Abstract
Uterine fibroids (UFs) are the most common benign tumors in women of reproductive age, frequently associated with pain symptoms and heavy menstrual bleeding (HMB), leading to impaired quality of life. Thus, the aim of the study was to evaluate the global perception of stress and the menstrual distress in patients with UFs. A cross-sectional observational study was conducted on a group (n = 69) of fertile age women with UFs compared to age-matched controls, by administering two questionnaires: the perceived stress scale (PSS) and the Menstrual Distress Questionnaire (MEDI-Q). The PSS, MEDI-Q Total Score and 3 subscales—menstrual symptoms (MS), menstrual symptoms distress (MSD), and menstrual specificity index (MESI)—were evaluated. Patients with UFs showed higher PSS than controls (18.5 ± 5.0 vs. 13.8 ± 5.0, p < 0.001) and PSS values were very high in those with HMB, severe dysmenorrhea, and impaired social and working life. Patients with UFs also showed significantly higher score for MEDI-Q Total Score (16.51 ± 12.99 vs. 10.86 ± 12.36) (p < 0.01) as well as for the subscales MSD (2.54 ± 1.07 vs. 1.57 ± 0.98) (p < 0.001) and MESI (0.76 ± 0.30 vs 0.60 ± 0.39) (p < 0.05). The menstrual distress was associated to being uncomfortable about uterine bleeding; in fact, MEDI-Q Total Score was significantly higher in women with HMB compared to those with moderate/normal bleeding. UF characteristics (number, type, and size) did not correlate with perceived stress or menstrual distress. In conclusion, women with UFs have significantly higher levels of perceived stress and menstrual distress than controls and HMB plays a major role in determining such conditions.
Keywords: Dysmenorrhea, Heavy menstrual bleeding, Menstruation, Menstrual distress, Stress, Uterine fibroids
Introduction
Uterine fibroids (UFs) are the most common benign tumors in women of childbearing age and the leading cause of hysterectomy among premenopausal women [1–3]. Patients with UFs suffer a variety of different symptoms: abnormal uterine bleeding (heavy menstrual bleeding (HMB) and irregular bleeding), pain (dysmenorrhea and dyspareunia) symptoms associated with fibroid growth (pelvic pressure, increased urinary frequency, constipation, and abdominal bloating), and infertility [4, 5]. The presence of these symptoms is not constant in all patients and some cases may be asymptomatic; however, a number of studies has revealed that UFs have a relevant impact on women’s health and on quality of life (QoL) [6–9]. Indeed, patients with UFs may show an impairment of sexuality, self-image, social life, emotional and physical well-being, and work productivity [8]. Notably, UFs are associated with levels of disability similar to those of other chronic diseases: affected women show low scores in the domain of vitality and social functioning, highlighting the significant psychosocial burden of UFs [10]. Moreover, a significant improvement in QoL after both surgical (myomectomy, hysterectomy) [11–14], medical [15–19], or physical treatments [20, 21] is reported.
Chronic stress plays a significant role in determining a decrease of QoL because it causes psychological wear and tear to some extent [22], and distress defines a maladaptive response to a stressor, mirrored by an impairment of QoL [23]. Limited data are available on distress in patients with UFs [24], which may contribute to stress-related depression [25].
Thus, the present study is aimed at evaluating the global perception of stress and the menstrual distress in patients with UFs compared to a control group and their possible correlation with clinical symptoms and UF characteristics.
Material and Methods
An observational cross-sectional study was conducted in a group of patients with UFs (n = 69, age range between 29 and 49 years) referred to the Obstetrics and Gynecology Division of the University of Florence (Careggi University Hospital) for a consultation for UFs between September 2020 and June 2021. The inclusion criteria for the study population were reproductive age, first diagnosis of UFs by ultrasound or magnetic resonance imaging (MRI), and no current medical treatment for UFs. An age-matched group of fertile age women (n = 69) without gynecological disorders referring to the hospital for routine gynecological checkup was used as control group. In patients with UFs and in controls data on menarche and characteristics of the menstrual cycle (time interval between menstruation and amount and duration of menstrual bleeding) were collected (Table 1). Furthermore, in patients with UFs information on location, number and size of UFs, pain symptoms, and bleeding pattern were reported (Table 2).
Table 1.
