Abstract
We report on amino acid substitutions in the quinolone resistance-determining region of type II topisomerases and the prevalence of reserpine-inhibited efflux for 70 clinical isolates of S. pneumoniae for which the ciprofloxacin MIC is ≥4 μg/ml and 28 isolates for which the ciprofloxacin MIC is ≤2 μg/ml. The amino acid substitutions in ParC conferring low-level resistance (MICs, 4 to 8 μg/ml) included Phe, Tyr, and Ala for Ser-79; Asn, Ala, Gly, Tyr, and Val for Asp-83; Asn for Asp-78; and Pro for Ala-115. Isolates with intermediate-level (MICs, 16 to 32 μg/ml) and high-level (MICs, 64 μg/ml) resistance harbored substitutions of Phe and Tyr for Ser-79 or Asn and Ala for Asp-83 in ParC and an additional substitution in GyrA which included either Glu-85-Lys (Gly) or Ser-81-Phe (Tyr). Glu-85-Lys was found exclusively in isolates with high-level resistance. Efflux contributed primarily to low-level resistance in isolates with or without an amino acid substitution in ParC. The impact of amino acid substitutions in ParE was minimal, and no substitutions in GyrB were identified.
Streptococcus pneumoniae is a leading cause of bacterial respiratory tract infections and meningitis (3). Due to the increasing resistance of this bacterium to commonly used antimicrobials, fluoroquinolones with enhanced activity against gram-positive bacteria have been recommended for the treatment of pneumococcal infections (2). Concerns have been raised regarding the emergence of resistance to the fluoroquinolones in S. pneumoniae, as clinical isolates with reduced susceptibility to this class of agents have already been reported (5, 13).
The fluoroquinolones inhibit DNA replication by forming cleavage complexes with type II topoisomerases: topoisomerase IV and DNA gyrase (for a review, see reference 7). In S. pneumoniae, topoisomerase IV and DNA gyrase are tetrameric enzymes encoded for by parC-parE and gyrA-gyrB, respectively. Several in vitro studies have shown that fluoroquinolone resistance in S. pneumoniae occurs in a stepwise fashion (11, 12, 17, 27). In the case of ciprofloxacin, low-level resistance occurs first with a mutation in parC and progresses to higher levels with an additional mutation in gyrA (19). Specific amino acid substitutions in the quinolone resistance-determining regions (QRDRs) have been reported and typically include amino acid substitutions at Ser-79 and Asp-83 in ParC and Ser-81 and Glu-85 in GyrA (19). Although mutations in ParE and GyrB have been reported, their contributions to resistance and in particular to high-level resistance are unclear (6, 23, 24). In addition, efflux, mediated by the presumed membrane protein PmrA, has also been shown to contribute to resistance; however, its role in high-level resistance remains uncertain (10, 29).
The knowledge of fluoroquinolone resistance in S. pneumoniae, in particular, the contribution afforded by specific type II topoisomerase mutations, has been primarily based on in vitro selection studies and to a lesser extent on the genetic characterization of resistant clinical isolates. Few studies have identified large numbers of clinical isolates for which ciprofloxacin MICs are ≥8 μg/ml, thereby making it difficult to draw conclusions as to what mutations or combinations thereof contribute to resistance (1, 13, 14). In our study, we characterized the QRDRs of the type II topoisomerase genes in 98 previously reported (5) Canadian clinical isolates of S. pneumoniae for which the ciprofloxacin MICs were ≥4 μg/ml (n = 70 isolates) and ≤2 μg/ml (n = 28 isolates). This allowed us to examine the possible mechanisms of resistance acquired by S. pneumoniae which have potentially been exposed to ciprofloxacin, ofloxacin, or norfloxacin. The in vitro activities of the newer fluoroquinolones, to which these clinical isolates had not been previously exposed, were also examined in order to evaluate the effects of the existing type II topoisomerase mutations.
MATERIALS AND METHODS
Characterization of bacterial strains.
A total of 98 S. pneumoniae isolates were collected in 1988 and from October 1993 to September 1998. Isolates were collected from 40 different laboratories located in eight provinces as part of a Canada-wide surveillance program. The MICs of ciprofloxacin, sparfloxacin, levofloxacin, grepafloxacin, gatifloxacin, trovafloxacin, moxifloxacin, and gemifloxacin were determined for each isolate. The drugs were supplied by their respective manufacturers. Susceptibility testing was performed by the broth microdilution method according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines (18).
