Abstract
Objectives
The correlate(s) of protection against SARS-CoV-2 remain incompletely defined. Additional information regarding the combinations of antibody and T cell-mediated immunity which can protect against (re)infection is needed.
Methods
We conducted a population-based, longitudinal cohort study including 1044 individuals of varying SARS-CoV-2 vaccination and infection statuses. We assessed spike (S)- and nucleocapsid (N)-immunoglobulin(Ig)G and wildtype, Delta, and Omicron-neutralizing antibody (N-Ab) activity. In a subset of 328 individuals, we evaluated S, membrane (M), and N-specific T cells. Three months later, we reassessed Ab (n = 964) and T cell (n = 141) responses and evaluated factors associated with protection from (re)infection.
Results
At the study start, >98% of participants were S-IgG seropositive. N-IgG and M/N-T-cell responses increased over time, indicating viral (re)exposure, despite existing S-IgG. Compared to N-IgG, M/N-T cells were a more sensitive measure of viral exposure. High N-IgG titers, Omicron-N-Ab activity, and S-specific-T-cell responses were all associated with a reduced likelihood of (re)infection over time.
Conclusion
Population-level SARS-CoV-2 immunity is S-IgG-dominated, but heterogeneous. M/N-T-cell responses can distinguish previous infection from vaccination, and monitoring a combination of N-IgG, Omicron-N-Ab, and S-T-cell responses may help estimate protection against SARS-CoV-2 (re)infection.
Keywords: SARS-CoV-2, Hybrid immunity, Seroprevalence, Neutralizing antibodies, T cell responses, Interferon-gamma release assay
Introduction
It is now well-understood that exposure to SARS-CoV-2 elicits robust antibody (Ab) and T cell-mediated immune responses to multiple viral proteins—in particular spike (S), nucleocapsid (N), and membrane (M) proteins [1], [2], [3], [4], [5]. In contrast to infection, the messenger RNA-based COVID-19 vaccines used widely in the United States and Europe elicit responses to the viral S protein; the only antigenic component of these vaccines [6,7]. As the correlate(s) of protection needed to prevent infection or severe illness have yet to be clearly defined [8], data on population-level humoral and cellular immune responsiveness to SARS-CoV-2 remain important for understanding (i) the scope of viral exposure and (ii) what proportion of the population possesses some degree of virus-specific immunity.
Although much is now known regarding population-level Ab responses to SARS-CoV-2 infection, our understanding of T cell-mediated immunity is much less comprehensive. T-cell responses have been described following both vaccination [9], [10], [11], [12], [13] and infection, including mild or asymptomatic cases even without seroconversion [1], [2], [3],5,[13], [14], [15], [16]. However, extensive studies of T-cell responses, particularly at the population level, are lacking, partially due to the labor-intensive and relatively low-throughput nature of assays designed to evaluate them, such as enzyme-linked immunospot (ELISpot) and flow cytometry-based assays. To address this, adaptation of interferon (IFN)-gamma release assays (IGRAs), such as those used in Mycobacterium tuberculosis and Cytomegalovirus screening [17,18], may aid in the detection of SARS-CoV-2-specific T cells in a larger number of samples. Importantly, as both humoral and cellular responses contribute to immunity against SARS-CoV-2, a better understanding of the heterogeneous combinations of immune memory which can protect against disease may help to inform vaccination strategies, including the administration of additional booster vaccine doses.
Here, we conducted a population-based cohort study evaluating Ab and T-cell responses to SARS-CoV-2 among individuals aged 16+ in Zurich, Switzerland, including individuals of varying vaccination and infection statuses. In March 2022, for all study participants (n = 1044) we evaluated total SARS-CoV-2 S- and N-immunoglobulin(Ig)G Ab levels, as well as neutralizing Ab (N-Ab) activity to wildtype (WT) virus, Delta, and Omicron variants using a surrogate neutralization assay. In a randomly selected subset of individuals (n = 328), we further assessed T-cell responses to S, M, and N proteins by IGRA. To investigate longitudinal changes in immune responses over time we reassessed Ab (n = 964) and T cell (n = 141) responses 3 months later, in June 2022. Overall, we found distinct immune response patterns among participants depending on the reported infection and vaccination statuses. Already at the beginning of the study, nearly all participants had detectable S-IgG responses. In contrast, N-IgG and M/N-specific T-cell responses increased significantly over time, despite existing S-IgG, indicating viral (re)exposure. Importantly, participants with the highest N-IgG titers and Omicron-N-Ab activity, and those with IFN-gamma-producing S-reactive T cells all had significantly reduced likelihood of (re)infection between March and June 2022. Together, our results indicate that population-level immune responses to SARS-CoV-2 are S-IgG-dominated but heterogeneous. They suggest a role for assessing M/N-specific T cells in estimating previous viral exposure and further suggest that monitoring a combination of N-IgG, Omicron-N-Ab, and S-reactive T-cell responses may help to predict population-level protection against Omicron SARS-CoV-2 (re)infection.
Abbreviated methods
Detailed methods and information on statistical analyses can be found in the supplementary materials.
Participant recruitment and sample collection
Individuals aged 16+ residing in the canton of Zurich, Switzerland were randomly selected by age-stratified intervals from a population registry and invited to participate. In total, 4875 individuals were contacted and 1044 enrolled (21.4% participation, Supplementary Figure 1). Initial study visits were conducted from March 1st through 31st, 2022 and second study visits (964/1044, 92.3% participation, Supplementary Figure 1) were conducted from June 7th through July 11th, 2022. At each visit, participants provided information regarding previous COVID-19 vaccination and positive SARS-CoV-2 tests. From each participant, 10 ml of venous blood was collected and plasma was cryopreserved before analysis of S-Ig and N-Ig levels and WT, Delta, and Omicron SARS-CoV-2 N-Ab activity. For participants selected for T-cell assessment, an additional 5 ml of venous blood was collected and immediately used for IGRA analysis.