Menstrual cycle characteristics of UFs and control groups
| UFs (n = 69) | Controls (n = 69) | p | |
|---|---|---|---|
| Age of menarche (years) | 12.5 ± 1.6 | 12.4 ± 2.0 | 0.785 |
| Menstrual cycle length (days) | 27.7 ± 7.5 | 28.8 ± 6.1 | 0.165 |
| Regular menstrual cycle | 46 (66.7%) | 53 (76.8%) | 0.256 |
| Menstrual bleeding | |||
| Light | 6 (8.7%) | 12 (17.3%) | 0.001 |
| Moderate | 19 (27.5%) | 42 (60.8%) | |
| Heavy | 44 (63.8%) | 15 (21.7%) | |
| Duration of menstrual bleeding | 6.3 ± 4.5 | 5.1 ± 2.1 | 0.0001 |
Table 2.
UF characteristics (n = 69)
| Number of fibroids | |
| Single | 26.6% |
| Multiple | 73.4% |
| Type of fibroids | |
| Subserosal | 67.4% |
| Intramural | 79.3% |
| Submucosal | 34.8% |
| Size | |
| < 3 cm | 6.6% |
| 4–6 cm | 64.1% |
| > 7 cm | 30.3% |
| Clinical presentation | |
| At least one symptom | 57.9% |
| Pelvic pain | 35% |
| Dysmenorrhea | 22.5% |
| Dyspareunia | 7.5% |
| Urinary disorders | 10% |
| Pelvic pressure | 15% |
| HMB | 63.8% |
| Infertility | 12.5% |
Two different questionnaires, the Menstrual Distress Questionnaire (MEDI-Q) [26] and the Italian version of the perceived stress scale (PSS-10) [27, 28], were administered to both cases and control. The PSS-10 is a widely used psychological instrument for measuring the global perception of stress. It is a 10-item questionnaire that evaluates the degree to which an individual has perceived life as unpredictable, uncontrollable, and overloading over the previous month before the interview. The questions ask about feelings and thoughts during the last month. In each case, respondents are asked how often they felt a certain way on a five-point scale from “never” (= 0) to “very often” (= 4). The PSS score is then obtained by summing across all scale items. The score is divided into four categories (range, 0–40 points): 0–6, low level of stress; 7–19, medium level of stress; 20–25, high level of stress; and ≥ 26, very high level of stress.
The MEDI-Q aims to assess and score the distress related to menstruation across the last 12 months. The instrument investigates 25 items, covering the following areas: pain, discomfort, psychic/cognitive changes, gastrointestinal symptoms, and changes in physiological functions. The MEDI-Q Total Score evaluates the global menstruation-related distress, whereas 3 additional subscales investigate the number of menstrual symptoms (MS) that exacerbate during menstruation, the average distress related to menstrual symptoms (menstrual symptom distress, MSD), and the specificity of menstrual symptoms (menstrual specificity index, MESI) [26].
The study protocol was approved by the Ethics Committee of the Institution (n.14558_oss approved on 28 May 2019). A written informed consent was requested for participation to the study. Exclusion criteria were overt menopause, non-Italian native-speakers, illiteracy, or inability to provide informed consent.
Statistical Analysis
The data extrapolated from the questionnaires and baseline characteristics of the cases and controls were entered in an electronic database and analyzed using the software SPSS (Statistical Package for Social Science) (IBM SPSS Statistics 23, IBM Corporation). A descriptive analysis was conducted with the evaluation of position measures (mean, median) and dispersion indices (standard deviation, range) for the quantitative variables. The binomial variables were described by calculating the absolute and percentage frequencies. Pearson’s chi-square test or Fisher’s exact test was used to compare the qualitative variables, depending on the size of the sample. For the quantitative variables, the t-test or the Mann–Whitney test was used, depending on whether or not the distribution of the data was normal. One-way ANOVA was also used to compare MEDI-Q Total Score, MS, MSD, and MESI according to menstrual bleeding (normal, moderate, and heavy). Regression analysis was used to test the association between MEDI-Q scores and UF characteristics and clinical presentation. Statistical significance was considered in case of p < 0.05.
Results
Regarding the characteristics of menstrual cycle, patients with UFs reported more frequently HMB (63.8% vs. 21.7%, p = 0.001) and prolonged menstruation than controls (6.3 ± 4.5 vs. 5.1 ± 2.1, p = 0.0001) (Table 1).
Patients with UFs showed significantly higher levels of PSS than controls (18.5 ± 5.0 vs. 13.8 ± 5.0, p < 0.001) (Fig. 1). The evaluation of PSS in patients with UFs showed that 59% had medium level of stress, whereas 20% and 21% had high and very high level of stress, respectively. Women with high and very high PSS reported significantly more HMB, severe dysmenorrhea, and impaired social and working life (Table 3).