Seventy isolates for which ciprofloxacin MICs were ≥4 μg/ml were chosen for characterization of the mechanisms of fluoroquinolone resistance. Serotyping and pulsed-field gel electrophoresis had previously demonstrated (5) that these isolates were of multiple clones and serotypes, including serotypes 11A, 23F, 9V, 6A, 6B, 9N, 22F, and 14. For comparative purposes, a random sample of 28 strains for which the ciprofloxacin MIC was ≤2 μg/ml was also characterized.
Isolation of DNA and PCR amplification.
Crude cell lysates were used as DNA templates for PCR. Briefly, a loop of overnight growth on Columbia nutrient agar supplemented with 5% sheep blood was suspended in 100 μl of lysis buffer (100 mM NaCl, 10 mM Tris-HCl [pH 8.3], 1 mM EDTA, 1% Triton X-100) and the mixture was boiled for 10 min; 10 μl of the supernatant was used as the template in a 50-μl reaction volume. The QRDRs of parC, parE, and gyrA of all strains were amplified by PCR. The QRDR of gyrB was analyzed for 21 strains for which the ciprofloxacin MIC was ≥16 μg/ml and for 5 strains for which the ciprofloxacin MIC was 4 μg/ml. Primers were based on published sequences (19, 23). Amplification products were purified with either the QIAquick PCR purification kit (Qiagen Inc., Mississauga, Ontario, Canada) or the Concert Rapid PCR purification kit (Life Technologies, Burlington, Ontario, Canada).
Sequencing and identification of mutations.
DNA sequencing was performed by ABI Prism Big Dye terminator cycle sequencing with the ABI 377 automated sequencer (PE Applied Biosystems, Mississauga, Ontario, Canada). Nucleotide and amino acid sequence comparisons were performed with the multiple-alignment sequence function of Vector NTI Suite software (InforMax Inc., Bethesda, Md.). The GenBank accession numbers for the wild-type sequences used for comparison purposes were Z67739 for parC and parE (20), AB010387 for gyrA and Z67740 for gyrB (16).
Active efflux.
Isolates for which ciprofloxacin MICs were ≥2 μg/ml were examined for active efflux by agar dilution on Mueller-Hinton agar containing 5% sheep blood in the presence of ciprofloxacin with or without 10 mg of the alkaloid reserpine (Sigma Chemical Co., St. Louis, Mo.) per ml (4). Strains for which there was a fourfold or greater decrease in the ciprofloxacin MIC in the presence of reserpine were considered in this study to be positive for reserpine-inhibited efflux.
RESULTS
Bacterial strains.
For the purposes of this study, the isolates were categorized according to the ciprofloxacin MIC. Six isolates were classified as having high-level resistance (MIC, 64 μg/ml), 15 were classified as having intermediate-level resistance (MICs, 16 to 32 μg/ml), 49 were classified as having low-level resistance (MICs, 4 to 8 μg/ml), and 28 were classified as susceptible (MIC, ≤2 μg/ml).
Type II topoisomerase amino acid substitutions and levels of resistance.
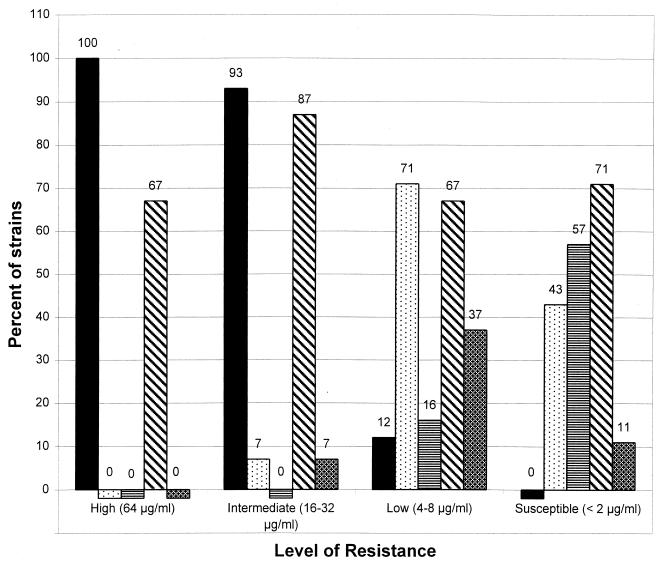
All isolates with high- and intermediate-level resistance had at least one amino acid substitution in ParC and all but one, an isolate with intermediate-level resistance, had an additional substitution in GyrA. In contrast, only 6 of the 49 (12%) strains with low-level resistance had amino acid substitutions in both ParC and GyrA (Fig. 1). Amino acid substitutions in ParC, but not in GyrA, were identified in 35 of the 49 (71%) isolates with low-level resistance and 12 of 28 (43%) susceptible isolates. Strains lacking ParC and GyrA substitutions were found only among isolates for which the ciprofloxacin MICs were ≤4 μg/ml (8 of 49 [16%] and 6 of 8 [57%] for the low-level resistance and susceptible groups, respectively) (Fig. 1). ParE substitutions were detected in 4 of the 6 (67%) isolates with high-level resistance isolates, 13 of the 15 (87%) isolates with intermediate-level resistance, 33 of the 49 (67%) isolates with low-level resistance, and 20 of the 28 (71%) susceptible isolates. In the case of GyrB, no amino acid substitutions in the QRDR were detected for any of the 26 strains tested (the ciprofloxacin MICs for the isolates characterized ranged from 4 to 64 μg/ml).
