Abstract
Programmed death-ligand 1 (PD-L1) expression has now been implicated in gastric cancer (GC). This study was conducted to determine the impact of clinicopathological characteristics on PD-L1 expression and its association with survival in GC patients receiving standard-of-care. In total, 268 GC patients receiving upfront surgery were enrolled at Chiang Mai University Hospital. PD-L1 expression was assayed by immunohistochemistry staining using the Dako 22C3 pharmDx. The rates of PD-L1 positivity by combined positive score (CPS) at a cutoff value of 1 and 5 were 22% and 7%. PD-L1 positivity was significantly higher in patients younger than 55 than those older than 55 (32.6% vs. 16.5%, p = 0.003; 11.6% vs. 4.4%, p = 0.027). PD-L1 positivity was observed more frequently in GC with metastases than without (25.2% vs. 17.1%, p = 0.112; 7.2% vs. 6.7%, p = 0.673). Patients with PD-L1 positive had a significantly shorter median overall survival than those with PD-L1 negative (32.7 vs. 41.6 months, p = 0.042, 27.6 vs. 40.8 months, p = 0.038). In conclusion, PD-L1 expression has been associated with young age, short survival, and metastases, although unrelated to the tumor stage. For GC patients, PD-L1 testing is recommended, especially among young patients with metastases.
Subject terms: Cancer, Biomarkers, Gastroenterology
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide, and gastric adenocarcinoma accounts for most GC cases1. Also, GC is the third leading cause of cancer-related deaths worldwide. The prognosis of GC remains poor, especially in advanced stages, even with multidisciplinary therapies that improve treatment outcomes2. One of the most challenging problems in the clinical treatment of GC is that only a part of GC patients benefits from traditional chemical treatment strategies, indicating that other considerations, such as the human immune reaction, also affect the clinical outcome.
Immunotherapeutic agents targeting immunosuppressive proteins have been recognized as potential treatments for cancer due to their favorable curative effect and improved survival time. Among these agents, anti-programmed death protein-1/ligand 1 (PD-1/PD-L1) antibodies are considered the most exciting advancements in cancer immunotherapy3. PD-1/PD-L1 checkpoint inhibitors have shown promising results in treating many types of cancer, including recurrent locally advanced or metastatic GC4,5. Furthermore, the PD-L1 protein expression in viable cancer cells determined by immunohistochemistry (IHC) correlates with the therapeutic effect of immune checkpoint inhibitors. The Food and Drug Administration (FDA) recently approved PD-L1 IHC as a predictive biomarker for the anti-PD-L1 response for some solid tumors, including GC6. In addition, the combined positive score (CPS) is validated as a robust and reproducible method to score PD-L1 protein expression for GC patients treated with pembrolizumab7,8. In CheckMate-649, compared with chemotherapy, the survival benefits of first-line nivolumab combined with chemotherapy increased with the CPS cutoff value9. There are many studies on the clinicopathological and prognostic significance of PD-L1 expression in GC. However, the PD-L1 expression in the Thai population with GC has yet to be evaluated. Furthermore, there is limited data about the prognostic significance of PD-L1 expression among GC patients receiving standard-of-care. Therefore, the primary objective of this study was to examine the rate of PD-L1 expression in Thai patients with GC. Other purposes were to characterize PD-L1 expression and its association with clinicopathological features and the survival of patients with GC.
Materials and methods
Patients and data collection
We retrospectively enrolled GC patients who underwent upfront surgery at Chiang Mai University Hospital, Thailand, from January 1, 2018, to December 31, 2021. All patients were diagnosed with gastric adenocarcinoma by the pathological results of H&E staining specimens. Two experienced pathologists reviewed all cases and confirmed the histological diagnoses without discrepancy. The exclusion criteria were those who received neoadjuvant therapy and those with tumors that were not gastric adenocarcinoma. In addition, patients who died postoperatively due to surgical-related complications were also excluded. Clinical characteristics, including age, sex, tumor location, tumor size, pathologic stage (pTNM), histologic type based on Lauren classification, lymph node status, and vascular invasion, were obtained from hospital medical records and extracted from Chiang Mai University Hospital's electronic database. All patients received standard-of-care for GC therapy. Telephone interviews and medical records were used as follow-up procedures. This study was registered at thaiclinicaltrials.org (number TCTR20221031001). Written informed consent was obtained from all participants included in the study. The study was approved by the Research Ethics Committee Faculty of Medicine, Chiang Mai University (MED-2562–06300) and conducted in accordance with the Helsinki Declaration and its later amendments or comparable ethical standards.
