Abstract
Objective:
A sizeable minority of patients with binge-eating disorder (BED) do not fully respond to evidence-based treatments. Evidence to guide refinements of treatments is needed. Conceptualizing BED as arising from a network of symptom-to-symptom interactions allows for identification of the most strongly connected symptoms, which could inform intervention targets. This study estimated networks of BED features at pretreatment and posttreatment to assess whether cognitive behavioral therapy (CBT) and interpersonal psychotherapy (IPT) differentially impacted the interrelationships of BED symptoms/features.
Methods:
Participants were 392 adults (83% women, 88% white) with BED who received CBT (n=236) or IPT (n=156) and assessed at pretreatment and posttreatment. Networks were estimated across timepoints and treatments. Expected influence (EI) was calculated; symptoms with the highest EI have the most strong and frequent associations with other symptoms. We also assessed whether the symptom with the highest EI predicted posttreatment BED symptoms.
Results:
In the CBT and IPT networks, shape concern, weight concern, and eating concern had the highest EI at pretreatment and posttreatment. EI significantly increased from pretreatment to posttreatment for some symptoms in CBT but did not change for any symptoms in IPT. Shape concern significantly and positively predicted BED symptoms at posttreatment in CBT and IPT.
Conclusions:
CBT and IPT similarly impacted interrelations among BED features. Pretreatment EI predicted posttreatment BED symptom change, indicating that pretreatment centrality could signal meaningful intervention targets. Clinical implications and avenues for future research are discussed including how personalized network analysis may advance the understanding of the clinical utility of centrality.
Keywords: binge eating, eating disorders, network analysis, cognitive behavioral therapy, interpersonal psychotherapy
Introduction
Binge-eating disorder (BED) is defined by recurrent episodes of binge eating, marked distress, and absence of inappropriate weight-compensatory behaviors (American Psychiatric Association, 2013). BED is associated with elevated rates of psychiatric/medical comorbidity and functional impairments (Udo & Grilo, 2018, 2019).
Several psychological and pharmacological treatments are effective for BED (Hilbert, Petroff, & Herpertz, 2019). Cognitive behavioral therapy (CBT) and interpersonal psychotherapy (IPT) have received strong and reliable support, with studies reporting significant reductions in binge-eating frequency and eating-disorder psychopathology, and improved psychological functioning (Grilo, Masheb, Wilson, Gueorguieva, & White, 2011; Wilson, Wilfley, Agras, & Bryson, 2010). Although leading evidence-based BED interventions (including CBT and IPT) produce meaningful improvements that are well-maintained through two-year follow-up (Wilson et al., 2010), roughly 50% of patients do not attain binge-eating abstinence (Linardon, 2018). While binge-eating abstinence may not be necessary to achieve good quality of life nor necessarily reflect full BED recovery (Slof-Op’t Landt et al., 2019), the fact that 50% of people continue to experience binge eating following treatment highlights the need for treatments to be improved.
It has been long suggested that identifying mediators and predictors/moderators of treatment outcomes could inform intervention refinement (Kraemer, Wilson, Fairburn, & Agras, 2002; Wilson, Grilo, & Vitousek, 2007). To date, however, research with BED has identified no mediators, no reliable predictors (other than rapid response and body-image disturbances) and mainly nonsignificant findings for moderators of treatment outcomes (Linardon, de la Piedad Garcia, & Brennan, 2017). The very few studies that reported significant moderator findings (Linardon et al., 2017) did so for limited outcomes (e.g., Anderson et al., 2020; Grilo, Masheb, & Crosby, 2012) and require broader replication prior to being viewed as reliable indicators of the characteristics for whom a specific treatment works best (Poldrack, Huckins, & Varoquaux, 2020). An alternative approach to understanding BED and its response to treatments might prove informative.
While we know what evidence-based treatments do (i.e., they reduce symptom intensities; Hilbert et al., 2019), we do not yet understand how treatments exert their influences on psychopathology. Viewing psychopathology through the lens of the network theory of psychopathology may advance our understanding of how treatments impact symptoms. The network theory of psychopathology views disorders as a network of interconnected symptoms that influence and maintain one another (Borsboom, 2017; Borsboom & Cramer, 2013). By quantifying symptom interactions, ideally with longitudinal data, it is possible to identify those symptoms that are most central to a disorder. The most central symptoms in a network are thought to be those that either cause most other symptoms or are caused by most other symptoms (Levinson et al., 2022). Theoretically, if an intervention targets a disorder’s most central symptom(s), this may reduce the symptom’s “input/influence” on other symptoms, causing those symptoms to lessen, which would thereby disrupt the symptom network (Borsboom & Cramer, 2013).
Assessing interconnections among psychiatric symptoms before and after treatment can address the question of how treatments impact symptoms and psychopathology as a whole, which may, in turn, contribute to greater understanding of treatment mechanisms and potential targets for refinement (Levinson et al., 2022). For example, if a particular symptom transitioned from being highly central before treatment to not highly central after treatment, this could mean that treatment (1) effectively targeted this central symptom and/or (2) disrupted the symptom-to-symptom interconnections in a potentially meaningful way. It is possible, however, that symptom centrality changes could also occur due to measurement error (Fried et al., 2016); this is discussed further in the Discussion.
To date, network analyses have been applied to BED treatment outcomes in two studies (Hilbert et al., 2020; Forrest & Grilo, 2022). Hilbert and colleagues (2020) explored change in a network of BED and general psychopathology symptoms in 178 patients with subthreshold and/or full BED before and after CBT and at 6-month follow-up. Eating disorder-related impairment and self-esteem were the most central features. Network connectivity overall increased significantly from pretreatment to posttreatment (i.e., the posttreatment network had more and/or stronger edges than the pretreatment network), and binge eating and shape concerns had the greatest increases in strength centrality, meaning these two symptoms had the strongest and most frequent interconnections with other symptoms. Forrest and Grilo (2022) examined network structure and changes in 191 patients with BED before and after behaviorally-based weight-loss treatments (BBWLT) and at 12-month follow-up. At pretreatment, overvaluation of shape/weight and preoccupation with shape/weight and with food/eating had highest strength centrality. At posttreatment and 12-month follow-up, dissatisfaction with shape/weight had highest strength centrality, which significantly increased from pretreatment. Importantly, the network structure following BBWLT resembled networks of people without eating disorders (e.g., Forrest et al., 2019). This shift in centrality—where overvaluation was the most central at pretreatment but dissatisfaction was the most central at posttreatment and follow-up—could indicate that even though CBT proposes that treatment exerts its impact via weakening connections between overvaluation and other symptoms (Fairburn et al., 2003), BBWLT may have amplified connections between dissatisfaction and other eating-disorder symptoms (Forrest & Grilo, 2022). Collectively, these two initial applications of network analyses to CBT (Hilbert et al., 2020) and BBWLT (Forrest & Grilo, 2022) for BED highlight the potential importance of understanding how treatments impact relationships, not just intensities, of symptoms.
