Micro-Abstract
The associations between SARS-CoV-2 infection, vaccination and total serum PSA levels are unknown. In a retrospective big-data analysis, we found higher PSA elevation in the group of patients who exposed to SARS-CoV-2 infection and/or vaccination compared to the controlled group (0.04 vs. 0.02, P < .001). The relative risk for PSA elevation ≥1 ng/dL was 1.22 (95% CI 1.1-1.35). The clinical significance of this elevation appears to be minimal.
Keywords: Cancer Screening, COVID-19 Vaccines, Prostate Cancer, SARS-CoV-2 Infection, TMPRSS2 protein
Abstract
Introduction
The associations among SARS-CoV-2 infection, vaccination and total serum prostate serum antigen (PSA) levels in men undergoing screening for prostate cancer are unknown.
Methods
A retrospective analysis of data from a large health maintenance organization. Records of individuals aged 50 to 75 years with two serum PSA tests taken between March 2018 and November 2021 were included. Individuals with prostate cancer were excluded. Changes in PSA levels were compared between individuals who had at least 1 SARS-CoV-2 vaccination and/or infection between the two PSA tests and individuals who did not have an infection and were not vaccinated between the two PSA tests. Subgroup analyses were performed to assess the impact of the elapsed time between the event and the second PSA test on the results.
Results
The study and control groups included 6,733 (29%) and 16 286 (71%) individuals, respectively. Although the median time between PSA tests was shorter in the study vs. the control group (440 vs. 469 days, P<.001), PSA elevation between the tests was higher in the study group (0.04 vs. 0.02, P<.001). The relative risk for PSA elevation ≥1 ng/dL was 1.22 (95% CI 1.1, 1.35). Among individuals who were vaccinated, PSA increased by 0.03 ng/dL (IQR -0.12, 0.28) and 0.09 ng/dL (IQR -0.05, 0.34) after 1 and 3 doses, respectively (P<.001). Multivariate linear regression showed that SARS-CoV-2 events (β 0.043; 95% CI 0.026-0.06) were associated with a greater risk for PSA elevation, after adjusting for age, baseline PSA and days between PSA tests.
Conclusion
SARS-CoV-2 infection and vaccinations are associated with a slight increase in PSA, with the third anti-COVID vaccine dose having a more prominent impact, but its clinical significance is unknown yet. Any significant increase in PSA must be investigated and cannot be dismissed as secondary to SARS-CoV-2 infection or vaccination.
Introduction
In late 2019, a new strain of coronavirus (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2) was identified as the root cause of a series of pneumonia cases that emerged in Wuhan, China. This strain rapidly spread, resulting in a global pandemic. The common symptoms of Coronavirus Disease 2019 (COVID-19) have been extensively reported. A small percentage of patients developed acute respiratory distress syndrome and multiorgan dysfunction syndrome. Additionally, some patients presented with new symptoms involving most organ systems.1
Although initially the lungs were considered the only target of SARS-CoV-2, it was later divulged that all tissues expressing angiotensin-converting enzyme 2 (ACE2) are potential targets of SARS-CoV-2, as the virus uses this enzyme to dock with the host cell.2 SARS-CoV-2 also uses transmembrane protease serine 2 (TMPRSS2) to invade the host cell.3 As TMPRSS2 is expressed in human prostate epithelial cells, the prostate is a putative target of the SARS-CoV-2.4
In addition to the effect of SARS-CoV-2 infection, messenger RNA (mRNA)-based vaccines against COVID-19 were associated with systemic and organ-specific inflammation. Increasing incidences of uveitis, myocarditis, pericarditis, colitis, appendicitis, cholecystitis, and lymphadenopathy were reported.5 , 6
Prostate-specific antigen (PSA) is a single-chain glycoprotein molecule that is found mainly in acinar cells and the ductal epithelium of normal and malignant prostate cells.7 In clinical practice, increased serum PSA levels are used for early detection of prostate cancer.8 , 9 Increased PSA levels are also observed in benign conditions that disrupt prostate tissue structure, including prostatitis, benign prostatic hyperplasia, and prostate infarction. Thus, it is accepted that PSA is an organ-specific protein rather than a cancer-specific one.10, 11, 12
We hypothesized that SARS-CoV-2 infection and/or vaccination may cause PSA elevation via shared receptor-based pathways. Therefore, we aimed to examine the association between SARS-CoV-2 infection or vaccination and total serum PSA levels in a large cohort of men undergoing screening for prostate cancer.