Immunohistochemistry (IHC) staining and evaluation
IHC was performed on 4 -µm-thick tissue sections using an automated IHC Stainer (Ventana, Tucson, AZ, USA). The assessment of PD-L1 protein expression in GC is a qualitative IHC assay that uses anti-PD-L1 antibodies (Dako, 22C3) to detect PD-L1 protein in formalin-fixed, paraffin-embedded tissues from gastric adenocarcinomas. A minimum of 100 tumor cells must be present in the PD-L1 stained slide for the specimen to be considered adequate for PD-L1 evaluation. Expression of PD-L1 was reported as CPS, defined as the total number of PD-L1 positive cells (lymphocytes, macrophages, and tumor cells) divided by the total number of viable tumor cells10. The CPS ≥ 1 and ≥ 5 were chosen to define PD-L1 positive. A monoclonal antibody against Latent Membrane Protein (LMP)-1 (CS1-4; Dako, Glostrup, Denmark) was used to detect EBV-specific protein to identify EBV status for GC with CPS ≥ 1. IHC for LMP-1 was done according to the method previously described11. Brown granular cytoplasmic and membrane staining was interpreted as positive for EBV LMP-1, whereas bluish staining of the cytoplasm and membrane was interpreted as negative for EBV LMP-1. A positive control included a tissue known to have EBV infection, whereas, for negative controls, the test antibody was omitted and replaced by phosphate-buffered saline.
Statistical analysis
All statistical analyses were performed using Stata software, version 15.1 MP (Stata Corporation, College Station, Texas, USA). Data for categorical variables was shown by frequency and percentage. As appropriate, the comparison between PD-L1 expression and clinicopathological features of GC was analyzed using the Chi-squared test or Fisher's exact test. Overall survival was defined as the time from the initial diagnosis to death by any cause or last follow-up. The relationship between PD-L1 expression and overall survival was analyzed using the Kaplan–Meier method and log-rank tests, with PD-L1 negative as the reference. A two-tailed p value < 0.05 was considered statistically significant.
Ethical approval
The study conformed to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. It was approved by the Research Ethics Committee Faculty of Medicine, Chiang Mai University (MED-2562–06300).
Consent to participate
Patients received written and oral information on the study and gave their consent to participate and use their medical data for research purposes.
Results
Clinicopathological characteristics
A total of 268 patients with GC were included in this study. None of the patients received chemotherapy or radiation before surgery. There were 132 (49%) males and 136 (51%) females with a mean age of 59.0 ± 10.2 years (range 37–87 years) at diagnosis. Tumor location was in the lower part of the stomach for 51%, the middle part for 28%, and the upper part for 21%. A tumor diameter of less than 5 cm accounted for 49% of patients, whereas a tumor diameter of more than 5 cm accounted for 51%. Lauren classification was diffuse for 55% and intestinal type for 45%. According to the pTNM classification, the disease was stage I, II, III, IV, and undetermined at 3%, 12%, 25%, 52%, and 8%, respectively. Lymph node metastasis was 44%, and vascular invasion was 23%. More detailed clinicopathological characteristics are summarized in Table 1.
Table 1.
