To the Editor,
The pathogenesis of atopic dermatitis (AD) involves an impairment of the skin barrier by an interplay of genetic and environmental factors. The resulting inappropriate defense against allergens, microbes, and pollutants results in a chronic, mainly T-helper (Th) 2 cell-driven skin inflammation.1 Environmental factors that may increase the risk for AD are airborne particulate matter (PM) and commonly associated polycyclic aromatic hydrocarbons (PAHs).1,2 However, the available epidemiological data provide a heterogeneous picture. While some studies found a significant association between PM exposure and AD symptoms, in particular in children, other studies reported null associations.1,2 This data inconsistency in airborne PM exposure-related AD may depend on interindividual genetic susceptibilities.3
A gene that is highly expressed in lesional AD skin4 and upregulated in PAH-exposed keratinocytes in an aryl hydrocarbon receptor-dependent manner5 encodes aldo-keto reductase (AKR)1C3. AKR1C3 reduces prostaglandin (PG)D2 to 9α,11β-PGF2, a metabolically stable stimulator of Th2 cells that serves as a systemic biomarker for allergen-induced mast cell activation.6 Herein, we demonstrate the functional and clinical relevance of the AKR1C3 gene variant rs12529 for the PM exposure-associated development of AD.
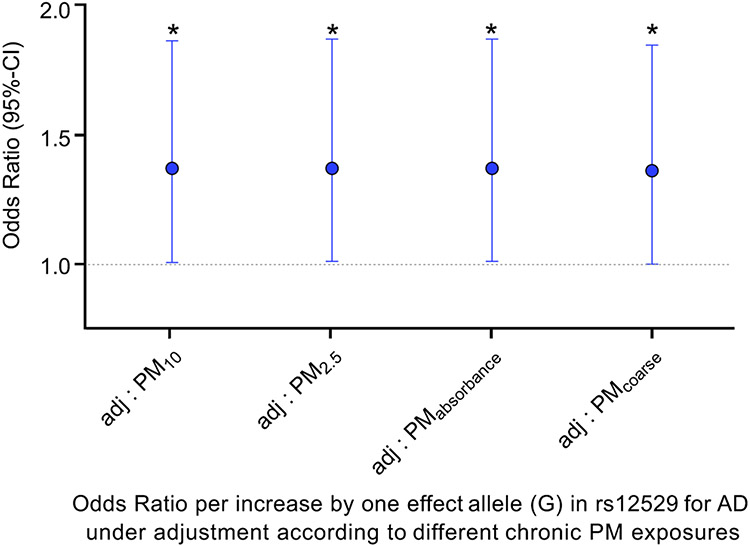
Study individuals enrolled in the GINIplus/LISA birth cohort were restricted to 457 participants (49.5% male; age: mean = 15.1 years, sd = 0.2; BMI: mean = 21.2, sd = 3.3) with available AD diagnosis at the 15-year follow-up examination, air pollution, and genetic data. AD, defined as ever diagnosed by a physician, was present in 174 individuals. Median chronic exposures to PMs with interquartile ranges were for PM2.5 17.3 μg/m3 (0.9), for PM10 25.2 μg/m3 (1.5), for PM2.5 absorbance 1.16 10−5/m (0.2), and for PMcoarse 8.4 μg/m3 (0.6). The single nucleotide polymorphism (SNP) rs12529 was genotyped with sufficient quality (estimated R2 = 0.997), and the minor/effect allele frequency (EAF) was G: 0.400. We found consistent effects for all PM exposures showing a higher chance for adolescent carriers of the rs12529 effect allele (G) to develop AD as compared to rs12529 major allele (C) carriers under constant airborne PM exposure (Figure 1). With the increase per one effect allele, the odds ratio for developing AD significantly increases by 38% (PM10, PM2.5, PM2.5 absorbance) and 37% (PMcoarse), respectively.
FIGURE 1.
Carriers of the effect allele (G) in rs12529 have a higher chance to develop AD when adjusting to different chronic PM exposures. Individual chronic exposure to particulate matter with a median aerodynamic diameter of ≤2.5/ ≤10 μm (PM2.5/PM10), diameters of 2.5–10 μm (PMcoarse), and the reflectance of PM2.5 filters (PM2.5 absorbance/ in figure: PMabsorbance) was considered. 417 (for PM2.5, PM10, PM2.5 absorbance) and 420 (for PMcoarse) participants were included in the regression model. With the increase per one effect allele G, the odds ratio (OR) for developing AD significantly increases by a factor of 1.38, hence per 38% (p = 0.043, 95% confidence intervals (CI) = 1.011;1.879) under constant chronic exposure to PM10 (PM2.5: OR = 1.38, 95% CI = 1.015;1.885, p = 0.040; PM2.5 absorbance: OR = 1.38, 95% CI = 1.015;1.886, p = 0.040; PMcoarse: OR = 1.37, 95% CI = 1.005;1.861, p = 0.046). * = p < 0.05.
