Abstract
Purpose:
To assess whether MUC1 peptide vaccine produces an immune response and prevents subsequent colon adenoma formation.
Patients and Methods:
Multicenter, double blind, placebo-controlled randomized trial in individuals age 40–70 with diagnosis of an advanced adenoma ≤1 year from randomization. Vaccine was administered at 0, 2, and 10 weeks with a booster injection at week 53. Adenoma recurrence was assessed ≥1 year from randomization. The primary endpoint was vaccine immunogenicity at 12 weeks defined by anti-MUC1 ratio ≥2.0.
Results:
53 participants received the MUC1 vaccine and 50 placebo. 13/52 (25%) of MUC1 vaccine recipients had a ≥2-fold increase in MUC1 IgG (range 2.9–17.3) at week 12 vs. 0/50 placebo recipients (1-sided Fisher’s exact P<0.0001). Of 13 responders at week 12, 11 (84.6%) responded to a booster injection at week 52 with a ≥2-fold increase in MUC1 IgG measured at week 55. Recurrent adenoma was observed in 31 of 47 (66.0%) in the placebo group vs. 27 of 48 (56.3%) in the MUC1 group (adjusted relative risk (aRR) = 0.83 [95% CI, 0.60–1.14], P=0.25). Adenoma recurrence occurred in 3/11 (27.3%) immune responders at week 12 and week 55 (aRR = 0.41 [95% CI, 0.15–1.11], P=0.08 compared to placebo). There was no difference in serious adverse events.
Conclusion:
An immune response was observed only in vaccine recipients. Adenoma recurrence was not different than placebo, but a 38% absolute reduction in adenoma recurrence compared to placebo was observed in participants who had an immune response at week 12 and with the booster injection.
Keywords: colorectal cancer, adenomatous polyps, immunoprevention, vaccines, MUC1
Introduction:
Endoscopic removal of adenomatous polyps, the precursor lesion of colorectal cancer, reduces subsequent colorectal cancer (CRC) incidence (1). However, removing all adenomas is an inefficient means of preventing cancer, because there are many more adenomas than cancers, and most adenomas will not evolve to malignancy. Moreover, adenomatous polyp recurrence rates are high, and repeated colonoscopic surveillance to monitor and remove recurrent adenomas is expensive, invasive, and associated with medical risk. Methods that would either prevent adenomas from forming or prevent them from evolving into malignancy would be welcome.
Clinical trials of chemoprevention to prevent recurrence of adenomatous polyps with agents such as aspirin (2), calcium (3) or folic acid supplements (4), difluoromethylornithine (DMFO) (5), dietary manipulation (6), have been performed. Most have demonstrated limited or no benefit. The most promising agents, including aspirin or DFMO, are limited by side effects and by the burden of compliance, as chemoprevention agents must be taken regularly.
Immunoprevention with vaccines, to target and eliminate pre-malignant precursors, is a potentially safe and effective approach to cancer control (7). Moreover, because of the specificity of the immune response and its long-term memory, immunoprevention offers the potential for prolonged protection. Targeting antigens aberrantly expressed on cancers and their precursors offers the potential for a relatively non-invasive and non-toxic risk-reduction strategy.
MUC1 mucin is a high molecular weight transmembrane glycoprotein expressed in normal epithelial cells, polarized to the apical surface, and extensively glycosylated (8). Abnormal or neoplasia-associated MUC1, that is overexpressed and severely hypoglycosylated compared to MUC1 on normal epithelial cells is found on the vast majority of colorectal adenocarcinomas and on their precursors colorectal adenomas (8–10), and increased MUC1 expression corelates with increasing dysplasia (9). Cancer patients with MUC1 positive tumors produce MUC1-specific antibodies and T cells at low levels (11, 12). MUC1-based vaccines could raise the immune response to therapeutic levels (13, 14). However, immunosuppressive forces in the tumor microenvironment blunt the immune response (15–17), suggesting that vaccines administered in the pre-malignant phase when immunosuppression is not expected, might generate a more robust immune response.
Individuals with a history of advanced adenomatous polyps are at a 3-fold increased risk of CRC compared to those without adenomas (18). Colonic adenomas, like CRC, express the abnormal form of MUC1 (10). In a pilot study, MUC1 vaccine in participants with a history of advanced adenomas resulted in a 2- to 40-fold increase in anti-MUC1 IgG in 44% (17/39), with a robust 1 year memory response, and without significant toxicity (13). We performed a trial of MUC1 vaccine versus placebo in individuals with advanced adenomas within 1 year of removal. The objective was to evaluate the immune response to a MUC1 vaccine and its effect on recurrence of adenomatous polyps.
Methods:
Study Design
This was a randomized, double-blind, placebo controlled, multicenter trial. Eligible participants with ≥1 advanced adenoma within 1 year were randomly assigned in a 1:1 ratio to receive vaccine or placebo. Participants were enrolled at six clinical centers, the Mayo Clinic, Rochester MN; the University of Pittsburgh Medical Center, Pittsburgh PA; the University of Puerto Rico, San Juan PR; the Veterans Administration, Kansas City, KS; Thomas Jefferson University, Philadelphia PA; and Massachusetts General Hospital, Boston MA. All participants provided written informed consent and the protocol was approved by the institutional review board at each site. The trial was administratively coordinated by the National Cancer Institute Division of Cancer Prevention through the Cancer Prevention Network at the Mayo Clinic. All authors had access to the study data and reviewed and approved the final manuscript. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Inclusion Criteria:
Participants were 40 – 70 years of age at the time of randomization, had no prior history of colorectal cancer, no history of heritable colorectal cancer syndromes, no history of malignancy within the previous 5 years other than non-melanoma skin cancer, no nonalcoholic steatohepatitis (NASH) with a nonalcoholic fatty liver disease (NAFLD) activity score ≥ 5 (19), no history of auto-immune disease nor current or planned use of immunomodulators, and no corticosteroid use within the previous 12 weeks. All participants had an advanced adenoma (AA) within 1 year prior to randomization, defined as: ≥ 1 cm in size, with villous or tubulovillous histology, or with severe or high-grade dysplasia. Complete removal of all adenomatous lesions and normal blood testing within defined parameters for hematologic, renal and liver function, and anti-nuclear antibody was required.
MUC1 Vaccine
The MUC1 vaccine consisted of a 100-amino acid synthetic MUC1 (20) (Methods supplement M1) admixed with an adjuvant, toll-like receptor 3 (TLR3) agonist, polyinosinic-polycytidylic acid (Poly ICLC - Hiltonol®), supplied by Oncovir Inc. (Washington, DC). Poly ICLC is a synthetic, non-replicating double-stranded ribonucleic acid (dsRNA), with no specific genetic message. It acts as a viral mimic with broad innate and adaptive immune enhancing, adjuvant, antiviral and antiproliferative effects (21, 22). The placebo was normal saline.
Vaccine or placebo were administered subcutaneously, blinded to content, in the upper thigh. Vaccine or placebo was administered at week 0, 2 and 10 with a booster dose administered at week 53. Injections were administered after blood testing results for toxicity were returned, as per the protocol. Blood was drawn prior to vaccination at week 0, 2, 10, and 52, at week 12 and 55 for assessment of immune response, and within ± 4 weeks of the colonoscopy to evaluate for adenoma recurrence.
Immune and Colonoscopy Endpoints
The primary endpoint was MUC1 IgG levels at week 12 in vaccine vs. placebo recipients. An anti-MUC1 IgG response requires activation of MUC1-specific helper T cells to promote isotype switching from IgM to IgG, hence indirectly measures T cell immunity. An anti-MUC1 IgG ratio of ≥2.0 at 12 weeks (week 12/week 0) was defined as an immune response. Anti-MUC1 IgG levels at week 55 (T55/T52) were also measured after booster injection at week 53. Individuals having an anti-MUC1 IgG ratio of ≥2.0 at 12 weeks and at 55 weeks were considered immune responders.
Adenoma recurrence at the first colonoscopy >1 year post initial vaccination was the primary clinical outcome. The standard for individuals with advanced adenoma is to undergo surveillance colonoscopy at 3 years, but follow-up colonoscopy timing was determined for each participant by the treating endoscopist.