One ongoing question within network science is to what extent central symptoms at pretreatment are suggestive of important treatment targets. Four studies have examined this issue, and all suggest that centrality could be an important indicator of treatment outcomes in anorexia nervosa (Elliott et al., 2020a, 2020b), social anxiety disorder (Rodebaugh et al., 2019), PTSD (Papini et al., 2020), and complicated grief (Robinaugh et al., 2016). The association between centrality and treatment outcomes merits investigation in BED both for informing treatment refinements and for guiding nosological issues, given the tension between evidence of the importance of body image as a severity signal in BED vs. the DSM-5 BED diagnostic criteria, which does not include a body-image criterion (Grilo, 2013). For example, if overvaluation is the most central BED symptom (DuBois et al., 2017; Wang et al., 2019), does this suggest that targeting overvaluation (i.e., reducing the symptom’s intensity and/or interconnectivity with other symptoms) is a necessary treatment goal? Moreover, an ongoing question concerning treatment for BED is how interventions that are seemingly so distinct, such as CBT, BBWLT, and IPT, result in relatively similar BED courses and outcomes, given their differential foci and attention devoted to binge eating and body image. Examining symptom centrality change following different BED treatments is needed to understand (1) how treatments impact connectivity of BED symptom networks and (2) how centrality at pretreatment may be associated with BED outcomes.
The current study used data from “Clinical Trials of BED” (CT-BED), which aggregated data from randomized controlled trials testing psychosocial treatments for BED to examine racial/ethnic differences in BED (Franko et al., 2012) and demographic (Shingleton et al., 2015; Thompson-Brenner et al., 2013) and clinical variables (Grilo et al., 2021) as predictors/moderators of outcomes. The current study analyzed data from patients who received CBT or IPT, given the treatments’ conceptual and procedural differences (i.e., cognitive versus interpersonal models of binge-eating; CBT focuses exclusively on eating-related behaviors and cognitions whereas IPT focuses on interpersonal factors while proscribing attention to eating), yet comparable outcomes (Wilfley et al., 2002; Wilson et al., 2010). Aim 1 compared pretreatment and posttreatment centrality of BED features between CBT and IPT to assess whether these two treatments differentially impacted the interrelationships among BED features. Aim 2 assessed whether the symptom with highest centrality at pretreatment predicted indicators of remission status at posttreatment (e.g., Brown et al., 2020). These models also included the symptom with the lowest centrality at pretreatment as a predictor in order to determine the extent to which more central symptoms are more predictive of outcomes compared to least central symptoms. This aim has implications not only for understanding BED treatments but also for network methodology, as there currently exists very limited evidence regarding whether centrality relates meaningfully to treatment outcomes (McNally, 2021; Robinaugh et al., 2020).
Methods
Participants
N=392 patients with BED from the CT-BED database (Franko et al., 2012) received either CBT (n=236; five sites) or IPT (n=156; two sites). Other treatments in CT-BED, including briefer guided-self-help CBT, were excluded. Table 1 summarizes demographic characteristics, which were self-reported. Methods have been previously described (Franko et al., 2012); only a brief overview follows. Institutional Review Boards for each site approved studies.
Table 1.
Sample descriptive statistics summarized by treatment type and timepoint, including results of mixed effects models assessing symptom intensity changes from pretreatment to posttreatment
| CBT N = 236 |
IPT N = 156 |
|||||||
| n (%) or M (SD) | n (%) or M (SD) | |||||||
|
| ||||||||
| Sex | ||||||||
| Male | 41 (17.4) | 25 (16.0) | ||||||
| Female | 195 (82.6) | 131 (84.0) | ||||||
| Race and ethnicity | ||||||||
| White | 212 (89.8) | 132 (84.6) | ||||||
| African American | 14 (5.9) | 16 (10.3) | ||||||
| Native American | 2 (0.8) | 1 (0.6) | ||||||
| Hispanic | 6 (2.5) | 7 (4.5) | ||||||
| Additional racial/ethnic identity* | 2 (0.8) | 0 (0) | ||||||
| Education | ||||||||
| Some high school or less | 5 (2.1) | 1 (0.6) | ||||||
| High school graduate or GED | 24 (10.2) | 21 (13.5) | ||||||
| Some college | 74 (31.4) | 45 (28.8) | ||||||
| College graduate | 86 (36.4) | 28 (17.9) | ||||||
| Graduate degree | 44 (18.6) | 59 (37.8) | ||||||
| Not reported | 3 (1.3) | 2 (1.3) | ||||||
| Age | 44.9 (9.82) | 46.72 (10.55) | ||||||
|
| ||||||||
| CBT | IPT | |||||||
|
|
||||||||
| Pre | Post | Pre | Post | |||||
|
|
|
|||||||
| M (SD) | M (SD) | t | p | M (SD) | M (SD) | t | p | |
|
| ||||||||
| BMI | 38.09 (6.82) | 38.07 (6.97) | −0.16 | .88 | 36.73 (5.04) | 36.55 (5.26) | −1.04 | .30 |
| Restraint | 1.79 (1.32) | 1.21 (0.94) | −5.80 | < .001 | 1.92 (1.20) | 1.51 (1.13) | −3.37 | < .001 |
| Shape concerns | 3.80 (0.98) | 2.68 (1.30) | −14.35 | < .001 | 3.90 (0.95) | 2.51 (1.27) | −13.81 | < .001 |
| Weight concerns | 3.41 (1.09) | 2.48 (1.21) | −11.43 | < .001 | 3.31 (1.03) | 2.23 (1.20) | −10.07 | < .001 |
| Eating concerns | 2.30 (1.26) | 0.95 (0.94) | −15.60 | < .001 | 2.19 (1.39) | 0.65 (0.79) | −13.96 | < .001 |
| Binge-eating frequency | 21.04 (13.69) | 2.98 (6.63) | −20.22 | < .001 | 19.72 (11.54) | 3.03 (13.27) | −18.64 | < .001 |
| Depression | 1.04 (0.75) | 0.56 (0.78) | −10.70 | < .001 | 1.25 (0.70) | 0.42 (0.73) | −13.53 | < .001 |
Note. CBT = cognitive behavioral therapy, IPT = interpersonal psychotherapy, GED = general educational development, BMI = body mass index. Mixed models were estimated with random intercepts to account for non-independence of observations between timepoints. t and p values indicate the fixed effects of time on the given node.
Describing specific racial/ethnic identities is preferable to categorizing any racial/ethnic identity as “other” or “additional.” Regrettably, these data were originally merged such that people who reported a racial/ethnic identity other than White, African American, Native American, or Hispanic were coded only as “other” and we do not have the ability to determine these people’s specific racial/ethnic identities.
