Abstract
Objectives:
E-cigarettes are the most commonly used nicotine containing products among youth. In vitro studies support the potential for e-cigarettes to cause cellular stress in vivo; however, there have been no studies to address whether exposure to e-liquid aerosols can induce cell transformation, a process strongly associated with pre-malignancy. We examined whether weekly exposure of human bronchial epithelial cell (HBEC) lines to e-cigarette aerosols would induce transformation and concomitant changes in gene expression and promoter hypermethylation.
Materials and methods:
An aerosol delivery system exposed three HBEC lines to unflavored e-liquid with 1.2% nicotine, 3 flavored products with nicotine, or the Kentucky reference cigarette once a week for 12 weeks. Colony formation in soft agar, RNA-sequencing, and the EPIC Beadchip were used to evaluate transformation, genome-wide expression and methylation changes.
Results:
Jamestown e-liquid aerosol induced transformation of HBEC2 and HBEC26, while unflavored and Blue Pucker transformed HBEC26. Cigarette smoke aerosol transformed HBEC4 and HBEC26 at efficiencies up to 3-fold greater than e-liquids. Transformed clones exhibited extensive reprogramming of the transcriptome with common and distinct gene expression changes observed between the cigarette and e-liquids. Transformation by e-liquids induced alterations in canonical pathways implicated in lung cancer that included axonal guidance and NRF2. Gene methylation, while prominent in cigarette-induced transformed clones, also affected hundreds of genes in HBEC2 transformed by Jamestown. Many genes with altered expression or epigenetic-mediated silencing were also affected in lung tumors from smokers.
Conclusions:
These studies show that exposure to e-liquid aerosols can induce a pre-malignant phenotype in lung epithelial cells. While the Food and Drug Administration banned the sale of flavored cartridge-based electric cigarettes, consumers switched to using flavored products through other devices. Our findings clearly support expanding studies to evaluate transformation potency for the major categories of e-liquid flavors to better inform risk from these complex mixtures.
Keywords: flavored e-liquids, transformation, human bronchial epithelial cell line, pre-malignancy, methylation, transcriptional reprogramming
1. Introduction
Electronic cigarettes (e-cigarettes) are the most commonly used nicotine containing product among youth with 4.7% and 19.6% of U.S. middle-school and high school students, respectively reporting use in 2020 in the past 30 days [1]. In addition, in 2019, 3.2% of U.S. adults (non-Hispanic White, African American, and Hispanics) were current e-cigarette users [2]. While generally thought to be a safer alternative to cigarettes, e-cigarettes contain nicotine and users have access to over 7,000 unique flavorings that along with nicotine liquid can be added to the humectants propylene glycol (PG) and vegetable glycerin (VG) to comprise the complex e-liquid that is then vaporized and inhaled into the lungs [3]. Heating the e-liquid also causes thermal decomposition of the PG and VG to generate carbonyls that include the carcinogens formaldehyde and acetaldehyde and the toxicant acrolein [4, 5].
In vitro studies have compared total particulate matter (TPM) or complete aerosol generated from cigarettes and e-cigarettes for a multitude of effects on normal cell functions [reviewed in 6]. Scheffler et al. [7] exposed two primary undifferentiated bronchial epithelial cell lines at the air-liquid interface to aerosol from a cigarette (60 puffs) or an e-cigarette (200 puffs) and observed 24 h after exposure significant levels of cytotoxicity and oxidative stress for both products, albeit much greater effects with the cigarette. E-cigarette effects were similar with and without nicotine. Similar studies using H292 cells, a human pulmonary carcinoma cell line showed reduced cell viability and release of inflammatory cytokines [8]. The Calu3 adenocarcinoma cell line showed dose-dependent reductions in proliferation and viability in response to 13 different flavored e-liquids following direct administration or aerosol delivery of 0–35 puffs 24 h after exposure [9]. The magnitude of response varied by e-liquid likely reflecting differences in chemical profile across products. Sundar et al. [10] showed that aerosol exposure to e-cigarettes, particular those with flavoring, induce inflammation and pro-senescence responses in oral epithelial cells. Our recent study showed heterogeneity across ten diverse flavored e-liquids in producing cytotoxicity and genotoxicity in three immortalized oral epithelial cell lines exposed to the generated aerosols [11]. Exposures across studies were lower than daily consumption by many e-cigarette users, 23% and 8% who report consuming 4–5 mls or > 8 mls of e-liquid per day that equates to 1200 and 2000 puffs [12]. All these findings support the potential for e-cigarettes to cause cellular stress in vivo, but there have been no studies to address whether exposure to e-liquid aerosols can induce cell transformation, a process strongly associated with pre-malignancy.
Our group developed an in vitro pre-malignancy model to study genotoxicity, transformation, and associated changes in cell regulation using immortalized HBEC lines. Those studies treated HBECs weekly (12 wk) with genotoxic, but not cytotoxic doses of the carcinogens methylnitrosourea (MNU) and benzo(a)pyrene-diolepoxide 1 (BPDE) to identify key molecular changes that drive transformation and clonal outgrowth of pre-neoplastic cells during exposure to specific carcinogens in tobacco smoke [13-17]. These direct acting carcinogens mirror the DNA damage profiles occurring with NNK and B[a]P.
This pre-malignancy model was used to extend our work to examine potential health effects of flavored e-cigarette products by evaluating whether weekly exposure of HBEC lines to e-cigarette aerosols induces transformation and concomitant changes in gene expression and promoter hypermethylation. Comparative studies were conducted using an aerosol delivery system that exposed three immortalized HBEC lines to unflavored e-liquid with 1.2% nicotine, 3 flavored products with nicotine, or the Kentucky reference cigarette.
Materials and methods
2.1. Cell culture
HBEC2, HBEC4, and HBEC26 immortalized with hTERT and CDK4 were obtained from Drs. Shay and Minna, Southwestern Medical Center, Dallas, TX. The A549 cell line was obtained from American Type Culture Collection. Cell culture conditions have been described [13]. Cells tested negative for mycoplasma using MycoProbe Detection Kit (R&D Systems).
