Abstract
Alcohol expectancies (AEs) are associated with likelihood of alcohol initiation and subsequent alcohol use disorders. It is unclear whether genetic predisposition to alcohol use and/or related traits contributes to shaping how one expects to feel when drinking alcohol. We used the Adolescent Brain Cognitive Development study to examine associations between genetic propensities (i.e., polygenic risk for problematic alcohol use, depression, risk-taking), sociodemographic factors (i.e., parent income), and the immediate social environment (i.e., peer use and disapproval toward alcohol) and positive and negative AEs in alcohol-naïve children (max analytic N=5,352). Mixed-effect regression models showed that age, parental education, importance of the child’s religious beliefs, adverse childhood experiences, and peer disapproval of alcohol use were associated with positive and/or negative AEs, to varying degrees. Overall, our results suggest several familial and psychosocial predictors of AEs but little evidence of contributions from polygenic liability to problematic alcohol use or related phenotypes.
Keywords: Alcohol expectancies, polygenic risk scores, adverse childhood experiences, peer deviance, educational attainment, religiosity
INTRODUCTION
Alcohol expectancies (AEs) are personal beliefs about how one anticipates or expects that alcohol will impact behavior, mood, and physiology1,2. Typically assessed using self-report questionnaires3,4, psychometric approaches broadly categorize these beliefs as positive (e.g., “drinking alcohol makes a person feel good or happy”; “drinking makes people worry less”) or negative (e.g., “alcohol can make people more careless”) expectations about how alcohol will make them feel or behave. Expectancies (anticipated effects of alcohol) are distinct from drinking motives (experienced effects of alcohol5), and thus, are ideally evaluated in alcohol-naïve samples or in recent drinkers. In such youth cohorts, positive AEs have been associated with the initiation and early stages of alcohol use6, as well as greater alcohol use and future alcohol use disorders7-9; in contrast, negative AEs are generally associated with decreased alcohol use and cessation8,10, although some studies have found negative AEs to be predictive of alcohol use problems11. Rooted in expectancy theory, AEs are presumed to index memories, environmental impacts and personality attributes that shape cognitions surrounding the effects of alcohol, and thus, are considered key proximal contributors to the onset and maintenance of alcohol use1,2,12.
Given the cognitive component of AEs, and evidence for heritable influences on cognition as well as aspects of alcohol use, including frequency and quantity of drinking and alcohol use disorder13,14, researchers have speculated whether AEs have a heritable basis. Interestingly, twin studies have yielded mixed results. While some studies found evidence for genetic influences on AEs15-17, others have reported null heritability18,19 and instead, documented a substantial role of familial environment (i.e., those non-genetic factors that makes members of twin and sibling pairs more similar to each other). The heightened influence of familial environment is highly plausible as alcohol-naïve youth are often “learning” of the subjective effects of alcohol from observing alcohol use in family members and peers. Indeed, both parents and peers play a pivotal role in developing and reinforcing expectations towards drinking20-22. Likewise, familial factors, such as religiosity23, parental drinking patterns or views and practices regarding alcohol use24,25, as well as macro-level environmental factors (e.g., alcohol availability, liquor restrictions, taxation26) are likely contributors to AEs.
Expectancy theory posits that an individual’s beliefs about an anticipated behavior are likely to shape their level of future engagement in that behavior. Yet, most studies of AEs have been conducted in samples where few participants, if any, are alcohol naïve, thus confounding expectations regarding the anticipated effects of alcohol with any experienced effects of alcohol which may be influenced by genetic and environmental factors to a differing degree (e.g., 18). The Adolescent Brain Cognitive DevelopmentSM (ABCD) Study provides an ideal sample (N = 11,235) in which to characterize AEs with respect to genetic, familial, and other psychosocial factors as the sample consisted of youth, 26% of whom reported ever sipping alcohol at the time at which their AEs were assessed. In the subsample of youth who had not initiated alcohol use (i.e., not even had a sip of alcohol; N = 8,319), we examined whether polygenic liability scores, sociodemographic factors, parent history of alcohol use, adverse childhood experiences, and peer use and attitudes toward alcohol were associated with positive and negative AEs. These variables were chosen based on prior associations in the literature (e.g., 17,27-30) or hypothesized importance for shaping AEs prior to alcohol use. The examination of the extent to which polygenic liability to problematic alcohol use, as well as risk-taking (related to anticipated positive reinforcement from alcohol) and depression (related to anticipated negative effects of alcohol), were associated with AEs was novel to the study; we hoped this would advance our understanding of whether AEs are merely a product of familial and peer-related environmental factors or whether genetic propensities also shape their development.
METHODS
Target sample description
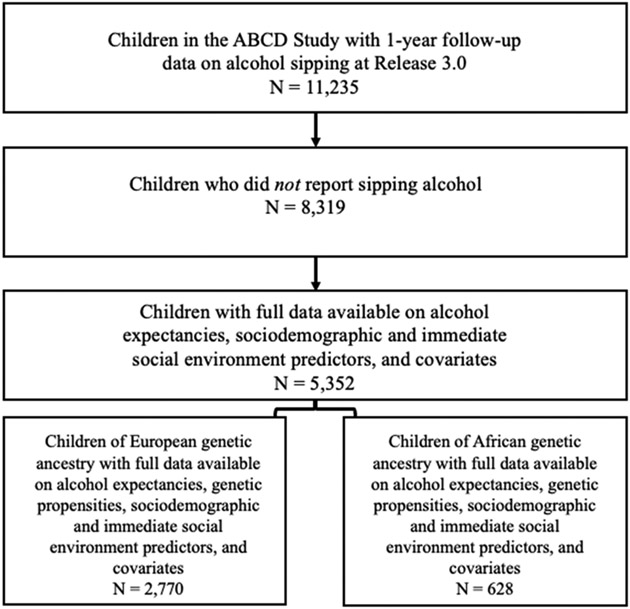
The ABCD Study® is a long-term study of brain development through adolescence in over 11,000 children31. Furthermore, one of the key objectives of the ABCD Study was to identify risk and resilience factors associated with the initiation of substance use, making this an ideal sample in which to characterize AEs before the onset of alcohol use31. Data were initially collected in children ages 9-11 across 22 sites and have subsequently been collected each year. Genetic samples were collected from the children in addition to demographic and phenotypic data on both the children and their parents. For the current study, genetic and phenotypic data were obtained from the ABCD Study Release 3.0. We excluded children who reported having sipped alcohol at baseline or the 1-year follow-up (N sipped = 2,916; N not sipped = 8,319); however, the analytic Ns were smaller due to incomplete data on alcohol expectancies, sociodemographic and immediate social environment predictors, genetic propensities, and/or covariates (maximum analytic N = 5,352). See Figure 1 for more details on sample size.
Figure 1.
Analytic sample size flowchart for the present study.