Pre-specified, alternative clinical endpoints included adenoma outcome in participants with an immune response at week 12 and at week 55. Recurrent advanced adenomas that were in the same segment as the baseline advanced adenoma that could represent a residual as opposed to a new recurrence were excluded, and recurrence was restricted to adenomas >5mm since diminutive adenomas can be missed (23).
Adverse Events (AEs)
The NCI common terminology criteria for adverse events (CTCAE) version 4.0 was used to monitor toxicity.
Measurement of Anti-MUC1 IgG, Myeloid-Derived Suppressor Cells (MDSC), and Cytokines
Enzyme-linked Immunosorbent Assay (ELISA) was used to measure plasma anti-MUC1 IgG levels (13). MDSC subpopulations in peripheral blood mononuclear cells (PBMC) were characterized based on their cell surface markers as polymorphonuclear (PMN)-MDSC, monocytic (M)-MDSC and early (e)-MDSC (24). Bead-based multiplex cytokine assays were used to measure IL-1β, INF-α, IL-6, INF-γ, MCP1, TNF-α, IL-8, IL-10, IL-12, IL-17, IL-18, IL-23, IL-33. The cytokines in this panel were chosen based on their previously reported roles in inflammation and cancer (25, 26). IL-1β, INF-α, IL-6, INF-γ, MCP1, TNFα and IL-8 (or CXCL8) are proinflammatory cytokines that are known to suppress immunity and IL-10 can suppress both innate cell-mediated and adaptive immunity. IL-12, IL-17, IL-18 and IL-23 promote type 1 immunity while IL-33 promotes type 2 immunity (Methods supplement M2).
Immunohistochemistry (IHC) Staining of Polyps for MUC1 Expression
Formalin-fixed, paraffin-embedded sections were stained using a rabbit monoclonal antibody against MUC1 (1:100 dilution). Stained tissues were evaluated for the extent, intensity, and localization of staining (27) (Methods supplement M2).
Immunofluorescence Staining, Image Collection and Analysis
Uniplex immunofluorescence staining was performed manually in randomly selected participants with available tissue of interest, using the Opal 6-Plex kit (Akoya Biosciences, Marlborough, MA), which uses individual tyramide signal amplification (TSA)-conjugated fluorophores to detect targets. The slides were scanned using the Vectra Polaris spectral imaging system (Akoya Biosciences) (28). A total of 466 regions of interest (ROI) were identified using Phenochart under the supervision of a gastrointestinal-trained pathologist (ADS). Five marker-positive cells were annotated, including CD4+ T cells, CD8+ T cells, CD15+ myeloid cells, CD20+ B cells, and FOXP3+ regulatory T cells (Methods supplement M2).
Statistical Analysis
Participants were randomized at the Mayo coordinating center in a 1:1 fashion to MUC1 or placebo using the Pocock-Simon dynamic allocation procedure (29), which balances the marginal distributions of the stratification (30). Stratification factors included number of adenomatous polyps removed (>=3 vs. < 3), gender, and treatment site.
The primary endpoint was to compare the ratio of the week 12 to week 0 IgG levels between MUC1 vaccine and placebo in this phase II study. Assuming equal standard deviations (i.e. 10) across the MUC1 and placebo groups, and assuming up to a 10% drop-out rate by year 1, retaining at least 50 evaluable participants per arm yields at least 86% power to detect an increase in the mean IgG ratio from 1 to 6.5 for placebo vs. MUC1 (effect size = 0.55), using a 1-sided t-test with a significance level of 0.05.
Univariate log-binomial models were used to compute estimates of the relative risk (RR). Multivariate logistic regression models were used to compute the adjusted estimates of the odds ratio (OR), which then in turn were used to approximate estimates of the relative risk (RR) by the following formula: , where indicates incidence of the varying types of adenoma recurrence in the placebo group. Variables included in the multivariate models were body mass index (BMI), gender (male vs. female), nonsteroidal anti-inflammatory drug (NSAID) use (yes vs. no), aspirin use (yes vs. no), multiple baseline adenomas (yes vs. no), and multiple baseline advanced adenomas (yes vs. no). Multivariate associations were assessed by the Wald test.
We also compared adverse events, MUC1 expression on original and recurrent adenomas, serum cytokines, IgG and MDSC levels, imaging results, etc. using standard statistical tests, including the Wilcoxon rank-sum test for continuous data and the chi-square or Fisher’s exact tests for categorical data.
All tests were performed with use of a two-sided alpha level of 0.05, unless otherwise specified. SAS version 9.4 (SAS Institute, Inc.) was used for statistical analysis.
Data Availability Statement
Data from this study (#MAY2013-01-01) will be made available by the National Cancer Institute on request on the Cancer Data Access System site, https://cdas.cancer.gov/eppt/.
Results:
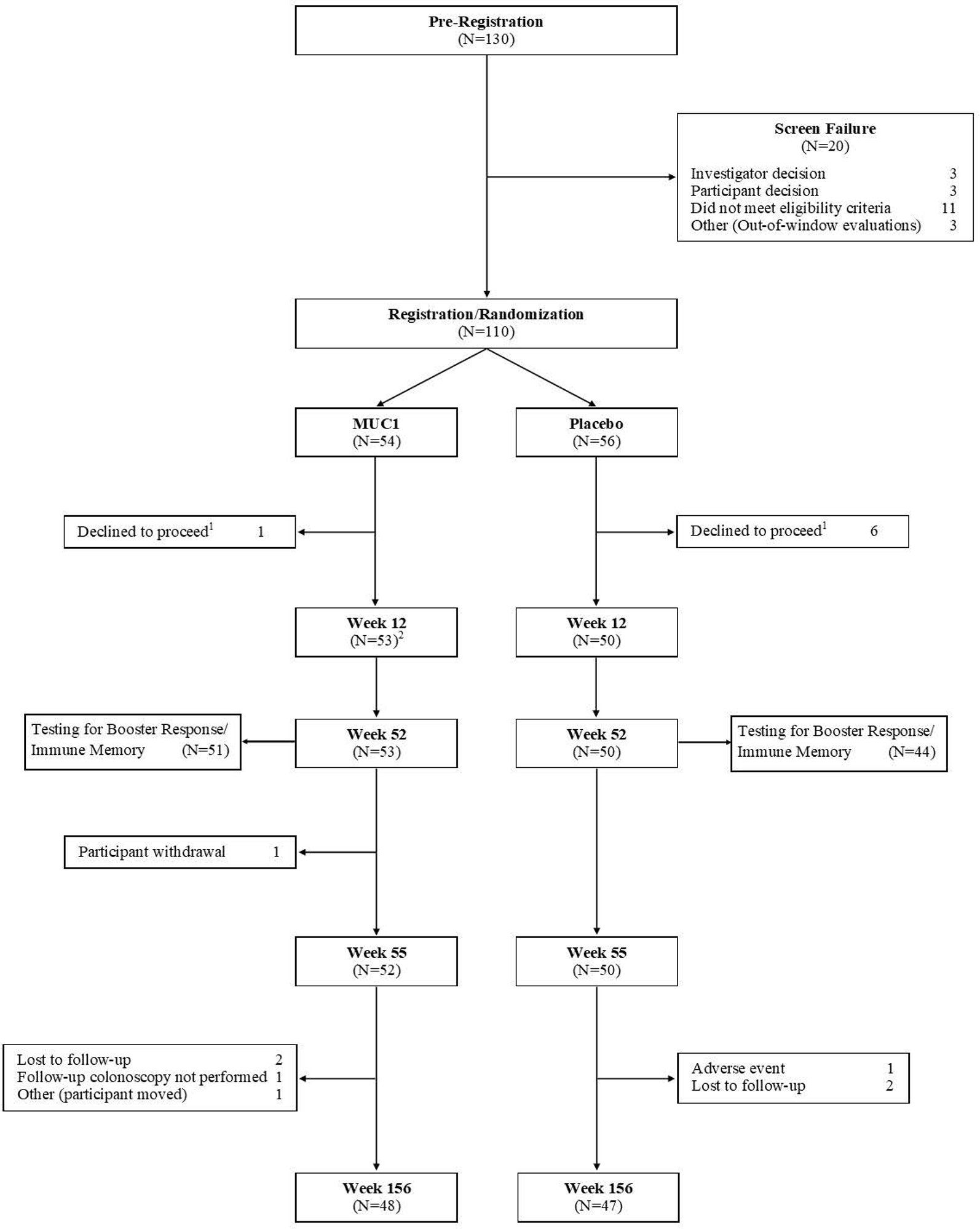
The Consort diagram for participant flow is shown in Figure 1. A total of 130 participants were pre-registered and signed informed consent. Consented participants underwent additional laboratory testing and evaluation and 110 were confirmed eligible. With both investigator and participant blinded to randomization arm, 7 eligible participants declined to proceed. Of the remaining 103 participants, one withdrew from subsequent study visits at week 2 but remained in the study, leaving 102 (52 in the MUC1 group and 50 in the placebo group) evaluable at week 12. Ninety-five participants (51 in the MUC1 group and 44 in the placebo group) completed testing for booster response/immune memory at 52 and 55 weeks. One participant withdrew after 52 weeks. Ninety-five participants (48 in the MUC1 group and 47 in the placebo group) had an endpoint colonoscopy.
