Abstract
Coronary computed tomography angiography has become a mainstay in diagnosing coronary artery disease and is increasingly used in screening symptomatic patients. Recently, photon-counting computed tomography (PCCT) has been introduced into clinical practice, offering higher spatial and temporal resolution. As the applied radiation dose is highly dependent on the choice of scan mode and is lowest using the ultra-fast high-pitch (FLASH) mode, guidelines for their application are needed. From a retrospective study investigating the properties of a novel photon-counting computed tomography, all patients who underwent FLASH-mode PCCT angiography were selected between January and April 2022. This resulted in a study population of 46 men and 27 women. We recorded pre- and intrascan ECG readings and calculated heart rate (maximum heart rate 73 bpm) as well heart rate variability (maximum HRV 37 bpm) as measured by the standard deviation of the heart rate. Diagnostic quality and motion artifacts scores were recorded for each coronary artery segment by consensus between two readers. We found a highly significant association between heart rate variability and image quality (p < 0.001). The heart rate itself was not independently associated with image quality. Both heart rate and heart rate variability were significantly associated with the presence of motion artifacts in a combined model. Scan heart rate variability—but not heart rate itself—is a highly significant predictor of reduced image quality on high-pitch coronary photon-counting computed tomography angiography. This may be due to better scanner architecture and an increased temporal resolution compared to conventional energy-integrating detector computed tomography, which has to be addressed in a comparison study in the future.
Keywords: Photon-counting detector CT, Heart rate, FLASH mode, Dose reduction, Image quality
Introduction
Cardiac computed tomography has become a mainstay in the noninvasive diagnosis and assessment of coronary heart disease. Coronary computed tomography angiography (CCTA) is recommended for initial testing of patients suspected of having obstructive coronary artery disease (CAD) and may reduce the need for invasive coronary angiography (ICA) [1]. Adding CCTA to routine testing has been shown to lower the rate of a combined endpoint of cardiovascular death or myocardial infarction [2]. Initial CCTA instead of ICA has a similar risk of major adverse cardiovascular events, but a lower rate of major procedure-related complications [3]. However, CCTA is not recommended for patients with irregular heart rates or other conditions that may lead to lower image quality [1].
CCTA can be performed using different scan modes, with mode choice depending on heart rate and heart rate variability. Premedication with nitrates or beta-blockers can improve image quality significantly [4]. The lowest radiation dose is generally obtained by using the high-pitch mode, which has been made possible by the introduction of dual-source CT scanners and termed FLASH mode [5, 6]. By performing a single ECG-synchronized high-pitch scan of the whole heart radiation exposure is kept minimal. FLASH-mode CCTA can be performed if three requirements are met: the patient has both a low heart rate and a regular heart rhythm, as well as a high temporal resolution of the CT scanner. If these requirements are not met, the sequential scan mode is chosen, which demonstrates higher signal-to-noise ratios (SNR) but much higher dose exposures [7].
That thresholds for heart rate or heart rate variability above FLASH-mode scanning become inadvisable has been the subject of considerable research interest, with a broad range of found values [8–12]. A possible explanation are differences in scanner design and scanning parameters, as even third-generation CT scanners demonstrated higher SNR in FLASH mode than second-generation devices.
Recently, photon-counting detector computed tomography (PCCT) scanners have been introduced into clinical practice. The differences in detector design and resulting advantages over conventional energy-integrating detectors (EID) have been described in detail elsewhere [13, 14]. They can briefly be summarized as photon-counting detectors (PCD) having a higher inherent spatial resolution and photon efficiency, which can be used either to increase image quality or reduce radiation dose.
It is, therefore, necessary to reevaluate under which conditions FLASH-mode CCTA can be performed using PCCT scanners and how to optimize image quality and dose exposure. Herein, we investigated the influence of heart rate and heart rate variability on image quality and motion artifacts scores of FLASH-mode CCTA.
