Abstract
Collagen is one of the most abundant proteins in animals and a major component of the extracellular matrix (ECM) in tissues. Besides playing a role as a structural building block of tissues, collagens can modulate the behavior of cells, and their deregulation can promote diseases such as cancer. In tumors, collagens and many other ECM molecules are mainly produced by fibroblasts, and recent evidence points towards a role of tumor-derived collagens in tumor progression and metastasis. In this review, we focus on the newly discovered functions of collagens in cancer. Novel findings have revealed the role of collagens in tumor dormancy and immune evasion, as well as their interplay with cancer cell metabolism. Collagens could serve as prognostic markers for cancer patients, and therapeutic strategies targeting the collagen ECM have the potential to prevent tumor progression and metastasis.
INTRODUCTION
Tissues are composed of a cellular and an acellular component, the extracellular matrix (ECM). The main categories of ECM proteins are collagens, glycoproteins and proteoglycans that are organized in a three-dimensional meshwork. While one of the major functions of the ECM is to provide physical support to cells in the tissues, it also plays a crucial role in regulating cellular behavior and dysregulation of the ECM can cause diseases such as cancer.
Collagens are among the most abundant ECM proteins in tissues. Aberrant deposition of collagen matrix contributes to tumor formation(1), and mutations in collagens cause diseases such as collagenopathies(2) (osteogenesis imperfecta, Ehlers–Danlos syndrome) and the Alport syndrome(3). There are 28 different types of collagens organized in different categories: Fibrillar collagens, FACIT (fibril-associated collagens with interrupted triple helices), basement membrane collagens, filamentous collagens, short chain collagens, long chain collagens, multiplexins and MACIT (membrane-associated collagens with interrupted triple helices)(4). Collagens are organized in a triple helix with a repeat consensus amino acid sequence, Gly-X-Y, where X and Y can be any amino acid. After translation of the procollagen molecule, post-translational modifications (PTM) (proline and lysine hydroxylation and glycosylation) take place in the endoplasmic reticulum and the collagen chains are assembled into a triple helix. The procollagen triple helix is secreted through vesicles to the extracellular space. Then, procollagen is cleaved by proteinases in the N- and C- terminus and lysyl oxidases crosslink collagens in the extracellular space for assembly into fibrils. In order to interact with different collagens in the extracellular space, cells display collagen receptors on their surface(5): a) integrin α1β1 is expressed in many mesenchymal cell types and it is important for the action of many inflammatory cells, including T lymphocytes; integrin α2β1 plays a role in cell migration, proliferation and survival(6) ; integrin α10β1 is expressed in cartilage tissues(7) and plays a role in bone development(8); integrin α11β1 is abundant in many mesenchymal tissues during development and is required for the maintenance of bone mass(9). b) Discoidin Domain Receptors (DDRs) are tyrosine kinases widely expressed in different tissues and their downstream signals regulate cell proliferation, differentiation and the response to the extracellular matrix(10).
c) Glycoprotein VI (GPVI, p62) is present on platelets and regulates their function during thrombosis(11). d) LAIR-1 is a member of the immunoglobulin superfamily, expressed on peripheral blood mononuclear cells and involved in innate immunity(12). e) OSCAR is an osteoclast-associated receptor, and its activation by collagen costimulates the Fc receptor common γ-chain to regulate osteoclastogenesis; it is also involved in a variety of other cellular processes like adhesion, activation and enhancement of proinflammatory cascades, cellular recruitment, and prevention of apoptosis(13). f) G protein-coupled receptor 56 (GPR56), recently shown to have an important role facilitating changes in platelet shape and integrin activation, both of which are necessary for efficient hemostasis and thrombosis(14). Collagen can also be endocytosed by cells through the endocytic receptor endo180 and degraded into fragments that serve as a source of different amino acids(15,16) (see Table 1 for a summary of collagen receptors). In this review, we will discuss the functions and impact of collagens on immune regulation, metabolism, and epigenetic reprogramming in the context of cancer (Figure 1).
Table 1.
Types of collagens, receptors and their expression in different cell types.
| Receptor | Collagen Type | Cell Type |
|---|---|---|
| Integrins α1β1 | Collagens IV,VI, Fibril-forming collagens | Mesenchymal cells, Inflammatory cells (T-lymphocytes), Epithelial cell types, Platelets |
| Integrin α2β1 | Fibril-forming collagens | Mesenchymal cells, Epithelial cell types, Platelets |
| Integrin α10β1 | Collagen IV and VI | Chondrocytes |
| Integrin α11β1 | Fibril-forming collagens and collagen X | Mesenchymal cells |
| Discoidin domain receptor 1 (DDR1) | Fibril-forming collagens (Collagen I,II,III), Collagen IV and VIII | Epithelial cells |
| Discoidin Domain Receptor 2 (DDR2) | Fibril-forming collagens and collagen X | Mesenchymal cells |
| Glycoprotein VI (GPVI) | Fibril-forming collagens | Platelets |
| LAIR-1; LAIR-2 | Collagen I, transmembrane collagens XIII, XVII, XXIII | Leukocytes |
| OSCAR | Fibril-forming collagens (Collagen I, II,III) | Vascular endothelial cells, Osteoclast, Macrophages |
| GPR56 | Fibril-forming collagens (i.e. Collagen III) | Platelets |
| Mannose receptor family (MR,PLA2R, DEC-205, Endo180) | Fibril-forming collagens and collagen IV | Fibroblasts |
Figure 1. Multiple functions of Collagens in the context of cancer progression.
Used with permission from ©Mount Sinai Health System.