Spike- and nucleocapsid-specific immunoglobulin G and SARS-CoV-2 neutralizing antibody activity
Cryopreserved plasma samples were thawed and analyzed for S- and N-specific IgG by Luminex assay as described [19]. Mean fluorescence intensity (MFI) values for each sample were divided by the mean value of negative control samples to yield an MFI ratio. Individuals were considered seropositive if the MFI ratio exceeded a lower limit of detection (LOD) of 6.0 [19]. Plasma samples were further evaluated for WT, Delta, and Omicron SARS-CoV-2 N-Ab activity using a cell- and virus-free surrogate neutralization assay as described [20]. Half maximal inhibitory concentration (IC) values of 50.0 and 2430.0 were set as lower and upper LODs, respectively.
Interferon-gamma release assay
T-cell responses were assessed by IGRA from whole blood stimulated overnight with overlapping 15-mer peptide pools spanning the entire M and N proteins (M/N pool) or the S1 domain of the S protein and a mix of the predicted immunodominant peptides from S containing most of the S2 domain (S pool) (M, N, S1, and S PepTivator peptide pools, respectively; Miltenyi Biotec). After incubation, stimulated plasma was collected and IFN-gamma-assessed using the Human IFN-gamma enzyme-linked immunosorbent assay (ELISA) assay (Human IFN-gamma DuoSet ELISA kit, R&D Systems, Catalog DY285B, and DuoSet ELISA Ancillary Reagent Kit 2, R&D Systems, Catalog DY008) according to manufacturer's instructions.
Results
Participant demographics and overall antibody and T-cell immune responses
Of the March 2022 study participants (n = 1044, Supplementary Figure 1, Supplementary Table 1), 45.5% were male and 54.3% were female. Of them, 73.7% were aged 16-64 years and 26.3% were 65+ years. A total of 93.5% reported previous SARS-CoV-2 vaccination; 90.8% were fully vaccinated (2+ vaccine doses) and 72.1% had received at least one booster (3+ vaccine doses) [21,22]. In total, 32.6% of participants reported a previous SARS-CoV-2 infection (defined as having received a positive polymerase chain reaction [PCR] or antigen test result) at some point from the pandemic start until the study visit. Older participants (65+ years) were more likely to report being immunized against COVID-19 (odds ratio [OR] 2.87, 95% confidence interval [CI] 1.36-6.09, P = 0.006) and less likely to report previous infection (OR 0.44, 0.32-0.61, P <0.0001) compared to participants aged 16-64, possibly reflecting both the emphasis on vaccination for those 65+, as well as the preventive effect of vaccination on subsequent infection.
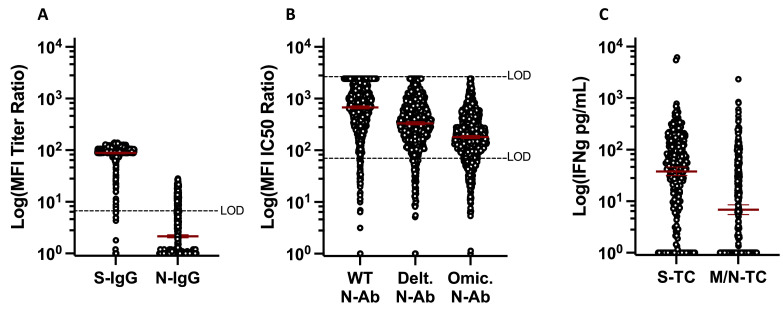
In total, 98.4% of participants were S-IgG seropositive and 23.2% were N-IgG seropositive (Figure 1 a) [21,22]. A total of 96.8%, 93.7%, and 89.5% of participants had detectable neutralization IC50 values to WT, Delta, and Omicron viral variants, respectively. Geometric mean IC50 values, however, differed significantly between variants, being highest for WT and lowest for Omicron (P <0.0001 for all comparisons, Friedman test with Dunn's multiple comparisons, Figure 1b). In a subset of study participants (n = 328), circulating T-cell responses to S, or a combination of M and N proteins [14], were assessed. In total, 89.6% had detectable S-specific T-cell responses, while 57.3% had detectable M/N-specific T-cell responses. Geometric mean IFN-gamma production was also greater for S-stimulation, as compared to M/N-stimulation (Figure 1c). Taken together, these data indicate that, as of March 2022, nearly 99% of the population had previous SARS-CoV-2 antigen exposure (either through vaccination, infection, or both). As M/N proteins are not components of the vaccines available in Switzerland at the time [6,7] but are present during infection, and, as M- and N-T-cell responses are longer-lasting than N-IgG [1,3,14], these findings further suggest that at least 57% of the population had been previously infected by this time.
Figure 1.
Quantitative antibody and T cell Responses among participants, March 2022. (a) anti-S- and N-IgG geometric mean MFI titer ratios (n = 1044), assay LOD = 6.0. (b) anti-WT, Delta, and Omicron geometric mean neutralizing Ab titers (n = 1044), assay LOD 50.0-2430.0. (c) Geometric mean IFN-gamma production following S or M/N peptide stimulation of whole blood (n = 328). Ab, antibody; IC, inhibitory concentration; IFN, interferon; Ig, immunoglobulin; LOD, limit of detection; MFI, mean fluorescence intensity; M, membrane; N, nucleocapsid; N-Ab, neutralizing antibody; S, spike; TC, T cell; WT, wildtype.