FIG. 1.
Percentage of clinical isolates with amino acid substitutions in ParC only (dotted bars), ParC and GyrA (solid bars), neither ParC nor GyrA (bars with horizontal lines), and ParE (bars with diagonal lines) for each ciprofloxacin resistance group (high-level, intermediate-level, and low-level resistance and susceptible). The percentage of isolates for which the ciprofloxacin MIC decreased by fourfold or greater when grown in the presence of reserpine is also shown (cross-hatched bars).
Specific amino acid substitutions in QRDRs of ParC and GyrA for isolates with intermediate- and high-level resistance (MICs, ≥16 μg/ml).
All 21 isolates for which the ciprofloxacin MIC was ≥16 μg/ml had at least one substitution in the QRDR of ParC. Amino acid substitutions within this group were detected at positions 52, 79, 83, and 137 (Table 1). However, 18 of these isolates (86%) had a substitution at Ser-79 to either Phe or Tyr. The Ser-79-Phe substitution was more prevalent, with 5 of the 6 (83%) isolates with high-level resistance and 8 of the 15 (53%) isolates with intermediate-level resistance harboring this substitution. Other less frequent substitutions were also detected in both groups, including Ser-52-Gly (two isolates with high-level resistance), Lys-137-Asn (three and two isolates with high- and intermediate-level resistance, respectively), Asp-83-Asn (two isolates with intermediate-level resistance), and Asp-83-Ala (one isolate with intermediate-level resistance) (Table 1).
TABLE 1.
Number of clinical isolates with specific amino acid substitutions in the QRDRs of ParC, GyrA, and ParE
| Resistance group | No. of isolates with amino acida substitutions at the indicated positions of type II topoisomerases:
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ParC
|
GyrA
|
ParE
|
|||||||||||||||||||
| S79
|
D83
|
S52, G | K137, N | A115, P | D78, N | Y129, S | S81
|
E85
|
I460
|
D435
|
R447
|
A468
|
|||||||||
| Y | F | A | N | A | Y | G | V | F | Y | K | G | V | N | S | V | ||||||
| High level (MIC, 64 mg/liter) (n = 6) | 1 | 5 | 2 | 3 | 2 | 4 | 4 | ||||||||||||||
| Intermediate level (MICs, 16 to 32 mg/liter) (n = 15) | 4 | 8 | 2 | 1 | 2 | 11 | 2 | 1 | 13 | ||||||||||||
| Low level (MICs, 4 to 8 mg/liter) (n = 49) | 6 | 18 | 1 | 5 | 3 | 1 | 1 | 1 | 18 | 1 | 1 | 5 | 1 | 33 | 2 | 1 | |||||
| Susceptible (MIC ≤2 mg/liter) (n = 28) | 2 | 3 | 1 | 6 | 1 | 18 | 2 | ||||||||||||||
Y, tyrosine; F, phenylalanine; K, lysine; G, glycine; A, alanine; N, asparagine; V, valine; P, proline; S, serine; R, arginine; D, aspartic acid; E, glutamic acid; I, isoleucine.
GyrA substitutions were detected in all isolates for which the ciprofloxacin MIC was ≥32 μg/ml and in all but one of the isolates for which the ciprofloxacin MIC was 16 μg/ml (Table 1). Unlike ParC, the amino acid substitutions in GyrA occurred at only two amino acid positions (Ser-81 and Glu-85). Thirteen of the 20 isolates with GyrA substitutions (11 of the 14 [79%] isolates with intermediate-level resistance and 2 of the 6 [33%] isolates with high-level resistance) harbored a Phe substitution at position 81, and only 2 of the 14 (14%) isolates with intermediate-level resistance had a Ser-81-Tyr substitution. Interestingly, only strains with high-level resistance harbored the Glu-85-Lys substitution (four of the six [67%] isolates). Although a substitution at Glu-85 was noted in one isolate for which the ciprofloxacin MIC was 16 μg/ml, the amino acid change was to Gly and not Lys.