Target sample phenotypes
Alcohol expectancy scores
Alcohol expectancy (AE) scores were generated from the 7-item Alcohol Expectancy Questionnaire- Adolescent, Brief32,33. For each item, participants rated how much they agreed or disagreed with statements about the effects of alcohol on a 5-point scale (1 = Strongly disagree; 5 = Strongly agree). These data were collected at the 1-year follow-up, when the participants were 10-12 years old. Consistent with past work30,32, we created a positive AE sum score by summing responses to questions 1, 2, 4, and 6, and created a negative AE sum score by summing responses to questions 3, 5, and 7 (questions available in Supplemental Table 1, correlations available in Supplemental Figure 1). As the AE data were collected at the 1-year follow-up, all other assessments described below were also taken from this timepoint, with the exception of demographic measures and parental history of alcohol problems which were only collected at baseline.
Child alcohol sipping
We assessed whether children had sipped alcohol at baseline and/or the 1-year follow-up using data from the ABCD Youth Substance Use Interview. Specifically, children were asked “…Have you ever tried a sip of alcohol such as beer, wine or liquor (rum, vodka, gin, whiskey) at any time in your life?” We excluded children who responded “yes” to this question from all subsequent analyses.
Demographics
Child’s race was collected from the parent/caregiver report at the baseline assessment in the ABCD Parent Demographics Survey with the question “What race do you consider the child to be? Please check all that apply.” We collapsed some of the response categories to create 7 categories in total: White, Black, Asian, American Indian and Alaska Native (AIAN), Native Hawaiian and Pacific Islander (NHPI), “Other” (note: this was a caregiver-endorsed category), and Multiracial (for children whose caregivers indicated more than one racial category). Child’s ethnicity was also collected at baseline in the same survey with the question, “Do you consider the child Hispanic/Latino/Latina?” We recoded responses as “yes” = 1 and “no” = 0. Following the example of a prior study30, parents’ marital status was recoded into two categories: married and not married. Family income was coded as a categorical variable with 3 categories: less than $35,000, $35,000 to $99,999, and $100,000 and above. Parental education levels were averaged across the two parents/caregivers and analyzed as a continuous variable. Child’s religious denomination preference was recoded to create a binary measure of “religious affiliation”, such that responses of “atheist”, “agnostic”, or “nothing in particular” were coded as −1, and any other denomination was coded as +1. A measure of how important a child’s religious beliefs were to their identity (“In general, how important are your child's religious and spiritual beliefs in his/her daily life?”) was coded on a scale ranging from 1 (“Not at all”) to 4 (“Very”).
Family history
Data on self-reported parent alcohol problems were available from the Parent Family History Summary Scores. We included both biological father’s history of alcohol problems and biological mother’s history of alcohol problems as predictors, both coded as binary variables (“yes” = 1, “no” = 0).
Total number of adverse childhood experiences
Data on the total number of adverse childhood experiences34 were available from the Mental Health Youth summary scores. Specifically, we analyzed the “ple_y_ss_total_bad” overall summary score from the PhenX toolkit; this was a summation of all the events that the child judged and reported to be negative events.
Peer attitudes and use of alcohol
Three items on peer tolerance of alcohol use were taken from the ABCD Youth Substance Use Attitudes questionnaire35. These items included the following questions: “How do you think your CLOSE FRIENDS feel (or would feel) about YOU doing each of the following things?
Trying one or two drinks of an alcoholic beverage (beer, wine, liquor)
Taking one or two alcohol drinks nearly every day
Having five or more alcoholic drinks once or twice each weekend
These items were assessed on a 3-point scale, with possible responses ranging from 0 (“Not Disapprove”) to 2 (“Strongly Disapprove”). Because of the strong correlations between these measures (rs ranging from 0.59 - 0.75), we created a sum score of all three peer tolerance measures (hereafter referred to as “peer disapproval” due to higher scores indicating greater peer disapproval of alcohol use).
Additionally, we assessed three items related to peer alcohol use36, also from the ABCD Youth Substance Use Attitudes questionnaire, which queried: “How many of your friends currently
Drink alcohol (full beer, wine, or liquor)?
Get drunk?
Have problems with alcohol or other drugs?
Responses were on a 5-point scale, ranging from 0 (“None”) to 4 (“All”). We created a sum score from these three variables (correlations ranging from 0.38 - 0.48) with higher scores denoting more peers with greater use of alcohol.
Target sample genotyping QC
We used the Rapid Imputation and COmputational PIpeLIne for Genome-Wide Association Studies (RICOPILI37) to perform quality control (QC) on the 11,099 individuals with available ABCD Study phase 3.0 genotypic data, using RICOPILI’s default parameters. The 10,585 individuals who passed QC checks were matched to broad self-report racial groups using the ABCD Study parent survey. 6,787 parents/caregivers indicated that their child’s race was only “white”, and 5,561 of those individuals did not endorse any Hispanic ethnicity/origin. Further, we identified 1,675 parents/caregivers who indicated that their child’s race was only “black”, and 1,584 of those individuals did not endorse any Hispanic ethnicity/origin. After performing a second round of QC on these sub-samples, 5,556 non-Hispanic White and 1,584 non-Hispanic Black individuals were retained in the analyses. Principal component analysis (PCA) in RICOPILI was used to confirm the genetic ancestry of these individuals by mapping onto the 1000 Genomes reference panel, resulting in PCA-selected European- and African-ancestry subsets.
Each ancestry subset was then imputed to the TOPMed imputation reference panel38. Imputation dosages were converted to best-guess hard-called genotypes, and only SNPs with Rsq > 0.8 and MAF > 0.01 were kept for PRS analyses.
Discovery samples for polygenic risk scores
Along with a polygenic score for problematic alcohol use, we also decided to include PRS for depression (as a proxy for negative affect) and risk-taking (as a proxy for reward sensitivity), as both of these phenotypes may be thought to represent stage-based constructs of addiction (e.g., withdrawal/negative emotionality, risk-taking/binging39). We selected phenotypes for which there existed a GWAS in both European and African genetic ancestry samples to create maximally-predictive PRS for both ancestry groups. Polygenic risk scores (PRS) were created using the following discovery GWAS for problematic alcohol use, depression, and risk-taking:
Problematic alcohol use:
We used a European-ancestry GWAS meta-analysis of alcohol use disorder, alcohol dependence, and the problem subscale of the alcohol use disorders identification test from Zhou et al.40 (N = 435,563). To create PRS in the African-ancestry subset of ABCD, we meta-analyzed a GWAS of alcohol use disorder (derived using ICD codes) in the African ancestry subset of the Million Veteran Program41 with a GWAS of alcohol dependence (based on DSM diagnoses) in African ancestry individuals from the PGC42 (meta-analyzed N = 62,928).
Depression:
We meta-analyzed two European-ancestry GWAS of depression (total N 750,414, N case= 254,566), one from the Million Veteran Program43 and the other from a previous meta-analysis of the UK Biobank and Psychiatric Genomics Consortium44. We also used summary statistics from an African ancestry GWAS of depression from the Million Veteran Program (N = 59,60043).