2.2. Flavor chemicals
Three e-liquids were obtained from AVAIL VAPOR LLC who provides research grade products that come with certificate of analysis confirming nicotine content and lack of diacetyl (< 38 ppm) and its alternative acetyl propionyl (< 100 ppm, [18]). The ratio of PG and VG selected was 30%:70% as the vehicle along with a freebase nicotine content of 1.2% (12 mg/ml). This PG/VG ratio achieves a good vapor as characterized by a visible cloud and the nicotine content is common with never smoker first-time users [19]. This vehicle with nicotine also served as the unflavored product for all studies. The selection of the e-liquids was based on the diversity of chemical constituents in the products determined previously [19]. The selected flavored e-liquids in 30%PG/70%VG with nicotine were Blue Pucker (fruit flavor), Jamestown (tobacco flavor), and Mardi Gras (fruit flavor).
2.3. Aerosol Exposure
The aerosol generation system consists of an aerosol exposure chamber, a Joyetech eGo ONE 1100 mAh e-cigarette with a 2.5 ml tank capacity and a 0.5-ohm stainless-steel atomizer (the battery was replaced by a DC regulated power supply), a mixing chamber, and a solenoid valve controlled by a relay [11]. The concentration in the exposure chamber is controlled by the flow rate of dilution air, while the puffing protocol is controlled by the solenoid valve and its relay. The aerosols were generated at 5 volts using a puffing protocol based on the topography results of e-cigarette users consisting of ~52 ml puffs of 2.6 second duration at intervals of 18 seconds [20]. The amount and concentration of aerosol delivered was quantified by collection of TPM onto a filter. Cells were exposed to HEPA-filtered air only or e-liquid for 20 minutes (reflecting an average smoking period for an e-cigarette user) to a target aerosol dose of 450 mg TPM/m3. This exposure system accommodates culture dishes up to 100 mm and cells are attached to the surface of the plate covered with a small volume of serum free RPMI media during the exposure for the various assays and endpoints. The tops of the culture dishes are removed prior to placement in the exposure box. An orbital shaker facilitates exchange between the exposure atmosphere and cell media. The use of undifferentiated cells allows the assessment of endpoints such as growth in soft agar following exposure. The Kentucky reference 3R4F cigarette was used as a positive control for all assays. Target dose for exposure was reduced 200 mg TPM/m3 to avoid overt toxicity at the higher doses.
2.4. Soft agar colony formation
Soft agar assays were conducted as described previously [13]. Cultures were photographed and the colonies with diameters larger than 100 μm were counted using ImageJ software.
2.5. Oxidative Stress
Oxygen species are highly reactive (e.g., superoxide, singlet oxygen) and have a short half-life in solution with most being converted to H2O2 [21]. The ROS-Glo™ assay (Promega) uses an H2O2 substrate that directly reacts with H2O2 to produce a luciferin precursor that is then reacted with a detection solution to convert the precursor to luciferin. This provides luciferase to generate a light signal proportional to the level of H2O2 present. Cells at approximately 60% confluence in 48-well dishes containing 100 μl of RPMI serum free media were exposed in quadruplicate for 20 min. The exposure media was removed (no wash) and replaced with fresh media and incubated for 90 min for detection and H2O2 was then quantified as relative light units (RLU). The number of viable cells quantified by Cell-Titer assay (Promega) was used to normalize the RLUs detected.
2.6. DNA Strand Breaks
The alkaline comet assay is used for the detection of DNA strand breaks in cells or nuclei following exposure to potentially genotoxic materials [22]. Under alkaline conditions (>pH 13), the comet assay can detect single and double stranded breaks, resulting, for example, from direct interactions with DNA, alkali labile sites or as a consequence of transient DNA strand breaks resulting from DNA excision repair [22]. Cells at approximately 60% confluence in 6-well dishes containing 1 ml of RPMI serum free media were exposed in triplicate, removed from the plate by scraping, and the Trevigen Alkaline Comet Assay kit was used to conduct the comet assay. Comet images were captured at 4× magnification on a microscope equipped with epifluorescence and a CCD camera. All slides for analysis were randomly coded and scored “blinded” to prevent scoring bias. At least 150 scoreable comets without overlapping tails and not located at the edge of the slides per treatment per cell line were analyzed using Trevigen Comet Analysis Software. The percent tail DNA (also known as percent tail intensity) was used for the evaluation and interpretation of results, and was determined by the DNA fragment intensity in the tail expressed as a percentage of the cell's total intensity.
2.7. Cytochrome P450 reductase assay
A colorimetric assay (Abcam) was used to determine cytochrome P450 reductase activity in HBEC lines and the A549 adenocarcinoma cell line. Cytochrome P450 reductase activity was measured in a kinetic mode over 30 minutes using cell lysates incubated with NADPH and the addition of glucose-6-phosphate to start the reaction as described in the kit. Reductase activity (nm/mg protein) was determined by comparing absorbance reading to a standard curve generated with known concentrations of glucose-6-phosphate and NADPH.
2.8. Gene expression
Total RNA was isolated from transformed clones and air exposed HBEC lines and quantified using a NanoDrop 2000 spectrophotometer and Qubit 2.0 Fluorometer (Life Technologies). RNA (1 μg) was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Life Technologies). RT-qPCR was carried out with inventoried TaqMan assays (Life Technologies). Experiments were performed in triplicate and normalized to β-Actin using the 2(−ΔΔCt) method.
2.9. Gene transcriptome profiling
RNA integrity for sequencing was assessed with an Agilent Tape Station and an RNA Integrity Number and DV200 index was used to determine quality. RNA libraries with PolyA selection were prepared and sequenced at 150 bp paired-end runs at a target depth of 30 million reads per sample using an Illumina HiSeq (Genewiz, San Diego, CA).
2.10. Gene methylation profiling
DNA isolated from transformed clones and air exposed HBEC lines was bisulfite modified and hybridized to the Illumina Infinium Methylation EPIC Beadchip. Idat files were exported from Genome Studio (Illumina) and preprocessed using the normal-exponential out-of-band method for background correction with dye-bias normalization from the minfi package in R (3.3.2) to generate β-values for 863,804 probes with a detection p-value <0.01.
2.12. Identification of methylated genes with reduced expression from the TCGA dataset
We analyzed the TCGA dataset generated with the Illumina HM450K Beadchip array that interrogated 811 adenocarcinomas and/or squamous cell carcinomas for genome-wide methylation changes. The approach described previously identified 3,759 methylated genes with loss of expression ≥2-fold for comparison to methylated genes in transformed HBECs [17].