Material and Methods
Setting
We have retrospectively analyzed medical records obtained from the central data warehouse of 2 districts of the largest health maintenance organization (HMO) in Israel
(Clalit Health Services), and a tertiary medical center run by the same HMO.13 Data regarding SARS-CoV-2, including all polymerase-chain-reaction test results, COVID-19 vaccinations (BNT162b2 vaccine, Pfizer–BioNTech) and diagnoses were collected centrally by the Israeli Ministry of Health and shared with the HMO.
The study was approved by the institutional ethics committees of Clalit Health Services (approval number 2020-1436) and the medical center (approval number 0079-22 RMC). The requirement for individual informed consent was waived.
Study Design
Records of individuals aged 50 to 75 years who had 2 serum PSA test results between March 2018 (2 years before the pandemic) and November 2021 were included and divided into 2 groups. The study group included individuals who had received at least 1 anti-COVID-19 vaccination and/or had a SARS-CoV-2 infection (classified as “events”) between the 2 PSA tests. The control group included individuals who did not have a record of a SARS-CoV-2 infection and were not vaccinated against COVID-19 in the period between the 2 PSA tests. The cases included in the control group had an interval of at least 1 year between the 2 tests to allow the quantification of the natural increase in PSA over time.
We assumed that the net effect of SARS-CoV-2 infection and vaccination can be deduced by comparing the natural increase in PSA over time in the control group to the change in PSA levels in the group exposed to SARS-CoV-2 infection and/or vaccination.
Individuals with prostate cancer, as determined by International Classification of Diseases (ICD) codes, or those with PSA< 0.1 ng/dL or > 20 ng/dL were excluded from the analysis. Because confounding could not be adequately addressed, individuals who had prostate surgery, prostatitis, acute urinary retention, or started a 5α-reductase inhibitor during the study period were also excluded.
Statistical Analysis
Changes in PSA levels between the first and second PSA test results were compared between the groups by event type (infection and/or vaccination) and by the number of events. Subgroup analyses were performed to assess the impact of the elapsed time between the event and the second PSA test on the results.
The data were analyzed by descriptive statistics. Continuous variables were reported as median and interquartile range (IQR) and categorical variables were reported as number and frequency. The study groups were compared using Pearson's chi-squared test for categorical variables and Mann–Whitney test for continuous variables. The relative risk (RR) for PSA elevation ≥ 1 and ≥ 0.35 ng/dL were calculated with a 95% confidence interval (CI).14 Finally, a multivariate linear regression analysis was used to identify variables associated with PSA change in the whole study population. All statistical analyses were performed using the SPSS software (SPSS 27.0; IBM Corp. Armonk, NY), with a 2-sided significance level set at P < .05.