Risk-taking:
We used the risk tolerance GWAS meta-analysis of the UK Biobank and 10 replication cohorts from Karlsson Linnér et al.45 (N = 466,571) to create PRS in the European ancestry subset of ABCD. This GWAS was based on a single item querying whether someone is a risk-taker: “Would you describe yourself as someone who takes risks? Yes/No”. For an African ancestry-matched GWAS, we used the Pan UK Biobank GWAS of the same item of risk tolerance, which was derived from 6,101 individuals (N cases = 2,523) of African descent who were residents of the United Kingdom (https://pan.ukbb.broadinstitute.org).
Educational attainment:
We also created a PRS for educational attainment using summary statistics from a GWAS by Lee et al.46 (N = 766,345). This was included as an additional predictor in a follow-up analysis described in more detail in the Results section below. N.B., this PRS was only scored in the children of European ancestry in ABCD, as we were unaware of a GWAS of educational attainment in other ancestry groups.
Statistical analyses
We used PRS-CS47 to calculate polygenic risk scores in the European ancestry subset of the ABCD sample, using effect sizes from the discovery GWAS summary statistics. We used the ‘auto’ function of PRS-CS, allowing the software to learn the global shrinkage parameter from the data. To maximize prediction in the African ancestry subset of ABCD, we used PRS-CSx’s ‘meta’ option48 to create polygenic risk scores that leveraged the larger sample size of the European ancestry version of the discovery GWAS by meta-analyzing those weights along with weights from the smaller, ancestry-matched discovery GWAS.
After deriving SNP weights using PRS-CS and PRS-CSx, we then used PLINK 1.9’s49 --score command to produce PRS in the ABCD sample. All subsequent analyses were performed using R Statistical Software50. We scaled the PRS to a mean of zero and standard deviation of one before including them in regression models. All regression models included age and sex as fixed covariates, while family ID and recruitment site were included as random intercepts. Parent-reported race and Hispanic ethnicity were included as covariates in Models 1 & 2 (see below), while ten genetic ancestry principal components were included as covariates in Models 3-6. For the meta-analyses reported in Models 3-6, we used the metafor package51 in R and employed a fixed-effects meta-analysis across ancestry groups. We performed Bonferroni corrections within each model (see below), correcting for the number of model-wise predictors that were interpreted.
We analyzed positive and negative expectancies separately due to their relatively small correlation (Spearman’s ρ = −0.11), their separate treatment in prior literature (e.g.,20,30), and their potentially distinct pattern of associations with future alcohol use and alcohol use disorders8-10. We analyzed the ABCD data using a series of six models:
Model 1: Sociodemographic and immediate social environment predictors of positive AEs.
Regressing positive alcohol expectancy sum scores onto sociodemographic factors and immediate social environment (parent history, adverse childhood experiences, and peer disapproval and use) predictors and covariates (age, sex, race, and ethnicity) in the full sample of children who have not sipped alcohol.
Model 2: Sociodemographic and immediate social environment predictors of negative AEs.
Regressing negative alcohol expectancy sum scores onto sociodemographic factors and immediate social environment (parent history, adverse childhood experiences, and peer disapproval and use) predictors and covariates (age, sex, race, and ethnicity) in the full sample of children who have not sipped alcohol.
Model 3: Genetic propensity predictors of positive AEs.
Regressing positive alcohol expectancy sum scores onto all three PRS - the problematic alcohol use PRS, the risk-taking PRS, and the depression PRS - along with covariates (age, sex, and ten genetic principal components); models were run separately in samples of genetically-determined European ancestry and African ancestry children who had not sipped alcohol, and the results were then meta-analyzed across ancestry groups.
Model 4: Genetic propensity predictors of negative AEs.
Regressing negative alcohol expectancy sum scores onto all three PRS - the problematic alcohol use PRS, the risk-taking PRS, and the depression PRS - along with covariates (age, sex, and ten genetic principal components); models were run separately in samples of genetically-determined European ancestry and African ancestry children who had not sipped alcohol, and the results were then meta-analyzed across ancestry groups.
Model 5: Genetic propensities, sociodemographic, and immediate social environment predictors of positive AEs.
Regressing positive alcohol expectancy sum scores onto all three PRS, all sociodemographic and immediate social environment predictors, and covariates (age, sex, and ten genetic principal components); models were run separately in samples of genetically-determined European ancestry and African ancestry children who had not sipped alcohol, and the results were then meta-analyzed across ancestry groups.
Model 6: Genetic propensities, sociodemographic, and immediate social environment predictors of negative AEs.
Regressing negative alcohol expectancy sum scores onto all three PRS, all sociodemographic and immediate social environment predictors, and covariates (age, sex, and ten genetic principal components); models were run separately in samples of genetically-determined European ancestry and African ancestry children who had not sipped alcohol, and the results were then meta-analyzed across ancestry groups
RESULTS
Demographic and psychosocial measures and PRS in ABCD
Descriptive statistics are provided in Table 1. Correlations between the three PRS ranged from 0.16 to 0.20 and from 0.004 to 0.05 in the European and African ancestry subsets of ABCD, respectively.
Table 1. Descriptive statistics of the ABCD sample of children who had not sipped alcohol at the 1-year follow-up.
Mean (SD) provided for continuous variables, number (% of analytic sample) provided for categorical variables.
| Variable | Mean (SD) or N (%) |
|---|---|
| Age (years) | 10.9 (0.64) |
| Female | 4,074 (49.1%) |
| Race | |
| AIAN | 46 (0.6%) |
| Asian | 178 (2.2%) |
| Black | 1,434 (17.6%) |
| Multiracial | 880 (10.8%) |
| NHPI | 12 (0.1%) |
| Other | 518 (6.3%) |
| White | 5,092 (62.4%) |
| Hispanic ethnicity | 1,737 (21.2%) |
| Caregivers married | 5,551 (67.5%) |
| Parents’ income | |
| <$35k | 1,705 (22.5%) |
| $35k - $100k | 2,900 (38.3%) |
| >$100k | 2,976 (39.3%) |
| Years of parental educational attainment | 16.2 (2.8) |
| Religious affiliation (not atheist, agnostic, or “nothing in particular”) | 5,883 (74.7%) |
| Importance of religious beliefs (scale of 1, “not at all”, to 4, “very”) | 2.9 (1.1) |
| Father history of alcohol problems | 1,010 (12.7%) |
| Mother history of alcohol problems | 349 (4.3%) |
| Total number of adverse childhood experiences | 2.4 (2.3) |
| Peer disapproval of alcohol sum scores (range: 0-6) | 5.6 (1) |
| Peer alcohol use sum scores (range: 0-12) | 0.1 (0.53) |
AIAN = American Indian and Alaska Native; NHPI = Native Hawaiian and Pacific Islander.