Figure 1: Consort Diagram.
Among the 103 intervention participants, the mean age was 59.4 ± 7.0 years, 62.1% were male, 88.3% were white, and 18.4% were Hispanic. There were no significant clinical differences by study arm (Table 1), and the advanced adenoma findings at the qualifying, baseline colonoscopy were also similar (Supplementary Table 1).
Table 1.
Baseline Participant Characteristics
| MUC1 | Placebo | Total | ||
|---|---|---|---|---|
| Baseline Participant Characteristics | P value | |||
| (N=53) | (N=50) | (N=103) | ||
|
| ||||
| Age | 0.98421 | |||
| Mean (SD) | 59.3 (7.56) | 59.5 (6.45) | 59.4 (7.01) | |
| Median | 59.0 | 59.5 | 59.0 | |
| Range | 40.0, 70.0 | 47.0, 70.0 | 40.0, 70.0 | |
| Ethnicity, n (%) | 0.89822 | |||
| Hispanic or Latino | 10 (18.9%) | 9 (18.0%) | 19 (18.4%) | |
| Not Hispanic or Latino | 43 (81.1%) | 40 (80.0%) | 83 (80.6%) | |
| Unknown | 0 (0.0%) | 1 (2.0%) | 1 (1.0%) | |
| Race, n (%) | 0.63492 | |||
| White | 48 (90.6%) | 43 (86.0%) | 91 (88.3%) | |
| Black or African American | 4 (7.5%) | 5 (10.0%) | 9 (8.7%) | |
| Asian | 0 (0.0%) | 1 (2.0%) | 1 (1.0%) | |
| More than one race | 0 (0.0%) | 1 (2.0%) | 1 (1.0%) | |
| Unknown | 1 (1.9%) | 0 (0.0%) | 1 (1.0%) | |
| Gender, n (%) | 0.66423 | |||
| Male | 34 (64.2%) | 30 (60.0%) | 64 (62.1%) | |
| Female | 19 (35.8%) | 20 (40.0%) | 39 (37.9%) | |
| BMI | 0.92381 | |||
| Mean (SD) | 30.0 (5.14) | 30.2 (5.64) | 30.1 (5.36) | |
| Median | 29.7 | 30.2 | 30.2 | |
| Range | 19.1, 44.1 | 19.9, 47.0 | 19.1, 47.0 | |
| NSAID Use4, n (%) | 0.56643 | |||
| No | 31 (58.5%) | 32 (64.0%) | 63 (61.2%) | |
| Yes | 22 (41.5%) | 18 (36.0%) | 40 (38.8%) | |
| Aspirin Use4, n (%) | 0.37693 | |||
| No | 44 (83.0%) | 38 (76.0%) | 82 (79.6%) | |
| Yes | 9 (17.0%) | 12 (24.0%) | 21 (20.4%) | |
| Multiple adenomas, n (%) | 0.29363 | |||
| No | 20 (37.7%) | 14 (28.0%) | 34 (33.0%) | |
| Yes | 33 (62.3%) | 36 (72.0%) | 69 (67.0%) | |
| Total number advanced adenomas/participant, n (%) | 0.76622 | |||
| 1 | 47 (88.7%) | 41 (82.0%) | 88 (85.4%) | |
| 2 | 3 (5.7%) | 6 (12.0%) | 9 (8.7%) | |
| 3 | 1 (1.9%) | 1 (2.0%) | 2 (1.9%) | |
| 4 | 2 (3.8%) | 2 (4.0%) | 4 (3.9%) | |
Abbreviations: SD=Standard Deviation; BMI=Body Mass Index; NSAID= Nonsteroidal Anti-Inflammatory Drug
Wilcoxon rank-sum test
Fisher’s exact test
Chi-square test
Use defined as at least three times a week for a duration of at least 60 days starting prior to Week 0
Vaccine immunogenicity
After injections at 0, 2, and 10 weeks, the mean ± standard deviation (SD) of week 12/week 0 IgG ratio was significantly higher in MUC1 vaccine vs. placebo recipients (3.0 ± 4.31 vs. 1.0 ± 0.19, P=0.0004). An anti-MUC1 IgG ratio of ≥2.0 at 12 weeks was observed in 13/52 (25%) MUC1 vaccine recipients vs. 0/50 placebo recipients (1-sided Fisher’s exact P<0.0001.
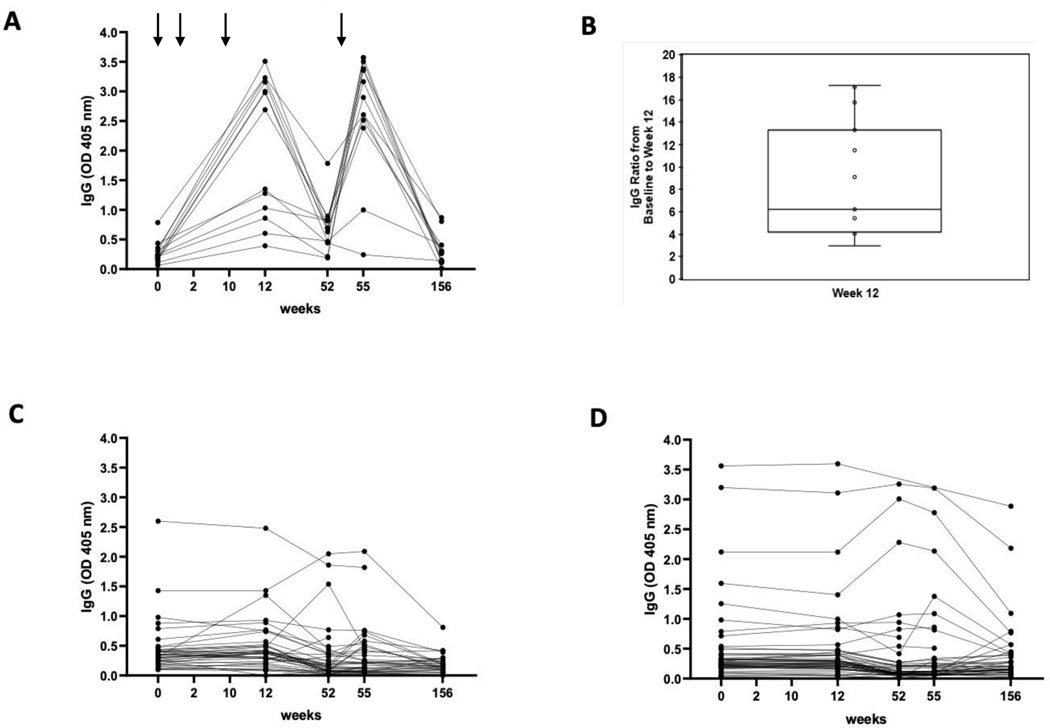
After the booster injection, an anti-MUC1 IgG ratio of ≥2.0 at week 55/week 52 was observed in 17/51 (33.3%) MUC1 vaccine recipients versus 2/44 (4.5%) in the placebo group (P=0.0003). In the 13 MUC1 vaccine recipients who had an immune response at week 12, 11 (84.6%) had a ≥2-fold increase in MUC1 IgG at week 55, whereas 0/44 participants in the placebo group responded at both week 12 and at week 15. Figure 2A shows the kinetics of the antibody generation in 13 participants who had an immune response at week 12. Figure 2B shows fold elevation in anti-MUC1 IgG at week 12 (N=13, range 2.9 – 17.3, mean 8.9). Figure 2C shows pre and post vaccination anti-MUC1 IgG levels in the MUC1 vaccine recipients who did not manifest an immune response and figure 2D shows anti-MUC1 IgG levels in the placebo group. Endpoint titers of plasma antibodies at week 12 amongst those with an immune response is shown in Supplementary Figure 1.