Results
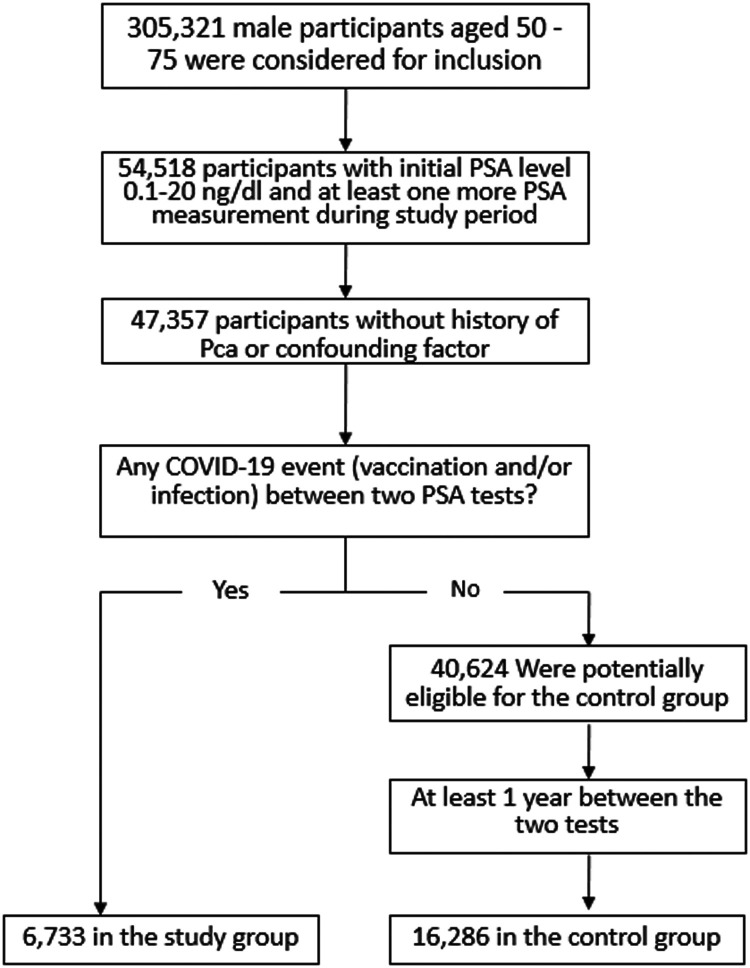
A total of 23,019 individuals with a median age of 65 years (IQR 59-70) were included in the analysis: 6733 (29%) were included in the study group and 16,286 (71%)—in the control group. The study flow chart is shown in Figure 1 . In the study group, 868 individuals (12.9%) had a single event between the 2 PSA tests: 659 (75.8%) received a vaccine against COVID-19, and 209 (24.2%) had a SARS-CoV-2 infection. In addition, 4414 (65.5%), 1448 (21.5%), and 3 (0.04%) individuals had 2, 3, and 4 events, respectively. By definition, none of the individuals in the control group had an event.
Figure 1.
Study flow chart.
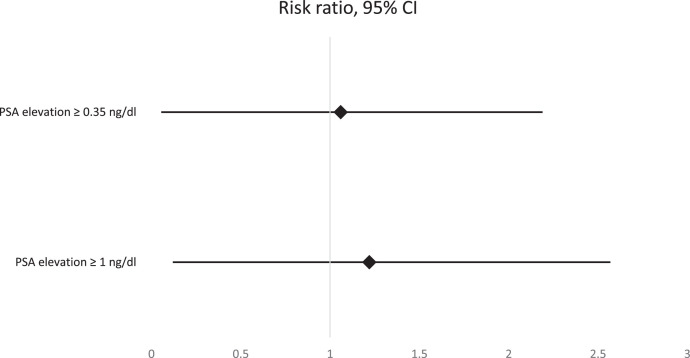
The baseline characteristics of both groups are presented in Table 1 . The median period between PSA tests was statistically significantly longer in the control group compared to the study group (469 vs. 440 days, P < .001). The increase in PSA levels between the first and second PSA tests was significantly greater in the study vs. the control group (0.04 ng/dL vs. 0.02 n/dL, P < .001; Table 2 ). A PSA increase of ≥1 ng/dL was observed in 7.3% and 6% of individuals in the study and control group, respectively (P < .001). A PSA increase of ≥0.35 ng/dL was observed in 20% and 18.8% of individuals in the study and control group, respectively (P = .028). The RRs for PSA elevation ≥1 ng/dL and ≥0.35 ng/dL were 1.22 (95% CI 1.1, 1.35) and 1.06 (95% CI 1.01, 1.13), respectively (Figure 2 ).
Table 1.
Baseline Characteristics of the Study Population
| Study group N = 6733 | Control group N = 16,286 | P-value* | ||
|---|---|---|---|---|
| Age, years, median (IQR) | 64.6 (59, 69) | 65.2 (60, 70) | <.001 | |
| Baseline PSA, ng/dL median (IQR) | 1.09 (0.6, 2) | 1.13 (0.6, 2) | 0.08 | |
| Number of SARS-CoV-2 events (infection and/or vaccination), n (%) | 0 | 0 | 16,286 (100) | NA |
| 1 vaccine dose | 659 (9.8) | NA | ||
| 2 vaccine doses | 4344 (64.5) | |||
| 3 vaccine doses | 1405 (20.9) | |||
| 1 infection | 209 (3.1) | |||
| 1 infection & 1 vaccine dose | 70 (1) | |||
| 1 infection & 2 vaccine doses | 43 (0.6) | |||
| 1 infection & 3 vaccine doses | 3 (0.04) | |||
| Number of days between PSA tests, median (IQR) | 440 (323, 583) | 469 (405, 564) | <.001 | |
| Number of days between the event to PSA test, n (%) | ≤ 30 d | 1743 (26) | NA | NA |
| > 30 d | 4990 (74) |
Abbreviations: IQR = interquartile range; NA = not applicable; PSA = prostate-specific antigen
Mann–Whitney U test
Table 2.
