surveys from 1993–2014. Respir Med. 2015;109(8):982–990. doi: 10.1016/j.rmed.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 2.Canonica GW, Colombo GL, Rogliani P, et al. Omalizumab for Severe Allergic Asthma Treatment in Italy: A Cost-Effectiveness Analysis from PROXIMA Study. Risk Manag Healthc Policy. 2020;13:43–53. doi: 10.2147/RMHP.S211321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3(1):1. doi: 10.1186/s40733-016-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zervas E, Samitas K, Papaioannou AI, Bakakos P, Loukides S, Gaga M. An algorithmic approach for the treatment of severe uncontrolled asthma. ERJ Open Res. 2018;4(1):00125–02017. doi: 10.1183/23120541.00125-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–454. doi: 10.1038/nature07204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin K The role of mast cells in allergic inflammation. Respir Med. 2012;106(1):9–14. doi: 10.1016/j.rmed.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 7.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. doi:http://dx.doi.org.prox.lib.ncsu.edu/10.1038/nm.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambayashi T, Koretzky GA. Proximal signaling events in FcɛRI-mediated mast cell activation. J Allergy Clin Immunol. 2007;119(3):544–552. doi: 10.1016/j.jaci.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 9.Ashmole I, Duffy SM, Leyland ML, Morrison VS, Begg M, Bradding P. CRACM/Orai ion channel expression and function in human lung mast cells. J Allergy Clin Immunol. 2012;129(6):1628–1635.e2. doi: 10.1016/j.jaci.2012.01.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: A Multi-Functional Master Cell. Front Immunol. 2015;6:620. doi: 10.3389/fimmu.2015.00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y, Tedder TF. Identification of a CD20-, FcϵRIβ-, and HTm4-Related Gene Family: Sixteen New MS4A Family Members Expressed in Human and Mouse. Genomics. 2001;72(2):119–127. doi: 10.1006/geno.2000.6472 [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Buckley TR, Tu L, Langdon SD, Tedder TF. Structural organization of the human MS4A gene cluster on Chromosome 11q12. Immunogenetics. 2001;53(5):357–368. doi: 10.1007/s002510100339 [DOI] [PubMed] [Google Scholar]
- 13.Sandford AJ, Shirakawa T, Moffat MF, et al. Localisation of atopy and β subunit of high-affinity IgE receptor (Fc∈RI) on chromosome 11q. The Lancet. 1993;341(8841):332–334. doi: 10.1016/0140-6736(93)90136-5 [DOI] [PubMed] [Google Scholar]
- 14.Stafford AN, Rider SH, Hopkin JM, Cookson WO, Monaco AP. A 2.8 Mb YAC contig in 11q12 – q13 localizes candidate genes for atopy: FcɛRIβ and CD20. Hum Mol Genet. 1994;3(5):779–785. doi: 10.1093/hmg/3.5.779 [DOI] [PubMed] [Google Scholar]
- 15.Lympany P, Welsh KI, Cochrane GM, Kemeny DM, Lee TH. Genetic analysis of the linkage between chromosome 11q and atopy. Clin Exp Allergy. 1992;22(12):1085–1092. doi: 10.1111/j.1365-2222.1992.tb00134.x [DOI] [PubMed] [Google Scholar]
- 16.Cruse G, Kaur D, Leyland M, Bradding P. A novel FcεRIβ-chain truncation regulates human mast cell proliferation and survival. FASEB J. 2010;24(10):4047–4057. doi: 10.1096/fj.10-158378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruse G, Beaven MA, Ashmole I, Bradding P, Gilfillan AM, Metcalfe DD. A truncated splice-variant of the FcεRIβ receptor subunit is critical for microtubule formation and degranulation in mast cells. Immunity. 2013;38(5):906–917. doi: 10.1016/j.immuni.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singleton TE, Platzer B, Dehlink E, Fiebiger E. The first transmembrane region of the β-chain stabilizes the tetrameric FcɛRI complex. Mol Immunol. 2009;46(11):2333–2339. doi: 10.1016/j.molimm.2009.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruse G, Yin Y, Fukuyama T, et al. Exon skipping of FcεRIβ eliminates expression of the high-affinity IgE receptor in mast cells with therapeutic potential for allergy. Proc Natl Acad Sci. 2016;113(49):14115–14120. doi: 10.1073/pnas.1608520113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931 [DOI] [PubMed] [Google Scholar]