Figure 2: anti-MUC1 IgG Levels:
(A) among MUC1 vaccine recipients with an immune response (N=13); (B) Fold elevation in anti MUC1 IgG among vaccine recipients with an immune response; (C) among vaccine recipients without an immune response (N=39); (C) among placebo controls (N=50). Arrows indicate timing of vaccine administration. Blood sampling occurred at the weeks enumerated on the X-axis. Results are shown for 1:80 plasma dilution.
Correlates of Vaccine Responsiveness
Myeloid derived suppressor cells (MDSC)
Three different MDSC subpopulations, M-MDSC, PMN-MDSC and e-MDSC in pre-vaccination PBMC were evaluated in 47 participants in relation to immune response at week 12 (Table S2). Non-responders (N=34) had a higher mean percentage (±SD) level of circulating PMN-MDSC pre-vaccination than responders (N=13), (8.0 ± 11.0 vs. 2.0 ± 0.9, P=0.0004), and of e-MDSC (4.0 ± 3.0 vs. 2.0 ± 1.1, P=0.02) (Supplementary Table S2). There was no association between pre-vaccination levels of M-MDSC and response to the vaccine.
Cytokines
Cytokine concentrations in pre-vaccination plasma from those with an immune response at week 12 (N=13) were compared with non-responders (N=39) (Figure 3). Circulating levels of IL-6 (p=0.0194) and IL-8 (p=0.001) were significantly higher in non-responders. Other differences did not reach statistical significance.
Figure 3: Pre-vaccination levels of circulating proinflammatory cytokines in MUC vaccine recipients.
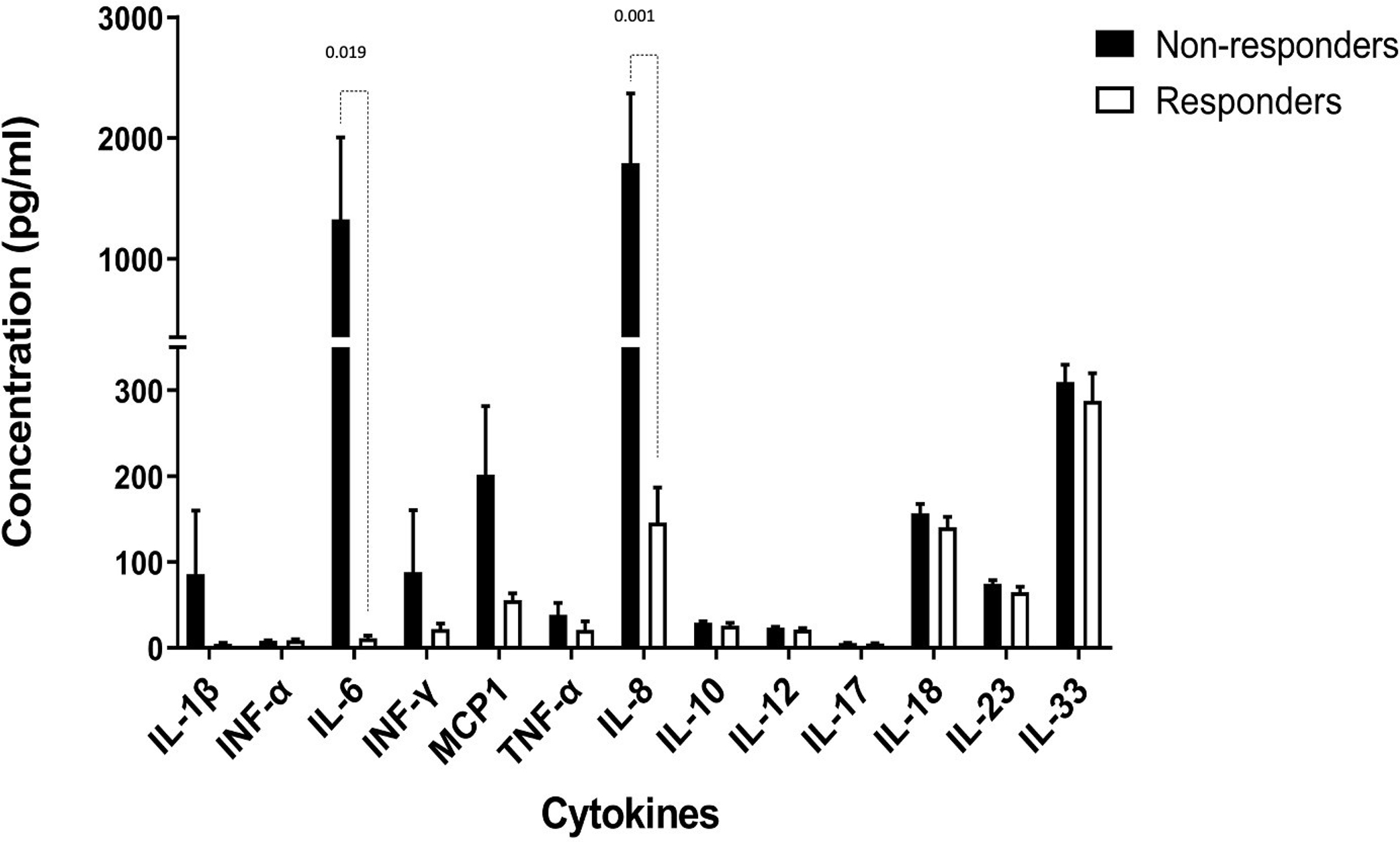
White bars: vaccine recipients with an immune response (N=13); black bars, vaccine recipients without an immune response (N=39). The Y-axis shows cytokine concentrations measured by bead-based multiplex cytokine assay (LEGENDplex™, Biolegend, San Diego, CA, USA) at 1:2 plasma dilution.
Factors Associated with Immune Response at Week 12
Women were more likely to manifest an immune response to the vaccine (9/20, 45%) than men (4/32, 12.5%, P=.009). In a univariate logistic regression model examining demographic factors, NSAID or aspirin use, adenoma characteristics and MDSC populations, females had higher odds of immune response than males (OR=6.5 [95% CI, 1.6–25.9], P=0.005), and PMN-MDSC and e-MDSC as continuous variables were associated with a lower odds of immune response (OR=0.54 [95% CI, 0.33 – 0.88], P=<0.001), and (OR=0.003 [95% CI, <.001 – 0.486], P=0.002), respectively. In a multivariate logistic regression, only female gender was significantly associated with a higher likelihood of having an immune response (OR=5.79 [95% CI, (1.09 – 30.8)], P=0.04), and a 1 unit increase in PMN-MDSC led to a borderline significant lower odds of immune response (OR=0.004 [95% CI, (<0.001 – 1.55], P=0.07).
Adenoma Recurrence
The mean ± SD time to follow up colonoscopy from initial vaccination was 886.1 ± 248.9 days for the MUC1 vaccinated group versus 923.0 ± 258.7 days in the placebo group (P=0.36). The unadjusted and adjusted multivariate relative risk association of various types of adenoma recurrence including any adenoma recurrence, advanced or multiple adenoma recurrence, adenoma recurrence restricted to an adenoma >5mm, and an advanced adenoma recurrence restricted to a different anatomic segment than the baseline advanced adenoma in relation to receipt of vaccine and to being an immune responder at week 12 and at week 55, are shown in Table 2. A recurrent adenoma was observed in 31 of 47 (66.0%) participants receiving placebo vs. 27 of 48 (56.3%) participants in the MUC1 group (adjusted relative risk (aRR) = 0.83 [95% CI, 0.60–1.14], P=0.25). In immune responders at week 12 and at week 55 (N=11), any adenoma recurrence was observed in 3 of 11 (27.3%) (aRR= 0.41 [95% CI, 0.15–1.11], P=0.08 compared to placebo). When restricting recurrence to adenomas >5mm, the adjusted RR point estimate for recurrence was lower in both the MUC1 group (RR=0.79 [95% CI, 0.46–1.36], P=0.41) and in the immune responders group at week 12 and at week 55 (RR=0.17 [95% CI, 0.02–1.11], P=0.06) (Table 2). There was no statistically significant difference in the mean number of adenomas per participant between the MUC1 and the placebo groups (1.0 ± 1.26 vs. 1.5 ± 2.19, respectively; P=0.29), but there was a difference in total number of recurrent adenomas in the placebo group (N=71) vs. the MUC1 group (N=47), P=0.05 (Supplementary Table S1).