Impacts of infection and vaccination on antibody and T-cell responses
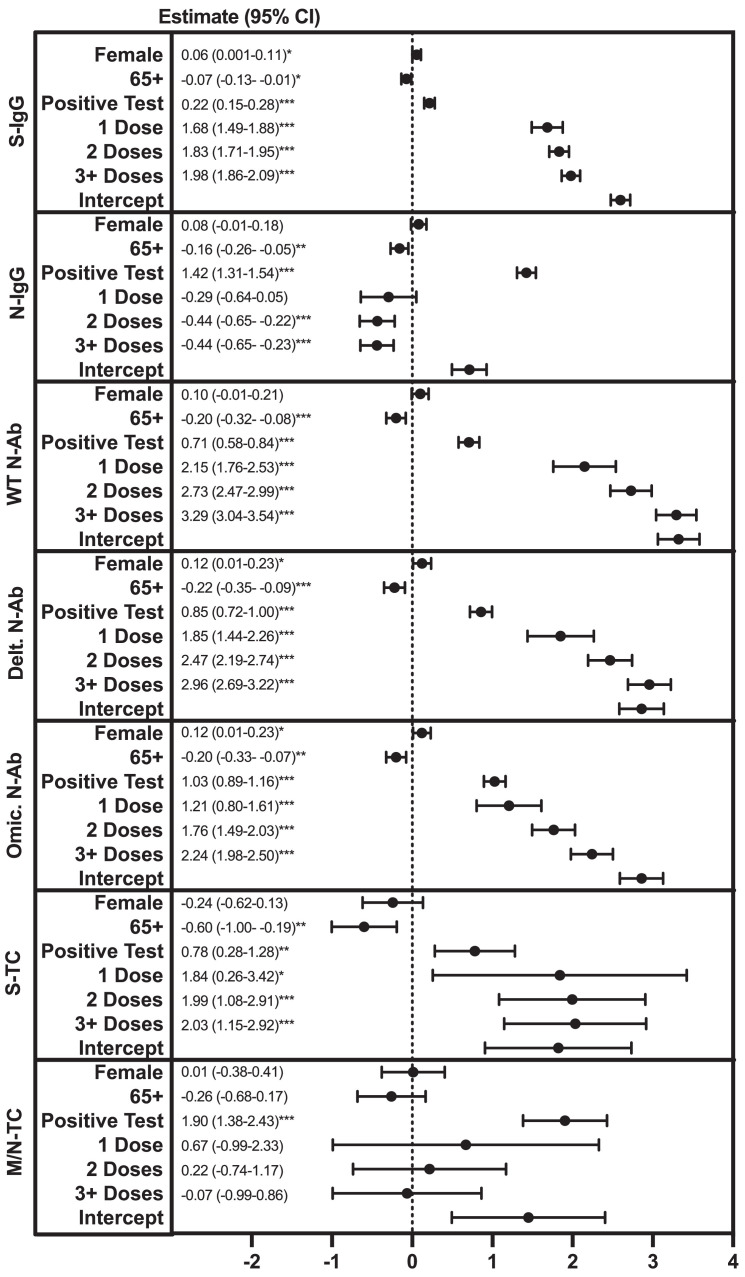
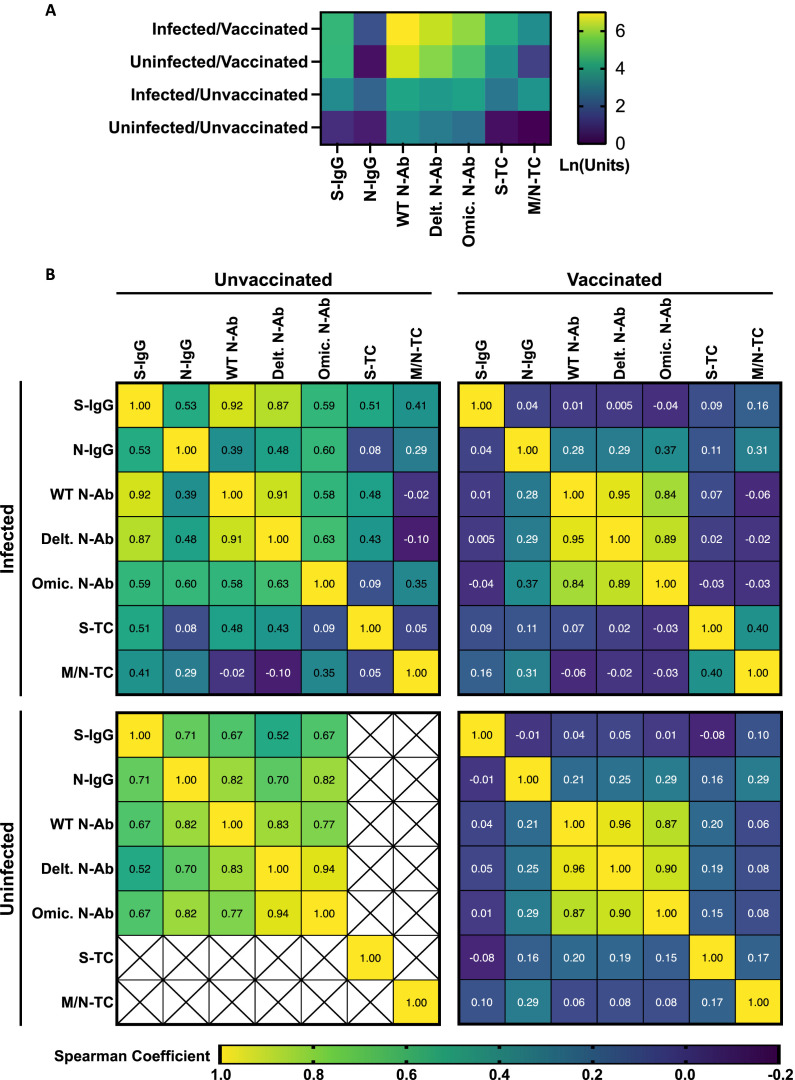
We next assessed the impacts of infection and vaccination on Ab titers and T-cell responses by multivariable linear regression. Increasing age (65+ vs 16-64) was associated with lower S- and N-IgG titers, lower anti-WT, -Delta, and -Omicron-N-Ab activity, and lower S-T cell responses (Figure 2 ). Previous SARS-CoV-2 infection and receiving an increasing number of vaccine doses were both associated with increased S-IgG titers, anti-WT, Delta, and Omicron-N-Ab activity, and S-T-cell responses (Figure 2). Previous infection was also associated with increased N-IgG titers and M/N-T-cell responses (Figure 2). Participants were stratified into four groups: infected/vaccinated (n = 285 Ab, 80 T cell tested), uninfected/vaccinated (n = 686 Ab, 229 T cell tested), infected/unvaccinated (n = 53 Ab, 14 T cell tested), and uninfected/unvaccinated (n = 15 Ab, 3 T cell tested) (Figure 3 a and b). In general, Ab and T-cell response patterns were more similar between vaccinated participants, compared to infected participants. S-IgG and N-Ab tended to be higher in vaccinated individuals (both previously infected and uninfected, Figure 3a), and these did not correlate with N-IgG responses (Figure 3b). In contrast, N-IgG and M/N-T-cell responses were higher among previously infected individuals (both vaccinated and unvaccinated, Figure 3a and b). As expected, the lowest overall responses were observed in uninfected/unvaccinated individuals. Due to the low sample number, there were insufficient data to assess the T-cell correlation patterns for this group (Figure 3a and b).
Figure 2.
Factors associated with March 2022 S- or N-IgG, N-Ab titers, or S- or M/N-T cell IFN-gamma levels. Multivariable linear regression modeling was used to assess the relationship between gender (female vs male), age group (65+ vs 16-64 years), reporting a previous SARS-CoV-2 infection (positive polymerase chain reaction or antigen test) (yes vs no), and the number of COVID-19 vaccine doses received (1, 2, 3+ vs 0), and S- or N-IgG, N-Ab mean fluorescence intensity ratio titers, or S- or M/N-T cell IFN-gamma levels (natural logarithm-transformed). *P >0.05, ** P >0.01, *** P >0.005. CI, confidence interval; IFN, interferon; Ig, immunoglobulin; M, membrane; N, nucleocapsid; N-Ab, neutralizing antibody; S, spike; TC, T cell; WT, wildtype.