Specific amino acid substitutions in QRDRs of ParC and GyrA for low-level resistance (MICs, 4 to 8 μg/ml).
Twenty-five of the 41 (61%) isolates with an amino acid substitution in ParC harbored a substitution for Ser-79 (Table 1). Of these isolates, 18 (44%) had Ser-79-Phe substitutions and 6 (15%) had Ser-79-Tyr substitutions. Substitutions were also detected at positions Ser-52, Asp-83, and Lys-137. However, unlike the isolates with intermediate- and high-level resistance, the following substitutions were also noted: Ser-79-Ala, Asp-83-Gly, Asp-83-Val, Asp-83-Tyr, Ala-115-Pro, and Asp-78-Asn (Table 1).
Although GyrA substitutions were detected in only a small proportion of the isolates for which the ciprofloxacin MIC was 4 to 8 μg/ml (6 of the 49 [12%] isolates) compared with the proportions of isolates with high- and intermediate-level resistance with GyrA substitutions, identical amino acid substitutions were noted. These included Ser-81-Phe (five of six [83%] isolates) and Glu-85-Gly (one of six [17%] isolates) (Table 1).
Specific amino acid substitutions in QRDRs of ParC and GyrA for susceptible isolates (MICs, ≤2 μg/ml).
Twelve of the 28 (43%) susceptible isolates had a substitution for residues in the QRDR of ParC (Table 1). Of these, for nine the ciprofloxacin MIC was 2 μg/ml, for one the ciprofloxacin MIC was 1 μg/ml, and for two the ciprofloxacin MIC was 0.5 μg/ml. Mutations in parC resulting in amino acid substitutions of Ser-79-Phe (3 [25%] isolates) or Ser-79-Tyr (two [17%] isolates) were detected only in isolates for which ciprofloxacin MICs were 2 μg/ml. Other substitutions included Lys-137-Asn (six [50%] isolates), Ser-52-Gly (one [8%] isolate), and Tyr-129-Ser (one [8%] isolate). Of the isolates for which the ciprofloxacin MIC was ≤1 μg/ml, only Ser-52-Gly and Lys-137-Asn substitutions were noted.
Specific amino acid substitutions in QRDRs of GyrB and ParE.
Amino acid substitutions in ParE were detected in 67% of the isolates with high-level resistance, 87% of the isolates with intermediate-level resistance, 67% of the isolates with low-level resistance, and 71% of the susceptible strains (Table 1). In particular, four of nine isolates (44%) for which the ciprofloxacin MIC was ≤0.5 μg/ml had ParE substitutions. With the exception of two isolates with an Ala-468-Val substitution (ciprofloxacin MICs, 0.25 and 2 μg/ml, respectively), all strains harboring a ParE substitution had an Ile-to-Val substitution at position 460. In addition, Arg-447-Ser and Asp-435-Asn substitutions were also found in combination with the Ile-460-Val substitution in two isolates with low-level resistance. No amino acid substitutions in the QRDR of GyrB were detected in any of the 26 strains tested (21 isolates for which the ciprofloxacin MICs were ≥16 μg/ml and 5 isolates for which the ciprofloxacin MIC was 4 μg/ml).
Comparative ciprofloxacin susceptibilities and specific ParC-GyrA amino acid substitution combinations.
In order to determine if particular amino acid substitution combinations in the QRDRs would correlate with particular ciprofloxacin MICs, all 98 isolates were grouped according to their ParC-GyrA substitutions together with the MIC range (Table 2). Of the 13 isolates which harbored a substitution for Asp-83, only 4 harbored an additional substitution in GyrA and the ciprofloxacin MIC range never exceeded 16 μg/ml. In contrast, for isolates harboring an identical GyrA substitution but in combination with a substitution for Ser-79 instead of Asp-83, ciprofloxacin MICs were as high as 64 μg/ml. Ciprofloxacin MICs for isolates harboring a substitution in ParC (for either Ser-79 or Asp-83) but not in GyrA ranged from 0.5 to 16 μg/ml; however, of these isolates the MIC was 16 μg/ml for only one isolate, and that isolate was also positive for reserpine-inhibited efflux. Substitutions of Asn for Lys-137 and Gly for Ser-52 in isolates for which the ciprofloxacin MIC was ≥16 μg/ml were found only in combination with an additional substitution at either Ser-79 or Asp-83. For eight isolates which harbored only the Lys-137-Asn substitution and no GyrA substitution, the ciprofloxacin MICs were in the range of 0.5 to 4 μg/ml. Incidentally, for four of these eight isolates the ciprofloxacin MIC was 4 μg/ml, but the isolates were also classified as positive for reserpine-inhibited efflux.