Table 2.
Relative Risk of Recurrent Adenoma(s)
| N with Recurrent | Unadjusted Relative | Adjusted2 Relative | |||
|---|---|---|---|---|---|
| Recurrent Adenoma(s) | P-value1 | P-value1 | |||
| Adenoma / Total N (%) | Risk (95% CI) | Risk (95% CI) | |||
|
| |||||
| Any adenoma | |||||
| Placebo (reference) | 31/47 (66.0%) | 1.00 | – | 1.00 | – |
| MUC1 | 27/48 (56.3%) | 0.85 (0.62–1.18) | 0.3395 | 0.83 (0.60–1.14) | 0.2486 |
| Immune Responder3 | 3/11 (27.3%) | 0.41 (0.15–1.11) | 0.0791 | 0.41 (0.15–1.11) | 0.0794 |
| Advanced adenoma | |||||
| Placebo (reference) | 4/47 (8.5%) | 1.00 | – | 1.00 | – |
| MUC1 | 7/48 (14.6%) | 1.71 (0.54–5.47) | 0.3693 | 1.69 (0.53–5.39) | 0.3826 |
| Immune Responder3 | 1/11 (9.1%) | 1.07 (0.13–8.64) | 0.9551 | 0.86 (0.11–7.00) | 0.8999 |
| Multiple adenomas | |||||
| Placebo (reference) | 13/47 (27.7%) | 1.00 | – | 1.00 | – |
| MUC1 | 12/48 (25.0%) | 0.90 (0.46–1.77) | 0.7812 | 0.87 (0.44–1.71) | 0.7004 |
| Immune Responder3 | 0/11 (0.0%) | NE | – | NE | – |
| Advanced adenoma, in different location from baseline 5 | |||||
| Placebo (reference) | 3/47 (6.4%) | 1.00 | – | 1.00 | – |
| MUC1 | 1/48 (2.1%) | 0.33 (0.04–3.03) | 0.3295 | 0.13 (0.01–1.22) | 0.0743 |
| Immune Responder3 | 0/11 (0.0%) | NE | – | NE | – |
| Adenoma > 5 mm in size6 | |||||
| Placebo (reference) | 19/47 (40.4%) | 1.00 | – | 1.00 | – |
| MUC1 | 15/48 (31.3%) | 0.77 (0.45–1.33) | 0.3600 | 0.79 (0.46–1.36) | 0.4068 |
| Immune Responder3 | 1/11 (9.1%) | 0.22 (0.03–1.50) | 0.1239 | 0.17 (0.02–1.11) | 0.0630 |
Abbreviations: CI=Confidence Interval; NE = Not Estimable
Wald test
Risk ratios has been adjusted for body mass index, gender, nonsteroidal anti-inflammatory drug use, aspirin use, multiple baseline adenomas, and multiple baseline advanced adenomas
Participants who responded at both Week 12 and Week 55
Participants who did not respond at both Week 12 and Week 55
Excluding advanced adenomas as recurrences for those detected in the same segment of the bowel as baseline advanced Adenomas
Excluding adenomas ≤5 mm as recurrences
The mean PMN-MDSC level within the vaccine group at baseline was higher among those with an adenoma recurrence compared to those without an adenoma recurrence (0.7 ± 0.99) vs. (0.6 ± 1.07), P=0.06. Within the placebo group, there was no difference in mean PMN-MDSC between those with adenoma recurrence (0.8 ± 0.54) vs. those without adenoma recurrence (1.0 ± 1.59), P=0.65.
We measured levels of anti-MUC1 IgG within ± 4 weeks of follow-up colonoscopy in 76 individuals. In the MUC1 group (N=36) the time of blood draw was 922 days ± 196 versus 946 days ± 222 in the placebo group (N=40), P=0.36. Antibody levels were low, not different from baseline, and there was no association with adenoma recurrence. The mean IgG OD ±SD at the time of follow-up colonoscopy was 0.34 ± 0.42 for those that had an adenoma recurrence and 0.31 ± 0.51 for those that did not (P=0.63).
Vaccine safety
Grade 1 or higher adverse events (AEs) were more common in vaccine recipients compared with placebo (96.2% vs. 76.0%, respectively; P=0.003), as were grade 2 or higher AEs (84.9% vs. 52.0%, respectively; P=0.0003), primarily due to greater injection site reactions in the MUC1 group. Grade 1 injection site reactions occurred in 47/53 (88.7%) of MUC1 recipients vs. 10/50 (20.0%) in the placebo group (P<0.001), and grade 2 injection site reactions occurred in 37/53 (69.8%) of MUC1 recipients vs. 2/50 (4%) in the placebo group (P<0.001). There was no difference in grade 3 or higher AE’s between the MUC1 and the placebo groups (20.8% vs. 20.0%, respectively; P=0.92) and none of the serious adverse events (grade 3 or higher) were attributable to the vaccine. A detailed accounting of all adverse events is provided in supplementary tables S3–S5.
Expression of MUC1 and the immune microenvironment of baseline and recurrent adenomas
MUC1 expression by immunohistochemistry, was evaluated in paired baseline and recurrent adenomas in 8 participants, six selected randomly from the placebo group and from two of the three MUC1 vaccine immune responders at week 12 and at week 55 who recurred. In both groups, there was preserved MUC1 expression in both the baseline and the recurrent adenomas, with extent and intensity of staining rated at the highest value of 4. Localization was predominantly apical and cytoplasmic with no change of pattern (Supplementary Figure S2).
Multi-spectral Imaging
Using multi-spectral imaging, we examined the immune microenvironment of adenomas. We characterized the infiltrating immune cells in 43 matched adenoma pairs (baseline and recurrent), 26 from the placebo group and 17 from the MUC1 vaccinated group (15 non-responders and 2 vaccine responders at week 12 and at week 55, though recurrent adenoma was not available from one of the vaccine responders). Figure 4A is a bioinformatic summary of the landscape of immune infiltrates. The cell density score expressed as a heat map demonstrates that the stroma was more heavily infiltrated than the epithelial component in both the baseline and the recurrent adenomas. The epithelium of recurrent adenomas was more heavily infiltrated than in baseline adenomas, primarily due to CD4 T cells that increased in recurrent adenomas in both the placebo (P=0.058) and the MUC1 group (P=0.059) (Figure 4B). B cell (CD20) infiltration in the epithelium was low in both groups (Figure 4B). FoxP3 regulatory T cells and CD15 myeloid cell immunosuppressive populations which include MDSCs, were significantly higher in the recurrent adenomas in the placebo group (FoxP3 p=0.044; CD15 p=0.0024), but were not significantly different in the MUC1 non-responder group (Foxp3 p=0.20; CD15 p=0.37).
Figure 4: Immune infiltrates in colon adenomas.