The matrisome and cancer progression: The role of collagens
The matrisome is a collection of all ECM and ECM-related proteins in the human genome(17–19). There are two types of proteins within the matrisome: core matrisome proteins (including collagens, proteoglycans and glycoproteins) and matrisome-associated proteins (including secreted factors, ECM-regulators and ECM-affiliated molecules)(19). Recent studies have shown that matrisome-related gene signatures can be used as a predictive marker of cancer outcomes in several tumor types such as ovarian, lung, gastric, and colon cancer. In these tumor types, a nine-gene ECM signature is associated with poor prognosis(20).
In high-grade serous ovarian cancer, ECM composition dynamically transforms as the tumor develops and in response to chemotherapy, which later leads to an upregulation of COL VI(21). Also, a study analyzing 30,000 cancer patient samples showed that changes in matrisome composition can be used as markers(22) to predict response to immunotherapy.
Mutations in collagen proteins have been identified in cancer. For example, in gastric cancer, analysis of TCGA signatures indicates that collagen genes harbor somatic mutations at a higher rate when compared to the background mutation data. In this study, the authors showed that somatic mutations in COL7A1 predict improved survival(23). Recently, a pan-cancer analysis unfolded the mutational landscape of the matrisome. The analysis shows that the mutational burden in core matrisome genes impacts cancer patient overall survival(24). The authors highlight that copy number alterations and mutations in matrisome genes are very frequent, even more than in the rest of the genome.
Metastasis.
ECM molecules also play a critical role in the metastasis cascade (Figure 2a). Intravital imaging studies in breast tumors have shown that, during migration, cancer cells use collagen fibers as tracks to leave the primary tumor(25). Recent work by the Oudin lab showed that chemotherapy-induced COL IV drives breast cancer cell motility(26) and COL VI regulates motility of breast cancer cells in obese models(27). In pancreatic ductal carcinoma (PDAC), tumor-derived ECM was identified as a driver of tumorigenesis and metastasis(28,29), and tends to correlate with poor patient survival. Profiling of the ECM showed that the matrisome composition at metastasis sites differs from the one at the primary site and it is also different between metastatic sites(30). In breast cancer, it has also been shown that tumors with different metastatic potential exhibit unique ECM composition(31). All together, these results raise the interesting question of whether adaptation of cancer cells to metastatic organs is mediated by these changes in the composition of the extracellular matrix regulated by the cancer cells.
Figure 2. Role of collagens in the metastatic cascade.
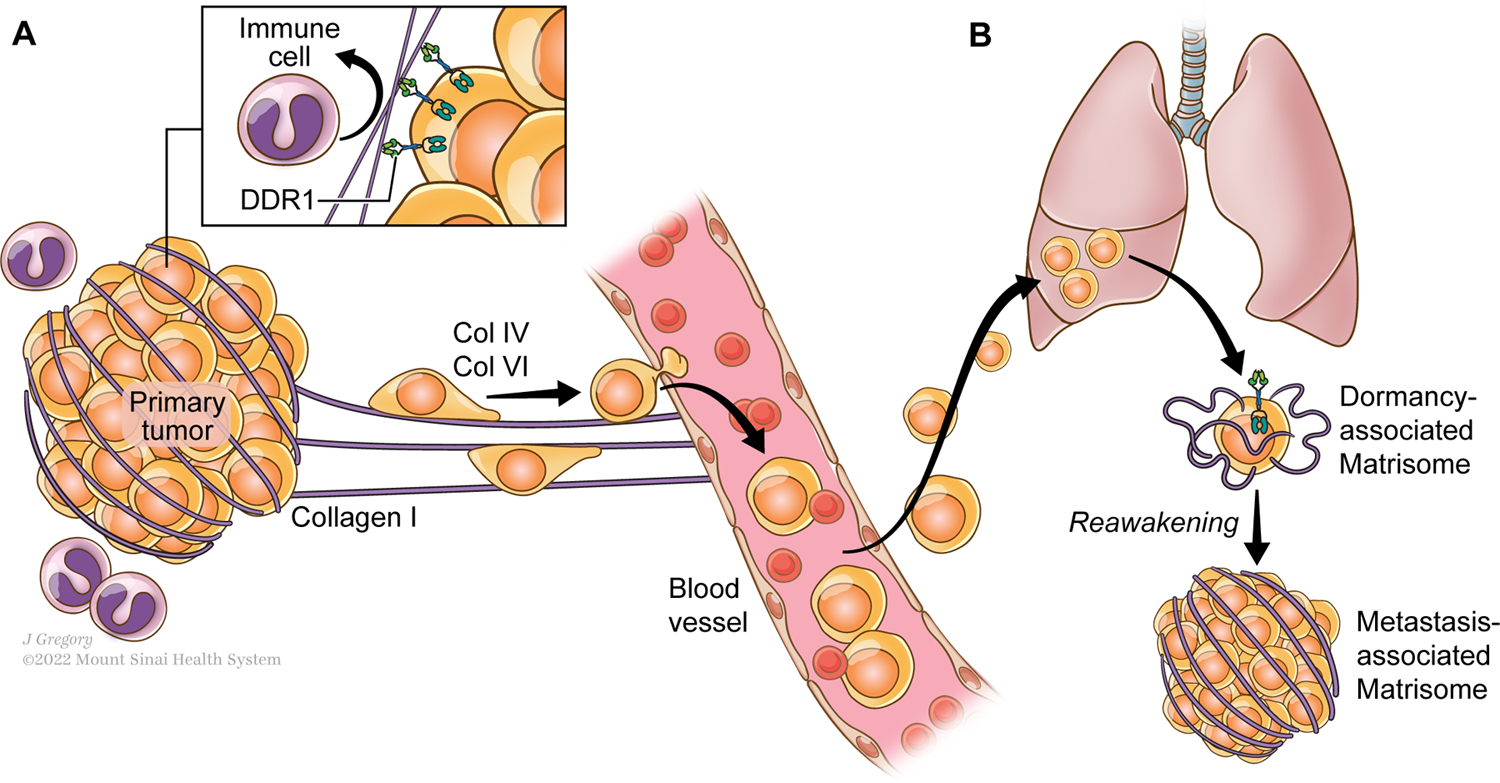
A. Aligned collagens fibrils contribute to protect the primary tumor from immune cells cytotoxic effects and support cancer cell migration into the circulation and towards distant organs.