TABLE 2.
Identified amino acid substitution combinations in QRDRs of ParC and GyrA and the associated ciprofloxacin MICs
| No. of isolates with amino acida substitution at the indicated position in ParC (ciprofloxacin MIC range [μg/ml])
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid substitution in GyrA | S79
|
D83
|
K137
|
A115P | D78N | Y129S | NSb | |||||||||||
| Y | Y + K137N | F | F+ K137N | F + S52G, K137N | A | N | N + K137N | A | G | V | Y + K137N | N | N + S52G | |||||
| S81 | ||||||||||||||||||
| F | 4 (8–32) | 6 (8–32) | 1 (64) | 1 (64) | 1 (16) | 2 (8–16) | 1 (16) | 1 (8) | 1 (4) | |||||||||
| Y | 1 (32) | 1 (16) | ||||||||||||||||
| E85 | ||||||||||||||||||
| K | 1 (64) | 2 (64) | 1 (64) | |||||||||||||||
| G | 1 (16) | 1 (8) | ||||||||||||||||
| NS | 6 (2–8) | 1 (4) | 14 (2–16) | 6 (4–8) | 1 (8) | 1 (8) | 3 (4) | 1 (8) | 1 (4) | 3 (4) | 8 (0.5–4) | 2 (0.5–4) | 1 (2) | 24 (0.25–4) | ||||
Y, tyrosine; F, phenylalanine; K, lysine; G, glycine; A, alanine; N, asparagine; V, valine; P, proline; S, serine; R, arginine; D, aspartic acid; E, glutamic acid.
NS, no substitution.
Active efflux phenotype.
Active efflux, which was evaluated indirectly by reserpine reduction of the ciprofloxacin MIC, was tested for all isolates for which the ciprofloxacin MIC was ≥2 μg/ml. As shown in Fig. 1, all strains for which the ciprofloxacin MIC was ≥32 μg/ml were found to be negative for reserpine-inhibited efflux and only 1 of the 11 strains with intermediate-level resistance for which the ciprofloxacin MIC was 16 μg/ml was positive for reserpine-inhibited efflux. In fact, a 16-fold decrease in the ciprofloxacin MIC was noted for this strain when it was grown in the presence of reserpine. Interestingly, this strain with intermediate-level resistance had amino acid substitutions only in ParC and not in GyrA. In contrast, 18 of the 49 (37%) isolates with low-level resistance (MICs, 4 to 8 μg/ml) were positive for reserpine-inhibited efflux (Fig. 1). Moreover, of these 18 strains, 8 isolates for which the ciprofloxacin MIC was 4 μg/ml had no substitutions in either ParC or GyrA. Only 3 of the 28 (11%) susceptible strains (ciprofloxacin MIC, ≤2 μg/ml) were positive for reserpine-inhibited efflux.
Comparative fluoroquinolone susceptibilities and their association with amino acid substitutions in ParC and GyrA.
Table 3 shows that of the other fluoroquinolones tested, all displayed improved activity against S. pneumoniae relative to the activity of ciprofloxacin. However, for isolates harboring both a ParC and GyrA substitution the MICs of these fluoroquinolones were higher than those for isolates with a substitution in ParC only. One isolate harbored neither a ParC nor a GyrA mutation, but the gemifloxacin MIC for the isolate was 0.25 μg/ml and the isolate had an amino acid substitution of Ser for Arg-447 in ParE (Table 3). This is in contrast to the nine additional isolates for which the gemifloxacin MIC was 0.25 μg/ml, which had amino acid substitutions in the QRDRs of both ParC and GyrA but did not harbor the Arg-447-Ser substitution in the QRDR of ParE.
TABLE 3.