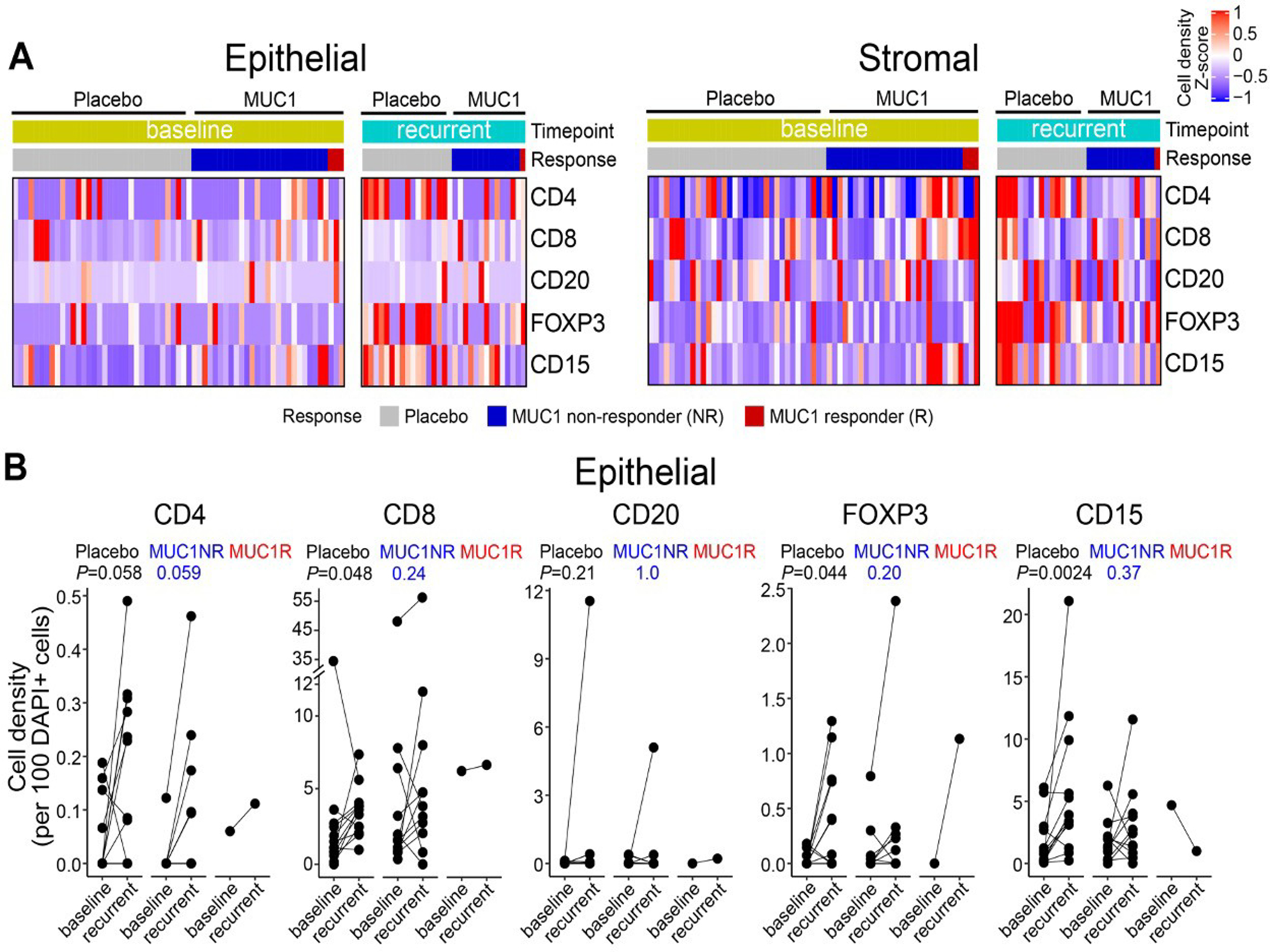
(A) Epithelial and Stromal distribution of CD4+ T cells, CD8+ T cells, CD20+ B cells, FOXP3+ regulatory T cells, and CD15+ myeloid cells in adenomas. Results are grouped by randomization (MUC1 vaccine or placebo), by baseline or recurrent adenomas, and by immune response to the vaccine. In the heat maps, each row depicts a cell population, and each column represents one trial patient. Data are shown for individuals from the baseline adenoma in placebo group (N=25), baseline adenoma in MUC1 recipients (N=17), recurrent adenoma in placebo group (N=14), and recurrent adenoma in MUC1 group (N=12). Cell density was calculated as the number of marker-positive cells divided by each compartment’s total number of cells. Cell density of each sample across all regions of interest (ROIs) per compartment was collapsed at sample level by median and then collapsed at patient level by median when multiple samples per individual were tested. The dendrogram shows clustering of the samples based on cell density with Euclidean distance.
(B) Comparison of immune infiltrates in the epithelial compartment between baseline and recurrent adenomas within individuals from the placebo and MUC1 vaccine groups. Data are shown for placebo (N=13), MUC1 non-responder (MUC1NR) (N=11), and a MUC1 responder at week 12 and week 52 (MUC1R) (N=1). Samples from the baseline and recurrent adenoma within an individual are connected by a line and are compared via a two-sided paired t-test.
Discussion
This is the first randomized, double-blind, multicenter trial of a vaccine for the prevention of human non-viral neoplasia, based on a shared, non-mutated tumor associated antigen. Twenty-five percent of vaccine recipients developed an immune response at week 12 compared with 0% in the placebo group, a highly significant difference. Using at a minimum a doubling of anti-MUC1 IgG as a marker of response to both the initial vaccination and a memory response to the booster dose at 1 year, 22% of MUC1 vaccine recipients (11/51) were immune responders at both week 12 and week 55. For the clinical outcome of adenoma recurrence, 66% in the placebo group recurred compared with 56% in the vaccine arm, a non-significant difference. Among participants who were immune responders at week 12 and at week 55, 27% (3/11) had a recurrent adenoma, a 38% absolute reduction compared to the placebo group (66% recurrence), which approached, but did not reach statistical significance (P=0.08).
In a pilot study of this same vaccine in individuals with advanced adenoma, administered in a similar fashion and schedule (13), there was a 44% (17/39) response at week 12 compared to 25% in this trial. The smaller than expected percentage of participants who met criteria for being an immune responder in this randomized trial limited the power of the study to demonstrate a significant effect on the clinical efficacy endpoint of adenoma recurrence.
In terms of the magnitude of the immune response, in the pilot study we observed an increase in the ratio of anti-MUC1 IgG at week 12/week 0 of up to 36-fold with a mean ratio increase of 13.5, whereas in this study the ratio increase went to 17-fold with a mean ratio increase of 8.9 (Figure 2B).
The reasons why the response rate to the initial vaccination and the magnitude of the immune response were reduced in this trial compared to the pilot study are unclear. One potentially important difference between the two studies was the time between the advanced adenoma removal and the administration of the vaccine. In the pilot study, participants could have had an advanced adenoma at any time prior to enrollment, and some had their adenoma removed up to 9 years prior. In the current study, vaccine administration occurred within 1 year of adenoma removal.
The multispectral images of the immune infiltrate in the baseline advanced adenomas demonstrate a presence of suppressive regulatory T cells (FoxP3+ Tregs) and MDSC (CD15+). Evidence is beginning to accumulate that these cells can suppress systemic as well as local immune responses (31–33). Even though the baseline adenoma was removed prior to vaccination, Tregs could remain in the circulation and MDSCs could continue to be generated due to the continuous presence of proinflammatory cytokines produced by both innate and adaptive immune activation.
Evidence of continued systemic immunosuppression post-adenoma removal is demonstrated in our analysis of both MDSC and serum cytokines. IL-6 and IL-8 in particular are proinflammatory cytokines that are biomarkers of ongoing inflammation and known to negatively affect adaptive immune responses. They have been shown previously to be elevated in the sera of cancer patients (34, 35) and to modulate response to immunotherapy (36, 37). More recently they have also been found in premalignant disease, such as in individuals with cervical epithelial neoplasms (38), oral premalignant lesions (39, 40), and Barrett’s esophagus (41). The increased levels of these cytokines in those without an immune response at week 12 compared to responders, suggests that even in the setting of premalignancy, vaccines may need to be combined with or preceded by other non-toxic approaches that can alleviate immunosuppression.
In some individuals the baseline levels of anti-MUC1 antibodies were relatively high and did not increase after vaccination. One reason why those with high antibody titers at baseline did not respond to the vaccine could be that the pre-existing antibodies cleared the administered vaccine antigen, preventing its presentation for additional immune priming or reactivation of a memory response. Alternatively, the antibodies at baseline were generated when the individuals were immunocompetent but immunosuppressive mechanisms developed in the presence of the adenoma prevented a renewed response to the vaccine. We did not observe a correlation between high baseline anti-MUC1 antibody levels and adenoma recurrence, but we did observe a lower adenoma recurrence rate in those who generated both an initial and a memory response.