The children who were excluded from the present study for having sipped alcohol (N = 2,916) were significantly older than the children who had not sipped (mean age = 11.0 years, SD = 0.64; p = 1.4e-9) and significantly fewer were female (43.5% vs. 49.1%; p = 1.9e-7). The distribution of self-reported race differed across sipping status (p < 2e-16), with a greater proportion of children who reported sipping identifying as “White”. Specifically, 0.07% of children who reported sipping identified as being Native Hawaiian or Pacific Islander, 0.38% of these children identified as being American Indian or Alaskan Native, 1.9% identified as Asian, 7.9% identified as Black, 10.9% identified as Multiracial, 74.2% identified as White, and 4.8% identified as belonging to an “Other” category. The proportion of children with fathers who reported a history of alcohol problems (13.5%) or mothers who reported a history of alcohol problems (3.5%) were not significantly different from the subset of children who had not sipped. Children who were of European genetic ancestry and reported having sipped alcohol (analytic N = 1,669) had a higher mean risk-taking PRS (mean = 0.05) compared to children of European genetic ancestry who had not sipped alcohol (analytic N = 3,713; mean = −0.02; p = 0.03). No other polygenic risk scores significantly differed by sipping status. Mean positive alcohol expectancy scores were higher in children who reported sipping compared to those who had not sipped alcohol (means = 7.9 vs. 7.0, p < 2e-16), while the difference in mean negative alcohol expectancy scores was smaller yet significant (means = 12.2 vs. 12.0, p = 0.008).
Alcohol expectancies
In the sample of children who had not sipped alcohol, positive AE scores ranged from 4 to 20, with a mean of 7.03, while negative AE scores ranged from 3 to 15 with a mean of 12.04 (see distributions in Supplemental Figure 2). As mentioned above, the correlation between positive and negative AEs was relatively small and negative (Spearman’s ρ = −0.11; see Supplemental Figure 1 for correlations between individual items). There was no significant difference in mean positive AEs between males and females but mean negative AEs were significantly higher in males (male mean = 12.1 vs. female mean = 11.9, t-test p = 0.002). For both positive AEs and negative AEs, the mean scores were lower in children of Hispanic ethnicity than those who did not report being of Hispanic ethnicity (positive AE mean = 6.8 vs. 7.1, t-test p = 7.9e-5; negative AE mean = 11.8 vs. 12.1, t-test p = 6.0e-5). There was a significant difference in mean positive AEs by race (F(6, 8001) = 3.7, p = 0.001); in post-hoc tests, significant differences were found between the “Other” and “Multiracial” groups (p = 1.3e-4; “Other” mean = 6.6 vs. “Multiracial” mean = 7.4) and the “Multiracial” and “White” groups (p = 0.02; “Multiracial” mean = 7.4 vs. “White” mean = 7.0). We also found a significant difference in mean negative AE scores by race (F(6, 8001) = 13.7, p = 1.8e-15). When we conducted post-hoc tests, we found significant mean differences between “White” and “AIAN” groups (p = 0.006; “White” mean = 12.2 vs. “AIAN” mean = 10.6), between the “White” and “Black” groups (p < 2e-16; “White” mean = 12.2 vs. “Black” mean = 11.5), between the “White” and “Other” groups (p = 0.02; “White” mean = 12.2 vs. “Other” mean = 11.8), between the “Multiracial” and “AIAN” groups (p = 0.02; “Multiracial” mean = 12.1 vs. “AIAN” mean = 10.6), and between the “Multiracial” and “Black” groups (p = 2.5e-5; “Multiracial” mean = 12.1 vs. “Black” mean = 11.5).
Models 1 & 2: sociodemographic and immediate social environment predictors of AEs
Age (betas = 0.02; 0.03, 95% confidence intervals = [0.01 to 0.03]; [0.02 to 0.04], ps < 1.5e-5), parental educational attainment (betas = 0.07; 0.10, 95% confidence intervals = [0.03 to 0.11]; [0.06 to 0.15], ps < 4.2e-4), and peer disapproval of alcohol use (betas = −0.21; 0.18, 95% confidence intervals = [−0.29 to −0.13]; [0.09 to 0.26], ps < 3.8e-5) were all significantly associated with both positive and negative AEs after correcting for the number of predictors tested (Table 2). Interestingly, the directions of associations for age and education remained consistent for both positive and negative AEs, such that older age and greater parental education were associated with greater positive and negative AEs. Greater peer disapproval of alcohol drinking was positively associated with negative AEs and negatively associated with positive AEs. Overall, the sociodemographic factors and immediate social environment predictors (and covariates) explained an additional 3.6% variance in positive AEs, compared to a null model. These predictors explained an additional 2.4% variance in negative AEs.
Table 2. Results (Beta [95% confidence interval]) from models regressing alcohol expectancy sum scores on sociodemographic, immediate social environment, and genetic propensity predictors.
| Model 1 Sociodemographi c and immediate social environment predictors of positive AEs in full sample N = 5,352 |
Model 2 Sociodemographi c and immediate social environment predictors of negative AEs in full sample N = 5,352 |
Model 3 Genetic propensity predictors of positive AEs meta-analyzed across both genetic ancestry groups N = 4,860 |
Model 4 Genetic propensity predictors of negative AEs meta-analyzed across both genetic ancestry groups N = 4,860 |
Model 5 Genetic propensities, sociodemographic , and immediate social environment predictors of positive AEs meta-analyzed across both genetic ancestry groups N = 3,398 |
Model 6 Genetic propensities, sociodemographic , and immediate social environment predictors of negative AEs meta-analyzed across both genetic ancestry groups N = 3,398 |
||
|---|---|---|---|---|---|---|---|
| Covariates | Age | 0.03 [0.02, 0.04] * | 0.02 [0.01, 0.03] * | 0.03 [0.02, 0.04] * | 0.03 [0.02, 0.04] * | 0.04 [0.02, 0.05]* | 0.03 [0.02, 0.04] * |
| Sex | 0.06 [−0.09, 0.22] | 0.14 [−0.02, 0.31] | 0.17 [−0.003, 0.34] | 0.17 [0.001, 0.34] | 0.11 [−0.09, 0.31] | 0.07 [−0.13, 0.26] | |
| Race | |||||||
| Asian | −0.03 [−1.40, 1.33] | 1.06 [−0.37, 2.50] | - | - | - | - | |
| Black | −0.16 [−1.42, 1.10] | 0.59 [−0.74, 1.91] | - | - | - | - | |
| Multiracial | 0.11 [−1.15, 1.38] | 0.97 [−0.35, 2.30] | - | - | - | - | |
| NHPI | 0.43 [−1.88, 2.75] | −0.87 [−3.30, 1.56] | - | - | - | - | |
| Other | −0.20 [−1.49, 1.09] | 0.94 [−0.41, 2.29] | - | - | - | - | |
| White | −0.09 [−1.33, 1.16] | 1.00 [−0.31, 2.31] | - | - | - | - | |
| Hispanic ethnicity | −0.13 [−0.38, 0.12] | −0.07 [−0.34, 0.19] | - | - | - | - | |
| Sociodemographic background | Caregivers not married | −0.12 [−0.33, 0.10] | −0.02 [−0.25, 0.21] | - | - | 0.06 [−0.24, 0.36] | −0.02 [−0.32, 0.28] |