Measures
All BED features were assessed at pretreatment and posttreatment. Eating Disorder Examination (EDE; Fairburn & Cooper, 1993) is a semi-structured investigator-based interview that assesses binge-eating frequency and eating-disorder psychopathology, which is reflected in four subscales: restraint, eating concern, weight concern, and shape concern. Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960; Reynolds & Kobak, 1995) assessed depression levels; HAM-D depression severity categories are as follows: 0=no depression, 1=mild depression, 2=mild-to-moderate depression, and 3=severe depression. Body mass index (BMI; kg/m2) was calculated from measured weights and heights.
Our node selection process proceeded as follows. First, only the EDE four subscales and binge-eating frequency were available, so these five variables—which are important CBT constructs—were included as nodes. Second, we included a HAM-D depression variable because depression/negative affect frequently co-occurs with BED and, if present, may signal a more disturbed variant (Grilo, Masheb, & Wilson, 2001). In addition, depression scores correlate moderately with measures of self-esteem (Hrabosky et al., 2007), and self-esteem is relevant in IPT; thus, including a depression node was an imperfect but relevant variable to ensure that networks included measures of constructs relevant in both treatments. Third, consistent with previous BED network studies (e.g., Hilbert et al., 2019; Wang et al., 2020) we included BMI as a node. Although BMI and the issue of weight loss is viewed by some within the eating-disorder field as controversial, the empirical reality is that BED is associated strongly with obesity (Udo & Grilo, 2018), most of the RCT datasets used in these aggregated data either required obesity as an inclusion or comprised participant groups that met criteria for obesity (Franko et al., 2012), and many patients with BED report both wanting to reduce binge eating and lose weight (Brody, Masheb, & Grilo, 2005). With that said about the logic of including BMI data in the network analyses, we highlight that network findings for BED to date have reported BMI to be among the least central BED symptoms (Wang et al., 2019), whereas body image concerns are among the most central symptoms. This supports the position, reviewed by Grilo (2013), that body image concerns in BED are important psychological and subjective constructs that do not simply reflect concerns commensurate with higher BMIs or excess weight.
Indicators of Remission Status
We included three dichotomous indicators of remission status. The first indicator was whether participants had zero binge-eating episodes during the past 28 days. The second indicator was whether participants’ EDE Global scores were within one standard deviation (SD) of community norms (M = 1.41, SD = 1.15; Mond, Hay, Rodgers, & Owen, 2006), which we refer to hereafter as normative EDE Global. The third indicator was whether participants attained both binge-eating abstinence and normative EDE Global.
Analytic Plan
Analyses were performed in R software environment (R Core Team, 2021), using the following packages: mice (van Buuren & Groothuis-Oudshoorn, 2011), lme4 (Bates, Maechler, Bolker, & Walker, 2015), lmerTest (Kuznetsova, Brockhoff, & Christensen, 2017), bootnet (Epskamp, Borsboom, & Fried, 2018), qgraph (Epskamp, Cramer, Waldorp, Schmittmann, & Borsboom, 2012), NetworkComparisonTest (van Borkulo et al., 2017), dplyr (Wickham, François, Henry, & Müller, 2020), and ggplot2 (Wickham, 2016).
Missing Data
The maximum proportion of missing data for EDE variables and BMI was 11%. The depression variable was missing for 41.6% at pretreatment (CBT = 34.7%, IPT = 51.39%) and 52.0% at posttreatment (CBT = 52.1%, IPT = 51.39%), because one of the five CBT sites and one of the two IPT sites did not include the HAM-D. Missing data were imputed (m=50) using predictive mean matching and aggregated for analyses.
Symptom Changes Throughout Treatment
To supplement interpretation of any network changes, we used linear mixed models with random intercepts to evaluate how network symptoms changed throughout treatment.
Network Estimation
Pretreatment and posttreatment BED symptom networks were estimated for CBT and IPT through the graphical least absolute shrinkage and selection operator (LASSO) method. LASSO method includes a tuning parameter to reduce small partial correlations to 0, reducing the likelihood of false positive edges (i.e., partial correlations) from emerging. LASSO’s tuning parameter was set to .50. Given the differing scales of nodes, networks were estimated via Spearman correlations, where edges represent partial Spearman correlation between two nodes (i.e., symptoms).
Centrality
Network centrality was indicated by calculating expected influence, which indicates the sum of the edges present for each node. Expected influence is similar to strength centrality but differs in that strength indicates the sum of the absolute values of the edges present for each node whereas expected influence is calculated by raw values. Given that positive vs. negative edges imply differential impact and that several edges were negative (i.e., red lines in Figure 1), we chose to use expected influence in the current study.
Figure 1.
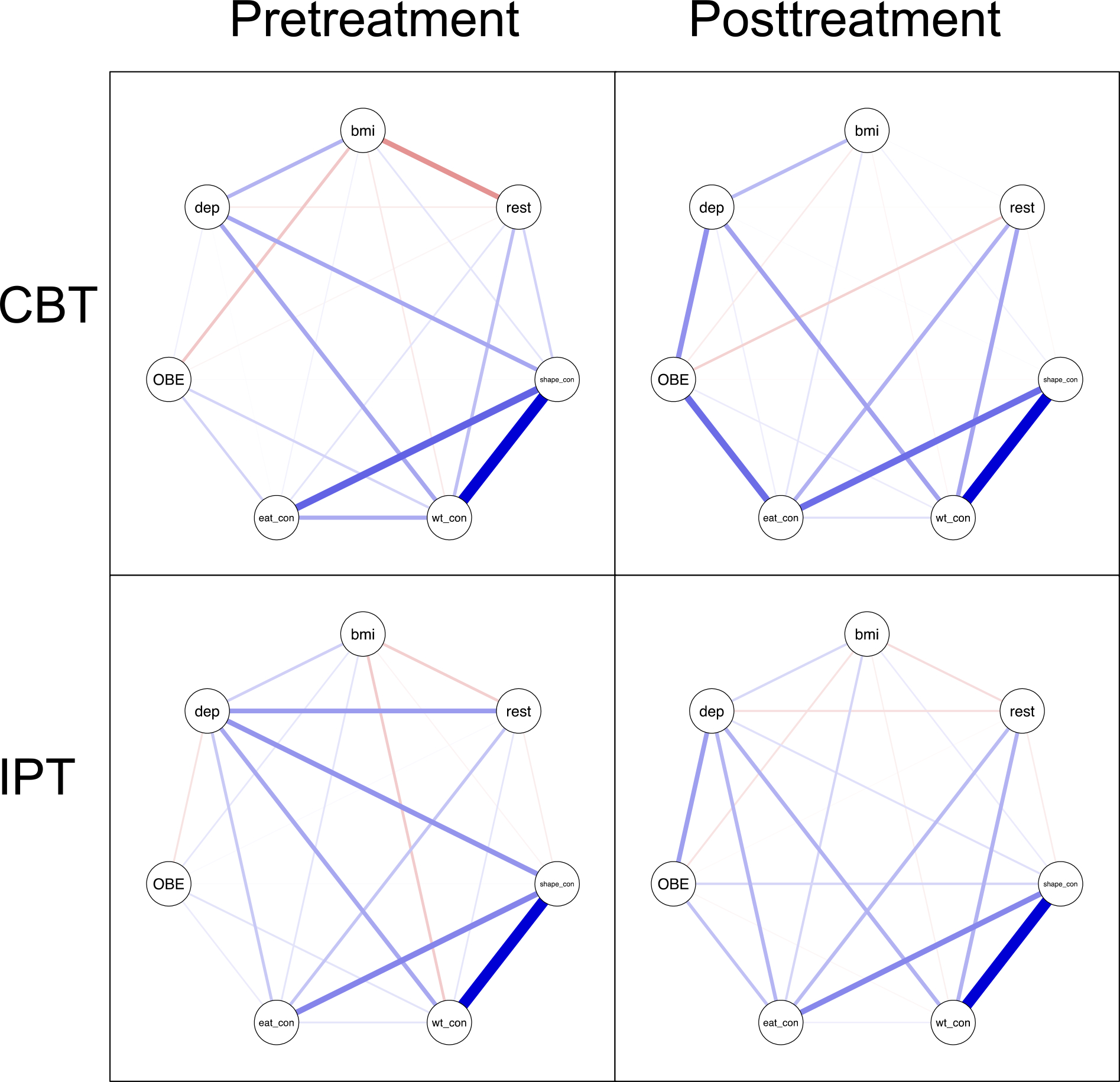
Binge-eating disorder symptom networks by treatment type and timepoint. Note. CBT = cognitive behavioral therapy, IPT = interpersonal psychotherapy, bmi = body mass index, rest = restraint Eating Disorder Examination (EDE) subscale, shape_con = shape concerns EDE subscale, wt_con = weight concerns EDE subscale, eat_con = eating concern EDE subscale, OBE = binge-eating frequency, dep = depression.