2.13. Statistical analysis
ROS and comet values for each cell line was analyzed separately, and fold change for each product was calculated by normalizing to the average of the air control for that cell line. T-test was used to evaluate significance compared to air and between products (n=12/exposure).
Due to the strong association between methylation of CpGs around the transcriptional start site (TSS) and gene silencing, our analytic strategy for methylation data focused on this region to assess the methylation status of 211,289 CpG oligonucleotide probes within 200 base pairs 5' of the TSS and extending through the first exon as described [23]. Briefly, average signal intensity between methylated and unmethylated probes was determined, and β-values from 0-1 (fully methylated) were calculated. Methylation analysis was restricted to probes in vehicle treated cell lines with β-value <0.2. Probes with delta β-values ≥ 0.2 (or 20%) in transformed HBECs were considered to be methylated.
RNA sequencing data were analyzed using Illumina’s cloud-based genomics-computing environment. Sequencing reads were filtered and trimmed using Bowtie, aligned to a human reference genome HG38 using STAR, and quantified using Illumina’s DRAGEN RNA Pipeline v3.6.3. The count-based statistical method DESeq2 in Illumina’s DRAGEN Differential Expression app v3.6.3 normalizes sequencing values for gene length and number of reads and was used to identify differences (fold changes) between transformed clones relative to controls. DeSeq2 uses the Wald test for significance testing and Benjamini-Hochberg method to determine the false discovery rate (FDR). An FDR <0.05 was used as the cut-off for identifying differentially expressed genes.
Qiagen Ingenuity Pathway Analysis software was used to identify pathways and networks statistically over-represented in the lists of differentially expressed genes from the RNA-seq. Statistical analyses were conducted in SAS 9.4 and R 3.3.2
3. Results
3.1. Cytochrome P450 profile and reductase activity of HBECs
The focus of this study was to evaluate the potency of e-liquid aerosols with respect to inducing transformation and gene expression profiles with comparison to cigarette smoke aerosol. Because cigarette smoke aerosol contains procarcinogens requiring metabolism, RNA sequencing was performed on selected HBEC lines (HBEC2, HBEC4, and HBEC26) to characterize the expressed cytochrome P450s (CYP). Counts from duplicate sequencing were used to display the results that showed 31, 41, and 34 of the 47 annotated CYPs expressed in HBEC2, HBEC4, and HBEC26, respectively. Table 1 compares relative expression across cell lines for the 26 CYP commonly expressed. Briefly, CYP1A1 and CYP1B1 that metabolize PAHs and aromatic amines showed a 10-fold and 2-fold difference in expression across HBECs (Table 1 [24]). CYP2E1 that metabolizes nitrosamines was expressed at relatively low levels, but present in all HBEC lines (Table 1 [24]). CYP2A6 and CYP2A13 that also metabolize nitrosamines were only expressed in HBEC4.
Table 1.
Relative Expression of Cytochrome P450s in HBEC Lines
| Cell Lines | |||
|---|---|---|---|
| Gene | HBEC2 | HBEC4 | HBEC26 |
| (Counts) | |||
| CYP1A1 | 10 | 41 | 127 |
| CYP1B1 | 1142 | 536 | 657 |
| CYP20A1 | 223 | 153 | 117 |
| CYP24A1 | 52 | 18 | 14 |
| CYP26B1 | 7 | 59 | 13 |
| CYP27A1 | 1 | 5 | 39 |
| CYP27B1 | 10 | 218 | 267 |
| CYP27C1 | 365 | 5 | 4 |
| CYP2B7P | 6 | 48 | 1 |
| CYP2D7 | 10 | 50 | 9 |
| CYP2E1 | 2 | 9 | 5 |
| CYP2J2 | 1 | 34 | 28 |
| CYP2R1 | 88 | 310 | 185 |
| CYP2S1 | 161 | 151 | 430 |
| CYP2T1P | 1 | 6 | 4 |
| CYP2U1 | 341 | 195 | 300 |
| CYP39A1 | 9 | 130 | 19 |
| CYPA5 | 4 | 5 | 5 |
| CYP4B1 | 1 | 49 | 11 |
| CYP4F11 | 11 | 932 | 70 |
| CYP4F12 | 3 | 3 | 20 |
| CYP4F26P | 9 | 37 | 37 |
| CYP4F3 | 2 | 6 | 136 |
| CYP4V2 | 98 | 410 | 10 |
| CYP4X1 | 49 | 14 | 59 |
| CYP51A1 | 887 | 5108 | 902 |
Cytochrome P450 reductase, required for electron transfer of NADPH to CYP in xenobiotic metabolism, is often lost in cell lines established from malignant tumors. Enzymatic activity determined in cell lysates showed reductase activity was detected and similar (7.8–8.5 nM/mg protein) across HBECs and not detected in the A549 lung adenocarcinoma cell line (Suppl. Fig. 1). P450 reductase levels in HBECs were also similar to that observed in normal lung tissue [25].
3.2. Effect of E-liquid and cigarette aerosols on DNA damage and oxidative stress
Our prior studies exposing oral epithelial cell lines to e-liquid aerosols showed non-significant increases in DNA damage and oxidative stress at the 450 mg TPM/m3, the highest level studied that approximates exposures by some users [11]. The alkaline comet chip assay, that detects DNA strand breaks, was used to assess DNA damage in HBECs exposed to 450 mg TPM/m3. There was no significant increase in DNA damage by unflavored or any e-liquid across the three HBEC lines (Suppl. Table 1). In contrast, exposure of HBECs to the Kentucky reference cigarette aerosol at this dose generated comets in 100% of cells indicative of high levels of genotoxicity and accompanied by cytotoxicity. A dose of 200 mg TPM/m3 was found to induce a 4–11-fold increase in DNA strand breaks across cell lines in the absence overt cytotoxicity (Suppl. Table 1). Similar studies evaluating oxidative stress by the ROS-Glo assay showed modest, albeit significant increases of 1.3–2.0 fold across e-liquid products for HBEC4 and HBEC26 (Table 2). In contrast, 4.5–7-fold increase in oxidative stress was seen in response to cigarette smoke aerosol (Table 2).
Table 2.