| Income | - | - | |||||
| >$100k | 0.01 [−0.19, 0.20] | −0.10 [−0.31, 0.10] | - | - | 0.09 [−0.16, 0.33] | −0.11 [−0.35, 0.13] | |
| <$35k | −0.09 [−0.34, 0.17] | −0.26 [−0.53, 0.01] | - | - | 0.13 [−0.25, 0.50] | −0.48 [−0.86, −0.09] | |
| Educational attainment | 0.07 [0.03, 0.11] * | 0.10 [0.06, 0.15] * | - | - | 0.10 [0.04, 0.16] * | 0.12 [0.06, 0.18] * | |
| Religious affiliation | −0.09 [−0.21, 0.02] | −0.09 [−0.21, 0.04] | - | - | −0.17 [−0.33, −0.01] | −0.07 [−0.22, 0.09] | |
| Importance of religious beliefs | −0.17 [−0.27, −0.08]* | 0.06 [−0.04, 0.16] | - | - | −0.13 [−0.26, −0.004] | 0.03 [−0.11, 0.17] | |
| Immediate social environment | Father alcohol problems | 0.16 [−0.08, 0.41] | 0.01 [−0.25, 0.26] | - | - | 0.18 [−0.14, 0.51] | 0.10 [−0.22, 0.43] |
| Mother alcohol problems | 0.14 [−0.28, 0.57] | 0.12 [−0.32, 0.56] | −0.33 [−0.87, 0.22] | 0.15 [−0.40, 0.69] | |||
| Total number of adverse childhood experiences | 0.13 [0.09, 0.17] * | 0.05 [0.01, 0.09] | - | - | 0.17 [0.12, 0.22]* | 0.07 [0.02, 0.12] | |
| Peer disapproval sum score | −0.21 [−0.29, −0.13] * | 0.18 [0.09, 0.26] * | - | - | −0.22 [−0.32, −0.11] * | 0.16 [0.06, 0.27] * | |
| Peer use sum score | 0.04 [−0.10, 0.19] | −0.01 [−0.16, 0.14] | - | - | 0.03 [−0.17, 0.24] | −0.03 [−0.23, 0.18] | |
| Genetic propensities | PAU PRS | - | - | 0.05 [−0.04, 0.14] | −0.003 [−0.09, 0.08] | 0.09 [−0.02, 0.19] # | 0.01 [−0.09, 0.11] |
| Dep PRS | - | - | −0.05 [−0.13, 0.04] | 0.04 [−0.04, 0.13] | −0.03 [−0.13, 0.08] | 0.11 [0.01, 0.21] | |
| Risk-taking PRS | - | - | 0.05 [−0.03, 0.14] | 0.10 [0.01, 0.19] | −0.003 [−0.10, 0.10] | 0.07 [−0.03, 0.17] | |
| % Change in pseudo-R2 | 3.6% | 2.4% | 1.3% (European ancestry); 2.2% (African ancestry) | 1.3% (European ancestry); 1.6% (African ancestry) | 4.8% (European ancestry); 7.1% (African ancestry) | 2.3% (European ancestry); 7.8% (African ancestry) | |
Betas [95% confidence interval] from all six analytic models are presented, along with the change in pseudo-R2 for each model (estimated by subtracting the pseudo-R2 of a null, intercept-only model from the pseudo-R2 of the full model). For Models 3-6, the change in pseudo-R2 was estimated separately for each genetic ancestry group. Models 1 and 2 included only sociodemographic and immediate social environment predictors; analyses were conducted in the full sample (N = 5,352). Models 3 and 4 included only genetic propensities (PRS) as predictors, and present the coefficients after being meta-analyzed across both genetic ancestry groups (meta-analyzed N = 4,860). Models 5 & 6 included both the genetic propensities and sociodemographic and immediate social environment predictors, and present the coefficients after being meta-analyzed across both genetic ancestry groups (meta-analyzed N = 3,398). For brevity, the coefficients for the 10 genetic principal components (included in Models 3-6) are not shown, nor were they interpreted. Bolded and starred estimates represent coefficients that passed a model-wise multiple testing correction (i.e., for Model 1, we corrected for 20 tests; for Model 3, we corrected for 5 tests; for Model 5, we corrected for 16 tests). The reference category for sex was female, the reference category for race was AIAN, and the reference category for income was $35k-100k. AEQ = alcohol expectancies questionnaire; AIAN = American Indian and Alaska Native; NHPI = Native Hawaiian and Pacific Islander; PAU = problematic alcohol use; Dep = depression; PRS = polygenic risk score.
significant test for heterogeneity across ancestry groups in meta-analysis [Q(df = 1)] at p < 0.05.
The more important the child’s religious beliefs, the less likely that the child was to endorse positive expectations about alcohol (beta = −0.17, 95% confidence interval = [−0.27 to −0.08], p = 5.2e-4). In a follow-up model, we tested whether religious affiliation and importance of religious beliefs interacted to influence AEs; the interaction was not significant. Lastly, a larger number of adverse childhood experiences was associated with greater likelihood of positive AEs (beta = 0.13, 95% confidence interval = [0.09 to 0.17], p = 3.0e-12).
Models 3 & 4: genetic propensity predictors of AEs
After correction for multiple testing, there were no significant associations between the PRS and negative or positive AEs. The strongest association was between the risk-taking PRS and negative AEs (beta = 0.10, 95% confidence interval = [0.01 to 0.19], p = 0.02). Taken together, the PRS and covariates explained an additional 1.3-2.2% variance in positive AEs, compared to a null model, and an additional 1.3-1.6% variance in negative AEs.
Models 5 & 6: genetic propensities, sociodemographic, and immediate social environment predictors of AEs
Including independent variables from Models 1-4 (both PRS and non-PRS measures) in the same model did not substantively alter the findings, except that importance of religious beliefs was no longer significantly associated with positive AEs. Age (betas = 0.03; 0.04, 95% confidence intervals = [0.02 to 0.04];[0.02 to 0.05], ps < 9.6e-6), parental educational attainment (betas = 0.10; 0.12, 95% confidence intervals = [0.04 to 0.16]; [0.06 to 0.18], ps < 0.002), and peer disapproval of alcohol use (betas = −0.22; 0.16, 95% confidence intervals = [−0.32 to −0.11]; [0.06 to 0.27], ps < 0.002) were associated with both positive and negative AEs, while total number of adverse childhood experiences was associated only with positive AEs (beta = 0.17, 95% confidence interval = [0.12 to 0.22], p = 2.8e-12). All together, the PRS, sociodemographic and immediate social environment predictors, and additional covariates (e.g., genetic PCs) explained an additional 4.8-7.1% variance in positive AEs, and an additional 2.3-7.8% variance in negative AEs, compared to a null model.
Given the robust associations between AEs and parental educational attainment, and the moderate heritability of educational attainment, we tested follow-up models in the European ancestry subset of ABCD where a PRS for educational attainment was included as an additional predictor to estimate the extent to which polygenic contributions to educational attainment account for this association. We found that the educational attainment PRS did not substantively alter the associations between parental educational attainment and positive AEs or negative AEs, though the associations were somewhat attenuated (positive AEs: beta = 0.086, 95% confidence interval = [0.02, 0.16] vs. beta = 0.077, 95% confidence interval = [0.01, 0.15]; negative AEs: beta = 0.10, 95% confidence interval = [0.04, 0.17] vs. beta = 0.087, 95% confidence interval = [0.02, 0.16]).