Centrality is sometimes related to node variance (Elliott et al., 2020b; Terluin et al., 2016), which could mean that centrality may be an unnecessary metric. To examine this possibility, we assessed Spearman’s rho and Pearson’s r correlations between expected influence and standard deviation in both treatment groups cross-sectionally and longitudinally, with and without controlling for outliers (Supplemental Table 2). Of the 32 correlations, only two were significant, and one of these was in negative direction, which is the opposite direction of Terluin et al.’s (2016) findings.
Centrality Difference Tests
Centrality difference tests were conducted via nonparametric bootstrapping. These are a useful supplement to centrality analyses because centrality is relative. Centrality difference tests identified whether a given node’s centrality is significantly greater than centrality for other nodes.
Stability
Network stability was indicated by the correlation-stability (CS) coefficient. The CS coefficient is obtained by re-estimating expected influence using progressively smaller subsets of the original sample via bootstrapping. The correlation between the full sample’s expected influence and the bootstrapped subsets’ expected influence is calculated. The CS coefficient indicates the maximum proportion of the sample that can be dropped while retaining an expected influence correlation >0.70 with the original sample. CS coefficients ≥0.50 are considered good (Epskamp et al., 2018).
Network Comparison Tests
Network comparison tests were performed to assess pretreatment to posttreatment comparisons within treatments (dependent samples) and timepoint comparisons between treatments (e.g., pretreatment networks compared for CBT vs. IPT; independent samples). For each comparison, we assessed whether the two networks differed in global strength (i.e., overall network connectivity) and expected influence for each node. Given the number of expected influence comparisons, node centrality comparison p-values were adjusted (Benjamini and Hochberg method).1
Pretreatment Expected Influence Predicting Indicators of Remission Status
We used logistic regressions to assess whether the symptoms with the highest and lowest expected influence in each treatment predicted indicators of remission status at posttreatment (binge-eating abstinence, normative EDE Global, and binge-eating abstinence plus normative EDE Global).
Results
Symptom Changes
Table 1 shows that for both CBT and IPT, all BED features except BMI were significantly lower at posttreatment than pretreatment.
Symptom Networks
CS coefficients indicated that all networks were estimated reliably (pretreatment CBT=0.75, posttreatment CBT=0.75, pretreatment IPT=0.67, posttreatment IPT=0.75).
Table 1 includes descriptive statistics for all nodes. Figure 1 displays the four networks. Table 2 and Figure 2 show expected influence. Supplemental Figure 1 shows the centrality difference test results. At CBT pretreatment, shape concern had the highest expected influence, which was significantly higher than expected influence for all other nodes, except for weight concern. At CBT posttreatment, weight concern and eating concern had the highest expected influence, which were significantly higher than expected influence for all other nodes at posttreatment.
Table 2.
Expected influence centrality by treatment type and timepoint, with comparisons by pretreatment vs. posttreatment within cognitive behavioral therapy (CBT) and interpersonal psychotherapy (IPT) and comparing pretreatment centrality between CBT and IPT and posttreatment centrality between CBT and IPT
| CBT | IPT | p for centrality differences between networks | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Pre | Post | Pre | Post | CBT pre vs. CBT post | IPT pre vs. IPT post | Pre CBT vs. pre IPT | Post CBT vs. post IPT | |
|
| ||||||||
| BMI | −0.14 | 0.20 | −0.02 | 0.06 | .03 | 1.00 | .61 | .25 |
| Restraint | 0.00 | 0.28 | 0.26 | 0.13 | .11 | .71 | .47 | .25 |
| Shape concerns | 1.20 | 0.97 | 0.99 | 1.10 | .49 | .77 | .77 | .82 |
| Weight concerns | 1.00 | 1.10 | 0.80 | 0.99 | .89 | .77 | .47 | .23 |
| Eating concerns | 0.67 | 1.10 | 0.67 | 0.95 | .01 | .77 | .59 | .04 |
| Binge-eating frequency | 0.08 | 0.49 | 0.12 | 0.39 | .01 | .47 | .47 | .61 |
| Depression | 0.51 | 0.69 | 0.79 | 0.70 | .01 | .71 | .47 | .82 |
Note. CBT = cognitive behavioral therapy, IPT = interpersonal psychotherapy, BMI = body mass index.
Figure 2.