B. Collagen ECM composition exerts a selective pressure on the disseminated tumor cells (DTCs) at distant organs sites. The presence of Collagen III in particular contributes to maintain the tumor cells in a dormant status, while Collagen I fibers drives their awakening that results in the progression of the disease.
Used with permission from ©Mount Sinai Health System.
Collagens and quiescence, an old link.
In 1993, Coppock et al., discovered that upon exiting the cell cycle and entering quiescence, lung fibroblasts express a series of genes named quiescins (quiescence-inducible genes)(32). They identified 8 genes that are upregulated in quiescent fibroblasts, including decorin, C1r, Q6, Q10 and several collagen chains: COL6A1, COL3A1, COL1A1 and COL1A2. Interestingly, the authors observed a 10-fold increase of COL3A1 when the cells enter into quiescence in comparison with the other collagens. These early studies raise the interesting hypothesis of whether collagens contribute to maintaining cells, including cancer cells, in a quiescent status and if the transcriptional regulation of certain collagen genes in disseminated cancer cells could have a role in balancing proliferative/quiescence signals during the metastatic cascade. Upon dissemination, cancer cells remain in a non-proliferative quiescent state, called tumor dormancy, for many years before they restore growth(33). ECM proteomic analysis of dormant cells revealed a matrisome highly enriched in collagens(34), that go through changes in its composition between dormant and proliferative states. The analysis of the dormancy-associated matrisome or “quiesome” has also outlined very interesting features of the ECM composition of dormant tumor cells and how the stroma-derived matrisome adapts to the presence of a dormant cancer cell. This study showed that an increase in tumor-derived COL III in the matrisome of dormant cells is required to sustain quiescence and upon depletion of COL3A1 in dormant cells, tumor growth is restored (Figure 2b). Other collagens have been shown to be involved in sustaining quiescence of other cell types such as stem cells. For example, satellite stem cells in the muscle produce COL V to maintain their quiescence status through CALCR(35) and COL VI regulates satellite cell self-renewal(36). Whether COL V or other collagens play a role in tumor cell quiescence could represent an interesting area of investigation that may reveal new features of collagens in the context of dormancy.
The collagen receptor DDR1 and the control of the matrisome
In 1993 Johnson et al., identified a tyrosine phosphoprotein in breast cancer cells called Discoidin Domain Receptor I (DDR1) that contains a unique extracellular proline/glycine-rich domain. This extracellular domain confers an unusual geometry of interactions with ligands or its substrates (37). In this study, the authors defined the discoidin domains as “a class of domains that may interact with cell surface molecules”. In the same year Di Marco et al., in normal keratinocytes and several human cell lines, isolated and cloned a new member of the Trk family, TrkE(38). Zerlin et al., also in 1993, isolated murine cDNA encoding a receptor like tyrosine kinase(39). All these reports identified the collagen receptor DDR1. A few years later in 1997, Vogel et al. described collagen as an activator of the DDR1 receptor, defining it as a potential regulator of the response to the extracellular matrix(40). After those early studies, the biology of DDR1 has been vastly explored and while our understanding of the receptor biology is quite ample(10), new functions of DDR1 have been discovered in the context of cancer.
The DDR1 receptor is widely expressed in human tissues and plays diverse roles, being involved in cell proliferation and differentiation processes, but also, as a collagen sensor, its activity is crucial for cell migration, adhesion, and invasion in different cell types including tumor cells. In breast tumors, Takai et al., demonstrated that in MMTV-PyMT mice, Ddr1 knockout promotes spontaneous mammary gland tumorigenesis by elevating epithelial tension and matricellular fibrosis, revealing a clear basal phenotype and an enhancement of metastatic lung disease(41). More recently Sun et al. showed that in breast cancer patients high levels of DDR1 mRNA are associated with poor prognosis and this direct correlation is maintained in the group of patients with triple negative breast cancer (TNBC) when analyzed alone(42). In other tumors such as lung cancer, knockout of Ddr1 attenuates tumorigenesis(43). In the context of tumor dormancy, DDR1 has been shown to sustain tumor cell quiescence through Collagen III and STAT1(34) in head and neck and breast cancer models. After a dormancy period, DDR1 is also required to restore growth of breast tumor cells through Collagen I, TM4SF1, JAK2 and STAT3(44). All these results emphasize that depending on the tumor stage and tumor type, DDR1 function may be pro- or anti-tumorigenic and these different outputs may be dependent on the downstream signaling regulated and the type of collagen that binds to it.
There are a few interesting aspects of the biology of DDR1 that point to a crucial role in controlling the biology of the ECM. In 2003, Faraci et al. performed an ECM microarray study to find target genes downstream of DDR1(45). They used the human cell line HT1080 with or without overexpressing DDR1 and found several ECM genes that were either activated or inactivated upon DDR1 overexpression. This was the first report showing that the collagen receptor can regulate the composition of the matrisome. Recent work from our lab showed that the matrisome of dormant cells changes upon their awakening through DDR1 depletion(34). In head and neck models, the study of ECM composition of the awake/proliferating cancer cells revealed an important rearrangement of the matrisome composition that vastly differs from the one surrounding dormant cancer cells. Several collagen chains (COL6A1, COL22A1, COL4A1) accumulate in the awakened tumors and a decrease in Collagen type III is observed. In these models, STAT1 activation downstream of DDR1 regulates transcription of COL3A1 in dormant cells. Interestingly, recent work in kidney cells done by the Pozzi lab has shown that DDR1, even without a nuclear translocation signal, can translocate to the nucleus to regulate transcription upon collagen binding and through interaction with SEC61B, a component of the Sec61 translocon and non-muscle myosin IIA and β-actin. In the nucleus, DDR1 binds to chromatin and increases transcription of Col IV by binding to the COL IV promoter(46). The impact of DDR1 translocation to the nucleus and the regulation of collagen expression in dormant cells is not known but it is an interesting mechanism that could be explored to further understand its complex role during tumor progression.