Methods
Study design
For this retrospective, single-center study, we included all patients who underwent, according to European Society of Cardiology (ESC) guidelines [1], clinically indicated cardiac computed tomography using a novel photon-counting computed tomograph between January and April 2022, who gave informed consent to be included in this study and for which the ultra-fast high-pitch mode (FLASH) was chosen. Patients were excluded in case of metal artifacts in the scan volume (n = 1) or in cases of active or former cardiac malignancies (n = 1). All investigations were conducted as part of a larger, IRB-approved study (ID 2021–659) investigating the properties of the novel PCCT scanner over a variety of use cases and were conducted according to the Declaration of Helsinki.
Computed tomography imaging
All patients were scanned on a novel, first-generation dual-source photon-counting computed tomography scanner (Naeotom Alpha, Siemens Healthineers, Forchheim, Germany). All scans were performed using the electrocardiographic (ECG)-gated ultra-fast high-pitch (FLASH) mode with an effective rotation time of 0.25 s, a tube voltage of 120 kV, and automatic dose modulation. The choice of scan mode was left to the performing physician, guided by international guidelines and previous studies using conventional EID-CT scanners [8, 9]. If no contraindications were present, patients received 5–10 mg of intravenous metoprolol and 0.4—0.8 mg of sublingual nitroglycerine before the scan. First, a non-contrast-enhanced low-dose cardiac CT was performed for coronary calcium quantification. This scan was followed, independent of the result of the calcium quantification, by a contrast-enhanced CT angiography of the coronary arteries. For this, 80 ml of iodinated contrast agent (Imeron 400, Bracco Imaging Deutschland GmbH, Konstanz, Germany) were injected at a flow rate of 4—5 ml / s via an antecubital venous catheter followed by a 20 ml saline chaser (0.9% NaCl). Images were reconstructed using a vascular Bv40 kernel, a matrix size of 512 × 512, a variable FOV covering the whole heart and coronary vessels, a slice thickness of 0.6 mm, and a slice increment of 0.4 mm.
Heart rate and heart rhythm assessment
Using the ECG readings provided by the scanner, heart rate was determined by the value directly during the scan. The heart rate variability was determined by the standard deviation of the five heartbeats prior to the scan.
Image quality and motion artifact scores
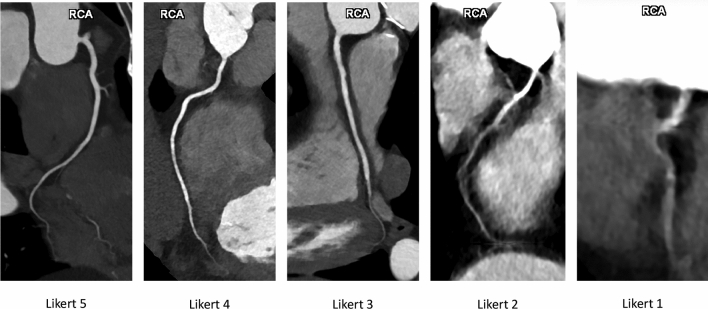
Coronary arteries were divided into ten segments: left main (LM) coronary artery, proximal (pLAD), middle (mLAD), and distal left anterior descending (dLAD) coronary artery, proximal (pCX), middle (mCX), and distal circumflex (dCX) coronary artery, as well as proximal (pRCX), middle (mRCX), and distal right (dRCX) coronary artery. For each scan and each segment, both a diagnostic quality score (DQS) and a motion artifact score (MAS) were collected. The diagnostic quality score was graded according to the clinical adequacy of the coronary visualization. Scores of five to three corresponded to excellent, good, and moderate image quality with excellent, good and sufficient image quality, respectively. Scores of two corresponded to nondiagnostic quality with a visible coronary segment, and scores of one to nondiagnostic quality with poorly visible segments. An exemplary visualization of the DQS scores can be found in Fig. 1.
Fig. 1.