In vitro activities of selected fluoroquinolones against 44 pneumococcal isolates (ciprofloxacin MICs, ≥4 μg/ml) with or without QRDR ParC-GyrA amino acid substitutions
| Fluoroquinolone tested | QRDR substitution
|
No. of isolates for which the MIC (μg/ml) was:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ParC | GyrA | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | 16.0 | 32.0 | 64.0 | |
| Ciprofloxacin | + | − | 28 | 7 | 1 | |||||||||
| + | + | 1 | 5 | 10 | 4 | 6 | ||||||||
| − | − | 8 | ||||||||||||
| Levofloxacin | + | − | 5 | 30 | 1 | |||||||||
| + | + | 2 | 10 | 10 | 4 | |||||||||
| − | − | 5 | 3 | |||||||||||
| Sparfloxacin | + | − | 28 | 7 | 1 | |||||||||
| + | + | 1 | 6 | 13 | 2 | 3 | 1 | |||||||
| − | − | NAa | NA | |||||||||||
| Grepafloxacin | + | − | 6 | 21 | 8 | 1 | ||||||||
| + | + | 1 | 3 | 11 | 6 | 5 | ||||||||
| − | − | 2 | 5 | 1 | ||||||||||
| Trovafloxacin | + | − | 4 | 28 | 4 | |||||||||
| + | + | 1 | 2 | 7 | 9 | 6 | 1 | |||||||
| − | − | 7 | 1 | |||||||||||
| Gatifloxacin | + | − | 3 | 29 | 4 | |||||||||
| + | + | 8 | 13 | 5 | ||||||||||
| − | − | 8 | ||||||||||||
| Moxifloxacin | + | − | 2 | 30 | 3 | 1 | ||||||||
| + | + | 5 | 13 | 6 | 2 | |||||||||
| + | − | 1 | 7 | |||||||||||
| Gemifloxacin | + | − | 14 | 20 | 2 | |||||||||
| + | + | 1 | 8 | 12 | 1 | 4 | ||||||||
| − | − | 2 | 5 | |||||||||||
NA, data not available.
DISCUSSION
Our study provides a detailed analysis of the type II topoisomerase mutations that arise in clinical isolates that potentially have been exposed only to quinolones that preferentially target topoisomerase IV and in particular the ParC subunits in S. pneumoniae. In accordance with the predicted stepwise generation of mutations that confer resistance, none of the isolates characterized harbored DNA gyrase QRDR mutations in the absence of topoisomerase IV QRDR mutations. Consistent also with in vitro selection studies (11, 12, 17, 19, 27) was our finding that low-level resistance typically arises following an amino acid substitution for Ser-79 or Asp-83 in ParC and that intermediate- and high-level resistance is achieved following a subsequent substitution for Ser-81 or Glu-85 in GyrA. Furthermore, our findings suggest that the acquisition of a GyrA substitution, in combination with a ParC substitution, is sufficient to increase the MICs of fluoroquinolones that are more active than ciprofloxacin against gram-positive organisms.
Of the ParC substitutions associated with resistance in this study (Ser-79-[Phe, Tyr, Ala], Asp-83-[Ala, Asn, Gly, Tyr, Val], Asp-78-Asn, and Ala-115-Pro), all but the substitutions of Ala for Ser-79 and Pro for Ala-115 have previously been reported for S. pneumoniae (9, 13, 19, 21, 22, 24–26, 28). In addition, we also found amino acid substitutions such as Lys-137-Asn (13, 17, 26) and Ser-52-Gly which were not associated with increased MICs. Of note was the finding that the substitutions of Ser-79-Ala, Asp-83-(Gly, Tyr, Val), Asp-78-Asn, and Ala-115-Pro were associated only with low-level resistance. These findings suggest that both the location of the ParC residue mutated and the specific amino acid substituted for that residue determine whether an isolate has the potential of attaining high levels of resistance. For example, isolates with the ParC amino acid substitutions of Asp-78-Asn and Ala-115-Pro exhibited only low-level resistance, even though they had QRDR substitutions in GyrA identical to those found in isolates with intermediate- and high-level resistance. In addition, the likelihood of Ser(TCT)-79-Ala(GCT) ever being converted to the more critical substitution of Ser(TCT)-79-Phe(TTT) is rare since a double nucleotide change would be required, thereby suggesting that the amino acid substitution in ParC determines the level of resistance attainable by a particular isolate.