Collagens and the DDR1 receptor in tumor immune regulation
In the past few years, efforts have focused on understanding the molecular processes underlying tumor immunity, leading to the development of new therapeutic strategies to treat cancer. The 3D architecture of the ECM acts as a physical barrier regulating immune cell migration. In order to access tissues, immune cells have to actively migrate through the ECM. The highly porous collagen matrix of healthy tissues facilitates migration of T-lymphocytes and natural killer cells and their physiological immune surveillance activity within the tissues. Collagen fibril size and density define the external resistance which immune cells are subjected to and influence their access to the tissues. Work by Friedl et al., showed that immune cells are able to deform their nuclei and move along the loose and well-aligned collagen matrix in a crawling-like fashion, typical of the amoeboid movement(47). In addition, it has been demonstrated that the ECM plays an important role both in modulating the immune response within the primary tumor and in the activation or repression of innate immunity at the distant sites of metastasis. The increased stiffness of the ECM, due to the presence of hyper-crosslinked collagen and overproduction of glycoproteins surrounding the cancer cells, limits immune cell infiltration and motility, prevents their interaction with cancer cells, and inhibits their cytotoxic activity, a process known as immune exclusion. Hartmann et al. demonstrated that in pancreatic cancer, even in presence of high levels of CXCL10 or CXCL4 (T-cells chemoattractant), T cells infiltration in the tumor cells nests was inhibited due to the presence of high density collagen fibers(48). Moreover, a study by Salmon et al., showed that high collagen density in the vascular region and surrounding the tumor epithelial cells can direct the migratory trajectory of the T cells and limit their access to the tumor islets(49). An interesting study by LaRue et al., in pancreatic cancer has described a mechanism in which collagen synthesis together with degradation have a direct effect on the production of a highly desmoplastic and immunosuppressive microenvironment. This study demonstrated that tumor-associated macrophages remodel the ECM through collagen production and degradation, mediated by mannose MRC1 receptor, with consequent increase of intracellular arginine levels, upregulation of inducible nitric oxide synthase (iNOS) and the production of reactive nitrogen species (RNS). The RNS, in a paracrine mechanism, stimulate the pancreatic stellate cells in the production and extracellular deposition of high quantities of collagen, therefore augmenting tumor fibrosis(50).
As mentioned before, DDR1 function concerning the immune system can vary depending on the tumor stage and context. For example, DDR1-expressing immune cells become very responsive to the presence of collagen within the ECM and are highly motile. Chetoui at al., provided evidence of how DDR1 expression can enhance the binding of T cells to collagen fibers, promoting their migratory phenotype(51). In support of this concept, in vitro 3D collagen I migration assays show that the blockage of DDR1 with a soluble recombinant protein (DDR1:Fc) interferes with T cells binding to biotinylated collagen I. In models of pancreatic ductal adenocarcinoma (PDAC), the collagen organization and topography modulate the migration of tumor-activated T-cells at the metastatic sites and the expression of the collagen receptor DDR1 is directly correlated to the metastatic potential of pancreatic cancer cells. Furthermore, the high expression of DDR1 on PDAC cells favors DDR1-collagen interaction in the ECM, and triggers CXCL5 production. As a result, CXCL5 induces the recruitment of tumor-associated neutrophils and the formation of neutrophil extracellular traps, thereby promoting cancer cell invasion and metastasis(52).
Different studies demonstrate how DDR1 exerts a pro-tumor activity by regulating the immune microenvironment. In triple negative breast cancer, DDR1 modulates the infiltration of CD4+ and CD8+ T cells regulating the tumor growth(53). In breast tumors, Sun et al.(42) showed that DDR1 mRNA and protein levels negatively correlate with genes that define anti-tumor immunity. Also, depletion of DDR1 in breast tumor cells facilitates immune cells penetration into the tumors (Figure 2a). The authors show that the ectodomain of DDR1 remodels the collagen matrix and reorganizes it into an aligned structure that prevents immune penetration. Treatment of tumors with anti-DDR1 antibodies prevents collagen alignment and allows the immune cells to penetrate into the tumor.
Collagens, wound healing and the tumor microenvironment
The presence of collagens in the ECM is essential to confer the mechanical strength and elasticity to the tissues and contributes to the formation of a natural substrate for cellular attachment, proliferation, and differentiation(54). Moreover, in the event of a tissue injury, collagens play a key role in the complex process of wound repair. The wound healing process requires four precise and highly programmed biological steps which need to occur in the proper sequence and time frame: hemostasis, inflammation, proliferation, and maturation/remodeling. Collagens are involved in all these critical phases.
In the 80s, Dvorak described the connection between the tumor microenvironment and wound healing, and how tumor stroma forms through abnormal activation of wound healing pathways(55). During the last decade it has been noted that tumors behave as wounds that fail to heal. Interestingly, recent work by the Massague lab showed that metastasis-initiating cells exploit wound healing signaling pathways for metastasis regrowth. The authors found that L1CAM (L1 cell adhesion molecule), a protein involved in intestinal wound healing, is required for liver metastasis colonization and chemoresistance(56).
The tumor microenvironment is a dynamic space in which remodeling of the ECM by cancer associated fibroblasts (CAFs) is crucial for tumor progression, similar to the process of wound healing in which remodeling of the collagen ECM by fibroblasts promotes scar maturation. CAFs can regulate the collagen matrix stiffness by regulating its crosslinking affecting migration of cancer cells(57). CAFs can also proteolyze COL IV at the basement membrane which can favor tumor invasion(58).