A Comparison of PSA Levels in the Study and Control Groups, and Subgroups Analyses
| Number of individuals | Age, years, median (IQR) | Baseline PSA, ng/dL, median (IQR) | Days between PSA tests, median days (IQR) | PSA change, ng/dL median (IQR) | P value for PSA change* | ||
|---|---|---|---|---|---|---|---|
| Overall | Study group | 6,733 | 64.6 (59, 69) | 1.09 (0.6, 2) | 440 (323, 583) | 0.04 (-0.1, 0.3) | <.001 |
| Control group | 16,286 | 65.2 (60, 70) | 1.13 (0.6, 2) | 469 (405, 564) | 0.02 (-0.1, 0.2) | ||
| Vaccination vs. infection | 1 vaccine dose | 659 | 64.2 (58, 70) | 1.07 (0.6, 2) | 420 (280, 578) | 0.03 (-0.12, 0.28) | .6 |
| 1 infection | 209 | 62.1 (58, 67) | 1.12 (0.55, 2) | 467 (336, 601) | 0.03 (-0.1, 0.24) | ||
| Number of vaccine doses (without an infection) | 1 | 659 | 64.2 (58, 70) | 1.07 (0.6, 2) | 420 (280, 578) | 0.03 (-0.12, 0.28) | <.001 for 1 & 2 vaccine doses vs. 3 vaccine doses |
| 2 | 4344 | 64.6 (59, 70) | 1.09 (0.6, 2) | 441 (298, 586) | 0.03 (-0.11, 0.23) | ||
| 3 | 1405 | 65 (59, 70) | 1.08 (0.6, 2) | 441 (360, 574) | 0.09 (-0.05, 0.34) | ||
| Number of events (infection and/or vaccination) | 1 | 868 | 63.8 (58, 69) | 1.08 (0.6, 2) | 433 (301, 582) | 0.03 (-0.12, 0.27) | <.001 for 1 & 2 events vs. 3 events |
| 2 | 4413 | 64.6 (59, 69) | 1.09 (0.6, 2) | 442 (299, 586) | 0.03 (-0.11, 0.23) | ||
| 3 | 1448 | 65.1 (59, 70) | 1.08 (0.6, 2) | 441 (360, 576) | 0.09 (-0.06, 0.33) | ||
| PSA increase according to the interval between an event and second PSA test | Event to PSA test ≤30 d | 1743 | 65 (59, 70) | 1.11 (0.6, 2.1) | 429 (301, 581) | 0.04 (-0.1, 0.28) | .7 |
| Event to PSA test >30 d | 4990 | 64.4 (58, 69) | 1.08 (0.6, 2) | 445 (328, 583) | 0.04 (-0.1, 0.25) | ||
| Event to PSA test ≤14 d | 907 | 64.9 (59, 70) | 1.06 (0.6, 2) | 426 (308, 580) | 0.05 (-0.1, 0.27) | .7 | |
| Event to PSA test >14 d | 5826 | 64.5 (59, 69) | 1.09 (0.6, 2) | 442 (325, 583) | 0.04 (-0.1, 0.26) | ||
| PSA increase according to the interval between infection and second PSA test | Infection to PSA test ≤30 d | 42 | 63.2 (58, 68) | 1.02 (0.5, 2) | 447 (310, 616) | 0.05 (-0.05, 0.36) | .3 |
| Infection to PSA test >30 d | 283 | 62.1 (58, 67) | 1.11 (0.6, 2) | 476 (337, 608) | 0.03 (-0.09, 0.26) |
Abbreviations: IQR = interquartile range; PSA = prostate-specific antigen.