In contrast to the heterogeneity of the amino acid substitutions in ParC, GyrA substitutions occurred at only two amino acid positions: Ser-81-(Phe, Tyr) and Glu-85-(Lys, Gly) (19, 21, 22, 26, 28). The substitution of Lys for Glu-85 in GyrA was identified only in isolates with high-level resistance, while its substitution to Gly was associated only with intermediate-level resistance (ciprofloxacin MIC, 16 μg/ml). The equivalent residue in Escherichia coli forms part of the α-helix in GyrA, which lies adjacent to what is thought to be the active site for DNA breakage-reunion reactions and the site for fluoroquinolone binding (15). We speculate that the bulkier side chain of the Lys residue, in contrast to the hydrogen atom of Gly, would significantly reduce quinolone-binding affinity by steric hindrance. Moreover, the positively charged amino group of the Lys side chain at this position may also reduce the binding affinity and hence the effectiveness of the fluoroquinolone.
The significance of the substitutions identified in ParE was less clear than the significance of those identified in ParC and GyrA. A previously reported substitution of Asp-435-Asn in ParE (23, 24) was observed in this study in two isolates with low-level resistance; however, neither isolate harbored the conventional QRDR ParC amino acid substitution, thereby suggesting that Asp-435 may be important for quinolone-topoisomerase IV-DNA binding. Moreover, it also demonstrates that while amino acid substitutions generally occur first in ParC, the drug recognizes not just a single subunit but, rather, the topoisomerase IV complex as a whole (8). In contrast to Asp-435-Asn, the substitution of Val for Ile-460, as described previously (24), appears to play no role in resistance, as it was identified at all levels of resistance and in similar percentages of isolates. In addition, the substitution of Ser for Arg-447 was associated with an elevated gemifloxacin MIC (0.25 μg/ml) compared to those for isolates with no documented mutations. This particular isolate did not have a substitution in the QRDR of either ParC or GyrA, thereby suggesting that this substitution may result in the decreased activity of gemifloxacin against S. pneumoniae.
Our findings suggest that reserpine-inhibited efflux contributes primarily to lower levels of ciprofloxacin resistance and may do so either alone or to a lesser extent by complementing QRDR substitutions in ParC. Interestingly, of the isolates with low-level resistance identified that demonstrated reserpine-inhibited efflux with ParC substitutions, the majority harbored amino acid substitutions not associated with resistance (Ser-52-Gly and Lys-137-Asn) or substitutions that are associated exclusively with low-resistance (Asp-83-[Gly, Tyr]). No isolates with both reserpine-inhibited efflux and a gyrA mutation were identified. Although it is tempting to speculate that efflux is down-regulated following the acquisition of an amino acid substitution in the QRDR of GyrA, it is also possible that the doubling dilution technique used in this study was an inaccurate measure of efflux in isolates for which the ciprofloxacin MICs were ≥32 μg/ml. Although further studies are needed to support the hypothesis of down-regulation, it would seem likely that an organism that has achieved resistance to a particular drug by chromosomal mutations would not require such an energetically costly process.
Our study supports the predicted stepwise generation of mutations in S. pneumoniae. For the most part, isolates for which the ciprofloxacin MIC was greater than or equal to 16 μg/ml have both ParC and GyrA substitutions of Ser-79 or Asp-83 and Ser-81 or Glu-85, respectively. However, ciprofloxacin MICs cannot always be predicted for isolates with given QRDR amino acid substitutions. For example, the ciprofloxacin MICs for two isolates harboring identical QRDR mutations in ParC and GyrA had a fourfold difference. This suggests the presence of unidentified mutations in regions outside the currently recognized QRDR and/or additional mechanisms yet to be identified.
ACKNOWLEDGMENTS
This work was supported by a grant from the Canadian Bacterial Diseases Network. D.J.B. is a recipient of a Medical Research Council of Canada postdoctoral research fellowship.
We thank Tiffany Tam and Heather MacKenzie for help with the PCR.
