Abstract
Introduction
Variability in adherence rates to the Seattle protocol and to surveillance interval recommendations, established quality indicators (QIs) in Barrett’s esophagus (BE), are unknown.
Methods
We evaluated endoscopist and site-based adherence rates to these QIs from 1/2018–5/2021 using the GI Quality Improvement Consortium (GIQuIC) national registry with matched endoscopy and pathology data.
Results
Across 153 practices with 572 endoscopists performing 20,155 endoscopies, adherence to Seattle protocol varied by endoscopists (median 93.8%, IQR 18.9%) and by site (median 90.0%, IQR 20.1%). Adherence to appropriate surveillance intervals for nondysplastic BE also varied by endoscopist (median 82.4%, IQR 36.3%) and site (median 77.2%, IQR 29.8%). Overall dysplasia detection rate was 3.1% and varied among endoscopists and sites.
Conclusion
These US population-based results can serve as a benchmark for quality initiatives and intervention trials aimed at improving outcomes for BE patients.
Introduction
Barrett’s esophagus (BE) is the only identifiable precursor lesion to esophageal adenocarcinoma (EAC), a highly lethal cancer with a rising incidence in the United States (US).1 Early detection of BE and enrollment in surveillance programs, along with endoscopic eradication therapy for dysplastic BE are critical to prevent progression to EAC.2 Several quality indicators (QIs) highlighting best practices in BE have been described, including 1) adherence to the Seattle biopsy protocol for sampling the BE segment (4-quadrant biopsies every 2 cm), and 2) adherence to a surveillance interval of 3–5 years in non-dysplastic BE (NDBE).3 Prior studies demonstrated poor adherence to these QIs but only provided overall estimates without consideration for individual endoscopist or different sites.4–6 In addition to these metrics, the neoplasia or dysplasia detection rate (NDR, DDR) have been proposed as potential outcome measures for quality analogous to the adenoma detection rate (ADR) in colonoscopy. There is abundant data to support the high penetrance of ADR in gastroenterology practice and substantial variation in this quality measure among endoscopists.7 Variation in adherence to proposed QIs in BE has not been previously explored. Using a national registry, we evaluated adherence to QIs in BE among a large group of endoscopists practicing in multiple endoscopy centers across the US.
Methods
We analyzed data from the GI Quality Improvement Consortium (GIQuIC) Registry, a national repository of endoscopy data with matched pathology powered by the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy. Upper endoscopies performed on patients with suspected or established BE from 1/2018 – 5/2021 were included. Endoscopists and sites that did not meet a predetermined volume threshold (≥10 and ≥20 endoscopy records, respectively) were excluded (Supplementary Figure 1). Adherence to Seattle protocol was assessed by dividing the BE length by number of pathology jars; a ratio of ≤2.0 with rounding down was considered adherent. Adherence to recommended surveillance for NDBE was calculated by assessing the proportion recommended to undergo a repeat endoscopy between 3–5 years per professional society guidelines.8 Due to limitations in capturing EAC within GIQuIC, DDR, defined as detection of indefinite for dysplasia (IND), low-grade dysplasia (LGD) or high-grade dysplasia (HGD) on screening or surveillance examinations among BE patients, was chosen as a more appropriate endpoint. The primary outcome was adherence to QIs (Seattle protocol, surveillance intervals) and secondary outcomes were DDR by endoscopist and by site and correlation between Seattle protocol and DDR.