Illustration of diagnostic quality scores of the right coronary artery (RCA) on radial reconstructions of coronary photon-counting computed tomography angiography
Motion artifact scores of zero corresponded to no motion artifacts, one to low to moderate and two to severe artifacts. Scores were determined as consensus between two radiologists (one consultant with nine years and one resident with three years of experience). The detailed score categories can be found in Table 1.
Table 1.
Detailed score definition descriptions
| Diagnostic quality score | |
| 1 | Non diagnostic image quality, segment is poorly visible |
| 2 | Non diagnostic image quality, segment is visible |
| 3 | Moderate image quality with sufficient intraluminal visibility |
| 4 | Good image quality with good intraluminal visibility |
| 5 | Excellent image quality with excellent intraluminal visibility |
| Motion artifact score | |
| 0 | No motion artifacts |
| 1 | Low to moderate motion artifacts |
| 2 | Severe motion artifacts |
Statistics
Summary statistics are reported as mean ± standard deviation for numerical and category percentages of categorical variables. Pearsons correlation coefficients were used to quantify the linear correlation between two variables. Linear mixed-effects models were used to control for patient identity and for vessel segment to account for making multiple measurements per scan. We calculated the Bayesian Information Criterion (BIC) and the log-likelihood as a model quality parameters.
All statistics were performed using R 4.2.1 (R Foundation, Vienna, Austria).
Results
Demographics
We included 73 patients (27 women), mean age 57 ± 12 years at exam, based on the inclusion criteria. The mean computed tomography dose index (CTDIvol) was 3.0 ± 1.1 mGy and the mean dose-length product (DLP) 56.5 ± 24.8 mGy cm for the coronary PCCT angiography scan. Further patient demographics and summary statistics can be found in Table 2.
Table 2.
Study population and score distributions
| Study population | ||
|---|---|---|
| Number | 73 | – |
| Age | 57 ± 12 | Years |
| Sex (w) | 27 (37%) | – |
| Scan parameters | ||
| CTDIvol | 3.0 ± 1.1 | mGy |
| DLP | 56.5 ± 24.8 | mGy cm |
| Coronary CTA | ||
| HR | 58 ± 7 | bpm |
| HRV | 3.57 ± 5.50 | bpm |
| Diagnostic quality score | ||
| 1 | 40 [5.6%] | – |
| 2 | 107 [14.9%] | – |
| 3 | 186 [25.9%] | – |
| 4 | 240 [33.4%] | – |
| 5 | 146 [20.3%] | – |
| Motion artifact score | ||
| 0 | 423 [58.9%] | – |
| 1 | 207 [28.8%] | – |
| 2 | 88 [12.3%] | – |
CTA computed tomograpy angiography, CTDIvol computed tomography dose index, DLP dose-length product, HR heart rate, HRV heart rate variability
Parameter and score distributions
Mean HR at CTA was 58 ± 7 beats per minute (bpm) with a range of 39–73 bpm. 15.0% of patients had an HR below or equal to 50 bpm and 5.4% above or equal to 70 bpm. Mean HRV was 3.5 ± 6.0 bpm with a range of 0.4–37 bpm.
For a total number of 719 vessels a DQS and for 718 vessels a MAS could be obtained. 40 (5.6%) vessels had a DQS of one, 107 (14.9%) of two, 186 (25.9%) of three, 240 (33.4%) of four, and 146 (20.3%) of five. 423 (58.9%) vessels had a MAS of zero, 207 (28.8%) of one, and 88 (12.3%) of two. Detailed parameter distribution per vessel can be found in Table 3.
Table 4.
Correlations between variables and p-values for association significance
| HR | HRV | MAS | DQS | |
|---|---|---|---|---|
| Correlations between variables | ||||
| HR | 1.00 | |||
| HRV | − 0.36 | 1.00 | ||
| MAS | 0.10 | 0.28 | 1.00 | |
| DQS | − 0.02 | − 0.18 | − 0.55 | 1.00 |
| Holms-corrected two-sided pairwise p-values | ||||
| HR | – | |||
| HRV | < 0.001 | – | ||
| MAS | 0.0086 | < 0.001 | – | |
| DQS | 0.57 | < 0.001 | < 0.001 | − |
HR heart rate; HRV, heart rate variability; MAS, motion artifact score; DQS, diagnostic quality score
Table 3.