Clinicopathological features of gastric cancer.
| Characteristics | n (%) | |
|---|---|---|
| Sex | Male | 132 (49.3) |
| Female | 136 (50.7) | |
| Age (yr.) | < 40 | 22 (8.2) |
| 40–60 | 114 (42.5) | |
| 60–80 | 122 (45.5) | |
| > 80 | 10 (3.7) | |
| Stage | I | 8 (3.0) |
| II | 32 (11.9) | |
| III | 66 (24.6) | |
| IV | 140 (52.2) | |
| Undetermined | 22 (8.2) | |
| Lauren classification | Intestinal | 120 (44.8) |
| Diffuse | 148 (55.2) | |
| Tumor location | Upper part | 56 (20.9) |
| Middle part | 76 (28.4) | |
| Lower part | 136 (50.7) | |
| Tumor diameter | ≤ 5 cm | 132 (49.3) |
| ≥ 5 cm | 136 (50.7) | |
| Lymphatic invasion | 118 (44.0) | |
| Vascular invasion | 62 (23.1) | |
Correlation between PD-L1 expression and clinicopathological characteristics
The positive rates of CPS with a cutoff value of 1 and 5 were 22% (58/268) and 7% (18/268), respectively. The relationship between clinicopathological characteristics and PD-L1 expression are summarized in Table 2. Among all clinicopathological characteristics, PD-L1, CPS ≥ 1, and PD-L1, CPS ≥ 5 were significantly higher in patients younger than 55 than those older than 55 (32.6% vs. 16.5%, p = 0.003; 11.6% vs. 4.4%, p = 0.027). PD-L1 expression is age-related in patients with GC. PD-L1 positivity was observed more frequently in GC with metastases than without (25.2% vs. 17.1%, p = 0.112; 7.2% vs. 6.7%, p = 0.673). However, no significant correlations were observed between PD-L1 expression and gender, tumor location, tumor diameter, pTNM stage, Lauren classification, lymphatic and vascular invasion, or metastases. Among GC with CPS ≥ 1, 30 (52%) of 58 patients had IHC positive for EBV LMP-1.
Table 2.
PD-L1 expression by study subgroup.
| n (%) | CPS ≥ 1 | CPS < 1 | p value | CPS ≥ 5 | CPS < 5 | p value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 28 (21.2) | 104 (78.8) | 0.867 | 8 (6.7) | 124 (93.3) | 0.673 |
| Female | 30 (22.1) | 106 (77.9) | 10 (7.4) | 126 (92.6) | ||
| Age | ||||||
| ≤ 55 | 28 (32.6) | 58 (67.4) | 0.003 | 10 (11.6) | 76 (88.4) | 0.027 |
| > 55 | 30 (16.5) | 152 (83.5) | 8 (4.4) | 174 (95.6) | ||
| Stage | ||||||
| Early (I & II) | 8 (20.0) | 32 (80.0) | 0.837 | 4 (10.0) | 36 (90.0) | 0.476 |
| Advanced (III & IV) | 44 (21.4) | 162 (78.6) | 14 (6.8) | 192 (93.2) | ||
| Lauren classification | ||||||
| Intestinal | 30 (25.0) | 90 (75.0) | 0.096 | 13 (10.8) | 107 (89.2) | 0.057 |
| Diffuse | 16 (13.7) | 101 (86.3) | 5 (4.3) | 112 (95.7) | ||
| Tumor location | ||||||
| Lower part | 26 (19.1) | 110 (80.9) | 0.308 | 8 (5.9) | 128 (94.1) | 0.579 |
| Other parts | 32 (24.3) | 100 (75.7) | 10 (7.6) | 122 (92.4) | ||
| Tumor diameter | ||||||
| ≤ 5 cm | 28 (21.1) | 105 (78.9) | 0.816 | 9 (6.8) | 124 (93.2) | 0.974 |
| > 5 cm | 30 (22.2) | 105 (77.8) | 9 (6.7) | 126 (93.3) | ||
| Lymphatic invasion | ||||||
| Positive | 20 (16.9) | 98 (83.1) | 0.098 | 8 (6.8) | 110 (93.2) | 0.971 |
| Negative | 38 (25.3) | 112 (74.7) | 10 (6.7) | 140 (93.3) | ||
| Vascular invasion | ||||||
| Positive | 12 (19.4) | 50 (80.6) | 0.618 | 5 (8.1) | 57 (91.9) | 0.629 |
| Negative | 46 (22.3) | 160 (77.7) | 13 (6.3) | 193 (93.7) | ||
| Metastases | ||||||
| Positive | 38 (25.2) | 113 (74.8) | 0.112 | 11 (7.2) | 140 (92.8) | 0.673 |
| Negative | 20 (17.1) | 97 (82.9) | 7 (6.3) | 110 (93.7) | ||
Statistically significant p values are in bold (p < 0.05).