Results
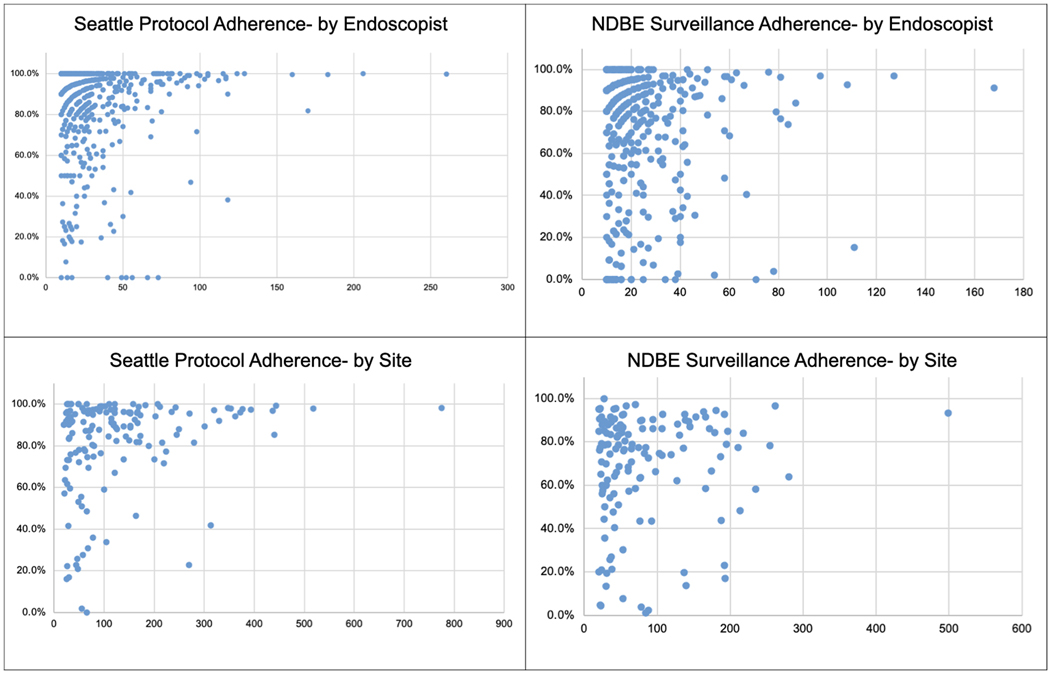
A total of 415,883 upper endoscopies were assessed and 20,155 (4.8%) were included [mean age 62.5 years (SD 12.7), 61.3% male, 91.5% Caucasian, mean BE length 2.3 (SD 2.8)]. These cases represented 255 practices and 1195 endoscopists nationwide. The distribution of endoscopists based on US census region was South (n=436, 36.5%), West (n=329, 27.5%), Northeast (n=266, 22.3%), Midwest (n=163, 13.6%). The overall adherence to the Seattle protocol was 86.1%. Among endoscopists meeting inclusion criteria (n=572), there was wide variability in adherence rates to Seattle protocol (median 93.8%, IQR 18.9%). Similar results were noted for adherence to Seattle protocol (median 90.0%, IQR 20.1%) in site-based analysis (n=153). Of the 12,100 EGDs with documented NDBE, 8517 (70.4%) had a guideline concordant recommended surveillance interval of 3–5 years. When analyzing adherence to appropriate surveillance interval in NDBE, there was variability at the endoscopist level (median 82.4%, IQR 36.3%) and the site level (median 77.2%, IQR 28.9%) (Figure 1).
Figure 1.
Adherence to Seattle Protocol and Surveillance Interval Recommendations by Endoscopist and by Site
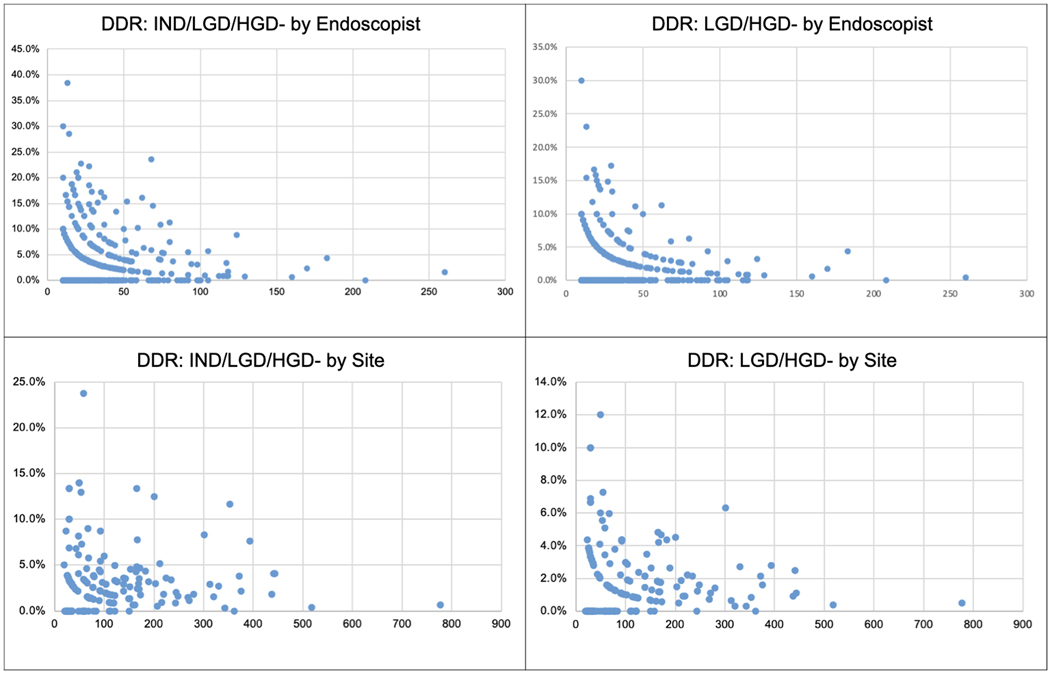
Overall DDR was 3.1% (323/20,155), LGD and HGD 1.6%. DDR varied among endoscopists [mean DDR: 3.3% (SD 5.2); LGD/HGD: 1.8% (SD 3.5)] and participating sites [mean DDR: 3.3% (SD 3.7), LGD/HGD: 1.9% (SD 2.2)] (Figure 2). The 95% confidence intervals for each provider for DDR were highly variable and ranged from −20.0% to 119.3%. Notably, increasing EGD volume had an inconsistent effect on adherence rates and DDR by endoscopists and sites (Table 1). There was no correlation between overall DDR and Seattle protocol adherence among sites (r= −0.098, P=0.230). There was a weak yet statistically significant negative correlation between DDR and Seattle protocol adherence among individual endoscopists (r= −0.083, p<0.05).