- 21.Küster H, Zhang L, Brini AT, MacGlashan DW, Kinet JP. The gene and cDNA for the human high affinity immunoglobulin E receptor beta chain and expression of the complete human receptor. J Biol Chem. 1992;267(18):12782–12787. doi: 10.1016/S0021-9258(18)42344-7 [DOI] [PubMed] [Google Scholar]
- 22.Kraft S, Rana S, Jouvin MH, Kinet JP. The Role of the FcεRI β-Chain in Allergic Diseases. Int Arch Allergy Immunol. 2004;135(1):62–72. doi: 10.1159/000080231 [DOI] [PubMed] [Google Scholar]
- 23.Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J Exp Med. 1994;179(2):745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platzer B, Baker K, Vera MP, et al. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol. 2015;8(3):516–532. doi: 10.1038/mi.2014.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greer AM, Wu N, Putnam AL, et al. Serum IgE clearance is facilitated by human FcεRI internalization. J Clin Invest. 2014;124(3):1187–1198. doi: 10.1172/JCI68964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer D, Fiebiger S, Ebner C, et al. Peripheral blood dendritic cells express Fc epsilon RI as a complex composed of Fc epsilon RI alpha- and Fc epsilon RI gamma-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol. 1996;157(2):607–616. [PubMed] [Google Scholar]
- 27.Human epidermal Langerhans cells express the high affinity receptor for immunoglobulin E (Fc epsilon RI). J Exp Med. 1992;175(5):1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung DS, Ehlenbach SJ, Kitchens RT, et al. Cutting Edge: CD49d+ Neutrophils Induce FcεRI Expression on Lung Dendritic Cells in a Mouse Model of Postviral Asthma. J Immunol. 2010;185(9):4983–4987. doi: 10.4049/jimmunol.1002456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehlink E, Baker AH, Yen E, Nurko S, Fiebiger E. Relationships between Levels of Serum IgE, Cell-Bound IgE, and IgE-Receptors on Peripheral Blood Cells in a Pediatric Population. PLoS One. 2010;5(8):e12204. doi: 10.1371/journal.pone.0012204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasudev M, Cheung DS, Pincsak H, et al. Expression of High-Affinity IgE Receptor on Human Peripheral Blood Dendritic Cells in Children. PLoS One. 2012;7(2):e32556. doi: 10.1371/journal.pone.0032556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holloway JA, Holgate ST, Semper AE. Expression of the high-affinity IgE receptor on peripheral blood dendritic cells: Differential binding of IgE in atopic asthma. J Allergy Clin Immunol. 2001;107(6):1009–1018. doi: 10.1067/mai.2001.115039 [DOI] [PubMed] [Google Scholar]
- 32.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8(3):205–217. doi: 10.1038/nri2273 [DOI] [PubMed] [Google Scholar]
- 33.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7(5):365–378. doi: 10.1038/nri2072 [DOI] [PubMed] [Google Scholar]
- 34.Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, Kinet JP. Allergy-Associated FcRβ Is a Molecular Amplifier of IgE- and IgG-Mediated In Vivo Responses. Immunity. 1998;8(4):517–529. doi: 10.1016/S1074-7613(00)80556-7 [DOI] [PubMed] [Google Scholar]
- 35.Alber G, Miller L, Jelsema CL, Varin-Blank N, Metzger H. Structure-function relationships in the mast cell high affinity receptor for IgE. Role of the cytoplasmic domains and of the beta subunit. J Biol Chem. 1991;266(33):22613–22620. doi: 10.1016/S0021-9258(18)54615-9 [DOI] [PubMed] [Google Scholar]
- 36.Cruse G, Beaven MA, Music SC, Bradding P, Gilfillan AM, Metcalfe DD. The CD20 homologue MS4A4 directs trafficking of KIT toward clathrin-independent endocytosis pathways and thus regulates receptor signaling and recycling. Mol Biol Cell. 2015;26(9):1711–1727. doi: 10.1091/mbc.E14-07-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arthur GK, Ehrhardt-Humbert LC, Snider DB, et al. The FcεRIβ homologue, MS4A4A, promotes FcεRI signal transduction and store-operated Ca2+ entry in human mast cells. Cell Signal. 2020;71:109617. doi: 10.1016/j.cellsig.2020.109617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanmugalingam D, Wardlaw AJ, Bradding P. Adhesion of human lung mast cells to bronchial epithelium: evidence for a novel carbohydrate-mediated mechanism. J Leukoc Biol. 2000;68(1):38–46. doi: 10.1189/jlb.68.1.38 [DOI] [PubMed] [Google Scholar]