Expression of PD-L1 and clinical outcomes
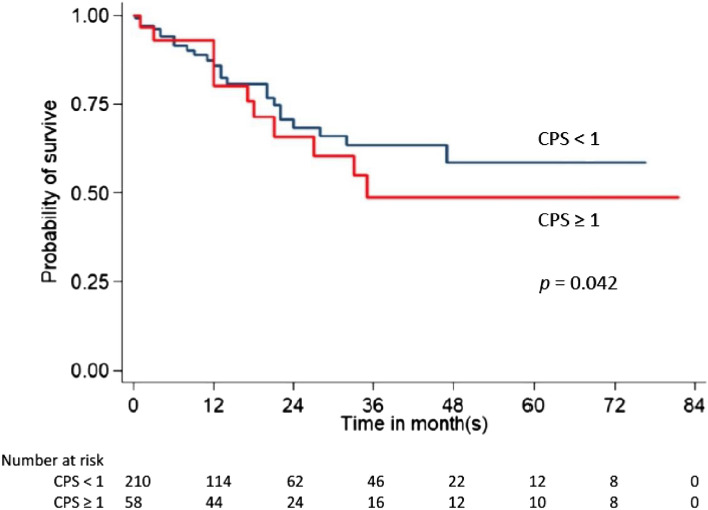
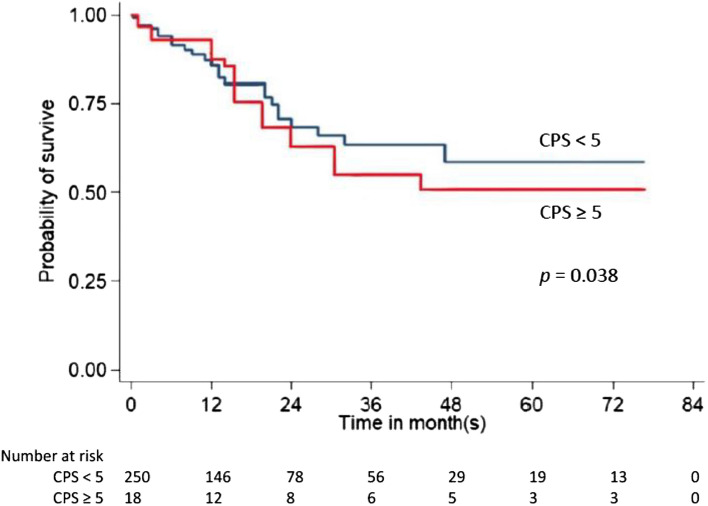
We investigated the prognostic significance of PD-L1 expression concerning overall survival. Based on CPS, overall survival is represented by the Kaplan–Meier curve in Figs. 1 and 2. Our study revealed that the median overall survival was significantly shorter in patients with PD-L1 positive (CPS ≥ 1 and ≥ 5) than in those with PD-L1 negative (32.7 vs. 41.6 months, p = 0.042, 27.6 vs. 40.8 months, p = 0.038).
Figure 1.
Kaplan–Meier analysis of CPS ≥ 1 and overall survival.
Figure 2.
Kaplan–Meier analysis of CPS ≥ 5 and overall survival.
Discussion
PD-1/PD-L1 immune checkpoint inhibitors are now approved for treating patients with advanced GC12,13. PD-L1 expression, evaluated by IHC, is accepted as a predictive biomarker for the effectiveness of PD-1/PD-L1 inhibitors14. This present study is the first evaluation of PD-L1 expression in Thai patients with GC. The prevalence of PD-L1 expression with a CPS cutoff value of 1 and 5 in patients with GC was 22% and 7%, respectively. Patients with PD-L1 positive were typically younger and had significantly shorter survival than those with PD-L1 negative. PD-L1 expression is common in GC patients with metastases. PD-L1 overexpression appears to be an unfavorable prognostic factor in GC.