Figure 2.
Dysplasia detection rate by Endoscopist and by Site
Table 1.
Adherence Rates to Established Quality Indicators (Seattle Protocol, Surveillance) and potential metrics (Dysplasia detection rate)
| Endoscopist Adherence Rates to Seattle Biopsy Protocol | |||
|---|---|---|---|
| EGD Volume | Number of endoscopists | Mean Adherence Rate (SD) | 25th / 50th / 75th Percentile |
| 10–19 | 230 | 83.3% (23.2) | 76.9% / 92.3% / 100% |
| 20–29 | 146 | 85.6% (17.5) | 80.0% / 92.0% / 100% |
| ≥30 | 196 | 86.8% (22.3) | 83.9% / 96.5% / 100% |
| Endoscopist Adherence Rates to Appropriate 3–5 year Surveillance in NDBE | |||
| 10–14 | 129 | 66.6% (SD 33.8) | 50.0% / 80.0% / 91.7% |
| 15–24 | 151 | 75.5% (26.3) | 64.7% / 84.2% / 94.4% |
| ≥25 | 120 | 69.8% (29.6) | 55.2% / 80.6% / 92.5% |
| Site Adherence Rates to Seattle Biopsy Protocol | |||
| EGD Volume | Number of sites | Mean Adherence Rate (SD) | 25th / 50th / 75th Percentile |
| 20–49 | 43 | 76.0% (26.8) | 61.5% / 90.0% / 95.8% |
| 50–99 | 40 | 77.3% (26.4) | 73.3% / 87.2% / 97.0% |
| 100–149 | 23 | 86.7% (15.6) | 84.4% / 90.8% / 96.4% |
| ≥150 | 47 | 88.1% (15.6) | 82.7% / 94.2% / 97.6% |
| Site Adherence Rates to Appropriate 3–5 year Surveillance in NDBE | |||
| 20–29 | 29 | 68.8% (28.6) | 56.0% / 78.6% / 90.5% |
| 30–49 | 32 | 65.1% (23.9) | 52.7% / 69.4% / 83.6% |
| 50–99 | 37 | 67.6% (27.1) | 63.2% / 76.4% / 86.3% |
| ≥100 | 38 | 73.3% (23.1) | 63.9% / 81.1% / 89.9% |
| Endoscopist Dysplasia Detection Rate | |||
| EGD Volume | Number of endoscopists/sites | DDR Mean (SD) Range | LGD and HGD (SD) Range |
| 10–19 | 230 | 3.28% (6.11) 0–38.46% |
1.78% (4.13) 0–30% |
| 20–29 | 145 | 3.92% (5.12) 0–22.73% |
2.23% (3.63) 0–17.24% |
| ≥30 | 197 | 2.91% (4.08) 0–23.53% |
1.47% (2.35) 0–13.33% |
| Site Dysplasia Detection Rate by volume of endoscopies | |||
| 20–49 | 42 | 3.32% (3.69) 0–13.33% |
2.23% (2.72) 0–10% |
| 50–99 | 40 | 4.01% (4.90) 0–23.73% |
2.15% (2.59) 0–12% |
| 100–149 | 23 | 2.16% (1.55) 0–6% |
1.18% (1.10) 0–3.5% |
| ≥150 | 47 | 3.34% (3.11) 0–13.33% |
1.7% (1.45) 0–6.31% |
Discussion
Using a national quality registry, this population-based study demonstrated variability in the two established QIs for management of BE patients: adherence to the Seattle biopsy protocol and to recommended endoscopic surveillance intervals for NDBE. In the present study which includes data from 2018–2021, adherence to the Seattle protocol was 86%, considerably higher than the 51% reported from 2002–2007.4 Our results also demonstrate higher rates of adherence to surveillance recommendations compared to prior analyses (70.4% versus 55% on pooled data from 20 studies).9 The differences may be related to the different registries and/or populations being studied, but may also reflect improved knowledge and adoption of guidelines among endoscopists in recent years as GI professional societies have placed more emphasis on quality metrics. As the GIQuIC registry represents the ‘best-case scenario’ for adherence, since sites and endoscopists enrolled in this quality registry are aware that their practices are being monitored, these results indicate that there is still room for improvement and better consistency. This is the first study to highlight variation in adherence to these measures at a center and endoscopist level. Possible explanations for this variability include lack of knowledge, personal disagreement with guidelines, or financial incentives. Patient factors such as racial differences may also contribute to variable rates as prior research has shown that Blacks are less likely than Whites to have appropriate surveillance intervals.10