DISCUSSION
In a sample of predominantly alcohol-naïve youth, positive and negative AEs were associated with older age, greater parental education, and greater peer disapproval of alcohol use, while adverse childhood experiences and greater importance of religious beliefs were only associated with positive AEs. These findings were broadly consistent with a prior study by Murphy et al.30 that was conducted in the ABCD sample, but some distinctions also emerged, discussed in more detail below. Notably, none of the genetic propensities tested – polygenic scores for problematic alcohol use, depression, or risk-taking – were associated with negative or positive AEs. Our data suggest that AEs, particularly in alcohol-naïve youth, are more strongly shaped by familial and environmental experiences and influences than polygenic risk scores for problematic alcohol use, risk-taking, or depression.
As in Murphy et al.30, we found that older age was significantly associated with both positive and negative AEs. While the link between age and increasingly positive AEs is well-documented, the relationship between age and negative AEs has been more mixed; however, our findings are consistent with a recent systematic review that found an overall positive association between older age and an increase in negative AEs as well as positive AEs52. Other findings that might appear counterintuitive were also supported by the extant literature. For instance, we found that higher parental educational attainment was related to both greater positive and greater negative AEs, and this was not substantively attenuated when we accounted for a polygenic score of educational attainment. Greater educational attainment has consistently been linked to greater likelihood of daily alcohol use53,54. Furthermore, parental education is often used as a proxy for socio-economic status, and such socio-economically advantaged environments may depict and promote socially accepted drinking, thus providing a context for positive expectancies. At the same time, greater parental education may also relate to greater insight into the harms associated with heavy alcohol use and thus, correspond with less permissive attitudes towards alcohol or greater caution and oversight surrounding youth access to alcohol55.
As mentioned above, an earlier paper from Murphy et al.30 examined the associations between AEs and alcohol sipping in the ABCD Study® when a subset of the 1-year follow-up data (n = 4,951) had been released. The authors included demographic variables (e.g., race, religiosity) and parental history of alcohol problems as predictors in their models and found that positive AEs in the ABCD sample were positively associated with age, ethnicity, and alcohol sipping and negatively associated with religious affiliation, while negative AEs were only significantly associated with age. We also saw that alcohol sipping was correlated with AEs in our study, with positive AEs showing a larger association with sipping than negative AEs (although unlike Murphy et al., we did not include other predictors in this test). Like Murphy et al., we were also interested in examining the factors that shape positive and negative AEs in the ABCD Study, but we focused on associations that were present before the onset of alcohol use. Interestingly, and in contrast to Murphy et al.30, we did not find a significant association between religious affiliation and AEs. However, we did note a significant association between greater importance of a child’s religious beliefs, a measure not evaluated by Murphy et al, and less endorsement of positive AEs. One possibility is that the relative importance of the child’s religious beliefs is a stronger predictor of positive AEs, and as it was significantly correlated with religious affiliation (r = 0.65), the importance variable suppressed the variance explained by religious affiliation in our regression models. Indeed, when we tested a model where positive AEs were regressed on all the demographic and psychosocial variables except for importance of religious beliefs, religious affiliation was significantly associated with positive AEs, such that religious beliefs were associated with lower positive AEs (beta = −0.21, p = 4.0e-6). The relative importance of religious beliefs over religious affiliation has been documented in the literature56,57. It is also worth noting that the religious affiliation measure did not account for denominations with greater drinking proscriptions and thus is a heterogeneous representation of religious beliefs surrounding alcohol. Future studies with more fine-grained assessments of individuals’ religious and spiritual beliefs surrounding alcohol may find an influence of specific denominations or beliefs on AEs58.
Unlike previous studies59,60, we did not find significant associations between history of paternal or maternal alcohol problems and child’s AEs, even in follow-up analyses that included father’s alcohol problems or mother’s alcohol problems as predictors in separate models. There are several potential explanations for this observation. First, the prevalence of family history of alcohol problems was rather low in the sample, suggesting either volunteer bias (i.e., those without alcohol problems are more likely to enroll their children and consistently participate in longitudinal studies) or perhaps limitations of the assessment instrument. Second, studies suggest that observation of parental alcohol use, rather than family history, may be more relevant in shaping childrens’ AEs61,62. This finding also resonates with the limited role of genetic influences on AEs. Indeed, if familial effects on AEs are enacted via memories of drinking episodes, rather than via inheritance of genetic predispositions, then parents in remission or those who conceal their problem drinking may not be actively influencing their offspring’s AEs.
Youth who thought that their peers would disapprove of drinking were, overall, less likely to have positive AEs and more likely to report negative AEs, as noted previously63,64. By contrast, respondent report of observed or perceived peer alcohol use was not significantly associated with positive or negative AEs. We suspect this is largely due to the relatively low endorsement of peers who use alcohol at this time in this sample (~6%); it is likely that youth are not yet exposed to the experiences that their peers may have with alcohol.
Similar to findings from a previous study of adolescents in Taiwan65, we found that a greater total number of adverse childhood experiences was associated with greater positive AEs. One possible explanation is that adversity in early life may make someone more likely to view alcohol as a future source of tension reduction65 (helps a person relax, feel less tense) or empowerment in a vulnerable context (do things better, feel more powerful). This hypothesis regarding the expectation that drinking facilitates coping with negative mood or trauma is well-supported, such that persons reporting tension reduction as a drinking motive have been found to be particularly vulnerable to later drinking problems66,67. Liquid courage (the idea that alcohol bestows strength, power, cognitive improvements) has also been linked to college binge drinking68,69 and other risky behaviors that are also elevated in individuals with prior trauma exposure70,71.
In general, our null results for the PRS predictors are in line with the findings from twin and family studies, which have generally reported no significant contribution of additive genetic factors, or, if significant, a relatively modest heritability15-19. The strongest association was between the risk-taking PRS and greater negative AEs (beta = 0.10, 95% confidence interval = [0.01, 0.19], p = 0.02), but this association was attenuated in a model that included the sociodemographic and immediate social environment predictors (beta = 0.07, 95% confidence interval = [−0.03, 0.17], p = 0.19). We suspect that this attenuation is largely due to the inclusion of the peer disapproval of alcohol and alcohol use variables, based on evidence from follow-up analyses. Specifically, when we analyzed only the European ancestry subset of ABCD (which showed the strongest association between risk-taking PRS and negative AEs in the PRS-only model: beta = 0.10, p = 0.03) and we included all sociodemographic and immediate social environment variables except peer disapproval and use of alcohol, the association increased to beta = 0.12, p = 0.02 (compared to beta = 0.08, p = 0.14 in the full model). This suggests that peer tolerance for alcohol use and peer alcohol use and polygenic liability for risk-taking may share some amount of variance that is related to negative AEs; indeed, this is supported by genetic correlations between alcohol use and risk-taking72.