Pearson's chi-squared.
Figure 2.
Risk ratios and 95% confidence interval for PSA elevation greater than 1 and 0.35 ng/dL.
Subgroup analysis showed no statistically significant differences between PSA elevation in individuals who had a SARS-CoV-2 infection compared to those who were vaccinated (0.03 ng/dL [IQR -0.1, 0.24] vs. 0.03 ng/dL [IQR -0.12, 0.28], P = .6).
Among individuals who did not have a SARS-CoV-2 infection, the increase in PSA was 0.03 ng/dL (-0.12, 0.28), 0.03 ng/dL (-0.11, 0.23), and 0.09 ng/dL (-0.05, 0.34) after 1, 2, and 3 vaccination doses, respectively. The increase in PSA after the third vaccine dose was statistically significantly higher than the increase observed after 1 and 2 vaccine doses (P<.001, Table 2). Among individuals who had a SARS-CoV-2 infection and/or were vaccinated against COVID-19, the increase in PSA was 0.03 ng/dL (-0.12, 0.27), 0.03 ng/dL (-0.11, 0.23), and 0.09 ng/dL (-0.06, 0.33) after 1, 2, and 3 events, respectively. The increase in PSA after the third event (SARS-CoV-2 infection or vaccination), was statistically significantly higher than the increase observed after 1 and 2 events (P<.001, Table 2).
Temporal changes in PSA levels according to the intervals between an event (infection/vaccination) and the subsequent PSA levels are reported in Table 2. No differences in PSA levels were found between individuals who were tested for PSA within 30 or 14 days of the event and those who were tested later.
A multivariate linear regression was performed to examine if age, baseline PSA level, the interval between PSA tests and the number of SARS-CoV-2 events (infection or vaccination) predict the change in PSA levels. The results showed that all 4 independent variables were significant predictors of the change in PSA levels (F(77, 39 746) = 11.2, P<.001, R2 = 0.002). The most prominent unstandardized regression coefficient (β) was the number of events. After adjusting for age, baseline PSA and the number of days between PSA tests, each SARS-CoV-2 infection/vaccination event led to an increase of 0.043 ng/dL in PSA (Table 3 ).
Table 3.
Multivariate Linear Regression Model Predicting PSA Change (ng/ml) in the Whole Study Population (n = 23,019)
| β | P value | 95% CI | |
|---|---|---|---|
| Age | 0.003 | .019 | 0.001-0.006 |
| Baseline PSA | -0.014 | .011 | -0.024 to -0.003 |
| Days between PSA tests | 0.0003 | <.001 | 0.0001-0.0004 |
| Number of events | 0.043 | <.001 | 0.026-0.06 |
Abbreviations: CI = confidence interval; PSA = prostate specific antigen.
Discussion
Using a dataset of more than 50,000 individuals from an HMO, we found an association between SARS-CoV-2 infections or vaccinations and increased serum PSA levels. Although PSA levels statistically significantly increased following the third vaccine dose, compared to PSA levels after the first and second vaccines, the clinical significance of this change has not yet been established.