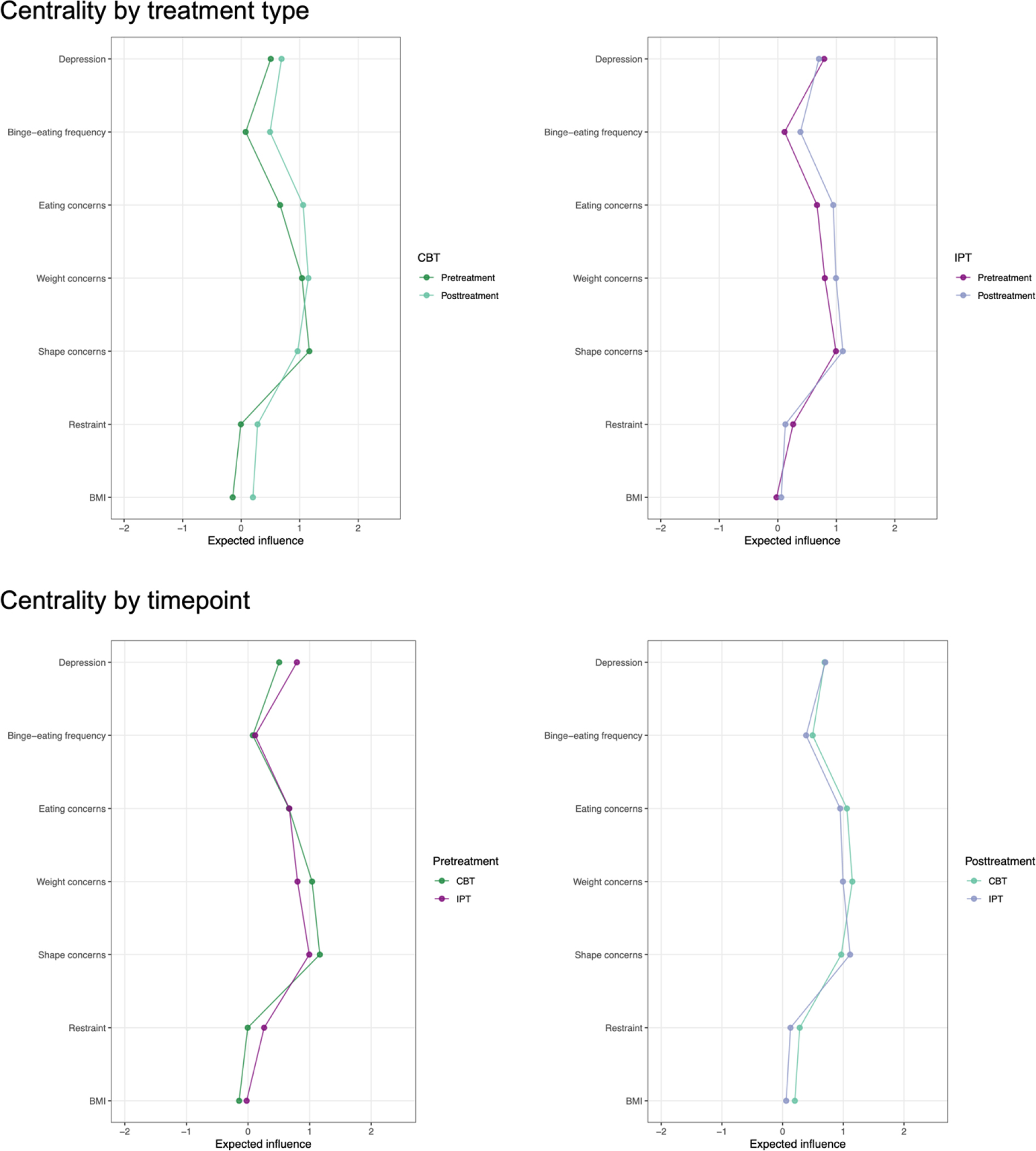
Centrality by treatment type and timepoint Note. CBT = cognitive behavioral therapy, IPT = interpersonal psychotherapy, BMI = body mass index.
At IPT pretreatment, shape concern, weight concern, eating concern, and depression had the highest expected influence. These values were significantly higher than expected influence for the three other nodes but did not differ from one another. At IPT posttreatment, shape concern, weight concern, and eating concern had the highest expected influence. These values were significantly higher than expected influence for the three other nodes but did not differ from one another.
Network Comparison Tests
CBT by Timepoint
Global strength did not significantly differ between pretreatment and posttreatment in CBT (2.01 vs. 2.55, p=.08). After adjusting for multiple comparisons, centrality comparisons revealed that expected influence for BMI, eating concern, binge-eating frequency, and depression was significantly higher at posttreatment vs. pretreatment in CBT.
IPT by Timepoint
Global strength did not significantly differ between pretreatment and posttreatment in IPT (1.49 vs. 1.66, p=.53). After adjusting for multiple comparisons, expected influence did not differ for any nodes between pretreatment and posttreatment in IPT.
Pretreatment by CBT vs. IPT.
Global strength at pretreatment did not differ between CBT and IPT (2.01 vs. 1.49, p=.27). After adjusting for multiple comparisons, expected influence did not differ for any nodes at pretreatment between CBT and IPT.
Posttreatment by CBT vs. IPT
Global strength at posttreatment was significantly higher in CBT vs. IPT (2.55 vs. 1.66, p=.03). After adjusting for multiple comparisons, expected influence for eating concern was significantly higher at posttreatment in CBT vs. IPT.
Centrality Predicting Indicators of Remission Status
Given that shape concern was the node with the highest centrality and BMI was the node with the lowest centrality, shape concern and BMI were entered as predictors of the three indicators of remission status (see Table 3). In CBT, higher shape concern (most central) significantly predicted lower likelihood of attaining binge-eating abstinence, normative EDE Global, and both binge-eating abstinence plus normative EDE Global. BMI (least central) did not significantly predict likelihood of binge-eating abstinence or attaining both binge-eating abstinence plus normative EDE Global, but it did significantly predict normative EDE Global. Even though both the most central and least central symptom predicted normative EDE Global, the effect was stronger for shape concern vs. BMI.
Table 3.
Most and least central symptoms at pretreatment predicting indicators of remission status in both samples
| CBT | IPT | |||||
|---|---|---|---|---|---|---|
| OR (SE) | z | p | OR (SE) | z | p | |
| Outcome = Binge-eating abstinence | ||||||
| Pre EDE shape concern (most central) | 0.68 (0.14) | −2.65 | .008 | 0.92 (0.18) | −0.48 | .63 |
| Pre BMI (least central) | 0.99 (0.02) | −0.66 | .51 | 1.00 (0.03) | 0.01 | .99 |
| Outcome = EDE Global within 1 SD of norm | ||||||
| Pre EDE shape concern (most central) | 0.45 (0.20) | −4.12 | < .001 | 0.53 (0.25) | −2.53 | .01 |
| Pre BMI (least central) | 0.95 (0.02) | −2.01 | .04 | 0.95 (0.04) | −1.09 | .28 |
| Outcome = Binge-eating abstinence + EDE Global within 1 SD of norm | ||||||
| Pre EDE shape concern (most central) | 0.62 (0.14) | −3.29 | .001 | 0.88 (0.17) | −0.72 | .47 |
| Pre BMI (least central) | 0.98 (0.02) | −1.12 | .26 | 1.01 (0.03) | 0.40 | .69 |
Note. CBT = cognitive behavioral therapy, IPT = interpersonal psychotherapy, EDE = Eating Disorder Examination.
In IPT, higher shape concern (most central) significantly predicted lower likelihood of attaining normative EDE Global. Shape concern was not associated with likelihood of attaining binge-eating abstinence or both binge-eating abstinence plus normative EDE Global. BMI (least central) did not significantly predict likelihood of binge-eating abstinence, normative EDE Global, or both binge-eating abstinence plus normative EDE Global.