Diagnostic quality and motion artifact scores per vessel
| pRCA | mRCA | dRCA | LM | pLAD | mLAD | dLAD | pCX | mCX | dCX | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic quality score | ||||||||||
| 1 | 2 (2.78%) | 3 (4.17%) | 9 (12.50%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (11.11%) | 0 (0%) | 3 (4.17%) | 15 (20.83%) |
| 2 | 3 (4.17%) | 7 (9.72%) | 7 (9.72%) | 0 (0%) | 1 (1.39%) | 8 (11.11%) | 40 (55.56%) | 2 (2.78%) | 10 (13.89%) | 29 (40.28%) |
| 3 | 8 (11.11%) | 29 (40.28%) | 11 (15.28%) | 3 (4.23%) | 4 (5.56%) | 40 (55.56%) | 17 (23.61%) | 18 (25.00%) | 38 (52.78%) | 18 (25.00%) |
| 4 | 31 (43.06%) | 20 (27.78%) | 29 (40.28%) | 26 (36.62%) | 43 (59.72%) | 23 (31.94%) | 6 (8.33%) | 36 (50.00%) | 18 (25.00%) | 8 (11.11%) |
| 5 | 28 (38.89%) | 13 (18.06%) | 16 (22.22%) | 42 (59.15%) | 24 (33.33%) | 1 (1.39%) | 1 (1.39%) | 16 (22.22%) | 3 (4.17%) | 2 (2.78%) |
| Motion artifact score | ||||||||||
| 0 | 47 (65.28%) | 22 (30.56%) | 46 (63.89%) | 62 (87.32%) | 59 (81.94%) | 39 (54.17%) | 31 (43.06%) | 52 (72.22%) | 35 (48.61%) | 30 (42.25%) |
| 1 | 12 (16.67%) | 29 (40.28%) | 12 (16.67%) | 8 (11.27%) | 11 (15.28%) | 26 (36.11%) | 34 (47.22%) | 16 (22.22%) | 25 (34.72%) | 34 (47.89%) |
| 2 | 13 (18.06%) | 21 (29.17%) | 14 (19.44%) | 1 (1.41%) | 2 (2.78%) | 7 (9.72%) | 7 (9.72%) | 4 (5.56%) | 12 (16.67%) | 7 (9.86%) |
pRCA proximal right coronary artery, mRCA middle right coronary artery, dRCA distal right coronary artery, LM left main coronary artery, pLAD proximal left anterior descending coronary artery, mLAD middle left anterior descending coronary artery, dLAD distal left anterior descending coronary artery, pCX proximal circumflex coronary artery, mCX middle circumflex coronary artery, dCX distal circumflex coronary artery
There were significant correlations between HR and HRV and between DQS and MAS (both p < 0.05), as can be seen in Table 4
Diagnostic quality score
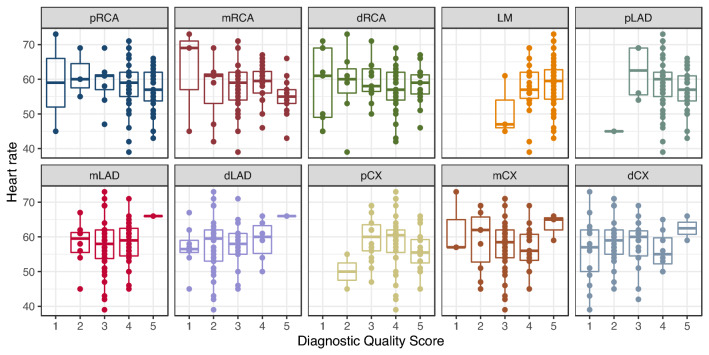
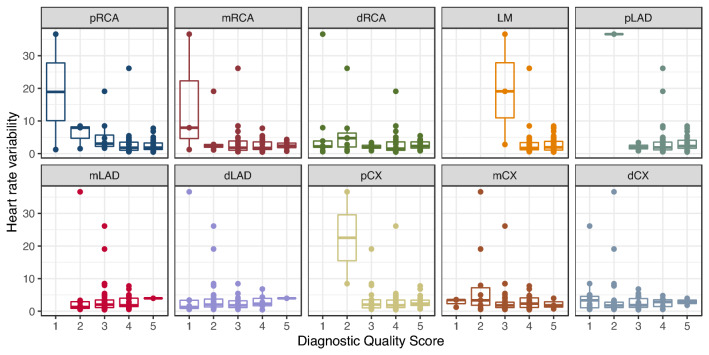
The distribution of the DQS over the segments depending on the HR is shown in Fig. 2 and on the HRV in Fig. 3.