In an analysis conducted on a small-sized cohort, Cinislioglu et al. showed that during an acute symptomatic SARS-CoV-2 infection, serum PSA levels were higher than those measured 3-6 months before the infection as well as higher than levels measured 3 months after the infection.15 In contrast to this reported transient increase in PSA, we did not find any differences in PSA levels measured within 14 or 30 days of the infection and those measured later (Table 2). As Cinislioglu et al. measured PSA levels on the first day of COVID-19 diagnosis, it is possible that the greater increase in PSA levels was due to the active infection, which was less noticeable in our subgroup. Additionally, although they did not find statistically significant differences between baseline PSA levels and PSA levels 3 months after a SARS-CoV-2 infection, mean PSA levels 3 months after the infection were actually higher by 0.51 ng/dL than baseline levels, which is in accordance with our findings. The lack of statistical power may be attributed to the relatively small sample size (n = 91) in that study.15
The association between SARS-CoV-2 infection and the increase in PSA levels may be explained by the renin-angiotensin-aldosterone system (RAAS) and the opposing activity of ACE and ACE2.16 The RAAS is a hormonal cascade mainly regulating blood pressure and cardiovascular function. ACE cleaves angiotensin I into angiotensin II (Ang-II), subsequently increasing blood pressure. Additionally, Ang-II receptor activation induces inflammation and fibrogenesis, and activates vascular and cellular growth mechanisms.17 ACE2, is a part of the RAAS protective phase, degrades Ang-II into angiotensin-(1-7), thereby lowering blood pressure and inducing anti-fibrotic and anti-inflammatory activity.18
ACE2 is a type-I membrane protein19 expressed in many human tissues, such as upper bronchial and nasal epithelia, the intestines, the prostate, and the testes. Some coronaviruses, including SARS-CoV-2, use ACE2 to enter cells.20 , 21 The serine protease TMPRSS2 is necessary for priming the SARS-CoV-2 spike protein, and coexpression of TMPRSS2 and ACE2 is crucial for its cell entry.3 , 22 SARS-CoV-2 invasion using ACE2 for binding and membrane fusion, leads to ACE2 loss of function and diminished ACE2 expression in the cellular membranes of prostate tissue.23 ACE2 downregulation leads to Ang-II accumulation, preventing its degradation to angiotensin-(1-7) levels and accelerating inflammatory processes.24 These processes can cause structural deterioration in the prostate, including basal cell loss, impaired basement membrane integrity, and damaged luminal structure which is reflected by increased PSA levels in serum.25
The relatively high ACE2 expression levels in male reproductive organs, suggest that the testes and the prostate are potentially vulnerable to SARS-CoV-2 infection.26 , 27 However, there is no clinical evidence thus far that SARS-CoV-2 affects the testes directly, nor are there reports on clinically relevant acute orchitis or sexual transmission, and evidence on the presence of SARS-CoV-2 in semen remains scarce.28 A possible explanation is that despite high levels of ACE2 expression in testis cells, less than 0.1% of them coexpress both ACE2 and TMPRSS2, decreasing the likelihood of the virus to enter these cells.29 Similarly, 0.3% of all prostate epithelial cells express ACE2, 18.6% express TMPRSS2, and less than 0.1% coexpress ACE2 and TMPRSS2.4 , 30 Our findings also disprove extensive prostate damage. The absolute risk increase for PSA elevation ≥1 ng/dL and ≥0.35 n/dL were only 1.3% and 1.2%, respectively, which may correspond with low coexpression of ACE2 and TMPRSS2.
Another possible mechanism underlying prostate damage following SARS-CoV-2 infection may be related to the virus’ well-established systemic procoagulant effect, which affects many organs and is one of the leading causes for multiple organ dysfunction and death.31 The first report of prostate involvement in this context was published by Duarte et al.32. They described a 71-year-old patient who was in a critical condition due to a SARS-CoV-2 infection and presented with acute urinary retention. His histological examination showed thrombi in small vessels and extensive ischemic prostatic infarction. While this report did not provide data regarding changes in PSA levels, it is reasonable to assume that these processes involve prostatic tissue destruction and increased PSA levels.
To the best of our knowledge, this is the first study to examine the association between the COVID-19 vaccine and changes in PSA levels. Vaccination against COVID-19 resulted in a statistically significant increase in PSA levels, with the third dose having a notable impact. Two large studies on the safety of the BNT162b2 vaccine have reported increased organ-specific inflammation, however, prostatitis was not mentioned in either one of them.5 , 6 These studies have also reported increased risk (RR = 1.6) for coagulation disorder, including immune thrombocytopenia and disseminated intravascular coagulation,6 which could explain prostate tissue damage as described above. The higher impact observed following the third vaccine dose is due to its greater reactogenicity and clinical effectiveness compared to the 2 primary vaccine doses and is consistent with reports in the literature.33 , 34 Notably, individuals who had no seroconversion after 2 doses, presented detectable anti-SARS-CoV-2 IgG after a third dose.35 This heightened immune response may include subclinical prostatitis or coagulation disorders, and could result in prostate tissue damage.