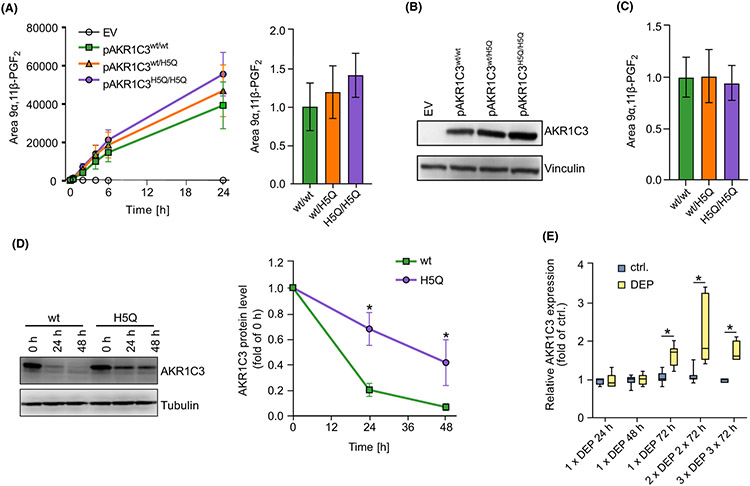
Next, we investigated whether the rs12529 effect allele, causing an amino acid exchange in codon 5 from His to Gln, affects the catalytic activity of AKR1C3. In comparison with the major allele variant, the overexpression of an effect allele-resembling AKR1C3 variant in CRISPR/Cas9-generated AKR1C3-knockout (HaCaT-AKR1C3-KO) keratinocytes (Figure S1A-C) resulted in an enhanced 11-ketoreduction of PGD2 to 9α,11β-PGF2 (Figure 2A). However, after normalization of the LC–MS data to the protein level, this effect was diminished (Figure 2B,C), indicating that the rs12529 effect allele affects AKR1C3 enzyme activity indirectly by enhancing its protein stability. Accordingly, treatment of transfected HaCaT-AKR1C3-KO cells with the translation blocker cycloheximide revealed a delayed degradation of the effect allele-resembling AKR1C3 enzyme over time (Figure 2D), indicating that the SNP-related amino acid exchange indeed enhances protein stability.
FIGURE 2.
The SNP rs12529 increases AKR1C3 protein stability and airborne PM upregulates AKR1C3 in human skin explants. A) LC–MS analyses of supernatants from HaCaT-AKR1C3-KO keratinocytes transfected with pAKR1C3wt, pAKR1C3H5Q, pAKR1C3wt/pAKR1C3H5Q (50:50), or empty vector. After 24 h, cells were treated with PGD2 (1 μM) for up to 24 h. Shown as mean ± SEM of n = 3 (left) and normalized to pAKR1C3wt/wt at 24 h (right). (B) AKR1C3 protein level of 24 h samples shown in (A). Representative blot of n = 3. (C) LC–MS quantification of 9α,11β-PGF2 after 24 h depicted in (A) normalized to AKR1C3 protein level shown in B). Shown as mean ± SEM of n = 3. (D) Keratinocytes were either transfected with pAKR1C3wt or pAKR1C3H5Q. After 24 h, cells were treated with 10 μM cycloheximide for up to 48 h. Representative blot (left) and mean quantification ± SEM of n = 4 (right). (E) Six μg/cm2 diesel exhaust particles (DEP) were topically applied to cultured human skin explants at days 1, 4, and 7. Skin samples were harvested after single, double, or triple DEP application for 3 days. Gene expression of AKR1C3 was analyzed by qPCR and normalized to 18 S rRNA level. *p ≤ 0.05.
Importantly, AKR1C3 expression is not only inducible by PAHs, such as benzo[a]pyrene, but also by PAH-rich PM. In fact, treatment of HaCaT keratinocytes with an organic extract of PM2.5 collected from traffic-related air pollution and a repetitive topical exposure of human ex vivo skin with diesel exhaust particles increased the expression of AKR1C3 (Figure 2E, Figure S2A) and the prototypic aryl hydrocarbon receptor target gene cytochrome P450 (CYP)1A1 (Figure S2).
Taken together, our data show that under constant chronic PM exposure, the increase per one AKR1C3 SNP rs12529 effect allele increases the chance to develop AD significantly by approx. 37%–38%. PM exposure induces AKR1C3 expression in human skin; therefore, this observation might be due to an enhanced protein level and corresponding catalytic activity of AKR1C3. The allele frequency of rs12529 varies markedly across continental populations.7 Hence, we speculate that due to a higher rs12529 EAF, some populations (e.g., Asians, EAF = 0.861) might be more susceptible to PM/PAH exposure-induced or -exacerbated AD than others (e.g., Europeans, EAF = 0.405).
Supplementary Material
ACKNOWLEDGMENTS
FH was supported by the Jürgen Manchot Foundation. CFAV was supported by the National Institute of Environmental Health Sciences and the National Institutes of Health under Award Numbers R01ES029126 and R01ES032827.