Fig. 2.
Heart rate depending on the diagnostic quality score broken down per vessel segment
Fig. 3.
Heart rate variability depending on the diagnostic quality score broken down per vessel segment
In linear mixed-effects models containing only each variable, HRV (model 2), but not HR (model 1) was a significant predictor of DQS (p < 0.001). In a combined mixed-effects model with both predictors (model 3), again only HRV was a significant predictor (p < 0.001). Model 2 had the lowest BIC, indicating that adding HR does not improve prediction accuracy. It can be seen from Fig. 3 that the association hinges mostly on outliers with high HRV. Detailed model parameters can be found in Table 5.
Table 5.
Linear mixed-effects models for the diagnostic quality score controlled for vessel segment and patient identity
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Coefficients | p-Values | Coefficients | p-Values | Coefficients | p-Values | |
| HR | − 0.0028 | 0.76 | – | – | − 0.016 | 0.087 |
| HRV | – | – | − 0.039 | 0.00090 | − 0.046 | 0.00021 |
| BIC | 1771.2 | – | 1760.0 | – | 1763.6 | – |
| logLikelihood | − 869.1 | – | − 863.5 | – | − 862.0 | – |
HR heart rate, HRV heart rate variability, BIC Bayes information criterion
Motion artifact score
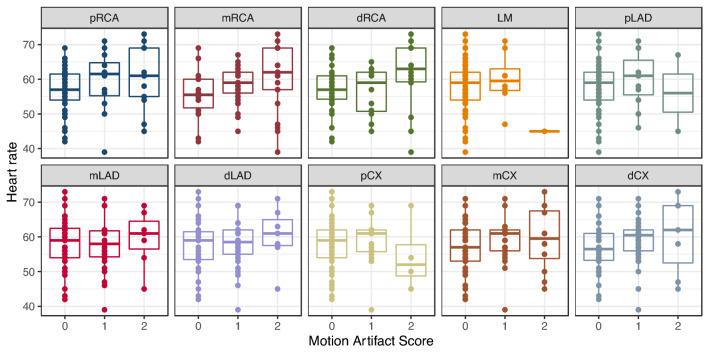
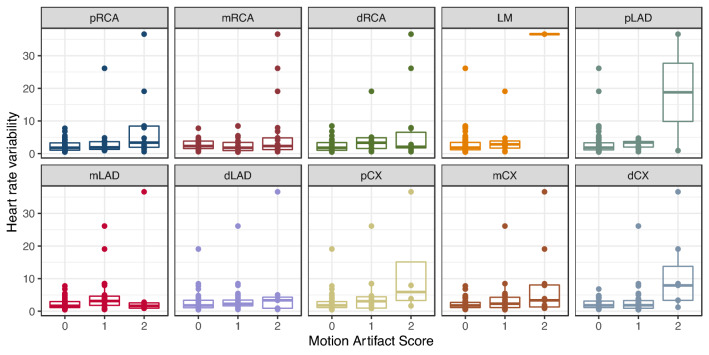
The distribution of the MAS over the segments depending on the HR is shown in Fig. 4 and on the HRV in Fig. 5.