Discussion
This study estimated networks of BED features at pretreatment and posttreatment to assess whether CBT and IPT differentially impacted interrelationships of BED features. We also assessed whether pretreatment BED centrality corresponded to change in BED symptoms at posttreatment. Findings have relevance to both the eating-disorders field specifically and the network theory of psychopathology more broadly.
Shape concern, weight concern, and eating concern were the most central symptoms at both timepoints in both treatments (CBT and IPT). This is broadly in line with the CBT model of eating disorders (DuBois et al., 2017; Fairburn et al., 2003). These centrality findings also converge with those of other network studies of eating disorders that constructed networks using EDE subscales (Hilbert et al., 2020; Mares et al., 2022).
In terms of prognostic significance, the symptom with the highest expected influence at pretreatment (shape concern) significantly and positively predicted all three indicators of remission status in CBT and predicted one of the three indictors of remission status (normative EDE Global) in IPT. The least central symptom (BMI) was associated with only one indicator of remission status (normative EDE Global) in CBT only, though the effect was stronger for the most central symptom vs. the least central symptom. Overall, this pattern of findings might suggest that the most central symptom in pretreatment BED networks might inform targets for BED treatment, where interventions specifically designed to reduce the most central symptom (e.g., shape overvaluation [Wang et al., 2019]) may result in higher likelihood of experiencing various indicators of remission status. These findings add to those of the few emerging studies suggesting that pretreatment symptom centrality be associated with treatment outcomes (Brown et al., 2020; Elliott et al., 2020a, 2020b; Papini et al., 2021; Rodebaugh et al., 2019). These findings also extend those of Brown et al (2020), by assessing whether the most central symptom is more predictive of outcomes compared to the least central symptom. Two caveats, however, should be noted. One, in some studies, pretreatment centrality did not predict change in symptoms measured by assessments that were not included in the original network (e.g., Rodebaugh et al., 2019). Although our three indicators of remission status were not included in the network, they were all derived from nodes that were included in the network (binge-eating abstinence was derived from binge-eating frequency, EDE Global scores were derived from the four EDE subscales). Ideally, studies determining whether symptom centrality corresponds to treatment outcomes would operationalize treatment outcomes using measures that are completely distinct from measures used to estimate networks. Whether pretreatment centrality predicts change in BED-relevant outcomes not included in the networks here remains an empirical question for future research. Two, symptom centrality may change throughout treatment (Nemesure et al., preprint). Much remains to be learned about network dynamics during treatment and how matching treatments to network dynamics may influence treatment outcomes.
In both treatment samples, pretreatment and posttreatment networks did not differ in global strength. These findings were unexpected and contrast with those reported by Forrest & Grilo (2022) and Hilbert et al. (2020), which found that global strength significantly increased following treatment. Research comparing symptom networks before and after treatment is in its early stages, and we do not yet know whether significant global or node-wise centrality changes have clinical relevance. Indeed, some authors question whether centrality is a meaningful metric overall (Bringmann et al., 2019). Global centrality decreases could mean a significantly disrupted symptom network (Borsboom, 2017) while global centrality increases could indicate increased likelihood for a significant transition toward symptom change (Groen et al. 2019), both of which could have clear clinical relevance. However, global centrality increases could also indicate less clinically relevant information, such as effects of repeated measurements (Fried et al., 2016). Similarly, changes in node centrality following treatment could signal that the symptom network transitioned from an “active disease” state, demarcated by one symptom being highly central, to a “recovered disease” state, demarcated by a different symptom being highly central (c.f. Borsboom, 2017), or could have no association with treatment response or symptom severity. We—and these previous studies—are unable to address these questions, as our data only allow for estimating between-subjects networks. However, these questions could be answered by estimating idiographic networks, constructed from intensive longitudinal data collected throughout treatment (e.g., DuBois et al., in press). This would allow for comparison of treatment outcomes and prognosis between patients whose global centrality and pattern of centrality did vs. did not change throughout treatment. We contend that idiographic networks hold great promise in clarifying the current debate surrounding the clinical utility of centrality (McNally, 2021; Robinaugh et al., 2020).
Comparisons of symptom networks between CBT and IPT revealed no significant differences in global strength or centrality at pretreatment. At posttreatment, the CBT network was more strongly connected than the IPT network but only the eating concern node had significantly different expected influence between samples. One potential interpretation of these quite similar node centrality findings is that CBT and IPT impact relationships amongst BED symptoms in a similar fashion. In other words, the partial correlations for how weight concern relates to depression, shape concern, and restraint are roughly similar in CBT and IPT. This parallels findings that CBT and IPT reduce the intensities of the symptoms quite similarly in time course and outcome (Wilfley et al., 2002; Wilson et al., 2010). We view our findings as very preliminary because our data are not able to address mechanisms of change. We encourage future research to include individual items (as compared to subscales) in networks to allow for more fine-grained understanding of how CBT vs. IPT impact connectivity among specific types of symptoms emphasized in each treatment (e.g., overvaluation vs. dissatisfaction in CBT, negative social evaluation vs. negative self-evaluation in IPT).
Study strengths include having two treatment samples. Our measures of BED symptoms and depression are validated, which increases confidence in the measurement of symptoms. The CBT sample size of 236 participants is the largest sample to compare pretreatment and posttreatment BED symptom networks to date, which further increases confidence in those findings. Although the aggregated treatment data allowed us to perform this first analysis of how distinct treatments impact BED symptom connectivity, the data also have limitations. One, although we included a node assessing depression severity, which has relevance as an IPT construct, an IPT-specific measure was not available across studies. The lack of an IPT-specific measure may limit the interpretation of the IPT sample results. Similarly, the large amount of missingness on depression is a limitation of the study. Two, we did not have access to individual EDE items/constructs, which provide more fine-grained information on how treatment impacts specific BED symptom connectivity (e.g., Forrest & Grilo, 2022). Three, the sample was primarily educated, White women. Findings should not be assumed to be generalizable to people with other intersectional identities. Similarly, we recognize that describing specific racial/ethnic identities is preferable to categorizing any racial/ethnic identity as “other.” Regrettably, these data were originally merged such that people who reported a racial/ethnic identity other than White, Black, Native American, or Hispanic were coded only as “other” and we do not have the ability to determine these people’s specific racial/ethnic identities. Four, one correlation between centrality and variance was significant and positive, though 31 of the other correlations did not support a positive relation between centrality and node variance.
In summary, there were many similarities in global network connectivity and node centrality before and after CBT and IPT for BED. Pretreatment network centrality significantly predicted posttreatment BED symptom severity. Conceptualizing BED as a network of interconnected symptoms sets the stage for new research to understand how distinct treatments impact interrelations among symptoms and what symptoms may be the best targets within and across treatments.
Supplementary Material
Public Health Significance.