A recent paper by Fischer et al. showed a very interesting link between the process of wound healing, the ECM, and immune cells(59). By tagging collagen I with a fluorescent protein, the authors show that matrix can be transferred across organs into wounds upon injury. This transport is mediated by neutrophils that travel from the visceral and parietal mesothelial layers surrounding internal organs into injured tissues. The transfer of matrix that comes from distal mobile reservoirs provides material for tissue repair. Whether this biology can have implications in cancer remains to be explored. Interestingly, sustained lung inflammation caused by tobacco awakens dormant cells(60) mediated by neutrophil extracellular traps. Given the function of neutrophils in wound healing by moving matrix, it will be interesting to explore if neutrophils recruited to the lungs upon sustained inflammation also deposit matrix around dormant cells that may favor tumor cell awakening.
Collagens and their role in tumor metabolism and epigenetic remodeling
Extracellular matrix turnover is important for tissue homeostasis. The ECM can be degraded by metalloproteases in the extracellular space but it can also be internalized through α2β1 integrin(61), delivered to the lysosomes for degradation, and used as a source of energy by cells. Intact and cleaved collagen has been shown to be internalized by integrins(62) and Endo180(16,63). Cleaved collagen is transported inside the cells more efficiently than intact collagen through Endo180-dependent mechanisms.
In the context of cancer, it has been suggested that the ECM is a source of nutrients that helps to sustain tumor growth and invasion. In collagen, proline represents 25% of the amino acid residues, and studies showed that collagen can be a reservoir of proline and can be used by cancer cells to maintain proline metabolism(64). Another critical factor is tissue stiffness. The stiffness of the collagen ECM can regulate breast cancer metabolism and affect its progression. High density collagen induces the expression of TCA cycle genes and enhances glutamate metabolism in breast cancer(65). Also, as breast cancer cells migrate through dense collagen matrix, the ATP:ADP ratio increases facilitating their invasion(66) as visualized by imaging techniques and fluorescent biosensors (67,68). In addition, the degradation of collagen by metalloproteinases and collagenases in the tumor microenvironment serves as a reservoir for free extracellular proline which can be used for protein and collagen neo synthesis, feeding the collagen biosynthesis-maturation-degradation cycle and promoting the modification of the epigenetic landscape in cancer cells(69). These changes in metabolism in response to the ECM context suggest the ability of cancer cells to easily adapt in order to meet the energy demands required for the invasion process through the ECM.
One of the most important step of collagen maturation is carried out by the prolyl-4-hydroxylase (P4H) in the endoplasmic reticulum(70). Proline hydroxylation in collagens is a critical PTM which is necessary to ensure the stabilization of the triple helix(71). The transfer of hydroxyl residues on proline in collagens requires two essential cofactors: Vitamin C (VitC) and α−Ketoglutarate. High expression of P4H enzymes are linked to changes in the cancer cell metabolism and directly correlated to poor prognosis in various types of tumors, such as glioma, breast cancer, and cervical cancer among others(69,72,73). Gene expression of the isoform P4HA2 enzyme is induced by hypoxia, suggesting a direct correlation between collagen hydroxylation and metastasis initiation promoted by lack of oxygen in the primary tumor(74). D’aniello et al., showed in breast cancer how activity of P4HA2 and sequestering of VitC reduced the activity of the JumonjjC-domain containing (JmjC) histone dioxygenases and the Ten-eleven Translocation (Tet) DNA demethylases, also dependent on Vit C for their function. Inhibition of JmjC and Tet modifies the epigenetic landscape of tumor cells through a genome-wide increase in DNA methylation of histones. Hence, overexpression of P4H in a variety of human cancers suggests its significance as a promising target for diagnostic and therapeutic strategies.
Potential roles of the ECM as a prognostic factor and therapeutic target
Analysis of the 3D architecture of collagen matrix has been used as a prognostic factor in many tumor types. Pioneering work by Patricia Keely showed that in mammary tumors, alignment of the collagen matrix is a prognostic factor for disease progression(75). Similar measurements have been used in head and neck tumors(34), as well as prostate tumors(76), highlighting the importance of exploiting ECM imaging as a detective tool to identify tumor lesions and define their stages. A recent study by Jones at al. describes a novel imaging methodology to assess and quantify the spatial architecture of collagen fibers in the stroma compartment of breast tumor microenvironment. The imaging technology is based on the use of polarized light microscopy to create a stromal architecture signature (SAS) to differentiate between myxoid and sclerotic stroma in unstained human breast cancer specimens(77). The stroma organization and morphology can be used as prognostic factor not only in breast cancer but also in other solid tumors(78,79).
Recent work has also shown that modulating the collagen microenvironment may have a therapeutic effect on tumor growth. Brisson et al. demonstrated that collagen III pro-peptides inhibit fibroblast activation and decrease breast cancer tumor growth(80). Di Martino et al. showed that using sponges incubated with polymerized collagen III and applied to tumor-resected areas can prevent the recurrence and growth of residual cancer cells after primary tumor removal(34). The properties of the collagen matrix can also be leveraged to improve anti-tumor therapies. Monin et al. developed an elegant ECM-based approach to potentiate the effects of immunotherapy. The authors developed collagen anchoring cytokines and showed that the antitumor cytokines IL-2 and IL-12 fused to the collagen-binding protein lumican can potentiate the effect of systemic immunotherapies by prolonging their intratumoral retention(81). Interfering with collagen remodeling can also affect tumor growth, as shown in melanomas, in where DDR1 and DDR2 inhibition delay tumor relapse(82). Few collagen-related clinical trials has been conducted in cancer patients(83). A recent trial in uterine fibroids in where collagenase enzyme was injected in tumors showed reduction of matrix stiffness and cell proliferation(84).