Figure 3.
Antibody and T cell responses among participants by infection and vaccination status, March 2022. (a) Quantitative Ab and T cell responses. Log10 anti-S- and N-IgG geometric mean fluorescence intensity titer ratios, anti-WT, Delta, and Omicron geometric mean neutralizing Ab titers, and geometric mean IFN-gamma production following S or M/N peptide stimulation of whole blood. (b) Correlation between Ab and T cell responses. Top left: infected and unvaccinated individuals (n = 53 Ab tested, 14 T cell tested), top right: infected and vaccinated (n = 285 Ab tested, 80 T cell tested), bottom left: uninfected and unvaccinated (n = 15 Ab tested, 3 T cell tested), bottom right: uninfected and vaccinated (n = 686 Ab tested, 229 T cell tested). Values represent Spearman correlation coefficients for indicated Ab and T cell response pairs. Crosses indicate pairs with insufficient data for analysis. Ab, antibody; IFN, interferon; Ig, immunoglobulin; M, membrane; N, nucleocapsid; N-Ab, neutralizing antibody; S, spike; TC, T cell; WT, wildtype.
Longitudinal responses and protection from (re)infection
A total of 964 participants returned for a second study visit, 3 months later, in June 2022 (Supplementary Figure 1, Supplementary Table 1). In total, 141 were assessed for T-cell responses (118 longitudinally from March and an additional 23 not evaluated for T cell responses in the March round; Supplementary Table 1). At this time, 6.4% of participants were unvaccinated, 2.4% had received a single vaccine dose, 17.1% had received two doses, and 74.1% had received three or more doses. Nineteen individuals (2.0% of the study population) received an additional vaccination between March and June, all of which were second or booster doses. Of returning participants, 16.0% (154/964) reported a positive SARS-CoV-2 PCR or antigen test (infection) between March and June. Of participants reporting infection between March and June (n = 154), 14.3% (n = 22) were repeated infections (the same participant also reported infection before March) and 85.7% (n = 132) were new infections (the same participant did not report infection before March). In total, 45.4% (n = 438) of the population reported at least one SARS-CoV-2 infection from the beginning of the pandemic through June 2022.