There are several important limitations to note regarding the current study. First, we only looked at the composite “positive” and “negative” AE scores and did not analyze specific AE items (e.g., “liquid courage”, increased sociability). Second, we note that we did not assess parental motives for drinking, which are likely relevant to childrens’ AEs. Relatedly, we did not have parental genotype data to parse direct genetic effects on AEs from “genetic nurture”, or indirect effects on AEs 73. Third, while we could have assessed many more predictors putatively linked to alcohol use, we ultimately chose to focus on the immediate social environment and sociodemographic variables, especially those that had been previously associated with AEs. Future studies that assess a wider range of phenotypes may provide insight into additional predictors of AEs. Finally, we note that the ancestry-matched discovery GWAS used to create the PRS in children of African genetic ancestry in the ABCD sample were much smaller in general than the equivalent GWAS in individuals of European ancestry (the largest GWAS in individuals of African ancestry n = 62,928, compared to the equivalent European ancestry GWAS n > 435,000). Combined with the smaller target sample of children in ABCD of African genetic ancestry (n = 628 with non-missing data in Models 5 & 6), our PRS models were likely underpowered in the subset of ABCD children with African genetic ancestry.
The ABCD Study is longitudinal and as the children in ABCD begin to initiate alcohol use, and even more involved drinking behaviors, future analyses could examine the predictive role of AEs in these drinking milestones. Our study documents that such associations are unlikely to be due to shared genetic predispositions but rather, shaped by personal, familial and other psychosocial factors that provide youth with the framework for their expectations for alcohol.
Supplementary Material
Funding
ECJ was supported by K01DA051759. AA and RB receive support from R01DA054750. ASH was supported by K01AA030083. AJG was supported by DGE-213989. NRK was supported by K23MH12179201. SEP was supported by F31AA029934.
Ethics approval
This study was approved by the local Institutional Review Board.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/Consortium_Members.pdf. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI 10.15154/1523041.
Footnotes
Conflicts of interest/Competing interests
No conflicts of interest to declare.
Consent to participate
All participants in ABCD provided informed consent (or assent).
Availability of data and material
Genetic and phenotypic data in the ABCD sample are available for download by approved researchers from the NIMH Data Archive.
Code availability
Available at https://github.com/emmacj/ABCD_alc_expectancies
REFERENCES
- 1.Leonard KE & Blane HT Psychological theories of drinking and alcoholism. (Guilford Press, 1999). [Google Scholar]
- 2.Goldman MS, del Boca FK & Darkes J Alcohol expectancy theory: The application of cognitive neuroscience. (1999). [Google Scholar]
- 3.Brown SA, Christiansen BA & Goldman MS The Alcohol Expectancy Questionnaire: an instrument for the assessment of adolescent and adult alcohol expectancies. J Stud Alcohol 48, 483–491 (1987). [DOI] [PubMed] [Google Scholar]
- 4.Fromme K & D’Amico EJ Measuring adolescent alcohol outcome expectancies. Psychology of Addictive behaviors 14, 206 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Cooper ML Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychol Assess 6, 117 (1994). [Google Scholar]
- 6.Dick D et al. Spit for Science: launching a longitudinal study of genetic and environmental influences on substance use and emotional health at a large US university. Front Genet 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamboanga BL From the eyes of the beholder: Alcohol expectancies and valuations as predictors of hazardous drinking behaviors among female college students. Am J Drug Alcohol Abuse 32, 599–605 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Fromme K & D’Amico EJ Measuring adolescent alcohol outcome expectancies. Psychology of Addictive behaviors 14, 206 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Ham LS, Stewart SH, Norton PJ & Hope DA Psychometric assessment of the Comprehensive Effects of Alcohol Questionnaire: Comparing a brief version to the original full scale. J Psychopathol Behav Assess 27, 141–158 (2005). [Google Scholar]
- 10.Nicolai J, Demmel R & Moshagen M The comprehensive alcohol expectancy questionnaire: confirmatory factor analysis, scale refinement, and further validation. J Pers Assess 92, 400–409 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Zamboanga BL, Schwartz SJ, Ham LS, Borsari B & van Tyne K Alcohol expectancies, pregaming, drinking games, and hazardous alcohol use in a multiethnic sample of college students. Cognit Ther Res 34, 124–133 (2010). [Google Scholar]
- 12.Jones BT, Corbin W & Fromme K A review of expectancy theory and alcohol consumption. Addiction 96, 57–72 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Palmer RHC et al. Shared additive genetic influences on DSM-IV criteria for alcohol dependence in subjects of European ancestry. Addiction 110, 1922–1931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polderman TJC et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 47, 702–709 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Perry A The effect of heredity on attitudes toward alcohol, cigarettes, and coffee. Journal of Applied Psychology vol. 58 275–277 Preprint at 10.1037/h0035527 (1973). [DOI] [Google Scholar]
- 16.Vernon PA, Lee D, Harris JA & Jang KL Genetic and environmental contributions to individual differences in alcohol expectancies. Personality and Individual Differences vol. 21 183–187 Preprint at 10.1016/0191-8869(96)00068-2 (1996). [DOI] [Google Scholar]
- 17.Samek DR, Keyes MA, Iacono WG & McGue M Peer Deviance, Alcohol Expectancies, and Adolescent Alcohol Use: Explaining Shared and Nonshared Environmental Effects Using an Adoptive Sibling Pair Design. Behav Genet 43, 286–296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agrawal A et al. Drinking expectancies and motives: a genetic study of young adult women. Addiction 103, 194–204 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Slutske WS et al. Genes, environment, and individual differences in alcohol expectancies among female adolescents and young adults. Psychol Addict Behav 16, 308–317 (2002). [PubMed] [Google Scholar]
- 20.Ting T-T, Chen WJ, Liu C-Y, Lin Y-C & Chen C-Y Peer influences on alcohol expectancies in early adolescence: A study of concurrent and prospective predictors in Taiwan. Addictive Behaviors 40, 7–15 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Simons-Morton B Social influences on adolescent substance use. Am J Health Behav 31, 672–684 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Boyd SJ, Sceeles EM, Tapert SF, Brown SA & Nagel BJ Reciprocal relations between positive alcohol expectancies and peer use on adolescent drinking: An accelerated autoregressive cross-lagged model using the NCANDA sample. Psychology of addictive behaviors 32, 517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauer-Zavala S, Burris JL & Carlson CR Understanding the Relationship Between Religiousness, Spirituality, and Underage Drinking: The Role of Positive Alcohol Expectancies. J Relig Health 53, 68–78 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Ouellette JA, Gerrard M, Gibbons FX & Reis-Bergan M Parents, peers, and prototypes: Antecedents of adolescent alcohol expectancies, alcohol consumption, and alcohol-related life problems in rural youth. Psychology of Addictive Behaviors 13, 183 (1999). [Google Scholar]
- 25.Brown SA, Creamer VA & Stetson BA Adolescent alcohol expectancies in relation to personal and parental drinking patterns. J Abnorm Psychol 96, 117 (1987). [DOI] [PubMed] [Google Scholar]