Overall, these results highlight the potential of using strategies that modify the collagen microenvironment to affect tumor growth and show how these approaches can have beneficial effects impacting tumor progression. The more knowledge we acquire on the role of collagens in the different stages of tumor progression, the better approaches and interventions could be developed to eliminate tumors and metastasis by targeting the collagen microenvironment.
Acknowledgments
We want to thank Erin Bresnahan and Swagata Basu for critical reading and editing of the manuscript and Jill Gregory for her illustrations. This work was supported by an NCI R01 (CA244780) and the Tisch Cancer Institute NIH Cancer Center grant (P30 CA196521).
References
- 1.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer 2014;15:1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobling R, D’Souza R, Baker N, Lara-Corrales I, Mendoza-Londono R, Dupuis L, et al. The collagenopathies: review of clinical phenotypes and molecular correlations. Curr Rheumatol Rep. United States; 2014;16:394. [DOI] [PubMed] [Google Scholar]
- 3.Pescucci C, Longo I, Bruttini M, Mari F, Renieri A. Type-IV collagen related diseases. J Nephrol. Italy; 2003;16:314–6. [PubMed] [Google Scholar]
- 4.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol [Internet] Nature Publishing Group; 2014;15:771–85. Available from: 10.1038/nrm3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leitinger B Transmembrane Collagen Receptors 2011; [DOI] [PubMed] [Google Scholar]
- 6.Madamanchi A, Santoro SA, Zutter MM. α2β1 Integrin. Adv Exp Med Biol United States; 2014;819:41– 60. [DOI] [PubMed] [Google Scholar]
- 7.Camper L, Hellman U, Lundgren-Åkerlund E. Isolation, Cloning, and Sequence Analysis of the Integrin Subunit α10, a β1-associated Collagen Binding Integrin Expressed on Chondrocytes*. J Biol Chem [Internet] 1998;273:20383–9. Available from: https://www.sciencedirect.com/science/article/pii/S0021925818490709 [DOI] [PubMed] [Google Scholar]
- 8.Lundgren-Åkerlund E, Aszòdi A. Integrin α10β1: a collagen receptor critical in skeletal development. Adv Exp Med Biol United States; 2014;819:61–71. [DOI] [PubMed] [Google Scholar]
- 9.Shen B, Vardy K, Hughes P, Tasdogan A, Zhao Z, Yue R, et al. Integrin alpha11 is an Osteolectin receptor and is required for the maintenance of adult skeletal bone mass. Stainier DY, Rosen CJ, Leesshepard J, editors. Elife [Internet] eLife Sciences Publications, Ltd; 2019;8:e42274. Available from: 10.7554/eLife.42274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitinger B Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol 2014;310:39–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuentes E Modulation of Glycoprotein VI and Its Downstream Signaling Pathways as an Antiplatelet Target. Int J Mol Sci Switzerland; 2022;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyaard L LAIR and collagens in immune regulation. Immunol Lett Netherlands; 2010;128:26–8. [DOI] [PubMed] [Google Scholar]
- 13.Nedeva IR, Vitale M, Elson A, Hoyland JA, Bella J. Role of OSCAR Signaling in Osteoclastogenesis and Bone Disease. Front cell Dev Biol Switzerland; 2021;9:641162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeung J, Adili R, Stringham EN, Luo R, Vizurraga A, Rosselli-Murai LK, et al. GPR56/ADGRG1 is a platelet collagen-responsive GPCR and hemostatic sensor of shear force. Proc Natl Acad Sci U S A United States; 2020;117:28275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun [Internet] 2017;8:16031. Available from: 10.1038/ncomms16031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelholm LH, Ingvarsen S, Jürgensen HJ, Hillig T, Madsen DH, Nielsen BS, et al. The collagen receptor uPARAP/Endo180. Front Biosci (Landmark Ed. Singapore; 2009;14:2103–14. [DOI] [PubMed] [Google Scholar]
- 17.Socovich AM, Naba A. The cancer matrisome: From comprehensive characterization to biomarker discovery. Semin. Cell Dev. Biol 2019. [DOI] [PubMed] [Google Scholar]
- 18.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol Cell Proteomics [Internet] 2012;11:M111.014647-M111.014647. Available from: 10.1074/mcp.M111.014647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naba A “ The Cancer Matrisome Project : Proteomic Profiling the Tumor Extracellular Matrix Identifies Novel Metastatic Promoters ” 2019;2. [Google Scholar]
- 20.Yuzhalin AE, Urbonas T, Silva MA, Muschel RJ, Gordon-Weeks AN. A core matrisome gene signature predicts cancer outcome. Br J Cancer [Internet] Nature Publishing Group; 2018;15:1–6. Available from: 10.1038/bjc.2017.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietilä EA, Gonzalez-Molina J, Moyano-Galceran L, Jamalzadeh S, Zhang K, Lehtinen L, et al. Co-evolution of matrisome and adaptive adhesion dynamics drives ovarian cancer chemoresistance. Nat Commun 2021;12:3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bin Lim S, Chua MLK, Yeong JPS, Tan SJ, Lim W-T, Lim CT. Pan-cancer analysis connects tumor matrisome to immune response. NPJ Precis Oncol 2019;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodsky AS, Khurana J, Guo KS, Wu EY, Yang D, Siddique AS, et al. Somatic mutations in collagens are associated with a distinct tumor environment and overall survival in gastric cancer. BMC Cancer 2022;22:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izzi V, Davis MN, Naba A. Pan-Cancer Analysis of the Genomic Alterations and Mutations of the Matrisome. Cancers (Basel) 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahai E, Wyckoff J, Philippar U, Segall JE, Gertler F, Condeelis J. Simultaneous imaging of GFP, CFP and collagen in tumors in vivousing multiphoton microscopy. BMC Biotechnol [Internet] 2005;5:14. Available from: 10.1186/1472-6750-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatherree JP, Guarin JR, McGinn RA, Naber SP, Oudin MJ. Chemotherapy-Induced Collagen IV Drives Cancer Cell Motility through Activation of Src and Focal Adhesion Kinase. Cancer Res United States; 2022;82:2031–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wishart AL, Conner SJ, Guarin JR, Fatherree JP, Peng Y, McGinn RA, et al. Decellularized extracellular matrix scaffolds identify full-length collagen VI as a driver of breast cancer cell invasion in obesity and metastasis. Sci Adv 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chenxi T, R. CK, Daniel Ö, Steffen R, Ying H, Mala G, et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci [Internet]. Proceedings of the National Academy of Sciences; 2019;116:19609–18. Available from: 10.1073/pnas.1908626116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian C, Öhlund D, Rickelt S, Lidström T, Huang Y, Hao L, et al. Cancer-cell-derived matrisome proteins promote metastasis in pancreatic ductal adenocarcinoma. Cancer Res 2020;canres.2578.