Induction of Oxidative Stress in HBEC Lines Exposed to Cigarette or E-liquid Aerosols
| Cell Lines | |||
|---|---|---|---|
| Exposure | HBEC2 | HBEC4 | HBEC26 |
| (Fold Change Compared to Air) | |||
| Cigarette1 | 7.0 ± 1.7* | 4.5 ± 1.0* | 4.8 ± 1.2* |
| E-Liquid | |||
| Unflavored | 1.5 ± 0.5 | 1.3 ± 0.2* | 1.5 ± 0.2* |
| Blue Pucker | 1.5 ± 0.7 | 1.5 ± 0.2* | 1.7 ± 0.4* |
| Jamestown | 1.6 ± 0.4 | 1.6 ± 0.2* | 2.0 ± 0.5* |
| Mardi Gras | 1.5 ± 0.5 | 1.6 ± 0.3* | 1.8 ± 0.4* |
Values are mean ± SD for n=12/exposure
Kentucky reference cigarette
p <0.05 compare to air
3.3. Transformation of immortalized human bronchial epithelial cell lines
Doses used to characterize DNA damage and oxidative stress were selected for the transformation studies. Cell lines were exposed in triplicate once a week for 12 weeks to air, unflavored e-liquid with 1.2% nicotine, the three flavored e-liquids containing 1.2% nicotine, or the Kentucky reference cigarette. Jamestown induced colony formation across all replicates in HBEC2 (average of 36 colonies) and HBEC26 (average of 21 colonies [Table 3]). An average of 15 and 22 colonies were seen for unflavored and Blue Pucker in HBEC26 exposed cells, while no colonies were seen with Mardi Gras. In addition, none of the products induced transformation in HBEC4. Cigarette smoke aerosol did not transform HBEC2, but induced colony formation in HBEC4 and HBEC26 at efficiencies up to 3-fold greater than e-liquids (Table 3).
Table 3.
Effect of Cigarette and E-liquid Aerosol on Transformation of HBEC Lines
| Cell Lines | |||
|---|---|---|---|
| Exposure2 | HBEC2 | HBEC4 | HBEC26 |
| (Number of Soft Agar Colonies) | |||
| Air | 0 | 0 | 0 |
| Cigarette1 | 0 | 99 ±16 | 53 ± 3 |
| E-Liquid | |||
| Unflavored | 0 | 0 | 15 ± 4 |
| Blue Pucker | 0 | 0 | 22 ± 2 |
| Jamestown | 36 ± 16 | 0 | 21 ± 3 |
| Mardi Gras | 0 | 0 | 0 |
Values are mean ± SD from n=3
Kentucky reference cigarette
Mean ± SD for actual exposure dose achieved over the 12 weeks were 204±8, 463±23, 454±21, 447±24, and 454±23 for the Cigarette, Unflavored, Blue Pucker, Jamestown, and Mardi Gras, respectively.
3.4. Global reprogramming of the transcriptome in transformed clones
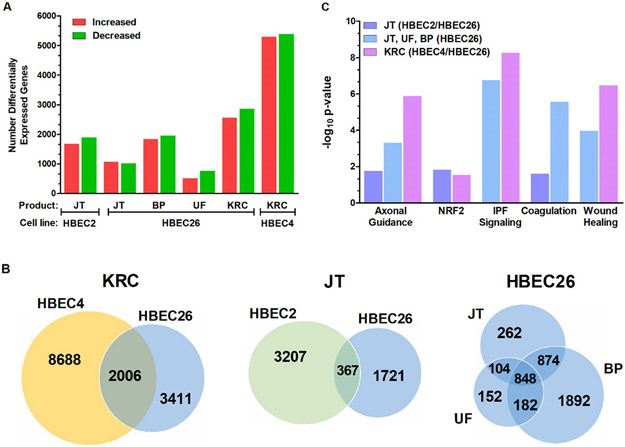
The effect of transformation on global gene expression (FDR ≤ 0.05) was assessed in HBEC lines using RNA sequencing. Cigarette smoke-induced transformed clones had the highest number of differentially expressed genes with 10,694 and 5,416 for HBEC4 and HBEC26, while 2,088 – 3,796 genes with altered expression were seen across the flavored e-liquid transformed HBEC lines (Fig. 1A). Only 1,286 differentially expressed genes were seen for HBEC26 transformed by unflavored e-liquid.
Figure 1.
Differentially expressed genes and common canonical pathways associated with transformation of HBEC lines by the Kentucky reference cigarette (KRC) and e-liquids. A. Number of genes with increased or decreased expression across HBEC2, HBEC4, and HBEC26 transformed by Jamestown (JT), Blue Pucker (BP), Unflavored (UF) or the KRC. B. Venn diagrams showing common and distinct genes differentially expressed between transformed clones from HBEC4/HBEC26 exposed to KRC, HBEC2/HBEC26 exposed to JT, and HBEC26 exposed to JT, BP, or UF. C. Significantly altered pathways comprised of common genes from transformed clones from HBEC4/HBEC26 exposed to KRC, HBEC2/HBEC26 exposed to JT, and HBEC26 exposed to JT, BP, or UF.
Venn diagrams depict the overlap for gene expression changes between cigarette (HBEC4/HBEC26), Jamestown (HBEC2/26), and HBEC26 e-liquid (unflavored, Jamestown, Blue Pucker) transformed clones (Fig. 1B). There were 2006, 367, and 848 genes in common across cigarette, Jamestown, and HBEC26 transformed clones, respectively. Pathway analysis was performed using the common differentially expressed genes. The top five categories for altered molecular and cellular functions were predictive for cancer risk across transformed clones and included cell movement, death, and survival for all groups (Table 4). In addition, cellular development, function, maintenance, and organization were altered by cigarette smoke-induced transformation, while one or more of these cellular functions was also seen in Jamestown and HBEC26 transformed clones. The canonical pathway altered by all products was axonal guidance signaling that comprises genes involved in control of cell migration, tissue development, and vascular network (Fig. 1C [26]). The families of genes and their corresponding receptors residing in this signaling network include the semaphorins/neuropilins/plexins, ephrins and Eph receptors, netrin/DCC/UNC5, Slit/Robo and Notch/Delta (Suppl. Table 2 [26, 27]). Other canonical pathways with highest significance altered in cigarette and HBEC26 transformed clones (comprising Jamestown, Blue Pucker, unflavored) were idiopathic pulmonary fibrosis and wound healing (Fig. 1C). Coagulation pathways were affected only in association with e-liquid-induced transformation.
Table 4.