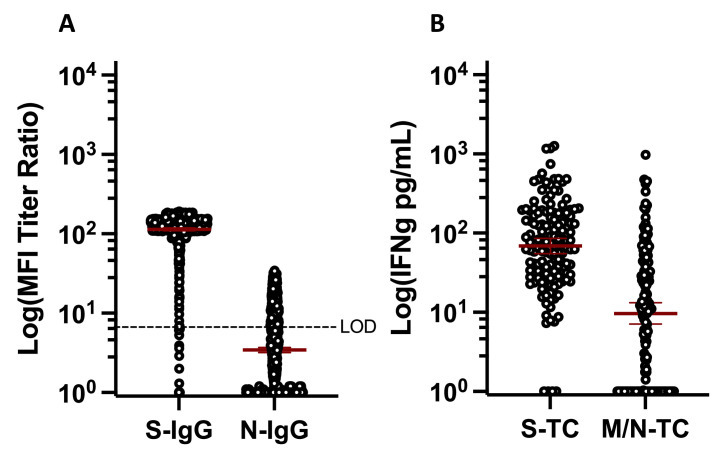
In total, 98.8% of participants were S-IgG seropositive (similar to March) and 36.7% were N-IgG seropositive (increasing from March; P <0.0001, two-sample test of proportions). Geometric mean MFI ratio titers for both S-IgG and N-IgG increased between March and June (Figure 4 a; S-IgG P <0.0001, N-IgG P <0.0001, Wilcoxon matched-pairs signed rank test). A total of 97.2% and 72.3% of participants had detectable S- and M/N-T cell responses, respectively; significantly more than in March (Figure 4a and b, S P = 0.043, M/N P = 0.001, Fisher's exact test) and geometric mean IFN-gamma production among the overall population was higher for both in June (Figure 4b, S P = 0.05, M/N P = 0.053, Mann-Whitney test). M/N-T cell responses tended also to be higher in the longitudinal subset, though this was not statistically significant (P = 0.109, Wilcoxon matched-pairs signed rank test). Between March and June, 0.4% (4/948) of those who were seropositive for S-IgG became seronegative while 50% (8/16) of those who were seronegative became seropositive. For N-IgG, 32.3% (72/223) of those who were seropositive became seronegative and 27.4% (203/741) of those who were seronegative became seropositive. Of individuals tested longitudinally for T cell responses, 1.9% (2/107) of those positive for S-T cells in March were negative in June, while 81.8% (9/11) of those negative in March were positive in June. In total, 13.3% (8/60) of those positive for M/N-T cells in March were negative in June, while 58.6% (34/58) of those negative in March were positive in June. Of note, only 59.1% (120/203) of individuals that became N-IgG seropositive and 29.4% (10/34) of individuals that became M/N-T cell positive reported infection. As N-IgG seropositivity and/or M/N-T cell positivity could only be due to infection in this population, these findings indicate substantial underreporting of infections and highlight the importance of immune monitoring efforts in understanding SARS-CoV-2 exposures.
Figure 4.
Quantitative antibody and T cell Responses, June 2022. (a) S- and N-IgG geometric mean MFI ratio titers (n = 964). (b) Geometric mean IFN-gamma production following S or M/N peptide stimulation of whole blood (n = 141). IFN, interferon; Ig, immunoglobulin; M, membrane; MFI, mean fluorescence intensity; N, nucleocapsid; S, spike; TC, T cell; WT, wildtype.
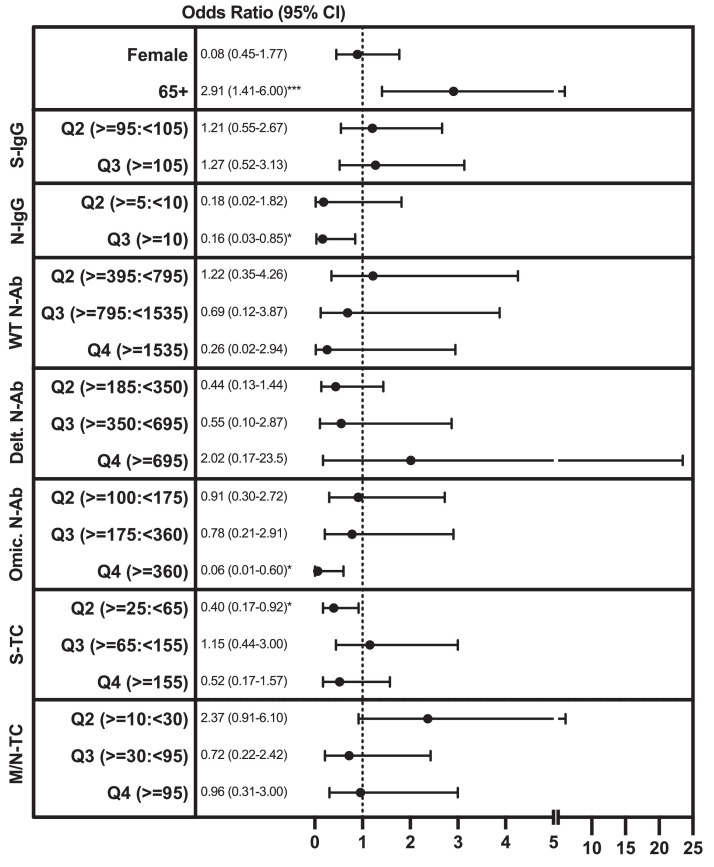
Overall, we observed three dominant immune response patterns, representing over 90% of study participants, which were: group I: S-IgG+/N-IgG+/S-T cell+/M/N-T cell+ (positive for all factors), group II: S-IgG+/N-IgG-/S-T cell+/M/N-T cell+ (positive for everything except N-IgG;), and group III: S-IgG+/N-IgG-/S-T cell+/M/N-T cell- (only S-IgG and S-T cell positive). In March, we observed 17.7%, 36.3%, and 34.2% of participants in group I, group II, and group III, respectively. This was similar in June, with 30.5%, 39.0%, and 23.4% of participants in group I, group II, and group III, respectively (Supplementary Figure 2A and B). Interestingly, only 5.2% of those in group I reported an infection between March and June compared to 26.6% of those in group II and 21.9% of those in group III, potentially suggesting superior protection by the group I combination of immune responses. To evaluate which immune response components might be capable of providing protection against (re)infection, we assessed whether an individual's levels of S- and N-IgG, N-Ab, and S- and M/N-T cells in March were associated with infection between March and June (Figure 5 , Supplementary Table 2). As vaccination is expected to influence SARS-CoV-2-specific immune responses, individuals vaccinated between March and June (n = 19) were excluded from the analysis. Individually, increasing S-IgG (area under curve [AUC] 0.57, 95% 0.52-0.61), N-IgG (AUC 561, 95% 0.59-0.64), WT-N-Ab (AUC 0.66, 95% 0.62-0.70), Delta-N-Ab (AUC 0.67, 95% 0.63-0.71), Omicron-N-Ab (AUC 0.67, 95% 0.63-0.72), and S-T cell (AUC 0.54, 95% 0.51-0.66) responses were all significant but not strong predictors for remaining uninfected (i.e., not reporting a positive SARS-CoV-2 PCR or antigen test) between March and June (Supplementary Table 2). Using a multivariable logistic regression model, we found that individuals