Fig. 4.
Heart rate depending on the motion artifact score broken down per vessel segment
Fig. 5.
Heart rate variability depending on the motion artifact score broken down per vessel segment
In linear mixed-effects models containing only each variable, only HRV (model 2), but not HR (model 1) was a significant predictor of the MAS (p < 0.001). In a combined mixed effects model (model 3), both predictors were significant (both p < 0.001). The combined model had a lower BIC than the other models, indicating that taking both into account improves prediction of the MAS compared to univariate models. Detailed model parameters can be found in Table 6.
Table 6.
Linear mixed-effects models for the motion artifact score controlled for vessel segment and patient identity
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Fixed effects | p-values | Fixed effects | p-values | Fixed effects | p-values | |
| HR | 0.010 | 0.13 | – | – | 0.024 | 0.00017 |
| HRV | – | – | 0.036 | 2.03e−5 | 0.048 | 4.8e−8 |
| BIC | 1340.8 | 1324.4 | 1316.4 | |||
| logLikelihood | − 653.9 | − 645.7 | − 638.4 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Coefficients | p-Values | Coefficients | p-Values | Coefficients | p-Values | |
| HR | 0.010 | 0.13 | – | – | 0.024 | 0.00017 |
| HRV | – | – | 0.036 | 2.03e−5 | 0.048 | 4.8e−8 |
| BIC | 1340.8 | 1324.4 | 1316.4 | |||
| logLikelihood | − 653.9 | − 645.7 | − 638.4 | |||
HR heart rate, HRV heart rate variability, BIC Bayes information criterion
Discussion
In this study, we investigated the influence of HR and HRV on diagnostic quality and presence of motion artifacts in cardiac photon-counting computed tomography angiography. We found no evidence of a significant association of HR with image quality scores (p > 0.05) but observed a strong influence of the HRV (p < 0.001) in a combined mixed-effects model. Both HR and HRV were significantly associated with the presence of motion artifacts (both p < 0.001) in a combined model.
We chose to define heart rate variability in terms of the standard deviation of the five heartbeats preceding the acquisition of the CT angiography. This is equivalent to the commonly applied standard deviation of the normal R-R intervals (SDNN). We obtained a mean SDNN of 55.2 ± 68.1 ms, which is in line with previous studies in patients with compromised cardiac health [15, 16].
The influence of HRV on both diagnostic quality and motion artifact scores is plausible from a mechanistic point of view. Variations in the R-R intervals prior to the scan lead to mistiming of the acquisition, which then falls, instead of the ideal mid-diastole, in intervals of the heart cycle with increased cardiac wall movement. Elevated HR itself does not inherently lead to mistimed acquisitions but shortens the ideal time interval. Accordingly, while the HR is a significant predictor of the motion artifact score, the correlation coefficient is very low (Pearson’s correlation coefficient 0.10). Furthermore, due to this weak association, HR does not become a predictor of reduced image quality, despite the significant correlation between MAS and DQS.
The lack of an association between image quality scores and HR may also be due to the high temporal resolution of the CT scanner architecture. This is supported by a study showing that FLASH-mode image quality increased significantly between second- and third-generation dual-source CT scanners [17]. These improvements may have increased the threshold above which HR starts impacting diagnostic quality above the highest HR included in our study. It is also possible that properties of the PCD itself may lead to better image quality at a given HR. At the same dose, the higher photon efficiency leads to lower image noise [13], possibly shifting the threshold above which unacceptable decreases in image quality occur. Furthermore, the generated virtual monoenergetic images improve iodine contrast, which may allow better differentiation between cardiac vessels and tissue [18]. Only 5.4% of the included patients had a HR of 70 or more, and the highest recorded heart rate at the scan was 73. Therefore, further studies including patients with higher HR are indicated.