Top Five Categories of Molecular and Cellular Functions Altered in Transformed Cigarette and E-cigarette Clones
| Product(s) | Molecular & Cellular Function1 | −log10 p-value | #Genes |
|---|---|---|---|
| Jamestown (HBEC2/HBEC26) | Cell Movement | 15.0 | 136 |
| Cell Death & Survival | 11.1 | 147 | |
| Cellular Development | 8.8 | 132 | |
| Cell Growth & Proliferation | 7.3 | 134 | |
| Lipid Metabolism | 5.9 | 54 | |
| Jamestown Blue Pucker Unflavored (HBEC26) | Cell Movement | 44.5 | 321 |
| Cell Death & Survival | 21.0 | 325 | |
| Cell Assembly & Organization | 16.8 | 242 | |
| Cell Function & Maintenance | 16.8 | 277 | |
| Cell-to-Cell Signaling | 16.5 | 248 | |
| Kentucky Reference Cigarette (HBEC4/HBEC26) | Cell Movement | 66.0 | 656 |
| Cell Death & Survival | 47.5 | 748 | |
| Cell Assembly & Organization | 42.0 | 535 | |
| Cell Function & Maintenance | 42.0 | 473 | |
| Cell Development | 33.5 | 810 |
Derived from common differentially expressed genes across product(s).
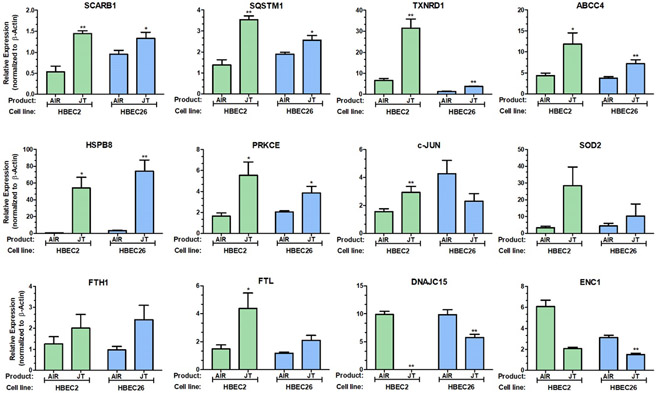
In addition, the NRF-2-mediated oxidative stress response pathway was altered by Jamestown (HBEC2/HBEC26) and cigarette (HBEC4/HBEC26) exposures with 12 and 30 genes, respectively showing differential expression (Suppl. Tables 3 & 4). Activation of NRF2 in lung cancer has been linked to metabolic reprogramming of catalytic and anabolic pathways supporting proliferation and survival [28]. Thus, quantitative RT-PCR was performed to validate the expression changes detected by RNA-seq for the 12 genes predicted to be altered in this pathway by exposure to Jamestown. Consistent with RNA-seq, RT-qPCR showed significant increase in expression for 10 genes (SCARB1, SQSTM1, TXNRD1, ABCC4, HSPB8, PRKCE, c-Jun, SOD2, FTH1, and FTL), while expression was reduced for DNAJC15 and ENC1 in HBEC2 Jamestown transformed cells compared to passage control (Fig 2.). This pattern of expression was also seen for HBEC26 with exception for c-Jun where expression was decreased (Fig. 2).
Figure 2.
Altered expression of NRF2-regulated genes in transformed HBEC lines exposed to Jamestown e-liquid. RT-qPCR for expression of SCARB1, SQSTM1, TXNRD1, ABCC4, HSPB8, PRKCE, DNAJC15, ENC1, JUN, SOD2, FTH1, and FTL in Jamestown transformed clones from HBEC2 and HBEC26 compared to air and normalized to β-actin. *p< 0.05, **p<0.01 compared to vehicle (n = 3).
3.5. Gene promoter hypermethylation and associated gene silencing in transformed clones
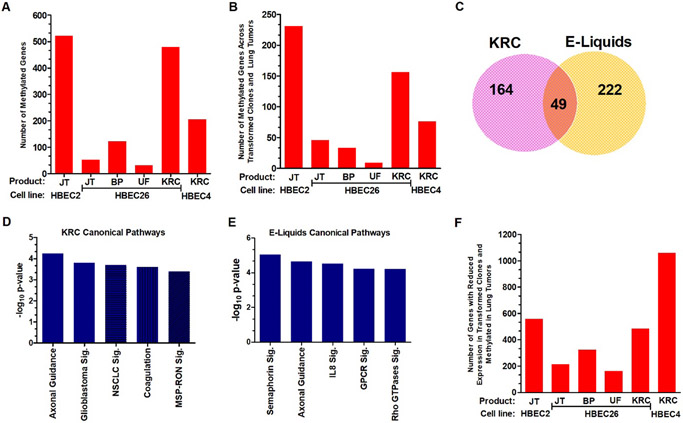
Epigenetic deregulation involving the methylation of cytosine to form 5-methylcytosine in conjunction with histone modifications in gene promoters to silence transcription is a key step in initiation and pre-malignancy, is detected in our prior HBEC transformation studies, and affects genes involved in all aspects of cell regulation [17]. There were 206 and 480 genes with hypermethylation in their promoter regions in conjunction with reduced expression in HBEC4 and HBEC26 cigarette induced transformed clones (Fig. 3A). HBEC2 transformed by Jamestown had the highest number of methylated genes with 522, while 31–122 genes were methylated across HBEC26 e-liquid transformed clones (Fig. 3A). We previously analyzed the TCGA dataset generated with the Illumina HM450K Beadchip that interrogated 811 adenocarcinomas and/or squamous cell carcinomas for genome-wide methylation changes and identified 3,759 methylated genes with loss of expression ≥2-fold [23]. Approximately one-third of genes methylated in HBEC transformed clones were also methylated in TCGA malignant tumors at prevalence ≥15% (Fig. 3A versus 3B). A Venn diagram depicts the number of TCGA methylated genes distinct between cigarette and e-liquid transformed clones and in common across all transformants (Fig. 3C).
Figure 3.