Fig. 1.
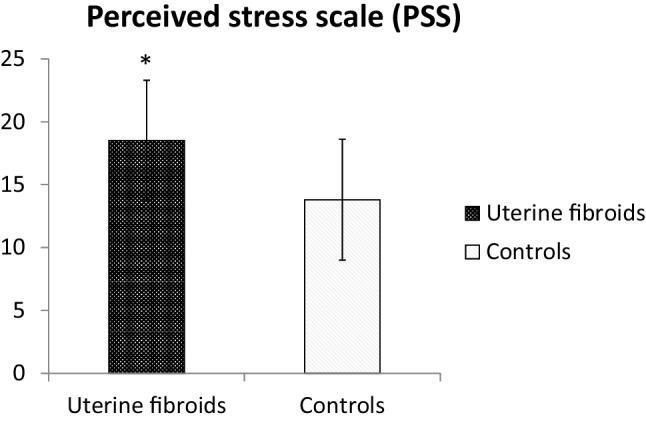
Perceives stress scale among patients with uterine fibroids and controls (18.5 ± 5.0 vs. 13.8 ± 5.0, p < 0.001)
Table 3.
Comparison between women with UFs with medium vs high/very high level of stress according to PSS in terms of menstrual cycle characteristics and symptoms
| PSS—medium level of stress (n = 38) | PSS—high/very high level of stress (n = 31) | p | |
|---|---|---|---|
| Irregular period | 12 (31.6%) | 14 (45.1%) | 0.220 |
| HMB | 18 (47.3%) | 26 (83.8%) | 0.0024 |
| Pelvic pain | 12 (31.5%) | 13 (41.9%) | 0.453 |
| Pelvic pressure | 6 (15.7%) | 5 (16.1%) | 1 |
| Severe dysmenorrhea | 6 (15.7%) | 12 (38.7%) | 0.05 |
| Severe dyspareunia | 3 (7.9%) | 3 (9.6%) | 1 |
| Impaired working/social life | 6 (15.7%) | 14 (45.1%) | 0.015 |
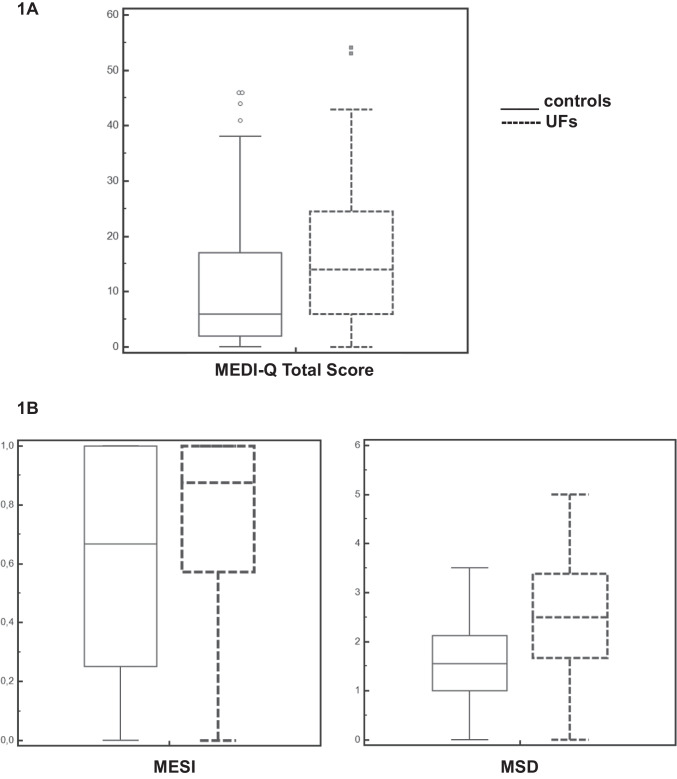
Patients with UFs had significantly higher score for MEDI-Q Total Score (16.51 ± 12.99 vs. 10.86 ± 12.36) (p < 0.01) (Fig. 2A) as well as for the subscales MSD (2.54 ± 1.07 vs. 1.57 ± 0.98) (p < 0.001) and MESI (0.76 ± 0.30 vs. 0.60 ± 0.39) (p < 0.05) (Fig. 2B).
Fig. 2.