In the literature, varying results for associations between image quality and HR or HRV have been found. A recent study demonstrated FLASH-mode scanning is possible for the majority of pediatric patients with heart rates < 100 bpm with adequate proximal coronary visualization [8]. In another study, average HR and HRV were significantly higher in patients with at least one nondiagnostic segment, and all patients with HR < 64 bpm and HRV < 13 bpm, as defined by the difference between the maximum and minimum HR, had diagnostic image quality in all segments [10]. Another study found that HR and motion artifacts on the preceding coronary artery calcium quantification scan are independent predictors of image quality [11]. In a combined phantom-patient study, distortion of coronary arteries was less than 1 mm for HR up to 75 bpm in the phantom and the depiction of patient coronary segments was of diagnostic image quality for all patients with HR up to 73 bpm [9]. The latter study, in which the phantom part was performed using a third-generation dual-source CT system (Somatom Force, Siemens Healthineers. Forchheim, Germany), is most in line with our study. Especially their results of the influence of the simulated HR on the distortion vector of the coronary arteries (see Fig. 3 there) show almost no influence up to 75 bpm, after which the magnitude increases. Given that the range of HR seen in our study is below this threshold, it should come as no surprise that no significant association with HR was observed.
Our study has several limitations. The main limiting factor is the retrospective nature and the restriction on the analysis of patients who received only FLASH scans. As the choice of scan mode was left to the performing physician, patients with significant arrhythmia or elevated heart rates were naturally excluded, leading to significant selection bias. However, as no fixed threshold was set beforehand, borderline cases were indeed included, as can be seen in the relatively high number of patients with HR for which normally a different scan mode would be chosen. Additionally, possible changes in HR and HRV between the timepoint of mode selection and the scan would inherently fuzzy such a threshold. Another factor is the inherent subjectivity of image quality and motion artifact scoring. While the scores were determined in consensus, a study with multiple readers would possibly reduce reading bias. Minor limiting factors were the relatively low patient number, possible temporal changes in heart rhythm due to the different amount of administered premedication, and the inclusion of patients both with and without known coronary, structural or functional heart disease. Furthermore, we did not perform a direct comparison between cardiac CTs performed on either EID-CT or PCCT scanners, which would provide further information on possible differences in the HR threshold.
This preliminary study represents the first investigation regarding the possible selection of scan mode in photon-counting cardiac CT imaging. Due to the retrospective and preliminary nature, changes in mode selection or threshold values can and should not be drawn. Further investigations with possible randomization or comparison between scan modes and inclusion of patients with higher heart rates are urgently necessary to improve the strength of evidence and provide clinical guidelines.
Conclusion
We could demonstrate that the scan heart rate variability, but not the mean heart rate during FLASH-mode PCCT coronary angiography is significantly associated with reduced subjective diagnostic quality and increased motion artifacts in a preliminary study with a maximum HR of 73 bpm and maximum HRV of 37 bpm. This has implications for the selection of scan mode choice and subsequently possibly reductions in mean applied radiation dose. The results are in line with previous results investigating last-generation conventional energy-integrating CT, and it is possible that PCCT scanners may push the HR threshold above which motion artifacts begin influencing image quality even higher.