Our study describes findings from the clinical audit of PD-L1 expression in GC, providing the first insight into the rate of PD-L1 positivity in gastric adenocarcinoma in Thailand. Based on 268 cases of GC analyzed for PD-L1 expression, patients with PD-L1, CPS ≥ 1, and PD-L1, CPS ≥ 5 accounted for 22% and 7% of participants, respectively. The rate of PD-L1 positivity was lower than that reported in the literature from different populations (43% to 63%)15–19. This low expression rate of PD-L1 may be attributed to correlated factors, including a patient cohort, ethnic differences, different types of tumor samples or staging, IHC staining method, and positive cutoff levels for PD-L1 expression. Our study used the IHC 22C3 pharmDx, the only companion diagnostic assay approved by the FDA, at the CPS ≥ 1 and ≥ 5 cutoffs to assess the PD-L1 expression in GC20. Moreover, we used surgical resection samples to avoid intratumoral heterogenicity from biopsy specimens and for precise pathological staging. Although, our study found no statistically significant correlation between PD-L1 positivity and gender, pTNM stage, Lauren classification, tumor location, tumor size, lymphatic invasion, vascular invasion, or metastases. However, PD-L1, CPS ≥ 1 and PD-L1, CPS ≥ 5 had a statistically significant correlation with age lower than 55 (32.6% vs. 16.5%, p = 0.003; 11.6% vs. 4.4%, p = 0.027). Consistent with the previous report, PD-L1 expression was more common in young-onset than average-onset GC patients (31% vs. 3%, p < 0.05)21.
GC is an epithelial tumor associated with Epstein-Barr virus (EBV) infection confirmed by EBV type A and wild-type LMP1 variants in GC lesions in the Thai population22. Based on epidemiological data, 95% of adult Thais have immunity to EBV from childhood infection23. Thus, EBV-positive GC is found in younger patients more often than in EBV-negative gastric tumors24. More than half of the GC patients in our study have been infected with EBV. Likewise, in the previous studies from Brazil and Turkey, the positivity of EBV was 50% to 60% in gastric cancer tissues25,26. EBV induces intra- or peri-tumoral immune cell infiltration and shows genomic amplification of the chromosome 9 locus containing the genes encoding PD-L127. In addition, EBV has upregulated expression levels of PD-L1 in cancer and immune cells28. Consequently, overexpression of PD-L1 is observed in young patients with EBV-associated GC29,30. Moreover, elderly patients have low levels of PD-L1 expression due to immune senescence caused by thymic involution and decreased synthesis of T cell progenitors from bone marrow31. These reasons explain the results of our study showing that PD-L1 positivity was more common in young Thai patients than in elderly patients with GC. We hypothesize that EBV plays a role in the pathogenesis of GC by enhancing PD-L1 expression and provides potentially relevant biomarkers for selecting patients who may derive more significant benefits from PD-1/PD-L1 checkpoint inhibitors, an emerging novel treatment option for GC.
The impact of PD-L1 expression on prognosis remains controversial in several malignancies19,32–35. In our study, PD-L1 positivity in Thai patients with GC was associated with poor prognosis and higher mortality, reducing the chances of overall survival. These findings are related to the PD-L1 positivity, which was more common in patients with metastases than without. Supporting our findings, a meta-analysis on GC patients revealed that PD-L1 positivity corresponded to a poor prognosis for overall survival36,37. Patients with PD-L1 expression should receive immunotherapy instead of standard-of-care for GC. Therefore, PD-L1 expression can be used as a reliable indicator for monitoring the clinical prognosis of GC patients.