MEDI-Q Total Score (1A) and subscales MSD and MESI (1B) in patients with UFs and controls
By performing a sub-analysis of each single item determining the global menstrual distress, women with UFs showed a significant distress related to being uncomfortable for menstrual blood loss. Furthermore, lower abdominal pain, nausea, and pain at defecation were found to have a relevant impact in contributing to menstrual distress (Table 4). The relevance of menstrual bleeding as a stressful factor was also supported by the observation in the study population that MEDI-Q Total is significantly higher in patients with heavy bleeding compared to those with moderate and normal bleeding (p < 0.0001); similarly, MEDI-Q Total is significantly different in women with moderate bleeding compared to those with normal amount of bleeding (p < 0.0001) (Fig. 3). The regression analysis investigating the potential link between the UF characteristics (number, type and size) and the distress scores did not show any significant result.
Table 4.
Distress score for each item determining the global menstrual distress. *p < 0.05; **p < 0.01; ***p < 0.001
| Item number | Item description | Controls (n = 69) | UFs (n = 69) | Statistic |
|---|---|---|---|---|
| Item 1 | Lower abdominal pain | 1.07 ± 1.44 | 1.86 ± 1.90 | -2.72** |
| Item 2 | Urinary pain | 0.04 ± 0.27 | 0.12 ± 0.56 | -0.97 |
| Item 3 | Pain at defecation | 0.03 ± 0.17 | 0.19 ± 0.62 | -2.05* |
| Item 4 | Muscle/osteoarticular pain | 0.71 ± 1.32 | 0.68 ± 1.13 | 0.14 |
| Item 5 | Breast tenderness/widespread swelling sensation | 0.46 ± 0.96 | 0.72 ± 1.21 | -1.40 |
| Item 6 | Nausea | 0.07 ± 0.26 | 0.58 ± 1.28 | -3.23** |
| Item 7 | Headache | 0.86 ± 1.44 | 1.20 ± 1.69 | -1.30 |
| Item 8 | Pain during sexual intercourse | 0.03 ± 0.17 | 0.19 ± 0.90 | -1.45 |
| Item 9 | Digestive problems | 0.19 ± 0.49 | 0.30 ± 0.83 | -1.00 |
| Item 10 | Diarrhea | 0.36 ± 0.84 | 0.48 ± 1.09 | -0.70 |
| Item 11 | Constipation | 0.20 ± 0.65 | 0.23 ± 0.71 | -0.25 |
| Item 12 | Feeling uncomfortable about vaginal blood loss | 0.78 ± 1.37 | 1.96 ± 1.97 | -4.06*** |
| Item 13 | Feeling of being impure | 0.07 ± 0.49 | 0.22 ± 0.91 | -1.17 |
| Item 14 | Sadness | 0.75 ± 1.35 | 0.83 ± 1.37 | -0.31 |
| Item 15 | Emotional lability | 0.88 ± 1.29 | 1.22 ± 1.54 | -1.38 |
| Item 16 | Irritability/anger | 1.17 ± 1.48 | 1.32 ± 1.59 | -0.55 |
| Item 17 | Impulsiveness | 0.20 ± 0.76 | 0.49 ± 1.24 | -1.65 |
| Item 18 | Anxiety | 0.33 ± 0.85 | 0.42 ± 1.01 | -0.55 |
| Item 19 | Increased appetite | 0.58 ± 1.08 | 0.83 ± 1.48 | -1.12 |
| Item 20 | Decreased appetite | 0.10 ± 0.65 | 0.10 ± 0.43 | 0.00 |
| Item 21 | Insomnia | 0.13 ± 0.77 | 0.28 ± 0.95 | -0.98 |
| Item 22 | Hypersomnia | 0.25 ± 0.58 | 0.25 ± 0.81 | 0.00 |
| Item 23 | Fatigue | 0.91 ± 1.33 | 1.10 ± 1.56 | -0.76 |
| Item 24 | Decreased sexual drive | 0.41 ± 0.96 | 0.49 ± 1.23 | -0.46 |
| Item 25 | Concentration impairment | 0.25 ± 0.88 | 0.46 ± 1.21 | -1.21 |
Fig. 3.