Additionally, this is the first US population-based study to report the proposed QI DDR. This metric demonstrated marked variability across endoscopists and sites with a wide spread of values for each provider. This finding suggests that especially low volume centers might not find this QI particularly useful. The reasons for a lack of a volume-outcomes effect with EGD numbers and adherence to QIs/DDR needs to be explored in future studies. Quality metrics are vitally important as they foster value-based care that improves adherence to best practices and drives high-quality care and cost savings. These measures serve as a scorecard for quality care and allow for care across facilities and endoscopists to be compared. There has been a recent push to establish NDR as a QI in upper endoscopy since it is a surrogate for a high-quality examination and sampling technique. Available data indicates estimates of NDR (HGD/EAC) vary from 4–4.9%.11, 12 Our study is limited by the fact that EAC is underreported in GIQuIC and therefore not included as an endpoint. We therefore chose to focus on a closely related metric DDR which includes IND and LGD, estimated in prior studies at 11% and 5% respectively.13 Our results indicate DDR was 3.1%; a rate that is lower than previously published studies. The lower rates of DDR in this study may represent updated real-life population-based data that circumvent the limitation of selection and referral bias from studies conducted at expert centers. Thus, these data using the GIQuIC database, most accurately represent the real-world experience in Barrett’s endoscopy. We also cannot exclude the possibility of this being an underestimate due to missed lesions in routine clinical practice which may be a result of an inadequate exam (not using high-definition white light, virtual chromoendoscopy, insufficient inspection time or since cases were not exclusively performed at expert centers.14 This is the first study to compare DDR among endoscopists and endoscopy sites and to demonstrate variability. Given the parallels between NDR/DDR and ADR, this variation warrants exploration to further characterize its utility as a quality measure in the management of BE. Our results do not demonstrate a positive association between DDR and adherence to Seattle protocol by endoscopist or site level, a finding with unclear explanation that deserves exploration in future studies. Whether the weak negative correlation between DDR and adherence to Seattle protocol by endoscopist level is related to target sampling of visible lesions needs confirmation.
Improving rates of adherence to important QI measures and ensuring more consistent clinical behavior across practice groups and endoscopists is a critical first step to ensure high quality examinations and ultimately make an impact on the overall mortality of EAC. Several important barriers may prevent implementation of QIs for BE.15 Upper endoscopies have a host of indications and applying uniform requirements is not realistic given the diverse nature of the procedures. Furthermore, the sheer number of QIs that currently exist is becoming more burdensome for physicians, and the large amount of data required to develop QIs is often impractical to obtain. Nonetheless, practical avenues to implement these measures may include appealing to professional societies to emphasize these metrics to their members and streamlining the reporting systems for QIs within the electronic health records used across various practice settings. Additionally, practical solutions to improve examination quality may include educational interventions such as online learning platforms that teach dysplasia detection16 or that highlight best practices. These educational tools should be user-friendly, simple, and emphasize quality improvement measures. Future efforts are warranted to identify and extinguish predictors of this variability, and to determine whether these interventions can improve DDR and adherence rates to QIs among endoscopists doing these exams with the goal to improve EAC outcomes.