We reproduced the observation made in the pilot study that circulating MDSCs, but not circulating Tregs, were an immune correlate of vaccine non-responsiveness. The availability of the placebo group in this trial, allowed us to distinguish between the MDSC role in suppressing immune responses to the vaccine, versus their role in preventing adenoma recurrence. MDSC levels were similar in placebo participants with and without adenoma recurrence. We do not know why some individuals with advanced adenoma have higher levels of circulating MDSC. Being that response to the vaccine is compromised by the presence of these cells, PMN-MDSC levels could be used to select participants who would be more likely to respond to the MUC1 vaccine. Development of safe and effective approaches for MDSC neutralization of their immunosuppressive effect are also potential considerations. Supplemental arginine, an amino acid important for T cell survival (42), could replace arginine depleted by the MDSC-produced arginase (43). Phosphodiesterase inhibitors inhibit MDSC (44), or priming with systemic poly IC-LC (21), could be considered as combination treatments with the vaccine. Administration of cytokines such as IL-7 and IL-15 could promote T cell expansion and function (45) that, when added to the vaccine might increase antibody response levels.
Women were more likely to respond to the vaccine than men. Sex differences in response to vaccination and various immunotherapies have been previously observed and explanations proposed (46–49). MUC1 vaccine may be more immunogenic in females than in males because females experience a larger number of epithelial inflammatory events such as ovulatory cycles, endometriosis, and use of intrauterine devices, that affect epithelial tissues and transiently change MUC1 expression from normal to the tumor-like form, which can lead to spontaneous, albeit low level, immune priming and immune memory for abnormal MUC1 (50, 51). This immune memory could facilitate the response to the MUC1 vaccine resulting in a stronger immune response.
We used anti-MUC1 IgG as the marker for response to the vaccine. IgG is a highly stable molecule that is simple and cost effective to measure. Because MUC1-specific B cells require MUC1-specific T cell help to produce IgG, this is also an indirect measure of the T cell response. Anti-MUC1 IgG antibodies decreased over time, as reflected in week 52 and 156 antibody levels. However, the response to the week 52 booster confirmed the presence of vaccine-elicited memory T cells that could be activated by the booster and stimulate antibody production.
MUC1 is a cell surface antigen and anti-MUC1 antibodies could be important effector molecules for elimination of epithelial tissue expressing tumor-associated MUC1 through antibody dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) (52). Tumor (or a premalignant lesion) destruction by ADCC and ADCP results in antigen release and antigen presenting cell activation, leading to increased antigen presentation and generation of new immune responses, known as epitope spreading (53). Inducing immunity to one immunogenic antigen can create a favorable environment for the generation of immune responses to other antigens that may have an even stronger protective effect.
Considering that the use of non-mutated tumor-associated antigens as immunotherapy can potentially generate autoimmunity (54), it is notable that the MUC1 vaccine was well tolerated without significant toxicity other than injection site reactions that resolved without treatment. To confirm the tumor MUC1 specificity of the vaccine-elicited MUC1 immunity, we had cloned multiple anti-MUC1 IgG antibodies from vaccine responders in our pilot study and demonstrated that they reacted with MUC1 on tumor cells and not on corresponding healthy tissues (55). Thus, autoimmunity and other immune-mediated toxicities were not a limiting factor. Most importantly, we demonstrated the feasibility of testing non-mutated, shared tumor-associated antigen vaccines in the preventive setting, permitting an understanding of vaccine immunogenicity and safety without the confounding issues of cancer and cancer therapy. Our study demonstrates that individuals at increased risk for cancer are amenable to participating in vaccine trials to reduce their risk for cancer.
In this placebo controlled, randomized trial of MUC1 vaccine, immune responses to MUC1 were limited to vaccine recipients. There was a non-significant, but suggestive reduction in adenoma recurrence amongst those with a significant immune response to both the priming and to the booster vaccine. Efforts to further improve the immune response to the vaccine are needed to better assess its potential for prevention of colorectal cancer and other adenocarcinomas that express abnormal MUC1.
Supplementary Material
Translational Relevance:
Removal of adenomatous polyps, the precursor lesion of colorectal cancer (CRC), prevents CRC; however, most adenomas will not evolve to malignancy. Moreover, adenoma recurrence rates are high, and repeated colonoscopy surveillance to remove recurrent adenomas is expensive, invasive, and associated with medical risk. New methods that would prevent adenomas from forming or evolving into malignancy would be welcome. Antigens expressed on colonic adenomas are potential targets for the immune system. Vaccines that stimulate immunity against these antigens could potentially prevent recurrent adenoma formation or advancement. In a multicenter, double blind, placebo-controlled randomized trial in individuals with a recent diagnosis of an advanced adenoma, we assessed whether a MUC1 peptide vaccine produces an immune response and whether it could prevent recurrent adenoma formation. We observed a 38% absolute reduction in adenoma recurrence (P=0.08) compared to the placebo group in those who were immune responders at week 12 and at week 55. Immunoprevention through vaccines is a new frontier for colorectal cancer prevention.
Grant support:
This work is supported by contract HHSN261201200042I to the Mayo Clinic. Bioinformatics analysis was performed by Cancer Bioinformatics Services (CBS), supported in part by NCI through the UPMC Hillman Cancer Center CCSG award (P30CA047904).
Footnotes
Disclosures of Potential conflict of interest:
RES: Research support from Freenome, Immunovia, and Exact Sciences
LAB: None
MCC: None
AB: Research support from Freenome and Stella Diagnostics
DK: Consultant Medtronic, Research support - Exact Sciences and Freenome
CH: None
LD: None
SFK: None
LMR: None
ER: None
AU: None
ES: None
AS: Founder, CEO and CSO of Oncovir
JM: None
PB: None
RKP: Consultant for Alimentiv
ADS: None
CMJ: None
RB: RB declares patents: (all provisional) PCT/US15/612657 (Cancer Immunotherapy), PCT/US18/36052 (Microbiome Biomarkers for Anti-PD-1/PD-L1 Responsiveness: Diagnostic, Prognostic and Therapeutic Uses Thereof), PCT/US63/055227 (Methods and Compositions for Treating Autoimmune and Allergic Disorders)
BD: None
RPM: None
CS: None
NRF: None
DMZ: None
PJL: Chief Medical Officer for Screening at Exact Sciences through a contracted services agreement with the Mayo Clinic. Dr. Limburg and Mayo Clinic have contractual rights to receive royalties through this agreement.