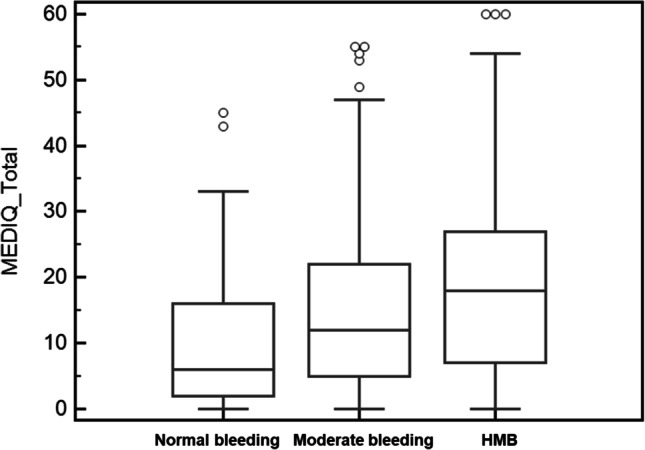
MEDI-Q Total Score according to the amount of menstrual bleeding (normal bleeding 10.36 ± 10.49; moderate bleeding 14.68 ± 12.21; and HMB 19.38 ± 14.39, p < 0.0001)
Discussion
The present study showed that patients with UFs have higher level of global perception of stress and menstrual distress compared to healthy controls, as measured by PSS and MEDI-Q, respectively. HMB plays a crucial role in determining such disabling conditions, impairing QoL.
PSS is a measure of the response evocated when an interaction between the environmental factors and the individual is perceived as beyond the resources of the person to manage. The use of this instrument in the UFs patients showed high level of stress, suggesting a perception of insufficient coping resources. Women with the highest levels of perceived stress referred to have HMB and severe dysmenorrhea, confirming the results obtained with the MEDI-Q. The high levels of stress may also explain the high incidence of mental health disorders in patients with UFs, such as anxiety, depression, and panic disorder, which are considered stress-related disorders [25]. Besides, mood disorders and self-directed violence have been shown to be particularly high among women with UFs who experienced pain symptoms or who underwent hysterectomy [29].
The relevant role of menstrual symptoms on stress was supported by the results obtained with the MEDI-Q. In fact, the level of distress resulted to be associated with menstruation-related characteristics, supporting that symptoms interfere with the person’s quality of life, recreational or work activity, and social relationships [26].
Several studies showed a significant impact of UFs on QoL [30, 31], highlighting the burden of UFs symptoms on emotional and psychosocial health [8, 32]. A diagnosis of UFs may be considered per se a relevant psychosocial stressor, with significant patient-reported health disabilities related to bodily pain, mental health, social functioning, and satisfaction with sex life [10]. The instruments normally used for evaluating the QoL are the Short Form-36 (SF-36) or a fibroid-specific symptom and QoL measure, named Uterine Fibroid Symptom and Quality of Life (UFS-QOL) questionnaire [8, 33]. However, these questionnaires are not designed to assess the menstrual distress.
So far, most of the studies have explored the role of stress as a risk factor for UF development [24, 34–37] and not as a consequence. The underlying mechanisms by which stress would be associated with UFs are under discussion [38]. HPA axis hyperactivation may play a role in the genesis of UFs, because it may influence LH and adrenal progesterone secretion, stimulating UFs growth [14]. On primary cultures of human uterine leiomyoma cells [39], it was shown that adrenergic receptor agonists modulate estrogen receptor (ER), progesterone receptor (PR), vascular endothelial growth factor (VEGF), and fibroblast growth factors (FGF), influencing cell proliferation. Moreover, a product of HPA axis, norepinephrine, promotes the synthesis of pro-inflammatory cytokines (e.g., IL (interleukin)-6, IL-1β, IL-10, and tumor necrosis factor-α) involved in the pathogenesis of UFs [38, 40–42].
The present study supports the concept that the presence of UFs contributes to determine distress, as a maladaptive response to the impairment of QoL and of various domains of daily life [23]. The MEDI-Q [26], an instrument designed to assess the menstrual distress, showed significantly higher scores in women with UFs than controls. This measure allows to evaluate the MEDI-Q Total Score, which assesses the global menstruation-related distress, and the MSD and MESI, which measures the distress related to menstrual symptoms and the specificity of menstrual symptoms. Our patients with UFs showed high distress related to the menstruation-related symptoms, as suggested by the indices MSD and MESI, which are specific for the menstruation. HMB is critical in determining this uncomfortable feeling and the amount and duration of menstrual bleeding play the key role in determining menstrual distress. HMB per se is associated with low QoL, because of the resultant anemia and chronic fatigue [43]. Although QoL among women with UFs is significantly impacted by both bleeding and non-bleeding UFs symptoms [6], there is some evidence that menstrual bleeding has the greater effect on QoL than other symptoms [44–46]. Our study showed that the MEDI-Q Total Score raises as the amount of bleeding increases from normal, to moderate, to heavy, further supporting a link between HMB and menstrual distress. Conversely, the UFs number, type and size did not influence the menstrual distress, suggesting the crucial role of symptoms in determining the impact on QoL and stress.