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hebert JD, Myers SA, Naba A, Abbruzzese G, Lamar JM, Carr SA, et al. Proteomic Profiling of the ECM of Xenograft Breast Cancer Metastases in Different Organs Reveals Distinct Metastatic Niches. Cancer Res 2020;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naba A, Clauser KR, Lamar JM, Carr SA, Hynes RO. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. Elife 2014;2014:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coppock DL, Kopman C, Scandalis S, Gilleran S. Preferential gene expression in quiescent human lung fibroblasts. Cell Growth Differ 1993;4:483–93. [PubMed] [Google Scholar]
- 33.Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat cancer 2020;1:672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Martino JS, Nobre AR, Mondal C, Taha I, Farias EF, Fertig EJ, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer [Internet] 2022;3:90–107. Available from: 10.1038/s43018-021-00291-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baghdadi MB, Castel D, Machado L, Fukada S, Birk DE, Relaix F, et al. Reciprocal signalling by Notch– Collagen V–CALCR retains muscle stem cells in their niche. Nature [Internet] 2018;557:714–8. Available from: http://www.nature.com/articles/s41586-018-0144-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 2013;4:1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci U S A 1993;90:5677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Marco E, Cutuli N, Guerra L, Cancedda R, De Luca M. Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues. J Biol Chem United States; 1993;268:24290–5. [PubMed] [Google Scholar]
- 39.Zerlin M, Julius MA, Goldfarb M. NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene England; 1993;8:2731–9. [PubMed] [Google Scholar]
- 40.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell United States; 1997;1:13–23. [DOI] [PubMed] [Google Scholar]
- 41.Takai K, Drain AP, Lawson DA, Littlepage LE, Karpuj M, Kessenbrock K, et al. Discoidin domain receptor 1 (DDR1) ablation promotes tissue fibrosis and hypoxia to induce aggressive basal-like breast cancers. Genes Dev 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X, Wu B, Chiang H-C, Deng H, Zhang X, Xiong W, et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 2021;599:673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambrogio C, Gómez-López G, Falcone M, Vidal A, Nadal E, Crosetto N, et al. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med United States; 2016;22:270–7. [DOI] [PubMed] [Google Scholar]
- 44.Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, et al. Multi-organ Site Metastatic Reactivation Mediated by Non-canonical Discoidin Domain Receptor 1 Signaling. Cell [Internet] Elsevier Inc.; 2016;166:47–62. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867416307383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faraci E, Eck M, Gerstmayer B, Bosio A, Vogel WF. An extracellular matrix-specific microarray allowed the identification of target genes downstream of discoidin domain receptors. Matrix Biol Netherlands; 2003;22:373–81. [DOI] [PubMed] [Google Scholar]
- 46.Chiusa M, Hu W, Liao H-J, Su Y, Borza CM, de Caestecker MP, et al. The Extracellular Matrix Receptor Discoidin Domain Receptor 1 Regulates Collagen Transcription by Translocating to the Nucleus. J Am Soc Nephrol 2019;ASN.2018111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedl P, Entschladen F, Conrad C, Niggemann B, Zänker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol Germany; 1998;28:2331– 43. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, et al. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin cancer Res an Off J Am Assoc Cancer Res United States; 2014;20:3422–33. [DOI] [PubMed] [Google Scholar]
- 49.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean M-C, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012;122:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaRue MM, Parker S, Puccini J, Cammer M, Kimmelman AC, Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc Natl Acad Sci U S A United States; 2022;119:e2119168119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chetoui N, El Azreq M-A, Boisvert M, Bergeron M-È, Aoudjit F. Discoidin domain receptor 1 expression in activated T cells is regulated by the ERK MAP kinase signaling pathway. J Cell Biochem United States; 2011;112:3666–74. [DOI] [PubMed] [Google Scholar]
- 52.Deng J, Kang Y, Cheng C-C, Li X, Dai B, Katz MH, et al. DDR1-induced neutrophil extracellular traps drive pancreatic cancer metastasis. JCI insight 2021;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong X, Zhang W, Sun T. DDR1 promotes breast tumor growth by suppressing antitumor immunity. Oncol Rep Greece; 2019;42:2844–54. [DOI] [PubMed] [Google Scholar]
- 54.Schultz GS, Chin GA, Moldawer L, Diegelmann RF. Principles of Wound Healing In: Fitridge R, Thompson M, editors. Adelaide (AU); 2011. [PubMed] [Google Scholar]
- 55.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med United States; 1986;315:1650–9. [DOI] [PubMed] [Google Scholar]