The limitations of this study include its retrospective nature. Although the medical records included in the study are extremely accurate with regard to the number and timing of the vaccine doses, cases of SARS-CoV-2 infection, especially asymptomatic infections, may not have been documented. To limit such cases in our analysis, the data cut-off date was before the spread of the SARS-CoV-2 Omicron variant, due to its high contagiousness and the utilization of home diagnosis kits became very widespread and may have contributed to undocumented infection events. Despite these limitations, using a large dataset we were able to provide novel data about the association between vaccination against COVID-19, SARS-CoV-2 infection and PSA dynamics.
Conclusions
It appears that the prostate is not a susceptible organ for SARS-CoV-2 infection in most individuals. SARS-CoV-2 infection and vaccinations are associated with a slight elevation in PSA levels and its clinical significance is yet to be defined. Thus, cases showing a significant increase in PSA must be investigated and cannot be dismissed as secondary to SARS-CoV-2 infection or vaccination.
Clinical Practice Points
-
•
Prostate-specific antigen (PSA) level is the most frequently used screening tool for early detection of prostate cancer.
-
•
The associations between SARS-CoV-2 infection, vaccination and total serum PSA levels in men undergoing screening for prostate cancer are unknown.
-
•
One small-size cohort showed that serum PSA level during an acute symptomatic SARS-CoV-2 infection is both higher than PSA levels measured 3 to 6 months before the infection and PSA measured 3 months after the infection.
-
•
Using a dataset of more than 50,000 individuals from a health maintenance organization, we found an association between SARS-CoV-2 infection or vaccination and serum PSA elevation.
-
•
While this elevation increases with the number of events, its clinical significance appears to be minimal. Any case of significant PSA elevation requires investigation and cannot be dismissed as secondary to SARS-CoV-2 infection or vaccination.
Authors' contributions
Michael Frumer – conceptualization, data curation, project administration, methodology, formal analysis, investigation, writing - original draft. Shachar Moshe Aharony – investigation, writing - review and editing. Ohad Shoshany - investigation, writing - review and editing. Daniel kedar - investigation, writing - review and editing. Jack Baniel - investigation, writing - review and editing, supervision. Shay Golan – conceptualization, resources, investigation, validation, writing - review and editing, supervision. All authors read and approved the submitted version.
Ethical Approval
This retrospective study was approved by the Institutional Review Board of Rabin Medical Center (0079-22 RMC) and Clalit Health Services (2020-1436). The Ethics Committee waived the requirement to obtain informed consent.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank Shiri Kushnir and Shira Hazon from the Data Science Research Unit of Rabin Medical Center for helping with this research project.
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Lampl BMJ, Buczovsky M, Martin G, Schmied H, Leitzmann M, Salzberger B. Clinical and epidemiological data of COVID-19 from Regensburg, Germany: a retrospective analysis of 1084 consecutive cases. Infection. 2021;49(4):661–669. doi: 10.1007/s15010-021-01580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40(5):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song H, Seddighzadeh B, Cooperberg MR, Huang FW. Expression of ACE2, the SARS-CoV-2 Receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol. 2020;78(2):296–298. doi: 10.1016/j.eururo.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraiman J, Erviti J, Jones M, et al. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine. 2022;40(40):5798–5805. doi: 10.1016/j.vaccine.2022.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MC, Papsidero LD, Kuriyama M, Valenzuela LA, Murphy GP, Chu TM. Prostate antigen: a new potential marker for prostatic cancer. Prostate. 1981;2(1):89–96. doi: 10.1002/pros.2990020109. [DOI] [PubMed] [Google Scholar]
- 8.Matti B, Xia W, van der Werf B, Zargar-Shoshtari K. Age-adjusted reference values for prostate specific antigen—a systematic review and meta-analysis. Clin Genitourin Cancer. 2022;20(2):e114–e125. doi: 10.1016/j.clgc.2021.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190(2):419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald AC, Vira MA, Vidal AC, Gan W, Freedland SJ, Taioli E. Association between systemic inflammatory markers and serum prostate-specific antigen in men without prostatic disease - the 2001-2008 National Health and Nutrition Examination Survey. Prostate. 2014;74(5):561–567. doi: 10.1002/pros.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jr Neal DE, S Clejan, Sarma D, Moon TD. Prostate specific antigen and prostatitis. I. Effect of prostatitis on serum PSA in the human and nonhuman primate. Prostate. 1992;20(2):105–111. doi: 10.1002/pros.2990200205. [DOI] [PubMed] [Google Scholar]
- 12.Lunacek A, Tischler M, Mrstik C, et al. Effects of cycling and rowing on serum concentrations of prostate-specific antigen: a randomized study of 101 male subjects. Prostate. 2022;82(7):804–808. doi: 10.1002/pros.24322. [DOI] [PubMed] [Google Scholar]
- 13.Cohen R, Rabin H. National Insurance Institute of Israel. Membership in sick funds 2016. August 2017. (In Hebrew) Available at: https://www.btl.gov.il/Publications/survey/Documents/seker289/seker_289.pdf.