Global methylation changes associated with transformation and recapitulated in lung tumors. A. Number of methylated genes across HBEC2, HBEC4, and HBEC26 transformed by Jamestown (JT), Blue Pucker (BP), Unflavored (UF) or the KRC. B. Number of methylated genes with reduced expression from HBEC2, HBEC4, and HBEC26 transformed clones that are also epigenetically silenced in lung tumors. C. Venn diagram showing common and distinct genes that are methylated in transformed clones from cigarette (KRC) and e-liquids and also methylated in lung tumors. D. Significantly altered canonical pathways comprising genes methylated in cigarette transformed clones and lung tumors. E. Significantly altered canonical pathways comprising genes methylated in e-liquid transformed clones and lung tumors. F. Number of genes with reduced expression from HBEC2, HBEC4, and HBEC26 transformed clones that become methylated in lung tumors. Abbreviation: Sig, signaling
Epigenetic dysregulation of gene expression plays an important role in lung pre-malignancy and malignancy [29]. Thus, we were interested in identifying the canonical pathways altered in the methylated genes identified in the cigarette and e-liquid transformed clones that were also present in primary tumors from TCGA. The analysis was performed using the distinct and common genes for cigarette smoke (n=213) and e-liquid (n=271) transformed clones. There were five significant canonical pathways for cigarette and e-liquids with axonal guidance being in common for both product types (Fig. 3D & E). Glioblastoma, non-small cell lung cancer, and MSP-RON signaling along with coagulation comprised the other pathways altered during cigarette-induced transformation. Reduced expression of the tumor suppressor p16 (CDKN2A), RAR-β, and EGFR, key genes implicated in NSCLC highlighted the list of genes for this pathway (Suppl. Table 5 [30]). The additional canonical pathways identified for the e-liquids include semaphorin, IL8, G-protein coupled receptor, and Rho family GTPases signaling (Fig. 3E, Suppl. Table 6).
Our group showed that chromatin remodeling involving histone methylation represses gene transcription and likely predisposes some genes to acquiring promoter hypermethylation [17]. Fifteen to 20% of genes with reduced expression following transformation by cigarette aerosol were found to be methylated in TCGA tumors at prevalence ≥15% (Fig. 1A & 3F). Similarly, 11–30% of genes with reduced expression in HBECs transformed by e-liquids were also methylated in tumors (Fig. 1A & Fig. 3F).
4.0. Discussion
These are the first studies to show with repeated exposure the induction of lung epithelial cell transformation by unflavored and flavored e-liquids aerosols. The validity of these findings was substantiated through transformation of two of the three HBEC lines by the flavored e-liquid Jamestown. Consistent with the greater genotoxicity and oxidative stress seen with the Kentucky reference cigarette, potency for transformation was greater for this cigarette than the e-liquid products. Transformed clones exhibited extensive reprogramming of the transcriptome with common and distinct gene expression changes observed between the cigarette and e-liquids. These changes were reflected by alterations in canonical pathways implicated in lung cancer that included axonal guidance and NRF2. In addition, epigenetic-mediated gene silencing by promoter hypermethylation, while prominent in cigarette-induced transformed clones, also affected hundreds of genes in HBEC2 transformed by Jamestown. The fact that many of the genes with altered expression or epigenetic-mediated silencing were affected in malignant lung tumors characterized through the TCGA supports our conclusion that exposure to e-liquids aerosols can induce a pre-malignant phenotype in lung epithelial cells.
Prior studies with our in vitro HBEC pre-malignancy model used the direct acting carcinogens MNU and BPDE that create DNA damage associated with NNK and benzo(a)pyrene [13-17]. This strategy obviated the need for xenobiotic metabolism, but excluded the contribution of other genotoxins in the particle and gas phases of the aerosol. The current study extends this model not only through demonstrating transformation by e-cigarettes, but also by the Kentucky reference cigarette aerosol. All HBEC lines expressed many of the CYP isoforms and retained cytochrome P450 reductase activity comparable to normal lung tissue [25]. However, the lack of transformation of HBEC2 compared to HBEC4 and HBEC26 may stem from its lower expression of CYPs metabolizing PAHs and nitrosamines. Studies by others used cigarette smoke condensate (largely comprised of particulates) to demonstrate acute effects of exposure on gene and microRNA expression or chronic exposure over six months to induce lung cancer-associated epigenetic changes in the absence of transformation [31-33]. Our cigarette aerosol-induced model for transformation was also associated with epigenetic changes seen in primary lung tumors that included reduced expression of p16 (CDKN2A), a tumor suppressor gene that regulates the cell cycle and is silenced at the earliest histological stages of adenocarcinoma and squamous cell carcinoma [34, 35]. In addition to global transcriptional reprogramming, other important epigenetically regulated genes with reduced expression and seen in primary lung tumors included RAR-β and EGFR [30, 34]. Thus, this model faithfully recapitulates many of the altered cellular functions and canonical pathways described for lung cancer, supporting the studies herein that evaluated the potency and genome-wide changes underlying transformation by e-liquid aerosols.
The e-liquid flavoring had an impact on transformation potency and effects on the genome. This was evident from comparing outcomes to unflavored (PG/VG + nicotine) liquid where the number of colonies, gene expression changes, and methylated genes were lower than the flavored e-liquids. However, there was considerable heterogeneity with respect to sensitivity and potency for transformation and effects on the genome. HBEC26 was most sensitive with three of four e-liquids inducing transformation while HBEC4 was resistant to transformation by these products. Interestingly, while cigarette smoke did not transform HBEC2, this cell line was sensitive to transformation by Jamestown and exhibited a comparable number of methylated genes to that observed for cigarette transformed HBEC26. The induction of oxidative stress was similar across cell lines, thus other unidentified inherent factors are contributing to the differences between cell lines for e-liquid aerosol-induced transformation. Axonal guidance, a well-characterized canonical pathway implicated in lung cancer, was altered through differential expression of 103 genes across e-liquid and cigarette-transformed clones and mediated in part by epigenetic repression. Axonal guidance molecules that include the semaphorins affect the cytoskeleton, actin filament organization, microtubules, and cell adhesion [26, 27]. The Ephrins, receptor tyrosine kinases, can influence diverse signaling patterns to affect for example cell-cell adhesion, proliferation, and migration [36]. Supporting these in vitro findings, reduced expression of axon guidance genes NCK2, SEMA5A, and SLIT2 were also seen in bronchial epithelial cells collected from cigarette and e-cigarette users [37]. The NRF2 pathway serves a major role in protecting cells from oxidative and/or electrophilic stress through regulating more than 200 genes functioning in part as antioxidant proteins, growth factors, transcription factors, and detoxification enzymes. Expression changes for genes regulated by NRF2 pathway activation were altered in Jamestown and cigarette transformed clones. Deregulation of this pathway has been associated with lung tumor progression, cyto-protection, chemoresistance, and poor prognosis [28].