REFERENCES
- 1.Barry A L, Brown S D, Fuchs P C. Fluoroquinolones resistance among recent clinical isolates of Streptococcus pneumoniae. J Antimicrob Chemother. 1999;43:428–429. doi: 10.1093/jac/43.3.428. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett J G, Breiman R F, Mandell L A, File T M., Jr Community-acquired pneumonia in adults: guidelines for management. The Infectious Diseases Society of America. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett J G, Mundy L M. Community-acquired pneumonia. N Engl J Med. 1995;333:1618–1624. doi: 10.1056/NEJM199512143332408. [DOI] [PubMed] [Google Scholar]
- 4.Brenwald N P, Gill M J, Wise R. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2032–2035. doi: 10.1128/aac.42.8.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D K, McGeer A, de Azavedo J C, Low D E. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 6.Davies T A, Pankuch G A, Dewasse B E, Jacobs M R, Appelbaum P C. In vitro development of resistance to five quinolones and amoxicillin-clavulanate in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:1177–1182. doi: 10.1128/aac.43.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fass D, Bogden C E, Berger J M. Quaternary changes in topoisomerase II may direct orthogon movement of two DNA strands. Nat Struct Biol. 1999;6:322–326. doi: 10.1038/7556. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda H, Hiramatsu K. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:410–412. doi: 10.1128/aac.43.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill M J, Brenwald N P, Wise R. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:187–189. doi: 10.1128/aac.43.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gootz T D, Zaniewski R, Haskell S, Schmieder B, Tankovic J, Girard D, Courvalin P, Polzer R J. Activity of the new fluoroquinolone trovafloxacin (CP-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob Agents Chemother. 1996;40:2691–2697. doi: 10.1128/aac.40.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janoir C, Zeller V, Kitzis M D, Moreau N J, Gutmann L. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob Agents Chemother. 1996;40:2760–2764. doi: 10.1128/aac.40.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones M E, Sahm D F, Martin N, Scheuring S, Heisig P, Thornsberry C, Kohrer K, Schmitz F J. Prevalence of gyrA, gyrB, parC, and parE mutations in clinical isolates of Streptococcus pneumoniae with decreased susceptibilities to different fluoroquinolones and originating from worldwide surveillance studies during the 1997–1998 respiratory season. Antimicrob Agents Chemother. 2000;44:462–466. doi: 10.1128/aac.44.2.462-466.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen J H, Weigel L M, Ferraro M J, Swenson J M, Tenover F C. Activities of newer fluoroquinolones against Streptococcus pneumoniae clinical isolates including those with mutations in the gyrA, parC, and parE loci. Antimicrob Agents Chemother. 1999;43:329–334. doi: 10.1128/aac.43.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morais Cabral J H, Jackson A P, Smith C V, Shikotra N, Maxwell A, Liddington R C. Crystal structure of the breakage-reunion domain of DNA gyrase. Nature. 1997;388:903–906. doi: 10.1038/42294. [DOI] [PubMed] [Google Scholar]
- 16.Munoz R, Bustamante M, De La Campa A G. Ser-127-to-Leu substitution in the DNA gyrase B subunit of Streptococcus pneumoniae is implicated in novobiocin resistance. J Bacteriol. 1995;177:4166–4170. doi: 10.1128/jb.177.14.4166-4170.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz R, De La Campa A G. ParC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob Agents Chemother. 1996;40:2252–2257. doi: 10.1128/aac.40.10.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. M100-S8. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 19.Pan X S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan X S, Fisher L M. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J Bacteriol. 1996;178:4060–4069. doi: 10.1128/jb.178.14.4060-4069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan X S, Fisher L M. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan X S, Fisher L M. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perichon B, Tankovic J, Courvalin P. Characterization of a mutation in the parE gene that confers fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1166–1167. doi: 10.1128/aac.41.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pestova E, Beyer R, Cianciotto N P, Noskin G A, Peterson L R. Contribution of topoisomerase IV and DNA gyrase mutations in Streptococcus pneumoniae to resistance to novel fluoroquinolones. Antimicrob Agents Chemother. 1999;43:2000–2004. doi: 10.1128/aac.43.8.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piddock L J, Johnson M, Ricci V, Hill S L. Activities of new fluoroquinolones against fluoroquinolone-resistant pathogens of the lower respiratory tract. Antimicrob Agents Chemother. 1998;42:2956–2960. doi: 10.1128/aac.42.11.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taba H, Kusano N. Sparfloxacin resistance in clinical isolates of Streptococcus pneumoniae: involvement of multiple mutations in gyrA and parC genes. Antimicrob Agents Chemother. 1998;42:2193–2196. doi: 10.1128/aac.42.9.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tankovic J, Perichon B, Duval J, Courvalin P. Contribution of mutations in gyrA and parC genes to fluoroquinolone resistance of mutants of Streptococcus pneumoniae obtained in vivo and in vitro. Antimicrob Agents Chemother. 1996;40:2505–2510. doi: 10.1128/aac.40.11.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varon E, Janoir C, Kitzis M D, Gutmann L. ParC and GyrA may be interchangeable initial targets of some fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:302–306. doi: 10.1128/aac.43.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeller V, Janoir C, Kitzis M D, Gutmann L, Moreau N J. Active efflux as a mechanism of resistance to ciprofloxacin in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1973–1978. doi: 10.1128/aac.41.9.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]