However, there are certain limitations of this study. This study was a retrospective analysis that used archived tissue specimens from tissue blocks which likely influenced the amount of PD-L1 expression that may change over time. In addition, since this was a single-center study, selection bias may have existed. Given these limitations, it is probably improper to consider our results as a wholly accurate representation of the prevalence of PD-L1 expression in GC. A well-conducted prospective randomized multicenter trial can give us the exact prevalence of PD-L1 expression and its clinicopathological correlation with GC in Thailand. However, our study can provide insights for improving the selection of patients eligible for anti-PD-1/PD-L1 therapy.
Conclusion
Accurate assessment of PD-L1 expression in GC in the Thai population provides valuable data unique to Thai patients and allows for the cost-effective management of cancer in this population. PD-L1 expression was evident in one-fourth of Thai patients with GC. Furthermore, the expression of PD-L1 has been associated with young age, short survival, and promoting metastases, although unrelated to the tumor stage. Therefore, PD-L1 testing is recommended, especially among young GC patients with metastases, to select patients eligible for anti-PD-1/PD-L1 therapy.
Acknowledgements
The authors thank Mrs. Patumrat Sripan, Research Institute for Health Sciences, Chiang Mai University, for her guidance in regression analysis and statistical computing.
Abbreviations
- CPS
Combined positive score
- EBV
Epstein-Barr virus
- GC
Gastric cancer
- IHC
Immunohistochemistry
- PD-1
Programmed death protein-1
- PD-L1
Programmed death-ligand 1
Author contributions
T.C. contributed to the study conception and design, acquisition, analysis, and interpretation of data; drafted the manuscript and revised it critically for valuable intellectual content; and gave the final approval for publication. P.G. performed data collection and statistical analysis. S.K., K.W., and N.L. helped with the pathologic evaluations. All authors have seen and approved the final version of the report.
Funding
This study was supported by the Faculty of Medicine, Chiang Mai University, grant number MED-025–2563.
Data availability
Datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomarkers Prev. 2014 doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012 doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robainas M, Otano R, Bueno S, Ait-Oudhia S. Understanding the role of PD-L1/PD1 pathway blockade and autophagy in cancer therapy. OncoTargets The.r. 2017 doi: 10.2147/OTT.S132508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, et al. Efficacy and safety of anti-PD-1/anti-PD-L1 antibody therapy in treatment of advanced gastric cancer or gastroesophageal junction cancer: A meta-analysis. World J. Gastrointest. Oncol. 2020;12(11):1346. doi: 10.4251/wjgo.v12.i11.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. ImmunoTher. Cancer. 2018 doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulangara K, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch. Pathol. Lab. Med. 2019;143(3):330. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 8.Emancipator K, et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Modern Pathol. 2021;34(3):532. doi: 10.1038/s41379-020-00710-9. [DOI] [PubMed] [Google Scholar]
- 9.Janjigian YY, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie T, et al. Appropriate PD-L1 cutoff value for gastric cancer immunotherapy: A systematic review and meta-analysis. Front. Oncol. 2021 doi: 10.3389/fonc.2021.646355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salano VE, Mwakigonja AR, Abdulshakoor A, Kahinga AA, Richard EM. Epstein-barr virus latent membrane protein-1 expression in nasopharyngeal Carcinoma. JCO Glob. Oncol. 2021 doi: 10.1200/go.21.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shitara K, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 13.Boku N, et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer. 2021;24(4):946. doi: 10.1007/s10120-021-01173-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sughayer MA, Dabbagh TZ, Battah AH. PD-L1 expression is a favorable prognostic marker in gastric carcinoma. Appl. Immunohistochem. Mol. Morphol. 2020;28(10):748. doi: 10.1097/PAI.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, D., Xu, Y. yun, Li, F., Xu, B. & Zhang, X. guang. (2014).The role of B7-H1 in gastric carcinoma: clinical significance and related mechanism. Med Oncol 31, 123-133 [DOI] [PubMed]