Supplementary Material
Supplemental Figure 1. GiQuIC Registry Record Inclusion Criteria Flowchart
The “new format” records use GIQuIC’s latest specifications. Most sites converted to these around April 2020. NDBE: records with pathology confirmed non dysplastic Barrett’s esophagus. Seattle: records with a documented potential Barrett’s length. DDR: records with a documented finding consistent with Barrett’s esophagus. Dysplasia detection rate includes indefinite for dysplasia, low grade dysplasia, and high-grade dysplasia
References
- 1.Kolb JM, Han S, Scott FI, et al. Early-Onset Esophageal Adenocarcinoma Presents With Advanced-Stage Disease But Has Improved Survival Compared With Older Individuals. Gastroenterology 2020;159:2238–2240.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277–88. [DOI] [PubMed] [Google Scholar]
- 3.Wani S, Muthusamy VR, Shaheen NJ, et al. Development of quality indicators for endoscopic eradication therapies in Barrett’s esophagus: the TREAT-BE (Treatment with Resection and Endoscopic Ablation Techniques for Barrett’s Esophagus) Consortium. Gastrointest Endosc 2017;86:1–17.e3. [DOI] [PubMed] [Google Scholar]
- 4.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009;7:736–42; quiz 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wani S, Han S, Kushnir V, et al. Recurrence Is Rare Following Complete Eradication of Intestinal Metaplasia in Patients With Barrett’s Esophagus and Peaks at 18 Months. Clin Gastroenterol Hepatol 2020;18:2609–2617 e2. [DOI] [PubMed] [Google Scholar]
- 6.Wani S, Williams JL, Falk GW, et al. An Analysis of the GIQuIC Nationwide Quality Registry Reveals Unnecessary Surveillance Endoscopies in Patients With Normal and Irregular Z-Lines. Am J Gastroenterol 2020;115:1869–1878. [DOI] [PubMed] [Google Scholar]
- 7.Ricci E, Hassan C, Petruzziello L, et al. Inter-centre variability of the adenoma detection rate: a prospective, multicentre study. Dig Liver Dis 2013;45:1022–7. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am J Gastroenterol 2022;117:559–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roumans CAM, van der Bogt RD, Steyerberg EW, et al. Adherence to recommendations of Barrett’s esophagus surveillance guidelines: a systematic review and meta-analysis. Endoscopy 2020;52:17–28. [DOI] [PubMed] [Google Scholar]
- 10.Jones B, Williams JL, Komanduri S, et al. Racial Disparities in Adherence to Quality Indicators in Barrett’s Esophagus: An Analysis Using the GIQuIC National Benchmarking Registry. Am J Gastroenterol 2021;116:1201–1210. [DOI] [PubMed] [Google Scholar]
- 11.Parasa S, Desai M, Vittal A, et al. Estimating neoplasia detection rate (NDR) in patients with Barrett’s oesophagus based on index endoscopy: a systematic review and meta-analysis. Gut 2019;68:2122–2128. [DOI] [PubMed] [Google Scholar]
- 12.Dhaliwal L, Codipilly DC, Gandhi P, et al. Neoplasia Detection Rate in Barrett’s Esophagus and Its Impact on Missed Dysplasia: Results from a Large Population-Based Database. Clin Gastroenterol Hepatol 2021;19:922–929.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai M, Lieberman DA, Kennedy KF, et al. Increasing prevalence of high-grade dysplasia and adenocarcinoma on index endoscopy in Barrett’s esophagus over the past 2 decades: data from a multicenter U.S. consortium. Gastrointestinal endoscopy 2019;89:257–263.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wani S, Yadlapati R, Singh S, et al. Post-Endoscopy Esophageal Neoplasia in Barrett’s Esophagus: Consensus Statements from an International Expert Panel. Gastroenterology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Parasa S, Shaheen N. Developing Quality Metrics For Upper Endoscopy. Gastroenterology 2020;158:9–13. [DOI] [PubMed] [Google Scholar]
- 16.Bergman J, de Groof AJ, Pech O, et al. An Interactive Web-Based Educational Tool Improves Detection and Delineation of Barrett’s Esophagus-Related Neoplasia. Gastroenterology 2019;156:1299–1308.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. GiQuIC Registry Record Inclusion Criteria Flowchart
The “new format” records use GIQuIC’s latest specifications. Most sites converted to these around April 2020. NDBE: records with pathology confirmed non dysplastic Barrett’s esophagus. Seattle: records with a documented potential Barrett’s length. DDR: records with a documented finding consistent with Barrett’s esophagus. Dysplasia detection rate includes indefinite for dysplasia, low grade dysplasia, and high-grade dysplasia