- 39.Cruse G, Cockerill S, Bradding P. IgE alone promotes human lung mast cell survival through the autocrine production of IL-6. BMC Immunol. 2008;9(1):2. doi: 10.1186/1471-2172-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirshenbaum AS, Akin C, Wu Y, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk Res. 2003;27(8):677–682. doi: 10.1016/S0145-2126(02)00343-0 [DOI] [PubMed] [Google Scholar]
- 41.Kuek LE, Leffler M, Mackay GA, Hulett MD. The MS4A family: counting past 1, 2 and 3. Immunol Cell Biol. 2016;94(1):11–23. doi: 10.1038/icb.2015.48 [DOI] [PubMed] [Google Scholar]
- 42.Manne BK, Badolia R, Dangelmaier C, et al. Distinct Pathways Regulate Syk Protein Activation Downstream of Immune Tyrosine Activation Motif (ITAM) and hemITAM Receptors in Platelets. J Biol Chem. 2015;290(18):11557–11568. doi: 10.1074/jbc.M114.629527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alshahrani MM, Yang E, Yip J, et al. CEACAM2 negatively regulates hemi (ITAM-bearing) GPVI and CLEC-2 pathways and thrombus growth in vitro and in vivo. Blood. 2014;124(15):2431–2441. doi: 10.1182/blood-2014-04-569707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura T, Kihara H, Bhattacharyya S, Sakamoto H, Appella E, Siraganian RP. Downstream Signaling Molecules Bind to Different Phosphorylated Immunoreceptor Tyrosine-based Activation Motif (ITAM) Peptides of the High Affinity IgE Receptor *. J Biol Chem. 1996;271(44):27962–27968. doi: 10.1074/jbc.271.44.27962 [DOI] [PubMed] [Google Scholar]
- 45.Broide DH, Gleich GJ, Cuomo AJ, et al. Evidence of ongoing mast cell and eosinophil degranulation in symptomatic asthma airway. J Allergy Clin Immunol. 1991;88(4):637–648. doi: 10.1016/0091-6749(91)90158-k [DOI] [PubMed] [Google Scholar]
- 46.Berlin AA, Hogaboam CM, Lukacs NW. Inhibition of SCF attenuates peribronchial remodeling in chronic cockroach allergen-induced asthma. Lab Invest. 2006;86(6):557–565. doi: 10.1038/labinvest.3700419 [DOI] [PubMed] [Google Scholar]
- 47.Ishizawa M, Shibasaki M, Yokouchi Y, et al. No association between atopic asthma and a coding variant of FcεR1β in a Japanese population. J Hum Genet. 1999;44(5):308. doi: 10.1007/s100380050166 [DOI] [PubMed] [Google Scholar]
- 48.Donnadieu E, Jouvin MH, Kinet JP. A Second Amplifier Function for the Allergy-Associated FcεRI-β Subunit. Immunity. 2000;12(5):515–523. doi: 10.1016/S1074-7613(00)80203-4 [DOI] [PubMed] [Google Scholar]
- 49.Furumoto Y, Hiraoka S, Kawamoto K, et al. Polymorphisms in FcϵRI β Chain Do Not Affect IgE-Mediated Mast Cell Activation. Biochem Biophys Res Commun. 2000;273(2):765–771. doi: 10.1006/bbrc.2000.2989 [DOI] [PubMed] [Google Scholar]
- 50.Ferenchak K, Deitch I, Huckfeldt R. Antisense Oligonucleotide Therapy for Ophthalmic Conditions. Semin Ophthalmol. 2021;36(5–6):452–457. doi: 10.1080/08820538.2021.1914116 [DOI] [PubMed] [Google Scholar]
- 51.Liao W, Dong J, Peh HY, et al. Oligonucleotide Therapy for Obstructive and Restrictive Respiratory Diseases. Molecules. 2017;22(1):139. doi: 10.3390/molecules22010139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scoles DR, Pulst SM. Oligonucleotide therapeutics in neurodegenerative diseases. RNA Biol. 2018;15(6):707–714. doi: 10.1080/15476286.2018.1454812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollingworth P, Harold D, Sims R, et al. Common variants in ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–435. doi: 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proitsi P, Lee SH, Lunnon K, et al. Alzheimer’s disease susceptibility variants in the MS4A6A gene are associated with altered levels of MS4A6A expression in blood. Neurobiol Aging. 2014;35(2):279–290. doi: 10.1016/j.neurobiolaging.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 55.Lacher SE, Alazizi A, Wang X, et al. A hypermorphic antioxidant response element is associated with increased MS4A6A expression and Alzheimer’s disease. Redox Biol. 2017;14:686–693. doi: 10.1016/j.redox.2017.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou XH, Bi YL, Tan MS, et al. Genome-wide association study identifies Alzheimer’s risk variant in MS4A6A influencing cerebrospinal fluid sTREM2 levels. Neurobiol Aging. 2019;84:241.e13–241.e20. doi: 10.1016/j.neurobiolaging.2019.05.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.