OJF: Consultant: PDS Biotech, GeoVax, Immodulon, Ardigen, Invectys, Sparc, Isa Therapeutics
References
- 1.Miller EA, Pinsky PF, Schoen RE, Prorok PC, Church TR. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. The Lancet Gastroenterology & Hepatology. 2018;28:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas.[comment]. New England Journal of Medicine. 2003;348(10):891–9. [DOI] [PubMed] [Google Scholar]
- 3.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group [see comments]. New England Journal of Medicine. 1999;340(2):101–7. [DOI] [PubMed] [Google Scholar]
- 4.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Jama. 2007;297(21):2351–9. [DOI] [PubMed] [Google Scholar]
- 5.Meyskens FL Jr., McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila). 2008;1(1):32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schatzkin A, Lanza E, Corle D, Lance P, Iber F, Caan B, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group [see comments]. New England Journal of Medicine. 2000;342(16):1149–55. [DOI] [PubMed] [Google Scholar]
- 7.Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18(3):183–94. [DOI] [PubMed] [Google Scholar]
- 8.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–93. [DOI] [PubMed] [Google Scholar]
- 9.Ajioka Y, Watanabe H, Jass JR. MUC1 and MUC2 mucins in flat and polypoid colorectal adenomas. Journal of Clinical Pathology. 1997;50(5):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner MS, McKolanis JR, Ramanathan RK, Whitcomb DC, Finn OJ. Mucins in gastrointestinal cancers. Cancer Chemotherapy & Biological Response Modifiers. 2003;21:259–74. [DOI] [PubMed] [Google Scholar]
- 11.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Research. 1994;54(11):2856–60. [PubMed] [Google Scholar]
- 12.Pandey JP, Namboodiri AM, Wolf B, Iwasaki M, Kasuga Y, Hamada GS, et al. Endogenous antibody responses to mucin 1 in a large multiethnic cohort of patients with breast cancer and healthy controls: Role of immunoglobulin and Fcgamma receptor genes. Immunobiology. 2018;223(2):178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, et al. MUC1 Vaccine for Individuals with Advanced Adenoma of the Colon: A Cancer Immunoprevention Feasibility Study. Cancer Prevention Research. 2013;6(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheid E, Major P, Bergeron A, Finn OJ, Salter RD, Eady R, et al. Tn-MUC1 DC Vaccination of Rhesus Macaques and a Phase I/II Trial in Patients with Nonmetastatic Castrate-Resistant Prostate Cancer. Cancer Immunol Res. 2016;4(10):881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott EN, Gocher AM, Workman CJ, Vignali DAA. Regulatory T Cells: Barriers of Immune Infiltration Into the Tumor Microenvironment. Front Immunol. 2021;12:702726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duhan V, Smyth MJ. Innate myeloid cells in the tumor microenvironment. Curr Opin Immunol. 2021;69:18–28. [DOI] [PubMed] [Google Scholar]
- 17.Sivagnanalingam U, Beatty PL, Finn OJ. Myeloid derived suppressor cells in cancer, premalignancy and inflammation: A roadmap to cancer immunoprevention. Mol Carcinog. 2020;59(7):852–61. [DOI] [PubMed] [Google Scholar]
- 18.Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. Jama-Journal of the American Medical Association. 2018;319(19):2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Mariappan SV, Catasti P, Domenech N, Finn OJ, Gupta G. Structure of a tumor associated antigen containing a tandemly repeated immunodominant epitope. J Biomol Struct Dyn. 1995;13(2):245–60. [DOI] [PubMed] [Google Scholar]
- 21.Sultan H, Salazar AM, Celis E. Poly-ICLC, a multi-functional immune modulator for treating cancer. Semin Immunol. 2020;49:101414. [DOI] [PubMed] [Google Scholar]
- 22.Sultan H, Wu J, Fesenkova VI, Fan AE, Addis D, Salazar AM, et al. Poly-IC enhances the effectiveness of cancer immunotherapy by promoting T cell tumor infiltration. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies [see comments]. Gastroenterology. 1997;112(1):24–8. [DOI] [PubMed] [Google Scholar]
- 24.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korneev KV, Atretkhany KN, Drutskaya MS, Grivennikov SI, Kuprash DV, Nedospasov SA. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine. 2017;89:127–35. [DOI] [PubMed] [Google Scholar]
- 26.Rossi JF, Lu ZY, Massart C, Levon K. Dynamic Immune/Inflammation Precision Medicine: The Good and the Bad Inflammation in Infection and Cancer. Front Immunol. 2021;12:595722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashash JG, Beatty PL, Critelli K, Hartman DJ, Regueiro M, Tamim H, et al. Altered Expression of the Epithelial Mucin MUC1 Accompanies Endoscopic Recurrence of Postoperative Crohn’s Disease. J Clin Gastroenterol. 2021;55(2):127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. [DOI] [PubMed] [Google Scholar]
- 29.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 30.Therneau TM. How many stratification factors are “too many” to use in a randomization plan? Control Clin Trials. 1993;14(2):98–108. [DOI] [PubMed] [Google Scholar]
- 31.Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5(52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliver AJ, Darcy PK, Trapani JA, Kershaw MH, Slaney CY. Cross-talk between tumors at anatomically distinct sites. FEBS J. 2021;288(1):81–90. [DOI] [PubMed] [Google Scholar]
- 33.Deshmukh SK, Srivastava SK, Poosarla T, Dyess DL, Holliday NP, Singh AP, et al. Inflammation, immunosuppressive microenvironment and breast cancer: opportunities for cancer prevention and therapy. Ann Transl Med. 2019;7(20):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmer R, Goumas FA, Waetzig GH, Rose-John S, Kalthoff H. Interleukin-6: a villain in the drama of pancreatic cancer development and progression. Hepatobiliary Pancreat Dis Int. 2014;13(4):371–80. [DOI] [PubMed] [Google Scholar]
- 35.Pawlik W, Pawlik J, Kozlowski M, Luczkowska K, Kwiatkowski S, Kwiatkowska E, et al. The Clinical Importance of IL-6, IL-8, and TNF-alpha in Patients with Ovarian Carcinoma and Benign Cystic Lesions. Diagnostics (Basel). 2021;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M, Zhai X, Li J, Guan J, Xu S, Li Y, et al. The Role of Cytokines in Predicting the Response and Adverse Events Related to Immune Checkpoint Inhibitors. Front Immunol. 2021;12:670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Principe S, Zapater-Latorre E, Arribas L, Garcia-Miragall E, Bagan J. Salivary IL-8 as a putative predictive biomarker of radiotherapy response in head and neck cancer patients. Clin Oral Investig. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vahedpour Z, Abedzadeh-Kalahroudi M, Sehat M, Piroozmand A, Memar M. Comparison of Cervical Levels of Interleukins-6 and −8 in Patients with and without Cervical Intraepithelial Neoplasia. Asian Pac J Cancer Prev. 2021;22(4):1225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dineshkumar T, Ashwini BK, Rameshkumar A, Rajashree P, Ramya R, Rajkumar K. Salivary and Serum Interleukin-6 Levels in Oral Premalignant Disorders and Squamous Cell Carcinoma: Diagnostic Value and Clinicopathologic Correlations. Asian Pac J Cancer Prev. 2016;17(11):4899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khyani IAM, Qureshi MA, Mirza T, Farooq MU. Detection of interleukins-6 and 8 in saliva as potential biomarkers of oral pre-malignant lesion and oral carcinoma: A breakthrough in salivary diagnostics in Pakistan. Pak J Pharm Sci. 2017;30(3):817–23. [PubMed] [Google Scholar]
- 41.Dvorak K, Dvorak B. Role of interleukin-6 in Barrett’s esophagus pathogenesis. World J Gastroenterol. 2013;19(15):2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167(3):829–42.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodríguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21(1):30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai C, Zhou L, Tang J, He J, Han J, Niu H, et al. Fusion Cytokines IL-7-Linker-IL-15 Promote Mycobacterium Tuberculosis Subunit Vaccine to Induce Central Memory like T Cell-Mediated Immunity. Vaccines (Basel). 2020;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein SL, Morgan R. The impact of sex and gender on immunotherapy outcomes. Biol Sex Differ. 2020;11(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aaby P, Benn CS, Flanagan KL, Klein SL, Kollmann TR, Lynn DJ, et al. The non-specific and sex-differential effects of vaccines. Nat Rev Immunol. 2020;20(8):464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41(2):239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capone I, Marchetti P, Ascierto PA, Malorni W, Gabriele L. Sexual Dimorphism of Immune Responses: A New Perspective in Cancer Immunotherapy. Front Immunol. 2018;9:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cramer DW, Titus-Ernstoff L, McKolanis JR, Welch WR, Vitonis AF, Berkowitz RS, et al. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(5):1125–31. [DOI] [PubMed] [Google Scholar]
- 51.Jacqueline C, Lee A, Frey N, Minden JS, Finn OJ. Inflammation-Induced Abnormal Expression of Self-molecules on Epithelial Cells: Targets for Tumor Immunoprevention. Cancer Immunol Res. 2020;8(8):1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller ML, Finn OJ. Flow cytometry-based assessment of direct-targeting anti-cancer antibody immune effector functions. Methods Enzymol. 2020;632:431–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brossart P The Role of Antigen Spreading in the Efficacy of Immunotherapies. Clin Cancer Res. 2020;26(17):4442–7. [DOI] [PubMed] [Google Scholar]
- 54.Turk MJ, Wolchok JD, Guevara-Patino JA, Goldberg SM, Houghton AN. Multiple pathways to tumor immunity and concomitant autoimmunity. Immunol Rev. 2002;188:122–35. [DOI] [PubMed] [Google Scholar]
- 55.Lohmueller JJ, Sato S, Popova L, Chu IM, Tucker MA, Barberena R, et al. Antibodies elicited by the first non-viral prophylactic cancer vaccine show tumor-specificity and immunotherapeutic potential. Sci Rep. 2016;6:31740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study (#MAY2013-01-01) will be made available by the National Cancer Institute on request on the Cancer Data Access System site, https://cdas.cancer.gov/eppt/.