Abbreviations
- AIC
Akaike Information Criterion
- BIC
Bayesian Information Criterion
- bpm
Beats per minute
- CHD
Coronary heart disease
- CT
Computed tomography
- CTDIvol
Computed tomography dose index
- CVD
Cardiovascular disease
- DICOM
Digital Imaging and Communications in Medicine
- DLP
Dose-length product
- DQS
Diagnostic quality score
- ECG
Echocardiography
- EID
Energy-integrating detector
- ESC
European Society of Cardiology
- HR
Heart rate
- HR-SD
Standard deviation of the heart rate
- MAS
Motion artifact score
- PCCT
Photon-counting computed tomography
- PCD
Photon-counting detector
- SD
Standard deviation
- SNR
Signal-to-noise ratio
Author contributions
L.T.R. and I.A. wrote the main manuscript text. C.R. and L.T.R. performed the statistical analysis. C.Z. and L.T.R. prepared all figures, M.F.F. and P.R. prepared all tables. S.O.S. and H.S. supervised the project administration and were responsible for funding. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 2.The SCOT-HEART Investigators Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 3.The DISCHARGE Trial Group. Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591–1602. doi: 10.1056/NEJMoa2200963. [DOI] [PubMed] [Google Scholar]
- 4.Schlett CL, Banerji D, Siegel E, et al. Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMICAT trial. JACC Cardiovasc Imaging. 2011;4(5):481–491. doi: 10.1016/j.jcmg.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achenbach S, Marwan M, Schepis T, et al. High-pitch spiral acquisition: a new scan mode for coronary CT angiography. J Cardiovasc Comput Tomogr. 2009;3(2):117–121. doi: 10.1016/j.jcct.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Hausleiter J, Bischoff B, Hein F, et al. Feasibility of dual-source cardiac CT angiography with high-pitch scan protocols. J Cardiovasc Comput Tomogr. 2009;3(4):236–242. doi: 10.1016/j.jcct.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Seppelt D, Kolb C, Kühn JP, et al. Comparison of sequential and high-pitch-spiral coronary CT-angiography: image quality and radiation exposure. Int J Cardiovasc Imaging. 2019;35(7):1379–1386. doi: 10.1007/s10554-019-01568-y. [DOI] [PubMed] [Google Scholar]
- 8.Malone LJ, Olson A, Barker AJ, Mong DA, Weinman JP, Browne LP. Visualization of proximal coronary arteries on high-pitch electrocardiogram-triggered computed tomography in pediatric congenital heart disease: effects of heart rate and body surface area. Pediatr Radiol. 2020;50(10):1375–1380. doi: 10.1007/s00247-020-04730-0. [DOI] [PubMed] [Google Scholar]
- 9.Morsbach F, Gordic S, Desbiolles L, et al. Performance of turbo high-pitch dual-source CT for coronary CT angiography: first ex vivo and patient experience. Eur Radiol. 2014;24(8):1889–1895. doi: 10.1007/s00330-014-3209-7. [DOI] [PubMed] [Google Scholar]
- 10.Scharf M, Bink R, May MS, et al. High-pitch thoracic CT with simultaneous assessment of coronary arteries: effect of heart rate and heart rate variability on image quality and diagnostic accuracy. JACC Cardiovasc Imaging. 2011;4(6):602–609. doi: 10.1016/j.jcmg.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Stolzmann P, Goetti RP, Maurovich-Horvat P, et al. Predictors of image quality in high-pitch coronary CT angiography. AJR Am J Roentgenol. 2011;197(4):851–858. doi: 10.2214/AJR.10.6072. [DOI] [PubMed] [Google Scholar]
- 12.Vonder M, Vliegenthart R, Kaatee MA, et al. High-pitch versus sequential mode for coronary calcium in individuals with a high heart rate: potential for dose reduction. J Cardiovasc Comput Tomogr. 2018;12(4):298–304. doi: 10.1016/j.jcct.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Willemink MJ, Persson M, Pourmorteza A, Pelc NJ, Fleischmann D. Photon-counting CT: technical principles and clinical prospects. Radiology. 2018;289(2):293–312. doi: 10.1148/radiol.2018172656. [DOI] [PubMed] [Google Scholar]
- 14.Willemink MJ, Noël PB. The evolution of image reconstruction for CT—from filtered back projection to artificial intelligence. Eur Radiol. 2019;29(5):2185–2195. doi: 10.1007/s00330-018-5810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 16.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin CT, Chu LCH, Zimmerman SL, Fishman EK. High-pitch non-gated scans on the second and third generation dual-source CT scanners: comparison of coronary image quality. Clin Imaging. 2020;59(1):45–49. doi: 10.1016/j.clinimag.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed Z, Campeau D, Gong H, et al. High-pitch, high temporal resolution, multi-energy cardiac imaging on a dual-source photon-counting-detector CT. Med Phys. 2022 doi: 10.1002/mp.16124. [DOI] [PMC free article] [PubMed] [Google Scholar]