- 14.Ørsted DD, Bojesen SE, Kamstrup PR, Nordestgaard BG. Long-term prostate-specific antigen velocity in improved classification of prostate cancer risk and mortality. Eur Urol. 2013;64(3):384–393. doi: 10.1016/j.eururo.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Cinislioglu AE, Demirdogen SO, Cinislioglu N, et al. Variation of serum PSA levels in COVID-19 infected male patients with benign prostatic hyperplasia (BPH): a prospective cohort studys. Urology. 2022;159:16–21. doi: 10.1016/j.urology.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy JL, Lambert DW, Warner FJ, Hooper NM, Turner AJ. Membrane-associated zinc peptidase families: comparing ACE and ACE2. Biochim Biophys Acta. 2005;1751(1):2–8. doi: 10.1016/j.bbapap.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169(3):477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 20.Dong M, Zhang J, Ma X, et al. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renu K, Prasanna PL, Valsala Gopalakrishnan A. Coronaviruses pathogenesis, comorbidities and multi-organ damage - A review. Life Sci. 2020;255 doi: 10.1016/j.lfs.2020.117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almehdi AM, Khoder G, Alchakee AS, Alsayyid AT, Sarg NH, Soliman SSM. SARS-CoV-2 spike protein: pathogenesis, vaccines, and potential therapies. Infection. 2021;49(5):855–876. doi: 10.1007/s15010-021-01677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta PK, Liu F, Fischer T, Rappaport J, Qin X. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10(16):7448–7464. doi: 10.7150/thno.48076. Published 2020 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21(2):383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 26.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1-2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 27.Bhowmick NA, Oft J, Dorff T, et al. COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr Relat Cancer. 2020;27(9):R281–R292. doi: 10.1530/ERC-20-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallak J, Teixeira TA, Bernardes FS, et al. SARS-CoV-2 and its relationship with the genitourinary tract: implications for male reproductive health in the context of COVID-19 pandemic. Andrology. 2021;9(1):73–79. doi: 10.1111/andr.12896. [DOI] [PubMed] [Google Scholar]
- 29.Pan F, Xiao X, Guo J, et al. No evidence of SARS-CoV-2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry GH, Malewska A, Joseph DB, et al. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 2018;25(12):3530–3542. doi: 10.1016/j.celrep.2018.11.086. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdelaal A, Abu-Elfatth A, Bakkar LM, El-Azeem HGA, Hetta HF, Badawy ER. Assessment of COVID -19 associated coagulopathy and multiple hemostatic markers: a single center study in Egypt. Infection. 2022;51(3):655–664. doi: 10.1007/s15010-022-01917-5. [published online ahead of print, 2022 Sep 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duarte SAC, Pereira JG, Iscaife A, Leite KRM, Antunes AA. Is prostate infarction and acute urinary retention a possible complication of severe COVID-19 infection? Pathology. 2020;52(7):818–821. doi: 10.1016/j.pathol.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascaso-Del-Rio A, García-Pérez J, Pérez-Olmeda M, et al. Immune response and reactogenicity after immunization with two-doses of an experimental COVID-19 vaccine (CVnCOV) followed by a third-fourth shot with a standard mRNA vaccine (BNT162b2): RescueVacs multicenter cohort study. EClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isnardi CA, Cerda OL, Landi M, et al. Immune response to SARS-CoV-2 third vaccine in patients with rheumatoid arthritis who had no seroconversion after primary 2-dose regimen with inactivated or vector-based vaccines. J Rheumatol. 2022;49(12):1385–1389. doi: 10.3899/jrheum.220469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.