The factors driving transformation by these e-liquids likely include the generation of reactive carbonyls formaldehyde, acetaldehyde, and acrolein and some chemical constituents characterized previously in these products [19]. These reactive carbonyls produce DNA crosslinks, single and double-strand breaks which could contribute to initiation of transformation [38-41]. Our previous characterization of 30 compounds frequently seen in e-liquids revealed heterogeneity with respect to presence and individual levels [19]. Ethyl maltol was present in Jamestown and Mardi Gras (positive and negative for inducing transformation, respectively) at levels found to be highly cytotoxic in the MTT assay [42]. Ethyl butyrate, a respiratory irritant, was found to be highest in Blue Pucker, present in Mardi Gras and at trace amounts in Jamestown and unflavored. These examples illustrate the complexity of trying to ascribe a specific constituent or combinations therein as contributing to transformation and will ultimately require reconstitution studies. While the Food and Drug Administration banned the sale of flavored cartridge-based electric cigarettes, consumers switched to flavored products through electronic delivery systems (ENDS) that include tanks or disposables [43, 44]. Our findings support a clear need for expansion of studies to evaluate transformation potency for the major varieties of e-liquid flavors, the choice for 85% of consumers, through evaluating a larger set of HBEC and small airway epithelial cell lines to better inform risk from these complex mixtures.
Supplementary Material
Highlights.
Human bronchial epithelial cell lines are transformed by flavored e-liquid aerosols.
E-liquid transformed epithelial cells exhibit extensive transcriptional reprogramming.
E-liquid transformed epithelial cells exhibit vast gene promoter hypermethylation.
Axonal guidance and NRF2 pathways are altered in e-liquid transformed epithelial cells.
Funding
This work was largely by the National Institute of Health grant (R01 DE026013 to SAB) and in part by (P30CA11800 to C. Willman) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors have nothing to disclose.
References
- [1].Wang TW, Gentzke AS, Neff LJ, Glidden EV, Jamal A, Park-Lee E, Ren C, Cullen KA, King BA, Hacker KA, Characteristics of e-cigarette use behaviors among US youth, 2020, JAMA Network Open 4 (2021) e2111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dai, H. H, Leventhal AM, Prevalence of e-cigarette use among adults in the United States, 2014-2018, JAMA 322 (2019) 1824–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF, Flavour chemicals in electronic cigarette fluids, Tob Control 25(e1) (2015) e10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Farsalinos KE, Gillman G, Carbonyl emissions in E-cigarette aerosol: A systematic review and methodological considerations, Front Physiol 8 (2018) 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khlystov A, Samburova V, Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping, Env Sci & Tech 50 (2016) 13080–13085. [DOI] [PubMed] [Google Scholar]
- [6].Merecz-Sadowska A, Sitarek P, Zielinska-Blizniewska H, Malinowska K, Zajdel K, Zakonnik L, Zajdel R, A summary of in vitro and in vivo studies evaluating the impact of e-cigarette exposure on living organisms and the environment, Int J Mol Sci 20 (2020) 652–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Scheffler S, Dieken H, Krischenowski O, Forster C, Branscheid D, Aufderheide M, Evaluation of e-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells, Int J Environ Res Public Hlth 12 (2015)3915–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML, Flavourings significantly affect toxicity of aerosol generated from electronic nicotine delivery systems (ENDS), Tob Control 25 (2016) ii81–ii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rowell, T TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, Glish GL, Tarran R, Flavored e-cigarette liquids reduce proliferation and viability in the Calu3 airway epithelial cell line Am J Physiol Lung Cell Mol Physiol 313 (2017) L52–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sundar IK, Javed F, Romanos GE, Rahman I, E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts, Oncotarget 7 (2016) 77196–77204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tellez CS, Phillips L, Thomas CL, Juri D, Do K, Dye WW, Wu G, Kishida S, Kiyono T, Belinsky SA, Cytotoxicity and genotoxicity of e-cigarette generated aerosols containing diverse flavoring products and nicotine in oral epithelial cell lines, Toxicol. Sci 179 (2012) 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McLaren N, Big Survey 2014–Initial findings e-liquid. Available online: http://vaping.com/data/big-survey-2014-initial-findings-eliquid (assessed on 27 February 2015). [Google Scholar]
- [13].Damiani LA, Yingling CM, Leng S, Walker DM, Nakumura J, Belinsky SA, Carcinogen-induced gene promoter hypermethylation is mediated by DNMT1 and causal for transformation of immortalized bronchial epithelial cells, Cancer Res 68 (2008) 9005–9014. [DOI] [PubMed] [Google Scholar]
- [14].Tellez CS, Juri DE, Do K, Bernauer A, Thomas C, Damiani LA, Tessema M, Leng S, Belinsky SA, Carcinogen exposure induces EMT through epigenetic silencing of miR-205 and miR-200 family and promotes stem-like phenotype during transformation of lung epithelial cells. Cancer Res 71 (2001) 3087–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen W, Bai L, Padilla MT, Gott K, Leng S, Tellez C, Wilder J, Belinsky SA, Scott B, Lin Y. Low-dose gamma-irradiation inhibits IL-6 secretion from human lung fibroblasts that promotes bronchial epithelial cell transformation by cigarette smoke carcinogen, Carcinogenesis 33 (2012) 1368–1374. [DOI] [PubMed] [Google Scholar]
- [16].Teneng I, Tellez CS, Picchi MA, Liu Y, Belinsky SA, Global identification of genes targeted by DNMT3b for epigenetic silencing in lung cancer, Oncogene 34 (2015) 621–630. [DOI] [PubMed] [Google Scholar]