with the highest N-IgG titers (MFI ratio titers above 10; the top 33% of the population) had an 84% reduced odds of infection between March and June (OR 0.16, 95% 0.03-0.85, P = 0.031; compared to the lowest 33%). Those with the highest Omicron-N-Ab activity (IC50 titers above 360; the top 25% of the population) had a 94% reduced odds of infection (OR 0.06, 0.006-0.60, P = 0.017; compared to the lowest 25%), while having S-T cells was associated with a 60% reduced likelihood of infection (production of ≥25 to <65 pg/ml IFN-gamma, OR 0.39, 0.17-0.92, P = 0.030; compared to the lowest 25%). Together, increasing N-IgG titers, Omicron-N-Ab, and S-T cell responses were predictive of not reporting an infection between March and June (AUC 0.73, 95% 0.66-0.79, Supplementary Table 2). Therefore, we find that N-IgG, Omicron-N-Ab, and S-specific T cells are associated with protection from Omicron (re)infection, and monitoring a combination of these responses may aid in the assessment of population-level immunity against Omicron SARS-CoV-2 (re)infection.
Figure 5.
Factors associated with reporting a SARS-CoV-2 infection between March and June 2022. Multivariable logistic regression modeling was used to assess the relationship between gender (female vs male), age group (65+ vs 16-64 years), quantiles of S- or N-IgG, N-Ab Titers, or S- or M/N-T cell IFN-gamma levels in March 2022, and reporting a previous SARS-CoV-2 infection (positive polymerase chain reaction or antigen test) (yes vs no) in June/July 2022. For S- and N-IgG MFI ratio titers, individuals were assigned to one of three expression quantiles (<33%, 33-67%, 67+% of all participants); for N-Ab IC50 values, and S- and M/N-T cell IFN-gamma levels, individuals were assigned to one of four expression quantiles (<25%, 25->50%, 50->75%, 75+% of all participants). Corresponding MFI ratios/IC50 titers/IFN-gamma levels are listed next to each variable. *P >0.05, ***P >0.005. CI, confidence interval; IC, inhibitory concentration; IFN, interferon; Ig, immunoglobulin; M, membrane; MFI, mean fluorescence intensity; N, nucleocapsid; N-Ab, neutralizing antibody; S, spike; TC, T cell; WT, wildtype.
Discussion
Although Ab responses among individuals in the Zurich area, and throughout Switzerland have been well-described [21], [22], [23], [24], much less is known regarding population-level T cell responsiveness to SARS-CoV-2. Here, we utilized an IGRA based on a short-term culture of whole blood with SARS-CoV-2-specific peptides to assess T cell responses, which demonstrated good concordance with an ELISpot assay that we used previously [14]. We found that by June 2022, 97% and 72% of study participants had S- and M/N-specific T cells, respectively. In comparison, 99% of participants were S-IgG seropositive, and slightly less than 40% were N-IgG seropositive. That S-specific Ab and T cell responses were higher in general than N-specific Ab and M/N-specific T cell responses is consistent with the high vaccination coverage in the population (>90% fully vaccinated), as vaccines available in Switzerland contained S, but not M or N antigens [6,7]. Furthermore, the half-life of S-IgG is substantially longer than that of N-IgG [14,25,26], consistent with our observation that the fraction of participants who were initially seropositive in March but became seronegative by June was greater for N-IgG (14.1%) compared to S-IgG (1.2%). The higher percentage of M/N-T cell positivity compared to N-IgG positivity is worth noting and may be due to (i) the use of both M- and N-peptides in the IGRA, (ii) that the half-lives of circulating M- and N-specific T cells are longer than that of N-IgG [1,3,14], (iii) that some individuals develop only M- and N-T cell responses after infection [14], and, (iv) that previous exposure to endemic human coronaviruses (HCoV)-229E, -NL63, -OC43, and -HKU1 can generate low levels of T cells cross-reactive to SARS-CoV-2 [2,4,5]. Our findings, however, indicate that assessing SARS-CoV-2 M/N-T cells is likely a more sensitive method for evaluating viral exposure compared to N-IgG and that monitoring M/N-T cells may help to assess population-level “hybrid immunity” in areas where vaccines based solely on S (as opposed to whole-virus vaccines which contain all proteins [27]) are predominately used.