- 56.Ganesh K, Basnet H, Kaygusuz Y, Laughney AM, He L, Sharma R, et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat cancer 2020;1:28–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pankova D, Chen Y, Terajima M, Schliekelman MJ, Baird BN, Fahrenholtz M, et al. Cancer-Associated Fibroblasts Induce a Collagen Cross-link Switch in Tumor Stroma. Mol Cancer Res 2016;14:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maneva-Radicheva L, Ebert U, Dimoudis N, Altankov G. Fibroblast remodeling of adsorbed collagen type IV is altered in contact with cancer cells. Histol Histopathol Spain; 2008;23:833–42. [DOI] [PubMed] [Google Scholar]
- 59.Fischer A, Wannemacher J, Christ S, Koopmans T, Kadri S, Zhao J, et al. Neutrophils direct preexisting matrix to initiate repair in damaged tissues. Nat Immunol 2022;23:518–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice 2018;4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arora PD, Wang Y, Bresnick A, Dawson J, Janmey PA, McCulloch CA. Collagen remodeling by phagocytosis is determined by collagen substrate topology and calcium-dependent interactions of gelsolin with nonmuscle myosin IIA in cell adhesions. Mol Biol Cell 2013;24:734–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rainero E Extracellular matrix endocytosis in controlling matrix turnover and beyond: emerging roles in cancer. Biochem Soc Trans [Internet] 2016;44:1347–54. Available from: 10.1042/BST20160159 [DOI] [PubMed] [Google Scholar]
- 63.Melander MC, Jürgensen HJ, Madsen DH, Engelholm LH, Behrendt N. The collagen receptor uPARAP/Endo180 in tissue degradation and cancer (Review). Int J Oncol 2015;47:1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phang JM, Donald SP, Pandhare J, Liu Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids Austria; 2008;35:681–90. [DOI] [PubMed] [Google Scholar]
- 65.Morris BA, Burkel B, Ponik SM, Fan J, Condeelis JS, Aguirre-Ghiso JA, et al. Collagen Matrix Density Drives the Metabolic Shift in Breast Cancer Cells. EBioMedicine 2016;13:146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Y, Zanotelli MR, Zhang J, Reinhart-King CA. Matrix-driven changes in metabolism support cytoskeletal activity to promote cell migration. Biophys J 2021;120:1705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zanotelli MR, Zhang J, Ortiz I, Wang W, Chada NC, Reinhart-King CA. Highly motile cells are metabolically responsive to collagen density. Proc Natl Acad Sci U S A United States; 2022;119:e2114672119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zanotelli MR, Goldblatt ZE, Miller JP, Bordeleau F, Li J, VanderBurgh JA, et al. Regulation of ATP utilization during metastatic cell migration by collagen architecture. Mol Biol Cell 2018;29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Aniello C, Cermola F, Palamidessi A, Wanderlingh LG, Gagliardi M, Migliaccio A, et al. Collagen Prolyl Hydroxylation-Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. Cancer Res United States; 2019;79:3235–50. [DOI] [PubMed] [Google Scholar]
- 70.Prolyl Myllyharju J. 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol Netherlands; 2003;22:15–24. [DOI] [PubMed] [Google Scholar]
- 71.Rappu P, Salo AM, Myllyharju J, Heino J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem England; 2019;63:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Q, Wang Q, Zhang Q, Zhang J, Zhang J. Collagen prolyl 4-hydroxylase 2 predicts worse prognosis and promotes glycolysis in cervical cancer. Am J Transl Res 2019;11:6938–51. [PMC free article] [PubMed] [Google Scholar]
- 73.Lin J, Jiang L, Wang X, Wei W, Song C, Cui Y, et al. P4HA2 Promotes Epithelial-to-Mesenchymal Transition and Glioma Malignancy through the Collagen-Dependent PI3K/AKT Pathway. J Oncol 2021;2021:1406853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilkes DM, Bajpai S, Wong CC, Chaturvedi P, Hubbi ME, Wirtz D, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res 2013;11:456–66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 2011;178:1221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pavlova IP, Nair SS, Lundon D, Sobotka S, Roshandel R, Treacy P-J, et al. Multiphoton Microscopy for Identifying Collagen Signatures Associated with Biochemical Recurrence in Prostate Cancer Patients. J Pers Med 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones B, Thomas G, Westreich J, Nofech-Mozes S, Vitkin A, Khorasani M. Novel quantitative signature of tumor stromal architecture: polarized light imaging differentiates between myxoid and sclerotic human breast cancer stroma. Biomed Opt Express United States; 2020;11:3246–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ueno H, Jones AM, Wilkinson KH, Jass JR, Talbot IC. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut England; 2004;53:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang LM, Silva MA, D’Costa Z, Bockelmann R, Soonawalla Z, Liu S, et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget United States; 2016;7:4183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brisson BK, Stewart DC, Burgwin C, Chenoweth D, Wells RG, Adams SL, et al. Cysteine-rich domain of type III collagen N-propeptide inhibits fibroblast activation by attenuating TGFβ signaling. Matrix Biol 2022;109:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Momin N, Mehta NK, Bennett NR, Ma L, Palmeri JR, Chinn MM, et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci Transl Med United States; 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berestjuk I, Lecacheur M, Carminati A, Diazzi S, Rovera C, Prod’homme V, et al. Targeting Discoidin Domain Receptors DDR1 and DDR2 overcomes matrix-mediated tumor cell adaptation and tolerance to BRAF-targeted therapy in melanoma. EMBO Mol Med England; 2022;14:e11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu S, Xu H, Wang W, Li S, Li H, Li T, et al. The role of collagen in cancer: from bench to bedside. J Transl Med [Internet] 2019;17:309. Available from: 10.1186/s12967-019-2058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Islam MS, Afrin S, Singh B, Jayes FL, Brennan JT, Borahay MA, et al. Extracellular matrix and Hippo signaling as therapeutic targets of antifibrotic compounds for uterine fibroids. Clin Transl Med United States; 2021;11:e475. [DOI] [PMC free article] [PubMed] [Google Scholar]