GINIplus/LISA birth cohort: The authors thank all the families for their participation in the GINIplus study. Furthermore, we thank all members of the GINIplus Study Group for their excellent work. The GINIplus Study group consists of the following: Institute of Epidemiology, Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg (Heinrich J, Brüske I, Schulz H, Flexeder C, Zeller C, Standl M, Schnappinger M, Ferland M, Thiering E, Tiesler C); Department of Pediatrics, Marien-Hospital, Wesel (Berdel D, von Berg A); Ludwig-Maximilians-University of Munich, Dr von Hauner Children's Hospital (Koletzko S); Child and Adolescent Medicine, University Hospital rechts der Isar of the Technical University Munich (Bauer CP, Hoffmann U); IUF-Environmental Health Research Institute, Düsseldorf (Schikowski T, Link E, Klümper C, Krämer U, Sugiri D). The authors thank all the families for their participation in the LISA study. Furthermore, we thank all members of the LISA Study Group for their excellent work. The LISA Study group consists of the following: Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Epidemiology, Munich (Heinrich J, Schnappinger M, Brüske I, Ferland M, Schulz H, Zeller C, Standl M, Thiering E, Tiesler C, Flexeder C); Department of Pediatrics, Municipal Hospital “St. Georg”, Leipzig (Borte M, Diez U, Dorn C, Braun E); Marien-Hospital Wesel, Department of Pediatrics, Wesel (von Berg A, Berdel D, Stiers G, Maas B); Pediatric Practice, Bad Honnef (Schaaf B); Helmholtz Centre of Environmental Research—UFZ, Department of Environmental Immunology/Core Facility Studies, Leipzig (Lehmann I, Bauer M, Röder S, Schilde M, Nowak M, Herberth G, Müller J); Technical University Munich, Department of Pediatrics, Munich (Hoffmann U, Paschke M, Marra S); Clinical Research Group Molecular Dermatology, Department of Dermatology and Allergy, Technische Universität München (TUM), Munich (Ollert M, J. Grosch). Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The GINIplus study was mainly supported for the first 3 years of the Federal Ministry for Education, Science, Research and Technology (interventional arm) and Helmholtz Zentrum Munich (former GSF) (observational arm). The 4-year, 6-year, 10-year, and 15-year follow-up examinations of the GINIplus study were covered from the respective budgets of the 5 study centres (Helmholtz Zentrum Munich (former GSF), Research Institute at Marien-Hospital Wesel, LMU Munich, TU Munich, and from 6 years onwards also from IUF—Leibniz Research Institute for Environmental Medicine at the University of Düsseldorf), and a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Further, the 15-year follow-up examination of the GINIplus study was supported by the Commission of the European Communities, the 7th Framework Program: MeDALL project, and as well by the companies Mead Johnson and Nestlé.
The LISA study was mainly supported by grants from the Federal Ministry for Education, Science, Research and Technology and in addition from Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research—UFZ, Leipzig, and Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef for the first 2 years. The 4-year, 6-year, 10-year, and 15-year follow-up examinations of the LISA study were covered from the respective budgets of the involved partners (Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research—UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef, IUF—Leibniz Research Institute for Environmental Medicine at the University of Düsseldorf) and in addition by a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Further, the 15-year follow-up examination of the LISA study was supported by the Commission of the European Communities, the 7th Framework Program: MeDALL project.
The research leading to the ESCAPE results has received funding from the European Community's Seventh Framework Program (FP7/2007–2011) under grant agreement number: 211250.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Luger T, Amagai M, Dreno B, et al. Atopic dermatitis: role of the skin barrier, environment, microbiome, and therapeutic agents. J Dermatol Sci. 2021;102(3):142–157. [DOI] [PubMed] [Google Scholar]
- 2.Dijkhoff IM, Drasler B, Karakocak BB, et al. Impact of airborne particulate matter on skin: a systematic review from epidemiology to in vitro studies. Part Fibre Toxicol. 2020;17(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks JD, Stanek LW, Luben TJ, et al. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119(4):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantel A, Carpenter-Mendini AB, Vanbuskirk JB, De BA, Beck LA, Pentland AP. Aldo-keto reductase 1C3 is expressed in differentiated human epidermis, affects keratinocyte differentiation, and is upregulated in atopic dermatitis. J Invest Dermatol. 2012;132(4):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogeley C, Sondermann NC, Woeste S, et al. Unraveling the differential impact of PAHs and dioxin-like compounds on AKR1C3 reveals the EGFR extracellular domain as a critical determinant of the AHR response. Environ Int. 2022;158:106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oymar K, Aksnes L. Urinary 9alpha,11beta-prostaglandin F(2) in children with atopic eczema/dermatitis syndrome: an indicator of mast cell activation? Acta Derm Venereol. 2004;84(5):359–362. [DOI] [PubMed] [Google Scholar]
- 7.The 1000 Genomes Project Consortium, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.