An additional takeaway from our findings is that most individuals had more than one type of virus-specific memory response and that protection was not clearly mediated only by a single subset. The most common patterns, representing over 90% of the study population, belonged to three groups: group I, positive for all assessed responses, group II, positive for everything except N-IgG, and group III, positive only for S-IgG and S-T cells. All of these patterns included S-IgG, but nearly 95% of those in group I did not report an infection between March and June compared to 73-78% of those in groups II and III. Furthermore, despite high S-IgG seropositivity already in March 2022, the percentage of participants with detectable N-IgG titers and M/N-T cells increased significantly by June 2022, indicating continued viral (re)infections, even among individuals with some level of S-specific immunity. In assessing potential mediators of protective immunity, we found that having high N-IgG titers and/or high Omicron-N-Ab activity were both protective against Omicron SARS-CoV-2 infection. Because the half-life of N-IgG is relatively short at approximately 60-90 days [14,25,26], individuals with high titers were likely recently infected—perhaps in the 3-6 months before March. It would also make sense that these individuals were infected with the Omicron variant, which was responsible for >99% of reported COVID-19 cases in late January 2022 in Switzerland [28]. As recent infection may contribute to a state of “trained immunity” [29] with enhanced baseline activation of the innate immune system, we speculate that N-IgG is likely not a sole mediator of protection in and of itself but may serve as a marker for a persisting “antiviral” state which, in turn, limits reinfection. We additionally found that S-T cells were associated with a reduced likelihood of infection. S-reactive T cells are known to be generated following SARS-CoV-2 infection and vaccination [[1], [2], [3],[9], [10], [11], [12], [13], [14], [15]], and individuals can possess pre-existing memory T cell responses generated from previous endemic human coronavirus exposure [2,4,5]. Though the role of S-T cell responses as a correlate of protection against SARS-CoV-2 is not completely clear [8], it is known that T cell-mediated immunity is more cross-reactive than corresponding Ab responses [30]. Furthermore, it has been observed in animal models that, in the absence of Ab responses, protection from SARS-CoV-2 can be mediated solely by T cell immunity [31], and, similarly, we recently observed that individuals can clear SARS-CoV-2 infection in the absence of detectable Ab responses [14], highlighting the importance of this subset in protection from infection.
Some limitations to our study include, first, that we relied on self-reported SARS-CoV-2 infections based on receiving a positive PCR or antigen test result. Although false positive results are possible, it is also likely, because many individuals use self-tests, which have limited sensitivity especially early in infection, that true infections are under-reported. Similarly, we observed that a substantial fraction—20% (3/15)—of participants that reported being uninfected/unvaccinated had detectable S- or N-IgG titers, which we would expect only in response to SARS-CoV-2 antigen exposure. Due to this misclassification, the associations between immunological markers and infections and their discriminative properties (AUC) are likely biased toward the null and, thus, underestimated. An additional limitation was the low number of uninfected/unvaccinated individuals, and as we collected T cell data only from a subset of individuals, we did not have sufficient data to thoroughly assess this group, which represents an interesting immune “baseline”. Furthermore, in terms of the assays used, we assessed only IFN-gamma as a measure of T cell activity. Studies have demonstrated that interleukin (IL)-2-producing T cell responses are also generated in SARS-CoV-2 infection and vaccination [5,16]. It would be valuable to test the IGRA approach in evaluating IL-2 responses in further studies. In addition, we limited our T cell analysis to the three dominant antigens for cellular immune responses (S, M, and N), and furthermore, for experimental feasibility, S1 and S2 domains, as well as M and N responses, were pooled, although they have been shown in other studies to exhibit some distinct behaviors [1,2,14,16]. We cannot exclude the importance of subdominant T cell responses against other viral antigens in some of the participants, which may have led to an underestimation of T cell responses. Another limitation is that, although the IGRA results had a high degree of concordance with ELISpot assay (Supplementary Methods, Supplementary Figure 3A and B), they did not strongly correlate. This is not unexpected, though, as IGRA measures total IFN-gamma output in pg/ml which could be produced by few specific T cells, while ELISpot assesses only the number of IFN-gamma-producing cells without taking the amount of IFN-gamma produced by individual cells into account, making it difficult to compare values from these two assays directly. Furthermore, we used a surrogate assay to indirectly quantify neutralizing activity by measuring competitive inhibition of trimeric SARS-CoV-2 S protein binding to the angiotensin-converting enzyme 2 receptor. However, this assay showed high sensitivity compared to live virus assays during validation [20] and permitted simultaneous assessment of neutralization against WT-SARS-CoV-2, Delta, and Omicron variants.
Nevertheless, we provide here population-level estimates of cellular immunity as well as factors that may be associated with protection from Omicron SARS-CoV-2 infection. Our results suggest that, while most individuals possess anti-S-IgG, these responses in and of themselves are likely not a good predictor of protection from (re)infection. In terms of estimating what fraction of the population has been infected with SARS-CoV-2, monitoring M/N-reactive T cells responses may be helpful. However, to assess what fraction of the population might be protected from (re)infection with Omicron SARS-CoV-2, our data suggest that monitoring a combination of anti-N-IgG, Omicron-N-Ab, and S-reactive T cells may be beneficial. Our findings indicate a pattern where co-correlates of protection, rather than simply S-IgG, are likely important for mediating long-term protective immunity against SARS-CoV-2 and future variants and provide important information for policymakers regarding vaccination strategy in the case of changing disease epidemiology.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Author Contributions
Conception and design: KDZ, CM, MAP, and AF. Development of methodology: KDZ and DLC. Funding acquisition: JSF and MAP. Supervision: CM, JSF, and MAP. Project administration: MAP and AF. Data acquisition: KDZ, DLC, and AF. Analysis and interpretation of data: KDZ, DM, CM, JSF, MAP, and AF. Data visualization: KDZ. Writing of original manuscript draft: KDZ. Review and editing of manuscript: KDZ, DLC, DM, CM, JSF, MAP, and AF. All contributing authors approved the submitted manuscript.