This is the first study to investigate the global perceived stress and the menstruation-related distress in a selected population of women with UFs, according to their symptoms. One of the strengths is the use of a new instrument, the MEDI-Q, which investigates specifically the impact of menstruation on multiple areas, identifying those relevant to determine distress. The study included only women newly diagnosed with UFs, not undergoing any specific medical (hormonal/non-hormonal) or surgical treatment yet, to avoid bias from the therapeutic approach. In addition, cases were matched to controls according to age, in order to get a more homogeneous study population for baseline menstrual pattern related to age itself and parity. However, some limitations should be acknowledged, as the sample size is small and a larger population may be more representative of UF characteristics, in terms of number, size, type, and associated symptoms. In addition, a wider sample would allow to better explore the role of associated gynecological and non-gynecological diseases in contributing to perceived stress. Available evidences show that iron deficiency (ID) has multiple non-erythropoietic effects, including those on mood and neuro-function [47]. Neuro-bioavailability and brain capture of blood iron are necessary for an appropriate synthesis of neurotransmitters (serotonin, dopamine, and noradrenaline) and may explain the effect of ID on mood changes and emotional behavior [48]. Thus, information on health issues related to HMB, such as ID, anemia, and fatigue, may provide further inputs to better understand the link between UF-related symptoms and menstrual distress.
In conclusion, patients with UFs refer significant perceived stress levels and report higher menstrual distress scores compared to controls. On this background, HMB plays a major role in determining the menstrual distress, whereas UF characteristics do not contribute to make this condition as a stressor. Thus, menstruation-related symptoms are key factors to contribute to the global perception of stress among women with UFs.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Declarations
Ethics Approval
Ethics Committee of the Institution approved the study protocol on 28 May 2019 (n.14558_oss approved).
Consent to Participate
All subjects were asked the consent to participate in the study.
Consent for Publication
All subjects were asked the consent to publish the study results.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Prim. 2016;2:16043. [DOI] [PubMed]
- 2.Giuliani E, As‐Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynecol Obstet. 2020;149:3–9. [DOI] [PubMed]
- 3.Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2018;46:3–11. doi: 10.1016/j.bpobgyn.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Ciebiera M, Bariani MV, Ali M, Elkafas H, Boyer TG, et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr Rev. 2022;43:678–719. 10.1210/endrev/bnab039. [DOI] [PMC free article] [PubMed]
- 5.Donnez J, Dolmans M-M. Uterine fibroid management: from the present to the future. Hum Reprod Update 2016;22:665–86. [DOI] [PMC free article] [PubMed]
- 6.Soliman AM, Margolis MK, Castelli-Haley J, Fuldeore MJ, Owens CD, Coyne KS. Impact of uterine fibroid symptoms on health-related quality of life of US women: evidence from a cross-sectional survey. Curr Med Res Opin England. 2017;33:1971–1978. doi: 10.1080/03007995.2017.1372107. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hendy A, Myers E, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473–80. [DOI] [PMC free article] [PubMed]
- 8.Fortin C, Flyckt R, Falcone T. Best practice & research clinical obstetrics and gynaecology alternatives to hysterectomy : the burden of fi broids and the quality of life. Best Pract Res Clin Obstet Gynaecol Elsevier Ltd. 2018;46:31–42. doi: 10.1016/j.bpobgyn.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Hervé F, Katty A, Isabelle Q, Céline S. Impact of uterine fibroids on quality of life: a national cross-sectional survey. Eur J Obstet Gynecol Reprod Biol Ireland. 2018;229:32–7. doi: 10.1016/j.ejogrb.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Go VAA, Thomas MC, Singh B, Prenatt S, Sims H, Blanck JF, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol United States. 2020;223:674–708.e8. doi: 10.1016/j.ajog.2020.05.044. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson WK, Wegienka G, Zhang S, Wallace K, Stewart E, Laughlin-Tommaso S, et al. Short-term health-related quality of life after hysterectomy compared with myomectomy for symptomatic leiomyomas. Obstet Gynecol United States. 2019;134:261–269. doi: 10.1097/AOG.0000000000003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace K, Zhang S, Thomas L, Stewart EA, Nicholson WK, Wegienka GR, et al. Comparative effectiveness of hysterectomy versus myomectomy on one-year health-related quality of life in women with uterine fibroids. Fertil Steril United States. 2020;113:618–626. doi: 10.1016/j.fertnstert.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Llarena N, Falcone T. Does hysterectomy result in better quality of life than myomectomy? Fertil Steril United States. 2020;113:549–50. doi: 10.1016/j.fertnstert.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin-Tommaso SK, Lu D, Thomas L, Diamond MP, Wallace K, Wegienka G, et al. Short-term quality of life after myomectomy for uterine fibroids from the COMPARE-UF Fibroid Registry. Am J Obstet Gynecol United States. 2020;222:345.e1–345.e22. doi: 10.1016/j.ajog.2019.09.052. [DOI] [PubMed] [Google Scholar]
- 15.Lukes AS, Soper D, Harrington A, Sniukiene V, Mo Y, Gillard P, et al. Health-related quality of life with ulipristal acetate for treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2019;133:869. doi: 10.1097/AOG.0000000000003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surrey E, Taylor HS, Giudice L, Lessey BA, Abrao MS, Archer DF, et al. Long-term outcomes of elagolix in women with endometriosis results from two extension studies. Obstet Gynecol. 2018;132:147–160. doi: 10.1097/AOG.0000000000002675. [DOI] [PubMed] [Google Scholar]
- 17.Stewart EA, Diamond MP, Williams ARW, Carr BR, Myers ER, Feldman RA, et al. Safety and efficacy of the selective progesterone receptor modulator asoprisnil for heavy menstrual bleeding with uterine fibroids: pooled analysis of two 12-month, placebo-controlled, randomized trials. Hum Reprod. 2019;34:623–634. doi: 10.1093/humrep/dez007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolmans M-M, Cacciottola L, Donnez J. Conservative management of uterine fibroid-related heavy menstrual bleeding and infertility: time for a deeper mechanistic understanding and an individualized approach. J Clin Med. 2021;10:4389. [DOI] [PMC free article] [PubMed]
- 19.Donnez J, Dolmans M-M. Fibroids and medical therapy: bridging the gap from selective progesterone receptor modulators to gonadotropin-releasing hormone antagonist. Fertil Steril. 2020;114:739–41. [DOI] [PubMed]
- 20.Voogt MJ, De Vries J, Fonteijn W, Lohle PNM, Boekkooi PF. Sexual functioning and psychological well-being after uterine artery embolization in women with symptomatic uterine fibroids. Fertil Steril. 2009;92:756–761. doi: 10.1016/j.fertnstert.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Li Y, Wang Z, McCulloch P, Hu L, Chen W, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG England. 2018;125:354–364. doi: 10.1111/1471-0528.14689. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev United States. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 23.American Psychological Association. Distress. In APA Dictionary of Psychology. https://dictionary.apa.org/distress. Accessed 20 Mar 2022.
- 24.Vines AI, Ta M, Esserman DA. The association between self-reported major life events and the presence of uterine fibroids. women’s heal issues. Jacobs Institute Women’s Health. 2010;20:294–8. doi: 10.1016/j.whi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen T-C, Yang C-Y, Huang Y-J, Lin C-L, Sung F-C. Risk of depression in patients with uterine leiomyoma: a nationwide population-based cohort study. J Affect Disord Netherlands. 2017;213:126–130. doi: 10.1016/j.jad.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Vannuccini S, Rossi E, Cassioli E, Cirone D, Castellini G, Ricca V, et al. Menstrual Distress Questionnaire (MEDI-Q): a new tool to assess menstruation-related distress. Reprod Biomed Online. 2021;43:1107. doi: 10.1016/j.rbmo.2021.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed]
- 28.Mondo M, Sechi C, Cabras C. Psychometric evaluation of three versions of the Italian Perceived Stress Scale. Curr Psychol Current Psychol. 2021;40:1884–1892. doi: 10.1007/s12144-019-0132-8. [DOI] [Google Scholar]