Cognitive behavioral therapy and interpersonal therapy for binge-eating disorder, which are two leading evidence-based treatments for binge-eating disorder that are quite different in their models and approaches, similarly impacted interrelations among binge-eating disorder symptoms. In addition, the most strongly interconnected symptom predicted change in binge-eating disorder symptom severity. Studying the interrelations among symptoms may provide new insight on how treatments impact symptom relationships and inform intervention targets.
Funding:
This research was supported by NIH grant (HT-B, R03 MH083987). Dr Grilo was also supported, in part, by NIH grants R01 DK49587 and R01 DK114075. Funding agencies played no role in the content of this paper.
Footnotes
Potential Conflicts of interest: The authors declare no conflicts of interest. Dr. Grilo reports broader interests unrelated to this research, including honoraria for lectures and CME activities, and Royalties from Guilford Press and Taylor & Francis Publishers for academic books.
Although outside of a network analysis context there are multiple options to correct for multiple comparisons (e.g., Bonferroni correction), the NetworkComparisonTest package provides only the Benjamini Hochberg method to correct for multiple comparisons.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Anderson LM, Smith KM, Schaefer LM, Crosby RD, Cao L, Engel SG,…& Peterson CB (2020). Predictors and moderators of treatment outcome in a randomized clinical trial for binge-eating disorder. Journal of Consulting and Clinical Psychology, 88, 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. [Google Scholar]
- Borsboom D (2017). A network theory of mental disorders. World Psychiatry, 16, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D & Cramer AO (2013). Network analysis: an integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. [DOI] [PubMed] [Google Scholar]
- Borsboom D, Mellenbergh GJ & van Heerden J (2003). The theoretical status of latent variables. Psychology Review, 110, 203–219. [DOI] [PubMed] [Google Scholar]
- Bringmann LF, Elmer T, Epskamp S, Krause RW, Schoch D, Wichers M, Wigman JTW, & Snippe E (2019). What do centrality measures measure in psychological networks? Journal of Abnormal Psychology, 128, 892–903. [DOI] [PubMed] [Google Scholar]
- Brody ML, Masheb RM, & Grilo CM (2005). Treatment preferences of patients with binge eating disorder. International Journal of Eating Disorders, 37, 352–356. [DOI] [PubMed] [Google Scholar]
- Brown TA, Vanzhula IA, Reilly EE, Levinson CA, Berner LA, Krueger A, … & Wierenga CE (2020). Body mistrust bridges interoceptive awareness and eating disorder symptoms. Journal of Abnormal Psychology, 129, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois RH, Rodgers RF, Franko DL, Eddy KT, & Thomas JJ (2017). A network analysis investigation of cognitive-behavioral theory of eating disorders. Behaviour Research and Therapy, 97, 213–221. [DOI] [PubMed] [Google Scholar]
- DuBois RH, Rodgers RF, Fuller-Tyszkiewicz M, Shiyko M, Franko DL (in press). The relationship between moment-by-moment symptom connectivity and global symptom severity: An exploratory network analysis among eating disorder symptoms. International Journal of Eating Disorders. [DOI] [PubMed] [Google Scholar]
- Elliott H, Jones PJ & Schmidt U (2020a). Central symptoms predict posttreatment outcomes and clinical impairment in anorexia nervosa: A network analysis. Clinical Psychological Science, 8, 139–154. [Google Scholar]
- Elliott H, Jones PJ, & Schmidt U (2020b). Corrigendum: central symptoms predict posttreatment outcomes and clinical impairment in anorexia nervosa: A network analysis. Clinical Psychological Science, 8, 388–389. [Google Scholar]
- Epskamp S, Borsboom D, & Fried EI (2018). Estimating psychological networks and their accuracy: A tutorial paper. Behavior Research Methods, 50, 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S, Cramer AOJ, Waldorp LJ, Schmittmann VD, & Borsboom(2012). qgraph: network visualizations of relationships in psychometric data. Journal of Statistical Software, 48(4), 1–18. [Google Scholar]
- Fairburn CG, & Cooper Z (1993). The eating disorder examination. In Fairburn CG & Wilson GT (Eds.), Binge eating: Nature, assessment, and treatment (pp. 317–360). NY: Guilford Press. [Google Scholar]
- Fairburn CG, Cooper Z, & Shafran R (2003). Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behaviour Research and Therapy, 41, 509–528. [DOI] [PubMed] [Google Scholar]
- Forrest LN, & Grilo CM (2022). Change in eating-disorder psychopathology network structure in patients with binge-eating disorder: findings from treatment trial with 12-month follow-up. Journal of Consulting and Clinical Psychology, 90, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LN, Perkins NM, Lavender JM & Smith AR (2019). Using network analysis to identify central eating disorder symptoms among men. International Journal of Eating Disorders, 52, 871–884. [DOI] [PubMed] [Google Scholar]
- Franko DL, Thompson-Brenner H, Thompson DR, Boisseau CL, Davis A, Forbush KT, et al. (2012). Racial/ethnic differences in adults in randomized clinical trials of binge eating disorder. Journal of Consulting and Clinical Psychology, 80, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, van Borkulo CD, Epskamp S, Schoevers RA, Tuerlinckx F & Borsboom D (2016). Measuring depression over time . . . Or not? Lack of unidimensionality and longitudinal measurement invariance in four common rating scales of depression. Psychological Assessment, 28, 1354–1367. [DOI] [PubMed] [Google Scholar]
- Grilo CM (2013). Why no cognitive body image feature such as overvaluation of shape/weight in the binge eating disorder diagnosis? International Journal of Eating Disorders, 46, 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM & Crosby RD (2012). Predictors and moderators of response to cognitive behavioral therapy and medication for the treatment of binge eating disorder. Journal of Consulting and Clinical Psychology, 80, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, & Wilson GT (2001). Subtyping binge eating disorder. Journal of Consulting and Clinical Psychology, 69, 1066–1072. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Wilson GT, Gueorguieva R, & White MA (2011). Cognitive-behavioral therapy, behavioral weight loss, and sequential treatment for obese patients with binge-eating disorder: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 79, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Thompson-Brenner H, Shingleton RM, Thompson DR, & Franko DL (2021). Clinical moderators and predictors of cognitive-behavioral therapy by guided self-help versus therapist led for binge-eating disorder: analysis of aggregated clinical trials. International Journal of Eating Disorders, 54, 1875–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, White MA, Masheb RM, Ivezaj V, Morgan PT & Gueorguieva R (2020). Randomized controlled trial testing the effectiveness of adaptive “SMART” stepped-care treatment for adults with binge-eating disorder comorbid with obesity. American Psychologist, 75, 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen RN, Snippe E, Bringmann LF, Simons CJP, Hartmann JA, Bos EH, & Wichers M (2019). Capturing the risk of persisting depressive symptoms: A dynamic network investigation of patients’ daily symptom experiences. Psychiatry Research, 271, 640–648. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert A, Herpertz S, Zipfel S, Tuschen-Caffier B, Friederich H-C, Mayr A, & de Zwaan M (2020). Psychopathological networks in cognitive-behavioral treatments for binge-eating disorder. Psychotherapy and Psychosomatics, 89, 379–385. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Petroff D, Herpertz S, Pietrowsky R, Tuschen-Caffier B, Vocks S & Schmidt R (2019). Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. Journal of Consulting and Clinical Psychology, 87, 91–105. [DOI] [PubMed] [Google Scholar]