Funding
The Corona Immunitas research network is coordinated by the Swiss School of Public Health (SSPH+) and funded by fundraising of SSPH+, including funds of the Swiss Federal Office of Public Health and private funders (ethical guidelines for funding stated by SSPH+ were respected), by funds of the cantons of Switzerland (Vaud, Zurich, and Basel), and by institutional funds of the Universities.
Ethical approval
We obtained written, informed consent from all participants upon study enrollment. Participants were compensated with a flat fee for any travel expenses related to study visits but otherwise did not receive any compensation for their participation. The study protocol was approved by the Cantonal Ethics Committee of Zurich (BASEC Registration No. 2020-01247) and registered (ISRCTN registry 18181860, date of registration 13 July 2020, retrospectively registered).
Acknowledgments
The authors would like to thank Danusia Vanoaica and Osman Yoztekin for their support with the T cell assays, and the study administration team and the study participants for their dedicated contribution to this research project.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.04.407.
Appendix. Supplementary materials
References
- 1.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181 doi: 10.1016/j.cell.2020.05.015. 1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 5.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 7.Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldblatt D, Alter G, Crotty S, Plotkin SA. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol Rev. 2022;310:6–26. doi: 10.1111/imr.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Mateus J, Coelho CH, Dan JM, Moderbacher CR, Gálvez RI, et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell. 2022;185:2434–2451.e17. doi: 10.1016/j.cell.2022.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mateus J, Dan JM, Zhang Z, Rydyznski Moderbacher C, Lammers M, Goodwin B, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science. 2021;374:eabj9853. doi: 10.1126/science.abj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859.e11. doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev. 2022;310:27–46. doi: 10.1111/imr.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menges D, Zens KD, Ballouz T, Caduff N, Llanas-Cornejo D, Aschmann HE, et al. Heterogenous humoral and cellular immune responses with distinct trajectories post-SARS-CoV-2 infection in a population-based cohort. Nat Commun. 2022;13:4855. doi: 10.1038/s41467-022-32573-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS-CoV-2-Specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thieme CJ, Anft M, Paniskaki K, Blazquez-Navarro A, Doevelaar A, Seibert FS, et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giulieri S, Manuel O. QuantiFERON®-CMV assay for the assessment of cytomegalovirus cell-mediated immunity. Expert Rev Mol Diagn. 2011;11:17–25. doi: 10.1586/erm.10.109. [DOI] [PubMed] [Google Scholar]
- 19.Fenwick C, Croxatto A, Coste AT, Pojer F, André C, Pellaton C, et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence Studies. J Virol. 2021;95 doi: 10.1128/JVI.01828-20. e01828–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenwick C, Turelli P, Pellaton C, Farina A, Campos J, Raclot C, et al. A high-throughput cell- and virus-free assay shows reduced neutralization of SARS-CoV-2 variants by COVID-19 convalescent plasma. Sci Transl Med. 2021;13:eabi8452. doi: 10.1126/scitranslmed.abi8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amati R, Frei A, Kaufmann M, Sabatini S, Pellaton C, Fehr J, et al. Functional immunity against SARS-CoV-2 in the general population after a booster campaign and the Delta and Omicron waves, Switzerland, March 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.31.2200561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frei A, Kaufmann M, Amati R, Dettwiler AB, Wyl Vv, Annoni AM, et al. Development of hybrid immunity during a period of high incidence of infections with Omicron subvariants: a prospective population based multi-region cohort study. medRxiv 2022. doi: 10.1101/2022.10.14.22281076v1. [Accessed 24 January 2023]. [DOI]
- 23.West EA, Kotoun OJ, Schori LJ, Kopp J, Kaufmann M, Rasi M, et al. Seroprevalence of SARS-CoV-2 antibodies, associated factors, experiences and attitudes of nursing home and home healthcare employees in Switzerland. BMC Infect Dis. 2022;22:259. doi: 10.1186/s12879-022-07222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringhini S, Zaballa ME, Pullen N, Perez-Saez J, de Mestral C, Loizeau AJ, et al. Seroprevalence of anti-SARS-CoV-2 antibodies 6 months into the vaccination campaign in Geneva, Switzerland, 1 June to 7 July 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.43.2100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumley SF, Wei J, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis. 2021;73:e699–e709. doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheatley AK, Juno JA, Wang JJ, Selva KJ, Reynaldi A, Tan HX, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12:1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oronsky B, Larson C, Caroen S, Hedjran F, Sanchez A, Prokopenko E, et al. Nucleocapsid as a next-generation COVID-19 vaccine candidate. Int J Infect Dis. 2022;122:529–530. doi: 10.1016/j.ijid.2022.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swiss Federal Office of Public Health (FOPH) Switzerland and Liechtenstein; 2022. COVID-19 Switzerland Information on the current situation: Epidemiological course.https://www.covid19.admin.ch/en/epidemiologic/virus-variants [accessed 12 June 2022] [Google Scholar]
- 29.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sewell AK. Why must T cells be cross-reactive? Nat Rev Immunol. 2012;12:669–677. doi: 10.1038/nri3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kingstad-Bakke B, Lee W, Chandrasekar SS, Gasper DJ, Salas-Quinchucua C, Cleven T, et al. Vaccine-induced systemic and mucosal T cell immunity to SARS-CoV-2 viral variants. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2118312119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.