- 16.Hou J, et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7–H1 in the tumor tissues of gastric cancer. Exp. Mol. Pathol. 2014;96:284–291. doi: 10.1016/j.yexmp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Qing Y, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des. Devel. Ther. 2015;9:901–909. doi: 10.2147/DDDT.S75152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, et al. Programmed cell death ligand 1 (PD-L1) expression on gastric cancer and its relationship with clinicopathologic factors. Int. J. Clin. Exp. Pathol. 2015;8(9):11084. [PMC free article] [PubMed] [Google Scholar]
- 19.Eto S, et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer. 2016;19:466–471. doi: 10.1007/s10120-015-0519-7. [DOI] [PubMed] [Google Scholar]
- 20.Park Y, et al. PD-L1 testing in gastric cancer by the combined positive score of the 22c3 pharmdx and sp263 assay with clinically relevant cutoffs. Cancer Res. Treat. 2020;52(3):661. doi: 10.4143/crt.2019.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore A, et al. Young-onset gastric cancer and Epstein-Barr Virus (EBV)—A major player in the pathogenesis? BMC Cancer. 2020;20:1. doi: 10.1186/s12885-020-6517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanvimonsuk S, Thitiwanichpiwong P, Keelawat S, Mutirangura A, Kitkumthorn N. Distribution of the epstein-barr virus in the normal stomach and gastric lesions in Thai population. J Med Virol. 2019;91(3):444. doi: 10.1002/jmv.25318. [DOI] [PubMed] [Google Scholar]
- 23.Suntornlohanakul R, et al. Seroprevalence of anti-EBV IgG among various age groups from Khon Kaen province, Thailand. Asian Pacific J. Cancer Prev. 2015;16(17):7583. doi: 10.7314/APJCP.2015.16.17.7583. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, et al. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. J. Gastroenterol. Hepatol. (Australia) 2009 doi: 10.1111/j.1440-1746.2009.05775.x. [DOI] [PubMed] [Google Scholar]
- 25.de Aquino PF, et al. Epstein-Barr virus DNA associated with gastric adenocarcinoma and adjacent non-cancerous mucosa in patients from Manaus. Brazil. Genet. Mol. Res. 2012;11(4):4442. doi: 10.4238/2012.October.15.3. [DOI] [PubMed] [Google Scholar]
- 26.Durmaz R, Aydin A, Köroglu M, Durmaz B, Çiralik H. Investigation of the relationship between Epstein-Barr virus and ordinary gastric carcinoma using the nested polymerase chain reaction. Acta Virol. 1998;42:359–363. [PubMed] [Google Scholar]
- 27.Bass AJ, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:7517. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derks S, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925. doi: 10.18632/oncotarget.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, et al. Relationship between programmed death ligand 1 expression and other clinicopathological features in a large cohort of gastric cancer patients. Front. Immunol. 2022 doi: 10.3389/fimmu.2022.783695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Z, Yoon HH. The promise of PD-1 inhibitors in gastro-esophageal cancers: Microsatellite instability vs. PD-L1. J. Gastrointest. Oncol. 2016;7(5):771. doi: 10.21037/jgo.2016.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henson SM, Riddell NE, Akbar AN. Properties of end-stage human T cells defined by CD45RA re-expression. Curr. Opin. Immunol. 2012 doi: 10.1016/j.coi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage i pulmonary squamous cell carcinoma. Eur. J. Cancer. 2016;57:91. doi: 10.1016/j.ejca.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Hamanishi J, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. U.S.A. 2007;104(9):3360. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JW, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19(1):42. doi: 10.1007/s10120-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 35.Taube JM, et al. Colocalization of inflammatory response with B7–H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Trans. Med. 2012;4:127. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu Z, Du Y. Clinicopathological and prognostic significance of programmed death ligant-1 expression in gastric cancer: A meta-analysis. J. Gastrointest. Oncol. 2021;12(1):112. doi: 10.21037/jgo-20-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassen G, et al. Programmed death-ligand 1 (PD-L1) positivity and factors associated with poor prognosis in patients with gastric cancer: An umbrella meta-analysis. Cureus. 2022;14:e23845–e23845. doi: 10.7759/cureus.23845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets analyzed during the current study are available from the corresponding author upon reasonable request.