- Hrabosky JI, Masheb RM, White MA, & Grilo CM (2007). Overvaluation of shape and weight in binge eating disorder. Journal of Consulting and Clinical Psychology, 75, 175–180. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG & Agras WS (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59, 877–83. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82, 1–26. [Google Scholar]
- Levinson CA, Hunt RA, Christian C, Williams BM, Keshishian AC, Vanzhula IA, & Ralf-Nearman C (2022). Longitudinal group and individual networks of eating disorder symptoms in individuals diagnosed with an eating disorder. Journal of Psychopathology and Clinical Sciences, 131, 58–72. [DOI] [PubMed] [Google Scholar]
- Linardon J (2018). Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: Meta-analysis. International Journal of Eating Disorders, 51, 785–797. [DOI] [PubMed] [Google Scholar]
- Linardon J, de la Piedad Garcia X & Brennan L (2017). Predictors, Moderators, and mediators of treatment outcome following manualised cognitive-behavioural therapy for eating disorders: a systematic review. European Eating Disorders Review, 25, 3–12. [DOI] [PubMed] [Google Scholar]
- Mares SHW, Burger J, Lemmens LHJM, van Elburg AA, & Vroling MS (2022). Evaluation of the cognitive behavioural theory of eating disorders: A network analysis investigation. Eating Behaviors, 44: 101590. [DOI] [PubMed] [Google Scholar]
- Mason TB, Smith KE, Anderson LM, Schaefer L, M., Engel SG, Crow SJ, … & Wonderlich SA (2021). Affective response to binge eating as a predictor of binge eating disorder treatment outcome. Clinical Psychological Science, 9, 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ (2021). Network analysis of psychopathology: Controversies and challenges. Annual Review of Clinical Psychology, 17, 31–53. [DOI] [PubMed] [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, & Owen C (2006). Eating Disorder Examination Questionnaire (EDE-Q): Norms for young adult women. Behaviour Research and Therapy, 44, 53–62. [DOI] [PubMed] [Google Scholar]
- Nemesure MD, Collins AC, Price G, Griffin TZ, Pillai A, Nepal S, … Jacobson NC (preprint). Depressive symptoms as a heterogeneous and constantly evolving dynamical system: idiographic depressive symptom networks of rapid symptom changes among persons with major depressive disorder. 10.31234/osf.io/pf4kc [DOI] [PMC free article] [PubMed]
- Papini S, Rubin M, Telch MJ, Smits JAJ, & Hien DA (2020). Pretreatment posttraumatic stress disorder symptom network metrics predict the strength of the association between node change and network change during treatment. Journal of Traumatic Stress, 33, 64–71. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Huckins G, Varoquaux G (2020). Establishment of best practices for evidence for prediction: A review. JAMA Psychiatry, 77, 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, & Herman CP (2006). An evolutionary perspective on dieting. Appetite, 47, 30–35. [DOI] [PubMed] [Google Scholar]
- Putterman E, & Linden W (2004). Appearance versus health: Does the reason for dieting affect dieting behavior? Journal of Behavioral Medicine, 27, 185–204. [DOI] [PubMed] [Google Scholar]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Robinaugh DJ, Hoekstra RHA, Toner ER, & Borsboom D (2020). The network approach to psychopathology: A review of the literature 2008 – 2018 and an agenda for future research. Psychological Medicine, 50, 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinaugh DJ, Millner AJ, & McNally RJ (2016). Identifying highly influential nodes in the complicated grief network. Journal of Abnormal Psychology, 125, 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh TL, Tonge NA, Piccirillo ML, Fried E, Horenstein A, Morrison AS, Goldin P, Gross JJ, Lim MH, Fernandez K, & Blanco C (2019). Does centrality in a cross-sectional network suggest intervention targets for social anxiety disorder? Journal of Consulting and Clinical Psychology, 86(10), 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingleton RM, Thompson-Brenner H, Thompson DR, Pratt EM, & Franko DL (2015). Gender differences in clinical trials of binge eating disorder: an analysis of aggregated data. Journal of Consulting and Clinical Psychology, 83, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slof-Op’t Landt MCT, Dingemans AE, de la Torre Y Rivas J, van Furth EF (2019). Self-assessment of eating disorder recovery: Absence of eating disorder psychopathology is not essential. International Journal of Eating Disorders, 52, 956–961. [DOI] [PubMed] [Google Scholar]
- Terluin B, de Boer MR, & de Vet HCW (2016). Differences in connection strength between mental symptoms might be explained by differences in variance. Reanalysis of network data did not confirm staging. PLoS ONE, 11: e1055205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Brenner H, Franko DL, Thompson DR, Grilo CM, Boisseau CL, Roehrig JP, et al. (2013). Race/ethnicity, education, and treatment parameters as moderators and predictors of outcome in binge disorder. Journal of Consulting and Clinical Psychology, 81, 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T & Grilo CM (2018). Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biological Psychiatry, 84, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T & Grilo CM (2019). Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. International Journal of Eating Disorders, 52, 42–50. [DOI] [PubMed] [Google Scholar]
- van Borkulo CD, Boschloo L, Kossakowski JJ, Tio P, Schoevers RA, Borsboom D, & Waldorp LJ (2021). Comparing network structures on three aspects: A permutation test. Psychological Methods. DOI: 10.1037/met0000476 [DOI] [PubMed] [Google Scholar]
- van Buuren S, & Groothuis-Oudshoorn K (2011). mice: Multivariate imputation by chained equations in R, Journal of Statistical Software, 45, 1–67. [Google Scholar]
- Wang SB, Jones PJ, Dreier M, Elliott H & Grilo CM (2019). Core psychopathology of treatment-seeking patients with binge-eating disorder: a network analysis investigation. Psychological Medicine, 49, 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant graphics for data analysis. Springer-Verlag: New York. [Google Scholar]
- Wickham H, François R, Henry L, & Müller K (2021). dplyr: A grammar of data manipulation. R package version 1.0.5. https://CRAN.R-project.org/package=dplyr [Google Scholar]
- Wilfley DE, Welch RR, Stein RI, Spurrell EB, Cohen LR, Saelens B,…& Matt GE (2002). A randomized comparison of group cognitive-behavioral therapy and group interpersonal psychotherapy for the treatment of overweight individuals with binge-eating disorder. Archives of General Psychiatry, 59, 713–721. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Grilo CM, & Vitousek KM (2007). Psychological treatment of eating disorders. American Psychologist, 62, 199–216. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Wilfley DE, Agras WS, & Bryson SW (2010). Psychological treatments of binge eating disorder. Archives of General Psychiatry, 67, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