- 29.Chiuve SE, Huisingh C, Petruski-Ivleva N, Owens C, Kuohung W, Wise LA. Uterine fibroids and incidence of depression, anxiety and self-directed violence: a cohort study. J Epidemiol Community Health. 2022;76:92–9. [DOI] [PMC free article] [PubMed]
- 30.Williams VSL, Jones G, Mauskopf J, Spalding J, Duchane J. Uterine fibroids: a review of health-related quality of life assessment. J Women’s Heal. 2006;15:818–29. [DOI] [PubMed]
- 31.Marsh EE, Al-Hendy A, Kappus D, Galitsky A, Stewart EA, Kerolous M. Burden, prevalence, and treatment of uterine fibroids: a survey of U.S. women. J Women’s Heal. 2018;27:1359–67. [DOI] [PMC free article] [PubMed]
- 32.Ghant MS, Sengoba KS, Recht H, Cameron KA, Lawson AK, Marsh EE. Beyond the physical: a qualitative assessment of the burden of symptomatic uterine fibroids on women’s emotional and psychosocial health. J Psychosom Res. 2015;78:499–503. [DOI] [PubMed]
- 33.Spies J, Coyne K, Guaou Guaou N, Boyle D, Skynarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290–300. [DOI] [PubMed]
- 34.He Y, Zeng Q, Dong S, Qin L, Li G, Wang P. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: a case-control study in China. Asia Pac J Clin Nutr Australia. 2013;22:109–117. doi: 10.6133/apjcn.2013.22.1.07. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y, Xu Q, Xu J, Ren ML, Cai YL. Environmental exposure and risk of uterine leiomyoma: an epidemiologic survey. Eur Rev Med Pharmacol Sci Italy. 2013;17:3249–3256. [PubMed] [Google Scholar]
- 36.Wise LA, Palmer JR, Cozier YC, Hunt MO, Stewart EA, Rosenberg L. Perceived racial discrimination and risk of uterine leiomyomata. Epidemiology. 2007;18:747–757. doi: 10.1097/EDE.0b013e3181567e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wise LA, Palmer JR, Rosenberg L. Lifetime abuse victimization and risk of uterine leiomyomata in black women. Am J Obstet Gynecol. 2013;208:272.e1–272.e13. doi: 10.1016/j.ajog.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin H, Lin Z, Vásquez E, Xu L. The association between chronic psychological stress and uterine fibroids risk : a meta-analysis of observational studies. Stress Health. 2019;35:585–94. 10.1002/smi.2895. [DOI] [PubMed]
- 39.Xia T, Li S, Ma R, Guan S, Li J, Li H, et al. Taiwanese Journal of Obstetrics & Gynecology Effects of liver depression and psychological stress on human uterine leiomyoma cells by an AR e cAMP e PKA signal transduction pathway. Taiwan J Obstet Gynecol Elsevier Ltd. 2017;56:291–301. doi: 10.1016/j.tjog.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciebiera M, Włodarczyk M, Zgliczyńska M, Łukaszuk K, Męczekalski B, Kobierzycki C, et al. The role of tumor necrosis factor α in the biology of uterine fibroids and the related symptoms. Int J Mol Sci. 2018;19:3869. doi: 10.3390/ijms19123869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciebiera M, Włodarczyk M, Wrzosek M, Męczekalski B, Nowicka G, Łukaszuk K, et al. Role of transforming growth factor β in uterine fibroid biology. Int J Mol Sci. 2017;18:2435. doi: 10.3390/ijms18112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kocaoz S, Cirpan R, Degirmencioglu AZ. The prevalence and impacts heavy menstrual bleeding on anemia, fatigue and quality of life in women of reproductive age. Pakistan J Med Sci. 2019;35:365–370. doi: 10.12669/pjms.35.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pron G, Cohen M, Soucie J, Garvin G, Vanderburgh L, Bell S. The Ontario Uterine Fibroid Embolization Trial. Part 1. Baseline patient characteristics, fibroid burden, and impact on life. Fertil Steril United States. 2003;79:112–9. doi: 10.1016/S0015-0282(02)04539-9. [DOI] [PubMed] [Google Scholar]
- 45.Spies JB, Warren EH, Mathias SD, Walsh SM, Roth AR, Pentecost MJ. Uterine fibroid embolization: measurement of health-related quality of life before and after therapy. J Vasc Interv Radiol United States. 1999;10:1293–1303. doi: 10.1016/S1051-0443(99)70235-6. [DOI] [PubMed] [Google Scholar]
- 46.Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol United States. 1999;93:915–921. doi: 10.1016/s0029-7844(98)00541-9. [DOI] [PubMed] [Google Scholar]
- 47.Musallam KM, Taher AT. Iron deficiency beyond erythropoiesis: should we be concerned? Curr Med Res Opin. 2018;34:81–93. doi: 10.1080/03007995.2017.1394833. [DOI] [PubMed] [Google Scholar]
- 48.Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3:263–275. doi: 10.1002/jha2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.