- [17].Tellez CS, Picchi MA, Juri D, Do K, Desai D, Amin SG, Hutt JA, Filipczak PT, Belinsky SA, Chromatin remodeling by the histone methyltransferase EZH2 drives lung pre-malignancy and is a target for prevention, Clin Epigenetics 13 (2021) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL, Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant, New Engl J Med 347 (2002) 330–338. [DOI] [PubMed] [Google Scholar]
- [19].Zhou Y, Irshad H, Dye WW, Wu G, Tellez CS, Belinsky SA, Voltage and e-liquid composition affect nicotine deposition with the oral cavity and carbonyl formation, Tob Control 30 (2021) 485–491. [DOI] [PubMed] [Google Scholar]
- [20].Behar RZ, Hua M, Talbot P, Puffing topography and nicotine intake of electronic cigarette users, PLoS One 10 (2015) e0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lushchak VI, Free radicals, reactive oxygen species, oxidative stress and its classification, Chemico-Bio Interac 224 (2014) 164–175. [DOI] [PubMed] [Google Scholar]
- [22].McKelvey-Martin VJ, Green MHL, Schmezer P, Pool-Zobel BL, DeMeo MP, Collins A, The single cell gel electrophoresis assay (comet assay): A European review, Sci Direct 288 (1993) 47–63. [DOI] [PubMed] [Google Scholar]
- [23].Teneng I, Picchi MA, Leng S, Dagucon C, Ramalingam S, Tellez CS, Belinsky SA, DNA-PKc deficiency drives pre-malignant transformation by reducing DNA repair capacity in concert with reprogramming the epigenome in human bronchial epithelial cells, DNA Repair 79 (2019) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anttila S, Raunio H, Hakkola J, Cytochrome P450-mediated pulmonary metabolism of carcinogens: Regulation and cross-talk in lung carcinogenesis, Am. J. Respir. Cell Mol. Biol 44 (2011) 583–590. [DOI] [PubMed] [Google Scholar]
- [25].de Cerain AL, Marin A, Idoate MA, Tunon MT, Bellow J, Carbonyl reductase and NADPH cytochrome P450 reductase activities in human tumoral versus normal tissues, Eur. J. Cancer 35 (1999) 320–324. [DOI] [PubMed] [Google Scholar]
- [26].Nasarre P, Potiron V, Drabkin H, Roche J, Guidance molecules in lung cancer, Cell Adhesion Migration 4 (2009) 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Potiron VA, Roche J, Drabkin HA, Semaphorins and their receptors in lung cancer, Cancer Lett 273 (2009) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Barrera-Rodriguez R, Importance of the Keap1-Nrf2 pathway in NSCLC: Is it a possible biomarker? (Review), Biom Reports 9 (2018) 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Belinsky SA, Unmasking the lung cancer epigenome, Annu Rev Physiol 77 (2015) 1.1–1.22. [DOI] [PubMed] [Google Scholar]
- [30].Belinsky SA, Silencing of genes by promoter hypermethylation: key event in rodent and human lung cancer, Carcinogenesis 26 (2005) 1481–1487. [DOI] [PubMed] [Google Scholar]
- [31].Xi S, Yang M, Tao Y, Xu H, Shan J, Inchauste S, Zhang M, Mercedes L, Hong JA, Rao M, Schrump DS DS, Cigarette smoke induces C/EBP-β-mediated activation of miR-31 in normal human respiratory epithelial and lung cancer cells, PLoS One 5 (2010) e13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xi S, Xu H, Shan J, Tao Y, Hong JA, Inchauste S, Zhang M, Kunst TR TR, Mercedes L, Schrump DS, Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis, J Clin Invest 123 (2013) 1241–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vaz M, Hwang SY, Kagiampakis I, Phallen J, Patil A, O’Hagan HM, Murphy L, Zahnow CA, Gabrielson E, Velculescu VE, Easwaran HP, Baylin SB. Chronic cigarette smoke-induced epigenomic changes precede sensitization of bronchial epithelial cells to single-step transformation by KRAS mutations, Cancer Cell 32 (2017) 360–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Licchesi JD, Westra WH, Hooker CM, Herman JG, Promoter hypermethylation of hallmark genes in atypical adenomatous hyperplasia of the lung, Clin Cancer Res 14 (2008) 2570–2578. [DOI] [PubMed] [Google Scholar]
- [35].Belinsky SA, Nikula KJ, Palmisano WA, Michels R, Saccomanno G, Gabrielson E, Baylin SB, Herman JG, Aberrant methylation of p16INK4a is an early event in lung cancer and a potential biomarker for early diagnosis, Proc Natl Acad Sci (USA) 95 (1998)11891–11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Buckens OJ, Hassouni BE, Giovannetti E, Peters GJ, The role of Eph receptors in cancer and how to target them: novel approaches in cancer treatment, Expert Opin Invest Drugs 29 (2020) 567–581. [DOI] [PubMed] [Google Scholar]
- [37].Corbett SE, Nitzberg M, Moses E, Kleerup E, Wang T, Perdomo C, Liu G, Xiao X, Liu H, Elashoff DA, Brooks DR, O’Connor GT, Dubinett SM, Spira A, Lenburg ME, Gene expression alterations in the bronchial epithelium of e-cigarette users, Chest 156 (2019) 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li L, Jiang L, Geng C, Cao J, Zhong L, The role of oxidative stress in acrolein-induced DNA damage in HepG2 cells, Free Radical Res 42 (2008) 354–361. [DOI] [PubMed] [Google Scholar]
- [39].Zhang S, Chen H, Wang A, Liu Y, Hou H, Hu Q, Combined effects of co-exposure to formaldehyde and acrolein mixtures on cytotoxicity and genotoxicity in vitro, Environ Sci Pollution Res 25 (2018) 25306–25314. [DOI] [PubMed] [Google Scholar]
- [40].Farsalinos KE, Gillman G, Carbonyl emissions in E-cigarette aerosol: A systematic review and methodological considerations, Front. Physiol 8 (2018) 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Khlystov A, Samburova V, Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping, Env Sci & Tech 50 (2016) 13080–13085. [DOI] [PubMed] [Google Scholar]
- [42].Hua M, Omaiye EE, Luo W, McWhirter KJ, Pankow JF, Talbot P, Identification of cytotoxic flavor chemicals in top-selling electronic cigarette refill fluids, Sci Rep 9 (2019) 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gravely S, Meng G, Hammond D, Reid JL, Seo YS, Hyland A, Cummings KM, Rivard C, Fong GT, Kasza KA, Electronic nicotine delivery systems (ENDS) flavours and devices used by adults before and after the 2020 US FDA ENDS enforcement priority: findings from the 2018 and 2020 US ITC smoking and vaping surveys, Tob Control 31 (2022) s167–s175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li D, Ossip DJ, Bansal-Travers M, Xie Z Z, Impact of the FDA flavour enforcement policy on flavoured electronic cigarette use behaviour changes, Tob Control 31 (2022) s176–s183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.