- 26.Elder RW et al. The effectiveness of tax policy interventions for reducing excessive alcohol consumption and related harms. Am J Prev Med 38, 217–229 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown SA, Tate SR, Vik PW, Haas AL & Aarons GA Modeling of alcohol use mediates the effect of family history of alcoholism on adolescent alcohol expectancies. Experimental and Clinical Psychopharmacology vol. 7 20–27 Preprint at 10.1037/1064-1297.7.1.20 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Chen A-J et al. Association between childhood negative life events with alcohol expectancies in early adolescence: Cumulative risk and latent class approaches. Drug Alcohol Depend 226, 108853 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Martino SC, Collins RL, Ellickson PL, Schell TL & McCaffrey D Socio-environmental influences on adolescents’ alcohol outcome expectancies: a prospective analysis. Addiction 101, 971–983 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Murphy MA, Dufour SC & Gray JC The association between child alcohol sipping and alcohol expectancies in the ABCD study. Drug Alcohol Depend 221, 108624 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Jernigan TL, Brown SA & Dowling GJ The Adolescent Brain Cognitive Development Study. J Res Adolesc 28, 154–156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisdahl KM et al. Adolescent brain cognitive development (ABCD) study: overview of substance use assessment methods. Dev Cogn Neurosci 32, 80–96 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein LAR et al. Validity and Reliability of the Alcohol Expectancy Questionnaire-Adolescent, Brief. J Child Adolesc Subst Abuse 16, 115–127 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albertina EA, Barch DM & Karcher NR Internalizing Symptoms & Adverse Childhood Experiences Associated with Functional Connectivity in A Middle Childhood Sample. Biol Psychiatry Cogn Neurosci Neuroimaging (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston LD, O’Malley PM, Miech RA, Bachman JG & Schulenberg JE Monitoring the Future Results on Drug Use: 1975–2013: Overview, Key Findings on Adolescent Drug Use, 2013. Ann Arbor Inst. Soc. Res. Univ. Michigan (2014). [Google Scholar]
- 36.Freedman D, Thornton A, Camburn D, Alwin D & Young-DeMarco L The life history calendar: A technique for collecting retrospective data. Sociol Methodol 37–68 (1988). [PubMed] [Google Scholar]
- 37.Lam M et al. RICOPILI: Rapid Imputation for COnsortias PIpeLIne. bioRxiv 587196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taliun D et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstein RZ & Volkow ND Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159, 1642–1652 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci 23, 809–818 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kranzler HR et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun 10, 1499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walters RK et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci 21, 1656–1669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levey DF et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat Neurosci 24, 954–963 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard DM et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 22, 343–352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linnér RK et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet 51, 245 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JJ et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 50, 1112–1121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge T, Chen C-Y, Ni Y, Feng Y-CA & Smoller JW Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun 10, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruan Y et al. Improving polygenic prediction in ancestrally diverse populations. Nat Genet 54, 573–580 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang CC et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R Core Team. R: A language and environment for statistical computing. Preprint at https://www.r-project.org/ (2017). [Google Scholar]
- 51.Viechtbauer W Conducting Meta-Analyses in R with the metafor Package. J Stat Softw 36, 1–48 (2010). [Google Scholar]
- 52.Jones SC & Gordon CS A systematic review of children’s alcohol-related knowledge, attitudes and expectancies. Prev Med (Baltim) 105, 19–31 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Heckley G, Jarl J & Gerdtham U-G Frequency and intensity of alcohol consumption: new evidence from Sweden. The European Journal of Health Economics 18, 495–517 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huerta MC & Borgonovi F Education, alcohol use and abuse among young adults in Britain. Soc Sci Med 71, 143–151 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Ryan SM, Jorm AF & Lubman DI Parenting factors associated with reduced adolescent alcohol use: a systematic review of longitudinal studies. Australian & New Zealand Journal of Psychiatry 44, 774–783 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Kendler KS, Gardner CO & Prescott CA Religion, psychopathology, and substance use and abuse: A multimeasure, genetic-epidemiologic study. Am J Psychiatry (1997). [DOI] [PubMed] [Google Scholar]
- 57.MILLER L, DAVIES M & GREENWALD S Religiosity and Substance Use and Abuse Among Adolescents in the National Comorbidity Survey. J Am Acad Child Adolesc Psychiatry 39, 1190–1197 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Kendler KS et al. Dimensions of Religiosity and Their Relationship to Lifetime Psychiatric and Substance Use Disorders. American Journal of Psychiatry 160, 496–503 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Brown SA, Tate SR, Vik PW, Haas AL & Aarons GA Modeling of alcohol use mediates the effect of family history of alcoholism on adolescent alcohol expectancies. Experimental and Clinical Psychopharmacology vol. 7 20–27 Preprint at 10.1037/1064-1297.7.1.20 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Brown SA, Creamer VA & Stetson BA Adolescent alcohol expectancies in relation to personal and parental drinking patterns. J Abnorm Psychol 96, 117 (1987). [DOI] [PubMed] [Google Scholar]
- 61.Smit K, Voogt C, Otten R, Kleinjan M & Kuntsche E Exposure to Parental Alcohol Use Rather Than Parental Drinking Shapes Offspring’s Alcohol Expectancies. Alcohol Clin Exp Res 43, 1967–1977 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Waddell JT, Blake AJ, Sternberg A, Ruof A & Chassin L Effects of Observable Parent Alcohol Consequences and Parent Alcohol Disorder on Adolescent Alcohol Expectancies. Alcohol Clin Exp Res 44, 973–982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb JA, Baer PE, Francis DJ & Caid CD Relationship among social and intrapersonal risk, alcohol expectancies, and alcohol usage among early adolescents. Addictive Behaviors 18, 127–134 (1993). [DOI] [PubMed] [Google Scholar]
- 64.Mason MJ, Mennis J, Linker J, Bares C & Zaharakis N Peer attitudes effects on adolescent substance use: The moderating role of race and gender. Prevention science 15, 56–64 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Chen A-J et al. Association between childhood negative life events with alcohol expectancies in early adolescence: Cumulative risk and latent class approaches. Drug Alcohol Depend 226, 108853 (2021). [DOI] [PubMed] [Google Scholar]
- 66.Corbin WR, Waddell JT, Ladensack A & Scott C I drink alone: Mechanisms of risk for alcohol problems in solitary drinkers. Addictive Behaviors 102, 106147 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Hasking P, Lyvers M & Carlopio C The relationship between coping strategies, alcohol expectancies, drinking motives and drinking behaviour. Addictive Behaviors 36, 479–487 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Zamboanga BL, Bean JL, Pietras AC & Pabón LC Subjective evaluations of alcohol expectancies and their relevance to drinking game involvement in female college students. Journal of Adolescent Health 37, 77–80 (2005). [DOI] [PubMed] [Google Scholar]
- 69.LaBrie JW, Grant S & Hummer JF “This would be better drunk”: Alcohol expectancies become more positive while drinking in the college social environment. Addictive Behaviors 36, 890–893 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shorey RC, McNulty JK, Moore TM & Stuart GL Being the victim of violence during a date predicts next-day cannabis use among female college students. Addiction 111, 492–498 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Read JP et al. Trauma and posttraumatic stress symptoms predict alcohol and other drug consequence trajectories in the first year of college. J Consult Clin Psychol 80, 426 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu M et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 51, 237–244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kong A et al. The nature of nurture: Effects of parental genotypes. Science (1979) 359, 424–428 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.